Intensive Care Management of Medical and Surgical Complications
The purpose of this chapter is to describe some common complications seen in patients who are critically ill that have implications for physical therapists practicing in the intensive care unit (ICU). Complications arising from the following conditions are included: respiratory failure, surgery, acute lung injury and ARDS, shock, sepsis, and multiorgan system failure (MOSF). Furthermore, the implications for cardiovascular and pulmonary physical therapy are presented. Complications add further complexity to the diagnosis of the multiple factors contributing to impaired oxygen transport and to the challenge of prescribing treatment parameters effectively. Understanding the pathophysiological deficits in these complex conditions is the basis for delivering effective management, reducing the risk of an untoward treatment response, and preventing worsening of the patient’s condition. In addition, risks such as age, comorbidity, severity of trauma, extent of surgery, obesity, deconditioning, smoking, and FiO2 greater than 0.5 have been reported.1 Compared with nonsurvivors, survivors of these conditions have increased right ventricular ejection fraction, PaO2, FiO2, and oxygen consumption (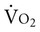 ).2 With respect to vasoactive substances, atrial natriuretic peptide, catecholamines, renin, and vasopressin are lower in survivors. Cardiac index is not different between survivors and nonsurvivors, and there is no correlation between hemodynamics and circulating vasoactive substances. Impending life threat is associated with global energetic failure secondary to cellular oxygen deficits. Despite the severity of illness of patients in the ICU, physical therapy has a well defined role in this setting.3 More recently, the integration of physical therapy has been included in a quality control model of ICU care.4 Although treatment options may be more restricted in patients with complications and greater severity of illness, judiciously prescribed physical therapy can have an important role in reducing further complications, enhancing weaning from mechanical ventilation, and reducing ICU stay.5
).2 With respect to vasoactive substances, atrial natriuretic peptide, catecholamines, renin, and vasopressin are lower in survivors. Cardiac index is not different between survivors and nonsurvivors, and there is no correlation between hemodynamics and circulating vasoactive substances. Impending life threat is associated with global energetic failure secondary to cellular oxygen deficits. Despite the severity of illness of patients in the ICU, physical therapy has a well defined role in this setting.3 More recently, the integration of physical therapy has been included in a quality control model of ICU care.4 Although treatment options may be more restricted in patients with complications and greater severity of illness, judiciously prescribed physical therapy can have an important role in reducing further complications, enhancing weaning from mechanical ventilation, and reducing ICU stay.5
Common Complications of Medical and Surgical Etiologies
In severe cases of heart and lung failure, extracorporeal life support with artificial heart and lung, commonly called extracorporeal membrane oxygenation (ECMO), may be indicated. Rather than being a treatment, ECMO is a temporary means of providing partial or total life support. ECMO stabilizes the patient by regulating gas exchange and perfusion and facilitates recovery from the primary problem.6 The major limitations to widespread applications are the need for anticoagulation and bleeding complications. Advances in ECMO have minimized the risk of bleeding and need for anticoagulants. They hold promise for prolonged extracorporeal circulation in patients with severe respiratory and cardiac failure.
Metabolic Dysfunction
Complications associated with respiratory failure that can further impair tissue oxygenation are described in Box 36-1. The metabolic consequences of these complications and impairment of oxygen transport are life-threatening for the patient. Thus prevention of their development is a priority. Should complications develop, however, early detection and definitive management become the priorities if the patient is to survive.
A hallmark of these complications is the impairment of multiple steps in the oxygen transport pathway, which adds to the complexity of management.7 The three major components of oxygen transport (i.e., oxygen delivery, consumption, and extraction) can be affected individually or in combination.8,9
In healthy individuals, the ratio of oxygen consumption to delivery is low (i.e., 23%, which ensures an oversupply of oxygen as a safety margin) (see Chapter 2). This safety margin also ensures that most patients are able to recover from insults to the oxygen transport system. If the insult is extreme, however, such as that resulting from complications of respiratory failure, surgery, acute lung injury and acute respiratory distress syndrome, shock, sepsis, and MOSF, metabolic dysfunction secondary to tissue hypoxia can result.
The relationship between oxygen consumption and delivery has elucidated our understanding of hemodynamic and metabolic changes observed in critical illness.10 The phenomenon of oxygen-delivery dependence of oxygen consumption occurs when a patient’s oxygen transport system is unable to supply sufficient oxygen to meet basal oxygen demand.11 Oxygen delivery below 300 mL/min/m2 limits the oxygen diffusion gradient and reduces oxygen extraction and usage at the cellular level. This is termed the critical level of oxygen delivery. When oxygen delivery exceeds 300 mL/min/m2, 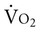 does not depend on delivery. Thus the greater the delivery in relation to
does not depend on delivery. Thus the greater the delivery in relation to 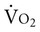 , the greater the safety margin. When oxygen transport is so severely compromised that oxygen delivery falls below the critical level, anaerobic metabolism is triggered. Anaerobic metabolism, however, may also be triggered at levels of oxygen delivery that exceed the normal critical threshold for anaerobic metabolism.12 This so-called “pathological dependence” of oxygen consumption on oxygen delivery occurs when the cells are inadequately extracting and using oxygen even in the presence of supranormal oxygen delivery levels. This phenomenon is observed in patients with ARDS and shock (discussed later this chapter).
, the greater the safety margin. When oxygen transport is so severely compromised that oxygen delivery falls below the critical level, anaerobic metabolism is triggered. Anaerobic metabolism, however, may also be triggered at levels of oxygen delivery that exceed the normal critical threshold for anaerobic metabolism.12 This so-called “pathological dependence” of oxygen consumption on oxygen delivery occurs when the cells are inadequately extracting and using oxygen even in the presence of supranormal oxygen delivery levels. This phenomenon is observed in patients with ARDS and shock (discussed later this chapter).
Given that physical therapy is one of the most metabolically demanding ICU interventions,13,14 the physical therapist needs to be able to calculate this safety margin to prescribe the type of treatment and its parameters (i.e., intensity, duration, and frequency) such that treatment is maximally beneficial and associated with the least risk to the patient.
The ultimate treatment outcome measures are markers of oxygen tissue metabolism.8,15,16 In addition, continuous assessment of oxygen delivery, consumption, and extraction provide the basis for directing management of oxygen transport deficits.
Pulmonary Dysfunction
Complications of the cardiovascular and pulmonary systems can lead to respiratory failure (see Box 36-1).17,18 Some of these, such as ventilator-associated pneumonia, relate to being mechanically ventilated. Certain technical problems related to the cuffs used in conjunction with artificial airways may occur (e.g., overinflation, distortion, and herniation of the orifice of the tube). Mucus plugs can occlude the endotracheal tube or tracheostomy and impede ventilation. The common complications can be reduced if the tube is changed frequently and if minimal amounts of air are used for cuff inflation.
Acid-Base Abnormalities
Any combination of acid-base imbalance may occur either acutely or chronically during respiratory failure. Severe alkalemia associated with potassium and chloride losses may occur after mechanical ventilation and can precipitate serious cardiovascular and neurological complications (see Chapter 16). Significantly impaired oxygen delivery to peripheral tissue may contribute to increased anaerobic metabolism and metabolic acidosis.12
Cardiac Dysfunction
When the heart fails to the point of cardiac output being compromised, intracardiac volumes and pressures can be monitored. Flow-directed pulmonary artery catheters (i.e., Swan-Ganz catheters) are commonly used in the ICU for monitoring patients who develop such hemodynamic complications. Although inserted through the venous side of the circulation through the right atrium and ventricle to lodge in a branch of the pulmonary artery, they provide useful measures and indices of right-sided and left-sided heart function and the adequacy of fluid resuscitation and pharmacological support. These catheters are also associated with some complications (see Chapter 16). Infection may lead to bacteremia and septicemia. Judicious selection and application of any invasive procedure is warranted to minimize undue hazard. The presence of these catheters limits head and neck positions and requires mobilization be carried out cautiously within the patient’s hemodynamic tolerance.
Thromboembolism
A high incidence of pulmonary thrombosis or embolism exists in patients in acute respiratory failure. Early diagnosis and management of pulmonary thromboembolism have been greatly facilitated by the use of serial ultrasound procedures and scans. Physical therapy has a key role in preventing the development of thromboemboli by promoting frequent changes in position; specific bed exercises, particularly of the lower limbs; and passive range-of-motion exercises if indicated. It is essential that movement and repositioning be performed regularly to maximize their cardiovascular and pulmonary protective benefits. Pneumatic extremity cuffs apply pressure intermittently over the lower legs to minimize venous pooling and assist venous return (see Chapter 33). Compression stockings also may be applied over the feet and legs to increase circulatory transit time in the dependent areas and reduce circulatory stasis.
Muscular and Neurological Dysfunction
Critical illness neuropathy and critical illness myopathy are serious complications of critical illness and are associated with metabolic disturbance during illness, paralysis, neuromuscular blockade, recumbency, and restricted mobility.19,20 Prevention is a primary goal and includes early detection. Detection of risk factors is an important component of the physical therapy assessment. Additional risk factors include ICU stays over 7 days, sepsis and systemic inflammatory response syndrome (SIRS), multiorgan system dysfunction, a high Acute Physiology and Chronic Health Evaluation III (APACHE III) score, the use of high-dose steroids, and patients who have had organ transplantation, patients with severe neurological or muscle disease, and those with hyperglycemia.21
Periodic nerve electrical stimulation has been one means of establishing nerve conduction integrity during an assault of critical illness requiring neuromuscular blockade and sedation. Medical management is focused on addressing its causes and reversing them. Because rehabilitation potential is threatened in the presence of critical illness neuropathy and critical illness myopathy, physical therapy goals focus not only on reducing the possibility of neuropathy and myopathy and their functional sequelae but also on facilitating weaning and improving clinical outcomes overall including functional independence.21
Renal Dysfunction
Lung and kidney dysfunction are closely related.22 The development of renal failure greatly compromises the chances of the patient’s survival. Renal failure can result from gastrointestinal bleeding, sepsis associated with shock, drug-induced nephrotoxicity, and hypotension. Urinary outputs are maintained with adequate fluid and diuretics, with care not to induce pulmonary edema. Dialysis may need to be instituted if more conservative management fails.23 If dialysis is anticipated, the physical therapist should review existing treatment goals to modify treatment accordingly.
Systemic Inflammatory Response Syndrome
Localized inflammation is a physiological protective response. Typically this response is controlled by the body at the site of injury.24 Loss of local control or an overly active response results in an exaggerated systemic response that is known as systemic inflammatory response syndrome (SIRS). Compensatory mechanisms and outcome (such as resolution, or potentially multiple organ dysfunction syndrome or death) depend on the balance of SIRS and the effectiveness of the compensatory mechanisms. The effectiveness of therapies to date remains equivocal.
Common Complications of Surgical Etiology
Respiratory failure in a patient postoperatively is usually associated with a low PaO2 and a high PaCO2. This situation is likely to be more common than generally appreciated. If the patient is in good general health and is free from underlying cardiovascular and pulmonary conditions, recovery is usually rapid. Otherwise, more severe complications and cardiovascular and pulmonary failure may result and progress to a life-threatening situation. The effects of surgery on oxygen transport and on the various organ systems are described in Chapter 23. Common perioperative complications and their causes are listed in Boxes 36-2 and 36-3. With severely reduced arterial oxygen content, hence oxygen delivery, oxygen extraction increases.25
Hypoxemia
The most common postoperative complication is hypoxemia secondary to alveolar hypoventilation, reduced functional residual capacity (FRC), airway closure, and postsurgical atelectasis.26,27 Adequate oxygenation, however, can be present despite hypoventilation when oxygen is being administered. The presence or absence of cyanosis may be an unreliable sign because peripheral cyanosis can occur despite adequate arterial PO2. Morbidity and mortality have been reported to be reduced in patients with severe respiratory failure and system involvement when supranormal levels of oxygen delivery are achieved.21,28
Principles of Physical Therapy Management
On the basis of patient assessment, arterial blood gases, fluid and electrolyte balance, hemodynamic status, and radiographs, a decision is made as to which treatments on the physiological hierarchy will optimize oxygen transport and what parameters will be used for each treatment. Positioning these patients upright and mobilizing them whenever possible will maximize FRC and reduce closing volumes and hence enhance gas exchange and oxygenation. What precludes mobilizing these patients even minimally is their lack of alertness, which must be explained. If the patient is unable to respond to treatment because of narcotics, for example, this can be discussed at rounds, and other medications should be considered so that the patient is able to cooperate more. Thus even extreme body positioning will achieve more favorable results.29–31
Endotracheal intubation and mechanical ventilation may be indicated if blood gases fail to improve with conservative management. The treatment priorities for the ventilated patient before and during weaning are presented in Chapter 33. Special attention in the postoperative patient is given to the pulmonary complications associated with diminished ability to move spontaneously, surgical pain, restrictions imposed by dressings and binders, and diminished ability to cooperate and to periodically hyperventilate the lungs.
Pain management is integral to the management of the surgical patient. Noninvasive and nonpharmacological pain control strategies need to be exploited for all surgical patients to augment or reduce the need for potent analgesics, especially narcotics. Chapter 30 describes some physical therapy pain-control strategies for surgical patients that can be applied with modification to the patient with surgical complications. Of these, use of electrotherapy modalities, such as transcutaneous electrical nerve stimulation, may be limited in the ICU because of electrical interference with monitoring devices.
Complications Resulting in Secondary Cardiovascular and Pulmonary Dysfunction
Acute Lung Injury and Acute Respiratory Distress Syndrome
Acute lung injury results from damage to the alveolar epithelium.32 The extent of the damage reflects damage to the type I and type II alveolar cells. Damage to the type I cells results in alveolar edema, atelectasis, and loss of lung compliance secondary to loss of structural integrity of the alveoli provided by the type I alveolar cells. Damage to the type II cells also contributes to atelectasis and loss of lung compliance, but the mechanism relates to impairment of the production of surfactant and pulmonary fluid that covers the alveolar epithelium.
Acute lung injury is characterized as a clinical spectrum of parenchymal cell dysfunction. Mild injury reflects predominantly endothelial cell dysfunction and noncardiogenic edema. Severe injury reflects a progression to both endothelial and epithelial cell dysfunction and ARDS. The clinical spectrum of acute lung injury and the clinical manifestations of mild and severe injury are shown in Figure 36-1. The clinical presentation of moderate injury falls between these two extremes.
The signs and symptoms of ARDS may take up to 48 hours to fully manifest. Survival rate has increased over the past decade from 50% to 80%.33 The explanation for this improvement is unclear; however, the shift to an integrated management approach may be responsible.34,35 Hypoxemia is a principal feature of the syndrome, resulting from ventilation-perfusion mismatch and from a right-to-left shunt, whereby fluid-filled alveoli are ineffectively ventilated.36 Hyperventilation and labored respiration can be expected in conjunction with hypoxemia. Oxygen therapy has little effect in the presence of shunting. Hypercapnia is not usually a major problem in the patient with ARDS.
Recent advances in the medical management of ARDS include the use of partial liquid ventilation. Although not widely adopted, this form of ventilation may be more effective in recruiting dependent alveoli than conventional mechanical ventilation and may augment the benefits of exogenous surfactant replacement, inhaled nitric oxide, and prone positioning.37 In addition to improving oxygenation and respiratory mechanics, partial liquid ventilation may reduce ventilator-induced lung damage.38
Over the past decade there has been greater interest in long-term outcomes of patients after a course of ICU care, with evidence to support poor long-term functional outcomes. Herridge and colleagues,39 for example, followed 109 survivors of ARDS at 3, 6, and 12 months after hospital discharge. The patient characteristics were mean age 45 (36 to 58 years); mean APACHE II score 23 (17 to 27); mean ventilator days, 21 (12 to 40); and mean ICU stay, 25 days (15 to 45). All patients reported poor function and attributed this to the loss of muscle bulk, proximal weakness, and fatigue. Only 49% reported working at 12 months. Thus survivors of ARDS may have persistent functional disability 1 year after discharge from the ICU. Furthermore, extrapulmonary conditions such as muscle wasting and weakness were common.
Principles of Physical Therapy Management
Initially, if the patient is sedated, body positioning is exploited to maintain blood gases, and optimal range of motion and skin care are the focus of management. In severe ARDS the patient usually requires a high degree of ventilatory support with high FiO2, and sedation. Severely affected patients may require neuromuscular blockade to reduce their oxygen demand and enable them to respond to ventilatory assistance more effectively. Handling and positioning patients on neuromuscular blockade requires particular care because these patients lack muscle tone to protect their muscles and joints. Rotating beds can be extremely beneficial for patients who are either too hemodynamically unstable or difficult to turn manually. These mechanical beds slowly rotate side to side through an arc, thus changing the patient’s body position continuously.40,41 When the effects of continuous axial rotation on a kinetic bed are compared with the effects in prone position, they have been shown to be comparable.42
The prone position can have immediate benefit in remediating hypoxemia in patients with ARDS,43,44 and oxygenation can be significantly improved over supine.45 This position and semirecumbent positions can augment the effects of mechanical ventilation.46 The prescriptive parameters of the prone position in patients with ARDS, however, need to be based on a careful analysis of the pros and cons for each patient, and no single position will be maximally beneficial all the time. There are other physiological benefits to shifting the patient’s position rather than maintaining a single position (see Chapter 20), provided that oxygenation is not compromised. With improvement of ARDS (e.g., less ventilator support, reduced FiO2, and satisfactory gases), the patient may be able to be mobilized with careful monitoring of vital signs, ECG, saturation, and subjective tolerance. Body positioning is initiated as soon as patients can tolerate it without significant desaturation. Special attention is given to body positioning to promote ventilation and perfusion matching and mucociliary transport and to minimize the effect of restriction of diaphragmatic and chest wall excursion. Some patients, for example, benefit from side-lying positions in which excursion of the inferior hemidiaphragm is favored. Other patients, however, seem to deteriorate from apparent restriction of the inferior lung in side-lying positions.
The underlying pathophysiology associated with ARDS is primarily related to respiratory mechanics, which could explain why improvements in oxygenation have not been associated with secretion clearance.47 Each patient’s condition and specific areas of lung involvement must be taken into consideration when a turning regimen is prescribed. The effect of the patient’s body position on blood gases helps to establish a suitable regimen on a rational basis. Optimal positioning can result in reduced supplemental oxygen needs.48 The sitting position optimizes lung capacity even when a patient is intubated and mechanically ventilated. The airway can dislodge, however, so caution must be observed and the artificial airway properly secured before any mobility activity. The use of a reclining chair at the bedside should be considered in the management of patients with acute lung injury. Theoretically, the potential function of all lung fields will be benefited with the lungs in a more upright position. Patients who are too unstable to tolerate upright positions and whose oxygenation is compromised in this position49 may respond favorably to extreme body positions and the prone position.50,51 The clinical benefits of the prone position in the management of the majority of patients with ARDS have been well documented over the past 20 years; thus physical therapists need to consider positioning these patients prone as a common practice barring contraindications.
Improvements in oxygenation in patients with acute lung injury and ARDS with the prone position, including prone abdomen free, have been well documented in adult and pediatric populations.33,52–55 The benefits can persist when the patient is returned to the supine position, and furthermore, the prone position does not adversely affect hemodynamics.56 The use of the prone position in the management of ARDS has been reported to be independently associated with survival.57 The literature is inconsistent, however, regarding whether patients respond differently depending on the underlying ARDS pathophysiology (pulmonary or nonpulmonary). Some investigators report no differences,58 whereas others have reported marked differences with respect to radiographic changes, respiratory mechanics, and time course of oxygenation.54 These differences may be explained by pathophysiological differences in the primary disease insult responsible for ARDS, stage and severity, and comorbidity.
The mechanisms for improved gas exchange in prone positions in these severely ill patients have been the subject of much interest. Hypotheses have included improved alveolar recruitment and lung volumes; homogeneous distributions of ventilation and perfusion59; increased static lung compliance60; and reduced compressive forces compared with the supine position.61,62 In the posterior lung fields in the prone position, in the areas where atelectasis, shunt, and ventilation-perfusion mismatch are most prevalent, transpulmonary pressure exceeds airway opening pressure without apparently compromising anterior lung fields.63 A decrease in PaCO2 with prone positioning predicts survival.64
Problems with positioning patients in prone have been reported to be rare. Studies have been focusing on the differences between patients who respond favorably and those who do not (i.e., responders versus nonresponders). Patients may respond better in the early stages in which pulmonary edema is present versus the later stages in the presence of pulmonary fibrosis.65 Alveolar recruitment procedures are more effective in improving PaO2 in the prone position, and lower PEEP levels are needed to sustain improved PaO2 compared with the supine position.66 In addition, positive inspiratory pressure, hence barotrauma, and FiO2 can be reduced.59 The effects of mechanical ventilation including PEEP,67 airway pressure release ventilation,68 and certain pharmacological agents (inhaled nitric oxide) may be augmented in the prone position.69 Other studies have reported a particular beneficial effect of the prone position. One study reported that the prone position rather than PEEP improved oxygenation.70 Another study reported that prone positioning improves oxygenation more than inhaled nitric oxide in patients with severe ARDS.71
Although considerable evidence supports the clinical effectiveness of the prone position to improve oxygenation, this position should be considered for the management of hypoxemia associated with other conditions as a means of simulating the normal effects of gravity on cardiovascular and pulmonary function. Favorable results have been reported in the management of hypoxemia associated with acute respiratory failure in acute myeloid leukemia,72 pulmonary hemorrhage,73 and subarachnoid hemorrhage.74
Few complications have been reported with the use of body positioning, and in particular the prone position. Normal precautions are taken to ensure that ventilator tubing and lines and leads are not compromised. Despite beneficial effects of oxygenation in patients with ARDS related to trauma,75 complications associated with prone positioning have been reported for such patients, including facial and chest wall skin necrosis, wound dehiscence, and cardiac arrest.76 Special precautions are necessary in this subgroup. Brachial plexopathy has also been reported when positioning patients prone in the ICU.19 In addition to frequent body position changes, the semiprone position may help to avoid the adverse effects of prone positioning in some patients.77
Shock
Failure of cellular function secondary to shock can result from a deficiency of substrate for energy production, a reduced ability to use the nutrients for energy production, or both. The pathophysiological mechanisms responsible include hypoperfusion of the tissues, hormonal and metabolic cellular changes, and the toxic effects of the metabolic changes. Collectively, these produce cellular damage. With hypoperfusion and decreased oxygen delivery and other nutrients, the production of adenosine triphosphate is reduced. The maintenance and repair of cell membranes is disrupted, resulting in swelling of the endoplasmic reticulum and eventually the mitochondria. Persisting cellular hypoxia contributes to rupture of the lysosomes, which releases enzymes that contribute to intracellular digestion and calcium deposition. Once the lysosomes have ruptured and intracellular digestion has been triggered, irreversible cell damage ensues, impairing oxygen extraction and uptake.9
The dependence between oxygen consumption and delivery is a marker for septic shock and thus provides justification for increasing oxygen delivery (see Chapter 2).78 The relationship of oxygen consumption and delivery has been used to evaluate patients who are critically ill with SIRS and predict metabolic stress on this basis.79
Septic shock is a serious condition; patients manifest hypotension despite intensive medical treatment. Persistent hypotension leads to decreased blood flow to vital organs and can result in acute MOSF. Poulsen and colleagues (2009)80 followed 174 patients in the ICU to evaluate the physical outcomes of survivors 1 year after septic shock. Survivors were interviewed about their physical function and socioeconomic status. Seventy-eight patients were invited to participate, and 70 replied. After 12 months, two thirds of the patients reported that they had not regained their preadmission physical status, and 81% attributed this to loss of muscle mass. Physical function is substantially reduced in survivors of septic shock 1 year after discharge; thus recovery of physical function warrants being identified as a long-term goal.
Sepsis and Multiorgan System Failure
Sepsis is the response to bacteremia or other byproducts of bacteria in the blood. The clinical features of sepsis include fever, tachycardia, tachypnea, and respiratory alkalemia. Metabolic abnormalities are also a common feature of sepsis. Sepsis is the most common predisposing factor contributing to MOSF, which typically involves failure of more than two organ systems.81,82 Table 36-1 shows the major organs affected and their clinical manifestations (i.e., pulmonary, gastrointestinal, hepatic, renal, cardiovascular, hematological, and central nervous systems). The cascade of pathophysiological features of MOSF is precipitated by multiple mediator systems. The release of these mediators impairs oxygen delivery and usage of oxygen by the cells. Inadequate tissue oxygenation may be a mechanism underlying MOSF. Thus, the supply of the major energy source to the cell, adenosine triphosphate, is reduced, which leads to structural and functional damage of the various organ systems. The mortality rate ranges from 60% to 80%.
Table 36-1
Presentation of Multiorgan System Failure
| Organ | Clinical Presentation | Syndrome |
| Lungs | Hypoxemia, lung compliance, diffuse infiltrates | Acute lung injury, ARDS |
| Kidneys | Creatine >2 mg/dL | |
| Urine output <500 mL/24 h | Oliguric ARF | |
| Urine output >500 mL/24 h | Nonoliguric ARF | |
| Liver | Bilirubin 2 mg/dL, SGOT and LDH | Jaundice |
| Intractable hyperglycemia or hypoglycemia | Hepatocyte failure | |
| Cholecystitis | Acalculous cholecystitis | |
| Gut | Upper gastrointestinal bleed | Stress ulceration |
| Coagulation | Thrombocytopenia, prolonged PT and PTT | Hypofibrinogenemia, DIC |
| Heart | Hypotension, CI | Heart failure |
| CNS | Response only to painful stimuli | Obtundation |
Modified from Kirby RR, Taylor RW, Civetta JM: Critical care, ed 2, Philadelphia, 1996, Lippincott Williams & Wilkins.
Sepsis causes damage to peripheral nerves and muscles as well as organs and is termed critical illness polyneuropathy (CIP).83 Although the pathogenesis is not understood, these effects must be detected early and addressed to avoid clinical manifestations including muscle weakness, prolonged recovery, and delayed weaning. The systemic inflammatory response and MOSF have been implicated, and neuromuscular blockers and steroids can exacerbate these manifestations. The use of steroids and muscle relaxants should be minimized. Stabilizing the underlying critical condition and avoiding sepsis are of major importance in preventing CIP.
General guidelines for current medical strategies for sepsis and septic shock have been documented.84 These include life support measures including fluid resuscitation, blood product administration, vasopressor and inotropic support, bicarbonate therapy, and hemodynamic stabilization. To control the sepsis process requires identification and management of the source of sepsis, as well as antibiotic and steroid administration. Sedation, analgesia, neuromuscular blockade, and glucose control are instituted to reduce excess metabolic demand. In the event of renal dysfunction, hemofiltration may be required, along with intermittent hemodialysis. The management of acute renal failure in patients who are hemodynamically unstable with fluid overloaded may include continuous renal replacement therapy (CRRT). CRRT is a dialysis treatment provided as a continuous 24-hour-per-day therapy. Intermittent dialysis treatments are treatments that are provided for brief intervals, usually every day or every 2 or 3 days as needed.
Principles of Physical Therapy Management
The patient with sepsis and MOSF, like the patient in shock, is gravely ill and unlikely to be able to cooperate with treatment (Figure 36-2). The principles of management are comparable to those for managing the patient in shock; however, oxygen delivery is likely to be consistently compromised in these patients. If oxygen delivery is critically low, 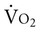 depends on oxygen delivery and the patient is in a state of metabolic acidosis (see Chapter 2). In this situation—when oxygen delivery is compromised to the point of not meeting tissue oxygen demands—the goal of treatment is to maximize oxygen delivery85 and minimize oxygen demand so that oxygenation of vital organs is threatened to the least extent. Thus the physical therapist must estimate the oxygen reserve capacity (i.e., the balance between oxygen demand and oxygen supply) in every assessment to select optimal treatment that is associated with the least risk. Treatments are selected to improve the efficiency of oxygen transport and usage and thereby reduce the work of the heart and of breathing. Above all, treatment should not worsen the patient’s oxygen transport status. Selective body positioning, including the prone position, can augment oxygen transport and maximize the effective FiO2.86 Even though the patient will likely benefit more from some positions than others, frequent body position changes, preferably a 360-degree turning regimen, are still necessary to avoid the sequelae of static body positioning. Semiprone positions can substitute well for full prone positions if the patient is too hemodynamically unstable. Semiprone positions may be tolerated better by the patient and may be safer. Even though hourly position changes may not be feasible in these severely ill patients, prolonged periods in a static position (more than 2 hours) are deleterious. Thus a balance between these two concerns must be achieved.
depends on oxygen delivery and the patient is in a state of metabolic acidosis (see Chapter 2). In this situation—when oxygen delivery is compromised to the point of not meeting tissue oxygen demands—the goal of treatment is to maximize oxygen delivery85 and minimize oxygen demand so that oxygenation of vital organs is threatened to the least extent. Thus the physical therapist must estimate the oxygen reserve capacity (i.e., the balance between oxygen demand and oxygen supply) in every assessment to select optimal treatment that is associated with the least risk. Treatments are selected to improve the efficiency of oxygen transport and usage and thereby reduce the work of the heart and of breathing. Above all, treatment should not worsen the patient’s oxygen transport status. Selective body positioning, including the prone position, can augment oxygen transport and maximize the effective FiO2.86 Even though the patient will likely benefit more from some positions than others, frequent body position changes, preferably a 360-degree turning regimen, are still necessary to avoid the sequelae of static body positioning. Semiprone positions can substitute well for full prone positions if the patient is too hemodynamically unstable. Semiprone positions may be tolerated better by the patient and may be safer. Even though hourly position changes may not be feasible in these severely ill patients, prolonged periods in a static position (more than 2 hours) are deleterious. Thus a balance between these two concerns must be achieved.
The assessment of neuromuscular function is challenging and inaccurate in patients who are severely ill. Patients may have marked weakness related to CIP and myopathy87,88 and from interventions themselves (e.g., resting of the respiratory muscles with mechanical ventilation; pharmacological agents including glucocorticoids, some antibiotics, and neuromuscular blockers) (Figure 36-3). Thus these interventions may be risk factors, and their use needs to be assessed.
















































































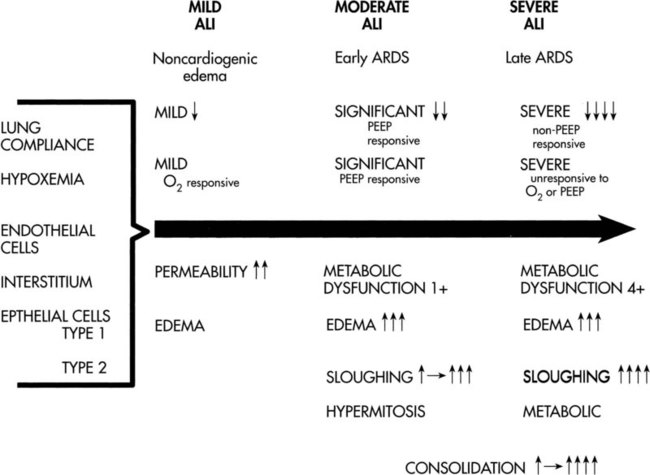
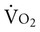 may become oxygen supply dependent; thus peripheral extraction may be increased.
may become oxygen supply dependent; thus peripheral extraction may be increased.