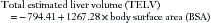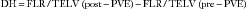Chapter 38 Interventional Imaging in the Oncologic Patient
Image-Guided Tissue Ablation
Technical Background
Radiofrequency Ablation
Image-guided percutaneous RFA is typically guided by computed tomography (CT), ultrasound (US), or a combination of both. RFA is performed by connecting a generator that provides an electric to a metallic applicator probe. Thermal energy is applied onto tissues through the tip of the probe. The tissues surrounding the tip are destroyed within seconds as temperatures reach 55°C and destroyed immediately at temperatures greater than 60°C. Care is taken to avoid charring tissues, which limits heat propagation. During RFA, an alternating electrical current (frequency range 480-500 kHz) is deposited within the tissues via an electrode placed directly into the tumor. The resulting ionic agitation within the lesion and surrounding tissues generates heat in the immediate vicinity of the electrode. The heat is then conducted to the surrounding environment, resulting in ablation of a finite volume of tissue. Ideally, the ablation zone should encompass the tumor and a 5- to 10-mm margin of normal tissue. Thus, the periphery of the ablation zone consists of a rim of hyperemia and inflammatory reaction involving the surrounding liver parenchyma. The size and shape of the ablation zone will vary depending on the amount of energy, type of electrode, duration of ablation, and inherent tissue characteristics.1 Image-guided RFA has been used to treat tumors in a wide variety of organs such as liver, kidneys, lung, and bone, among many others. Initial RFA indications included the treatment of small lesions in patients who were not surgical candidates or for palliation of large lesions. However, owing to the efficacy and safety profile of the technique, its use has greatly expanded in certain diseases that now also include patients who are surgical candidates with comparable outcomes.2 The limitations of the technique include the “heat-sink effect,” in which adjacent blood vessels larger than 3 mm lead to perfusion-mediated attenuation of thermal energy deposition, potentially leading to incomplete ablation; large (>5 cm) complex lesions; and proximity to delicate structures, such as gastrointestinal wall, gallbladder, diaphragm, nerves; among other effects.
Cryoablation
Cryoablation is typically guided by CT or magnetic resonance imaging (MRI). Cryoablation consists of the application of freezing temperatures onto tumors in order to cause tissue destruction. This technique has been used to treat tumors in a variety of tissues such as liver, kidney, prostate, lung, and cervix.3–5 In order to perform cryoablation, a metallic probe is directly inserted into the target lesion. Argon gas circulates through the probe, causing a rapid drop of the local temperature. The ensuing low temperatures cause disruption of the cellular membrane and local ischemia. Ice crystals form within the cells and the adjacent interstitium, causing cell dehydration and surrounding vascular thrombosis. Subsequently, when the tissues thaw, vascular occlusion leads to further ischemic injury.6 Consistent tumor cell death is accomplished when the tissues are exposed to temperatures of at least –20°C, corresponding to the area approximately 3 mm inside the margins of the ice ball. The temperatures along the interface between the ice ball and the adjacent tissues, as well as in the ice ball’s peripheral rim, are suboptimal for tissue necrosis and represent only a reference for the interventional oncologist. As with RFA, the main limitations of cryoablation include proximity to blood vessels, gastrointestinal organs, nerves, and skin. Treatment of large tumor volumes with cryoablation can lead to the development of important systemic complications, such as cryoshock, a cytokine-mediated inflammatory response associated with coagulopathy and multiorgan failure; myoglobinuria; and severe thrombocytopenia.7–9 Otherwise, most complications of cryotherapy—such as hemorrhage and injury to adjacent organs—are generally similar to those of RFA.
Clinical Applications
Liver Tumors
RFA is routinely performed worldwide for the curative or palliative treatment of liver tumors. There is ample literature documenting the successful use of minimally invasive RFA for both resectable and nonresectable liver lesions. Hepatocellular carcinoma (HCC) and metastatic colorectal carcinoma are the most common malignancies that affect the liver. The literature supports the use of percutaneous RFA in patients with HCC and either a single lesion smaller than 5 cm in diameter or up to three lesions smaller than 3 cm in diameter, if partial hepatic resection or transplantation is not available.10 Recent extensive review of the literature regarding RFA in the treatment of colorectal liver metastasis by Mulier and coworkers11 indicates a similar rate of local recurrences after open RFA versus surgical resection for colorectal liver metastasis smaller than 3 cm. In addition, RFA can be used to treat a wide variety of other unresectable liver tumors or theoretically resectable lesions in patients who are not surgical candidates.
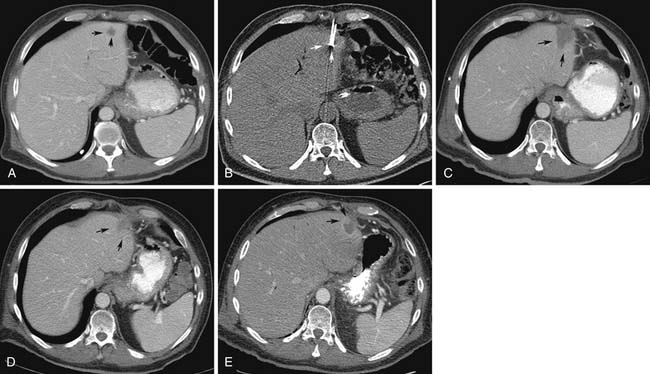
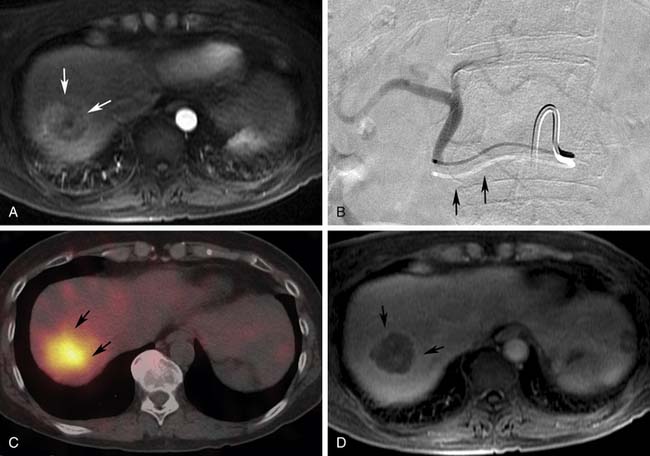
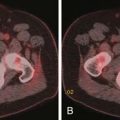
During the initial postprocedure follow-up imaging, the completeness of the ablation has to be carefully scrutinized. Dual-phased imaging is recommended to optimally characterize postintervention findings. The early arterial phase is essential for hypervascular lesions, such as HCC and neuroendocrine tumors. The portal venous phase is ideal for all other lesions and the combination increases the sensitivity of the study. The zone of ablation can be readily identified as an area showing lack of contrast enhancement and overlapping the location of the original lesion. This area has to have least 0.5 cm of its margins extending beyond the original confines of the target lesion on all sides. If this safety margin is not present, the radiologist needs to have a high index of suspicion for residual disease. When CT is the modality used for guidance, a contrast-enhanced study can be performed at the end of the treatment and any suspicious areas subjected to additional ablation. The presence of a uniform, rather than a nodular, rim of enhancement around the ablated area shortly after the procedure is usually benign and represents inflammatory reaction.12–15 Likewise, a central area of increased attenuation on CT scans immediately after ablation is usually benign in nature.12,13 Other expected findings commonly seen shortly after ablation that should not raise concern for complications include portal venous gas and air bubbles within the treatment cavity. Over time, the adequately ablated lesion becomes well-circumscribed and shows gradual size involution. Any areas showing enlarging nodular peripheral enhancement are concerning for residual or recurrent disease (Figure 38-1). A review of comparison prior studies is usually sufficient to establish the diagnosis. However, when an area is deemed questionable, but not definite, for recurrent or residual disease, a logical next diagnostic step is to proceed with fluoro-2-deoxy-D-glucose (FDG)–positron-emission tomography (PET) or PET/CT, which have been shown to be more accurate than CT in this particular scenario.16,17
The most common complications after hepatic RFA include: postprocedural hemorrhage, liver abscess with an incidence of approximately 2%, bile duct injury with biloma formation, hepatic infarction, and injury to adjacent organs.18,19 Hemorrhage is readily identified in the immediate postprocedural CT scans as a hyperattenuating fluid collection on nonenhanced images. Abscess formation should be suspected when new gas bubbles in the treatment bed are identified that were not present on the initial post RFA scan. Focal dilatation of bile ducts peripheral to the ablation cavity is commonly observed, but usually requires no further intervention. Along the same lines, development of a low-attenuation fluid-filled cavity within the parenchyma is suggestive of biloma formation. Bilomas tend to have a benign course, rarely necessitating drainage.
Renal Tumors
The widespread use of US, CT, and MRI for the investigation of abdominal pathologies has led to the diagnosis of a large number of incidental renal tumors. Renal nodules displaying postcontrast enhancement of cross-sectional images are assumed to be malignant until proved otherwise. Because these lesions are increasingly being discovered, when they are still small in size, therapeutic options that spare renal function are highly desirable. Minimally invasive percutaneous procedures offer a safe alternative to partial nephrectomies.20 The thermal ablation techniques typically employed in the treatment of such tumors, include RFA and cryoablation. The ideal lesion for RFA should be small in diameter (<3 cm) and peripheral in location. Central lesions are usually better treated with cryotherapy, owing to the decreased risk of ureteral stricture. A meta-analysis of 47 studies that compared cryoablation with RFA found that repeat ablation was required more frequently with RFA (8.5% of cases vs. 1.3% for cryoablation) and that local tumor progression occurred more frequently with RFA (12.9% vs. 5.2%).21 Metastatic disease was also more common in the RFA group (2.5% of cases vs. 1%). One series comparing cryoablation with RFA found less tumor persistence or recurrence with cryoablation (11.1% of cases vs. 1.8%).22
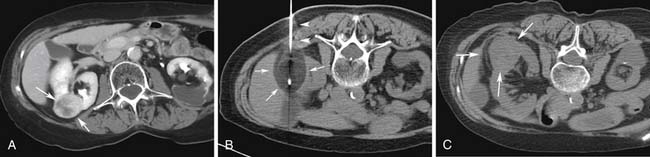
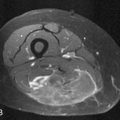
The radiologist has to be familiar with the different appearances of the zone of ablation in the treated kidney (Figure 38-2). In order to do so, it is imperative to understand the gradual evolution of the completed area, potential complications, and signs of residual or recurrent disease. The expected natural course of a successfully treated renal mass is to slowly decrease in size over 24 months, finally reaching approximately half of its original size. A caveat may be observed on the initial follow-up images after treatment of small lesions when a slight increase in lesional volume is occasionally noted. This finding may be secondary to ablation of normal adjacent renal parenchyma. The typical zone of ablation does not show contrast enhancement. A focal area of enhancement should raise suspicion for residual or recurrent disease, particularly if nodular in morphology. During the precontrast phase, the ablation bed tends to be hyperattenuating compared with the adjacent renal parenchyma on CT scans. This feature indicates the presence of proteinaceous material in the treated area. Similarly, these products lead to increased signal on the nonenhanced MRI T1-weighted sequences. Particular to MRI is a thin rim of peripheral enhancement due to the presence of postablation inflammatory changes. This finding is usually not identified on CT scans, probably owing to the lower signal-to-noise ratio.
Lung Tumors
CT and PET scans are the imaging modalities most commonly employed to image lung lesions after ablation. Follow-up imaging is usually performed approximately 1 month after the initial treatment, subsequently at 3- to 6-month intervals up to 24 months. Typically, the ablation zone is identified on follow-up CT scans as a nonenhancing hypoattenuating lesion, whereas areas of tumor progression tend to exhibit enhancement greater than 15 Hounsfield units (HU). A rim of peripheral enhancement on the initial posttreatment images is usually secondary to the inflammatory reaction around the ablation cavity rather than residual or recurrent disease. In addition, ground-glass opacities may be observed around the zone of ablation in the immediate postprocedural scans.23 Owing to these early postablative changes surrounding the target lesion, interpretation of initial posttreatment CT scans may be difficult in cases in which the target lesion appears larger in size but no obvious areas of enhancement are visualized. In this particular scenario, the use of FDG-PET/CT is useful to help distinguish areas of residual viable tumor cells. Although initial inflammatory changes around the zone of ablation may lead to early false-positive results, the presence of areas of showing increased FDG uptake after 3 months should be considered suspicious for residual disease.24
The most common complications of lung ablation include pleural effusion, pneumothorax, bronchopleural fistula, hemoptysis, infection, and potential worsening of the underlying lung disease. The incidence of pneumothorax revolves approximately 28% with approximately 10% of patients requiring chest tube insertion.25
The estimated stage I lung cancer survival rates are 70%, 57%, 36%, 27%, and 27% for 1, 2, 3, 4, and 5 years, respectively, after initial lung RFA. These numbers compare favorably with those from a recent study investigating external beam radiation therapy alone for patients with inoperable stages I to II non–small cell lung cancer.25
Transcatheter Arterial Hepatic Embolization and Chemoembolization
Technical Background
Hepatic arterial embolization for the treatment of liver tumors was first performed in the 1970s for local disease control. The rationale behind this approach emerges from the particularities of blood flow to liver neoplasms, which are supplied preferentially through the hepatic artery, whereas supply to the normal liver parenchyma occurs predominantly through the portal vein.26,27
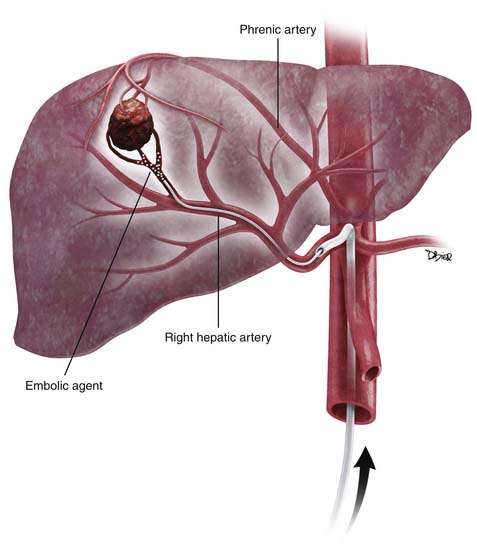
Arterial chemoembolization consists of transcatheter intra-arterial delivery of a combination of embolic agents and chemotherapy drugs into a liver tumor. The principles behind chemoembolization are based on the theory that tumor ischemia caused by embolization of the dominant arterial supply has a synergistic effect with the chemotherapeutic drugs. This technique was first pioneered by Yamada and colleagues in 1977.28 The introduction of ethiodol (iodized oil), an iodinated ester derived from poppy-seed oil, greatly advanced the technique. Ethiodol is well suited for chemoembolization because of its preferential tumoral uptake by certain hepatic tumors. This unique behavior is explained by the concept of enhanced permeability and retention suggested by Maeda and associates.29 In this original study, the authors state that newly formed tumor vessels are more permeable. This increased permeability coupled with a lack of lymphatic vessels in the neoplasm leads to retention of molecules of higher molecular weight within the tumor interstitium for a longer period. This retention may explain, in part, the accumulation of iodized oil or the increase in concentration of polymer conjugates of chemotherapeutic agents in neoplasms. In addition, the iodized oil acts as both a distal embolic agent and a vehicle for the chemotherapy drugs.30,31 It is critical to note that slow infusion of iodized oil into the hepatic artery will eventually reach the portal vein branches via the peribiliary plexus. This observation is important because the operator may inadvertently cause dual embolization, which may lead to parenchymal infarction.
Clinical Applications
Two randomized clinical trials showed a survival advantage when chemoembolization was performed in selected HCC patients.32–34 Colorectal carcinoma patients with liver-dominant disease that did not respond to systemic chemotherapy are candidates for palliative transcatheter chemotherapy. A recently published study by Soulen and associates evaluated the role of TACE in 121 patients with unresectable colorectal liver metastases after failure of second-line systemic therapy. The study showed partial response in 2% of the patients, 41% had stable disease, and 57% had disease progression. Survival was significantly improved when chemoembolization was performed after first- or second-line systemic therapy (11-12 mo) than after third- to fifth-line therapies (6 mo).35 In 2006, Gupta and coworkers published a study analyzing 85 patients with metastatic gastrointestinal stromal tumors.35a Twelve patients (14%) demonstrated partial responses, 63 (74%) demonstrated stable disease, and 10 (12%) demonstrated progressive disease. It is important to point out that this study was done when imatinib first became available and a substantial number of patients in the study did not receive any treatment before the chemoembolization. Other, less frequent, metastatic tumors have also been successfully treated with TACE including among others, ocular melanoma and neuroendocrine, thyroid, and breast carcinoma.
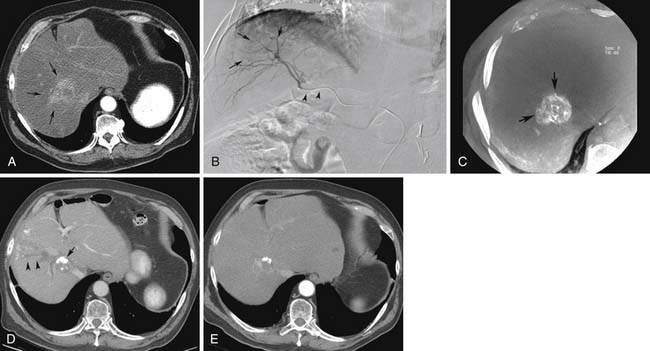
Traditionally, CT scans are used to evaluate patients after hepatic embolization (Figure 38-3). Interpretation is relatively simple, given that areas of necrosis will not show contrast enhancement, whereas viable tumors usually do. However, when iodized oil is administered concomitantly with other embolic agents and chemotherapy, assessment of residual viable tumor is complicated by the oil accumulation within the tumor because the oil is highly radiopaque. Although diffuse homogeneous accumulation of the iodized oil within the lesion portends a good general prognosis, it is important to note that the accumulation of iodized oil within the treated tumor does not always correspond to tissue death.36
Takayasu and associates37 compared CT findings after chemoembolization with iodized oil for treatment of HCC with resected surgical specimens. The study found lack of correlation between tumor size reduction and the histopathologic necrosis rate in resected specimens. This finding is supported by additional studies that show complete retention of the oil within the tumors as a more specific indicator of necrosis rather than overall size reduction.36,38,39 (Figure 38-4). The interpretation becomes difficult when the distribution of iodized oil within the lesion is nonuniform. In this scenario, the studies have shown that the degree of tumor necrosis cannot be accurately predicted. Owing to this limitation, many authors advocate the use of MRI in the assessment of viability of lesions that contain iodized oil.39 Iodized oil appears hyperintense on T1-weighted sequences during the first few months after embolization. The signal of the tumor does not change on the T2-weighted sequences, even in the presence of the oil.40 Shortly after TACE, decrease of tumor contrast enhancement is typically seen in both the arterial and the portal venous phases. Several studies have also evaluated the role of diffusion-weighted images (DWI) and apparent diffusion coefficient (ADC) maps in the assessment of tumor viability after locoregional therapy of liver tumors. The diffusion of water within viable tumors is restricted by the intact cell membranes, particularly because these lesions are typically highly cellular compared with the surrounding liver parenchyma. After treatment, the membranes are typically disrupted, which allows free water diffusion. Therefore, in the setting of tumor necrosis, ADC values are increased. However, the lesions become dehydrated after approximately 4 weeks with subsequent decrease in apparent diffusion.41,42 Unfortunately, these measurements are difficult to obtain in small lesions (<1 cm in diameter). Recently, a new chemoembolization device consisting of a drug-eluting bead has been developed. These beads are biocompatible, nonresorbable hydrogel particles that can be loaded with certain specific chemotherapic agents, such as doxorubicin and irinotecan. Theoretically, owing to a better pharmacokinetic profile, this new device allows for delivery of higher intralesional drug concentrations with concomitant lower systemic toxicity and a prolonged intratumoral retention time of the chemotherapy. Preclinical and clinical studies have demonstrated higher and prolonged retention of doxorubicin within tumors after treatment with drug-eluting bead.43,44 Unlike TACE using iodized oil, this modality of chemoembolization does not lead to retention of highly radiopaque material within the target lesions.
Radioembolotherapy
Technical Background
Radioembolotherapy consists in the TACE delivery of microspheres loaded with radioactive elements, most commonly yttrium-90 (90Y). Using the same principle as other locoregional liver therapies, radioembolization relies on the preferential arterial supply to liver tumors.26,27 Conventional radiation therapy does not have a central role in the management of patients with liver tumors, primarily because of the low tolerance of the whole liver to external beam radiation.45 The risk of radiation-induced liver disease (RILD) after whole liver radiation therapy delivering between 28 gray (Gy) and 35 Gy over 3 weeks is approximately 5%,46,47 and these doses are far less than those needed to adequately treat these lesions. Hence, delivery of the microspheres into the hepatic artery allows deposition of the particles predominantly within the tumor vascularity leading to tissue damage, while preserving the surrounding liver parenchyma. Hence, this critical feature allows delivery of substantially higher radiation doses than what can be safely accomplished by external beam radiotherapy. 90Y is a beta emitter with a 64.1-hr half-life. The beta radiation travels an average of 2.5 mm (maximum 11 mm), a desirable feature because it helps minimize untoward damage.
Clinical Applications
Multiple studies have demonstrated the safety of radioembolization with 90Y for the treatment of unresectable HCC and metastatic colorectal cancer.48–50 Initial studies evaluated CT findings after radioembolotherapy. The treated areas tend to exhibit decreased attenuation on postcontrast images. The extent and heterogeneity of these areas of decreased attenuation logically vary according to the arterial level of microsphere infusion (whole liver, lobar, or selective) as well as with the size and vascularity of the target lesions. Larger hypervascular masses display preferential accumulation of the device, thus lessening the distribution of spheres to the surrounding parenchyma. These changes reach their peak 2 months after treatment and may spontaneously resolve after 6 months. The findings are nonspecific and can also be noted with external beam radiotherapy.51,52 As with other forms of hepatic embolotherapy, lack of tumor enhancement is a potential indicator of favorable response. MRI offers some important advantages over CT. MRI sequences can assess lesion functionality, which may increase specificity for true tumor response because reliance on simple size measurements tends to underestimate treatment response, owing to the presence of intratumoral edema.53 DWI studies are routinely employed to assess tumor response after TACE. In a similar fashion, after radioembolotherapy, accurate functional imaging techniques that increase detection of treatment failures have the potential to allow early re-intervention. ADC measurements have been shown to increase 1 month after radioembolization, thus preceding anatomic changes.53 Metabolic imaging with FDG-PET is also emerging as an accurate way to evaluate response to radioembolization.54 A consideration unique to 90Y embolization is the acquisition of bremsstrahlung scans, which are broad-spectrum secondary gamma-ray emissions produced as a result of the interaction of the high-energy beta emissions with tissue. Review of these scans combined with anatomic CT scans can depict extrahepatic deposition of microspheres or help elucidate the distribution of microspheres in the tumors (Figure 38-5).
As mentioned previously, liver exposure to larger radiation doses can lead to RILD. This condition is characterized by the development of ascites, hepatomegaly, and elevated liver function tests weeks to months after therapy. The incidence of RILD after radioembolization is approximately 4%, most commonly observed between 4 weeks and 4 months after injection.55 Accurate interpretation of diagnostic studies is critical to differentiate RILD from disease progression. Changes in whole liver morphology, signs of hepatic vascular congestion, and ascites suggest RILD as the underlying etiology.
Portal Vein Embolization
Technical Background
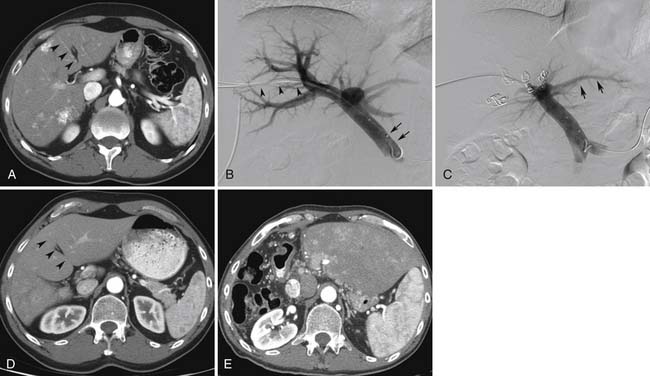
Surgical resection remains the best curative option for patients with primary and metastatic disease confined to the liver.56,57 However, extensive hepatic resection requires sufficient amounts of remaining liver tissue in order to avoid postsurgical hepatic failure. Thus, patients with limited liver remnant volumes may not be considered as candidates for resection owing to the increased risk of posthepatectomy complications.58,59 Portal vein embolization (PVE) has emerged as an important tool in the preoperative management of patients with small anticipated future liver remnant (FLR) prior to major hepatectomy.60,61 Embolization of the portal vein branches supplying the liver segments to be resected redirects blood flow to the nondiseased liver. This redistribution induces hypertrophy of the FLR, making it possible for these patients, not previously considered as candidates, to safely undergo major hepatectomy. Extension of the preoperative embolization to segment IV to optimize hypertrophy is now performed in the majority of patients and should be anticipated by the diagnostic imager.62
The range of reported mean absolute FLR increase for PVE in general was 46% to 70%, depending on the particle type used for embolization.62 Whereas TACE leads to cell necrosis, PVE causes mostly apoptosis, so patients do not experience postembolization syndrome. Fibrin glue, Gelfoam, thrombin, particles, coils, and absolute ethanol all have been successfully used as embolic agents for PVE. In the United States, the most commonly utilized agents include a combination of particles and embolization coils.62 Recent studies have confirmed improvements in the postoperative course after PVE. Because of these improvements, an increased number of patients previously considered to have unresectable disease have become candidates for potentially curative hepatic resection. For this reason, PVE prior to major hepatectomy is now considered the standard of care in many comprehensive hepatobiliary centers worldwide.
Clinical Applications
PVE is indicated for patients with primary or metastatic liver disease who are otherwise hepatic resection candidates but are estimated to have small FLRs. These include patients with cirrhosis or advanced fibrosis with an FLR/total liver volume (TLV) less than 40%,63,64 patients with a history of extensive previous exposure to chemotherapy and an FLR/TLV less than 30%,65,66 and those with no underlying disease and an FLR/TLV less than 20%.61,67,68 It becomes obvious that, for optimal utilization of the technique, accurate determination of liver volumes is essential. Volumetric three-dimensional (3D) contrast-enhanced CT is essential for planning hepatic resection.69
3D-CT volumetric measurements are calculated by outlining the hepatic segmental contours and estimating surface measurements from each slice. Measurements should be standardized according to individual patient size because larger patients require larger FLR than smaller patients.70 The TLV is estimated by using the following formula70:
Patients whose DH is less than 5% have been shown to have a higher postoperative complication rate than patients whose DH is greater than 5%. DH, therefore, may be seen as a prognostic test to help determine whether or not the patient is ultimately resected61 (Figure 38-6). Particularly in those patients who do not show sufficient hypertrophy after PVE, the radiologist can help determine the need for additional embolization by identifying recanalized portal vein branches, which will show enhancement between the pre- and postcontrast CT sequences. Finally, the FLR should be carefully scrutinized for evidence of disease progression that would preclude surgical resection.
Key Points Portal vein embolization
• PVE is indicated for patients with primary or metastatic liver disease who are otherwise hepatic resection candidates but are estimated to have small FLRs.
• Volumetric 3D-CT is essential for optimal planning.
• The presence of patent vessels on follow-up in a patient with insufficient hypertrophy should prompt repeat embolization.
1. Ahrar K., Matin S., Wood C.G., et al. Percutaneous radiofrequency ablation of renal tumors: technique, complications, and outcomes. J Vasc Interv Radiol. 2005;16:679-688.
2. Cho Y.K., Kim J.K., Kim W.T., Chung J.W. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284-1290.
3. Erce C., Parks R.W. Interstitial ablative techniques for hepatic tumours. Br J Surg. 2003;90:272-289.
4. Lee J.M., Stitik F.P., Carter D., Baker R. Local ablative procedures designed to destroy squamous-cell carcinoma. Thorax. 1975;30:152-157.
5. Murphy D.P., Gill I.S. Energy-based renal tumor ablation: a review. Semin Urol Oncol. 2001;19:133-140.
6. Gage A.A., Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171-186.
7. Bageacu S., Kaczmarek D., Lacroix M., et al. Cryosurgery for resectable and unresectable hepatic metastases from colorectal cancer. Eur J Surg Oncol. 2007;33:590-596.
8. Seifert J.K., France M.P., Zhao J., et al. Large volume hepatic freezing: association with significant release of the cytokines interleukin-6 and tumor necrosis factor a in a rat model. World J Surg. 2002;26:1333-1341.
9. Sheen A.J., Poston G.J., Sherlock D.J. Cryotherapeutic ablation of liver tumours. Br J Surg. 2002;89:1396-1401.
10. Chen M.S., Li J.Q., Zheng Y., et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321-328.
11. Mulier S., Ruers T., Jamart J., et al. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg. 2008;25:445-460.
12. Goldberg S.N., Gazelle G.S., Compton C.C., et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88:2452-2463.
13. Goldberg S.N., Grassi C.J., Cardella J.F., et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728-739.
14. Kim S.K., Lim H.K., Kim Y.H., et al. Hepatocellular carcinoma treated with radio-frequency ablation: spectrum of imaging findings. Radiographics. 2003;23:107-121.
15. Lim H.K., Choi D., Lee W.J., et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447-454.
16. Goldberg S.N., Solbiati L., Hahn P.F., et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371-379.
17. Rhim H., Dodd G.D.3rd. Radiofrequency thermal ablation of liver tumors. J Clin Ultrasound. 1999;27:221-229.
18. de Baere T., Risse O., Kuoch V., et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695-700.
19. Livraghi T., Solbiati L., Meloni M.F., et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451.
20. Bandi G., Hedican S.P., Nakada S.Y. Current practice patterns in the use of ablation technology for the management of small renal masses at academic centers in the United States. Urology. 2008;71:113-117.
21. Kunkle D.A., Uzzo R.G. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008;113:2671-2680.
22. Hegarty N.J., Gill I.S., Desai M.M., et al. Probe-ablative nephron-sparing surgery: cryoablation versus radiofrequency ablation. Urology. 2006;68(Suppl 1):7-13.
23. Jin G.Y., Lee J.M., Lee Y.C., et al. Primary and secondary lung malignancies treated with percutaneous radiofrequency ablation: evaluation with follow-up helical CT. AJR Am J Roentgenol. 2004;183:1013-1020.
24. Higaki F., Okumura Y., Sato S., et al. Preliminary retrospective investigation of FDG-PET/CT timing in follow-up of ablated lung tumor. Ann Nucl Med. 2008;22:157-163.
25. Simon C.J., Dupuy D.E., DiPetrillo T.A., et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268-275.
26. Breedis C., Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969-977.
27. Ridge J.A., Bading J.R., Gelbard A.S., et al. Perfusion of colorectal hepatic metastases. Relative distribution of flow from the hepatic artery and portal vein. Cancer. 1987;59:1547-1553.
28. Yamada R., Nakatsuka H., Nakamura K., et al. Hepatic artery embolization in 32 patients with unresectable hepatoma. Osaka City Med J. 1980;26:81-96.
29. Maeda H., Seymour L.W., Miyamoto Y. Conjugates of anticancer agents and polymers: advantages of macromolecular therapeutics in vivo. Bioconjug Chem. 1992;3:351-362.
30. Nakakuma K., Tashiro S., Hiraoka T., et al. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer. 1983;52:2193-2200.
31. Kan Z. Dynamic study of iodized oil in the liver and blood supply to hepatic tumors. An experimental investigation in several animal species. Acta Radiol Suppl. 1996;408:1-25.
32. Llovet J.M., Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429-442.
33. Llovet J.M., Real M.I., Montana X., et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739.
34. Lo C.M., Ngan H., Tso W.K., et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171.
35. Albert M., Kiefer M.V., Sun W., et al. Chemoembolization of colorectal liver metastases with cisplatin, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer. 2011;117:343-352.
35a. Kobayashi K., Gupta S., Trent J.C., et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: response, survival, and prognostic factors. Cancer. 2006;107:2833-2841.
36. Jinno K., Moriwaki S., Tanada M., et al. Clinicopathological study on combination therapy consisting of arterial infusion of lipiodol-dissolved SMANCS and transcatheter arterial embolization for hepatocellular carcinoma. Cancer Chemother Pharmacol. 1992;31(Suppl):S7-S12.
37. Takayasu K., Arii S., Matsuo N., et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol. 2000;175:699-704.
38. Choi B.I., Kim H.C., Han J.K., et al. Therapeutic effect of transcatheter oily chemoembolization therapy for encapsulated nodular hepatocellular carcinoma: CT and pathologic findings. Radiology. 1992;182:709-713.
39. Kamel I.R., Bluemke D.A., Eng J., et al. The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2006;17:505-512.
40. De Santis M., Alborino S., Tartoni P.L., et al. Effects of lipiodol retention on MRI signal intensity from hepatocellular carcinoma and surrounding liver treated by chemoembolization. Eur Radiol. 1997;7:10-16.
41. Yu J.S., Kim J.H., Chung J.J., Kim K.W. Added value of diffusion-weighted imaging in the MRI assessment of perilesional tumor recurrence after chemoembolization of hepatocellular carcinomas. J Magn Reson Imaging. 2009;30:153-160.
42. Kamel I.R., Liapi E., Reyes D.K., et al. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250:466-473.
43. Lammer J., Malagari K., Vogl T., et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52.
44. Poon R.T., Tso W.K., Pang R.W., et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100-1108.
45. Dawson L.A., Guha C. Hepatocellular carcinoma: radiation therapy. Cancer J. 2008;14:111-116.
46. Emami B., Lyman J., Brown A., et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122.
47. Lawrence T.S., Robertson J.M., Anscher M.S., et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237-1248.
48. Geschwind J.F., Salem R., Carr B.I. et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S194-S205.
49. Salem R., Lewandowski R.J., Atassi B., et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627-1639.
50. Stubbs R.S., Cannan R.J., Mitchell A.W. Selective internal radiation therapy (SIRT) with 90Yttrium microspheres for extensive colorectal liver metastases. Hepatogastroenterology. 2001;48:333-337.
51. Marn C.S., Andrews J.C., Francis I.R., et al. Hepatic parenchymal changes after intra-arterial Y-90 therapy: CT findings. Radiology. 1993;187:125-128.
52. Murthy R., Nunez R., Szklaruk J., et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005;25(Suppl 1):S41-S55.
53. Rhee T.K., Naik N.K., Deng J., et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008;19:1180-1186.
54. Flamen P., Vanderlinden B., Delatte P., et al. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with yttrium-90 labeled resin microspheres. Phys Med Biol. 2008;53:6591-6603.
55. Kennedy A.S., McNeillie P., Dezarn W.A., et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494-1500.
56. Poon R.T., Fan S.T., Lo C.M., et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70.
57. Imamura H., Seyama Y., Kokudo N., et al. Single and multiple resections of multiple hepatic metastases of colorectal origin. Surgery. 2004;135:508-517.
58. Shirabe K., Shimada M., Gion T., et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309.
59. Shoup M., Gonen M., D’Angelica M., et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-330.
60. Madoff D.C., Abdalla E.K., Vauthey J.N. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779-790.
61. Ribero D., Abdalla E.K., Madoff D.C., et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394.
62. Madoff D.C., Abdalla E.K., Gupta S., et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215-225.
63. Farges O., Belghiti J., Kianmanesh R., et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217.
64. Kubota K., Makuuchi M., Kusaka K., et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181.
65. Azoulay D., Castaing D., Krissat J., et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665-672.
66. Adam R., Pascal G., Castaing D., et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052-1061. discussion 1061–1064
67. Abdalla E.K., Barnett C.C., Doherty D., et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-680. discussion 680–681
68. Vauthey J.N., Pawlik T.M., Abdalla E.K., et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722-730. discussion 730–732
69. Vauthey J.N., Chaoui A., Do K.A., et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519.
70. Vauthey J.N., Abdalla E.K., Doherty D.A., et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233-240.