21 Interventions for Failing Hemodialysis Access
Hemodialysis Access Anatomy
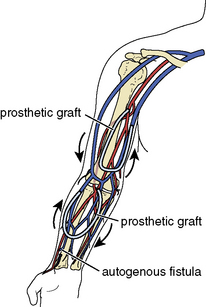
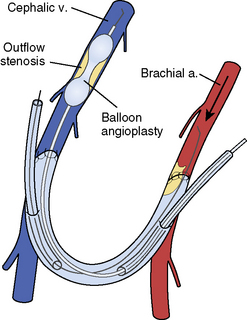
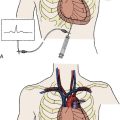
An autogenous arteriovenous access is surgically created by directly anastomosing a native outflow vein (Fig. 21-1) to a native inflow artery (Fig. 21-2), usually in the form of an end-to-side anastomosis. A prosthetic arteriovenous access is constructed by surgically interposing a segment of polytetrafluoroethylene (PTFE) between a native artery and a native vein in either a straight or looped configuration. Common patterns include the brachial-cephalic configuration in the forearm or the brachial-basilic configuration in the upper arm (Fig. 21-3). For the purposes of this chapter, an autogenous arteriovenous access will be referred to as a fistula, a prosthetic arteriovenous access as a graft, and when mentioned together, both types will be referred to as accesses.
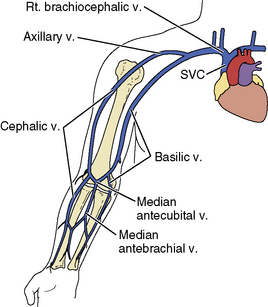
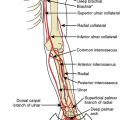
Figure 21-1 Venous anatomy of the upper extremity. Rt, right; SVC, superior vena cava; v, vein.
(Reprinted from Bittl JA. Catheter interventions for hemodialysis fistulas and grafts. JACC Cardiovasc Interv 2010;3:1–11, with permission from Elsevier.)
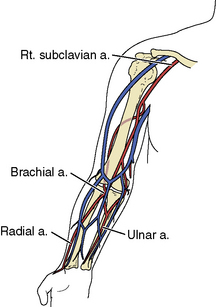
Figure 21-2 Pertinent arterial anatomy of the upper extremity. a, artery; Rt., right.
(Reprinted from Bittl JA. Catheter interventions for hemodialysis fistulas and grafts. JACC Cardiovasc Interv 2010;3:1–11, with permission from Elsevier.)
Mechanisms of Hemodialysis Access Failure
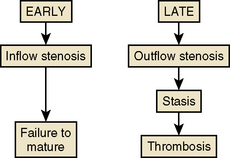
Two failure modes of fistulas and grafts are amenable to interventional treatment (Fig. 21-4). An inflow stenosis in newly placed fistulas may inhibit physiologic hypertrophy and maturation. An outflow stenosis in chronically used fistulas and grafts may cause high pressures and thrombosis. Although 50% of malfunctioning accesses ultimately undergo thrombosis, this is not the primary cause of failure. Instead, shear stress and fibromuscular hyperplasia of the outflow vein causes a progressively worsening stenosis, which then leads to stasis and eventual thrombosis (see Fig. 21-4).
Procedures
Thrombosed Accesses
A four-step procedure is recommended.
1. Thrombectomy
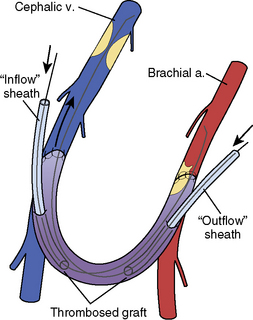
Two 6F sheaths are placed within the access: one in the direction of the venous outflow and one in the direction of the arterial inflow (Fig. 21-5). Sheath insertion is carried out by inserting 18-gauge needles into the occluded fistula or graft near the usual entry sites, which are identified by needle tracks and induration. As the thrombosed access is entered, a “pop” may be felt as the needle penetrates the dura. Although no flashback is seen and no blood can be aspirated from a thrombosed access, intravascular entry is confirmed by smooth guidewire advancement under fluoroscopy. It is important to avoid puncturing the back wall of the graft, because an extrinsic hematoma may cause extrinsic compression. It is important to avoid injecting contrast into a thrombosed access, because thrombus may dislodge and embolize. An alternative approach is to begin with a 4F micropuncture set (Cook, Bloomington, IN).
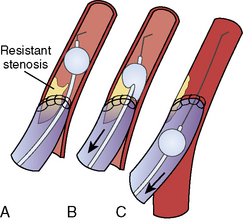
Although the method is called the “cross-sheath” technique, the tips of the sheaths face each other but do not actually overlap (Fig. 21-5). Two 150-cm 0.018-inch V-18 hydrophilic control wires (Boston Scientific Medi-Tech, Miami, FL) are advanced through the sheaths under fluoroscopic guidance without contrast injections, one in the outflow direction and one in the inflow direction. If it is difficult to identify or advance the wire beyond the outflow stenosis or to enter the inflow artery, a 65-cm 5F multipurpose A1 catheter (Cordis, Miami Lakes, FL) can be inserted for additional maneuverability. The resistant stenosis can usually be penetrated with a 0.035-inch hydrophilic curved wire and replaced with a 0.018-inch guidewire for thrombectomy.
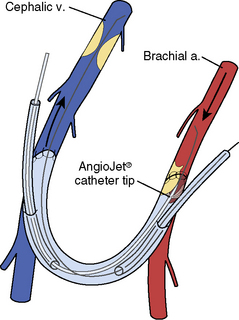
Thrombectomy is carried out first in the outflow direction (Fig. 21-6) and then in the inflow direction (Fig. 21-7). When the blunt edge of the opposing sheath blocks advancement of the rheolytic thrombectomy catheter, transient insertion of the sheath dilator in the opposing sheath may present a smoother transition for passage of the thrombectomy catheter.
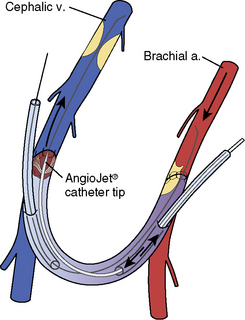
Figure 21-6 Rheolytic thrombectomy of venous outflow. a, artery; v, vein.
(Reprinted from Bittl JA. Catheter interventions for hemodialysis fistulas and grafts. JACC Cardiovasc Interv 2010;3:1–11, with permission from Elsevier.)
2. Angioplasty
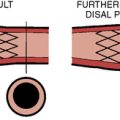
Venous angioplasty of the culprit outflow stenosis entails the use of 4- to 10-mm balloons (Fig. 21-8). The venous stenoses tend to be fibrotic, may be resistant to dilatation, and occasionally require pressures >20 atm. High-pressure, noncompliant balloons with rated burst pressures of 20 to 24 atm can be used (Conquest or Dorado, Bard Peripheral Vascular, Tempe, AZ). Cutting balloons can be used when high-pressure balloons are unsuccessful (Boston Scientific), but the use of peripheral cutting balloons in one study was associated with an increased risk of rupture. Stents are usually reserved for severe recoil, venous perforations, or stenoses in surgically inaccessible veins, but the use of stent grafts will likely increase in an effort to reduce restenosis.
3. Fogarty Thrombectomy
Fogarty thrombectomy using an over-the-wire 4F Thru-Lumen Embolectomy Catheter (Edwards Lifesciences, Irvine, CA) is recommended in almost all cases to extract resistant thrombus at the arterial inflow (Fig. 21-9). The catheter must be withdrawn forcefully to dislodge the resistant thrombus. Persistent thrombosis is most successfully treated with repeat Fogarty thrombectomy of the inflow. If this fails and the access continues to have low pressure, balloon angioplasty of the arterial inflow anastomosis with a 6-mm dilatation catheter can be tried. An approach of last resort is to perform balloon angioplasty along the entire length of the access.
Beathard G.A. Angioplasty for arteriovenous grafts and fistulae. Semin Nephrol. 2002;22:202–210.
Beathard G.A. Management of complications of endovascular dialysis access procedures. Semin Dial. 2003;16:309–313.
Beathard G.A., Arnold P., Jackson J., et al. Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487. 1484
Beathard G.A., Litchfield T. Effectiveness and safety of dialysis vascular access procedures performed by interventional nephrologists. Kidney Int. 2004;66:1622–1632.
Bittl J.A. Venous rupture during percutaneous treatment of hemodialysis fistulas and grafts. Catheter Cardiovasc Interv. 2009;74:1097–1101.
Bittl J.A. Catheter interventions for hemodialysis fistulas and grafts. JACC Cardiovasc Interv. 2010;3:1–11.
Bittl J.A., Feldman R.L. Cutting balloon angioplasty for undilatable venous stenoses causing dialysis graft failure. Catheter Cardiovasc Interv. 2003;58:524–526.
Bittl J.A., Feldman R.L. Prospective assessment of hemodialysis access patency after percutaneous intervention: Cox proportional hazards analysis. Catheter Cardiovasc Interv. 2005;66:309–315.
Collins A.J., Foley R.N., Herzog C., et al. Excerpts from the United States Renal Data System 2007 annual data report. Am J Kidney Dis. 2008;51(suppl 1):S1–S320.
Haskal Z.J., Trerotola S., Dolmatch B., et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med. 2010;362:494–503.
NKF-K/DOQI clinical practice guidelines for vascular access. update 2000. Am J Kidney Dis. 2001;37:S137–S181.
Sidawy A.N., Gray R., Besarab A., et al. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35:603–610.
US Renal Data System. USRDS 2004 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2005;45(suppl 1):S1–S280.
Valji K. Transcatheter treatment of thrombosed hemodialysis access grafts. AJR Am J Roentgenol. 1995;164:823–829.
Weiswasser J.M., Sidawy A.N. Strategies of arteriovenous dialysis access. In: Rutherford R.B., ed. Vascular surgery. Philadelphia: Saunders; 2005:1669–1676.