CHAPTER 22 INTERVENTIONAL RADIOLOGY: DIAGNOSTICS AND THERAPEUTICS
Radiology has always been an important component of a Level I trauma center. This relationship has increased over the last two decades such that it is almost impossible to conceive of caring for a trauma patient without the ability to perform trauma imaging. Interventional radiology techniques, including angiography, angioembolization, and stent placement, have evolved from infrequently used adjuncts in the care of trauma patients into pivotal adjuncts in the nonoperative management of solid organ injury and hemorrhage associated with pelvic trauma. Historically, these techniques have only been available in a dedicated angiographic suite that was physically separate from the resuscitation area and operating room. This required that patients were hemodynamically normal so that they could tolerate the transportation to the angiographic suite. In addition, commitment to availability 24 hours a day from angiographic technologists and staff was necessary to ensure that these techniques would be available. This distinction among resuscitation area, operating room, and angiographic suite has been gradually dissolving over the past decade. Many centers have built angiographic suites into or next to their emergency department so that the risk of transportation has been decreased. In addition, the development of better radiolucent operating room tables and portable fluoroscopy machines with digital subtraction capabilities has enabled some interventional radiology techniques to be performed in the operating room. Several institutions have built endovascular suites in their operating room suites for the performance of endovascular techniques by vascular surgeons. This ever increasing fusion of resuscitation area, operating room, and angiographic suite has made interventional radiology techniques available to more trauma patients than ever before.
BLUNT CEREBROVASCULAR INJURY
Several screening triggers have been suggested in the literature including cervical spine fracture, neurologic findings not explained by radiographic findings, Horner’s syndrome, LeFort II or III facial fractures, skull base fractures involving the foramen lacerum, and neck soft tissue injury. A good screening test should be relatively inexpensive, have a low morbidity rate, and a high sensitivity rate. It should find all the true positive results with some false positives and no false negatives. In a comparison of magnetic resonance angiography (MRA), computed tomographic angiography (CTA), and four-vessel cerebral angiography between 2000 and 2002, the sensitivity of MRA and CTA for BCVI was 47%–53%. These rates are too low for a test to be an effective screening modality. Four-vessel cerebral angiography has been identified as the gold standard for the diagnosis of BCVI. However, its cost and major complication rate of 1%–3% in large series make it a less than ideal screening test. The development of the multidetector CT scanner has increased the resolution of the CT scanner. Two recent studies demonstrated that CTA performed on multidetector CT scanners has dramatically improved ability to diagnose these injuries. A head-to-head comparison of CTA with multidetector CT scanners and four-vessel cerebral angiography has yet to be done. As a result, four-vessel cerebral angiography remains the gold standard for diagnosis and screening of these injuries. It will be important to continue to monitor improvements in CTA, MRA, and possibly even ultrasound technologies for less invasive, cheaper, and safer screening modalities for BCVI.
THORACIC INJURY
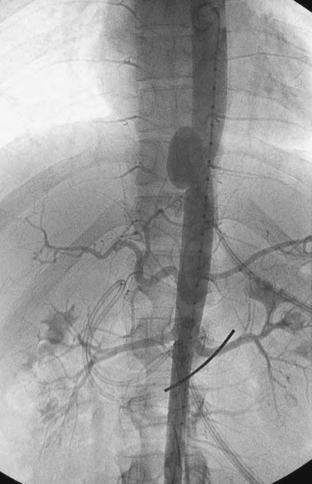
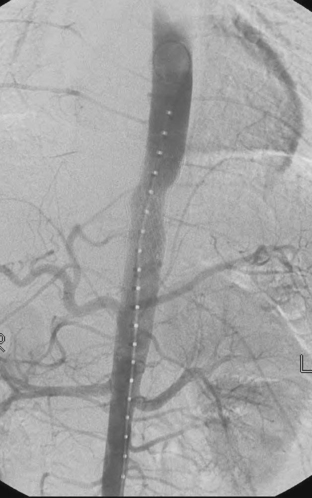
The management of BAI has been changing over the last several years. If the patient is hemodynamically normal, the patient is admitted to the ICU and conservatively managed until the associated injuries are resolved. Conservative management includes the use of beta-blockers and vasodilators to prevent tachycardia and hypertension. Several studies have demonstrated a decreased rate of in-hospital mortality and paraplegia with delayed repair of BAI. Endovascular stents have been used to repair BAI in both the emergent and delayed settings. The reported cases have all demonstrated few acute complications and 30-day mortality rates below 15%. While these results are encouraging, long-term results are unknown. One long-term study on endovascular repair of aortic aneurysms demonstrated a 65% endoleak rate, 35% graft migration, and 78% graft deformation. In fairness, this study involved some of the first generation of endovascular stents, and hopefully, the results for newer generation of stents will be better. Figure 1 demonstrates a traumatic pseudoaneurysm of the descending aorta. Figure 2 demonstrates the completion aortogram after placement of an endovascular stent. The first commercially available endovascular stents for the thoracic aorta only became available in the United States in 2005. There is currently a prospective analysis of the use of these grafts in the management of BAI being conducted by the American Association for the Surgery of Trauma (AAST). The ultimate success of these devices will be based on their long-term performance. It is important to remember that the average age of a trauma patient is much lower than that of patients undergoing aortic aneurysm or dissection repair. These grafts may need to last 40–60 years when placed in a young trauma patient. These results are simply unknown at this time.
ABDOMINAL TRAUMA
The role of angioembolization in the management of blunt hepatic trauma has developed over the last 25 years as well. Initially, angioembolization was reported as being useful in the management of hemobilia after blunt hepatic trauma and iatrogenic injury. These case reports and small series demonstrated that angioembolization of the liver could be used to successfully manage hemobilia. The operative management of hepatic trauma, whether the mechanism is blunt or penetrating, has evolved over last 15 years as well. Historically, the operative mortality for grade IV and grade V liver injuries has been reported as between 50% and 80%. About 10 years ago, the concept of damage control laparotomy was developed to try and avoid the “triad of death”: hypothermia, acidosis, and coagulopathy. This concept revolves around minimizing the length of the initial laparotomy in severely injured patients so that they arrive at the ICU for further resuscitation as soon as possible. This technique involves packing liver injuries rather than performing extensive operative maneuvers to gain hemostasis, repairing vascular injuries, and resecting bowel injuries, but leaving the bowel in discontinuity and temporary abdominal closure. This operative strategy has been reported to lower the operative mortality rate of grades IV and V liver injuries to 25%–40%. Angioembolization has been successfully used as an adjunct to damage control laparotomy to further decrease mortality and achieve hemostasis. Today, most authors would recommend a damage control laparotomy with packing of the injured liver for severe (AAST grade IV and V) hepatic trauma with angioembolization as an adjunct to further achieve hemostasis rather than extensive operative attempts to gain hemostasis. Angioembolization is also used as an adjunct to the nonoperative management of blunt hepatic injuries. Those patients who are seen to have active contrast extravasation from a liver injury on CT scan can undergo angioembolization to control their hemorrhage. This approach has improved the success rate of nonoperative management of blunt hepatic trauma to over 85%. The complications of hepatic embolization include hepatic necrosis, hepatic abscess, and bile leaks. If the patient has an intact portal vein with hepatopetal flow, the risk of hepatic necrosis is markedly reduced. For victims of both penetrating and blunt hepatic trauma, angioembolization can be a very useful adjunct to control the hemorrhage associated with significant liver trauma regardless of whether the patient is managed operatively or nonoperatively.
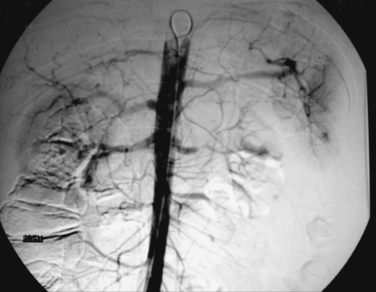
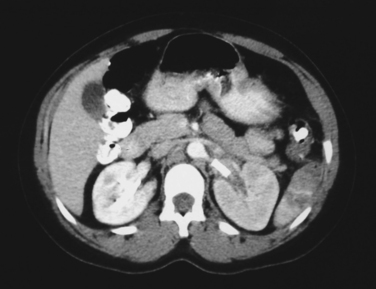
Angioembolization and endovascular stents have also been used in the management of renal trauma. Blunt renal injuries with evidence of active contrast extravasation on CT scan have been managed with arteriography and embolization of associated renal artery branches to achieve hemostasis. Endovascular stents have been used to manage both blunt and penetrating injuries to the renal arteries. The kidney is very sensitive to warm ischemia and that restoration of blood flow to the kidney needs to occur within the first 4–6 hours after injury. Due to time spent in transportation from the scene, resuscitation and evaluation in the trauma room and possibly CT scan, it is often 2–3 hours after injury that the diagnosis of a renal artery injury is made. As a result, the operative kidney salvage rate for exploration of the renal artery has been reported to be 5%–10%. This rate is so low that some authors do not recommend attempting to revascularize a unilateral blunt renal artery occlusion. There have been several small series and case reports of using endovascular stents in blunt renal artery injuries, which suggest that the kidney salvage rate for this approach may be as high as 25%. It is important to remember that all of the patients in these studies were hemodynamically normal and did not require operative exploration for associated injuries. Figure 3 demonstrates a blunt dissection of the left renal artery after a motor vehicle collision. Figure 4 is a CT scan after placement of 17 mm × 6 mm renal artery stent with restoration of perfusion to the left kidney. Further study of the use of endovascular stents in the management of both blunt and penetrating renal artery injuries is clearly warranted.
EXTREMITY TRAUMA
Arteriography of the extremity, whether intraoperative or in the interventional radiology suite, can be used to evaluate the injured extremity. In penetrating trauma patients who have “hard” signs of vascular injury, which includes expanding hematoma, pulse discrepancy, and pulsatile bleeding, usually surgical exploration is all that is performed. However, for patients with multiple penetrating wounds to the same extremity, it may be desirable to perform arteriography to more precisely locate the injury. In blunt trauma patients with posterior knee dislocations, floating knees (distal femur fracture with tibial plateau fracture), supracondylar knee fractures and tibial plateau fractures, there is an increased incidence of blunt popliteal injury. If there is a pulse discrepancy in an extremity with one of these injuries, arteriography is usually performed prior to or at the time of operative exploration to confirm the diagnosis of blunt popliteal injury. This assumes that obtaining the arteriogram will not significantly delay appropriate treatment of the vascular injury. Duplex scanning is increasing in specificity and sensitivity for these injuries but the gold standard remains arteriography. Development of the multidetector CT scanner and resultant ability to perform CTA may ultimately replace traditional catheter arteriography.
Andrassy J, et al. Stent versus open surgery for acute and chronic traumatic injury of the thoracic aorta: a single-center experience. J Trauma. 2006;60:765.
Asensio JA, et al. Approach to the management of complex hepatic injuries. J Trauma. 2000;48:66.
Biffl WL, et al. The unrecognized epidemic of blunt carotid arterial injuries—early diagnosis improves neurologic outcome. Ann Surg. 1998;228:462.
Biffl WL, et al. The devastating potential of blunt vertebral artery injuries. Ann Surg. 2000;231:672.
Castelli P, et al. Endovascular repair of traumatic injuries of the subclavian and axillary arteries. Injury. 2005;36:778.
Cooney R, et al. Limitations of splenic angioembolization in treating blunt splenic injury. J Trauma. 2005;59:926.
Davis KA, et al. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J Trauma. 1998;44:1008.
Dent D, et al. Blunt splenic injuries-high nonoperative management rate can be achieved with selective embolization. J Trauma. 2004;56:1063.
Fabian TC, et al. Blunt carotid injury—importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223:513.
Haan JM, et al. Splenic embolization revisited: a multicenter review. J Trauma. 2004;56:542.
Johnson JW, et al. Hepatic angiography in patients undergoing damage control laparotomy. J Trauma. 2002;52:1102.
Leurs LJ, et al. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom thoracic endograft registries. J Vasc Surg. 2004;40:670.
Martin MJ, et al. Functional outcome after blunt and penetrating carotid artery injuries—analysis of the national trauma data bank. J Trauma. 2005;59:860.
Miller PR, et al. Blunt cerebrovascular injuries—diagnosis and treatment. J Trauma. 2001;51:279.
Miller PR, et al. Prospective screening for blunt cerebrovascular injuries—analysis of diagnostic modalities and outcomes. Ann Surg. 2002;226:386.
Pacini D, et al. Traumatic rupture of the thoracic aorta—ten years of delayed management. J Thorac Cardiovasc Surg. 2005;129:880.
Shapiro M, et al. The role of repeat angiography in the management of pelvic fractures. J Trauma. 2005;58:227.
Wahl WL, et al. The need for early angiographic embolization in blunt liver injuries. J Trauma. 2002;52:1097.
Wheatley GHIII, et al. Midterm outcome in 158 consecutive Gore TAG thoracic endoprostheses: single center experience. Ann Thorac Surg. 2006;81:1570.
Wong YC, et al. Mortality after successful transcatheter arterial embolization in patients with unstable pelvic fractures: rate of blood transfusion as a predictive factor. J Trauma. 2000;49:71.