54 Interspinous Spacers for Minimally Invasive Treatment of Dynamic Spinal Stenosis and Low Back Pain
KEY POINTS
 It increases the size and areas of the spinal canal as well as of the subarticular zones and the foramen and thus has an indirect “decompression” effect on neural structure.
It increases the size and areas of the spinal canal as well as of the subarticular zones and the foramen and thus has an indirect “decompression” effect on neural structure. It unloads the facet joints as well as the posterior part of the disc and thus has a potential effect on low back pain arising from pathologic load pattern on these anatomical structures. The different implants that are currently on the market or in clinical studies provide these biomechanical effects. They can be categorized in two groups:
It unloads the facet joints as well as the posterior part of the disc and thus has a potential effect on low back pain arising from pathologic load pattern on these anatomical structures. The different implants that are currently on the market or in clinical studies provide these biomechanical effects. They can be categorized in two groups:
Introduction: Interspinous Spacers – How Do They Work?
Indirect enlargement of the spinal canal through interspinous distraction devices has become popular for the treatment of dynamic spinal canal stenosis of the lumbar spine.1–5 Biomechanical data acquired with the first implant on the market (X-Stop, Medtronic, Memphis, TN, USA), could show that interspinous distraction induces segmental slight flexion, reduces segmental lordosis, and limits extension.6 Thus, the spinal canal and neural foramen areas and diameters are enlarged.7 These findings are considered to be the most important primary effects that justify the clinical use of the device for the treatment of dynamic spinal stenosis. Randomized controlled trials could confirm the therapeutic efficiency and proved that the implantation of an interspinous spacer leads to clinical results superior to conservative treatment.5
In ex vivo experiments it could also be demonstrated that interspinous distraction can lead to a significant unloading of the facet joints8,9 and the posterior annulus fibrosus, as well as the nucleus pulposus in neutral position and predominantly in extension.8,10,11 Kinematics of the adjacent segments seem not to be affected.6,12
The “Extension Stoppers”
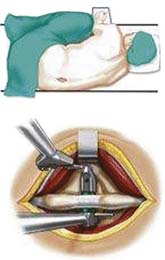
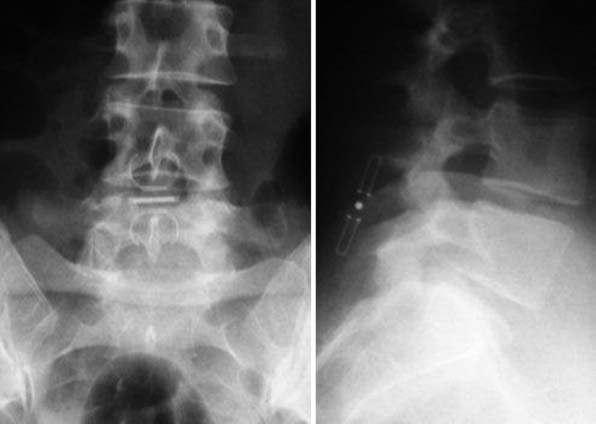
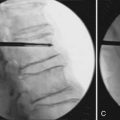
X – Stop (Medtronic) (Figure 54-1)
The main indication is neurogenic claudication with leg/buttock pain due to dynamic degenerative lumbar spinal stenosis, which is relieved upon flexion of the lumbar spine.5
Surgical Technique
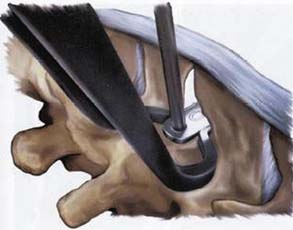
It is implanted through a posterior approach. The patient is in a prone position. The dorsolumbar fascia is split on both sides of the spinous processes, the paravertebral muscles are retracted, and the interspinous ligament is pierced. The spinous processes are then actively distracted with a distraction forceps and the X-Stop is implanted from one side. The wing on the contralateral side is then attached (Figure 54-2).
Results
In a randomized controlled trial, it could be shown that the results of the treatment of dynamic spinal stenosis are superior to those in conservative therapy .1,5,13 Whereas in initial reports its usefulness was also documented for degenerative spondylolisthesis not greater than grade I, recent data could not confirm this.14
Summary
Although the implantation of the X-Stop device is claimed to be minimally invasive, it occasionally requires a larger skin incision and a wider bilateral muscular dissection as compared to modern microsurgical direct decompression techniques.15,16 Due to the iatrogenic alteration of the dorsolumbar fascia and the paraspinal muscles, it also cannot be considered as a treatment option for discogenic or arthrogenic low back pain. Moreover, bisegmental or multilevel implantations require larger surgical approaches.
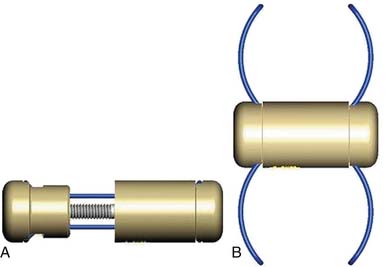
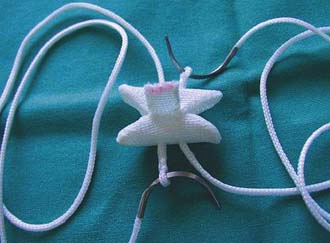
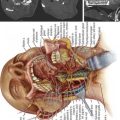
InSpace (Synthes, Paoli, PA, USA) (Figure 54-3)
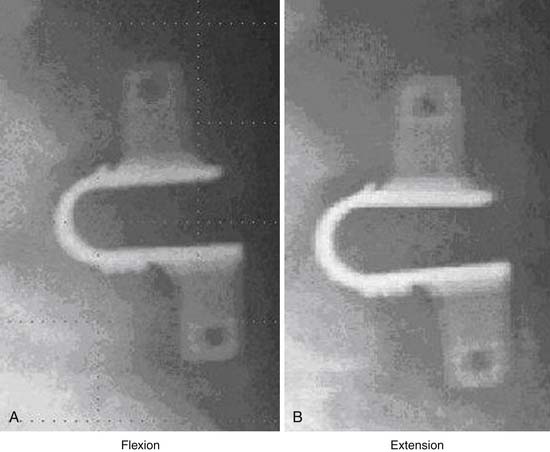
In order to solve the problem of invasiveness, a new cylindrically shaped PEEK interspinous implant with a central titanium screw and four wings that can be deployed once the implant is placed into the interspinous space, has been presented recently. Biomechnical tests have shown that the implant effectively reduces extension without affecting lateral bending of the segment.17,18 Cyclic loading tests have shown that the functionality of the implant is preserved and that the integrity of anatomic structures is not impaired through 15,000 loading cycles.19,20 The effects are thus comparable to the ones described for the X-Stop implant.
The indications are also identical with those described for X-Stop. There are preliminary reports on its potential usefulness for the treatment of discogenic and/or arthrogenic low back pain.21,22
Surgical Technique
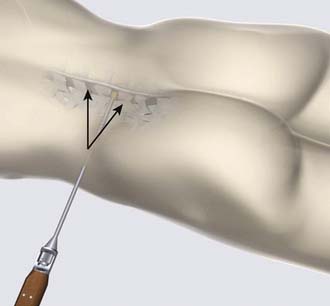
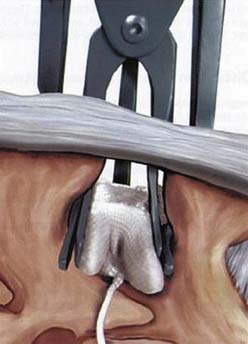
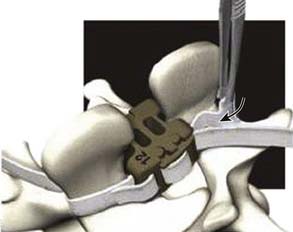
The surgical procedure can be performed under local or general anesthesia. The patient is placed in a prone position on a flat soft-frame on an adjustable operating table or on a Wilson frame. Passive distraction of the interspinous space is achieved and adjusted by tilting the foot end of the surgical table until maximum “opening” of the interspinous space is reached (Figure 54-4). The implant is placed through a lateral percutaneous approach (Figure 54-5). Piercing of the interspinous ligament is performed with a K-wire; enlargement of the interspinous space is achieved with blunt distractors of increasing sizes. After removal of the distractors, the implant can be introduced through an application sleeve and the implant wings are deployed under AP fluoroscopic control. Once the wings are deployed completely, the implant is uncoupled from the implant holder, which, together with the application sleeve, is then removed en bloc, leaving the implant in place (Figure 54-6).
Results
The first operation worldwide was performed on March 15, 2006. Preliminary results in 41 patients show a good reduction of pain level as well as of the Oswestry Disability Index in patients with low back pain as well as in patients with dynamic degenerative lumbar spinal stensosis.22
Summary
InSpace is significantly less invasive as compared to all other extension stoppers currently on the market. The average intraoperative blood loss was less than 5 cc. Surgical time for a single level is usually less than 15 minutes in uncomplicated cases. There were no clinically relevant intraoperative complications. Other advantages of this lateral approach are the short learning curve and no significant blood loss. It can be performed as an outpatient procedure. Postoperative magnetic resonance imaging does not show any evidence of muscular damage or hematoma. The technical limitations are at L5-S1 or in patients with a high iliac crest, due to the angulation required to access the interspinous space. There are still few clinical data available. The implant is currently used in a prospective randomized controlled IDE trial in the United States for the treatment of dynamic lumbar spinal stenosis.
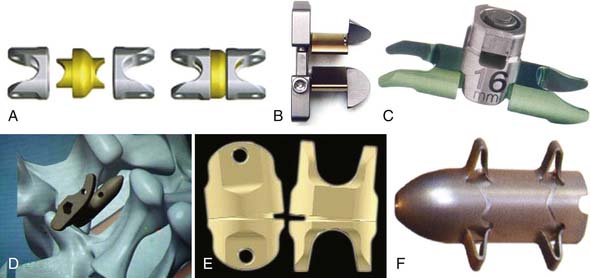
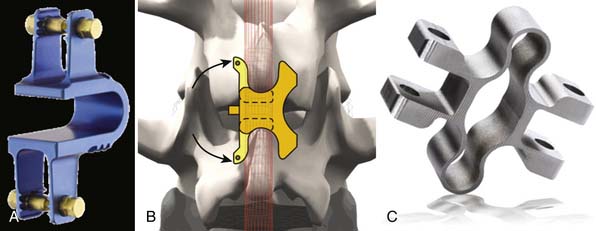
Other Implant Types (Figure 54-7)
Coflex (Paradigm Spine, New York, NY, USA) (Figure 54-8)
This is a U-shaped titanium implant with two bendable wings on its cranial and caudal parts. The Coflex is a dynamic extension stopper that acts like a spring in such a way that extension leads to an elastic compression of the “U” (Figure 54-9). It is either used as an adjunct to open decompression in spinal stenosis cases to unload the facet joints and to “keep the spinal canal open,” or following discectomy to “protect” the disc from excessive load. It thus represents a low back pain treatment concept, i.e., a dynamic stabilization to reduce the load on the facet joints and/or the disc space, and/or to keep the spinal canal “open” by interspinous distraction following decompression procedures.
Surgical Technique
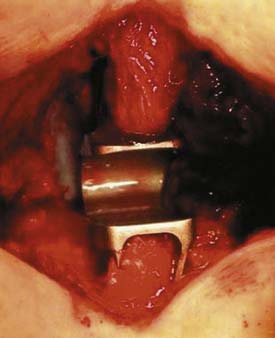
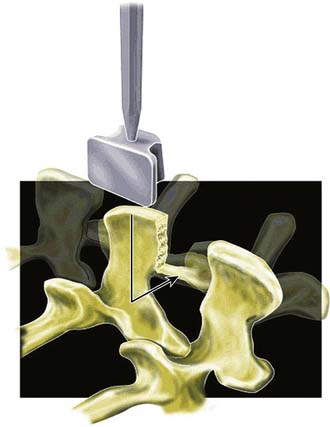
The patient positioning is the same as for open decompression (knee-chest or prone). After segmental decompression the surfaces of the spinous processes are “shaped” to achieve a good press fit of the implant (Figure 54-10). The interspinous ligament is completely resected, and the supraspinous ligament is detached from the spinous processes and reattached with transosseous sutures after the implantation. The size of the implant is determined with templates. The implant is inserted and press fit between the spinous processes as far anterior as possible, leaving 2 to 3 mm space between the dura and the bottom of the U.
Results
First results have been presented by Adelt et al.23 The implant was used as an adjunct to open decompression in a series of more than 200 patients with spinal stenosis. After a follow-up period of an average of 2 years, more than 90% of the patients reported subjective satisfaction. In 429 patients followed for 1 year postoperatively, the authors found an improvement in low back pain in 75%, an improvement in leg pain in 87%, and an improvement in intermittent neurogenic claudication in 87%. Ninety-three percent answered “yes” when asked whether they would again decide to have this type of operation if they were in the same situation. The complication rate in their series was 6%. Satisfactory results were also published recently by Brussee et al in a series of 65 patients with degenerative lumbar spinal stenosis, 74.2% of whom were very or moderately satisfied.24 However, considering all domains of the Zurich Claudication Questionnaire, an overall good result could only be achieved in 30.6% of the patients.24 In a prospective study, the Coflex was used in 18 patients with segmental lumbar instability and compared to 24 patients in whom a PLIF procedure was applied.25 After 1 year follow-up, both groups showed significant improvement on the Visual Analog Scale (VAS); however, the range of motion in the segment above the index level increased significantly following the fusion procedure as compared to the dynamic stabilization with Coflex. The authors conclude that Coflex can be a good alternative to fusion, posing less stress in the adjacent level.
Dynamic/Rigid Interspinous Stabilizers
The rationale behind this second group of interspinous distraction devices is to achieve an interspinous stabilization to avoid or to augment fusion. Whereas the extension stoppers described above do not provide stabilization, these implants can achieve an interspinous distraction as well as an increased dynamic or rigid stability. They are thus nearly exclusively promoted to be used as an adjunct to open decompression procedures in patients with spinal stenosis or as an alternative to other types of lumbar fusion in cases of low back pain. The indications thus do not overlap with most of the extension stoppers, with perhaps the exception of the Coflex.
The DIAM Implant (Medtronic, Minneapolis, MN, USA) (Figure 54-11)
The DIAM is a soft implant that consists of an H-shaped silicone core covered by a polyethylene sheath (Figure 54-11). It can be fixed to the spinous processes with two synthetic ligaments. The biomechanical effect, aside from interspinous distraction, is shock absorption, as well as dynamic neutralization of the motion segment.26,27 The indications promoted by the protagonists are facet joint pain, for postdiscectomy patients, and spinal and foraminal stenosis with low back pain.28–30 The product has been mainly used as an alternative to rigid fixation with pedicle screws.
Surgical Technique
The implantation can be performed with or without resection of the supraspinous ligament. The interspinous ligament is resected and the implant size is determined with a template after interspinous distraction with a distraction forceps (Figure 54-12). Within a special implant holder, the elastic implant is “folded” and inserted into the interspinous space. The ligaments are passed around the spinous processes and fixed (Figure 54-13).
Results
First results were reported by Mariottini et al,31 who reported satisfactory outcomes in 97 % of 43 patients. In an Italian multicenter trial, high rates of satisfaction as well as low complication rates were reported by Guizzardi in 2005.32 Results with the DIAM implant have been reported by Taylor et al.28 in a multicenter series of 104 patients with herniated discs, and foraminal or central spinal canal stenosis. The median follow-up was 18.1 months. There was significant pain relief in 83.8 % of patients.
Kim et al. used the implant in patients suffering from disc herniations. They compared the results of simple microdiscectomy with microdiscectomy followed by the implantation of DIAM in patients suffering from radicular as well as low back pain symptoms.29
Summary
DIAM is a stabilizing interspinous implant that provides soft interspinous distraction and tension banding. The biomechanical behavior leads to a dynamic neutralization of the motion segment. Although the technique seems to be less aggressive as compared to lumbar fusion techniques, good clinical data are lacking, and the evidence is still poor. The implant is currently in an FDA-IDE trial in the United States.
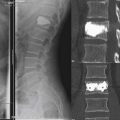
The Wallis Implant (Abbott Spine, Austin, TX, USA) (Figure 54-14)
The Wallis implant was invented by Senegas in the mid-1980s.33,34 It is an H-shaped interspinous spacer made from PEEK. It can be fixed at the spinous processes with woven Dacron bands that contain radiodense tantalum markers (Figure 54-14). The spacer blocks extension and the bands limit flexion of the motion segment. It is mainly used to increase intersegmental stability after decompression procedures.35 Thus the main indication is low back pain that accompanies disc herniation, spinal stenosis, recurrent disc herniation, degenerative disc disease with or without Modic type I changes, as well as degenerative disc disease in a level adjacent to fusion.34
Surgical Technique
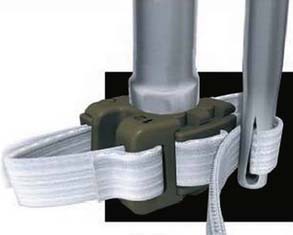
The patient is usually placed in a prone position. After the decompression operation or discectomy procedure, the supraspinous ligament is detached from the spinous processes and the interspinous ligament is resected (Figure 54-15). The size of the interspinous spacer is determined with a template, after the surfaces of the spinous processes are trimmed. The concave surface of the superior spinous process is flattened, as is the junctional zone between the spinous process and the laminae. The spacer is inserted and the bands are passed around the superior and inferior spinous process (Figure 54-16). They are then passed through a clip that is snapped into the spacer. Next, the tightness of the band can finally be adjusted (Figure 54-17) to achieve a good compression. Finally, the supraspinous ligament is reattached and fixed.
Results
In 2007, Senegas first reported a long-term survivorship of the implant in a series of 241 patients who had been treated between 1987 and 1995.34 The survivorships were 75.9% for “any subsequent lumbar operation,” and 81.3% for “implant removal.”
Overall reoperation rate was 21.1%. In 2007 Floman et al reported a series of 37 patients who underwent lumbar discectomy followed by fixation with the Wallis implant.35 The follow-up was 16 months. The indication included patients with low back pain and patients with large voluminous disc herniations. The intention was to “protect” the segment from collapse and thus to prevent recurrent disc herniation and/or low back pain postdiscectomy.
Summary
The Wallis implant is probably the strongest interspinous implant and the one with the greatest capability to “stabilize” the segment. It is, likewise, the implant that requires the most aggressive surgical approach and is thus no less invasive than lumbar fusion techniques. Its protective effect for the disc has not been proven yet, and whether it can be an alternative to other less invasive interspinous spacers or to fusion procedures remains to be determined.
1. Anderson P.A., Tribus C.B., Kitchel S.H., Hartjen C.A. Treatment of neurogenic claudication by interspinous decompression: application of the X-Stop device in patients with lumbar degenerative spondylolisthesis. J. Neurosurg. Spine. 2006;4:463-471.
2. Hsu K.Y., Zucherman J.F., Mehalik T.F., Implicito D.A., Martin M.J., Johnson D.R., Skidmore G.A., Vessa P.P., Dwyer J.W., Cauthen J.C., Ozuna R.M. Quality of life of lumbar stenosis-treated patients in whom the X-Stop interspinous device was implanted. J. Neurosurg. Spine. 2006;5:500-507.
3. Kondrashov D.G., Hannibal M., Hsu K.Y., Zucherman J.F. Interspinous process decompression with the X-Stop device for lumbar spinal stenosis: a 4-year follow-up study. J. Spinal Disord. Tech.. 2006;19:323-327.
4. Lauryssen C. Appropriate selection of patients with lumbar spinal stenosis for interspinous process decompression. Neurosurg. Focus. 2007;22:121-126.
5. Zucherman J.F., Hus K.Y., Hartjen C.A., Mehalic T.F., Implicito D.A., Martin M.J., Johnson D.R., Skidmore G.A., Vessa P.P., Dwyer J.W., Puccio S.T., Cauthen J.C., Ozuna R.M. A multicenter, prospective randomized controlled trial evaluating the X-Stop interspinous process decompression system for the treatment of neurogenic intermittend claudication. Spine. 2005;30:1351-1358.
6. Lindsey D.P., Swanson K.E., Fuchs P., Hsu K.Y., Zucherman J.F., Yerby S.A. The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine. 2003;28:2192-2197.
7. Richards J.C., Majumbar S., Lindsey D.P., Beaupré G.S., Yerby S.A. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittend claudication. Spine. 2005;30:744-749.
8. Siddiqui M., Nicol M., Karadimas E., Smith F., Wardlaw D. The positional magnetic resonance imaging changes in the lumbar spine following insertion of a novel interspinous process distraction device. Spine. 2005;30:2677-2682.
9. Wiseman C.M., Lindsey D.P., Fredrick A.D., D, S.A. Yerby The effect of an interspinous process implant on facet loading during extension. Spine. 2005;30:903-907.
10. Siddiqui M., Karadimas E., Nicol M., Smith F.W., Wardlaw D. Influence of X-Stop on neural foramina and spinal canal area in spinal stenosis. Spine. 2006;31:2958-2962.
11. Swanson K.E., Lindsey D.P., Hsu K.Y., Zucherman J.F., Yerby S.A. The effect of an interspinous implant on intervertebral disc pressures. Spine. 2003;28:26-32.
12. Siddiqui M., Karadimas E., Nicol M., Smith F.W., Wardlaw D. Effects of X-Stop device on sagittal lumbar spine kinematics in spinal stenosis. J. Spinal Disord. Tech. 2006;19:328-333.
13. Idler C., Zucherman J.F., Yerby S., Hsu K.Y., Hannibal M., Kondrashov D. A novel technique of intra-spinous process injection of PMMA to augment the strength of an interspinous process device such as the X-Stop. Spine. 2008;33:452-456.
14. Verhoof O.J., Bron J.L., Wapstra F.H., van Royen B.J. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur. Spine J.. 2008;17:188-192.
15. Mayer H.M. Microsurgical decompression for acquired central and lateral spinal canal stenosis. In Mayer H.M., editor: Minimally invasive spine surgery, second ed., Berlin – Heidelberg – New York: Springer-Verlag, 2005.
16. McCulloch A. Microsurgery for lumbar spinal canal stenosis. In: McCulloch J.A., Young P.H., editors. Essentials of spinal microsurgery. Philadelphia, PA: Lippincott-Raven, 1998.
17. J. Lim, J. Park, Biomechanical study of InSpace interspinous device, Spine Arthroplasty Society, Annual Meeting 08, Miami Poster 162, 2008.
18. L.I. Voronov, R.M. Havey, S.M. Renner, G. Carandang, C. Abjornson, A.G. Parwadhan, Biomechanics of novel posterior dynamic stabilization device (InSpace), Spine Arthroplasty Society, Annual Meeting 08, Miami Poster 148, 2008.
19. G.A. Goel, V.K. Goel, A. Mehta, D. Dick, A. Khere, C. Abjornson, Cyclic loading does not compromise functionality of interspinous spacer or any damage to the segment, Spine Arthroplasty Society, Annual Meeting 08, Miami Poster 212, 2008.
20. B. Kelly, E. Sander, N. Zufelt, D. DiAngelo, Low endurance testing of a novel spinous process spacer under coupled loading conditions, Spine Arthroplasty Society, Annual Meeting 08, Miami Poster 197, 2008.
21. Mayer H.M., Mehren C., Skidmore G., et al. A new percutaneous lateral approach for the insertion of an interspinous spacer. San Diego: Annual Meeting of the American Academy of Neurological Surgeons (AANS); May 2-4, 2009.
22. H.M. Mayer, C. Mehren, C. Siepe, et al: A new interspinous spacer for minimally invasive treatment of dynamic lumbar spinal stenosis and low back pain Annual Meeting of the American Academy of Neurological Surgeons (AANS), San Diego, May 2-4, 2009.
23. Adelt D., Samani J., Kim W.K., Eif M., Lowery G., Chomiak R.J. Coflex interspinous stabilization: clinical and radiographic results from an international multicenter retrospective study. Paradigm Spine J.. 2007;1:1-4.
24. Brussee P., Hauth J., Donk R.D., Verbeek A.L.M., Bartels R.H.M. Self-rated evaluation of outcome of the implantation of interspinous process distraction (X-Stop) for neurogenic claudication. Eur. Spine J.. 2008;17(2):200-203.
25. Kong D.S., Kim E.S., Eoh W. One-year outcome evaluation after interspinous implantation for degenerative spinal stenosis with segmental instability. J. Korean Med. Sci. 2007;22:330-335.
26. Schmoelz J.F., Nydegger T., Claes L., Wilke H.J. Dynamic stabilization of the lumbar spine: an in vitro experiment. J. Spinal Disord. Tech. 2003;16:418-423.
27. Phillips F.M., Voronov L.I., Gaitanis I.N., Carandang G., et al. Biomechanics of posterior dynamic stabilization device (DIAM) after facetectomy and discectomy. Spine J. 2006;6:714-722.
28. Taylor J., Pupin P., Delajoux S., Palmer S. Device for intervertebral assisted motion: technique and initial results. Neurosurg. Focus. 2007;22(1):E6.
29. Kim K.A., McDional M., Pik J.H.T., Khoueir P. Dynamic intraspinous spacer technology for posterior stabilization: clinical safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg. Focus. 22, 2007.
30. Kim D. T. Albert: Interspinous process spacers. J. Am.Acad. Orthop. Sur. 2007;15:200-207.
31. Mariottini A., Pieri S., Giachi S., et al. Preliminary results of a soft novel lumbar intervertebral prosthesis (DIAM) in the degenerative spinal pathology. Acta Neurochir. Suppl. 2005;92:129-131.
33. Guizzardi G., Petrioni P., Fabrizi A.P., et al. The use of DIAM (interspinous stress-breaker device) for the DDD: Italian multicenter experience. Spine Arthroplasty Society Meeting. 2005.
34. Senegas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur. Spine J. 2002;11(Suppl. 2):S164-S169.
35. Senegas J., Vital J.M., Pointillard V., Mangione P. Long-term survivorship analysis of an interspinous stabilization system. Eur. Spine J. 2007;16:1279-1287.
36. Floman Y., Millgram M.A., Smorgick Y., Rand N., Ashkenazi E. Failure of the Wallis Interspinous Implant to lower the incidence of recurrent lumbar disc herniations in patients undergoing primary disc excision. J. Spinal Disord. Tech. 2007;20:337-341.