CHAPTER 136 Interventional Neuroradiology of the Skull Base, Head, and Neck
Materials and Techniques
Angiographic Equipment
It is essential during endovascular interventional procedures to define the blood supply of a lesion with the highest possible resolution in all projections. Arteries or their collateral channels act as conduits for embolic material to the lesion. Of equal importance is the visualization of the many dangerous collateral vessels leading to the intracranial circulation or to the blood supply of the cranial nerves. Superb visualization and an excellent knowledge of the vascular anatomy are of paramount importance, as is an understanding of the potential clinical symptoms from embolization into an unwanted vascular territory and occlusion of the blood supply to normal tissues.1
Embolic Agents
Two commonly used particulate materials are Gelfoam (Upjohn Pharmaceuticals, Kalamazoo, MI) and polyvinyl alcohol (PVA) (PVA Foam, Cook, Inc., Bloomington, IN; Trufill, Cordis Endovascular Systems, Miami Lakes, FL; Ivalon, Inc., San Diego, CA; Contour Emboli, Boston Scientific/Target Therapeutics, Inc., Fremont, CA).2,3 Gelfoam breaks down 72 hours after embolization, and this lack of permanence detracts from its efficacy if surgery is not performed within a few days after the procedure. Gelfoam has been used as a preoperative embolization material for neoplasms that are to be operated on within 48 hours, and for patients with epistaxis in whom the goal is to slow the bleeding sufficiently so that the body’s normal hemostatic mechanisms stop the hemorrhage. Gelfoam powder always should be used with care because its particles (approximately 50 µm) in solution act as a liquid, easily passing through tiny arteries, which may result in skin necrosis or damage to cranial nerves, or through collateral channels communicating with the intracranial circulation.
PVA is more permanent than Gelfoam, but much of the efficacy of the vascular occlusion is a result of a combination of PVA plus intravascular thrombus. The stellate-shaped particles slow the intravascular flow, and thrombus forms.2–4 This thrombus may be metabolized before fibrosis occurs, however, resulting in partial or complete recanalization over weeks to months. PVA is easy to use, being supplied as uniform particles within a narrow range of size (150 to 1250 µm). In most patients with neoplasms, the smallest size (150 µm) is used because the particles can be easily injected through a small microcatheter placed selectively into tiny feeding arteries and penetrate into the tumoral vascular bed.
Because of the stellate shape of PVA particles, they do not form a tightly packed embolic mass, allowing recanalization as the interspersed thrombus undergoes lysis. Various types of beads and spheres have been produced to overcome that limitation, such as Embosphere Clear (Biosphere Medical, Inc., Rockland, MA). Their more uniform size and spherical geometry theoretically produce more complete and more permanent vascular occlusion. Similar spheres might also be filled with a chemotherapeutic or other agent for more prolonged treatment of a nonsurgical neoplasm. Microfibrillary collagen (Avitene; Avicon, Inc., Fort Worth, TX) is a hemostatic agent that may be mixed with contrast material for embolization5 or mixed with other embolic agents such as PVA and ethanol.6
Detachable balloons with a valve mechanism to keep the balloon inflated are currently unavailable in the United States, but should be available again in late 2009. They are used primarily for fistulas with a single artery-to-vein connection. Most experience was with intracranial post-traumatic carotid-cavernous sinus fistulas, but this technique was also used for vertebral artery–vertebral venous fistulas (usually post-traumatic) or for any other type of fistula in the face or neck.7–9 They were also used for occlusion of a parent artery leading to an unclippable aneurysm, a dissected carotid or vertebral artery producing embolization into intracranial vessels, or a carotid artery to be sacrificed at tumor surgery. Electrolytically detachable coils are used now instead of the currently unavailable detachable balloons. Many coils are usually necessary, however, to do what one balloon could accomplish, increasing the complexity and expense of the procedure.
There are numerous liquid embolic agents, the most commonly used being absolute alcohol (100% ethanol) and various tissue adhesives, including the cyanoacrylates and Onyx (MicroTherapeutics, Inc., Irvine, CA). Absolute ethanol is extremely toxic to the endothelium,10 and is highly effective at producing sclerosis of vascular lesions, such as venous and lymphatic malformations.11–13 Although it has also been used to treat arteriovenous malformations and dural arteriovenous fistulas, the problem with these fast-flowing lesions is the need to increase the “dwell time” of the ethanol to interact with the intima, often requiring temporary balloon occlusion more proximally. It has also been used for tumors, via endovascular and percutaneous access, particularly recurrent tumors that are surgically inaccessible.
Cyanoacrylates such as isobutyl-2-cyanoacrylate (IBCA) or N-butyl-2-cyanoacrylate (NBCA) produce polymerization of rapidly flowing blood within seconds. They not only produce immediate thrombosis and have tissue adhesive properties, but they also initiate a giant cell inflammatory reaction of the vessel wall.14 These embolic liquids are excellent for lesions with rapidly flowing blood, such as an arteriovenous malformation or an arteriovenous fistula. Neoplasms have slow flow, so there is no need to use these agents, which are associated with more risk than particulate materials injected intra-arterially or other agents injected percutaneously.
The newest “liquid” agent is Onyx, which is an ethylene vinyl alcohol copolymer containing dimethyl sulfoxide as the agent facilitating absorption through endothelial barriers.15 This tissue adhesive is a needed new addition to the armamentarium of the neurointerventionalist because it slowly permeates into tiny vessels feeding a vascular lesion, “creeping” into these feeders during fluoroscopic visualization and control, without the rapid setup time of the cyanoacrylates.15,16
Any embolization procedure with a liquid agent should be approached with trepidation because occlusion of the end-arteries to the face, tongue, and cranial nerves may lead to necrosis, and intracranial embolization may occur as the liquid passes through tiny collateral channels to vessels feeding normal structures. Liquid embolization should be performed only when superselective catheterization can be done directly into the direct feeders to the lesion, or with percutaneous puncture of the lesion and direct visualization of the flow of the agent,17 to prevent unwanted extension to the blood supply of vital structures.
Provocative Testing to Ensure the Safety of Embolization
Two important techniques help to ensure the safety of vascular occlusion at the skull base. The first involves the injection of lidocaine (Xylocaine) 1% without preservatives into an arterial feeder that is considered a candidate for embolization, to predict the potential of permanent cranial nerve palsy from the embolization procedure.18 This provocative test anesthetizes the cranial nerve if there is blood supply leading to it from the vessel catheterized. Critics of this test suggest that a false-positive test may occur because a liquid anesthetic can be injected into the capillary bed, whereas particles used for embolization stop short of terminal arterioles so that devascularization is rare. It is likely that a negative test result is truly negative and reassuring.
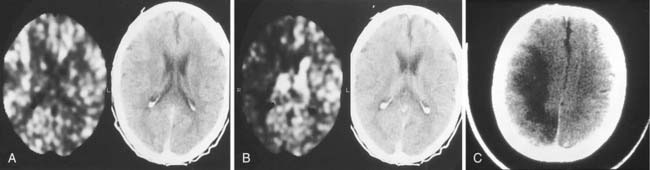
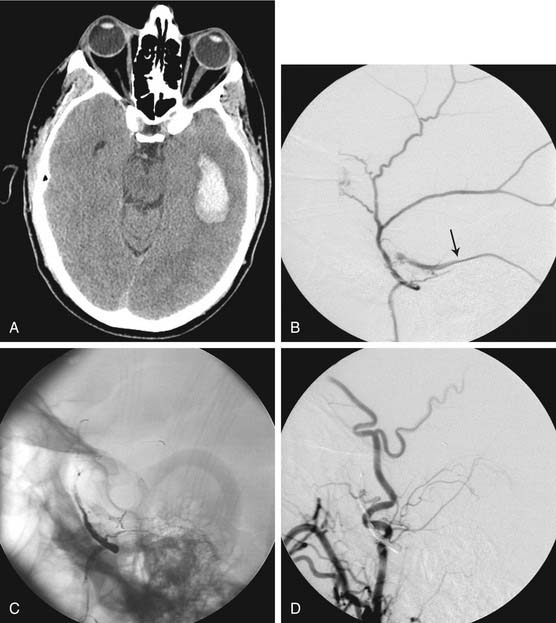
Different methods have been used during temporary occlusion to evaluate the physiologic effects of the BOT on cerebral blood flow to predict the risk of cerebral infarction after definitive occlusion. These methods include electric studies such as evoked potentials or electroencephalography,19 measurements of arterial stump pressures distal to the site of temporary occlusion,20 induced hypotensive challenges, and transcranial Doppler studies. Also, several cerebral blood flow imaging methods, including xenon-enhanced CT (Fig. 136-1),21 single photon emission computed tomography (SPECT), positron emission tomography, and MRI and CT perfusion studies, have been used to evaluate cerebral blood flow during BOT to determine the potential risk of ischemia after permanent occlusion.22,23 We prefer to use SPECT because it adds little to the basic BOT and easily provides the needed information. After the balloon has been inflated for a short time, the radionuclide is injected intravenously, and the patient is scanned a few hours after the completion of the angiographic study (the radiopharmaceutical “sticks” to the brain tissue during its initial circulation in proportion to the blood flow to that tissue).
Endovascular Treatment of Neoplasms
Paragangliomas
Paragangliomas, also known as chemodectomas or glomus tumors, are neoplasms related to chemoreceptor tissue. They usually are benign, but locally invasive and highly vascularized. Most glomus tumors originate within the temporal bone (48%), including lesions along the promontory of the middle ear (glomus tympanicum tumors) and lesions related to chemoreceptor tissue in the jugular bulb (glomus jugulare tumors). Tumors related to the vagus body (11%) in the high cervical region are called glomus vagale tumors, and tumors related to the carotid body (35%) at the common carotid artery bifurcation in the neck are called carotid body tumors. Multiple tumors are found in approximately 10% of patients (Fig. 136-2), and a familial form exists.24
Glomus Tympanicum Tumor
The glomus tympanicum is a tiny tumor that usually manifests with pulsatile tinnitus and otoscopically is seen as a reddish blue mass behind the tympanic membrane. CT scanning allows the differentiation of this small tumor from an extension into the middle ear cavity of a larger glomus jugulare tumor. It also is necessary to exclude an aberrant ICA. In the former, the bony plate between the jugular foramen and the middle ear is destroyed, whereas in the latter, the posterior bony margin of the carotid canal is missing.25 This tumor is usually small enough for surgery to be performed without embolization.
Glomus Jugulare Tumor
A patient with a glomus jugulare tumor usually presents with dysfunction of cranial nerves IX, X, or XI, and XII if the tumor is large. Pulsatile tinnitus also is common. Contrast-enhanced CT or MRI usually is the first diagnostic test performed, with the diagnosis generally made and the extension of the tumor shown. The tumor begins in the region of the jugular foramen and may extend inferiorly into the upper neck, superolaterally into the middle ear by destroying bone, posteromedially and posterolaterally into the posterior fossa, and anteriorly to envelop the petrous ICA. Bone destruction can be extensive, simulating a malignant tumor, as the chemodectoma spreads through the skull base. Destruction around the foramen magnum may occur, with compression of the brainstem.26
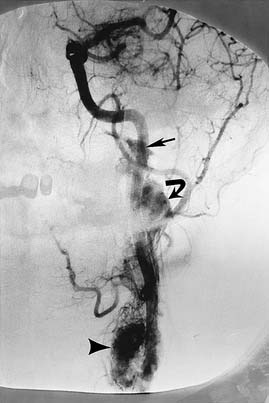
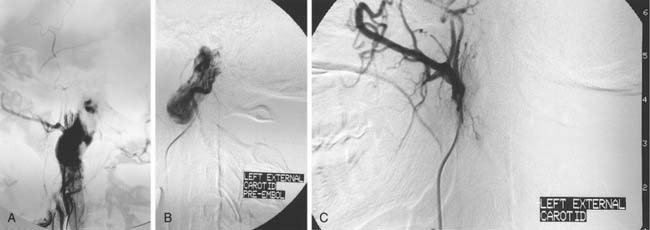
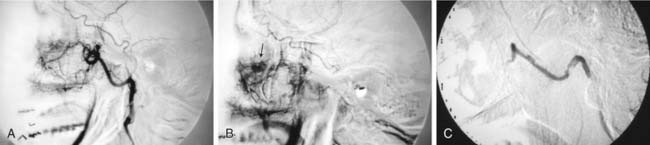
The tumor is fed by multiple external carotid artery (ECA) branches, with each of these arteries feeding a specific compartment of tumor. The ascending pharyngeal (bilaterally at times) and middle meningeal (posterior division) arteries are the most commonly involved, with the stylomastoid branch of the occipital and the posterior auricular arteries providing supply less frequently (Fig. 136-3). If the tumor extends posteriorly around the foramen magnum, supply may be from the anterior and posterior meningeal branches of the vertebral artery. Intradural tumor within the posterior fossa may be fed from the anterior and posterior inferior cerebellar arteries. Tumor surrounding the high cervical or petrous portions of an ICA may parasitize tiny branches of these segments (see Fig. 136-3).27–29
The glomus jugulare tumor is highly vascular, which makes surgery difficult in this confined bony area. Embolization may be of great help.30 Because the diagnosis generally has been made on the basis of CT or MRI before angiography, the patient is prepared with consent forms for angiography and embolization in the same sitting. The arterial feeders are mapped with high-resolution digital subtraction angiography in multiple planes, as previously described (Fig. 136-4). Selective catheterization of the multiple feeders is performed, followed by embolization of particulate material, most commonly PVA of small size (e.g., 150 µm) (see Fig. 136-4). Before injecting external carotid branches that may supply cranial nerve VII, particularly the stylomastoid artery, 1% lidocaine is injected as a provocative test to ensure that the cranial nerve is not devascularized during the embolization procedure, as previously discussed.18
Glomus Vagale Tumor
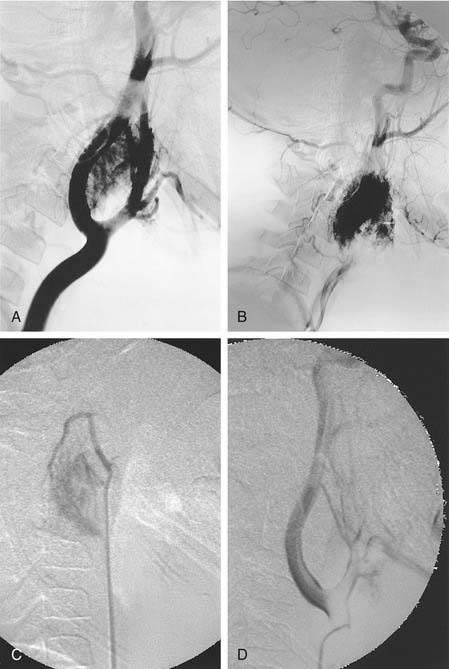
The glomus vagale tumor originates from the vagus body high in the neck at the C1-2 level. The tumor may extend superiorly so that differentiation from a glomus jugulare tumor is difficult and may be moot. The typical appearance is that of anterior displacement of the high cervical ICA. Blood supply is from multiple branches of the ECA, particularly the ascending pharyngeal artery. The arterial supply is mapped as for the glomus jugulare tumor, although there generally are fewer sources of supply (Fig. 136-5). Potential supply from ascending and deep cervical branches of the thyrocervical and costocervical trunks and of neuromuscular branches from the ipsilateral vertebral artery also should be evaluated. The feeding vessels are catheterized and embolized to the point of fluoroscopic devascularization, particularly with tiny PVA particles (see Fig. 136-5). The lidocaine provocative test is used if there is any question regarding potential cranial nerve insult during the procedure.
Carotid Body Tumor
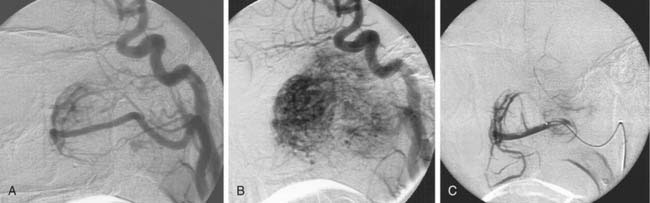
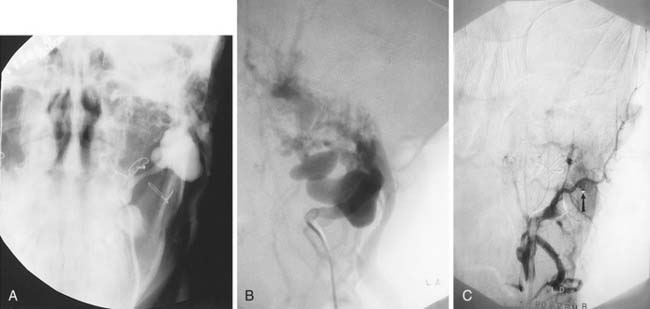
A carotid body tumor is a highly vascular mass occurring in the crotch between the origins of the internal and external carotid arteries, splaying them apart. There are commonly many tiny feeders from the distal common carotid and proximal internal carotid arteries that are too small to be catheterized selectively to the degree necessary to avoid reflux of particles into the ICA, although there usually are major feeders from the ECA, particularly the ascending pharyngeal, superior thyroidal, lingual, facial, and carotid body arteries (Fig. 136-6). These can be selectively catheterized, and much of the tumor may be appropriately embolized with tiny PVA particles to make surgery easier with less blood loss (see Fig. 136-6). Some surgeons do not want preoperative embolization, believing that a potential secondary inflammatory reaction from embolization makes it more difficult to define tumor planes for removal.31
Juvenile Angiofibroma
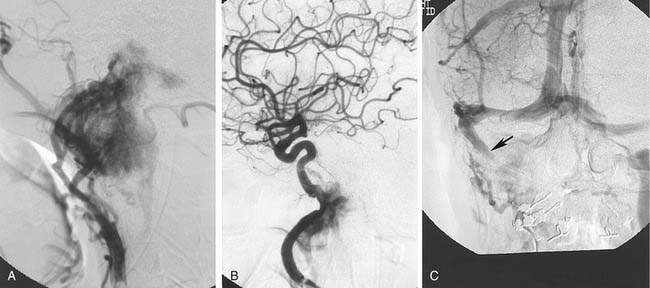
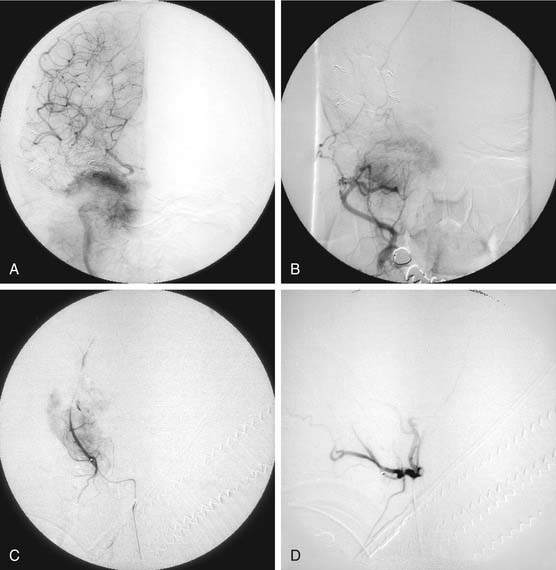
Typically, blood supply is from the ascending pharyngeal artery (which may be bilateral and may require common carotid rather than ECA injections to visualize because of the possible origin of this vessel near the common carotid bifurcation), and the internal maxillary artery and its branches, particularly the ascending and descending palatine arteries (Fig. 136-7). Tumor may remain extracranial and receive supply from collaterals of the petrous and cavernous segments of the ICA.32–34 Intracranial extension may be supplied from the middle meningeal and accessory meningeal branches of the internal maxillary artery, and from branches of the petrous and cavernous internal carotid arteries. Because the middle meningeal, accessory meningeal, and foramen rotundum arteries supply cranial nerves within the cavernous sinuses,33 it is preferable to catheterize the internal maxillary artery beyond the origin of the accessory meningeal artery for particulate material embolization (150 µm) of tumors that are purely extracranial (see Fig. 136-7). Supply from the middle meningeal and accessory meningeal arteries should be embolized with care, usually preceded by a lidocaine test. Other arteries are catheterized and embolized as necessary. Such embolization usually dramatically decreases the blood loss at the time of surgery.32–35
Meningiomas
Whether to embolize a meningioma in a given location depends on the risk-to-benefit ratio of the intervention and the ability to catheterize the appropriate blood supply selectively.36,37 Frontobasal meningiomas involve the orbital roof, the olfactory groove, and the planum sphenoidale. Their blood supply is primarily from ethmoidal branches of the ophthalmic arteries. Because of the potential risk to vision, selective catheterization of an ophthalmic artery and subsequent embolization usually is not performed. Sphenoid wing and middle cranial fossa tumors may derive significant blood supply from the middle meningeal artery. If so, selective embolization of the middle meningeal artery, taking care to place the microcatheter above the origins of the branches supplying the cranial nerves, may be a valuable preoperative intervention, especially because the location of this tumor may make control of the blood supply difficult at surgery.
Cavernous sinus meningiomas have various potential sources of blood supply from branches supplying the dura in this region. The inferolateral trunk from the C4 portion of the ICA frequently represents a major supply to meningiomas in this region, and, if enlarged, may be selectively catheterized with a microcatheter. If catheterization is impossible, a nondetachable balloon may be placed in the ICA above the origins of the dural branches, and embolization with tiny particles performed.38,39 Other sources of blood supply include the accessory meningeal and the middle meningeal arteries. Both of these latter vessels provide blood supply to the cranial nerves, so there is the potential of cranial nerve injury if these vessels are embolized. Provocative testing with the use of 1% lidocaine can be performed, and catheterization beyond the origins of the cranial nerve blood supply.
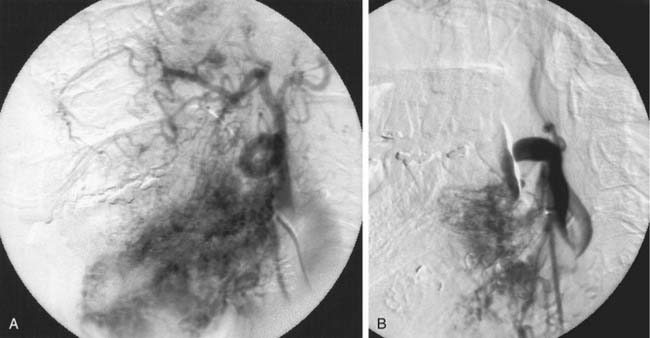
If a microcatheter with an inner diameter of 0.014 inch is used, 150-µm particles can be used to penetrate the meningioma; these particles usually are too large to penetrate the vessels supplying the cranial nerves. Gelfoam powder with particles of 40 to 60 µm behaves more like a liquid, and has a greater potential of producing cranial nerve palsies and passing through dangerous anastomoses. True liquids, such as cyanoacrylates and ethanol, carry too high a risk for cranial nerve injury or passage into anastomotic channels to be used as a preoperative agent. Occasionally, for patients with recurrent, nonoperative tumors requiring shrinkage for palliation of mass effect, absolute ethanol can be used, acknowledging the higher risk of cranial nerve injury (Fig. 136-8). Meningiomas in this region may infiltrate the ICA or middle cerebral artery. If en bloc resection of the tumor, including the ICA, is contemplated, preoperative occlusion of the ICA with a detachable balloon may be performed.39
Intra-arterial Chemotherapy
Endovascular techniques permit the selective perfusion of high concentrations of chemotherapeutic agents directly into a tumor, while minimizing systemic side effects. This technique has been used for advanced primary head and neck tumors, and metastatic tumor to cervical lymph nodes.40 A new delivery technique involves intra-arterial injection of ethylcellulose microspheres containing chemotherapeutic agents. These biodegradable microspheres can remain in the tissue for weeks while they slowly release their anticancer drugs.41
Lesions of Vascular Etiology
Epistaxis
In all patients, bilateral selective internal and external carotid angiography should be performed to visualize the feeders to the bleeding site (Fig. 136-9).42,43 Superselective catheterization of internal maxillary artery, facial, and ascending pharyngeal vessels is performed with a microcatheter for angiography. If a bleeding site is found, all feeders and potential collaterals to the site are embolized by moving the microcatheter distal to branches potentially feeding cranial nerves such as the middle meningeal and accessory meningeal arteries. If no bleeding site is found, distal internal maxillary artery and facial artery embolization is performed bilaterally to ensure hemostasis (see Fig. 136-9).32,44–47 Particles 150 µm provide excellent hemostasis, and there is no risk of devascularizing normal facial tissues. Distal rather than proximal vascular occlusion decreases the chance of recurrent bleeding from collateral circulation. With the development of coaxial microcatheter systems and the heightened awareness of functional facial vascular anatomy, success rates of 91% to 97% with complication rates of 0% to 3% have been reported.46
Facial Arteriovenous Fistulas
Arteriovenous fistulas may occur after direct facial trauma or from iatrogenic trauma such as facial surgery or image-guided biopsy.48 Temporomandibular joint or maxillary sinus surgery may result in an arteriovenous fistula (Fig. 136-10). Arteriovenous fistulas also may be congenital in origin. Whatever the cause, selective angiography defines the site of the fistula, which generally is easy to occlude with an appropriately sized detachable balloon (see Fig. 136-10),7 or with detachable coils. Metallic coils also may be used to pack the venous and arterial sides of a fistula via superselective arterial or venous approaches. To do so, a catheter with a nondetachable balloon is introduced from one femoral artery and placed proximal to the fistula, to be inflated to provide flow control so that the coils do not get swept downstream. A second catheter is introduced via the other femoral artery and placed proximal to the fistula. This second catheter is a guiding catheter with a lumen that accommodates a microcatheter that is manipulated across the fistula into the venous side. Coil occlusion is performed during proximal balloon inflation, progressively pulling the microcatheter back to occlude the arterial side.
Dural Arteriovenous Fistulas
Transverse and sigmoid sinus dural arteriovenous fistulas are acquired lesions, probably resulting after sinus thrombosis and increased venous pressure, which opens tiny arteriovenous shunts in the dura, with or without subsequent sinus recanalization.49 Increased flow in the transverse and sigmoid sinuses produces a subjective bruit. The prolonged high flow may lead to intimal hyperplasia of the sinus wall, stenosis, and recurrent occlusion. Persistent or recurrent occlusion of the transverse and sigmoid sinuses produces increased outflow in the contralateral sinuses. Compromise of this venous drainage produces retrograde flow into cortical venous channels, venous hypertension, and tissue congestion, resulting in neurologic deficits.50 Further venous hypertension may lead to intraparenchymal or subarachnoid hemorrhage.51 The arterial supply to a transverse and sigmoid sinus dural arteriovenous fistula is from multiple ECA branches bilaterally, particularly the occipital arteries and the tentorial branches of the internal carotid arteries. Meningeal branches of the vertebral arteries also may be involved.52
Arterial embolization alone may be only palliative, with multiple other dural sources increasing in size as the primary feeders are occluded. Embolization with particles is particularly discouraging; if only the arterial side is embolized, a tissue adhesive such as Onyx (Fig. 136-11), or alcohol, may be very effective. Another solution is a combination of arterial and venous approaches. After decreasing the arterial flow rate with particle embolization, coils are deposited in the transverse and sigmoid sinuses via a transvenous approach (usually transfemoral venous catheterization of the jugular bulb with a large guiding catheter, through which a smaller catheter is manipulated into the transverse sinus for coil deposition along the length of the transverse and sigmoid sinuses).53 If transfemoral venous access is impossible, intraoperative exposure of the sinus for coil packing may be necessary. Some transverse and sigmoid sinus dural arteriovenous fistulas are complex; recurrences are frequent, and therapy may require the combined expertise of the interventionalist and the surgeon over multiple sessions.
Facial Vascular Malformations
Venous malformations of the face manifest as bluish lesions that may distend with the Valsalva maneuver. Although they are congenital, they usually are dormant until some type of factor, such as local trauma, causes them to enlarge.54,55 MRI studies show lesional hyperintensity on T2-weighted sequences, and the size and the extent of the lesion. Management is for cosmetic purposes. Percutaneous puncture allows the deposition of sclerosing agents. Absolute ethanol is more effective than sodium tetradecyl sulfate, but produces more swelling and is much more painful on injection, requiring deep sedation or anesthesia.10–13
Arteriovenous malformations of the face are rare. If surgery or irradiation of a facial arteriovenous malformation is impossible for cosmetic reasons, embolization may be undertaken as a palliative treatment. In such a case, ethanol can be injected intra-arterially, particularly with flow control to keep the agent in proximity to the endothelium for as long as possible (Fig. 136-12). The major complication is flow of the ethanol into the tiny arteries feeding the skin and cranial nerves, producing necrosis. Even with flow control, the rapid flow in the arteries feeding the malformation usually sweeps the ethanol into the venous system for dilution, preventing such complications.
Hemangiomas of the Face and Neck
Hemangiomas have a proliferative phase followed by involution, so that 90% of hemangiomas that are present at birth regress by early childhood.55 Therapy for infantile or early childhood hemangioma is necessary only if there is airway obstruction, such as from hemangioma of the tongue or subglottic tissues. Even if the hemangioma is in the periorbital region and interferes with normal eye function, time should be allowed for regression, followed by eye muscle and cosmetic surgery. In adolescents or adults, problems may be simply cosmetic, although the hemangioma may infiltrate surrounding tissues such as the alveolar ridge, resulting in hemorrhage when eating, or narrow the airway or esophagus. If there is a slow arterial component, embolization with PVA particles can be performed. If there is primarily a capillary or venous component, direct puncture of the lesion with the instillation of a sclerosing agent, such as absolute ethanol or sodium tetradecyl sulfate, may be effective. In most patients, embolization is followed by surgical removal.
Pseudoaneurysms
Rapid hyperextension of the neck with contralateral head rotation forces the high cervical ICA against the lateral mass of C2, or stretches a vertebral artery at C1-2 between its contained segments within the C2 foramen transversarium and the dura at the foramen magnum,56 resulting in arterial dissection and subsequent pseudoaneurysm formation. Surgical repair may be difficult, necessitating the use of endovascular techniques. Because the pseudoaneurysm lacks a true arterial wall, frequently has a wide neck, and may contain semisoft clot of variable age, successful occlusion with intra-aneurysmal balloons or coils usually is impossible. The endovascular placement of a stent across the neck of a pseudoaneurysm is the preferred method of management; the combination of stenting and intra-aneurysmal coil placement may be even more successful. If necessary, occlusion of the parent artery with balloons57 or coils placed above and below the aneurysm is definitive, but should be preceded with the BOT and assessment of cerebral blood flow after carotid occlusion via collateral channels.
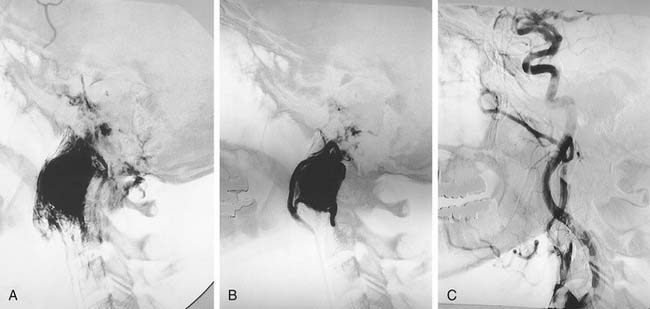
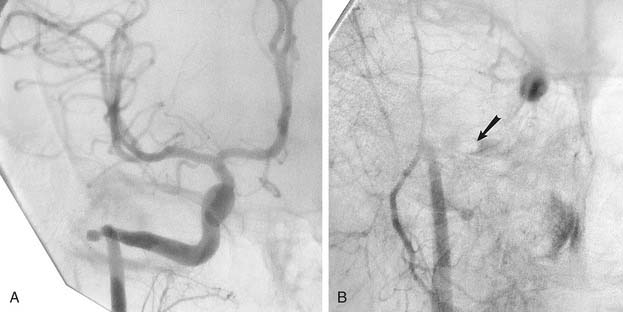
Inadvertent biopsy of an aberrant carotid artery in the middle ear cavity has been reported, leading to severe otorrhagia and massive epistaxis. These lesions generally are best handled by trapping with balloons or coils proximal and distal to the aneurysm (Fig. 136-13).58
Tinnitus-Producing Fibromuscular Dysplasia of the Carotid Artery
Fibromuscular dysplasia of the ICA, which usually occurs at the level of C2, is one of the many causes of pulsatile tinnitus. Vascular webs that characterize this entity may be stretched by angioplasty,59 and stent placement may be necessary to prevent recurrence.
Bederson JB. Pathophysiology and animal models of dural arteriovenous malformations. In: Awad A, Barrow D, editors. Dural Arteriovenous Malformations. Park Ridge, IL: AANS; 1993:23-33.
Casasco A, Herbreteau D, Houdart E, et al. Devascularization of craniofacial tumors by percutaneous tumor puncture. AJNR Am J Neuroradiol. 1994;15:1233-1239.
Davies MA, TerBrugge K, Willinsky R, et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830-837.
Erba SM, Horton JA, Latchaw RE, et al. Balloon test occlusion of the internal carotid artery with stable xenon/CT cerebral blood flow imaging. AJNR Am J Neuroradiol. 1988;9:533-538.
Gandhi D, Gemmete JJ, Ansari SA, et al. Interventional neuroradiology of the head and neck. AJNR Am J Neuroradiol. 2008;29:1806-1815.
Garcia-Monaco R, Lasjaunias P, Alvarez H, et al. Embolization of vascular lesions of the head and neck. In: Valavanis A, editor. Interventional Neuroradiology. Berlin: Springer; 1993:221-253.
Gobin YP, Murayama Y, Englund E, et al. Head and neck hypervascular lesions: embolization with ethylene vinyl alcohol copolymer—laboratory evaluation in swine and clinical evaluation in humans. Radiology. 2001;221:309-317.
Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989;20:385-392.
Latchaw RE. Imaging and endovascular therapy of intracranial arteriovenous fistulae. In: Latchaw RE, Kucharczyk J, Moseley ME, editors. Imaging of the Nervous System—Diagnostic and Therapeutic Applications. Philadelphia: Mosby; 2005:629-668.
Latchaw RE. Preoperative intracranial meningioma embolization: technical considerations affecting the risk-benefit ratio. AJNR Am J Neuroradiol. 1993;14:583.
Latchaw RE, Rai AT, Branstetter BF, Hogg JP. Extra-axial tumors of the head: diagnostic imaging, physiologic testing, and embolization. In: Latchaw RE, Kucharczyk J, Moseley ME, editors. Imaging of the Nervous System—Diagnostic and Therapeutic Applications. Philadelphia: Mosby; 2005:771-851.
Mulliken JB, Young AE. Vascular Birthmarks: Hemangiomas and Malformations. Philadelphia: Saunders; 1988.
Wakhloo AK, Juengling FD, Van Velthoven V, et al. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol. 1993;14:571-582.
Valavanis A. Embolization of intracranial and skull base tumors. In: Valavanis A, editor. Interventional Neuroradiology. Berlin: Springer; 1993:165-220.
Valavanis A. Preoperative embolization of the head and neck: indications, patient selection, goals, and precautions. AJNR Am J Neuroradiol. 1986;7:943.
Yakes WF, Haas DK, Parker SH, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology. 1989;170:1059-1066.
Yakes WF, Luethke JM, Merland JJ, et al. Ethanol embolization of arteriovenous fistulas: a primary mode of therapy. J Vasc Interv Radiol. 1990;1:89-96.
Zanella FE, Valavanis A. Interventional neuroradiology of the lesions of the skull base. Neuroimaging Clin North Am. 1994;4:619.
1. Garcia-Monaco R, Lasjaunias P, Alvarez H, et al. Embolization of vascular lesions of the head and neck. In: Valavanis A, editor. Interventional Neuroradiology. Berlin: Springer; 1993:221-253.
2. Latchaw RE, Gold LHA. Polyvinyl foam embolization for vascular and neoplastic lesions of the head, neck, and spine. Radiology. 1979;131:669.
3. Berenstein A, Kricheff II. Catheter and material selection for transarterial embolization: technical considerations. Radiology. 1979;132:631.
4. Horton JA, Marano GD, Kerber CW, et al. Polyvinyl alcohol foam and Gelfoam for therapeutic embolization: a synergistic mixture. AJNR Am J Neuroradiol. 1983;4:143-147.
5. Kumar AJ, Kaufman SL, Patt J, et al. Preoperative embolization of hypervascular head and neck neoplasms using microfibrillar collagen. AJNR Am J Neuroradiol. 1982;3:163-168.
6. Dion J, Vinuela F, Nombella L, et al. Radiological-pathological correlations of experimental superselective ethanol embolization: analysis of effects of proximal intimal wall damage and end-organ necrosis. AJNR Am J Neuroradiol. 1984;5:664.
7. Berenstein A, Scott J, Choi IS, et al. Percutaneous embolization of arteriovenous fistulas of the external carotid artery. AJNR Am J Neuroradiol. 1986;7:937-942.
8. Debrun G, Lacour P, Vinuela F, et al. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981;55:678-692.
9. Halbach VV, Higashida RT, Hieshima GB. Treatment of vertebral arteriovenous fistulas. AJNR Am J Neuroradiol. 1987;8:1121.
10. Dion J, Vinuela F, Nombella L, et al. Radiological-pathological correlations of experimental superselective ethanol embolization: analysis of effects of proximal intimal wall damage and end-organ necrosis. AJNR Am J Neuroradiol. 1984;5:664.
11. Berenstein A, Choi IS. Treatment of venous angiomas by direct alcohol injection. AJNR Am J Neuroradiol. 1983;4:1144.
12. Yakes WF, Haas DK, Parker SH, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology. 1989;170:1059-1066.
13. Yakes WF, Luethke JM, Merland JJ, et al. Ethanol embolization of arteriovenous fistulas: a primary mode of therapy. J Vasc Interv Radiol. 1990;1:89-96.
14. Brothers MF, Kaufmann JC, Fox AJ, et al. n-Butyl-2-cyanoacrylate: substitute for IBCA in interventional neuroradiology: histopathologic and polymerization time studies. AJNR Am J Neuroradiol. 1989;10:777-786.
15. Gobin YP, Murayama Y, Englund E, et al. Head and neck hypervascular lesions: embolization with ethylene vinyl alcohol copolymer—laboratory evaluation in swine and clinical evaluation in humans. Radiology. 2001;221:309-317.
16. Quadros RS, Gallas S, Delcourt C, et al. Preoperative embolization of a cervicodorsal paraganglioma by direct percutaneous injection of onyx and endovascular delivery of particles. AJNR Am J Neuroradiol. 2006;27:1907-1909.
17. Casasco A, Herbreteau D, Houdart E, et al. Devascularization of craniofacial tumors by percutaneous tumor puncture. AJNR Am J Neuroradiol. 1994;15:1233-1239.
18. Horton JA, Kerber CW. Lidocaine injection into external carotid branches: provocative test to preserve cranial nerve function in therapeutic embolization. AJNR Am J Neuroradiol. 1986;7:105.
19. Momma F, Wang A, Symon L. Effects of temporary arterial occlusion on somatosensory responses in aneurysm surgery. Surg Neurol. 1987;27:343.
20. Barker DW, Jungreis CA, Horton JA, et al. Balloon test occlusion of the internal carotid artery: change in stump pressure over 15 minutes and its recognition with xenon CT cerebral blood flow. AJNR Am J Neuroradiol. 1993;14:587-590.
21. Yonas H, Gur D, Latchaw RE, et al. Xenon-computed tomographic blood flow mapping. In: Wood JH, editor. Cerebral Blood Flow: Physiologic and Clinical Aspects. New York: McGraw-Hill; 1987:220-242.
22. Monsein L, Jeffery PJ, van Heerden BB, et al. Assessing adequacy of collateral circulation during balloon test occlusion of the internal carotid artery with 99mTc-HMPAO SPECT. AJNR Am J Neuroradiol. 1991;12:1045-1051.
23. Erba SM, Horton JA, Latchaw RE, et al. Balloon test occlusion of the internal carotid artery with stable xenon/CT cerebral blood flow imaging. AJNR Am J Neuroradiol. 1988;9:533-538.
24. Spector GJ, Ciralsky R, Maisel RH, et al. Multiple glomus tumors in the head and neck. Laryngoscope. 1975;85:1066-1075.
25. Larson TC, Reese DF, Baker HLJr, et al. Glomus tympanicum chemodectomas: radiographic and clinical characteristics. Radiology. 1987;163:801-806.
26. Latchaw RE, Rai AT, Branstetter BF, et al. Extra-axial tumors of the head: diagnostic imaging, physiologic testing, and embolization. In: Latchaw RE, Kucharczyk J, Moseley ME, editors. Imaging of the Nervous System—Diagnostic and Therapeutic Applications. Philadelphia: Mosby; 2005:771-851.
27. Moret J, Delvert G, Lasjaunias P. Vascular architecture of tympanojugular glomus tumors: its application regarding therapeutic angiography. J Neuroradiol. 1982;9:237.
28. Moret J, Lasjaunias P, Theron J. Vascular compartments and territories of tympanojugular glomic tumors. J Belge Radiol. 1980;63:321.
29. Valavanis A. Preoperative embolization of the head and neck: indications, patient selection, goals, and precautions. AJNR Am J Neuroradiol. 1986;7:943.
30. Simpson GT, Konrad HR, Takahashi M, et al. Immediate postembolization excision of glomus jugulare tumors: advantages of new combined techniques. Arch Otolaryngol Head Neck Surg. 1979;105:639-643.
31. Myers EN, Johnson JT. Otolaryngology–Head and Neck Surgery: Neck Neoplasms. St. Louis: Mosby; 1993.
32. Davis KR. Embolization of epistaxis and juvenile nasopharyngeal angiofibromas. AJR Am J Roentgenol. 1987;148:209.
33. Lasjaunias P. Nasopharyngeal angiofibromas: hazards of embolization. Radiology. 1980;136:119.
34. Lasjaunias P, Picard L, Manelfe C, et al. Angiofibroma of the nasopharynx. J Neuroradiol. 1990;7:73-95.
35. Pletcher JD, Newton TH, Dedo HH, et al. Preoperative embolization of juvenile angiofibromas of the nasopharynx. Ann Otol. 1975;84:740-746.
36. Latchaw RE. Preoperative intracranial meningioma embolization: technical considerations affecting the risk-benefit ratio. AJNR Am J Neuroradiol. 1993;14:583.
37. Wakhloo AK, Juengling FD, Van Velthoven V, et al. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol. 1993;14:571-582.
38. Valavanis A. Embolization of intracranial and skull base tumors. In: Valavanis A, editor. Interventional Neuroradiology. Berlin: Springer; 1993:165-220.
39. Zanella FE, Valavanis A. Interventional neuroradiology of the lesions of the skull base. Neuroimaging Clin North Am. 1994;4:619.
40. Robbins K, Storniolo AM, Kerber C, et al. Phase I study of highly selective supradose cisplatin infusions for advanced head and neck cancer. J Clin Oncol. 1994;12:2113-2120.
41. Yang J, Ma XC, Zou ZJ, et al. Experimental maxillo-facial arterial chemoembolization with encased-cisplatin ethylcellulose microspheres. AJNR Am J Neuroradiol. 1995;16:1037-1041.
42. Kurata A, Kitahara T, Miyasaka Y, et al. Superselective embolization for severe traumatic epistaxis caused by fracture of the skull base. AJNR Am J Neuroradiol. 1993;14:343-345.
43. Wurman LH, Sack JG, Flannery JVJr, et al. The management of epistaxis. Am J Otolaryngol. 1992;13:193-209.
44. Lasjaunias P, Marsot-Dupuch K, Doyon D. The radioanatomical basis of arterial embolization for epistaxis. J Neuroradiol. 1979;6:45.
45. Riche MC, Chiras J, Melki JP, et al. The role of embolization in the treatment of severe epistaxis. J Neuroradiol. 1979;6:207-220.
46. Sinlluoto TMJ, Leinonen AS, Karttunen AI, et al. Embolization for the treatment of posterior epistaxis. Arch Otolaryngol Head Neck Surg. 1993;119:837-841.
47. Ernst R, Bulas RV, Gaskill-Shipley M, et al. Endovascular therapy of intractable epistaxis complicated by carotid artery occlusive disease. AJNR Am J Neuroradiol. 1995;16:1463-1468.
48. Walker AT, Chaloupka JC, Putman CM, et al. Sentinel transoral hemorrhage from a pseudoaneurysm of the internal maxillary artery: a complication of CT-guided biopsy of the masticator space. AJNR Am J Neuroradiol. 1996;17:377-381.
49. Houser OW, Campbell JK, Campbell RJ, et al. Arteriovenous malformation affecting the transverse dural venous sinus: an acquired lesion. Mayo Clin Proc. 1979;54:651-661.
50. Bederson JB. Pathophysiology and animal models of dural arteriovenous malformations. In: Awad A, Barrow D, editors. Dural Arteriovenous Malformations. Park Ridge, IL: AANS; 1993:23-33.
51. Davies MA, TerBrugge K, Willinsky R, et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996;85:830-837.
52. Latchaw RE. Imaging and endovascular therapy of intracranial arteriovenous fistulae. In: Latchaw RE, Kucharczyk J, Moseley ME, editors. Imaging of the Nervous System—Diagnostic and Therapeutic Applications. Philadelphia: Mosby; 2005:629-668.
53. Halbach VV, Higashida RT, Hieshima GB, et al. Transvenous embolization of dural fistulas involving the transverse and sigmoid sinuses. AJNR Am J Neuroradiol. 1989;20:385-392.
54. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412.
55. Mulliken JB, Young AE. Vascular Birthmarks: Hemangiomas and Malformations. Philadelphia: Saunders; 1988.
56. Schellhas KP, Latchaw RE, Wendling LR, et al. Vertebrobasilar injuries following cervical manipulation. JAMA. 1980;244:1450-1453.
57. Mehringer CM, Hieshima GB, Grinnell VS, et al. Therapeutic embolization for vascular trauma of the head and neck. AJNR Am J Neuroradiol. 1983;4:137-142.
58. Reilly JJJr, Caparosa RJ, Latchaw RE, et al. Aberrant carotid artery injured at myringotomy: control of hemorrhage by a balloon catheter. JAMA. 1983;249:1473-1475.
59. Hasso AN, Bird CR, Zinke DE, et al. Fibromuscular dysplasia of the internal carotid artery: percutaneous transluminal angioplasty. AJNR Am J Neuroradiol. 1981;2:955-960.