Integrating technology into clinical practice in neurological rehabilitation*
KATIE BYL, PhD, NANCY N. BYL, PT, MPH, PhD, FAPTA, MARTEN BYL, PhD, BRADLEY W. STOCKERT, PT, PhD, SEBASTIAN SOVERO, MS, CLAYTON D. GABLE, PT, PhD and DARCY A. UMPHRED, PT, PhD, FAPTA
After reading this chapter the student or therapist will be able to:
1. Summarize the need, demand and principles for integrating advanced robotic technology in neurological rehabilitation.
2. Define common terminology used in the field of rehabilitation robotics and technology.
3. Classify the different types of advanced technology used in neurorehabilitation.
a. Rehabilitation robots and assistive technology including:
i. Service robots for movement
ii. Service robots for physical assistance and indoor and outdoor navigation
iii. Nonwearable robotic assistive device for mobility, unweighting, and object manipulation
iv. Wearable robotic assistive device for upper-limb object manipulation
v. Wearable robotic assistive device for lower-limb mobility and gait training
vi. Communication robotics to enable interpersonal interaction
vii. Interactive entertainment robotics for companionship and emotional support
b. Advanced clinical technology including:
i. Virtual reality training systems for improved neural recovery of upper- and lower-limb function
ii. Computerized learning-based gaming systems for home training of individuals with physical disabilities and memory impairments
iii. Computerized patient simulators for teaching clinical diagnoses and intervention strategies to medical professionals
iv. Computer technology for teaching home exercise programs to patients
4. Use the guidelines for integrating robotics and assistive technology into a patient’s rehabilitation program.
5. Summarize the challenges and basic engineering principles involved in creating rehabilitation robotics and interfacing with advanced technology to help individuals to design:
a. Robots that operate independently
b. Controllers, actuators, and sensors required for service and assistive rehabilitation robots
c. Human interfaces (physical, sensory physical, cognitive, and brain machine)
d. User-friendly interfaces and controllers to maximize kinematics (e.g., force, velocity, timing)
e. Rehabilitation robotics based on the materials and control technology currently available
f. Safe robotics for rehabilitation
6. Discuss the benefits of performing a cost-effectiveness analysis when considering the application of robotic technology in rehabilitation.
7. Describe the challenges of commercializing robotic devices.
8. Discuss the future of advanced technology and rehabilitation.
Introduction to the application of robotics and technology in rehabilitation
General overview
The objective of rehabilitation technology is to empower clinicians and individuals to take responsibility and control of the environment, facilitate physical and cognitive recovery, and comply with learning-based practice to drive neural adaptation and neural reorganization. The principles underlying technology and rehabilitation are summarized in Box 38-1. Since the early 1990s, medical science has been able to minimize damage to the nervous system postinjury. It is known that the central nervous system (CNS) possesses the potential for spontaneous healing and recovery. Learning-based sensory and motor training can be used to drive recovery of function. Rehabilitation robotics are a logical addition to supervised, one-on-one therapeutic interventions.1–9
Robotic technology can provide service, unweighting, passive assistance, active assistance, variable and on-demand assistance, or a combination of service and assistance.10 Computerized and robotic technology provides the foundation for patients to practice and attend to purposeful, goal-oriented, progressive tasks spaced over time. This technology can also minimize the risk of injury during retraining. Robotic interfaces, actuators, and controllers can convert sensory, physical, and cognitive signals to control robots, permit perception of spatial relationships, mobilize individuals in space, assist in object manipulation, provide emotional support, and allow individuals to call for help and communicate with others. In addition, through creative virtual training environments and gaming technology, patients can improve memory, motor skills, and movement quality. In addition, patient simulators can help medical professionals learn diagnostic processes, treatment interventions, and manual techniques. Computer-assisted technology can also improve our ability to teach home exercise programs to patients. Over the next 10 years, robotic technology will expand the opportunities for clinicians to assist patients to achieve maximum independence and quality of life with less dependence on others.
History supporting the use of technology in neurological rehabilitation
The idea of interfacing technology with rehabilitation was introduced into practice by George J. Kelin in the 1940s. Kelin was a productive inventor from Canada who invented the power wheelchair for patients with quadriplegia, the microsurgical staple gun, and a wide range of industrial gearing systems. He also contributed to internationally important innovations in aviation and space technology. During the early 1970s, a new field emerged known as mechatronics, which combines mechanical, electrical, and control engineering design principles to produce a diverse range of useful practical devices.11,12 The science of biomechatronics then developed as a unique engineering discipline responsible for integrating neuromusculoskeletal appliances with biological systems to control and facilitate human-machine interactions as well as developing interfaces, sensors, actuators, and energy supplies to create functional devices for human use.13
The first conference on rehabilitation robotics was held in 1990. There are now multiple conferences each year on rehabilitation robotics. In 1999 the Robotics and Automation Society created the Rehabilitation Robotics Technical Committee to improve definitions and understanding about rehabilitation and assistive robotics.14 The scope of this technical committee has been recently specified as rehabilitation and assistive robotics. This modification is the direct outcome of the scientific progress and maturity reached in this broad research area. The goal of rehabilitation robotics is to investigate the application of robotics to therapeutic procedures for achieving the best possible motor, cognitive, and functional recovery for persons with impairments associated with aging, disease, or trauma (e.g., stroke, neuromotor disorders, brain trauma, orthopedic trauma, cognitive disease).
Some clinicians have been skeptical of robotics in rehabilitation. Some health care providers worry that robots will replace therapists; others worry that robots are unsafe.8 However, researchers have persisted in developing innovative hardware, new control strategies, improved compliance, and feed-forward and adaptive control systems, as well as computerized modeling. In addition, new assistive, wearable robotic arm devices have been developed (e.g., MIT-Manus, the MIME, the ARM, and the iARM) to more carefully outline and address the engineering challenges related to what the robot can do, the logical physical targets for active assistance, and the joints and the types of movements that can safely be assisted.
The field of rehabilitation robotics is still considered to be in its infancy. However, with the increasing demand for effective rehabilitative strategies, many new and exciting innovations are being developed. There are many robotic systems in various stages of research and development, but only a few are commercially available. Improvements in engineering, materials, human physical interfaces, software, and robotic designs will require constant analysis and adjustment in the future. It is projected that the market for personal robotic devices will be worth $15 billion by the year 2015.15,16 The challenges of robotic engineering are broad. Clinicians will need to participate in research to help document cost-effective outcomes as well as to develop efficient screening criteria to match patient needs with available robotic devices. One of these challenges will be to bridge the gap between the mechanical attributes of robotic sensors, actuators, controls, microprocessors, force, velocity, friction, unweighting, pressure tolerance, software design, and flexibility with the human limb, brain, and nervous system. Important issues related to safety, materials, technology, and the quality of matching machine and human movements must constantly be considered. These engineering issues are discussed later in this chapter.
Classification of rehabilitation robots
General principles
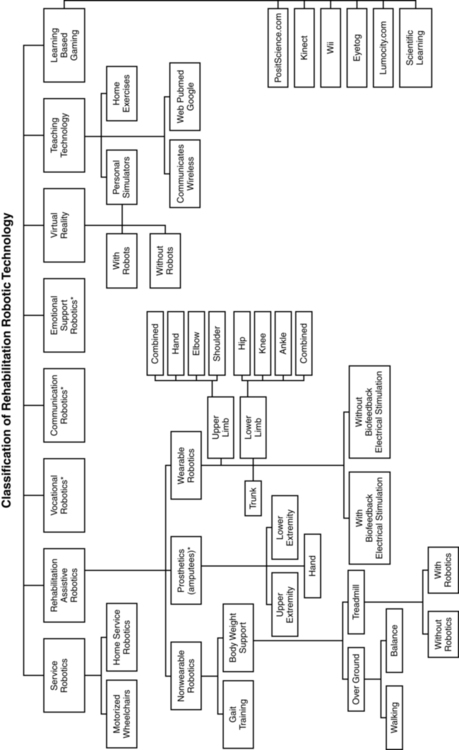
There is a variety of ways to classify computerized technology for rehabilitation. For this chapter, we will group robotic technology first in terms of how robotics are used with or by the client relative to rehabilitation. This classification system is summarized in Figure 38-1 and Box 38-2. Rehabilitation technology can be further classified by a variety of variables summarized in Box 38-3. Rehabilitation robotics can also be classified by type of interface used. Some classification systems classify technology by multiple parameters.
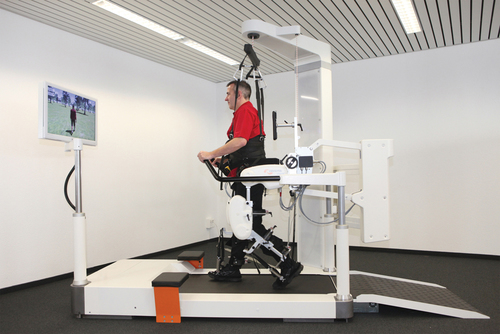
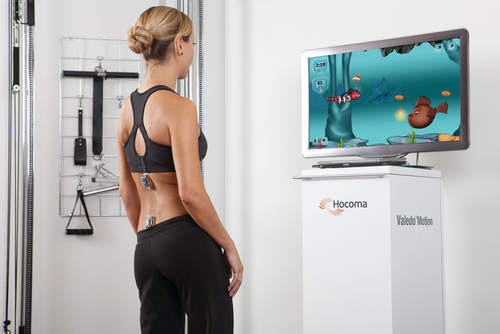
Service robots usually focus on task performance, movement assistance, and stability. These devices can be fixed, can be movable, or can be attached to a wheelchair (Box 38-4). Assistive robotic devices help patients perform a task with direct or indirect assistance. Some of the assistive robotics are nonwearable but assist through unweighting or movement assistance (Box 38-5). Wearable robotics are specifically designed to be worn by patients to assist movements. These are designed for the upper or lower limb (Box 38-6). There are some new assistive training devices for the spine such as the Valedo Shape, Valedo Motion, and Hocoma devices (Figure 38-2). Prosthetic devices help patients maintain function despite the loss of a limb. Vocational robotics can enhance performance at work either in terms of repetitive motions or high-force task production that would otherwise be dangerous to humans. Communication robotic devices are designed to improve communication potential for subjects who cannot adequately speak or hear. Emotional support robotics are designed to provide emotional support for isolated individuals at home.
VR training technology (with and without robotics) provides the opportunity to simulate simple and complex environmental and clinical situations to facilitate learning (Box 38-7). Game-oriented computerized learning systems are currently popular for fun and recreation, but they can also facilitate memory as well as sensory and motor skill development. Finally, computerized technology can also enhance teaching home exercises to patients.
In this chapter, we will not address prosthetics for amputees, vocational robotics, communication robotics, emotional support robotics, or socially assistive devices,17,18 as these areas are considered specialty oriented and may or may not be included in traditional neurorehabilitation programs coordinated by physical or occupational therapists. However, information about the impact of the sound of the robot voice on patient motivation and compliance may be relevant to effectiveness. It is also important to acknowledge there are a number of motorized chairs, lifts, and walkers available that can be used to transition a patient from sitting to standing, or provide unweighting while walking or working on balance. Examples can be found in Box 38-8. Many of these systems are electromechanical systems controlled by the patient or the therapist. These devices are not usually programmable and are not classified as “rehabilitation robotics” or “advanced technology.” However, these types of devices are very beneficial for helping patients maintain walking and training to improve safety and quality of gait at home and with supervision. It is important for therapists to be sure these types of assistive devices have been integrated into a patient’s rehabilitation program and at home before recommending more sophisticated technology.
Description of robotic systems by type
Service robotic systems that provide movement assistance
Service robotics assist individuals with severe disabilities. Most commonly, the robot performs everyday activities (e.g., assisting with eating, drinking, object replacing, ambulating). There are three main types of schemes: desktop-mounted robots, wheelchair-mounted robots, and mobile autonomous robots. In general, these robots are used in the home, are interconnected to a variety of control systems, and are programmed to the environment and consequently are not very portable.19–21
Several examples of service robotics are described in Box 38-9.22–30 A major issue is patient control options for service robotic devices. For example, through the use of headpieces on robotic devices, information can be detected from flexion and extension, rotation, and side bending of the head to operate wheelchairs, TV sets, telephones, doors, and security systems. There are also some new interfaces that are sensitive to facial movements and optoelectronic detection of light-reflective head movements.31 Other interfaces are sensitive to eye movements or use voice recognition, brain control,32,33 and gesture recognition.34 These interfaces not only may allow control of the robot but also may be applied to move a limb or perform a task.
Assistive robotics
Nonwearable assistive robotic devices.
Types of nonwearable assistive robotic devices.
There is a variety of nonwearable assistive robotic devices. Some of these nonwearable assistive robotic devices are summarized in Box 38-10.35–43 This group of robotic devices primarily includes powered wheelchairs with autonomous intelligence, body-weight–supported mobile walking aids, robots for body support with indoor and outdoor navigation, hands-off service robotic devices, and body-weight–supported treadmill systems (BWSTSs) with and without robotics.37,41–45
Purpose of nonwearable assistive robotic devices.
Nonwearable assistive robotic devices were designed primarily to facilitate mobility and gait training. More specifically, body-weight–supported gait training systems were initially designed to unweight the body, decrease ground reaction forces (GRFs), and protect against falling (Figure 38-3). Under these assisted conditions, it is easier for patients to achieve intense levels of exercise such as walking, skipping, and running with less pain and less trauma to the joints.46 These systems also allow runners to exceed the speed of overground running while being protected from falling.47 Unweighting also allows patients to increase their heart rate more slowly while running or jogging at a higher speed.47 For patients with spinal cord injuries, body unweighting over a treadmill was a translation of basic science findings to clinical practice. In animal studies, walking ability could be restored after induced spinal cord injury by facilitating the spinal generator for stepping through movement of the treadmill belt.48–50 Through unweighting, it is also possible to maintain metabolic activity while allowing healing and recovery after joint inflammation, muscle injuries, degenerative joint conditions, bone fractures, surgical repairs, joint replacements, osteoporosis, stroke, or head trauma. Most of the research on bone density is based on studies involving patients with spinal cord injuries. In these studies, walking on a BWSTS does not significantly increase bone density. However, those who walk on the BWSTS lose less bone density than those who do not exercise on the treadmill.51,52 On the other hand, although body-weight–supported treadmill training (BWSTT) may not necessarily increase bone density, particularly in patients with spinal cord injuries, exercise (with or without BWSTT) can increase muscle mass and/or prevent atrophy. On the other hand, in one study the use of shorter, frequent mechanical loading sessions was correlated with enhanced bone mass.53 More research is needed to determine the time and frequency of standing and walking exercises and their beneficial effects on bone density.54
Key features of unweighting.
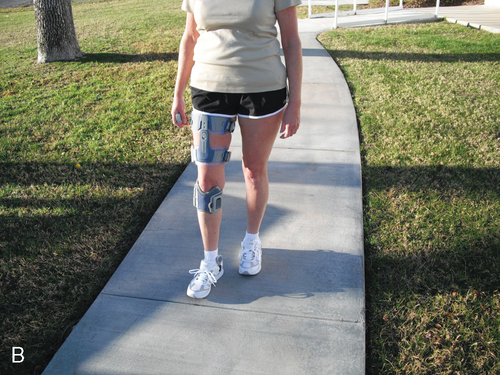
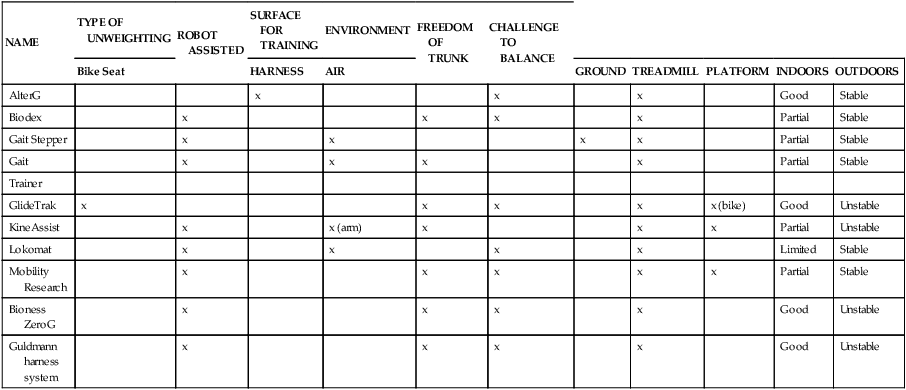
A key feature of BWSTT is the degree, comfort, and convenience of adjustable support.55 Whereas harness systems are the most commonly used for unweighting, when high levels of body support are needed or patients are jogging, these harnesses can be uncomfortable and cause pressure chaffing. This has led to the development of new body-weight–support systems such as the AlterG trainer (Figure 38-4), which uses a lower-body air distribution system, or the GlideTrak and Glide Cycle, which use a suspended bicycle seat–type unweighting system (Figure 38-5). The movement of the treadmill support surface provides an automatic stretch stimulus for the individual to step. In addition, the speed can be modified to stimulate faster or slower lower-extremity movements, which is very beneficial for individuals who need to vary the rate and responsiveness of their motor movements, such as clients with Parkinson disease, aging adults, or individuals with balance impairments. See Table 38-1 for a summary of the characteristics of some of the current unweighting systems.
TABLE 38-1 
NONWEARABLE ASSISTIVE UNWEIGHTING GAIT TRAINING SYSTEMS
| NAME | TYPE OF UNWEIGHTING | ROBOT ASSISTED | SURFACE FOR TRAINING | ENVIRONMENT | FREEDOM OF TRUNK | CHALLENGE TO BALANCE | |||||
| Bike Seat | HARNESS | AIR | GROUND | TREADMILL | PLATFORM | INDOORS | OUTDOORS | ||||
| AlterG | x | x | x | Good | Stable | ||||||
| Biodex | x | x | x | x | Partial | Stable | |||||
| Gait Stepper | x | x | x | x | Partial | Stable | |||||
| Gait | x | x | x | x | Partial | Stable | |||||
| Trainer | |||||||||||
| GlideTrak | x | x | x | x | x (bike) | Good | Unstable | ||||
| KineAssist | x | x (arm) | x | x | x | Partial | Unstable | ||||
| Lokomat | x | x | x | x | Limited | Stable | |||||
| Mobility Research | x | x | x | x | x | Partial | Stable | ||||
| Bioness ZeroG | x | x | x | x | Good | Unstable | |||||
| Guldmann harness system | x | x | x | x | Good | Unstable |
Effectiveness of body-unweighting treadmill training.
Various biomechanical studies have been done to confirm the parameters of unweighting. After use of early lower-body positive pressure support (LBPPS) prototypes, Grabowski46,47 reported that GRFs were reduced while metabolic power demands were maintained during running. Individuals could achieve faster running speeds when under conditions of unweighting. With slow-speed running on the LBPPS (1.0 to 1.5 mph), it is possible to reduce GRFs by 50% when the body is unweighted to 27% to 48% of normal body weight. At faster speeds (3 to 5 m/s; 6.6 to 11 mph), individuals must be unweighted to 25% of their body weight to maintain a GRF similar to that during walking over ground.
During progressive levels of unweighting, Ruckstuhl55 compared cardiorespiratory performance, gait parameters, and comfort between a laboratory prototype device similar to the LBPPS AlterG trainer and a traditional harness BWSTS. Subjects reported significantly greater comfort using the AlterG trainer and greater tolerance for high unloading. Subjects had significantly lower heart rates when training on the LBPPS versus the harness-based body support system. Other clinical studies have been carried out to confirm the benefits of body unweighting for patients with neurological problems (Box 38-11).
The outcomes studies on BWSTT (in patients after stroke, after spinal cord injury, and with Parkinson disease) have generally positive findings. BWSTT enhances gait speed, endurance, and potentially quality.56–58 It is important to force patients to walk at community-level velocities of at least 0.8 m/s (faster than 2.0 mph).59–63 With forced intensity, patients are more likely to achieve physiological and possibly neuroprotective benefits.64,65 For those who make the greatest gains, the improvement has been noted as increased single-limb support, particularly on the most affected limb of individuals after stroke.66 Although there are positive mobility benefits of BWSTT for patients with spinal cord injuries,58,59,67–73 guidelines still have not been generated regarding the best parameters for training (e.g., speed, amount of unweighting, time of intervention). Furthermore, one randomized clinical trial found that intense task-specific gait training over ground was equally as effective as intense body-weight–supported gait training for patients after stroke and after spinal cord injury.74 In another study, BWSTT produced similar gains in mobility compared with locomotor training at home.56 However, there were more falls in those patients who were started on locomotor training early rather than late. In addition, although the outcomes of improved gait performance are similar for BWSTT and aggressive bracing-assisted walking over ground for patients poststroke, the patients with the most severe impairments made the greatest gains with BWSTT.75 Other researchers have reported statistically significant gains in balance, balance confidence, and quality of life after chronic poststroke patients were trained on a BWSTS. However, over the long term the gains were not necessarily considered to meet the criteria for minimal detectable change.76 It appears that the gains in speed after BWSTT should be 0.16 m/s to achieve a minimal clinically important difference.77 Furthermore, there may be additional training activities that can be done to increase the effectiveness of BWSTT, such as VR training,78 electrical stimulation,79 constraint-induced therapy and/or robotics,70 or dual-task learning-based training.80
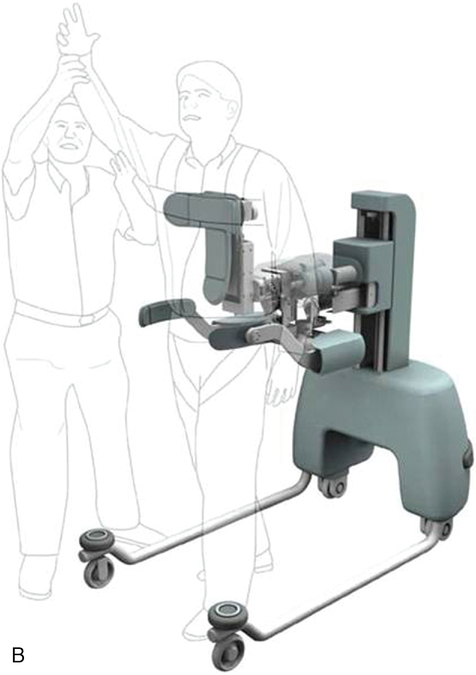
One BWSTS device allows the individual to walk overground while having one arm attached to the apparatus. The KineAssist gait trainer–destabilizer is an example of this type of body-supported gait trainer (Figure 38-6). Its primary function is to retrain balance to prevent falling. It is considered a BWSTS because the attached arm perturbs the individual while walking and can also provide some support. It perturbs the individual to fall and then catches the individual before he or she falls to the ground.40
Key elements of wearable assistive robotic devices.
Two major issues in wearable robotic devices are safety and dependability. A third major issue is the quality of the interface with the patient and the control of the assistive robotic device. In designing a wearable robotic device, biological principles must be followed. It is important for the robot to be adaptable with minimal weight, muscle fatigue, and energy consumption. In addition, there should be minimal damage imposed on the tissues while the biological systems have high functionality. The extremities can be used to perform multiple tasks, and the redundancy of the joints and degrees of freedom (shoulder, elbow, and wrist) allow human subjects to perform tasks in a variety of ways, creating high functionality. The robot must be adaptable, have minimal weight, be slim, create minimal muscle fatigue, conserve energy, and be easy to control. In addition, there should be minimal damage imposed on the tissues while the biological systems have high functionality. Consistent with biomechanical function of the upper limb, the upper limb must be able to perform multiple tasks with redundancy of the joints and degrees of freedom from the shoulder to the elbow to the wrist. This redundancy, integrated within different environments, provides the opportunity for variation in movement, high functionality, and minimum energy costs.81
Classification of wearable assistive robotic devices.
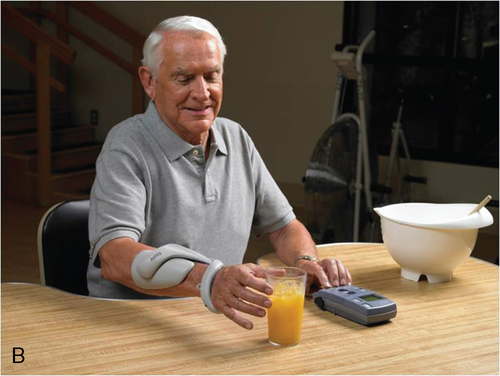
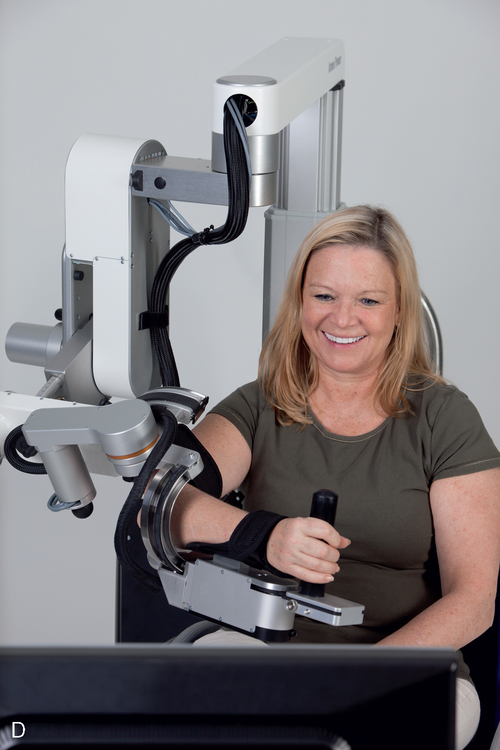
Some common types of wearable assistive devices are summarized in Box 38-1210,11,82–114 for the upper extremities (Figure 38-7) and Box 38-1310,11,35,42,115–121 for the lower extremities (Figure 38-8). In general, they are divided into three categories of wearable robots: single-task robots, workstation robots, and wheelchair-mounted manipulators (see Figure 38-7). They can also be classified by whether they use neuromuscular stimulation or biofeedback to assist the movement (Figure 38-9). Some patients use these wearable assistive robotics for training and recovery. Those who have reached a plateau in recovery may continue to use these assistive robotic devices for ongoing functional assistance and safety.
In 2012, there were a variety of wearable upper-limb robotic devices on the market, and most were unilateral (see Box 38-12). At the same time, there were fewer lower-limb wearable robotic devices on the market; however, a variety of lower-limb exoskeletons are under development10,11,35,94 (see Box 38-13).
Effectiveness of wearable assistive robotic devices.
The effectiveness of wearable assistive robotic devices is promising. Assistive robotic devices for the lower limb have been positively associated with short- and long-term gains in gait speed, quality of gait, endurance, time in single leg stance, toe clearance, and balance.35,115,119,120 There is also positive evidence supporting the effectiveness of training with a neuroprosthetic ankle-foot gait trainer, with short- and long-term gains reported in gait speed, endurance, safety, and quality.122–126
For upper-limb assistive robotic training, there are positive trends for improving motor control, particularly in patients poststroke.84,96,105,106,127–143 Unfortunately, based on a systematic review of upper-limb robotics,132,133 improved function in task performance and object manipulation was not necessarily correlated with improved motor control. The gains in motor control appear to be greatest when one-on-one care is supplemented with robotic training.
Research studies also report improved motor control with the integration of a neuroprostheses in functional arm training (e.g., includes neuromuscular stimulation or biofeedback).82,144–146 However, although there are greater gains in motor control, these gains are not necessarily associated with improved function.146
Emotional interactive entertainment and friendly robots.
Emotional interactive entertainment robots (EIARs) are similar to VR systems. EIAR systems are designed for communication and emotional support.147 EIAR devices can increase emotional comfort and give emotional relief to people who live alone.
Entertainment robots are mechatronic devices that exhibit animal-like behaviors.
For example, within the project Home Information Infrastructure House (HII) house, National Panasonic introduced a user-friendly home interface for older people as a memory jogger (e.g., cuddly toys). The speech synthesis can reproduce phrases and can be programmed to remind users to take their medications at a particular time. Failure to respond can activate a direct call to a caregiving staff member who can then check on the user. BECKY,148 a friendly robot from Korea, demonstrates different behaviors according to the emotional status of the user. The seal robot and the cat robots from Japan149,150 provide interactions between hospitalized human patients and the pet robots. The Autonomous Robotic Remedial Activity (AuRoRA) Project151,152 applied robots to education and therapy for children with autism.
Screening patients for the integration of robotic technology
General screening
An objective evaluation is needed to match a patient with a commercially available robotic device. This evaluation must include a thorough assessment of anatomical, physiological, cognitive, and sensory impairments. Whenever possible, standardized tests should be used to document strength, flexibility, endurance, balance, coordination, synergistic responses, hypertonicity, gait, balance, posture, and postural righting skills. These impairments need to be integrated into functional and task-specific assessments of motor learning, motor control, activities of daily living (ADLs), work requirements, and recreational needs. Then each patient should be screened by defined objectives relative to outcomes in terms of quality of life and independence. Subjective and emotional issues such as attention, motivation, history of positive health behaviors, durability, depression, desire for independence, and commitment to learning must also be considered. Many of these assessment tools are described in Chapter 8. The challenge is to determine if achieving rehabilitation goals requires integration of advanced technology and whether the patient has the potential to be trained to benefit from the prescribed technology.
Specific screening by type of robotic device
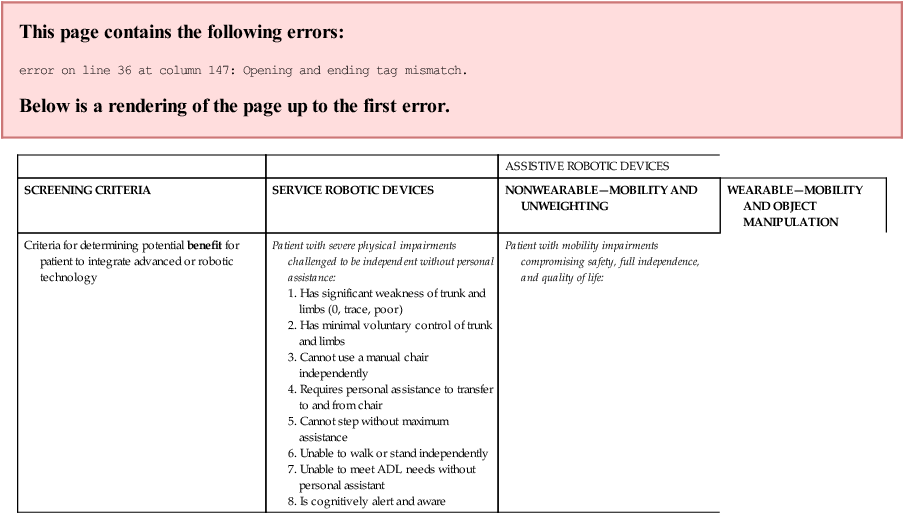
Table 38-2 summarizes some screening criteria that could be used by the therapist to determine if a patient can include rehabilitation technology into the plan of care in order to maximize function. This table also summarizes the level of function required by the patient to be able to effectively use robotic technology to improve independence and quality of life. The criteria to assess patient needs and abilities are classified by type of robotic technology. The criteria are neither all-inclusive nor exclusive but serve as a starting point for a therapist to make a recommendation for this type of technology to be considered.
TABLE 38-2 
| ASSISTIVE ROBOTIC DEVICES | |||
| SCREENING CRITERIA | SERVICE ROBOTIC DEVICES | NONWEARABLE—MOBILITY AND UNWEIGHTING | WEARABLE—MOBILITY AND OBJECT MANIPULATION |
| Criteria for determining potential benefit for patient to integrate advanced or robotic technology | Patient with severe physical impairments challenged to be independent without personal assistance:
1. Has significant weakness of trunk and limbs (0, trace, poor) 2. Has minimal voluntary control of trunk and limbs 3. Cannot use a manual chair independently 4. Requires personal assistance to transfer to and from chair 5. Cannot step without maximum assistance 6. Unable to walk or stand independently |
1. Has impaired ability to walk
2. Has poor or slow balance responses
3. Demonstrates unstable single-limb support (unilateral or bilateral)
5. Could benefit from fall protection when standing
6. Is weak (has poor strength)
7. Has difficulty initiating stepping
8. Lacks high-quality voluntary control for walking
9. Has involuntary synergistic movements
11. Uses an assistive mobility device
12. Needs to reduce ground reaction forces when walking or during intense exercise owing to pain, inflammation, osteoporosis, joint replacement, incoordination, and so on
13. Walks very slowly (household ambulatory but limited community ambulation)
14. Must maintain cardiopulmonary and metabolic health
15. Could benefit from stimulation of neurotransmitters and endorphins
1. Is unable to perform common daily tasks for independence without an assistive device or personal assistance
2. Needs assistance at one or more joints
3. Needs assistance to complete a task
4. Takes excessive time to complete task
5. Needs assistive device to ensure stability to walk
6. Has experienced a fall or an injury when performing a daily task (e.g., toileting)
7. Cannot live safely alone without personal assistance
8. Cannot cross the street in time
9. Needs more controlled forced task practice
10. Needs assistance to improve quality
11. Is not afraid of computerized or electromechanical devices
12. Is learning “abnormal” movements
13. Lives alone, but safety is in question
14. Drives but needs some assistance with transfers and/or arm or leg movements
1. Has cognitive ability to control motorized and robotic devices
2. Has the sensory, physical, and cognitive abilities to control a motorized chair (e.g., via joystick, head movement, button press, breath, piezoelectricity, voice) without human assistance
3. Can activate an automated harness system to transfer into a wheelchair
4. Can sit in a chair with or without positional assistance
5. Has sufficient understanding and ability to activate robotic service and emergency devices to be independent without human assistance (smart house designs)
1. Has cognitive ability to participate in training
2. Has sufficient attention and understanding to cooperate in gait training
3. Has adequate head and trunk control to maintain postural uprightness when legs moving
4. Has sufficient strength in legs to stand up when unloaded or protected from falling
5. Has some sensation in lower limbs or can see legs
6. Has partial movement in major muscle groups in lower limb (hip, knee, ankle)
7. Has sufficient range of motion to get into standing or walking position
8. Can transfer onto the treadmill or transfer from chair to standing
9. Tone does not prevent the feet from staying on the ground or stepping when unloaded
10. Has ability to step when standing over ground or over treadmill
11. Steps after perturbation of the treadmill
12. Can swing leg through clearing floor or treadmill (with or without AFO).
1. Has intellectual ability to understand how to use the wearable assistive orthotic device
2. Has the ability to don the wearable assistive device (or has someone at home to help)
3. Is motivated to use a wearable assistive robotic device to improve independence
4. Has basic stability of the head and trunk to move the limb(s) (even if positioning device is required)
5. Involuntary movements do not interfere with robotic assistance
6. Has adequate standing balance (with or without cane or walker) to work with a wearable assistive orthotic gait training exoskeleton
7. Has the range of motion to allow full or partial task function
8. Has adequate sensation to sense rubbing and chaffing of orthotic device
9. If inadequate sensation, has sufficient vision, audition, touch, or cognition to monitor wear and control of interface
10. Has adequate strength to lift the weight of the exoskeleton
11. Has sufficient voluntary control to assist the exoskeleton
12. Able to use the robotic assistive device several hours per day
13. Has the physical, sensory, sensorimotor, and/or cognitive skills to control the interface of the assistive robotic device
14. If unable to meet 13, may be eligible for advanced technology using a brain interface
1. Goals and objectives to be functionally independent without personal assistance
2. Predictable disease- or impairment-specific issues that are not going to change or may get worse
3. A support system (family or community) to check on status at home
4. Home that can be modified to accommodate robotic equipment
1. Goals and objectives to be functionally independent without personal assistance
2. Predictable disease- or impairment-specific issues that necessitate regular exercise to maintain independence
3. A progressive neurodegenerative condition that could be slowed with regular exercise
4. A support system (family or community) to check on status at home
5. Access to public transportation to access resources
6. The ability to drive (e.g., take driver’s training or simulation; car modification) to maintain independent access to needed resources
1. Goals and objectives to be functionally independent without personal assistance
2. Predictable disease- or impairment-specific issues that require regular exercise to maintain and maximize independence despite injury or disease
3. Ability to maintain if not maximize independence despite injury
4. Ability to use robotic technology to improve functional recovery
5. Ability to put on and remove the assistive devices
6. Ability to slow down progression or maintain or improve function despite neurodegenerative condition that could be slowed with protected, guarded, stress-reduced intense exercise
7. A support system (family or community) to check on status at home
8. Access to public transportation to achieve community independence
9. Ability to maintain independent community driving with regular training, simulation, car modification
1. Safely step on and off the treadmill
2. Tolerate the tension of the harness or unweighting system
3. Spontaneously step as triggered by the moving treadmill
4. Express concern if exercising beyond his or her limits
5. Manipulate the parameters of the treadmill
6. Tolerate the end range parameters for performance (e.g., high speed)
1. Adequate sensation to notice increased pressure or chaffing or skin sensitivity to electrodes
2. Ability to decrease the hypertonicity that interferes with function or causes pain
3. Sufficient cognitive awareness to recognize abnormal robotic behaviors (e.g., forced movement in abnormal directions)
4. Ability to stop excessive, abnormal movements
5. Ability to initiate self-directed movements with robotic assistance
1. Access to financial resources to afford advanced technology
2. Access to technology that is currently commercially available
3. Access to technology that is currently accessible in his or her geographical area
4. Opportunity to arrange for technical help to maintain equipment
1. Objective outcomes as measured by decreased personal assistance, which can be documented in terms of hourly costs saved
2. That resource investment costs can be amortized across 5 years
3. That patients could gain 5-10 years of independent function at home
4. That the patient would be able to sustain independence at home (versus in skilled living environment) in the last few months of life
1. Objective outcomes focused on maintaining independence at home in lieu of an extended care facility
2. Patient can minimize falls and decrease risks for inpatient stay resulting from fall injuries
3. Patient able to sustain safe, independent indoor navigation without personal assistance
4. Patient able to get out of house and move in community independently or with public transportation
1. Objective outcomes focused on maintaining independence at home
2. Patient can perform ADLs and community activities safely
3. Patient uses assistive devices to minimize risk of falls
4. Patient remains committed to brain and physical fitness
5. Patient maintains safe, independent indoor and outdoor navigation without personal assistance
6. Patient able to get out of house and move in community independently or with public transportation
Specific screening of patients for assistive nonwearable robotic technology: example of body-weight–supported gait training technology systems
The patient needs to go through general and specific screenings to determine the appropriateness of integrating a nonwearable assistive robotic device into the rehabilitation program (see Table 38-2). The patient who is most likely to benefit from body-weight–supported gait training is the patient who has the prognosis for functional independence but needs to train to improve quality, endurance, speed, and stability of gait. Thus many patients with problems described within this text might benefit from this type of training. Those who do not have the potential for independent ambulation could still benefit from training on a BWSTS. In these cases, the training would be directed toward enhancing metabolic health, providing a sense of well-being, increasing circulation, and minimizing secondary impairments associated with excessive sitting such as decubitus ulcers and bone demineralization. These patients may need robotic, human, or harness assistance to achieve standing or stepping as well as bracing of the neck and trunk when upright. Patients with joint pain in the back or knee may also benefit from wearing supports when standing or exercising.
In spite of advancements in rehabilitation robotic technology, some patients may still have difficulty taking advantage of this type of therapeutic assistance (Box 38-14). In patients with challenging impairments, for safety the use of an overhead harness to access the treadmill may be required, in addition to close supervision by one or more therapists.
Specific screening of patients for wearable assistive devices
Most patients with temporary or permanent sensory, motor, or structural impairments can benefit from training with a dynamic wearable assistive device. However, there are currently no guidelines that can be applied to assist a clinician with objective screening. Some screening criteria have been developed to try and match patients to devices, with a focus on safety.10,11,86,94,153–157
Screening must be sensitive to the characteristics of the individual and consistent with factors in Table 38-2. After identifying the parameters of performance and the potential benefits of assistive wearable technology, the patient, the family, the therapist, and sometimes the orthotist should determine what assistive robotic devices or advanced technological equipment are available to meet the patient’s needs. The team also has to assess where the devices are located, whether they are accessible, whether the device should be rented or purchased, and whether the insurance company will help pay for the rental or purchase of the device.
In addition to the general and specific screening criteria, it is important to note that some assistive robotic devices may target control of one specific joint. However, given the biomechanical links, flexibility and sensory and motor characteristics must be assessed at each major joint above and below the primary assisted joint. For example, the Tibion Bionic Leg (see Figure 38-8, A) focuses on assisting the knee, but the patient’s hip and ankle also should be assessed. The less affected side also needs to be evaluated. To maximize the benefit of a wearable assistive device, patients should ideally have some ability to voluntarily initiate movement and a grade of poor or greater strength to be able to assist in the movement.
Wearable robotics must be programmed to assist patient function but also to stop to avoid harm. This requires a balance between the dynamic nature of the wearable assistive device and a patient’s weakness, lack of voluntary motor control, and the presence of involuntary muscle activity including hypertonicity (e.g., spasticity, dystonia, rigidity, tremor) (Box 38-14). For example, how much force would the assistive device need to provide to overcome involuntary tone? Other relevant questions must then be asked: If a robotic device is programmed to assist with flexion and the patient initiates movement into extension or abduction, will the robot have to stop assisting the limb to prevent harm? If the robot stops assisting, is there a negative effect caused by the mismatch of force between the patient and the robot? Does the patient need consistent assistance or variable assistance? How will the therapist, the robot, or the patient determine how much assistance is needed? In one case a patient may need unweighting of only a limb, and in another case both unweighting and assistance may be needed. In theory, the amount of assistance needed should decrease with recovery of function. Thus it is helpful if the wearable assistive device can easily be adjusted by the therapist or the patient.
Despite the advancements in rehabilitation robotics, some patients will still have difficulty wearing an assistive orthotic. For example, wearable assistive orthotic devices may not work well for patients with severe sensory impairments, severe balance problems, a fear of falling, or inadequate assets to control the device or for elderly patients who are afraid of computerized technology (see Box 38-15). Sophisticated rehabilitation robotic devices may also not be recommended for patients who are disoriented, who have severe pain or neural hypersensitivity, or who cannot don the apparatus independently (Box 38-16).




































 ed (
ed ( pper •imb)
pper •imb)














 ’ Go wa•ker
’ Go wa•ker