CHAPTER 7 Interventional endoscopy
7.1 Stricture dilation
Key Points
1 Clinical and endoscopic assessment
1.1 Esophageal strictures
![]() Clinical Tips
Clinical Tips
1.2 Gastric, pyloric, and small bowel strictures
![]() Clinical Tips
Clinical Tips
3 Endoscopic techniques
3.1 General principles
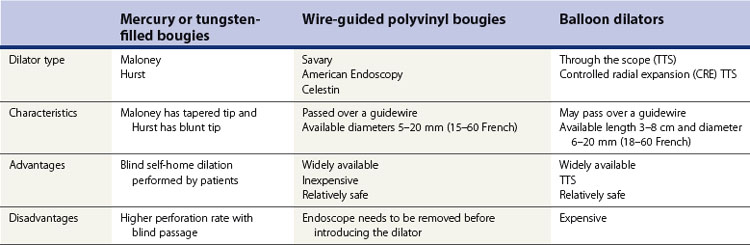
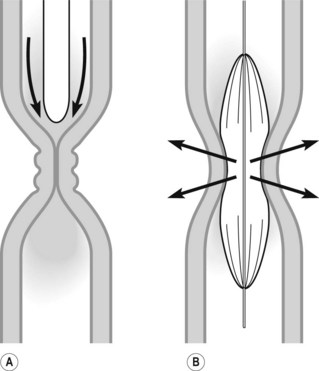
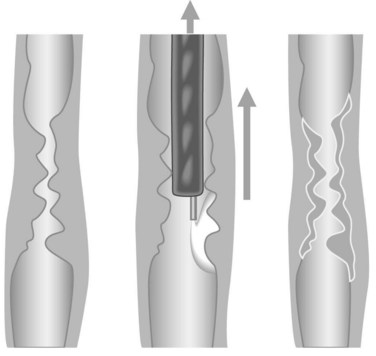
3.1.1 Dilation using bougies
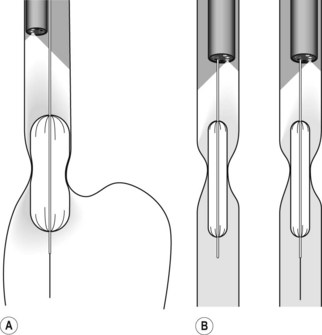
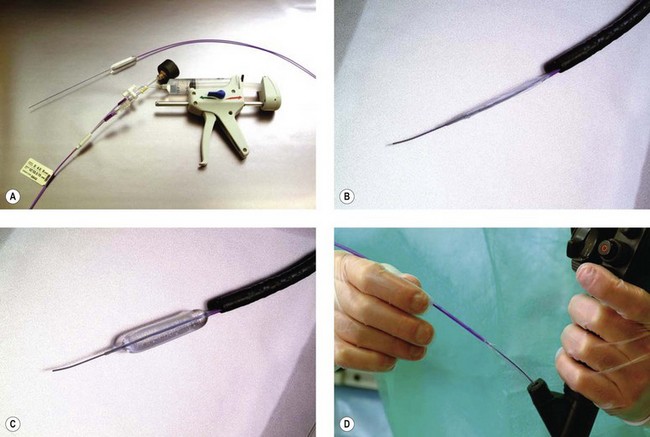
3.1.2 Dilation using balloons
![]() Clinical Tips
Clinical Tips
3.2 Esophageal strictures
Box 4 ASGE guidelines for the performance of esophageal dilation
3.3 Achalasia
3.4 Gastric/pyloric strictures
3.5 Small intestinal/colonic strictures
4 Complications
Borotto E, Gaudric M, Danel B, et al. Risk factors of oesophageal perforation during pneumatic dilatation for achalasia. Gut. 1996;39:9-12.
Hernandez LV, Jacobson JW, Harris MS. Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures. Gastrointest Endosc. 2000;51:460-462.
Kochhar R, Makharia GK. Usefulness of intralesional triamcinolone in treatment of benign esophageal strictures. Gastrointest Endosc. 2002;56:829-834.
Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc. 1995;41:15-17.
Lemberg B, Vargo JJ. Balloon dilation of colonic strictures. Am J Gastroenterol. 2007;102:2123-2125.
Pereira-Lima JC, Ramires RP, Zamin IJr, et al. Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. Am J Gastroenterol. 1999;94:1497-1501.
Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: what is the clinical significance? Endoscopy. 2006;38:515-520.
7.2 Emergency endoscopy in benign gastrointestinal obstruction
Key Points
Information about the emergency management of gastroduodenal or colonic obstruction due to stricture or malignancy can be found in Chapter 7.3.
1 Volvulus
1.1 Gastric volvulus
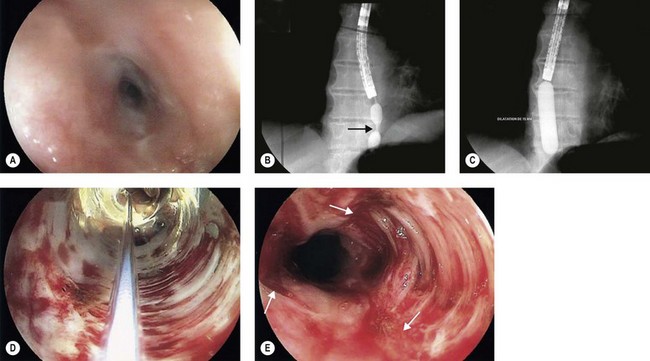
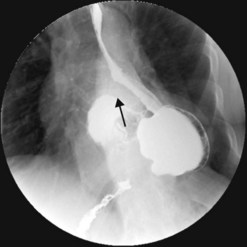
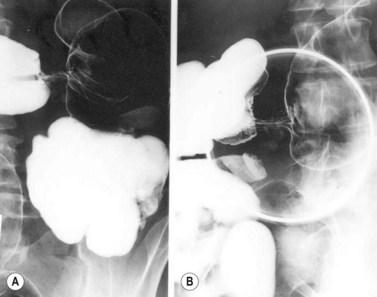
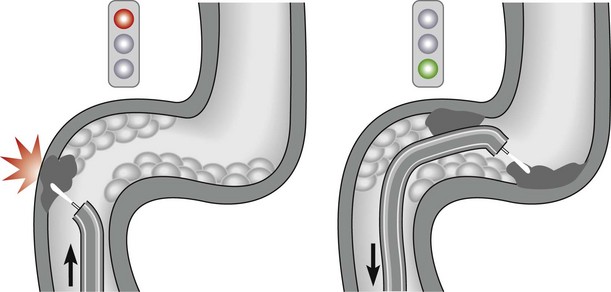
Gastric volvulus is a rare, potentially life-threatening entity that occurs when the stomach twists upon itself (Fig. 1). By definition, gastric volvulus is rotation of the entire or part of the stomach more than 180°. It is supra-diaphragmatic and associated with a paraesophageal or a mixed diaphragmatic hernia in two-thirds of the cases, and is subdiaphragmatic in the remaining one third. The volvulus is organoaxial in 60% of cases where the axis passes through the gastroesophageal and gastropyloric junctions, and mesenteroaxial in 40% of cases where the axis bisects the lesser and greater curvatures.
![]() Warning!
Warning!
![]() Clinical Tips
Clinical Tips
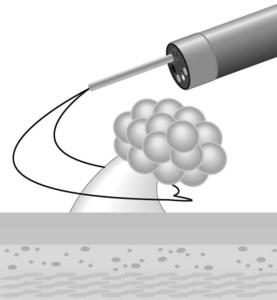
The alpha-loop maneuver (Fig. 2)
1.2 Colonic volvulus
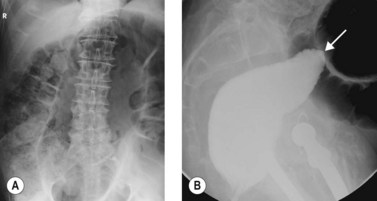
Colonic volvulus is the third most frequent cause of large bowel obstruction after neoplasms and diverticulitis. Whereas colonic neoplasms and diverticulitis usually result in an open-loop obstruction where the lumen is occluded at a single point along the bowel segment, colonic volvulus occurs when a colonic segment becomes twisted on its mesenteric axis and occludes both ends of the bowel segment resulting in a closed-loop obstruction (Fig. 4). The mesentery gets trapped and the blood supply to the bowel segment becomes strangulated, potentially leading to gut ischemia, necrosis and perforation. Delay in diagnosis and decompression compromises viability of the bowel and is a major cause of mortality.
The sigmoid colon (Fig. 4) and cecum (Fig. 5) are the most frequent sites of colonic volvulus, accounting for 75% and 22% of all cases, respectively. Patients with acute colonic volvulus present most commonly with acute abdominal distension and may have other non-specific symptoms of abdominal pain, nausea, vomiting, and constipation. The diagnosis of colonic volvulus can be made with plain abdominal films (supine and upright) or water-soluble contrast enemas in 85% of the cases.
![]() Clinical Tips
Clinical Tips
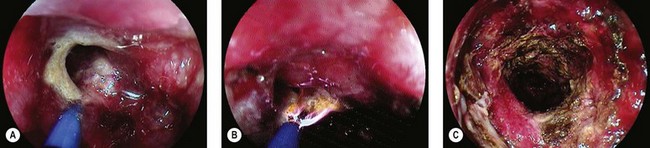
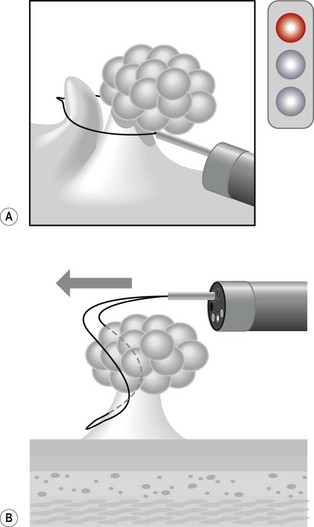
Acute colonic volvulus should be managed on an emergent basis. Patients with colonic necrosis/perforation should be managed surgically. A more conservative approach with an initial attempt at endoscopic detorsion and decompression can be followed in more stable patients (Fig. 6). The benefits of such a strategy are:
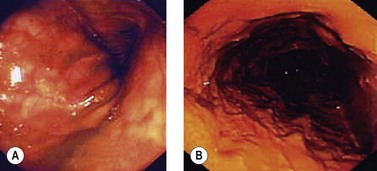
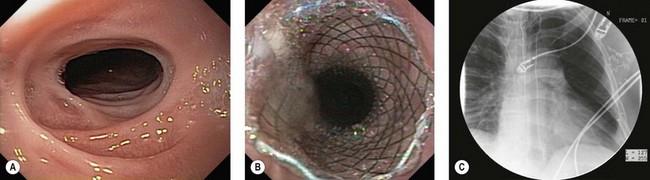
Figure 6 Endoscopic view of a sigmoid volvulus. (A) Before reduction. (B) Following endoscopic reduction.
![]() Clinical Tips
Clinical Tips
Endoscopic treatment of colonic volvulus
2 Acute colonic pseudo-obstruction
Acute colonic pseudo-obstruction, also known as Ogilvie’s syndrome, is a disorder characterized by massive colonic dilation in the absence of colon obstruction. This definition excludes toxic colitis, which occurs in the setting of severe colitis secondary to inflammatory bowel disease or infection. It occurs most often in the setting of surgery and severe medical illnesses, and thus is a disorder of institutionalized patients. Acute colonic pseudo-obstruction is believed to result from autonomic imbalance with suppressed large bowel parasympathetic tone. This results in decreased colonic motility, accumulation of gas and fluid in the colon, increased intraluminal pressure, colonic distension and rising wall tension. Wall tension is highest in the cecum where the colonic diameter is the largest. This may result in the impediment of cecal capillary circulation and lead to ischemia, gangrene, and subsequent perforation. Plain abdominal radiographs show diffuse dilatation of the colon. A cutoff in the colonic gas is often seen at the hepatic flexure, splenic flexure, or sigmoid region with minimal air distal to the cutoff (collapsed left colon). Unlike toxic colitis, preserved haustral markings, smooth inner colonic contour, and thin colonic wall are present. In contrast to mechanical obstruction, air fluid levels are absent and distension is gaseous (Figs 7, 8). Water-soluble contrast enema is usually needed to rule out a true mechanical obstruction.
![]() Clinical Tips
Clinical Tips
Box 1 Treatment of acute colonic pseudo-obstruction: conservative measures
![]() Warning!
Warning!
Box 2 Facts about neostigmine
Box 3 Facts about colonoscopic decompression in patients with acute colonic pseudo-obstruction
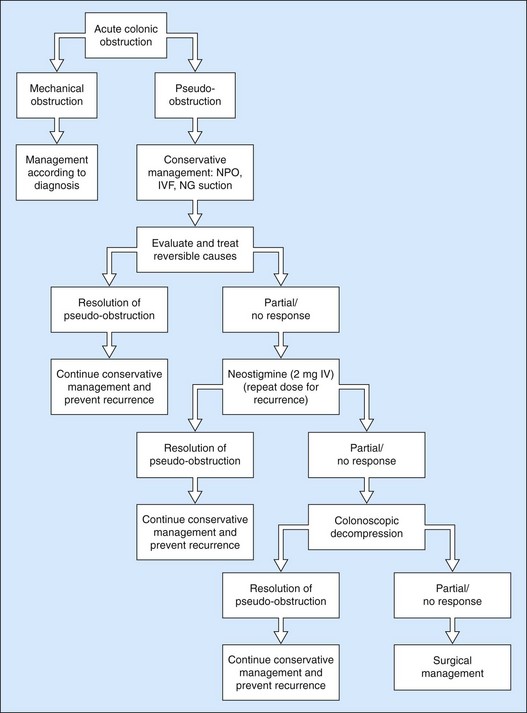
Patients who fail medical and endoscopic treatment and those with signs of colonic perforation/necrosis should be treated surgically with cecostomy or colectomy. Figure 9 illustrates an algorithm suggested by the ASGE for treating patients with ACPO.
Eisen GM, Baron TH, Dominitz JA, et al. Acute colonic pseudo-obstruction. Gastrointest Endosc. 2002;56:789-792.
Godshall D, Mossallam U, Rosenbaum R. Gastric volvulus: case report and review of the literature. J Emerg Med. 1999;17:837-840.
Loftus CG, Harewood GC, Baron TH. Assessment of predictors of response to neostigmine for acute colonic pseudo-obstruction. Am J Gastroenterol. 2002;97:3118-3122.
Madiba TE, Thomson SR. The management of cecal volvulus. Dis Colon Rectum. 2002;45:264-267.
Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999;341:137-141.
Renzulli P, Maurer CA, Netzer P, Buchler MW. Preoperative colonoscopic derotation is beneficial in acute colonic volvulus. Dig Surg. 2002;19:223-229.
Tejler G, Jiborn H. Volvulus of the cecum. Report of 26 cases and review of the literature. Dis Colon Rectum. 1988;31:445-449.
Tsang TK, Walker R, Yu DJ. Endoscopic reduction of gastric volvulus: the alpha-loop maneuver. Gastrointest Endosc. 1995;42:244-248.
7.3 Esophageal, duodenal and colorectal stenting
Key Points
1 General principles
2 Esophageal stenting
2.1 General concepts
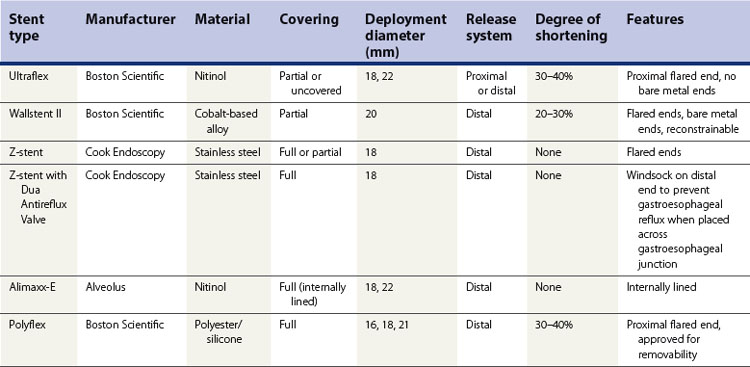
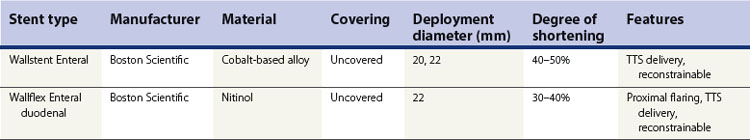
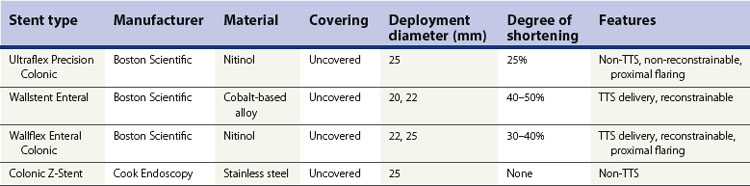
2.2 FDA-approved expandable esophageal stents (Table 1)
2.2.1 Ultraflex stent
2.2.2 Wallstent II
2.2.3 Z-stent
2.2.4 Alimaxx-E stent
2.2.5 Polyflex stent
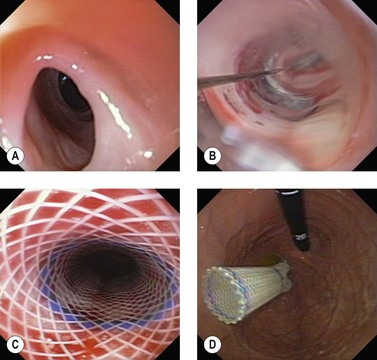
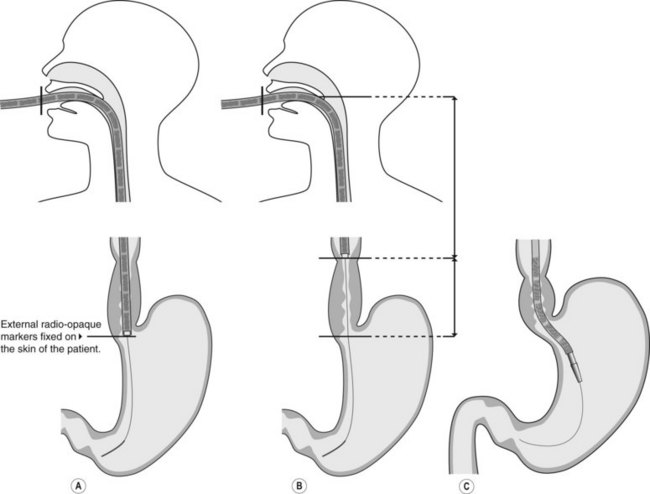
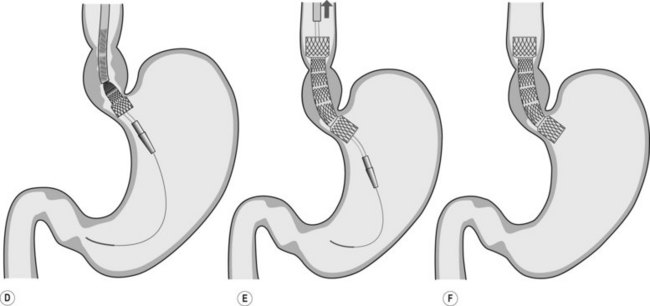
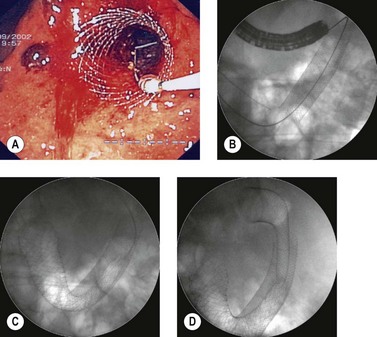
2.3 Technique of stent placement
![]() Clinical Tips
Clinical Tips
![]() Clinical Tips
Clinical Tips
2.4 Stent retrieval techniques
2.5 Outcome data (efficacy)
Box 2 Take home points
2.5.1 Fistula formation
2.5.2 Parallel stent placement
2.5.3 Proximal esophageal carcinoma
3 Duodenal stenting
3.1 General concepts
3.3 Technique of stent placement
3.4 Outcome data (efficacy) and complications
Jejunal stents may also be placed. These can sometimes be placed with a pediatric colonoscope; however, augmented enteroscopy with double balloon, single balloon or spiral enteroscope (Fig. 6) provide a stable platform for stent insertion.
4 Colonic stenting
4.1 General concepts
 Bridge to surgery in patients with colorectal cancer (CRC) who present with total or subtotal colonic obstruction.
Bridge to surgery in patients with colorectal cancer (CRC) who present with total or subtotal colonic obstruction.Box 3 Rationale for colorectal stenting as a bridge to surgery
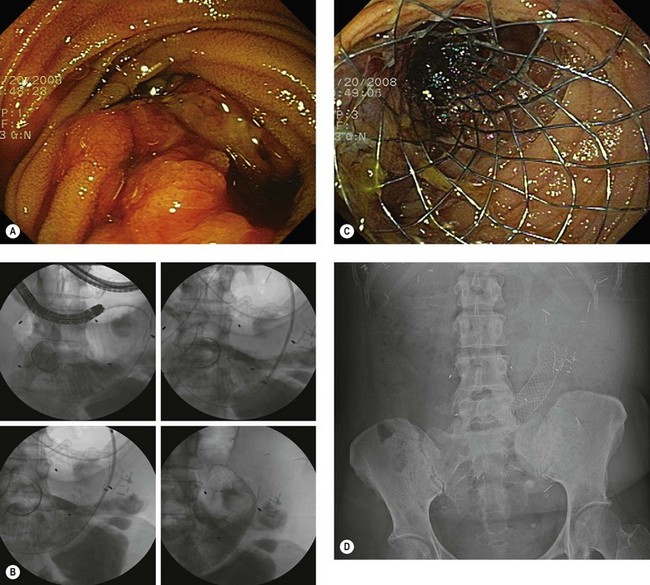
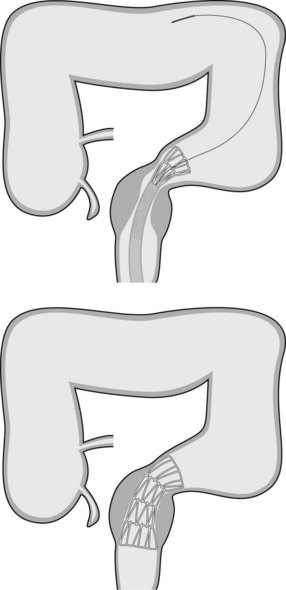
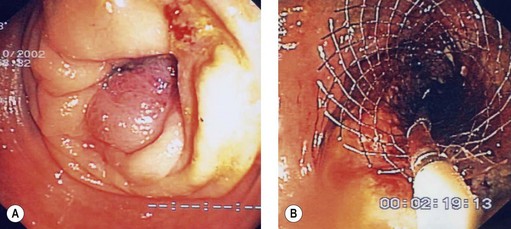
4.3 Technique of stent placement (Figs 7, 8)
![]() Clinical Tips
Clinical Tips
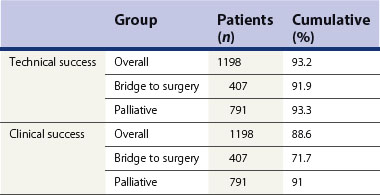
4.4 Outcome data (efficacy) and complications
![]() Clinical Tips
Clinical Tips
Table 5 Preoperative and palliative colorectal stents complications (n = 826)
| Mortality | 0.3% |
| Morbidity | 5–10% |
| Perforation | 3% |
| Migration | 9% |
| Bleeding/pain/tenesmus | 10% |
| Re-obstruction | 9% (tumor ingrowth/overgrowth 74%, fecal impaction 20%, migration 6%) |
Baerlocher MO, Asch MR, Dixon P, et al. Interdisciplinary Canadian guidelines on the use of metal stents in the gastrointestinal tract for oncological indications. Can Assoc Radiol J. 2008;59:107-122.
Baron TH. Expandable gastrointestinal stents. Gastroenterology. 2007;133:1407-1411.
Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681-1687.
Dormann A, Meisner S, Verin N, et al. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543-550.
Eloubeidi MA, Lopes TL. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol. 2009;104:1374-1381.
Mougey A, Adler DG. Esophageal stenting for the palliation of malignant dysphagia. J Support Oncol. 2008;6:267-273.
Nathwani RA, Kowalski T. Endoscopic stenting of esophageal cancer: the clinical impact. Curr Opin Gastroenterol. 2007;23:535-538.
Sebastian S, Johnston S, Geoghegan T, et al. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057.
Tierney W, Chuttani R, Croffie J, et al. Enteral stents. Gastrointest Endosc. 2006;63:920-926.
7.4 Argon plasma coagulation
Key Points
1 General principles
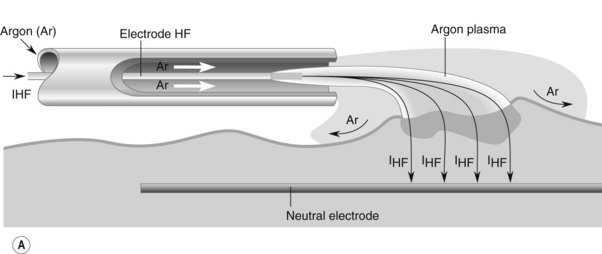
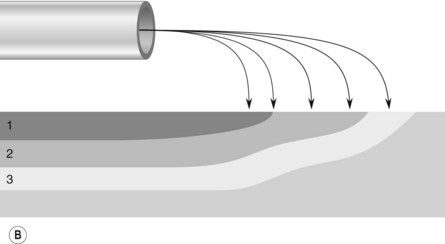
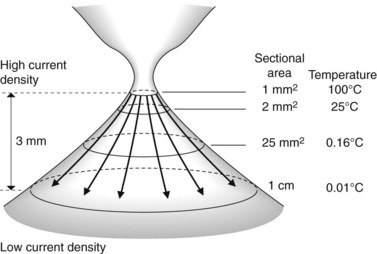
The probe of the delivery catheter contains a tungsten electrode through which a high frequency electric current is delivered to the target tissue using ionized argon gas (argon plasma). This results in the coagulation of the target tissue. The depth of coagulation is dependent on the power of the electrosurgical generator, the distance between the probe and the target tissue, and the duration of application. The physical properties of the treated tissue also affects the depth of tissue injury. Treated tissue demonstrates three zones of effect. The outermost zone, closest to the probe, is the dessication zone followed by the coagulation and devitalization zone (Fig. 1).
2 Equipment
There are two manufacturers of APC generators (Conmed, Utica, NY and ERBE Electromedizen, Tubingen, Germany). Power can be adjusted between 0 and 150 W and gas flow rates between 0.5 and 7.0 L/min. The flexible delivery catheters are disposable and are available in a 1.5 mm, 2.3 mm, and 3.2 mm diameters with lengths of 220 cm and 300 cm. The most commonly used is the 2.3 mm diameter, 220 cm long catheter. The wider diameter catheters are used if a larger treatment area is required. The 300 cm catheters are used for treatment of lesions in the small bowel during push enteroscopy. Catheters can direct current in a parallel or ‘forward firing’ versus perpendicular or ‘side firing’ manner, in relation to the longitudinal axis of the catheter. The ‘side firing’ probe can be used for lesions which are difficult to access, such as those located behind a fold or around a sharply-angled corner (Fig. 2). The foot activation pedal synchronizes delivery of the current and argon gas which ionizes the argon gas and allows current to be delivered to the target tissue.
3 Technique
![]() Clinical Tips
Clinical Tips
How to decrease risk of perforation
4 Clinical applications
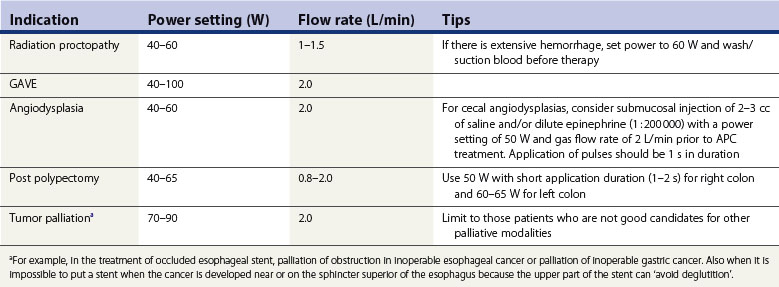
4.1 Radiation proctopathy
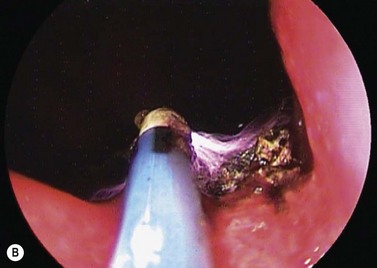
APC has been shown to be an effective treatment for gastrointestinal bleeding associated with radiation proctopathy in several case series studies (Fig. 3).
4.2 Vascular lesions
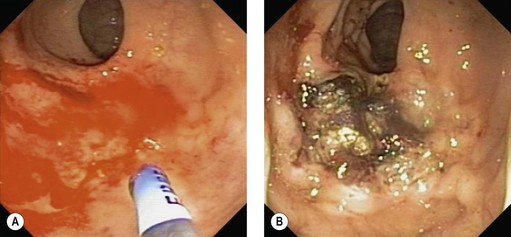
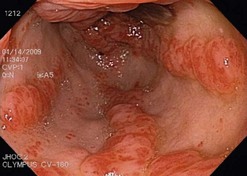
APC is an effective treatment for gastric antral vascular ectasia (GAVE) (Fig. 4) and angiodysplasia (Fig. 5), and prevents recurrent bleeding associated with these lesions.
GAVE (Fig. 4) is an uncommon cause for upper gastrointestinal blood loss associated with cirrhosis and other chronic diseases (Box 1). Involvement of the antrum can be patchy or diffuse. Patients often present with iron deficiency anemia. Improvements in hemoglobin levels and a decrease in transfusion requirements are seen in most patients. However, recurrence can be seen in 30–40% of patients between 20 and 30 months after therapy, which often requires further APC treatment.
Angiodysplasias (Fig. 5) can be found throughout the gastrointestinal tract and are effectively treated with APC. Angiodysplasias can be found throughout the gastrointestinal tract and are effectively treated with APC.
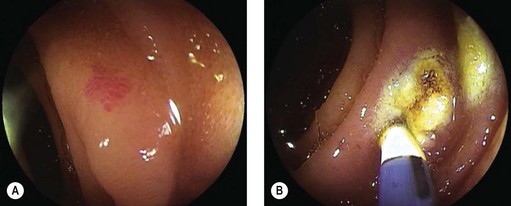
4.4 Other applications
APC has been used for palliative debulking of obstructive tumors of the esophagus (Figs 6, 7), stomach, ampulla, and rectum (Figs 8, 9). While symptomatic improvement is seen in >90% of patients, multiple treatment sessions are often required. While there have been few randomized studies, a large series of patients with obstructive esophageal and cardia tumors underwent APC with maintenance of luminal patency in 64% of patients until death.
5 Complications
Canard JM, Fontaine H, Vedrenne B. Electrocoagulation par plasma d’Argon: première expérience française rapportée [Argon plasma electrocoagulation: first French experience reported]. Gastroenterol Clin Biol. 1997;21:A36.
Canard JM, Vedrenne B. Clinical applications of Argon plasma coagulation in gastro-intestinal endoscopy: has the time come to replace the laser? Endoscopy. 2001;33(4):353-357.
Canard JM, Vedrenne B, Bors G, et al. Résultats à long terme du traitement des rectites radiques hémorragiques par la coagulation au plasma d’Argon [Long-term results of the treatment of hemorrhagic radiation proctitis by argon plasma coagulation]. Gastroenterol Clin Biol. 2003;27:455-459.
Farin G, Grund KE. Technology of argon plasma coagulation with particular regard to endoscopic applications. Endosc Surg Allied Technol. 1994;2:71-77.
Dumot JA, Greenwald BD. Argon plasma coagulation, bipolar cautery, and cryotherapy: ABCs of ablative techniques. Endoscopy. 2008;40:1026-1032.
Ginsberg GG, Barkun AN, Bosco JJ, et al. The argon plasma coagulator. Gastrointest Endosc. 2002;55:807-810.
Manner H. Argon plasma coagulation therapy. Curr Opin Gastroenterol. 2008;24:612-616.
Morris ML, Tucker RD, Baron TH, et al. Electrosurgery in gastrointestinal endoscopy: principles to practice. Am J Gastroenterol. 2009;104:1563-1574.
Postgate A, Saunders B, Tjandra J, et al. Argon plasma coagulation in chronic radiation proctitis. Endoscopy. 2007;39:361-365.
Selinger CP, Ang YS. Gastric antral vascular ectasias (GAVE): an update on clinical presentation, pathophysiology, and treatment. Digestion. 2008;77:131-137.
Suzuki N, Arebi N, Saunders BP. A novel method of treating colonic angiodysplasias. Gastrointest Endosc. 2006;64:424-427.
Vargo JJ. Clinical applications of the argon plasma coagulator. Gastrointest Endosc. 2004;54:81-88.
7.5 Management of ingested foreign bodies
Introduction
Once in the stomach, most foreign bodies pass through the GI tract without complication within 1–2 weeks (Box 1). Exceptions to this rule are objects longer than 5 cm or wider than 2 cm, which may not pass through the pylorus or duodenum.
1 Clinical assessment
1.1 Foreign body lodged in the respiratory tract
This should be suspected if the patient has difficulty breathing or has wheezing.
2 Imaging
Plain radiographs in two planes should be performed promptly. Anteroposterior and lateral radiographs of the chest and abdomen should be performed. This allows for localization of the foreign body and will also detect the presence of pneumomediastinum, pleural effusion or subcutaneous air, which are associated with perforation. Anteroposterior and lateral films of the neck and chest should be performed if there is a suspicion of a foreign body in the esophagus versus the trachea (Fig. 1).
Not all foreign bodies are visible on a plain radiograph. Radio-opaque objects are visible; however, non-radio-opaque objects will not be seen (Table 1). Thus, plain radiographs will miss 50–80% of swallowed bones later identified on endoscopy. Computerized tomography is superior to plain radiographs, and will identify the location of 80–100% of foreign bodies. Barium studies should not be performed due to risk of acute pulmonary edema if aspiration occurs.
| Radiolucency | Foreign body |
|---|---|
| Radio-opaque | Metal (such as coins), batteries, needles and pins |
| Not always visualised | Cartilage, meat or fish bones, pieces of plastic and occasionally glass or alloy are not always visualized on plain films |
| Radiolucent | Food |
3 Timing of endoscopy
The majority of foreign bodies which reach the stomach can be watched. Exceptions to this rule are:
4 Equipment
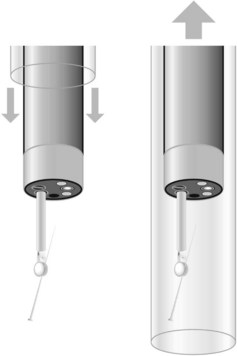
Figure 4 Removal of a sharp foreign body. An overtube should be used to withdraw a sharp foreign body.
5 Endoscopy technique
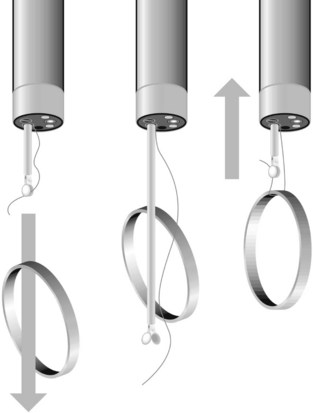
Overtubes can be used to extract foreign bodies whose removal could cause bleeding or perforation, such as pointed, sharp or long foreign bodies (Fig. 4). Standard overtubes extend past the upper esophageal sphincter, while longer overtubes (45–60 cm) pass the lower esophageal sphincter and are used to remove sharp objects from the stomach. Overtubes are also useful where there is a risk of aspiration or where multiple attempts will be made to remove a foreign body (i.e. food bezoars in non-intubated patient). Overtubes are rarely used in children, given their diameters.
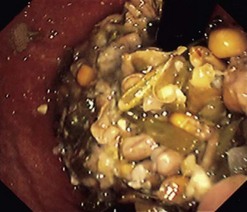
5.2 Bezoars
When endoscopy is required, an attempt should be made to pass the endoscope between the esophageal wall and the bezoars and into the stomach. The bezoar will often follow the endoscope into the stomach (Fig. 6). If this fails, or if the bezoar is large, it should be broken into smaller fragments, which can then be extracted with a basket or Roth net.
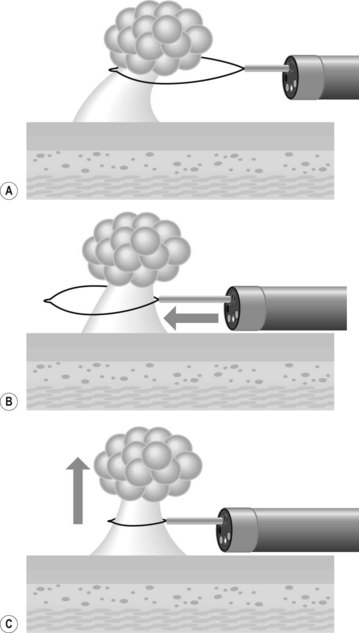
5.3 Round blunt foreign bodies
The management of round, blunt foreign bodies (i.e. coins) depends on their size, the patient’s symptoms and the location. Objects which measure 2.5 cm or less will pass through the pylorus and may be watched (Table 2). If they are located in the esophagus, they should be removed if they have not passed within 24 h. If they are located in the stomach, they should be removed if they have failed to pass after 4–6 days. If the foreign body has passed through the pylorus, patients may be placed on a regular diet, with plain radiographs every week to confirm passage through the gut. Prokinetic medication has not been proved to be effective.
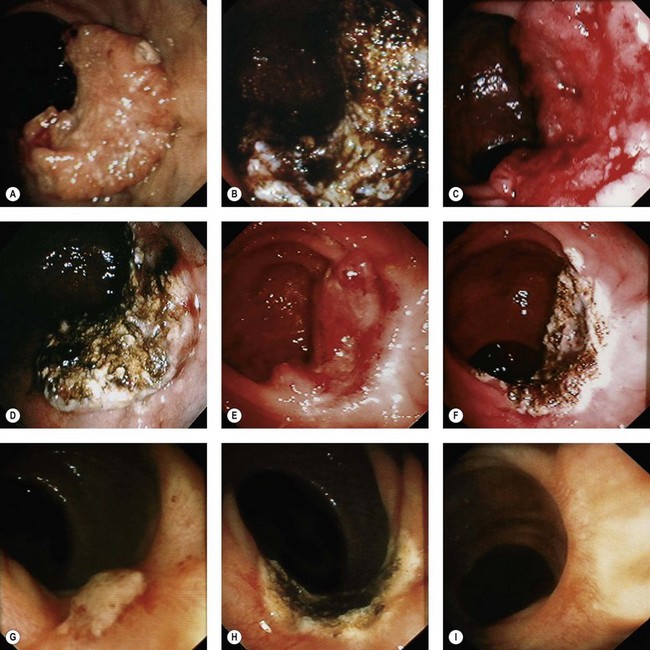
Table 2 Classification of lesions seen up to 24 h after caustic burn
| Stage | Endoscopic findings | Management and outcome |
|---|---|---|
| 0 | Normal mucosa | Excellent |
| I | Mucosal erythema and edema | Spontaneous recovery in 1–3 days |
| IIa | Ulceration of the mucous membrane with exudates and bleeding | Commence liquid diet and progress to solid after 48 h |
| No endoscopic follow-up necessary | ||
| IIb | Circumferential or deep ulceration | Major risk of stricture formation |
| Initiate nasoenteric feeding after 24 h with liquid at 48 h if clinically well | ||
| III | Extensive necrosis | High risk of perforation |
| Monitor carefully for 10 days | ||
| Surgery indicated |
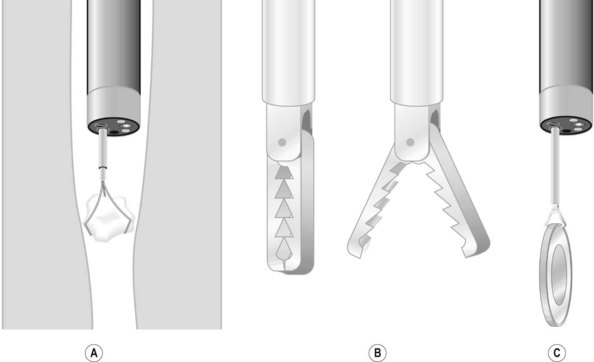
If endoscopy is required, most round, blunt objects can be removed using a Dormia basket, a Roth net, a polyp snare or forceps (Fig. 7). It is essential to ensure that the airway is protected from aspiration or inadvertent loss of the foreign body into the upper airways (i.e. coin).
5.4 Batteries
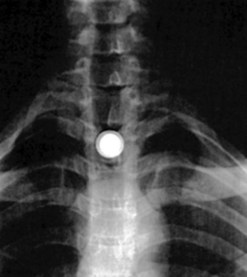
Batteries are blunt objects which yield a ‘halo’ image on standard radiographs (Fig. 1), because they have two sides with different diameters. Their ingestion requires emergency treatment, as there is a high risk of perforation. Mucosal damage can occur in the esophagus in 1 h, muscular damage in 2–4 h, with perforation occurring in 8–12 h.
5.5 Sharp foreign bodies
To extract a sharp object, ensure that the blunt end of the object is closest to the endoscope, with the sharp end following. This is done to avoid damaging or perforating the mucosa while extracting the foreign body. These objects can usually be removed using a forceps, polypectomy snare, Dormia basket or Roth net. If there is significant risk of trauma, an overtube or rubber hood should be used (Fig. 4). An alternative that has been described uses a simple latex glove: the tip is cut off and secured with a thread to the distal end of the endoscope. The foreign body is grasped and the latex glove inverts around the foreign body as it comes through the lower esophageal sphincter.
5.6 Long foreign bodies
Long objects (>5 cm) need urgent endoscopy if they are in the esophagus or stomach. They are usually pens, spoons or toothbrushes. These should be maneuvered using a polypectomy snare so that the widest part is adjacent to the endoscope. The endoscope and the long foreign body should then be withdrawn. This should be done in combination with either an overtube or a rubber hood. An overtube is recommended if the object is >45 cm long (Fig. 5).
6 Complications
7 Ingestion of toxins
7.1 Ingestion of caustic toxins
Take a detailed history and examine the patient to assess:
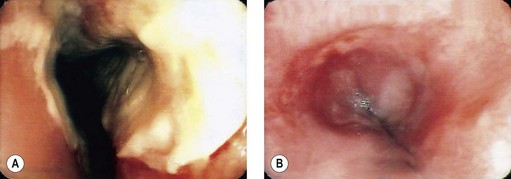
Basic (caustic cleaners, dishwasher products or hair conditioner), acidic products or bleaching agents are caustic to the pharynx, larynx, esophagus and also the stomach and duodenum (Fig. 8).
7.3 Contraindications and requirement for upper endoscopy
7.4 Endoscopy
7.5 Post-endoscopy
7.6 Special cases
Cotton PB. Overtubes (sleeves) for upper gastrointestinal endoscopy. Gut. 1983;24(9):863-866.
Kay M, Wyllie R. Pediatric foreign bodies and their management. Curr Gastroenterol Rep. 2005;7:212-218.
Webb WA. Management of foreign bodies of the upper gastrointestinal tract: update. Gastrointest Endosc. 1995;41:39-51.
Wells CD, Fleischer DE. Overtubes in gastrointestinal endoscopy. Am J Gastroenterol. 2008;103(3):745-752.
7.6 Endoscopy in obesity
Key Points
Introduction
Obesity is excess body fat and has a harmful effect on health resulting from an increased risk of cardiovascular disease (hypertension and cerebrovascular accidents), hyperlipidemia and type 2 diabetes. All cause mortality is also significantly higher in obese people. It is defined in clinical practice by the body mass index (BMI) which is calculated as weight/height2 (kg/m2). The WHO classification of obesity is shown in Table 1.
| BMI | Description | Obesity grade |
|---|---|---|
| <18.5 | Thin | |
| 18.5–24.9 | Normal | |
| 25–29.9 | Overweight | |
| 30–39.9 | Obese | I (30–34.9), II (35–39.9) |
| ≥40 | Morbidly obese | III |
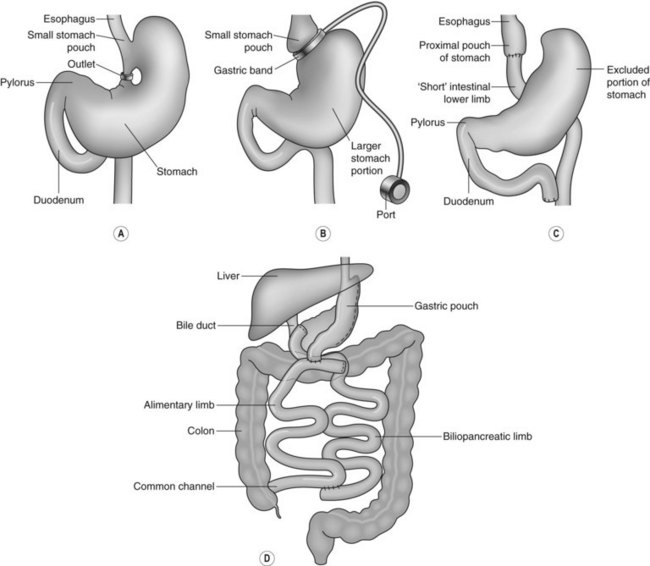
1 Endoscopy and bariatric surgery
Two types of surgical procedures are performed:
It is important for endoscopists to understand the different types of operation, the resulting anatomical alterations and the different complications that can arise following these procedures (Fig. 1).
5 Endoscopic placement of intragastric balloons
5.1 Equipment and technique
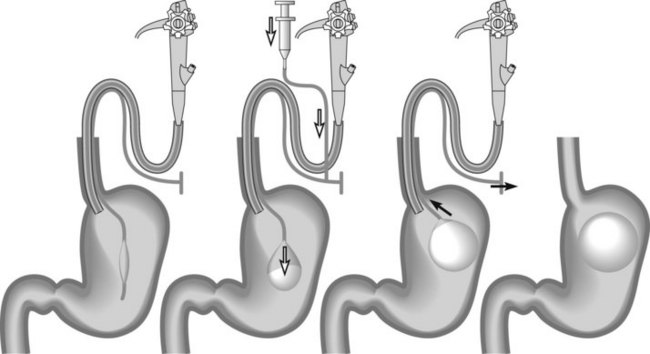
The water-filled balloon is made of silicone, equipped with an antireflux valve, and is round so it is easily positioned after an endoscopic examination of the digestive tract under anaesthesia (Fig. 2). It is released into the stomach after filling with 500–600 mL of physiological saline containing methylene blue (Figs 3, 4). The IGB is released by traction, against the gastric cardia. The covering membrane frees itself gradually, releasing the balloon from its constraint.
5.3 Contraindications
There is no consensus about contraindications (Box 1), but the presence of a large hiatus hernia, malignancy or active gastric or duodenal ulceration, may postpone the procedure or alter the approach. General anesthesia and intubation of the patient is advisable when balloons are inserted, because of the risk of aspiration. Given current knowledge of IGB, their use in adolescents is not recommended at present.
5.4 Monitoring
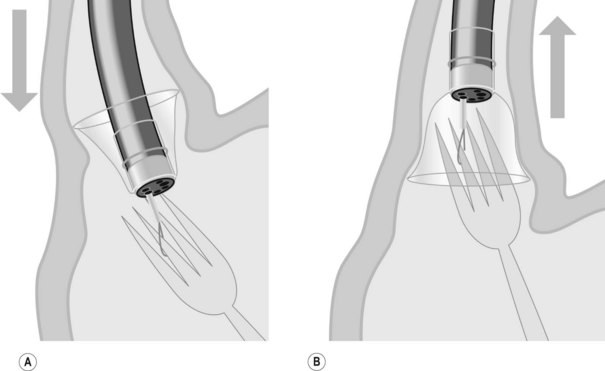
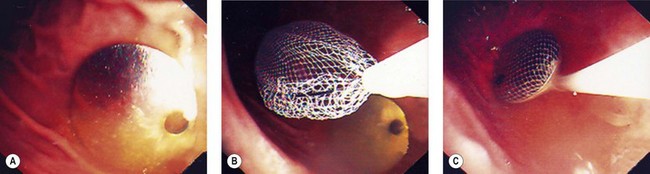
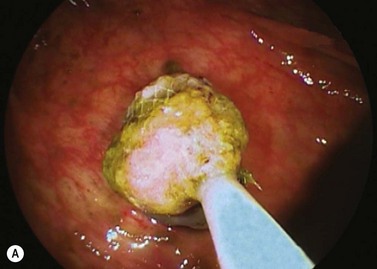
Patients should undergo clinical monitoring weekly or monthly after insertion of an IGB (laboratory tests in the event of vomiting, X-ray or ultrasound if fracture or migration is suspected), and regular consultations with a dietitian are an essential supplement of therapy for the duration of treatment. A mean weight loss of 10–12% over 6 months can be expected. According to the manufacturer’s recommendations, the IGB is left in position for 6 months and removed by puncture and suction of the physiological saline using a removable trocar mounted in a plastic catheter. Once flattened, the IGB is extracted using tripod forceps or a polypectomy snare (Figs 5, 6). As few perforations as possible should be made in the IGB because this may cause it to empty partially like a watering-can rose, forming pockets of fluid, which make it more difficult to remove.
ASGE standards of practice committee. Guideline. Role of endoscopy in the bariatric surgery patient. Gastroinest Endosc. 2008;68:1-9.
Coté GA, Edmundowicz SA. Emerging technology: endoluminal treatment of obesity. Gastrointest Endosc. 2009;70(5):991-999.
Feitoza AB, Baron TH. Endoscopy and ERCP in the setting of previous upper GI tract surgery. Part I: Reconstruction without alteration of pancreaticobiliary anatomy. Gastroinest Endosc. 2001;54:743-749.
Feitoza AB, Baron TH. Endoscopy and ERCP in the setting of previous upper GI tract surgery. Part II: postsurgical anatomy with alteration of the pancreaticobiliary tree. Gastrointest Endosc. 2002;55(1):75-79.
Sauerland S, Angrisani L, Belachew M, et al. European Association for Endoscopic Surgery. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc. 2005;19:200-221.
Tsesmeli N, Coumaros D. Review of endoscopic devices for weight reduction: old and new balloons and implantable prostheses. Endoscopy. 2009;41(12):1082-1089.
Wright BE, Cass OW, Freeman ML. ERCP in patients with long-limb Roux-en-Y gastrojejunostomy and intact papilla. Gastrointest Endosc. 2002;56:225-232.
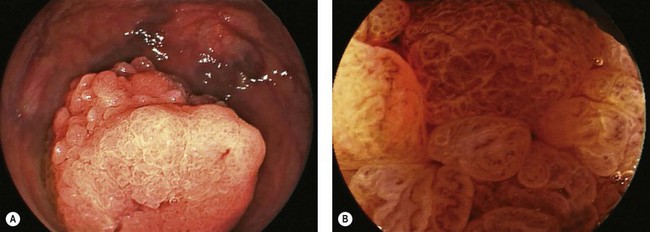
7.7 Polypectomy
Key Points
1 Optical enhancement techniques
Optical enhancement techniques, such as narrow-band imaging (NBI), chromoendoscopy (CE), autofluorescence (AF), Fuji Intelligent Chromo Endoscopy (FICE), and I-Scan, may be used to improve polyp detection and/or predict polyp histology in real time (Fig. 1) (see Ch. 6.2). The discussion of the former use is beyond the scope of this chapter. However, understanding the use of such techniques to predict polyp histology may assist in determining the need for polypectomy.
NBI and CE are the most studied optical enhancing technologies. CE involves spraying of dye, and is cumbersome and time-consuming. NBI has been called digital chromoendoscopy, does not involve spraying of dye, and is an efficient means of predicting colon polyp histology. Table 1 depicts the endoscopic features that are predictive of adenomatous and hyperplastic histology when viewed by NBI. Experienced endoscopists can correctly predict adenomatous and hyperplastic histology of diminutive polyps (≤5 mm) in 90–95% of cases. NBI may, thus, identify small distal hyperplastic polyps that need not be resected (or resected and discarded). All proximal hyperplastic polyps, however, should be resected as they may represent serrated adenomas, which are now recognized as precancerous lesions.
Table 1 Endoscopic features predictive of polyp histology when viewed by NBI
| Adenomatous polyps | Hyperplastic polyps |
|---|---|
2 Equipment
3 Small polyp removal
3.1 General concepts
3.2 Cold biopsy forceps
3.3 Hot biopsy forceps
3.4 Cold snare
3.5 Hot snare
| Current type | Early bleeding risk | Delayed bleeding risk |
|---|---|---|
| Coagulation | ↓ | ↑ |
| Cutting | ↑ | ↓ |
| Blended | ↑ | ↓ |
The type of current affects the risk of early or late bleeding. Thus coagulation is associated with an increased risk of delayed bleeding, while using a cutting current is associated with a decreased risk of delayed bleeding but an increased risk of early bleeding.