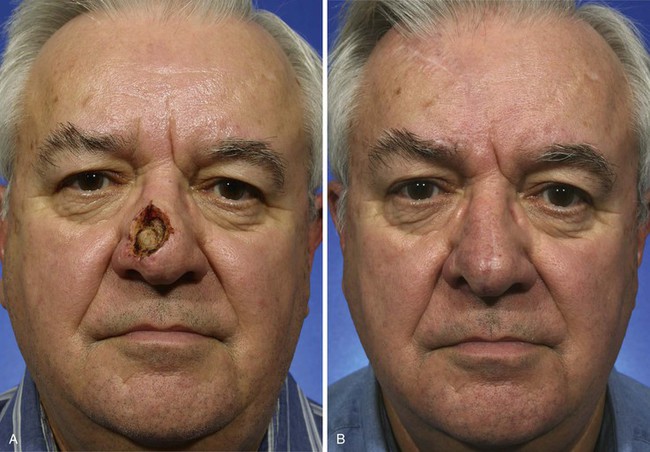
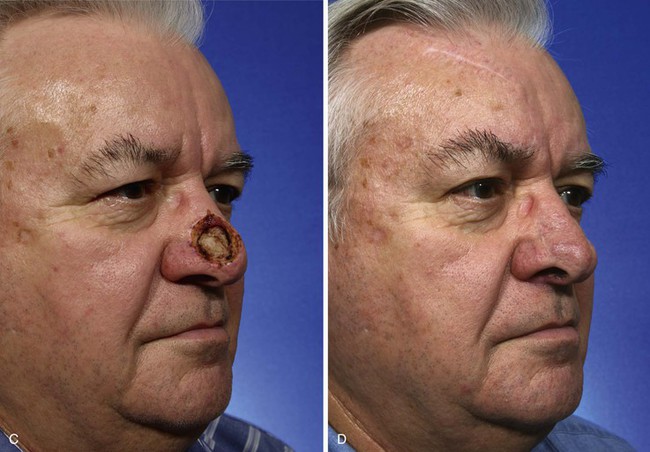
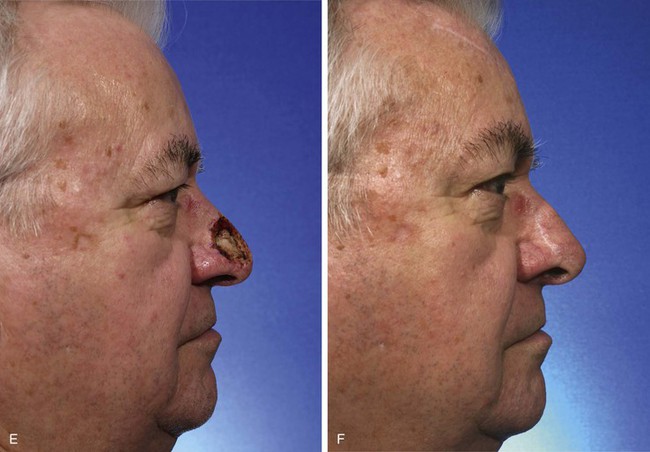
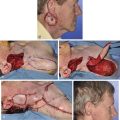
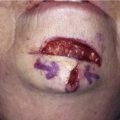
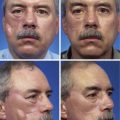
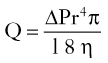Interpolated Paramedian Forehead Flaps
Introduction
Median forehead flaps were first described in an Indian medical treatise, the Sushruta Samita, in approximately 700 BC.1–3 The operation was performed by members of a caste of potters known as the Koomas. The need for this operation arose from the common Indian practice of amputating the tip of the nose as punishment for a variety of crimes ranging from robbery to adultery.4,5 The first reported use of the median forehead flap outside of India was by Antonio Branca of Italy. Based on an Arabic translation of the Sushruta Samita, Branca performed a nasal reconstruction using the midforehead flap in the 15th century.6,7 In the 16th and early 17th centuries, little advancement was made in the use of the median forehead flap because plastic and reconstructive surgery fell into disrepute.4,5,7 The flap had a revival in 1794, when J. C. Carpue read an editorial in the Gentleman’s Gazette of London describing the flap’s use for nasal reconstruction.8–10 Initially, Carpue practiced the median forehead flap operation on cadavers. Twenty years passed before he performed the operation on two patients. He reported his successful results in a monograph entitled “An Account of Two Successful Operations for Restoring a Lost Nose from the Integuments of the Forehead.” His article was widely circulated throughout Europe and served to popularize the operation.6,9 In the 1830s, Ernst Blausius (Chief of Ophthalmologic Surgery of Berlin), Johann Friedrich Dieffenbach (Chief of Surgery at Munich Hospital), and Natale Petrali of Milan simultaneously reported on uses and variations of the median forehead flap for reconstruction of the face and nose. On the basis of their influence as respected surgeons at large European teaching hospitals, the use and popularity of the forehead flap grew.6 In the late 1830s, use of the median forehead flap for nasal and facial reconstruction crossed the Atlantic when J. M. Warren performed the operation in the United States.4,11
By the early 1900s, the forehead flap was used to reconstruct losses of the nose secondary to battle, scrofula, syphilis, and cancer. Many American surgeons, such as Pancoast, Mutter, Buck, Davis, and Fomon, wrote about the use of the forehead flap in nasal reconstruction. Little modification of flap design, use, harvest, or donor site closure occurred until articles authored by Kazanjian appeared in the plastic surgery literature of the 1930s. This pioneer plastic surgeon was the first to determine that the primary blood supply of the flap is from the supratrochlear and supraorbital arteries. Kazanjian described a forehead flap designed precisely in the midline, allowing primary closure of the donor site. This technical modification minimized the forehead donor scar, which up to that point represented the major morbidity of the operation. Before Kazanjian’s modification, the donor site of the forehead flap had either been skin grafted or left open to heal secondarily by granulation and wound contraction. This practice commonly left the patient more scarred and disfigured than before the reconstructive surgery.12
Kazanjian carried incisions from the hairline to a point immediately above the level of the nasofrontal angle. By his time, surgeons had recognized that the use of unlined forehead flaps to repair full-thickness nasal defects predictably resulted in contraction of the flap and compromise of the nasal passage. The contour of the external nose also deformed as the airway constricted from progressive contracture of scar developing on the undersurface of the flap. In response to this, surgeons developed a number of modifications in the design of the forehead flap to achieve additional length so the flap could be folded on itself for internal lining. These designs included oblique and horizontally oriented flaps. Gillies13 described a U-shaped flap with an ascending and descending component that he called the up-and-down flap. The ascending portion of the flap was positioned over the axis of the supraorbital artery on one side and the descending portion over the contralateral supraorbital artery. Converse14 harvested lateral forehead skin based on a long pedicle of hair-bearing scalp, which became known as the scalping flap. The designs by Gillies and Converse circumvented the need to include hair-bearing scalp to provide additional length so that the flap could be folded on itself. Unfortunately, these modifications of the original design of the median forehead flap left marked deformities of the forehead. It also became apparent that folding the flap on itself created a great deal of tissue bulk that caused the nose to collapse. In addition, partial necrosis of the portion turned internally was common.
In the 1960s, Millard11,15 designed a large modified median forehead flap called the seagull flap, in which lateral extensions were used to cover the nasal alae. Incisions for the pedicle of the flap extended below the bony orbital rim to gain additional flap length. Millard also described methods of donor site repair and techniques for constructing nasal support, all of which improved the outcome of nasal reconstruction.
Labat7,16 was the first surgeon to design a median forehead flap with the base centered over a unilateral supratrochlear artery. He curved the incisions of the proximal pedicle so the base of the flap rested immediately above the medial brow and canthus on one side. This reduced the standing cutaneous deformity resulting from pivoting of the flap and increased the effective length, making more tissue available for reconstruction. Millard shifted the entire vertical axis of the central forehead flap to a paramedian position, demonstrating that the flap could survive without including the central glabellar skin in the pedicle.7 Menick modified Millard’s design of the paramedian flap by making the pedicle narrower.7 This offered a greater freedom of tissue movement, a smaller standing cutaneous deformity, and a longer effective length to the flap.
In the 1980s, Burget and Menick17–19 found that extending incisions for the pedicle of the paramedian flap below the bony orbital rim provided additional flap length. This often avoided the need to extend the flap to hair-bearing scalp for sufficient length to reach the nasal tip. They noted that the end arterioles of the supratrochlear artery are located immediately under the dermis, superficial to the frontalis muscle. The authors determined that the frontalis muscle could be safely removed from the distal flap without impairing the vascularity of the skin.1
Studies by Mangold, McCarthy, and Shumrick better defined the vascular anatomy of the forehead. In 1980, Mangold et al20 demonstrated that the blood supply to forehead skin is from the dorsal nasal (a terminal branch of the angular artery), supratrochlear, supraorbital, and superficial temporal arteries. Each of these vessels provides a primary blood supply to a particular region of the forehead, but all demonstrate numerous interconnecting anastomoses. Mangold’s injection studies and dissections of cadavers showed that the forehead could be divided into regions based on their predominant vascular supply (Fig. 13-1). On the basis of these vascular regions, Mangold determined that median and paramedian vertically oriented forehead flaps are nourished primarily by the supratrochlear artery and secondarily by the dorsal nasal and supraorbital arteries. McCarthy and others21,22 confirmed Mangold’s work in clinical experiences with patients. McCarthy injected the facial artery after ligation of the supraorbital and supratrochlear arteries and showed sufficient filling of the forehead vasculature to supply vertically oriented flaps in the region of the central forehead.21 This suggested that a paramedian forehead flap could survive even when the supratrochlear artery on the side of the flap is not present.
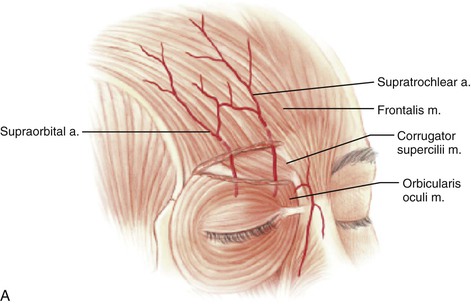
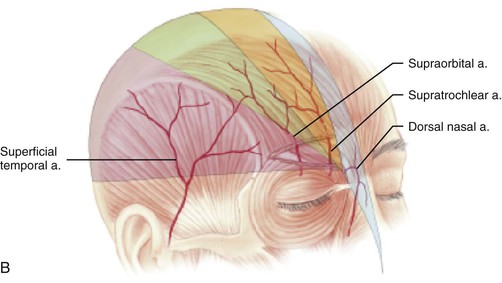
FIGURE 13-1 A, Supratrochlear artery exits orbit approximately 2 cm lateral to midline, passing under orbicularis oculi and over corrugator supercilii. At level of eyebrow, supratrochlear and supraorbital arteries pass through orbicularis and frontalis muscles and continue superiorly in superficial subcutaneous tissue plane. B, Vascular territories of arteries supplying forehead skin. (From Baker SR: Interpolated paramedian forehead flaps. In Baker SR, editor: Principles of nasal reconstruction, ed 2, New York, Springer, 2011.)
In 1992, Shumrick and Smith10 performed detailed anatomic studies of the forehead using techniques of latex injection, radiography, and microdissection to determine the precise vascular anatomy of the central forehead. Examination of the radiographic data confirmed a clinically apparent fact: the forehead region contains an intricate system of anastomosing vessels among the angular, supratrochlear, supraorbital, and superficial temporal arteries (Fig. 13-2). The paired supratrochlear arteries connected with each other by several horizontal unnamed arteries that cross the midline. Moreover, the supratrochlear arteries consistently demonstrated anastomotic branches with the angular and supraorbital arteries in the medial canthal region. Microdissection of the forehead vasculature confirmed these radiographic findings. The supratrochlear artery was consistently found to exit the superior medial orbit approximately 1.7 to 2.2 cm lateral to the midline and continued its course vertically in a paramedian position approximately 2 cm lateral to the midline. This position closely corresponds to the location of the medial border of the eyebrow. The supratrochlear artery was found to exit the orbit by piercing the orbital septum, passing under the orbicularis oculi and over the corrugator supercilii.10 At approximately the level of the eyebrow, the artery passes through the orbicularis and frontalis muscles and continues superiorly in the superficial subcutaneous tissue. This transition of the artery from a deeper to a more superficial tissue plane was confirmed by histologic examination of cross sections of forehead skin at various levels. Doppler examinations of healthy volunteers helped confirm the findings of the cadaver studies.10
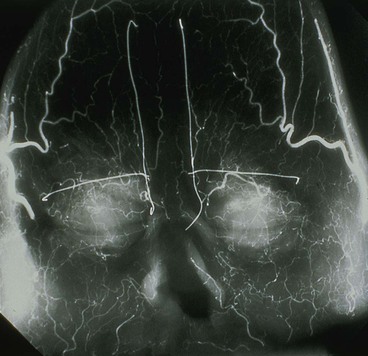
FIGURE 13-2 Radiograph showing skin vasculature after injection of contrast medium. Horizontal wire markers delineate superior bony orbital rims. Vertical wires mark medial end of eyebrows. Note intricate system of anastomosing vessels. (From Baker SR: Interpolated paramedian forehead flaps. In Baker SR, editor: Principles of nasal reconstruction, ed 2, New York, Springer, 2011.)
Studies of the vascular anatomy of the forehead confirm that the supratrochlear artery serves as the axial blood supply of median and paramedian vertically oriented forehead flaps. The studies also confirm a rich anastomotic network in the medial canthal region. Surgical techniques that preserve this regional blood flow have allowed surgeons to harvest paramedian forehead flaps based on pedicles narrower than those used for median forehead flaps. The narrower pedicle provides the flap with greater freedom of transposition about its pivotal point and greater effective length. The pedicle may be as narrow as 1.2 cm, reducing deformity of the inferior forehead area and always enabling the surgeon to achieve primary closure of the lower half of the donor defect (Fig. 13-3).7,23
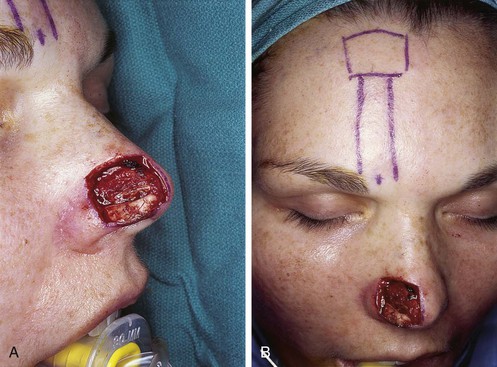
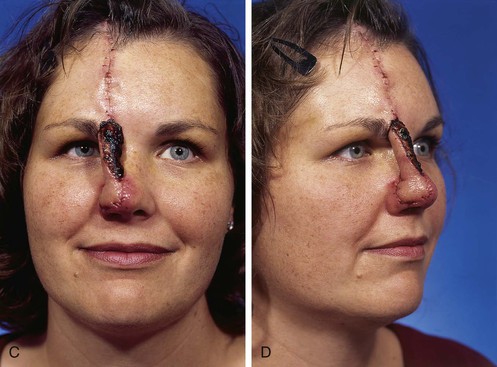
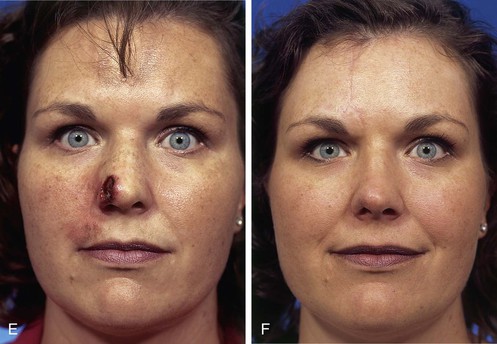
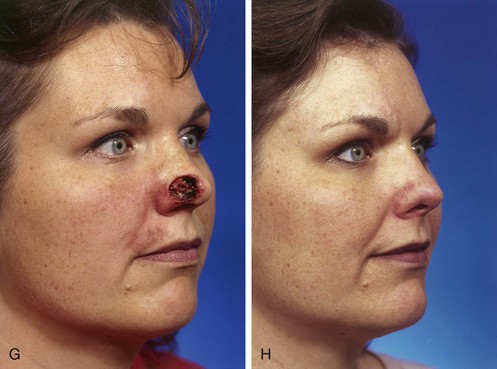
FIGURE 13-3 A, Heminasal cutaneous defect. Auricular cartilage graft placed along nostril rim for structural support. B, Paramedian forehead flap designed for reconstruction of nose. Centering of pedicle over vertical axis of supratrochlear artery enables narrow pedicle 1.2 cm wide. C, D, Narrow pedicle provides greater effective length and smaller standing cutaneous deformity than wider pedicle. E-H, Preoperative and 1.5-year postoperative views. No revision surgery performed.
Paramedian Forehead Flap
Midforehead flaps, which include the median and paramedian flaps and their many variations, have proved to be dependable flaps for midfacial reconstruction.2,3,24 However, the paramedian forehead flap based on a single supratrochlear artery has replaced the median forehead flap for nasal reconstruction because it has a more axial design, narrower base, and greater effective length. The design also enables the simultaneous or sequential use of two vertically oriented forehead flaps. The paramedian flap has an abundant blood supply, providing for revascularization of cartilage and bone grafts covered by the flap. Removal of muscle and subcutaneous fat from the distal portion can make the paramedian forehead flap thin, pliable, and easily contoured to fit any defect of the nose. Maintaining the attachment of the frontalis muscle to the flap is occasionally useful when more bulk is required to fill defects of considerable depth.25 In most instances, however, freeing the flap from the frontalis muscle and most of its subcutaneous fat is essential when the forehead flap is used as covering for the nose. Proper thinning of the flap will ensure that the shape and contour of the underlying nasal framework are visually manifested. Flap thinning may be accomplished without concern for compromise of the flap’s vasculature because the supratrochlear artery travels superiorly in the subcutaneous/subdermal tissue plane from a point 1 cm superior to the level of the eyebrow.10 The superficial location of the artery enables thinning of the distal portion of the flap by removal of the fascia and frontalis muscle and, if necessary, nearly all of the subcutaneous tissue. The ability to modulate the thickness of a flap so that it may exactly match the thickness of the nasal defect at the recipient site greatly enhances the aesthetic result.25
Paramedian forehead flaps used as interpolated flaps for nasal reconstruction require a second operation to separate the pedicle.26 The pedicle may safely be detached as soon as 10 to 14 days after flap transfer. The author prefers a 3-week interval between the time of flap transfer and flap inset for all patients, especially those who use tobacco products. At 3 weeks, the distal portion of the flap has developed sufficient collateral blood supply from the nose to allow thinning and sculpturing of the more proximal portion of the flap left attached to the recipient site. If the nasal defect is small, the portion of the forehead flap covering the defect may be completely thinned at the time of initial transfer.
A minor disadvantage of the paramedian forehead flap is the donor site scar and the necessity for a two-stage procedure. However, forehead scars are rarely unsightly because primary wound closure is easily achieved in the inferior portion of the forehead owing to the narrow pedicle required (Fig. 13-4). Although the narrow pedicle enables primary repair of the inferior aspect of the donor site wound, the width of the superior portion of the flap may be several centimeters, precluding complete wound approximation. In general, flaps wider than 4.5 cm are too large to allow complete primary closure of the donor site. In such circumstances, the superior portion of the donor site wound must, in part, be left to heal by secondary intention. Fortunately, the resulting midline or paramedian scar in the superior portion of the forehead is extremely forgiving and only occasionally requires revision. The acceptable scar is related to the immobile skin and convex contour of the superior central forehead (Fig. 13-5).
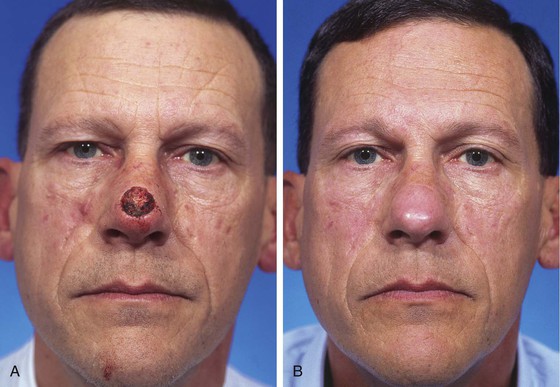
FIGURE 13-4 A, A 1.5 × 1.8-cm cutaneous defect of nasal tip. B, At 8 months after repair of defect with paramedian forehead flap. C, Donor site forehead scars are rarely unsightly. No revision surgery performed.
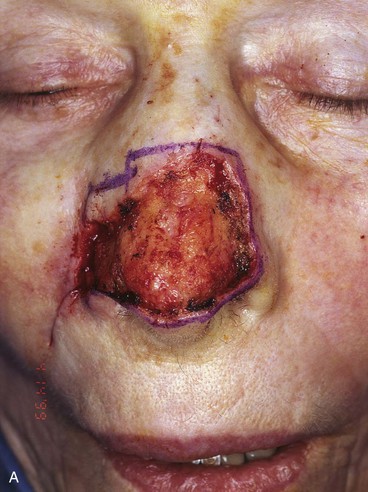
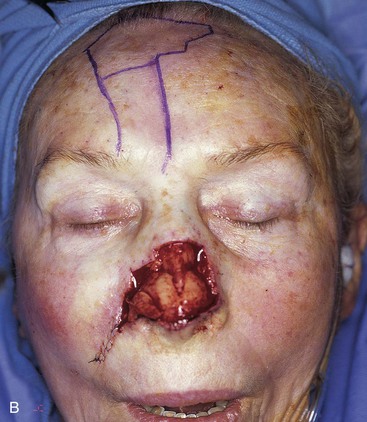
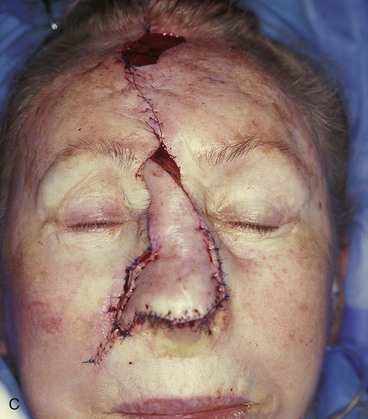
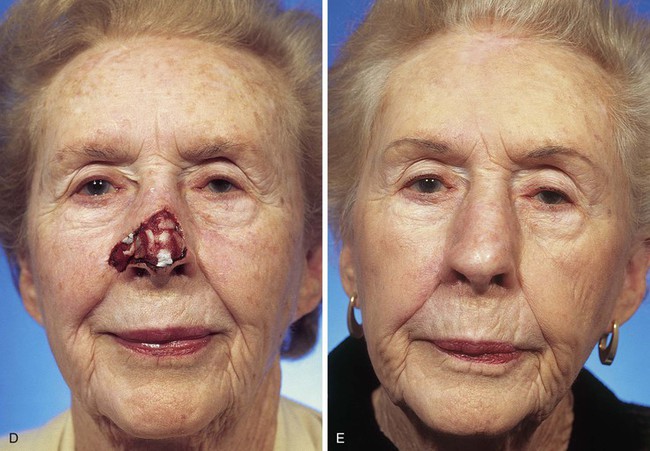
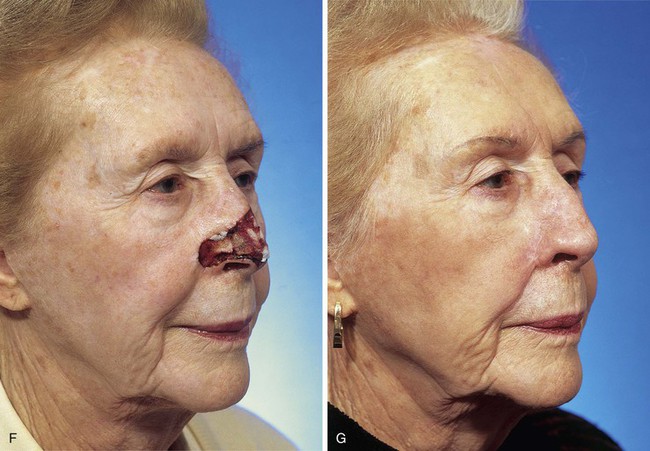
FIGURE 13-5 A, A 5 × 4-cm cutaneous defect of nasal tip, caudal dorsum, and right ala. B, Paramedian forehead flap designed for repair. C, Flap in place. Flaps wider than 4.5 cm are too large to allow complete wound closure of donor site. Portion of superior donor site wound left to heal by secondary intention. D-G, Preoperative and 9-month postoperative views. Contouring procedure to restore alar groove performed 6 months postoperatively. No revision surgery performed on forehead scar.
The fine vellus hair that is prominent in some patients just in front of the forehead hairline is even more difficult to eliminate from a forehead flap because the hair follicles are not visible by the human eye and are located in the dermis rather than in the subdermal plane. This hair may be treated by electrolysis with limited success. The best treatment is to have the patient periodically use a depilatory cream for removal. Laser hair removal may be used in place of electrolysis for dark-pigmented scalp hair, but it is not effective for vellus hair.
To avoid multiple procedures directed at depilation, paramedian forehead flaps should be designed not to include scalp hair whenever possible. Avoiding scalp hair may be possible by extending the incision for the pedicle through the eyebrow to the level of the bony orbital rim. The pedicle is skeletonized on the soft tissue surrounding the supratrochlear artery as it exits the orbit. This requires complete sectioning of the corrugator supercilii to achieve free tissue movement. If this step is unlikely to lend sufficient length to the flap, another helpful approach is to obliquely angle the flap from the midline laterally toward the temporal recession just beneath the hairline (Fig. 13-6).27,28 This modification in design of the paramedian forehead flap is possible only for smaller flaps measuring less than 3 cm in maximum width. Harvesting of flaps 3 cm or greater in width from the lateral portion of the forehead may occasionally cause more apparent scars than centrally located donor sites. It may also cause excessive upward displacement of the central portion of the eyebrow on the donor side. Thus, flaps wider than 3 cm are usually extended to the hair-bearing scalp rather than designed in an oblique fashion when the necessary length of the flap requires such extensions (Fig. 13-7). When the oblique forehead design is used, the distal portion of the flap does not have the advantage of an axial vascular pattern. However, the author has been successful in transferring forehead skin based on a supratrochlear artery but positioned several centimeters lateral to the axis of the artery. This success is related to the rich vascular anastomotic network of forehead skin.
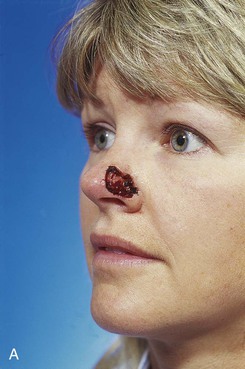
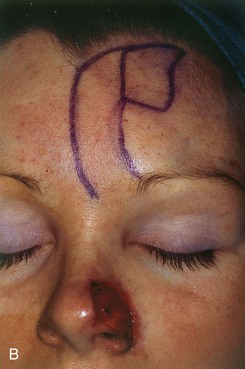
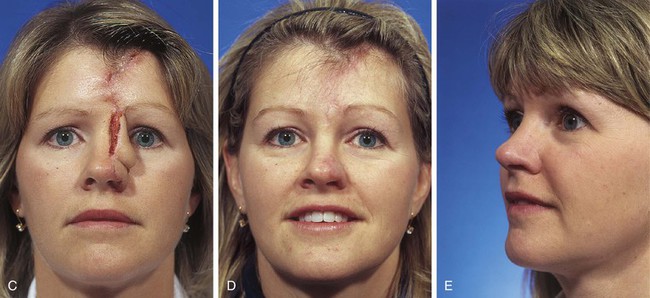
FIGURE 13-6 A, A 2 × 1-cm cutaneous defect of nasal tip and ala. B, Paramedian forehead flap designed for repair. Distal flap angled toward temporal recession beneath anterior hairline to provide additional length to flap while avoiding transfer of scalp hair to nose. C, Flap in place. D, At 2 months after flap inset. E, Postoperative view at 1.5 years. No revision surgery performed.
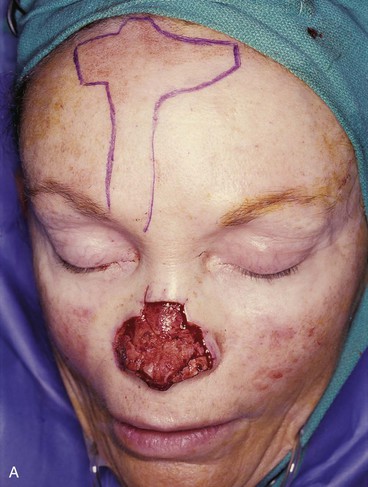
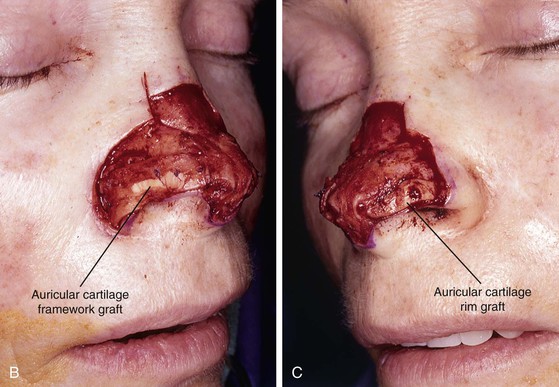
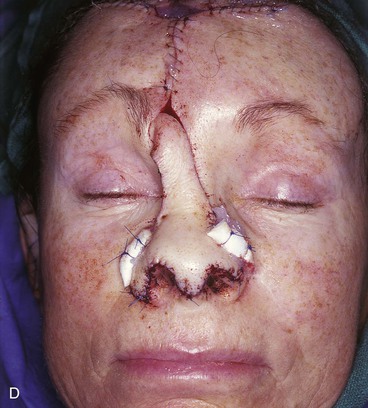
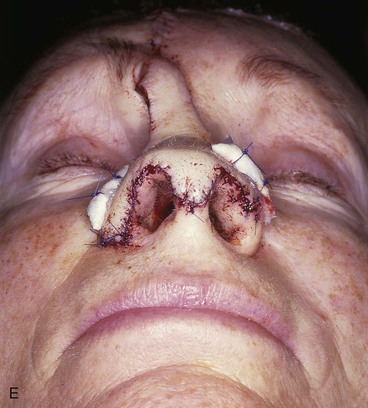
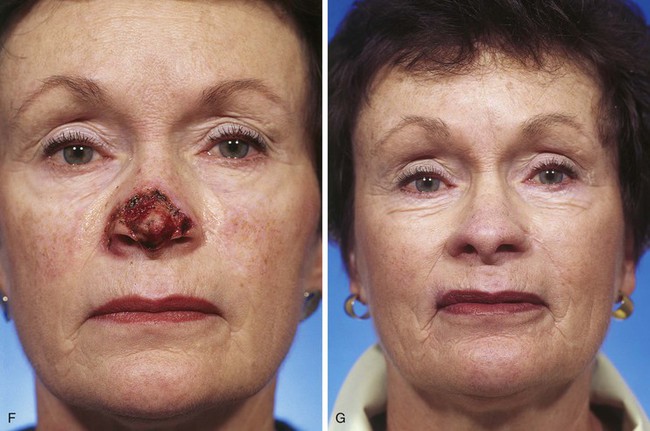
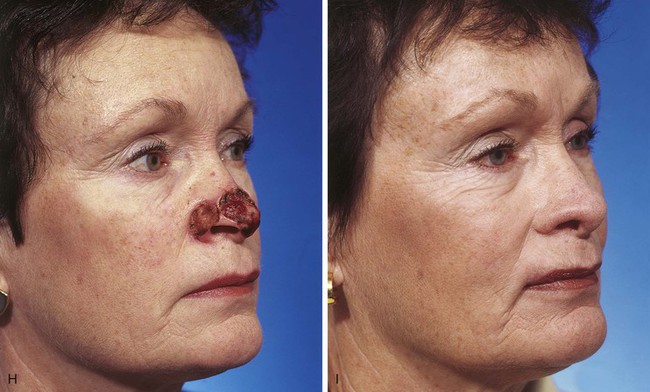
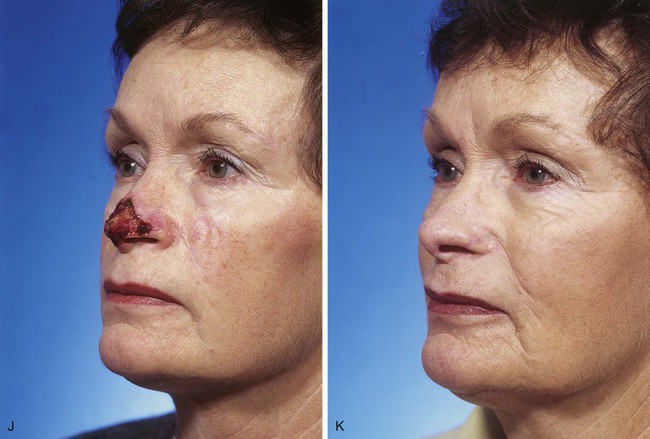
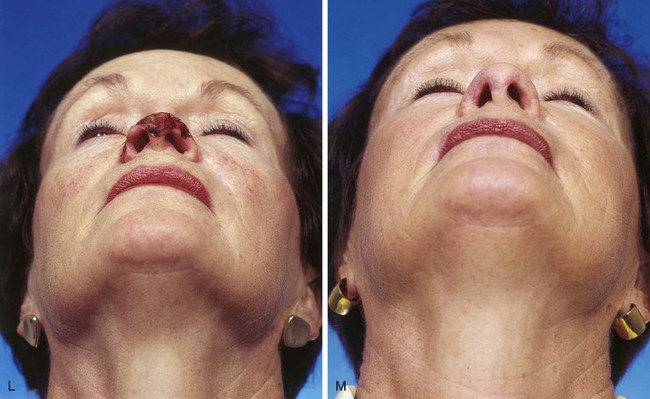
FIGURE 13-7 A, A 5 × 2-cm cutaneous defect of nasal tip, caudal dorsum, and right ala. Paramedian forehead flap designed for repair. Size of defect necessitated extending forehead flap into hair-bearing scalp. B, C, Auricular cartilage grafts in place to provide structural support to right ala and left nostril margin. D, E, Flap in place. Portion of superior donor site wound left to heal by secondary intention. Bolster dressings used to appose flap to cartilage grafts. F-M, Preoperative and 5-year postoperative views. Contouring procedure and nasal base reduction performed.
Historically, the paramedian forehead flap has been used for reconstruction of larger defects of the nose. It is the preferred method for covering nasal defects too large to be repaired with full-thickness skin grafts, nasal cutaneous flaps, composite auricular grafts, or interpolated melolabial flaps.4,5,12 In general, nasal defects larger than 2 cm in width in the horizontal axis are best repaired with a paramedian forehead flap (see Fig. 13-7).12 In addition, this flap is best for repair of nasal defects with exposed bone and cartilage deficient of periosteum or perichondrium and in instances in which the central face has been irradiated.5,12
Surgical Technique
The cutaneous defect is outlined by squaring off the corners of the defect with a skin marker. Giving the defect angular rather than curvilinear borders reduces the propensity for development of trap-door deformity (see Fig. 13-5). If the defect occupies more than 50% of the surface area of a convex-shaped nasal aesthetic unit (tip and alae), the remaining skin of the unit is marked for removal (Fig. 13-8). When a unilateral cutaneous defect encompasses half or less of the surface area of the nasal tip, the defect is usually enlarged only to the degree that the enlargement creates a hemi-tip defect (Fig. 13-9). This will limit the size of the forehead flap necessary for reconstruction, which in turn minimizes the deformity of the forehead. The aesthetic result of resurfacing a heminasal defect with a paramedian forehead flap is as pleasing as resurfacing the entire nasal tip, provided the remaining nasal skin is of thin or medium thickness.
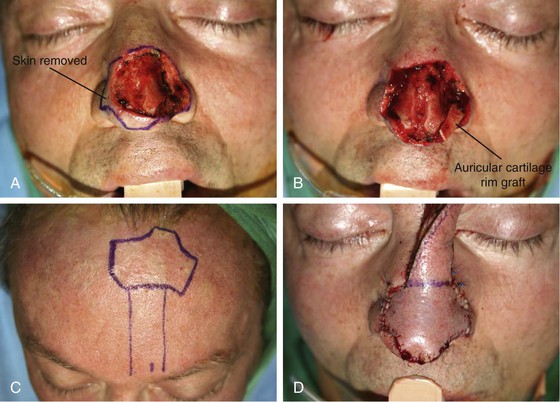
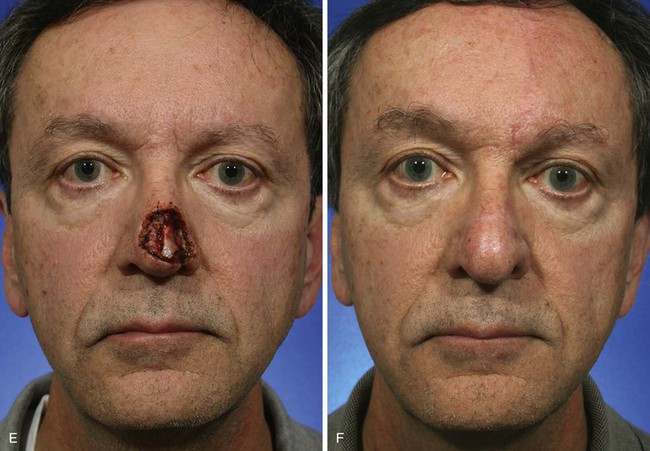
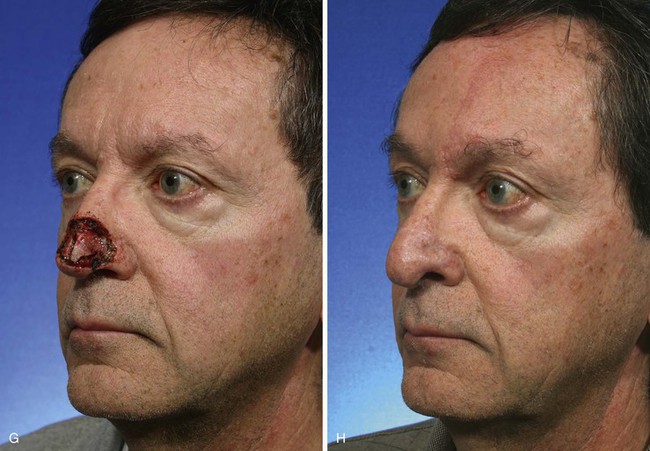
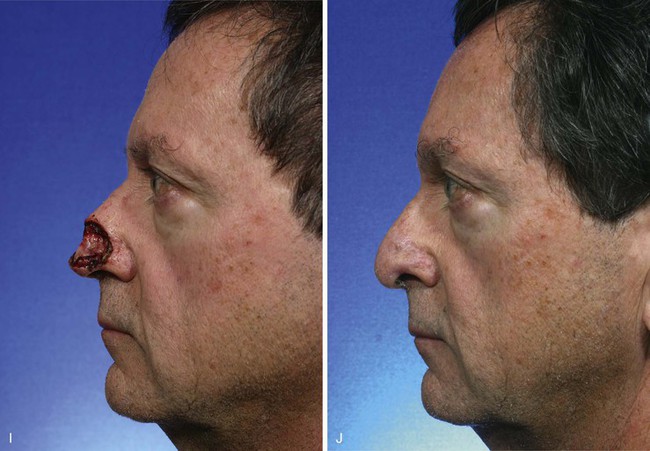
FIGURE 13-8 A, A 3 × 2.5-cm cutaneous defect of nasal tip and small portion of left ala. Defect involves left nostril margin. Remaining skin of tip marked for excision. B, Skin excised. Auricular cartilage rim graft in place to prevent nostril retraction. C, Paramedian forehead flap designed for repair. D, Flap in place. E-J, Preoperative and 9-month postoperative views. Patient had contouring procedure and forehead scar revision 7 months postoperatively.
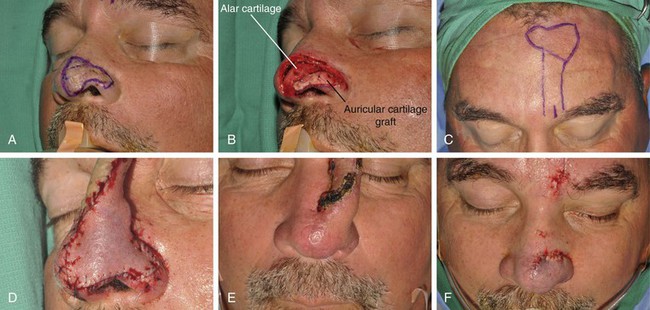
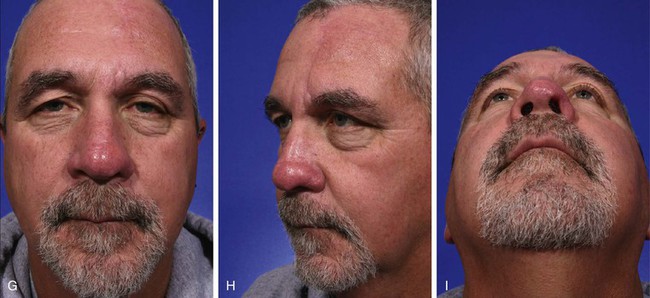
FIGURE 13-9 A, A 2.8 × 3.5-cm melanoma of nasal hemi-tip marked for excision. B, When a unilateral skin defect encompasses half or less of surface area of nasal tip, defect is usually enlarged only to create hemi-tip defect. This will limit size of forehead flap necessary for reconstruction, which in turn minimizes deformity of forehead. Ear cartilage graft in place for structural support. C, Paramedian forehead flap designed for repair. D, Flap in place. E, F, Flap inset 3 weeks after flap transfer. G-I, Postoperative views at 5 months. No revision surgery performed.
An exception to the rule of resurfacing the entire nasal unit when the defect occupies half or more of the surface area of the unit is the dorsum. When cutaneous defects occur on the dorsum, the thin skin of the rhinion is never removed unless it is required for tumor ablation. In most patients, the intrinsic thickness of forehead skin is greater than that of the native skin covering the rhinion. Even with removal of all the subcutaneous tissue from a forehead flap, the thickness of the flap will not match the thinness of the in situ skin and muscle of the rhinion. In this region, contour outweighs the advantage of placing the borders of the flap along the junction of aesthetic units. It is wiser to leave this skin intact and to resurface only the caudal dorsum than to resurface the entire dorsal nasal aesthetic unit (Fig. 13-10). The same principle applies to skin of the superior nasal sidewall, which is very thin and is not replaced with forehead skin unless the defect involves this region of the nose.
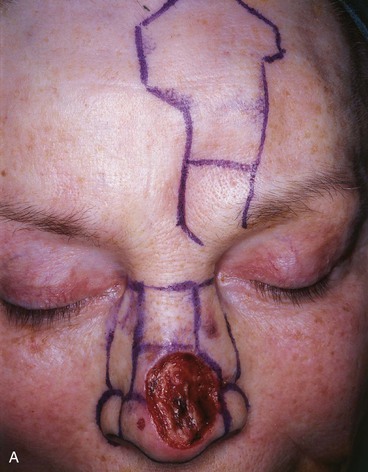
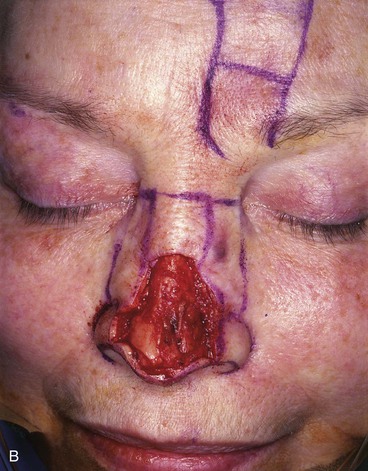
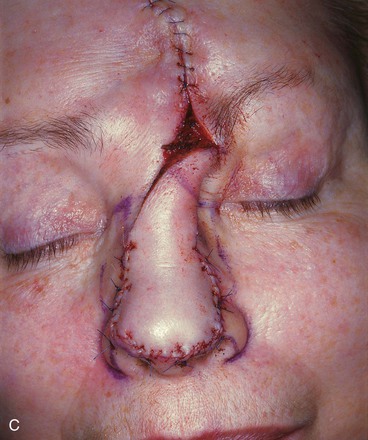
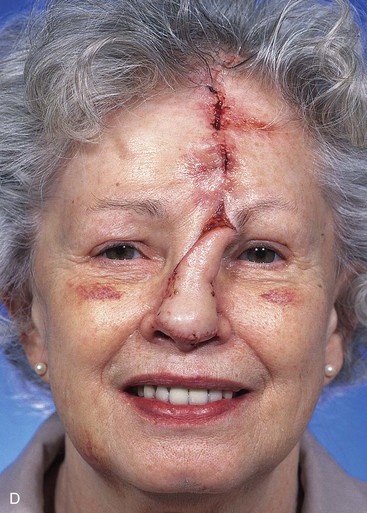
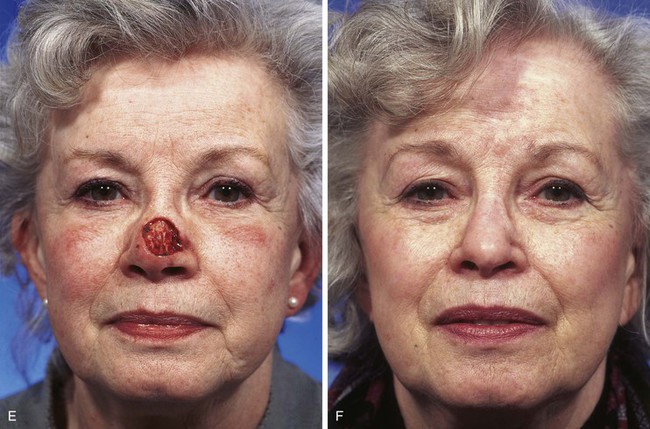

FIGURE 13-10 A, A 2 × 2-cm cutaneous defect of nasal tip and caudal dorsum. B, Remaining skin of tip aesthetic unit removed. Defects of dorsal aesthetic unit are never enlarged to remove thin skin of rhinion unless it is required for tumor ablation. Intrinsic thickness of forehead skin is greater than native skin covering rhinion. C, D, Immediately and 1 week after flap transfer. E, Preoperative view. F, G, Postoperative views at 1 year, 9 months. No revision surgery performed. Note smooth transition between skin of flap and native skin of rhinion.
Similar to the rhinion, the skin of the nasal facets is delicate and thin. It is supported only by fibroconnective tissue. Forehead skin cannot replicate the delicate nostril margin in this area of the nose. Nasal tip defects requiring a forehead flap as a cover should be enlarged only to a line along the upper border of the facet (Fig. 13-11). This line corresponds to the caudal border of the intermediate crura of the lower lateral cartilages.
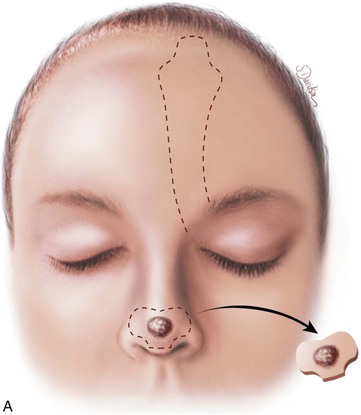
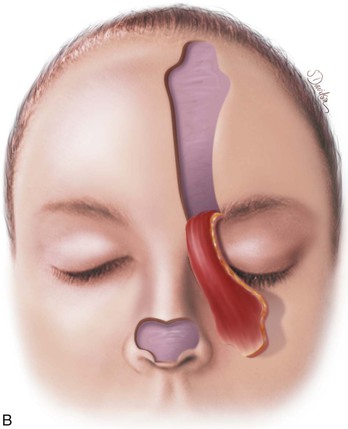
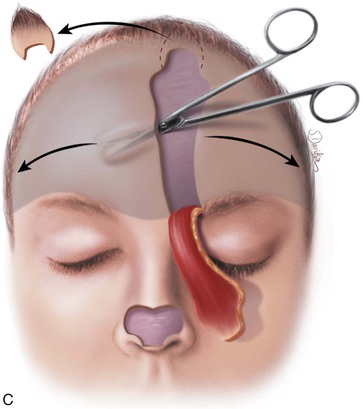
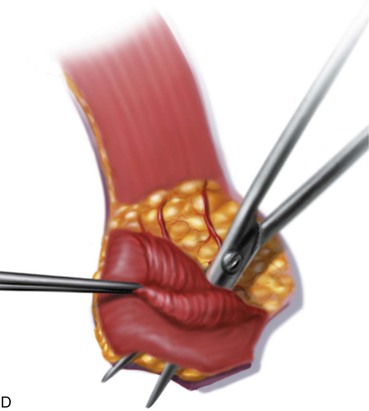
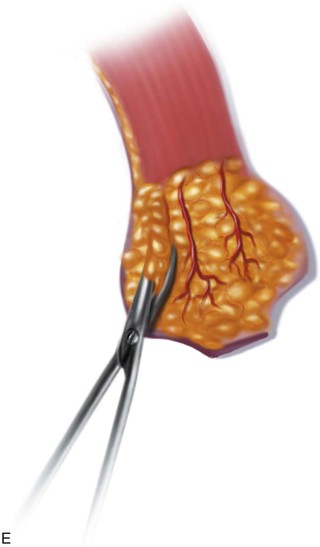
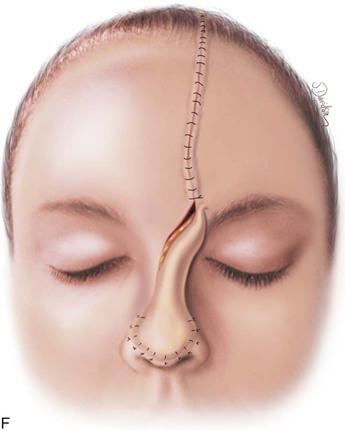
FIGURE 13-11 A, Base of paramedian forehead flap is 1.5 cm wide centered over medial end of eyebrow. Axis of pedicle is vertical and 2 cm from midline. B, Flap is elevated from underlying periosteum. C, Donor site closure is achieved by undermining in subfascial plane from one temporalis muscle to another. Standing cutaneous deformity of scalp from advancement of forehead skin is removed vertically. D, Galea and frontalis muscle completely removed from distal flap. E, Majority of subcutaneous fat is removed from distal flap. F, Flap is sutured at recipient site with vertical mattress cutaneous sutures. Subcuticular sutures are not used. Forehead donor site is closed in two layers: interrupted suture approximation of muscle and galea and continuous cutaneous suture. (From Baker SR: Interpolated paramedian forehead flaps. In Baker SR, editor: Principles of nasal reconstruction, ed 2, New York, Springer, 2011.)
A three-dimensional template exactly duplicating the surface area and contour of the region to be resurfaced is fashioned from foil or a thin sheet of foam rubber. The author prefers foam rubber because it has the flexible qualities of skin and easily conforms to the convex and concave contours of the nasal topography (Fig. 13-12). The final defect to be resurfaced is marked on the nasal skin, outlining additional skin removal to square off the defect or to remove the remaining cutaneous portions of the aesthetic unit. The template is designed before the defect is enlarged because once the additional skin is removed, the wound will spread and the defect will appear larger than it is. For this reason, if the nasal defect involves an aesthetic unit that has an intact counterpart, the intact unit is used to design the template because it will give a more accurate measurement of surface area. The template is then reversed to design the flap. When the defect involves an aesthetic unit that is unpaired, the template is designed as an ideal size for the specific patient. In dealing with large surface defects, it is helpful to suture the rubber foam template to the margins of the defect to fashion the template more precisely (see Fig. 13-12). Cartilage grafts are required to replace missing framework, and the template is designed after the grafts are in place. Templates should not be oversized but tailored exactly.
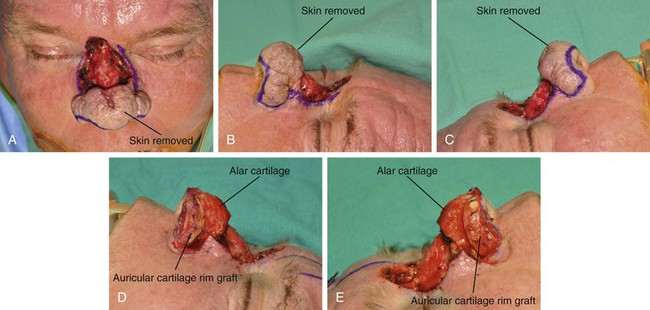
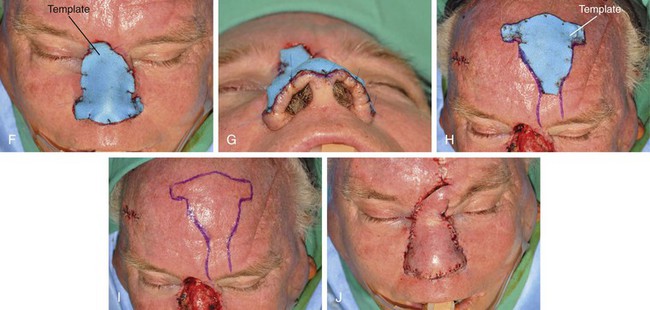
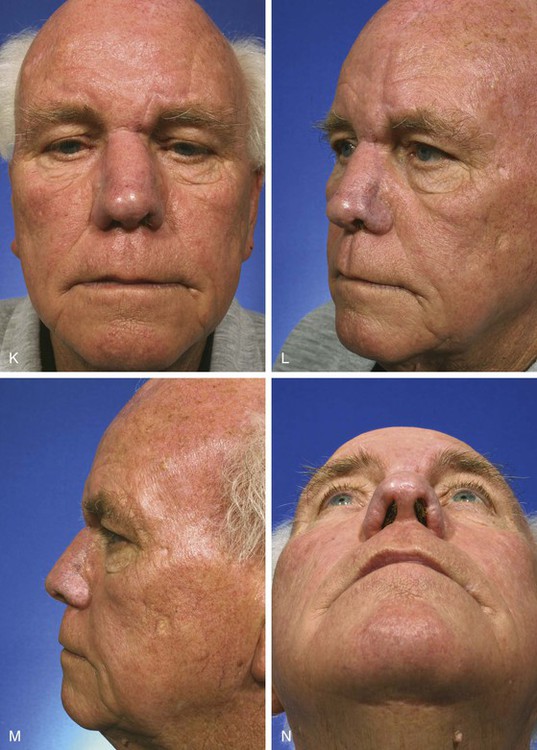
FIGURE 13-12 A-E, A 2.5 × 3-cm cutaneous defect of dorsum after micrographic surgery for basal cell carcinoma. Rhinophyma of tip and alae removed. Auricular cartilage rim grafts placed caudal to alar cartilages to prevent nostril retraction and to provide contour to alae. F, G, Foam rubber sheet sutured to nasal defect to create template. H, Template used to design paramedian forehead flap. I, J, Forehead flap designed and transferred to nose. K-N, Postoperative views at 11 months. Two contouring procedures, Z-plasty of nostril and forehead scar revision, performed at 5 and 8 months postoperatively.
The template is used to design the flap on the forehead skin (see Fig. 13-12). The center of the template is positioned approximately 2 cm lateral to the midline. At a minimum, the upper border of the template is positioned at the frontal hairline unless the patient has a receding hairline or frontal balding. The length of the flap is measured by a length of suture extending from the distal end of the positioned template to the level of the medial eyebrow. Holding it at the eyebrow, the suture is rotated 180° in the coronal plane toward the midline to the most distal recipient site on the nose. If the suture does not reach this point, the template must be repositioned higher on the forehead or the pedicle must be lowered by extending it below the level of the eyebrow. By use of this method of determining flap length, a decision can be made concerning the necessity of placing a portion of the template over hair-bearing scalp. The flap is then precisely outlined on the forehead with a skin marker, following the exact shape of the template (see Fig. 13-12).
Cutaneous defects that involve the nose and significant portions of the medial cheek or upper lip are reconstructed in stages. The first surgical stage is directed toward repair of the cheek to provide a stable foundation for subsequent reconstruction of the nose. The surgical plan of first restoring the foundation on which to place the constructed nose, before initiating nasal reconstruction, is used whenever a sizeable full-thickness defect of the cheek or lip is associated with a full-thickness nasal defect. For example, when there is a full-thickness loss of the ala or columella and adjacent upper lip, it is prudent to delay reconstruction of the nose until the lip is repaired and scars have contracted to their maximum propensity.
Figures 13-13 and 13-14 show two examples of cutaneous defects that extend from the nasal sidewall into the cheek. The patient shown in Figure 13-13 had a limited extension of the cutaneous defect into the cheek. The adjacent cheek skin was advanced and secured to the nasofacial sulcus without the necessity of making incisions in the cheek skin. Small standing cutaneous deformities resulting from advancement of the cheek skin were removed in the nasofacial sulcus superiorly and alar-facial sulcus inferiorly. In contrast, the patient shown in Figure 13-14 suffered a more extensive defect of the cheek necessitating an incision that was made inferior to the defect, paralleling the melolabial crease. In both cases, the remaining skin of the nasal sidewall involved with the defect was removed to resurface the entire aesthetic unit. Likewise, the remaining skin of the nasal dorsum was removed from the nose of the patient shown in Figure 13-14 so that the aesthetic unit could be covered with the paramedian forehead flap used to reconstruct the nose.
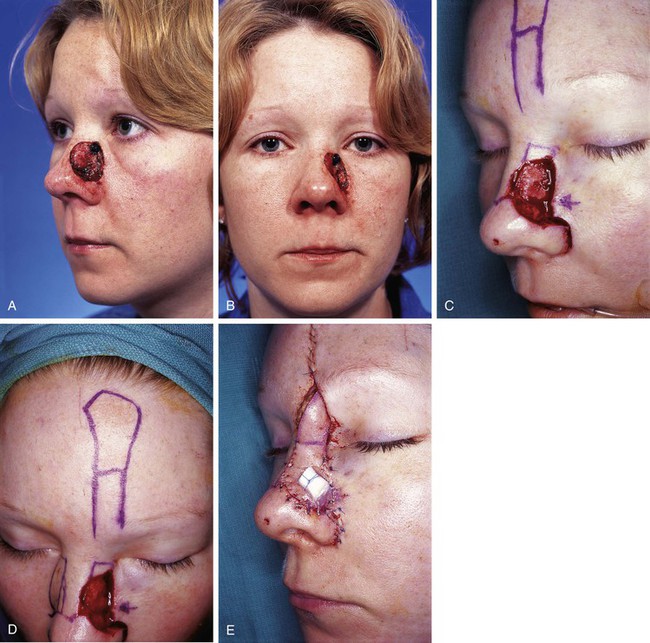
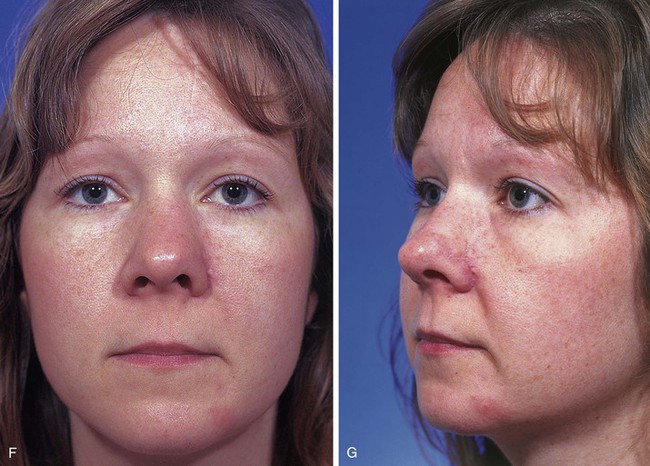
FIGURE 13-13 A, B, A 3 × 2-cm cutaneous defect of nasal sidewall with minimal extension into cheek. C, D, Cheek skin advanced to nasofacial sulcus. Paramedian forehead flap designed for repair of nasal sidewall. E, Flap in place. Bolster dressing used to reduce bleeding beneath flap. F, G, Postoperative views at 1 year, 10 months. Contouring procedure performed 6 months postoperatively.
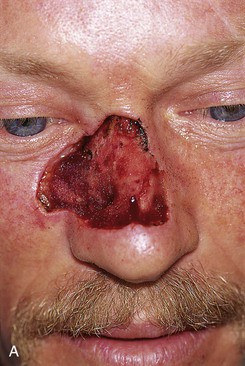
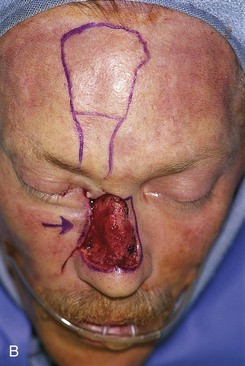
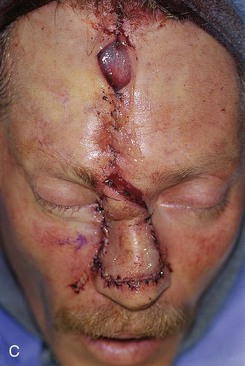
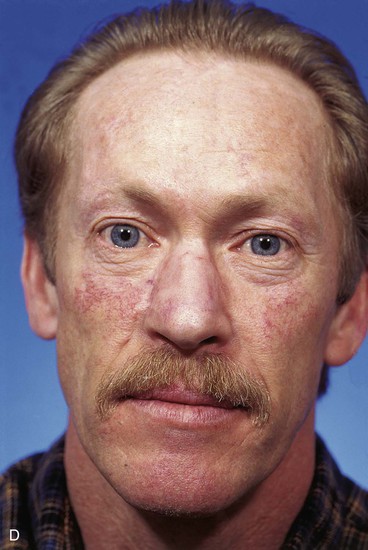
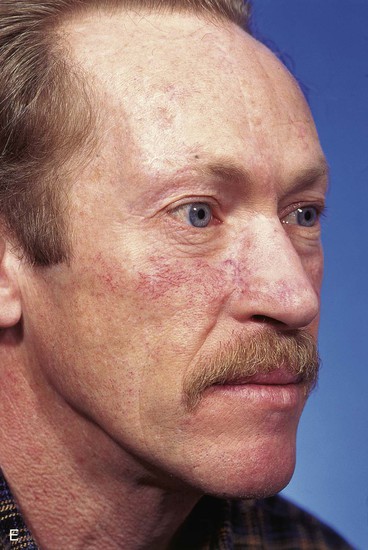
FIGURE 13-14 A, A 3.5 × 2.5-cm cutaneous defect of nasal dorsum, sidewall, and medial cheek. B, Cheek advancement flap used to repair cheek component of defect. Leading border of flap secured to periosteum of nasofacial sulcus to prevent lateral migration of flap during healing. Paramedian forehead flap designed for repair of nose. C, Forehead flap in place. Portion of superior donor site wound left to heal by secondary intention. D, E, Postoperative views at 2 years. No revision surgery performed.
Paramedian forehead flaps are usually dissected with use of local anesthetic and intravenous sedation. Lidocaine 0.5% with 1:100,000 concentration of epinephrine mixed in equal parts with bupivacaine 0.25% without epinephrine is injected circumferentially and deeply about the surgical defect. The entire forehead from the level of the lateral canthus to its counterpart is injected with 15 mL of similar anesthetic solution. The skin along the entire length of the supraorbital bony rims is infiltrated to the level of the periosteum. Particular attention is given to a broad vertical band of skin in the axes of the supratrochlear and supraorbital nerves. The anesthetic is injected into the subcutaneous tissue plane because this is the location of the nerves and vessels supplying the forehead skin. The base of the flap and skin over the root of the nose are also injected. Dolasetron (Anzemet) is administered intravenously to control postoperative nausea commonly associated with the procedure. A template used to design the forehead flap is constructed as previously described. The margins of the nasal defect are made perpendicular with a scalpel. If indicated, the remaining skin of the aesthetic unit is removed to the level of the perichondrium or periosteum. Until the pedicle of the flap is detached and the flap is inset, it is not necessary to excise the skin of the more extreme cephalic portion of the dorsum and sidewall aesthetic units when defects involve these units. The skin bordering the nasal defect is undermined below the muscle layer for 1 to 2 cm. Undermining of adjacent nasal skin may help prevent trap-door deformity.
The template is centered over the axis of the supratrochlear artery on either side of midline. With use of the template, the flap is outlined with a skin marker (Fig. 13-15). To avoid oversizing the flap, incisions are made inside the lines of the designed flap. The flap is incised through the skin, subcutaneous tissue, muscle, and fascia. The flap is elevated from superior to inferior in the subfascial plane, just superficial to the periosteum of the frontal bone. Rapid dissection may be performed in this plane until the corrugator supercilii muscle is encountered, at which point the muscle is dissected away from the underlying periosteum bluntly with scissors or a periosteal elevator. If it is necessary to extend incisions of the pedicle below eyebrow level, it is accomplished with a scalpel cutting through skin only. Blunt dissection by spreading tissue with a hemostat is then performed to mobilize the pedicle away from the medial bony orbit. The supratrochlear artery may sometimes be visually identified in the area on the deep surface of the frontalis muscle just as it exits over or through the corrugator supercilii muscle and before passing deep to the orbicularis oculi muscle to enter the orbit. Adequate flap mobilization usually requires complete sectioning of the corrugator muscle to achieve sufficient flap length. Blunt and sharp dissections are used to continue flap elevation downward into the root of the nose (see Fig. 13-10) or until sufficient pedicle length and flap mobility have been attained to allow tension-free wound closure. Hemostasis along the border of the flap is achieved with an electrocautery applied judiciously.
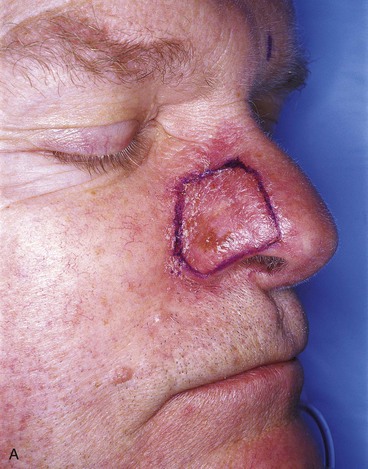
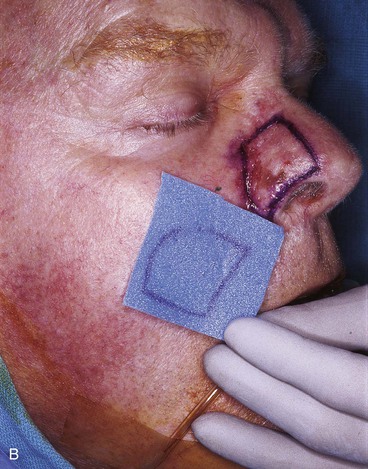
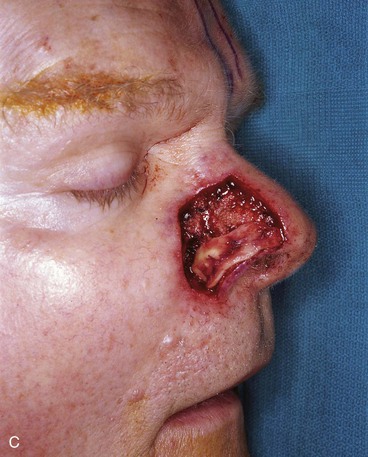

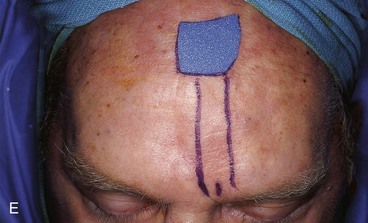
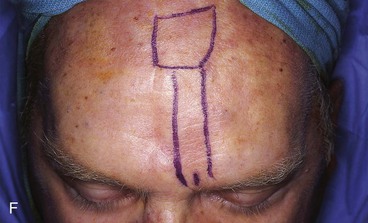
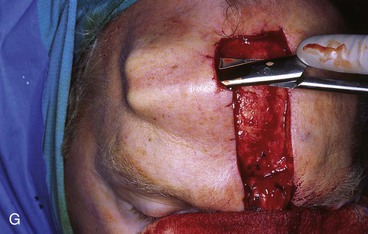
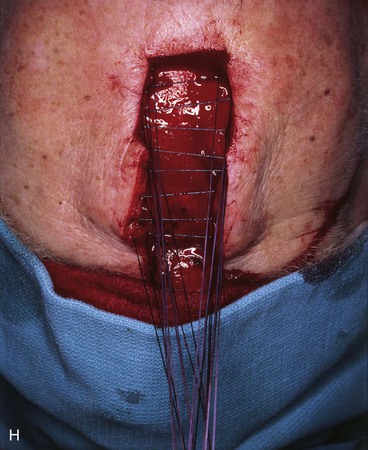
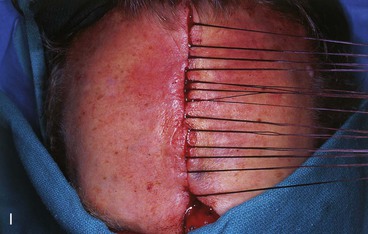
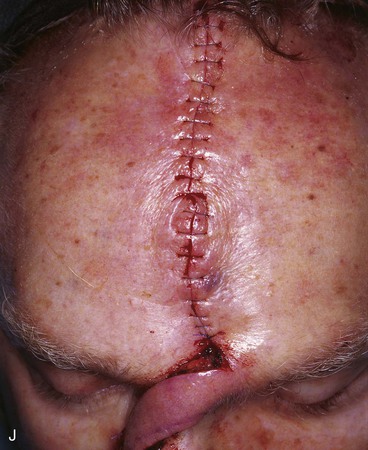
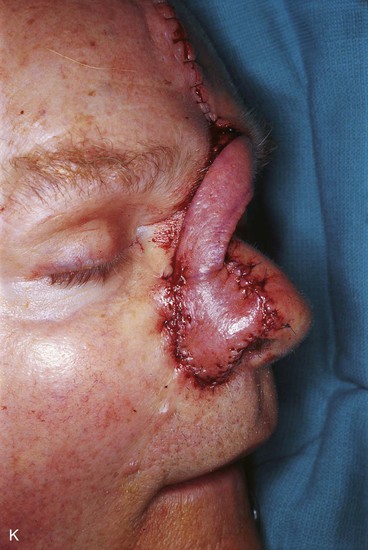
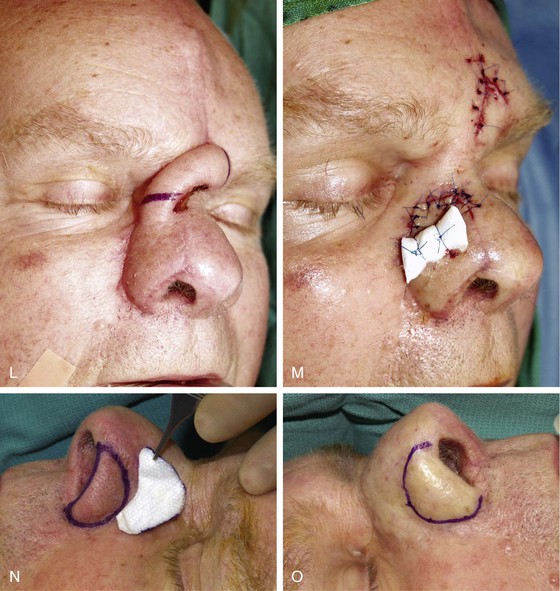
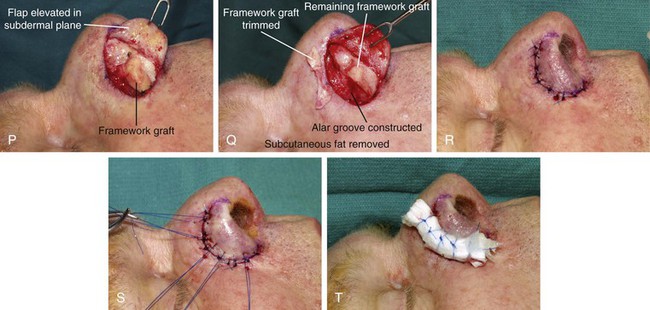
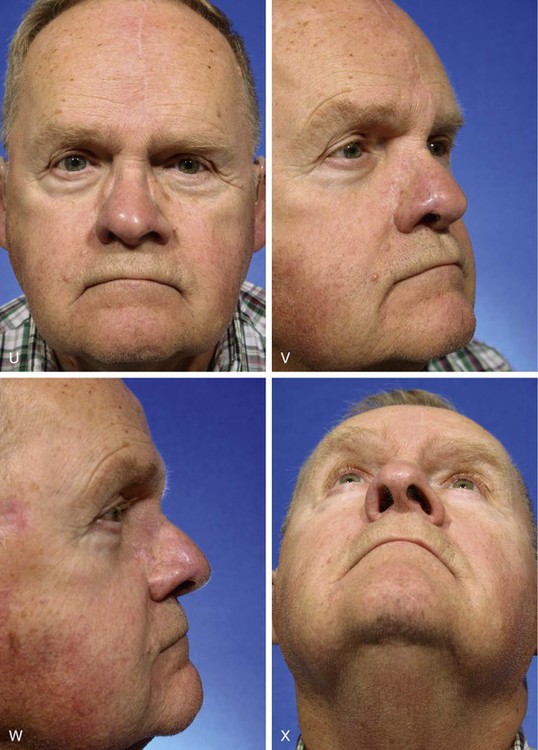
FIGURE 13-15 A, Melanoma in situ marked for excision. B, Template of planned excision. C, Melanoma removed. Auricular cartilage graft placed along nostril margin for structural support. D-F, Template created and used to design paramedian forehead flap. G, Donor defect closed by wide undermining of remaining skin in subfascial plane. H, I, Muscle and galea apposed by interrupted sutures. J, Skin closed with running cutaneous sutures. K, After thinning, forehead flap is transferred to nasal defect and secured with vertical mattress cutaneous sutures. L, M, Flap inset 3 weeks later. N, At 4 months after flap inset. Alar groove constructed by contouring procedure. Template of contralateral ala fashioned. O, Template used to mark planned alar groove of reconstructed ala. P, Cutaneous hinge flap elevated in subdermal plane. Q, Excess fat and early scar removed. Framework graft trimmed in area of planned alar groove. R, Hinge flap sutured in place. S, Bolster sutures straddle constructed alar groove. T, Bolster dressing in place. U-X, Six months after contouring procedure. (From Baker SR: Interpolated paramedian forehead flaps. In Baker SR, editor: Principles of nasal reconstruction, ed 2, New York, Springer, 2011.)
The forehead flap is covered with a damp sponge and reflected downward to allow repair of the donor site. Donor site closure is accomplished by extensive undermining of the forehead skin in the subfascial plane from the anterior border of one temporalis muscle to the other (see Figs. 13-11 and 13-15G). A few parallel vertical galeotomies 2 to 3 cm apart may be made to facilitate primary repair of the superior portion of the donor site if the donor defect is large. Galeotomies should be made just through the galea to the level of the muscle and removed from the vertical corridor of the supraorbital and supratrochlear nerves. The deep branch of the supraorbital nerve can readily be seen through the galea as it travels superiorly just medial to the temporal line, and it should be protected when galeotomies are performed.
Horizontal incisions along the hairline to facilitate closure of the donor site are not performed. This would increase anesthesia of the anterior scalp by cutting through the distal branches of the supraorbital nerves and create an additional visible scar on the forehead. As the wound edges are advanced, a standing cutaneous deformity of scalp tissue occurs at the superior apex of the flap donor site defect. This is excised completely by extending an incision sufficiently superiorly in the scalp to enable excision of the tissue cone (see Fig. 13-15).
The muscle and galea of the forehead are approximated as a single layer with 2-0 polyglactin suture. These are left untied until all have been placed (see Fig. 13-15). The skin incision is repaired with a simple running suture of 5-0 polypropylene. A drain is not employed. Primary approximation of the inferior forehead wound is rarely a problem because the pedicle width is 2 cm or less. However, superior portions of the donor site may not close completely. Any remaining open wound is filled with an antibacterial ointment. The patient is instructed to keep the wound moist with petroleum ointment until healing is complete. Healing by secondary intention takes 4 to 6 weeks.
The forehead donor site wound is repaired before the flap is sutured in place. The thickness of the flap is tailored by thinning the distal portion. This usually requires removal of the muscle and most of the subcutaneous fat to match the thickness of the nasal defect (Fig. 13-16; see also Fig. 13-11). When necessary, all but the subcutaneous fat immediately attached to the dermis may be removed. If the vertical height of the nasal defect is less than 2 cm, the portion of the flap covering the entire defect may be thinned at the time of initial transfer. For larger defects, the proximal flap covering the more cephalic portion of the defect is left with all of its muscle and subcutaneous fat intact and is thinned at the time of pedicle division. If necessary, the muscle and galea may be removed from the entire length of the flap beginning 1 cm superior to the point at which the supratrochlear artery pierces the frontalis muscle. Any hair follicles transferred with the flap should be individually cauterized with a fine-pointed electric cautery or removed manually. The distal flap, appropriately thinned, will be sufficiently supple and thin to conform to the nasal framework and manifest its contour.

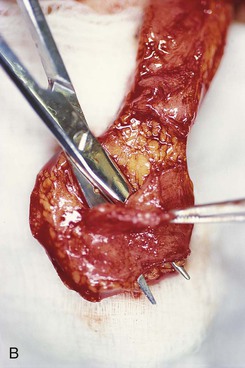
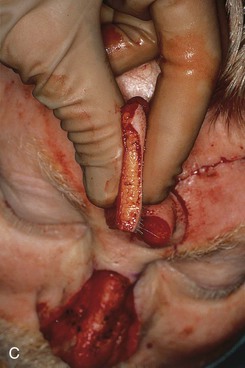
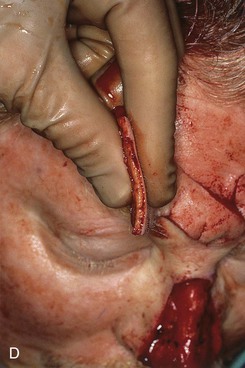
FIGURE 13-16 Forehead flap thickness is tailored to match depth of recipient site. This usually requires complete removal of frontalis muscle and majority of subcutaneous fat of distal flap. If vertical height of nasal defect is 2 cm or less, portion of flap covering entire nasal defect may be thinned at time of initial transfer. For larger defects, proximal flap covering cephalic portion of defect is not thinned until time of flap inset. A, Incision to thin flap made immediately beneath skin of distal forehead flap to create plane of dissection. B, Frontalis muscle and subcutaneous fat dissected in subdermal plane. C, D, Another forehead flap before and after thinning.
The practice of removing the frontalis muscle and the majority of subcutaneous fat from a paramedian forehead flap used to cover the nose begs the question of why the frontalis muscle is transferred with the flap. Including the muscle in the flap inferiorly near the eyebrow protects the supratrochlear artery as it ascends from the eyebrow between the muscle and skin. The superior aspect of a paramedian forehead flap may be dissected in the deep subcutaneous tissue plane, leaving the frontalis muscle in place. However, elevation in the subcutaneous tissue plane causes more bleeding than elevation beneath the muscle and places the axial blood supply to the distal flap at some risk for injury. Unfortunately, leaving the frontalis muscle in situ when a paramedian forehead flap is transferred to the nose does not preserve the supratrochlear nerve, which must be sacrificed when the pedicle of the flap is divided. Transferring the muscle as part of the flap does not impair the motor function of the more lateral aspect of the frontalis muscle. In addition, removal of the muscle beneath the flap facilitates subgaleal dissection of the remaining forehead skin during closure of the flap donor site. Because most donor wounds of paramedian forehead flaps are closed primarily, any muscle preserved in the depths of the donor wound would require excision to prevent bunching and redundancy of the muscle during advancement of the remaining forehead skin. For all of these reasons, the frontalis muscle immediately beneath the flap is included in the flap during dissection.
Postoperative wound care consists of cleaning the suture lines with hydrogen peroxide and application of the antibacterial ointment twice daily for 3 days, after which the ointment is exchanged for petroleum ointment. The patient is allowed to shampoo and shower on the first postoperative day. After the shower, ointment is reapplied to the suture line and exposed raw edges of the proximal flap. On the sixth to seventh postoperative day, sutures are removed. The wound is carefully checked for flap viability and signs of infection. If all appears well and healing is occurring as expected, the patient is scheduled for pedicle detachment approximately 3 weeks after the date of initial flap transfer. Although the pedicle may be safely divided 2 weeks postoperatively, the shorter interval may limit the ability to thin the more proximal flap remaining at the recipient site when the flap is inset. Patients are allowed to wear their eyeglasses if necessary; however, proper positioning of the eyeglasses may be a problem. Eyeglasses may require temporary readjustment by an optometrist. Devices are also available for suspending eyeglasses from the forehead, circumventing the need to rest them on the nose.
Pedicle separation is accomplished under local anesthesia. Lidocaine 1% containing epinephrine is injected into the base of the pedicle and circumferentially around the flap where it attaches to the nose, followed by the usual sterile preparation and draping. The pedicle is separated with a scalpel at the superior margin of the defect or higher if additional nasal skin is to be removed from the superior aspect of the aesthetic unit (Figs. 13-17 and 13-18). An incision is made in the cephalic portion of the old scars between the flap and adjacent nasal skin on either side of the pedicle. The extent of this incision should be such that it releases the cephalic quarter of the flap from the nose. This is necessary to provide sufficient exposure for thinning and proper trimming and inset of the flap. The skin margins surrounding the skin defect created by the flap release are undermined 1 cm. Thinning is performed of any portion of the flap left attached to the recipient site that was not adequately thinned at the time of the initial flap transfer. In the case of reconstructing skin-only nasal defects that involve the rhinion, it may be necessary to remove all subcutaneous fat to more accurately replicate the naturally thin skin of the rhinion. It is often necessary to remove early scar tissue under the flap to facilitate proper tailoring. Deep-layer closure is unnecessary because there is no wound closure tension. The flap is inset with interrupted vertical mattress and simple 5-0 fast-absorbing plain gut cutaneous sutures. On occasion, a cotton bolster dressing with percutaneous sutures to secure the bolster is used to ensure that the flap conforms to the skeletal framework of the nose. An overnight compression dressing is applied if a bolster dressing is not employed.
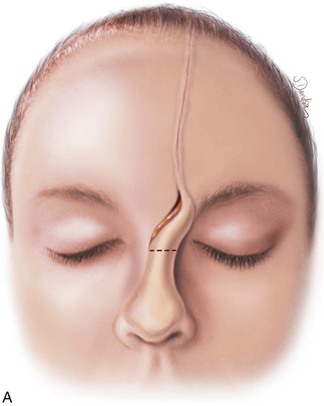
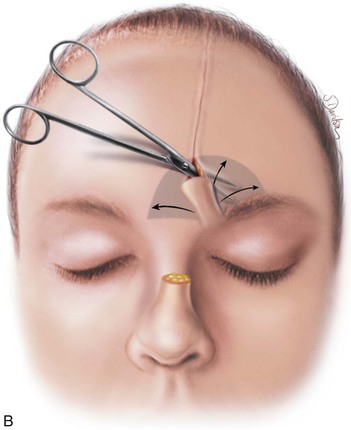
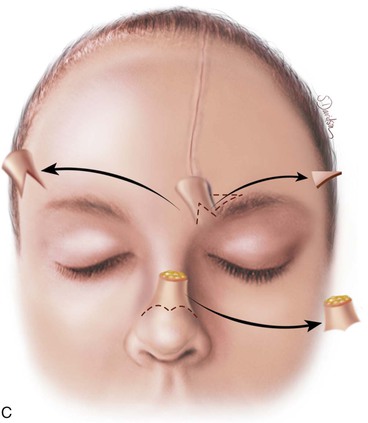
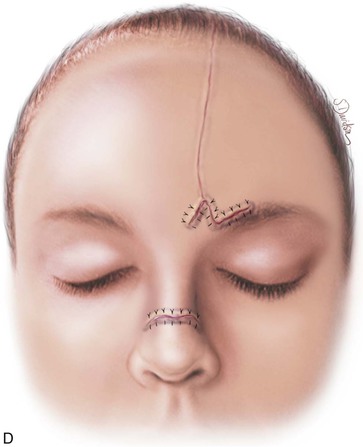
FIGURE 13-17 A, Pedicle of paramedian forehead flap separated 3 weeks after transfer. B, Inferior donor site wound opened and area between eyebrows widely undermined to release wound contracture. C, Proximal pedicle converted to small triangular flap that is advanced superiorly to restore original position of eyebrow on donor side. It is sometimes necessary to excise a Burow triangle immediately above medial eyebrow on donor side. Flap inset at nose by trimming of redundant tissue. D, Wound repair accomplished with vertical mattress cutaneous sutures. (From Baker SR: Interpolated paramedian forehead flaps. In Baker SR, editor: Principles of nasal reconstruction, ed 2, New York, Springer, 2011.)
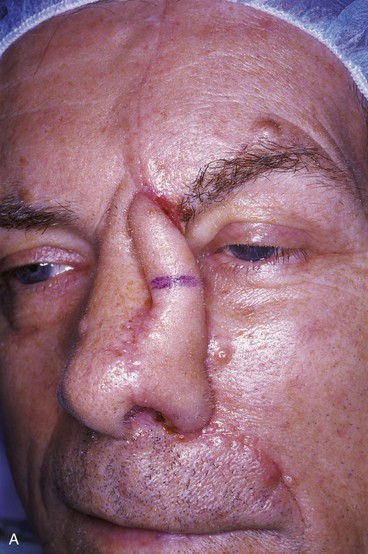
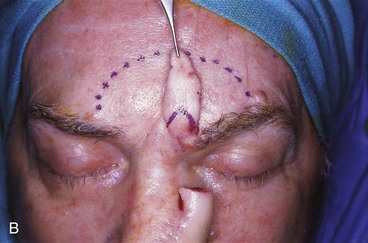
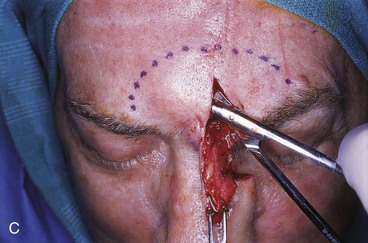
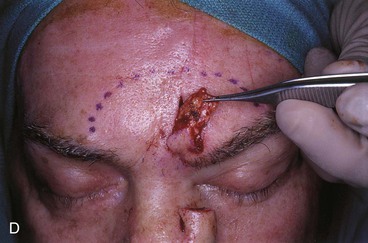
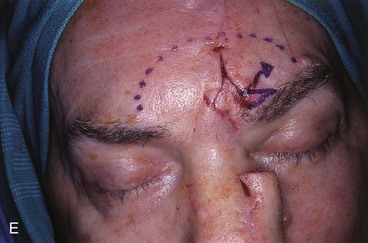
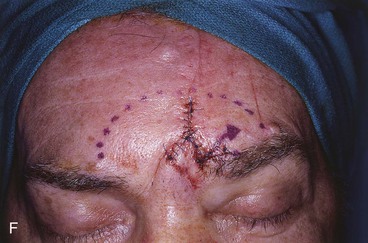
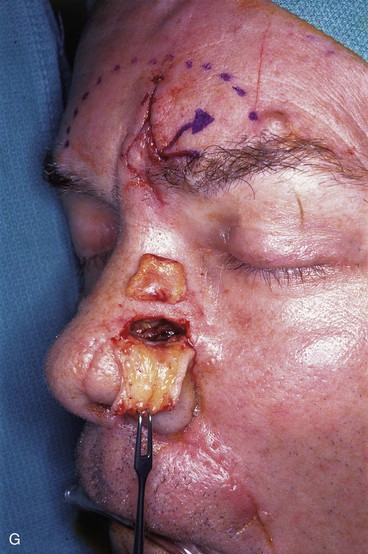
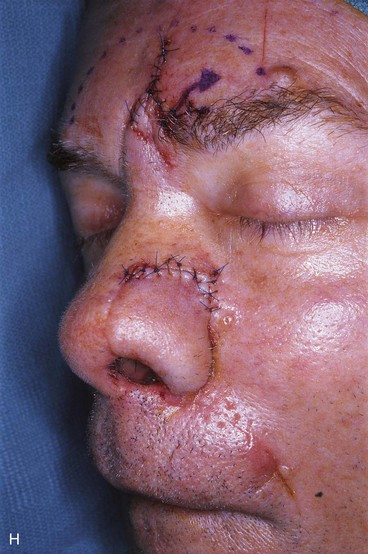
FIGURE 13-18 A, At 3 weeks after transfer of paramedian forehead flap to reconstruct full-thickness alar defect. B, Pedicle divided. Inverted V marked on proximal pedicle indicates location for amputation of redundant pedicle tissue. C, Dotted line indicates area that is undermined to restore normal anatomic relationship of medial aspect of eyebrows. D, Redundant pedicle amputated while preserving peninsula of muscle and fat that is tunneled under inferior aspect of donor site scar to prevent depressed contour. E, Peninsula sutured beneath inferior aspect of forehead scar. Triangle of skin above medial aspect of eyebrow marked for excision to elevate position of eyebrow to level of contralateral eyebrow. F, Triangle of skin excised and wound closure completed. G, H, Flap inset after thinning cephalic portion and trimming flap so wound closure is without tension. Soft tissue resting on nasal sidewall represents tissue removed during process of flap contouring.
The base of the pedicle is returned to its donor site to restore the normal inter-eyebrow distance. Just as with the inset of the flap at the recipient site, it is often necessary to remove early scar deposition in the donor area to enable the pedicle to lie flat between the eyebrows. The medial aspect of both eyebrows is undermined for several centimeters to release all contractions (Fig. 13-18). This maneuver enables the surgeon to position the medial aspect of the eyebrows in proper relationship to each other and to the superior bony orbital rims. Typically, the medial aspect of the eyebrow on the flap side is displaced inferiorly as a result of secondary movement from flap transfer. The brow must be mobilized sufficiently to correct this displacement. This is accomplished by converting the proximal pedicle of the flap into a small triangle-shaped flap incorporating in its base the medial aspect of the eyebrow on the donor side. To accommodate scar contraction and inferior migration of the eyebrow during healing, the flap is mobilized upward until the level of the medial eyebrow is positioned 2 mm above the level of the opposite eyebrow. Tissue should not be returned to the forehead above the level of this point. Excess pedicle tissue above the portion of the triangular flap necessary for securing the medial eyebrow in proper position is resected and discarded. To facilitate superior advancement of the triangular flap and thus the medial eyebrow on the donor side, it is sometimes necessary to excise a small crescent or triangular segment of skin just lateral to the base of the flap along the superior medial border of the eyebrow (see Figs. 13-17 and 13-18). This maneuver removes the standing cutaneous deformity that forms from advancement. This in essence serves as a direct browlift limited to the extreme medial portion of the eyebrow. The muscle on the deep aspect of the triangular flap is preserved to prevent a postoperative depressed contour. In addition, it is often helpful to create a tongue of muscle and subcutaneous tissue extending 0.5 cm beyond the apex of the triangular flap (see Fig 13-18D). This tissue is tunneled under the most inferior portion of the forehead scar where it meets the apex of the flap triangle. The tissue tongue prevents contour depression at this point. The deep layers of the wound are closed with 4-0 absorbable sutures, and the skin is closed with 5-0 fast-absorbing plain gut sutures placed in vertical mattress fashion.
Postoperative care after pedicle detachment consists of cleaning the suture lines with a hydrogen peroxide solution and application of an antibacterial ointment twice daily for 3 days, followed by petroleum ointment for another 3 days. Sutures are removed in 5 to 7 days. Full physical activities may be resumed 1 week after surgery. The patient is advised to avoid excessive sunlight exposure to the forehead and face region for 3 months to prevent postinflammatory hyperpigmentation of the scars. Patients are instructed that extremes of heat or cold may cause temporary color changes in the flap skin at the recipient site for several months. Revision surgery, such as thinning of the flap, is delayed 3 to 4 months to allow complete wound healing, wound contracture, and the beginning of scar maturation. Revision surgery is occasionally necessary to create or to refine an alar groove (see Fig. 13-15) or to remove persistent hair follicles transferred from the scalp. These revisions are accomplished with use of local anesthesia. The author has not found it necessary or advantageous to perform an intermediate stage consisting of more proximal thinning of the flap before pedicle division. Although an intermediate stage may be advisable for a patient addicted to tobacco, in most patients it is not necessary and does not enhance the final aesthetic appearance of the reconstruction. Depending on the circumstance, the majority of the distal portion of a paramedian forehead flap may be thinned to the subdermis at the time of flap transfer. The remainder of the flap left attached to the nose can be contoured at the time of pedicle division. Performing a contouring procedure as an intermediate stage before the pedicle of the flap is divided subjects the patient to another 2 to 3 weeks of deformity as a result of the flap’s crossing from the eyebrow to the nose. This delays the patient from returning to work and resuming social activities.
The Paramedian Forehead Flap as a Lining Flap
Forehead skin is rarely required for lining full-thickness nasal defects. Septal mucoperichondrium and turbinate mucosa are preferred for this purpose and are generally in adequate supply. However, in total or near-total nasal defects that include the nasal septum, the surgeon must look to other sources of lining. A microsurgical flap of skin or temporoparietal fascia or fascia lata29,30 is a source of internal lining for reconstruction of major full-thickness nasal defects. Another alternative is the simultaneous use of two paramedian forehead flaps. One flap provides internal lining, and the other provides external cover (Fig. 13-19).31 Bone from the calvarium and cartilage grafts from rib or auricle are placed between the two flaps to provide a supporting framework. A template is fashioned that will provide ample skin to line the reconstructed nose, including the dorsum, sidewalls, tip, and alae. Unlike the forehead flap used for covering the exterior of the nose, a forehead flap used as a lining flap is incised and hinged downward without pivoting the base of the flap. This enables the raw undersurface of the flap to face outward. The pedicle may be tunneled under the glabellar skin or delivered superficial to the intervening skin between forehead and defect in such a fashion that it will not prevent the plating of bone grafts to the nasal process of the frontal bone (Fig. 13-20). When the nasal bones are intact, the flap may on occasion be transferred to the nasal defect from a lateral approach, which may necessitate a temporary nasal fistula. The sidewall dehiscence allowing the admittance of the lining flap is closed in layers when the flap is inset. The entire lining flap, except for the base of the pedicle, is thinned to the level of the subcutaneous tissue plane.
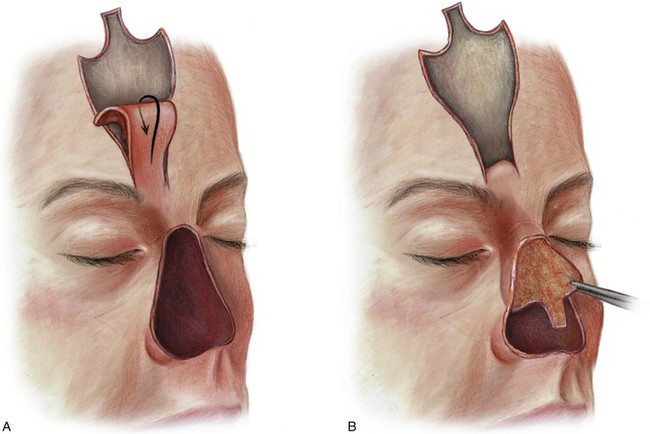
FIGURE 13-19 In cases of total or near-total loss of nose where nasal septum is absent, a paramedian forehead flap may be used to provide internal nasal lining. (From Baker SR: Interpolated paramedian forehead flaps. In Baker SR, editor: Principles of nasal reconstruction, ed 2, New York, Springer, 2011.)
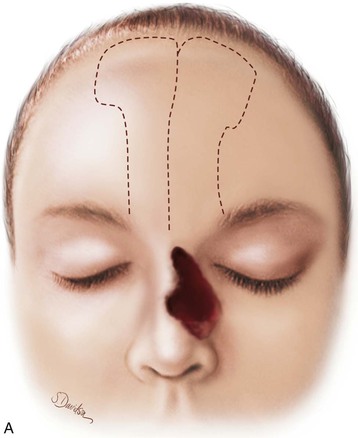
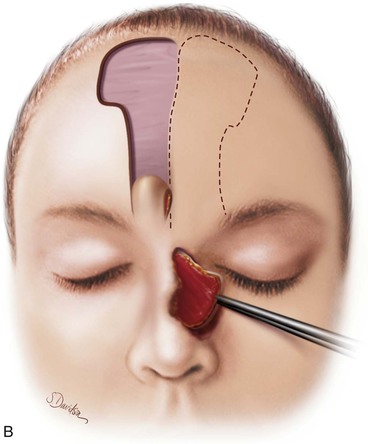
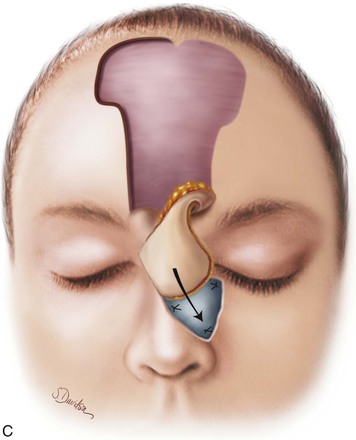
FIGURE 13-20 A, Bilateral paramedian forehead flaps designed for repair of full-thickness defect of nasal dorsum and sidewall. B, Contralateral forehead flap used to line defect delivered to nasal passage through tunnel beneath glabellar skin. C, Cartilage or bone used for structural support placed over exposed raw surface of lining flap. Ipsilateral paramedian forehead flap transferred as covering flap. (From Baker SR: Interpolated paramedian forehead flaps. In Baker SR, editor: Principles of nasal reconstruction, ed 2, New York, Springer, 2011.)
The lining flap may be transferred to the nose before or after construction of the nasal framework, depending on the circumstance. It is usually preferable to transfer the lining flap to the nose before attaching the framework. An incision is made at the mucocutaneous junction around the perimeter of the nasal defect. Adjacent skin is reflected sufficiently to expose the bone of the frontal processes of the maxillae. This provides access to attach bone grafts for skeletal support of the upper and middle nasal vaults. The mucosa along the perimeter is reflected sufficiently to provide a flap of tissue. The mucosal margins are approximated to the margins of the forehead lining flap with 3-0 running polyglactin suture. Bone or costal cartilage grafts are shaped and contoured to provide a framework for the dorsum and sidewalls. In cases in which the nasal bones are absent, a dorsal bone graft can be plated to the frontal bone at the level of the planned nasal-frontal junction. This creates strong stable skeletal support for the upper and middle nasal vaults. Additional cranial bone is fashioned into two rectangular grafts. The length of the grafts is sufficient to extend from the maxilla to the level of the lower nasal vault. The grafts are plated to the dorsal bone graft and to the adjacent maxillae and serve as the framework for the nasal sidewalls. Several holes are drilled through the bone grafts, and horizontal mattress polyglactin sutures are placed through the holes and the lining flap to appose the exposed raw surface of the lining flap against the undersurface of the bone grafts. Cartilage grafts are then used to create the framework for the lower nasal vault. The grafts may be stabilized to the caudal end of the bony sidewall grafts with sutures passed through holes drilled in the bone. Once the framework for the lower vault has been constructed, mattress sutures are used to approximate the forehead lining flap against the undersurface of the cartilage grafts. It is important that the lining flap completely cover the undersurface of the bone and cartilage grafts so they are not exposed to the nasal passage.
When the framework has been constructed and secured to the maxillae and the lining flap has been apposed to the framework, a template is fashioned to design a covering flap using a second paramedian forehead flap. The second flap is usually based on the ipsilateral supratrochlear artery (Fig. 13-21). The second flap is pivoted toward the midline, and the distal two-thirds of the flap is thinned and used to cover the entire framework. The caudal border of this flap is sutured to the caudal border of the lining flap, completely enveloping the cartilage grafts used for the lower nasal vault framework. The lateral borders of the covering flap are sutured to the surrounding skin edges of the nasal defect. A few bolster sutures passing through both the covering and lining flaps may be used to coapt the undersurfaces of the two flaps, eliminating dead space between the flaps. The forehead donor site is large when bilateral paramedian forehead flaps are used and can only partially be closed. The remaining forehead wound is allowed to heal by secondary intention.
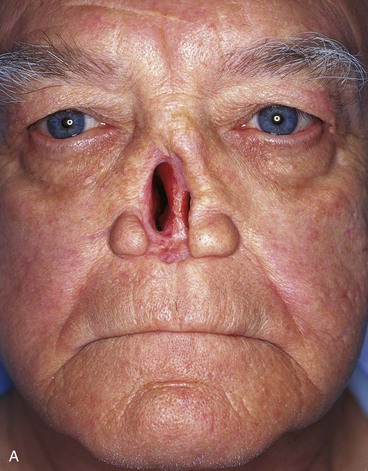
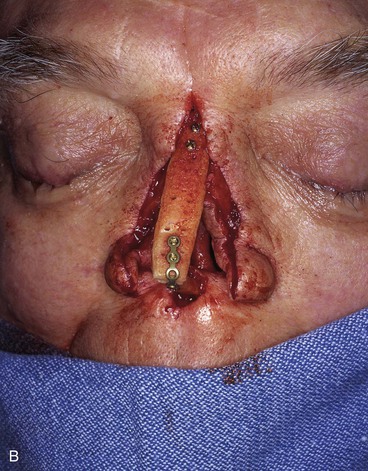
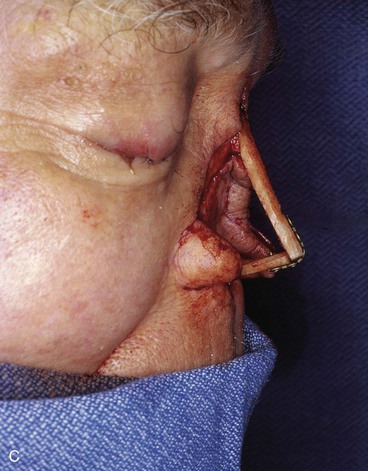
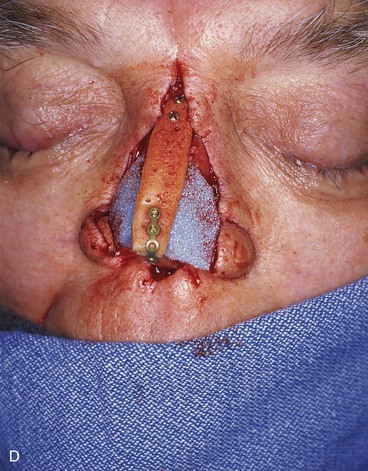
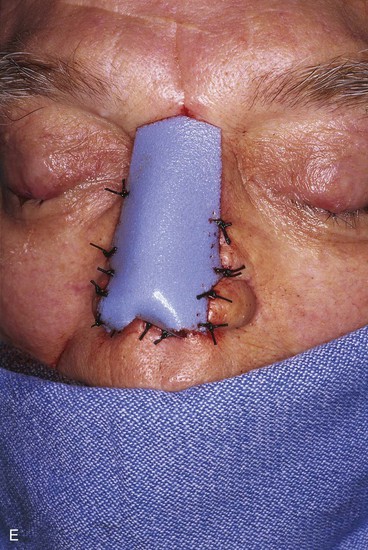
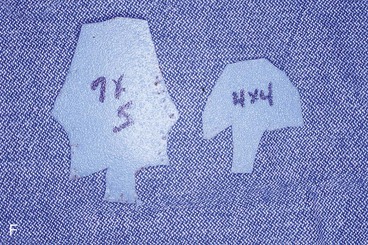
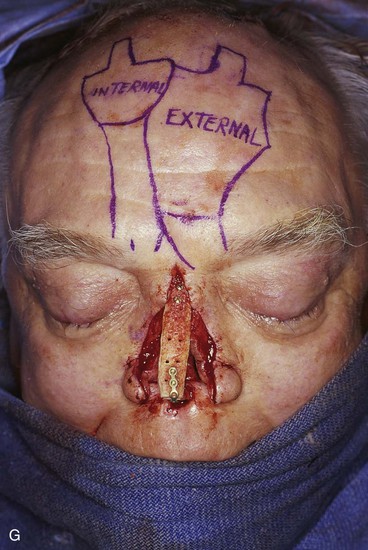
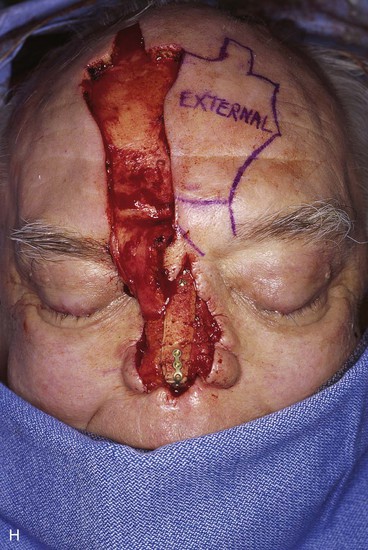
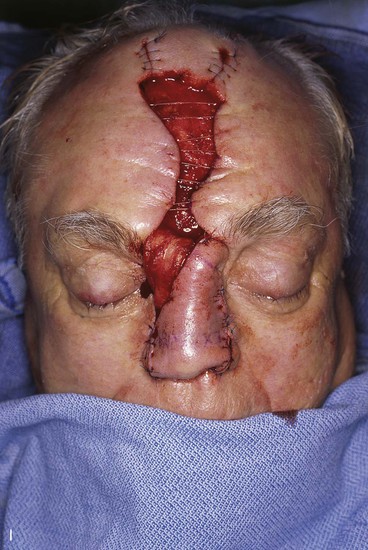
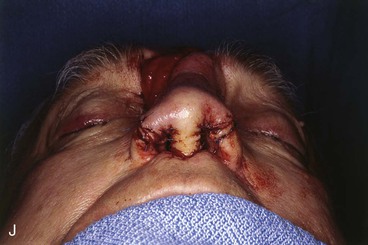
FIGURE 13-21 A, Patient with near-total rhinectomy. B, C, Cranial bone grafts secured in place for nasal framework. D, Foam rubber used to fashion template for internal lining flap. E, Foam rubber used to fashion template for external covering flap. F, A 7 × 5-cm template for external covering flap and 4 × 4-cm template for internal lining flap. G, Bilateral paramedian forehead flaps designed. H, Internal lining flap delivered to nose through temporary opening in cephalic sidewall. Borders of flap sutured to borders of mucosal defect. Flap suspended to deep surface of bone grafts with sutures passing through holes drilled in bone. I, External flap transferred to cover bone grafts. Forehead donor site partially closed. J, Internal lining flap sutured to external covering flap along nostril margin.
When dual paramedian forehead flaps are used to provide lining and cover to the nose, pedicle detachment is delayed for 2 months as both flaps depend on vascularization across a circumferential scar at the perimeter of the reconstructed nose. The pedicle of the lining flap is detached first, and the nasal fistula is closed. It is important to remove all of the skin of the portion of the pedicle that is beneath the covering flap and not exposed to the reconstructed nasal passage. This is to prevent development of skin line cysts and subsequent infection and drainage. The covering flap is inset 2 months after inset of the lining flap. At the time of pedicle division, the proximal pedicles of the two forehead flaps are returned to the forehead in their entirety and without trimming. Returning all of the skin and soft tissue composing the proximal pedicles of the flaps restores all of the forehead skin between the eyebrows as well as most of the skin of the central forehead. This will provide the inferior central forehead with a natural appearance (Fig. 13-22).
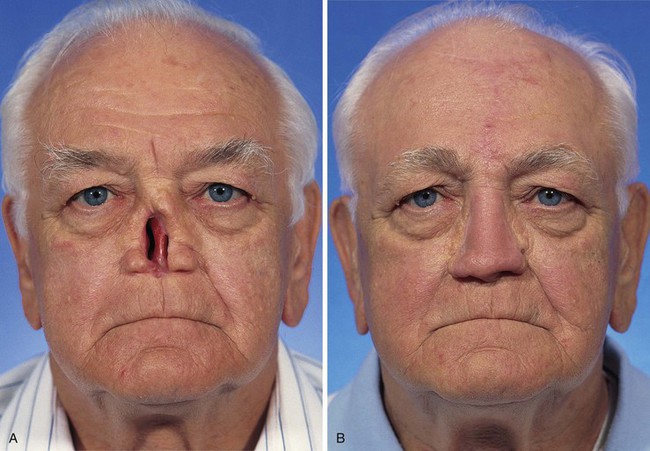
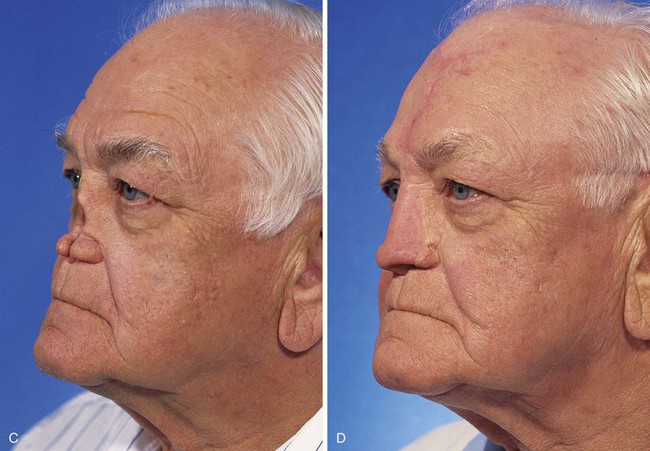
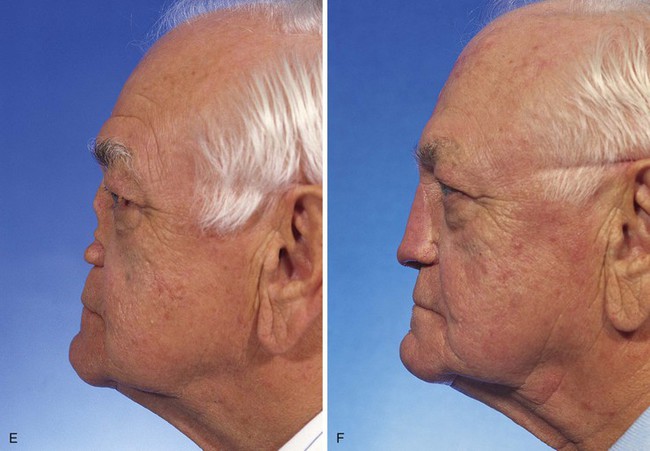
FIGURE 13-22 Same patient as shown in Figure 13-21. A-F, Preoperative and 1.5-year postoperative views. No revision surgery performed.
Forehead Expansion
Tissue expansion of the forehead in anticipation of using a paramedian forehead flap is not recommended because of the additional morbidity of the expansion process. In addition, forehead donor wounds that cannot be closed primarily heal by secondary intention and result in an acceptable-appearing scar. However, tissue expansion may have a role in patients who require dual paramedian forehead flaps, which will require removal of the majority of the central forehead skin.32 The patient must be informed that expansion of the forehead will be an additional surgical procedure and will cause an increasingly noticeable deformity of the forehead for several weeks before nasal reconstruction can be initiated.
Inflation begins 2 weeks after implantation of the expander. After the injection site is prepared with an alcohol swab, saline is infused by percutaneous puncture of the injection port with a 23-gauge scalp needle attached to a 50-mL syringe. The volume of injection depends on the tensile strength and tension of the skin overlying the expander and the amount of the patient’s discomfort. If it is not precluded by the patient’s discomfort, the forehead is expanded until slight blanching is observed in the skin overlying the expander. Saline is then withdrawn until the blanching disappears. However, tightness or pain is usually the limiting factor. Usually 25 to 30 mL of saline may be injected into a 250-mL volume expander on a weekly interval. The discomfort from inflation resolves within 24 to 48 hours, and the patient remains comfortable until the next inflation. Inflation is conducted once a week; more frequent expansion is associated with a greater risk of expander extrusion.
As expansion proceeds, the dermis becomes thinner, and a capsule forms around the expander. This often results in a reversible blue or red hue of the expanded skin. Dilated subcutaneous veins are frequently observed. The presence of the veins is not an indication of cyanosis or infection. Expansion continues until the circumference of the dome of the expanded skin measures two or three times the width of the anticipated defect. For nasal reconstruction, 6 to 8 weeks of expansion are required to achieve this goal.
Complications
Complications arising from the use of paramedian forehead flaps are rare as this is the “golden” flap of nasal reconstruction. The superficial axial blood supply of the flap provides ample nourishment, making distal flap necrosis unlikely. The superb vascularity of the flap also markedly reduces the risk of wound infection. However, this vascularity accounts for the most common complication observed with paramedian forehead flaps: the development of small hematomas under the distal flap. Hematomas develop at the time of initial flap transfer. The rich vascularity of the flap creates a tendency for the undersurface of the portion of the flap thinned of its muscle and subcutaneous fat at the time of transfer to bleed postoperatively. The potential dead space between the flap and the underlying nasal framework will accommodate small hematomas because there are usually no adhesions or sutures that attach the covering flap to the framework. To reduce the development of hematomas, careful hemostasis is achieved over the entire raw undersurface of the thinned portion of the flap. This portion requires special attention because it may ooze blood continually for a few hours after surgery. Compression dressings secured with a few bolster sutures passing full thickness through the flap to the nasal passage and back again may be placed judiciously if bleeding is profuse (see Fig. 13-13). Bolster dressings, however, are not used routinely because they risk impairment of the circulation to the distal flap.
Advantages
The advantages of the excellent blood supply of the paramedian forehead flap far exceed the problems of an occasional postoperative hematoma beneath the flap. In a review of the University of Michigan Mohs database from 1993 to 1999, 147 patients underwent forehead flap repair of nasal defects after micrographic (Mohs) surgery.33 Secondary procedures after flap inset were performed in 79 patients (54%), with 1 procedure in 56 patients, 2 procedures in 22 patients, and 3 procedures in 1 patient. These procedures consisted of contouring of the flap in 68 patients, dermabrasion in 40, Z-plasty scar revision in 11, additional cartilage grafting in 2, and suspension suture for opening of the nasal valve in 1. High aesthetic and functional goals were achieved in all patients (Figs. 13-23 and 13-24). No tumor recurrence or significant episodes of local bleeding or infection occurred. Perhaps the most significant finding of this study was that not a single case of significant flap necrosis occurred. Two patients (1%) did develop superficial partial-thickness necrosis. One case was related to a lengthy flap that was markedly thinned for the purpose of destroying hair follicles in an extended portion of the flap used to reconstruct the columella. The other case occurred in an oblique forehead flap that was harvested 3 to 4 cm lateral to the axis of the supratrochlear artery. Epidermolysis and superficial necrosis occurred in the center of the distal flap. Fortunately, the area healed without impairing the appearance of the reconstructed nose.
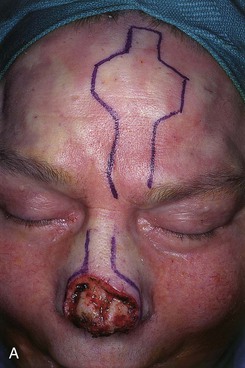
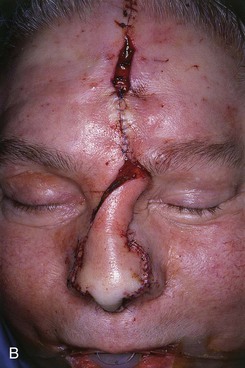
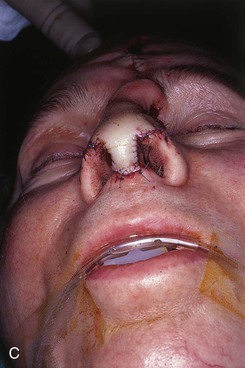
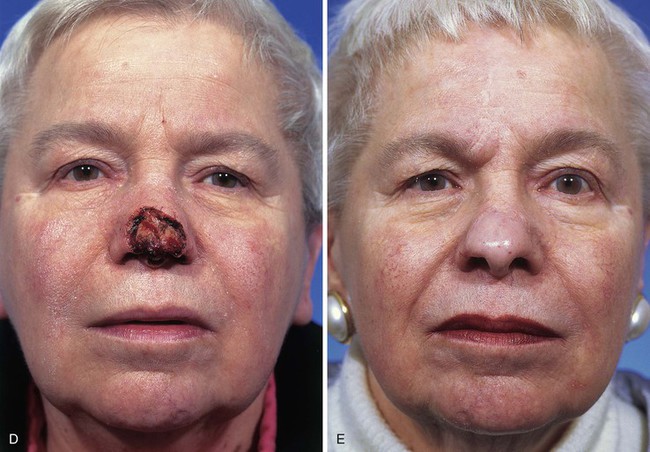
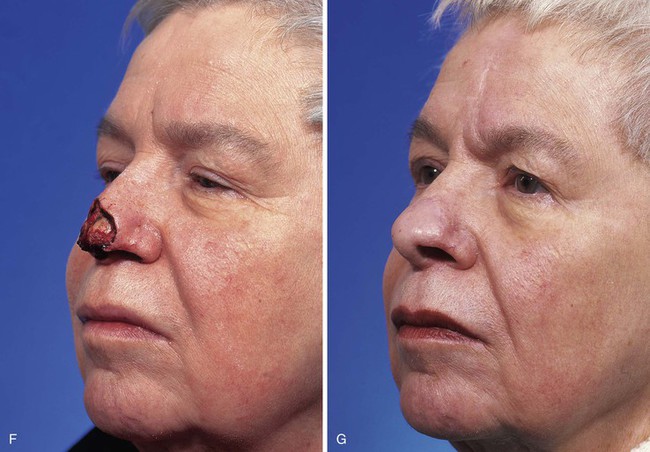
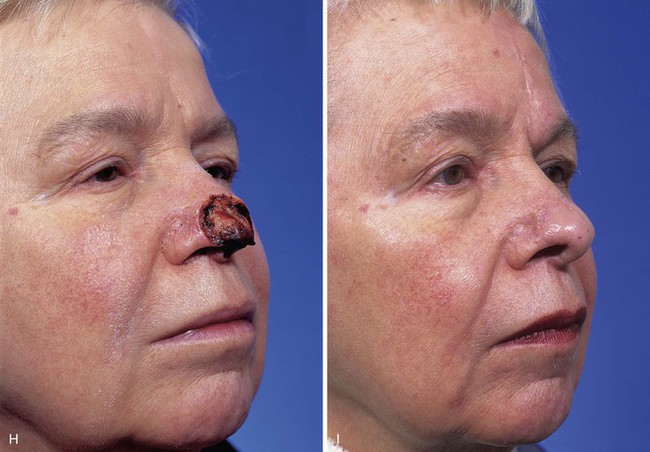
FIGURE 13-23 A, Cutaneous defect of nasal tip and columella. Paramedian forehead flap designed for repair. Remaining skin of aesthetic tip unit marked for excision. B, C, Flap in place. Portion of superior donor site left to heal by secondary intention. D-I, Preoperative and 9-month postoperative views. No revision surgery performed.
References
1. Burget, GC, Menick, FJ. Nasal support and lining: the marriage of beauty and blood supply. Plast Reconstr Surg. 1989; 84:189.
2. Conley, JJ, Price, JC. Midline vertical forehead flap. Otolaryngol Head Neck Surg. 1981; 89:38.
3. Jackson, IT. Local flaps in head and neck reconstruction. St. Louis: Mosby; 1985.
4. Converse, JM. Reconstructive plastic surgery. Philadelphia: WB Saunders; 1964.
5. Converse, JM. Reconstructive plastic surgery, 2nd ed. Philadelphia: WB Saunders; 1977.
6. Mazzola, RF, Marcus, S. History of total nasal reconstruction with particular emphasis on the folded forehead flap technique. Plast Reconstr Surg. 1983; 72:408.
7. Menick, FJ. Aesthetic refinements in use of the forehead flap for nasal reconstruction: the paramedian forehead flap. Clin Plast Surg. 1990; 17:607.
8. Baker, SR. Regional flaps in facial reconstruction. Otolaryngol Clin North Am. 1990; 23:925.
9. McDowell, F, Valone, JA, Bronn, JB. Bibliography and historical note on plastic surgery of the nose. Plast Reconstr Surg. 1952; 10:149.
10. Shumrick, KA, Smith, TL. The anatomic basis for the design of forehead flaps in nasal reconstruction. Arch Otolaryngol Head Neck Surg. 1992; 118:373.
11. Millard, DR. Total reconstructive rhinoplasty and a missing link. Plast Reconstr Surg. 1966; 37:167.
12. Kazanjian, VH. The repair of nasal defects with the median forehead flap: primary closure of the forehead wound. Surg Gynecol Obstet. 1946; 83:37.
13. Gillies, HD. Plastic surgery of the face. London: Frowde, Hodder, Stoughton; 1920.
14. Converse, JM. Reconstructive plastic surgery, 2nd ed. Philadelphia: WB Saunders; 1977.
15. Millard, DR. Hemi-rhinoplasty. Plast Reconstr Surg. 1967; 40:440.
16. Labat, M, De la rhinoplastie, art de restaurer ou de refaire completement la nez [dissertation] Paris, 1834.
17. Burget, GC, Menick, FJ. The subunit principle in nasal reconstruction. Plast Reconstr Surg. 1985; 76:239.
18. Burget, GC. Aesthetic reconstruction of the nose. Clin Plast Surg. 1985; 12:463.
19. Burget, GC, Menick, FJ. Nasal reconstruction: seeking a fourth dimension. Plast Reconstr Surg. 1986; 78:145.
20. Mangold, V, Lierse, W, Pfeifer, G. The arteries of the forehead as the basis of nasal reconstruction with forehead flaps. Acta Anat (Basel). 1980; 107:18.
21. McCarthy, JG, Lorenc, ZP, Cuting, L, et al. The median forehead flap revisited: the blood supply. Plast Reconstr Surg. 1985; 76:866.
22. McCarthy, JG. Acquired deformities of the nose in plastic surgery, 3rd ed. Philadelphia: WB Saunders; 1990.
23. Burget, GC. Current therapy in plastic and reconstructive surgery. New York: BC Decker; 1989.
24. Barton, FE. Aesthetic aspects of nasal reconstruction. Clin Plast Surg. 1988; 15:155.
25. Hart, NB, Goldin, JM. The importance of symmetry in forehead flap rhinoplasty. Br J Plast Surg. 1984; 37:477.
26. Converse, JM, Wood-Smith, D. Experiences with the forehead island flap with a subcutaneous pedicle. Plast Reconstr Surg. 1963; 31:521.
27. Baker, SR. Oblique forehead flap for total reconstruction of the nasal tip and columella. Arch Otolaryngol Head Neck Surg. 1985; 111:425.
28. Baker, SR, Alford, EL. Mid-forehead flap. Op Tech Otolaryngol Head Neck Surg. 1993; 4:24.
29. Winslow, CP, Cook, TA, Burke, A, et al. Total nasal reconstruction: utility of the free radial forearm fascial flap. Arch Facial Plast Surg. 2003; 5:159.
30. Seth, R, Revenaugh, PC, Scharpf, J, et al. Free anterolateral thigh fascia lata flap for complex nasal lining defects. JAMA Facial Plast Surg. 2013; 15:21.
31. Baker, SR. Principles of nasal reconstruction, 2nd ed. New York: Springer; 2011.
32. Baker, SR, Swanson, NA. Tissue expansion of the head and neck: indications, techniques and complications. Arch Otolaryngol Head Neck Surg. 1990; 116:1147.
33. Boyd, CM, Baker, SR, Fader, DJ, et al. The forehead flap for nasal reconstruction. Arch Dermatol. 2000; 136:1365.