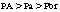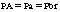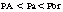20 Inhalation anesthesia
Adjustable Positive-Pressure Relief (APR) Valve: Used for release of excessive gas on the circle system of an anesthesia machine.
Amnesia: A component of anesthesia in which the patient is unable to recall the events that occurred during the administration of the inhalational anesthetic.
Analgesia: A component of anesthesia in which the patient is unable to experience pain.
CO2 Absorption Canisters: Located in a circle system on an anesthesia machine that clears rebreathed gas that contains carbon dioxide by passing through a canister containing a chemical carbon dioxide absorbent.
Delirium: A portion of a stage of anesthesia in which the patient has a transient disturbance during a loss of consciousness accompanied by a change in cognition that has a fluctuating course.
Diffusion Hypoxia: Sometimes referred to as the Fink phenomenon; refers to the rapid exit of nitrous oxide and thus partial reduction of the percentage of oxygen that can be inhaled during the immediate (first 4 to 5 minutes) emergence phase of recovery from anesthesia. Supplemental oxygen and the use of the modified stir-up regime negate this effect of nitrous oxide on emergence.
Effective Dose (ED): The dose of a drug necessary to produce a certain effect in a certain percentage of patients. For example, the ED50 is the term for when a drug produces a particular effect in 50% of patients.
Hypnosis: A component of anesthesia in which the patient becomes unconscious.
Inhalation Anesthesia: Anesthetic substances, in either volatile or gaseous form, that are inhaled via an anesthesia machine.
Lacrimation: Tears from the lacrimal glands on the medial side of the tissue surrounding the eyes.
Minimal Alveolar Concentration (MAC): A measure of potency of inhalation anesthetic agents; occurs when the equilibrium end-tidal anesthetic concentrations, expressed as a fraction of 1 atm, prevent movement in response to surgical skin incision in 50% of human subjects.
Muscle Relaxation: A component of anesthesia in which the patient has reduced tension of the skeletal muscle.
Nociception: A component of anesthesia in which the patient is unable to sense pain.
Scavenger System: Used to reduce exposure to escaping gases from the anesthesia machine; a waste gas suction tube (scavenger) is connected to the adjustable positive-pressure relief valve and anesthesia ventilator relief valve, and the gases are then vented to the outside atmosphere via an operating room suction system.
Solubility Coefficient: The ratio of the concentration of an anesthetic in blood or other tissue to that in a gas phase when the two are in equilibrium.
Sympatholysis: A component of anesthesia in which the patient is blocked from having an autonomic response to nociceptive (painful) stimuli.
Vaporizer: A device on the anesthesia machine that converts liquid anesthetics into metered amounts of vapor that are added to the fresh gas mixture to produce a known concentration of the vaporized form of the inhalational anesthetic agent.
The inhalation anesthetic agents in use today have survived the many examinations of research, and clinically they have been shown to add significant safety factors and improved outcomes for perianesthesia patients. Research continues in this area; for example, xenon is under evaluation as an inhalation anesthetic agent.1 Other volatile agents are undergoing evaluation, all in an effort to find the inhalational agent that best represents all the facets of anesthesia, such as muscle relaxation, sedation, analgesia, amnesia, with minimal effects on the major organ systems of the body.
Basic concepts
Evolution of the signs and stages of anesthesia
The five components of anesthesia are hypnosis, analgesia, muscle relaxation, sympatholysis, and amnesia. In the past, when diethyl ether was the primary general anesthetic, assessment of anesthetic depth with the signs and stages of anesthesia was simple; the patient could be monitored by assessing the pupils, respiratory activity, muscle tone, and various reflexes. The ether signs and stages were devised to provide a means of assessing the depth of anesthesia. The first three stages were described by Plomley in 1847; 1 year later, John Snow added a fourth stage: overdose.2 During World War I, Guedel more accurately defined and described the signs and stages of anesthesia. A graphic representation of these signs and stages is provided in Fig. 20-1.
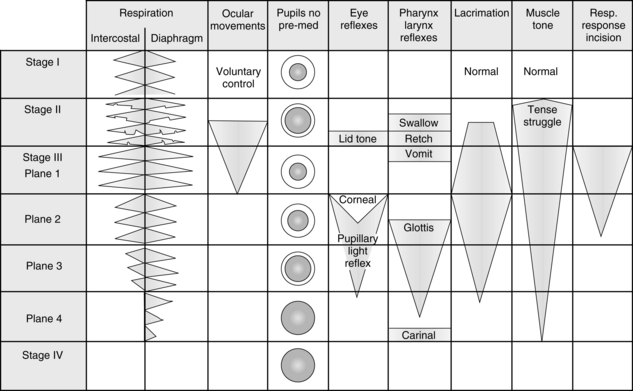
FIG. 20-1 Signs and reflex reactions of stages of anesthesia.
(Adapted from Gillespie NA: Signs of anesthesia, Anesth Analg 22:275, 1943.)
Stage I begins with the initiation of anesthesia and ends with the loss of consciousness. It is commonly called the stage of analgesia. This stage has been described as the lightest level of anesthesia and represents sensory and mental depression. Stage I is the level of anesthesia used with nitrous oxide. Patients are able to open their eyes on command, breathe normally, maintain protective reflexes, and tolerate mild painful stimuli.
Stage III is the stage of surgical anesthesia. With ether anesthesia, this stage is defined as lasting from the onset of a regular pattern of breathing to the cessation of respiration. At this stage of anesthesia, response to surgical incision is absent. The modern concept of minimal alveolar concentration (MAC) is predicated in part with the signs and stages of surgical anesthesia. MAC is exceeded by a factor of 1.3 in stage III because most patients do not respond to surgical incision at this level of anesthesia. Patients who receive 1.3 MAC anesthesia3 have a depression in all elements of nervous system function—that is, sensory depression, loss of recall, reflex depression, and some skeletal muscle relaxation. From this point, with the modern anesthetics, increased MAC results in further respiratory, cardiovascular, and central nervous system (CNS) depression. The difficulty is that each of the newer agents affects the clinical signs, such as blood pressure, differently. Consequently, monitoring of the level of anesthesia depends on the particular properties of each agent.
Most surgical procedures in which ether anesthesia was used were performed at this stage of anesthesia, which is divided into four planes.4 Plane 1 is entered when the lid reflex is abolished and respiration becomes regular. During this plane, the vomiting reflex is gradually abolished. The nurse working in the PACU must know that swallowing, retching, and vomiting reflexes tend to disappear in that order during induction and reappear in the same order during emergence from anesthesia.
Some of the more reliable indicators of depth of anesthesia for the more modern inhalation anesthetics include changes in breathing pattern, eye movement, lacrimation, and muscle tone. Because the ventilation is under autonomic control, it is the most sensitive indicator of depth of anesthesia. In the PACU, a patient who uses diaphragmatic ventilation without the intercostal muscles should be considered to be in surgical anesthesia. As the ventilatory pattern returns to a more normal rate, rhythm, and pattern, the patient can be considered to be in light anesthesia and about to have total emergence. Eye movement as opposed to pupillary size is a good indicator of anesthetic depth. Light anesthesia is present with eye movement. Deeper anesthesia is present when the eyes are close together in a cross-eyed position. Lacrimation does not occur during surgical anesthesia when a patient receives desflurane (Suprane), isoflurane (Forane), or sevoflurane (Ultane). Conversely, if a patient received one of those drugs and has tearing, light anesthesia can be considered to be present. As the depth of anesthesia is increased, the amount of muscle tone decreases. Therefore, if a patient in the PACU lacks muscle tone, especially in the jaw and abdomen, the patient should be considered to be in a surgical depth of anesthesia. With the assessment of the degree of muscle tone, the perianesthesia nurse must critically assess the degree of reversal of skeletal muscle relaxants (see Chapter 23) before determining the depth of anesthesia with the criterion of muscle tone. Finally, because the determinants of anesthesia depth have such a high degree of variability, all possible assessment tools should be incorporated into the care of the patient in the PACU. The bottom line is constant vigilance of the patient’s physiologic parameters during emergence from anesthesia and the institution of appropriate nursing interventions based on an ongoing assessment.
Pharmacokinetics of inhalation anesthetics
The pharmacokinetics of inhalation anesthetics involve uptake, distribution, metabolism, and elimination. Basically, this involves a series of partial pressure gradients starting in the anesthesia machine to the patient’s brain for induction, and vice versa for emergence. The object of anesthesia is a constant and optimal partial pressure in the brain. The key to attainment of anesthesia is the alveolar partial pressure (PA) in equilibrium with the arterial partial pressure (Pa) and brain partial pressure (Pbr) of the inhaled anesthetic.4 The partial pressure of an inhalation anesthetic in the brain is used to determine the depth of anesthesia. The more potent the anesthetic, the lower the partial pressure of the agent needed to produce a certain depth of anesthesia.
Movement of inhalation anesthetic from alveoli to arterial blood
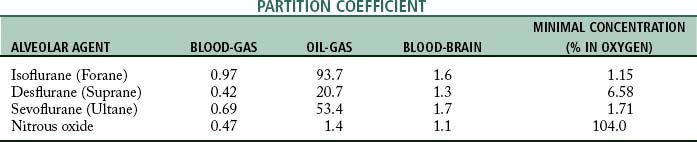
The movement of the inhalation anesthetic agent from the alveoli to the arterial blood depends on the blood-gas partition coefficient and the cardiac output. The rate at which the anesthetic is taken up by the blood and tissues is governed in part by the solubility of the agent in blood. This is expressed as the blood-gas partition coefficient, or the Oswald solubility coefficient, and is defined as the ratio of the concentration of an anesthetic in blood to that in a gas phase when the two are in equilibrium (Table 20-1). This concept is difficult to understand because the more soluble the anesthetic agent is, the slower the agent is in producing anesthesia. This effect is because the blood serves as a reservoir and a large volume of the agent must be introduced to attain an equilibrium between the blood partial pressure and the partial pressure in the lungs.
The blood conveys the anesthetic agent to the tissues. Consequently, a normal cardiac output is needed for facilitation of the movement of the inhalation anesthetic through the tissues to the brain. The partial pressure increases most rapidly in the tissues with the highest rates of blood flow. Of interest is the great variation in blood perfusion of certain tissues in the body. The body tissue compartments can be divided into the following major groups:
• The vessel-rich group, which consists of the heart, brain, kidneys, hepatoportal system, and endocrine glands
• The intermediate group of perfused tissues, which consists of muscle and skin
• The fat group, which includes marrow and adipose tissue
• The vessel-poor group, which has the poorest circulation per unit volume and comprises tendons, ligaments, connective tissue, teeth, bone, and other avascular tissue
The tissue tensions of the inhaled anesthetic increase and approach the arterial blood tension and ultimately the PA. One of the tissue groups that affects both the induction and the emergence from anesthesia is the fat group. The oil-gas partition coefficient best exemplifies the process involved with the affinity of anesthesia agents to adipose tissue and ultimately the emergence from anesthesia. The oil-gas partition coefficient is defined as the ratio of the concentration of the anesthetic agent in oil (adipose tissue) to that in a gaseous phase when the two are in equilibrium (see Table 20-1). The oil-gas partition coefficients seem to parallel anesthetic requirements. In fact, it is possible to calculate the MAC by knowing the oil-gas partition coefficient. With the constant of 150, the calculated MAC for an anesthetic with an oil-gas partition coefficient of 100 is 1.5%.
Halothane (Fluothane) was the classic inhalation anesthetic from the 1960s to the 1990s. Inclusion of halothane in the discussion is helpful because all the inhalation anesthetic agents currently in use have almost the same partition coefficients. Halothane has an oil-gas partition coefficient that is approximately twice that of isoflurane, desflurane, or sevoflurane; therefore, with use of halothane as the marker, the newer inhalation agents are twice as fast during induction and emergence as before. All three of the inhalational anesthetics currently in use are used in a variety of settings, and all possess a blood-gas partition coefficient less than 1 (see Table 20-1).
Movement of inhalation anesthetic from arterial blood to the brain
The transfer of the inhalation anesthetic from the arterial blood to the brain depends on the blood-brain partition coefficient of the agent and the cerebral blood flow. The blood-brain partition coefficient for most of the inhalation anesthetics is between 1.3 and 2 (see Table 20-1). The concentration gradient during induction of anesthesia is as follows:
Emergence of inhalation anesthesia
This gradient favors the removal of the anesthetic agent from the brain tissue. The partial pressure in the tissues declines first and is followed by that in the arterial blood. The agent returns to the lungs and is then eliminated into the atmosphere. The factors that affect the rate of elimination of the agent are the same ones that determine how rapidly an anesthetic agent takes a patient to surgical anesthesia. If a short procedure is performed (less than 1 hour), complete equilibrium among PA, Pa, and Pbr might not have occurred, and the recovery from anesthesia is more rapid. The reverse is true; during long procedures in which equilibrium occurs, a prolonged emergence may be anticipated.
Potency of inhalation anesthetic agents
Potency is determined by factors such as absorption, distribution, metabolism, excretion, and affinity for a receptor. The potency of the anesthetic agent refers to its ability to take the patient through all the stages of anesthesia to respiratory and circulatory arrest without the occurrence of hypoxia or the use of preanesthetic medication. Certainly, circulatory and respiratory arrests are not desired outcomes of the use of anesthetic agents; these features are used merely to describe the potency of anesthetic agents that are used clinically. For example, isoflurane is 100% potent compared with nitrous oxide, which is 15% potent. Isoflurane, when administered with oxygen to meet the patient’s metabolic needs and when given without premedication, takes the patient to circulatory and respiratory arrest, whereas nitrous oxide administered with oxygen takes the patient only to the first portion of surgical anesthesia and no further. Therefore, clinically speaking, potency of a drug makes little difference as long as the drug that is to be administered has an effective dose for a particular patient, which is why the concept of ED was developed. The ED is the dose of a drug necessary to produce certain effects in a certain percentage of patients. For example, an ED50 means that a drug produces a particular effect in 50% of the patients.4
Another method of determination of potency is with the use of the MAC. The MAC is found with determining the alveolar concentration (at 1 atm) needed for prevention of gross muscular movement in response to painful stimuli in 50% of anesthetized patients. The lower the MAC value, the more anesthetic potency of the inhalation anesthetic. Consequently, the MAC defines the therapeutic effect of inhalational anesthetics as the prevention of movement in response to surgical stimulation.4
The potency of an inhalation anesthetic agent with the MAC as the tool of assessment varies with the patient’s age, state of health, clinical conditions, and concurrent use of other drugs (with depressant effects). The factors that modify the MAC are presented in Box 20-1. MAC awake is the anesthetic dose at which a patient responds to commands; it is also the dose of anesthetic at which most patients lose consciousness and recall. MAC awake usually corresponds with stage I of anesthesia. Another term used with MAC is MAC-Block Adrenergic Response, which is the MAC needed to block the adrenergic and cardiovascular responses to incision; it corresponds to stage III, plane III anesthesia.
Techniques of administration
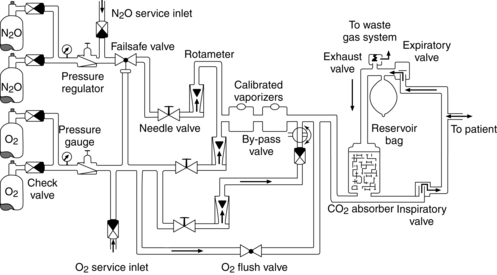
The inhalation anesthetics are usually administered by means of an anesthesia machine (Fig. 20-2). The anesthesia machine is essentially a breathing circuit that conveys the agent and oxygen to the patient. It consists of a mask, corrugated tubing, an absorber for removal of expired carbon dioxide, a reservoir bag, unidirectional valves, adjustable positive-pressure relief (APR) valve, and vaporizers (Fig. 20-3).5
Circle systems
A variety of techniques can be used to deliver gaseous agents with the anesthesia machine by adding or removing certain features. The most common technique used is the semiclosed circle method, in which some rebreathing of expired gases occurs by opening the APR valve to vent some of the gas to the atmosphere. The closed circle method is used when low gas flows are desired. In this technique, the APR valve is completely closed and complete rebreathing of expired gases occurs. A carbon dioxide absorber is used in both the semiclosed and the closed techniques.
Inhalation agents
Modern inhalation anesthetics
Isoflurane (forane)
Isoflurane, an analogue of enflurane, is also a halogenated methyl ethyl ether; it produces a dose-related depression of the CNS.6 In contrast with enflurane, this anesthetic agent does not produce convulsive electroencephalographic abnormalities. Isoflurane reduces the systemic arterial blood pressure and total peripheral resistance; however, during isoflurane anesthesia, the heart rate is usually increased and the cardiac output usually remains within normal limits. This agent produces respiratory depression and skeletal muscular relaxation in a dose-related fashion, because isoflurane markedly potentiates the actions of the nondepolarizing muscle relaxants. Of interest to the perianesthesia nurse is the fact that isoflurane does not sensitize the myocardium to catecholamines to the same extent as halothane does. Thus, the chance of dysrhythmias is reduced when the patient has received isoflurane anesthesia.
The recovery phase is rapid because of isoflurane’s low blood-gas partition coefficient of 0.97.3,6 The patient awakens promptly and is lucid within 15 to 30 minutes after termination of the anesthetic. However, clinical observation indicates that if the anesthesia time with isoflurane is longer than 45 to 60 minutes, the patient will probably have a slower emergence phase than expected, given that the drug has such a low blood-gas partition coefficient.
Sevoflurane (ultane)
Sevoflurane is a 100% potent inhalation anesthetic agent that has a blood-gas solubility coefficient of 0.69,6 which is near nitrous oxide and thus makes it an extremely rapid-acting agent. Consequently, patients emerge from sevoflurane anesthesia in a matter of minutes when they have received this drug as the sole agent. One must remember that a rapid recovery from an inhalation anesthetic usually mandates the need for analgesic drugs in the immediate postoperative period.7
The drug is not irritating to the respiratory tract, and the degree of patient acceptance is high. Sevoflurane can be used in place of halothane for the induction of anesthesia in children8; it tends to decrease the blood pressure by decreasing the systemic vascular resistance. Like all other inhalation agents, this drug is a respiratory depressant and blunts the ventilatory response to an increased PaCO2. This drug undergoes some metabolism at approximately the same degree as does enflurane. The metabolites of sevoflurane include fluoride and hexafluoroisopropanol. On the basis of a number of studies, no evidence of toxicity has been shown in regard to the biodegradation of this agent, probably because of sevoflurane’s rapid ventilatory excretion, in which the metabolic byproducts do not seem to be significantly detrimental to the patient.
Desflurane (suprane)
Desflurane is a fluorinated ether that is similar to isoflurane. This drug has a blood-gas partition coefficient that is the same as cyclopropane (0.42) and even less than nitrous oxide, which makes it extremely rapid acting.6 As with sevoflurane, patient emergence is extremely rapid and analgesia is needed in the immediate postoperative period. This drug produces a dose-related decrease in blood pressure and cardiac output that is slightly greater than the depression seen with equivalent doses of isoflurane. Because this drug is an ether-type inhalation agent, the incidence rate of cardiac dysrhythmias when epinephrine is administered is extremely low.
The pungency of desflurane irritates the respiratory tract and causes coughing, breathholding, and laryngospasm. Consequently, the drug is not recommended as an inhalation induction agent, especially in the pediatric age group. This drug depresses respiration in the same fashion as does sevoflurane and thus blunts the response to an increased PaCO2. Desflurane produces a dose-related reduction in cerebrovascular resistance. Desflurane also enhances the neuromuscular blockade produced by skeletal muscle relaxants. However, like sevoflurane, this action is not of consequence for the patient in the PACU because of its extremely rapid ventilatory excretion during emergence.8 Finally, as opposed to sevoflurane, this drug resists biodegradation and is almost totally eliminated by the respiratory system and therefore does not have a negative effect on the kidney or liver.
Nitrous oxide
Nitrous oxide is a 15% potent agent; therefore the maximum depth of anesthesia that can be produced while the patient’s metabolic need for oxygen is supplied is the middle of plane 1 of stage III anesthesia. This agent has no side effects unless hypoxia is present. It is nontoxic and nonirritating; however, nitrous oxide can cause postoperative nausea and vomiting, particularly in the ambulatory surgical setting when the procedure lasts for 1 hour, and probably for 2 hours or more.9 Nitrous oxide is a rapid-acting agent in part because of its blood-gas partition coefficient of 0.47.6 This agent does not combine with hemoglobin but is carried in physical solution in the blood. It is excreted mostly unchanged by the lungs, although a small fraction is excreted through the skin. It does not sensitize the heart to epinephrine, and it provides a fair amount of analgesia. Even in subanesthetic concentrations, nitrous oxide has an analgesic effect in humans, and 20% concentrations of the gas have been claimed to be as effective as 15 mg of morphine sulfate. If this agent were more potent, it would probably be considered an almost perfect anesthetic.
In current anesthesia practice, nitrous oxide serves an important role because it is administered alone and in combination with various agents. Recently, the balanced technique of anesthesia has been favored because of the number of negative factors associated with some of the more potent volatile inhalation anesthetics. The balanced technique consists of the administration of opioids that may or may not be in combination with a tranquilizer, a muscle relaxant, nitrous oxide, oxygen, and barbiturates.3 All the elements of anesthesia or nervous system depression are met: sensory block (analgesia), motor block (muscle relaxation), reflex block, and mental block (narcosis). When nitrous oxide is administered with a potent volatile inhalation anesthetic such as desflurane, it acts as a carrier and provides an additional analgesic effect. The second gas effect occurs because of nitrous oxide’s rapid uptake, after which the potent volatile agent takes the patient to the desired surgical plane. The reverse takes place at termination of the anesthetic.
Diffusion hypoxia after nitrous oxide anesthesia is another area of concern for the perianesthesia nurse. This effect is sometimes called the Fink phenomenon. It occurs when not enough nitrous oxide is removed from the lungs at the end of the surgical procedure. Normally, 100% oxygen is administered at the end of the procedure for removal of the nitrous oxide, which is called nitrous oxide washout. Diffusion hypoxia is directly related to the dilution of alveolar gas by the rapid diffusion of the nitrous oxide out of the blood. This outpouring of nitrous oxide into the alveoli occurs during the first 1 to 5 minutes after the nitrous oxide has been discontinued. In addition, the rapid movement into the alveoli can cause a dilutional effect of the PACO2 and ultimately a reduction in the stimulus to breathe. Therefore administration of oxygen via mask to all patients who are admitted to the PACU is highly advisable. This maneuver forestalls the development of severe hypoxia if some unpredicted airway problem occurs. Another measure for avoiding this complication is the provision of adequate verbal and physical stimulation to the patient to promote good ventilatory effort. This approach should include encouraging the patient to sigh every 5 minutes to ensure adequate removal of the anesthetic gases.
Assessment of the effects of inhalation agents in the perianesthesia care unit
In assessment of the patient’s degree of emergence from inhalation anesthesia, the nurse must understand the pharmacologic effects of each anesthetic agent and of the preoperative medications used. In addition, the rate of recovery from inhalation anesthesia is predictable based on the solubility of the anesthetic agent, alveolar ventilation, and duration of the anesthetic. Each anesthetic agent is essentially a depressant drug. Certain volatile agents, such as sevoflurane, desflurane, and isoflurane, possess a high degree of myocardial and respiratory depressant properties. One parameter for monitoring of the emergence phase with administration of these agents is the vital signs. Preanesthetic baseline vital sign readings are reliable postoperative indicators of the patient’s cardiorespiratory status and can be used in the assessment of the patient’s stage of recovery. When this assessment is made, however, all other factors of the patient’s condition must also be considered.10 Total assessment of the patient recovering from anesthesia is discussed in Chapter 27. Most inhalation anesthetics cause some degree of depression of the respiratory system. Consequently, the PaCO2 increases in a dose-related manner, frequency increases, and tidal volume is reduced. Because of the respiratory depression that all patients have after anesthesia and surgery, the perianesthesia nurse must use the stir-up regimen that encourages the patient to perform the sustained maximal inspiration maneuver (see Chapter 27).
Along with the factors attributed to the blood-gas partition coefficient, those attributed to the oil-gas partition coefficient should be considered in an evaluation of length of time of emergence from the anesthetic. When the intraoperative phase is of long duration, an agent with a high oil-gas partition coefficient redistributes into the adipose tissue. As mentioned previously, because the vascular supply to adipose tissue is sparse, the release of the agent to the blood is slow and the emergence is prolonged. Both coefficients must be kept in mind in prediction of the length of the emergence phase from an inhalation anesthetic agent. Isoflurane, for example, has the low blood-gas partition coefficient of 0.97, and a rapid recovery from its administration would be expected. However, isoflurane has a high oil-gas partition coefficient of 93.7; therefore, when it is administered for longer than 2 hours, the adipose tissue is saturated and emergence from the anesthetic agent is prolonged. Nitrous oxide, desflurane, and isoflurane have low blood-gas and oil-gas partition coefficients.7
Inhalation agents, because of their depressant effect on the hypothalamus, disrupt the regulation of body temperature that may be manifested by either a reduction or an elevation, depending on the environmental temperature.10 In the recovery phase, the emerging patient should be monitored for hypothermia or hyperthermia. Serious heat loss can occur in newborns and create difficulties in the reestablishment of adequate ventilatory effort after surgery. Body temperature should be monitored in patients who were febrile before surgery and who received atropine before or during the operative procedure. Agents such as isoflurane that have a direct vasodilatory effect on vascular smooth muscle can cause a temperature drop of 1° C in esophageal temperature. Shivering and tremors have been reported during the postoperative period after isoflurane anesthesia, although these phenomena have mostly been associated with a generalized loss of muscle tone during surgery and anesthesia.
Water and electrolyte balance is affected by inhalation anesthesia.1 Pituitary and adrenocortical systems appear to be affected in such a way that water and sodium retention and potassium loss occur after anesthesia. This balance is also affected in part by the stress of surgical trauma. Decreased glomerular filtration, increased tubular reabsorption, and varying degrees of oliguria exist in the recovery phase because of renal vasoconstriction. If renal blood flow is not impaired, glomerular function quickly returns to normal after the operation. The increased tubular reabsorption of water usually persists for 36 to 48 hours, but may continue for several days in elderly patients.
Summary
The inhalation anesthetic agents have moved from the first anesthetic discovered in 1846, ether, to derivatives of ether (methoxyflurane); to Penthrane, which was introduced in the early 1960s; to halothane (Fluothane), which was a halogenated hydrocarbon type drug introduced in the late 1960s; and to the current inhalational drugs described in this chapter. The anesthetic agents available for the anesthesia practitioner has in some ways become simplified, and the current selection of agents offers many advantages to the older drugs. For the perianesthesia nurse, this movement in the use of inhalational anesthetic agents has been positive. Previously, the patient would emerge rather slowly. With the current inhalation agents, patients wake up rather quickly. With the advent of these faster acting agents, the perianesthesia nurse is now confronted with new or more profound nursing care issues, such as pain, confusion, and awareness during anesthesia. Consequently, Chapter 31 has been devoted to pain and its physiology, and Chapters 28 and 29 are devoted to the nursing interventions for the sequelae of confusion and awareness with anesthesia.
The administration of inhalation agents has also become more simplified, and gone is the equipment used during the ether to Penthrane days. However, for the assessment of potency, the MAC is still used; and because many practitioners still use the signs and stages first described by Guedel, a description is included in this chapter. In addition, the prediction of emergence of the patient recovering from an inhalational anesthetic can be aided with a firm knowledge of the movement of the anesthetic through the various vascular groups along with the various partition coefficients. Finally, an overview of the assessment of a patient recovering from inhalational anesthesia is provided with a detailed discussion presented in Chapter 27.
Inhalation anesthetic agents used in modern practice have become more simplified, but that is not to be taken out of context. These highly rapid-acting agents have in fact justified the fact that advance practice critical care nursing is the level of care provided in the PACU. These rapid-acting agents require nurses in the PACU to possess a strong knowledge base and the ability to rapidly react to critical situations that can and do occur in the PACU.
1. Jordan B, Wright E. Xenon as an anesthetic agent. AANA J. 2010;78(5):387–392.
2. Longnecker D, Murphy F. Dripps/Eckenhoff/Vandam introduction to anesthesia, ed 9. Philadelphia: Saunders; 1997.
3. Stoelting R. Pharmacology and physiology in anesthetic practice, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
4. Miller R, Pardo M. Basics of anesthesia, ed 6. Philadelphia: Saunders; 2011.
5. Dorsch J, Dorsch S. Understanding anesthesia equipment, ed 5. Philadelphia: Lippincott Williams & Wilkins; 2007.
6. Brunton L, et al. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill Professional; 2010.
7. Pasero C, McCaffery M. Pain assessment and pharmacologic management. St. Louis: Mosby; 2011.
8. Jones R. Desflurane and sevoflurane: inhalation anesthetics for this decade. Br J Anaesth.1990;65:527–536.
9. Stancer-Smiley B, Paradise N. Does the duration of N2O administration affect postoperative nausea and vomiting. Nurse Anesth. 1991;2(1):13–20.
10. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2010.
American Association of Critical-Care Nurses: Core curriculum for progressive care nursing. Philadelphia: Saunders; 2010.
Aitkenhead A, et al. Textbook of anesthesia, ed 5. Philadelphia: Churchill Livingstone; 2007.
Alspach J. Core curriculum for critical care nursing, ed 6. Philadelphia: Saunders; 2005.
Atlee J. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
Barash P, et al. Clinical anesthesia, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2009.
Barrett K, et al. Ganong’s review of medical physiology, ed 23. New York: McGraw-Hill Medical; 2009.
Bready L, et al. Decision making in anesthesiology, ed 4. St. Louis: Mosby; 2007.
Brunton L, et al. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill Professional; 2010.
Conlay L, et al. Case files anesthesiology. New York: McGraw Hill Medical; 2011.
Davis P, et al. Smith’s anesthesia for infants and children, ed 8. St. Louis: Mosby; 2011.
Drake R, et al. Gray’s anatomy for students, ed 2. Philadelphia: Churchill Livingstone; 2009.
Deutschman C, Netigan P. Evidence-based practice of critical care. Philadelphia: Saunders; 2010.
Dorsch J, Dorsch S. Understanding anesthesia equipment, ed 5. Philadelphia: Lippincott Williams & Wilkins; 2007.
Fisher L. Anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2007.
Gallager C, Issenberg B. Simulation in anesthesia. Philadelphia: Saunders; 2007.
Hall J. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
Hines R, Marschall K. Handbook for Stoelting’s anesthesia and co-existing disease, ed 3. Philadelphia: Saunders; 2009.
Hines R, Marschall K. Stoelting’s anesthesia and co-existing disease, ed 5. Philadelphia: Saunders; 2008.
Kaplan J, et al. Cardiac anesthesia. New York: Churchill Livingstone; 2011.
Kier L, Dowd C. The chemistry of drugs for nurse anesthetists. Chicago: AANA Publishing, Inc; 2004.
Kulli J, Koch C. Does anesthesia cause loss of consciousness. Trends Neurosci. 1991;14(1):6–10.
Longnecker D, et al. Anesthesiology. New York: McGraw Hill Medical; 2007.
Miller R, et al. Miller’s anesthesia, ed 7. New York: Churchill Livingstone; 2010.
Miller R, Pardo M. Basics of anesthesia, ed 6. Philadelphia: Saunders; 2011.
Moos D, Cuddeford D. Implications of obstructive sleep apnea syndrome for the perianesthesia nurse. J Perianesth Nurs. 2006;21(2):103–118.
Mason R. Murray and Nadel’s textbook of respiratory medicine, ed 5. Philadelphia: Saunders; 2011.
Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2010.
Passannante A, et al. Anesthestic management of patients with obesity and sleep apnea. Anesthesiol Clin North Am. 2005;23:479–490.
Sandberg W, et al. The MGH textbook of anesthetic equipment. New York: Churchill Livingstone; 2011.
Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing, ed 2. Philadelphia: Saunders; 2010.
Shorten G, et al. Postoperative pain management: an evidence-based guide to practice. Philadelphia: Saunders; 2006.
Sieber F. Geriatric anesthesia. New York: McGraw-Hill Medical; 2006.
Shorten G, et al. Postoperative pain management: an evidence-based guide to practice. Philadelphia: Saunders; 2006.
Stoelting R. Pharmacology and physiology in anesthetic practice, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
Townsend C, et al. Sabiston’s textbook of surgery, ed 18. Philadelphia: Saunders; 2008.
Vincent J, et al. Textbook of critical care, ed 6. Philadelphia: Saunders; 2011.
White P. Perioperative drug manual, ed 2. Philadelphia: Saunders; 2005.