CHAPTER 2 Integrating cells into tissues
Cells evolved as single, free-living organisms, but natural selection favoured more complex communities of cells, multicellular organisms, where groups of cells specialize during development to carry out specific functions for the body as a whole. This allowed the emergence of larger organisms with greater control over their internal environment and to the evolution of highly specialized organic structures such as the brain. The human body contains more than 200 different cell types, sharing the same genome but with different patterns of gene expression.
There is molecular evidence that this structure-based scheme of classification has validity. Thus the intermediate filament proteins (p. 15) characteristic of all epithelia are keratins; those of connective tissue are vimentins; those of muscle are desmins; and those of nervous tissue are neurofilament and glial fibrillary acidic proteins. However, cells such as myofibroblasts, neuroepithelial sensory receptors and ependymal cells of the central nervous system have features of more than one tissue type. Despite its anomalies, the scheme is useful for descriptive purposes and widely used, and will be adopted here.
In this section, two of the major tissue categories, epithelia and general connective and supporting tissues, will be described. Specialized skeletal connective tissues, i.e. cartilage and bone, together with skeletal muscle, are described in detail in Chapter 5 as part of the musculoskeletal system overview. Smooth muscle and cardiac muscle are described in Chapter 6. Nervous system tissues are described in Chapter 3. Specialized defensive cells, which also form a migrant population within the general connective tissues, are considered in more detail in Chapter 4, with blood, lymphoid tissues and haemopoiesis.
EPITHELIA
The term epithelium is applied to the layer or layers of cells that cover the body surfaces or line the body cavities that open on to it. Developmentally, epithelia are derived from all three layers of the early embryo (p. 198). The ectoderm gives rise to the epidermis, glandular tissue of the breast, cornea and the junctional zones of the buccal cavity and anal canal. The endoderm forms the epithelial lining of the alimentary canal and its glands, most of the respiratory tract and the distal parts of the urogenital tract. Mesodermal derivatives include the epithelia of the kidney, the suprarenal (adrenal) cortex and endocrine cells of the ovary and testis. These endocrine cells are atypical epithelia in that they differentiate from embryonic mesenchyme and, in common with endocrine cells in general, they lack a free surface that communicates with the exterior. This atypical category also includes endothelia that line blood vessels and lymphatics (p. 135), and the epithelium-like cell layers of mesodermal (and mesenchymal) origin that line internal cavities of the body and are usually classified separately as mesothelia: they line the pericardial, pleural and peritoneal cavities.
Epithelia function generally as selective barriers that facilitate, or inhibit, the passage of substances across the surfaces they cover. In addition, they may: protect underlying tissues against dehydration, chemical or mechanical damage; synthesize and secrete products into the spaces that they line; function as sensory surfaces. In this respect, many features of nervous tissue can be regarded as those of a modified epithelium and the two tissue types share an origin (p. 186) in embryonic ectoderm.
Epithelia (Fig. 2.1) are predominantly cellular and the little extracellular material they possess is limited to the basal lamina. Intercellular junctions, which are usually numerous, maintain the mechanical cohesiveness of the epithelial sheet and contribute to its barrier functions. A series of three intercellular junctions forms a typical epithelial junctional complex: in sequence from the apical surface, this consists of a tight junctional zone, an adherent (intermediate) junctional zone and a region of discrete desmosome junctions (p. 6). Epithelial cell shape is most usually polygonal and partly determined by cytoplasmic features such as secretory granules. The basal surface of an epithelium lies in contact with a thin layer of filamentous protein and proteoglycan termed the basal lamina, which is synthesized predominantly by the epithelial cells. The basal lamina is described on p. 33.
Epithelia can usually regenerate when injured. Indeed, many epithelia continuously replace their cells to offset cell loss caused by mechanical abrasion (reviewed in Blanpain et al 2007). Blood vessels do not penetrate typical epithelia and so cells receive their nutrition by diffusion from capillaries of neighbouring connective tissues. This arrangement limits the maximum thickness of living epithelial cell layers. Epithelia, together with their supporting connective tissue, can often be removed surgically as one layer, which is collectively known as a membrane. Where the surface of a membrane is moistened by mucous glands it is called a mucous membrane or mucosa (p. 39), whereas a similar layer of connective tissue covered by mesothelium is called a serous membrane or serosa (p. 39).
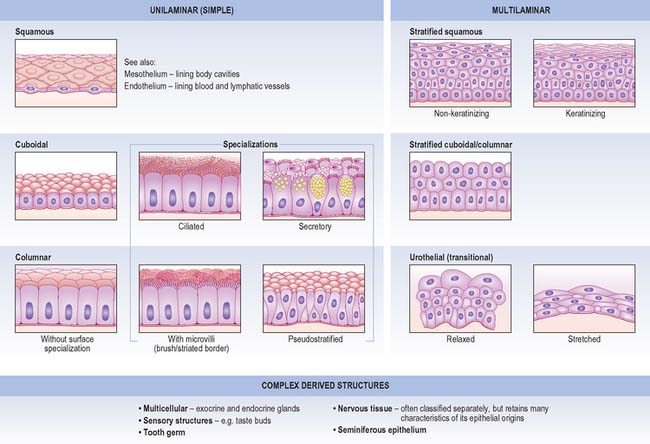
CLASSIFICATION
Unilaminar (simple) epithelia
Squamous epithelium
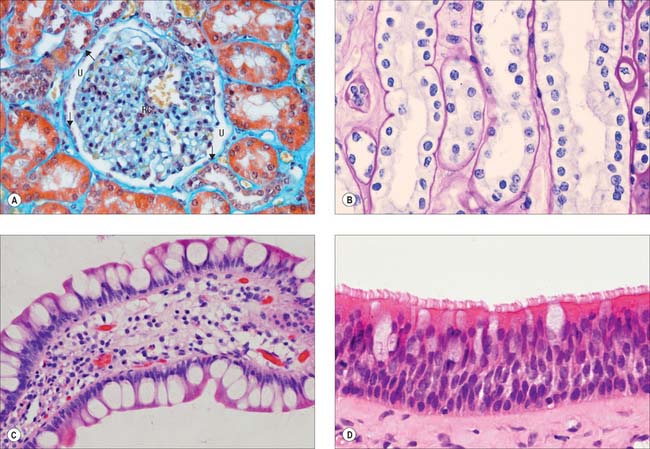
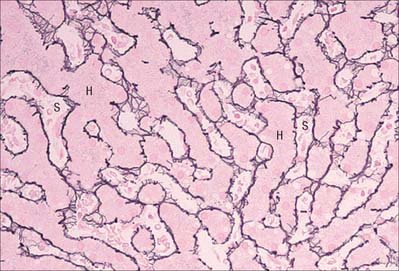
Simple squamous epithelium is composed of flattened, tightly apposed, polygonal cells (squames). This type of epithelium is described as tessellated when the cells have complex, interlocking borders rather than straight boundaries. The cytoplasm may in places be only 0.1 μm thick and the nucleus usually bulges into the overlying space (Fig. 2.2A). These cells line the alveoli of the lungs, where their surface area is huge and cytoplasmic volume correspondingly large, and they form the outer capsular wall of renal corpuscles, the thin segments of the renal tubules and various parts of the inner ear. Because it is so thin, simple squamous epithelium allows rapid diffusion of gases and water across its surface; it may also engage in active transport, as indicated by the presence of numerous endocytic vesicles in these cells. Tight junctions between adjacent cells ensure that materials pass primarily through cells, rather than between them.
Cuboidal and columnar epithelia
Cuboidal and columnar epithelia consist of regular rows of cylindrical cells (Fig. 2.2B, Fig. 2.2C). Cuboidal cells are approximately square in vertical section, whereas columnar cells are taller than their diameter, and both are polygonal when sectioned horizontally. Commonly, microvilli (Ch. 1) are found on their free surfaces, which considerably increases the absorptive area, e.g. in the epithelia of the small intestine (columnar cells with a striated border of very regular microvilli), the gallbladder (columnar cells with a brush border) and proximal convoluted tubules of the kidney (large cuboidal to low columnar cells with brush borders).
Ciliated columnar epithelium lines most of the respiratory tract, except for the lower pharynx and vocal folds, and it is pseudostratified (Fig. 2.2D) as far as the larger bronchioles; some of the tympanic cavity and auditory tube; the uterine tube; the efferent ductules of the testis. Submucosal mucous glands and mucosal goblet cells secrete mucus on to the luminal surface of much of the respiratory tract and cilia sweep a layer of mucus and trapped dust particles etc., from the lung towards the pharynx in the mucociliary rejection current, which clears the respiratory passages of inhaled particles. Cilia in the uterine tube assist the passage of oocytes and fertilized ova to the uterus (Ch. 77).
Some columnar cells are specialized for secretion and aggregates of such cells may be described as glandular tissue. Their apical domains (p. 4) typically contain mucus- or protein-filled (zymogen) vesicles, e.g. mucin-secreting and chief cells of the gastric epithelium. Where mucous cells lie among non-secretory cells, e.g. in the intestinal epithelium, their apical cytoplasm and its secretory contents often expand to produce a characteristic cell shape, and they are known as goblet cells (Fig. 2.2D). For further details of glandular tissue, see page 31 and for the characteristics of mucus, see page 39.
Pseudostratified epithelium
Pseudostratified epithelium is a single-layered (simple) columnar epithelium in which nuclei lie at different levels in a vertical section (Fig. 2.2D). All cells are in contact with the basal lamina throughout their lifespan, but not all cells extend through the entire thickness of the epithelium. Some constitute an immature basal cell layer of smaller cells, which are often mitotic and able to replace damaged mature cells. Migrating lymphocytes and mast cells within columnar epithelia may also give a similar, pseudostratified appearance because their nuclei are found at different depths. Much of the ciliated lining of the respiratory tract is of the pseudostratified type, and so is the sensory epithelium of the olfactory area.
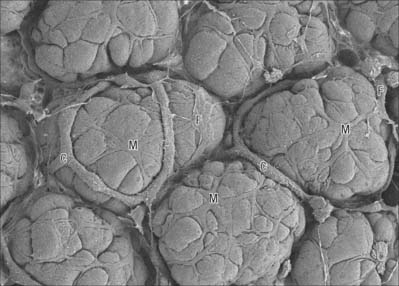
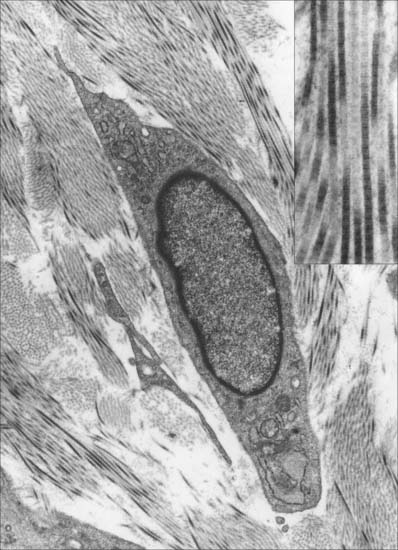
Myoepithelial cells
Myoepithelial cells, which are also sometimes termed basket cells, are fusiform or stellate in shape (Fig. 2.3), contain actin and myosin filaments, and contract when stimulated by nervous or endocrine signals. They surround the secretory portions and ducts of some glands, e.g. mammary, lacrimal, salivary and sweat glands, and lie between the basal lamina and the glandular or ductal epithelium. Their contraction assists the initial flow of secretion into larger conduits. Myoepithelial cells are ultrastructurally similar to smooth muscle cells in the arrangement of their actin and myosin, but differ from them because they originate, like the glandular cells, from embryonic ectoderm or endoderm. They can be identified immunohistochemically on the basis of the co-localization of myofilament proteins (which signify their contractile function), and keratin intermediate filaments (which accords with their epithelial lineage).
Multilaminar (stratified) epithelia
Stratified squamous epithelia
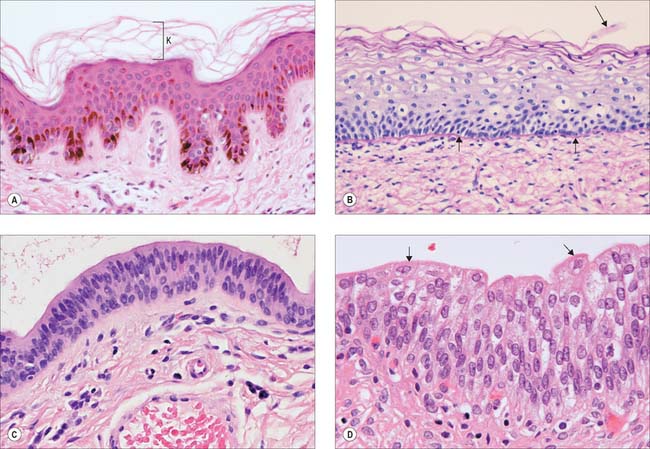
Stratified squamous epithelia are multilayered tissues in which the formation, maturation and loss of cells is continuous, although the rates of these processes can change, e.g. after injury. New cells are formed in the most basal layers by the mitotic division (p. 21) of stem cells and transit (or transient) amplifying cells. The daughter cells move more superficially, changing gradually from a cuboidal shape to a more flattened form and are eventually shed from the surface as a highly flattened squame. Typically, the cells are held together by numerous desmosomes to form strong, contiguous cellular sheets that provide protection to the underlying tissues against mechanical, microbial and chemical damage. Stratified squamous epithelia may be broadly subdivided into keratinized and non-keratinized types.
Keratinized epithelium
Keratinized epithelium (Fig. 2.4A) is found at surfaces that are subject to drying or mechanical stresses, or are exposed to high levels of abrasion. These include the entire epidermis and the mucocutaneous junctions of the lips, nostrils, distal anal canal, outer surface of the tympanic membrane and parts of the oral lining (gingivae, hard palate and filiform papillae on the anterior part of the dorsal surface of the tongue). Their cells, keratinocytes, are described in more detail on page 146. A distinguishing feature of keratinized epithelia is that cells of the superficial layer, the stratum corneum, are anucleate, dead, flattened squames that eventually flake off from the surface. In addition, the tough keratin intermediate filaments become firmly embedded in a matrix protein. This unusual combination of strongly coherent layers of living cells and more superficial strata made of plates of inert, mechanically robust protein complexes, interleaved with water-resistant lipid, makes this type of epithelium an efficient barrier against different types of injury, microbial invasion and water loss.
Non-keratinized epithelium
Non-keratinized epithelium is present at surfaces that are subject to abrasion but protected from drying (Fig. 2.4B). These include: the buccal cavity (except for the areas noted above); oropharynx and laryngopharynx; oesophagus; part of the anal canal; vagina; distal uterine cervix; distal urethra; cornea; inner surfaces of the eyelids; the vestibule of the nasal cavities. Cells go through the same transitions in general shape as are seen in the keratinized type, but they do not fill completely with keratin or secrete glycolipid, and they retain their nuclei until they desquamate at the surface. In sites where considerable abrasion occurs, e.g. parts of the buccal cavity, the epithelium is thicker and its most superficial cells may partly keratinize, so that it is referred to as parakeratinized, in contrast to the orthokeratinized state of fully keratinized epithelium. Diets deficient in vitamin A may induce keratinization of such epithelia, and excessive doses may lead to its transformation into mucus-secreting epithelium.
Stratified cuboidal and columnar epithelia
Two or more layers of cuboidal or low columnar cells (Fig. 2.4C) are typical of the walls of the larger ducts of some exocrine glands, e.g. the pancreas, salivary glands and the ducts of sweat glands and they presumably provide more strength than a single layer. Parts of the male urethra are also lined by stratified columnar epithelium. The layers are not continually replaced by basal mitoses and there is no progression of form from base to surface, but they can repair themselves if damaged.
Urothelium (urinary or transitional epithelium)
Urothelium (Fig. 2.4D) is a specialized epithelium that lines much of the urinary tract and prevents its rather toxic contents from damaging surrounding structures. It extends from the ends of the collecting ducts of the kidneys, through the ureters (p. 1242) and bladder (p. 1250), to the proximal portion of the urethra. In males it covers the urethra as far as the ejaculatory ducts, then becomes intermittent and is finally replaced by stratified columnar epithelium in the membranous urethra. In females it extends as far as the urogenital membrane. During development, part of it is derived from mesoderm and part from ectoderm and endoderm.
The epithelium appears to be four to six cells thick, and lines organs that undergo considerable distension and contraction. It can therefore stretch greatly without losing its integrity. In stretching, the cells become flattened, without altering their positions relative to each other, as they are firmly connected by numerous desmosomes. However, the urothelium appears to be reduced to two or three cells thick. The epithelium is called transitional because of the apparent transition between a stratified cuboidal epithelium and a stratified squamous epithelium, which occurs as it is stretched to accommodate urine, particularly in the bladder. The basal cells are basophilic, with many ribosomes, uninucleate (diploid), and are cuboidal when relaxed. More apically, they form large binucleate, or, more often, polyploid uninucleate cells. The surface cells are largest and may even be octaploid: in the relaxed state, they typically bulge into the lumen as dome-shaped cells with a thickened, eosinophilic glycocalyx or cell coat (p. 4). Their luminal surfaces are covered by a specialized plasma membrane in which plaques of intramembranous glycoprotein particles are embedded. These plaques stiffen the membrane. When the epithelium is in the relaxed state, and the surface area of the cells is reduced, the plaques are partially internalized by the hinge-like action of the more flexible interplaque membrane regions. They re-emerge onto the surface when it is stretched.
Seminiferous epithelium
Seminiferous epithelium is a highly specialized, complex stratified epithelium. It consists of a heterogeneous population of cells that form the lineage of the spermatozoa (spermatogonia, spermatocytes, spermatids), together with supporting cells (Sertoli cells). It is described in detail in Chapter 76 (p. 1266).
GLANDS
Glands may be subdivided into exocrine glands and endocrine glands. Exocrine glands secrete, usually via a duct, onto surfaces that are continuous with the exterior of the body, including the alimentary tract, respiratory system, urinary and genital ducts and their derivatives, and the skin. Endocrine glands are ductless and secrete hormones directly into interstitial fluid and thence the circulatory system, which conveys them throughout the body to affect the activities of other cells. In addition to strictly epithelial glands, some tissues derived from the nervous system, including the suprarenal medulla (p. 1200) and neuro-hypophysis (p. 321), are neurosecretory.
Paracrine glandular cells are similar to endocrine cells, but their secretions diffuse locally to cellular targets in the immediate vicinity; many of these are classed as neuroendocrine cells since they secrete molecules used elsewhere in the nervous system as neurotransmitters or neuromodulators (Ch. 3). Modes of signalling by secretory cells are illustrated in Fig. 1.6.
EXOCRINE GLANDS
Types of secretory process
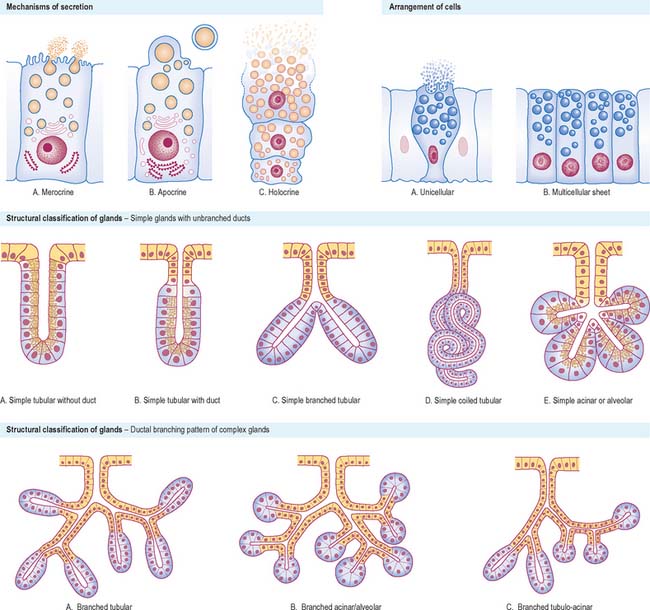
The mechanism of secretion varies considerably. If the secretions are initially packaged into membrane-bound vesicles, these are conveyed to the cell surface (p. 9), where they are discharged. In merocrine secretion, which is by far the most common secretory mechanism, vesicle membranes fuse with the plasma membrane to release their contents to the exterior (Fig. 2.5). Specialized transmembrane molecules in the secretory vesicle wall recognize marker proteins on the cytoplasmic side of the plasma membrane and bind to them. This initiates interactions with other proteins that cause the fusion of the two membranes and the consequent release of the vesicle contents. The stimulus for secretion varies with the type of cell, but often appears to involve a rise in intracellular calcium. Glands such as the simple sweat glands of the skin, where ions and water are actively transported from plasma as an exudate, were once classified as eccrine glands. They are now known to synthesize and secrete small amounts of protein by a merocrine mechanism, and are thus reclassified as merocrine glands.
In apocrine glands, some of the apical cytoplasm is pinched off with the contained secretions, which are stored in the cell as membrane-free droplets (Fig. 2.5). The best understood example of this is the secretion of milk fat by mammary gland cells (p. 936), in which a small amount of cytoplasm is incorporated into the plasma membrane-bound lipid globule as it is released from the cell. Larger amounts of cytoplasm are included in secretions by specialized apocrine sweat glands in the axilla and anogenital regions of the body. In some tissues there is a combination of different types of secretion, e.g. mammary gland cells secrete milk fat by apocrine secretion and milk protein, casein, by merocrine secretion.
In holocrine glands (Fig. 2.5), e.g. sebaceous glands in the skin, the cells first fill with secretory products (lipid droplets or sebum, in this instance) and then the entire cell disintegrates to liberate the accumulated mass of secretion into the duct or, more usually, hair follicle.
Structural and functional classification
Exocrine glands are either unicellular or multicellular. The latter may be in the form of simple sheets of secretory cells, e.g. the lining of the stomach, or may be structurally more complex and invaginated to a variable degree. Such glands (Fig. 2.5) may be simple units or their connection to the surface may be branched. Simple unbranched tubular glands exist in the walls of many of the hollow viscera, e.g. the small intestine and uterus, whereas some simple glands have expanded, flask-like ends (acini or alveoli). Such glands may consist entirely of secretory cells, or may have a blind-ending secretory portion that leads through a non-secretory duct to the surface, in which case the ducts may modify the secretions as they pass along them.
ENDOCRINE GLANDS
Endocrine glands secrete directly into connective tissue interstitial fluid and the circulation. Their cells are grouped around beds of capillaries or sinusoids (p. 133) which typically are lined by fenestrated endothelia (Ch. 6) to allow the rapid passage of macromolecules through their walls. Endocrine cells may be arranged in clusters within vascular networks, in cords between parallel vascular channels or as hollow structures (follicles) surrounding their stored secretions. In addition to the cells of specialized ductless endocrine glands (e.g. pituitary, pineal, thyroid and parathyroid), hormone-producing cells also form components of other organ systems. These include: the cells of the pancreatic islets; thymic epithelial cells; renin-secreting cells of the kidney juxtaglomerular apparatus; erythropoietin-secreting cells of the kidney; circumventricular organs; interstitial testicular (Leydig) cells; interstitial follicular and luteal ovarian cells; in pregnancy, placental cells. Some cardiac myocytes, particularly in the walls of the atria, also have endocrine functions. These are all described within the appropriate regional sections.
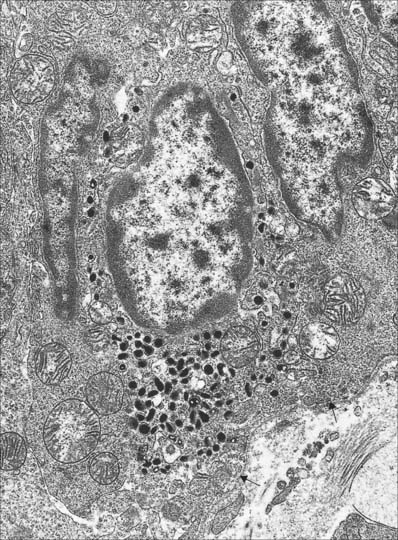
Isolated endocrine cells also exist scattered amongst other tissues as part of the dispersed (diffuse) neuroendocrine system (p. 56), e.g. throughout the alimentary and respiratory tracts. Neuroendocrine cells are generally situated within a mucosal epithelium and their bases often rest on the basal lamina (see below). In response to an external stimulus, they secrete their product basally into interstitial fluid. A typical neuroendocrine cell is shown in Fig. 2.6. The secretory granules vary in shape, size and ultrastructure in the different cell types, many of which take the name of their secretion, e.g. gastrin-secreting G cells of the small intestine. Neuroendocrine cells share many of their secretory products with chemical mediators (p. 47) in the nervous system.
CONTROL OF GLANDULAR SECRETION
The activities of cells in the various tissue and organ systems of the body are tightly regulated by the coordinated activity of the endocrine and autonomic nervous systems (Ch. 15). Endocrine (and paracrine) signals (Ch. 1) reach target cells in interstitial fluid, often via blood plasma, and together with autonomic nervous signals they ensure that the body responds to normal physiological stimuli and adjusts to changes in the external environment. Hormone secretion is itself controlled in a number of ways, e.g. by neural control, regulatory feedback loops or according to various cyclical, rhythmical or pulsatile patterns of release. Endocrine glands have a rich vascular supply and their blood flow is controlled by autonomic vasomotor nerves, which can thus modify glandular activity.
Glandular activity may also be controlled directly by autonomic secretomotor fibres, which may either form synapses on the bases of gland cells (e.g. in the suprarenal medulla) or release neuromediators in the vicinity of the glands, to reach them by diffusion. Alternatively, the autonomic nervous system may act indirectly on gland cells, e.g. on neuroendocrine G cells via histamine, released neurogenically from another neuroendocrine cell in the gastric lining (Ch. 65). Such paracrine activities of neuroendocrine cells (Ch. 1) are important in the respiratory, as well as the gastrointestinal system. Circulating hormones from the adenohypophysis stimulate synthesis and secretion by target cells in many endocrine glands. Such signals, mostly detected by receptors at the cell surface and mediated by second messenger systems, may increase the synthetic activity of gland cells, and may cause them to discharge their secretions by exocytosis. Secretions from certain exocrine glandular cells are expressed rapidly from those glands by the contraction of associated myoepithelial cells (Fig. 2.3) that enclose the secretory units and smaller ducts. Myoepithelial cells may be under direct neural control, as in the salivary glands, or they may respond to circulating hormones, e.g. such cells in the mammary gland respond to the concentration of circulating oxytocin.
Feedback loops and endocrine axes
The hypothalamus of the brain and the adenohypophysis are central to most regulatory feedback loops within the endocrine system. Loops can be either positive or negative, e.g. the hypothalamus stimulates release of follicle stimulating hormone (FSH) by the adenohypophysis, which in turn stimulates ovarian follicular maturation and secretion of oestradiol, which acts on breast and endometrial target tissues. Oestradiol, in this case, also acts back on the adenohypophysis and hypothalamus to reinforce their function positively in a feedback loop. In contrast, hypothalamic and adenohypophysial stimulation of testicular production of testosterone, which acts on targets such as skeletal muscle, are negatively regulated in a feedback loop generated by circulating testosterone. Such negative feedback regulation is a widely utilized physiological mechanism.
BASEMENT MEMBRANE AND BASAL LAMINA
There is a narrow layer of extracellular matrix (p. 36), which stains strongly for carbohydrates, at the interface between connective and other tissues, e.g. between epithelia and their supporting connective tissues. In early histological texts this layer was termed the basement membrane. As almost all of its components are synthesized by the epithelium or other tissue (e.g. muscle), rather than the adjacent connective tissue, it will be discussed here.
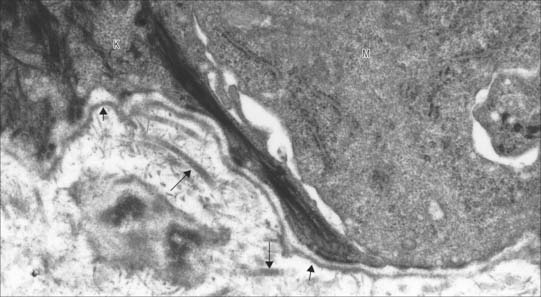
Electron microscopy revealed that the basement membrane is composed of two distinct components. A thin, finely fibrillar layer, the basal lamina, is associated closely with the basal cell surface (Fig. 2.7). A variable reticular lamina of larger fibrils and glycosaminoglycans of the extracellular matrix underlies this layer and is continuous with the connective tissue proper, although it is much reduced or largely absent in some tissues, e.g. surrounding muscle fibres, Schwann cells and capillary endothelia. In other tissues, the basal lamina separates two layers of cells and there are no intervening typical connective tissue elements. This occurs in the thick basal lamina of the renal glomerular filter (p. 1238) and the basal lamina of the thin portions of the lung interalveolar septa across which gases exchange between blood and air (p. 998).
The basal lamina is usually about 80 nm thick, varying between 40 and 120 nm, and consists of a sheet-like fibrillar layer, the lamina densa (20–50 nm wide), separated from the plasma membrane of the cell it supports by a narrow electron-lucent zone, the lamina lucida. The lamina lucida is absent from tissues prepared by rapid freezing and so may be an artefact. In many tissues this zone is crossed by integral plasma membrane proteins, e.g. keratinocyte hemidesmosomes (Chs 1 and 7) are anchored into the lamina densa in the basal lamina of the epidermis. The basal lamina is a delicate felt-like network composed largely of two glycoprotein polymers, laminin and type IV collagen, which self-assemble into two-dimensional sheets interwoven with each other. Early embryonic basal lamina is formed only of the laminin polymer. Two other molecules cross-link and stabilize the network: entactin (nidogen) and perlecan (a large heparan sulphate proteoglycan).
In Descemet’s membrane in the cornea, collagen type VIII replaces collagen type IV in the much thickened (increasing with age, up to 10 μm,) endothelial basal lamina. The basal lamina of the neuromuscular junction (p. 62) contains agrin, a heparan sulphate proteoglycan, which plays a part in the clustering of muscle acetylcholine receptors in the plasma membrane at these junctions.
FUNCTIONS OF BASAL LAMINA
Basal laminae perform a number of important roles (Iozzo 2005). They form selectively permeable barriers (anionic filters) between adjacent tissues, e.g. in the glomerular filter of the kidney; they anchor epithelial and connective tissues and so stabilize and orientate the tissue layers; they may exert instructive effects on adjacent tissues, and so determine their polarity, rate of cell division, cell survival etc.; they regulate angiogenesis. In addition, they may act as pathways for the migration and pathfinding of growing cell processes, both in development and in tissue repair, e.g. in guiding the outgrowth of axons and the reestablishment of neuromuscular junctions during regeneration after injury in the peripheral nervous system. Changes in basal lamina thickness are often associated with pathological conditions, e.g. the thickening of the glomerular basal lamina in glomerulonephritis and diabetes.
CONNECTIVE AND SUPPORTING TISSUES
The connective tissues are defined as those composed predominantly of intercellular material, the extracellular matrix, which is secreted mainly by the connective tissue cells. The cells are therefore usually widely separated by their matrix, which is composed of fibrous proteins and a relatively amorphous ground substance (Fig. 2.8). Many of the special properties of connective tissues are determined by the composition of the matrix, and their classification is also largely based on its characteristics. In some types of connective tissue, the cellular component eventually dominates the tissue, even though the tissue originally has a high matrix:cell ratio, e.g. adipose tissue. Connective tissues are derived from embryonic mesoderm or, in the head region, largely from neural crest.
Structural connective tissues are divided into ordinary (or general) types, which are widely distributed, and special skeletal types, i.e. cartilage and bone, which are described in Chapter 5. A third type, haemolymphoid tissues, consists of peripheral blood cells, lymphoid tissues and their precursors; these tissues are described in Chapter 4. They are often grouped with other types of connective tissue, because of their similar mesenchymal origins and because the various defensive cells of the blood also form part of a typical connective tissue cell population. They reach connective tissues via the blood circulation and migrate into them through the endothelial walls of vessels.
CELLS OF GENERAL CONNECTIVE TISSUES
Resident cells
Fibroblasts
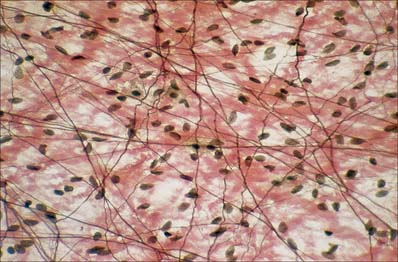
Fibroblasts are usually the most numerous resident cells. They are flattened and irregular in outline, with extended processes, and in profile they appear fusiform or spindle-shaped (Fig. 2.9; see also Fig. 2.11). Fibroblasts synthesize most of the extracellular matrix of connective tissue (Fig. 2.9); accordingly they have all the features typical of cells active in the synthesis and secretion of proteins. Their nuclei are relatively large and euchromatic and possess prominent nucleoli. In young, highly active cells, the cytoplasm is abundant and basophilic (reflecting the high concentration of rough endoplasmic reticulum), mitochondria are abundant and several sets of Golgi apparatus are present. In old and relatively inactive fibroblasts (often termed fibrocytes) the cytoplasmic volume is reduced, the endoplasmic reticulum is sparse and the nucleus is flattened and heterochromatic.
Fibroblasts are particularly active during wound repair (Ch. 7) following traumatic injury or inflammation, when tissue mass is lost through cell death. They proliferate and lay down a fibrous matrix that becomes invaded by numerous blood vessels (granulation tissue). Contraction of wounds is at least in part caused by the shortening of specialized contractile fibroblasts (myofibroblasts) with smooth muscle-like properties, which differentiate from fibroblasts in granulation tissue (reviewed in McAnulty 2007). In cases where the specialized cells of the damaged region cannot divide and regenerate functional tissue, e.g. cardiac muscle cells after infarction, connective tissue fibroblasts and their extracellular matrix fill the void to form a scar. An exception is the central nervous system, where glial scars are formed. Fibroblast activity is influenced by various factors such as steroid hormone concentrations, dietary content and prevalent mechanical stresses. In vitamin C deficiency, there is an impairment of collagen formation.
Adipocytes (lipocytes, fat cells)
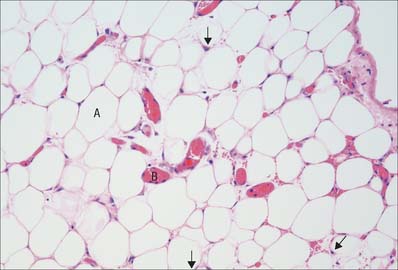
Adipocytes occur singly or in groups in many, but not all, connective tissues. They are numerous in adipose tissue (Fig. 2.10). Individually, the cells are oval or spherical in shape, but when packed together they are polygonal. They vary in diameter, averaging 50 μm. Each cell consists of a peripheral rim of cytoplasm, in which the nucleus is embedded, surrounding a single large central globule of fat, which consists of glycerol esters of oleic, palmitic and stearic acids. There is a small accumulation of cytoplasm around the nucleus, which is oval in shape and compressed against the cell membrane by the lipid droplet, as is the Golgi complex. Many cytoskeletal filaments, some endoplasmic reticulum and a few mitochondria lie around the lipid droplet, which is in direct contact with the surrounding cytoplasm and not enclosed within a membrane. In sections of tissue not specially treated to preserve lipids, the lipid droplet is usually dissolved out by the solvents used in routine preparations, so that only the nucleus and the peripheral rim of cytoplasm surrounding a central empty space remain.
Mesenchymal stem cells
Mesenchymal stem cells are normally inconspicuous cells in connective tissues. They are derived from embryonic mesenchyme and are able to differentiate into the mature cells of connective tissue during normal growth and development, in the turnover of cells throughout life and, most conspicuously, in the repair of damaged tissues in wound healing (p. 162). There is evidence that, even in mature tissues, mesenchymal stem cells remain pluripotent and able to give rise to all the resident cells of connective tissues in response to local signals and cues.
Migrant cells
Macrophages
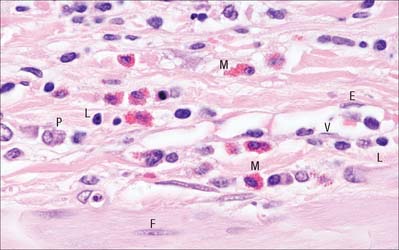
Macrophages are typically numerous in connective tissues (Fig. 2.11), where they are either attached to matrix fibres or are motile and migratory. They are relatively large cells, 15–20 μm in diameter, with indented and relatively heterochromatic nuclei and a prominent nucleolus. Their cytoplasm is slightly basophilic, contains many lysosomes (p. 12) and typically has a foamy appearance under the light microscope. Macrophages are important phagocytes, and form part of the mononuclear phagocyte system (p. 78). They can engulf and digest particulate organic materials, such as bacteria, and are also able to clear dead or damaged cells from a tissue. They are also the source of a number of secreted cytokines that have profound effects on many other cell types. Macrophages are able to proliferate in connective tissues to a limited extent, but are derived and replaced primarily from haemopoietic stem cells (p. 75) in the bone marrow, which circulate in the blood as monocytes before migrating through vessel walls into connective tissues.
Lymphocytes
Lymphocytes are typically present in small numbers and are numerous in general connective tissue only in pathological states, when they migrate in from adjacent lymphoid tissue or from the circulation. The majority are small cells (6–8 μm) with highly heterochromatic nuclei, but they enlarge when stimulated. Two major functional classes exist, termed B and T lymphocytes (p. 70). B lymphocytes originate in the bone marrow, then migrate to various lymphoid tissues, where they proliferate. When antigenically stimulated, they undergo further mitotic divisions, then enlarge as they mature, commonly in general connective tissues, to form plasma cells that synthesize and secrete antibodies (immunoglobulins). Mature plasma cells are rounded or ovoid, up to 15 μm across, and have an extensive rough endoplasmic reticulum. Their nuclei are spherical, often eccentrically situated and have a characteristic ‘clock-face’ configuration of heterochromatin (see p. 70 and Fig. 4.12) that is regularly distributed in peripheral clumps. The prominent Golgi complex is visible with a light microscope as a pale region to one side of the nucleus and the remaining cytoplasm is deeply basophilic because of the abundant endoplasmic reticulum. Mature plasma cells do not divide.
T lymphocytes originate from precursors in bone marrow haemopoietic tissue, but later migrate to the thymus, where they develop T-cell identity, before passing into the peripheral lymphoid system where they continue to multiply. When antigenically stimulated, T cells enlarge and their cytoplasm becomes filled with free polysome clusters. The functions of T lymphocytes are numerous: different subsets recognize and destroy virus-infected cells, tissue and organ grafts, or interact with B lymphocytes and several other defensive cell types (p. 71).
Mast cells
The major granule components, many of them associated with inflammation, are the proteoglycan heparin, histamine, tryptase, superoxide dismutase, aryl sulphatase, β-hexosaminidase and various other enzymes, together with chemotactic factors for neutrophil and eosinophil granulocytes. Mast cells may be disrupted to release some or all of their contents, either by direct mechanical or chemical trauma, or after contact with particular antigens to which the body has previously been exposed. The consequences of granule release include alteration of capillary permeability, smooth muscle contraction, and activation and attraction to the locality of various other defensive cells. Responses to mast cell degranulation may be localized, e.g. urticaria, or there may occasionally be a generalized response to the release of large amounts of histamine into the circulation (anaphylactic shock). Mast cells closely resemble basophil granulocytes of the general circulation but are thought to develop as distinct descendants of an earlier myeloid lineage precursor (Ch. 4). It is believed that they are generated in the bone marrow and circulate to the tissues as immature basophil-like cells, migrating through the capillary and venule walls to their final destination. For further reading, see Bischoff (2007).
Granulocytes (polymorphonuclear leukocytes)
Neutrophil and eosinophil granulocytes are immigrant cells from the circulation. Relatively infrequent in normal connective tissues, their numbers may increase dramatically in infected tissues, where they are important components of cellular defence. Neutrophils are highly phagocytic, especially towards bacteria. The functions of eosinophils are less well understood. These cells are described further in Chapter 4.
CELLS OF SPECIALIZED CONNECTIVE TISSUES
Skeletal tissues, namely cartilage and bone, are generally classified with the connective tissues, but their structure and functions are highly specialized; they are described in Chapter 5. As with the general connective tissues, these specialized types are characterized by their extracellular matrix, which forms the major component of the tissues and is responsible for their properties. The resident cells are different from those in general connective tissues. Cartilage is populated by chondroblasts, which synthesize the matrix, and by mature chondrocytes. Bone matrix is elaborated by osteoblasts. Their mature progeny, osteocytes, are embedded within the matrix, which they help to mineralize, turn over and maintain. A third cell type, the osteoclast, has a different lineage origin and is derived from haemopoietic tissue; osteoclasts are responsible for bone degradation and remodelling in collaboration with osteoblasts.
EXTRACELLULAR MATRIX
The term extracellular matrix is applied collectively to the extracellular components of connective and supporting tissues. Essentially it consists of a system of insoluble protein fibres, adhesive glycoproteins and soluble complexes composed of carbohydrate polymers linked to protein molecules (proteoglycans and glycosaminoglycans), which bind water. The extracellular matrix distributes the mechanical stresses on tissues and also provides the structural environment of the cells embedded in it, forming a framework to which they adhere and on which they can move (reviewed in Even-Ram & Yamada 2005). With the exception of bone matrix, it provides a highly hydrated medium, through which metabolites, gases and nutrients can diffuse freely between cells and the blood vessels traversing it or, in the case of cartilage, passing nearby. There are many complex interactions between connective tissue cells and the extracellular matrix. The cells continually synthesize, secrete, modify and degrade extracellular matrix components, and themselves respond to contact with the matrix in the regulation of cell metabolism, proliferation and motility. Tissue remodelling depends on the controlled degradation of extracellular matrix by secreted metalloproteinases (reviewed in Mott & Werb 2004), regulated by their specific inhibitors, as occurs for instance during wound healing and involution, e.g. of the postpartum uterus. Pathological, uncontrolled degradation of matrix can lead to disease states such as emphysema and arthritis. In the process of matrix degradation, bioactive peptides are liberated that act as growth factors, cytokines and other signalling molecules to change the behaviour of cells in the vicinity.
The insoluble fibres are mainly of two types of structural protein: members of the collagen family, and elastin (Fig. 2.12). The interfibrillar matrix (ground substance) includes a number of adhesive glycoproteins that perform a variety of functions in connective tissues, including cell–matrix adhesion and matrix–cell signalling. These glycoproteins include fibronectin, laminin, tenascin and vitronectin, in addition to a number of other less well characterized proteins. The glycosaminoglycans of the interfibrillar matrix are, with one notable exception, post-translationally modified proteoglycan molecules in which long polysaccharide side chains are added to short core proteins during transit through the secretory pathway between the rough endoplasmic reticulum and the trans-Golgi network. The exception, the polymeric disaccharide, hyaluronan, has no protein core and is synthesized entirely by cell surface enzymes. For further reading on extracellular matrix molecules, see Pollard and Earnshaw (2007). Functional attributes of connective tissues vary and depend on the abundance of its different components. Collagen fibres resist tension, whereas elastin provides a measure of resilience to deformation by stretching. The highly hydrated, soluble polymers of the interfibrillar material (proteoglycans and glycosaminoglycans, mainly hyaluronan) generally form a stiff gel resisting compressive forces. Thus tissues that are specialized to resist tensile forces (e.g. tendons) are rich in collagen fibrils, tissues that accommodate changes in shape and volume (e.g. mesenteries) are rich in elastic fibres and those that absorb compressive forces (e.g. cartilages) are rich in glycosaminoglycans and proteoglycans. In bone, mineral crystals take the place of most of the soluble polymers, and endow the tissue with incompressible rigidity.
Fibrillar matrix
Collagens
Biochemically, all collagens have a number of features in common. Unlike most other proteins, they contain high levels of hydroxyproline and all are composed of three polypeptides that form triple helices and are substantially modified post-translationally. After secretion, individual molecules are further cross-linked to form stable polymers. Functionally, collagens are structural proteins with considerable mechanical strength. Just a few of their distinguishing structural features are described below. For further reading on the molecular structure and functions of the collagens, see Pollard and Earnshaw (2007).
Type I collagen
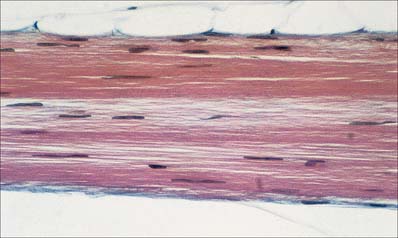
Type I collagen is very widely distributed. It forms inextensible fibrils in which collagen molecules (triple helices) are aligned side by side in a staggered fashion, with three-quarters of the length of each molecule in contact with neighbouring molecules. The fibril has well-marked bands of charged and uncharged amino-acids arranged across it; these stain with heavy metals in a banding pattern that repeats every 65 nm in longitudinal sections viewed in the electron microscope (Fig. 2.9 insert).
Fresh type I collagen fibres are tightly packed assemblies of parallel fibrils and are white and glistening. They form variably wavy (crimped) bundles of various sizes that are generally visible at the light microscope level. The component fibres may leave one bundle and interweave with others. In some situations, collagen fibrils are laid down in precise geometrical patterns, in which successive layers alternate in direction, e.g. corneal stroma, where the high degree of order is essential for transparency. Tendons, aponeuroses and ligaments are also highly ordered tissues (Ch. 5).
Types II, III, V and XI collagens
Reticular fibres
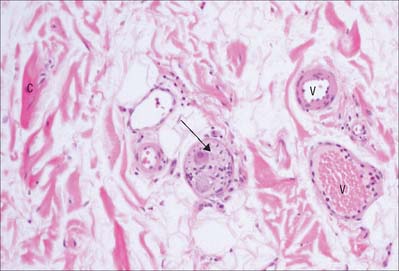
Fine branching and anastomosing reticular fibres form the supporting mesh framework of many glands, including the liver (Fig. 2.13), the kidney and lymphoreticular tissue (lymph nodes, spleen, etc.). Classically, these fibres stained intensely with silver salts, although they are poorly stained using conventional histological techniques. They associate with basal laminae and are often found in the neighbourhood of collagen fibre bundles. Reticular fibres are formed principally of type III collagen.
Elastin
Elastin is a 70 kDa protein, rich in the hydrophobic amino-acids valine and alanine. Elastic fibrils, which also contain fibrillin, are highly cross-linked via two elastin-specific amino-acids, desmosine and iso-desmosine, which are formed extracellularly from lysine residues. They are less widely distributed than collagen, yellowish in colour, typically cross-linked and are usually thinner (10–20 nm) than collagen fibrils. They can be thick, e.g. in the ligamenta flava and ligamentum nuchae. Unlike collagen type I, they show no banding pattern in the electron microscope. They stain poorly with routine histological stains, but are stained with orcein-containing preparations (see Fig. 2.12). They sometimes appear as sheets, as in the fenestrated elastic lamellae of the aortic wall. Elastin-rich structures stretch easily with almost perfect recoil, although they tend to calcify with age and lose elasticity. Elastin is highly resistant to attack by acid and alkali, even at high temperatures.
Interfibrillar matrix
Adhesive glycoproteins
These proteins include molecules that mediate adhesion between cells and the extracellular matrix, often in association with collagens, proteoglycans or other matrix components. All of them are glycosylated and they are, therefore, glycoproteins. General connective tissue contains the well known families of fibronectins (and osteonectin in bone), laminins and tenascins; there is a rapidly growing list of other glycoproteins associated with extracellular adhesion (Pollard & Earnshaw 2007). They possess binding sites for other extracellular matrix molecules and for cell adhesion molecules (p. 5), especially the integrins; in this way they enable cells selectively to adhere to appropriate matrix structures (e.g. the basal lamina). They also function as signalling molecules, which are detected by cell surface receptors and initiate changes within the cytoplasm (e.g. to promote the formation of hemidesmosomes or other areas of strong adhesion; reorganize the cytoskeleton; promote or inhibit locomotion and cell division).
CLASSIFICATION OF CONNECTIVE TISSUES
Irregular connective tissues
Loose (areolar) connective tissue
Loose connective tissue consists of a meshwork of thin collagen and elastin fibres interlacing in all directions (see Fig. 2.12) to give a measure of both elasticity and tensile strength. The large meshes contain the soft, semi-fluid interfibrillar matrix or ground substance, and different connective tissue cells, which are scattered along the fibres or in the meshes. It also contains adipocytes, usually in small groups, and particularly around blood vessels.
Adipose tissue
A few adipocytes occur in loose connective tissue in most parts of the body. However, they constitute the principal component of adipose tissue (see Fig. 2.10), where they are embedded in a vascular loose connective tissue, usually divided into lobules by stronger fibrous septa carrying the larger blood vessels. Adipose tissue only occurs in certain regions. In particular it is found in: subcutaneous tissue; the mesenteries and omenta; the female breast; bone marrow; as retro-orbital fat behind the eyeball; around the kidneys; deep to the plantar skin of the foot; as localized pads in the synovial membrane of many joints. Its distribution in subcutaneous tissue shows characteristic age and sex differences. Fat deposits serve as energy stores, sources of metabolic lipids, thermal insulation (subcutaneous fat) and mechanical shock-absorbers (e.g. soles of the feet, palms of the hands, gluteal region and synovial membranes).
Regular connective tissues
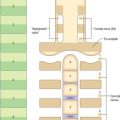
Regular connective tissues include highly fibrous tissues in which fibres are regularly orientated, either to form sheets such as fasciae and aponeuroses, or as thicker bundles such as ligaments or tendons (Fig. 2.14). The direction of the fibres within these structures is related to the stresses which they undergo, moreover, fibrous bundles display considerable interweaving, even within tendons, which increases their structural stability and resilience.
Although regular connective tissue is predominantly collagenous, some ligaments contain significant amounts of elastin, e.g. the ligamenta flava of the vertebral laminae and the vocal folds. The collagen fibres may form precise geometrical patterns, as in the cornea (p. 678).
Mucoid tissue
Mucoid tissue is a fetal or embryonic type of connective tissue, found chiefly as a stage in the development of connective tissue from mesenchyme. It exists in Wharton’s jelly, which forms the bulk of the umbilical cord, and consists substantially of extracellular matrix, largely made up of hydrated mucoid material and a fine meshwork of collagen fibres, in which nucleated, fibroblast-like cells with branching process are found. Fibres are usually rare in typical mucoid tissue, although the full-term umbilical cord contains perivascular collagen fibres. Postnatally, mucoid tissue is seen in the pulp of a developing tooth, the vitreous body of the eye (a persistent form of mucoid tissue that contains few fibres or cells) and the nucleus pulposus of the intervertebral disc.
TRANSDIFFERENTIATION AND METAPLASIA
Transitions occur between populations of cells forming an epithelium (sheets of polarized cells) and mesenchymal types (where the cells lack polarity) during normal development (Section 3), but all well-described transitions between morphologically different cell types in postnatal life do not cross the epithelial–mesenchymal boundary. They are transitions between types of epithelial cell or, less frequently, between mesenchymal (connective tissue) cell types. Most instances of such transdifferentiation (metaplasia) are adaptive, to changing environmental conditions or trauma, and almost all are pathological; the altered cells are termed metaplastic. A very common and physiologically normal example is the squamous metaplasia of columnar secretory epithelium of the distal endocervical canal (Section 8), when exposed to the hormonally-stimulated vaginal environment. Gastric metaplasia of the lower oesophagus may occur when chronic reflux of gastric juices exposes its stratified squamous epithelial lining to acid, and the original epithelium is replaced by a mucus-secreting columnar epithelium typical of the stomach (Barrett’s oesophagus): this is pathological and susceptible to malignant change. Similarly, the respiratory epithelium (see Fig. 2.2D) of the upper airway often develops foci of stratified squamous metaplasia in response to irritants in cigarette smoke. Mesenchymal (osseous) metaplasia can occur, for example, in the fibrous connective tissue of muscles subjected to repeated damage, where trabeculi of bone develop. It is thought that stem cells (rather than the differentiated cells) in the affected tissue respond to changes in their environment by altering their differentiation pathway, a process which may be reversible if the stimulus is removed.
FASCIA
SUPERFICIAL FASCIA
Superficial fascia is a layer of loose connective tissue of variable thickness that merges with the deep aspect of the dermis; it is thus also known as the hypodermis. It is often adipose, particularly between muscle and skin. It allows increased mobility of skin, and the adipose component contributes to thermal insulation and constitutes a store of energy for metabolic use. Subcutaneous nerves, vessels and lymphatics travel in the superficial fascia; their main trunks lie in its deepest layer, where adipose tissue is sparse. In the head and neck, superficial fascia also contains a group of striated muscles – collectively termed the muscles of facial expression (Ch. 29) – which are a remnant of more extensive sheets of skin-associated musculature found in other mammals (panniculus carnosus).
Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93-104.
Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445-458.
Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17:524-532.
Hinz B, Gabbiani G. Cell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodeling. Thromb Haemost. 2003;90:993-1002.
Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646-656.
McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem & Cell Biol. 2007;39:666-671.
Mott JD, Werb Z. Curr Opin Cell Biol. 2004;16:558-564.
Pollard TD, Earnshaw WC. Cell Biology. Philadelphia: Saunders, 2007. Section VIII, Cell adhesion and the extracellular matrix