11 INTEGUMENTARY SYSTEM
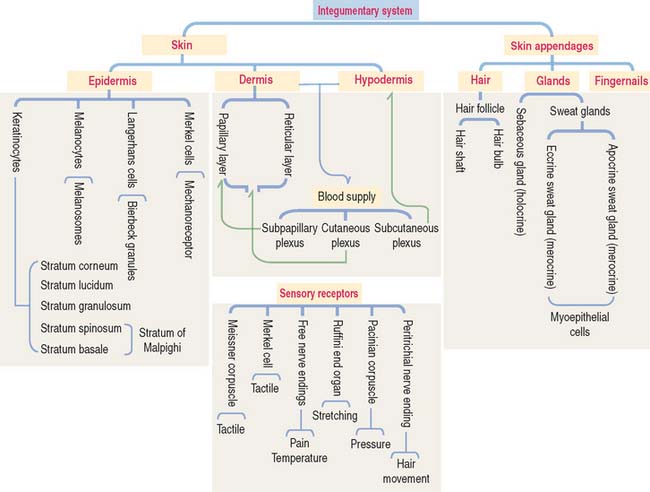
The integument is the largest organ of the body. It consists of two components: (1) the skin and (2) the epidermal derivatives, such as nails, hair, and glands (sweat and sebaceous glands and the mammary gland).
General organization and types of skin
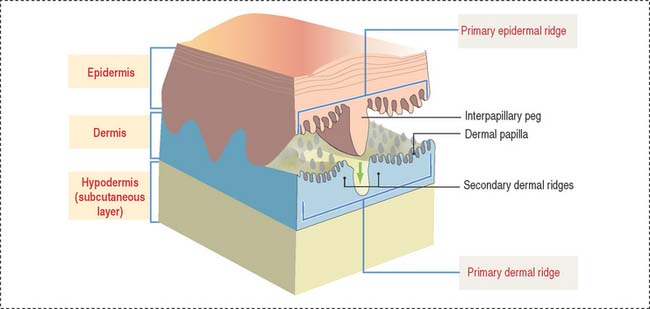
The skin consists of three layers firmly attached to one another (Figure 11-1): (1) the outer epidermis—derived from ectoderm; (2) the deeper dermis—derived from mesoderm; and (3) the hypodermis or subcutaneous layer—corresponding to the superficial fascia of gross anatomy.
Skin is generally classified into two types: (1) thick skin and (2) thin skin.
The epidermis and dermis display a tight fit interface at the dermal-epidermal junction, where a basal lamina and hemidesmosomes are located. A primary epidermal ridge interlocks with a subjacent primary dermal ridge (see Figure 11-1). An epidermal interpapillary peg, projecting downward from the primary epidermal ridge, interlocks with the primary dermal ridge, which is subdivided into two secondary dermal ridges. A number of dermal papillae project upward from the surface of each secondary dermal ridge into the epidermal region, interlocking with downward projections of the epidermis. This arrangement is predominant in hairless thick skin. Dermal papillae are numerous and branched. In thin skin, papillae are low and their number is reduced.
EPIDERMIS
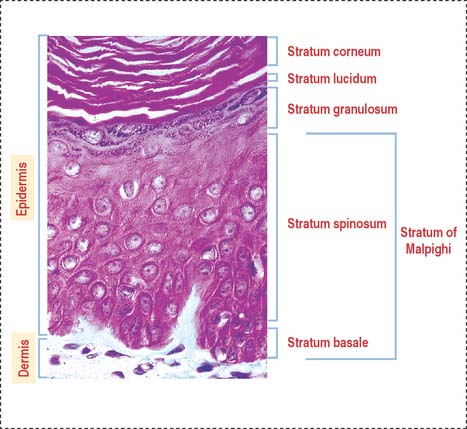
The stratified squamous epithelial layer of the epidermis consists of four distinct cell types (Figure 11-2):
Clinical significance: Wound healing
Wound healing starts with the formation of a blood clot covering temporarily the open wound. We discussed in Chapter 6, Blood and Hematopoiesis, that the blood clot consists of platelets embedded in a fibrous mesh of cross-linked fibrin molecules formed when thrombin cleaves fibrinogen.
We discussed in Chapter 6 that platelets contain platelet-derived growth factor (PDGF) stored in alpha granules. PDGF and other growth factors are released when platelets degranulate and leukocytes arrive at the wound site. Keratinocytes and endothelial cells express cytokine CXC (for cysteine-x-cysteine) and CXC receptor, which recruit neutrophils, monocytes, and lymphocytes to the wound site. A deletion of CXC receptor gene results in delayed wound healing.
Reepithelialization starts when keratinocytes of the stratum basale layer migrate from the edges of the wound by the formation of F-actin–containing lamellopodia. We discuss in Chapter 1, Epithelium, that hemidesmosomes anchor basal cells to the basal lamina. Leading edge keratinocytes facilitate their displacement by disrupting hemidesmosome attachment to the basal lamina and by dissolving the fibrin clot barrier. To accomplish the dissolution of the fibrin clot, keratinocytes up-regulate the expression of plasminogen activator to convert plasminogen within the clot into the fibrinolytic enzyme plasmin. Keratinocytes become free from hemidesmosome anchorage with the help of members of the matrix metalloproteinase family produced by fibroblasts in the dermis. We discussed the importance of matrix metalloproteinases in Chapter 4, Connective Tissue.
Within 3 to 4 days after the wound injury, the underlying connective tissue of the dermis contracts, bringing the wound margins toward one another. Stimulated by local levels of PDGF and transforming growth factor-β, dermal fibroblasts begin to proliferate, infiltrate the blood clot, and deposit type III collagen and extracellular matrix. About 1 week after wounding, a number of wound fibroblasts change into myofibroblasts (resembling smooth muscle cells), wound contraction takes place, and healing with a scar occurs.
Clinical significance: Psoriasis
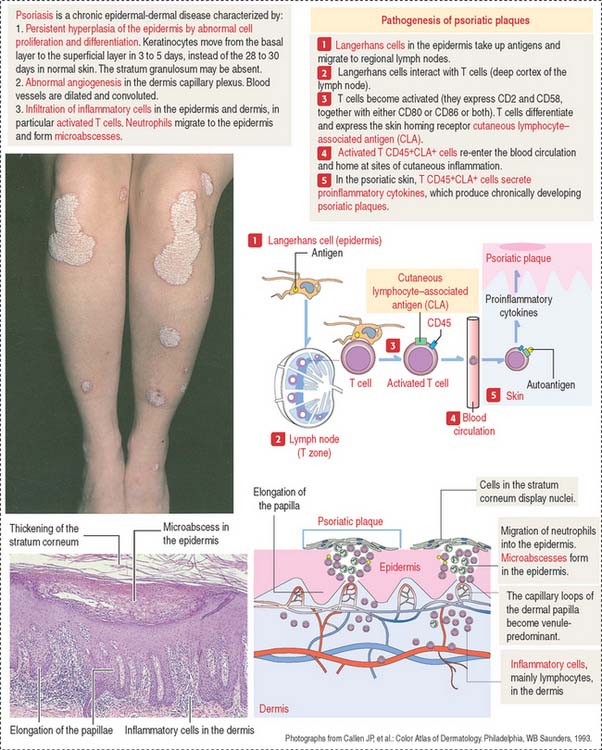
The histologic characteristics of the psoriatic plaque include excessive proliferation of epidermal keratinocytes (caused by an accelerated migration of keratinocytes from the stratum basale to the stratum corneum), presence of inflammatory cells (T cells and neutrophils) in the dermis and epidermis (microabscesses), elongation of epidermic papillae, and prominent angiogenesis (Figure 11-4).
Langerhans cells initiate the psoriatic process. The role of Langerhans cells in the activation of T cells in regional lymph nodes is summarized in Figure 11-4.
Differentiation of a keratinocyte
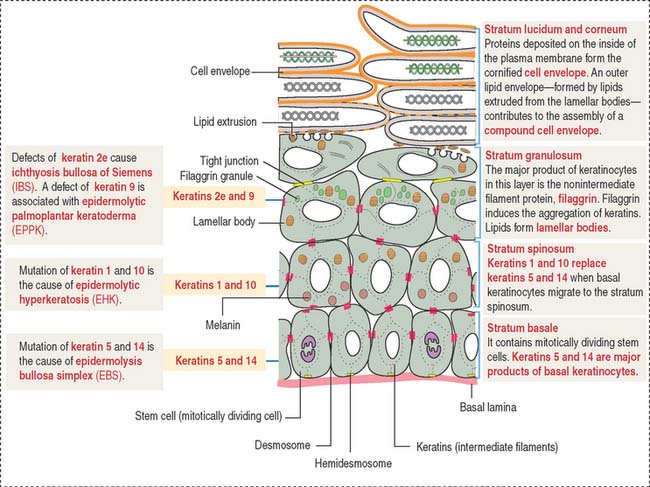
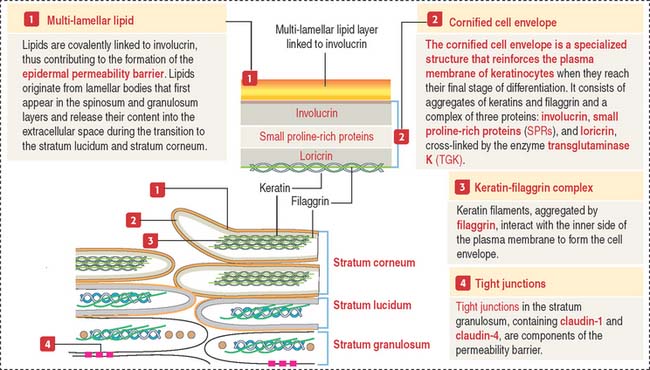
The stratum granulosum consists of a multilayered assembly of flattened nucleated keratinocytes with characteristic, irregularly shaped keratohyalin granules without a limiting membrane and associated with the tonofilaments. The lamellar bodies, which first appear in keratinocytes of the stratum spinosum, increase in number in the stratum granulosum, and the lamellar product, the glycolipid acylglucosylceramide, is released into the intercellular spaces (Figure 11-5). Tight junctions, containing claudin-1 and claudin-4, are found in the stratum granulosum (Figure 11-6). In the intercellular space, the lamellar lipid material forms a multilayered structure arranged in wide sheets, coating the surface of keratinocytes of the upper layer, the stratum lucidum. The glycolipid coating provides the water barrier of the epidermis.
Both the stratum lucidum and stratum corneum consist of several layers of keratinocytes without nuclei and a cytoplasm containing aggregated intermediate filaments of keratin cross-linked with filaggrin (see Figure 11-6) by a process catalyzed by transglutaminases. Filaggrin aggregates keratin intermediate filaments into tight bundles, leading to cell flattening, a characteristic of the stratum corneum.
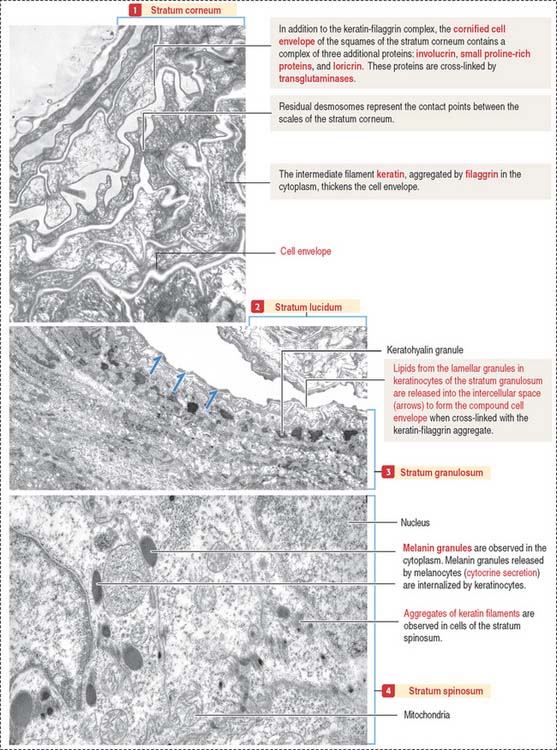
The keratin-filaggrin complex is deposited on the inside of the plasma membrane forming a structure called the cornified cell envelope (Figure 11-7). Additional proteins—involucrin, small proline–rich proteins (SPRs), and loricrin—are cross-linked by several transglutaminases and reinforce the cornified cell envelope just beneath the plasma membrane. On the outside of the cell, a complex of lipids extruded from lamellar bodies cross-link the cell envelope, forming the compound cornified cell envelope.
In summary, keratinocytes of the stratum corneum consist of a keratin-filaggrin matrix surrounded by a reinforcing involucrin–SPRs–loricrin complex that provides elasticity and mechanical resistance. Extracellular insoluble lipids, cross-linked to involucrin, make the cell membrane impermeable to fluids (permeability barrier). See Box 11-A.
Box 11-A Cornified cell envelope disorders
The terminally differentiated keratinocytes of the stratum corneum consist of flattened squames with a highly resistant compound cell envelope. Squames are sloughed from the surface of the epidermis and are continually replaced by keratinocytes of the inner strata.
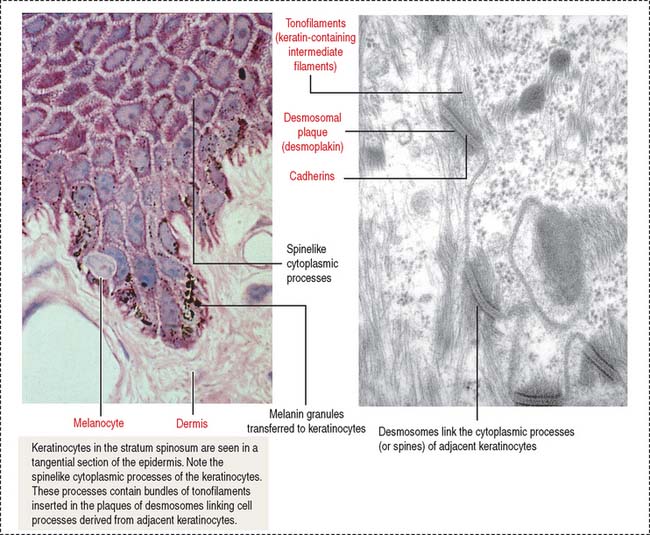
Two additional characteristics of the epidermis are (1) the cell layer–specific expression of keratins observed during differentiation of keratinocytes (see Figure 11-5) and (2) the presence of tight junctions and desmosomes in the epidermis. The maintenance of a three-dimensional lattice of tightly attached keratinocytes is essential for the protective nature of the permeability barrier.
In Chapter 1, Epithelium, we discussed the structure and components of tight junctions, desmosomes, and intermediate filament keratins, including pathologic conditions such as blistering, epidermolytic, and proliferative diseases (Box 11-B).
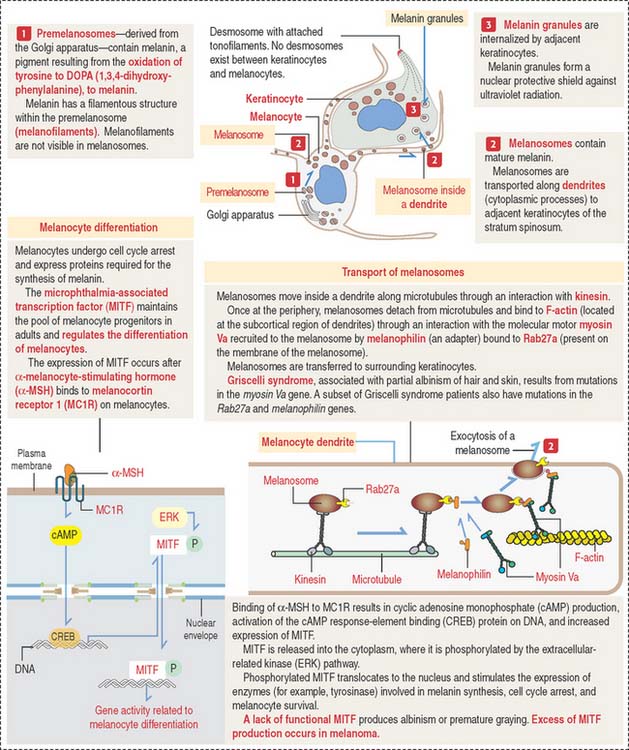
Melanocytes
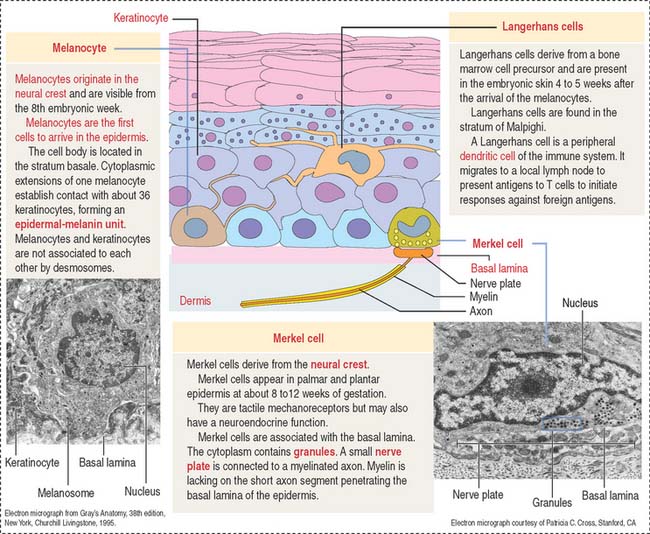
Melanocytes are branching cells located in the stratum basale of the epidermis (Figure 11-8; see Figure 11-3). Melanocytes derive from melanoblasts, a cell precursor migrating from the neural crest.
Melanocytes enter the developing epidermis and remain as independent cells without desmosome attachment to the differentiating keratinocytes. The turnover of melanocytes is slower than that of keratinocytes.
Melanocytes produce melanin, contained in melanosomes, which are transferred to neighboring keratinocytes through their branching cell processes, called melanocyte dendrites, and released by cytocrine secretion (Figure 11-9; Box 11-C).
Box 11-C Melanocyte differentiation
Melanin is initially stored in a membrane-bound premelanosome derived from the Golgi apparatus. Melanin is produced by oxidation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) by the enzyme tyrosinase. DOPA is then transformed to melanin, which accumulates in melanosomes, the mature melanin granules that are distributed along the melanocyte dendrites.
Cytocrine secretion is preceded by the transport of melanosomes along cytoplasmic microtubules by the motor protein kinesin. Melanosomes are then transferred to a network of F-actin tracks located beneath the plasma membrane. Melanosome transfer occurs when melanophilin, an adapter protein, binds to Rab27a, a protein inserted in the melanosome membrane. The F-actin–based molecular motor myosin Va binds to the Rab27a-melanophilin complex and transports the melanosome to the plasma membrane. Extruded melanin by exocytosis is captured by adjacent keratinocytes and internalized by endocytosis. The molecular characteristics of the unconventional myosin V are discussed in Chapter 1, Epithelium.
In addition to melanocytes, melanin-producing cells are present in the choroid plexus, retina, and ciliary body of the eye. Albinism results from the inability of cells to form melanin. Griscelli syndrome is determined by mutations of the myosin Va gene. Patients with Griscelli syndrome have silvery hair, partial albinism, occasional neurologic defects, and immunodeficiency (due to a defective vesicular transport and secretion in cytolytic T cells). Similar pigmentation disorders are determined by mutations in the Rab27a and melanophilin genes.
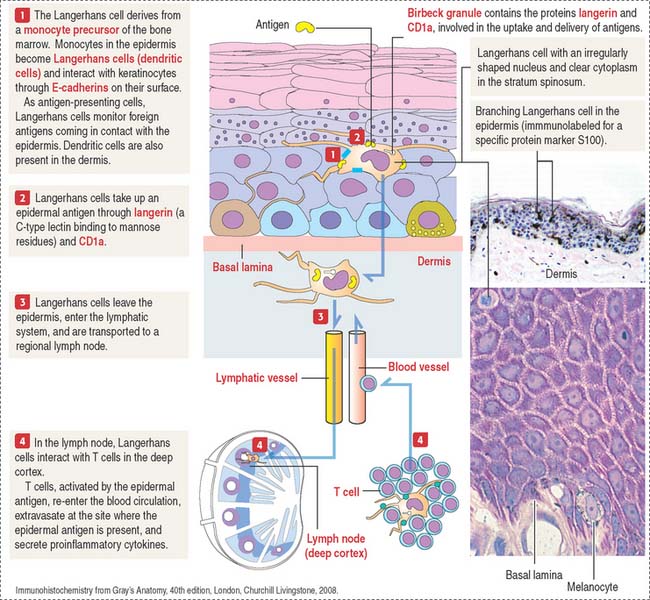
Langerhans cells (dendritic cells)
Langerhans cells are bone marrow–derived cells present in the epidermis as immunologic sentinels, involved in immune responses, in particular the presentation of antigens to T cells (Figure 11-10).
Langerhans cells use CD1a and langerin to trigger cellular immune responses to Mycobacterium leprae, the causative agent of leprosy, also known as Hansen’s disease, a neurologic disease affecting the extremities. Myelin-producing Schwann cells are the primary target. In the early stages, infected individuals have skin nodules on the face and all over the body, followed by paralysis or loss of sensation in the affected areas, and eventually loss of fingers and toes. Blindness occurs in advanced stages of the disease. Multidrug therapy, consisting of rifampicin, clofazimine, and dapsone, is used to treat all cases of leprosy.
Merkel cells
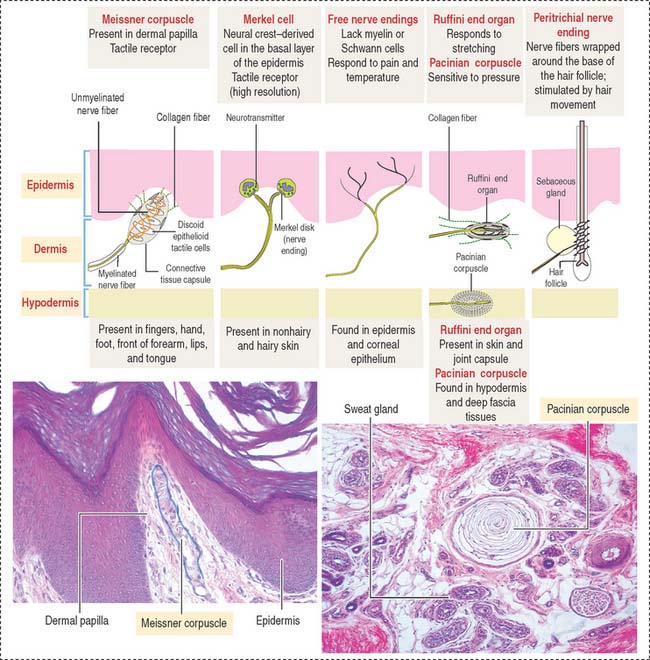
Merkel cells resemble modified keratinocytes, are found in the stratum basale, and are numerous in the fingertips. Merkel cells are mechanoreceptor cells linked to adjacent keratinocytes by desmosomes and in contact with an afferent myelinated nerve fiber projecting from the dermis into the epidermis. The nerve fiber becomes unmyelinated after passing through the basal lamina of the epidermis and expands into a platelike sensory ending, the nerve plate, in contact with the Merkel cell (see Figure 11-3).
DERMIS
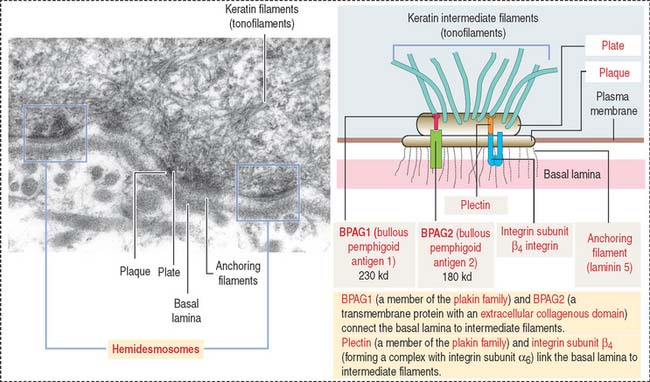
The dermis is formed by two layers without distinct boundaries: (1) the papillary layer, consisting of numerous papillae interdigitating with epidermal pegs forming the dermal-epidermal junction. The junctional interface is stabilized by hemidesmosomes anchoring basal keratinocyte cells to the basal lamina. Loose connective tissue (fibroblasts, collagen fibers, and thin elastic fibers) provides mechanical anchorage and nutrients to the overlying epidermis. (2) The reticular layer, containing thick bundles of collagen fibers and coarse elastic fibers. Hemidesmosomes on the basal domain of keratinocytes of the stratum basale attach the epidermis to the basement membrane and the papillary layer of the dermis by a plate/plaque–anchoring filament complex summarized in Figure 11-11. The molecular and structural components of the hemidesmosome are of considerable importance for understanding the cause of blistering diseases of the skin. We discussed in Chapter 1, Epithelium, the clinical significance of hemidesmosomes and intermediate filaments (see Figures 1-36 and 1-37, and Box 11-B).
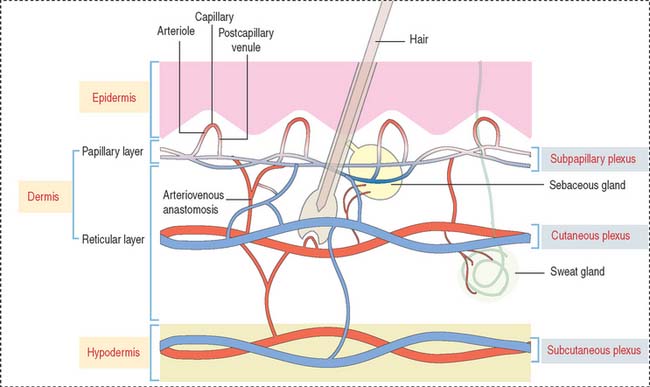
BLOOD AND LYMPHATIC SUPPLY
Three interconnected networks are recognized in the skin (Figure 11-12):
A special form of arteriovenous shunt occurring in the periphery is the glomus apparatus. The glomus consists of an endothelial-lined channel surrounded by cuboidal glomus cells and a rich nerve supply.
Clinical significance: Vascular diseases
Acute urticaria is a transient reaction caused by increased vascular permeability associated with edema in the dermis. In Chapter 4, Connective Tissue, we discussed the mechanism of degranulation of mast cells and release of histamine as determinants. Vasculitis includes a group of disorders in which there is inflammation of and damage to blood vessel walls. Most cases of cutaneous vasculitis affect small vessels, predominantly venules.
SENSORY RECEPTORS
Three categories of sensory receptors are present in the skin and other organs (Figure 11-13): (1) exteroceptors, (2) proprioceptors, and (3) interoceptors.
Mechanoreceptors respond to mechanical deformation of the tissue or the receptor itself (for example, stretch, vibration, pressure, and touch). The mechanoreceptors include both exteroceptors and proprioceptors.
Thermoreceptors respond to warmth or cold.
The second type of mechanoreceptor is the Merkel disk. The nerve ending of this receptor discriminates touch and forms a flattened discoid structure attached to the Merkel cell found in the stratum basale of the epidermis.
SKIN APPENDAGES: HAIR
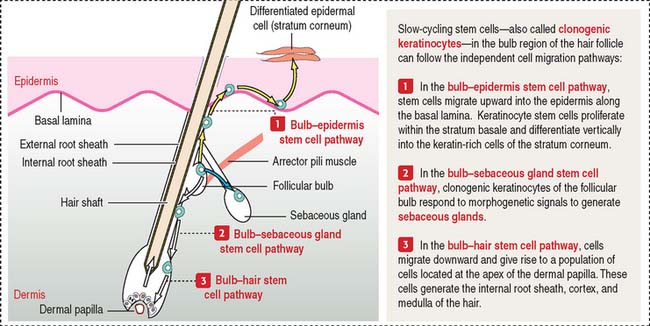
A bulbous swelling (called the follicular bulb) on the side of the hair germ contains stem cells—clonogenic keratinocytes—that can migrate and regenerate the hair shaft, the epidermis, and sebaceous glands (Figure 11-14) in response to morphogenetic signals.
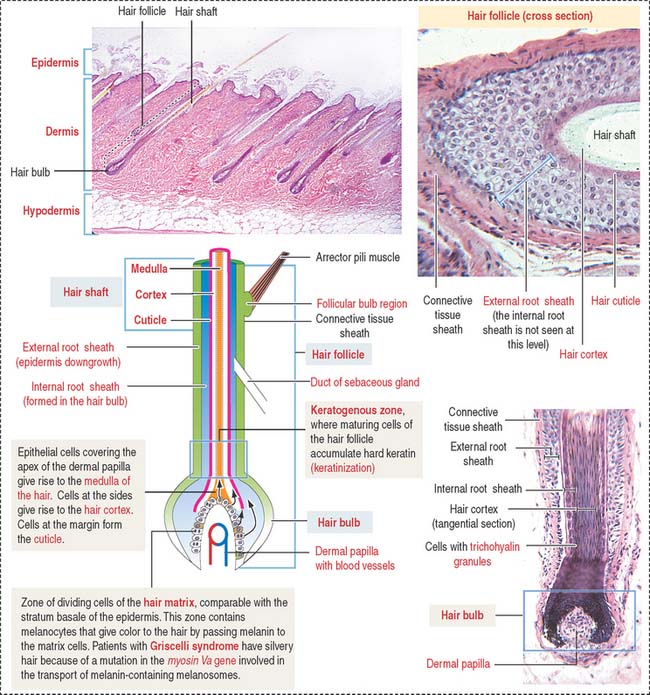
Each hair follicle consists of two parts (Figure 11-15): (1) the hair shaft and (2) the hair bulb.
The hair shaft is a filamentous keratinized structure present almost all over the body surface, except on the thick skin of the palms and soles, the sides of the fingers and toes, the nipples, and the glans penis and the clitoris, among others. A cross section of the hair shaft of thick hair reveals three concentric zones containing keratinized cells: (1) the cuticle, (2) the cortex, and (3) the medulla (the last is absent in thin hair). The hair shaft consists of hard keratin.
A structure that is not recognized in routine histologic sections of hairs is the peritrichial nerve endings wrapped around the base of the hair follicle. The nerve is stimulated by hair movement (see Figure 11-13).
Keratinocyte stem cells and the hair follicle
The epidermis is contiguous with the external root sheath of the hair follicle, a structure responsible for developing the hair shaft. When the epidermis is lost in severely burned patients, keratinocyte stem cells migrate upward from the follicular bulb to reestablish the epidermis by populating the highly proliferative and self-renewing cells of the stratum basale (see Figure 11-14). These stem cells can also give rise to hair follicles and sebaceous glands.
GLANDS
The glands of the skin are (1) the sebaceous glands (Figure 11-16), (2) the sweat glands (eccrine and apocrine sweat glands) (Figures 11-17 and 11-18), and (3) the mammary glands. The mammary gland is discussed in Chapter 23, Fertilization, Placentation, and Lactation.
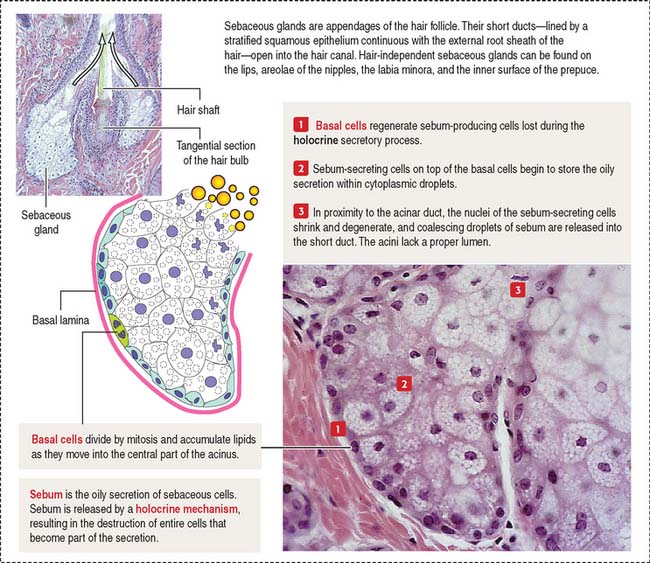
The sebaceous gland is a holocrine simple saccular gland extending over the entire skin except for the palms and soles. The secretory portion of the sebaceous gland lies in the dermis, and the excretory duct opens into the neck of the hair follicle. Sebaceous glands can be independent of the hairs and open directly on the surface of the skin of the lips, the corner of the mouth, the glans penis, the labia minora, and the mammary nipple.
Sweat glands
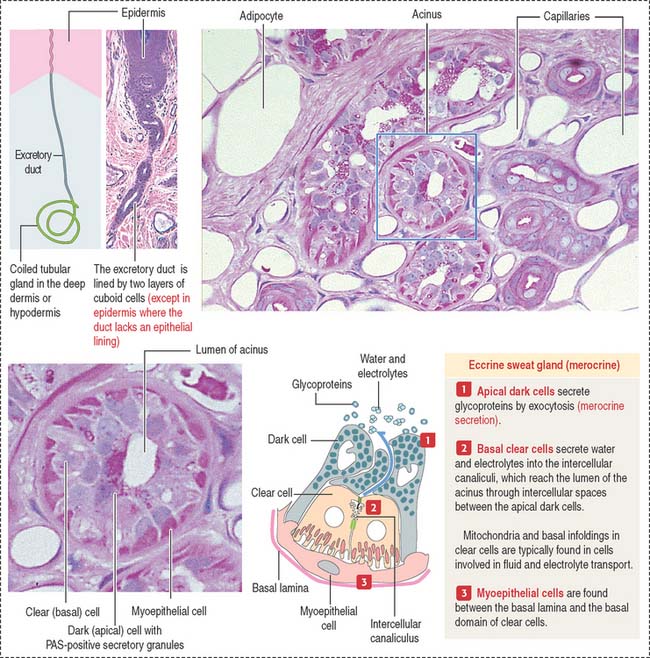
There are two types of sweat glands: (1) eccrine (merocrine) sweat glands (see Figure 11-17) and (2) apocrine sweat glands (see Figure 11-18).
The eccrine sweat gland is a simple coiled tubular gland with a role in the control of body temperature. Eccrine sweat glands are innervated by cholinergic nerves. The secretory portion of the eccrine sweat gland (see Figure 11-17) is a convoluted tube composed of three cell types: (1) clear cells, (2) dark cells, and (3) myoepithelial cells.
The dark cells rest on top of the clear cells. Dark cells secrete glycoproteins.
Myoepithelial cells are found between the basal lamina and the clear cells.
The excretory portion of the eccrine sweat gland is lined by a bilayer of cuboid cells that partially reabsorb NaCl and water under the influence of aldosterone. The reabsorption of NaCl by the excretory duct is deficient in patients with cystic fibrosis (see next section). The duct follows a helical path when it approaches the epidermis and opens on its surface at a sweat pore. Within the epidermis, the excretory duct loses its epithelial wall and is surrounded by keratinocytes.
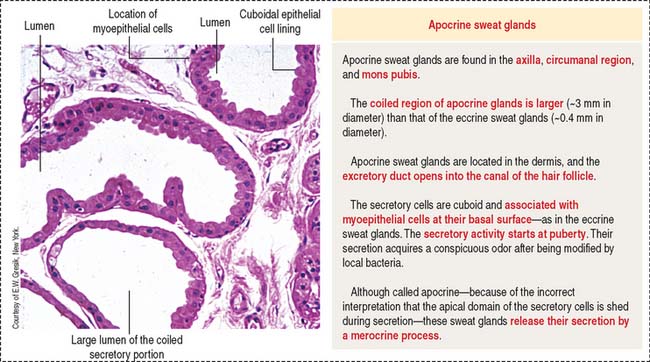
Apocrine sweat glands (see Figure 11-18) are coiled and occur in the axilla, mons pubis, and circumanal area. Apocrine sweat glands contain secretory acini larger than those in the eccrine sweat glands. The secretory portion is located in the dermis and hypodermis. The excretory duct opens into the hair follicle (instead of into the epidermis as in the eccrine sweat glands). Apocrine sweat glands are functional after puberty and are supplied by adrenergic nerves.
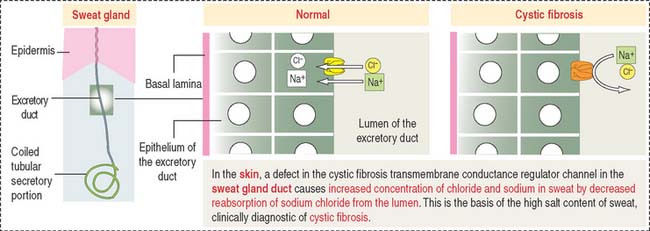
Clinical significance: Sweat glands and cystic fibrosis
The excretory ducts of sweat glands are lined by epithelial cells containing CFTR involved in the transport of Cl− (Figure 11-19). The CFTR channel opens when an agonist, such as acetylcholine, induces an increase in cyclic adenosine monophosphate (cAMP), followed by activation of protein kinase A, production of adenosine triphosphate (ATP) (see Chapter 3, Cell Signaling), and binding of ATP to two ATP-binding domains of CFTR.
In the respiratory epithelium (see Chapter 13, Respiratory System), a defect in CFTR results in a reduction or loss of chloride secretion into the airways, active reabsorption of sodium and water, and a consequent decrease in the water content of the protective mucus blanket. Dehydrated mucus causes defective mucociliary action and predisposes to recurrent pulmonary infections.
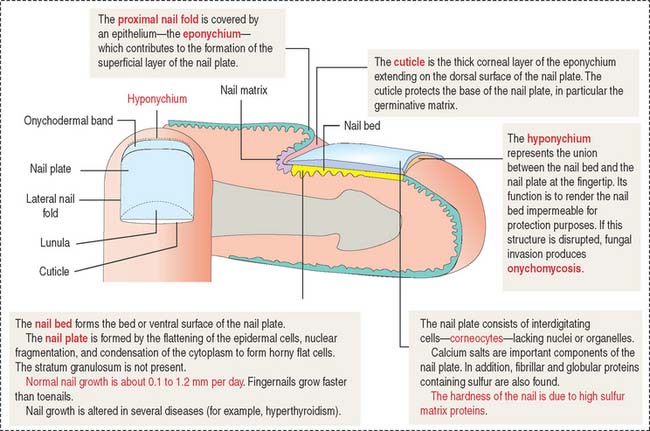
FINGERNAILS
The nails are hard keratin plates on the dorsal surfaces of the terminal phalanges of the fingers and toes (Figure 11-20). The nail plate covers the nail bed, the surface of the skin that consists of the stratum basale and stratum spinosum only.
The body of the plate is surrounded by lateral nail folds with a structure similar to that of the adjacent epidermis of the skin. When the lateral nail folds break down, an inflammatory process develops. This process is called onychocryptosis and is frequently observed in the nail of the first toe (ingrown nail).
Integumentary System
Based on the type of stimulus, sensory receptors can be classified as (1) mechanoreceptors (respond to mechanical stimulation from outside or inside the body; this group includes nerve free endings, the Merkel cell, the encapsulated Meissner corpuscle and pacinian corpuscle), and the peritrichial nerve endings of the hair; (2) thermoreceptors (respond to temperature changes); and (3) nociceptors (respond to pain).