20 Interventional Cardiology
THE USE OF CATHETERIZATION in the care of children with congenital heart disease (CHD) was first described by Dexter and colleagues in 1947.1 It has evolved from a physiologic assessment tool to a technique to define anatomic relationships and has become a therapeutic modality. The first interventional procedure, balloon atrial septostomy, was described by Rashkind and Miller in 1966,2 and since then, the discipline of interventional cardiology has continued to evolve.
As echocardiography and magnetic resonance imaging (MRI) diagnostic capabilities have increased, the need for purely diagnostic cardiac catheterization has declined.3,4 However, technologic advances and more sophisticated equipment have increased the scope for interventional procedures, and the patient population has changed as more children with CHD are surviving longer. As the surgical management of CHD has evolved, it has introduced a new spectrum of surgical complications. Some surgical operations have been replaced altogether by interventional procedures, and some interventional procedures have facilitated more complex heart surgery.5 The shifts in practice and population have affected the anesthetic management of these children.6 Procedures are more diverse, and patients can vary from moribund neonates to healthy adolescents. There are no simple anesthesia recipes for all children and all heart conditions; the type of anesthesia must fit each child and each procedure.
Types of Procedures Performed
Diagnostic Catheterization
Diagnostic catheterization allows accurate documentation of pressure and oxygen content from all regions of the circulation. Interpretation of these hemodynamic data allows quantification of the degree of intracardiac shunting and calculation of the vascular bed resistances. This information is necessary to assess the suitability of a child to undergo palliative or reparative surgery for congenital heart lesions. Expected values for hemodynamic variables are listed in Table 20-1. There are no absolute values for these variables, and they vary according to the age of the child. The use of angiocardiography to define anatomy is waning because of the widespread use of noninvasive imaging modalities such as echocardiography, computed tomography, and MRI.
TABLE 20-1 Normal Values in Diagnostic Cardiac Catheterization
| Structure | Value (mm Hg) |
|---|---|
| Right atrium | 3-5 (mean) |
| Right ventricle | 20-25/3-5 (systolic/end-diastolic) |
| Pulmonary artery | 12-15 (mean) |
| Left atrium | 7-10 (mean) |
| Left ventricle | 65-110/3-5 (systolic/end-diastolic) |
| Aorta | 65-110/35-65 (systolic/diastolic) |
Interventional Catheterization
Atrial Septostomy
Atrial septostomy improves mixing of oxygenated and deoxygenated blood at the atrial level in neonates with d-transposition of the great vessels. Children with this disorder are born with ventriculoarterial discordance. The right ventricle pumps deoxygenated blood to the aorta, and the left ventricle pumps oxygenated blood to the main pulmonary artery. Enlargement of the foramen ovale by atrial septostomy improves mixing of oxygenated and deoxygenated blood, resulting in increased systemic oxygen saturation. This procedure can be performed in the catheterization laboratory with fluoroscopic guidance, or it can be easily and safely undertaken at the bedside in the intensive care unit using echocardiographic guidance.7 A femoral venous or umbilical venous approach can be used. The drawback with the umbilical venous route is the often-encountered difficulty in traversing the ductus venosus to secure access to the inferior vena cava. The major risks with this procedure are vessel injury, paradoxical embolism, arrhythmia, and cardiac perforation.
Atrial Septal Defect Closure
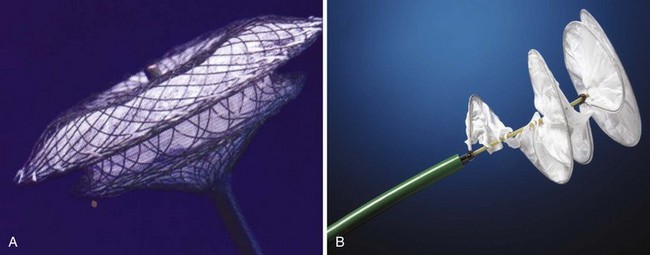
To warrant closure, children need to demonstrate clear evidence of volume loading of the right heart structures and a defect that is unlikely to close spontaneously in the short to medium term. The choice of closure device depends on the size and the margins of the defect. The two types of closure devices have either a centering or a noncentering design (Fig. 20-1). The choice of one design over another is based more on the clinician’s preference than scientific performance, although the Amplatzer Septal Occluder (AGA Medical Corporation, Golden Valley, Minn.) can close a wider range of defect sizes.8–10 Daily aspirin in a dose of 3 to 5 mg/kg is recommended for a minimum 6 months after implantation of either type of device. The main complications associated with ASD closure include vessel injury, cardiac arrhythmia, cardiac perforation, and device embolization.11 Atrial septal closure devices have also been used to close surgically created fenestrations between the atrium and venous conduits after a Fontan operation. This is undertaken only when the fenestration is no longer required.
Ventricular Septal Defect Closure
Ventricular septal defect (VSD) closure presents a technically greater challenge than ASD closure and is associated with a greater risk. The VSDs most suitable for device closure are those in the midmuscular septum or those closer to the apex.12 With further refinement of the implantation technique and equipment, this approach has been undertaken with perimembranous defects.13,14
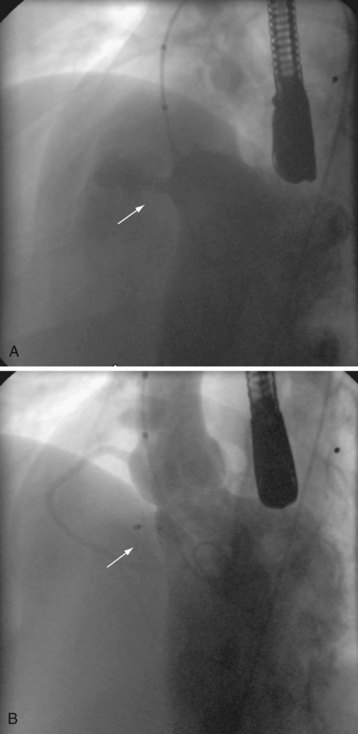
During device closure of VSDs, a snare is placed in the right side of the heart to capture a guidewire that has been passed across the VSD from the left ventricle. The guidewire is brought outside the body to form an arteriovenous rail. The delivery sheath for the VSD device is then advanced over the wire to approach the VSD from the right side of the heart. For anterior and high muscular defects, the wire is best snared and exteriorized through a femoral vein approach, whereas for defects in the middle to low muscular septum, the wire is best snared and exteriorized through a jugular venous approach (Fig. 20-2). Complications include dysrhythmias, blood loss, valve dysfunction, and device embolization.15,16 At our institution,17 device closure of perimembranous VSDs with the Amplatzer Membranous VSD Occluder had an unacceptable incidence of complete heart block and is therefore not currently performed. However, other medical units have continued to undertake this intervention, and alternative devices are being developed with the goal of implantation that does not cause complete heart block.
Patent Ductus Arteriosus Closure
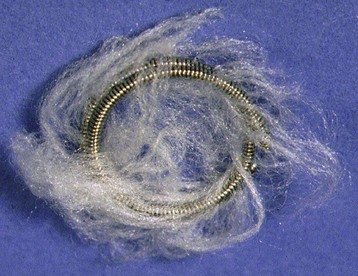
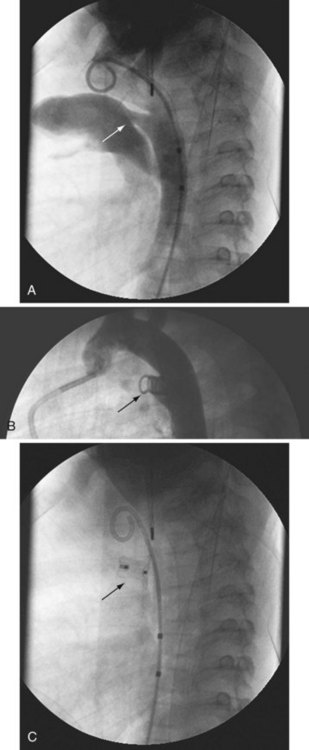
Closure of patent ductus arteriosus (PDA) was the second specific intervention developed for children with CHD, and it continues to be a common procedure performed using techniques that are similar to the original methods pioneered by Rashkind and associates.18 The customary approach is to perform an aortogram to define the size and geometry of the PDA. Based on this information, a choice is made between using a stainless steel coil or an occluder device.19 Most interventional cardiologists implant a stainless steel coil to close a small PDA (no greater than 3 mm) using a retrograde or antegrade approach.20 To lessen the chance of coil embolization during implantation, several techniques can control release of the coils.21–23 For the larger PDA, most interventional cardiologists implant an occluder device because it lessens the risk of a significant residual shunt. The major risks associated with this procedure are vessel injury and device or coil embolization. Coils are also used to close major aortopulmonary collateral vessels (Figs. 20-3 and 20-4).
Balloon Dilation and Stent Implantation
Balloon angioplasty techniques are used to dilate stenotic aortic, mitral, tricuspid, and pulmonary valves and stenotic segments of the aorta or of the pulmonary arteries. In neonates, membranous atresia of the pulmonary valve may be crossed with the stiff end of a guidewire24 or with radiofrequency catheters.25 After both techniques, the valve is dilated with a balloon that is approximately 120% the size of the annulus. Balloon angioplasty of stenotic pulmonary valves in children beyond infancy is often a curative procedure, whereas balloon valvuloplasty of critical pulmonary stenosis in the neonate often requires intervention again in later infancy. The potential hemodynamic behavior of the child depends on the nature of the lesion. A neonate with duct-dependent critical stenosis and little antegrade flow can tolerate balloon dilation well because there is little disruption of the cardiac output, whereas neonates and infants with less critical stenosis can suffer significant reductions in cardiac output when the balloon is inflated, especially if the ductus arteriosus is not patent. Older children tend to tolerate balloon valvuloplasty surprisingly well,26 and life-threatening hypotension is uncommon.27
In contrast to pulmonary balloon valvuloplasty, aortic balloon valvuloplasty is usually only a palliative procedure, with most children eventually requiring surgery. Balloon dilation of aortic stenosis in the neonate is a high-risk procedure. These infants often present in a low cardiac output state requiring ventilation, inotropic support, and prostaglandin E1 (PGE1) infusion to maintain ductal patency. Catheterization can be complicated by arrhythmias (including asystole), the development of significant aortic regurgitation (which may require surgical intervention), and sudden death due to acute coronary ischemia.26 The complication rate in older children is less than in younger children, and transient hypotension, bradycardia, and left bundle branch block are commonly reported.
Nonsurgical Pulmonary Valve Replacement
It is more than a decade since Bonhoeffer and colleagues28 first described the technique of replacing a dysfunctional valve in a right ventricle to pulmonary artery conduit with a catheter-implanted valve. The technology used has matured, and the Melody Valve (Medtronic Inc, Minneapolis, Minn.) is available for use in Europe and is undergoing clinical trials in the United States. The Edwards Sapien Transcatheter Heart Valve (Edwards Lifesciences LLC, Irvine, Calif.) has also been used but is currently not licensed for this indication. Other results29 have confirmed a high procedural success rate and satisfactory short-term valve function with implantation of the Melody Valve. The need for careful patient selection and for adequate relief of right ventricular outflow tract obstruction at the time of valve implantation are paramount in achieving results comparable to those of surgical replacement of dysfunctional conduits. Unfortunately, most children after tetralogy of Fallot repair with a transannular patch are currently unsuitable for implantation of a stented valve because of an aneurysmal right ventricular outflow tract.
Alternative devices are being sought to overcome these problems and to reduce the size of the delivery systems to enable use of these technologies in younger and smaller children. A novel use of Melody devices in an animal model that replicates the clinical situation of an aneurysmal right ventricular outflow tract has been reported.30 Implantation of Melody Valves in branch pulmonary arteries appears to favorably reduce the regurgitant fraction in this animal model.
Electrophysiologic Catheterization
Endocardial catheters that record an electrocardiogram first became available in the early 1960s. Endovascular techniques were developed as an alternative to surgery for certain forms of tachycardia. These therapies initially used direct current energy and subsequently have made use of radiofrequency energy and localized freezing techniques. Tachycardia may be treated by this technique in children with structurally normal hearts and those who have dysrhythmias after surgery for CHD.26 Ablative procedures account for about 20% of all cardiac catheterization procedures.
The technique requires specialized equipment, specially trained staff, and use of multiple catheters to measure the electrical signals within the heart at any given time. These procedures are often more time consuming than other catheterization procedures. The success of the therapy depends on the mechanism of the tachycardia, the location of the aberrant pathway, and the technique for interrupting the aberrant pathway. Ablation of ectopic foci has a good success rate and small complication rate.31,32 The main risks with this procedure are heart block, cardiac perforation, vessel injury, and stroke.
Choice OF Vessel Access
The most common approach for cardiac catheterization is the femoral route. Femoral venous catheterization avoids the risk of pneumothorax, and the vein is easier to access than the internal jugular vein in the unanesthetized child. In children who are likely to have a cavopulmonary shunt placed surgically for palliation, avoiding routine cannulation of the internal jugular vein can decrease the risk of compromising the superior vena cava. However, internal jugular vein access sometimes is required, such as during VSD closures, investigation of cavopulmonary connections in children, and when the cardiac interventionalist is unable to obtain access through the femoral veins. In neonates, the umbilical vein may be used, although it can sometimes be difficult to cross the ductus venosus. Patency of the ductus venosus can be assessed by ultrasound before the procedure to avoid unnecessary manipulation of the umbilical vein. An alternative is transhepatic puncture. This route has been used for temporary access during catheterization and for long-term vascular access.33
Complications and Limitations of Procedures
Interventional cardiology can be associated with significant morbidity and mortality. Complications attributable to the procedure or the physiology of the child occur far more frequently than purely anesthesia-related problems. An important prerequisite for providing quality anesthesia care is understanding the diagnosis and management of anticipated complications. Complications such as tamponade, dysrhythmia, embolism, and rupture may occur suddenly and without warning. The anesthesiologist must be vigilant and maintain communication and rapport with the cardiologist throughout the procedure. The availability of backup from surgeons, cardiologists, and anesthesiologists is preferable, and standard procedures for emergencies should be in place. Some clinicians advocate the availability of cardiopulmonary bypass or extracorporeal membrane oxygenation (ECMO) for children with unanticipated difficulties.16 Although this type of therapy is available in major referral hospitals, it may not be an option in some centers. Institutions must develop policies regarding the procedures that can be undertaken locally (based on experience and infrastructure) and those that require referral of children to centers that are better equipped to address more complex procedures.
Overall Mortality
Despite the increased complexity of interventional procedures, the mortality rate is steadily decreasing. A report from the early 1960s found an overall mortality rate (neonates through to adulthood) of 0.44%,34 but more recent data show overall mortality rates of 0.08%,35 0.14%,36,35 and 0.39%.27 All reviews describe a relatively high mortality rate among infants and neonates in particular.27,36,37 Explanations include a reduced physiologic reserve, presence of uncorrected or partially palliated congenital heart defects, increased risk of obstruction to great vessels and cardiac chambers, and greater susceptibility to catheter-induced damage in infancy.27 However, neonatal mortality rates are diminishing; one institution reported a decrease from 6.7% to 0.9% during a span of 20 years.36 The explanations cited for this decrease were noninvasive imaging that reduced the number of neonates who required cardiac catheterization, improved management of the critically ill child, correction of metabolic abnormalities, use of PGE1, and use of improved catheters and better support equipment such as temperature control.36
Overall Morbidity
Complications are frequently categorized as major, minor, and incidental.27,37 Major complications are potentially life-threatening events that require surgical intervention or are significant permanent lesions resulting from the procedure (e.g., cerebral infarct). Minor complications are transient and resolve with specific treatment (e.g., transient arterial thrombosis, temporary loss of a pulse or decreased perfusion after arterial puncture). An incidental complication has no effect on the patient’s condition and requires minimal or no treatment (e.g., transient hypotension responding to volume infusion, catheter-induced arrhythmia). The incidence of major complications is between 1.4%37 and 2.6%,38 and the incidence of minor complications is between 6.8%37 and 7.5%.38 The overall complication rate has remained stable for the past 20 years.38
Three groups of children are at substantial risk for complications: those who are young, those with low weight, and those undergoing interventional rather than diagnostic procedures; balloon interventions are associated with the greatest risk.36–38 The incidence of vascular access complications after interventional procedures is three times greater than the rate for diagnostic procedures36; this may reflect the use of larger-diameter catheters during interventional procedures than during purely diagnostic procedures. Closure of a PDA and balloon atrial septostomy carries a small overall risk.27,36
Vascular Complications
Vascular complications are the most common and broadest category of complications.36 They may be acute, leading to unexpected hemodynamic instability, or delayed, leading to longer-term morbidity. Many factors contribute to unexpected hemodynamic instability, including the child’s condition, blood loss, dysfunction of a valve, arrhythmias, tamponade, vessel rupture, balloon dilation, catheter-induced interruptions in blood flow or coronary perfusion, and device malposition.
Arterial Thrombosis and Occlusion
Femoral artery occlusion due to thrombosis is a common complication after femoral cannulation.36 The true incidence of arterial compromise is unknown,39 although 32% of infants had compromised blood flow to the leg as measured by Doppler after femoral arterial cannulation in one study.40 The clinical incidence of arterial compromise is 2.4% to 3.7%.36,37 Infants undergoing dilational interventional procedures are at a greater risk.41 The incidence of these complications can be reduced by minimizing the size of the sheath, use of systemic heparinization, and avoidance of arterial entry by using alternative techniques to enter the left side of the heart.36 Despite the widespread use of intravenous heparin for prophylaxis against arterial occlusion, there is no agreement on the appropriate dosage. Commonly, 50 to 100 IU/kg heparin is used, but schedules vary.26 Larger doses than these do not reduce the incidence of arterial compromise.39
In most cases where the pulse is reduced or absent after catheterization, the occlusion either spontaneously resolves or is managed with anticoagulation or thrombolytic therapy.36,37,41 Guidelines are available for the management and prevention of femoral artery thrombosis, but advice should be sought from a pediatric hematologist.42 Although surgical intervention is rarely required, it is most commonly indicated for an arterial tear or avulsion, arterial thrombosis (including iliac arteries), and arterial pseudoaneurysm.41 Occasionally, despite medical therapy and a well-perfused lower limb, a pulse may be persistently reduced. There is little evidence to predict how and when a reduced pulse may cause delay in limb growth,36 although cases have been reported.41 Although femoral cannulation for balloon intervention is associated with vascular compromise of the superficial femoral artery, one small study (43 children between 1 day and 15 years of age) failed to demonstrate significant limb growth discrepancy after a median follow-up of 3.5 years.43
Venous Thrombosis and Occlusion
Although venous thrombosis is a well-known complication of central venous access, the incidence after cardiac catheterization is unclear. Isolated cases of femoral or iliofemoral venous occlusions with limb edema have been published as part of large series,36,37 in which the incidence of symptomatic venous occlusion was less than 0.3%. All of these children responded to heparin therapy without the need for further intervention.37 As in arterial thrombosis, the use of smaller catheters and heparin prophylaxis during catheterization procedures may reduce the incidence of venous thrombosis.
Vessel Rupture, Perforation, and Dissection
Vessel rupture can occur at the site of vessel entry or at the site of intervention. It is a rare but potentially catastrophic event. One death due to intraabdominal hemorrhage after rupture of a femoral vein in a neonate was reported in a series of 4454 catheterizations.27 Arterial or venous perforation was responsible for four major complications and six minor complications in a series of 4952 procedures, and significant groin hematoma occurred in 25 cases.36 Femoral artery injuries may require surgical consultation and exploration.
Vessel perforation has occurred at the site of intervention, particularly during balloon interventions. Ruptures have been reported most often after balloon dilation of branch pulmonary arteries,4,26 but they also have occurred along the ascending aorta and arch after balloon dilation of the aortic valve. Depending on the site of the tear, rupture may cause hemopericardium or hemothorax, or both.41 Intrapulmonary hemorrhage is usually self-limited. If rupture or hemorrhage occurs, hypertension should be avoided, the trachea should be intubated (if the airway is not already secured), and any circulating heparin should be reversed. Pulmonary artery disruption after balloon dilation may manifest as hemoptysis. Increased blood flow after balloon dilation of pulmonary vessels may lead to unilateral pulmonary edema, which may also present as hemoptysis. Occasionally, arterial dissection,41 aneurysm, and pseudoaneurysm formation may occur.
Cardiac Tamponade and Perforation
Cardiac tamponade is an uncommon complication of cardiac catheterization, but when it occurs, it can be responsible for significant morbidity and mortality. The incidence of cardiac tamponade in three large series was 0.1%,36 0.04%,27 and 0%.37 In one series of 4952 patients, tamponade was responsible for two deaths: one neonate after a balloon atrial septostomy and one 4-year-old child after a recent Fontan procedure for stent insertion in a branch pulmonary artery.
Although tamponade is uncommon, perforation is not. The atrial appendage and right ventricular outflow tract are the sites most commonly perforated,41 whereas the left ventricle has been punctured less commonly.42 Perforation of the heart is described during many procedures, including balloon and blade atrial septostomy, balloon dilation of the mitral valve,4 and attempted radiofrequency perforation of membranous pulmonary atresia.5
Damage or Dysfunction of a Valve
Damage to a valve is not particularly common, although it is more likely to occur with a balloon valvuloplasty procedure than with other procedures.41 The primary complication is creation of excessive regurgitation. The hemodynamic consequences of such a defect are more significant on the systemic side of the circulation than on the pulmonary side.5,41 The mechanism of injury is most commonly leaflet avulsion during dilation, although the leaflet can be inadvertently perforated by the guidewire, and there is the likelihood of significant further damage to the leaflet with advancement and inflation of the angioplasty catheter. Emergency repair is occasionally required.41 Injuries to the atrioventricular valves have rarely been reported. Placement of wires and large sheaths across atrioventricular valves and septal defects can cause severe hemodynamic disturbance. This is particularly true during implantation of VSD occlusion devices.15,26 ASD and PDA occlusions are less likely to produce significant hemodynamic disturbance.26 Rarely, the implanted ASD and VSD devices adversely affect the functioning of an atrioventricular valve.
Blood Loss
Blood loss may be sudden with rupture of vessels, but more often, it is slow and insidious due to multiple blood samples and blood loss associated with catheter exchanges. Anemia may be difficult to detect in a dark room and a covered child. Significant loss is more likely to occur during insertion of a closure device. Blood transfusion was required in 54% of 86 VSD closure devices in one early study.15 Because interventions have the potential for blood loss, it is reasonable to have a unit of blood and blood administration equipment in the cardiac catheterization laboratory.
Dysrhythmias and the Catheterization Laboratory
Transient dysrhythmias are common during cardiac catheterization.27 Most dysrhythmias are mechanically induced, and repositioning the wire or catheter usually resolves the dysrhythmia. Other causes of rhythm abnormality include coronary air embolism, electrolyte imbalance, and hypercarbia. Although they are usually minor complications, dysrhythmias are one of the most common causes of major complications, with a frequency of 2.6%36 to 3.6%.37 Infants have the greatest incidence of rhythm disturbance. A defibrillator, a pacing device, and antiarrhythmic agents must be present in the cardiac catheterization suite. Doses of any unfamiliar antiarrhythmic drugs should always be double-checked or determined before the procedure with the cardiologist.
Types of Dysrhythmias
Dysrhythmias may be atrial or ventricular in origin or involve degrees of heart block. Atrial arrhythmias such as supraventricular tachycardia or atrial flutter frequently resolve spontaneously, but persistent atrial dysrhythmias can be treated pharmacologically or with overdrive pacing. They rarely progress to major events.36,37 Atrioventricular block occurs in 0.4% of cases, of which one fourth required pacing, although all children were in sinus rhythm by the time of discharge from hospital.37 First- or second-degree atrioventricular block is well tolerated at all ages. When complete heart block occurs, it usually resolves shortly after the procedure and rarely persists.36 Device closure for VSD has a high incidence (10.5%) of severe junctional bradycardia or complete heart block, and almost half of these children require pacing or isoproterenol.15 Transient left bundle branch block has been reported after dilation of the aortic valve.26
Overall, the incidence of ventricular tachycardia or fibrillation is approximately 0.2%.36,37 However, 30% of children who underwent VSD device placement had serious dysrhythmias and hypotension requiring catheter withdrawal, and 8.5% of them had ventricular arrhythmias requiring lidocaine or cardioversion.15 Even relatively low-risk procedures are associated with dysrhythmias. Balloon atrial septostomy is frequently accompanied by rhythm disturbances. Typically, they are transient, but rarely, they can be permanent or even fatal.4
Cardioversion
Atrial, supraventricular, and ventricular tachyarrhythmias; bradycardias; atrioventricular block; and bundle branch blocks have been described after cardioversion. Factors that influence the incidence of tachyarrhythmias include the underlying rhythm disturbance and cardiac disease, metabolic derangement, drugs (e.g., digoxin), and the strength of shock. Histologic injury to the myocardium is rare when the starting power for cardioversion is set at 0.5 J/kg.26 Systemic and pulmonary emboli are rare in children compared with adults, for whom the incidence is 1% to 2%. Children with pacemakers are becoming more common, and these patients may require defibrillation. This can be done safely if the electrode pads are placed a distance from the generator and the pacemaker circuits and programming mode are checked afterward.
Cyanosis
When cyanosis occurs in the catheterization laboratory, it may be respiratory or circulatory in origin. A transesophageal echocardiographic (TEE) probe can cause desaturation by compressing the bronchi or vessels, pressing on the trachea, or precipitating bronchospasm. These events are more common in children weighing less than 10 kg.26 Pneumothorax is rare but documented.37 Hypercarbia, acidosis, excessive positive-pressure ventilation, contrast media, and hypoxia can increase pulmonary vascular resistance, which may lead to increased shunting and cyanosis. Hypercyanotic episodes are frequently observed,36 particularly in infants with uncorrected tetralogy of Fallot. In one study, 12% of children with tetralogy of Fallot exhibited a hypercyanotic episode within 12 hours of catheterization despite adequate hydration, sedation, and the use of nonionic contrast media.37
Embolization
Device and Balloon
In a 1998 report of 1457 interventional cases, 18 devices embolized; 3 required surgical removal, 8 were removed in the catheterization laboratory, and 7 were of no hemodynamic consequence and were left in situ.36 Devices that embolized included coils, duct umbrellas, an atrial defect occlusion device, and endovascular stents.36 Improvements in device design have reduced the risk for embolization; for example, for ASD devices the risk has decreased from 11.1% to less than 1.1%.4,44
Balloon rupture was common in the past, although it rarely produced intimal damage or embolic phenomena.41 Balloon fragmentation has become uncommon because of technologic improvements in materials and design. To minimize this risk, an inflation device with an attached manometer is recommended to ensure that the pressure does not exceed the burst pressure of the balloon.
Thrombus or Dislodged Material
Thrombus may be dislodged from devices or catheters, and balloon dilation may dislodge calcium, intimal lining from conduits, and thrombus from surgical systemic-pulmonary shunts.4
Air
Gas emboli may occur in sheaths and catheters, burst balloons, or anesthetic infusion lines. Air embolus (as well as blood loss) is a known risk during interventional procedures in which there are many wire and catheter exchanges.26 Balloons are dilated with a weak contrast mixture, and in view of the occasional balloon rupture, it is important to ensure all gas bubbles are eliminated from the contrast mix syringe and catheter before dilation is undertaken. Balloons used for flotation tip catheters should be filled with carbon dioxide rather than air to minimize the potential embolic effect if the balloon bursts. All intravenous lines, injections, and infusions must be free of air bubbles because these sources can cause embolic occlusion of arterial vessels, producing cerebral or myocardial ischemia in children with right-to-left mixing. Nitrous oxide should be avoided because it may expand any air embolism.
Contrast Toxicity
Adverse reactions to intravascular contrast are relatively uncommon, but the anesthesiologist must diagnose and manage a contrast-mediated reaction early in order to minimize morbidity or mortality. Reactions are often classified as idiosyncratic (i.e., unpredictable reactions independent of dose or concentration such as anaphylaxis) or chemotoxic (i.e., related to dose and physiologic characteristics such as osmolality). The pathophysiology of most reactions, however, is complex.45
Acute Reactions
Acute reactions to contrast agents can vary from mild to severe. Flushing, nausea, pruritus, vomiting, headache, and urticaria occur in 1% to 3% of patients receiving nonionic contrast.46 These reactions are usually mild and self-limiting, requiring no specific treatment. Intermediate effects can manifest as moderate hypotension and bronchospasm and as more severe degrees of the mild reactions. Severe reactions can include convulsions, laryngeal edema, dysrhythmias, and cardiac arrest.
The likelihood of reaction varies with the type of contrast material; low osmolar (nonionic) solutions have considerably reduced risk. The incidence of severe reactions to high osmolar (ionic) contrast is 0.2% to 0.06%, whereas reaction to low osmolar contrast is five times less common.45 Reactions are more common when contrast medium is given through an arterial access compared with a venous access. Acute reactions should be managed according to anaphylaxis protocols (i.e., oxygen, intravenous fluid, epinephrine, corticosteroids, and histamine1– and histamine2-antagonist therapy). Prophylaxis with corticosteroids and antihistamines should be considered only if there is a well-documented history of acute reaction to a nonionic contrast material. If there is a history of reaction to nonionic contrast material, other imaging modalities, such as MRI, should be strongly considered. Occasionally, staining of the myocardium by contrast material has been observed, although this does not appear to confer any significant consequences.37
Delayed Reactions
Delayed reactions to ionic and nonionic media are well described, with an incidence in one study of 8%.47 Manifestations of these reactions include flulike illness, parotitis, nausea and vomiting, abdominal pain, headache, and rashes. The pathophysiology is unknown. Reactions (e.g., seizures, cerebral edema, electrolyte imbalance) to high- or low-osmolar solutions are possible if given in excessive doses.
Renal Adverse Reactions and Prevention
The term contrast media nephrotoxicity (CMN) refers to an increase in serum creatinine concentration by more than 25% or 0.5 mg/dL within 3 days of receiving intravenous contrast media in the absence of another cause.45,47 The underlying mechanism of the renal injury is unclear, although it is thought that contrast agents can reduce renal perfusion and are toxic to the tubular cells.
CMN occurs almost exclusively in children with preexisting renal damage. Children with CHD undergoing coronary angiography with low-osmolar contrast media may develop limited glomerular effects and reversible tubular dysfunction, but no long-term effects have been demonstrated. However, any child with reduced renal perfusion (e.g., dehydration, cardiac failure) should be regarded as at risk for CMN. It has been suggested that infants and children who receive more than 5 mL/kg of nonionic contrast agent are at increased risk for CMN.48
Many interventions have been given prophylactically to prevent CMN, including normal saline/half-normal saline hydration, administration of N-acetylcysteine (NAC), mannitol, theophylline, calcium channel blockers, diuretics, dopamine, dopamine1 (D1) receptor antagonists, endothelin receptor antagonists, atrial natriuretic peptide, angiotensin-converting enzyme inhibitors, and PGE1.49,50 Although preliminary studies with NAC have been promising, no interventions have been more effective than normal saline hydration. To minimize the risk of CMN, the minimal dose of contrast agent should be used.49,50 When possible, potentially nephrotoxic drugs should be stopped at least 24 hours before the procedure.51
Gadolinium-based contrast materials are considered non-nephrotoxic in the normal MRI dose of up to 0.3 mmol/kg.51 However, there is some evidence that the increased doses required for cardiac angiography may confer adverse renal effects.52
Neurologic Events
Central and peripheral neurologic damage can occur as a complication of the catheterization procedure. In one prospective study, 0.38% children suffered a neurologic complication, and the incidence is significantly greater after interventional procedures than diagnostic procedures.53
Central Nervous System
An ischemic cerebrovascular event may occur as a result of embolization, damage to the carotid artery, or acute low cardiac output states causing hypoxic-ischemic encephalopathy.37,53 Thrombotic emboli may originate from any site in which there is endovascular or endocardial damage from the inner surface of the catheter or an implanted device. Factors that increase the risk during interventional procedures include large catheter size, more numerous vascular punctures, and procedures of increased duration.53 However, embolic strokes also can follow an unremarkable catheterization procedure. The most common complications after an embolic stroke are convulsion and hemiplegia. Children with this type of stroke usually recover fully.53 Seizures have been associated with lidocaine toxicity.54 The outcome is more guarded after hypoxic-ischemic encephalopathy that occurs after a period of reduced cardiac output.53
Peripheral Nervous System
As with any prolonged procedure under general anesthesia, it is crucial to consider pressure areas and traction forces on nerves such as the brachial plexus. Reduced cardiac output states associated with cardiac catheterization can augment the risk. Frequently, the arms are extended above the head to improve the lateral views of the heart. Brachial plexus injury is a risk in these circumstances26,55 and is an important cause of malpractice lawsuits.56At-risk positions should be accepted only if the cardiologist clearly indicates it is required. If it is necessary to bring the arms above the head, the elbows should be flexed and elevated at least 15 cm above the level of the table to minimize traction on the brachial plexus. Head rotation should be minimized.55,57 Passive movement may help to reduce injury, but it increases the risk of dislodging monitoring equipment or the airway. If such a position is required, it should be documented in the anesthesia record that this was the demand of the cardiologist.
Radiation
Radiation poses a risk to the patient and staff. Radiation overexposure can lead to scarring and skin injury, cellular injury, gene mutation, cell death, leukemia, bone cancer, thyroid cancer, and birth defects.58 The principle with regard to radiation exposure is expressed by the acronym ALARA (or ALARP) which means as low as reasonably achievable (or practical). This principle must be applied in the context of obtaining adequate diagnostic images.
Radiation Exposure of Patients
Radiation exposure is a particularly important for children because of their greater radiosensitivity compared with adults. Moreover, a greater proportion of their body is irradiated during procedures. Complex interventional procedures require long fluoroscopy times with multiple angiographic or fluoroscopic acquisitions.59 Children undergoing electrophysiologic studies are particularly at risk for long fluoroscopy times.
When estimating the lifetime risk of cancer from radiation exposure, the child’s age and weight need to be considered along with the duration and effective dose of radiation exposure. The risk for adult coronary angiography is 6% per sievert (Sv), and the average dose is about 10 mSv, which gives an increased risk of 0.06%.60 In contrast, infants require smaller exposures due to their reduced body weights, but they have an increased sensitivity. An infant has a lifetime cancer risk of 11% to 15% per sievert. If an infant is exposed to approximately 20 mSv (e.g., 1 hour of fluoroscopy and seven digital acquisition runs), the lifetime cancer risk is estimated as 0.03%.60
Overcoming Limitations
The main limitation in the use of catheterization techniques in younger or smaller children has been the delay in development of equipment of appropriate size. To overcome this difficulty, clinicians have developed hybrid procedures to permit current catheter-based techniques to be used in infants and small children. Examples of this collaborative approach between surgeon and interventional cardiologist include perventricular closure of muscular ventricular septal defects61 and hybrid stage 1 palliation for hypoplastic left heart syndrome (i.e., off-pump placement of a PDA stent and creation of an unrestricted ASD).60
The next great challenge is to develop equipment and techniques that can be used in conjunction with MRI.62 This would minimize radiation exposure to children and staff. However, there are significant technical obstacles that need to be overcome before this approach can fully replace catheterization laboratories using ionizing radiation.
Anesthesia
Who and How?
The care of these children may be provided by a nurse anesthetist, general pediatric anesthesiologist, or specialized cardiac pediatric anesthesiologist, depending on the complexity of the child′s condition and qualifications of the practitioner. Because deep sedation can easily merge into general anesthesia, current guidelines sensibly suggest that deep sedation should be supervised by someone skilled at providing anesthesia and sedation.63 This person should be skilled at resuscitation of children with CHD and must not be the proceduralist. The choice of sedation or general anesthesia and the seniority of anesthesiologist should match the procedure and the child.
Increasingly, there are fewer diagnostic procedures and more interventional procedures. This change is reflected in a shift from sedation to general anesthesia and the expanding role of specialized cardiac pediatric anesthesiologists.64 Anesthesia providers for these children must have a high level of experience in pediatric anesthesia and a thorough understanding of pediatric cardiology and CHD. They must understand the physiology, the procedure, and the potential complications.
Preprocedural Assessment and Management
Up to 25% of children with CHD have syndromes or other anomalies that may affect their anesthesia care and require a thorough assessment of all relevant systems. The children might have had previous cardiac surgery or several other interventions. Obtaining intravenous access may be very difficult in some of them. Children with CHD are likely to have undergone anesthesia several times in the past, and the family may be well informed about the child’s condition, hospital process, and anesthesia. During the preoperative assessment, it is important to have a discussion about the sedative premedication, parental presence, and the mode of induction of anesthesia (see Chapters 1 and 4).
Anatomy and Function
THE Environment
The cardiac catheterization laboratory is a hostile environment for anesthesiologists. Access to the child may be limited by the anteroposterior and lateral x-ray cameras, sterile drapes, and radiation protection devices. The x-ray cameras are bulky and may be unexpectedly moved for oblique views, different fields, or greater magnification. The lighting is often subdued to enhance viewing of the radiographs (Fig. 20-5).
THE Cardiac Patient
The management of anesthesia for children with CHD is discussed further in Chapters 15 to 17. Important considerations include the potential for myocardial dysfunction and poor ventricular function reserve. This may be a particular problem in children with hypertrophied right ventricles operating at near-systemic pressures or with volume-loaded dilated ventricles.
 Patients may have increased reactivity, such as in neonates or children with primary pulmonary hypertension.
Patients may have increased reactivity, such as in neonates or children with primary pulmonary hypertension.
 Children may have chronically increased pulmonary artery pressures and minimal right ventricular reserve.
Children may have chronically increased pulmonary artery pressures and minimal right ventricular reserve.
 Children may have balanced circulations, such as after a Norwood procedure, in which increases or decreases in pulmonary vascular resistance may lead to spiraling hypoxia or systemic ischemia and acidosis.
Children may have balanced circulations, such as after a Norwood procedure, in which increases or decreases in pulmonary vascular resistance may lead to spiraling hypoxia or systemic ischemia and acidosis.
 Children may have low-pressure pulmonary circulations, such as cavopulmonary shunts or after Fontan surgery.
Children may have low-pressure pulmonary circulations, such as cavopulmonary shunts or after Fontan surgery.
Choice of Anesthesia
 Ensure the child is not distressed
Ensure the child is not distressed
 Provide optimal conditions for accurate diagnostic measures or successful completion of any intervention
Provide optimal conditions for accurate diagnostic measures or successful completion of any intervention
 Minimize any risk inherent in children with abnormal cardiovascular physiology
Minimize any risk inherent in children with abnormal cardiovascular physiology
Sedation
The difference between deep sedation and general anesthesia is imprecise and controversial, especially for small children in whom consciousness and memory are harder to measure (see Chapters 45 and 47). When considering the suitability of sedation or general anesthesia, several issues are important:
 Does immobility need to be guaranteed?
Does immobility need to be guaranteed?
 What will be the level of stimulation?
What will be the level of stimulation?
 Do oxygen and carbon dioxide tensions need to be controlled?
Do oxygen and carbon dioxide tensions need to be controlled?
 What will be the cardiovascular effects of the anesthetic agents?
What will be the cardiovascular effects of the anesthetic agents?
Sedation techniques have evolved over the past 2 decades, resulting in much more effective and titratable strategies today. Since the 1950s, the classic lytic cocktail, consisting of intramuscular meperidine, promethazine, and chlorpromazine, has been the standard for sedation.65 However, this cocktail has a high incidence of failure and oversedation, and is associated with sterile abscess formation.66 Oral ketamine and midazolam provide more reliable sedation, but occasionally, respiratory support is required.67 In theory, intravenous ketamine is an excellent choice for sedation because it provides a stable or increased heart rate and blood pressure and has little or no effect on pulmonary vascular resistance. However, prolonged recovery, vomiting, and dysphoric reactions may be problematic.68 Intravenous ketamine has been successfully used alone or in combination with midazolam69 or propofol.70 Propofol is also widely used for sedation, but compared with ketamine, it causes a greater reduction in systemic blood pressure and systemic vascular resistance, with no effect on pulmonary vascular resistance. This may increase a right-to-left shunt, or in diagnostic procedures, it may attenuate the gradient across a stenosis, making the decision to dilate the stenosis more difficult.68,71,72 Compared with propofol sedation, a combination of ketamine with propofol produces similar sedation with less cardiovascular depression.73 Dexmedetomidine has been proffered for sedation of children undergoing cardiac catheterization, although it may not provide sufficient sedation by itself.74 When combined with ketamine, dexmedetomidine was inferior to propofol and ketamine.75
In sedated children, local anesthesia with the use of a topical cream may facilitate venous access.76 Spinal anesthesia has been described as an alternative to sedation or general anesthesia in high-risk infants younger than 6 months of age when the procedure is expected to take less than 90 minutes.77
Sedation is often used to avoid the potential effects of general anesthesia on diagnostic measures. However, as the diagnosis shifts to intervention, this argument assumes less relevance. Deep sedation may be associated with significant respiratory changes67,78 and hypoxia72 in some children. These changes can have as much effect on the circulation as general anesthesia, limiting the theoretical advantage of sedation. General anesthesia and sedation may alter hemodynamics and intracardiac shunts.67,72
General Anesthesia
Etomidate may have a role in children with significant compromise because it has little hemodynamic effect on systemic or pulmonary pressures or resistances.79 Remifentanil may offer an advantage in cardiac procedures because of the short, context-sensitive half-time that allows rapid awakening on completion of the procedure, although it is an opioid and lacks the sedation qualities of other medications. Concerns remain about the possible risks of bradycardia, hypotension, and chest wall rigidity with this opioid.80,81 In some cases, postprocedural sedation may offer an advantage in reducing patient movement, thereby reducing the risk for dislodging a clot on the catheter or at the catheterization site.
Radiofrequency ablation procedures may be protracted and require an immobile child. They may also precipitate arrhythmias, requiring defibrillation. For these reasons, general anesthesia is preferred. General anesthetic agents have effects on conduction that may affect the generation of preexcitation and automatic tachycardia. Good clinical data are scant; however, it appears that for preexcitation, isoflurane and sevoflurane have little effect at less than 1 minimal alveolar concentration (MAC), whereas propofol and opioids have no demonstrable effects at any dose. In contrast, automatic tachycardia may be suppressed by large doses of opioids, propofol or dexmedetomidine.82 In a prospective, randomized trial, isoflurane- and propofol-based anesthesia resulted in a similar duration of anesthesia and effectiveness of ablation.83 Because of the protracted time course of these procedures, a forced-air warming device is indicated to maintain thermal homeostasis. Esophageal ulceration and atrioesophageal fistula have been reported in up to 3% of patients undergoing these procedures, particularly when excessive energy is applied during ablation (e.g., more than 25 W for extended periods).84 We recommend using energy levels less than 25 W during the ablation and to monitor the esophageal temperature by inserting an esophageal temperature probe to the level of the mid-esophagus. Position of the stethoscope may be verified by injecting contrast dye in the bulb of the stethoscope.
Principles of Technique
Attention to detail is essential for providing safe anesthesia for children with reduced ventricular reserve and critical pulmonary circulations. The most frequent error precipitating adverse events is providing inadequate anesthesia. Hemodynamic consequences of light anesthesia, such as hypoxia due to laryngospasm, are poorly tolerated in children with pulmonary hypertension. Hypoxia or hypercapnia may lead to increasing pulmonary vascular resistance, which may increase the shunt and further worsen the hypoxia. Increased pulmonary hypertension may also lead to significant decreases in pulmonary compliance, further increasing hypoxia and precipitating a downward spiral.85 Another cause of adverse outcomes is the use of unfamiliar anesthesia techniques. For example, it is imprudent to use remifentanil for the first time in a child with primary pulmonary hypertension. Although there are theoretical grounds to support the use of one drug or another, the most important principle of anesthesia in children with CHD is to carefully use techniques that are reliable and familiar. This reinforces the need for these children to be anesthetized only by those with sound knowledge and experience with the physiology of the CHD and the planned procedures. Careful anesthesia entails attention to preprocedural anxiolysis, fluid management, full monitoring, expert assistance, and taking extra time to deliver anesthetic drugs slowly or in incremental doses to avoid overpressure or excessive blood levels. A useful approach is to transduce the cardiologist’s arterial access onto the anesthesia monitor. This can be accomplished with a slave connection or by adding a second stopcock to the system with a second transducer for the anesthesia monitor.
Arnold PD, Holtby HM. Anesthesia for the cardiac catheterization laboratory. In: Andropoulos DB, Stayer SA, Russell IA, eds. Anesthesia for congenital heart disease. Malden, MA: Blackwell Futura; 2005:407–426.
Bennett D, Marcus R, Stokes M. Incidents and complications during pediatric cardiac catheterization. Paediatr Anaesth. 2005;15:1083–1088.
This paper describes the complications in pediatric cardiac catheterization.
Cassidy SC, Schmidt KG, Van Hare GF, et al. Complications of pediatric cardiac catheterization: a 3-year study. J Am Coll Cardiol. 1992;19:1285–1293.
The complications of pediatric cardiac catheterization are described.
Feltes TF, Bacha E, Beekman RH, III., et al. AHA scientific statement: indications for cardiac catheterization and intervention in pediatric cardiac disease. Circulation. 2011;123:2607–2652.
The American Heart Association has provided a comprehensive overview of the subject.
Friesen RH, Alswang M. Changes in carbon dioxide tension and oxygen saturation during deep sedation for paediatric cardiac catheterization. Paediatr Anaesth. 1996;6:15–20.
The authors review a significant issue for sedation techniques.
Reddy K, Jaggar S, Gillbe C. The anaesthetist and the cardiac catheterisation laboratory. Anaesthesia. 2006;61:1175–1186.
This is a good review of anesthesia for adult and pediatric cardiac catheterization.
Taylor CJ, Derrick G, McEwan A, Haworth SG, Sury MRJ. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth. 2007;98:657–661.
Vitiello R, McCrindle BW, Nykanen D, et al. Complications associated with pediatric cardiac catheterization. J Am Coll Cardiol. 1998;32:1433–1440.
This important paper describes the complications in pediatric cardiac catheterization.
1 Dexter L, Haynes FW, Burwell CS, Eppinger EC, Seibel RE. Studies of congenital heart disease: I. Technique of venous catheterization as a diagnostic procedure. J Clin Invest. 1947;26:547–553.
2 Rashkind WJ, Miller WW. Creation of an atrial septal defect without thoracotomy: a palliative approach to complete transposition of the great arteries. JAMA. 1966;196:991–992.
3 Grech V. The evolution of diagnostic trends in congenital heart disease: a population-based study. J Paediatr Child Health. 1999;35:387–391.
4 Allen HD, Beekman RH, 3rd., Garson A, Jr., et al. Pediatric therapeutic cardiac catheterization: a statement for healthcare professionals from the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 1998;97:609–625.
5 Andrews RE, Tulloh RM. Interventional cardiac catheterisation in congenital heart disease. Arch Dis Child. 2004;89:1168–1173.
6 Shim D, Lloyd TR, Crowley DC, Beekman RH, 3rd. Neonatal cardiac catheterization: a 10-year transition from diagnosis to therapy. Pediatr Cardiol. 1999;20:131–133.
7 Ward CJ, Hawker RE, Cooper SG, et al. Minimally invasive management of transposition of the great arteries in the newborn period. Am J Cardiol. 1992;69:1321–1323.
8 Masura J, Gavora P, Formanek A, Hijazi ZM. Transcatheter closure of secundum atrial septal defects using the new self-centering Amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn. 1997;42:388–393.
9 Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm Amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580–584.
10 Latson LA, Jones TK, Jacobson J, et al. Analysis of factors related to successful transcatheter closure of secundum atrial septal defects using the HELEX Septal Occluder. Am Heart J. 2006;151:1129. e7-11
11 Amin Z, Hijazi ZM, Bass JL, et al. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63:496–502.
12 Tofeig M, Patel RG, Walsh KP. Transcatheter closure of a midmuscular ventricular septal defect with an Amplatzer VSD occluder device. Heart. 1999;81:438–440.
13 Hijazi ZM, Hakim F, Haweleh AA, et al. Catheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: initial clinical experience. Catheter Cardiovasc Interv. 2002;56:508–515.
14 Holzer R, de Giovanni J, Walsh KP, et al. Transcatheter closure of perimembranous ventricular septal defects using the Amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv. 2006;68:620–628.
15 Laussen PC, Hansen DD, Perry SB, et al. Transcatheter closure of ventricular septal defects: hemodynamic instability and anesthetic management. Anesth Analg. 1995;80:1076–1082.
16 Allan CK, Thiagarajan RR, Armsby LR, et al. Emergent use of extracorporeal membrane oxygenation during pediatric cardiac catheterization. Pediatr Crit Care Med. 2006;7:212–219.
17 Dumitrescu A, Lane GK, Wilkinson JL, et al. Transcatheter closure of perimembranous ventricular septal defect. Heart. 2007;93:867.
18 Rashkind WJ, Mullins CE, Hellenbrand WE, Tait MA. Nonsurgical closure of patent ductus arteriosus: clinical application of the Rashkind PDA Occluder System. Circulation. 1987;75:583–592.
19 Wang JK, Hwang JJ, Chiang FT, et al. A strategic approach to transcatheter closure of patent ductus: Gianturco coils for small-to-moderate ductus and Amplatzer duct occluder for large ductus. Int J Cardiol. 2006;106:10–15.
20 Hijazi ZM, Lloyd TR, Beekman RH, 3rd., Geggel RL. Transcatheter closure with single or multiple Gianturco coils of patent ductus arteriosus in infants weighing < or = 8 kg: retrograde versus antegrade approach. Am Heart J. 1996;132:827–835.
21 Ing FF, Bierman FZ. Percutaneous transcatheter coil occlusion of the patent ductus arteriosus aided by the nitinol snare: further observations. Cardiovasc Intervent Radiol. 1995;18:222–226.
22 Kuhn MA, Latson LA. Transcatheter embolization coil closure of patent ductus arteriosus-modified delivery for enhanced control during coil positioning. Cathet Cardiovasc Diagn. 1995;36:288–290.
23 Grifka MR, Jones TK. Transcatheter closure of large PDA using 0.052-inch Gianturco coils: controlled delivery using a bioptome catheter through a 4-French sheath. Catheter Cardiovasc Interv. 2000;49:301–306.
24 Latson LA. Nonsurgical treatment of a neonate with pulmonary atresia and intact ventricular septum by transcatheter puncture and balloon dilation of the atretic valve membrane. Am J Cardiol. 1991;68:277–279.
25 Justo RN, Nykanen DG, Williams WG, Freedom RM, Benson LN. Transcatheter perforation of the right ventricular outflow tract as initial therapy for pulmonary valve atresia and intact ventricular septum in the newborn. Cathet Cardiovasc Diagn. 1997;40:408–413.
26 Javorski JJ, Hansen DD, Laussen PC, et al. Paediatric cardiac catheterization: innovations. Can J Anaesth. 1995;42:310–329.
27 Bennett D, Marcus R, Stokes M. Incidents and complications during pediatric cardiac catheterization. Paediatr Anaesth. 2005;15:1083–1088.
28 Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405.
29 McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US Melody Valve trial. Circulation. 2010;122:507–516.
30 Robb JD, Harrris MA, Minakawa M, et al. Melody valve implantation into the branch pulmonary arteries for treatment of pulmonary insufficiency in an ovine model of right ventricular outflow tract dysfunction following tetralogy of Fallot repair. Circ Cardiovasc Interv. 2011;4:80–87.
31 Kugler JD, Danford DA, Houston KA, Felix G. Pediatric radiofrequency catheter ablation registry success, fluoroscopy time, and complication rate for supraventricular tachycardia: comparison of early and recent eras. J Cardiovasc Electrophysiol. 2002;13:336–341.
32 Campbell RM, Strieper MJ, Frias PA, et al. Current status of radiofrequency ablation for common pediatric supraventricular tachycardias. J Pediatr. 2002;140:150–155.
33 Johnson JL, Fellows KE, Murphy JD. Transhepatic central venous access for cardiac catheterization and radiologic intervention. Cathet Cardiovasc Diagn. 1995;35:168–171.
34 Braunwald E, Gorlin R, McIntosh HD, et al. Cooperative study on cardiac catheterization. Circulation. 1968;37(Suppl 5):59–66. III
35 West R, Ellis G, Brooks N. Complications of diagnostic cardiac catheterisation: results from a confidential inquiry into cardiac catheter complications. Heart. 2006;92:810–814.
36 Vitiello R, McCrindle BW, Nykanen D, et al. Complications associated with pediatric cardiac catheterization. J Am Coll Cardiol. 1998;32:1433–1440.
37 Cassidy SC, Schmidt KG, Van Hare GF, et al. Complications of pediatric cardiac catheterization: a 3-year study. J Am Coll Cardiol. 1992;19:1285–1293.
38 Rhodes JF, Asnes JD, Blaufox AD, Sommer RJ. Impact of low body weight on frequency of pediatric cardiac catheterization complications. Am J Cardiol. 2000;86:1275–1278.
39 Bulbul ZR, Galal MO, Mahmoud E, et al. Arterial complications following cardiac catheterization in children less than 10 kg. Asian Cardiovasc Thorac Ann. 2002;10:129–132.
40 Kocis KC, Snider AR, Vermilion RP, Beekman RH. Two-dimensional and Doppler ultrasound evaluation of femoral arteries in infants after cardiac catheterization. Am J Cardiol. 1995;75:642–645.
41 McElhinney DB, Reddy VM, Moore P, et al. Surgical intervention for complications of transcatheter dilation procedures in congenital heart disease. Ann Thorac Surg. 2000;69:858–864.
42 Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S–801S.
43 Lee HY, Reddy CB, Rao S. Evaluation of superficial femoral artery compromise and limb growth retardation after transfemoral artery balloon dilatations. Circulation. 1997;95:974–980.
44 Meier B. Closure of patent foramen ovale: technique, pitfalls, complications, and follow up. Heart. 2005;91:444–448.
45 Thomsen HS, Moróos SK. Radiographic contrast media. BJU Int. 2000;86(Suppl 1):1–10.
46 Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5:28–31.
47 Morcos SK, Thomsen HS, Webb JA. Contrast media–induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol. 1999;9:1602–1613.
48 Niboshi A, Nishida M, Itoi T, et al. Renal function and cardiac angiography. Indian J Pediatr. 2006;73:49–53.
49 Cox CD, Tsikouris JP. Preventing contrast nephropathy: what is the best strategy? A review of the literature. J Clin Pharmacol. 2004;44:327–337.
50 Asif A, Garces G, Preston RA, Roth D. Current trials of interventions to prevent radiocontrast-induced nephropathy. Am J Ther. 2005;12:127–132.
51 Thomsen HS. How to avoid CIN. guidelines from the European Society of Urogenital Radiology. Nephrol Dial Transplant. 2005;20(Suppl 1):i18–i22.
52 Sam AD, 2nd., Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313–318.
53 Liu XY, Wong V, Leung M. Neurologic complications due to catheterization. Pediatr Neurol. 2001;24:270–275.
54 Ryan CA, Robertson M, Coe JY. Seizures due to lidocaine toxicity in a child during cardiac catheterization. Pediatr Cardiol. 1993;14:116–118.
55 Souza Neto EP, Durand PG, Sassolas F, Vial C, Lehot JJ. Brachial plexus injury during cardiac catheterisation in children: report of two cases. Acta Anaesthesiol Scand. 1998;42:876–879.
56 Kroll DA, Caplan RA, Posner K, et al. Nerve injury associated with anesthesia. Anesthesiology. 1990;73:202–207.
57 Hansen TG, Henneberg SW. Brachial plexus injury during cardiac catheterisation in children. Acta Anaesthesiol Scand. 1999;43:364–365.
58 Joe RR, Chen LQ. Anesthesia in the cardiac catheterization lab. Anesthesiol Clin North Am. 2003;21:639–651.
59 Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H. Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation. 2005;111:83–89.
60 Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol. 2005;26:190–199.
61 Bacha EA, Cao QL, Galantowicz ME, et al. Multicenter experience with perventricular device closure of muscular ventricular septal defects. Pediatr Cardiol. 2005;26:169–175.
62 Moore P. MRI-guided congenital cardiac catheterization and intervention: the future? Catheter Cardiovasc Interv. 2005;66:1–8.
63 Practice guidelines for sedation and analgesia by nonanesthesiologists. Anesthesiology. 2002;96:1004–1017.
64 Andropoulos DB, Stayer SA. An anesthesiologist for all pediatric cardiac catheterizations: luxury or necessity? J Cardiothorac Vasc Anesth. 2003;17:683–685.
65 Smith C, Rowe RD, Vlad P. Sedation of children for cardiac catheterization with an ataractic mixture. Can Anaesth Soc J. 1958;5:35–40.
66 Nahata MC, Clotz MA, Krogg EA. Adverse effects of meperidine, promethazine, and chlorpromazine for sedation in pediatric patients. Clin Pediatr (Phila). 1985;24:558–560.
67 Auden SM, Sobczyk WL, Solinger RE, Goldsmith LJ. Oral ketamine/midazolam is superior to intramuscular meperidine, promethazine, and chlorpromazine for pediatric cardiac catheterization. Anesth Analg. 2000;90:299–305.
68 Oklu E, Bulutcu FS, Yalcin Y, et al. Which anesthetic agent alters the hemodynamic status during pediatric catheterization? Comparison of propofol versus ketamine. J Cardiothorac Vasc Anesth. 2003;17:686–690.
69 Jobeir A, Galal MO, Bulbul ZR, et al. Use of low-dose ketamine and/or midazolam for pediatric cardiac catheterization. Pediatr Cardiol. 2003;24:236–243.
70 Kogan A, Efrat R, Katz J, Vidne BA. Propofol-ketamine mixture for anesthesia in pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2003;17:691–693.
71 Lebovic S, Reich DL, Steinberg LG, et al. Comparison of propofol versus ketamine for anesthesia in pediatric patients undergoing cardiac catheterization. Anesth Analg. 1992;74:490–494.
72 Williams GD, Jones TK, Hanson KA, Morray JP. The hemodynamic effects of propofol in children with congenital heart disease. Anesth Analg. 1999;89:1411–1416.
73 Akin A, Esmaoglu A, Guler G, et al. Propofol and propofol-ketamine in pediatric patients undergoing cardiac catheterization. Pediatr Cardiol. 2005;26:553–557.
74 Munro HM, Tirotta CF, Felix DE, et al. Initial experience with dexmedetomidine for diagnostic and interventional cardiac catheterization in children. Pediatr Anesth. 2007;17:109–120.
75 Tosun Z, Akin A, Guler G, et al. Dexmedetomidine-ketamine and propofol-ketamine combinations for anesthesia in spontaneously breathing pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2006;20:515–519.
76 Pirat A, Karaaslan P, Candan S, et al. Topical EMLA cream versus prilocaine infiltration for pediatric cardiac catheterization. J Cardiothorac Vasc Anesth. 2005;19:642–645.
77 Katznelson R, Mishaly D, Hegesh T, et al. Spinal anesthesia for diagnostic cardiac catheterization in high-risk infants. Paediatr Anaesth. 2005;15:50–53.
78 Friesen RH, Alswang M. Changes in carbon dioxide tension and oxygen saturation during deep sedation for paediatric cardiac catheterization. Paediatr Anaesth. 1996;6:15–20.
79 Sarkar M, Laussen PC, Zurakowski D, et al. Hemodynamic responses to etomidate on induction of anesthesia in pediatric patients. Anesth Analg. 2005;101:645–650.
80 Reyntjens K, Foubert L, De Wolf D, et al. Glycopyrrolate during sevoflurane-remifentanil-based anaesthesia for cardiac catheterization of children with congenital heart disease. Br J Anaesth. 2005;95:680–684.
81 Foubert L, Reyntjens K, De Wolf D, et al. Remifentanil infusion for cardiac catheterization in children with congenital heart disease. Acta Anaesthesiol Scand. 2002;46:355–360.
82 Mason KP, Lerman J. Dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113:1129–1142.
83 Erb TO, Kanter RJ, Hall JM, et al. Comparison of electrophysiologic effects of propofol and isoflurane-based anesthetics in children undergoing radiofrequency catheter ablation for supraventricular tachycardia. Anesthesiology. 2002;96:1386–1394.
84 Martinek M, Bencsik G, Aichinger Hassanein S, et al. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets and esophageal visualization. J Cardiovasc Electrophysiol. 2009;20:7326–7333.
85 Schulze-Neick I, Werner H, Penny DJ, et al. Acute ventilatory restriction in children after weaning off inhaled nitric oxide: relation to rebound pulmonary hypertension. Intensive Care Med. 1999;25:76–80.