CHAPTER 97 Instrumentation Complications
Classification of Complications
Biologic Failure
Spinal fixation and motion-preserving devices need to reside in a bioactive and mechanically challenging environment. Infection surrounding these devices can occur shortly after insertion or many years after the surgical procedure. Wound infection rates are slightly higher in the presence of instrumentation compared with noninstrumented procedures.1,2 For example, a posterior bone-only lumbar fusion may have a smaller infection rate than an instrumented fusion. The infection can be related to the presence of the hardware itself or the increased operative time associated with the instrumentation procedure. Early instrumentation systems were made from stainless steel. The advent of titanium systems theoretically will decrease the affinity of bacteria to the surface of the device.3 However, unintended wear debris may be greater with titanium implants in a developing pseudarthrosis with implant interface micromotion leading to a more robust inflammatory response.4,5 The issue of wear debris as it relates to disc arthroplasty does not seem to be as problematic compared with arthroplasty in a synovial environment.6,7 With a wide range of disc designs and arthroplasty interfaces in use in Europe and only recently approved in the United States, a definitive statement regarding wear debris cannot be made at this time.8 However, infection in the anterior lumbar interbody region can be extremely difficult to approach, drain, and reconstruct.9
The interface between spinal instrumentation systems depends on the quality of the host bone and/or vertebral endplate. Osteoporosis can lead to early fixation failure or implant loosening before an attempted arthrodesis procedure heals. Deficient vertebral endplates or subchondral osteoporosis can lead to interbody device loosening or subsidence.10–12 Motion-sparing devices rely on a solid point of fixation to the spine. Osteoporosis can compromise the interface leading to alterations in the biomechanical performance of the motion-sparing device.
Many other patient-related factors contribute to biologic failure and subsequent instrumentation complications. Steroid use, smoking, cancer, prior radiation therapy, multiple trauma, and poor nutrition are all factors than can either adversely affect a patient’s ability to heal a biologic procedure (fusion), diminish bone quality, and/or increase the risk of infection.13–21 Many of these such as steroid use, cancer, and trauma are unavoidable factors that one must encounter in the use of spinal instrumentation. Nutritional status can be assessed and improved if surgery is elective.22 Smoking cessation before spinal reconstructive procedures can improve fusion rates.16 The option should always be explored with patients. The decision of what to do if a patient cannot stop smoking before surgery is a social, ethical, and national health problem that is beyond the scope of this chapter. Needless to say, smoking is a deterrent to the success of a spinal fusion and may contribute to an increased incidence of instrumentation complications.
Biomechanical Failure
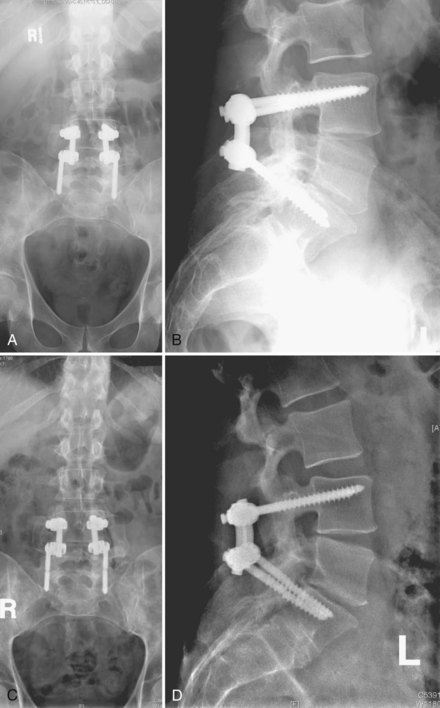
It is difficult to discuss biomechanical failure of spinal instrumentation without considering it along with an initiating problem. As mentioned earlier, an instrumented posterior fusion procedure resulting in a pseudarthrosis can lead to pedicle screw or rod breakage (Fig. 97–1).23 With any instrumented fusion, it is a race between failure of the instrumentation and healing of the fusion procedure.
Error in Thought Process
Accurate assessment of spinal anatomy leads directly to the next step of preoperative planning. Nearly every device placed in the interbody region will usually have a corresponding set of templates, corrected for varying degrees of magnification, to allow templating for the proper implant size. Threaded lumbar interbody cages are a typical implant that requires templating. Dual cages require adequate left to right spacing so that they will be well confined to the interbody region without encroachment of the cauda equina or lumbar root in the neuroforamina.24 They also need to adequately engage the superior and inferior endplates for stability and healing. Potential cage size can be determined from preoperative templating. More important, templating one size larger and smaller than the planned size will give the surgeon insight as to what may be necessary during surgery, what can be attempted should the planned cage size not be correct, and ensure that the proper implants are available at the time of surgery. Other implants/anatomic regions requiring templating on a routine basis are pedicle screws and pedicle diameter, anterolateral plates and vertebral body screws for the thoracolumbar spine, and disc replacements.
Error in Application
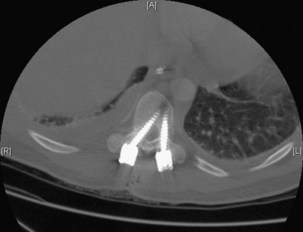
Execution of a well-designed and thought-out surgical plan is not without complication. Spinal instrumentation is no exception to this problem. Application errors can be directly linked to errors in thought process by deciding on the incorrect implant for a particular goal in a spinal reconstructive procedure. They can also include basic errors such as misplacing a pedicle screw in a pedicle. Hopefully, this would occur laterally into muscle rather than inferiorly into the foramen or medially into the spinal canal (Fig. 97–2).
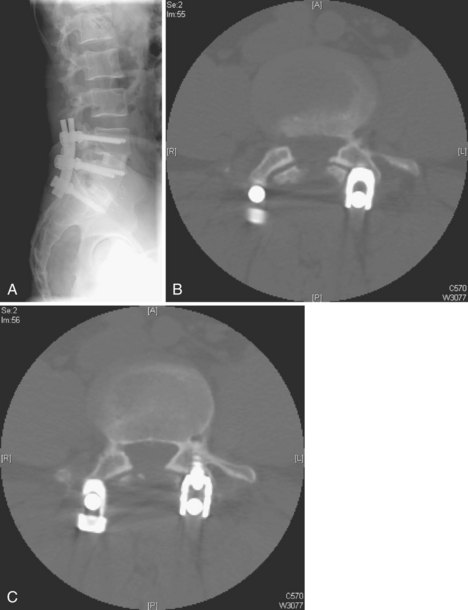
Lack of intraoperative orientation to the spine can lead to application errors as well. Applying an anterolateral plate at the thoracolumbar junction requires the patient to be in the true lateral position. If the patient has rolled slightly anterior or posterior, vertebral body screws run the risk of entering the spinal canal or injuring the great vessels.25 Loss of midline orientation in the anterior cervical spine can lead to malpositioning of an anterior cervical plate and even vertebral artery injury from vertebral body screws. The surgeon must maintain his or her own three-dimensional orientation while inserting spinal instrumentation in order to prevent injury to surrounding neural, vascular, visceral, and other soft tissues. Intraoperative feedback from live fluoroscopy or image-guidance systems are not substitutes for a thorough understanding of anatomy and surgical landmarks associated with inserting different types of spinal instrumentation systems (Fig. 97–3).
Complications by Spinal Region and Implant Type
Anterior Cervical Spine
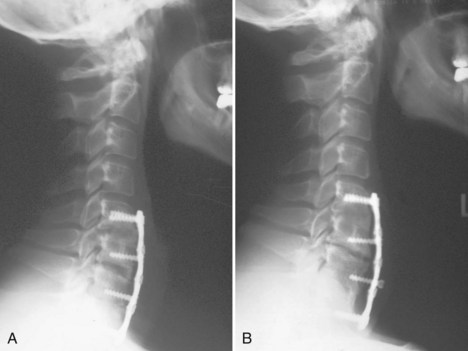
Cervical plates are the most common implants used in the anterior cervical spine. The use of buttress screws and wire has fallen out of favor over the past 20 years. Anterior plates have evolved from nonlocking, simple bone plates and bicortical screws to systems that have a locking mechanism between the plate and screw while allowing dynamic settling of the plate if the surgeon chooses this option. Contemporary systems tend to use unicortical fixation rather than bicortical screw fixation. Therefore screw penetration into the spinal canal with neurologic injury is a rare complication. Plate fracture is also quite rare. Screw back-out can still occur despite the locking mechanisms designed to prevent this problem. If this occurs, it typically represents abnormal motion at the fusion site leading to a pseudarthrosis (Fig. 97–4). Screws that back out should be removed to prevent esophageal injury, and pseudarthrosis repair should be undertaken if necessary. Occasionally, the screw-plate locking mechanism will remain intact and the plate will lift off the anterior vertebral body surface as the screw-bone interface loosens. This would also be indicative of a pseudarthrosis and should be addressed surgically. Screw breakage can occur with settling of a statically locked, anterior-plated fusion construct. If the plate remains flush with the vertebral body and the fusion heals, then a revision is typically not required.
Placement of the anterior plate can also be associated with complication. Placing the plate too lateral puts the vertebral artery at risk when drilling for and inserting screws on that side. This was particularly of concern when screws were inserted in a divergent direction. Most contemporary systems make use of convergence to minimize the risk of this occurrence. One must also evaluate each level to be instrumented for vertebral artery anomalies so as to avoid injuring the artery during drilling or screw insertion.26 Placing a plate too cephalad or caudal relative to an adjacent unaffected disc places that disc space at risk for adjacent-level ossification.27 Whether or not this represents a long-term problem remains to be demonstrated.
Anterior odontoid screws have a limited role in the overall treatment of odontoid fractures. Safe screw insertion requires a reducible fracture that is not the reverse oblique pattern, minimal bone comminution, reasonable bone quality for screw purchase, and the ability to keep the odontoid fragment from spinning while drilling and inserting the screw. Fluoroscopic visualization in the anterior-posterior and lateral planes needs to be established before the start of surgery. Patients with large/barrel chests may preclude the surgeon from obtaining a shallow enough angle to drill and insert the screw. Complications therefore include inability to insert the screw, screw cutout, spinning of the odontoid fragment and malreduction, fracture displacement, and spinal canal penetration.28
One set of complications of anterior cervical instrumentation cannot be defined on radiographs—those associated with the surgical approach and the use of power drills and taps. Care must be taken to safely mobilize the midline neck structures and protect the nerves associated with speech and swallowing function. Drill and tap sleeves should be used to prevent soft tissue injury. A common postoperative complication following anterior cervical spine surgery, dysphagia, has been linked to prolonged retractor use.29 Careful attention to adjusting retractors, working efficiently, and attempting to limit retractor time can help to reduce this complication.
Posterior Cervical Spine
The occiput-C1-C2 region has its own unique challenges with reference to the vertebral artery anatomy, unique bony characteristics, and proximity of the brain and spinal cord. Wiring procedures are relatively safe unless sublaminar wire fixation at C1 is considered. Care must be taken not to allow the wire to pass too ventral, causing spinal cord compression. Screw fixation across the C1-C2 facet joint places the vertebral artery at risk. C1 lateral mass screws require assessment of the vertebral and internal carotid artery position, the former as it relates to screw insertion point and the latter as it relates to the final position of the screw tip.30
Anterolateral Thoracolumbar Spine
Anterolateral vertebral body screw-rod systems and plating systems represent the bulk of instrumentation systems that are used in the anterolateral thoracolumbar spine. Here orientation and proper patient positioning are the first steps in avoiding aberrant screw placement with potential neurologic or great vessel injury. This is especially true in patients with rotational deformities. It is important before surgery to premeasure the vertebral widths on available imaging studies. In deformity cases, the ends of the construct are areas of stress concentration31 and can pull out or fail. It is important to accommodate adjacent curves and pay attention to sagittal balance in an effort to minimize the risk of transition syndromes.
Anterior plates should be long enough to capture the superior and inferior screws yet short enough so as not to overlap the mobile disc above and below the instrumented levels. Osteoporosis can lead to loss of fixation and screw cutout into the disc space, plowing through the vertebra, or lifting off/backing out of the vertebral body. Reconstruction of the corpectomy defect can be performed with structural autograft, allograft, composite spacers, linear cages, and expandable cages. They all must bear stress on the vertebral endplate. Nonstructural grafts are more likely to collapse with time. Osteoporosis or damage to the endplate can lead to excessive subsidence and loss of fixation.32,33
Posterior Thoracolumbar Spine
Pedicle screw-based systems are the workhorse instrumentation systems of this region. The benefits over hooks and sublaminar wires are many: three-column fixation allows better pull-out strength and greater control in the sagittal, coronal, and rotational planes due to increased stability to axial, bending, and rotational forces.34–37 Additionally, fewer vertebral motion segments are fused, thereby preserving more motion segments. The added fixation strength may lessen or obviate the need for postoperative bracing. Other benefits include secure fixation after laminectomy or in a situation where the posterior elements are otherwise incompetent and the ability to treat three-column injuries with adequate stability. However, hooks and wires can still be used with or without pedicle screws in modern segmental instrumentation systems and in some cases may be the only option for posterior fixation in a given spinal segment.
The complications related to hooks and sublaminar wires are well studied. Sublaminar wires run the risk of neurologic injury and cutting out of bone by sawing through the lamina. Sublaminar hooks also represent a source of neurologic injury. Hooks should not be placed in regions of canal narrowing secondary to degenerative, traumatic, or deformity-based stenosis.38 Pedicle and transverse process hooks can also be used with less risk of neurologic injury but with the latter not having the biomechanical strength of laminar hooks or pedicle screw systems.
When placing pedicle screws, especially in the thoracic spine, the proximity of the instrumentation to adjacent structures is the major concern. Vaccaro and colleagues39 advanced screws positioned accurately within the pedicle through the anterior cortex in cadaveric specimens to assess the proximity of important neurovascular structures. The following structures are endangered when screws are placed on the right side of the body: superior intercostal vessels (T4-T5), esophagus (T4-T9), azygous vein (T5-T11), inferior vena cava (T11-T12), and the thoracic duct (T4-T12). On the left side of the body, the following structures may be damaged by screws placed through the anterior cortex: esophagus (T4-T9) and aorta (T5-T12). When screws are placed laterally, the lung, segmental vessels, and sympathetic chain are in jeopardy on both sides. The aorta is endangered on the left (T5-T10) with laterally placed screws and the azygous vein on the right (T5-T11). In terms of nerve root and spinal cord proximity to the pedicle, there are consistent differences between the thoracic and lumbar spine. The thoracic nerve roots are approximately evenly spaced between the superior and inferior pedicles due to the more perpendicular angle off of the spinal cord. In the lumbar spine, there is a significant difference in pedicle-nerve root distance with the nerve roots having three to four times more space superior to the pedicle than inferior. The dura directly abuts the medial aspect of the thoracic pedicle in most studies, whereas there is 1 to 2 mm of space in the lumbar spine.
The complications associated with pedicle screws placed in the lumbar spine have been well documented. Reports vary in terms of whether the complication rates correspond to the rate per screw placed or the rate per patient. Lonstein and colleagues40 reviewed almost 4800 pedicle screws—more than 98% within the lumbar spine—with an overall complication rate of 2.4%. On the basis of radiographic analysis, 5.1% of screws extended outside the pedicle or vertebral body. A significant percentage of these screws was intentionally placed through the anterior cortex for bicortical fixation at S1. The most common complication was pain (23%) that was believed to be related to pseudarthrosis or the implant itself. Nerve root irritation was uncommon (0.2%) despite 1% of screws penetrating either medial or inferior to the pedicle wall. In other studies, the rate of implant removal due to pain is relatively low and nerve root irritation is also uncommon (range: 0.2% to 5% in various series).
In the thoracic spine, several studies have analyzed rates of complications. The majority of these studies review pediatric deformity patients. Suk and colleagues41 reported the largest series of thoracic pedicle screws to our knowledge. Of more than 4600 screws investigated, 1.5% of screws were determined to be malpositioned by radiographic analysis. Screw malpositions were lateral in 27%, medial in 6%, superior in 18%, and inferior in 49%. In this series there was one neurologic injury causing transient paraparesis due to a medial pedicle perforation causing a delayed epidural hematoma. Other complications cited include pedicle fractures (0.24%), screw loosening (0.76%), and infections (1.9%). Kim and colleagues42 reported the safety and reliability of a free-hand (anatomic) technique of pedicle screw placement in the thoracic spine. Of the 1118 screws placed without the help of fluoroscopy or radiographs, 400 were randomly evaluated by CT. Of the 400 screws reviewed by CT, 55.8% were totally contained within the pedicle. Three percent of the screws had penetrated the spinal canal by 2 to 4 mm. There were no neurologic or vascular complications.
Lumbar Interbody Fixation
Lumbar interbody devices can be placed via anterior or posterior approaches. All of these devices require preoperative templates to determine the implant size and extent of disc exposure to permit safe insertion. Interbody fusion devices placed via a posterior interbody route (posterior lumbar interbody fusion [PLIF]) place the cauda equina and nerve roots at risk during insertion because of the retraction required to insert the device (cage).43,44 Implants need to be large enough to engage the disc endplates but not too large to force over-retraction of the neurologic elements. Transforaminal cage insertion (transforaminal lumbar interbody fusion, TLIF) places the exiting and traversing nerve roots on the side of insertion at less risk than the PLIF approach. Both PLIF and TLIF procedures destabilize the posterior tension band supporting structures and require supplement posterior stabilization.45–47
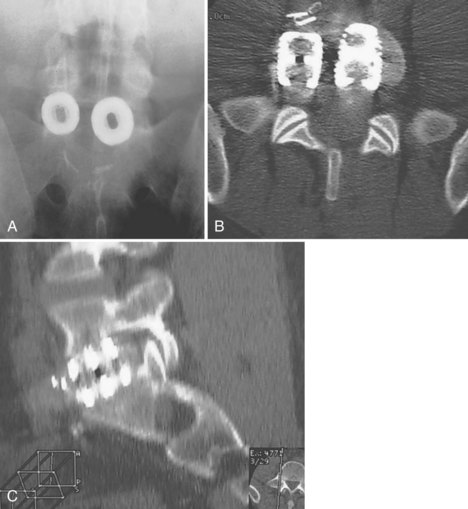
Anterior devices require mobilization of the abdominal contents and, most commonly, the left iliac vein. The use of paired, stand-alone interbody cages requires preoperative templating to ensure that cage height is sufficient to engage the vertebral endplates while the combined cage width does not exceed the margins of the disc space and protrude into the spinal canal/neuroforamina (Fig. 97–5).22 Depth of insertion depends on the implant: interbody cages are usually placed so that the anterior portion of the cage resides just under the anterior margin of the vertebral body. Countersinking the cage can result in subsidence and subsequent pseudarthrosis, as well as loss of foraminal height resulting in radicular symptoms. Disc arthroplasty usually requires that the prosthesis is placed posteriorly in the disc space—usually at the posterior vertebral margin so as to optimize motion. Excessive endplate resection or damage during the discectomy, as well as osteoporosis, can lead to subsidence of interbody devices as well.
Other Implant-Related Complications
Although this chapter is mainly dedicated to instrumentation complications, the authors feel that a review of complications associated with the use of bone morphogenetic proteins in spinal fusion surgery should be included. INFUSE (Medtronic), recombinant human bone morphogenetic protein-2 (rhBMP-2), was approved by the U.S. Food and Drug Administration (FDA) in 2002 for use in a threaded lumbar interbody cage for anterior lumbar interbody fusion. It was approved as a medical device. This is the only current FDA-approved indication for rhBMP-2 in spine surgery. Nonetheless, rhBMP-2 has been used for years in what has come to be called “off-label” applications. The potential benefits include increased fusion rates and minimizing and/or eliminating autologous bone graft harvest and its associated morbidity.48–50
With increasing use, however, have come some device-specific adverse events. rhBMP-2 used for interbody arthrodesis in cages inserted through a posterior approach (transforaminal lumbar interbody fusion/TLIF) has been linked to an increased incidence of radiculitis in both standard open and minimally invasive approaches.51,52 The exact mechanism creating the radiculitis remains unknown. Rihn and colleagues51 reported a reduction in the incidence of radiculitis in the rhBMP-2 TLIF cohort with the use of a hydrogel sealant.
Other adverse events include ectopic bone formation. This has been reported to be both asymptomatic and symptomatic. Joseph and Rampersaud reported the incidence of heterotopic bone formation following minimally invasive TLIF or PLIF with rhBMP-2 to be 20.8% versus 8.3% in the non-BMP group.53 In the rhBMP-2 group, one patient developed bone within the epidural space and the other four developed bone in the foramen. The authors did not detect any adverse clinical events associated with the development of heterotopic bone.
Recent retrospective case series suggest that ectopic bone formation following TLIF/PLIF with rhBMP-2 can be symptomatic. Wong and colleagues54 reported on five cases in which BMP was used with a PLIF/TLIF and resulted in ectopic bone within the spinal canal and potential neurologic compromise. The findings were confirmed by CT. Three of the five patients had revision surgery for symptomatic neural compression following initial surgery. Ectopic bone was found within the canal in each case, adherent to the neural structures. According to the authors of the study, each patient has at least partial improvement in symptoms postoperatively, suggesting that the ectopic bone formation was, at least in part, contributing to symptoms.54 Chen and colleagues55 reported on four cases following minimally invasive TLIF with BMP in which the patients developed delayed, symptomatic neural compression related to ectopic bone formation.
Adverse events associated with rhBMP-2 use in the anterior cervical spine have been well-documented. In 2006 Smucker and colleagues56 reported on clinically significant adverse events associated with rhBMP-2 use for anterior cervical arthrodesis. Of patients in the rhBMP-2 group, 27.5% developed significant swelling events (P < 0.0001) compared with 3.6% in the non-rhBMP-2 group. Swelling complications included delay in discharge, severe dysphagia, reintubation, and visible neck swelling. Swelling occurred, on average, on postoperative day 4.2. Most cases of swelling occurred in primary surgeries. Three patients were treated for irrigation and débridement for a swollen surgical site without evidence at time of exploration of hematoma or fluid collections. No patient demonstrated an actual compromised airway on exploration.
Since this study, other reports have surfaced regarding adverse events associated with swelling in the anterior cervical spine. Butterman57 reported a 50% incidence of neck swelling presenting as dysphagia in a series of anterior cervical fusions with allograft and BMP, compared with 14% in the iliac crest bone graft cohort. In a study citing the efficacy of rhBMP-2 in anterior cervical arthrodesis with use of a PEEK spacer, Tumialan and colleagues58 reported a 7% (14 patients) incidence of clinically significant dysphagia. The authors suggest lowering the dose of rhBMP-2 and placing it only within the PEEK spacer to limit symptomatic dysphagia.58
Instrumentation Complications and Standard of Care
Pearls
Pitfalls
Key Points
1 Arens S, Schlegel U, Printzen G, et al. Influence of materials for fixation implants on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br. 1996;78:647-651.
2 Andersen T, Christiensen FB, Laursen M, et al. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine. 2001;26:2623-2628.
3 Curylo LJ, Mason HC, Bohlman HH, Yoo JU. Tortuous course of the vertebral artery and anterior cervical decompression: a cadaveric and clinical case study. Spine. 2000;25:2860-2864.
4 Kim YJ, Lenke LG, Bridwell KH, et al. Freehand pedicle screw placement in the thoracic spine: is it safe? Spine. 2004;29:333-342.
5 Smucker JD, Rhee JM, Singh K, et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31(24):2813-2819.
1 Weinstein MA, McCabe JP, Cammissa FPJr. Post-operative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422-426.
2 Massie JB, Heller JG, Abitbol JJ, et al. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99-108.
3 Arens S, Schlegel U, Printzen G, et al. Influence of materials for fixation implants on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br. 1996;78:647-651.
4 Wang JC, Yu WD, Sandhu HS, et al. Metal debris from titanium spinal implants. Spine. 1999;24:899-903.
5 Cunningham BW, Orbegoso CM, Dmitriev AE, et al. The effect of spinal instrumentation particulate wear debris. An in vivo rabbit model and applied clinical study of retrieved instrumentation cases. Spine J. 2003;3:19-32.
6 Anderson PA, Sasso RC, Rouleau JP, et al. The Bryan Cervical Disc: wear properties and early clinical results. Spine J. 2004;4(6 Suppl):303S-309S.
7 Chang BS, Brown PR, Sieber A, et al. Evaluation of the biological response of wear debris. Spine J. 2004;4(6 Suppl):239S-244S.
8 Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter FDA IDE study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32:1155-1162.
9 Spivak JM, Petrizzo AM. Revision of a lumbar disc arthroplasty following late infection. Eur Spine J. 2010;19:677-681.
10 Truummees E, Demetropoulos CK, Yang KH, Herkowitz HN. Failure of human cervical endplates: a cadaveric experimental model. Spine. 2003;28:2204-2208.
11 Hasegawa K, Abe M, Washio T, et al. An experimental study of the interface strength between titanium mesh cage and vertebra in reference to vertebral bone mineral density. Spine. 2001;26:957-963.
12 Jost B, Cripton PA, Lund T, et al. Compressive strength of interbody cages in the lumbar spine: the effect of cage shape, posterior instrumentation, and bone density. Eur Spine J. 1998;7:132-141.
13 Sawin PD, Dickman CA, Crawford NR, et al. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg Spine. 2001;94:76-81.
14 Andersen T, Christiensen FB, Laursen M, et al. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine. 2001;26:2623-2628.
15 Hilibrand AS, Fye MA, Emery SE, et al. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg Am. 2001;83-A:668-673.
16 Glassman SD, Anagnost SC, Parker A, et al. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25:2608-2615.
17 McPhee IB, Williams RP, Swanson CE. Factors influencing wound healing after surgery for metastatic disease of the spine. Spine. 1998;23:726-733.
18 Wise JJ, Fischgrund JS, Herkowitz HN, et al. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine. 1999;24:1943-1951.
19 Klein JD, Garfin SR. Nutritional status in the patient with a spinal infection. Orthop Clin North Am. 1996;27:33-36.
20 Hu SS, Fontaine F, Kelly B, Bradford DS. Nutritional depletion in staged spinal reconstructive surgery. The effect of total parenteral nutrition. Spine. 1998;23:1401-1405.
21 McLain RF, Burkus JK, Benson DR. Segmental instrumentation for thoracic and thoracolumbar: prospective analysis of construct survival and five-year follow-up. Spine J. 2001;1:310-323.
22 Klein JD, Hey LA, Yu CS, et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine. 1996;21:2676-2682.
23 McAfee PC, Weiland DJ, Carlow JJ. Survivorship analysis of pedicle spinal instrumentation. Spine. 1991;16(Suppl):S422-S427.
24 Ghanayem AJ. “Cages: Clinical Indication and Augmentation” in Advanced Spinal Surgical Technologies. Quality Medical Publishing; 2004.
25 Ghanayem AJ, Zdeblick TA. “Anterior Z-Plate Thoracolumbar Instrumentation” in Spinal Instrumentation, 2nd edition. Philadelphia: Lippincott-Raven Publishers; 1999.
26 Curylo LJ, Mason HC, Bohlman HH, Yoo JU. Tortuous course of the vertebral artery and anterior cervical decompression: a cadaveric and clinical case study. Spine. 2000;25:2860-2864.
27 Park JB, Cho YS, Riew KD. Development of adjacent level ossification in patients with an anterior cervical plate. J Bone Joint Surg Am. 2005;87-A:558-563.
28 Platzer P, Thalhammer G, Ostermann R, et al. Anterior screw fixation of odontoid fractures comparing younger and elderly patients. Spine. 2007;32:1714-1720.
29 Mendoza-Lattes S, Clifford K, Bartelt R, et al. Dysphagia following anterior cervical arthrodesis is associated with continuous, strong retraction of the esophagus. J Bone Joint Surg Am. 2008;90:256-263.
30 Currier BL, Todd LT, Maus TP, et al. Anatomic relationship of the internal carotid artery to the C1 vertebra: a case report of cervical reconstruction for chordoma and pilot study to assess the risk of screw fixation of the atlas. Spine. 2003;28:E461-E467.
31 Kostuik JP, Valdevit A, Chang HG, Kanzaki K. Biomechanical testing of the lumbosacral spine. Spine. 1998;23:1721-1728.
32 SS Hu. Internal fixation in the osteoporotic spine. Spine. 1997;22(24 Suppl):43S-48S.
33 Glassman SD, Alegre GM. Adult spinal deformity in the osteoporotic spine: options and pitfalls. Instr Course Lect. 2003;52:579-588.
34 Koptan WM, Elmiligui YH, Elsebaie HB. All pedicle screw instrumentation for Scheuermann’s kyphosis: it is worth it? Spine J. 2009;9:296-302.
35 Watanabe K, Lenke LG, Bridwell KH, et al. Comparison of radiographic outcomes for the treatment of scoliotic curves greater than 100 degrees: wires versus hooks versus screws. Spine. 2008;33:1084-1092.
36 Lenke LG, Kuklo TR, Ondra S, Polly DW. Rationale behind current state-of-the-art treatment of scoliosis (in the pedicle screw era). Spine. 2008;33:1051-1054.
37 Lehman RA, Polly DW, Kuklo TR, et al. Straight-forward versus anatomic trajectory technique of thoracic pedicle screw fixation: a biomechanical analysis. Spine. 2003;28:2058-2065.
38 Polly DW, Potter BK, Kuklo TR, et al. Volumetric spinal canal intrusion: a comparison between thoracic pedicle screws and thoracic hooks. Spine. 2004;29:63-69.
39 Vaccaro AR, Rizzolo SJ, Balderston RA, et al. Placement of pedicle screws in the thoracic spine. Part II: An anatomical and radiographic assessment. J Bone Joint Surg Am. 1995;77:1200-1206.
40 Lonstein JE, Denis F, Perra JH, et al. Complications associated with pedicle screws. J Bone Joint Surg Am. 1999;81:519-1528.
41 Suk SI, Kim WJ, Lee SM, et al. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine. 2001;26:2049-2057.
42 Kim YJ, Lenke LG, Bridwell KH, et al: Thoracic pedicle screw placement in deformity: Is it safe? Poster presentation at the Scoliosis Research Society, September 17-21, 2001, Cleveland, Ohio, 2001.
43 Humphreys SC, Hodges SD, Patwardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine. 2001;26:567-571.
44 Brislin B, Vaccaro AR. Advances in posterior lumbar interbody fusion. Orthop Clin North Am. 2002;33:367-374.
45 Tencer AF, Hampton D, Eddy S. Biomechanical properties of threaded inserts for lumbar interbody spinal fusion. Spine. 1995;20:2408-2414.
46 Wang ST, Goel VK, Fu CY, et al. Posterior instrumentation reduces differences in spine stability as a result of different cage orientations: an in vitro study. Spine. 2005;30:62-67.
47 Harris BM, Hilibrand AS, Savas PE, et al. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine. 2004;29:E65-E70.
48 Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
49 Glassman SD, Dimar JR3rd, Burkus K, et al. The efficacy of rhBMP-2 for posterolateral lumbar fusion in smokers. Spine. 2007;32:1693-1698.
50 Dawson E, Bae HW, Burkus JK, et al. Recombinant human bone morphogenetic protein-2 on an absorbable collage sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91:1604-1613.
51 Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9:623-629.
52 Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusion: a series review. Spine. 2009;34:1480-1484.
53 Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32:2885-2890.
54 Wong DA, Kumar A, Jatana S, et al. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8:1011-1018.
55 Chen NF, Smith ZA, Stiner E, et al. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12:40-46.
56 Smucker JD, Rhee JM, Singh K, et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31:2813-2819.
57 Butterman GR. Prospective nonrandomized comparison of an allograft with bone morphogenetic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8:426-435.
58 Tumialan LM, Pan J, Rodts GE, Mummaneni PV. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8:529-535.