Intercurrent Disease and Anaesthesia
Intercurrent diseases may have a variety of effects on anaesthesia and surgery:
 the course of the disease may be modified by anaesthesia and surgery
the course of the disease may be modified by anaesthesia and surgery
 the disease may influence the effects of anaesthesia
the disease may influence the effects of anaesthesia
 concurrent drug therapy may influence the effects of anaesthesia
concurrent drug therapy may influence the effects of anaesthesia
 the patient’s physiological reserve or functional capacity
the patient’s physiological reserve or functional capacity
 the disease processes involved and whether they can be improved before surgery.
the disease processes involved and whether they can be improved before surgery.
Physiological Reserve
It is increasingly recognized that physiological reserve is an important predictor of outcome from major surgery. Cardiopulmonary exercise (CPEX) testing is a useful tool to allow preoperative assessment of cardiovascular and respiratory reserve and the ability to withstand the stresses of major surgery. More simply, or where CPEX testing is unavailable, the capacity of the cardiorespiratory system to respond adequately to perioperative stress can be estimated in terms of metabolic equivalents (METs). If a patient has no major cardiac risk factors (see below) and can achieve more than 4 METs of activity without significant cardiorespiratory symptoms then the perioperative risk of an adverse cardiac event is low (Table 18.1). It may be possible to improve cardiorespiratory reserve before surgery in some patients. Knowledge of physiological reserve will guide the choice of anaesthetic technique, the level of monitoring used and the requirement for Level 2 or Level 3 care postoperatively.
TABLE 18.1
Metabolic Equivalent (MET) Levels for Readily Assessed Activity Levels
| MET Score | Approximate Level of Activity |
| 1 | Dress, walk indoors |
| 2 | Light housework, slow walk |
| 4 | Climb one flight of stairs, run a short distance |
| 6 | Moderate sport, e.g. golf, doubles tennis or dancing |
| 10 | Strenuous sports or exercise |
One MET is approximately equivalent to an oxygen consumption of 3.5 mL kg−1 min−1.
Adapted from Fleisher LA, Beckman JA, Brown KA et al 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 50:1707–1732.
Extent of Surgery
This determines the level of physiological stress which the patient will experience. High-risk operations (cardiac morbidity > 5%) include aortic and other major vascular procedures; intermediate risk procedures include intraperitoneal, intrathoracic, major orthopaedic or urological surgeries, and also procedures anticipated to be prolonged and to involve significant fluid shifts and blood loss (Table 18.2). Following discussion with the patient and surgeon, it may be appropriate in some cases to consider alternatives to surgery or a less major operation if the patient is considered at too high a risk. In some cases the appropriate decision is not to undergo surgery.
TABLE 18.2
Cardiac Risk* Stratification for Non-Cardiac Surgical Procedures
| Risk Stratification | Procedure Examples |
| Vascular (reported cardiac risk often more than 5%) |
Aortic and other major vascular surgery Peripheral vascular surgery |
| Intermediate (reported cardiac risk generally 1% to 5%) |
Intraperitoneal and intrathoracic surgery Carotid endarterectomy Head and neck surgery Orthopaedic surgery Prostate surgery |
| Low † (reported cardiac risk generally less than 1%) |
Endoscopic procedures Superficial procedures Cataract surgery Breast surgery Ambulatory surgery |
*Combined incidence of cardiac death and non-fatal myocardial infarction.
†These procedures do not generally require non-invasive testing.
Adapted from Fleisher LA, Beckman JA, Brown KA et al 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 50:1707–1732.
CARDIOVASCULAR DISEASE
Preoperative Assessment
The aims of preoperative assessment in this group are to:
 define the fitness of the patient for the proposed anaesthetic and surgery
define the fitness of the patient for the proposed anaesthetic and surgery
 delineate the level of risk of the procedure
delineate the level of risk of the procedure
 decide on the most appropriate anaesthetic technique
decide on the most appropriate anaesthetic technique
 assess the requirement for preoperative therapy to be initiated, for example β-blockade or blood transfusion
assess the requirement for preoperative therapy to be initiated, for example β-blockade or blood transfusion
 assess the level of perioperative monitoring required
assess the level of perioperative monitoring required
 decide on the patient’s postoperative management, including where this should take place.
decide on the patient’s postoperative management, including where this should take place.
The Lee revised cardiac risk index for patients undergoing non-cardiac surgery identifies several intermediate risk factors (Table 18.3). The presence of two or more of these factors has been shown to identify patients with moderate (7%) and high (10%) risk of cardiac complications. There is evidence that this increased risk may continue for 6 months following surgery.
TABLE 18.3
Stratification of Risk Factors for Patients Undergoing Non-Cardiac Surgery
Active cardiac condition:
Unstable coronary syndrome (MI within 30 days, PCI within last 6 weeks)
Decompensated heart failure
Significant arrhythmias
Severe valvular disease
Intermediate Factors according to the Revised Cardiac Risk Index:
History of heart disease
History of compensated or prior heart failure
History of cerebrovascular disease
Diabetes mellitus*
Renal impairment
MI, myocardial infarction; PCI, percutaneous coronary intervention.
*The original Lee Revised Cardiac Risk Index included only diabetes treated with insulin, though it is now thought that Type II diabetes is also an intermediate risk factor.
Adapted from Fleisher LA, Beckman JA, Brown KA et al 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 50:1707–1732.
 high-risk surgery (defined as intraperitoneal, intrathoracic or suprainguinal vascular surgery)
high-risk surgery (defined as intraperitoneal, intrathoracic or suprainguinal vascular surgery)
 ischaemic heart disease diagnosed either from the history or investigation
ischaemic heart disease diagnosed either from the history or investigation
History: Symptoms of cardiovascular disease include chest pain, dyspnoea, palpitations, ankle swelling and intermittent claudication. Past medical history and medical records usually reveal the nature and severity of disease, as many patients with cardiovascular symptoms will already have undergone relevant investigations.
Examination: Preoperative cardiovascular examination should include measurement of heart rate, arterial pressure and assessment of peripheral pulses and perfusion. Signs of heart failure should be sought, including a third heart sound, elevated jugular venous pressure and fine basal crepitations on auscultation of the lung fields. The heart should be auscultated for murmurs indicative of valvular disease. In particular, it is vital to make the diagnosis of aortic stenosis pre-operatively.
Risk Stratification: Assessment of the patient’s risk of a perioperative cardiac event provides prognostic information. These issues may be discussed with the patient and appropriate written information provided. Adequate provision of information and the opportunity to ask questions has been shown to allay preoperative anxiety. Assessment also guides perioperative investigation and management.
In patients with an active cardiac condition defined as unstable coronary syndrome, decompensated heart failure, significant arrhythmias or severe valvular disease (Table 18.3), only emergency procedures should be considered. Elective procedures should be postponed for evaluation, testing and optimization of the patient’s active cardiac condition to minimize perioperative risk. The need for evaluation and further testing depends on the risks associated with a particular surgical procedure, the patient’s physiological reserve or functional capacity, and whether testing would change management. Patients undergoing low-risk surgery, or those with proven good functional capacity undergoing intermediate or higher risk surgery, can usually proceed to surgery. They will only require further invasive cardiac investigations if it would change management (i.e. they would require medical optimization or be a candidate for coronary revascularization) (Fig. 18.1).
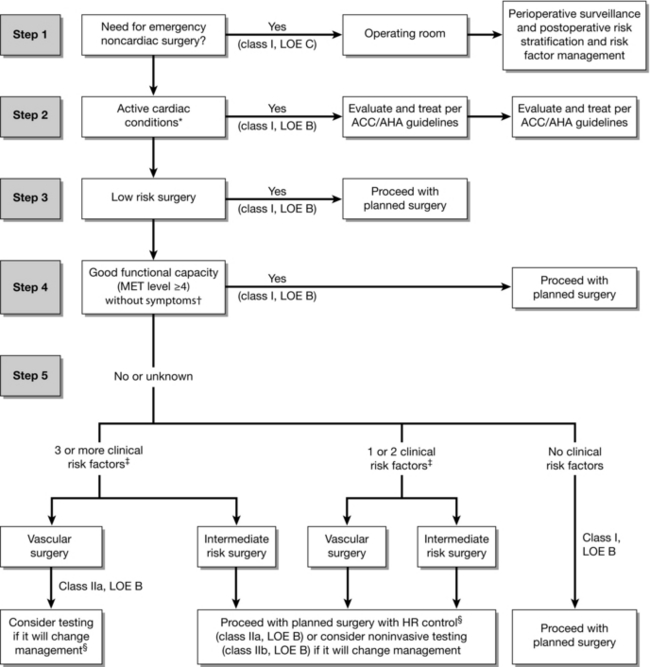
FIGURE 18.1 Cardiac evaluation and care algorithm for noncardiac surgery based on active clinical conditions, known cardiovascular disease, or cardiac risk factors for patients 50 years of age or greater. *See Table 18.3 for active cardiac conditions. † See Table 18.1 for estimated MET level equivalent. ‡ Clinical risk factors include ischaemic heart disease, compensated or prior heart failure, diabetes mellitus, renal insufficiency, and cerebrovascular disease (Table 18.3). §Consider perioperative beta blockade for populations in which this has been shown to reduce cardiac morbidity/mortality. ACC/AHA indicates American College of Cardiology/American Heart Association. HR, heart rate; LOE, level of evidence; and MET, metabolic equivalent. (Adapted from Fleisher LA, Beckman JA, Brown KA et al 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 50:1707–1732.)
For example, patients who have sustained a myocardial infarction (MI) within the 30 days before proposed surgery are a high-risk group. As a result of increased sympathetic stimulation and the coagulation activation secondary to surgery, such patients have a very high risk (up to 28%) of perioperative MI, which carries a high (10–15%) mortality. A history of uncomplicated MI more than 30 days before surgery is no longer considered an absolute contraindication to elective surgery, provided that the patient is symptom-free and has a good exercise capacity (see Table 18.1).
Increasing numbers of patients now present for non-cardiac surgery having undergone percutaneous coronary interventions (PCI), particularly intracoronary stenting (ICS). Guidelines for the optimal perioperative management of these patients have been produced (Fig. 18.2). There are a number of important points. Firstly, it is beneficial to discuss the patient’s management with an experienced cardiologist. The risk of non-cardiac surgery in patients with intracoronary stents depends on the timing of surgery related to insertion of the stent and the type of stent used. Bare metal stents have been largely superseded by drug-eluting stents, which contain a cytotoxic agent. This is slowly released from the ICS to limit endothelialization, which reduces the incidence of thrombosis and stenosis within the stent itself. However, more prolonged and intensive antiplatelet therapy is required for drug-eluting stents because they are at increased risk of thrombosis until re-endothelialization has occurred. Following insertion of any ICS, there is an initial requirement for dual antiplatelet therapy (e.g. aspirin and clopidogrel). Non-cardiac surgery should be avoided during this time if possible. If antiplatelet therapy is stopped, the risk of stent thrombosis (which carries a 7% mortality) is high, while continuing therapy increases the risk of perioperative bleeding. The duration of dual antiplatelet therapy should be a minimum of one month after bare metal stents and up to 12 months for drug-eluting stents. It is recommended that even urgent surgery should be postponed for at least 4–6 weeks after ICS insertion, and elective surgery deferred for 3 months after bare metal stent, and for 12 months after drug-eluting stent insertion. If possible, even beyond these times, aspirin should be continued throughout the perioperative period, particularly because abrupt cessation of aspirin increases thrombogenicity. In the future, the possibility of bridging therapy with short-acting glycoprotein IIb/IIa inhibitors such as tirofiban may be considered.
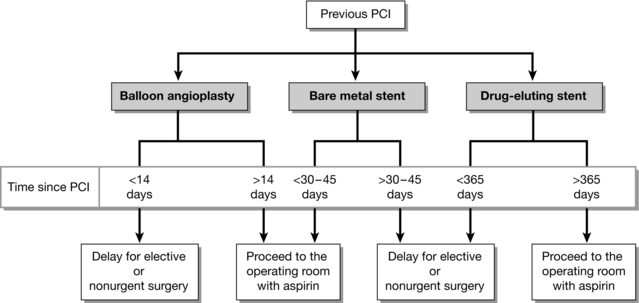
FIGURE 18.2 Recommended timing of noncardiac surgery following percutaneous coronary intervention (PCI) depends on whether a stent was placed and the type of stent used. (Adapted from Fleisher LA, Beckman JA, Brown KA et al 2007 ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 50:1707–1732.)
Investigations: Standard investigations including haematology, biochemistry, an ECG and chest X-ray are necessary in all patients with proven or suspected cardiovascular disease. A coagulation screen may be indicated.
Subsequent investigations depend on the assessed risk for the patient and the clinical findings.
Between these two extremes lies a group of patients at increased risk of perioperative cardiovascular events in whom further assessment is indicated because perioperative care is influenced by the results. Moreover, accurate determination of risk to the patient may help decision-making with regard to the need for surgery and/or the type of operation and anaesthetic (Fig. 18.1).
Patients who should be considered for further pre-operative testing include:
Preoperative Therapy
Pre-Existing Cardiovascular Disease: Ischaemic heart disease. Medical therapy should be reviewed and optimized if symptoms are poorly controlled.
Antihypertensive therapy should be continued as far as possible throughout the perioperative period.
Treatment and Additional Interventions: β-Blockers. Established β-blocker therapy should be maintained throughout the perioperative period either orally or intravenously if necessary. Sudden preoperative cessation may be associated with rebound effects such as angina, myocardial infarction, arrhythmias and hypertension. The dose of β-blocker may be reduced if there is undue bradycardia preoperatively (< 50 beat min−1). Intraoperative bradycardia usually responds to intravenous atropine or glycopyrrolate. Some studies have shown that institution of perioperative β-blockade reduces short and long term cardiovascular morbidity and mortality in patients with definite evidence of ischaemic heart disease undergoing high-risk surgery.
Premedication: Anxiety is a cause of sympathetic nervous system activation which may be detrimental in patients with cardiovascular disease. While not all patients require anxiolytic premedication, there should be a low threshold for use in these patients. A benzodiazepine such as temazepam is usually satisfactory. In patients with low or fixed cardiac output states, e.g. mitral or aortic stenosis, constrictive pericarditis or congestive cardiac failure, and other high-risk patients, it is important to avoid hypotension or excessive sedation, respiratory depression and hypoxaemia which could result from premedication, and in these situations it may be preferable to omit sedative premedication.
Anaesthesia: General Principles
 Anaesthesia should comprise a balanced technique aimed at maintaining cardiovascular stability. A variety of options may be suitable, including both general and regional anaesthesia or a combination.
Anaesthesia should comprise a balanced technique aimed at maintaining cardiovascular stability. A variety of options may be suitable, including both general and regional anaesthesia or a combination.
 Tachycardia should be avoided and an adequate arterial pressure maintained (there should not be a sustained reduction in arterial pressure of more than 20% of the patient’s normal pressure). Coronary perfusion and myocardial oxygen delivery are thus maintained without increasing myocardial work and oxygen requirements.
Tachycardia should be avoided and an adequate arterial pressure maintained (there should not be a sustained reduction in arterial pressure of more than 20% of the patient’s normal pressure). Coronary perfusion and myocardial oxygen delivery are thus maintained without increasing myocardial work and oxygen requirements.
 For patients identified as high risk, consideration should be given to stress reduction. Measures to achieve this are dictated by the patient and operative factors. These include the following:
For patients identified as high risk, consideration should be given to stress reduction. Measures to achieve this are dictated by the patient and operative factors. These include the following:
 Use of neuraxial blockade. This has been associated with reduced perioperative myocardial ischaemia and infarction. However, this must be balanced against the sympathetic block and associated hypotension. This may be pronounced, particularly with a high spinal block. Early judicious use of vasopressors coupled with maintenance of intravascular volume should limit this problem. However, neuraxial blockade, particularly spinal block, is relatively contraindicated if there is severely limited cardiovascular reserve and if maintenance of adequate arterial pressure is critical, e.g. severe aortic stenosis (see below).
Use of neuraxial blockade. This has been associated with reduced perioperative myocardial ischaemia and infarction. However, this must be balanced against the sympathetic block and associated hypotension. This may be pronounced, particularly with a high spinal block. Early judicious use of vasopressors coupled with maintenance of intravascular volume should limit this problem. However, neuraxial blockade, particularly spinal block, is relatively contraindicated if there is severely limited cardiovascular reserve and if maintenance of adequate arterial pressure is critical, e.g. severe aortic stenosis (see below).
 The level of intraoperative monitoring should be dictated by risk assessment. The following should be considered in addition to standard monitoring.
The level of intraoperative monitoring should be dictated by risk assessment. The following should be considered in addition to standard monitoring.
 Five-lead ECG. The usual ECG configuration for anaesthetic monitoring is standard limb lead II. Whilst this is useful for identifying arrhythmias, myocardial ischaemia occurs most commonly in the left ventricle and is detected more sensitively with a CM5 configuration (see Fig. 16.2).
Five-lead ECG. The usual ECG configuration for anaesthetic monitoring is standard limb lead II. Whilst this is useful for identifying arrhythmias, myocardial ischaemia occurs most commonly in the left ventricle and is detected more sensitively with a CM5 configuration (see Fig. 16.2).
 Direct arterial pressure recording.
Direct arterial pressure recording.
 CVP monitoring with or without central venous oxygen saturations.
CVP monitoring with or without central venous oxygen saturations.
 Oesophageal Doppler, providing a measurement of cardiac output and intravascular filling.
Oesophageal Doppler, providing a measurement of cardiac output and intravascular filling.
 Other minimally invasive cardiac output monitors are now available and may prove useful as intraoperative monitors. Examples include devices which derive cardiac output and other variables from the arterial pressure waveform using internal algorithms. Some devices (FloTrac/Vigileo or LiDCO) use a standard arterial catheter whereas others (PiCCO) require a dedicated thermistor tipped catheter in a proximal (femoral or axillary artery).
Other minimally invasive cardiac output monitors are now available and may prove useful as intraoperative monitors. Examples include devices which derive cardiac output and other variables from the arterial pressure waveform using internal algorithms. Some devices (FloTrac/Vigileo or LiDCO) use a standard arterial catheter whereas others (PiCCO) require a dedicated thermistor tipped catheter in a proximal (femoral or axillary artery).
 Pulmonary artery flotation catheter with continuous cardiac output and mixed venous oxygen saturation monitoring.
Pulmonary artery flotation catheter with continuous cardiac output and mixed venous oxygen saturation monitoring.
 Patients should be well oxygenated and normocapnic.
Patients should be well oxygenated and normocapnic.
 Close attention to fluid balance is mandatory. This begins preoperatively when fluid depletion secondary to factors such as excessive fasting times and bowel preparation should be corrected. As far as possible, normovolaemia should be maintained. Intravascular volume depletion is known to compromise organ perfusion and oxygen delivery but there is increasing evidence that postoperative recovery is also compromised by excessive volume and sodium loading in the immediate perioperative period.
Close attention to fluid balance is mandatory. This begins preoperatively when fluid depletion secondary to factors such as excessive fasting times and bowel preparation should be corrected. As far as possible, normovolaemia should be maintained. Intravascular volume depletion is known to compromise organ perfusion and oxygen delivery but there is increasing evidence that postoperative recovery is also compromised by excessive volume and sodium loading in the immediate perioperative period.
 Patients at high risk from cardiovascular disease do not tolerate anaemia. The optimal level of haemoglobin is the subject of much discussion but is probably around 10 g L−1.
Patients at high risk from cardiovascular disease do not tolerate anaemia. The optimal level of haemoglobin is the subject of much discussion but is probably around 10 g L−1.
 Patients should be actively warmed to avoid hypothermia, which activates the stress response, predisposes to arrhythmias and increases oxygen consumption postoperatively as a result of shivering.
Patients should be actively warmed to avoid hypothermia, which activates the stress response, predisposes to arrhythmias and increases oxygen consumption postoperatively as a result of shivering.
 Effective perioperative analgesia is essential. Pain is a potent stimulator of the stress response and uncontrolled sympathetic activation increases myocardial work and oxygen demand, predisposing to myocardial ischaemia or infarction.
Effective perioperative analgesia is essential. Pain is a potent stimulator of the stress response and uncontrolled sympathetic activation increases myocardial work and oxygen demand, predisposing to myocardial ischaemia or infarction.
 Before embarking on anaesthesia and surgery, consideration needs to be given to the patient’s management and destination postoperatively, e.g. would benefit be derived from a period of artificial ventilation or continued close monitoring in a high dependency or intensive care area postoperatively?
Before embarking on anaesthesia and surgery, consideration needs to be given to the patient’s management and destination postoperatively, e.g. would benefit be derived from a period of artificial ventilation or continued close monitoring in a high dependency or intensive care area postoperatively?
 Good communication between all of the relevant carers, including cardiology, critical care and the surgical team, is important.
Good communication between all of the relevant carers, including cardiology, critical care and the surgical team, is important.
Anaesthetic Agents: Most intravenous anaesthetic induction agents are cardiovascular depressants, causing both vasodilatation and myocardial depression. This is exaggerated in patients with low fixed cardiac output states and by concurrent hypovolaemia. Of the agents in regular use, etomidate is the least cardiac depressant. Care with dosing and rate of administration limits the hypotension caused by drugs such as propofol or thiopental. Co-induction with more than one agent may be beneficial in reducing the dose requirements of each and limiting hypotension. Concurrent administration of midazolam and a short-acting opioid (alfentanil or fentanyl) is often used. Remifentanil may be useful in these patients, in a low-dose infusion of 0.1–0.2 μg kg−1 min−1. It limits the dose of induction agent required and blunts the cardiovascular response to laryngoscopy and tracheal intubation. However, used in high doses, it may induce respiratory muscle stiffness and make bag and mask ventilation difficult before the onset of neuromuscular blockade.
Of the neuromuscular blocking agents, rocuronium and vecuronium are the most cardiostable.
Arrhythmias
Antiarrhythmic therapy should continue throughout the perioperative period.
Indications for preoperative temporary pacing include:
 bradyarrhythmia unresponsive to atropine if associated with syncope, hypotension or ventricular arrhythmias
bradyarrhythmia unresponsive to atropine if associated with syncope, hypotension or ventricular arrhythmias
 second-degree heart block (Mobitz 2)
second-degree heart block (Mobitz 2)
Intraoperative Arrhythmias: Arrhythmias are common in the perioperative period and are often self-limiting and require no specific treatment. However, precipitants of these arrhythmias should be sought and corrected if possible as they are more likely to occur and cause cardiovascular compromise in patients with underlying heart disease.
Factors predisposing to intraoperative arrhythmias include the following.
Management: This depends on the nature of the arrhythmia, likely causes and the degree of haemodynamic compromise.
 The precipitant should be removed if possible. Reflex arrhythmias tend to occur more commonly during light anaesthesia and may often be prevented by deepening anaesthesia.
The precipitant should be removed if possible. Reflex arrhythmias tend to occur more commonly during light anaesthesia and may often be prevented by deepening anaesthesia.
 Physiological abnormalities should be corrected, as specific antiarrhythmic therapy may be ineffective in the presence of uncorrected hypoxaemia, hypovolaemia or electrolyte abnormalities. Treatment of these should be concurrent with specific management of the arrhythmia.
Physiological abnormalities should be corrected, as specific antiarrhythmic therapy may be ineffective in the presence of uncorrected hypoxaemia, hypovolaemia or electrolyte abnormalities. Treatment of these should be concurrent with specific management of the arrhythmia.
Intraoperative Bradyarrhythmias: Bradyarrhythmias may often be prevented and may be treated using an intravenous anticholinergic, e.g. atropine or glycopyrrolate.
Intraoperative Tachyarrhythmias: These are either supraventricular or ventricular in origin. Generally, but not exclusively, supraventricular arrhythmias are narrow-complex, in distinction to broad-complex ventricular arrhythmias.
If the patient is not severely compromised, management depends on the individual arrhythmia:
 Atrial fibrillation. This is the commonest supraventricular arrhythmia seen intraoperatively. Often, a return to sinus rhythm cannot be achieved until the underlying precipitants are resolved. Improvements in oxygenation, volume status and analgesia may all improve the situation. However, ventricular rate control may also require treatment with either amiodarone or digoxin. Beta blockers or verapamil may also be used to slow ventricular rate. When surgery is complete, anticoagulation should be considered to avoid the embolic complications of atrial fibrillation.
Atrial fibrillation. This is the commonest supraventricular arrhythmia seen intraoperatively. Often, a return to sinus rhythm cannot be achieved until the underlying precipitants are resolved. Improvements in oxygenation, volume status and analgesia may all improve the situation. However, ventricular rate control may also require treatment with either amiodarone or digoxin. Beta blockers or verapamil may also be used to slow ventricular rate. When surgery is complete, anticoagulation should be considered to avoid the embolic complications of atrial fibrillation.
 Atrial flutter. This should be managed in the same way as atrial fibrillation if it occurs intraoperatively.
Atrial flutter. This should be managed in the same way as atrial fibrillation if it occurs intraoperatively.
 AV node/AV re-entry tachycardia and atrial tachycardia. Vagal manoeuvres, e.g. carotid sinus massage, may be tried, as can intravenous adenosine. Adenosine transiently slows AV conduction and may convert supraventricular tachycardia (SVT) to sinus rhythm. Alternatively, it may aid diagnosis by revealing flutter or fibrillation waves. A rapid i.v. bolus of 6 mg is given, followed by 12 mg a maximum of three times at 2-min intervals. Adenosine is contraindicated in patients with asthma, second- or third-degree heart block, patients receiving carbamazepine or dipyridamole and patients with a denervated heart, e.g. after cardiac transplant. Care must be taken with its use if the patient has Wolff-Parkinson-White syndrome. Verapamil, β-blockers and amiodarone may control the ventricular rate. Intravenous verapamil should never be given to a β-blocked patient.
AV node/AV re-entry tachycardia and atrial tachycardia. Vagal manoeuvres, e.g. carotid sinus massage, may be tried, as can intravenous adenosine. Adenosine transiently slows AV conduction and may convert supraventricular tachycardia (SVT) to sinus rhythm. Alternatively, it may aid diagnosis by revealing flutter or fibrillation waves. A rapid i.v. bolus of 6 mg is given, followed by 12 mg a maximum of three times at 2-min intervals. Adenosine is contraindicated in patients with asthma, second- or third-degree heart block, patients receiving carbamazepine or dipyridamole and patients with a denervated heart, e.g. after cardiac transplant. Care must be taken with its use if the patient has Wolff-Parkinson-White syndrome. Verapamil, β-blockers and amiodarone may control the ventricular rate. Intravenous verapamil should never be given to a β-blocked patient.
 Ventricular tachycardia. Synchronized DC cardioversion is the treatment of choice. Alternatively, amiodarone (300 mg i.v. over 20-60 min, followed by an infusion of 900 mg over 24 h) may be given if the arrhythmia is well tolerated. Lidocaine 1 mg kg− 1 may be used as an alternative if amiodarone is not available, but should not be given if amiodarone has been given already.
Ventricular tachycardia. Synchronized DC cardioversion is the treatment of choice. Alternatively, amiodarone (300 mg i.v. over 20-60 min, followed by an infusion of 900 mg over 24 h) may be given if the arrhythmia is well tolerated. Lidocaine 1 mg kg− 1 may be used as an alternative if amiodarone is not available, but should not be given if amiodarone has been given already.
Permanent Pacemakers: Many patients with these devices have underlying heart disease which should be managed accordingly.
Permanent pacemakers are inserted in an increasing number of patients and are becoming increasingly complex. Pacemakers are classified by a series of 5 letters relating to the functions they possess (Table 18.4).
Specific Issues in Anaesthetic Management:
 Preoperative assessment: the pacemaker clinic should be contacted to find out the indication for pacemaker insertion, its history and mode of action noted, and any evidence of malfunction sought. The underlying rhythm and rate should be determined and the consequences in case of pacemaker malfunction failure known to determine the need for backup support.
Preoperative assessment: the pacemaker clinic should be contacted to find out the indication for pacemaker insertion, its history and mode of action noted, and any evidence of malfunction sought. The underlying rhythm and rate should be determined and the consequences in case of pacemaker malfunction failure known to determine the need for backup support.
 The main intraoperative hazards are electromagnetic interference, which may reprogramme the pacemaker, cause inappropriate inhibition or trigger a defibrillator discharge, or damage the pacemaker circuitry.
The main intraoperative hazards are electromagnetic interference, which may reprogramme the pacemaker, cause inappropriate inhibition or trigger a defibrillator discharge, or damage the pacemaker circuitry.
 Routine investigations should include ECG, chest X-ray and electrolytes.
Routine investigations should include ECG, chest X-ray and electrolytes.
 The pacemaker should have been checked within 3 months of elective surgery. The battery life should be known; consider replacing any device near its elective replacement time.
The pacemaker should have been checked within 3 months of elective surgery. The battery life should be known; consider replacing any device near its elective replacement time.
 Due to the complexity of programming available, it is no longer acceptable practice to use a magnet to return the pacemaker to a fixed rate mode. Magnets should not be used, as they have an unpredictable effect on programming.
Due to the complexity of programming available, it is no longer acceptable practice to use a magnet to return the pacemaker to a fixed rate mode. Magnets should not be used, as they have an unpredictable effect on programming.
 Some pacemakers have a rate modulation facility. This implies that they can vary the rate of pacing with the patient’s activity detected usually by muscle activity or respiratory activity so that heart rate may be increased with exercise. In general, rate modulation features should be inactivated before anaesthesia and surgery as shivering and muscle fasciculation may be misinterpreted and lead to inappropriate increases in heart rate.
Some pacemakers have a rate modulation facility. This implies that they can vary the rate of pacing with the patient’s activity detected usually by muscle activity or respiratory activity so that heart rate may be increased with exercise. In general, rate modulation features should be inactivated before anaesthesia and surgery as shivering and muscle fasciculation may be misinterpreted and lead to inappropriate increases in heart rate.
 Central venous or pulmonary artery catheters may dislodge pacing leads, particularly if the pacemaker has only recently been inserted. Consideration should be given to use of the femoral vein for central venous access and to alternative monitors of cardiac output.
Central venous or pulmonary artery catheters may dislodge pacing leads, particularly if the pacemaker has only recently been inserted. Consideration should be given to use of the femoral vein for central venous access and to alternative monitors of cardiac output.
 Alternative pacing should be available in the event of pacemaker failure; external pacing is a rapid and effective back-up.
Alternative pacing should be available in the event of pacemaker failure; external pacing is a rapid and effective back-up.
 Pacemakers should be routinely checked postoperatively either before discharge or via an early appointment at the pacemaker clinic. Electromagnetic interference may unpredictably reprogramme the pacemaker or cause damage to it.
Pacemakers should be routinely checked postoperatively either before discharge or via an early appointment at the pacemaker clinic. Electromagnetic interference may unpredictably reprogramme the pacemaker or cause damage to it.
 Diathermy: bipolar diathermy should be used if possible. If unipolar diathermy is used, the diathermy and ground plate should be as far from the pacemaker as possible and the current pathway should be placed at right angles to the pacing wire(s).
Diathermy: bipolar diathermy should be used if possible. If unipolar diathermy is used, the diathermy and ground plate should be as far from the pacemaker as possible and the current pathway should be placed at right angles to the pacing wire(s).
 Lithotripsy: the lithotriptor should be at least 12 cm away from the pacemaker and rate modulation should be deactivated.
Lithotripsy: the lithotriptor should be at least 12 cm away from the pacemaker and rate modulation should be deactivated.
 Peripheral nerve stimulators and transcutaneous electrical nerve stimulators (TENS) should be kept at least 12 cm from the pacemaker.
Peripheral nerve stimulators and transcutaneous electrical nerve stimulators (TENS) should be kept at least 12 cm from the pacemaker.
 Defibrillator paddles should be 12 cm away from the pacemaker.
Defibrillator paddles should be 12 cm away from the pacemaker.
Implantable Cardioverter Defibrillators (ICDs): Increasingly, these devices are used for the management of patients with recurrent life-threatening episodes of VF and VT. ICDs may also have a pacemaker function. As with permanent pacemakers, they may be subject to electromagnetic interference and the same precautions apply.
Valvular Heart Disease
General Principles
 The patient’s functional reserve is a good indicator of the severity of a valve lesion
The patient’s functional reserve is a good indicator of the severity of a valve lesion
 Routine antibiotic prophylaxis is no longer recommended for all patients with valvular heart disease
Routine antibiotic prophylaxis is no longer recommended for all patients with valvular heart disease
 Patients with valvular heart disease may be receiving anticoagulants; perioperative heparinization is necessary
Patients with valvular heart disease may be receiving anticoagulants; perioperative heparinization is necessary
 No specific anaesthetic technique is preferred for valvular heart disease. The aim is to maintain cardiovascular stability. In severe disease, this is often best achieved using a general anaesthetic technique with opioids and controlled ventilation
No specific anaesthetic technique is preferred for valvular heart disease. The aim is to maintain cardiovascular stability. In severe disease, this is often best achieved using a general anaesthetic technique with opioids and controlled ventilation
Mitral Stenosis
Patients with mitral stenosis who present for surgery are frequently receiving digoxin, diuretics and anticoagulants. Preoperative control of atrial fibrillation, treatment of pulmonary oedema and management of anticoagulant therapy (see Ch 13) are necessary. During anaesthesia, control of heart rate is important. Tachycardia reduces diastolic ventricular filling and thus cardiac output, while bradycardia also results in decreased cardiac output because stroke output is limited. As with aortic stenosis, drugs which produce vasodilatation may cause severe hypotension. As a result of pre-existing pulmonary hypertension, patients are particularly vulnerable to hypoxaemia. Both hypoxaemia and acidosis are potent pulmonary vasoconstrictors and may produce acute right ventricular failure. Thus, opioid analgesics should be prescribed cautiously, and airway obstruction avoided.
Hypertrophic Cardiomyopathy
Diagnosis is confirmed by echocardiography.
 Acute changes in volume status cause severe haemodynamic consequences and hypovolaemia should be avoided
Acute changes in volume status cause severe haemodynamic consequences and hypovolaemia should be avoided
 Outflow obstruction is exacerbated by catecholamines so that inotropic agents should be avoided
Outflow obstruction is exacerbated by catecholamines so that inotropic agents should be avoided
 Patients are usually receiving a β-blocker which should be continued perioperatively
Patients are usually receiving a β-blocker which should be continued perioperatively
 Patients with previous malignant ventricular arrhythmias are likely to have an ICD in situ.
Patients with previous malignant ventricular arrhythmias are likely to have an ICD in situ.
RESPIRATORY DISEASE
Assessment
Investigations
Chest X-ray: The preoperative chest X-ray is a poor indicator of functional impairment but may be indicated in certain situations:
ECG: This may indicate right atrial enlargement or right ventricular hypertrophy (P pulmonale in II; dominant R wave in III, V1–3). Associated ischaemic heart disease is common, and ECG abnormalities may confirm this (Fig. 18.1).
Haematology: Polycythaemia occurs secondary to chronic hypoxaemia, while anaemia aggravates tissue hypoxia. Leucocytosis may indicate active infection.
Sputum Culture: Sputum culture is essential in patients with chronic lung disease or suspected acute infection.
Pulmonary Function Tests: Peak expiratory flow rate, forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) can be measured easily at the bedside. The FEV1:FVC ratio is decreased in obstructive lung disease and normal in restrictive disease. In the presence of obstructive disease, the test should be repeated 5–10 min after administration of a bronchodilator aerosol to provide an indication of reversibility. An FVC < 1–1.5 L is indicative of limited ability to take large sigh breaths, expand lung bases and clear secretions by coughing.
Blood Gas Measurement: Arterial blood gas measurement is indicated in patients with chronic respiratory disease scheduled to undergo significant surgery and also if there is suspected acute hypoxaemia. It is also advisable when pulmonary function tests are markedly abnormal, e.g. in obstructive disease where the FEV1 is less than 1.5 L. A raised PaCO2 with normal pH indicates chronic hypercapnia with renal compensation; a combined raised PaCO2 and acidosis indicates an acute event. Hypercapnia, particularly if acute, associated with acidosis, is likely to be associated with postoperative pulmonary complications. With a PaCO2 of 6.7 kPa (50 mmHg) or greater, elective controlled ventilation may be required after major surgery. The combination of a low preoperative arterial oxygen tension (PaO2) and dyspnoea at rest is also associated with a high likelihood of the need for planned ventilation after abdominal surgery.
Effects of Anaesthesia and Surgery
 mucosal irritation by anaesthetic agents
mucosal irritation by anaesthetic agents
 introduction of infection by aspiration or tracheal intubation
introduction of infection by aspiration or tracheal intubation
 respiratory depression by muscle relaxants, opioid analgesics or volatile anaesthetic agents.
respiratory depression by muscle relaxants, opioid analgesics or volatile anaesthetic agents.
Regional Anaesthesia: The use of appropriate regional anaesthetic techniques, where possible, confers several advantages in patients with respiratory disease both intra- and post-operatively, including:
 possible avoidance of tracheal intubation and controlled ventilation
possible avoidance of tracheal intubation and controlled ventilation
 reduced or absent requirement for respiratory depressant agents such as volatile agents and opioids
reduced or absent requirement for respiratory depressant agents such as volatile agents and opioids
 epidural analgesia may reduce postoperative hypoxaemia by diminishing the decrease in FRC associated with anaesthesia and abdominal surgery
epidural analgesia may reduce postoperative hypoxaemia by diminishing the decrease in FRC associated with anaesthesia and abdominal surgery
 effective analgesia, allowing the patient to undergo chest physiotherapy, to mobilize early and to avoid prolonged bed rest.
effective analgesia, allowing the patient to undergo chest physiotherapy, to mobilize early and to avoid prolonged bed rest.
Laparoscopic Surgery: The use of laparoscopic techniques for cholecystectomy, fundoplication and other abdominal procedures has markedly reduced postoperative pulmonary morbidity with the result that patients with severe pulmonary disease can usually undergo these procedures without the need for postoperative ventilatory support. The reasons for reduced morbidity include the relative lack of postoperative pain and the preservation of lung volumes postoperatively. These techniques should be encouraged in patients with chronic pulmonary disease. Nevertheless, cardiopulmonary function may be considerably compromised intraoperatively by raised intra-abdominal pressure, and judicious use of invasive haemodynamic monitoring has been recommended in patients with severe cardiorespiratory disease.
ASTHMA
Preoperative Management
The current state of the patient’s disease is assessed by:
 History – frequency and severity of attacks, factors provoking attacks, recent episodes of infection, drug history.
History – frequency and severity of attacks, factors provoking attacks, recent episodes of infection, drug history.
 Examination – presence or absence of wheeze, prolonged expiratory phase, overdistension, evidence of infection (cough, sputum, temperature, WBC).
Examination – presence or absence of wheeze, prolonged expiratory phase, overdistension, evidence of infection (cough, sputum, temperature, WBC).
 Pulmonary function tests – peak expiratory flow rate or FEV1/FVC before and after inhalation of bronchodilator.
Pulmonary function tests – peak expiratory flow rate or FEV1/FVC before and after inhalation of bronchodilator.
 Blood gas analysis including changes in PaCO2 to varying inspired oxygen concentrations.
Blood gas analysis including changes in PaCO2 to varying inspired oxygen concentrations.
Treatment of Airways Obstruction
Corticosteroids are also important in the prevention and treatment of bronchospasm in asthmatics as they modify the underlying inflammatory process. Patients prescribed long-term inhaled or systemic steroid therapy who are suboptimally controlled may require a course of augmented steroid therapy, e.g. prednisolone 40–60 mg daily or hydrocortisone 100 mg four times daily, to cover the anaesthetic and postoperative periods. Equivalent doses of steroid preparations are shown in Table 18.5. The steroid dose should be gradually reduced postoperatively, titrated against the severity of the asthma.
TABLE 18.5
Equivalent doses of Glucocorticoids
| Glucocorticoid | Dose (mg) |
| Betamethasone | 3 |
| Cortisone acetate | 100 |
| Dexamethasone | 3 |
| Hydrocortisone | 80 |
| Methylprednisolone | 16 |
| Prednisolone | 20 |
| Triamcinolone | 16 |
Weight Reduction
This should be encouraged before elective surgery in obese patients with respiratory disease.
Smoking
Patients should be strongly encouraged to stop smoking for at least 6 weeks before elective surgery.
Anaesthesia
An Approach with Minimal Intervention: Spontaneous ventilation with the option of local or regional anaesthesia is indicated for minor body surface operations. The use of a laryngeal mask airway (LMA) avoids tracheal intubation with its attendant risk of provoking bronchoconstriction, and if undue respiratory depression occurs, manifested by an increased PaCO2, ventilation may be readily assisted via the LMA. Volatile anaesthetic agents, being bronchodilators, are well tolerated in asthmatics. Plexus blocks and low subarachnoid or epidural anaesthesia enable limb, lower abdominal or pelvic surgery in patients with severe respiratory impairment. Sedation should be administered carefully to avoid respiratory compromise.
Elective Mechanical Ventilation: A decision may be made to undertake intermittent positive-pressure ventilation (IPPV) during anaesthesia and for a variable period after operation, at least until elimination of neuromuscular blockers and anaesthetic agents has occurred. This also permits optimal provision of analgesia without fear of opioid-induced depression of ventilation. This technique is usually preferred if the preoperative PaCO2 is greater than 6.7 kPa (50 mmHg) or if major thoracic or abdominal surgery is planned. Care should be taken with ventilator settings. A sufficiently long expiratory phase should be allowed to enable lung deflation and prevent gas trapping, while the inspiratory time should be adequate to avoid unduly high inflation pressures, with the attendant risk of pneumothorax.
Regional Anaesthesia: A combined general/epidural anaesthetic technique is often useful for major abdominal or thoracic surgery, as there is good evidence of a reduction in postoperative pulmonary complications with effective epidural analgesia. This approach may avoid a need for postoperative IPPV in some patients or may be usefully combined with noninvasive ventilation.
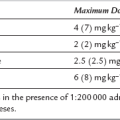
Anaesthetic Agents: Drugs which are associated with histamine release, e.g. atracurium and morphine, are perhaps best avoided, whilst rocuronium and fentanyl are preferred. β-Blocking drugs should also be avoided. If bronchospasm occurs during anaesthesia, it may result from easily remedied causes such as light anaesthesia or tracheal tube irritation and these should be corrected. If bronchospasm persists, nebulized salbutamol 2.5–5 mg should be administered into the anaesthetic breathing system and if this is not immediately beneficial, salbutamol 125–250 μg or aminophylline 250 mg should be administered by slow i.v. injection over at least 20 minutes, under ECG monitoring. The aminophylline dose should be modified if the patient is receiving oral theophylline. Thereafter, an infusion of aminophylline, up to 0.5–0.8 mg kg−1 h−1, or salbutamol, possibly in combination with nebulized salbutamol by positive-pressure ventilation (solution of 50–100 μg mL−1 of water, run at 3–20 μg min−1), should be maintained until improvement occurs. Hydrocortisone 200 mg i.v. should be given simultaneously, although it has no immediate effect. Inhaled volatile anaesthetic agents, e.g. sevoflurane, and intravenous ketamine have also been used with success when other agents have failed to relieve acute bronchospasm. Intravenous magnesium should also be considered in refractory cases.
Postoperative Care
Oxygen and Respiratory Care: Asthmatic patients rarely lose carbon dioxide responsiveness, and therefore high inspired oxygen concentrations are well tolerated and should be given. In patients with COPD, a controlled concentration of oxygen is generally required during spontaneous ventilation, using a 24% or 28% Venturi mask. Arterial blood gases should be checked frequently to ensure an adequate PaO2 (> 8 kPa) without excessive carbon dioxide retention (PaCO2 < 7.5–8 kPa). Using a pulse oximeter, the FiO2 may be titrated to achieve an SpO2 of around 90%. Hypoxaemia may seriously aggravate existing pulmonary hypertension and precipitate right ventricular failure.
Analgesia: Simple, non-opioid analgesics and/or local and regional techniques should be used where possible. Non-steroidal anti-inflammatory drugs (NSAIDs) such as diclofenac or ibuprofen are useful in reducing the opioid requirements following major surgery and may be adequate on their own after minor surgery. However, NSAIDs may aggravate bronchospasm in some asthmatics as a result of increased leukotriene production. These agents should not be given to patients with a history of aspirin hypersensitivity or to asthmatics who have never taken NSAIDs previously. Opioid analgesics are best administered, where necessary, in small i.v. doses, under direct supervision, or using patient-controlled analgesia. Physiotherapy, bronchodilators and antibiotics should be continued postoperatively.
GASTROINTESTINAL DISEASE
Gastrointestinal disease presents several problems for the anaesthetist:
LIVER DISEASE
Preoperative Assessment
Particular problems relevant to the anaesthetist include the following:
Initial Management Issues
 The airway and breathing may be compromised by impaired conscious level, diaphragmatic splinting by ascites or both. Intubation of the trachea and IPPV may be required, especially for transport.
The airway and breathing may be compromised by impaired conscious level, diaphragmatic splinting by ascites or both. Intubation of the trachea and IPPV may be required, especially for transport.
 Direct monitoring of arterial and central venous pressures is required. Hypotension requires correction, generally with a vasopressor, e.g. noradrenaline (noradrenaline). Intravascular hypovolaemia and poor cardiac output need to be considered as possible additional factors.
Direct monitoring of arterial and central venous pressures is required. Hypotension requires correction, generally with a vasopressor, e.g. noradrenaline (noradrenaline). Intravascular hypovolaemia and poor cardiac output need to be considered as possible additional factors.
 Conscious level: airway protection and IPPV may be required. Blood glucose concentration must be checked and hypoglycaemia corrected with intravenous glucose infusion. The presence of blood in the gastrointestinal tract following variceal or other haemorrhage is commonly a precipitating factor for encephalopathy; it is treated with lactulose by nasogastric tube.
Conscious level: airway protection and IPPV may be required. Blood glucose concentration must be checked and hypoglycaemia corrected with intravenous glucose infusion. The presence of blood in the gastrointestinal tract following variceal or other haemorrhage is commonly a precipitating factor for encephalopathy; it is treated with lactulose by nasogastric tube.
 Electrolyte problems such as hypokalaemia should be corrected.
Electrolyte problems such as hypokalaemia should be corrected.
 Active bleeding should, if possible, be controlled before transfer to a specialist centre, e.g. by banding of gastro-oesophageal varices, or insertion of a Linton or Sengstaken-Blakemore tube.
Active bleeding should, if possible, be controlled before transfer to a specialist centre, e.g. by banding of gastro-oesophageal varices, or insertion of a Linton or Sengstaken-Blakemore tube.
RENAL DISEASE
Measurement of blood urea and electrolyte concentrations should be undertaken before all major surgery and in all elderly or potentially unwell patients; a raised blood urea concentration demonstrated preoperatively may be the first indication of renal disease. Severity of renal dysfunction may be assessed further by measurement of serum creatinine concentration and creatinine clearance, urinary:plasma osmolality ratio and urinary urea and electrolyte excretion (Table 18.6).
TABLE 18.6
Urinary Measurements in Prerenal and Renal Failure
| Variable | Prerenal | Renal |
| Specific gravity | High > 1.020 | 1.010–1.012 |
| Sodium (mmol L−1) | Low < 20 | High > 40 |
| U:P urea ratio | High > 20 | Low < 10 |
| U:P creatinine ratio | High > 40 | Low < 10 |
| U:P osmolality ratio | High > 2.1 | Low < 1.2 |
| U, urine; P, plasma |
CKD 1&2: eGFR > 60 but with other evidence of kidney disease such as haematuria or proteinuria.
Pre-Anaesthetic Assessment
Electrolyte Disturbances
Sodium: Sodium retention occurs in renal failure, and through increased secretion of ADH, is associated with water retention, oedema and hypertension.
Potassium: Hyperkalaemia occurs typically in renal failure, frequently in association with metabolic acidosis. It causes delayed myocardial conduction and, if untreated, leads to cardiac arrest in asystole or ventricular fibrillation.
 calcium chloride 10% titrated up to 20 mL i.v. to antagonize the cardiac effects of hyperkalaemia, under ECG guidance
calcium chloride 10% titrated up to 20 mL i.v. to antagonize the cardiac effects of hyperkalaemia, under ECG guidance
 glucose 50%, 50 mL with 5–15 units of soluble insulin followed by an infusion of 20% glucose with insulin as required, depending on BM-test blood sugar estimation
glucose 50%, 50 mL with 5–15 units of soluble insulin followed by an infusion of 20% glucose with insulin as required, depending on BM-test blood sugar estimation
 nebulized salbutamol 5 mg repeated regularly
nebulized salbutamol 5 mg repeated regularly
 sodium bicarbonate 1.26% to improve the metabolic acidosis
sodium bicarbonate 1.26% to improve the metabolic acidosis
 an ion exchange resin, e.g. calcium polystyrene sulphonate, orally, provides longer-term control in chronic renal failure
an ion exchange resin, e.g. calcium polystyrene sulphonate, orally, provides longer-term control in chronic renal failure
 haemodialysis or haemofiltration. The former is more effective in lowering serum potassium concentration rapidly, but haemofiltration may be more easily set up as an emergency in a general intensive care unit.
haemodialysis or haemofiltration. The former is more effective in lowering serum potassium concentration rapidly, but haemofiltration may be more easily set up as an emergency in a general intensive care unit.
Cardiovascular Effects
Drug Treatment
This is important for several reasons:
 patients are frequently receiving concurrent medication for attendant problems, e.g. antihypertensive therapy
patients are frequently receiving concurrent medication for attendant problems, e.g. antihypertensive therapy
 many drugs are renally excreted; dosages require modification and plasma concentrations may require monitoring, e.g. aminoglycosides, digoxin
many drugs are renally excreted; dosages require modification and plasma concentrations may require monitoring, e.g. aminoglycosides, digoxin
 some drugs have active metabolites which are renally excreted, e.g. morphine, midazolam. Dosage requires careful titration, or use of alternative agents should be considered, e.g. fentanyl, oxycodone
some drugs have active metabolites which are renally excreted, e.g. morphine, midazolam. Dosage requires careful titration, or use of alternative agents should be considered, e.g. fentanyl, oxycodone
 some drugs adversely affect renal function, even in normal dosage. NSAIDs and the newer specific cyclo-oxygenase 2 inhibitors inhibit vasodilator prostaglandin production in the kidney and thus reduce glomerular blood flow and sodium excretion. This may be critical in septic or shocked patients, those with pre-existing renal dysfunction or those undergoing surgery associated with major blood loss. Their use should be avoided in such high-risk patients.
some drugs adversely affect renal function, even in normal dosage. NSAIDs and the newer specific cyclo-oxygenase 2 inhibitors inhibit vasodilator prostaglandin production in the kidney and thus reduce glomerular blood flow and sodium excretion. This may be critical in septic or shocked patients, those with pre-existing renal dysfunction or those undergoing surgery associated with major blood loss. Their use should be avoided in such high-risk patients.
DIABETES MELLITUS
 hyperglycaemia leading to increased risk of infectious complications and impaired healing (including anastomotic failure)
hyperglycaemia leading to increased risk of infectious complications and impaired healing (including anastomotic failure)
 hypoglycaemia, the clinical signs of which may be masked completely by anaesthesia
hypoglycaemia, the clinical signs of which may be masked completely by anaesthesia
Precise diabetic management depends upon:
 the nature of the diabetes and its treatment (insulin-dependent or non-insulin-dependent)
the nature of the diabetes and its treatment (insulin-dependent or non-insulin-dependent)
 the magnitude of the surgery contemplated, in particular duration of fasting
the magnitude of the surgery contemplated, in particular duration of fasting
 the time available for improving control of the diabetes preoperatively if necessary.
the time available for improving control of the diabetes preoperatively if necessary.
Preoperative Assessment
Control of Blood Glucose
Treatment Regimens: Oral hypoglycaemic agents:
 the sulphonylureas, e.g. glipizide and gliclazide, stimulate release of insulin from the pancreatic islets. Hypoglycaemia may be induced by these agents.
the sulphonylureas, e.g. glipizide and gliclazide, stimulate release of insulin from the pancreatic islets. Hypoglycaemia may be induced by these agents.
 biguanides, e.g. metformin, which increase peripheral uptake of glucose and decrease gluconeogenesis, are used either alone or in combination with sulphonylureas. These agents may cause lactic acidosis, usually, but not exclusively, in patients with a degree of renal or hepatic impairment. Guidelines for the administration of i.v. contrast media include the instructions to withhold metformin for 24 h before and 48 h after the investigation. Lactic acidosis carries a very high mortality. Metformin, the only biguanide now available, should usually be discontinued on the morning of surgery. Newer guidelines however suggest that it may be safely continued provided the patient does not have renal impairment and hypovolaemia is avoided.
biguanides, e.g. metformin, which increase peripheral uptake of glucose and decrease gluconeogenesis, are used either alone or in combination with sulphonylureas. These agents may cause lactic acidosis, usually, but not exclusively, in patients with a degree of renal or hepatic impairment. Guidelines for the administration of i.v. contrast media include the instructions to withhold metformin for 24 h before and 48 h after the investigation. Lactic acidosis carries a very high mortality. Metformin, the only biguanide now available, should usually be discontinued on the morning of surgery. Newer guidelines however suggest that it may be safely continued provided the patient does not have renal impairment and hypovolaemia is avoided.
 acarbose inhibits intestinal glucosidases, delaying carbohydrate digestion and reducing postprandial glucose surges.
acarbose inhibits intestinal glucosidases, delaying carbohydrate digestion and reducing postprandial glucose surges.
 Short-acting insulins. Soluble insulins, e.g. Humulin S and Actrapid, have an onset time of 30 min, peak effect 2–4 h and duration 8 h when given subcutaneously. Given intravenously, their effect is much shorter, with a half-life of around 2.5 min and a duration of action of 30 min. Insulin aspart (NovoRapid) and insulin lispro (Humalog) are human insulin analogues and have an even faster onset and shorter duration of action.
Short-acting insulins. Soluble insulins, e.g. Humulin S and Actrapid, have an onset time of 30 min, peak effect 2–4 h and duration 8 h when given subcutaneously. Given intravenously, their effect is much shorter, with a half-life of around 2.5 min and a duration of action of 30 min. Insulin aspart (NovoRapid) and insulin lispro (Humalog) are human insulin analogues and have an even faster onset and shorter duration of action.
 Intermediate, e.g. isophane insulin, insulin zinc suspension and the human insulin analogues: insulin detemir (levemir) and insulin glargine (lantus) have a more prolonged duration of action up to 16–35 hours. Onset time is 1–2 hours with peak effect at 4–12 hours. The longest acting agents, detemir and glargine, are often given once daily.
Intermediate, e.g. isophane insulin, insulin zinc suspension and the human insulin analogues: insulin detemir (levemir) and insulin glargine (lantus) have a more prolonged duration of action up to 16–35 hours. Onset time is 1–2 hours with peak effect at 4–12 hours. The longest acting agents, detemir and glargine, are often given once daily.
 Biphasic fixed mixtures, e.g. Mixtard (soluble and isophane insulin), Humalog (insulin lispro and insulin lispro protamine), NovoMix (insulin aspart and insulin aspart protamine). These are a combination of soluble and longer-acting insulins available in a variety of different proportions.
Biphasic fixed mixtures, e.g. Mixtard (soluble and isophane insulin), Humalog (insulin lispro and insulin lispro protamine), NovoMix (insulin aspart and insulin aspart protamine). These are a combination of soluble and longer-acting insulins available in a variety of different proportions.
Complications of Diabetes Mellitus
 Cardiovascular disorders (coronary artery, cerebrovascular and peripheral vascular) are common in diabetic patients and there is an increased risk of perioperative myocardial infarction. There may be significant ischaemic heart disease in the absence of warning symptoms and, as discussed earlier, this is a group which may merit further cardiovascular investigation before major surgery.
Cardiovascular disorders (coronary artery, cerebrovascular and peripheral vascular) are common in diabetic patients and there is an increased risk of perioperative myocardial infarction. There may be significant ischaemic heart disease in the absence of warning symptoms and, as discussed earlier, this is a group which may merit further cardiovascular investigation before major surgery.
 Renal disease. Microvascular damage produces glomerulosclerosis with proteinuria, oedema and eventually chronic renal failure. Anaesthetic implications of renal disease are discussed on page 401, and in Chapter 10.
Renal disease. Microvascular damage produces glomerulosclerosis with proteinuria, oedema and eventually chronic renal failure. Anaesthetic implications of renal disease are discussed on page 401, and in Chapter 10.
 Ocular problems. Cataracts, exudative or proliferative retinopathy, vitreous haemorrhage and retinal detachment may occur. In the long term, good blood glucose control has been shown to reduce the frequency of such complications.
Ocular problems. Cataracts, exudative or proliferative retinopathy, vitreous haemorrhage and retinal detachment may occur. In the long term, good blood glucose control has been shown to reduce the frequency of such complications.
 Infection. Diabetic patients are prone to infection and an increased risk of septicaemia, abscess formation and wound infection. Infection is associated with increased insulin requirements, which return to normal on its eradication.
Infection. Diabetic patients are prone to infection and an increased risk of septicaemia, abscess formation and wound infection. Infection is associated with increased insulin requirements, which return to normal on its eradication.
 Neuropathy. Chronic sensory peripheral neuropathies are common; mononeuropathies and acute motor neuropathies (amyotrophy) are associated with poor control of blood glucose. Loss of sensation together with peripheral vascular disease may lead to ulceration after trivial trauma. Consequently, care in positioning patients in the operating theatre is important. Local anaesthetic nerve or plexus blocks should be avoided in patients with an acute neuropathy, as neurological deficits may be attributed to the local anaesthetic solution.
Neuropathy. Chronic sensory peripheral neuropathies are common; mononeuropathies and acute motor neuropathies (amyotrophy) are associated with poor control of blood glucose. Loss of sensation together with peripheral vascular disease may lead to ulceration after trivial trauma. Consequently, care in positioning patients in the operating theatre is important. Local anaesthetic nerve or plexus blocks should be avoided in patients with an acute neuropathy, as neurological deficits may be attributed to the local anaesthetic solution.
 Autonomic neuropathy may cause postoperative urinary retention or vasomotor instability, e.g. postural hypotension or hypotension during anaesthesia. IPPV or subarachnoid or epidural block may be associated with significant hypotension; preoperative intravascular volume status should be assessed and fluids given to achieve normovolaemia before performing a block. Precise cardiovascular monitoring, use of vasopressors and careful anaesthetic management are essential.
Autonomic neuropathy may cause postoperative urinary retention or vasomotor instability, e.g. postural hypotension or hypotension during anaesthesia. IPPV or subarachnoid or epidural block may be associated with significant hypotension; preoperative intravascular volume status should be assessed and fluids given to achieve normovolaemia before performing a block. Precise cardiovascular monitoring, use of vasopressors and careful anaesthetic management are essential.
Perioperative Diabetic Management
Individual units have local protocols for the perioperative management of diabetes.
Blood transfusion may increase insulin requirements as citrate stimulates gluconeogenesis.
OTHER ENDOCRINE DISORDERS
Acromegaly: Acromegaly is caused by increased secretion of growth hormone from eosinophil cell tumours of the anterior pituitary gland. If this occurs before fusion of the epiphyses, gigantism results. Problems for the anaesthetist include the following:
 upper airway obstruction may result from an enlarged mandible, tongue and epiglottis, thickened pharyngeal mucosa and laryngeal narrowing. Maintenance of a clear airway and tracheal intubation may be difficult, and postoperative care of the airway must be meticulous. Consideration may be given to awake fibreoptic tracheal intubation.
upper airway obstruction may result from an enlarged mandible, tongue and epiglottis, thickened pharyngeal mucosa and laryngeal narrowing. Maintenance of a clear airway and tracheal intubation may be difficult, and postoperative care of the airway must be meticulous. Consideration may be given to awake fibreoptic tracheal intubation.
 cardiac enlargement, hypertension and congestive cardiac failure occur commonly and require preoperative treatment.
cardiac enlargement, hypertension and congestive cardiac failure occur commonly and require preoperative treatment.
 growth hormone increases blood sugar concentration. Hyperglycaemia should be controlled perioperatively.
growth hormone increases blood sugar concentration. Hyperglycaemia should be controlled perioperatively.
 thyroid and adrenal function may be impaired because of decreased release of thyroid-stimulating hormone (TSH) and ACTH. Thyroxine and steroid replacement may be required.
thyroid and adrenal function may be impaired because of decreased release of thyroid-stimulating hormone (TSH) and ACTH. Thyroxine and steroid replacement may be required.
Cushing’s Disease: Cushing’s disease results from basophil adenomas, which secrete ACTH (see below).
Hypopituitarism (Simmonds’ Disease): Causes include chromophobe adenoma, tumours of surrounding tissues, e.g. craniopharyngioma, skull fractures, infarction following postpartum haemorrhage and infection. Clinical features include loss of axillary and pubic hair, amenorrhoea, features of hypothyroidism and adrenal insufficiency, including hypotension, but with a striking pallor, in contrast to the pigmentation of Addison’s disease (see p. 408).
Diabetes Insipidus: This is caused by disease or damage affecting the hypothalamic posterior pituitary axis. Common causes are pituitary tumour, craniopharyngioma, basal skull fracture and infection, or it may occur as a sequel to pituitary surgery. In 10% of cases, diabetes insipidus is renal in origin.
Thyroid Disease
Goitre: Thyroid swelling may result from iodine deficiency (simple goitre), autoimmune (Hashimoto’s) thyroiditis, adenoma, carcinoma or thyrotoxicosis. Nodules of the thyroid gland may be ‘hot’ (secreting thyroxine) or ‘cold’.
Preparation for Thyroidectomy
Hypothyroidism: This may result from primary thyroid failure, Hashimoto’s thyroiditis, as a consequence of thyroid surgery, or secondary to pituitary failure. The diagnosis is suggested by tiredness, cold intolerance, loss of appetite, dry skin and hair loss. It may be confirmed by the finding of a low serum thyroxine concentration associated, in primary thyroid failure, with a raised serum TSH concentration.
Disease of the Adrenal Cortex
Clinical symptoms are associated with increased or decreased secretion of cortisol or aldosterone.
Hypersecretion of Cortisol (Cushing’s Syndrome): Most instances are caused by pituitary adenomas which secrete ACTH and thus cause bilateral adrenocortical hyperplasia (Cushing’s disease). In 20–30% of patients, an adrenocortical adenoma or carcinoma is present. Rarely, an oat-cell carcinoma of bronchus, secreting ACTH, is the cause. ACTH and corticosteroid therapy present similar pictures. Clinical features include obesity, hypertension, proximal myopathy and diabetes mellitus. Biochemically, there is a metabolic alkalosis with hypokalaemia. Depending on the cause, treatment may involve hypophysectomy or adrenalectomy.
Primary Hypersecretion of Aldosterone (Conn’s Syndrome): Conn’s syndrome is caused by an adenoma of the zona glomerulosa of the adrenal cortex and presents with hypertension, hypernatraemia, hypokalaemia and oliguria. Anaesthetic management involves preoperative treatment of hypertension, administration of spironolactone and potassium replacement; meticulous intra- and postoperative monitoring of arterial pressure is essential.
Adrenocortical Hypofunction: Primary adrenocortical insufficiency (Addison’s disease) may be caused by an autoimmune process, tuberculosis, HIV infection, amyloid, metastatic carcinoma, or bilateral adrenalectomy. Haemorrhage into the glands during meningococcal septicaemia may cause acute adrenal failure in association with septic shock. Secondary failure results from hypopituitarism or prolonged corticosteroid therapy. In secondary failure resulting from pituitary insufficiency, aldosterone secretion is maintained, and fluid and electrolyte disturbances are less marked.
All surgical procedures in these patients must be covered by increased steroid administration (see below). Patients with acute adrenal insufficiency require urgent fluid and sodium replacement with arterial pressure and CVP monitoring, glucose infusion to combat hypoglycaemia and hydrocortisone 100 mg 6-hourly i.v. They should be cared for in a high dependency or critical care area. Antibiotics are advisable to cover the possibility that infection has provoked the crisis. In cases of primary adrenal failure, mineralocorticoid replacement with fludrocortisone is required. If emergency surgery is required in acute adrenal failure, all precautions necessary for anaesthetizing the shocked patient should be taken (see Ch 37).
Congenital Adrenal Hyperplasia (Adrenogenital Syndrome): This is associated with overproduction of androgens as a result of deficiency of the hydroxylase enzyme required for production of cortisol. Hydrocortisone treatment overcomes adrenal insufficiency and, by suppressing ACTH production, decreases androgen accumulation. Augmented steroid cover is required for surgery in these patients.
Steroid Therapy
Replacement therapy in cases of primary adrenocortical failure and hypopituitarism is given as oral hydrocortisone 20 mg in the morning and 10 mg in the evening. Fludrocortisone 0.05–0.1 mg daily is given additionally to replace aldosterone in primary adrenocortical failure. Equivalent doses of other steroid preparations are shown in Table 18.5. Prednisolone and prednisone have less mineralocorticoid effect, while betamethasone and dexamethasone have none. Requirements increase following infection, trauma or surgery.
Steroid Cover for Anaesthesia and Surgery
 patients with pituitary adrenal insufficiency, receiving steroid replacement therapy
patients with pituitary adrenal insufficiency, receiving steroid replacement therapy
 patients undergoing pituitary or adrenal surgery
patients undergoing pituitary or adrenal surgery
 patients receiving systemic steroid therapy for more than 2 weeks before surgery
patients receiving systemic steroid therapy for more than 2 weeks before surgery
 patients no longer receiving systemic steroid therapy, but who received steroids within the three months before surgery.
patients no longer receiving systemic steroid therapy, but who received steroids within the three months before surgery.
 minor diagnostic procedures – usual morning dose of steroid OR i.v. hydrocortisone 25–50 mg at induction; recommence patient’s usual dose after surgery
minor diagnostic procedures – usual morning dose of steroid OR i.v. hydrocortisone 25–50 mg at induction; recommence patient’s usual dose after surgery
 intermediate operations, e.g. inguinal herniorrhaphy – usual morning dose of steroid AND i.v. hydrocortisone 25–50 mg at induction, then 25–50 mg 8-hourly for 24 h
intermediate operations, e.g. inguinal herniorrhaphy – usual morning dose of steroid AND i.v. hydrocortisone 25–50 mg at induction, then 25–50 mg 8-hourly for 24 h
 major surgery – usual morning dose of steroid AND i.v. hydrocortisone 25–50 mg at induction, then 8-hourly for 48–72 h.
major surgery – usual morning dose of steroid AND i.v. hydrocortisone 25–50 mg at induction, then 8-hourly for 48–72 h.
NEUROLOGICAL DISEASE
 a depressed level of consciousness may prejudice airway protection and result in depressed respiratory drive
a depressed level of consciousness may prejudice airway protection and result in depressed respiratory drive
 peripheral neuromuscular disease may lead to impaired ventilatory function and reduced ability to clear secretions
peripheral neuromuscular disease may lead to impaired ventilatory function and reduced ability to clear secretions
 autonomic dysfunction may result in blood pressure instability, cardiac arrhythmias and dysfunction of gastrointestinal motility
autonomic dysfunction may result in blood pressure instability, cardiac arrhythmias and dysfunction of gastrointestinal motility
 there may also be significant adverse effects from specific drug treatment, and there are several important drug interactions which need to be recognized.
there may also be significant adverse effects from specific drug treatment, and there are several important drug interactions which need to be recognized.
Assessment
Increased Intracranial Pressure
Elective surgery should be postponed if raised intracranial pressure is suspected, until investigation by CT scan and treatment have been undertaken. Anaesthetic agents which cause an increase in cerebral blood flow must be avoided. Hypercapnia must also be avoided, and controlled ventilation to a PaCO2 of approximately 4 kPa (30 mmHg) is indicated. This is discussed fully in Chapter 32.
Parkinson’s Disease
Gastrointestinal. There is an increased risk of reflux.
Medications. These may have cardiovascular side-effects and there are several potential interactions with anaesthetic agents, analgesics and neuromuscular blocking drugs. These are detailed in Table 18.7.
TABLE 18.7
Potential Drug Interactions in Patients with Parkinson’s Disease
Reproduced from Nicholson et al 2002 Parkinson’s disease and anaesthesia. British Journal of Anaesthesia 89(6):904–916. Copyright The Board of Management and Trustees of the British Journal of Anaesthesia. Reproduced by permission of Oxford University Press/British Journal of Anaesthesia.
Peripheral Neuropathies
Motor, sensory and autonomic fibres are involved. Causes include:
Motor Neurone Disease (Progressive Muscular Atrophy, Amyotrophic Lateral Sclerosis, Progressive Bulbar Palsy)
Dystrophia Myotonica
 Respiratory muscle weakness. Respiratory function should be assessed fully before operation. Respiratory depressant drugs, e.g. thiopental or opioids, should be used with care; there is sensitivity also to nondepolarizing neuromuscular blockers. Planned IPPV may be required after surgery. Postoperative care of the airway must be meticulous. Chest infections are common.
Respiratory muscle weakness. Respiratory function should be assessed fully before operation. Respiratory depressant drugs, e.g. thiopental or opioids, should be used with care; there is sensitivity also to nondepolarizing neuromuscular blockers. Planned IPPV may be required after surgery. Postoperative care of the airway must be meticulous. Chest infections are common.
 Cardiovascular effects. There may be a cardiomyopathy and conduction defects, including complete heart block. Patients may have a cardiac pacemaker in situ. Arrhythmias are common, particularly during anaesthesia, and may result in cardiac failure. Careful monitoring is essential.
Cardiovascular effects. There may be a cardiomyopathy and conduction defects, including complete heart block. Patients may have a cardiac pacemaker in situ. Arrhythmias are common, particularly during anaesthesia, and may result in cardiac failure. Careful monitoring is essential.
 Muscle spasm. This may be provoked by administration of depolarizing neuromuscular blockers or anticholinesterases; succinylcholine and neostigmine should thus be avoided. The spasm is not abolished by nondepolarizing relaxants.
Muscle spasm. This may be provoked by administration of depolarizing neuromuscular blockers or anticholinesterases; succinylcholine and neostigmine should thus be avoided. The spasm is not abolished by nondepolarizing relaxants.
 Gastrointestinal. Oesophageal dysmotility may predispose to regurgitation and aspiration.
Gastrointestinal. Oesophageal dysmotility may predispose to regurgitation and aspiration.
PSYCHIATRIC DISEASE
There are several considerations in the anaesthetic management of patients with psychiatric disease.
 Psychiatric patients are frequently depressed, with little understanding of, or interest in, anaesthesia.
Psychiatric patients are frequently depressed, with little understanding of, or interest in, anaesthesia.
 Patients are receiving a variety of medications with potential for serious drug interactions with anaesthetic agents.
Patients are receiving a variety of medications with potential for serious drug interactions with anaesthetic agents.
 Patients may have associated pathology as a result of drug and/or alcohol abuse.
Patients may have associated pathology as a result of drug and/or alcohol abuse.
 Repeated anaesthetics are required for electroconvulsive therapy.
Repeated anaesthetics are required for electroconvulsive therapy.
Electroconvulsive Therapy
Anaesthesia
 Cardiorespiratory function must be assessed in the light of the autonomic effects described.
Cardiorespiratory function must be assessed in the light of the autonomic effects described.
 Previous anaesthetic records – common in this group of patients.
Previous anaesthetic records – common in this group of patients.
 Antidepressants with anticholinergic effects slow gastric emptying so that an adequate fasting time (8 h) is important. However, patient reports of fasting time may be unreliable.
Antidepressants with anticholinergic effects slow gastric emptying so that an adequate fasting time (8 h) is important. However, patient reports of fasting time may be unreliable.
 Premedication is not usually given because it may influence seizure activity and prolong recovery time unnecessarily.
Premedication is not usually given because it may influence seizure activity and prolong recovery time unnecessarily.
 Anaesthesia is induced usually with either etomidate or propofol. The use of propofol is uncertain because, although it provides suitable anaesthesia, it may impair seizure activity.
Anaesthesia is induced usually with either etomidate or propofol. The use of propofol is uncertain because, although it provides suitable anaesthesia, it may impair seizure activity.
 A short-acting muscle relaxant is used to prevent trauma caused by seizure activity, and succinylcholine is the most frequently used agent.
A short-acting muscle relaxant is used to prevent trauma caused by seizure activity, and succinylcholine is the most frequently used agent.
 Hyperventilation by bag and mask before seizure induction lowers the seizure threshold and prolongs duration, whilst ventilation continued in the post-ictal phase avoids desaturation.
Hyperventilation by bag and mask before seizure induction lowers the seizure threshold and prolongs duration, whilst ventilation continued in the post-ictal phase avoids desaturation.
Drug Interactions
Tricyclic Antidepressants: Tricyclic antidepressants, e.g. amitriptyline and dothiepin, inhibit reuptake of noradrenaline into the presynaptic nerve terminals. These drugs have the following side-effects:
 MAOIs, e.g. phenelzine and tranylcypromine, inhibit intraneuronal metabolism of sympathomimetic amines.
MAOIs, e.g. phenelzine and tranylcypromine, inhibit intraneuronal metabolism of sympathomimetic amines.
 Tyramine (noradrenaline precursor) precipitates hypertensive crises (‘cheese reaction’).
Tyramine (noradrenaline precursor) precipitates hypertensive crises (‘cheese reaction’).
 They inhibit metabolism of indirect-acting sympathomimetic amines, e.g. ephedrine, resulting in severe hypertension if these drugs are given concurrently. There may also be an exaggerated sympathomimetic response if directly acting sympathomimetics are given concurrently.
They inhibit metabolism of indirect-acting sympathomimetic amines, e.g. ephedrine, resulting in severe hypertension if these drugs are given concurrently. There may also be an exaggerated sympathomimetic response if directly acting sympathomimetics are given concurrently.
 CNS excitation occurs with pethidine, manifest as agitation, hypertension, convulsions and hyperthermia. Other opioids appear to be safe although excessive sedation has been described. Note the antibacterial agent Linezolid is a reversible nonselective MAO inhibitor.
CNS excitation occurs with pethidine, manifest as agitation, hypertension, convulsions and hyperthermia. Other opioids appear to be safe although excessive sedation has been described. Note the antibacterial agent Linezolid is a reversible nonselective MAO inhibitor.
Selective Serotonin Reuptake Inhibitors: These selectively inhibit serotonin reuptake, e.g. fluoxetine, sertraline. They have fewer cardiac and anticholinergic effects than tricyclic antidepressants and may cause CNS toxicity in conjunction with tramadol.
Phenothiazines: This is a broad group of drugs and their therapeutic actions and side-effects vary considerably, e.g. chlorpromazine, thioridazine, prochlorperazine:
Lithium: Lithium inhibits release and increases reuptake of noradrenaline:
 lithium is used to treat manic states
lithium is used to treat manic states
 lithium acts as a sodium ion, and may potentiate muscle relaxants
lithium acts as a sodium ion, and may potentiate muscle relaxants
 it is renally excreted, and toxicity is enhanced by hyponatraemia because lithium is conserved along with sodium
it is renally excreted, and toxicity is enhanced by hyponatraemia because lithium is conserved along with sodium
 its toxic effects include tremor, ataxia, nystagmus, renal impairment and convulsions
its toxic effects include tremor, ataxia, nystagmus, renal impairment and convulsions
 it is recommended that lithium is discontinued 24 h before major surgery if possible.
it is recommended that lithium is discontinued 24 h before major surgery if possible.
CONNECTIVE TISSUE DISORDERS
Rheumatoid Arthritis
Conduct of Anaesthesia
Specific anaesthetic considerations include:
 consideration of general versus regional anaesthesia
consideration of general versus regional anaesthesia
 how to secure and maintain the patient’s airway if a general anaesthetic is required
how to secure and maintain the patient’s airway if a general anaesthetic is required
 steroid replacement where indicated
steroid replacement where indicated
 analgesia for a group of patients who may have significant preoperative pain and may be on regular opioids and other analgesics.
analgesia for a group of patients who may have significant preoperative pain and may be on regular opioids and other analgesics.
NUTRITIONAL PROBLEMS
Obesity poses several problems to the anaesthetist and surgeon.
Other Factors
Obese patients require careful preoperative respiratory and cardiovascular assessment (see Ch 17). The inspired oxygen fraction should be increased and positive end-expiratory pressure (PEEP) applied to maintain a satisfactory SpO2. Fluid balance should be monitored carefully. Elective postoperative artificial ventilation should be considered, especially after abdominal surgery. Pulmonary, thromboembolic and wound complications are more common, and appropriate prophylactic measures and/or early recognition and treatment are important.
ANAESTHETIC CONSIDERATIONS IN THE ELDERLY
Organ System Changes
 Autonomic dysfunction, leading to attenuated baroreceptor reflexes and susceptibility to hypotension, impaired temperature regulation and impaired gut motility.
Autonomic dysfunction, leading to attenuated baroreceptor reflexes and susceptibility to hypotension, impaired temperature regulation and impaired gut motility.
 Cognitive impairment, resulting in a reduced capacity to cope with new situations and increased likelihood of confusional states. This effect may be compounded by sensory deprivation associated with impaired hearing and vision.
Cognitive impairment, resulting in a reduced capacity to cope with new situations and increased likelihood of confusional states. This effect may be compounded by sensory deprivation associated with impaired hearing and vision.
Cardiovascular
 There is a loss of atrial pacemaker cells, resulting in an increasing tendency to develop atrial fibrillation.
There is a loss of atrial pacemaker cells, resulting in an increasing tendency to develop atrial fibrillation.
 Maximum heart rate decreases and the elderly are increasingly dependent on increased stroke volume to increase cardiac output. This renders them more sensitive to hypovolaemia and reduced preload.
Maximum heart rate decreases and the elderly are increasingly dependent on increased stroke volume to increase cardiac output. This renders them more sensitive to hypovolaemia and reduced preload.
 Peripheral vascular resistance is increased as a result of loss of blood vessel compliance, and compensatory left ventricular hypertrophy is common.
Peripheral vascular resistance is increased as a result of loss of blood vessel compliance, and compensatory left ventricular hypertrophy is common.
 Catecholamine receptors are downregulated, resulting in a less predictable response to adrenergic agents used therapeutically.
Catecholamine receptors are downregulated, resulting in a less predictable response to adrenergic agents used therapeutically.
Respiratory System
 Closing volume increases and encroaches on tidal volume such that, when supine, significant shunting, hypoxaemia and later atelectasis may occur.
Closing volume increases and encroaches on tidal volume such that, when supine, significant shunting, hypoxaemia and later atelectasis may occur.
 Obstructive sleep apnoea is more common with associated episodes of desaturation and these episodes may be potentiated by opioid analgesia.
Obstructive sleep apnoea is more common with associated episodes of desaturation and these episodes may be potentiated by opioid analgesia.
 Silent regurgitation and aspiration of gastric contents occurs more commonly.
Silent regurgitation and aspiration of gastric contents occurs more commonly.
HUMAN IMMUNODEFICIENCY VIRUS
With regard to anaesthesia, there is little specific information available.
 General anaesthesia is known to be immunosuppressant so that, theoretically, a regional technique, e.g. epidural anaesthesia, might be preferable. However, this must be balanced against the risk of pre-existing immunosuppression leading to epidural abscess and the potential to exacerbate pre-existing neuropathy. At present, individual decisions should be made at the discretion of the anaesthetist and the patient involved.
General anaesthesia is known to be immunosuppressant so that, theoretically, a regional technique, e.g. epidural anaesthesia, might be preferable. However, this must be balanced against the risk of pre-existing immunosuppression leading to epidural abscess and the potential to exacerbate pre-existing neuropathy. At present, individual decisions should be made at the discretion of the anaesthetist and the patient involved.
 Pain is a common symptom of late HIV infection and AIDS, and pain should be assessed and treated as part of the patient’s perioperative management.
Pain is a common symptom of late HIV infection and AIDS, and pain should be assessed and treated as part of the patient’s perioperative management.
 Drug interactions may occur with antiretroviral agents. For example, the protease inhibitors inhibit cytochrome P450, resulting in reduced metabolism of many drugs, including fentanyl and benzodiazepines. Conversely, non-nucleoside reverse transcriptase inhibitors induce cytochrome P450. Patients with HIV infection are usually receiving a combination of three agents so that interactions may be complex.
Drug interactions may occur with antiretroviral agents. For example, the protease inhibitors inhibit cytochrome P450, resulting in reduced metabolism of many drugs, including fentanyl and benzodiazepines. Conversely, non-nucleoside reverse transcriptase inhibitors induce cytochrome P450. Patients with HIV infection are usually receiving a combination of three agents so that interactions may be complex.
MYELOMA
 Widespread skeletal destruction occurs and careful handling of the patient on the operating table is essential. Pathological fractures are common.
Widespread skeletal destruction occurs and careful handling of the patient on the operating table is essential. Pathological fractures are common.
 Bone pain may be severe and often requires large doses of analgesics.
Bone pain may be severe and often requires large doses of analgesics.
 Hypercalcaemia occurs as a result of bone destruction and may precipitate renal failure.
Hypercalcaemia occurs as a result of bone destruction and may precipitate renal failure.
 Chronic renal failure may also result from direct nephrotoxicity.
Chronic renal failure may also result from direct nephrotoxicity.
 Anaemia is almost invariable, and preoperative blood transfusion is often necessary.
Anaemia is almost invariable, and preoperative blood transfusion is often necessary.
 Thrombocytopenia is common during cytotoxic therapy.
Thrombocytopenia is common during cytotoxic therapy.
 Patients are susceptible to infection, including chest infection, especially during chemotherapy.
Patients are susceptible to infection, including chest infection, especially during chemotherapy.
 Increased plasma immunoglobulin concentrations may increase blood viscosity, predisposing to arterial and venous thrombosis. Drug binding may be affected.
Increased plasma immunoglobulin concentrations may increase blood viscosity, predisposing to arterial and venous thrombosis. Drug binding may be affected.
 Neurological manifestations include spinal cord and nerve root compression.
Neurological manifestations include spinal cord and nerve root compression.
PORPHYRIA
 Gastrointestinal – abdominal pain and tenderness, vomiting, constipation and occasionally diarrhoea.
Gastrointestinal – abdominal pain and tenderness, vomiting, constipation and occasionally diarrhoea.
 Neurological – a motor and sensory peripheral neuropathy is common. It may involve bulbar and respiratory muscles. Epileptic fits and psychological disturbance may occur.
Neurological – a motor and sensory peripheral neuropathy is common. It may involve bulbar and respiratory muscles. Epileptic fits and psychological disturbance may occur.
 Cardiovascular – hypertension and tachycardia often occur during the attacks. Hypotension has also been reported.
Cardiovascular – hypertension and tachycardia often occur during the attacks. Hypotension has also been reported.
Anaesthesia in such patients is directed to avoiding drugs which may provoke attacks. Induction with propofol, followed by muscle relaxation with succinylcholine or vecuronium, ventilation with nitrous oxide, and oxygen, and analgesic supplementation with morphine or fentanyl is satisfactory (Table 18.8). If fits occur, diazepam is a suitable anticonvulsant, while chlorpromazine, promethazine or promazine are suitable sedatives.
TABLE 18.8
Safety of Drugs Commonly used in Clinical Anaesthesia for Patients with Acute Porphyrias
PS, possibly safe; S, safe; C, contentious; ND, no data; U, unsafe; PU, probably unsafe.
An up-to-date list of drugs considered safe in acute porphyria can be found at www.wmic.wales.nhs.uk/porphyria_info.php.
Avidan, M.S., Jones, N., Pozniak, A.L. The implications of HIV for the anaesthetist and the intensivist. Anaesthesia. 2000;55:344–354.
Benumof J.L., ed. Anesthesia and uncommon diseases, fourth ed., Philadelphia: WB Saunders, 1998.
British Journal of Anaesthesia. Postgraduate educational issue, cardiovascular disease in anaesthesia and critical care. Oxford: Oxford University Press, 2004.
British National Formulary, sixty third ed. 2012. British Medical Association and Royal Pharmaceutical Society of Great Britain.
Chassot, P.G., Delabays, A., Spahn, D.R. Preoperative evaluation of patients with, or at risk of, coronary artery disease undergoing non-cardiac surgery. Br. J. Anaesth. 2002;89:747–759.
Fleisher, L.A., Beckman, J.A., Brown, K.A., et al. ACC/AHA guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary. J. Am. Coll. Cardiol. 2007;50:1707–1732.
Management of adults with diabetes undergoing surgery and elective procedures. Available from: http://www.diabetes.nhs.uk/areas_of_care/emergency_and_inpatient/perioperative_management/
McAnulty, G.R., Robertshaw, H.J., Hall, M. Anaesthetic management of patients with diabetes mellitus. Br. J. Anaesth. 2000;85:80–90.
Nicholson, G., Pereira, A.C., Hall, G.M. Parkinson’s disease and anaesthesia. Br. J. Anaesth. 2002;89:904–916.
Priebe, H.J. The aged cardiovascular risk patient. Br. J. Anaesth. 2000;85:763–778.
Riddell, J.W., Chiche, L., Plaud, B., et al. Coronary stents and non cardiac surgery. Circulation. 2007;116:e378–e382.
Stoelting, R.K., Dierdorf, S.R. Anaesthesia and co-existing disease, third ed. London: Churchill Livingstone; 1993.