Interventional and Intraoperative Doppler
Introduction
Ultrasound is the preferred modality for performing imaging-guided interventional procedures. Advantages over other modalities such as CT include real-time guidance allowing precise needle placement, portability, relatively low cost, and lack of ionising radiation.1,2 The addition of colour Doppler has been shown to be of great utility during interventional procedures. The primary benefit is the enhanced ability to visualise blood vessels during needle placement. Other advantages include improved needle visualisation and timely identification of procedural complications (Box 16-1). Specific Doppler applications can be divided into pre-procedure planning, intra-procedural guidance, and post-procedure monitoring.
Pre-Procedure Planning
AVOIDANCE OF BLOOD VESSELS
The most important use of colour Doppler ultrasound in interventional procedures is visualisation of blood vessels along the needle trajectory. This allows the vessels to be avoided as the needle is advanced, thereby minimising the risk of bleeding.3 Alternatively, in some situations such as arteriography or venography, colour may be useful to localise vessels for puncture. The focus of this chapter will be the use of colour Doppler for non-vascular interventional procedures.
The addition of colour Doppler to imaging guidance has been shown to reduce the risk of bleeding in both percutaneous liver and kidney biopsies.4–6 Good guidance technique routinely incorporates colour Doppler imaging immediately prior to needle placement anywhere in the body. Specific vessels (Table 16-1) should be identified during certain common interventional procedures (Fig. 16-1).
TABLE 16-1
Specific Vasculature Structures That Doppler US can Identify and Help Avoid When Performing Biopsies, Aspirations, or Drainages in Various Body Regions
| Procedure site | Vessels to identify |
| Neck/thyroid | Carotid arteries/jugular veins |
| Mediastinum | Internal mammary artery |
| Chest/upper abdomen | Intercostal vessels |
| Left liver/epigastric region | Superior epigastric artery |
| Abdomen/pelvis | Inferior epigastric artery and abdominal wall varices |

FIGURE 16-1 Thick slab coronal MIP image from a CTA shows the positions of the internal mammary (black arrows), superior epigastric (white arrows), and inferior epigastric (block arrows) arteries. These must be identified and avoided during procedures involving an anterior approach.
In fine needle aspiration of thyroid nodules and lymph nodes, colour Doppler is used to avoid the internal jugular veins and carotid arteries and their major branches (Fig. 16-2). In the chest, colour Doppler ultrasound has been shown to improve vessel visualisation during transthoracic needle aspiration biopsies.7,8 During mediastinal biopsy, not only should the great vessels be visualised, but a specific search for the internal mammary arteries and veins must be performed. Intercostal vessels can be avoided by advancing the needle directly over ribs, as the vessels run along the inferior margin of the ribs. However, a search for intercostal vessels with colour Doppler is warranted, especially if the needle must be directed close to the inferior margin of the rib in order to target the lesion.

FIGURE 16-2 Thyroid fine needle aspiration biopsy. (A) Grey scale US image shows an isoechoic posterior right thyroid nodule (arrows). (B) The proximal needle (arrows) is visualised within hypoechoic muscle but the tip is difficult to identify as it enters more echogenic tissue. (C) Colour Doppler image shows colour flow at the tip of the needle (arrow) as it is gently jiggled back and forth, as well as demonstrating the internal jugular vein and carotid artery. (D) After the needle tip and vessels are identified, colour Doppler is turned off and the needle tip (arrow) is seen in the nodule while avoiding the vessels.
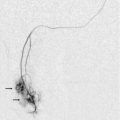
Ultrasound-guided percutaneous liver biopsy can be performed using a sub-xiphoid approach to access the left lobe, or an intercostal approach into the right lobe. Although both techniques are acceptable, some authors favour a sub-xiphoid approach to avoid damage to intercostal structures and avoid traversing the pleura.9 However, a recent study reported a 0.8% (6/776) rate of significant bleeding complications from percutaneous US-guided liver biopsy, all from the left lobe approach.10 Four of the six cases were attributed to superior epigastric artery injury. These vessels lie approximately 4 cm from midline at the level of the xiphoid process. Therefore the superior epigastric arteries must be identified and avoided during left lobe biopsies or any other procedure in the epigastric region. If they cannot be visualised, a midline approach is preferred in order to avoid these vessels. Likewise, the inferior epigastric arteries should be identified during any puncture in the lower abdomen or pelvis, such as paracentesis (Fig. 16-3). Additionally, as paracentesis is often performed in cirrhotic patients with portal hypertension, colour Doppler should be also used to identify enlarged abdominal wall varices.



FIGURE 16-3 CT- and US-guided pelvic mass biopsy. (A) CT was used for initial superficial needle placement during percutaneous biopsy of a large heterogeneous pelvic mass. (B) Colour Doppler was then utilised and revealed the needle trajectory (black arrow) was directed towards the inferior epigastric artery (white arrow). Slight repositioning allowed for the needle to be advanced avoiding the artery as shown in (C). Also note the tumour vessel (arrow) that was identified and avoided.
During liver biopsy colour Doppler can decrease bleeding risk by visualisation and avoidance of major intrahepatic vasculature. Fine needle aspiration biopsy under US guidance is an established technique for distinguishing between bland and malignant portal vein thrombus. Colour Doppler improves visualisation of the thrombus by identifying flow surrounding it or within it, and can guide needle placement directly into the thrombus without traversing patent portions of the portal vein and helping avoid the adjacent hepatic artery.11 Colour Doppler is useful to distinguish hepatic vessels from bile ducts when performing transhepatic cholangiography. Colour and spectral Doppler US can identify tumour neo-vascularity. It is important to avoid such vessels as they lack a normal coagulation response and have a higher propensity for bleeding (Fig. 16-4). Puncture of these vessels can result in aspiration of a large amount of blood during FNA which will degrade the specimen and reduce diagnostic yield.


FIGURE 16-4 Hypoechoic liver lesion biopsy in a patient with metastatic breast cancer. (A) Colour Doppler image shows a tumour vessel along the posterior aspect of the liver mass. (B) Colour Doppler image showing the biopsy trajectory (arrow) in the anterior portion of the mass in order to avoid this vessel.
IDENTIFICATION OF VASCULAR LESIONS
A less common but highly important use of colour Doppler ultrasound is the identification of vascular lesions such as pseudoaneurysms and arteriovenous fistulas (AVFs) prior to performing an intervention. On grey scale US, a pseudoaneurysm can appear to be a cystic lesion or fluid collection and aspiration or drainage may be requested. Puncture of such a lesion could result in a life-threatening haemorrhage. This scenario may be encountered in the setting of acute pancreatitis, where pseudocysts are common. However, splenic (or any branch of the celiac axis) artery pseudoaneurysm can mimic a pseudocyst and must be excluded before performing an intervention. Colour Doppler US can easily identify the vascular nature of these lesions by demonstrating the characteristic swirling blood in a ‘yin-yang’ pattern (Fig. 16-5). The use of colour Doppler in the treatment of pseudoaneurysms is discussed elsewhere in this book.



FIGURE 16-5 A 65-year-old man with a history of melanoma and neck mass. (A) Non-contrast CT through the neck reveals a soft tissue attenuation mass in the superior mediastinum displacing the trachea. A CT-guided biopsy was requested, however, the lesion was first studied with US. (B) Grey scale US reveals a hypoechoic mass, however colour Doppler (C) shows the classic ‘yin-yang’ pattern of a pseudoaneurysm and biopsy was deferred.
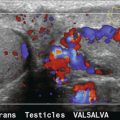
Arteriovenous fistulas have been reported as a complication of renal biopsies in 3–18%.12 Although often asymptomatic, they are of particular importance in renal transplant recipients who often undergo multiple biopsies. These lesions must be identified and avoided prior to repeat biopsy in order to minimise the risk of serious bleeding. Arteriovenous fistulas are rarely seen on grey scale US but can be easily identified on colour Doppler. Tissues surrounding the fistula vibrate due to the high-velocity blood flow and may show a flash of colour noise – the equivalent of a palpable thrill or audible bruit. The feeding artery will demonstrate aliasing unless the pulse repetition frequency is increased. Spectral Doppler tracings show high-velocity, low-resistance waveform in the feeding artery, and high-velocity, arterialised flow in the draining vein (see Chapter 10, Fig. 10-19).
Intra-Procedural Applications
IMPROVED NEEDLE VISUALISATION
Real time visualisation of the needle tip is an advantage of US-guided intervention. Generally, the needle should only be advanced when the tip can be seen in order to ensure successful access to the target lesion and to avoid inadvertent puncture of adjacent structures. Unfortunately, it can be difficult to clearly delineate the needle tip and that is a common source of frustration when performing US guided interventions. Visualisation of the needle is particularly difficult when it traverses tissues that have high echogenicity similar to that of the needle (fatty livers), when the needle is advanced parallel to the insonating beam, and with small-gauge needles. Many techniques have been described to improve needle tip visualisation, such as roughening the surface of the needle tip, Teflon coating, and the so-called ‘pump manoeuvre’ where the needle stylet is gently moved back and forth within the needle. The addition of colour Doppler US is a useful adjunct to improve needle visualisation.11,13 Colour Doppler detects motion of any kind and therefore the movement of the needle or inner stylet is more easily detected and the needle localised (Fig. 16-2).
MONITORING ASPIRATION, INJECTION, AND CATHETER PLACEMENT
During aspiration of fluid collections, the moving fluid through the needle will produce a line of colour on colour Doppler which can aid in needle localisation. Likewise a line of colour will be seen with injection of fluid during cyst sclerotherapy. Colour Doppler has also been shown to be useful in evaluating drainage catheter position and patency after placement.14 Pigtail catheters can be surprisingly difficult to visualise with grey scale US. Improved detection can be obtained by aspirating the catheter or injecting sterile saline, both of which will lead to colour signal with Doppler imaging (Fig. 16-6). Visualising colour signal at the side ports when injecting saline or aspirating will also confirm catheter patency. Saline should be injected slowly to avoid the snowstorm pattern which will obscure the catheter.
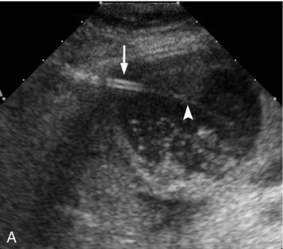
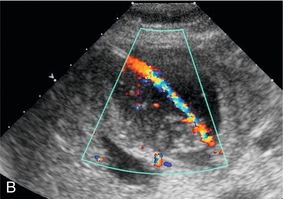
FIGURE 16-6 Percutaneous cholecystostomy. (A) Grey scale image shows inner stylet within the catheter (arrow) and the catheter (arrowhead) advancing over the stylet into the gallbladder though the tip is difficult to see within the sludge and stones. (B) Colour Doppler image obtained while gently aspirating the catheter clearly delineates the course of the catheter within the gallbladder.
Post-Procedure Monitoring
POST-PROCEDURE BLEEDING
US-guided percutaneous liver and renal biopsies are widely established techniques for diagnosing both diffuse parenchymal disease and focal lesions. The rate of serious bleeding complications has been reported at 0.5% for liver biopsies and 0.7% for kidney biopsies.15 However, the risk may be increased in patients with abnormal coagulation parameters such as low platelets, prolonged bleeding time, or elevated INR. In fact, many patients referred for these procedures have such coagulopathies due to their underlying hepatic or renal disease. Although appropriate correction of such deficiencies will reduce the risk: when it occurs, bleeding from these procedures can be life-threatening. Therefore prompt detection of post-biopsy bleeding is of paramount importance.
Recently colour Doppler US has been shown to be useful in identifying bleeding after both US-guided liver biopsy and RFA. After needle withdrawal, colour Doppler evaluation of the needle tract can reveal a line of colour signal (‘patent track’ or ‘colour line sign’), indicating bleeding through the needle track16,17 (Figs 16-7–16-9). This sign has been shown to indicate a significantly higher risk of clinically important post-procedure bleeding when it persists for five minutes after needle withdrawal following liver biopsy. The absence of this finding was predictive of a lack of significant bleeding with a negative predictive value of 99%.17 As this technique can be performed quickly and easily, we recommend using colour Doppler after all percutaneous biopsies of solid organs to assess for the line sign. If present, immediate pressure can be applied to the biopsy site to help tamponade the bleeding. The patient can be positioned with the biopsy site down forcing apposition of the biopsied organ against the body wall for five minutes. US can then be used to re-check for the absence or persistence of the line sign and further management decisions can be made at that point.
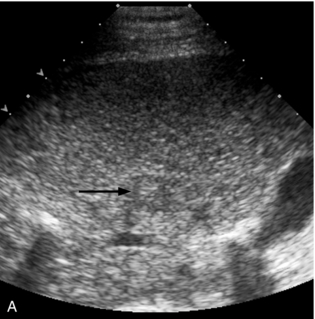
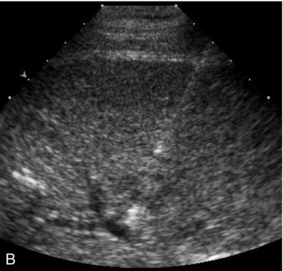
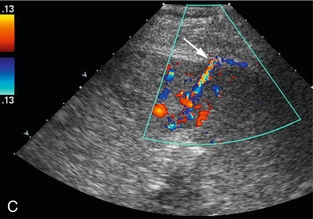
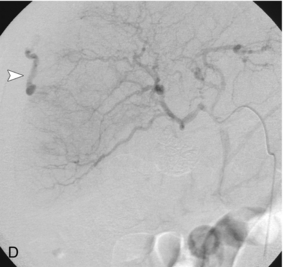
FIGURE 16-7 ‘Colour line sign’ after liver RFA. (A) Grey scale US image reveals a focal hepatic lesion (black arrow) consistent with hepatocellular carcinoma in this cirrhotic patient. (B) US-guided RFA was performed. (C) Colour Doppler image following probe removal reveals a ‘colour line’ along the track (white arrow) consistent with the ‘colour line sign.’ This persisted for 15 minutes and therefore the patient was referred for angiography (D) which revealed active arterial extravasation at the ablation site (white arrowhead). This was successfully coil embolised.
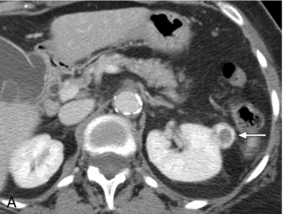
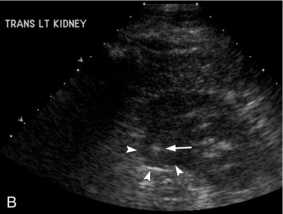
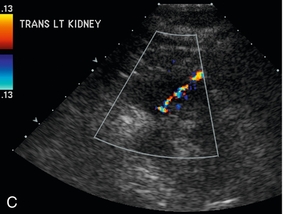
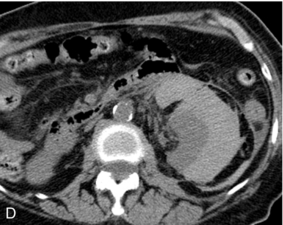
FIGURE 16-8 ‘Colour line sign’ after renal RFA. (A) Contrast-enhanced CT reveals a peripherally enhancing solid left renal mass (arrow). (B) Grey-scale US image shows the needle tip (white arrow) within the mass (arrowheads). (C) Colour Doppler image following ablation reveals the ‘colour line sign’ which persisted for greater than 5 minutes. (D) Non-contrast CT following the procedure reveals large left subcapsular haematoma.
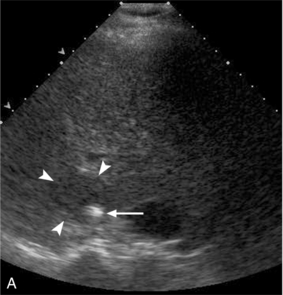
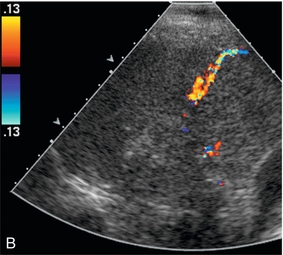
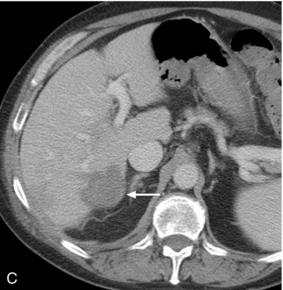
FIGURE 16-9 ‘Colour line sign’ after liver RFA without significant bleeding. (A) Grey scale US image reveals RFA probe (arrow) within the focal liver lesion (arrowheads). (B) Colour Doppler image following RFA shows the ‘colour line sign’. However, this persisted for less than 5 minutes and contrast-enhanced CT (C) following the procedure shows the ablation zone (arrow) but no significant haemorrhage.
VASCULAR COMPLICATIONS
Arteriovenous fistulas are a known complication of percutaneous renal biopsy. The true incidence is unknown, with estimates ranging from 3–18%.12 Although most are asymptomatic, some may cause complications such as hypertension or haematuria. Although these lesions are occult on grey scale imaging, colour Doppler US will easily identify these lesions as described above. One study found small localised flow disturbances consistent with AVFs in 12 of 77 patients using colour Doppler US immediately after renal biopsy.12 Importantly, most of these lesions resolved by 24 hours and none were symptomatic, therefore routine treatment is not indicated. However knowledge of their presence is important in the rare patient that does become symptomatic.
Intraoperative Colour Doppler Procedures
EQUIPMENT
Intraoperative ultrasound provides increased resolution compared to transcutaneous sonography as the signal degradation from overlying fat, muscle and bone is eliminated. For instance, with the increased resolution of intraoperative liver ultrasound additional small solid nodules which are not detected on transcutaneous ultrasound are often identified. It has been estimated that intraoperative ultrasound detects more focal liver lesions compared to preoperative ultrasound, CT or even MRI.18
USES OF INTRAOPERATIVE COLOUR DOPPLER ULTRASOUND
(b) Utilisation of colour Doppler during surgery.
(c) Predicting intraoperative outcome.
Many times intraoperative Doppler is utilised more than once during the course of a surgical procedure.
PLANNING SURGERY
Intraoperative Doppler ultrasound is very helpful in planning surgery. Within the abdomen either laparoscopic or open ultrasound imaging and Doppler can be utilised for surgical planning of the liver, pancreas and kidney. Ultrasound is used for defining tissue planes, identifying anomalous vessels or demonstrating tumour invasion.19,20 For instance, in surgical resection of the liver, identification of lobar and segmental anatomy is aided by the use of colour Doppler. Colour Doppler ultrasound is important to help define a plane for resection. Thus, colour Doppler may demonstrate major vessels to allow dissection in the most avascular plane in the liver or kidney. The middle hepatic vein is a critical landmark demarcating the plane between the right and left lobe of the liver (Fig. 16-10). Anomalous vasculature is readily identified by colour Doppler sonography. For instance, accessory arterial supply is frequently identified in the liver such as either a replaced right hepatic artery originating from the SMA or a replaced left hepatic artery from the left gastric artery. Intraoperative colour Doppler is also extremely helpful in predicting the extent of disease. For instance, while preoperative ultrasound or CT may not demonstrate vascular invasion by tumour, it can be precisely localised with colour Doppler ultrasound during surgery. Further research has shown added value of the use of contrast-enhanced intraoperative ultrasound in detecting increased number of colorectal liver metastases, as compared to preoperative imaging or intraoperative ultrasound.21
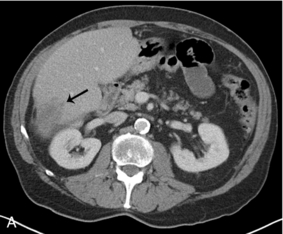
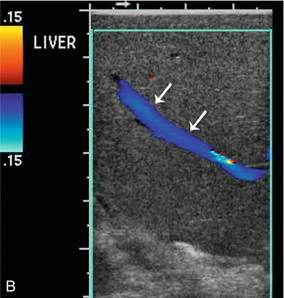
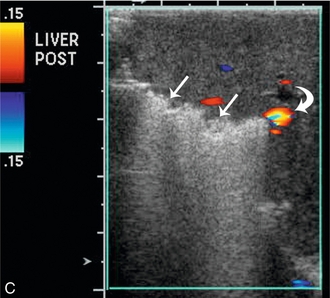
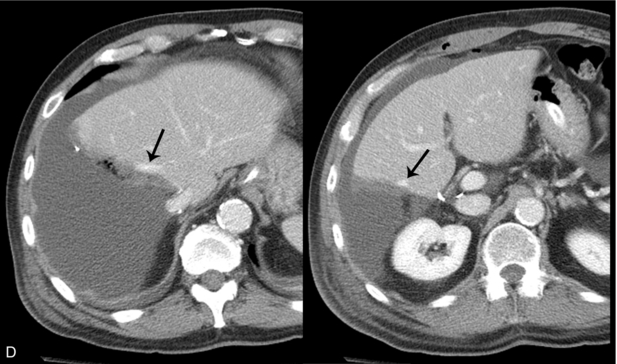
FIGURE 16-10 Metastatic colon cancer, right lobe of the liver with right hepatectomy and use of intraoperative ultrasound. (A) CT scan demonstrating one of the multiple hepatic metastases (arrow) noted within the right lobe of the liver. (B) Intraoperative ultrasound demonstrates the middle hepatic vein (arrows) used to define the plane between the right and left lobe of the liver. There was no tumour invasion into the middle hepatic vein. (C) During surgery, there was acoustic shadowing at the resection margin (arrow), but patency of the middle hepatic vein (curved arrow). (D) Postoperative CT demonstrates right hepatectomy with preservation of the middle hepatic vein (arrow) seen on two different regions within the liver.
Colour Doppler ultrasound has utility in numerous other surgical applications. For instance, intraoperative Doppler can be used to precisely localise arterial venous malformation of the brain, identifying the least disruptive surgical approach. Doppler has now replaced intraoperative angiography for confirmation of successful resection by not only demonstrating absence of colour flow of the AVM after resection; but also by identifying normalisation of resistance index in the feeding arteries22 (Fig. 16-11). Likewise, neurosurgical resection of large intracavernous paraclinoid aneurysms can be evaluated to assess blood flow of the parent and branch vessel with microvascular Doppler sonography.23 Colour Doppler ultrasound can be used in both preoperative and intraoperative planning and decision making in head and neck surgery.24
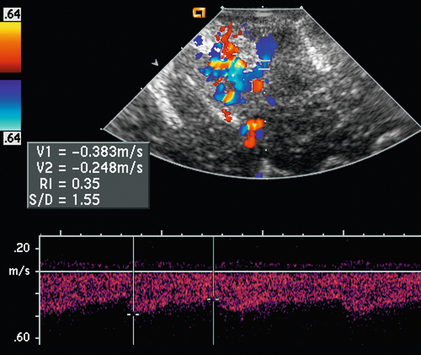
FIGURE 16-11 Doppler US to guide AVM resection. Colour and spectral Doppler evaluation identifies the arteriovenous malformation (AVM) nidus and its feeding artery. The direction of flow in the feeding artery is away from the transducer. A spectral Doppler tracing obtained over this artery shows low-resistance flow (RI 0.35) as would be expected with an AVM.
USE OF ULTRASOUND DURING SURGERY
Intraoperative Doppler ultrasound is very helpful to demonstrate success of surgery by re-evaluating vessel patency during difficult surgical dissection (Fig. 16-10). Also intraoperative blood flow to solid organs can be evaluated with intraoperative sonography.25,26 It can demonstrate ancillary findings, including critical vessel stenosis during surgery (Fig. 16-12).
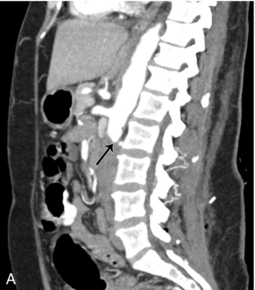
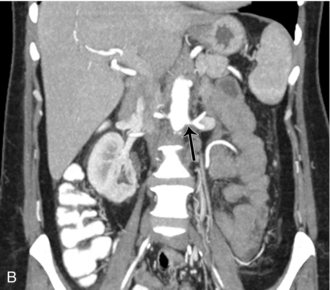
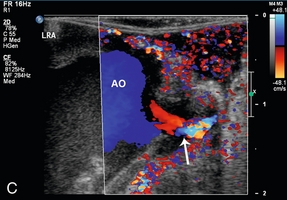
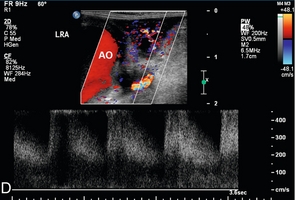
FIGURE 16-12 Intraoperative ultrasound of left renal artery stenosis and aortic occlusion. (A) Sagittal CT image of the abdomen demonstrates complete aortic occlusion (arrow). (B) Coronal CT image demonstrates narrowing of the left renal artery (arrow). (C) After aortic thrombectomy, intraoperative colour Doppler ultrasound confirms aortic patency, but identifies turbulence in the left renal artery (arrow). (D) Intraoperative spectral Doppler demonstrated peak systolic velocity in the left renal artery of greater than 600 cm per second, indicative of persistent high-grade left renal artery stenosis which became manifest after thrombectomy reestablished flow. (AO = aorta).
PREDICTING OUTCOMES
Microemboli can be a potential problem with any vascular surgical procedure. For example, small deposits of debris can develop at the surgical site during carotid endarterectomy or thoracic endovascular aortic repair (TEVAR) and may embolise to the brain. Intraoperative detection of these microemboli is critically important to the surgical outcome since microembolism during surgery increases the risk of perioperative stroke. Intraoperative Doppler ultrasound is used to monitor the signal from within the middle cerebral artery during the vascular surgery. A transcranial Doppler probe is placed on the thinnest portion of calvarium in the region of the temporal bone. The identification of increased spectral reflection from the microemboli, mimicking turbulence, guides intraoperative decision making, specifically increasing the use of platelet antagonists or anticoagulation.27–29
CONFIRMING SUCCESS OF SURGERY
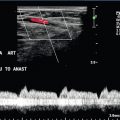
Intraoperative Doppler sonography may be used in a number of different circumstances to confirm an appropriate surgical outcome. For instance, after clipping of cerebral aneurysm, evaluation of the aneurysm and associated vessel after clip positioning is enabled by Doppler sonography. Doppler sonography, however, is limited in some cases especially when multiple clips are placed due to acoustic shadowing from them.30 Intraoperative Doppler sonography has been applied for surgical treatment of the median arcuate ligament syndrome. It can be utilised to demonstrate stenosis of celiac artery and post-stenotic dilatation before the release of the median arcuate ligament; and, afterwards, it can be used to confirm decompression of the celiac artery.31 It is used to predict success of vascular surgery, such as bypass grafts that may connect to vessels beyond an area of stenosis. Intraoperative confirmation of appropriate flow profiles and lack of flow restriction in the bypass graft is critical to successful surgery (Fig. 16-13).
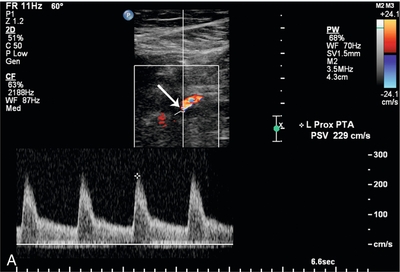
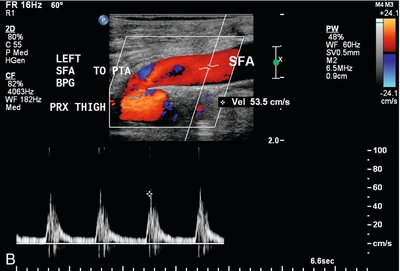
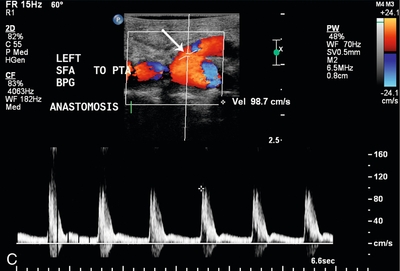
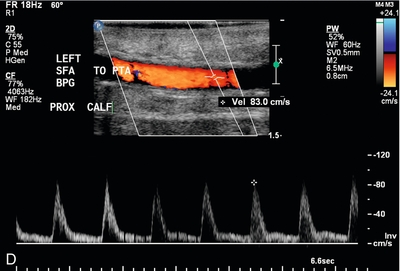
FIGURE 16-13 Superficial femoral artery (SFA) to posterior tibial artery (PTA) bypass graft, intraoperative evaluation. (A) Preoperative Doppler US examination demonstrates Doppler cursor placed on proximal posterior tibial artery (arrow) with peak systolic velocity of 229 cm per second. Proximal to this, velocity was in the range of 80–90 cm per second. (B) After surgical anastomosis of the SFA to the PTA, colour Doppler evaluation of the superficial femoral artery (SFA) demonstrates velocity in the range of 53 cm per second. (C) At the site of anastomosis (arrow) of the superficial femoral artery to the posterior tibial artery, velocities are 98 cm per second. (D) Downstream within the posterior tibial artery, velocity is 83 cm per second. Intraoperative ultrasound therefore confirmed successful anastomosis without stenosis.
IDENTIFYING COMPLICATIONS OF SURGERY
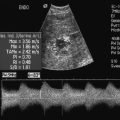
Probably the most important role of intraoperative colour Doppler is the timely identification of potential complications or inadequate outcomes. Identification of a critical arterial stenosis, an internal flap or a continued area of narrowing, allowing immediate surgical correction, is important to the success of carotid endarterectomy (Fig. 16-14). It obviates the need for reoperation. In one series 11% of cases of carotid endarterectomy were immediately revised because of persistent stenosis demonstrated with intraoperative duplex sonography.32
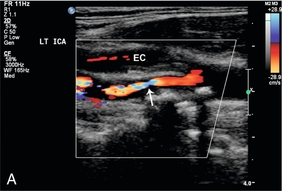
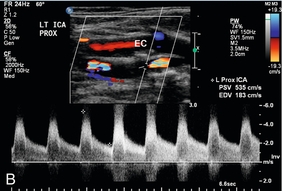
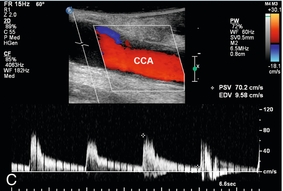
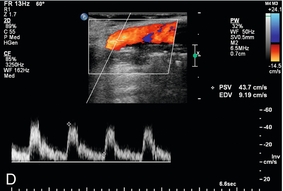
FIGURE 16-14 Intraoperative ultrasound to evaluate successful internal carotid endarterectomy. (A) Preoperative colour US examination demonstrates focal internal carotid artery stenosis (arrow). (B) Spectral Doppler confirms high-grade stenosis with peak systolic velocity of 535 cm per second and end diastolic velocity of 183 cm per second. (C) Intraoperative post-endarterectomy evaluation of the internal carotid artery along the previously stenotic area demonstrates a good surgical outcome with peak systolic velocity of 45 cm per second and without residual stenosis or flap.
Another major role for intraoperative Doppler sonography is identifying either arterial or venous thrombosis immediately before operative closure. This is especially important in the area of transplantation. For instance, intraoperative Doppler ultrasound can detect hepatic artery thrombosis allowing immediate revision of the anastomosis.33 Likewise, in renal transplantation, intraoperative colour duplex scanning can detect vascular complications such as renal vein thrombosis, arterial dissection, vasospasm, or arterial occlusion34,35 (Fig. 16-15).
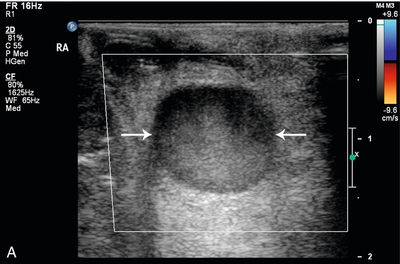
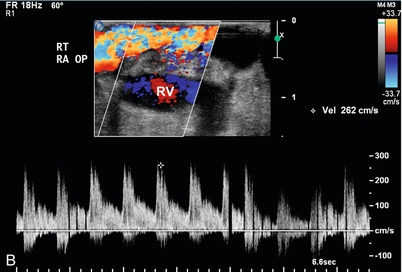
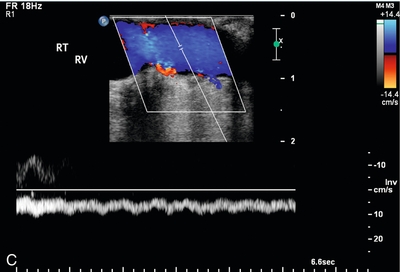
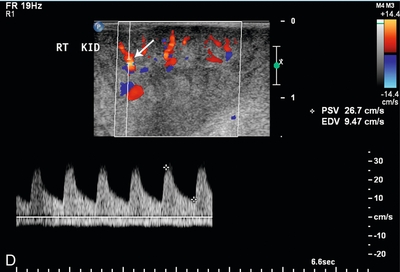
FIGURE 16-15 Intraoperative evaluation of occluded renal artery to transplanted kidney. (A) Intraoperative Doppler ultrasound of the main renal artery to renal transplant demonstrates no flow (arrows). The occluded artery was immediately opened and the thrombus removed. (B) Afterwards, the renal artery is patent with good Doppler flow but with elevation of the peak systolic velocity. (RV = renal vein). (C) Intraoperative Doppler of the main renal vein demonstrates normal venous flow. (D) Intraoperative Doppler cursor (arrow) placed on an inter-lobar artery of the transplanted kidney demonstrates good systolic and diastolic flow with appropriate resistance. Note brisk up-stroke during systole. This patient had a normal postoperative course.
REFERENCES
1. McGahan, J., Invasive Ultrasound Priciples (Biopsy, Aspiration, and Drainage)McGahan, J., Goldberg, B. B., eds. Diagnostic Ultrasound, 2nd ed., vol. 1. New York, NY: Informa Healthcare USA, Inc., 2008.
2. Matalon, T. A., Silver, B. US guidance of interventional procedures. Radiology. 1990; 174(1):43–47.
3. Rubens, D. J., Gottlieb, R. H., Fultz, P. L. Role of colour Doppler imaging in interventional sonography. J Clin Ultrasound. 1999; 27(5):259–271.
4. Lencioni, R., Caramella, D., Bartolozzi, C. Percutaneous biopsy of liver tumors with colour Doppler US guidance. Abdom Imaging. 1995; 20(3):206–208.
5. Polakow, J., Ladny, J. R., Dzieciol, J., et al. Ultrasound guided percutaneous fine-needle biopsy of the liver: efficacy of colour Doppler sonography. Hepatogastroenterology. 1998; 45(23):1829–1830.
6. Granata, A., Floccari, F., Ferrantelli, A., et al. Does systematic preliminar colour Doppler study reduce kidney biopsy complication incidence? Internat J Nephrol. 2011; 2011:419093.
7. Gorguner, M., Misirlioglu, F., Polat, P., et al. Colour Doppler sonographically guided transthoracic needle aspiration of lung and mediastinal masses. J Ultrasound Med. 2003; 22(7):703–708.
8. Yang, P. C. Ultrasound-guided transthoracic biopsy of the chest. Radiol Clin North Am. 2000; 38(2):323–343.
9. Nazarian, L. N., Feld, R. I., Herrine, S. K., et al. Safety and efficacy of sonographically guided random core biopsy for diffuse liver disease. J Ultrasound Med. 2000; 19(8):537–541.
10. Vijayaraghavan, G., Sheehan, D., Zheng, L., et al. Unusual complication after left-lobe liver biopsy for diffuse liver disease: severe bleeding from the superior epigastric artery. AJR Am J Roentgenol. 2011; 197(6):W1135–W1139.
11. Longo, J. M., Bilbao, J. I., Barettino, M. D., et al. Percutaneous vascular and nonvascular puncture under US guidance: role of colour Doppler imaging. Radiographics. 1994; 14(5):959–972.
12. Werner, M., Osadchy, A., Plotkin, E., et al. Increased detection of early vascular abnormalities after renal biopsies by colour Doppler sonography. J Ultrasound Med. 2007; 26(9):1221–1226.
13. Hamper, U. M., Savader, B. L., Sheth, S. Improved needle-tip visualisation by colour Doppler sonography. AJR Am J Roentgenol. 1991; 156(2):401–402.
14. Gerscovich, E. O., Budenz, R. W., Lengle, S. J. Assessment of catheter placement and patency by colour Doppler ultrasonography. J Ultrasound Med. 1994; 13(5):367–370.
15. Atwell, T. D., Smith, R. L., Hesley, G. K., et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010; 194(3):784–789.
16. McGahan, J. P., Wright, L., Brock, J. Occurrence and value of the colour Doppler ‘line sign’ after radiofrequency ablation of solid abdominal organs. J Ultrasound Med. 2011; 30(11):1491–1497.
17. Kim, K. W., Kim, M. J., Kim, H. C., et al. Value of ‘patent track’ sign on Doppler sonography after percutaneous liver biopsy in detection of postbiopsy bleeding: A prospective study in 352 patients. AJR Am J Roentgenol. 2007; 189(1):109–116.
18. Sahani, D. V., Kalva, S. P., Tanabe, K. K., et al. Intraoperative US in patients undergoing surgery for liver neoplasms: comparison with MR imaging. Radiology. 2004; 232(3):810–814.
19. Torzilli, G., Botea, F., Donadon, M., et al. Minimesohepatectomy for colorectal liver metastasis invading the middle hepatic vein at the hepatocaval confluence. Ann Surg Oncol. 2010; 17(2):483.
20. Sethi, A. S., Regan, S. M., Sundaram, C. P. The use of a Doppler ultrasound probe during vascular dissection in laparoscopic renal surgery. J Endourol. 2009; 23(9):1377–1382.
21. Ruzzenente, A., Conci, S., Iacono, C., et al. Usefulness of Contrast-Enhanced Intraoperative Ultrasonography (CE-IOUS) in patients with colorectal liver metastases after preoperative chemotherapy. J Ultrasound Med. 2012; 17(2):281–287. [Oct 11].
22. Dempsey, R. J., Moftakhar, R., Pozniak, M. Intraoperative Doppler to measure cerebrovascular resistance as a guide to complete resection of arteriovenous malformations. Neurosurgery. 2004; 55(1):155–160. [discussion 160–151].
23. Xu, B. N., Sun, Z. H., Jiang, J. L., et al. Surgical management of large and giant intracavernous and paraclinoid aneurysms. Chin Med J. 2008; 121(12):1061–1064.
24. Gravvanis, A., Tsoutsos, D., Delikonstantinou, I., et al. Impact of portable duplex ultrasonography in head and neck reconstruction. J Craniofac Surg. 2012; 23(1):140–144.
25. Mues, A. C., Okhunov, Z., Badani, K., et al. Intraoperative evaluation of renal blood flow during laparoscopic partial nephrectomy with a novel Doppler system. J Endourol. 2010; 24(12):1953–1956.
26. Ou, H. Y., Huang, T. L., Chen, T. Y., et al. Early modulation of portal graft inflow in adult living donor liver transplant recipients with high portal inflow detected by intraoperative colour Doppler ultrasound. Transplant Proc. 2010; 42(3):876–878.
27. Brosig, T., Hoinkes, A., Seitz, R. J., et al. Ultrasound turbulence index during thromboendarterectomy predicts postoperative cerebral microembolism. Cerebrovasc Dis. 2008; 26(1):87–92.
28. Bismuth, J., Garami, Z., Anaya-Ayala, J. E., et al. Transcranial Doppler findings during thoracic endovascular aortic repair. J Vasc Surg. 2011; 54(2):364–369.
29. Ogasawara, K., Suga, Y., Sasaki, M., et al. Intraoperative microemboli and low middle cerebral artery blood flow velocity are additive in predicting development of cerebral ischemic events after carotid endarterectomy. Stroke. 2008; 39(11):3088–3091.
30. Heiroth, H. J., Etminan, N., Steiger, H. J., et al. Intraoperative Doppler and Duplex sonography in cerebral aneurysm surgery. Br J Neurosurg. 2011; 25(5):586–590.
31. Tsujimoto, H., Hiraki, S., Sakamoto, N., et al. Laparoscopic treatment for median arcuate ligament syndrome: the usefulness of intraoperative Doppler ultrasound to confirm the decompression of the celiac artery. Surg Laparosc Endosc Percutan Tech. 2012; 22(2):e71–e75.
32. Ott, C., Heller, G., Odermatt, M., et al. Intraoperative duplex ultrasonography in carotid endarterectomy: the impact on indication for immediate revision and intermediate-term outcome. Vasa. 2008; 37(2):151–156.
33. Gu, L. H., Fang, H., Li, F. H., et al. Prediction of early hepatic artery thrombosis by intraoperative colour Doppler ultrasound in pediatric segmental liver transplantation. Clin Transplant. 2012; 26(4):571–576. [Feb 13].
34. Sadej, P., Feld, R. I., Frank, A. Transplant renal vein thrombosis: role of preoperative and intraoperative Doppler sonography. Am J Kidney Dis. 2009; 54(6):1167–1170.
35. Thalhammer, C., Aschwanden, M., Bilecen, D., et al. Intraoperative colour duplex ultrasound during renal transplantation. Ultraschall Med. 2008; 29(6):652–656.