92 Inotropic Therapy
 Rationale for Using Inotropic Therapy in the Critically Ill
Rationale for Using Inotropic Therapy in the Critically Ill
Use of Inotropes for Reversing Impaired Myocardial Contractility
The first category of situations where inotropic therapy is generally considered includes cardiogenic shock, acute heart failure, or acute exacerbation of chronic heart failure. However, although the use of such therapy in these clinical conditions seems logical on a purely pathophysiologic basis, no demonstration of a beneficial impact on morbidity and mortality can be found in the literature. Moreover, almost all the commercially available inotropes have been shown to be associated with increased mortality rates when given on a long-term basis to patients with chronic heart failure. It has been postulated that the long-term use of inotropes may lead to deterioration of left ventricular function through acceleration of myocardial cell apoptosis.1 Additionally, the beneficial effects on mortality of agents known to have negative inotropic effects, such as β-blockers, is now well established in patients with chronic heart failure.2,3 Therefore, inotropic therapy is generally reserved for patients with cardiogenic shock or for patients with advanced heart failure whose condition is refractory to standard therapy including diuretics, digoxin, β-blockers and angiotensin-converting enzyme (ACE) inhibitors. Under these conditions, clinicians can expect short-term positive effects of intravenous (IV) inotropic therapy, allowing cardiovascular stabilization. In patients with refractory heart failure who are candidates for cardiac transplantation, this therapy can be used as a bridge to transplantation. In those with potentially reversible causes of acute heart failure (such as myocardial infarction or acute myocarditis), short-term inotropic therapy must be considered as an appropriate bridge to coronary revascularization or recovery. The development of bedside echocardiography in the intensive care unit (ICU) should allow appropriate use of inotropic therapy, since this method provides a more accurate assessment of systolic cardiac function than traditional invasive methods like pulmonary artery catheterization.
Use of Inotropes for Achieving Supranormal Levels of Oxygen Delivery
High-Risk Surgical Patients
The concept of attempting to achieve supranormal hemodynamic endpoints emerged from studies in high-risk surgical patients. In a prospective study in high-risk patients undergoing surgery, Shoemaker et al. showed that the use of supranormal hemodynamic values as therapeutic endpoints was associated with a reduction in mortality from 33% to 4%.4 In the protocol group, dobutamine and dopamine were given as inotropic drugs—even in the absence of evidence of reduced cardiac contractility—when volume resuscitation (and packed red blood cells if necessary) failed to achieve supranormal values of myocardial oxygen delivery (DO2)4 (DO2 > 600 mL/min/m2). In other randomized studies performed in high-risk patients undergoing surgery, the deliberate perioperative increase in DO2 above supranormal values using fluid infusion and various inotropic drugs (dobutamine, dopamine, epinephrine, dopexamine) were associated with decreased mortality and postoperative complications.5 It remains unclear, however, whether the benefits were related to the increased DO2 per se or to other antiinflammatory effects of catecholamines.6 The issue of drug dose is also essential. A recent meta-analysis has suggested that in the setting of major surgery, dopexamine at low doses but not at high doses could improve outcome.7 From all these findings, it is reasonable to consider the increase of cardiac output and DO2 towards supranormal values during the perioperative period in high-risk patients undergoing elective major surgery.
Critically Ill Patients
Whether this therapeutic approach could also be applied to patients admitted to the ICU for established acute illnesses has been a matter of debate. On the one hand, a pathologic myocardial oxygen consumption/oxygen delivery (VO2/DO2) dependency, presumably due to impaired oxygen extraction capabilities, has been reported in various categories of acute illnesses such as sepsis8 and acute respiratory distress syndrome.9 Such a phenomenon was reported to correlate with the presence of increased blood lactate, a marker of global tissue hypoxia,8 and to be associated with a poor outcome.10 This so-called pathologic oxygen consumption/supply dependency would incite the clinician to increase DO2 towards supranormal values to overpass its critical level. However, such an aggressive therapeutic approach has been seriously questioned since the publication of randomized clinical trials performed in patients with acute illnesses that did not demonstrate any benefit from deliberate manipulation of hemodynamic variables toward values higher than physiologic values.11,12 In one of these studies, the mortality rate was even higher in the group of patients assigned to receive an aggressive treatment aimed at achieving supranormal values of DO2.11 It was postulated that deleterious consequences of the use of high doses of dobutamine in patients of the protocol group were responsible for the increased mortality. It has to be noted that (1) the patients of the protocol group received high doses of the inotropic agent despite the absence of evidence for an altered contractility, and (2) in most of these patients, the aggressive inotropic support failed to achieve the target value of VO2 (170 mL/min/m2). The analysis of the subgroup of septic patients of this study showed that the survivors were characterized by ability to increase both DO2 and VO2 regardless of their group of randomization.13 The non-survivors were characterized by an inability to increase their VO2 despite the increase in DO2, suggesting a more marked impairment of peripheral oxygen extraction in non-survivors than in survivors.13 In addition, the ability to increase cardiac output and DO2 was also significantly reduced in non-survivors in comparison with survivors, suggesting a decrease in cardiac reserve in those patients who will die.13 This is not a surprising finding, since the degree of myocardial dysfunction in septic shock correlates with increased risk of death. In this regard, it has been suggested that the response to a dobutamine challenge could have a prognostic value in septic patients. Indeed, in two prospective studies, survivors were able to increase both VO2 and DO2 in response to dobutamine, while non-survivors were unable to increase either DO2 or VO2 or both.14,15
From all the results of randomized controlled studies, the deliberative attempt to achieve supranormal hemodynamic targets in the general population of critically ill patients is no longer recommended.16,17 However, in the early phase of septic shock when blood flow and DO2 are generally low, an aggressive hemodynamic therapy including inotropes, aimed at rapidly normalizing DO2, was demonstrated to result in a better outcome in a randomized control trial.18 Thus, in the early phase of septic shock and maybe in other acute illnesses, it could be essential to rapidly restore normal global blood flow to avoid further deleterious consequences of systemic hypoperfusion. In later stages of the disease, with inflammatory processes and organ dysfunction already developed, no evidence of benefit from a further increase in DO2 has been shown. However, it seems likely that cardiac output should be kept in the normal range by using volume and/or inotropes to prevent worsening of the insult.
 Pharmacologic Properties of Inotropic Agents
Pharmacologic Properties of Inotropic Agents
Adrenergic Signaling
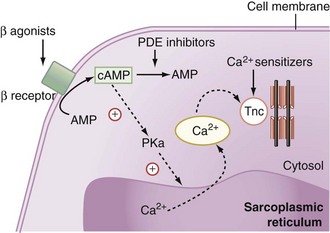
Natural as well as synthetic catecholamines enhance the Ca2+ cytosolic amount, which is directly related to the force of contraction (Figure 92-1). Ca2+ fixes on the troponin C Ca2+-specific binding site, inducing a conformational change that leads to the fixation of the myosin head to the actin filament. Hydrolysis of the adenosine monophosphate (ATP) molecule located on the myosin head to adenosine diphosphate (ADP) simultaneously induces the flexion of the myosin neck and the shortening of the contractile apparatus.
β1-Adrenergic Receptors
Pharmacologic Properties of the Inotropic Agents Used in Clinical Practice
Phosphodiesterase Inhibitors
Despite the major role of catecholamines in the management of critically ill patients with inadequate cardiac output, problems such as tachycardia, arrhythmias, increased myocardial VO2, excessive vasoconstriction, or loss of effectiveness with prolonged exposure to β-agonists may occur. Thus, other inotropic drugs such as phosphodiesterase inhibitors (milrinone and enoximone) have been proposed for the management of myocardial dysfunction. These synthetic drugs inhibit the peak III isoform of phosphodiesterase, which catalyses cAMP (see Figure 92-1). By increasing intracellular cAMP concentration, they induce a potent vasodilation of arterial and venous systems through relaxation of vascular smooth muscle. The left ventricular preload is reduced to a greater extent than with dobutamine. At the cardiac level, phosphodiesterase inhibitors induce an inotropic effect similar to that induced by dobutamine. The heart rate is increased only at high rates of administration. The resulting effect is an increase in cardiac output. Because the enhancement of cAMP intracellular concentration also promotes the reuptake of Ca2+ by the sarcoplasmic reticulum, phosphodiesterase inhibitors facilitate ventricular relaxation. Finally, since β-agonists exert their action by increasing the production of cAMP, phosphodiesterase inhibition could enhance their adrenergic effects. This is the pharmacologic basis for the synergic association of β-agonists and phosphodiesterase inhibitors.
Calcium Sensitizers
Calcium sensitizers increase the sensitivity of troponin C for Ca2+ and hence the force and duration of the cardiomyocytes’ contraction (see Figure 92-1). To date, levosimendan is the only calcium sensitizer approved for clinical use. The advantage of levosimendan over classical inotropes would be to increase the force of contraction without enhancing the influx of Ca2+ into the cytosol and thus without increasing the risk of arrhythmias related to this ionic alteration. Some degree of phosphodiesterase III inhibitory activity probably also contributes to the inotropic effect of these drugs. It also induces vasodilation by opening ATP-dependent K+ channels.19
Cardiac Myosin Activators
Cardiac myosin activators belong to a new class of inotropes. They increase the activity of the ATPase of the myofibrils, increasing the contractile force of the cardiomyocytes without increasing the amount of ATP molecules required for contraction—that is, without increasing the myocardial VO2.20 Additionally, these substances increase the cardiac contractile force without the potentially deleterious increase in intracytoplasmic Ca2+ concentration. Cardiac myosin activators have been tested in animal studies in which their inotropic properties have been well demonstrated. Pharmacologic studies in humans are ongoing.
Istaroxime
Istaroxime is a new drug that inhibits the Na+/K+-ATPase, increasing the activity of the sarcoendoplasmic reticulum calcium ATPase pump. It induces some inotropic and lusitropic effects.21 In animals, istaroxime was demonstrated to decrease the end-diastolic volume of the left ventricle and to increase the left ventricular ejection fraction. In patients with decompensated heart failure without hypotension, istaroxime decreased the pulmonary artery occlusion pressure and improved the diastolic function of the left ventricle.22 This drug is still under clinical evaluation.
 Hemodynamic Effects of Inotropic Agents in Critically Ill Patients
Hemodynamic Effects of Inotropic Agents in Critically Ill Patients
Effects on Cardiac Output
Dobutamine and Dopamine
In patients with acute heart failure, the effects of these two agents were compared in a crossover trial.26 Whereas dobutamine (2.5-10 µg/kg/min) increased cardiac output through an increase in stroke volume in a dose-response fashion, dopamine increased stroke volume and cardiac output at 4 µg/kg/min but not at higher doses, presumably because of an increase in left ventricular afterload. It was also reported that pulmonary artery occlusion pressure decreased with dobutamine while it increased with dopamine. Similar findings were observed in patients with respiratory failure in whom dopamine also increased the left ventricular end-diastolic volume measured using isotopes, while dobutamine did not.27 This suggests an increase in left ventricular preload only with dopamine.
In patients with septic shock, in addition to hypovolemia, severe systemic vasodilation is associated with a variable degree of depressed myocardial contractility.28 Dopamine at median or high doses has been proposed as one of the first-line catecholamines when arterial pressure remains low despite adequate volume resuscitation,19 as it can exert both an α-mediated increase in arterial tone and a β-mediated increase in myocardial contractility. However, it was reported that restoration of an adequate MAP with dopamine was mainly produced by the increase of cardiac output through an increase in stroke volume and, to a lesser extent, increase in heart rate; whereas minimal effects on systemic vascular resistance (SVR) were observed despite relatively high doses of this agent.29 Dopamine was even demonstrated to increase cardiac output markedly while SVR fell in septic patients without shock.30 Conversely, in another study in patients with severe septic shock, cardiac output did not increase significantly with dopamine at doses up to 25 µg/kg/min while SVR either did not change or significantly increased.31 This emphasizes the great heterogeneity in the response to dopamine among septic patients and hence the difficulty to predict clinical hemodynamic effects from pharmacologic properties because of interindividual differences in terms of severity of the insult, underlying diseases, comorbidities, integrity of the neurovegetative status, drugs concomitantly prescribed, and other factors.
In patients with septic shock and depressed myocardial function, dobutamine is expected to increase stroke volume and heart rate owing to its β1-adrenergic properties but a vasodilatory effect owing to its β2-adrenergic properties. Accordingly, an increase in cardiac output and a decrease in SVR with dobutamine were reported in septic patients.32,33 This emphasizes the need to give a potent vasopressive agent to septic shock patients when dobutamine is administered to support cardiac function in the presence of depressed myocardial contractility. One potential advantage of dobutamine is the decrease in cardiac filling pressures that could allow an additional volume infusion to improve further cardiac output when necessary. A change from dopamine to dobutamine was shown to result in lower right and left ventricular filling pressures and an increase in right ventricular ejection fraction for the same pulmonary artery pressure and right ventricular end-diastolic volume suggesting that dobutamine can exert a more favorable effect on cardiac contractility than dopamine.34 This has justified the recommendation to give dobutamine rather than dopamine when use of an inotropic drug is judged necessary in patients with severe sepsis or septic shock.17 However, because of the alteration of the β-adrenergic pathway in the septic heart, the effect on stroke volume and cardiac output of a β-agonist agent such as dobutamine may be attenuated in septic patients in comparison with nonseptic patients. In this regard, infusion of dobutamine at 5 µg/kg/min, a dose able to increase cardiac output substantially in patients with congestive heart failure,35 has been reported to exert variable effects in the context of sepsis. For example, dobutamine at 5 µg/kg/min was reported to induce a substantial increase in cardiac output in some studies in patients with severe sepsis32,36 and to have no significant effect on cardiac output in some studies investigating patients with septic shock.37–41 It is likely that these differences in response to dobutamine were related to various individual factors, including differences in the vasopressor treatment coadministered, in the degree of myocardial depression and/or β-receptor down-regulation. In this regard, Silverman and associates showed that incremental doses of dobutamine (0, 5, 10 µg/kg/min) produced a dose-related increase in cardiac output in septic patients without shock but no positive effect on cardiac output in patients with septic shock, even for the highest dose.23 Interestingly, they also found that post-β-adrenergic receptor signal transmission was impaired only in patients of the septic shock group and that impairment of β-adrenergic receptor responsiveness found in both groups was significantly more marked in the septic shock group.23 These findings which allow the divergent results of numerous studies to be reconciled32,36–43 emphasize the unpredictability of the effects of β-agonist agents in patients with sepsis. It must be stressed that the absence of positive cardiac response to dobutamine seems a marker of poor outcome in septic shock patients.14,15,40 Because dobutamine also has potentially harmful effects (e.g., myocardial ischemia, cardiac arrhythmias), monitoring its effects on cardiac output to check its efficacy is the minimum required. However, no high-level recommendation on which method of cardiac output monitoring (e.g., pulmonary artery catheter, transesophageal Doppler, pulse contour method) is the more appropriate in this setting is currently available.
Epinephrine and Norepinephrine
Although these agents have β1-adrenergic properties and thus are able to increase myocardial contractility, they are used as vasoconstrictive agents in cases of severe hypotension, since they also have potent α-adrenergic properties. Yet, significant increases in cardiac output with these drugs, consistent with potent inotropic effects, have been reported in septic patients.29,44,45 In this regard, norepinephrine was shown to increase cardiac output to the same extent as dopamine for the same increase in MAP.29 However, analysis of the existing literature indicates that the effects of norepinephrine on cardiac output are highly variable among septic patients.46,47 By contrast, epinephrine appeared to be a potent inotropic agent in most studies in septic patients.39,48–50
Dopexamine
The pharmacologic properties of dopexamine should result in a combination of inotropic, afterload-reducing, and renal-vasodilating effects which could be useful for the management of acute exacerbation of congestive heart failure. In this regard, dopexamine was reported to substantially increase cardiac output in patients with heart failure without altering blood pressure: at doses up to 4 µg/kg/min, the majority of the effects resulted from increase in stroke volume. At higher doses, the increase in heart rate made a greater contribution.51 In cases of human sepsis, dopexamine produced dose-dependent increases in stroke volume and heart rate but dose-dependent decrease in SVR.52 This underlines the marked vasodilating effect of this drug, which should not be administered in severe sepsis in the absence of a potent vasopressor. Under these conditions, dopexamine at doses ranging drom 1 to 4 µg/kg/min could still enhance cardiac output without altering blood pressure.53
Phosphodiesterase Inhibitors
In patients with heart failure, phosphodiesterase inhibitors significantly increased cardiac output and stroke volume, whereas blood pressure slightly decreased due to decrease in SVR, confirming the combined inotropic and vasodilating effects of these agents.54 Because of the ability of β-agonist agents to increase cAMP levels, thereby providing increased substrate for phosphodiesterase inhibitors, the combination of these two types of drugs would be attractive. Synergic effects on cardiac output of dobutamine and enoximone have been observed in patients with heart failure.55
Calcium Sensitizers
Levosimendan has stimulated many clinical studies during recent years. It is well demonstrated that it can induce some beneficial hemodynamic effects in patients with acute heart failure, enhancing cardiac output and decreasing pulmonary artery occlusion pressure.56 In the LIDO study, levosimendan was even demonstrated to improve hemodynamic performance more effectively in patients with low-output heart failure.56 Unlike dobutamine, levosimendan can keep its effects on cardiac performance in patients receiving β-blockers.56
Effects on Arterial Oxygen Content
The aim of inotropic therapy in critically ill patients with reduced cardiac contractility is not only to increase cardiac output but ultimately to improve DO2 to the tissues. Thus, attention should also be paid to the effects of these drugs on arterial oxygen content. Inotropes may affect arterial oxygen tension through several mechanisms. First, the reduction of lung filtration pressure resulting from improvement in cardiac function may decrease intrapulmonary shunt fraction and thus improve arterial oxygenation. Second, the increase in cardiac output may result in an increased venous admixture.57 On the other hand, the increased mixed venous blood oxygen tension resulting from increased cardiac output may improve arterial oxygenation in the presence of ventilation/perfusion mismatching and thus may compensate for the increased venous admixture. Accordingly, when looking at the published data, it appears that even if venous admixture increased with administration of an inotropic agent, no significant change in arterial oxygen tension was observed.58,59 Therefore, when an inotropic agent increases cardiac output in critically ill patients, it generally increases DO2 to the same extent.29,32,60
Effects on Tissue Oxygen Utilization
Septic Shock
The maldistribution of flow at the macrocirculatory level as well as the microcirculatory level mainly contributes to defective tissue utilization and eventually to tissue oxygen debt in sepsis, even when systemic oxygen transport is greater than normal. Besides sepsis-induced microthrombosis, sepsis-induced alteration in vascular reactivity is a major cause of altered distribution of blood flow between and within organs. In addition, severe sepsis can modify the impact of endogenous catecholamines and adrenergic drugs on regional blood flows, since a depressed vascular responsiveness to vasoactive agents is likely to occur in this setting. This hypothesis may account for the absence of reduction of renal blood flow observed during norepinephrine administration in bacteremic animals in comparison with controls.60
In cases of human sepsis, numerous studies examined the effects of adrenergic agents on splanchnic perfusion. Their findings have sometimes varied, either because of differences in the methods used for assessing this regional circulation (e.g., gastric tonometry, laser-Doppler flowmetry, indocyanine green dilution) or because of the heterogeneity of the studied populations (e.g., differences in the severity of the septic insult, in the underlying diseases, in the therapy coadministered). However, from findings of the majority of these studies, some reasonable conclusions can be drawn. First, dobutamine is likely to exert a beneficial effect on the gut mucosal perfusion,33,38,39,43,61 probably via a β2-adrenergic effect.62 Second, dopamine may have deleterious effects on gut mucosal perfusion29 despite its potential vasodilating action through mesenteric dopaminergic receptors. Third, epinephrine is probably the adrenergic agent with the least desirable effects on the splanchnic vasculature. Most studies showed a lower splanchnic blood flow with epinephrine than norepinephrine alone63 or in combination with dobutamine,38,39,64 even for similar global hemodynamic effects. Fourth, dopexamine can exert a favorable effect on splanchnic perfusion65 comparable to that of dobutamine37 and likely to be related to a β2-adrenergic effect.
Regarding the effects of inotropic agents on the renal circulation in septic patients, two major points must be kept in mind. First, an α-adrenergic agent such as norepinephrine is able to increase renal blood flow and urine output31,66–68 despite its potential vasoconstricting effect on the afferent glomerular arteries. This is probably due to the beneficial effect of increasing MAP when the renal blood flow is dependent on arterial pressure, as occurs in the presence of profound systemic hypotension. Otherwise, sepsis-induced depressed responsiveness of afferent glomerular arteries to the action of norepinephrine cannot be excluded. Accordingly, there is no evidence that norepinephrine decreases renal blood flow and urine output when given to septic patients to increase MAP toward normal values. Moreover, it has been demonstrated in patients with septic shock that elevating MAP up to 85 mm Hg with incremental doses of norepinephrine was not associated with a decrease in urine output.66,69,70 Second, although dopamine at low doses (<5 µg/kg/min) is pharmacologically able to vasodilate renal arteries through its action on dopaminergic receptors, the systematic administration of low doses of dopamine in critically ill patients, including patients with sepsis, does not result in improved outcome71 and must no longer be recommended.
Catecholamines can also exert proper effects on the microcirculation. Administration of 5 µg/kg/min of dobutamine was demonstrated to improve sublingual microvessel perfusion measured with orthogonal polarizing spectral imaging in patients with septic shock.72 Interestingly, these changes were independent of changes in systemic hemodynamic variables.72 Two studies showed no significant effect of increasing MAP with norepinephrine on sublingual microvessels in patients with septic shock who had already been resuscitated.44,45 However, a possible favorable effect of norepinephrine on microcirculation cannot be excluded when norepinephrine is used to reverse life-threatening hypotension. Finally, inotropic drugs may also exert non-hemodynamic effects that could affect cellular metabolism and/or organ function.6,73 For example, administration of epinephrine in patients with septic shock was demonstrated to increase blood lactate level independently of tissue hypoxia by stimulation of the skeletal muscle cell Na+/K+-ATPase, which accelerates aerobic glycolysis and thus the production of pyruvate and hence of lactate into the cell.74 This metabolic effect is assumed to be related to activation of the β2-adrenergic receptors located at the surface of the skeletal muscle cells.75 In addition, catecholamines may modulate cytokine response to sepsis, trauma, or major surgery through β-adrenergic receptor activation.6 Whether this effect (inhibition of proinflammatory cytokines and enhancement of proinflammatory cytokine production) plays a beneficial role in the reversal of tissue hypoxia and organ dysfunction remains to be evaluated.
 Main Indications for Inotropic Therapy in Patients with Circulatory Failure
Main Indications for Inotropic Therapy in Patients with Circulatory Failure
Acute Heart Failure and Cardiogenic Shock
In the American College of Cardiology Federation/American Heart Association guidelines, inotropic agents are indicated to improve symptoms and end-organ function in patients with low output syndrome, left ventricular systolic dysfunction, and systolic blood pressure below 90 mm Hg despite adequate filling pressure.76 In the European Society of Cardiology guidelines, inotropic agents are indicated in patients with values ≤ 100 mm Hg.77 These indications clearly limit use of inotropic agents only for those patients with acute heart failure and low systolic blood pressure, who are most likely to have increased mortality rates with a strong inverse correlation between systolic blood pressure and survival.78
Dopamine is classically recommended as the inotropic agent of choice in the presence of severe hypotension, whereas dobutamine is considered first-line therapy in the presence of predominant pump failure and volume overload but normal or moderately reduced blood pressure.79,80 Accordingly, the SHOCK trial registry (1190 patients) reported that dopamine and dobutamine were used in 89% and 70%, respectively, of patients with cardiogenic shock due to massive acute myocardial infarction.81 The combination of dopamine and dobutamine at low doses has been considered a therapy of interest when dobutamine alone fails to restore an adequate MAP in cardiogenic shock. Nowadays, however, the use of dopamine is a matter of debate. In a recent study comparing dopamine and norepinephrine as the first-line vasopressor agent in the treatment of shock, dopamine was associated with a greater number of cardiac arrhythmias.82 In addition, in a predefined subgroup analysis, the authors reported that dopamine was associated with increased risk of death in the subgroup of 280 patients with cardiogenic shock.82
It must be stressed, however, that IV administration of a catecholamine such as dobutamine is associated with an increased risk of death in acute heart failure patients.83,84 This emphasizes their restrictive use for those patients with severe hypotension and peripheral hypoperfusion.76,77
Phosphodiesterase inhibitors have been proposed as an alternative to β-adrenergic agents. However, results of trials of long-term oral phosphodiesterase inhibitor therapy in chronic heart failure and of the OPTIME-CHF study in acute decompensation of congestive heart failure85 have been disappointing. Thus, the use of these agents is limited to just a few categories of patients: (1) patients with advanced heart failure awaiting transplantation, in whom IV milrinone could be better tolerated than dobutamine, and its use may allow continuation of β-blocker therapy for controlling arrhythmias or myocardial ischemia86; (2) patients with acute decompensation of chronic heart failure unable to achieve stabilization with standard treatment; and (3) patients with long-term β-blocker use, in whom short-term IV milrinone may even be preferred to dobutamine.
There is now clear evidence that inotropic agents such as β-agonist agents and phosphodiesterase inhibitors can exert both short-term beneficial hemodynamic effects and serious adverse effects that make them even deleterious in terms of long-term outcome. It is likely their adverse effects (e.g., arrhythmias, increased risk of myocardial ischemia) are related to the increased cAMP concentration in the cytosol of the cardiomyocyte.87
The initial enthusiasm for calcium sensitizers in heart failure patients has also been attenuated in the recent years. In the LIDO study, compared to dobutamine, levosimendan significantly decreased mortality and improved the hemodynamic condition.56 Nevertheless, these positive results have been contradicted by two large-scale studies. In the REVIVE study,88 even though levosimendan improved a composite judgment criteria of clinical signs of heart failure at 5 days compared to placebo, the mortality rate was not significantly changed. In the SURVIVE study,89 levosimendan was not better than dobutamine for increasing the survival rate in patients with acute heart failure requiring an inotropic support. A recent meta-analysis concluded that levosimendan improved hemodynamic parameters when compared with placebo but without showing evidence of survival benefit.90 All these negative results have impeded the commercialization of levosimendan in many countries.
Nitric oxide synthase inhibitors have been proposed for use in patients with cardiogenic shock, in whom NO production is increased and may exert deleterious effects on cardiac function and vascular tone.80 Tilarginine is a nonselective NO synthase inhibitor developed for treating acute heart failure. However, in the TRIUMPH study, tilarginine was unable to improve the survival rate of patients with cardiogenic shock at 3 months in comparison with placebo.91 These negative results have interrupted clinical development of this new drug.
Septic Shock
In cases of septic shock, dobutamine is generally considered the inotropic drug of choice when myocardial contractility is severely depressed.19 Detection of a marked decrease in left ventricular ejection fraction using bidimensional echocardiography92 can help diagnose a severe decrease in cardiac contractility and thus suggest the use of dobutamine when signs of peripheral hypoperfusion persist despite volume resuscitation and restoration of perfusion pressure with vasopressors. However, bedside bidimensional echocardiography is not yet available in all general ICUs, so the recommendation for using an inotrope such as dobutamine in septic shock is still based on the presence of a low cardiac output and high cardiac filling pressures after fluid resuscitation and an adequate MAP.19 Since dobutamine can exert a vasodilatory effect, its use requires concomitant use of a vasopressor such as norepinephrine. Epinephrine is a potent inotrope with vasopressive properties that could be used as an alternative to the combination of dobutamine and norepinephrine. A randomized study in patients with septic shock and a presumed cardiac dysfunction found no significant difference in patient outcome between epinephrine alone and norepinephrine plus dobutamine.93 However, this study has been criticized for a lack of statistical power. In the condition of depressed vascular tone and reduced myocardial function, epinephrine was shown to be inferior to the combination of dobutamine and norepinephrine in terms of splanchnic perfusion, despite similar effects on systemic blood flow and pressure.38,39,64 For all these reasons, epinephrine is not recommended as the first-choice drug when treatment of impaired cardiac contractility is considered.19
Use of new inotropic drugs such as levosimendan has been proposed as an alternative to dobutamine in case of severe septic myocardial depression that no longer responds to dobutamine administration.94 The rationale for using levosimendan is that the sensitivity of calcium to myofilament is reduced during sepsis, probably because of an abnormal phosphorylation of the troponin complex at the site where the calcium ion binds to troponin C.95 Because levosimendan can improve not only left ventricular function but also right ventricular performance96,97 through pulmonary vasodilation,96 it might be useful in cases of septic myocardial depression with associated lung injury. However, more studies are needed to reach definitive conclusions about the utility of levosimendan in septic shock with myocardial depression.98
In summary, given all the available data, when inotropic therapy is used to reverse cardiac dysfunction in severe sepsis, the combination of norepinephrine and dobutamine is still recommended.19
Key Points
De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779-789.
Hayes MA, Timmins AC, Yau E, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717-1722.
Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883-1891.
Morelli A, De Castro S, Teboul JL, et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 2005;31:638-644.
Silverman HJ, Penaranda R, Orens JB, et al. Impaired beta-adrenergic receptor stimulation of cyclic adenosine monophosphate in human septic shock: association with myocardial hyporesponsiveness to catecholamines. Crit Care Med. 1993;21:31-39.
The TRIUMPH Investigators. Effect of tilarginine acetate in patients with acute myocardial infarction and shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657-1666.
1 Singh M, Roginskaya M, Dalal S, et al. Extracellular ubiquitin inhibits beta-AR-stimulated apoptosis in cardiac myocytes: role of GSK-3beta and mitochondrial pathways. Cardiovasc Res. 2010;86:20-28.
2 Jondeau G, Neuder Y, Eicher JC, et al. B-CONVINCED: Beta-blocker CONtinuation Vs. INterruption in patients with Congestive heart failure hospitalizED for a decompensation episode. Eur Heart J. 2009;30:2186-2192.
3 Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391-e479.
4 Shoemaker WC, Appel P, Kram HB, et al. Prospective trial of supranormal value of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176-1186.
5 Rampal T, Jhanji S, Pearse RM. Using oxygen delivery targets to optimize resuscitation in critically ill patients. Curr Opin Crit Care. 2010. Mar 18. [Epub ahead of print]
6 Uusaro A, Russell JA. Could anti-inflammatory actions of catecholamines explain the possible beneficial effects of supranormal oxygen delivery in critically ill surgical patients? Intensive Care Med. 2000;26:299-304.
7 Pearse RM, Belsey JD, Cole JN, et al. Effect of dopexamine infusion on mortality following major surgery: individual patient data meta-regression analysis of published clinical trials. Crit Care Med. 2008;36:1323-1329.
8 Vincent JL, Roman A, Kahn RJ. Oxygen uptake/supply dependency: effects of short term dobutamine infusion. Am Rev Respir Dis. 1990;142:2-8.
9 Ranieri VM, Giuliani R, Eissa NT, et al. Oxygen delivery-consumption relationship in septic adult respiratory distress syndrome patients: the effects of positive end-expiratory pressure. J Crit Care. 1992;7:150-157.
10 Bihari D, Smithies M, Gimson A, et al. The effects of vasodilation with prostacyclin on oxygen delivery and uptake in critically ill patients. N Engl J Med. 1987;317:397-404.
11 Hayes MA, Timmins AC, Yau E, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717-1722.
12 Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. N Engl J Med. 1995;333:1025-1032.
13 Hayes MA, Timmins AC, Yau EH, et al. Oxygen transport patterns in patients with sepsis syndrome or septic shock: influence of treatment and relationship to outcome. Crit Care Med. 1997;25:926-936.
14 Vallet B, Chopin C, Curtis SE, et al. Prognostic value of the dobutamine test in patients with sepsis syndrome and normal lactate values: A prospective, multicenter study. Crit Care Med. 1993;21:1868-1875.
15 Rhodes A, Lamb FJ, Malagon I, et al. A prospective study of the use of a dobutamine stress test to identify outcome in patients with sepsis, severe sepsis, or septic shock. Crit Care Med. 1999;27:2361-2366.
16 Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France 27-28 April 2006. Intensive Care Med. 2007;33:575-590.
17 Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296-327.
18 Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
19 Tavares M, Rezlan E, Vostroknoutova I, et al. New pharmacologic therapies for acute heart failure. Crit Care Med. 2008;36(1 Suppl):S112-S120.
20 Teerlink JR. A novel approach to improve cardiac performance: cardiac myosin activators. Heart Fail Rev. 2009;14:289-298.
21 Micheletti R, Palazzo F, Barassi P, et al. Istaroxime, a stimulator of sarcoplasmic reticulum calcium adenosine triphosphatase isoform 2a activity, as a novel therapeutic approach to heart failure. Am J Cardiol. 2007;99:24A-32A.
22 Shah SJ, Blair JE, Filippatos GS, et al. Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: results from the Hemodynamic, Echocardiographic, and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: a Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) trial. Am Heart J. 2009;157:1035-1041.
23 Silverman HJ, Penaranda R, Orens JB, et al. Impaired beta-adrenergic receptor stimulation of cyclic adenosine monophosphate in human septic shock: association with myocardial hyporesponsiveness to catecholamines. Crit Care Med. 1993;21:31-39.
24 Bernardin G, Kisoka RL, Delporte C, et al. Impairment of beta-adrenergic signaling in healthy peripheral blood mononuclear cells exposed to serum from patients with septic shock: involvement of the inhibitory pathway of adenylyl cyclase stimulation. Shock. 2003;19:108-112.
25 Matsuda N, Hattori Y, Akaishi Y, et al. Impairment of cardiac beta-adrenoceptor cellular signaling by decreased expression of G(s alpha) in septic rabbits. Anesthesiology. 2000;93:1465-1473.
26 Leier CV, Heban PT, Huss P, et al. Comparative systemic and regional hemodynamic effects of dopamine and dobutamine in patients with cardiomyopathic heart failure. Circulation. 1978;58:466-475.
27 Molloy DW, Ducas J, Dobson K, et al. Hemodynamic management in clinical acute hypoxemic respiratory failure. Dopamine vs dobutamine. Chest. 1986;89:636-640.
28 Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699-1713.
29 Marik PE, Mohedin M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA. 1994;272:1354-1357.
30 Day NP, Phu NH, Mai NT, et al. Effects of dopamine and epinephrine infusions on renal hemodynamics in severe malaria and severe sepsis. Crit Care Med. 2000;28:1353-1362.
31 Martin C, Papazian L, Perrin G, et al. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103:1826-1831.
32 Vincent JL, Roman A, Kahn RJ. Dobutamine administration in septic shock: addition to a standard protocol. Crit Care Med. 1990;18:689-693.
33 Joly LM, Monchy M, Cariou A, et al. Effects of dobutamine on gastric mucosal perfusion and hepatic metabolism in patients with septic shock. Am J Respir Crit Care Med. 1999;160:1983-1986.
34 Vincent JL, Reuse C, Kahn RJ. Effects of right ventricular function of a change from dopamine to dobutamine in critically ill patients. Crit Care Med. 1988;16:659-662.
35 Teboul JL, Boujdaria R, Graini L, et al. Cardiac index vs oxygen-derived parameters for rational use of dobutamine in patients with congestive heart failure. Chest. 1993;103:81-85.
36 Creteur J, De Backer D, Vincent JL. A dobutamine test can disclose hepatosplanchnic hypoperfusion in septic patients. Am J Respir Crit Care Med. 1999;160:839-845.
37 Levy B, Nace L, Bollaert PE, et al. Comparison of systemic and regional effects of dobutamine and dopexamine in norepinephrine-treated septic shock. Intensive Care Med. 1999;25:942-948.
38 Levy B, Bollaert PE, Lucchelli JP, et al. Dobutamine improves the adequacy of gastric mucosal perfusion in epinephrine treated septic shock. Crit Care Med. 1997;25:1649-1654.
39 Duranteau J, Sitbon P, Teboul JL, et al. Effects of epinephrine, norepinephrine or the combination of norepinephrine and dobutamine on gastric mucosa in septic shock. Crit Care Med. 1999;27:893-900.
40 Kumar A, Schupp E, Bunnell E, et al. Cardiovascular response to dobutamine stress predicts outcome in severe sepsis and septic shock. Crit Care. 2008;12:R35.
41 Jellema WT, Groeneveld AB, Wesseling KH, et al. Heterogeneity and prediction of hemodynamic responses to dobutamine in patients with septic shock. Crit Care Med. 2006;34:2392-2398.
42 De Backer D, Moraine JJ, Berre J, et al. Effects of dobutamine on oxygen consumption in septic patients. Direct versus indirect determinations. Am J Respir Crit Care Med. 1994;150:95-100.
43 Gutierrez G, Clark C, Brown SD, et al. Effect of dobutamine on oxygen consumption and gastric mucosal pH in septic patients. Am J Respir Crit Care Med. 1994;150:324-329.
44 Jhanji S, Stirling S, Patel N, et al. The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock. Crit Care Med. 2009;37:1961-1966.
45 Dubin A, Pozo MO, Casabella CA, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. 2009;13:R92.
46 Desjars P, Pinaud M, Tasseau F, et al. A reappraisal of norepinephrine therapy in human septic shock. Crit Care Med. 1987;15:134-137.
47 Meadows D, Edwards JD, Wilkins RG, et al. Reversal of intractable septic shock with norepinephrine therapy. Crit Care Med. 1988;16:663-667.
48 Bollaert PE, Bauer P, Audibert G, et al. Effects of epinephrine on hemodynamics and oxygen metabolism in dopamine-resistant septic shock. Chest. 1990;98:949-953.
49 Moran JL, O’Fathartaigh MS, Peisach AR, et al. Epinephrine as an inotropic agent in septic shock: A dose-profile analysis. Crit Care Med. 1993;21:70-77.
50 Le Tulzo Y, Seguin P, Gacouin A, et al. Effects of epinephrine on right ventricular function in patients with severe septic shock and right ventricular failure: a preliminary study. Intensive Care Med. 1997;23:664-670.
51 Smithies M, Yee TH, Jackson L, et al. Protecting the gut and the liver in the critically ill: effects of dopexamine. Crit Care Med. 1994;22:789-795.
52 Colardyn FC, Vandenbogaerde JF, Vogelaers DP, et al. Use of dopexamine hydrochloride in patients with septic shock. Crit Care Med. 1989;17:999-1003.
53 Kiefer P, Tugtekin I, Wiedeck H, et al. Effect of a dopexamine-induced increase in cardiac index on splanchnic hemodynamics in septic shock. Am J Respir Crit Care Med. 2000;161:775-779.
54 Ludmer PL, Wright RF, Arnold JM, et al. Separation of the direct myocardial and vasodilator actions of milrinone administered by an intracoronary infusion technique. Circulation. 1986;73:130-137.
55 Thuillez C, Richard C, Teboul JL, et al. Arterial hemodynamics and cardiac effects of enoximone, dobutamine, and their combination in severe heart failure. Am Heart J. 1993;125:799-808.
56 Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196-202.
57 Lynch JP, Mhyre JG, Dantzker DR. Influence of cardiac output on intrapulmonary shunt. J Appl Physiol. 1979;46:315-321.
58 Jardin F, Eveleigh MC, Gurdjian F, et al. Venous admixture in human septic shock: comparative effects of blood volume expansion, dopamine infusion and isoproterenol infusion on mismatching of ventilation and pulmonary blood flow in peritonitis. Circulation. 1979;60:155-159.
59 Regnier B, Safran D, Carlet J, et al. Comparative haemodynamic effects of dopamine and dobutamine in septic shock. Intensive Care Med. 1979;5:115-120.
60 Peng ZY, Critchley LA, Fok BS. The effects of increasing doses of noradrenaline on systemic and renal circulations in acute bacteraemic dogs. Intensive Care Med. 2005;31:1558-1563.
61 Neviere R, Mathieu D, Chagnon JL, et al. The contrasting effects of dobutamine and dopamine on mucosal perfusion in septic patients. Am J Respir Crit Care Med. 1996;154:1684-1688.
62 Shepherd AP, Riedel GL, Maxwell LC, et al. Selective vasodilators redistribute intestinal blood flow and depress oxygen uptake. Am J Physiol. 1984;247:G377-G384.
63 De Backer D, Creteur J, Silva E, et al. Effects of dopamine, norepinephrine and norepinephrine on the splanchnic circulation in septic shock: which is the best? Crit Care Med. 2003;31:1659-1667.
64 Meier-Hellman A, Reinhart K, Bredle DL, et al. Epinephrine impairs splanchnic perfusion in septic shock. Crit Care Med. 1997;25:399-404.
65 Seguin P, Laviolle B, Guinet P, et al. Dopexamine and norepinephrine versus epinephrine on gastric perfusion in patients with septic shock: a randomized study [NCT00134212]. Crit Care. 2006;10:R32.
66 Deruddre S, Cheisson G, Mazoit JX, et al. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. 2007;33:1557-1562.
67 Albanèse J, Leone M, Garnier F, et al. Renal effects of norepinephrine in septic and nonseptic patients. Chest. 2004;126:534-539.
68 Albanèse J, Leone M, Delmas A, et al. Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Crit Care Med. 2005;33:1897-1902.
69 LeDoux D, Astiz ME, Carpati CM, et al. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;8:2729-2732.
70 Bourgoin A, Leone M, Delmas A, et al. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med. 2005;33:780-786.
71 Bellomo R, Chapman M, Finfer S, et al. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139-2143.
72 De Backer D, Creteur J, Dubois MJ, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34:403-408.
73 Traber K, De Backer D, Radermacher P. Metabolic alterations in sepsis and vasoactive drugs-related metabolic effects. Curr Opin Crit Care. 2003;9:271-278.
74 Levy B, Gibot S, Franck P, et al. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: prospective study. Lancet. 2005;365:871-875.
75 Levy B, Desebbe O, Montemont C, et al. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock. 2008;30:417-421.
76 Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977-2016.
77 Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10:933-989.
78 Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217-2226.
79 Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004;110:e82-292.
80 Topalian S, Ginsberg F, Parrillo JE. Cardiogenic shock. Crit Care Med. 2008;36(1 Suppl):S66-S74.
81 Hochman JS, Buller CE, Sleeper LA, et al. Cardiogenic shock complicating acute myocardial infarction. Etiologies, management and outcome: A report from the SHOCK trial registry. J Am Coll Cardiol. 2000;36:1063-1070.
82 De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779-789.
83 Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol. 2005;46:57-64.
84 Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98-104.
85 Cuffe MS, Califf RM, Adams KFJr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541-1547.
86 Mehra MR, Ventura HO, Kapoor C, et al. Safety and clinical utility of long-term intravenous milrinone in advanced heart failure. Am J Cardiol. 1997;80:61-64.
87 Teerlink JR, Metra M, Zaca V, et al. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond. Heart Fail Rev. 2009;14:243-253.
88 Cleland JG, Freemantle N, Coletta AP, et al. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006;8:105-110.
89 Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883-1891.
90 Delaney A, Bradford C, McCaffrey J, et al. Levosimendan for the treatment of acute severe heart failure: a meta-analysis of randomised controlled trials. Int J Cardiol. 2010;138:281-289.
91 TRIUMPH Investigators. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657-1666.
92 Vieillard-Baron A, Caille V, Charron C, et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36:1701-1706.
93 Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet. 2007;370:676-684.
94 Morelli A, De Castro S, Teboul JL, et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 2005;31:638-644.
95 Tavernier B, Li JM, El-Omar MM, et al. Cardiac contractile impairment associated with increased phosphorylation of troponin I in endotoxemic rats. FASEB J. 2001;15:294-296.
96 Morelli A, Teboul JL, Maggiore SM, et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med. 2006;34:2287-2293.
97 Russ MA, Prondzinsky R, Carter JM, et al. Right ventricular function in myocardial infarction complicated by cardiogenic shock: Improvement with levosimendan. Crit Care Med. 2009;37:3017-3023.
98 De Backer D, Taccone FS, Radermacher P. Levosimendan in septic shock: another piece in the puzzle, but many pieces are still lacking. Intensive Care Med. 2007;33:403-405.