CHAPTER 92 Injection Procedures
BACKGROUND
The natural history of low back pain has been reported to be quite favorable in some studies and is often quoted to patients as such. It has been reported that 40–50% of patients are symptom free within 1 week and up to 90% have resolution of symptoms without medical attention in 6–12 weeks.1–4 However, recent studies suggest that lumbar axial pain is a more significant condition characterized by acute exacerbations and variable periods of remission. Deyo and Tsui-Wu5 reported 33.2% of patients with low back pain reported symptoms with a duration of less than 1 month, with 33% reporting pain for 1–5 months and 32.7% reporting pain for longer than 6 months. Recurrence rates from 60–85% have been reported in the first 2 years following an acute episode of low back pain.6,7 More recently, Von Korff et al.8 observed that over a 2-year follow-up period, 44% report chronic symptoms (defined as more than 90 days of back pain in the previous 6 months). In this study, most patients were experiencing low levels of back pain with 20% rating their pain as 4 or greater on a 0–10 scale; 13% rated their current pain as 5 or greater and only 8% reported pain of 6 or greater. Furthermore, Von Korff et al.8 reported that 15–20% of primary care patients with low back pain show moderate to severe activity limitations during a 1-year follow-up period after the initial episode has resolved.
PATHOPHYSIOLOGY
Descriptions of the treatment for low back pain dates to Hippocrates (460–370 BC), with the reporting of joint manipulation and the use of traction.9 It often is theorized that the onset of low back pain is associated with bipedal ambulation and that this transformation in the mechanics of locomotion is the inciting evolutionary event that has made the lumbar spine susceptible to a relatively accelerated rate of degenerative disease. Degeneration is universal to the structures that comprise the basic functional spinal unit, which is composed of two adjacent vertebral bodies, the intervertebral disc, and the two zygapophyseal (Z-) joints at the same segment functioning as a tri-joint complex. As humans age, endure various micro- and macrotraumas, and undergo changes in body habitus that alter and unevenly distribute biomechanical forces on the lumbar spine, there is a natural progression of degeneration of the lumbar motion segment with corresponding anatomic, biomechanical, radiologic, and clinical findings.10 It is this evolution that provides the insight into the root cause of lumbar spinal pain syndromes.
The posterior elements of the lumbar spinal functional unit bear less weight than the anterior elements in all positions. During sitting, the anterior elements bear over 90% of the forces transmitted through the lumbar spine, whereas during standing, this fraction decreases to approximately 80%.10 As the degenerative process progresses, the relative anterior to posterior force transmission approaches 50%.10 The spine functions best within a realm of static and dynamic stability. The bony architecture and associated specialized soft tissue structures, especially the intervertebral disc, provide static stability. Dynamic stability, however, is accomplished via a system of muscular and ligamentous supports which act in concert during various functional, occupational, and avocational activities. The overall mechanical effect of these structures maintains the histologic integrity of the tri-joint complex. The net shear and compressive forces must be maintained below respective critical minimums in order to maintain the integrity of the tri-joint articulation. Persistent, recurrent, nonmechanical and/or excessive forces to the motion segment beyond minimal thresholds leads to microtrauma to the disc and Z-joints, which triggers and continues the degenerative process.10 This degenerative cascade, as described by Kirkaldy-Willis,10 is the widely accepted pathophysiologic model describing the degenerative process and ensuing spinal segment dysfunction as it affects the lumbar spine and its individual motion segments. This process is described as occurring in three phases in a progressive longitudinal manner over time. These phases are a continuum with a gradual transition rather than three distinct, clearly definable stages. Although the etiology of spinally mediated lumbar axial pain is variable and not always clearly definable, there are a finite number of potentially painful structural components of the lumbar spine, and the aforementioned degenerative cascade provides insight on the process by which these components develop pathology and can become symptomatic.
BASIC SCIENCE EVIDENCE OF INFLAMMATORY MECHANISMS IN DISCOGENIC-MEDIATED PAIN SYNDROMES
The anterior elements of the lumbar spine, most notably the intervertebral disc, are currently thought to be the primary source of the vast majority of spinally mediated lumbar axial pain syndromes. Several theories cite a traumatically induced acute annular tear as the inciting pathologic event. Others theorize the natural result of aging is the sole etiology of pathology of the lumbar disc. However, these models do not explain spontaneously occurring annular tears and disc degeneration in the young. Therefore, the cause of lumbar disc disease is most likely multifactorial. Various genetic, environmental, autoimmune, inflammatory, traumatic, infectious, toxin-induced, and other factors may alone, or in various combinations, result in the initiation and progression of degeneration of the intervertebral disc in a way that has not yet been elucidated. However, to some degree, the basic science literature has been able to elucidate the inflammatory mechanisms of discogenic mediated lumbar axial pain syndromes. In 1987, McCarron et al.11 published a study in which they reported on the incitation of an inflammatory response upon injection of autologous nucleus pulposus into the epidural space of dogs. In addition, they reported that a delay in the treatment of this inflammatory process could result in tissue fibrosis and cellular damage. In 1990, Saal et al.12 demonstrated significantly increased level of PLA2, the rate-limiting enzyme in the arachidonic acid cascade, activity in lumbar HNP further demonstrating the inflammagenic properties of nuclear material. In 1996, further study helped elucidate the biochemical factors by which the nucleus pulposus elicits an inflammatory process. Takahashi et al.13 demonstrated the presence of inflammatory cytokines, including IL-1a+b, IL-6, TNF, GMCSF, and L-B4, in herniated nucleus pulposus (HNP). Kang et al.14 reported increased levels of MMPs, nitric oxide, IL-6, and PGF2 in the culture media of HNP. These properties may in part be explained by the fact that the nucleus pulposus, a remnant of the embryonic notochord, is sheltered from the bloodstream during embryologic development and may therefore be construed as an antigen upon presentation to the immune system when entering the ventral aspect of the epidural space.15 Subsequently, epidural steroid injections (ESI) have become commonly employed interventions in treating spinally mediated lumbar axial pain syndromes thought to be potentially due to an inflammatory mechanism based on the aforementioned basic science literature as it pertains to specific discogenic induced spinal syndromes.
EVOLUTION OF INTERVENTIONAL SPINE PROCEDURES
The initial reports of epidural injections almost a century ago reported the instillation of cocaine into the epidural space to treat lumbago and sciatica. In the early 1900s, epidural injection of local anesthetic was used to treat intractable sciatica.16 In 1952, Robecchi and Capra17 reported success with the first ESI in treating lumbar and associated sciatic pain. Presently, ESIs are a commonly utilized intervention in the management of spinally mediated lumbar axial syndromes both with and without associated radicular symptomatology. Several studies have shown that up to 40% of blind (nonfluoroscopically guided) ESIs may erroneously deliver the injectate superficially into the soft tissues or inadvertently deep into the subarachnoid space, which may result in serious complications.18–21 Therefore, we strongly advise that any type of ESI be performed under real-time fluoroscopic guidance with contrast-enhanced visualization of flow within the epidural space to the target site prior to the injection of medication unless otherwise contraindicated. In 1972, Winnie et al. emphasized the importance of placing the medication as close to the site of pathology as possible for the clinician to optimize outcome. They demonstrated improvement in 80% of patients in whom corticosteroids were infused at the site of pathology.22 This logical concept must be considered when determining which of the three commonly utilized epidural access routes, transforaminal, interlaminar, or caudal, is utilized when performing an ESI.
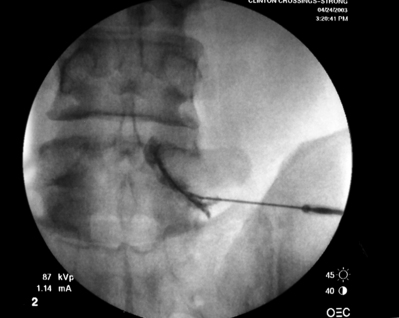
EFFICACY OF TRANSFORAMINAL ESI FOR LUMBAR AXIAL PAIN SYNDROMES WITH RADICULAR INVOLVEMENT
The optimal route for injection of corticosteroids into the epidural space at the site of pathology in patients with discogenic-mediated lumbar axial pain syndromes with corroborative radicular involvement is via the transforaminal route. This approach allows the clinician to deliver the injectate, composed of a combination of 6–12 mg of betamethasone and 0.5–1 cc of 1% lidocaine, precisely to eradicate the known inflammatory response emanating from the potentially inflammagenic HNP focally on the specific inflamed nerve root sleeve (Fig. 92.1). The efficacy of this approach has been demonstrated in four different randomized, prospective, double-blind, controlled clinical trials. Riew et al.23 reported results of a prospective, randomized, controlled, double-blinded study using fluoroscopically guided lumbar transforaminal injections in 55 patients with imaging evidence of nerve root compression and corroborative radicular symptoms. Twenty-eight patients received bupivacaine and betamethasone, and 27 received only bupivacaine. At follow-up (13–26 months) 33.3% of the bupivacaine group decided not to have surgery, versus 71.4% of the bupivacaine and betamethasone group. This difference in surgical rates was statistically significant (p<0.004). This controlled study demonstrates the beneficial effect of precisely delivered corticosteroids in obviating the need for operative treatment in patients with HNP and/or spinal stenosis. Kraemer et al.24 published a study in which they reported long-term pain relief with transforaminal ESI in a study in which 49 patients with lumbar radicular pain were randomized into a corticosteroid group and control group. Karppinen et al.25,26 reported on 160 consecutive patients with symptomatic herniated discs with no prior history of lumbar spine surgery randomized into either a corticosteroid group or normal saline group. Outcome measures obtained at 2 weeks, 3 months, and 6 months included pain relief, sick leave, medical costs, the Nottingham Health Profile, and the future requirement for surgical intervention. They reported that transforaminal ESI provided significant short- and long-term improvement in all of the aforementioned outcome measures. Thomas et al.27 reported results of a prospective, randomized, controlled, double-blinded study comparing the relative effectiveness of fluoroscopically guided lumbar transforaminal ESIs versus blind interlaminar ESIs in patients with radicular pain. They demonstrated the superiority of transforaminal ESIs in all of a variety of outcome measures including finger-to-floor lumbar flexion, daily activity including work and avocational function, and Dallas pain scores. This direct comparison study underscores the importance of fluoroscopic guidance as well as delivering medication accurately and precisely to the site of a potential ongoing inflammatory response. In a non-blinded, randomized, prospective study by Butterman,28 transforaminal ESIs provided efficacy measured by reducing symptomatology and disability and obviating surgery at a follow-up of up to 3 years in patients with large (>25% of the cross-sectional area of the spinal canal) symptomatic lumbar herniated discs. Butterman28 also reported that in those patients who had short-term improvement or ineffectiveness of transforaminal ESIs and required surgical discectomy, there was no adverse affect in the outcome of that surgery from the temporal delay caused by the trial of transforaminal ESIs.
In addition to the aforementioned randomized clinical outcome studies, several prospective nonrandomized clinical trials on the efficacy of transforaminal ESI also strongly suggest the beneficial effects of transforaminal ESIs for HNP causing lumbar axial pain with corroborative radicular pain. For example, Weiner and Fraser29 showed 21 out of 28 patients with a computed tomography (CT) documented HNP and corroborative lower extremity pain receiving a single transforaminal infusion of betamethasone and 1% Xylocaine injectate had moderate or complete pain relief not requiring surgery at an average of 3.4 years follow-up. Lutz et al.30 reported on 69 patients, with an average of 22 weeks of symptoms, with magnetic resonance imaging (MRI) evidence of an HNP and radicular pain. Patients underwent an average of 1.8 transforaminal injections of betamethasone and 1% Xylocaine followed by a 6–12-week course of lumbar spine stabilization therapy. They reported a successful outcome, as defined by a 50% or greater reduction in pain and return to near-previous level of functioning, in 75% of patients at an average of 80 weeks follow-up. In a retrospective evaluation, Wang et al.31 demonstrated significant symptom improvement in both the short and long term and the avoidance of discectomy in 77% of patients with lumbar disc herniations treated with one to six transforaminal ESIs.
With respect to the interventional treatment of lumbar axial pain syndromes with corroborative radicular pain due to spinal stenosis, the previously stated Riew et al.23 study reported on the effectiveness of transforaminal ESI as it incorporated spinal stenosis in addition to HNP as the underlying etiology to their subjects symptomatology. In addition, Delport et al.32 conducted a retrospective review of treating symptomatic spinal stenosis a subgroup of which underwent transforaminal ESI. They reported that of 140 subjects, over 70% had symptom improvement and were at least somewhat satisfied with their response, over 50% reported sustained improvement in their function, and only 29% of the 140 subjects ultimately underwent surgical decompression. Botwin et al.33 conducted a prospective cohort outcome study assessing the efficacy of transforaminal ESIs in the treatment of symptomatic degenerative spinal stenosis. Thirty-four subjects were followed for 1 year. They reported that 75% of the patients had a successful long-term outcome as defined as >50% symptom reduction. They also reported more than 50% of the subjects improved standing and/or walking tolerance.
The aforementioned literature strongly suggests that transforaminal ESI should be the standard of care index interventional spine procedure for patients with spinally mediated lumbar axial pain syndromes associated with radicular involvement due to HNP and/or spinal stenosis when more conservative measures have failed. Furthermore, in the case of the majority of HNPs, the known phagocytic immunologic response and consequent benign anatomic natural history assists in the relatively high rate and long-term success of transforaminal ESIs.34,35
CONCEPTUAL PATHOPHYSIOLOGIC MECHANISMS OF LUMBAR AXIAL PAIN SYNDROMES WITHOUT RADICULAR INVOLVEMENT DUE TO A DISCOGENIC-MEDIATED INFLAMMATORY RESPONSE
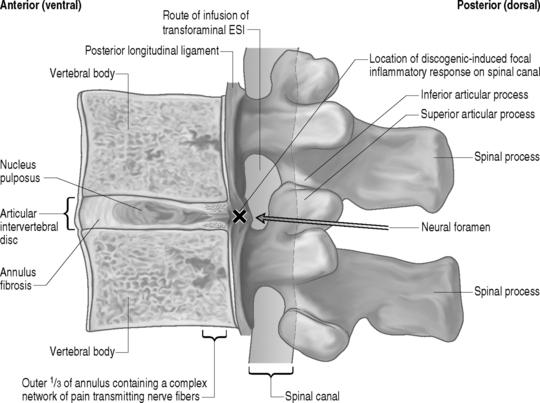
Some important inferences from the aforementioned literature on the successful long-term treatment of HNP-associated lumbar axial pain syndromes with corroborative radicular involvement may be extracted that, when combined with known previously stated basic science literature and lumbar spinal anatomy, suggest a possible utilization for transforaminal ESIs in the interventional treatment of discogenic-mediated lumbar axial pain without radicular involvement. The similarities in the basic ideologies by which the initial incorrect thought processes of compression as the sole mechanism of radicular pain due to HNP requiring discectomy as stated nearly a century ago by Mixter and Barr36 mirror recent theory that motion segment instability and/or structural disc pathology is the sole mechanism of discogenic-mediated lumbar axial pain without radicular involvement. In the decades subsequent to Mixter and Barr’s36 landmark article identifying HNP pathology as a key source of lumbar axial and radicular pain, there have been many attempts to identify the precise pathophysiologic mechanism of discogenic-mediated lumbar axial pain without radicular involvement. As stated earlier in this chapter, over the years, there have been key pieces of literature to suggest that a tear in the outer one-third of the anulus of the intervertebral disc may be the most common etiology of acute spinally mediated lumbar axial pain.10,37 As with the painful sequelae of most degenerative spinal conditions, improvement and eventual remission within 6 weeks is the natural history. However, subacute symptoms can persist and the mechanism for this may be inflammatory in nature.38 The basic science literature discussed earlier in this chapter has repeatedly and clearly demonstrated the inflammagenic properties of the intervertebral disc, particularly the nucleus pulposus when it is exposed to the ventral epidural space.13–17,38 This literature lends credence to the mechanisms by which transforaminal ESI effectuate symptom relief on a molecular basis. Therefore, the possibility of a focally contained inflammatory response to a centrally or paracentrally contained herniated disc is entirely possible and may be instrumental in pain generation as well as inhibition of the natural healing processes known to exist. In fact, a contained HNP may chemically sensitize the posterior longitudinal ligament, resulting in axial pain in a fashion similar to that of inflammatory radicular pain due to an HNP sensitizing a nerve root. Furthermore, patients with internal disc disruption syndrome in which an annular fissure extends from the nucleus pulposus to the outer one-third of the anulus (which may or may not be associated with a HNP) and may pierce through the outer anulus and intermittently or consistently communicate microscopic amounts of nuclear material to the ventral epidural space ensuing in an intermittent and/or ongoing focal discogenic-induced inflammatory response (Fig. 92.2). This pathophysiologic model may explain why a randomized, prospective, double-blind clinical trial performed by Simmons et al.39 failed to demonstrate a statistically significant benefit to intradiscal corticosteroid infusions when compared to placebo. The aforementioned hypothesis suggests the inflammatory response occurs in the ventral portion of epidural space on the floor of the spinal canal juxtapositioned to the afflicted intervertebral disc(s) rather than within nuclear material encased by the anulus fibrosus.
EFFICACY OF TRANSFORAMINAL ESI FOR LUMBAR AXIAL PAIN SYNDROMES WITHOUT RADICULAR INVOLVEMENT
To date, there have been only two nonrandomized, retrospective studies reporting on the outcome of transforaminal ESI on spinally mediated lumbar axial pain due to discogenic pathology without imaging evidence of nerve root involvement. One is a subgroup reported by Rosenberg et al.40 stating greater than 50% pain reduction after one year in 59% of patients. The other study reported by Manchikanti et al.41 on patients with spinally mediated lumbar axial pain treated by one of three interventions: (1) blind interlaminar ESI, (2) fluoroscopically guided caudal ESI, and (3) fluoroscopically guided transforaminal ESI, states that superior short- and long-term pain relief is achieved via the transforaminal route. This conclusion makes anatomical sense as transforaminal ESIs likely distribute injectate more focally to the ventral epidural space when compared to interlaminar and caudal route and therefore are more target specific when attempting the deliver medication to a possible focal posterior discogenic-induced inflammatory response.
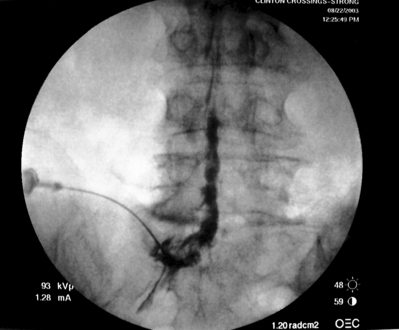
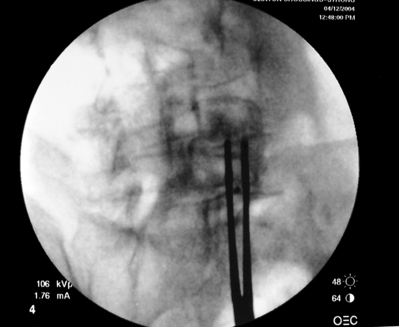
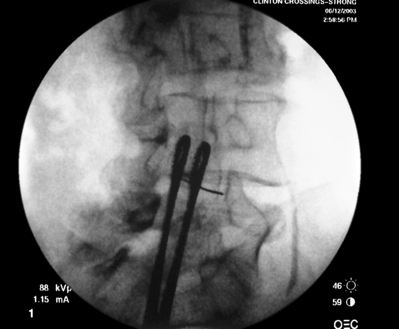
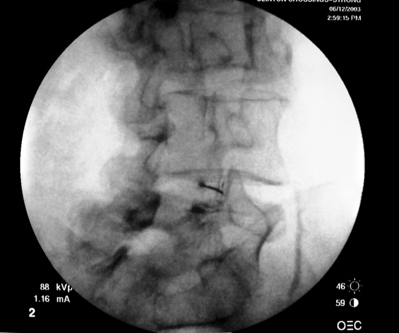
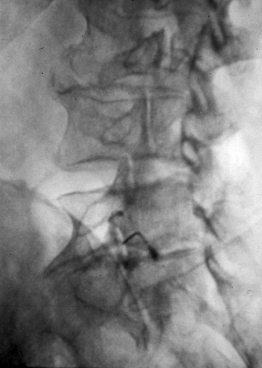
As previously implied, the best route for infusion of corticosteroids into the ventral epidural space in patients with a potential component of focal posterior discogenic inflammatory response is the transforaminal route. In many cases, the specific level of discogenic pathology has not yet been elucidated at the algorithmic point of consideration of ESIs for the treatment of lumbar axial pain thought to be potentially due to a discogenic inflammatory etiology. In fact, more than one segmental level may be contributing to inflammation in the ventral aspect of the lumbar epidural space. Therefore, unless imaging strongly suggests one segment, the S1 transforaminal ESI is recommended (Figs 92.3, 92.4). This route allows the clinician to mechanically drive the corticosteroid injectate, typically 12–18 mg of betamethasone, diffusely along the floor of the lumbar spinal canal via a pressure effect utilizing 3–5 cc of 1% lidocaine in an attempt to quell potential inflammation along the ventral epidural space incited by leakage of nuclear material. In those patients where one-level disease is strongly suspected, a smaller volume of local anesthetic may be infused after the corticosteroid dose at the foraminal level below the suspected level to deliver the corticosteroids in the cephalad direction more focally on the ventral aspect of the spinal canal at the posterior aspect of the suspicious intervertebral segment. In cases where a nonhealing annular tear(s) exists communicating nuclear material to the ventral epidural space, mediation of the inflammatory response may create a more optimal environment for spontaneous healing of the annular tear.
Technique of performing transforaminal ESI
Transforaminal ESI absolutely requires fluoroscopic guidance in order for the site of pathology to be precisely targeted. The transforaminal route is the optimal access for delivering an injectate on the lumbar nerve sleeve and/or the ventral portion of the epidural space. Transforaminal ESIs are performed with the patient prone on the fluoroscopy table. The approximated area of skin overlying the level of the lumbar spine to be injected is cleaned thoroughly with povidone-iodine and the region is draped in a sterile fashion. Real-time fluoroscopy is utilized to identify the pedicle corresponding to the foramina through which injectate is to be delivered. In patients with a transitional segment, either a lumbarized S1 vertebral body or sacralized L5 vertebral body, the interventional spine specialist will need to count the vertebral bodies beginning with L1 after the most caudal rib (T12) to establish that the appropriate pedicle is identified. Once the targeted pedicle is correctly identified, the c-arm should be rotated ipsilaterally until the superior articular process of the juxtapositioned caudal vertebral body comes into view and advances to the medial portion of the targeted pedicle on the created oblique view. The targeted pedicle should now take on an ovoid shape once the correct degree of rotation is achieved as opposed to the circular shape on a true anteroposterior (AP) image with the lumbar spinous processes in the midline. Then, a skin wheal utilizing a combination of 1–3 cc of 1% lidocaine and 0.1–0.3 cc of sodium bicarbonate42 is raised just inferior to the midline portion of the targeted pedicle on the oblique image. A long-handled sponge clamp is utilized to guide a 20-gauge, 3.5 inch spinal needle through the raised skin wheal in a bull’s-eye manner (the needle hub concentrically outlines the needle tip (Fig. 92.5) just inferior to the midline portion of the targeted pedicle until a change in tissue resistance is appreciated or until the spinal needle hub abuts the skin. If the needle abuts skin prior to a change in tissue resistance, the 3.5 inch spinal needle is then utilized as an introducer and is positioned to align its tip to the midline portion of the targeted pedicle and a 25-gauge, 6 inch needle is guided through the index needle until a change in tissue resistance is appreciated. In either instance, the needle tip should then feel stable or locked into position if it has entered the targeted foramen. Verification of needle tip placement is achieved by rotating the c-arm in a manner that provides a true midline AP image. The needle tip is then slowly advanced to the six-o’clock position, proximated just inferior to the targeted pedicle. The lumbar spinal nerves exit the spinal canal just inferior and medial to the midposition of the corroborative pedicle on the AP image. Needle placement just superior to the spinal nerve and just inferior to the pedicle is confirmed by visualization of contrast medium centrally hugging the pedicle both inferiorly and medially as well as spreading cephalad along the ipsilateral ventral portion of the spinal canal, bathing the posterior aspect of the intervertebral discs as well as peripherally along the lumbar spinal nerve sleeve. Care must be taken and technique must be precise throughout needle advancement to maintain the bull’s-eye approach at all times via real-time fluoroscopy to prevent straying of the needle away from the targeted foramen. Inadvertent curvature of the needle outside the confines of the bull’s-eye approach may result in the needle tip hitting periosteum, such as the targeted pedicle if the needle tip strays superiorly, punctured viscus if the needle tip strays anteriorly towards the retroperitoneal and/or abdominal cavity, or punctured spinal nerve extraforaminally if the needle tip strays inferiorly. In some instances when performing a transforaminal ESI through the L5–S1 foramen, the targeted inferior aspect of the midline of the L5 pedicle may be accessible only by ipsilateral rotation due to one or a combination of a high-riding pelvis, significant loss of disc space height, large L5 transverse process, L5–S1 fusion, or a L5 on S1 spondylolisthesis. These obstacles can be overcome with a variable degree, depending on the severity of the aforementioned anatomical variations, of cranial to caudal tilt which will in effect move the pelvis inferiorly, creating direct access to the inferior portion of the targeted L5 pedicle. In cases of performing transforaminal ESI through the L5–S1 foramen in patients with a partially or completely sacralized L5 vertebral body, the approach is similar to that utilized when performing sacral transforaminal ESIs. When performing S1 transforaminal ESI, the skin wheal is raised superior and lateral to the targeted S1 pedicle. The needle is advanced through the skin wheal via a long-handled sponge clamp in the direction of the X-ray beam at a 45° angle to gently abut sacral periosteum just lateral to the S1 pedicle. Then, the needle is slightly withdrawn, reoriented inferiorly and medially, and slowly advanced, sliding to just inferior to the midline of the S1 pedicle into the targeted foramen. A slight suction or vacuum phenomena may be felt as the needle tip enters the targeted sacral foramen. A similar approach is utilized for L5–S1 transforaminal ESI in patients with partial or complete sacralization of the L5 vertebral body; however, the skin wheal is raised lateral and inferior to the targeted L5 pedicle and the needle too is advanced superiorly and medially after depth is achieved by contacting periosteum.
Indications and efficacy of interlaminar ESI
The interlaminar route most likely continues to be the most widely utilized approach to accessing the lumbar epidural space for ESI in the treatment of spinally mediated lumbar axial pain syndromes; however, there is little in the published randomized literature to suggest long-term efficacy.43–49 Carette et al.43 demonstrated short-term improvement in radicular complaints in patients with HNP but no difference between control and treated groups at 3 months in a prospective, randomized, double-blind, controlled trial. In a prior prospective, randomized, double-blind, controlled trial, Cuckler et al.44 demonstrated no significant improvement in both the short and long terms in patients with lumbar axial pain due to HNP and/or spinal stenosis. Ridley et al.48 reported statistically significant long-term benefit (6-month follow-up) to interlaminar ESIs in patients with lumbar axial and radicular pain in a randomized, placebo-controlled outcomes trial. Lending credence to the variables influencing such outcomes studies are the previously mentioned studies that report up to 40% of blind interlaminar ESI do not enter the epidural space even in experienced hands.18–21 Furthermore, an intrinsic problem with interlaminar ESI is that even when the physician is able to correctly place the needle tip into the epidural space, the dorsal aspect of the spinal canal is infused. Therefore, interlaminar ESIs are likely delivering injectate dorsal to the thecal sac and at a site relatively distal to the ventrally located pain-generating structures.
Technique of performing interlaminar ESI
Interlaminar ESIs are performed under X-ray guidance with the patient prone or in the lateral decubitus position on the fluoroscopy table. The approximated area of skin overlying the level of the lumbar spine to be injected is cleaned thoroughly with povidone-iodine and the region is draped in a sterile fashion. The correct level, taking into account the possibility of a transitional segment, is verified by the process previously stated in the technique portion of the transforaminal ESI section. Then, with fluoroscopic imaging, the inferior ipsilateral aspect of the lamina corresponding to the site of pathology is visually confirmed. A skin wheal utilizing a combination of 1–3 cc of 1% lidocaine and 0.1–0.3 cc of sodium bicarbonate is raised.42 A 20- or 22-gauge, 3.5 inch spinal needle is carefully guided to abut the ligamentum flavum as resistance is felt. One of two techniques can be utilized for advancement into the dorsal epidural space. Firstly, the spinal needle can be slowly advanced using a loss of resistance technique. A ‘pop’ may be felt and/or heard when the ligamentum flavum is pierced and the needle tip has entered the epidural space. Otherwise, the stylet can be removed and a glass syringe filled with 5 cc of normal saline is attached to the needle hub. The spinal needle is slowly advanced with modest pressure being simultaneously applied to the plunger. Once a drop in resistance is encountered, the ligamentum flavum has been pierced and the dorsal epidural space has been infiltrated. The needle tip position is assessed on AP and lateral images and verified by the injection of contrast medium. Once needle confirmation is achieved, an injectate combination of 12–18 mg of betamethasone and 5–10 cc of lidocaine and/or normal saline is slowly infused.
Indications and efficacy of caudal ESI
The caudal ESI may be safer than other routes of ESI due to a substantially lower risk of inadvertent dural puncture and subsequent subarachnoid injection, as the dural sac rarely extends beyond the S1–2 level. The published randomized clinical outcomes literature as a whole provides a contradictory picture on both short- and long-term efficacy of caudal ESI in the treatment of lumbar axial pain with or without radicular involvement in patients both prior to and post surgical decompression.41,50–54
Technique of performing caudal ESI
Caudal ESIs are performed with the patient prone on the fluoroscopy table. The skin overlying the sacrococcygeal area is cleaned thoroughly with povidone-iodine and the region is draped in a sterile fashion. The clinician should then identify the right and left sacral cornu via palpation or fluoroscopic guidance, as the sacral hiatus is located between these two landmarks. A skin wheal utilizing a combination of 2–3 cc of 1% lidocaine and 0.1–0.3 cc of sodium bicarbonate is raised.42 A 22-gauge, 3.5 inch spinal needle is carefully guided at a 45° angle through the sacrococcygeal ligament and into the sacral canal hiatus near the superior portion of the gluteal cleft and then advanced slightly into the sacral canal no further than the inferior edge of S2. The needle tip position is assessed on AP and lateral images and verified by the injection of contrast medium and observance of cephalad flow. Once needle confirmation is achieved, 12–18 mg of betamethasone is injected followed by an infusion of 5–15 cc of lidocaine and/or normal saline to assist in the cephalad spread of the corticosteroid to the lumbar epidural space via a direct mechanical pressure effect in the cephalad direction.
Timing of ESI
The optimal timing for administration of ESIs has not yet been elucidated. It is standard practice for patients to undergo more conservative palliative measures (i.e. nonsteroidal antiinflammatory drugs (NSAIDs), lumbar spine stabilization physical therapy) prior to being considered for ESIs; however, the clinician must be wary not to delay ESIs when more conservative treatments do not seem to be helping. Delaying aggressive treatment may allow an ongoing inflammatory process to result in fibrosis and possibly cellular damage.13 It is unknown how often ESIs can be administered. Practitioners will often wait as long as 2 weeks prior to reassessing a patient for a response to an injection for possible re-injection. This practice became popular after Swerdlow and Sayle-Creer suggested that corticosteroids infused into the epidural space may remain in situ for up to 2 weeks.55
INTRADISCAL INJECTIONS OF BIOLOGICS FOR DISCOGENIC MEDIATED LUMBAR AXIAL PAIN SYNDROMES
In vitro studies have demonstrated a cellular response of intervertebral disc cells to exogenous bone morphogenetic protein (BMP). These studies have demonstrated improved disc height and signal changes on MRI suggestive of repair of the intervertebral disc following intradiscal administration of BMP to discs damaged via chemical and mechanical methods. In a rabbit model, Miyamoto et al.56 demonstrated that intradiscal infusion of osteogenic protein −1 (OP-1, BMP-7) restored the biomechanical properties of the degenerated intervertebral disc. Furthermore, An et al.57 demonstrated that infusion of OP-1 intradiscally resulted in increased disc height and proteoglycan content. In addition, Takegami et al.58 reported that intervertbral disc cells exposed to interleukin-1 alpha lost no intrinsic ability to upregulate proteoglycan synthesis in response to OP-1 stimulation. In fact, he went on to describe that the reformed matrix was actually richer in proteoglycans than that prior to interleukin-1 alpha exposure. Interestingly, Yoon et al.59 demonstrated that infusion of adenovirus carrying Lim Mineralization Protein − 1 increases disc production of proteoglycans as well as bone morphogenic proteins. In a rat model, Kawakami et al.60 demonstrated both enhancement of the extracellular matrix as well as inhibition of pain-related behavior with intradiscal OP-1 injection into painful degenerated discs. Based on these initial in vitro studies, it has been proposed that intradiscal infusion of BMP to a degenerated dessicated disc may initiate repair of the disc, manifested clinically as reduction in lumbar pain and concomitant improvement of physical function and potentially manifested structurally as a radiographic increase in disc height and/or an increase in MRI disc signal intensity. This author is a principal investigator in a multicenter phase 1 study evaluating infusion of a BMP into single level symptomatic degenerative disc disease in humans.
Although transforaminal ESIs targeted on the floor of the spinal canal at a focal discogenic mediated inflammatory response holds promise for some patients episodic chemically induced discogenic pain as previously described (Fig. 92.2), randomized prospective double-blinded studies done by both Simmons et al.61 as well as Khot et al.62 have demonstrated no statistically significant benefit of intradiscal infusion of corticosteroids for discogenic pain diagnosed by provocative lumbar discography. Other biologics that have been reported upon but whose role has not yet been elucidated include infusions of hematopoietic precursor stem cells, methylene blue and ozone (O3). For example, Haufe et al.63 reported no reduction in discogenic pain at one year follow-up in patients who underwent intradiscal infusion of hematopoietic precursor stem cells harvested from the pelvic bone marrow. Peng et al.64 demonstrated that 87% of patient with chronic discogenic pain who met inclusion criteria for lumbar interbody fusion surgery but were instead treated with intradiscal injection of methylene blue were able to report marked improvement in pain and physical function. In 2004, Muto et al65 reported on 2200 patients treated with intradiscal oxygen-ozone injection reporting no side effects and a 75–80% success rate at 6 and 18 months. Buric et al66 conducted a prospective case series reporting on chemonucleolysis via ozone injections in 30 patients with non-contained disc herniations. They stated 90% of patients had a statistically significant improvement in pain and function as measured by VAS and Roland Morris Disability Questionnaires. Of course, further study is required before any of the aforementioned intradiscal infusions of various biologics can be regarded as a generally acceptable or standard of care intervention for discogenic lumbar axial pain syndromes.
PATHOPHYSIOLOGY OF Z-JOINT-MEDIATED LUMBAR AXIAL PAIN SYNDROMES
The lumbar Z-joint is the primary posterior element structure thought to be a potential etiology of spinally mediated lumbar axial pain. The rationale is derived from conclusions drawn from the conceptual model of the lumbar spine degenerative cascade as initially described by Kirkaldy-Willis,10 as previously reported within this chapter, as well as properties intrinsic to the Z-joint. As outlined earlier in this chapter, the posterior elements take on more axial load in certain positions (standing) as well as with increasing age. Anatomically, the Z-joint is a true diarthrodial joint with articular and subchondral cartilage, a small meniscus, a capsule, and a fluid-filled synovium encased in synovial lining. The initiating event that is thought to implicate the Z-joint as an etiology of lumbar axial pain is synovitis and the resultant synovial reaction which occurs as a result of the shift in axial loading from the anterior elements to the posteriorly situated Z-joints due to the temporally related incompetence of the intervertebral disc. On the other hand, it is theorized that the aforementioned initiating pathology to the synovial capsule can result in a younger population when induced by sudden, unexpected flexion–extension moments as occurs commonly in motor vehicle trauma. The Z-joints are able to accommodate the increased load up to a critical maximum at which point synovial pathology ensues leading to eventual progressive destruction of the cartilaginous articular surface when facet joint weight bearing exceeds the critical maximum. Acute, recurrent, and/or chronic inflammation can result in these synovial joints filling with fluid and distending. This distention may result in pain perception because of mechanical and/or chemical stimulation of the richly innervated synovial capsule. Evidence suggests that the Z-joint’s synovial capsule has a complex innervation provided by small, C-type pain fibers.67 Furthermore, the majority of mechanosensitive somatosensory units found within the Z-joint were discovered to be group III high-threshold, slow-conduction units, which are thought to mediate nociception.68–70 Therefore, the Z-joint can result in nociception by osteochondral and/or capsular mechanisms similar to those found in other extensively studied degenerated joints, such as the hip or knee. Capsular laxity can result and the accompanying subluxation can stretch and/or tear the richly innervated capsule leading to one or a combination of inflammatory or structural lumbar axial pain syndromes emanating from the lumbar Z-joint. Another potential mechanism for nociception arising from lumbar Z-joint has not been recognized until recently. In most diarthrodial joints, prolonged immobilization can lead to a cascade of joint changes. These may include, among other things, capsular stiffness leading to contracture, decrease in synovial fluid production, and tightening of para-articular muscles, all leading to joint rigidity. In some cases, complete ankylosis may accompany extreme inactivity, immobilization, and inhibition of function. In such cases, mechanical loads applied to the rigid joints typically can produce pain, which may be related to capsular stretching/tearing, synovial irritation from cartilage fragmentation due to poor nutrition, and/or muscle spasm from sudden stretching of immobilized, debilitated muscle. In chronic lumbar axial pain conditions, patients often splint segments of the lumbar spine, and the chronic immobilization may lead to nociception. Stretching the involved joint–muscle complex is the obvious physiologic therapeutic modality. Unfortunately, pain and fear-avoidance may prevent some pain-sensitive patients from complying with such logical medical advice. Nevertheless, the Z-joint can be the source of pain associated with these mechanisms, particularly after chronic persistent or chronic episodic lumbar axial pain conditions have produced disability for longer than 6 months.71
Diagnosis of Z-joint-mediated lumbar axial pain syndromes
Revel et al. suggested certain clinical features including age greater than 65 years, pain not exacerbated by coughing, hyperextension, forward flexion, rising from forward flexion, or extension–rotation, or mitigated by recumbence, may be suggestive for a Z-joint etiology to lumbar axial pain.72,73 However, more recent studies demonstrate that no physical examination finding(s), including segmental rigidity, are specific for Z-joint-mediated lumbar axial pain.74,75 Although the aforementioned features on clinical presentation reported by Revel et al.72,73 may suggest the Z-joint as a possible etiology of lumbar axial pain and enter the Z-joint into the differential diagnosis, anesthetic blockade is currently the gold standard by which the diagnosis is ultimately made.76 Two methods are currently accepted: (1) a dual-block paradigm eliciting a positive response for the half-life duration of both a short- and long-acting local anesthetic performed on separate occasions, or (2) a single-block paradigm eliciting a positive response from a local anesthetic blockade with a negative response from a placebo control performed on separate occasions.
Efficacy of lumbar Z-joint injections in Z-joint-mediated lumbar axial pain syndromes
There has yet to be a study to determine the efficacy of intra-articular steroid injections of the lumbar Z-joints in patients diagnosed with Z-joint-mediated pain via a dual-block paradigm or placebo-controlled single-block paradigm. Carette et al.77 designed and implemented the best study to date assessing the efficacy of intra-articular corticosteroid injections of the lumbar Z-joints for Z-joint-mediated lumbar axial pain. They reported on 101 patients with lumbar axial pain diagnosed with Z-joint-mediated pain via a single intra-articular anesthetic blockade providing 50% pain reduction randomized into either a normal saline group and corticosteroid group. At 1 month, 42% of the corticosteroid group and 33% of the normal saline group reported significant pain relief (thus a one-third placebo response rate and requirement of a dual-block paradigm for diagnosis). There was a statistically significant difference in marked pain relief at 6-month follow-up between the corticosteroid group (46%) and normal saline group (15%). Carette et al.77 argued that intra-articular steroid Z-joint injections were not effective since the percentage of patients with long-term pain relief was low; however, one could retort that the inclusion criteria was detrimental to the study outcome because of the failure to exclude a placebo response. A more recent randomized, single-blind clinical trial by Mayer et al.75 found that fluoroscopically guided Z-joint injections with supervised stretching exercises when compared to exercise alone (control group) in patients with chronic disabling work-related lumbar spinal disorders demonstrated that incorporating Z-joint injections significantly increased lumbar range of motion (ROM) in all planes as well as lumbar mobility relative to the control group. However, Mayer et al.64 also observed that there was no increase in the improvement of pain and disability of the Z-joint injection group relative to the control group. The key point in this paradigm of segmental rigidity is that the purpose for the injection/exercise approach is to increase motion in spinal segments noted specifically to be rigid. The added goal of facilitating functional rehabilitation involving para-articular muscle strength, endurance, and coordination becomes possible only after joint mobility has been restored. This study demonstrated significant ROM gains were also present in the exercise-only control group, but that significantly greater improvements were noted when Z-joint injections were added to the standardized exercise program. It was also noted that only 17% of the patients meeting criteria for segmental rigidity also met criteria for Z-joint syndrome, as determined by the response to the Z-joint injections. As such, it appears that the entity of lumbar axial pain of Z-joint origin is noncongruent with the entity of segmental rigidity. More study of the relationships between these two phenomena is required, as comprehension will ultimately determine the prescription and efficacy of comprehensive treatment programs.
The nonrandomized outcome studies offer varying short- and long-term results in the utilization of intra-articular Z-joint corticosteroid injections in the treatment of lumbar axial pain. For example, Lynch and Taylor78 reported total pain relief in nine of 27 patients receiving intra-articular Z-joint injections and partial relief in another 16 of those 27 patients. However, none of the 15 patients receiving extra-articular corticosteroid injections reported complete pain relief and only eight of the 15 reported partial pain relief. Destouet et al.79 reported significant pain relief for 1–3 months in 62% of their patient sample and 38% reported 3–6 months of pain relief. Murtagh80 reported that 54% of his patient sample claimed up to 6 months significant pain relief. Mironer and Somerville81 only reported 28% of patients in their study reporting significant long-term pain relief. Two separate retrospective outcome studies demonstrated approximately 50% of patients reporting short-term pain relief with much lower percentages of patients (approaching 8%) reporting long-term relief at 12 months.82,83
Technique of performing lumbar intra-articular Z-joint interventions
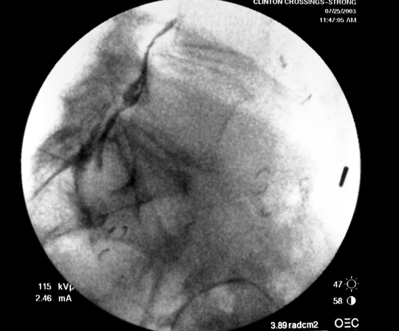
Lumbar intra-articular Z-joint injections are performed with the patient prone on the fluoroscopy table. The approximated area of skin overlying the level of the lumbar spine to be injected is cleaned thoroughly with povidone-iodine and the region is draped in a sterile fashion. The correct level, taking into account the possibility of a transitional segment, is verified by the process previously stated in the technique portion of the transforaminal ESI section. Real-time fluoroscopy is utilized to rotate the c-arm ipsilaterally until the medial and lateral edges of the targeted Z-joint are clearly visualized. Local anesthesia is achieved by infiltrating the skin, creating a skin wheal raised just medial to the midline or inferior aspect of the joint with a combination of approximately 1–3 cc of 1% lidocaine and 0.1–0.3 cc of sodium bicarbonate.42 Real-time fluoroscopy is utilized to advance a 22-gauge, 3.5 inch spinal needle in a bull’s-eye fashion so that the needle tip abuts the lateral edge of the middle to inferior portion of the Z-joint. The spinal needle is then slightly withdrawn and directed just medially into the capsule of the targeted Z-joint. Approximately 0.1–0.3 cc of contrast can be utilized to verify needle placement, visualized by the presence of an hourglass-shaped arthrogram or portion thereof (Figs 92.6–92.8). Once needle tip confirmation is achieved, a combination of 0.5–0.8 cc of betamethasone and 0.2–0.5 cc of 1% lidocaine can be injected, dependent on the degree of compromise of the synovial capsule and the ensuing capacity to accommodate an infusion of volume.
Efficacy of medial branch injections in Z-joint-mediated lumbar axial pain syndromes
Medial branch neural blockade is also performed in the treatment of lumbar axial pain of Z-joint etiology. Manchikanti et al.84 prospectively randomized 73 patients diagnosed with Z-joint mediated lumbar axial pain via a dual anesthetic block paradigm into one of two groups treated with therapeutic medial branch injections. One group received local anesthetic and sarapin whereas the other received a mixture local anesthetic, sarapin, and corticosteroid. Significant pain relief was achieved in both groups with a mean duration of relief greater than 6 months. In addition, physical, functional, psychological status, and return to work status were all improved. Of note, the efficacy of radiofrequency neurotomy of the medial branch of the corroborative spinal nerves has been demonstrated for patients diagnosed by a dual-block paradigm by Dreyfuss et al.76
In a nonrandomized study by Manchikanti et al,85 a retrospective analysis of 180 patients all diagnosed with Z-joint-mediated lumbar axial pain via a dual-anesthetic paradigm, three groups of 60 patients were evaluated, the groups receiving medial branch blocks with local anesthetic, local anesthetic and sarapin, or local anesthetic, sarapin, and corticosteroid. To summarize, the results of this retrospective study reported that diagnostic medial branch blocks alone had short-term therapeutic effects which were enhanced by the addition or sarapin and/or corticosteroid. In another nonrandomized clinical trial, North et al.86 demonstrated the short-term efficacy of medial branch blocks with a local anesthetic, but long-term improvement was infrequent.
Technique of performing medial branch injections
Medial branch neural blockade is performed with the patient in the prone position on the fluoroscopy table. The approximated area of skin overlying the level of the lumbar spine to be injected is cleaned thoroughly with povidone-iodine and the region is draped in a sterile fashion. The correct level, taking into account the possibility of a transitional segment, is verified by the process previously stated in the technique portion of the transforaminal ESI section. The c-arm is rotated until the eye of the ‘Scottie dog’ is clearly visualized on an oblique image. Local anesthesia is achieved by infiltrating the skin, creating a skin wheal raised over the eye of the ‘Scottie dog’ with a combination of approximately 1–3 cc of 1% lidocaine and 0.1–0.3 cc of sodium bicarbonate.42 A 22-gauge 3.5 inch spinal needle is advanced in a bull’s-eye manner to the eye of the ‘Scottie dog,’ representing the junction of the base of the corroborative transverse process and the superior articular process under fluoroscopic guidance. Injections must be performed at the level of the involved joint and the adjacent superior segmental level as both of these medial branches will contribute innervation to the targeted Z-joint. For example, the L4–5 Z-joint is innervated by the medial branches of the dorsal rami of both the L3 and L4 spinal nerves. Injectate consists of 0.5 cc of 1% or 2% lidocaine.
CONTRAINDICATIONS AND COMPLICATIONS OF INTERVENTIONAL SPINE PROCEDURES
Contraindications to interventional spine procedures include possible pregnancy (secondary to the potential adverse effects of fluoroscopic radiation on the fetus), hypersensitivity to any component of the injectate, bacteremia, full anticoagulation, and bleeding diathesis. Other concerns are elevations of serum glucose levels in diabetics, elevations of blood pressure in hypertensive patients, and fluid retention in congestive heart failure patients. The use of aspirin and other NSAIDs have not been demonstrated to predispose patients to any significant bleeding while receiving ESIs.87
Potential complications of fluoroscopically guided interventional spine procedures include increased pain, hypersensitivity reactions, dural puncture, subarachnoid injection, anaphylaxis, infection, unmasking of preexisting systemic infection, epidural abscess, increased pain, fever, epidural bleeding and/or hematoma, bladder dysfunction with urinary retention, weakness, permanent neural element damage via penetrating trauma, intravascular injection resulting in local anesthetic toxicity such as seizure, cardiac arrhythmia or arrest, and death. However, these events are quite rare. Botwin et al.88 reported no major complications and less than a 10% rate of minor complications, all of which resolved without hospitalization, in 207 patients undergoing 322 fluoroscopically guided lumbar transforaminal ESIs.
PHYSICAL REHABILITATION
Physical rehabilitation is a key modality on the treatment of patients with spinally mediated lumbar axial pain. Physical therapy programs prescribed specifically to address the primary site of injury and secondary sites of dysfunction can provide a means of treatment with or without adjuvant medications and interventional spine procedures. Back schools and other training programs that are not job specific have not been shown to be statistically effective; however, programs that integrate the job requirements into the training program show statistical significant results.89 Relative rest, which restricts all occupational and avocational activities, for up to the first 2 days following an acute episode may be indicated to help calm down initial pain symptoms and relieve fatigue. Rest for longer periods of time has not been shown to be beneficial, and may be harmful, potentially causing deconditioning, loss of bone density, decreased intradiscal nutrition, loss of muscle strength and flexibility, and increased segmental stiffness.90 Passive modalities are valuable during the initial 48 hours of relative rest to aid in pain relief, but protracted courses of passive treatments become counter-productive as they place the patient in a dependent role instead of participating in an active, function maximizing rehabilitation program. One of the most important components of any back care program is education. Education should include an explanation of the natural history of an acute, subacute, and chronic disc injury. In addition, education should include training in proper body mechanics and lumber ergonomics during various functional, occupational, and avocational activities. The greatest successes in health education can be seen in two great medical problems, heart disease91 and cancer.92 Education has had a direct effect on decreasing the prevalence of smoking, from 40% of the population in 1965 to 29% in 1987.93 An education-based low back paradigm is inexpensive and begins with providing reassuring information to the patient. The seeds of the educational approach exist in back schools, functional restorative programs, and innovative prevention and rehabilitation strategies. LaCroix found that 94% of patients with a good understanding of their condition returned to work, whereas only 33% of patients with a poor understanding of their condition returned to employment.94 Reassurance that activity is helpful promotes return to function. Back school provides information on spine anatomy and function and was found to be one of only three interventions identified by the Quebec Task Force on Spinal Disorders as effective for patients in randomized, controlled trials.95 Myofascial manual techniques may be employed to increase soft tissue pliability when secondary myofascial tightness is present. If the aforementioned measures are appropriate and completed, then an active, dynamic, outpatient lumbar spine stabilization rehabilitation program should be prescribed. In addition, rehabilitation of other associated components of the functional kinetic chain may be appropriate, as these structures may also be affected. A dynamic lumbar spine stabilization rehabilitation program is aimed at maintaining a neutral spine position throughout various daily activities. An extension bias is commonly employed to help reduce intradiscal pressure. This position allows for a balanced segmental force distribution between the intervertebral disc and Z-joints, provides functional stability with axial loading to help minimize the chance for acute dynamic overload upon the discs, minimizes tension on ligaments and fascia planes, and alleviates symptoms. Repetition is key in increasing flexibility, building endurance, and developing the required muscle motor programs that activate a series of key multimuscular contractions subconsciously that maintain the lumbar spine in a neutral position throughout static and dynamic activities. For athletes, the aforementioned program can be combined progressively with sport-specific polymetrics to help the lumbar spine maintain a neutral position during high-intensity, unpredictable, reaction-intensive sports. Another method for rehabilitation of athletes is to train them in maintaining a neutral spine position in the individual motions of their sport, then subsequently grouping these component motions into a new, safe, stable spine movement. Cardiovascular fitness training is an important component to a comprehensive rehabilitation program as it assists in providing the endurance necessary to prevent fatigue of the spine-stabilizing musculature.
CASE STUDIES
Case study 1
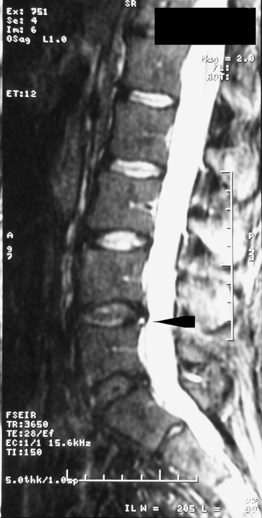
S.G. is a 36-year-old male with a history of occasional spontaneously resolving short-term (less than 1 week duration) low back pain episodes who presents to a spine specialist complaining of 12 weeks of worsening low lumbar axial pain initiated while lifting a heavy television set helping a friend move into a house. The patient states his symptoms are located across the width of the low back without lower extremity radiation. He qualifies his pain as a constant, deep, dull ache worsened with prolonged sitting, especially riding in his car, as well as with twisting motions when bent forward. The patient reports lumbar extension and staying active seem to provide some symptom mitigation. Physical examination reveals some tenderness and spasm to palpation over the bilateral low lumbar paraspinal musculature. Lumbar flexion is significantly limited due to worsening of the patient’s lumbar pain; however, lumbar extension alleviates the patient’s lumbar pain. In addition, the patient reported increasing lumbar pain with decreasing angle acuity during sustained hip flexion. Sacroiliac joint stress maneuvers and provocative maneuvers were not pain provoking. The lumbosacral spinal neurologic examination was normal. Plain films of the lumbar spine ordered by the patient’s primary care physician revealed moderate loss of disc height posteriorly at the L5–S1 segment. Physical therapy had been initiated 10 weeks previously with minimal improvement. The patient reports that mostly passive modalities had been employed in therapy for pain and spasm control, as several attempts at advancement to an active extension-biased McKenzie stabilization program proved too difficult due to pain provocation. An MRI of the lumbar spine was ordered, revealing focal degenerative disc desiccation at the L5–S1 segment with reactive endplate edema and a high-intensity zone lesion posteriorly (Figs 92.9, 92.10). A diagnosis of discogenic lumbar axial pain was made and the patient underwent two therapeutic bilateral S1 transforaminal ESIs. After the second procedure, the patient reported greater than 90% alleviation of his lumbar pain. Physical therapy was reinitiated, resulting in near-complete symptom resolution. The patient understood the key to obtaining long-term success with management of his disc disease included compliance with his home exercise program and proper lumbar spine mechanics during all activities. Although the patient continues to have occasional lumbar pain episodes, he reports that these episodes resolve within a few days with rest, activity modification and/or mild analgesics.
1 Anderson GBJ. Epidemiologic aspects of low back pain in industry. Spine. 1981;6:53.
2 Dixon ASJ. Diagnosis of low back pain – sorting the complainers. In: Jayson M, editor. The lumbar spine and back pain. New York: Grune & Stratton, 1976.
3 Fry J. Back pain and soft tissue rheumatism: advisory services colloquium proceedings. London; 1972.
4 White AWM. Low back pain in men receiving workmen’s compensation. Can Med Assoc J. 1966;95:50.
5 Deyo RA, Tsui-Wu Y-T. Descriptive epidemiology of low back pain and its related medical care in the United States. Spine. 1987;12:264-268.
6 Troup JDB, Martin JW, Lloyd DCEF. Back pain in industry: A prospective survey. Spine. 1981;6:61-69.
7 Valkenburg HA, Haanen HCM. The epidemiology of low back pain. In: White AAIII, Gordon SL, editors. American Academy of Orthopaedic Surgeons Symposium on Idiopathic Low Back Pain. St. Louis: Mosby, 1982.
8 Von Korff M, Deyo RA, Cherkin D, et al. Back pain in primary care: outcomes at one year. Spine. 1993;18:855-862.
9 Wiltse LL. The History of spinal disorders. In: Frymoyer JW, editor. The adult spine: principles and practice. Philadelphia: Lippincott-Raven; 1997:3-40.
10 Kirkaldy-Willis WH. The pathology and pathogenesis of low back pain. In: Kirkaldy-Willis WH, editor. Managing low back pain. New York: Churchill Livingstone; 1988:49.
11 McCarron RF, Wimpee MW, Hudkins PG, et al. The inflammatory effect on nucleus pulposus: a possible element in the pathogenesis of low back pain. Spine. 1987;12:760-764.
12 Saal JS, Franson RC, Dobrow R, et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine. 1990;15(7):674-678.
13 Takahashi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21(2):218-224.
14 Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6 and prostaglandin E2. Spine. 1996;21(3):271-277.
15 Verbout AJ. The development of the vertebral column. Adv Anat Embryol Cell Biol. 1985;90:1-122.
16 Viner N. Intractable sciatica – the sacral injections – an effective method of giving relief. Can Med Assoc J. 1925;15:630-634.
17 Robecchi A, Capra R. L’idrocortisone (composto F): prime esperienze cliniche in campo reumatologico. Minerva Med. 1952;98:1259-1263.
18 Fredman B, Nun MB, Zohar E, et al. Epidural steroids for treating ‘failed back surgery syndrome’: is fluoroscopy really necessary? Anesth Analg. 1999;88(2):367-372.
19 Watanabe AT, Nishimura E, Garris J. Image-guided epidural steroids injections. Tech Vasc Interv Radiol. 2002;5(4):186-193.
20 Renfrew DL, Moore TE, Kathol MH, et al. Correct placement of epidural steroid injections: fluoroscopic guidance and contrast administration. Am J Neuroradiol. 1991;12(5):1003-1007.
21 Johnson BA. Image-guided epidural injections. Neuroimaging Clin N Am. 2000;10(3):479-491.
22 Winnie AP, Hartman JT, Meyers HLJr, et al. Pain clinic II: intradural and extradural corticosteroids for sciatica. Anesth Analg. 1972;1:990-1003.
23 Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. J Bone Joint Surg [Am]. 2000;82:1589-1593.
24 Kraemer J, Ludwig J, Bickert U, et al. Lumbar epidural perineural injections: a new technique. Eur Spine J. 1999;8(1):81-82.
25 Karppinen J, Malmivaara A, Kurunlahti M, et al. Periradicular infiltration for sciatica. Spine. 2001;26:1059-1067.
26 Karppinen J, Ohinimaa A, Malmivaara A, et al. Cost effectiveness of periradicular infiltration for sciatica. Spine. 2001;26:2587-2595.
27 Thomas E, Cyteval C, Abiad L, et al. Efficacy of transforaminal versus interspinous corticosteroid injection in distal radiculalgia – a prospective, randomized, double-blind study. Clin Rheumatol. 2003;22(4–5):299-304.
28 Butterman GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy. A prospective, randomized study. J Bone Joint Surg [Am]. 2004;86(4):670-679.
29 Weiner BK, Fraser RD. Foraminal injection for lateral lumbar disc herniation. J Bone Joint Surg [Br]. 1997;79:804-807.
30 Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: An outcome study. Arch Phys Med Rehab. 1998;76:1362-1366.
31 Wang JC, Lin E, Brodke DS, et al. Epidural injections for the treatment of symptomatic lumbar herniated discs. J Spinal Disorders & Tech. 2002;15:269-272.
32 Delport EG, Cucuzzella AR, Marley JK, et al. Treatment of lumbar spinal stenosis with epidural steroid injections: a retrospective outcome study. Arch Phys Med Rehabil. 2004;85(3):479-484.
33 Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transforaminal epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;8(12):898-905.
34 Burke JG, Watson RW, McCormack D, et al. Spontaneous production of monocyte chemoattractant protein-1 and interleukin-8 by the human intervertebral disc. Spine. 2002;27(13):1402-1407.
35 Saal JA, Saal JS, Herzog RJ. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine. 1990;15(7):683-686.
36 Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
37 Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in Experimental Studies. Annulus tears and intervertebral disc degeneration: an experimental study using an animal model. Spine. 1990;15:762-767.
38 Burke JG, Watson RW, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Surg Joint. 2002;84(2):196-201.
39 Simmons JW, McMillin JN, Emery SF, et al. Intradiscal steroids: a prospective, double-blind clinical trial. Spine. 1992;17(6 Suppl):172-175.
40 Rosenberg SK, Grabinsky A, Kooser C, et al. Effectiveness of transforaminal epidural steroid injections in low back pain: A one year experience. Pain Physician. 2002;5:266-270.
41 Manchikanti L, Pampati V, Rivera JJ, et al. Caudal epidural injections with sarapin steroids in chronic low back pain. Pain Physician. 2001;4:322-335.
42 Nakayama M, Munemura Y, Kanaya N, et al. Efficacy of alkalinized lidocaine for reducing pain on intravenous and epidural catheterization. J Anesth. 2001;15(4):201-203.
43 Carette S, Lecaire R, Marcoux S, et al. Epidural corticosteroid injections for sciatica due to herniated nucleus pulposus. N Engl J Med. 1997;336:1634-1640.
44 Cuckler JM, Bernini PA, Wiesel SW, et al. The use of epidural steroid in the treatment of radicular pain. J Bone Surg Joint. 1985;67:63-66.
45 Snoek W, Weber H, Jorgensen B, et al. Double-blind evaluation of extradural methylprednisolone for herniated lumbar disc. Acta Orthop Scand. 1977;48:635-641.
46 Dilke TFW, Burry HC, Grahame R. Extradural corticosteroid injection in the management of lumbar nerve root compression. Br Med J. 1973;2:635-637.
47 Klenerman L, Greenwood R, Davenport HT, et al. Lumbar epidural injection in the treatment of sciatica. Br J Rheumatol. 1984;23:35-38.
48 Ridley MG, Kingsley GH, Gibson T, et al. Outpatient lumbar epidural corticosteroid injection in the management of sciatica. Br J Rheum. 1988;27:1003-1007.
49 Rogers P, Nash T, Schiller D, et al. Epidural steroids for sciatica. Pain Clinic. 1992;5:67-72.
50 Revel M, Auleley GR, Alaoui S, et al. Forceful epidural injections for the treatment of lumbosciatic pain with postoperative lumbar spinal fibrosis. Rev Rheum Engl Ed. 1996;63:270-277.
51 Helsa PE, Breivik H. Epidural analgesia and epidural steroid injection for treatment of chronic low back pain and sciatica. Tidsskr Nor Laegeforen. 1979;99:936-939.
52 Meadeb J, Rozenberg S, Duquesnoy B, et al. Forceful sacrococcygeal injections in the treatment of postdiscectomy sciatica. A controlled study versus glucocorticoid injections. Joint Bone Spine. 2001;68:43-49.
53 Breivik H, Hesla PE, Molnar I, et al. Treatment of chronic low back pain and sciatica. Comparison of caudal epidural injections of bupivacaine and methylprednisolone with bupivacaine followed by saline. In: Bonica JJ, Albe-Fesard D, editors. Advances in pain research and therapy. New York: Raven Press; 1976:927-932. (1)
54 Southern D, Lutz GE, Cooper G, et al. Are fluoroscopic caudal epidural steroid injections effective for managing chronic low back pain? Pain Physician. 2003;6:167-172.
55 Swerdlow M, Sayle-Creer W. A study of extradural medication in the relief of the lumbosciatic syndrome. Anesthesia. 1970;25:341-345.
56 Miyomoto K, Masuda K, Kim JG. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6(6):692-703.
57 An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30(1):25-31.
58 Takegami K, Thonar EJ, An HS. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27(12):1318-1325.
59 Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vito and in vivo. Spine. 2004;29(23):2603-2611.
60 Kawakami M, Matsumoto T, Hashizume H, et al. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine. 2005;30(17):1933-1939.
61 Simmons JW, McMillin JN, Emery SF, et al. Intradiscal Steroids. A Prospective Double-Blind Clinical Trial. Spine. 1992;17(6 suppl):s172-s175.
62 Khot A, Bowditch M, powell J, et al. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial. Spine. 2004;29(8):833-836.
63 Haufe SM, Mork AR. Intradiscal injectin of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15(1):136-137.
64 Peng B, Zhang Y, Hou S, et al. Intradiscal Methylene blue injection for the treatment of chronic discogneic low back pain. Eur Spine J. 2006.
65 Muto M, Andreula C, Leonardi M. Treatment of herniated lumbar disc by intradiscal and intraforaminal oxygen-ozone (O2-O3) injection. j Neuroradiol. 2004;31(3):183-189.
66 Buric J, Molino LR. Ozone chemonucleolysis in non-contained lumbar disc herniations: a pilot study with 12 months follow-up. Acta Neurochir Suppl. 2005;92:93-97.
67 Marks RC, Houston T, Thulbourne T. Facet joint injection and facet nerve block: a randomized comparison in 86 patients with chronic low back pain. Pain. 1992;49:325-328.
68 Ashtar IK, Aston BA, Gibson SJ, et al. Morphological basis for back pain: The demonstration of nerve fibers and neuropeptides in the lumbar facet joint capsule but not in ligamentum flavum. J Orthop Res. 1992;10:72-78.
69 Yamashita T, Cavanaugh JM, El-Boly AA, et al. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg [Am]. 1990;72:865-870.
70 Yamashita T, Cavanaugh JM, Ozaktay AC, et al. Effect of substance P on mechanosensitive units of tissues around the facet joint. J Orthop Res. 1993;11:205-214.
71 Mayer T, Robinson R, Pegues P, et al. Lumbar segmental rigidity: can its identification with facet injections and stretching exercises be useful? Arch Phys Med Rehabil. 2000;81:1143-1150.
72 Revel ME, Listrat VM, Chevalier XJ, et al. Facet joint block for low back pain: identifying predictors of a good response. Arch Phys Med Rehabil. 1992;73(9):824-828.
73 Revel M, Poiraudeau S, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. Proposed criteria to identify patients with painful facet joints. Spine. 1998;23(18):1972-1976.
74 Schwarzer AC, Aprill CN, Derby R, et al. Clinical features of patients with pain stemming from the lumbar zygapophyseal joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994;19(10):1132-1137.
75 Mayer TG, Gatchel RJ, Keeley J, et al. A randomized clinical trial of treatment of lumbar segmental rigidity. Spine. 2004;29(20):2199-2205.
76 Dreyfuss P, Halbrook B, Pauza K, et al. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophyseal pain. Spine. 2000;25:1270-1277.
77 Carette S, Marcoux S, Truchon R, et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med. 1991;325:1002-1007.
78 Lynch MC, Taylor JF. Facet joint injection for low back pain. A clinical study. J Bone Joint Surg [Br]. 1986;68:138-141.
79 Destouet JM, Gilula LA, Murphy WA, et al. Lumbar facet joint injection: Indication, technique, clinical correlation, and preliminary results. Radiology. 1982;145:321-325.
80 Murtagh FR. Computed tomography and fluoroscopy guided anesthesia and steroid injection in facet syndrome. Spine. 1988;13:686-689.
81 Mironer YE, Somerville JJ. Protocol for diagnosis and treatment of facet joint pain syndrome. A modified three-step approach. Pain Digest. 1999;9:188-190.
82 Lau LS, Littlejohn GO, Miller MH. Clinical evaluation of intra-articular injections for lumbar facet joint pain. Med J Aust. 1985;143:563-565.
83 Lippitt AB. The facet joint and its role in spine pain. Management with facet joint injections. Spine. 1984;9:746-750.
84 Manchikanti L, Pampati V, Bakhit CE, et al. Effectiveness of lumbar facet nerve blocks in chronic low back pain: a randomized clinical trial. Pain Physician. 2001;4:101-117.
85 Manchikanti L, Pampati V, Fellows B, et al. Prevalence of lumbar facet joint pain in chronic low back pain. Pain Physician. 1999;2:59-64.
86 North RB, Han M, Zahurak M, et al. Radiofrequency lumbar facet denervation: analysis of prognostic factors. Pain. 1994;57:77-83.
87 Horlocker TT, Wedel DJ, Offord KP. Does preoperative antiplatelet therapy increase the risk of hemorrhagic complications associated with regional anesthesia? Anesth Analg. 1990;70:631-634.
88 Botwin KP, Gruber RD, et al. Complications of fluoroscopically guided transforaminal lumbar epidural injections. Arch Phys Med Rehabil. 2000;81(8):1045-1050.
89 Shulenberger CC. Ergonomic intervention for the prevention and treatment of spinal disorders. In: White AH, Schofferman JH, editors. Spine care: diagnosis and conservative management. St. Louis: Mosby; 1995:472-485.
90 Deyo RA, Diehl AK, Rosenthal M. How many days of bed rest for acute low back pain? A randomized clinical trial. N Engl J Med. 1986;315:1064-1070.
91 National Center for Health Statistics. Vital statistics of the United States. Washington, DC: Government Printing Office, 1968–1988.
92 Department of Health and Human Services. Reducing the health consequences of smoking. 25 years of progress: a report of the Surgeon General. Washington, DC: Government Printing Office (DHHS pub. No. (CDC) 89-8411), 1989.
93 Manson J, Tosteson H, Ridker P, et al. The primary prevention of myocardial infarction. N Engl J Med. 1992;326:1406.
94 LaCroix J, Powell J, Lloyd G. Low back pain, factors of value in predicting outcome. Spine. 1990;15:495.
95 Spitzer W, LeBlanc F, Dupuis M. Scientific approach to the assessment and management of activity related spinal disorders: report of the Quebec Task Force on Spinal Disorders. Spine. 1987;12:51.