Section 1 Injection Therapy – The Evidence
The evidence base for injection therapy
Overview
Corticosteroid and local anaesthetic injection therapy has been in use for 60 years, and has stood the ‘test of time’.1 There is a wealth of anecdotal evidence for its efficacy, but few, if any, definitive studies,1–5 and few studies comparing injection therapy with other treatments; the comparative studies that do exist mainly concern the shoulder and elbow, and their conclusions are contradictory.6–20 Consequently, there are few facts and a mass of opinions – many of them dogmatic and contradictory – about almost every aspect of injection therapy21–24 and published guidelines for joint and soft tissue injections are based more on personal experience and anecdote than on evidence.1,4 This state of affairs is surprising, because injection therapy is the most common therapeutic intervention in rheumatological practice.25
Interpretation of injection therapy studies is compounded by a disconcerting lack of expert agreement about definitions, diagnosis, and outcome measures in musculoskeletal medicine,1,26–31 coupled with wide variations in methodology and quality between trials. Because of this, most authoritative reviews tend to be conservative in their estimates of the presence and size of treatment effects in injection therapy.3,5,32–43
Nonetheless, injection therapy is recommended for musculoskeletal (mainly knee and shoulder) disorders in national and international guidelines 3,44–48 and is used extensively for other musculoskeletal conditions.49,50 Given its relative safety,1,3,5,51–53 ease of application in trained hands and cost-effectiveness,3 plus the frequent lack of convincing systematic evidence for the effectiveness of alternatives,38 injection therapy is a very useful treatment modality.54 This is supported by the collective experience of the majority of clinicians in primary care and the locomotor specialties.55
In the first randomized study, patients with polyarticular disease who were treated with intra-articular injections of triamcinolone demonstrated significantly better pain control and range of motion than did those who were treated with the same total dosage of mini-pulse systemic steroids. Patient evaluation of disease activity, tender joint count, blood pressure, side effects, physician contacts, and hospital visits were significantly better for those treated with intra-articular steroids.56
The second study compared the efficacy and safety of intra-articular corticosteroid injection with systemic injection of the same dose of triamcinolone for the treatment of monoarthritis of the knee in rheumatoid arthritis patients. The intra-articular approach showed better results in terms of local inflammatory variables and improvement evaluation by the patient and physician.57
The definitive randomized trial to demonstrate the superiority of the intra-articular route of corticosteroid administration in inflammatory joint disease is still awaited. Nonetheless, authoritative international guidelines recommend that intra-articular corticosteroid injections should be considered for the relief of local symptoms in patients with inflammatory arthritis.58
As with other treatment modalities, the challenge for all clinicians delivering injection therapy is to implement evidence-based practice by applying the best research-based treatments, tempered by clinical experience and patients’ values.59 Where good research evidence is lacking, clinicians should become involved in research that will provide that evidence.
Delivery of injection therapy
Most general practitioners (GPs) in the UK carry out some joint and soft tissue injections, but limit themselves to knees, shoulders and elbows.60 A small highly active group receives referrals from colleagues.60,61 Most of the injections in the community are performed by just 5–15 % of GPs.61,62 The main perceived barriers to performing these injections are inadequate training, the inability to maintain injection skills and discomfort or lack of confidence with the performance of the technique.60–62 Training improves GPs’ injection activity and their level of confidence.63
In 1995 chartered physiotherapists in the UK were granted the right to use injection therapy, whereupon the authors of this textbook developed the first training programme in this field and were lead contributors to the only published injection therapy guidelines.64
Injections administered by physiotherapists have been shown to be part of a very effective way of managing orthopaedic65 and rheumatology 66 outpatients and patients in the community with musculoskeletal lesions.67 Extended Scope Practitioners in physiotherapy have been shown to be as effective as orthopaedic surgeons and to generate lower initial direct hospital costs.68 Podiatrists also deliver injection therapy for lower limb disorders and nurses have also been trained in musculoskeletal injection therapy.69,70
Current controversies in injection therapy
The research agenda in injection therapy
Certainly, newer agents may attract more interest because of their novelty value and (often unfulfilled) theoretical potential (see page 31, Other substances used for injection therapy).77 Perhaps research into novel treatments is generously funded by manufacturers (with the potential for partial reporting of results), while research into inexpensive, familiar treatments attracts little or no support from industry and academia. There are undoubtedly other reasons.
1. Ines L.P.B.S., da Silva J.A.P. Soft tissue injections. Best Pract Res Clin Rheumatol, 19. 2005: 303-527.
2. Peterson C., Holder J. Evidence-based radiology (part 2): Is there sufficient research to support the use of therapeutic injections into the peripheral joints? Skeletal Radiol. 2010;39:111-118.
3. National Collaborating Centre for Chronic Conditions. Osteoarthritis: national clinical guideline for care and management in adults. London: Royal College of Physicians; 2008. (NICE Guideline)
4. Speed C.A. Injection therapies for soft-tissue lesions. Best Pract Res Clin Rheumatol. 2007;21:2333-2347.
5. Cole B.J., Schumacher H.R. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;139:137-146.
6. Skedros J.G., Hunt K.J., Pitts T.C. Variations in corticosteroid/anesthetic injections for painful shoulder conditions: comparisons among orthopaedic surgeons, rheumatologists, and physical medicine and primary-care physicians. BMC Musculoskelet Disord. 2007;8:63. doi:10.1186/1471-2474-8-63
7. Gaujoux-Viala C., Dougados M., Gossec L. Efficacy and safety of steroid injections for shoulder and elbow tendonitis: a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2009;68(12):1843-1849.
8. Crashaw D.P., Helliwell P.S., Hensor E.M.A., et al. Exercise therapy after corticosteroid injection for moderate to severe shoulder pain: large pragmatic randomised trial. Br Med J. 2010;340:c3037.
9. Karthikayan S., Kwong H.T., Upadyhay P.K. A double-blind randomized controlled study comparing subacromial injection of tenoxicam or methylprednisolone in patients with subacromial impingement. J Bone Joint Surg Br. 2010;92(1):77-82.
10. Ryans I., Montgomery A., Galway R., et al. A randomized controlled trial of intra-articular triamcinolone and/or physiotherapy in shoulder capsulitis. Rheumatology. 2005;44(4):529-535.
11. Hay E.M., Thomas E., Paterson S.M., et al. A pragmatic randomised controlled trial of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Ann Rheum Dis. 2003;62:394-399.
12. van der Windt D.A.W.M., Bouter L.M. Physiotherapy or corticosteroid injection for shoulder pain? Ann Rheum Dis. 2003;62:385-387.
13. Carette S., Moffet H., Tardif J., et al. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: A placebo-controlled trial. Arthritis Rheum. 2003;48:829-838.
14. Winters J.C., Jorritsma W., Groenier K.H., et al. Treatment of shoulder complaints in general practice: long term results of a randomised, single blind study comparing physiotherapy, manipulation, and corticosteroid injection. Br Med J. 1999;318:1395-1396.
15. van der Windt D.A.W.M., Koes B.W., Deville W., et al. Effectiveness of corti-costeroid injections versus physiotherapy for treatment of painful stiff shoulder in primary care: randomised trial. Br Med J. 1998;317:1292-1296.
16. Winters J.C., Sobel J.S., Groenier K.H., et al. Comparison of physiotherapy manipulation and corticosteroid injection for treating shoulder complaints in general practice: randomised single blind study. Br Med J. 1997;314:1320-1325.
17. Tonks J.H., Pai S.K., Murali S.R. Steroid injection therapy is the best conservative treatment for lateral epicondylitis: a prospective randomised controlled trial. Int J Clin Pract. 2007;61:2240.
18. Bisset L., Beller E., Jull G., et al. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomized trial. Br Med J. 2006;333:939.
19. Hay E.M., Paterson S.M., Lewis M., et al. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. Br Med J. 1999;319:964-968.
20. Verhaar J.A.N., Walenkamp G.H.I.M., van Mameren H., et al. Local corticosteroid injection versus Cyriax type physiotherapy for tennis elbow. J Bone Joint Surg Br. 1995;77:128-132.
21. Charalambous C.P., Tryfonidis M., Sadiq S., et al. Septic arthritis following intra-articular glucocorticoid injection of the knee – a survey of current practice regarding antiseptic technique used during intra-articular glucocorticoid injection of the knee. Clin Rheumatol. 2003;22:386-390.
22. Haslock I., Macfarlane D., Speed C. Intraarticular and soft tissue injections: a survey of current practice. Br J Rheumatol. 1995;34:449-452.
23. Cluff R., Mehio A., Cohen S., et al. The technical aspects of epidural steroid injections: a national survey. Anesth Analg. 2002;95:403-408.
24. Masi A.T., Driessnack R.P., Yunus M.B., et al. Techniques for “blind” glucocorticosteroid injections into glenohumeral joints. J Rheumatol. 2007;34(5):1201-1202. [letter]
25. Bamji A.M., Dieppe P.A., Haslock D.I., et al. What do rheumatologists do? A pilot audit study. Br J Rheumatol. 1990;29:295-298.
26. Kassimos G., Panayi G., van der Windt D.A.W.M. Differences in the management of shoulder pain between primary and secondary care in Europe: time for a consensus and Author’s reply. Ann Rheum Dis. 2004;63:111-112.
27. Hoving J.L., Buchbinder R., Green S., et al. How reliably do rheumatologists measure shoulder movement?. Ann Rheum Dis, 7. 2002: 612-616.
28. Nørregaard J., Krogsgaard M.R., Lorenzen T., et al. Diagnosing patients with longstanding shoulder joint pain. Ann Rheum Dis. 2002;61:646-649.
29. Carette S. Adhesive capsulitis – research advances frozen in time? J Rheumatol. 2000;27:1329-1331.
30. Marx R.G., Bombardier C., Wright J.G. What do we know about the reliability and validity of physical examination tests used to examine the upper extremity? J Hand Surg. 1999;24A:185-193.
31. Bamji A.N., Erhardt C.C., Price T.R., et al. The painful shoulder: can consultants agree? Br J Rheumatol. 1996;35:1172-1174.
32. Gaujoux-Viala C., Dougados M., Gossec L. Efficacy and safety of steroid injections for shoulder and elbow tendonitis: a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2009;68:1843-1849.
33. Dorrestijn O., Stevens M., Winters J.C., et al. Conservative or surgical treatment for subacromial impingement syndrome: a systematic review. J Shoulder Elbow Surg. 2009;18(4):652-660.
34. Buchbinder R., Green S., Youd J.M. Corticosteroid injections for shoulder pain. Cochrane Database Sys Rev. (1):2003. Art. No.: CD004016. doi: 10.1002/14651858. CD004016. [Edited (no change to conclusions), published in Issue 1, 2009]
35. Shah N., Lewis M. Shoulder adhesive capsulitis: systematic review of randomised trials using multiple corticosteroid injections. Br J Gen Pract. 2007;57:662-667.
36. Koester M.C., Dunn W.R., Kuhn J.E., et al. The efficacy of subacromial corticosteroid injection in the treatment of rotator cuff disease: a systematic review. J Am Acad Orthop Surg. 2007;15(1):3-11.
37. Faber E., Kuiper J.I., Burdorf A., et al. Treatment of impingement syndrome: a systematic review of the effects on functional limitations and return to work. J Occup Rehabil. 2006;16(1):7-25.
38. Assendelft W., Green S., Buchbinder R., et al. Clinical review Extracts from Concise Clinical Evidence Tennis elbow. Br Med J. 2003;327:329.
39. Hepper C.T., Halvorson J.J., Duncan S.T. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: A systematic review of Level I Studies. J Am Acad Orthop Surg. 2009;17(10):638-646.
40. Bellamy N., Campbell J., Welch V., et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Sys Rev. (2):2006. Art. No.: CD005328. DOI: 10.1002/14651858. CD005328.pub2 [Edited – no change to conclusions – published in Issue 2, 2009]
41. Godwin M., Dawes M. Intra-articular steroid injections for painful knees: systematic review with meta-analysis. Can Fam Physician. 2004;50:241-248.
42. Arroll B., Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. Br Med J. 2004;328:869-870.
43. Gossec L., Dougados M. Review: Intra-articular treatments in osteoarthritis: from the symptomatic to the structure modifying. Ann Rheum Dis. 2004;63:478-482.
44. Geraets J.J., de Jongh A.C., Boeke A.J., et al. Summary of the practice guideline for shoulder complaints from the Dutch College of General Practitioners. Ned Tijdschr Geneeskd. 2009;153:A164. [Article in Dutch]
45. New Zealand Guidelines Group. Diagnosis and management of soft tissue shoulder injuries and related disorders. Best Practice Evidence Based Guideline. 2004.
46. American College of Rheumatology subcommittee on osteoarthritis guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905-1915.
47. Jordan M., Arden N.K., Doherty M., et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155.
48. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on Treatment of Carpal Tunnel Syndrome. Rosemont (IL): American Academy of Orthopaedic Surgeons (AAOS); 2008.
49. Creamer P. Intra-articular corticosteroid injections in osteoarthritis: do they work, and if so, how? Ann Rheum Dis. 1997;56:634-636.
50. Fanciullo G.J., Hanscom B., Seville J., et al. An observational study of the frequency and pattern of use of epidural steroid injection in 25,479 patients with spinal and radicular pain. Reg Anesth Pain Med. 2001;26(1):5-11.
51. Nichols A.W. Complications associated with the use of corticosteroids in the treatment of athletic injuries. Clin J Sport Med. 2005;15(5):E370.
52. Kumar N., Newman R. Complications of intra- and peri-articular steroid injections. Br J Gen Pract. 1999;49:465-466.
53. Seror P., Pluvinage P., Lecoq F., et al. Frequency of sepsis after local corticosteroid injection (an inquiry on 1,160,000 injections in rheumatological private practice in France). Rheumatology. 1999;38:1272-1274.
54. Holden J., Wooff E. Is our evidence-based practice effective? Review of 435 steroid injections given by a general practitioner over eight years. Clinical Governance: An International Journal. 2005;4:276-280.
55. Croft P. Admissible evidence. Ann Rheum Dis. 1998;57:387-389.
56. Furtado R.N., Oliveira L.M., Natour J. Polyarticular corticosteroid injection versus systemic administration in treatment of rheumatoid arthritis patients: a randomized controlled study. J Rheumatol. 2005;32:1691-1698.
57. Konai M.S., Vilar Furtado R.N., Dos Santos M.F., et al. Monoarticular corticosteroid injection versus systemic administration in the treatment of rheumatoid arthritis patients: a randomized double-blind controlled study. Clin Exp Rheumatol. 2009;27:214-221.
58. Combe B., Landewe R., Lukas C., et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:34-45.
59. Haynes R.B., Devereaux P.J., Guyatt G.H. Physicians’ and patients’ choices in evidence based practice. Br Med J. 2002;324:1350.
60. Liddell W.G., Carmichael C.R., McHugh N.J. Joint and soft tissue injections: a survey of general practitioners. Rheumatology. 2005;44(8):1043-1046.
61. Gormley G.J., Corrigan M., Steele W.K., et al. Joint and soft tissue injections in the community: questionnaire survey of general practitioners’ experiences and attitudes. Ann Rheum Dis. 2003;62:61-64.
62. Jolly M., Curran J.J. Underuse of intra-articular and periarticular corticosteroid injections by primary care physicians: discomfort with the technique. J Clin Rheumatol. 2003;9(3):187-192.
63. Gormley G.J., Steele W.K., Stevenson M. A randomised study of two training programmes for general practitioners in the techniques of shoulder injection. Ann Rheum Dis. 2003;62:1006-1009.
64. ACPOM. A Clinical Guideline for the Use of Injection Therapy by Physiotherapists. London: The Chartered Society of Physiotherapy; 1999.
65. Weale A., Bannister G.C. Who should see orthopaedic outpatients – physiotherapists or surgeons? Ann R Coll Surg Engl. 1994;77(suppl):71-73.
66. Dyce C., Biddle P., Hall K., et al. Evaluation of extended role of physio and occupational therapists in rheumatology practice. Br J Rheumatol, April, suppl. 1: abstracts. 1996: 130.
67. Hattam P., Smeatham A. An evaluation of an orthopaedic screening service in primary care. British Journal of Clinical Governance. 1999;42:45-49.
68. Daker-White G., Carr A.J., Harvey I., et al. A randomised controlled trial – shifting boundaries of doctors and physiotherapists in orthopaedic outpatient departments. J Epidemiol Community Health. 1999;53:643-650.
69. Edwards J., Hannah B., Brailsford-Atkinson K., et al. Intra-articular and soft tissue injections: assessment of the service provided by nurses. Ann Rheum Dis. 2002;61:656-657. (Letter)
70. Edwards J., Hassell A. Intra-articular and soft tissue injections by nurses: preparation for expanded practice. Nurs Stand. 2000;33(14):43-46.
71. Yelland M.J., Glasziou P.P., Bogduk N., et al. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized trial. Spine. 2004;29:9-16.
72. Rosseland L.A., Helgesen K.G., Breivik H., et al. Moderate-to-severe pain after knee arthroscopy is relieved by intra-articular saline: a randomized controlled trial. Anesth Analg. 2004;98:1546-1551.
73. Koes B.W. Corticosteroid injection for rotator cuff disease. Br Med J. 2009;338:a2599.
74. Ekeberg O.M., Bautz-Holter E., Tveita E.K., et al. Subacromial ultrasound guided or systemic steroid injection for rotator cuff disease: randomised double blind study. Br Med J. 2009;338:a3112.
75. Ghahreman A., Ferch R., Bogduk N. The efficacy of transforaminal injection of steroids for the treatment of lumbar radicular pain. Pain Medicine. 2010;11(8):1149-1168.
76. van der Windt D.A.W.M., Bouter L.M. Physiotherapy or corticosteroid injection for shoulder pain? Ann Rheum Dis. 2003;62:385-387.
77. Gerwin N., Hops C., Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;2:226-242.
Corticosteroids and local anaesthetics
Corticosteroids
Corticosteroids were first administered systemically in 1948 by Philip Hench in the USA7 and were hailed as the new ‘universal panacea’, but it soon became apparent that there were major side-effects that greatly limited their systemic use.1,2 In 1951 Hollander in the USA reported the first use of local hydrocortisone injections for arthritic joints.8
The commonly used injectable corticosteroids are synthetic analogues of the adrenal glucocorticoid hormone cortisol (hydrocortisone), which is secreted by the innermost layer (zona reticularis) of the adrenal cortex. Cortisol has many important actions including anti-inflammatory activity. Corticosteroids influence the cells involved in the immune and inflammatory responses primarily by modulating the transcription of a large number of genes. They act directly on nuclear steroid receptors to control the rate of synthesis of mRNA.3 However, they also reduce the production of a wide range of pro-inflammatory mediators including cytokines and other important enzymes.1,2,4–6
Rationale for using corticosteroids
We know surprisingly little about the precise pharmacological effects of corticosteroids when they are injected directly into joints and soft tissues.9–11
Local steroid injections are thought to work by:
Commonly used corticosteroids
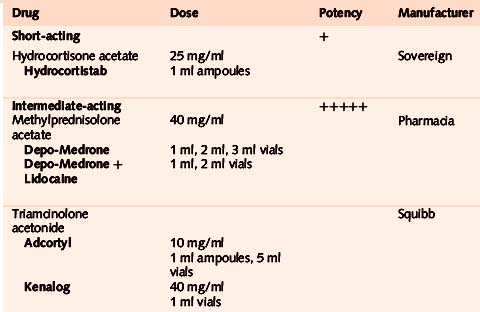
• Triamcinolone acetonide
Throughout the book we recommend Kenalog for ease of administration. This drug can be used in very small quantities so is ideal for small joints and tendons where distension may increase pain. Adcortyl, however, is useful where larger volume is required as in larger joints and bursae. The duration of action of the drug is approximately 2–3 weeks.34,35 (Triamcinolone hexacetonide – Lederspan – was the least soluble and longest lasting injectable drug previously available in the UK but the manufacturer withdrew it in 2001. It is still available from Sandoz in the USA as Aristospan).
• Methylprednisolone acetate
This drug may give more post-injection pain than triamcinalone acetonide.36 It is available also pre-mixed with local anaesthetic as Depo-Medrone (40 mg/1 ml) with Lidocaine (10 mg/ml) in 1 ml and 2 ml vials, which we do not recommend as it is a fixed-dose combination and therefore difficult to adjust.
• Hydrocortisone
Very soluble – this has the shortest duration of action of the steroids mentioned here, perhaps as little as 6 days.11 It may be particularly useful for superficial injections in thin, dark-skinned patients, where depigmentation or local fat atrophy may be more noticeable. 20 mg of Hydrocortistab is equivalent to 4 mg of triamcinolone or methylprednisolone (Table 1.1).
Local anaesthetics
These membrane-stabilizing drugs act by causing a reversible block to conduction along nerve fibres. The smaller nerve fibres are more sensitive, so that a differential block may occur where the small fibres carrying pain and autonomic impulses are blocked, sparing coarse touch and movement. Uptake into the systemic circulation is important for terminating their action and also for producing toxicity. Following most regional anaesthetic procedures, maximum arterial plasma concentrations of anaesthetic develop within 10–25 minutes, so careful surveillance for toxic effects is recommended for 30 minutes after injection if significant volumes are used.37
Rationale for using local anaesthetics
Commonly used local anaesthetics
Local anaesthetics vary widely in their potency, duration of action and toxicity.37 Those most commonly used for joint and soft-tissue injection are:
Lidocaine (under the brand name Xylocaine) and Marcain are also manufactured with added adrenaline (which causes vasoconstriction when used for skin anaesthesia, and so prolongs the local anaesthetic effect). These preparations are not recommended for procedures involving the appendages because of the risk of ischaemic necrosis.9 Xylocaine with adrenaline added is clearly marked in red. We recommend that clinicians who administer injection therapy avoid these combination products altogether.
Potential side-effects
Side-effects from injection therapy with corticosteroids and/or local anaesthetics are uncommon and when they do occur are usually mild and transient.45–47 Nonetheless it is incumbent upon the clinician practising injection therapy to be aware of the presentation and management of all the potential minor and more serious side-effects associated with this treatment. (Table 1.3)48
Table 1.3 Summary of potential side-effects of corticosteroid/local anaesthetic injection therapy
| Systemic side-effects | Local side-effects |
|---|---|
| Facial flushing | Post-injection flare of pain |
| Impaired diabetic control | Skin depigmentation, fat atrophy |
| Menstrual irregularity | Bleeding/bruising |
| Hypothalamic–pituitary axis suppression | Steroid ‘chalk’, calcification |
| Fall in ESR/CRP | Steroid arthropathy |
| Anaphylaxis (very rare) | Tendon rupture/atrophy |
| Joint/soft-tissue infection |
Injection of the wrong drug is a potentially serious and totally avoidable problem with severe consequences for all concerned.82 Strict attention to the preparation protocol should prevent this (Section 2).
Consider carefully before giving corticosteroid injections to pregnant or breastfeeding women; this therapy has been recommended for carpal tunnel syndrome and De Quervain’s tendovaginitis in these patients49,50 but these conditions usually resolve following delivery. If used, a detailed discussion of the pros and cons of injection therapy should be carefully documented.
Local side-effects
Local side-effects may occur when an injection is misdirected or too large a dose in too large a volume is injected too often. Subcutaneous placement of the steroid and the injection of a drug bolus at entheses must both be avoided. Serious local side-effects are rare.45,126
• Post-injection flare of pain
The quoted figures are from about 2 % to 10 % 47,51 but this is well in excess of our own experience. When it does happen it is usually after a soft-tissue injection, and rarely follows a joint injection.47 When corticosteroid is mixed with local anaesthetic the solution should be inspected carefully for flocculation/precipitation before injecting, as this may be related to post-injection flare of pain;11 this may also be caused by the rapid intracellular ingestion of the microcrystalline steroid ester and must always be distinguished from sepsis.52 There may be more frequent post-injection flares with methylprednisolone, but this may have more to do with the preservative in the drug than with the steroid itself.53 An early increase in joint stiffness following intra-articular corticosteroids is consistent with a transient synovitis.54
Multi-dose bottles of lidocaine contain parabens as a preservative. Many steroids will precipitate when added to it and this precipitate may be responsible for some cases of post-injection flare of pain and ‘steroid chalk’ (see below). Parabens may also be responsible for some allergic reactions to local injections. The use of multi-dose bottles increases the risk of cross infection and should be avoided.55 Single-dose vials of lidocaine do not contain parabens.
• Subcutaneous atrophy and/or skin depigmentation51,56
In one meta-analysis of shoulder and elbow injections ‘skin modification’ had a frequency of 4 %.51 Skin changes may be more likely to occur when superficial lesions are injected, especially in dark-skinned patients. The injected drugs should not be allowed to reflux back through the needle tract – pressure applied around the needle with cotton wool when withdrawing may help. In thin dark-skinned patients especially, it may be preferable to use hydrocortisone for superficial lesions. They must always be advised of the possibility of this side-effect, and the fact recorded. Local atrophy appears within 1–4 months after injection and characteristically proceeds to resolution 6–24 months later, but may take longer.57 Fat atrophy following corticosteroid injection may rarely have significant functional consequences.58,59
• Steroid ‘chalk’ or ‘paste’
This may be found on the surface of previously injected tendons and joints during surgery. Suspension flocculation, resulting from the mixture of steroid with a local anaesthetic containing preservative, may be responsible. The clinical significance of these deposits is uncertain.60
• Soft-tissue calcification
Corticosteroid injections into osteoarthritic interphalangeal joints of the hand may result in calcification or joint fusion, possibly because of pericapsular leakage of steroids due to raised intra-articular pressure.61 No deleterious effects have been ascribed to this calcification.
• Steroid arthropathy
A well known and much feared complication of local injection treatment – it is also largely a myth.62 In many instances injected steroid may be chondro-protective rather than destructive.20–28 There is good evidence linking prolonged high-dose oral steroid usage with osteonecrosis,46 but almost all the reports linking injected steroids with accelerated non-septic joint destruction are anecdotal, and mainly relate to joints receiving huge numbers of injections.62 A reasonable guide is to give injections into the major joints in the lower limbs at no less than 3–4 month intervals, although this advice is based on consensus rather than evidence.9,65 Reports of Charcot-like accelerated joint destruction after steroid injection in human hip osteoarthritis may reflect the disease itself rather than the treatment.61,64 Currently no evidence supports the promotion of disease progression by steroid injections.65 Repeat injections into the knee every 3 months seem to be safe over 2 years.65
One study determined the relationship between frequent intra-articular steroid injection and subsequent joint replacement surgery in patients with rheumatoid arthritis who had received 4 or more injections in an asymmetric pattern in a single year. A subset of 13 patients with an average of 7.4 years of follow up was established as the cohort of a 5-year prospective study. This highly selected cohort received 1622 injections; joint replacement surgery was not significantly more common in the injected joints. The authors concluded that frequent intra-articular steroid injection does not greatly increase the risk inherent in continued disease activity for these patients and may offer some chondroprotection.66
• Tendon rupture and atrophy
The literature does not provide precise estimates for complication rates following the therapeutic use of injected or systemic steroids in the treatment of athletic injuries but tendon and fascial ruptures are reported complications of injection.46 Tendon67–69 and fascial rupture70,71 or atrophy72 is probably minimized by withdrawing the needle a little if an unusual amount of resistance is encountered,67 and using a peppering technique at entheses with the smallest effective dose and volume of steroid.73 The whole issue of steroid-associated tendon rupture is controversial,68,69,71,74 disputed,75 anecdotal,46,67,76 and in humans not well supported in the literature,69 although it is widely accepted that repeated injection of steroids into load-bearing tendons carries the risk of rupture.77
The current climate of opinion is antithetical towards steroid injection in to and around the Achilles tendon. If this is being contemplated it is advisable to image the tendon first to confirm that it is a peritendinitis with no degenerative change (with or without tears) in the body of the tendon. Low dose peritendinous steroid injections appear to be safe78 and it might be safer to infiltrate with local anaesthetic alone. The patient should rest from provocative activity for 6–8 weeks.68 In rabbits, injections of steroid, both within the tendon substance and into the retro-calcaneal bursa, adversely affect the biomechanical properties of Achilles tendons. Additionally, rabbit tendons that received bilateral injections demonstrated significantly worse biomechanical properties compared with unilaterally injected tendons. Bilateral injections should be avoided as they may have a systemic effect in conjunction with the local effect, further weakening the tendon.79 Surgery for chronic Achilles tendonopathy has a complication rate of around 10% and should not be assumed to be a trouble free treatment option.80
• Delayed soft tissue healing
This may be associated with local steroid injection. In a study of rabbit ligaments the tensile strength of the injected specimens returned to a value that was equal to that of the non-injected controls; however, the peak load of the injected specimens remained inferior, with a lag in histological maturation.81 This has implications for the timing of return to activity following injection therapy.
• Sepsis
Joint sepsis is the most feared complication of steroid injection treatment;83 it may be lethal,84 but it is a rarity.45,85 Local infection occurs in only 1 in 17 000–162 000 patients when joint and soft tissue injections are performed as an ‘office’ procedure.61,86,104 In one study local sepsis following injection of a pre-packaged corticosteroid in a sterile syringe was 1 in 162 000 injections compared with 1 in 21 000 using a non-prepackaged syringe.86 Soft-tissue infections and osteomyelitis can also occur after local soft tissue injection.87,88
Prompt recognition of infection is essential to prevent joint and soft tissue destruction, although diagnosis may be delayed if symptoms are mistaken for a post-injection flare or exacerbation of the underlying arthropathy.89
In the case of a patient who developed septic arthritis following a shoulder joint injection by her GP, the expert opinion was that infection is a rare hazard of the procedure, for which the GP should not be blamed, but that failure to recognize and appropriately manage this side-effect is difficult to defend.48
Fragments of skin may be carried into a joint on the tip of a needle and may be a source of infection.90 Joint infections may also possibly occur by haematogenous spread, rather than by direct inoculation of organisms into the joint. Steroid injection may create a local focus of reduced immunity in a joint, thus rendering it more vulnerable to blood-borne spread. Rarely, injection of contaminated drugs or hormonal activation of a quiescent infection may be to blame. 83,89
All cases of suspected infection following injection must be promptly admitted to hospital for diagnosis and treatment.83 Blood tests (ESR, CRP, plasma viscosity, white blood cell differential count, blood cultures) should be taken along with diagnostic aspiration of the affected joint or any other localized swelling. The needle used for attempted aspiration may be sent for culture if no aspirate is obtained.87 X-ray changes may be absent in the early stages of joint infection and more sophisticated imaging techniques such as MRI and isotope bone scans may be helpful.
To avoid injecting an already infected joint have a high index of suspicion in rheumatoid patients,91 elderly osteoarthritic patients with an acute monarthritic flare (especially hip) and patients with coexistent infection elsewhere, e.g. chest, urinary tract and skin, especially the legs. Visualize and dipstick the urine and check the ESR.92
In the largest series of bacterial isolates reported from UK patients with septic arthritis, the commonest organisms were Staphylococcus aureus and Streptococci species. Others were E. coli, Haemophilus influenzae, Salmonella species, Pseudomonas species and Mycobacterium tuberculosis.93 M. tuberculosis may be particularly difficult to diagnose, and may require the study of synovial biopsy samples.89 Infection was most common in children and the elderly. Underlying risk factors were reported in one fifth of cases, the most frequent being a prosthetic joint (11 %). Others included haematological malignancy, joint disease or connective tissue disorder, diabetes, oral steroid therapy, chemotherapy, presence of an intravenous line, intravenous drug abuse and post-arthroscopy.93 Steroid injection may delay presentation of sepsis by 6–12 days.94
In one study, the incidence of septic arthritis increased over a 12-year period as more invasive procedures (arthroscopies and arthrocenteses) were performed on the study population, although the frequency of sepsis per procedure remained static, with sepsis after arthroscopy being almost four times as frequent.95
Joint infection has been reported as occurring between 4 days and 3 weeks after injection.87 Exotic infections may occur in immunocompromised patients following joint injection.96
Aggressive therapy, including powerful immunosuppressive and cytotoxic drugs, is increasingly used in the treatment of rheumatoid arthritis, and may confer increased susceptibility to infections. Septic arthritis is one infectious complication known to be overrepresented in this disease; in one small series of these patients with septic arthritis, 6 out of 9 had received an intra-articular injection into the infected joint within 3 months prior to the onset of the sepsis. Only one of these occurred immediately after joint injection. The annual frequency of septic arthritis was approximately 0.2 %; during the 4-year period studied the frequency was 0.5 %. A frequency of 1 per 2000 injections was found when late septic arthritis was included. The high frequency of delayed septic arthritis in rheumatoid patients after intra-articular steroid administration should alert clinicians to this complication.97
Concern has been raised that prior steroid injection of the knee and hip may increase the risk of a subsequent joint infection following joint replacement98,99 although this has been disputed.100 Some surgeons deprecate the routine use of intra-articular steroids following knee arthroscopy because of a perceived increased risk of infection,94 while others advocate this for post-procedural pain relief.101,102
If infection occurs following an injection, vigorous attempts must be made to isolate the causative organism. If this is Staphylococcus aureus the clinician should have nasal swabs taken and, if positive, should receive appropriate antibiotic treatment and not give any more injections until further swabs confirm clearance. A review of aseptic technique used should also be undertaken.87
Intra-articular corticosteroids may be effective following septic arthritis where pain and synovitis persist despite intravenous antibiotic treatment, and where lavage and repeat synovial fluid and blood cultures are sterile.103 Multidose bottles and vials should be avoided as they may become contaminated and act as a source of infection.55 Drugs for injection must be stored in accordance with the manufacturer’s instructions.
Systemic side-effects
Systemic complications are rare.11
• Facial flushing
This is probably the commonest systemic side-effect,63 occurring in from 5 % 107 of patients to less than 1 %. 60 It may come on within 24–48 hours after the injection and may last 1–2 days.
• Deterioration of diabetic glycaemic control
Diabetic patients must be warned about this possible temporary side effect.108 A common observation is that blood sugar levels undergo a modest rise for up to a week, rarely longer. Where larger doses of corticosteroid than recommended here for single site injection are given (or multiple sites are injected at one time, or over a few days), this may lead to a more prolonged (up to 3 weeks) elevation of blood sugar. This may require a short-term increase in diabetic drug dosage, so the patient should be informed about the steroid drug and dosage given. Systolic blood pressure may also be temporarily elevated by large doses of intra-articular corticosteroids.109,110
• Uterine bleeding (pre- and post-menopausal)
The exact mechanism is unknown but intra-articular steroid treatment causes a temporary, but considerable, suppression of sex steroid hormone secretion in women.111,112 In a post-menopausal woman post-injection uterine bleeding creates a difficult dilemma – is the bleeding related to the injection, or should she be investigated to exclude other, potentially serious causes? If this complication occurs it must always be taken seriously.
• Suppression of the hypothalamic–pituitary axis
This occurs following intra-articular and intra-muscular injection of corticosteroids113,114 but at the doses and frequencies described in this book this usually appears to be of no significant clinical consequence53 and we do not issue patients with a steroid card after injection.115 Rarely, however, systemic absorption of corticosteroid may evoke a secondary hypercortisolism similar to Cushing’s syndrome. Patients who develop a Cushingoid state about 2 weeks after injection therapy (and their clinicians) often do not associate this with the corticosteroid injection, with the potential for the patient to undergo unnecessary investigation and treatment for a presumed primary endocrine disorder (Table 1.2). Screening the urine for corticosteroid drug metabolites helps with diagnosis. There may also be a transient eosinopenia on the differential blood white cell count.128–130 Children may be particularly susceptible and display features of Cushing’s syndrome following intra-articular corticosteroid injection.118
Table 1.2 Suppression of the hypothalamic–pituitary axis by corticosteroid injection therapy128
| Probably under-recognized | |
| May occur with single or multiple injections within minimum of 5 weeks | |
| Onset 10–14 days after injection | |
| Clinical features | |
| Moon face/buffalo hump | Acne-like eruptions/flushing |
| Palpitations/tremors | Dyspnoea/weight gain 5–8 kg |
| Disturbed menstruation | |
| Outcome | |
| Spontaneous resolution at 3 months (one injection) and 6 months (2 injections) | |
Clinical improvement of distant joints in a polyarthritis is an early clinical feature suggestive of significant systemic absorption of locally administered corticosteroid. In one study, triamcinolone hexacetonide plasma levels reached their median serum peak 8 hours after injection into the rheumatoid knee.116 This may account for the common observation of symptomatic improvement in joints other than the one injected. Higher serum levels of the injectate have been found in patients in whom the dose was divided into two joints rather than administering it into a single joint; a putative potentiation effect of divided doses has been attributed to a greater absorptive surface area in the divided-doses.60 However, in one study comparing the treatment of rheumatoid arthritis with equivalent doses of intra-articular and intramuscular mini-pulse therapy with triamcinolone, less significant adrenocorticotropic hormone reduction was observed for the intra-articular group.117
• Significant falls in the ESR and CRP levels
The mean fall is about 50 %. Intra-articular corticosteroid injections can cause this in patients with inflammatory arthritis and this effect can last for up to 6 months. This needs to be taken into account when using these blood tests to assess the response of patients to disease-modifying drugs.119
• Anaphylaxis
Severe anaphylactic reactions to local anaesthetic injections are rare, but can be fatal.120 Anaphylactic reactions to corticosteroid injections are extremely rare and are probably a reaction to the stabilizers that the drug is mixed with, rather than the drug itself.53,121
• Other rare systemic side-effects
These include pancreatitis (patient presents with abdominal pain and the serum amylase is raised), nausea, dysphoria (emotional upset), acute psychosis, myopathy and posterior subcapsular cataracts.48,122,123 Complex regional pain syndrome has been reported after trigger thumb injection.124 In patients with sickle cell disease a crisis may be precipitated by intra-articular injection of corticosteroids; the mechanism is not clear, but it is suggested that this treatment be used with caution in these patients.125 Tibial stress fractures and multifocal osteonecrosis have been reported with systemic but not locally injected corticosteroids used for athletic injuries.46
Despite all the above, injection therapy for joints and soft tissues is a relatively safe form of treatment. Adverse events can be minimized by ensuring that well trained practitioners follow appropriate procedures.126
It is safe to mix corticosteroids with local anaesthetics prior to injection. High performance liquid chromatographic analysis to assess the stability of combinations of triamcinolone and hydrocortisone when mixed with combinations of lidocaine and bupivacaine shows that the combinations are stable when mixed together, supporting the continued use of these products in combination.105
Compared with the safety profile of oral non-steroidal anti-inflammatory drugs, the justification for using the minimum effective dose of injectable drugs in the correct place with appropriate preparation and aftercare becomes evident106 (Table 1.4).
Table 1.4 Numbers needed to harm for patients >60 prescribed oral NSAIDs > 2 months
| Number | Harm caused |
|---|---|
| 1 in 5 | Endoscopic ulcer |
| 1 in 70 | Symptomatic ulcer |
| 1 in 150 | Bleeding ulcer |
| 1 in 1200 | Death from bleeding ulcer |
(from Tramer et al106)
Costs
The injectable corticosteroids and local anaesthetics that we use are remarkably inexpensive (UK prices, BNF March 2010) (Table 1.5). Compare this with the cost of some commonly prescribed oral NSAIDs and also with the cost of hyaluronan injections (on-line pharmacy prices August 2010).
| Injectable drugs | |
|---|---|
| 1 ml ampoule of Adcortyl (10 mg of triamcinolone acetonide) | £0.91 |
| 1 ml vial of Kenalog (40 mg) | £1.52 |
| 10 ml of 1% lidocaine | £0.39 |
| Common oral NSAIDs | |
| Generic diclofenac – 1 month at 50 mg tds | £1.43 |
| Generic ibuprofen – 1 month at 400 mg tds | £1.87 |
| Generic naproxen – 1 month at 500 mg tds | £1.90 |
| Hyaluronan injections | |
| Hyalgan (one syringe 20 mg/2 ml, 5 injections) | £48.10 |
| Synvisc (one Hylan G-F20 syringe 24 mg/6 ml, 3 injections) | £266.50 |
Injection therapy may offer significant cost savings when compared with other treatment strategies for common musculoskeletal disorders.127
1. Pitzalis C. Corticosteroids – a case of mistaken identity? Br J Rheumatol. 1998;37:477-483.
2. Coombes G.M., Bax D.E. The use and abuse of steroids in rheumatology. Rep Rheum Dis. 1996. (Series 3). Practical Problems (No. 8) 1996
3. Creamer P. Intra-articular corticosteroid injections in osteoarthritis: do they work, and if so, how? Ann Rheum Dis. 1997;56:634-636.
4. af Klint E., Grundtman C., Engström M., et al. Intraarticular glucocorticoid treatment reduces inflammation in synovial cell infiltrations more efficiently than in synovial blood vessels. Arthritis Rheum. 2005;52(12):3880-3889.
5. Goulding N.J. Anti-inflammatory corticosteroids. Rep Rheum Dis. 1999. (Series 3). Topical Reviews (No. 18) 1999
6. Cutolo M. The roles of steroid hormones in arthritis. Br J Rheumatol. 1998;37:597-601.
7. Kirwan J.R., Balint G., Szebenyi B. Anniversary: 50 years of glucocorticoid treatment in rheumatoid arthritis. Rheumatology. 1999;38:100-102.
8. Hollander J.L., Brown E.M., Jester R.A., et al. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of a use of hydrocortisone as a local anti-arthritis agent. J Am Med Assoc. 1951;147:1269.
9. Speed C.A. Injection therapies for soft-tissue lesions. Best Pract Res Clin Rheumatol. 2007;21(2):333-347.
10. Ines L.P.B.S., da Silva J.A.P. Soft tissue injections. Best Pract Res Clin Rheumatol. 2005;19(3):503-527.
11. Cole B.J., Schumacher H.R. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;139(1):37-46.
12. Anon. Gout in primary care. Drug Ther Bull. 2004;42(5):39.
13. Gossec L., Dougados M. Intra-articular treatments in osteoarthritis: from the symptomatic to the structure modifying. Ann Rheum Dis. 63, 2004.
14. Kirwan J.R., Rankin E. Intraarticular therapy in osteoarthritis. Baillière’s Clin Rheumatol. 1997;11:769-794.
15. Franz J.K., Burmester G.-R. Antirheumatic treatment: The needle and the damage done. Ann Rheum Dis. 2005;64:798-800.
16. Jones A., Doherty M. Intra-articular corticosteroid injections are effective in OA but there are no clinical predictors of response. Ann Rheum Dis. 1996;55:829-832.
17. Brandt K.D., Radin E.L., Dieppe P.A., et al. Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis. 2006;65:1261-1264.
18. Dorman T., Ravin T. Diagnosis and Injection Techniques in Orthopaedic Medicine. Baltimore, Maryland: Williams and Wilkins. 1991:33-34.
19. Daley C.T., Stanish W.D. Soft tissue injuries: overuse syndromes. In: Bull R.C., editor. Handbook of Sports Injuries. New York: McGraw Hill; 1998:185.
20. Weitoft T., Larsson A., Ronnblom L. Serum levels of sex steroid hormones and matrix metalloproteinases after intra-articular glucocorticoid treatment in female patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:422-424.
21. Verbruggen G. Chondroprotective drugs in degenerative joint diseases. Rheumatology. 2006;45(2):129-138.
22. Weitoft T., Larsson A., Saxne T., et al. Changes of cartilage and bone markers after intra-articular glucocorticoid treatment with and without post-injection rest in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:1750-1753.
23. Larsson E., Harris H.E., Larsson A. Corticosteroid treatment of experimental arthritis retards cartilage destruction as determined by histology and serum COMP. Rheumatology. 2004;43(4):428-434.
24. Raynauld J.P. Clinical trials: impact of intra-articular steroid injections on the progression of knee osteoarthritis. Osteoarthritis Cartilage. 1999;7:348-349.
25. Hills B.A., Ethell M.T., Hodgson D.R. Release of lubricating synovial surfactant by intra-articular steroid. Br J Rheumatol. 1998;37(6):649-652.
26. Pelletier J.P., Mineau F., Raynauld J.P., et al. Intraarticular injections with methylprednisolone acetate reduce osteoarthritic lesions in parallel with chondrocyte stromelysin synthesis in experimental osteoarthritis. Arthritis Rheum. 1994;37:414-423.
27. Jubb R.W. Anti-rheumatic drugs and articular cartilage. Rep Rheum Dis. 1992. (Series 2). Topical Reviews (No. 20) 1992
28. Pelletier J.P., Pelletier J.M. Proteoglycan degrading metalloprotease activity in human osteoarthritis cartilage and the effect of intraarticular steroid injections. Arthritis Rheum. 1987;30(5):541-549.
29. Scott A., Khan K.M., Cook J.L., et al. What is ‘inflammation’? Are we ready to move beyond Celsus? Br J Sports Med. 2004;38:248-249.
30. Khan K.M., Cook J.L., Kannus P., et al. Time to abandon the “tendinitis” myth. Br Med J. 2002;324:626-627.
31. Khan K.M., Cook J.L., Maffulli N., et al. Where is the pain coming from in tendinopathy? It may be biochemical, not structural in origin. Br J Sports Med. 2000;34(2):81-83.
32. Gotoh M., Hamada K., Yamakawa H., et al. Increased substance P in subacromial bursa and shoulder pain in rotator cuff disease. J Orthop Res. 1998;16:618-621.
33. Johansson A., Hao J., Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990;34(5):335-338.
34. Derendorf H., Mollmann H., Gruner A., et al. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther. 1986;39:313-317.
35. Caldwell J.R. Intra-articular corticosteroids: guide to selection and indications for use. Drugs. 1996;52:507-514.
36. Piotrowski M., Szczepanski I., Dmoszynska M. Treatment of rheumatic conditions with local instillation of betamethasone and methylprednisolone: comparison of efficacy and frequency of irritative pain reaction. Rheumatologia. 1998;36:78-84.
37. . British National Formulary, No 59 Section 15.2. London: BMA/RPSGB. 2010. March
38. Kannus P., Jarvinen M., Niittymaki S. Long- or short-acting anesthetic with corticosteroid in local injections of overuse injuries? A prospective, randomized, double-blind study. Int J Sports Med. 1990;11(5):397-400.
39. Sölveborn S-A, Buck F, Mallmin H, et al. Cortisone injection with anaesthetic additives for radial epicondylalgia. Clin Orthop Relat Res. 1995;316:99-105.
40. Buchbinder R., Green S., Forbes A., et al. Arthrographic joint distension with saline and steroid improves function and reduces pain in patients with painful stiff shoulder: results of a randomised, double blind, placebo controlled trial. Ann Rheum Dis. 2004;63:302-309.
41. Gam A., Schydlowsky P., Rossel I., et al. Treatment of ‘frozen shoulder’ with distension and glucorticoid compared with glucorticoid alone: a randomised controlled trial. Scand J Rheumatol. 1998;27(6):425-430.
42. Mulcahy K.A., Baxter A.D., Oni O.O.A., et al. The value of shoulder distension arthrography with intra-articular injection of steroid and local anaesthetic: a follow-up study. Br J Radiol. 1993;67:263-266.
43. Jacobs L.G.H., Barton M.A.J., Wallace W.A., et al. Intraarticular distension and steroids in the management of capsulitis of the shoulder. Br Med J. 1991;302:1498-1501.
44. Creamer P., Hunt M., Dieppe P. Pain mechanisms in osteoarthritis of the knee: effect of intra-articular anesthetic. J Rheumatol. 1996;23:1031-1036.
45. Habib G.S., Saliba W., Nashashibi M. Local effects of intra-articular corticosteroids. Clin Rheumatol. 2010;29(4):347-356.
46. Nichols A.W. Complications associated with the use of corticosteroids in the treatment of athletic injuries. Clin J Sport Med. 2005;15(5):370-375.
47. Kumar N., Newman R. Complications of intra- and peri-articular steroid injections. Br J Gen Pract. 1999;49:465-466.
48. Dando P., Green S., Price J. Problems in General Practice – Minor Surgery. London: The Medical Defence Union; 1997.
49. Avci S., Yilmaz C., Sayli U. Comparison of non-surgical treatment measures for de Quervain’s disease of pregnancy and lactation. J Hand Surg. 2002;27(A):322-325.
50. Wallace W.A. (letter). Br Med J, 320. 2000, 4th March.
51. Gaujoux-Viala C., Dougados M., Gossec L. Efficacy and safety of steroid injections for shoulder and elbow tendonitis: a meta-analysis of randomised controlled trials. Ann Rheum Dis. 2009;68(12):1843-1849.
52. Berger R.G., Yount W.J. Immediate ‘steroid flare’ from intra-articular triamcinolone hexacetonide injection: case report and review of the literature. Arthritis Rheum. 1990;33(8):1284-1286.
53. Pullar T. Routes of drug administration: intra-articular route. Prescribers’ Journal. 1998;38(2):123-126.
54. Helliwell P.S. Use of an objective measure of articular stiffness to record changes in finger joints after intra-articular injection of corticosteroid. Ann Rheum Dis. 1997;56:71-73.
55. Kirschke D.L., Jones T.F., Stratton C.W., et al. Outbreak of joint and soft-tissue infections associated with injections from a multiple-dose medication vial. Clin Infect Dis. 2003;36:1369-1373.
56. Newman R.J. Local skin depigmentation due to corticosteroid injections. Br Med J. 1984;288:1725-1726.
57. Cassidy J.T., Bole G.G. Cutaneous atrophy secondary to intra-articular corticosteroid administration. Ann Intern Med. 1966;65(5):1008-1018.
58. Basadonna P.T., Rucco V., Gasparini D., et al. Plantar fat pad atrophy after corticosteroid injection for an interdigital neuroma: a case report. Am J Phys Med Rehabil. 1999;78:283-285.
59. Reddy P.D., Zelicof S.B., Ruotolo C., et al. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995;317:185-187.
60. Gray R.G., Gottlieb N.L. Basic science and pathology: intra-articular corticosteroids, an updated assessment. Clin Orthop Relat Res. 1982;177:235-263.
61. Gray R.G., Tenenbaum J., Gottlieb N.L. Local corticosteroid injection therapy in rheumatic disorders. Semin Arthritis Rheum. 1981;10:231-254.
62. Cameron G. Steroid arthropathy: myth or reality? Journal of Orthopaedic Medicine. 1995;17(2):51-55.
63. . British National Formulary, No 59 Section 10.1.2.2. London: BMA/RPSGB. 2010. March
64. Cooper C., Kirwan J.R. The risks of local and systemic corticosteroid administration. Baillière’s Clin Rheumatol. 1990;19(2):305-333.
65. Raynauld J., Buckland-Wright C., Ward R., et al. Safety and efficacy of long term intraarticular steroid injections in osteoarthritis of the knee. Arthritis Rheum. 2003;48:370-374.
66. Roberts W.N., Babcock E.A., Breitbach S.A., et al. Corticosteroid injection in rheumatoid arthritis does not increase rate of total joint arthroplasty. J Rheumatol. 1996;23:1001-1004.
67. Smith A.G., Kosygan K., Williams H., et al. Common extensor tendon rupture following corticosteroid injection for lateral tendinosis of the elbow. Br J Sports Med. 1999;33:423-425.
68. Shrier I., Gordon O. Achilles tendon: are corticosteroid injections useful or harmful? Clin J Sport Med. 1996;6:245-250.
69. Mahler F., Fritsch Y.D. Partial and complete ruptures of the Achilles tendon and local corticosteroid injections. Br J Sports Med. 1992;26:7-14.
70. Saxena A., Fullem B. Plantar fascia ruptures in athletes. Am J Sports Med. 2004;32:662-665.
71. Acevedo J.I., Beskin J.L. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19:91-97.
72. Fredberg U. Local corticosteroid injection in sport: review of literature and guidelines for treatment. Scand J Med Sci Sport. 1997;7:131-139.
73. Cyriax J.H., Cyriax P.J. Principles of treatment. In: Illustrated Manual of Orthopaedic Medicine. London: Butterworths; 1983:22.
74. McWhorter J.W., Francis R.S., Heckmann R.A. Influence of local steroid injections on traumatized tendon properties; a biomechanical and histological study. Am J Sports Med. 1991;19(5):435-439.
75. Read M.T.F. Safe relief of rest pain that eases with activity in achillodynia by intrabursal or peritendinous steroid injection: the rupture rate was not increased by these steroid injections. Br J Sports Med. 1999;33:134-135.
76. Mair S.D., Isbell W.M., Gill T.J., et al. Triceps tendon ruptures in professional football players. Am J Sports Med. 2004;32:431-434.
77. Mottram D.R., editor. Drugs in Sport, 2nd ed., London: E & FN Spon, 1996.
78. Gill S.S., Gelbke M.K., Matson S.L., et al. Fluoroscopically guided low-volume peritendinous corticosteroid injection for Achilles tendinopathy; a safety study. J Bone Joint Sur Am. 2004;86:802-806.
79. Hugate R., Pennypacker J., Saunders M., et al. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86:794-801.
80. Paavola M., Orava S., Leppilahti J., et al. Chronic achilles tendon overuse injury: complications after surgical treatment. An analysis of 432 consecutive patients. Am J Sports Med. 2000;28:77-82.
81. Wiggins M.E., Fadale P.D., Ehrlich M.G., et al. Effects of local injection of corticosteroids on the healing of ligaments; a follow-up report. J Bone Joint Surg Am. 1995;77(11):1682-1691.
82. Lanyon P., Regan M., Jones A., et al. Inadvertent intra-articular injection of the wrong substance. Br J Rheumatol. 1997;36:812-813.
83. Hughes R.A. Septic arthritis, (Series 3). Practical Problems (No. 7). Rep Rheum Dis. 1996: 1.
84. Yangco B.G., Germain B.F., Deresinski S.C. Case report: Fatal gas gangrene following intra-articular steroid injection. Am J Med Sci. 1982;283:294-298.
85. Charalambous C.P., Tryfonidis M., Sadiq S., et al. Septic arthritis following intra-articular glucocorticoid injection of the knee – a survey of current practice regarding antiseptic technique used during intra-articular glucocorticoid injection of the knee. Clin Rheumatol. 2003;22:386-390.
86. Seror P., Pluvinage P., Lecoq F., et al. Frequency of sepsis after local corticosteroid injection (an inquiry on 1,160,000 injections in rheumatological private practice in France). Rheumatology. 1999;38:1272-1274.
87. Grayson M. Three infected injections from the same organism. Br J Rheumatol. 1998;37:592-593.
88. Jawed S., Allard S.A. Osteomyelitis of the humerus following steroid injections for tennis elbow. Rheumatology. 2000;39:923-924. (Letter)
89. von Essen R., Savolainen H.A. Bacterial infection following intra-articular injection. Scand J Rheumatol. 1989;18:7-12.
90. Chustecka Z. Intra-articular injections may introduce skin into affected joint. Rheumawire; 7th March 2001. (Available online: www.jointandbone.org Accessed: 28 Dec 2010
91. Gardner G.C., Weisman M.H. Pyarthrosis in patient with rheumatoid arthritis; a report of 13 cases and a review of the literature from the past 40 years. Am J Med. 1990;88:503-511.
92. Knight D.J., Gilbert F.J., Hutchison J.D. Lesson of the week: septic arthritis in osteoarthritic hips. Br Med J. 1996;313:40-41.
93. Ryan M.J., Kavanagh R., Wall P.G., et al. Bacterial joint infections in England and Wales: analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36:370-373.
94. Gosal H.S., Jackson A.M., Bickerstaff D.R. Intra-articular steroids after arthroscopy for osteoarthritis of the knee. J Bone Joint Surg Br. 1999;81:952-954.
95. Geirsson A.J., Statkevicius S., Víkingsson A. Septic arthritis in Iceland 1990–2002: increasing incidence due to iatrogenic infections. Ann Rheum Dis. 2008;67:638-643.
96. Sohail M.R., Smilack J.D. Aspergillus fumigatus septic arthritis complicating intra-articular corticosteroid injection. Mayo Clin Proc. 2004:79478-79579.
97. Ostensson A., Geborek P. Septic arthritis as a non-surgical complication in rheumatoid arthritis: relation to disease severity and therapy. Br J Rheumatol. 1991;30:35-38.
98. Papavasiliou A.V., Isaac D.L., Marimuthu R., et al. Infection in knee replacements after previous injection of intra-articular steroid. J Bone Joint Surg Br. 2006;88-B(3):321-323.
99. Kaspar S., de Beer J de V. Infection in hip arthroplasty after previous injection of steroid. J Bone Joint Surg Br. 2005;87-B(4):454-457.
100. Chitre A.R., Fehily M.J., Bamford D.J. Total hip replacement after intra-articular injection of local anaesthetic and steroid. J Bone Joint Surg Br. 2007. 89-B266-168
101. Pang H.-N., Lo N.-N., Yang K.-Y., et al. Peri-articular steroid injection improves the outcome after unicondylar knee replacement. J Bone Joint Surg Br. 2008;90-B:638-744.
102. Wang J.-J., Ho S.-T., Lee S.-C., et al. lntra-articular triamcinolone acetonide for pain control after arthroscopic knee surgery. Anesth Analg. 1998;87:1113-1116.
103. Lane S.E., Merry P. Intra-articular corticosteroids in septic arthritis: beneficial or barmy? Ann Rheum Dis. 2000;59:240. (Letter)
104. Pal B., Morris J. Perceived risks of joint infection following intra-articular corticosteroid injections: a survey of rheumatologists. Clin Rheumatol. 1999;18(3):264-265.
105. Watson D.G., Husain S., Brennan S., et al. The chemical stability of admixtures of injectable corticosteroid and local anaesthetics. Scientific Commons. 2007. [Available online: accessed 27 December 2010]
106. Tramer M.R., Moore R.A., Reynolds J.M., et al. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain. 2000;85:169-182.
107. Anon. Articular and periarticular corticosteroid injection. Drug Ther Bull. 1995;33(9):67-70.
108. Black D.M., Filak A.T. Hyperglycemia with non-insulin-dependent diabetes following intra-articular steroid injection. J Fam Pract. 1989;28(4):462-463.
109. Younis M., Neffati F., Touzi M., et al. Systemic effects of epidural and intra-articular glucocorticoid injections in diabetic and non-diabetic patients. Joint Bone Spine. 2007;74:472-476.
110. Wang A.A., Hutchinson D.T. The effect of corticosteroid injection for trigger finger on blood glucose level in diabetic patients. J Hand Surg [Am]. 2006;31(6):979-981.
111. Mens J.M.A., De Wolf A.N., Berkhout B.J., et al. Disturbance of the menstrual pattern after local injection with triamcinolone acetonide. Ann Rheum Dis. 1998;57:700.
112. Weitoft T., Larsson A., Ronnblom L. Serum levels of sex steroid hormones and matrix metalloproteinases after intra-articular glucocorticoid treatment in female patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:422-424.
113. van Tuyl S.A.C., Slee P.H. Are the effects of local glucocorticoid treatment only local? Neth J Med. 2002;60:130-132.
114. Lazarevic M.B., Skosey J.L., Djordjevic-Denic G. Reduction of cortisol levels after single intra-articular and intramuscular steroid injection. Am J Med. 1995;99(4):370-373.
115. . British National Formulary, No 59 Section 6.3.2. London: BMA/RPSGB. March 2010.
116. Weitoft T., Rönnblom L. Glucocorticoid resorption and influence on the hypothalamic-pituitary-adrenal axis after intra-articular treatment of the knee in resting and mobile patients. Ann Rheum Dis. 2006;65:955-957.
117. Furtado R.N., Oliveira L.M., Natour J. Polyarticular corticosteroid injection versus systemic administration in treatment of rheumatoid arthritis patients: a randomized controlled study. J Rheumatol. 2005;32(9):1691-1698.
118. Kumar S., Singh R.J., Reed A.M., et al. Cushing’s syndrome after intra-articular and intradermal administration of triamcinolone acetonide in three pediatric patients. Paediatrics. 2004;113(6):1820-1824.
119. Taylor H.G., Fowler P.D., David M.J., et al. Intra-articular steroids: confounder of clinical trials. Clin Rheumatol. 1991;10(1):38-42.
120. Ewan P.W. Anaphylaxis (ABC of allergies). Br Med J. 1998;316:1442-1445.
121. Beaudouin E., Kanny G., Gueant J.L., et al. Anaphylaxis caused by carboxymethylcellulose: report of 2 cases of shock from injectable corticoids. Allerg Immunol (Paris). 1992;24(9):333-335. [Article in French]
122. Steroid psychosis after an intra-articular injection. Ann Rheum Dis. 2000;59:926. (Letter)
123. Boonen S., Van Distel G., Westhovens R., et al. Steroid myopathy induced by epidural triamcinolone injection. Br J Rheumatol. 1995;34:385-386.
124. Murphy A.D., Lloyd-Hughes H., Ahmed J. Complex regional pain syndrome (Type 1) following steroid injection for stenosing tenosynovitis. J Plast Reconstr Aesthet Surg. 2010 May 24. [Epub ahead of print]
125. Gladman D.D., Bombardier C. Sickle cell crisis following intraarticular steroid therapy for rheumatoid arthritis. Arthritis Rheum. 1987;30(9):1065-1068.
126. Brinks A., Koes B.W., Volkers L., et al. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord. 2010;11:206. doi: 10.1186/1471-2474-11-206
127. Kerrigan C.L., Stanwix M.G. Using evidence to minimize the cost of trigger finger care. J Hand Surg [Am]. 2009;34(6):97-1005.
128. Jansen T., van Roon E. Four cases of a secondary Cushingoid state following local triamcinolone acetonide injection. Neth J Med. 2002;60(3):151-153.
129. Lazarevic M.B., Skosey J.L., Djordjevic-Denic G., et al. Reduction of cortisol levels after single intra-articular and intramuscular steroid injection. Am J Med. 1995;99(4):370-373.
130. Lansang M.C., Farmer T., Kennedy L. Diagnosing the unrecognized systemic absorption of intra-articular and epidural steroid injections. Endocr Pract. 2009;15(3):225-228.
Other substances used for injection therapy
Overview
The first injectates, which yielded little benefit, were formalin, glycerin, lipodol, lactic acid, and petroleum jelly.1,2 Many other agents have been tried since (Table 1.6). For soft tissue lesions in particular there has long been a clear need for effective conservative therapies, and in recent times novel agents have been injected specifically to try to promote healing. Depending on your perspective, these treatments exist either on the fringe or the frontier of musculoskeletal therapeutics.
Table 1.6 Some substances injected into joints/soft tissues for therapeutic effect
| Adalimumab | Guanethidine | Phenol |
| Actovegin | Glycerin | Platelet rich plasma |
| Air | Hyaluronans & derivatives | Osmic acid |
| Anakinra | Infliximab | Radioactive materials |
| Aprotinin | Lactic acid | Sclerosing agents |
| Autologous whole blood | Lipodol | Traumeel® |
| Botulinum toxin A | Methotrexate | |
| Dextrose | Nonsteroidal anti-inflammatories | |
| Etanercept | Petroleum jelly | |
| Formalin | Polidocanol |
Hyaluronans
Endogenous (naturally occurring) hyaluronan (HA, previously known as hyaluronic acid) is a large, linear glycosaminoglycan and is a major non-structural component of both the synovial and cartilage extracellular matrix. It is also found in synovial fluid and is produced by the lining layer cells of the joint. These molecules produce a highly viscoelastic solution that is a viscous lubricant at low shear (during slow movement of the joint e.g. walking) and an elastic shock absorber at high shear (during rapid movement e.g. running). As well as conferring viscoelasticity the other key functions of HA in the joint are lubrication and the maintenance of tissue hydration and protein homeostasis through the prevention of large fluid movements by functioning as an osmotic buffer. HA is also considered a physiological factor in the trophic status of cartilage. HA has a very high water binding capacity; one gram dissolved in physiological saline occupies 3 litres of solution.3,4 In osteoarthritic joints the capacity of synovial fluid to lubricate and absorb shock is typically reduced. This is partly due to the production of abnormal HA with a reduction in the size and concentration of the molecules naturally present in the synovial fluid.4
Synthetic HA was isolated from roosters’ combs and umbilical cord tissue and developed for clinical use in ophthalmic surgery and arthritis in the 1960s. The rationale for joint injection therapy was to replace the normal physiological properties lost to the osteoarthritic joint as a consequence of the associated reduction in the volume and quality of HA, a concept known as viscosupplementation. Commercial preparations of HA have the same structure as endogenous HA although cross-linked HA molecules (known as hylans) were later engineered by molecular linkage in order to obtain greater elastoviscosity and intra-articular dwell-time.3
Osteoarthritic knees may be treated by intra-articular injection of HA, usually after any effusion is drained.3–6 The mode of action of exogenous (synthetic) HA and its derivatives is not clear, particularly when an effusion containing (endogenous) HA is removed and immediately replaced by (exogenous) HA which then stays in the joint cavity for only a few days at most. Perhaps these injections stimulate the synthesis of ‘better quality’ more physiologically normal endogenous HA and/or reduce inflammation.4 Given the relatively short intra-articular residency, any hypothesis for the mechanism of action must account for the long duration of clinical efficacy that has been reported.3
A number of commercial preparations are available for injection (Table 1.7); there is no evidence that any one preparation is superior.3 The licensed commercial formulations that have been available the longest in the UK are Hyalgan® and Synvisc®. Hyalgan has a lower molecular weight and is licensed as a medicinal product; it is injected once weekly for 5 weeks and is repeatable no more than 6-monthly. Synvisc has a higher molecular weight and is licensed as a medical device; it is injected once weekly for 3 weeks, repeatable once within 6 months, with at least 4 weeks between courses.
| Sodium hyaluronate | Hylan G-F20 |
|---|---|
| Durolane® | Synvisc® |
| Euflexxa® | |
| Fermathron™ | |
| Hyalgan® (not available for NHS prescription) | |
| Orthovisc® | |
| Ostenil® | |
| Suplasyn® | |
| Synocrom® | |
| Synopsis |
Research evidence on the efficacy of HAs is often difficult to interpret because of confounders, including: different molecular weights, different injection schedules (ranging from once to a series of five injections), and, despite large numbers of studies, generally poor trial design (lack of intention-to-treat analyses, and limitations in blinding).6 Intra-articular HA injection for osteoarthritic knees is endorsed by two authoritative guidelines7,8 but was rejected by the UK National Institute for Health and Clinical Excellence (NICE) on the grounds of cost.3
A recent systematic review and meta-analysis has concluded that from baseline to week 4, intra-articular corticosteroids appear to be relatively more effective for pain than intra-articular HA. By week 4, both therapies have equal efficacy, but beyond week 8, HA has greater efficacy. 9 A Cochrane review also suggests that the pain relief with HA therapy is achieved more slowly than with steroid injections, but the effect may be more prolonged.6
Two very recent 2010 prospective, double blind, randomized, placebo-controlled trials with large numbers of osteoarthritic knee patients have reached opposing conclusions. One study of 5 weekly injections of Hyalgan versus saline after one year showed no treatment effect in any outcome measure.10 The other compared a single injection of Synvisc with placebo and after 6 months showed clinically relevant pain relief. No safety issues were seen in either study.11
Overall, the evidence suggests that HA and hylan derivatives are superior to placebo in terms of pain reduction, efficacy and quality of life outcomes in patients with osteoarthritic knees although the effect size is generally small. Given this, and the cost of these therapies, together with the increased number of clinician visits required, NICE concluded that the benefits of HA injection therapy would have to be three to five times higher than the current estimates before efficacy reached the standard threshold for cost effectiveness to the NHS. NICE also concluded that clinical trials do not suggest that there are sub-groups of patients who may have greater benefit from HA treatment (which might improve cost effectiveness).3 Limited data are available concerning the effectiveness of multiple courses of HA therapy.12 Patients older than 65 and those with the most advanced radiographic stage of osteoarthritis are less likely to benefit.13
Some commercial HAs are licensed for use in the hip joint. No significant differences between HA and placebo were reported by a trial evaluating efficacy and function outcomes in patients with hip osteoarthritis;14 one systematic review noted methodological limitations in the literature, which were mainly the absence of a control group in most of the studies, overly short follow-up periods, and different ways of measuring outcomes. The review concluded that HA injection for hips should only be used under careful supervision and only in those cases where other treatments have failed.15 A second systematic review concluded that despite the relatively low level of evidence of the included studies, HA injection performed under fluoroscopic or ultrasound guidance seems to be effective, and appears to be safe and well tolerated but cannot be recommended as standard therapy in the wider population.16 A third review concluded that this therapy seems to be a valuable technique that may delay the need for surgical intervention, with no difference between products, but further studies are necessary.17
The use of HA injections in other joints is being investigated.18–23 Encouraging, but inconclusive results have been observed for the treatment of shoulder, carpometacarpal, and ankle osteoarthritis.24
The toxicity of intra-articular HA appears to be negligible. No major safety issues have been identified when compared with placebo, but a definitive conclusion is precluded due to sample-size restrictions.6 They may cause a short-term increase in knee inflammation.25 A small percentage may experience a transient mild to moderate increase in pain following injection, and some have a flare with marked effusion. Local reactions to hylan GF-20 occur more often in patients who have received more than one course of treatment. Following corticosteroid injection these reactions abate without apparent sequelae.26 As with any injection procedure, there is a very small risk of infection.3 The synergistic combination of corticosteroid and HA for simultaneous injection is an approach that has been investigated in a small number of studies.27,28
Sclerosants (prolotherapy)
Sclerosing therapy was used by Hippocrates, in the form of cautery at the shoulder, to prevent recurrent dislocation. In current medical practice sclerosing agents are mainly injected to treat varicose veins, oesophageal varices and piles. This therapy has been used to treat chronic low back pain for over 60 years29 and is also known as prolotherapy because it involves injecting a proliferant i.e. a substance that is intended to stimulate fibroblast proliferation.30
The musculoskeletal rationale for injection of sclerosants is to strengthen inadequate ligaments by exposing them to an irritant that will induce fibroblastic hyperplasia, seeking to stimulate connective tissue growth, and promote the formation of collagen.29 The treatment aims to cause soft tissue inflammation, the opposite objective to corticosteroid injection therapy,30 but the histological response may not be different from that caused by saline injections or needlestick procedures.31 Prolotherapy for musculoskeletal disorders is not widespread but it seems to be popular with some patients; a survey of 908 primary care patients receiving opioids for chronic pain in the USA, most commonly chronic low back pain (38 %), reported that 8 % had used prolotherapy in their lifetime, and 6 % had used it in the previous year.32
Although this treatment is mostly used for back pain,29,30,33–35 including the sacroiliac joint,36 it has also been tried for peripheral instability34,37 and in elite kicking-sport athletes with chronic groin pain from osteitis pubis and/or adductor tendinopathy.38 Intra-articular dextrose sclerotherapy for anterior cruciate ligament laxity has also been reported in a small study.39
Prolotherapy has been investigated in Achilles tendinopathy. In a study comparing effectiveness and cost-effectiveness of eccentric loading exercises (ELE) with prolotherapy or combined treatment, at 12 months results were better for prolotherapy and for combined treatment, which had the lowest incremental cost compared with ELE, but long-term results were similar.40 Sonography-guided intratendinous injection of 25 % hyperosmolar dextrose has also been used to treat chronic Achilles tendinopathy41 and plantar fasciitis.42
The term ‘prolotherapy’ encompasses a variety of treatment approaches rather than a specific protocol, and that there are a large number of sclerosants.35 One of the most common sclerosant solutions consists of a mixture of dextrose, glycerin, phenol and lidocaine (P2G).35 Some use just dextrose and lidocaine, which may be potentially less neurotoxic, although part of the pain-relieving effect of sclerosant injection may be from a toxic action on nociceptors.
In the most carefully conducted study of prolotherapy for back pain reported so far, there was no difference between the effect of injecting a sclerosant solution or injecting saline at key spinal ligament entheses, but as both groups of patients improved significantly, it is difficult to decide what the particular effect of sclerosant injections might be.43 A critical review concluded that prolotherapy may be effective at reducing spinal pain but great variation was found in the protocols used, precluding definite conclusions. It was recommended that future research should focus on those solutions and protocols that are most commonly used in clinical practice, and that have been used in trials reporting effectiveness, to help determine which patients are most likely to benefit.35
A Cochrane review concluded that there is conflicting evidence regarding the efficacy of prolotherapy for chronic low-back pain, and that when used alone it is not an effective treatment. When combined with spinal manipulation, exercise and other co-interventions prolotherapy may improve chronic low-back pain and disability. Conclusions were confounded by clinical heterogeneity amongst studies and by the presence of co-interventions.33
Polidocanol
Polidocanol (Ethoxysclerol) is a sclerosing local anaesthetic that has recently been injected to treat tendinopathies. The rationale for its use is that the pain from tendinopathy is related to the growth of new blood vessels (neovascularization) and their closely associated nerves. These vascular changes can be seen on colour Doppler ultrasound examination of tendons. In a pilot study polidocanol was injected under ultrasound control into the neovessels of patients with Achilles tendinopathy; 8 out of 10 subjects had significant reduction in their pain and returned to pain free tendon loading activities with benefit persisting at 6 months.44
A randomized controlled trial/cross-over study was conducted to investigate polidocanol in a group of elite athletes with patellar tendinopathy. The treatment group reported a significant improvement after 4 months; there was no change for the control group. After 8 months, when the control group had also received active treatment with polidocanol, they had a greater improvement than did the treatment group. There was no further improvement in either group at 12-month follow-up.45
Another prospective, randomized, controlled, double-blind, crossover trial compared guided intratendinous single injection of polidocanol with a single injection of local anaesthetic (lidocaine + epinephrine), in patients with tennis elbow. At the 3 month follow-up, additional injections with polidocanol were offered to both groups (crossover for group 2). At one year there was similar pain relief in both groups.46
In a retrospective study in which Achilles tendons received polidocanol injections for chronic midportion tendinopathy, pain correlated positively with neovessels on ultrasound. The authors concluded that their study did not confirm the postulated high beneficial value of sclerosing neovascularization injections in patients with this condition and stressed that polidocanol injection may not be as promising as was first thought.47
Autologous blood
It is postulated that tendon healing and regeneration may be improved by injecting autologous growth factors (AGF) obtained from the patient’s own blood48 and there is growing interest in the working mechanisms. The amount and mixture of growth factors produced using different cell separating systems are largely unknown and it is also uncertain whether platelet activation prior to injection is necessary. AGF can be injected with autologous whole blood or platelet-rich plasma (PRP) and this therapy is increasingly used with high expectations of regenerative effects. Chronic tendinopathies including wrist extensors, flexors, Achilles tendons (and plantar fascia), have been treated with this approach.49 PRP has also been injected into the knee to encourage the healing of cartilage.50
In a comparative, open study to evaluate the short-, medium-, and long-term effects of corticosteroid injection, AGF injection, and extracorporeal shock wave therapy in the treatment of tennis elbow, corticosteroid injection gave a high success rate in the short term. However, AGF and shock wave therapy gave better long-term results.51 A review of treatments for tennis elbow concluded that there is strong pilot-level evidence supporting treatment with prolotherapy, polidocanol, AGF and platelet-rich plasma injection, but that rigorous studies of sufficient sample size are needed to determine long-term effectiveness and safety, and whether these techniques can play a definitive role.52
In a systematic review, all studies showed that injections of AGF (whole blood and PRP) in chronic tendinopathy had a significant impact on improving pain and/or function over time. However, only three studies using autologous whole blood had a high methodological quality assessment, and none showed any benefit when compared with a control group. The review concluded that there is strong evidence that the use of injections with autologous whole blood should not be recommended. There were no high-quality studies found on PRP treatment and therefore limited evidence to support its use in the management of chronic tendinopathy.49
In a subsequent recent double blind, randomized, placebo-controlled trial of eccentric exercises plus either PRP or saline injection for chronic midportion Achilles tendinopathy, PRP injection did not result in greater improvement in pain and activity.53 Achilles tendinopathy in particular remains a frustratingly difficult therapeutic challenge.55
NICE guidance states that current evidence on the safety and efficacy of autologous blood injection for tendinopathy is inadequate and therefore this procedure should only be used with special arrangements for clinical governance, consent and audit, or for research. Clinicians wishing to undertake autologous blood injection for tendinopathy should take the following actions; inform the clinical governance leads in their Trusts; ensure that patients understand the uncertainty about the procedure’s efficacy, especially in the long term, make patients aware of alternative treatments, and provide them with clear written information – use of NICE’s information for patients (‘Understanding NICE guidance’) is recommended (available from www.nice.org.uk/IPG279publicinfo); audit and review clinical outcomes of all patients having autologous blood injection for tendinopathy.54
Aprotinin
In a case review and follow up questionnaire for consecutive patients with tendinopathy treated by aprotinin injection, 76 % had improved, 22 % reported no change, and 2 % were worse, 64 % found the injections helpful, while 36 % had neither a positive nor negative effect. Achilles patients were more successfully treated than patella tendon patients.56 In a prospective, randomized trial athletes with patella tendinopathy were injected with aprotinin, methyprednisolone or saline. At 12 months follow-up there were 72 % excellent or good responses in the aprotinin group, 59 % in the methylprednisolone group, and 28 % in the saline group, with respectively 7 %, 12 % and 25 % poor results.57
Another prospective, randomized, double blind, placebo-controlled trial comparing normal saline plus local anaesthetic injections and eccentric exercises, with aprotinin plus local anaesthetic injection and eccentric exercise demonstrated no significant benefit over placebo.58
Potential side-effects include allergy and anaphylaxis, although death has only been reported when used intravenously for cardiac surgery. Delay of 6 weeks between repeat aprotinin injections for tendinopathy reduces the risk of allergic reaction.59 The ‘test dose’ of 3–5 ml for major procedures is similar to the therapeutic dose for tendinopathy. Injecting aprotinin for tendon injuries is currently an ‘off-label’ indication.
Botulinum toxin
Botulinum toxin injection is used to treat various painful conditions including muscle spasticity, dystonia, headache and myofascial pain. A systematic review to assess the evidence for efficacy of botulinum toxin A (BTA) compared with placebo for myofascial trigger point injection found five clinical trials that met the inclusion criteria. One trial concluded that BTA was effective, and four concluded that it was not. The review concluded that the data is limited and clinically heterogeneous and that the current evidence does not support the use of BTA injection in trigger points for myofascial pain.60
More recently there has been a randomized, placebo controlled, crossover trial examining the efficacy of botulinum toxin type A (BoNT-A) injection (Dysport®) to the distal vastus lateralis muscle, plus an exercise programme for chronic anterior knee pain (AKP) associated with quadriceps muscle imbalance. BoNT-A injection produced a greater reduction in pain and disability than placebo injection in carefully selected patients.61
Actovegin®
Actovegin® is a deproteinized hemodialysate of calf’s blood postulated to improve cellular uptake and utilization of glucose and oxygen. It was initially licensed for intravenous use to improve tissue oxygen transport in patients with arterial disease. As a gel or cream it is also used to treat slow-healing skin lesions such as burns or skin-grafted wounds. In recent years it has gained notoriety for its use by elite cyclists as a performance-enhancing drug, with the consequence that the World Anti-Doping Agency (WADA) has banned its use in competition. Given the popularity of this treatment amongst professional athletes62 there is a dearth of clinical trials. A Cochrane review of treatments for Achilles tendinopathy63 concluded that, in the single small trial that compared Actovegin with a control injection, results were promising, but that the severity of patient symptoms was questionable.64 A small study has reported short-term improvement and no adverse effects when Actovegin was injected into OA knees.65 A pilot study of autologous blood injection for muscle strains used Actovegin plus Traumeel® as a control treatment and found the autologous blood to be superior.66
Radiosynovectomy
Radiosynovectomy (RSV, also known as radiosynoviorthesis) is the local intra-articular injection of radionuclides (beta particle emitters) in colloidal form for radiotherapy. First used in 1952 for medical synovectomy, the technique is for treatment of resistant synovitis of individual joints after failure of long-term systemic pharmacotherapy and intra-articular corticosteroid injections. RSV relieves pain and inflammation from rheumatoid arthritis, for which it initially was used, and is accepted as an alternative to surgical synovectomy in cases of this or other inflammatory joint diseases such as haemophiliac arthropathy. A systematic review and meta-analysis of the effectiveness of 169Erbium/186Rhenium-RSV (used predominantly in small joints) and 90Yttrium-RSV (used predominantly in knee joints) reported that success rates are high, but differences in effect with corticosteroid injection are less evident, although there is marked heterogeneity in the design of the small number of comparative studies. In comparison with surgical synovectomy, RSV produces equivalent results, costs less, allows the patient to remain ambulatory, and is repeatable. RSV has been proposed as the initial procedure of choice for the treatment of patients with haemarthrosis in haemophilia. In addition, local instillation of radiopharmaceuticals can effectively reduce effusions after implantation of a prosthesis.67,68