CHAPTER 73 Injection Procedures
INTRODUCTION
Perhaps because of a reduced potential for injury, the prevalence of painful disorders of the thoracic spine is significantly less than disorders of the cervical and lumbar spine. Protection against injury may be due to increased stability afforded by the rib cage1,2 and the steep orientation of the spinous processes that limits excessive extension. Stolker at al.3 reported a 5:2:20 ratio of cervical:thoracic:lumbar complaints of spinal pain in a series of patients seen at a pain clinic. Similarly, Occhipinti et al.4 surveyed factory workers and found a prevalence of thoracic spine symptoms of 5% compared to 24% for cervical and 33% for lumbar. In addition, Linton et al.5 reported the prevalence of spinal pain in Sweden in 35–45-year-olds to be 66.3% and found a 56% incidence of low back pain, a 44% incidence of neck pain, and a 15% incidence of upper back pain.
Bogduk6 logically postulated that for any structure to be a cause of back pain it should have a nerve supply, for without access to the nervous system it could not evoke pain. The structure should be capable of causing pain similar to that seen clinically. Ideally, this should be demonstrated in normal volunteers, for inferences drawn from clinical studies may be compromised by observer bias or poor patient reliability. Further, the structure should be susceptible to diseases or injuries that are known to be painful and should have been shown to be a source of pain in patients using diagnostic techniques of known reliability and validity.
The potential sources of thoracic pain therefore include intervertebral discs, zygapophyseal joints, costotransverse and costovertebral joints, dura mater, nerve roots, ligaments, and muscles. However, referred pain from visceral sources such as lung, pleura, heart, aorta, gallbladder, esophagus, and mediastinal pathology must not be missed.7,8
THORACIC ZYGAPOPHYSEAL JOINT PAIN
Although the thoracic zygapophyseal joint pain is under-reported, the cervical zygapophyseal joints have been extensively studied9–12 and are considered a common cause of spinal pain. There are, however, a few thoracic studies. Manchikanti et al. performed controlled comparative local anesthetic blocks of thoracic medial branches in 46 patients to determine the prevalence of zygapophyseal joint pain.13 Inclusion criteria required failure of conservative management with physical therapy, chiropractic management with physical therapy, absence of radicular pattern of pain, absence of disc herniation, and duration of pain for more than 6 months. Forty-eight percent of patients had positive response to double blocks. Interestingly, a false-positive rate of 58% was seen with single blocks.
Anatomical considerations
Nerve fibers converge into medial branch of the primary posterior ramus and relay in dorsal root ganglion prior to entering the spinal cord. Because intersegmental connections in the thoracic spine are not as pronounced as in its cervical and lumbar counterparts, the pain arising in any particular segment of the thoracic spine is more precisely localized than comparable segmental lesions in upper or lower regions of the vertebral column.14
Encapsulated mechanoreceptors and nociceptors in joint capsules of thoracic and lumbar spine have been demonstrated histologically15 and immunohistochemically.16 These mechanoreceptors respond to different states of excursion, provide proprioceptive sense, modulate protective muscular reflexes, and via nociception signal potential tissue damage in the event excessive force is applied. Consistent with neck mobility, need to position the head accurately in space, and the need for coordinated muscle control for protection and posture the cervical spine has more mechanoreceptors than the thoracic spine.
In the experimental study of healthy subjects pain was provoked in 72.5% of 40 tested thoracic T3–4 to T10–11 zygapophyseal joints when injected contrast medium distended the joint capsule.17 Referral pain patterns, although overlapping between segmental levels, were more localized compared to patterns obtained by stimulation of lumbar and cervical joints. The most intense pain was reported one segment below and lateral to the injected joint (Fig. 73.1). Precise borders could not be delineated. The largest inferior referral was 2.5 segments while lateral referral did not cross the posterior axillary line. Two subjects reported interesting referral patterns. In one case the T3–4 injection produced pain in the back and subject also stated that ‘pain went into my lung behind my sternum.’ In another case the T3–4 injection produced pain in the back and the subject also reported that pain ‘like a quarter-sized cylinder went toward my breast bone.’
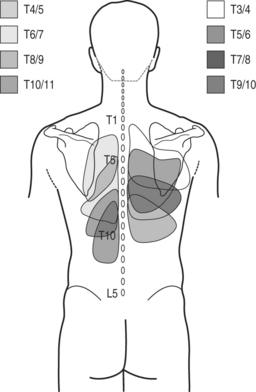
Fig. 73.1 Referral pain patterns from stimulation of zygapophyseal joints T3–4 to T10–11.
(Adapted from Dreyfuss et al. Thoracic zygapophyseal joint patterns: a study of normal volunteers. Spine 1994; 19(7):807–811)
Referral pain patterns from stimulation of zygapophyseal joints C7–T1 through T2–3 and T11–12 were described by Fukui et al. in 1997 (Fig. 73.2).18 A total of 21 joints were injected in 15 patients with previously documented zygapophyseal joint pain. At C7–T1 all patients described pain in paravertebral region extending towards the superior angle of scapula, into interscapular region, and to the inferior angle of scapula. Lateral extension toward the shoulder and suprascapular region was described by two patients. T1–2 stimulation referred into interscapular region and to the inferior angle of the scapula. In two subjects referral was reported into the superior angle of the scapula and suprascapular region. Stimulation at T2–3 joint provoked pain laterally toward the interscapular area and caudally toward the inferior angle of the scapula. T11–12 joint injection referred pain into localized area around the injection and one patient described pain over iliac crest. The authors conclude that referral maps from stimulation of joints at levels of C7–T1 to T2–3 provided such a large overlap that their clinical usefulness in tracking the origin of pain is limited. Anatomical dissection confirming the C7 and C8 medial branches traveling to T2 and T3 level17 may explain this observation.
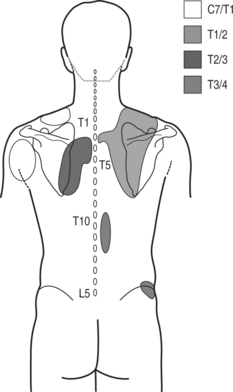
Fig. 73.2 Referral pain patterns from stimulation of zygapophyseal joints C7–T1 through T2–3 and T11–12.
(Adapted from Fukui et al. Regional Anesthesia 1997; 22(4):332–336)
Although lumbar facet denervation was introduced in 1974,19 the procedure was not properly performed until Bogduk20 described the anatomical course of the lumbar medial branches in 1979.
Percutaneous facet denervation was reported in thoracic Z-joints,3 but the study offered no data on the detailed surgical anatomy of the thoracic medial branches. The targeted points for the thoracic medial branches were at a location that was analogous to the position of the lumbar medial branches proposed previously20 at the junction between the superior articular process and the transverse process. In 1994, Stolker et al. published the data from the anatomical study of two thoracic cadaveric spines, where the cannula was placed under fluoroscopic guidance into a target point at the junction of the base of the superior articular process and the transverse process.21 The specimens were then frozen and sectioned with the heavy-duty cryomicrotome. It was observed that although the cannulas were placed reproducibly onto the osseous targets, the medial branch ‘stem’ was never found within the reach of the electrodes. The authors concluded that if the medial branch ‘stem’ is supposed to be the target, a more anterior, more cranial, and more lateral position would perhaps be more effective.
In contrast to the course of the lumbar medial branches, which are at the junction of superior articular process and the transverse process, the medial branch of the thoracic dorsal ramus has a different anatomical location (Figs 73.3, 73.4). Cadaveric dissection of 84 medial branches by Chua and Bogduk revealed the thoracic medial branch arose from the dorsal ramus typically 5 mm lateral to the intervertebral foramen, traversed laterally, dorsally, and inferiorly, and remained posterior to the superior costotransverse ligament.22 After leaving the intertransverse space the medial branch crossed the superolateral corner of the transverse process below (e.g. T3 medial branch and T4 transverse process) and then passed medially and inferiorly across the posterior surface of the transverse process. In its course over the dorsal aspect of the transverse process the nerve was sandwiched between the multifidus muscle anteriorly and the semispinalis thoracis posteriorly. This was the typical course at the levels of T1–4 and T9–10. The T11 medial branch crossed the lateral aspect of base of the superior articular process of T12 vertebra, the transverse process of which is much shorter that other transverse processes. The T12 medial branch had an analogous course to that of the lumbar medial branches at the junction of superior articular and the transverse process of L1. At midthoracic levels (T5–8) the medial branch did not always assume contact with the transverse process and often demonstrated cephalad displacement. The nerve, after making a turn medially in the intertransverse space, descended only slightly inferiorly and remained separated from the transverse process by the fascicles of multifidus muscle.22
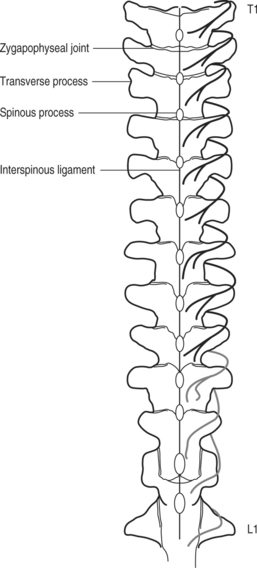
Fig. 73.3 Anatomical location of thoracic medial branches.
(Adapted from Chua and Bogduk. Acta Neurochir 1995; 136:140–144)
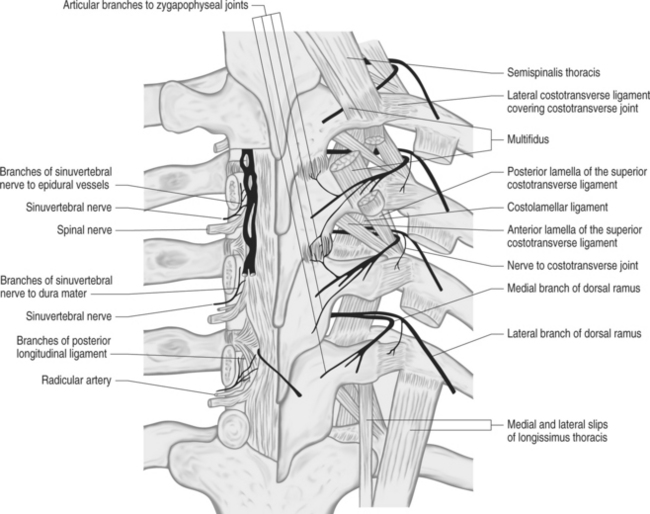
Fig. 73.4 Nerve supply of thoracic spine structures.
From Bogduk N. Innervation and pain patterns of the thoracic spine. In: Grant R, ed. Physical therapy of the cervical and thoracic spine, 3rd edn. New York: Churchill Livingstone; 2002:73–81)
Two articular branches were noted to arise from the medial branches. A short ascending branch separated from the medial branch inferior to the Z-joint and innervated the inferior capsule of the joint. A descending articular branch arose from the medial branch at the superolateral border of the transverse process and in its sinuous course through the multifidus muscle reached and innervated the superior capsule of the joint below.22
The medial branches in upper thoracic segments are musculocutaneous, while lower have only muscular distribution.7
Based on these data, the appropriate target for thoracic medial branch block at T1–3 and T9–10 levels is the superolateral corner of the transverse process where the nerve lies against the osseous structure. For radiofrequency denervation the probe, upon contacting the superolateral corner, should be passed over the edge of the transverse process to be in the contact with the medial branch. Targeting of midthoracic medial branches, due to their different positions, would be more challenging and less reliable. T11 and T12 medial branches are blocked in the same fashion as their lumbar counterparts (junction of the transverse process and the superior articular process). Interestingly, multiple interventional textbooks that have recently been published still describe the techniques of thoracic medial branch blocks and denervation not consistent with current knowledge of anatomy.
Thoracic zygapophyseal joint orientation
The articular surface of the joint is inclined anteriorly from the frontal plane at approximately 60 degrees to the horizontal plane, making the inferior portion of the joint more posterior and the cephalad pole more anterior. The joint plane is also rotated along the vertical axis about 20 degrees so that the lateral aspect of the joint is more anterior and the medial aspect of the joint is more posterior. This orientation changes in the low thoracic spine, which is the more vulnerable segment of the thoracic spine. While maintaining the frontal orientation at T10–11, the T11–12 level shows considerable variation with transition to sagittal orientation similar to orientation of zygapophyseal joints in lumbar spine.23 In a cadaveric study of 37 male spines by Malmivaara et al.,23 the authors also observed that at T10–11 level pathological changes were mostly disc degeneration, while at the level of T12–L1 level facet and costovertebral joint degeneration were dominant. The level of T11–12 demonstrated both disc and Z-joint and costovertebral joint degeneration.
Diagnostic injections
Diagnostic blocks used to temporarily denervate the Z-joints are performed using fluoroscopic guidance with local anesthetic injected into the intra-articular space, onto the medial branch, or both. Injecting 0.1–0.2 mL of nonionic water-soluble contrast agent such as iohexol or iopamidol will confirm the proper placement of the needle tip within the joint. False-positive or false-negative findings may result from extracapsular spread into epidural space during intra-articular injection or intravascular uptake during medial branch block. Extravasation of the local anesthetic beyond the intended target will decrease the specificity of the test by blocking the nociceptive transmission via the sinuvertebral nerve, nerve roots, or spinal nerve. Limiting the volume of the anesthetic agent will increase the specificity. Use of volumes larger than 1 mL or even 0.5 mL may be ‘empirically therapeutic,’ but will likely not have a diagnostic value.24
Wilson reported the outcome following intra-articular injection of bupivacaine and triamcinolone in 17 patients,25 of which two had thoracic kyphoscoliosis, seven had a history of spine injury, and eight had no apparent precipitating factors. After reproduction of their usual pain, 13 patients reported immediate benefit for the expected duration from the local anesthetic. Nine patients reported significant improvement for more than 1 month.
The technique of the intra-articular thoracic zygapophyseal joint injection has been described by Dreyfuss.17 With the monitored patient in a prone position on a fluoroscopy table the appropriate area is routinely prepped with Betadine and draped in a sterile fashion. A true posteroanterior (PA) projection of thoracic spine is obtained. If the T7–8 zygapophyseal joint, for example, is to be injected, the inferior aspect of this joint will be at the superior aspect of the T8 pedicle. A skin mark is then made at the mid to inferior portion of another pedicle below, i.e. T9. A 25-gauge, 3.5 inch needle is inserted in a 60° angle cephalad toward the target joint. Under intermittent fluoroscopic imaging in PA projection, the needle is advanced towards the superior articular process of T8. The needle should remain on the imaginary line connecting the centers of the T8 and T9 pedicles in order to avoid puncturing the pleura laterally or entering the epidural–subarachnoid space medially. Aiming the needle at the inferomedial aspect of the joint may facilitate the joint entry in difficult cases, as this is the most posterior aspect of the joint. As the tip is seen over the mid to superior aspect of the T8 pedicle, the image intensifier is rotated away from the side injected into almost full lateral view until the outline of the joint is clearly visible. The needle is then advanced into the inferior portion of the joint. If the needle is not seen adjacent to osseous structures, manipulation of the needle is performed in the anteroposterior (AP) projection to assure the needle has not strayed lateral or medial. Once the needle is in position, 0.1–0.15 mL of nonionic contrast is administered to confirm the proper placement of the needle. Contrast agent will be observed in superior and inferior recesses of the joint. Local anesthetic (0.5% bupivacaine or 2% lidocaine) with corticosteroid may be injected, but remember that the joint volume is only about 0.5 mL. When firm capsular endpoint is achieved, injection should be stopped to prevent joint capsule rupture. During capsular distension the patient is asked whether concordant pain is reproduced. After the expected onset of anesthesia the patient is asked about the pain relief and this is then further observed and recorded in a pain diary for up to 8–24 hours.
Dreyfuss reports repeating the joint injection 4 weeks later, up to three times a year if long-term relief is observed. If one can confirm that significant short-duration pain relief consistently occurs after local anesthetic denervation, radiofrequency denervation of the medial branches is the best treatment,17 if one assumes that there is an efficacy similar to that of cervical and lumbar medial branch neurotomies. However, the lack of anatomically based prospective randomized studies evaluating the efficacy of the thoracic medial branch neurolysis precludes the general acceptance of this treatment modality.
THORACIC DISCOGRAPHY
Anatomical considerations
Thoracic discs and vertebral bodies are innervated by two interconnected nerve plexuses. The ventral nerve plexus is associated with the anterior longitudinal ligament and has a bilateral supply from branches of the sympathetic trunk, rami communicantes, and perivascular nerve plexuses of segmental arteries. It is connected to the nerve plexuses of costovertebral joints. The dorsal nerve plexus is made up of the nerve plexus associated with the posterior longitudinal ligament. This nerve plexus is more irregular and receives contributions from the sinuvertebral nerves. Sinuvertebral nerves (recurrent nerves of Luschka) arise as branches from the superior or anterior aspect of the spinal nerves just distal to the dorsal root ganglion after exiting from the intervertebral foramen.8 After 2–3 mm of its course back towards the intervertebral foramen, this somatic root joins with the autonomic root that arises from the gray ramus communicans or sympathetic ganglion at the same segmental level. The sinuvertebral nerve passes through the intervertebral foramen anterior to the spinal nerve and nerve roots. Its branches innervate vertebral lamina, and periosteum of the costal neck. As a part of dorsal plexus it further innervates posterior longitudinal ligament, dura mater, and periosteum of vertebral bodies. Anatomical study of the intervertebral lumbar disc innervation26 also demonstrates distinctive innervation of posterior disc (sinuvertebral nerve) and lateral/anterior disc wall (branches of primary posterior rami and rami communicantes).
In a retrospective study of 100 symptomatic patients with magnetic resonance imaging (MRI) documented disc derangement who underwent provocative discography, the authors found discs with annular tears, intrinsic degeneration, and/or associated vertebral endplate infractions to be painful 75% of the time.27 Clinical concordance was about 50%. In this series, the authors observed that in addition to thoracic back pain, extraspinal pain, such as chest wall, intrathoracic and upper abdominal pain, was frequently provoked with thoracic disc injections. They note that location of pain upon provocation appears to relate to the anatomic location of annular tears and may refer to anterior extraspinal sites, such as ribs, chest wall, sternum, and visceral structures within the thorax or upper abdomen. Lateral annular defects often produced radicular-type pain either to visceral or musculoskeletal sites. Posterior annular defects produced back pain either locally or diffusely. The location of provoked sensation was not predictable. The authors stated that the disc with partial annular tears (nonprotruding disc derangements) may be painful and clinically significant, as more than 50% of painful discs fell into this category. The authors did not use antibiotics, and report no incidence of discitis in this series using a thin-needle technique.
As described by Schellhas and Pollei27 and complemented by Tibiletti,28 thoracic discography is performed using fluoroscopy with the patient in a prone position. The skin is prepped twice with iodine solution, followed by two rinses with isopropyl alcohol, and sterile drapes are applied. The disc is accessed from the side opposite to patient’s clinical pain. If the pain is in the midline, the side of injection is selected based on patient’s comfort or individual doctor preferences. A 25-gauge, 3.5 inch spinal needle is advanced through the skin under intermittent fluoroscopic imaging and needle bevel rotation is utilized to control direction. In large patients (over 300 lb) a 5 inch, 22-gauge needle is used. Care is taken to direct the needle lateral to the interpedicular line and medial to costovertebral joints in order to avoid puncture of either the pleura or dura. Needle placement into the upper thoracic discs can be at times technically difficult or even impossible. After annular puncture, the needle is positioned into the center of the disc. Nonionic (e.g. iohexol, iopamidol) contrast agent is injected under fluoroscopic imaging in AP or lateral projection until a firm endpoint is obtained, leakage of contrast medium through annulus is detected, distraction of vertebral bodies occurs, venous opacification is observed, or the patient’s pain response prompts the discographer to terminate the injection. The volume of injected contrast and characteristics of the endpoint (firm, gradual, or no endpoint) are recorded. The amount of contrast agent administered is typically 0.6–1.0 mL unless the disc is incompetent and the injectate leaks outside of the disc. In patients with allergy to contrast, sterile saline solution can be injected instead. Spot images are obtained in AP, lateral, and/or oblique projections at each level. The patient’s responses are recorded. Provoked sensation is judged concordant versus nonconcordant (familiar versus unfamiliar) relative to clinical pain. The patient quantifies the pain on a scale 0 to 10 (verbal rating scale or visual analogue scale). Local anesthetic can be injected into painful discs.
COSTOVERTEBRAL JOINT INJECTION
Anatomical considerations
The radiate ligament fans out superiorly, anteriorly, and inferiorly from the head of the rib and attaches the rib to the vertebral body (bodies). The intra-articular ligament bonds the apex of the rib head to the intervertebral disc and divides the synovial cavity into upper and lower halves. Neurohistological documentation of mechanoreceptors and nociceptors has been previously described in the articular tissue of costovertebral joints.29 Innervation of the costovertebral joint is provided by nerve plexuses receiving fibers from the sympathetic trunks and perivascular nerve plexuses in bisegmental fashion, i.e. from the same level and from the adjacent level above.30 Pain referral maps are not available for costovertebral joints. Radiographic changes in CV joints,31 symptomatic CV joint derangement in ankylosing spondylitis, seronegative spondyloarthropathy, rheumatoid arthritis, and functional costovertebral dysfunction was previously suggested in several reports.32–36
Costovertebral joint arthrography was described first in 1988.37 Five cases of CV arthropathy with pain referred into the abdomen and the loin are reported. At T11 and T12 levels, the authors performed costovertebral arthrography, which reproduced concordant pain. Injection of corticosteroid that followed resulted in long-term relief of pain.
Benhamou et al. studied 28 patients with costovertebral arthropathies who presented with pseudovisceral pain.36 The patients were worked up for chest, abdomen, and flank pain thought to represent the disease of genitourinary, gastrointestinal tract, heart, lung, and pleura. The initial working diagnoses were renal colic, angina pectoris, pulmonary embolism, and spinal tumor. After visceral pathology was ruled out, the musculoskeletal diagnosis was established by clinical examination, reproduction of pain with stressing of the CV joint mechanically, radiological studies, costovertebral arthrography, and anesthetic and corticosteroid injection of the joint. Pathology responsible for costovertebral arthropathy included ankylosing spondylitis, osteoarthritis, diffuse idiopathic skeletal hyperostosis, and degenerative arthropathies other than osteoarthritis.
COSTOTRANSVERSE JOINT INJECTION
Anatomical considerations
Articulation between the facet of the transverse process and the tubercle of the rib forms the costotransverse joint at the levels of T1–10. The lower levels of T11 and T12 lack this joint. T1–5 joint surfaces are reciprocally curved,6 while the lower levels are planar. The joint plane at the rib tubercle is convex and the facet on the transverse process is concave. The costotransverse joint is a synovial articulation and has a thin capsule. The joint is reinforced by the costotransverse, lateral costotransverse, and superior costotransverse ligaments. Innervation is from the articular branches of dorsal ramus and is not strictly segmental.29 Furthermore, each joint is provided with an accessory innervation through twigs derived from the segmentally related intercostal nerves and intramuscular branches of the nerves supplying the multifidus muscle.29 Neither studies in volunteers for the purpose of obtaining referral pain maps nor studies demonstrating pain relief after injecting the painful joint with local anesthetic are available to date.
As described by Lau and Littlejohn,38 a needle is directed into the joint using an oblique fluoroscopic imaging at 45–60 degrees ipsilaterally. Due to the high contrast between the spine and lung tissue, coning is recommended to improve visualization. After administration of local anesthesia into skin, a 22-gauge spinal needle is inserted into the joint. The patient is asked to rotate slowly from side to side and the needle tip is observed to be in the same relationship to the joint. After confirmation of proper position of the needle tip in the joint, 0.2 mL of contrast agent with 1 mL of local anesthetic with corticosteroid is administered.
EPIDURAL STEROID INJECTIONS
Complications are rare but include infection, hematoma, spinal cord injury, pneumothorax, and hiccups.39
1 Saumarez RC. An analysis of possible movements of human upper rib cage. J Appl Physiol. 1986;60(2):678-689.
2 Lewit K. Manipulacni lecba v ramci lecebne rehabilitace, 1st edn., Praha: NADAS; 1990:75-78.
3 Stolker RJ, Vervest AC, Groen GJ. Percutaneous facet denervation in chronic thoracic spinal pain. Acta Neurochir. 1993;122(1–2):82-90.
4 Occhipinti E, Colombini D, Grieco A. Study of distribution and characteristics of spinal disorders using a validated questionnaire in a group of male subjects not exposed to occupational spinal risk factors. Spine. 1993;18(9):1150-1159.
5 Linton SJ, Hellsing AL, Hallden K. A population-based study of spinal pain among 35–45-year-old individuals. Prevalence, sick leave, and health care use. Spine. 1998;23(13):1457-1463.
6 Bogduk N. Clinical anatomy of the lumbar spine and sacrum, 3rd edn., New York: Churchill Livingstone; 1997:191-192.
7 Bogduk N. Innervation and pain patterns of the thoracic spine. In: Grant R, editor. Physical therapy of the cervical and thoracic spine. 3rd edn. New York: Churchill Livingstone; 2002:73-81.
8 Wyke B. The neurological basis of thoracic spinal pain. Rheumatol Phys Med. 1970;10(7):356-367.
9 Lord SM, Barnsley L, Bogduk N. The utility of comparative local anesthetic blocks versus placebo-controlled blocks for the diagnosis of cervical zygapophysial joint pain. Clin J Pain. 1995;11(3):208-213.
10 Barnsley L, Lord S, Bogduk N. Comparative local anaesthetic blocks in the diagnosis of cervical zygapophysial joint pain. Pain. 1993;55(1):99-106.
11 Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns I: a study in normal volunteers. Spine. 1990;15:453-547.
12 Mooney V, Robertson J. The facet syndrome. Clin Orthop. 1976;115:149-156.
13 Manchikanti L, Singh V, Pampati V, et al. Evaluation of the prevalence of facet joint pain in chronic thoracic pain. Pain Phys. 2002;5:354.
14 Wyke B. The neurological basis of thoracic spinal pain. Rheumatol Phys Med. 1970;10(7):356-367.
15 McLain RF, Pickar JG. Mechanoreceptors endings in human thoracic and lumbar facet joints. Spine. 1998;23:168.
16 Giles LG, Harvey AR. Immunohistochemical demonstration of nociceptors in the capsule and synovial folds of human zygapophyseal joints. Br J Rheumatol. 1987;26(5):362-364.
17 Dreyfuss P. Thoracic zygapophyseal joint patterns: a review and description of an intra-articular block technique. Pain Dig. 1994;4:44-52.
18 Fukui S, Ohseto K, Shiotani M. Patterns of pain induced by distending the thoracic zygapophyseal joints. Reg Anesth. 1997;2(4):332-336.
19 Shealy CN. Facet denervation in the management of back and sciatic pain. Clin Orthopaed Rel Res. 1976;115:157-164.
20 Bogduk N, Long DM. The anatomy of the so-called ‘articular nerves’ and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg. 1979;51(2):172-177.
21 Stolker RJ, Vervest AC, Groen GJ. The management of chronic spinal pain by blockades: a review. Pain. 1994;58(1):1-20.
22 Chua WH, Bogduk N. The surgical anatomy of thoracic facet denervation. Acta Neurochir. 1995;136:140-144.
23 Malmivaara A, Videman T, Kuosma E, et al. Facet joint orientation, facet and costovertebral joint osteoarthrosis, disc degeneration, vertebral body osteophytosis and Schmorl’s nodes in the thoracolumbar junctional region of cadaveric spines. Spine. 1987;12:458-463.
24 Raymond J. Intra-articular facet block: diagnostic tests or therapeutic procedure? Radiology. 1984;151:333-336.
25 Wilson PR. Thoracic facet joint syndrome – a clinical entity? Pain Suppl. 1987;4:S87.
26 Bogduk N, Long DM. Percutaneous lumbar medial branch neurotomy: a modification of facet denervation. Spine. 1990;5(2):193-200.
27 Schellhas KP, Pollei SR. The role of discography in the evaluation of patients with spinal deformity. Orthop Clin N Am. 1994;25(2):265-273.
28 Tibiletti C. Practice guidelines: thoracic discography. In: Syllabus of ISIS 11th Annual Scientific Meeting, Orlando, FL, August 8, 2003.
29 Wyke B. Morphological and functional features of the innervation of the costovertebral joints. Folia Morphologica. 1975;23(4):296-305.
30 Groen GJ, Baljet B, Drukker J. Nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188(3):282-296.
31 Sanzhang C, Rothschild BM. Zygapophyseal and costovertebral/costotransverse joints: an anatomic assessment of arthritis impact. Br J Rheumatol. 1993;32(12):1066-1071.
32 Le T, Biundo J, Aprill C, et al. Costovertebral joint erosion in ankylosing spondylitis. Am J Phys Med Rehabil. 2001;80(1):62-64.
33 Ellrodt A, Goldberg D, Oberlin F, et al. Erosive arthritis of the costovertebral joint in seronegative spondyloarthropathy. J Rheumatol. 1986;13(2):452-454.
34 Pascual E, Castellano JA, Lopez E. Costovertebral joint changes in ankylosing spondylitis with thoracic pain. Br J Rheumatol. 1992;31(6):413-415.
35 Arroyo JF, Jolliet P, Junod AF. Costovertebral joint dysfunction: another misdiagnosed cause of atypical chest pain. Postgrad Med J. 1992;68(802):655-659.
36 Benhamou CL, Roux C, Tourliere D, et al. Pseudovisceral pain referred from costovertebral arthropathies. Twenty-eight cases. Spine. 1993;18(6):790-795.
37 Benhamou CL, Roux C, Gervais T, et al. Costovertebral arthropathy. Diagnostic and therapeutic value of arthrography. Clin Rheumatol. 1988;7(2):220-223.
38 Lau LS, Littlejohn GO. Costotransverse joint injection: description of technique. Austral Radiol. 1987;31(1):47-49.
39 Slipman CW, Shin CH, Patel RK, et al. Persistent hiccup associated with thoracic epidural injection. Am J Phys Med Rehabil. 2001;80(8):618-621.







