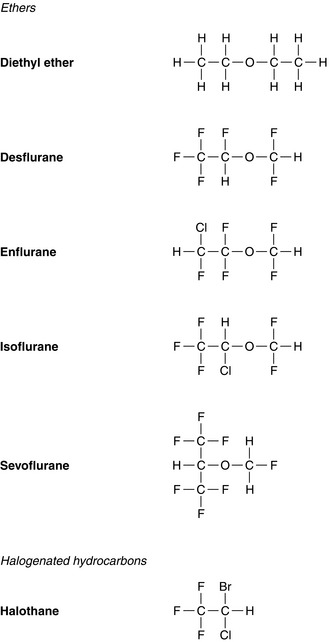
Inhalational Anaesthetic Agents
PROPERTIES OF THE IDEAL INHALATIONAL ANAESTHETIC AGENT
 It should have a pleasant odour, be non-irritant to the respiratory tract and allow pleasant and rapid induction of anaesthesia.
It should have a pleasant odour, be non-irritant to the respiratory tract and allow pleasant and rapid induction of anaesthesia.
 It should possess a low blood/gas solubility, which permits rapid induction of and rapid recovery from anaesthesia.
It should possess a low blood/gas solubility, which permits rapid induction of and rapid recovery from anaesthesia.
 It should be chemically stable in storage and should not interact with the material of anaesthetic circuits or with soda lime.
It should be chemically stable in storage and should not interact with the material of anaesthetic circuits or with soda lime.
 It should be neither flammable nor explosive.
It should be neither flammable nor explosive.
 It should be capable of producing unconsciousness with analgesia and preferably some degree of muscle relaxation.
It should be capable of producing unconsciousness with analgesia and preferably some degree of muscle relaxation.
 It should be sufficiently potent to allow the use of high inspired oxygen concentrations when necessary.
It should be sufficiently potent to allow the use of high inspired oxygen concentrations when necessary.
 It should not be metabolized in the body, be non-toxic and not provoke allergic reactions.
It should not be metabolized in the body, be non-toxic and not provoke allergic reactions.
 It should produce minimal depression of the cardiovascular and respiratory systems and should not interact with other drugs used commonly during anaesthesia, e.g. pressor agents or catecholamines.
It should produce minimal depression of the cardiovascular and respiratory systems and should not interact with other drugs used commonly during anaesthesia, e.g. pressor agents or catecholamines.
 It should be completely inert and eliminated completely and rapidly in an unchanged form via the lungs.
It should be completely inert and eliminated completely and rapidly in an unchanged form via the lungs.
 It should be easy to administer using standard vaporizers.
It should be easy to administer using standard vaporizers.
 It should not be epileptogenic or raise intracranial pressure.
It should not be epileptogenic or raise intracranial pressure.
None of the inhalational anaesthetic agents approaches the standards required of the ideal agent.
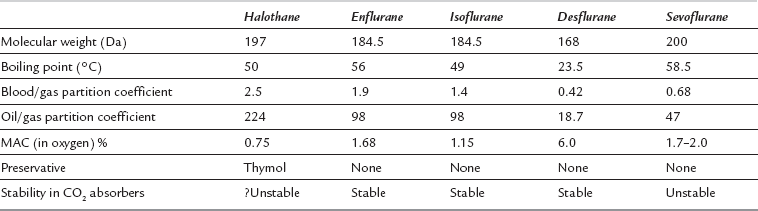
Minimum Alveolar Concentration (MAC)
The MAC values for the anaesthetic agents quoted in Table 2.1 were determined experimentally in humans (volunteers) breathing a mixture of the agent in oxygen. MAC values vary under the following circumstances:
Factors Which Lead to a Reduction in MAC:
 Sedative drugs such as premedication agents, analgesics
Sedative drugs such as premedication agents, analgesics
 Drugs which affect neurotransmitter release such as methyldopa, pancuronium and clonidine
Drugs which affect neurotransmitter release such as methyldopa, pancuronium and clonidine
 Higher atmospheric pressure, as anaesthetic potency is related to partial pressure – e.g. MAC for sevoflurane is 2.0% (2.03 kPa) at a pressure of 1 ata, but 1.0% (still 2.03 kPa) at 2 ata
Higher atmospheric pressure, as anaesthetic potency is related to partial pressure – e.g. MAC for sevoflurane is 2.0% (2.03 kPa) at a pressure of 1 ata, but 1.0% (still 2.03 kPa) at 2 ata
AGENTS IN COMMON CLINICAL USE
Isoflurane
Uptake and Distribution
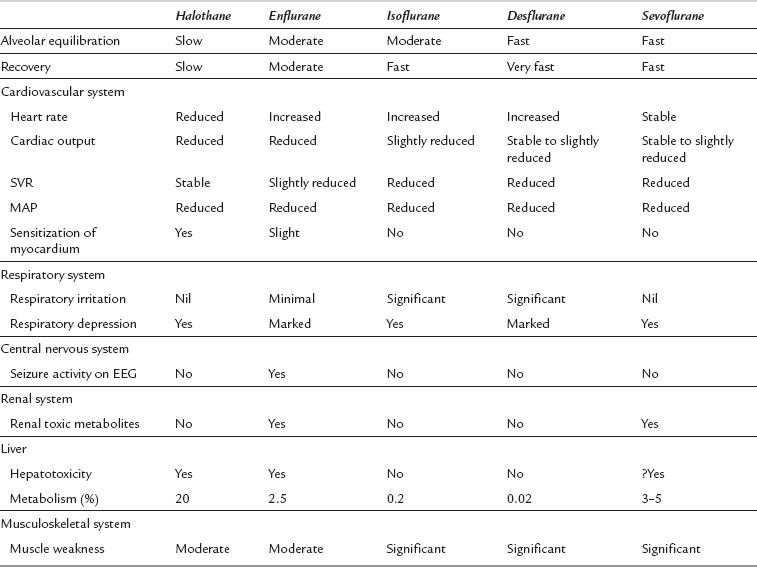
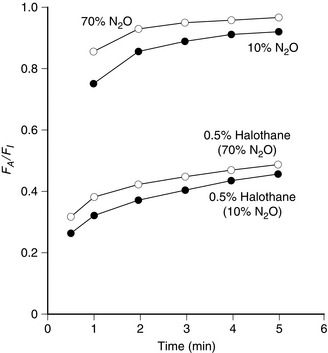
Isoflurane has a low blood/gas solubility of 1.4 and thus alveolar concentrations equilibrate fairly rapidly with inspired concentrations. The alveolar (or arterial) partial pressure of isoflurane increases to 50% of the inspired partial pressure within 4–8 min, and to 60% by 15 min (Fig. 2.2). However, the rate of induction is limited by the pungency of the vapour and in clinical practice may be no faster than that achieved with halothane. The incidence of coughing or breath-holding on induction is significantly greater with isoflurane than with halothane. It is not an ideal agent to use for inhalational induction. The rate of recovery is slower than that associated with desflurane or sevoflurane, but more rapid than after administration of halothane (Fig. 2.3).
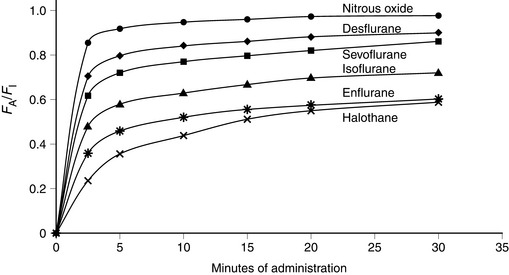
FIGURE 2.2 Ratio of alveolar (FA) to inspired (FI) fractional concentration of nitrous oxide, desflurane, sevoflurane, isoflurane, enflurane and halothane in the first 30 min of anaesthesia. The plot of FA/FI expresses the rapidity with which alveolar concentration equilibrates with inspired concentration. It is most rapid for agents with a low blood/gas partition coefficient.
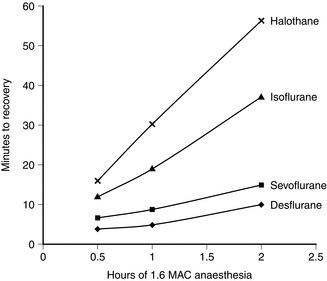
FIGURE 2.3 Rapidity of recovery from anaesthesia is inversely proportional to the solubility of the anaesthetic: the most rapid recovery is with the least soluble anaesthetic (desflurane). The difference is amplified by duration of anaesthesia. Note that the difference in time of recovery between the least (desflurane) and most soluble anaesthetic (halothane) is greater after 2 h of anaesthesia than after 0.5 h of anaesthesia.
Respiratory System
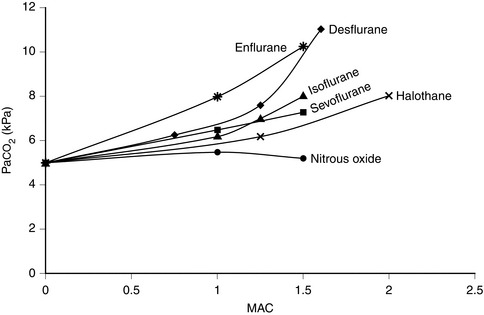
In common with other modern volatile agents, it causes dose-dependent depression of ventilation (Fig. 2.4); there is a decrease in tidal volume but an increase in ventilatory rate in the absence of opioid drugs. Isoflurane causes some respiratory irritation. This makes inhalational induction with isoflurane difficult.
Cardiovascular System
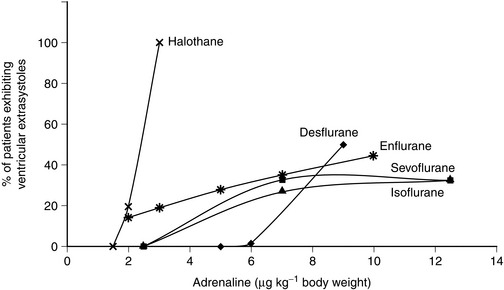
In vitro, isoflurane is a myocardial depressant, but in clinical use there is less depression of cardiac output than with halothane or enflurane (Fig. 2.5). Systemic hypotension occurs predominantly as a result of a reduction in systemic vascular resistance (Figs 2.6, 2.7). Arrhythmias are uncommon and there is little sensitization of the myocardium to catecholamines (Fig. 2.8).
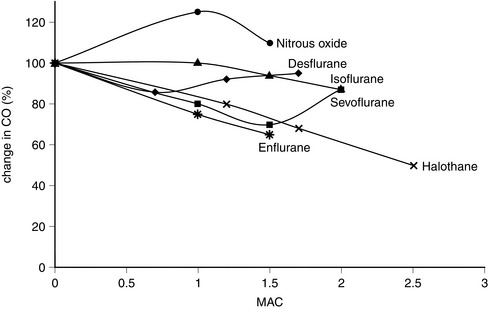
FIGURE 2.5 Comparative effects of nitrous oxide, isoflurane, halothane, enflurane, desflurane and sevoflurane on cardiac output (CO) in healthy volunteers.
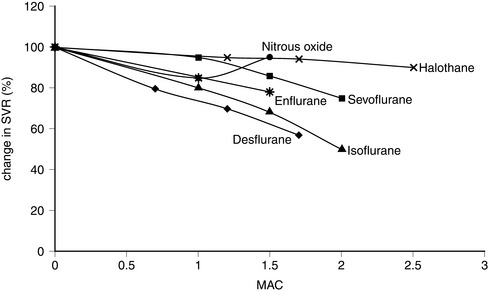
FIGURE 2.6 Comparative effects of nitrous oxide, halothane, enflurane, isoflurane, sevoflurane and desflurane on systemic vascular resistance (SVR) in healthy volunteers.
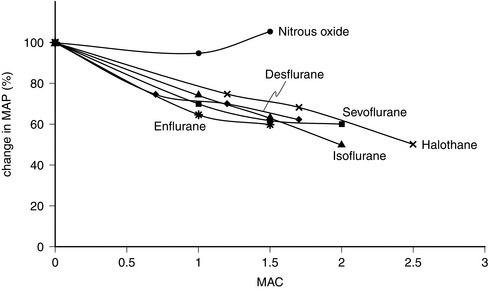
FIGURE 2.7 Comparative effects of nitrous oxide, halothane, enflurane, isoflurane, sevoflurane and desflurane on mean arterial pressure (MAP) in healthy volunteers.
Desflurane
Uptake and Distribution
Desflurane has a blood/gas partition coefficient of 0.42, almost the same as that of nitrous oxide. The rate of equilibration of alveolar with inspired concentrations of desflurane is virtually identical to that for nitrous oxide (Fig. 2.2). Induction of anaesthesia is therefore extremely rapid in theory but limited somewhat by its pungent nature. However, it is possible to alter the depth of anaesthesia very rapidly and the rate of recovery of anaesthesia is faster than that following any other volatile anaesthetic agent (Fig. 2.3).
Respiratory System
Desflurane causes respiratory depression to a degree similar to that of isoflurane up to a MAC of 1.5. It increases PaCO2 (Fig. 2.4) and decreases the ventilatory response to imposed increases in PaCO2. It is irritant to the upper respiratory tract, particularly at concentrations greater than 6%. It is therefore not recommended for gaseous induction of anaesthesia because it causes coughing, breath-holding and laryngospasm.
Cardiovascular Effects
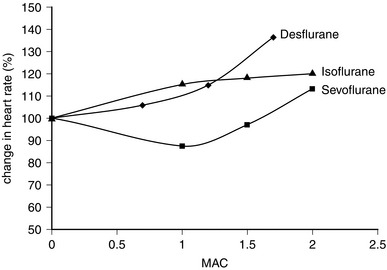
Desflurane appears to have two distinct actions on the cardiovascular system. Firstly, its main actions are those which are similar to isoflurane: dose-related decreases in systemic vascular resistance, myocardial contractility and mean arterial pressure (Figs 2.5–2.7). Heart rate is unchanged at lower steady-state concentrations, but increases with higher concentrations (Fig. 2.9). Addition of nitrous oxide maintains heart rate unchanged. Cardiac output tends to be maintained as with isoflurane. The second cardiovascular action occurs when its inspired concentration is increased rapidly to greater than 1 MAC. In the absence of premedicant drugs, this increases sympathetic activity, leading to increased heart rate and arterial pressure. Experimental studies in animals have not detected a coronary steal phenomenon. Desflurane, in common with isoflurane and sevoflurane, does not sensitize the myocardium to catecholamines (Fig. 2.8).
Musculoskeletal System
Therefore, in summary, desflurane offers some advantages over other agents:
 it has a low blood solubility; therefore it offers more precise control of maintenance of anaesthesia and rapid recovery.
it has a low blood solubility; therefore it offers more precise control of maintenance of anaesthesia and rapid recovery.
 it is minimally biodegradable and therefore non-toxic to the liver and kidney.
it is minimally biodegradable and therefore non-toxic to the liver and kidney.
However, it has some significant drawbacks:
 it cannot be used for inhalational induction because of its irritant effects on the airway.
it cannot be used for inhalational induction because of its irritant effects on the airway.
 it causes tachycardia at higher concentrations.
it causes tachycardia at higher concentrations.
 it requires a special vaporizer. Although the TEC-6 vaporizer is reasonably easy to use, it is more complex than the more conventional vaporizers and the potential for failure may be higher.
it requires a special vaporizer. Although the TEC-6 vaporizer is reasonably easy to use, it is more complex than the more conventional vaporizers and the potential for failure may be higher.
Sevoflurane
Uptake and Distribution
Sevoflurane has a low blood/gas partition coefficient and therefore the rate of equilibration between alveolar and inspired concentrations is faster than that for halothane or isoflurane but slower than that for desflurane (Fig. 2.2). It is non-irritant to the upper respiratory tract and therefore the rate of induction of anaesthesia should be faster than that with any of the other agents.
Because of its higher partition coefficients in vessel-rich tissues, muscle and fat than corresponding values for desflurane, the rate of recovery is slower than that after desflurane anaesthesia (Fig. 2.3).
Respiratory System
The drug is non-irritant to the upper respiratory tract. It produces dose-dependent ventilatory depression, reduces respiratory drive in response to hypoxaemia and increases carbon dioxide partial pressure to a similar degree to other volatile agents (Fig. 2.4). The ventilatory depression associated with sevoflurane may result from a combination of central depression of medullary respiratory neurones and depression of diaphragmatic function and contractility. It relaxes bronchial smooth muscle but not as effectively as halothane.
Cardiovascular System
The properties of sevoflurane are similar to those of isoflurane, with slightly smaller effects on heart rate (Fig. 2.9) and less coronary vasodilatation. It decreases arterial pressure (Fig. 2.7) mainly by reducing peripheral vascular resistance (Fig. 2.6), but cardiac output is well maintained over the normal anaesthetic maintenance doses (Fig. 2.5). There is mild myocardial depression resulting from its effect on calcium channels. Sevoflurane does not differ from isoflurane in its sensitization of the myocardium to exogenous catecholamines (Fig. 2.8). It is a less potent coronary arteriolar dilator and does not appear to cause coronary steal. Sevoflurane is associated with a lower heart rate and therefore helps to reduce myocardial oxygen consumption.
Halothane
Uptake and Distribution
Halothane has a blood/gas solubility coefficient of 2.5, which is the highest of all the modern agents. It is not irritant to the airway and therefore inhalational induction with halothane is relatively fast compared with either desflurane or isoflurane. However, it may take at least 30 min for the alveolar inspired concentration to reach 50% of the inspired concentration (Fig. 2.2); this is slower than for the other agents. As with all the volatile agents, it is customary to use the technique of ‘over-pressure’ and induce halothane anaesthesia with concentrations two to three times higher than the MAC value; the inspired concentration is reduced when a stable level of anaesthesia has been achieved. The MAC of halothane in oxygen is approximately 1.1% in the neonate, 0.95% in the infant, 0.9% at 1–2 years, 0.75% at 40 years (0.29 in 70% nitrous oxide) and 0.65% at 80 years.
Recovery from halothane anaesthesia is slower than with the other agents because of its high blood/gas solubility, and recovery is prolonged with increasing duration of anaesthesia (Fig. 2.3).
Respiratory System
Halothane is non-irritant and pleasant to breathe during induction of anaesthesia. There is rapid loss of pharyngeal and laryngeal reflexes and inhibition of salivary and bronchial secretions. In the unpremedicated subject, halothane anaesthesia is associated with an increase in ventilatory rate and reduction in tidal volume. PaCO2 increases as the depth of halothane anaesthesia increases (Fig. 2.4).
Cardiovascular System
Halothane is a potent depressant of myocardial contractility and myocardial metabolic activity as a result of inhibition of glucose uptake by myocardial cells. During controlled ventilation, halothane anaesthesia is associated with dose-related depression of cardiac output (by decrease in myocardial contractility) with little effect on peripheral resistance (Figs 2.5, 2.6). Thus, there is a reduction in arterial pressure (Fig. 2.7) and an increase in right atrial pressure. In spontaneously breathing patients, some of these effects may be offset by a small increase in PaCO2 which leads to a reduction in systemic vascular resistance and a shift in cardiac output back towards baseline values as a result of indirect sympathoadrenal stimulation.
The depressant effects of halothane on cardiac output are augmented in the presence of β-blockade.
 increased myocardial excitability augmented by the presence of hypercapnia, hypoxaemia or increased circulating catecholamines
increased myocardial excitability augmented by the presence of hypercapnia, hypoxaemia or increased circulating catecholamines
 avoid hypoxaemia and hypercapnia
avoid hypoxaemia and hypercapnia
 avoid concentrations of Adrenaline greater than 1 in 100 000
avoid concentrations of Adrenaline greater than 1 in 100 000
 avoid a dosage in adults exceeding 10 ml of 1 in 100 000 Adrenaline in 10 min (i.e. 100 μg) or 30 mL h–1 (300 μg).
avoid a dosage in adults exceeding 10 ml of 1 in 100 000 Adrenaline in 10 min (i.e. 100 μg) or 30 mL h–1 (300 μg).
Approximately 20% of patients breathing 1.25 MAC of halothane and who receive subcutaneous infiltration of 2 μg kg–1 Adrenaline exhibit ventricular ectopics. This increases to 100% of patients receiving 2.5–3 μg kg–1 (Fig. 2.8).
Gastrointestinal Tract
Gastrointestinal motility is inhibited. Postoperative nausea and vomiting are seldom severe.
Halothane-Associated Hepatic Dysfunction
 a careful anaesthetic history should be taken to determine previous exposure and any previous reaction to halothane.
a careful anaesthetic history should be taken to determine previous exposure and any previous reaction to halothane.
 repeated exposure to halothane within a period of 3 months should be avoided unless there are overriding clinical circumstances.
repeated exposure to halothane within a period of 3 months should be avoided unless there are overriding clinical circumstances.
 a history of unexplained jaundice or pyrexia after previous exposure to halothane is an absolute contraindication to its future use in that patient.
a history of unexplained jaundice or pyrexia after previous exposure to halothane is an absolute contraindication to its future use in that patient.
In summary, halothane is a useful inhalational anaesthetic agent. Its main advantages are:
Comparison of Isoflurane, Desflurane, Sevoflurane and Halothane
The rate of equilibration of alveolar with inspired concentrations is related to blood/gas solubility. The rate of uptake of desflurane is faster than that of any of the other volatile agents and similar to that of nitrous oxide (Fig. 2.2). Despite its low blood/gas solubility, the rate of induction of anaesthesia with desflurane (and isoflurane) may be reduced because of the pungent odour compared with the more pleasant odours of sevoflurane and halothane. Sevoflurane provides a smooth rapid induction and it has largely replaced halothane for induction in children. Potency, on the other hand, is related to the lower oil/water solubility. Sevoflurane and desflurane are less potent than the older agents, as reflected by their higher MAC values.
On recovery from anaesthesia, the rate of elimination of desflurane is faster than that for the other agents (Fig. 2.3).
Respiratory System
All inhalational agents cause dose-related respiratory depression. This results in reduced tidal volume, increased respiratory rate and reduced minute ventilation. PaCO2 increases (Fig. 2.4). In unstimulated volunteers, desflurane causes greater ventilatory depression than isoflurane, halothane or sevoflurane. Nitrous oxide does not cause hypercapnia. Thus the reduction in inspired volatile anaesthetic concentration permitted by addition of nitrous oxide is associated with less ventilatory depression. In addition, surgical stimulation is responsible for considerable antagonism of ventilatory depression during anaesthesia and PaCO2 does not normally reach the values shown in Figure 2.4 during surgery.
Halothane and, to a lesser extent, sevoflurane, causes bronchodilation.
Cardiovascular System
The data in Figures 2.5–2.7 and 2.9 were derived from studies in volunteers who were not subjected to surgical stimulation and in whom artificial ventilation was used to achieve normocapnia.
AGENTS IN OCCASIONAL USE
Uptake and Distribution
Enflurane has a blood/gas solubility coefficient of 1.9, which is between that of halothane and isoflurane. Thus, induction of and recovery from anaesthesia are faster than halothane but slower than isoflurane, desflurane and sevoflurane (Fig. 2.2).
Respiratory System
In common with all other volatile anaesthetic agents, enflurane causes dose-dependent depression of alveolar ventilation with a reduction in tidal volume and an increase in ventilatory rate in the unpremedicated subject. This results in an increase in arterial PCO2 (Fig. 2.4).
Cardiovascular System
Enflurane causes dose-dependent depression of myocardial contractility, leading to a reduction in cardiac output (Fig. 2.5). In association with a small reduction in systemic vascular resistance, this leads to a dose-dependent reduction in arterial pressure (Figs 2.6, 2.7). Because enflurane (unlike halothane) has no central vagal effects, hypotension leads to reflex tachycardia.
Enflurane anaesthesia is associated with a much smaller incidence of arrhythmias than halothane and much less sensitization of the myocardium to catecholamines, either endogenous or exogenous (Fig. 2.8).
Diethyl Ether
Central Nervous System
In common with all general anaesthetic agents, there is depression of cortical activity. Because induction of anaesthesia with ether is so slow, the classical stages of anaesthesia are seen; these are described in detail on page 448 and in Figure 21.2.
Respiratory System
Ether stimulates ventilation and minute volume is maintained with increasing depth of anaesthesia until surgical anaesthesia is achieved; thereafter, there is a gradual diminution in alveolar ventilation as plane 4 of stage 3 is approached (see Chapter 21).
ANAESTHETIC GASES
Physical Properties
Nitrous oxide is not flammable but it supports combustion of fuels in the absence of oxygen.
Pharmacology
Nitrous oxide has a low blood/gas solubility coefficient (0.47 at 37 °C) and therefore the rate of equilibration of alveolar with inspired concentrations is very fast (Fig. 2.2).
Nitrous oxide does not undergo metabolism in the body and is excreted unchanged.
The Concentration Effect
 the higher the inspired concentration of nitrous oxide, the greater is the concentrating effect on the nitrous oxide remaining in the alveolus.
the higher the inspired concentration of nitrous oxide, the greater is the concentrating effect on the nitrous oxide remaining in the alveolus.
 at high inspired concentrations of nitrous oxide, the reduction in alveolar gas volume causes an increase in PACO2. Equilibration with pulmonary capillary blood results in an increase in PaCO2.
at high inspired concentrations of nitrous oxide, the reduction in alveolar gas volume causes an increase in PACO2. Equilibration with pulmonary capillary blood results in an increase in PaCO2.
The result of the concentration effect on equilibration of nitrous oxide is illustrated in Figure 2.10.
The Second Gas Effect
When nitrous oxide is administered in a high concentration with a second anaesthetic agent, e.g. sevoflurane, the reduction in gas volume in the alveoli caused by absorption of nitrous oxide increases the alveolar concentration of sevoflurane, thereby augmenting the rate of equilibration with inspired gas. This is illustrated in the lower part of Figure 2.10. The second gas effect results also in small increases in PAO2 and PaO2.
Systemic Effects
Respiratory System: Nitrous oxide decreases tidal volume and increases respiratory rate and hence maintains minute ventilation. It reduces the ventilatory response to hypoxaemia and hypercapnia. It depresses tracheal mucociliary flow and neutrophil chemotaxis and may increase the incidence of post-operative respiratory complications.
Cardiovascular System: Nitrous oxide is a direct myocardial depressant, but in the normal individual this effect is antagonized by indirectly mediated sympathoadrenal stimulation (effects similar to those produced by carbon dioxide). Thus, healthy patients exhibit little change in the cardiovascular system during nitrous oxide anaesthesia. However, in patients with pre-existing high levels of sympathoadrenal activity and poor myocardial contractility, the administration of nitrous oxide may cause reductions in cardiac output and arterial pressure. For this reason (in addition to avoidance of the risk of doubling the size of air emboli), nitrous oxide is avoided in some centres during anaesthesia for cardiac surgery. Pulmonary vascular resistance is increased due to constriction of the pulmonary vascular smooth muscles and this may lead to increased right atrial pressure. For this reason, nitrous oxide is best avoided in patients with pulmonary hypertension.
Side-Effects of Nitrous Oxide
Postoperative Nausea and Vomiting: Nitrous oxide has been implicated in the aetiology of postoperative nausea and vomiting. The underlying mechanism for this is not well understood but it may be multifactorial involving changes in middle ear pressures, bowel distension and activation of dopaminergic neurones.
Diffusion Hypoxia: At the end of an anaesthetic, when the inspired gas mixture is changed from nitrous oxide/oxygen to nitrogen/oxygen, hypoxaemia may occur as the volume of nitrous oxide diffusing from mixed venous blood into the alveolus is greater than the volume of nitrogen taken up from the alveolus into pulmonary capillary blood (the opposite of the concentration effect). Thus, the concentration of gases in the alveolus is diluted by nitrous oxide, leading to reductions in PaO2 and PaCO2. In the healthy individual, diffusion hypoxia is relatively transient, but may last for up to 10 min at the end of anaesthesia; the extent of reduction in PaO2 may be of the order of 0.5–1.5 kPa. Administration of oxygen during this period is essential in order to avoid desaturation.
Effect on Closed Gas Spaces: When blood containing nitrous oxide equilibrates with closed air-containing spaces inside the body, the volume of nitrous oxide that diffuses into the cavity exceeds the volume of nitrogen diffusing out. Thus, in compliant spaces, such as the bowel lumen or the pleural or peritoneal cavities, there is an increase in volume of the space. If the space cannot expand (e.g. sinuses, middle ear) there is an increase in pressure. In the middle ear, this may cause problems with surgery on the tympanic membrane. When nitrous oxide is administered in a concentration of 75%, the volume of a cavity may increase to as much as three to four times the original volume within 30 min. If an air embolus occurs in a patient who is breathing nitrous oxide, equilibration with the gas bubble leads to expansion of the embolus within seconds; the volume of the embolus may double within a very short period of time. A similar problem arises during prolonged procedures where nitrous oxide diffuses into the cuff of the tracheal tube and may increase the pressure exerted on the tracheal mucosa. Either avoiding the use of nitrous oxide or inflating the cuff with saline or nitrous oxide may prevent this.
Effects on Blood and the Nervous System: Nitrous oxide inhibits the enzyme methionine synthetase which results in interference with DNA synthesis in both leucocytes and erythrocytes. It oxidizes the cobalt atom in vitamin B12 and interferes with folic acid metabolism. Prolonged exposure may cause agranulocytosis and bone marrow aplasia. Exposure of patients to nitrous oxide for 6 h or longer may result in megaloblastic anaemia. Occupational exposure to nitrous oxide may result in myeloneuropathy. This condition is similar to subacute combined degeneration of the spinal cord and has been reported in some dentists and also in individuals addicted to inhalation of nitrous oxide. Inhibition of methionine synthesis prevents production of methionine and tetrahydrofolate. Methionine is a precursor of S-adenosylmethionine which is incorporated into myelin and its absence leads to subacute combined degeneration of the cord.
Teratogenic Changes: Teratogenic changes have been observed in pregnant rats exposed to nitrous oxide for prolonged periods. The mechanism of this teratogenicity is not clear but it is thought to be multifactorial and may partially involve activation of the α1-adrenergic neurones. There is no evidence that similar effects occur in humans, but it has been suggested that nitrous oxide should be avoided in the first trimester of pregnancy.
Environment: Nitrous oxide is a greenhouse gas and it is 200–300 times more effective in trapping heat than carbon dioxide and therefore may contribute to global warming. However the contribution to the greenhouse effect of anaesthetic use of nitrous oxide is very small as anaesthesia only accounts for < 1% of total nitrous oxide emission.
Other Gases Used During Anaesthesia
Manufacture: Oxygen is manufactured commercially by fractional distillation of liquid air. Before liquefaction of air, carbon dioxide is removed and liquid oxygen and nitrogen separated by means of their different boiling points (oxygen, −183 °C; nitrogen, −195 °C).
Oxygen supports combustion, although the gas itself is not flammable.
Oxygen Concentrators: Oxygen concentrators produce oxygen from ambient air by absorption of nitrogen onto some types of alumina silicates. Oxygen concentrators are useful both in hospitals and in long-term domestic use in remote areas, in developing countries and in military surgery. The gas produced by oxygen concentrators contains small quantities of inert gases (e.g. argon) which are harmless.
Fire: Oxygen supports combustion of fuels. An increase in the concentration of oxygen from 21% up to 100% causes a progressive increase in the rate of combustion with the production of either conflagrations or explosions with appropriate fuels.
Cardiovascular depression: An increase in PaO2 leads to direct vasoconstriction, which occurs in peripheral vasculature and also in the cerebral, coronary, hepatic and renal circulations. This effect is not manifest at a PaO2 of less than 30 kPa and assumes clinical importance only at hyperbaric pressures of oxygen. Hyperbaric pressures of oxygen also cause direct myocardial depression. In patients with severe cardiovascular disease, elevation of PaO2 from the normal physiological range to 80 kPa may produce clinically evident cardiovascular depression.
Absorption atelectasis: Because oxygen is highly soluble in blood, the use of 100% oxygen as the inspired gas may lead to absorption atelectasis in lung units distal to the site of airway closure. Absorption collapse may occur in as short a time as 6 min with 100% oxygen, and 60 min with 85% oxygen. Thus, even small concentrations of nitrogen exert an important splinting effect and this accounts for current avoidance of 100% oxygen in estimation of pulmonary shunt ratio  in patients with lung pathology, in whom a greater degree of airway closure would result in greater areas of alveolar atelectasis. Absorption atelectasis has been demonstrated in volunteers breathing 100% oxygen at FRC; atelectasis is evident on chest radiography for a period of at least 24 h after exposure.
in patients with lung pathology, in whom a greater degree of airway closure would result in greater areas of alveolar atelectasis. Absorption atelectasis has been demonstrated in volunteers breathing 100% oxygen at FRC; atelectasis is evident on chest radiography for a period of at least 24 h after exposure.
Pulmonary oxygen toxicity: Chronic inhalation of a high inspired concentration of oxygen may result in the condition termed pulmonary oxygen toxicity (Lorrain-Smith effect), which is manifest by hyaline membranes, thickening of the interlobular and alveolar septa by oedema and fibroplastic proliferation. The clinical and radiological appearance of these changes is almost identical to that of the acute respiratory distress syndrome. The biochemical mechanisms underlying pulmonary oxygen toxicity probably include:
Central nervous system oxygen toxicity: Convulsions, similar to those of grand mal epilepsy, occur during exposure to hyperbaric pressures of oxygen.
Retrolental fibroplasia: Retrolental fibroplasia (RLF) is the result of oxygen-induced retinal vasoconstriction, with obliteration of the most immature retinal vessels and subsequent new vessel formation at the site of damage in the form of a proliferative retinopathy. Leakage of intravascular fluid leads to vitreoretinal adhesions and even retinal detachment. Retrolental fibroplasia occurs in infants exposed to hyperoxia in the paediatric intensive care unit and is related not to the FIO2 per se, but to an elevated retinal artery PO2. It is not known what the threshold of PaO2 is for the development of retinal damage, but an umbilical arterial PO2 of 8–12 kPa (60–90 mmHg) is associated with a very low incidence of RLF and no signs of systemic hypoxia. It should be stressed, however, that there are many factors involved in the development of RLF in addition to arterial hyperoxia.
Carbon Dioxide
Carbon dioxide is obtained commercially from four sources:
 as a byproduct of fermentation in brewing of beer
as a byproduct of fermentation in brewing of beer
 as a byproduct of the manufacture of hydrogen
as a byproduct of the manufacture of hydrogen
 by heating magnesium and calcium carbonate in the presence of their oxides
by heating magnesium and calcium carbonate in the presence of their oxides
Physiological Data: Variations in cardiovascular state induced by alterations in PaCO2 may be similar to those induced by pain or lightness of anaesthesia and the differential diagnosis is described in Table 40.2. The cardiovascular effects of CO2 are summarized in Table 2.3.
TABLE 2.3
| Arterial pressure Cardiac output Heart rate |
Biphasic response. Progressive increases in these variables with increase in PaCO2 up to approximately 10 kPa as a result of indirect sympathetic stimulation. At very high PaCO2, these variables decrease as a result of myocardial depression |
| Skin Coronary circulation Cerebral circulation Gastrointestinal circulation |
Dilatation with hypercapnia Constriction with hypocapnia |
Uses of Carbon Dioxide in anaesthesia: The use of carbon dioxide in anaesthetic practice has declined as appreciation of its disadvantages has increased. Because of reports of accidental administration of high concentrations of CO2, it is not available on most modern anaesthetic machines. Carbon dioxide is used mainly by surgeons for insufflation during laparoscopic procedures.
Xenon
Physical Properties: Xenon is a non-explosive, colourless and odourless gas. It is non-flammable and does not support combustion. Its blood/gas partition coefficient of 0.14–0.2 is lower than that of nitrous oxide (0.47). It therefore provides rapid induction of and recovery from anaesthesia. Xenon is more potent than nitrous oxide, with a MAC of 70%. It does not undergo biotransformation and it is harmless to the ozone layer.