CHAPTER 23 Injectable Agents for Dermal Soft-Tissue Augmentation of the Face: Options and Decision Making
Facial aesthetic enhancement to restore youth, beauty, and vitality has seen a rapid increase in popularity over the past few decades. The higher level of understanding of the components of facial aging has led us to the appreciation that volume restoration is integral to rejuvenation as surgical repositioning (the ‘lift’). Significant advances and refined techniques have allowed minimally invasive procedures and solutions to volume loss to flourish. Patients can now have these procedures performed in the comfort of a consulting room setting and a swift return to normal activity. Of these, dermal soft-tissue augmentation is unarguably one of the most popular due to its convenience and pleasing results. In addition to smoothing facial rhytids, injectable filling agents are often used in combination to improve the appearance of the periorbita, mid- and lower face, restoring a more youthful appearance with little inconvenience and few complications. In this chapter, we will discuss many of the currently available filling agents, their unique characteristics, and specific procedures most often requested.
Fillers: restoring the aging face
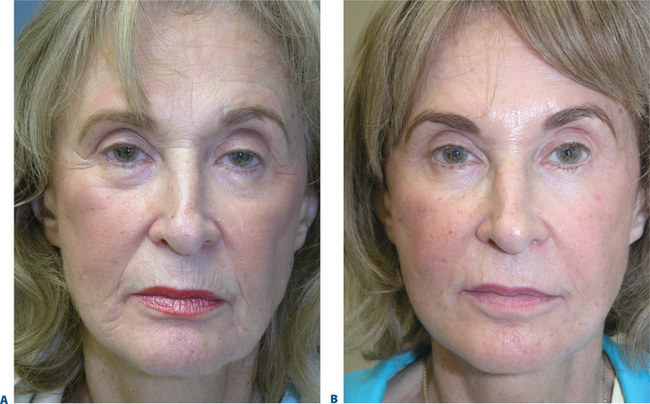
The aging face undergoes many alterations (Fig. 23-1), including changes to skin texture and color, wrinkles and furrows (due to overactive muscles and loss of elasticity), and sagging (in part, due to loss of underlying fat – see Chapter 2, and accompanying DVD clip). There are a number of options for the experienced clinician when choosing a filler for volume replacement, facial contouring, or simply to correct wrinkles and subtly restore the face to a more youthful appearance.
Injectable soft tissue options available
Collagen
Non-permanent biodegradable fillers:
Widely available and easy to use, bovine ‘collagen’ represents an excellent affordable option for temporary augmentation and is indicated for the correction of facial rhytids and scars, as well as lip augmentation. Although these agents are typically included in the long list of soft tissue fillers used for volume augmentation, their role (with the exception of lip augmentation) is primarily for line reduction/eradication. Zyderm and Zyplast (Allergan Inc, Irvine, CA). These agents were the first widely used commercially available products,1 whose names have become synonymous with the word ‘collagen’ despite the fact that there have been dozens of collagen-based injectable products commercially available worldwide since their introduction. These are composed of purified fragments of heterologous animal collagen fibrils derived from processed bovine skin, in a suspension of phosphate-buffered physiologic saline with 0.3 percent lidocaine. Supplied in prefilled syringes and stored at a temperature of 4°C, the dispersed collagen fibrils remain small and fluid. When implanted, the product rises in temperature, forming a more cohesive gel. Zyderm 1 was approved by the Food and Drug Administration (FDA) in 1981, contains a collagen concentration of 35 mg/ml, and is rapidly reabsorbed. Zyderm 2, approved in 1983, contains 65 mg/ml of collagen; reabsorption is consequently slower than with Zyderm 1. Zyplast, approved in 1985, is similar to Zyderm 1 and 2 but the fragmented collagen fibrils are chemically modified and stabilized via crosslinkage with glutaraldehyde. Zyplast is the most viscous of the three products, lasts longer than Zyderm, and although is theoretically less immunogenic than its predecessors has shown a similar rate of allergic reactions.
While early reports of bovine collagen were enthusiastic, claiming a duration of effect as long as 8–12 months, our experience has shown otherwise. Typically, bovine collagen requires two initial treatment sessions to produce the desired effect, which persists from 2–6 months, after which time maintenance treatments are required at least every 6 months or at times more often (Fig. 23-2). Single treatment sessions have been reported in some to last less than 6 weeks despite adequate technique. Good results with bovine collagen (as with all of the injectable filling agents) demand the injection of sufficient volume of product in the appropriate tissue plane; an overly conservative approach (injecting too little) will produce suboptimal effects of short duration. In addition, using sufficient volume ensures simpler maintenance, and less correction at follow-up. Deeper placement (i.e. sub- or deep dermal) produces more transient and less satisfactory effects as resorption with most dermal filling agents appears to be expedited when closer to the subcutaneous space. It has been suggested that the aesthetic improvement of facial lines rests more in the low-grade inflammation afforded by the heterologous (bovine) collagen2 rather than as a direct result of true augmentation of the dermis, but there is also a concern that may relate to potential complications arising from their use.
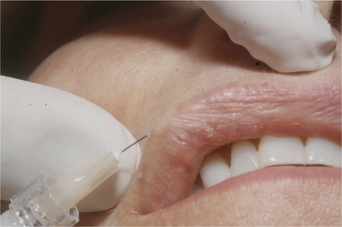
Injection technique is highly variable, but the preferred method is generally regionally dependent. The serial puncture technique is most commonly prescribed (Fig. 23-3) for use of CosmoDerm (for fine lines) and CosmoPlast (for deeper wrinkles and folds) (Allergan Inc, Irvine, CA). These are new bioengineered human collagen-replacement products approved for cosmetic use in Canada and the United States that contain human collagen purified from dermal tissue grown in the laboratory.3 These particular products are processed identically to their bovine counterparts (Zyderm/Zyplast). Although their use has been relatively limited, no allergic responses were observed in a study of 428 patients,4 and a great advantage is that a skin test is not required prior to treatment. Anecdotal reports with experienced injectors however, suggest that the persistence of these agents in some individuals might actually be even less than for bovine collagen. It is suggested that there is less antigenic stimulation, which may reduce the inflammatory effect and subsequent edema required to further reduce the appearance of rhytids.2 One advantage of all of these ‘collagen’ products is that the agents can mostly be delivered without the need for local anesthetic (already contained in each of the product mixtures) and minimal edema and bruising that allows for quicker return to normal activity and appearance.
Techniques for these agents are identical as those for Zyderm/Zyplast (Fig. 23-3).
The ‘collagens’ enjoyed the majority market-share of agents for injectable soft tissue augmentation for many years, primarily due to lack of availability of other agents and ease of administration. Additionally, they were available at a time when there was a lesser appreciation of the value of injectable soft tissue augmentation. Most patients expected that they would receive treatment and have positive effects that would last for a predictable, albeit abbreviated, time period (Figs 23-4 & 23-5). The standards had been set, and most individuals expected aesthetic improvement of the order of magnitude of several months at best. Other more labor-intensive agents introduced in the early 1990s were met with some resistance when they required multiple injection sessions to achieve optimal correction (Silicon, Fat, Dermalogen). Despite the introduction of new injectable filling agents with improved characteristics, these agents are still used by many for a host of reasons including lack of better options for particular applications (including the treatment of fine rhytids) and cost.
Complications
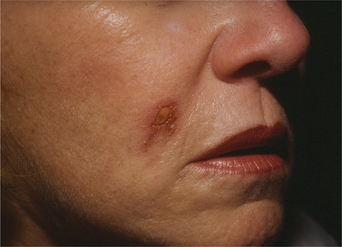
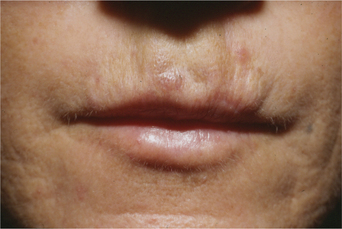
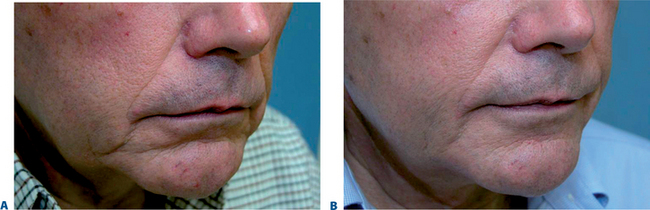
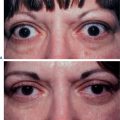
The transient nature of its clinical effect and the need for repeated treatments are injectable collagen’s main drawbacks. In addition, at least 3–5 percent of patients will experience an allergic reaction to bovine collagen5 (Fig. 23-6), necessitating double skin testing prior to treatment. Allergic responses, although rare, can also occur despite negative double skin testing and can be severe and/or long-lasting (Fig. 23-7). Adverse re-actions to bovine collagen injection in the lips are less common and transient. Redness at the injection site nearly always disappears within hours, and bruising will usually resolve within 3–7 days. The patient’s greatest dissatisfaction with ‘collagen’ relates mostly to its rapid resorption. The residence time, however, seems to be correlated with the depth of placement into the dermis, and superficial nodules (as a result of a bolus of products in the superficial dermis) or visible whitish particulate can last for months and even years. These are commonly seen in individuals who have received collagen on multiple occasions for the treatment of superficial rhytids. Another common finding in individuals who had received bovine collagen products to the lips (particularly the vermilion border) on multiple occasions is a progressive difficulty in finding the ‘vermilion plane’ that can usually (in the naïve patient) be easily found (and injected) by placing the needle in the subdermal plane parallel to the lip margin. In many patients, injection of product at the lateral lip margin with this technique can ‘roll’ the material sometimes clear across the midline to the contralateral side. It has been postulated, as well, that the obliteration of this plane is related to chronic inflammation and eventual scarring.2
Other ‘collagen’ products
Dermalogen
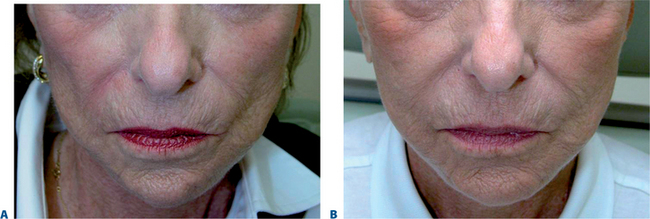
With all of the reported and anecdotal concerns relating to injectable bovine collagen, ophthalmologic pioneer, Charles D. Kelman, MD and collaborator biochemist Dale P. DeVore, Ph.D. began exploring the options and possibilities of using autologous human collagen as an injectable agent for facial soft tissue augmentation. This could potentially address the problems relating to allergy and inflammation, avoid a skin test prior to treatment, and hopefully would yield greater performance compared with the heterologous options. The concept essentially evolved into a process whereby patients undergoing cosmetic surgery (where skin was to be removed) would have their typically discarded skin ‘excess’ saved and transported via express overnight carrier to a central processing facility (Autogenesis Technologies, Acton, MA) where the collagen/dermal matrix could be extracted from the skin. The proprietary process from procurement to the final product delivered intact collagen fibers along with other constituents of the normal dermal matrix in an injectable form called Autologen.6–9 The approximate 3–5 percent sterile dispersion of dermal matrix was sent back to the injecting physician in 1 ml syringes that could be used at a defined period after receipt for the correction of dermal contour defects (Fig. 23-8). The process was a bit laborious and costly; however with appropriate injection technique persistence of wrinkle correction rivaled the bovine collagen experiences that essentially ‘owned’ the filler market at that time. The initial resistance to its use related to the limited applicability for only those individuals undergoing cosmetic procedures where skin was to be removed; at times poor yield and consistency (concentration) of the injectable product; requisite of more than one injection session for full correction in many; and the need for local anesthetic to improve patient comfort as the product contained no local anesthetic within. Shortly thereafter other human tissue products (such as Alloderm) were becoming accepted as care standards in other areas of cosmetic and reconstructive plastic surgery with a much reduced concern of the possibility of communicating disease with such products from a human donor source. There was also an improved understanding of rigorous donor tissue testing, cleansing, and sterilization/decontamination performed to insure safety. The idea of creating an allogeneic variety of human tissue collagen matrix was born whereby skin was obtained from approved tissue banks (like all other donor organs) that were certified by the American Association of Tissue Banks (AATB) and injectable human tissue collagen matrix could be created on a larger and more economical commercialized scale. Dermalogen (allogeneic human dermal matrix) was a result of this concept.2,8–10 The biologic behavior was identical to Autologen, but was far less costly and readily available, also without the requirement of skin testing (Figs 23-9 & 23-11).
The cost and time delay of procurement and custom processing, although ideal, could thereby be eliminated. Factors contributing to the resistance to its use also related to (as with Autologen) the need to use local anesthetic for comfort, as clinicians had only the bovine collagen experience (containing Xylocaine 0.3%). The product became unavailable after a few years of steady sales and satisfactory results by experienced injectors as a result of the demise of the parent company; however it will again become available (Collagen Matrix Technologies, Boca Raton, FL). As the product is highly biocompatible, it elicits almost no inflammatory response and the effects are mostly dependent on precision injection technique with delivery of product into the mid to superficial dermis. As we have all become more comfortable with the requirement for the use of local anesthetic and regional nerve blocks with our recent wealth of experiences with a host of hyaluronic acid products, its acceptance is now even greater. The advantages over the hyaluronic acid products, as with the other ‘collagens’ is its superiority specific for the treatment of finer and more superficial rhytids. Its advantage over the use of bovine is the lack of need for skin testing and the advantage over CosmoDerm/CosmoPlast also relates to persistence, lack of inflammatory reactions, and cost.
As with most of the injectable agents, preferred injection technique is highly variable and region dependent. Enhanced patient comfort is facilitated by local anesthesia. Once anesthetized a slow and deliberate application whether by serial puncture or threading can deliver excellent results with satisfactory persistence (Fig. 23-11).
Protocols for optimal treatment vary slightly from the Zyderm/CosmoDerm experiences whereby patients are treated initially and a second treatment is administered within 3–6 weeks to a satisfactory visual endpoint. Patients are seen again within the next 3–6 weeks to evaluate and consideration of a third treatment. In either scenario of a third (or even fourth) treatment or not, the experiences with skilled injectors is that the result can be up to 6 months.
More ‘collagens’
Over the past 20 years, a host of other collagen-like products have come and gone. The high level of interest in producing a collagen-based product for distribution has persisted since the introduction of bovine collagen in the early 1980s. Fascian, Cymetra, and a long list of other products have emerged after the introduction of Dermalogen, as a result of the demand for a collagen-based product for soft tissue augmentation that would not require skin testing. The nature of some of these agents however, yield suboptimal persistence, hence their waning popularity. A porcine collagen derivative, Evolence, will be introduced into the US market in the next couple of years.11 The theoretic advantages over the bovine collagens (beyond the growing European resistance to bovine products) relate to their proprietary cross-linking process that is supposed to aid against rapid degradation and resorption. A skin test, however, may also be required for this product.
Autologous fat
The oldest available filler and reportedly first used by Neuber in 1893,12 fat has the advantage of being auto-logous and abundant in the body,13 enabling the correction of contour defects without risk of allergy. The indications are most often for the correction of larger subcutaneous defects such as the nasolabial folds and senescent facial volume loss. However, the success of fat procedures depends on the method of harvesting, the type of fat used, and the injector’s level of experience, and disadvantages include donor site morbidity, calcification of the injected fat, and unpredictable resorption.13 Since the variable longevity of contour correction depends on technique, amount of fat injected, and location, improvements in harvesting, handling, and the injection process may have an impact on positive patient outcomes.14,15
Some believe autologous fat grafting to represent the ideal replacement for soft-tissue atrophy, replacing volume and contours lost in the aging process.14 Injections are delivered through large-bore needles requiring local or even general anesthesia. Techniques, handling, and instrumentation vary significantly and consensus has yet to be arrived upon. In 1997, Coleman promoted LipoStructure® – the placement of small amounts of autologous fat in multiple tunnels – for a safe, long-lasting method of recontouring the face.15 The fat autograft muscle injection (FAMI) technique, developed by Amar, involves the injection of adipose tissue within the muscles of facial expression in intricate layers of autologous fatty tissue with anatomic patented microcanulas and has shown improved long-term, aesthetic results for facial rejuvenation.16,17 In ‘fat rebalancing,’ fat is harvested from the patient, frozen, and transferred in less than 0.1 cc aliquots under low injection pressures, weaving fat in a crosshatched 3D design through the muscle to the subdermis or, when injected periorbitally, placed conservatively deep to the periocular musculature.18a,18b,19
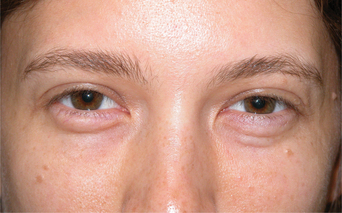
Despite improvements in technique, however, the fact remains that autologous fat grafting demonstrates unpredictable and sometimes temporary results.20,21 Relating to the use of injectable autologous fat for facial rejuvenation, possibly the largest ‘epidemic’ of disappointment by patients regarding their treatment is the indiscriminant use of fat as a tool for improving the volume depletion and hollowness of the lower periorbita (Fig. 23-12). Unfortunately, the attempts of ‘simple’ replacement of volume of the lower eyelid and cheek are met with several obstacles including the irregular fat survival after injection compounded by the atrophy of the lower periorbital structures that reveal the imperfect solution, and is not only distressful for the patient but at times difficult if not impossible to correct. Similar to experiences in the other facial regions, results also have been highly variable and somewhat unpredictable, often with gross visibility of the irregularity of fat survival through the thin peri-orbital skin. Surgical efforts to reverse these iatrogenic problems are difficult at best (Fig. 23-13).
Complications
Harvesting and reinjecting fat are both significant procedures that are associated with downtime and the possibility of serious complications. The main prob-lems associated with autologous fat are its variable resorption rate and unpredictability.13 Although largely technique-dependent, potential complications of autologous fat grafting include prolonged edema, bruising, undercorrection, overcorrection, clumping, irregularities, fat necrosis, migration, and infection, in addition to the other risks associated with surgical procedures.13
Hyaluronic acid
Acting as a space-filling and stabilizing molecule, natural hyaluronic acid is a key structural component of the skin. In gel form, hyaluronic acid binds to water and provides volume to easily fill in larger folds of skin around the mouth and cheeks. There are a number of hyaluronic acid derivatives from which to choose, including Restylane, Restylane Fine Lines, Perlane, and Perlane Plus (Q-Med, Uppsala, Sweden), Hylaform, Hylaform Plus, and Captique (Allergan Inc, Irvine, CA), and Juvéderm family of products (Allergan Inc, Irvine, CA). These stabilized hyaluronic acid (NASHA) gels appear to provide superior durability and aesthetic improvement than bovine collagen,22 may be useful adjuncts in facial cosmetic procedures,23,24 and rarely cause an allergic reaction.
There are a number of advantages associated with hyaluronans, including longer duration of effect requiring less frequent touch-ups than collagen, and a lack of hypersensitivity (ideal for patients who are allergic to bovine collagen). In addition, some patients may resist using animal-derived products, such as Zyderm and Zyplast, and may perceive greater safety with a non-animal product. As with collagen injections, treatment with hyaluronans is easy and quick and can be performed in as little as 30 minutes. In contrast, patient experience and possibly results25 may be significantly improved with the use of local or regional anesthetic (with epinephrine). The effects are immediate, there is little or no recovery time in most facial regions, and the clinical effects persist for 4–6 months, depending on the product, technique, volume, and density of the hyaluronic acid used. While hyaluronans provide greater persistence of effect, they are still similar to natural components of the skin and, as such, elements of the filler gradually break down and are absorbed, necessitating periodic touch-up treatments to maintain the desired outcome. Most treatment protocols involve the application of the hyalurons to slight overcorrection with the anticipated variable initial resorption or effect loss due to dissipation of the initial edema (that can be profound in some patients and facial regions [lips]). Occasionally, patients will require a second treatment session 2–4 weeks after the initial treatment to rebuild the tissue to the desired degree (Figs 23-14 to 24-16).
Complications
Transient adverse effects include pain on injection, and intermittent swelling, edema, and erythema at the injection site.3 Injection-related adverse effects, namely, bruising, erythema, pruritis, and discoloration are common, and delayed skin reactions (albeit rare) have been reported post-injection.26 Routine intramuscular or subperiosteal injections are not recommended for most regions, as much of the filler will be absorbed. Likewise, injections into dynamic areas associated with a great amount of movement, such as around the mouth, may lead to less satisfactory results, as the motion will encourage absorption and displacement.27 Enhanced effects can be achieved in mobile areas such as the lips with the adjuvant use of botulinum toxin in sufficient dosages to reduce movement yet maintain function.28 Residence time should also be considered when injecting in multiple planes as with most non-permanent injectable agents, persistence is greater in the more superficial layers of the dermis. If significant amounts of product are injected in the superficial dermis, the result may initially look quite satisfactory and this phenomenon may not be evident until the deeper product dissipates25 (Fig. 23-17). Recent publications have documented intermittent swelling and granulomatous reactions,27,29 and the use of impure or contaminated material can result in infection or foreign body reaction.30 Granulomas often respond to injected corticosteroids, topical antihistamines, and digital pressure or manipulation.27
Calcium hydroxylapatite beads
Radiesse (formerly Radiance) is an admixture of calcium hydroxylapatite beads in a carboxy-methylcellulose (CMC) vehicle (BioForm, Inc., Franksville, WI). It is now approved in the United States for the correction of moderate to severe facial folds and wrinkles around the nose and mouth, including nasolabial folds.31 Preliminary data suggest that with good technique and adequate volume of material that the persistence in most areas is of the order of magnitude of one year or greater.32 Once injected, the gel is absorbed; what remains is a matrix of material that will take on characteristics of the cells that populate it, requiring precise placement of the filler in order for the permanent correction to occur. Since the collagen produced is native and the material injected is inert, the filler formed has the potential to be stable and permanent. Skin testing is not required prior to use. Similar to other particulate fillers, this agent is less forgiving in areas of significant mobility, such as the lips, and is best reserved for injectors who have had great experience with its use with excellent injection technique (Fig. 23-18).
Complications
There is little data on the potential complications of Radiance when used in cosmetic procedures. To date, there have been no reports of antibody formation or hypersensitivity. Bruising and swelling are common. Pain associated with the injection is mild and can be treated with acetaminophen in most cases. Complications that are the most troubling to patients are persistence of palpable areas in the skin and nodule formation. Nodule formation can relate to an over-abundance of products placed in one region, superficial placement, and has also been implicated as a reaction to the carboxymethylcellulose. Placement in the mid- or superficial dermis (especially in light- or thin-skinned patients) will result in visible material. Nodule formation that is mild and self-limited occurs in a small percentage of patients injected, and some of these patients will have nodules that require treatment with either intralesional steroids or incision and drainage.32–34
Injectable poly-L-lactic acid
Sculptra (previously Nu-Fill; poly-L-lactic acid) has been used extensively in Europe especially for the treatment of facial volume loss as in patients with HIVassociated facial lipoatrophy. Due to its tremendous success in facial volume restoration in difficult situations such as HIV, the applications were readily extended to facial volume loss due to a variety of causes and specifically, age-related. Sculptra was FDA ap-proved for use in HIV-related facial lipoatrophy in August, 2004. It has been used ‘off label’ in the US for aesthetic purposes with good results in selected patients. The agent is provided in glass vials as a sterile lyophilized cake of micro-particles of poly-L-lactic acid (PLLA, similar to the constitution of Vicryl suture) with the average particle size (diameter) of approximately 50 microns. Carboxymethylcellulose and mannitol are two of the non-active ingredients. Most injectors now reconstitute the ma-terial in 5–8 ml of sterile water. Saline suspension is not recommended, because changing osmolality might interfere with the solution’s characteristics and hence the performance of PLLA. Biodegradation is by hydrolysis of the material to lactic acid monomers that are then metabolized via normal lactate metabolism. Injection techniques vary; however the premise is to disperse the suspension in the plane of the deep dermis/subcutaneous space by serial threading or fanning to actually deposit the material diffusely into the region to be affected. The attempt is not to achieve full correction with a single treatment, rather to cause a reaction which yields collagen production at the deep dermal layer. This actually increases dermal thickness while enhancing the appearance of regional volume. The immediate effect is simply mechanical, and related to the volume of fluid injected. The action is in the delayed effect that suggests the formation of new collagen despite the resorption of the PLLA particles. Most regions require two to three treatment sessions, spaced approximately one month (or greater) apart. Local anesthetic is not universally used; however some injectors prefer to give regional blocks while others use a small volume of anesthetic in the injectant itself. For aesthetic uses, after a series of treatments that vary in volume and in number, the results appear to last in the order of magnitude of at least one year35,36 (Figs 23-19 & 23-20).
Late granuloma formation is now seen less than the prior European experience, as a more dilute suspension appears to be more forgiving, whereas an attempt to ‘overcorrect’ and/or use a higher concentration in previous years is believed be a leading cause for this complication.
Permanent, non-biodegradable fillers
Polymethylmethacrylate (PMMA) microspheres
Artefill (previously Artecoll; Artes Medical Inc., San Diego, CA) is a suspension of 50 micron uniform, polished, polymethylmethacrylate (PMMA) microspheres in 3.5 percent bovine collagen solution. After implan-tation, the collagen vehicle eventually dissipates, leaving behind the non-biodegradable PMMA microspheres.37–39 Although the volume injected depends on the depth and size of the wrinkle, usually at least two injection sessions are required. Since the product is permanent, it is important to select patients carefully and treat cautiously. Partial correction, followed by reinjection after a few months, will produce a smoother, more natural appearance than overcorrection. Applied by a skilled clinician, Artecoll is a powerful and effective filling agent that yields excellent results and may not require repeated touch-up treatments (Fig. 23-21). However, its permanence demands that great care be taken in choosing the appropriate patients who are comfortable with the risks associated with permanent facial augmentation. Disadvantages to the current formula is that due to the bovine collagen carrier, a skin test is still required.
Complications
Injections are not recommended for areas of thin skin (e.g. lower eyelid) and with lip augmentation, or in any other areas of repetitive movement. Injudicious use of this agent may result in beading, palpability, and visibility of the implant. Artecoll facial injections have been reported to cause the delayed development of granulomatous reactions,40,41 and improper placement intradermally (rather than into the upper levels of the subdermis) or too aggressive injections can lead to beading, ridging, and nodule formation. This can cause disfigurement reversible only by surgical excision.27 Conservative amounts of Artecoll and a long interval between injections are recommended.42 Even with a conservative approach, granulomas can appear long after treatment. Fortunately with proper patient/region selection combined with good technique the incidence is low, and responds well to intralesional triamcinolone injections.27,41,43 Bovine collagen allergy has also been reported with the use of this product. As with most products, Artefill has evolved with a variety of product improvements that with careful patient selection and good injection technique can achieve satisfactory long-term results.
Acrylic hydrogel
DermaLive and DermaDeep (Euromedical Systems, Ltd., Nottingham, UK) are semi-permanent soft-tissue filler substances composed of acrylic hydrogel in a hyaluronic acid vehicle. After injection, the hyaluro-nic acid component is resorbed, while the nonbiodegradable acrylic hydrogel particles are left behind and induce a tissue response to yield long-lasting results of at least 12 months.44,45 DermaLive consists of smaller acrylic hydrogel particles and is injected less deeply into the tissue to fill medium to deep skin depressions, such as the nasolabial folds, glabellar lines, marionette lines, and facial scars; with its larger acrylic hydrogel particles, DermaDeep is reserved for pronounced tissue defects, such as deep nasolabial folds and sunken cheeks. Because of its longer duration of effect, Derma-Live/DermaDeep may be an acceptable alternative to hyaluronic acid or collagen in the carefully selected patient. Our experience with this product (JC, AC) is limited. More than one injection of Derma-Live/DermaDeep is often needed, with a maximum of three or four sessions spaced at least 3 months apart. Marketed in Europe primarily for long-term correction of facial lines and creating volume since 1998, Derma-Live/DermaDeep is currently not approved for cosmetic use in the United States.
DermaLive/DermaDeep should not be implanted in the mucosal part of the lips, the periorbital zone, horizontal lines across the forehead, or in vertical wrinkles on the upper lip. Contraindications include a history of hypertrophic scarring, immunotherapy, autoimmune or inflammatory diseases, multiple allergies, and (rarely) known allergy to sodium hyaluronate or hyaluronic acid. Since DermaLive/DermaDeep has no elements of animal origin and is generally non-allergenic, skin testing is not required.
Complications
Short-term side effects include pain on injection, itching, discoloration, tenderness, palpable lumpiness, redness, and edema, most of which resolve within 1–2 days or, in the case of lip injections, a week. The majority of complications seen with DermaLive/DermaDeep are considered due to improper injection techniques or use in the presence of contraindications.44 Long-term side effects include nodules, swelling or redness at the point of injection appearing an average of 6 months after injection. Serious complications, such as granuloma, superficial necrosis, and urticaria, are generally reported to be rare, although a few reports have suggested a much greater incidence of granuloma formation in a larger series of patients with longer (greater than 2 years) follow-up.27 At the first sign of a nodule or other complication, a series of corticosteroid injections with an interval of 1–3 weeks between each is usually effective. Surgical excision is a last resort and rarely necessary.
Silicone oil
Although injections of silicone oil are not approved by the FDA for cosmetic use (the approved indication is for its use in the repair of retinal detachments), their off-label use as a permanent soft-tissue filler for facial rejuvenation persists, despite the history of controversy. Injected through a special 30-gauge needle (RJ MaxFlo, Richard James Inc, Boston, MA) in microdroplets of 0.01 ml into the subdermis, each microdroplet induces its own fibroblastic response.13 Since fibrous capsules form around each silicone particle over several weeks, conservative injections with follow-up corrections 6 weeks apart are recommended to achieve adequate augmentation. This microdroplet serial puncture technique is believed responsible for dramatically lowering the risks previously associated with liquid silicone.45 Silicone oil is used most commonly in the perioral region (Fig. 23-22).
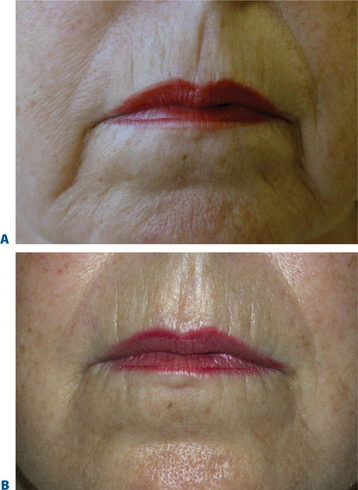
Figure 23-22 A, Pre-silicone. B, Post-silicone.
Photographs courtesy of Drs Alastair and Jean Carruthers.
Complications
Minor adverse reactions are similar to those seen with other injectable augmenting agents, including pain upon injection, edema, and discoloration.45 Although some believe complications of silicone oil to be largely due to improper injection technique or the quality of the silicone,46 reported complications include inflammation, discoloration, ulceration, migration, and granulomas.47 More disconcertingly, there are reported complications arising as long as 20 years after injection.48
Fillers: aesthetic facial applications
A youthful face is associated with smooth, even distribution of ample fat, in sharp contrast to the aging face, which dips and sags due to a host of factors19 including animation, environmental and hereditable factors, and volume loss.2,9,15 Recent research and clinical experience has supported the hypothesis that this sagging is caused not by gravity, as long believed, but by the atrophy of subcutaneous fat, suggesting that a redis-tribution of fat or volume replacement may restore vitality (see Chapter 2).18,19,21,49,50 With atrophy of subcutaneous soft-tissues, unsupported skin falls into wrinkles and folds, and often exaggerates the illusion of true soft tissue descent, which traditionally necessitated the surgical ‘lift’. Loss of volume, particularly around the eyes and cheeks, cannot always be adequately improved or corrected by traditional surgical procedures, which carry a variety of potential complications.51 A number of techniques aimed at restoring lost fat volume through the use of fillers have demonstrated that replacing volume to youthful proportions can restore the harmonious aesthetic of the face. Our early experiences with ‘fillers’ (primarily ‘collagen’) for the most part limited our application of these products to line reduction. With the establishment of the next generation of ‘off the shelf’ fillers that were useful for both line eradication and volume replacement, our ability to restore facial volume in an outpatient/consulting room setting has escalated to dramatic proportions. Fillers that have the capacity to enhance and restore volume can improve the appearance of brow, cheek and lower facial ptosis in selected patients.
Non-invasive ‘lifts’
Traditional surgical procedures in facial rejuvenation focus on identifying and correcting the signs of aging by tightening and lifting the soft tissue, with the underlying concept of gravitational aging in the brow and midface. However, there are a variety of compli-cations associated with forehead and brow-lifting procedures, as with any surgery.51 In addition, in many the results can be either unsatisfactory or not long-lasting. Alternatively, volume replacement with fillers in the brow and midface subtly restores the face to a more youthful aesthetic, and is particularly useful in those individuals who either are not quite ready for surgery, or already have embarked upon the surgical journey.
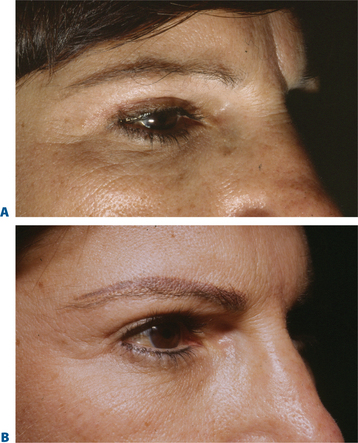
Temporal brow lift
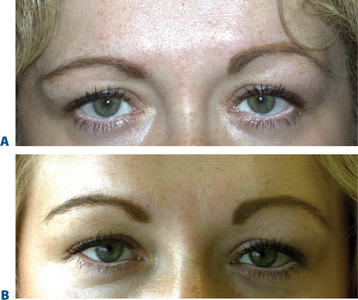
While brow ptosis has traditionally been approached via a surgical solution, experience has shown that a purely surgical lift cannot restore the same youthful appearance to the anterior projection of the lateral brow. Adequate pain management can be achieved either by anesthetizing the skin with topical ElaMax 5 percent or Betacaine ointment, or direct infiltrative anesthesia. For the first treatment, Restylane, Juvederm, and Hylaform Plus are ideal filler products; alternately, Artecoll may be used in patients with previous experience with a biodegradeable filler and a negative skin test. Using a marking pen to delineate the area to be treated, the product is injected using the ‘push-ahead’ technique (allowing the filling agent to dissect ahead of the needle) through a 30-gauge needle. The initial injection is placed at the temporal end of the lateral brow cilia (injection volume of approximately 0.2 cc), followed by a reinjection 7–10+ mm medial to the first at the lateral aspect of the supra-orbital notch (injection volume is 0.1–0.2 cc). After injection, the filler is molded gently to form the natural projection previously created by the brow fat pad, and ice is applied. An instantaneous lateral brow lift effect is observed (Figs 23-23, 23-24).
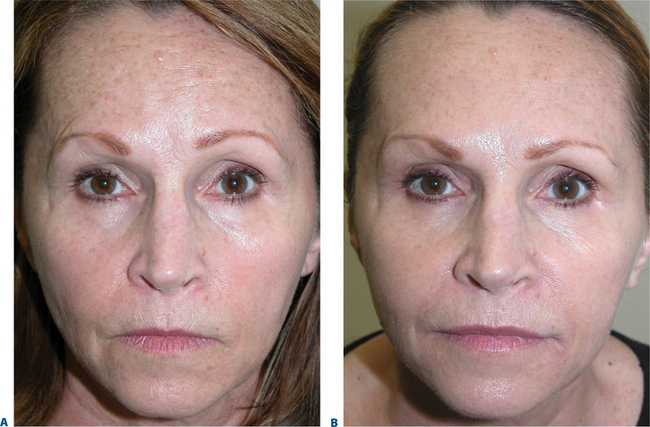
‘Tear trough deformity’
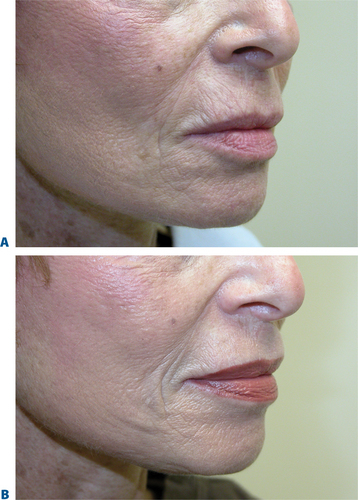
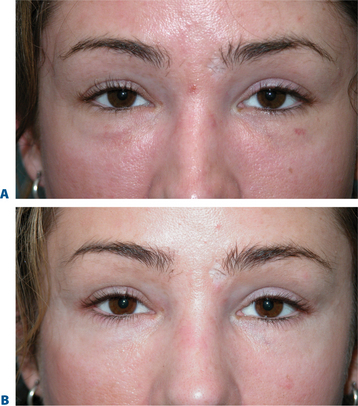
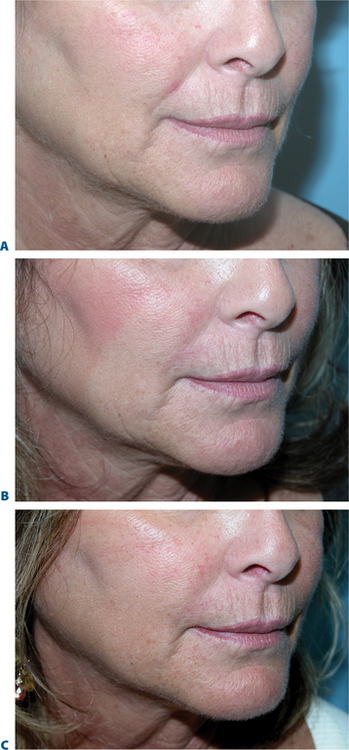
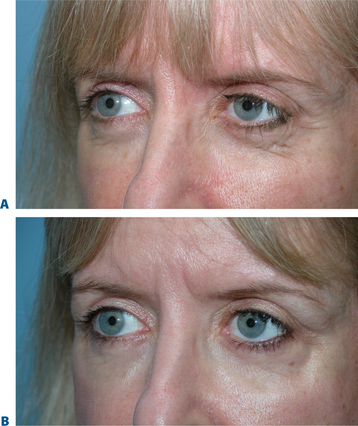
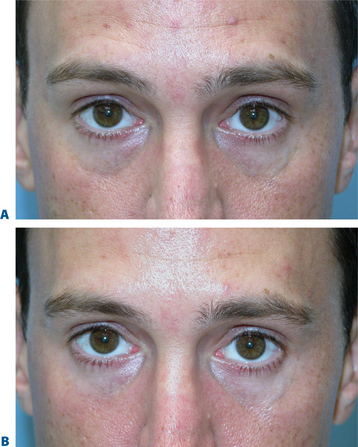
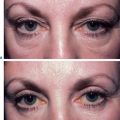
The causes of volume loss and skeletonization of the medial lower periorbita are multifold. This can be observed with overzealous fat excision after lower blepharoplasty, but is more commonly seen with age (in those who have never had surgery) and relates to soft tissue (fat) atrophy, skin-thinning, a mild degree of soft tissue descent and even fat herniation cephalad to this region. As the process is complex, simple monotherapy solutions, including soft tissue augmentation via injection, have been tried with varying success. The problems relating to injectables in this region is that the atrophy and thinning is unforgiving to the inevitable visibility of these products (Fig. 23-25). Nonetheless, with careful application using a variety of these agents in the appropriately selected patients, pleasing results and improvement can be achieved (see DVD). Treatment is best directed in a deeper, supraperiosteal plane to reduce visibility of the product bolus. The most forgiving are the hyaluronates; with this application, due to the common atrophy of the local soft tissue and thinning of the skin, a conservative application will yield subtle aesthetic improvement while lumpiness can be minimized (Fig. 23-26). The collagens are too rapidly resorbed to enhance the low risk/reward ratio. Artefill has shown satisfactory results with precision application and proceeding with utmost caution and conservatism. Fat can also gives good effects, but due to the variable resorption, yields surface contour irregularities even with experienced injectors (see pp. 283–284). Local anesthetic with epinephrine may be required for comfort and to minimize the inevitable bruising with injection to this highly vascular area. Deep plane injection bypassing the needle to periosteum will avoid intravascular injection in almost all situations (see DVD).
Midface ‘lift’
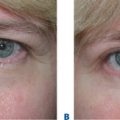
Hypoplasia of the zygoma or midface fat descent and atrophy robs the female face of the normal heart-shaped contour. Using a marking pen, the treatment area is demarcated. An evaluation to detect for facial asymmetry may indicate an individualized treatment on each side of the same individual to maximize results. Local or topical anesthetic is optional. Restylane, Juvederm, Hylaform Plus, and Perlane all work well in this region. Artecoll and Radiesse also work well, but clinicians should take care to inject a smaller volume and follow-up with a ‘touch-up’ treatment in approximately 8 weeks. After the chosen filler has been injected into the subdermal space in each treatment site, the patient can immediately inspect the aesthetic benefit (Fig. 23-27).
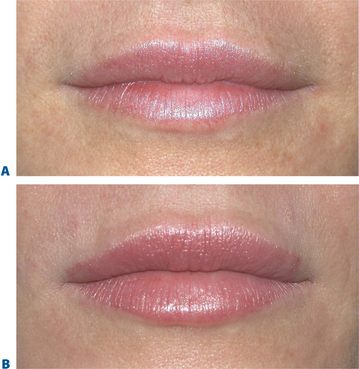
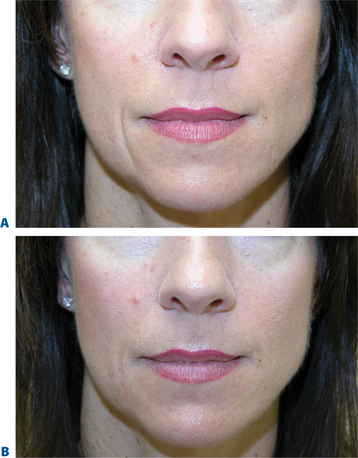
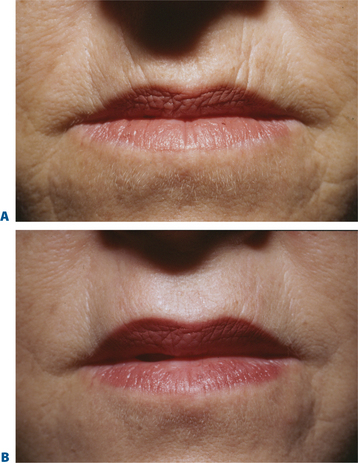
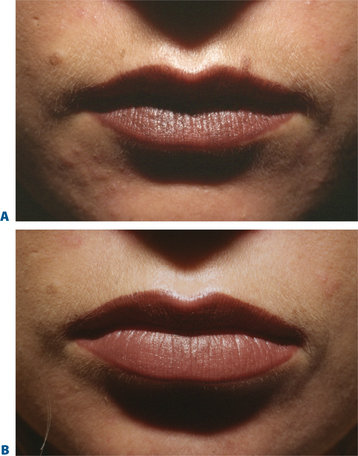
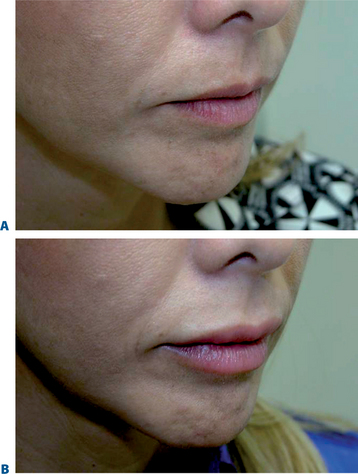
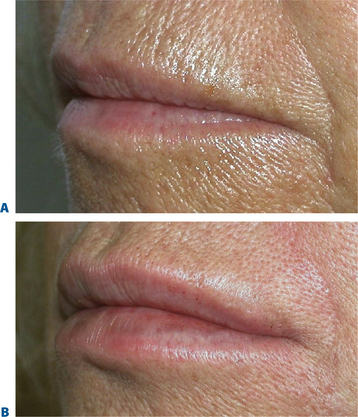
Lip augmentation
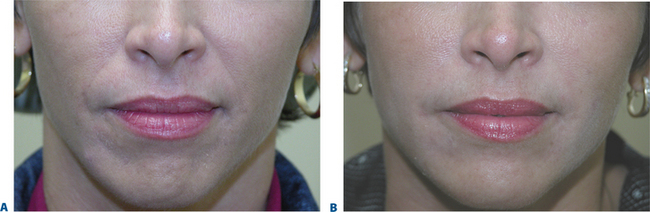
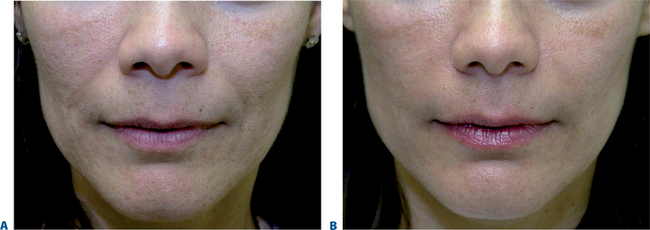
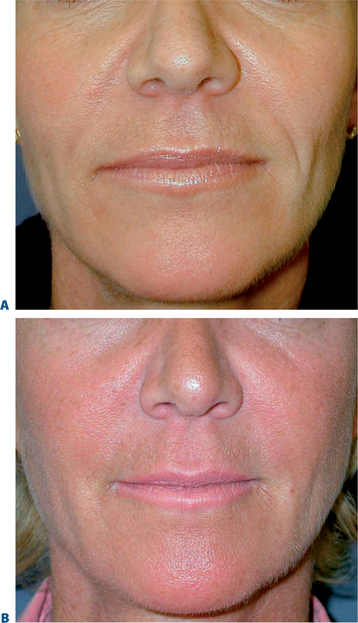
In patients seeking a more temporary augmentation of the lips, we use Zyderm 1, the least viscous of the bovine collagen injectables, for preliminary injections in the lips to define the area of treatment and to create a channel for subsequent Zyplast injection. We start in the outer corner and work our way across the length of each lip, just inside the vermilion border, with a total of five to six injections per lip and 1 cc of Zyderm 1. The lips should be gently massaged after injection to ensure that the filler is evenly distributed. Once Zyderm 1 has been injected, we may implant Zyplast in the channel formed by the Zyderm 1 injections beginning at the corner of the mouth and placing the injections at even intervals across the lips inside the vermilion border. Massage follows to break up any irregularities and to establish a smooth texture and appearance. Zyplast is then injected precisely along the vermilion border in the upper lip and directly into the upper lip just above the corners of the mouth, enhancing lip border definition, producing an aesthetically pleasing ‘ski-jump’ profile to the lips, and creating a slight upturn to the corners of the mouth (Fig. 23-2). The injection technique used with hyaluronans is similar to that used for collagen, with injections placed along the length of the lips within the vermilion border. We use Hylaform Plus, Perlane, and Reviderm for their extended duration of effect and effectiveness in imparting dramatic improvement to the shape and texture of the lips. Restylane, Juvederm or Hylaform Fineline injected more superficially along the vermilion border will create a line of firmness that will define the lip border and resist wrinkling. Juvederm and Restylane products are also superb for treating lip volume by direct injection into orbicularis and enhancing persistence by injecting low-dose Botox to the vermilion border (see Chapter 24) (Figs 23-28, 23-29).
In February 2003, the FDA recommended avoiding Artecoll for perioral injections due to reports of lumpiness. However, injection into the lips is possible, with some precautions. Ideally the orbicularis oris should be relaxed with botulinum toxin prior to injection to prevent filler migration and aggregation with movement of the lips. Since Artecoll is permanent, it is important to avoid overcorrection; instead, it is more prudent to use smaller volumes and assess the result before augmenting further. In addition, cautious and gradual treatment results in the most natural and pleasing effect. After injection, massage the lip gently and apply pressure to any perceptible lumps to ensure proper distribution of the filler. However, too-vigorous massage can cause diffusion, diminishing the effect of the product. A settling period occurs after treatment, as the collagen in Artecoll dissipates and is replaced by the body’s own collagen. To minimize the changes in appearance that take place during this period, we have found that the adjunctive use of a hyaluronan, placed more superficially, overlaying the Artecoll, produces an immediate cosmetic result (Fig. 23-30).
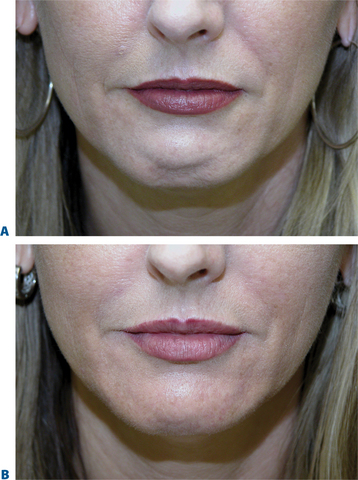
Chin ‘implant’ augmentation
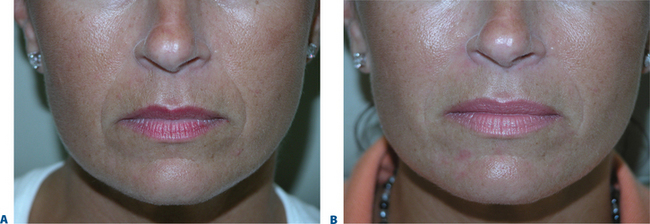
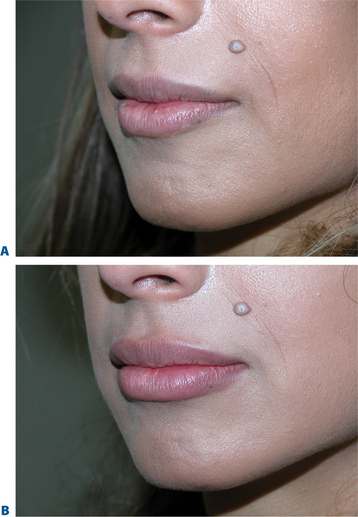
After photographing the face in primary position, as well as right and left lateral gaze, we inject the filler (typically, 0.2–0.4 cc) subdermally into the region superior to the mentum, producing a fuller chin that projects forward to line up with the lower lip when the face is in the Frankfort horizontal (Fig. 23-31).
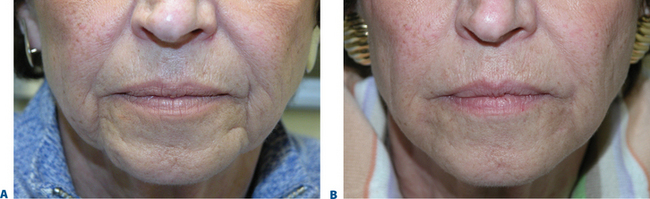
Peri-mental hollows
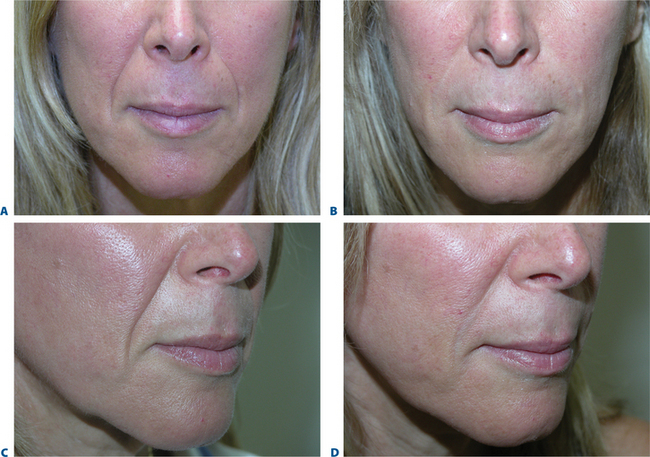
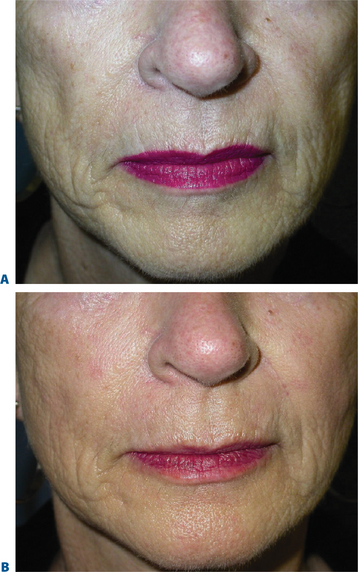
The signs of lower facial aging are commonly associated with ‘jowling’ along with the appearance of depressions or ‘hollows’ lateral to the chin (mentum) (Figs 23-32 & 23-33). The partial illusion of descent posterior to this depression is caused and exaggerated by the magnitude of this depression related to volume loss caused by life-long mobility of the lower lip/facial depressors (i.e. depressor anguli oris, etc.). In selected individuals improvement of this appearance can be afforded by applying injectable soft tissue augmentation agents along the jaw line overlying this depression. Agents commonly used are those that have more cap-acity for volume filling including the hyaluronics, Radiesse, and fat (see DVD).
Combination therapy: botulinum toxin and fillers
The combination of botulinum toxin type A (BTX-A; BOTOX, BOTOX Cosmetic; Allergan Inc., Irvine, CA) and soft-tissue augmentation is a highly synergistic approach used routinely to achieve more effective, longer lasting results, especially in the mid- and lower face, by simultaneously treating static and dynamic aspects of rhytids. Early anecdotal reports28,52,53 corroborated with clinical study54 found that BTX-A in patients undergoing soft-tissue augmentation in certain facial areas (i.e. deep glabellar furrows or lip augmentation) eliminated or reduced the muscular activity responsible for the wrinkles and increased the long-evity of the filling agent.28,54 Preceding the injection of filling agents by approximately 1 week, BTX-A may work on several levels, reducing the dynamic component of rhytid formation in newly remodeled skin and allowing more permanent eradication of wrinkles23,24,52 (Figs 23-28 to 23-33). For practical purposes, most combination therapy is given at the same office visit.
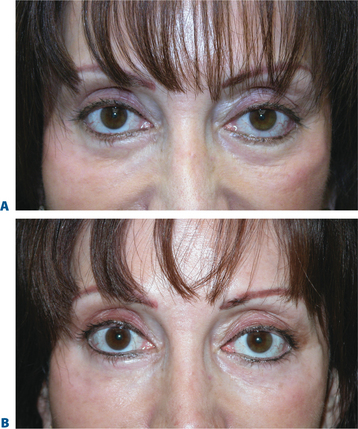
Several studies have also documented the synergistic effect of adjunctive BTX-A and fillers.55 In a study evaluating 65 patients with moderate-to-severe glabellar rhytids who received BTX-A, Zyderm II collagen, or a combination of both therapies55: after 1 month, patients who received combination therapy showed significantly greater improvement in furrows (79% vs. 56% and 50% in the BTX-A and Zyderm arms, respectively; p < 0.05). In addition, clinical effects were longer in duration and patient satisfaction higher in patients who received both BTX-A and collagen (Figs 23-34, 23-35). Likewise, in another study, patients with moderate-to-severe glabellar rhytids injected with both BTX-A and hylan B responded better than those treated with BTX-A alone.23 After injection of combination therapy, 6 percent had only moderate rhytids, while the rest (94%) had mild rhytids; of patients who received BTX-A alone, none (0%) had achieved no or mild rhytids. In a prospective, randomized study of 38 patients with moderate-to-severe glabellar rhytids, BTX-A plus NASHA (Restylane) led to a better response both at rest and on maximum frown than NASHA alone.24 In addition, combination therapy led to a longer duration of response: the median time for return to preinjection furrow status occurred at 18 weeks in the NASHA alone or BTX-A alone groups, compared to 32 weeks in patients treated with BTX-A plus NASHA. Combinations therapy is also advantageous in all other areas where BTX-A can be applied safely and effectively, such as the lips and perimental hollows,28,52,53 forehead and chin (Figs 23-32, 23-33, 23-36).
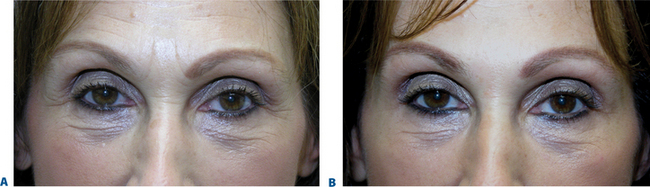
Figure 23-34 A, This patient presented after unsatisfactory resolution of glabellar and forehead furrows with Botox alone performed elsewhere. B, After CosmoDerm injections to the glabella and forehead furrows combined with Botox to these regions. See Chapter 24.
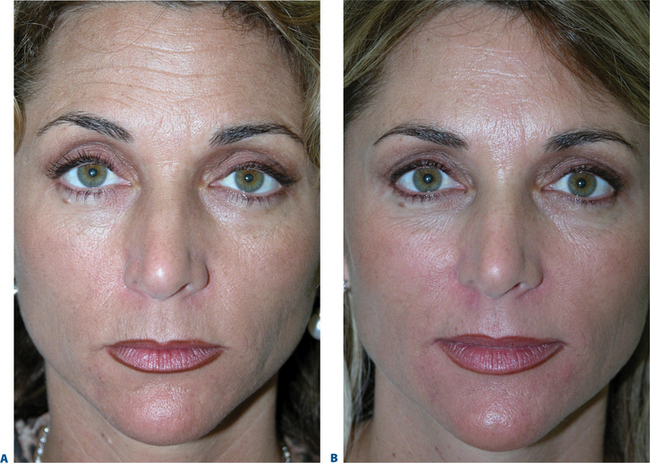
Figure 23-35 A, Before and B, after a combination of CosmoDerm and BTX to the glabella and forehead.
1 Knapp TR, Kaplan EN, Daniels JR. Injectable collagen for soft tissue augmentation. Plast Reconstr Surg. 1977;60:398-405.
2 Fagien S. Facial soft tissue augmentation with injectable autologous and allogeneic human tissue collagen matrix (Autologen and Dermalogen). Plast Reconst Surg. 2000;105:362.
3 Jordan DR. Soft-tissue fillers for wrinkles, folds and volume augmentation. Can J Ophthamol. 2003;38:285-288.
4 INAMED Esthetics. URL: www.inamed.com (accessed August 2004).
5 Klein AW. In favor of double testing. J Dermatol Surg Oncol. 1988;14(Suppl. 1):27.
6 DeVore DP, Fagien S, Kelman CD, Casson P. Autologous injectable dermal collagen. In: Bosniak S, editor. Principles and Practice of Ophthalmic Plastic and Reconstructive Surgery. Philadelphia: WB Saunders; 1993:670-675.
7 Fagien S. Autologous collagen injections to treat deep glabellar furrows. Letter to the Editor. Plastic Reconst Surg. 1994;93:642.
8 Fagien S. Facial soft tissue augmentation with autologous and homologous injectable collagen (Autologen and Dermalogen). In: Klein AW, editor. Tissue Augmentation in Clinical Practice, Procedures and Techniques. New York: Marcel Dekker; 1998:87-124.
9 Fagien S. Facial soft tissue augmentation with autologous injectable collagen. In: Putterman AM, editor. Cosmetic Oculoplastic Surgery: Eyelid, Forehead, and Facial Techniques, Third Edition, Chapter 32. Philadelphia: WB Saunders; 1998:367-375.
10 Fagien S, Elson ML. Facial soft tissue augmentation with allogeneic human tissue collagen matrix (Dermalogen and Dermaplant). In: Matarasso A, Matarasso SL, editors. Clinics in Plastic Surgery. Philadelphia: WB Saunders; 2000:63-81.
11 Monstrey SJ, Pitaru S, Hamdi M, et al. A two-stage phase I trial of EVOLENCE Collagen for soft tissue contour correction. Plast Reconstr Surg. 2007;120:303-311.
12 Neuber G. Fettransplantation. Bericht uber die Verhandlungen der Dt Ges f Chir Zentralblatt fur Chirurgie. 1893;22:66.
13 Cheng JT, Perkins SW, Hamilton MM. Collagen and injectable fillers. Otolaryngol Clin North Am. 2002;35:73-85.
14 Kanchwala SK, Bucky LP. Facial fat grafting: The search for predictable results. Facial Plast Surg. 2003;19:137-144.
15 Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24:347-367.
16 Amar RE. [Adipocyte microinfiltration in the face or tissue restructuration with fat tissue graft]. Ann Chir Plast Esthet. 1999;44:593-608.
17 Butterwick KJ, Lack EA. Facial volume restoration with the fat autograft muscle injection technique. Dermatol Surg. 2003;29:1019-1026.
18a Donofrio LM. Fat distribution: A morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
18b Donofrio LM. Structural autologous lipoaugmentation: A pan-facial technique. Dermatol Surg. 2000;26:1129-1134.
19 Donofrio LM. Fat rebalancing: The new ‘facelift.’. Skin Therapy Lett. 2002;7:7-9.
20 Chajchir A, Benzaquen I. Fat-grafting injections for soft tissue augmentation. Plast Reconstr Surg. 1989;84:921.
21 Coleman SR. Long-term survival of fat transplants: Controlled demonstrations. Aesthet Plast Surg. 1995;19:421.
22 Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588-595.
23 Carruthers J, Carruthers A, Maberley D. Deep resting glabellar rhytids respond to BTX-A and Hylan B. Dermatol Surg. 2003;29:539-544.
24 Carruthers J, Carruthers A. A prospective, randomized, parallel group study analyzing the effect of BTX-A (Botox) and nonanimal sourced hyaluronic acid (NASHA, Restylane) in combination compared with NASHA (Restylane) alone in severe glabellar rhytids in adult female subjects: Treatment of severe glabellar rhytids with a hyaluronic acid derivative compared with the derivative BTX-A. Dermatol Surg. 2003;29:802-809.
25 Fagien S: Injection and anesthetic techniques with Restylane. Focus on fillers. Inaugural Meeting of the International Filler Board and Faculty Training. The Brown Palace Hotel, Denver, Colorado, July 13, 2003.
26 Lowe NJ, Maxwell CA, Lowe P, Duick MG, Shah K. Hyaluronic acid skin fillers: Adverse reactions and skin testing. J Am Acad Dermatol. 2001;45:930-933.
27 Saylan Z. Facial fillers and their complications. Aesthetic Surg J. 2003;23:221-224.
28 Fagien S. Botox for the treatment of dynamic and hyperkinetic facial lines and furrows: Adjunctive use in facial aesthetic surgery. Plast Reconstr Surg. 2003;112:40S-52S.
29 Hoenig JF, Brink U, Korabiowska M. Severe granulomatous allergic tissue reaction after hyaluronic acid injection in the treatment of facial lines and its surgical correction. J Craniofac Surg. 2003;14:197-200.
30 Toy BR, Frank PJ. Outbreak of Mycobacterium abscessus infection after soft tissue augmentation. Dermatol Surg. 2003;29:971-973.
31 Sklar JA, White SM, Radiance FN. A new soft tissue filler. Dermatol Surg. 2004;30:764-768.
32 Jansen D, Graivier M. Soft tissue substitutes in perioral augmentation. Semin Plast Surg. 2003;17:181-197.
33 Gladstone HB, Morganroth GS: Evaluating calcium hydroxylapatite for facial augmentation. Presented at the Annual American Society for Dermatologic Surgery Meeting, New Orleans, LA, 2003.
34 McGrath MH, Nahai F, Airan L, Born T, Cohen SR, Coleman SR, Fagien S, Graivier M. Tissue fillers in plastic surgery. Roundtables in Plastic Surgery; Part II. Roundtable Discussion with the Experts. St. Louis, MO: Quality Medical Publishing, 2004.
35 Vleggaar D, Bauer U. Facial enhancement and the European experience with Sculptra (poly-L-lactic acid). J Drugs Dermatol. 2004;3:542-547.
36 Moyle GJ, Lysakova L, Brown S, et al. A randomized open-label study of immediate versus delayed polylactic acid injections for the cosmetic management of facial lipoatrophy in persons with HIV infection. HIV Med. 2004;5:82-87.
37 Lemperle G, Gauthier-Hazan N, Lemperle M. PMMA microspheres (Artecoll) for long lasting correction of wrinkles: Refinements and statistical results. Aesthetic Plast Surg. 1998;22:365.
38 Cohen SR, Holmes RE. Artecoll: A long-lasting injectable wrinkle filler material: Report of a controlled, randomized, multicenter clinical trial of 251 patients. Plast Reconstr Surg. 2004;114:964.
39 Fagien S, Born TM. Artecoll: A long-lasting injectable wrinkle filler material: Report of a controlled, randomized, multicenter clinical trial of 251 patients by Cohen SR and Holmes RE. Plast Reconstr Surg [Discussion]. 2004;114:977.
40 Alcalay J, Alkalay R, Gat A, Yorav S. Late-onset granulomatous reaction to Artecoll. Dermatol Surg. 2003;29:859-862.
41 Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27:354.
42 Pollack S. Some new injectable dermal filler materials: Hylaform, Restylane, and Artecoll. J Cutan Med Surg. 1999;3(Suppl 4):S27-S35.
43 Lemperle G, Gauthier-Hazan N, Lemperle M. PMMA-microspheres (Artecoll) for long-lasting correction of wrinkles: Refinements and statistical results. Aesthetic Plast Surg. 1998;22:356-365.
44 Bergeret-Galley C, Latouche X, Illouz Y-G. The value of a new filler material in corrective and cosmetic surgery: DermaLive and DermaDeep. Aesthetic Plast Surg. 2001;25:249-255.
45 Brown LH, Frank PJ. What’s new in fillers? J Drugs Dermatol. 2003;2:250-253.
46 Clark D, Hanke W, Swanson N. Dermal implants: Safety of products injected for soft tissue augmentation. J Am Acad Dermatol. 1989;21:992-998.
47 Spira M, Rosen T. Injectable soft tissue substitutes. Clin Plast Surg. 1993;20:181-188.
48 Rappaport MJ, Vinnik C, Zarem H. Injectable silicone: Cause of facial nodules, cellulites, ulceration and migration. Aesth Plast Surg. 1996;20:267-276.
49 Berman M. Rejuvenation of the upper eyelid complex with autologous fat transplantation. Dermatol Surg. 2000;26:1113-1116.
50 Lambros V: Facial aging. Presentation at the annual meeting of the American Society of Aesthetic Plastic Surgeons, April 30, 2005.
51 Tardy ME, Alex J, Hendrick D. Rejuvenation of the aging brow and forehead. In: Putterman AM, Warren LA, Lampert R, editors. Cosmetic Oculoplastic Surgery: Eyelid, Forehead, and Facial Techniques. Philadelphia: WB Saunders, 1999.
52 Fagien S, Brandt FS. Primary and adjunctive use of botox in facial aesthetic surgery: Beyond the glabella. In: Matarasso A, Matarasso SL, editors. Clinics in Plastic Surgery. Philadelphia: WB Saunders; 2000:127-148.
53 Fagien S. Extended use of botulinum toxin A in facial aesthetic surgery. Aesthet Surg J May/June;. 1998;18:215-219.
54 Carruthers J, Carruthers A. The adjunctive usage of botulinum toxin. Dermatol Surg. 1998;24:1244-1247.
55 Patel MP, Talmor M, Nolan WB. Botox and collagen for glabellar furrows: Advantages of combination therapy. Ann Plast Surg. 2004;52:442-447.