CHAPTER 31 Infratemporal and pterygopalatine fossae and temporomandibular joint
INFRATEMPORAL FOSSA
The infratemporal fossa has a roof, and lateral and medial walls, and is open to the neck posteroinferiorly, i.e. the fossa has no anatomical floor. The roof is formed by the infratemporal surfaces of the temporal bone and of the greater wing of the sphenoid, and contains the foramena ovale and spinosum and the petrotympanic fissure: it is open superiorly to the temporal fossa. The medial wall is formed anteriorly by the lateral pterygoid plate of the pterygoid process of the sphenoid, and more postero-medially by the pharynx and tensor and levator veli palatini. It contains the pterygomaxillary fissure across which structures pass between the infratemporal and pterygopalatine fossae (Fig. 31.1). The lateral wall is formed by the medial surface of the ramus of the mandible.
Lateral pterygoid provides a key to understanding the relationships of structures within the infratemporal fossa. This muscle lies in the roof of the fossa and runs anteroposteriorly in a more or less horizontal plane from the region of the pterygoid plates to the mandibular condyle (Fig. 31.1). Branches of the mandibular nerve and the main origin of medial pterygoid are deep relations and the maxillary artery is superficial. The buccal branch of the mandibular nerve passes between the two heads of lateral pterygoid. Medial pterygoid and the lingual and inferior alveolar nerves emerge below its inferior border and the deep temporal nerves and vessels emerge from its upper border. A venous network, the pterygoid venous plexus, lies around and within lateral pterygoid and is important in the spread of infection.
BONES
The sphenoid bone, the paired maxillae and temporal bones, and the mandible, collectively provide the skeletal framework to the infratemporal and pterygopalatine regions. The mandible and the two temporal bones articulate at the right and left temporomandibular joints. The disarticulated maxilla is described in Chapter 29, the temporal bone is described in Chapter 36, and the sphenoid and mandible are described here.
Sphenoid bone
The sphenoid bone lies in the base of the skull between the frontal, temporal and occipital bones. It has a central body, paired greater and lesser wings that spread laterally from the body, and two pterygoid processes that descend from the junction of the body and greater wings (Fig. 31.2).
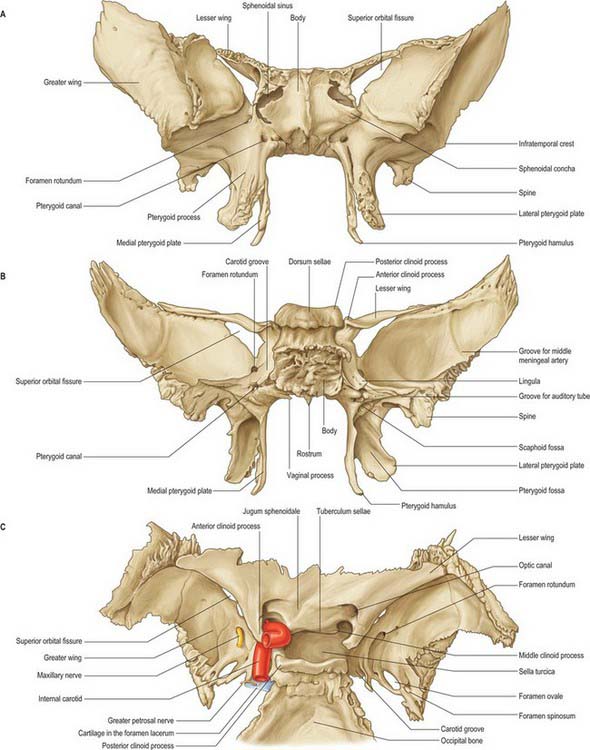
Fig. 31.2 Sphenoid bone. A, anterior view; B, posterior view; C, superior view.
(From Sobotta 2006.)
Body
The body of the sphenoid is cuboidal. It contains two air sinuses, separated by a septum (see Ch. 32). Its cerebral (superior) surface articulates in front with the cribriform plate of the ethmoid bone. Anteriorly is the smooth jugum sphenoidale, which is related to the gyri recti and olfactory tracts. The jugum is bounded behind by the anterior border of the sulcus chiasmatis, which leads laterally to the optic canals. Posteriorly is the tuberculum sellae, behind which is the deeply concave sella turcica. In life the sella contains the hypophysis cerebri in the hypophysial fossa. Its anterior edge is completed laterally by two middle clinoid processes, while posteriorly the sella turcica is bounded by a square dorsum sellae, the superior angles of which bear variable posterior clinoid processes. The diaphragma sella and the tentorium cerebelli are attached to the clinoid processes (see Ch. 27). On each side, below the dorsum sellae, a small petrosal process articulates with the apex of the petrous part of the temporal bone. The body of the sphenoid slopes directly into the basilar part of the occipital bone posterior to the dorsum sellae, together these bones form the clivus. In the growing child this is the site of the spheno-occipital synchondrosis: premature closure of this joint gives rise to the skull appearances seen in achondroplasia.
The lateral surfaces of the body are united with the greater wings and the medial pterygoid plates. A broad carotid sulcus accommodates both the internal carotid artery and the cranial nerves associated with the cavernous sinus above the root of each wing (see Ch. 27). The sulcus is deepest posteriorly. It is overhung medially by the petrosal part of the temporal bone and has a sharp lateral margin, the lingula, which continues back over the posterior opening of the pterygoid canal.
A median triangular, bilaminar sphenoidal crest on the anterior surface of the body of the sphenoid makes a small contribution to the nasal septum. The anterior border of the crest joins the perpendicular plate of the ethmoid bone, and a sphenoidal sinus opens on each side of it (see Ch. 32). In the articulated state the sphenoidal sinuses are closed anteroinferiorly by the sphenoidal conchae, which are largely destroyed when disarticulating a skull. Each half of the anterior surface of the body of the sphenoid possesses a superolateral depressed area joined to the ethmoid labyrinth which completes the posterior ethmoidal sinuses; a lateral margin which articulates with the orbital plate of the ethmoid above and the orbital process of the palatine bone below; and an inferomedial, smooth, triangular area, which forms the posterior nasal roof, and near whose superior angle lies the orifice of a sphenoidal sinus.
Superior orbital fissure
The superior orbital fissure connects the cranial cavity with the orbit. It is bounded medially by the body of the sphenoid, above by the lesser wing of the sphenoid, below by the medial margin of the orbital surface of the greater wing, and laterally, between the greater and lesser wings, by the frontal bone. The contents of the superior orbital fissure are described in Chapter 39.
Sphenoidal conchae
Anterior parts of the two bones meet in the midline, and protrude as the sphenoidal crest. The horizontal part appears in the nasal roof and completes the sphenopalatine foramen. Its medial edge articulates with the rostrum of the sphenoid and the ala of the vomer. Its apex, directed posteriorly, is superomedial to the vaginal process of the medial pterygoid plate and joins the posterior part of the ala. A small conchal part sometimes appears in the medial wall of the orbit, lying between the orbital plate of the ethmoid in front, the orbital process of the palatine bone below and the frontal bone above.
Mandible
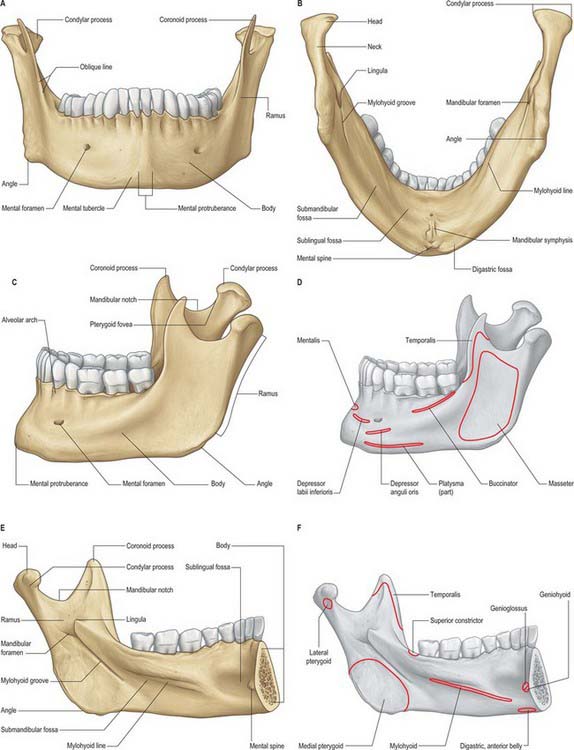
The mandible is the largest, strongest and lowest bone in the face. It has a horizontally curved body that is convex forwards, and two broad rami that ascend posteriorly (Fig. 31.3). The body of the mandible supports the mandibular teeth within the alveolar process. The rami bear the coronoid and condylar processes. Each condyle articulates with the adjacent temporal bone at the temporomandibular joint.
Body
The upper border, the alveolar part, contains 16 alveoli for the roots of the lower teeth. It consists of buccal and lingual plates of bone joined by interdental and inter-radicular septa. Near the second and third molar teeth the external oblique line is superimposed upon the buccal plate. Like the maxilla, the form and depth of the tooth sockets is related to the morphology of the roots of the mandibular teeth. The sockets of the incisor, canine and premolar teeth usually contain a single root, while those for the three molar teeth each contain two or three roots. The third molar is variable in its position and root presentation. It may be impacted vertically, horizontally, mesially or distally, and its roots may be bulbous, hooked, divergent or convergent, and occasionally embrace the mandibular (inferior dental) canal (see Ch. 30). The internal surface of the mandible is divided by an oblique mylohyoid line that gives attachment to mylohyoid (and, above its posterior end, to the superior pharyngeal constrictor, some retromolar fascicles of buccinator, and the pterygomandibular raphe behind the third molar). The mylohyoid line extends from a point approximately 1 cm from the upper border behind the third molar as far forwards as the mental symphysis; it is sharp and distinct near the molars, but faint further forwards. The mylohyoid groove extends downwards and forwards from the ramus below the posterior part of the mylohyoid line and contains the mylohyoid neurovascular bundle. The area below the line is a slightly concave submandibular fossa and is related to the submandibular gland. The area above the line widens anteriorly into a triangular sublingual fossa and is related to the sublingual gland: the bone is covered by oral mucosa above the sublingual fossa as far back as the third molar. In an edentulous subject it may be necessary to reduce any ridge-like prominence of the mylohyoid line in order that dentures may fit without traumatizing the overlying oral mucosa.
Ramus
The mandibular ramus is quadrilateral, and has two surfaces (lateral and medial), four borders (superior, inferior, anterior and posterior) and two processes (coronoid and condylar). The lateral surface is relatively featureless and bears the (external) oblique ridge in its lower part. The medial surface presents, a little above centre, the mandibular foramen, through which the inferior alveolar neurovascular bundle passes to gain access to the mandibular canal (see below). Anteromedially, the mandibular foramen is overlapped by a thin, sharp triangular spine, the lingula, to which the sphenomandibular ligament is attached, and which is also the landmark for an inferior alveolar local anaesthetic block injection. Below and behind the foramen, the mylohyoid groove runs obliquely downward and forward.
Ossification
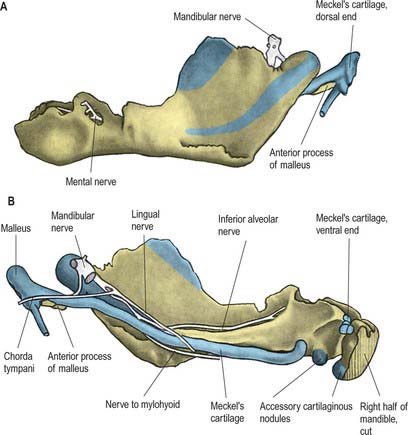
The mandible forms in dense fibromembranous tissue lateral to the inferior alveolar nerve and its incisive branch, and also in the lower parts of Meckel’s cartilage (first branchial arch). Each half is ossified from a centre that appears near the mental foramen about the sixth week in utero. From this site, ossification spreads medially and posterocranially to form the body and ramus, first below, and then around, the inferior alveolar nerve and its incisive branch. Ossification then spreads upwards, initially forming a trough, and later crypts, for the developing teeth. By the tenth week, Meckel’s cartilage below the incisor rudiments is surrounded and invaded by bone. Secondary cartilages appear later (Fig. 31.4): a conical mass, the condylar cartilage, extends from the mandibular head downwards and forwards in the ramus, and contributes to its growth in height. Although it is largely replaced by bone by midfetal life, its proximal end persists as proliferating cartilage under the fibrous articular lining until about the third decade. Another secondary cartilage, which soon ossifies, appears along the anterior coronoid border, and disappears before birth. One or two cartilaginous nodules also occur at the symphysis menti. At about the seventh month in utero these may ossify as variable mental ossicles in symphysial fibrous tissue: they unite with adjacent bone before the end of the first postnatal year.
Age changes in the mandible
At birth the two halves of the mandible are united by a fibrous symphysis menti (Fig. 31.5). The anterior ends of both rudiments are covered by cartilage, separated only by a symphysis. Until fusion occurs, new cells are added to each cartilage from symphysial fibrous tissue, and ossification on its mandibular side proceeds towards the midline. When the latter process overtakes the former, and ossification extends into median fibrous tissue, the symphysis fuses. At this stage the body is a mere shell which encloses the imperfectly separated sockets of deciduous teeth. The mandibular canal is near the lower border, and the mental foramen opens below the first deciduous molar and is directed forwards. The coronoid process projects above the condyle.
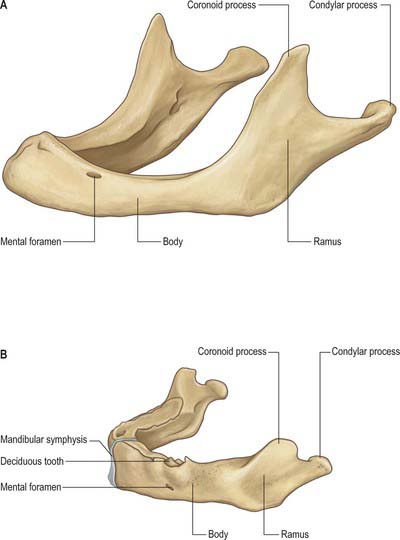
Fig. 31.5 A, Edentulous mandible; note position of mental foramen; B, Neonatal mandible.
(Redrawn with permission from Sobotta 2006.)
In general terms, increase in height of the body of the mandible is achieved primarily by formation of alveolar bone associated with the developing and erupting teeth, although some bone is also deposited on the lower border. Increase in length of the mandible is accomplished by deposition of bone on the posterior surface of the ramus and concomitant compensatory resorption on the anterior surface (accompanied by deposition of bone on the posterior surface of the coronoid process and resorption on the anterior surface of the condylar process). Increase in width of the mandible is produced by deposition of bone on the outer surface of the mandible and resorption on the inner surface. An increase in the comparative size of the ramus compared with the body of the mandible occurs during postnatal growth and tooth eruption.
In adults, alveolar and subalveolar regions are about equal in depth, and the mental foramen appears midway between the upper and lower borders. If teeth are lost, alveolar bone is resorbed, which means that the mandibular canal (which runs parallel to the mylohyoid line) and the mental foramen come to lie much nearer to the superior border (Fig. 31.5), indeed, sometimes they may both disappear, so that the nerves lie just beneath the oral mucosa.
TEMPOROMANDIBULAR JOINT
It is probably impossible to measure the pressure developed on the articular surfaces of the human jaw joint when biting, however direct measurement of loads across the joint in animals has demonstrated significant intermittent loading during mastication. There is also irrefutable theoretical evidence based on Newtonian mechanics that the jaw joint is a weight-bearing joint. With a vertical bite force of 500 N on the left first molar, the right condyle must support a load of well over 300 N (Osborn 1995a). The non-working condyle is more loaded than the condyle on the working side, which may help explain why patients with a fractured condyle choose to bite on the side of the fracture.
Fibrous capsule
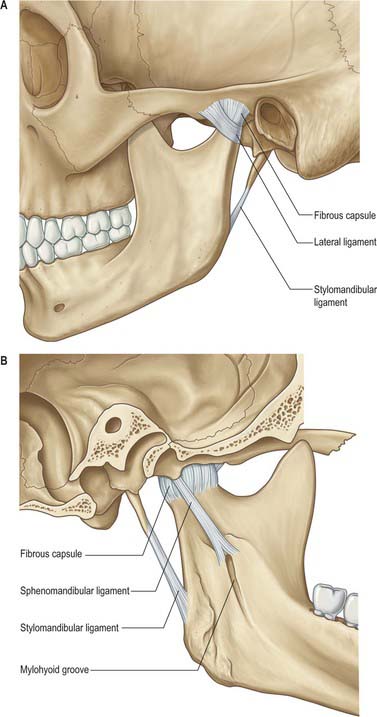
The lower part of the joint is surrounded by tight fibres which attach the condyle of the mandible to the disc. The upper part of the joint is surrounded by loose fibres which attach the disc to the temporal bone (Fig. 31.6). Thus the articular disc is attached separately to the temporal bone and to the mandibular condyle forming what could be considered two joint capsules. Longer fibres joining the condyle directly to the temporal bone may be regarded as reinforcing. The capsule is attached above to the anterior edge of the preglenoid plane, posteriorly to the lips of the squamotympanic fissure, between these to the edges of the articular fossa, and below to the periphery of the neck of the mandible.
Ligaments
Sphenomandibular ligament
The sphenomandibular ligament is medial to, and normally separate from, the capsule (Fig. 31.6). It is a flat, thin band that descends from the spine of the sphenoid and widens as it reaches the lingula of the mandibular foramen. Some fibres traverse the medial end of the petrotympanic fissure and attach to the anterior malleolar process. This part is a vestige of the dorsal end of Meckel’s cartilage.
Stylomandibular ligament
The stylomandibular ligament is a thickened band of deep cervical fascia that stretches from the apex and adjacent anterior aspect of the styloid process to the angle and posterior border of the mandible (Fig. 31.6). Its position and orientation indicate that it cannot mechanically constrain any normal movements of the mandible and does not seem to warrant the status of a ligament of the joint.
Temporomandibular (lateral) ligament
The broad temporomandibular ligament is attached above to the articular tubercle on the root of the zygomatic process of the temporal bone (Fig. 31.6). It extends downwards and backwards at an angle of approximately 45° to the horizontal, to attach to the lateral surface and posterior border of the neck of the condyle, deep to the parotid gland. A short, almost horizontal, band of collagen connects the articular tubercle in front to the lateral pole of the condyle behind. It may function to prevent posterior displacement of the resting condyle.
Development of the temporomandibular joint
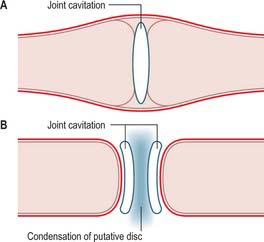
The bony components of the secondary jaw joint develop in membrane as two separate ectomesenchymal condensations between the 8th and 12th week in utero. This arrangement is different from that seen with most synovial joints, where a single blastema cavitates to form a joint space and the articulating bones are derived from the same condensation of tissue (see Ch. 51). The separate development of the temporal and condylar parts of the temporomandibular joint means that the articular surface of each bone is covered by a persisting layer of proliferative cells that is continuous with the periosteum surrounding each separate bony component (Fig. 31.7).
Articular surfaces
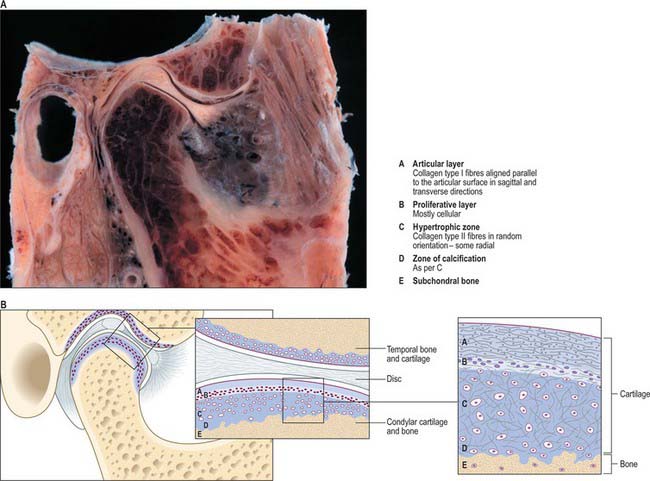
The fibrocartilaginous covering of the condyle is composed of four distinct layers (De Bont et al 1984). The most superficial layer consists of densely packed fibres of type I collagen that are arranged mostly parallel to the articular surface. This covers a thin cellular layer, the proliferative zone, that is continuous with the cambial layer of the periosteum beyond the margins of the joint. The third layer, of hypertrophic cartilage, is rich in intercellular matrix: it contains chondrocytes scattered throughout its depth, and randomly oriented fibres of collagen type II. The fourth layer, immediately above the subchondral bone, is the zone of calcification. Although the number of chondrocytes within the hypertrophic zone decreases with age, undifferentiated mesenchymal cells have been identified in post mortem specimens of all ages (Hansson 1977). This indicates that a capacity for proliferation persists in condylar cartilage, and may be the reason why condylar remodeling occurs throughout life (Robinson 1993, Toller 1974).
Articular disc
The transversely oval articular disc is composed predominantly of dense fibrous connective tissue with some chondrification in areas of maximum loading (Fig. 31.8). It has a thick margin which forms a peripheral anulus and a central depression in its lower surface that accommodates the articular surface of the mandibular condyle. The depression probably develops as a mechanical response to pressure from the condyle as it rotates inside the anulus. The disc is stabilized on the condyle in three ways. Its edges are fused with the part of the capsular ligament that tightly surrounds the lower joint compartment and is attached around the neck of the condyle; well-defined bands in the capsular ligament attach the disc to the medial and lateral poles of the condyle, and additionally, the thick anulus prevents the disc sliding off the condyle, provided that the condyle and disc are firmly lodged against the articular fossa (as is normally the case).
Relations
Superiorly a thin plate of temporal bone (2–3 mm) separates the upper joint space from the middle cranial fossa. This bony partition is occasionally breached inadvertently during surgery to the joint, particularly for release of ankylosis, or rarely by the condyle being driven superiorly by violent trauma to the mandible. The maxillary artery and its proximal branches, most notably the middle meningeal artery, lie medially, just beyond the joint capsule. The uppermost part of the parotid capsule enclosing the branches of the facial nerve that supply the muscles of the upper face, including obicularis oculi, are lateral to the joint capsule: the thread-like nerves are at risk during surgical approach to the joint. The upper part of the infratemporal space, which contains the two heads of lateral pterygoid, is anterior to the condyle (Fig. 31.9). It is into this space anterior to the articular eminence that the condyle is displaced in cases of dislocation of the TMJ or fracture dislocation of the condylar head. Posteriorly is the tegmen tympani and behind it the middle ear cavity. Perforation through this thin bony wall into the middle ear is a recognized complication of TMJ arthroscopy. Just below the joint, the maxillary artery winds around the posterior aspect of the condylar neck.
Vascular supply and innervation
The joint derives its arterial supply from the superficial temporal artery laterally and the maxillary artery medially. Penetrating vessels that supply lateral pterygoid may also supply the condyle. Veins drain the anterior aspect of the joint and associated tissues into the plexus surrounding lateral pterygoid, and posteriorly they drain into the vascular region that separates the two laminae of the bilaminar region of the disc. Pressure produced by forward and backward movement of the condyle shunts blood between these regions. Lymphatics drain deeply to the upper cervical lymph nodes surrounding the internal jugular vein.
Jaw movements
Symmetrical opening
There are conflicting views about the reason why forward slide occurs, probably because direct experimental testing is not possible. No other animal has an articular eminence constraint and ligaments comparable to man, which means that most supporting evidence for any theory is based on analyses of human joint dysfunction. It has been argued that a sensory input from the rotary movement, possibly from either the joint capsule or the jaw muscles, initiates a response that reflexly activates muscles that cause the slide. The fact that the condyle in a cadaver still slides forward when the jaw is rotated open suggests that it is not a neuromuscular response, but that it is the mechanical result of physical constraints. When the jaw is rotated open the temporomandibular ligament rapidly becomes taut (Osborn 1995b). The taut ligament acts as a constraint that allows the mandible only two rotary movements: it can swing about the upper attachment of the ligament and rotate about the lower attachment. The lower end of the taut ligament acts as a moving fulcrum that converts the downward and backward pull of the opening rotary force (created at the front by digastric and geniohyoid) into one that drives the condyle upward and forward into the concavity of the overlying articular disc. This now pushes the disc forward. Swing about the upper attachment creates space above for the disc to slide further forward which is possible because the upper part of the capsular ligament is loose. The two movements, rotation and swing, are inextricably linked by the taut ligament and, via the condyle, combine to keep the disc in firm contact with the articular eminence while the jaw is opened. The disc is stabilized by its tight attachment to the condyle and by the thickened margins of its anulus that prevent it sliding through the thinner compressed region between the centre of the condyle and the articular eminence.
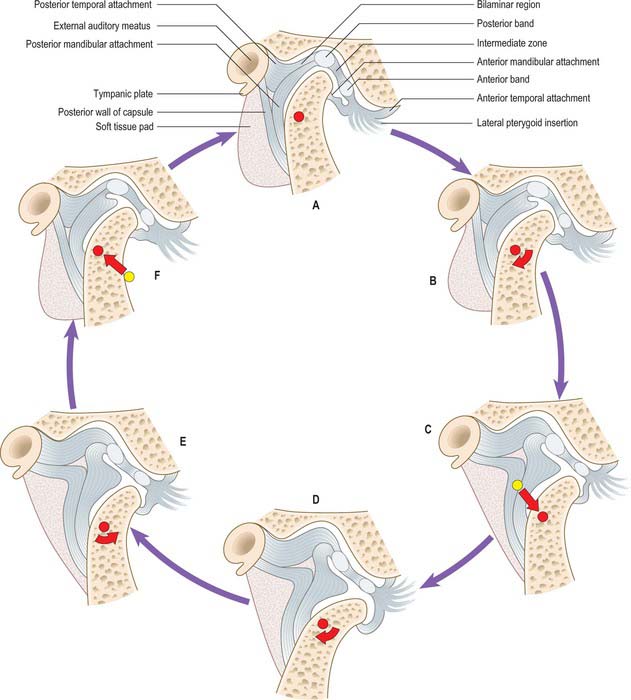
Changes in disc position during movement
With the teeth in occlusion, the condyle is in the glenoid fossa and the intra-articular disc sits on the condylar head; its posterior band above the summit of the condyle (Fig. 31.10A). As mouth opening begins (Fig. 31.10B), the condyle rotates within the lower joint space and the disc remains stationary. At about mid-opening, the condyle and disc begin to move forward together so that their relative position is maintained (Fig. 31.10C). At maximum gape (Fig. 31.10D) the condylar head slides further anteriorly than the disc so that the anterior band of the articular disc is above the summit of the condyle. Mouth closure involves the disc moving back in tandem with the condyle (Fig. 31.10E) which also reverses its angular rotation (Fig. 31.10F) to reassume the starting position of the cycle.
Commonly, the shifts in disc position become out of phase with those of the condyle, which causes obstruction to smooth jaw movement. The disc is typically pulled anteromedially by lateral pterygoid, so preventing forward translation of the condyle. As the force from the condyle on the disc increases, the elastic posterior attachments of the disc are stretched until the resistance to movement is overcome and the energy that had been stored is then released with an audible click (audible to the patient, and occasionally to others). The normal disc–condyle relation is then reestablished and the mouth is free to achieve maximum gape (Fig. 31.10C,D). The disc may then snap back in front of the condyle on mouth closure. This phenomenon is called reciprocal clicking or disc displacement with reduction.
MUSCLES
Masseter
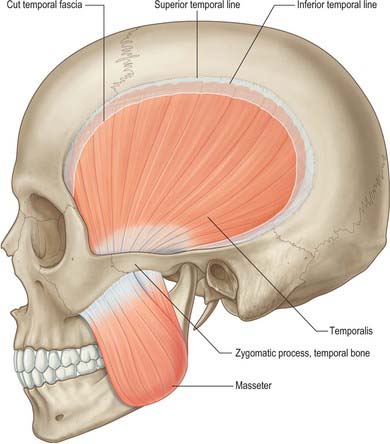
Masseter (Fig. 31.11) consists of three layers which blend anteriorly. The superficial layer is the largest. It arises by a thick aponeurosis from the maxillary process of the zygomatic bone and from the anterior two-thirds of the inferior border of the zygomatic arch. Its fibres pass downwards and backwards, to insert into the angle and lower posterior half of the lateral surface of the mandibular ramus. Intramuscular tendinous septa in this layer are responsible for the ridges on the surface of the ramus. The middle layer of masseter arises from the medial aspect of the anterior two-thirds of the zygomatic arch and from the lower border of the posterior third of this arch. It inserts into the central part of the ramus of the mandible. The deep layer arises from the deep surface of the zygomatic arch and inserts into the upper part of the mandibular ramus and into its coronoid process. There is still debate as to whether fibres of masseter are attached to the anterolateral part of the articular disc of the temporomandibular joint. Masseteric hypertrophy, e.g. in response to excessive use of masseter, may be treated by surgical reduction from the deep aspect of masseter or injections of botulinus toxin to paralyse the motor nerves.
Temporalis
Temporalis (Fig. 31.11) arises from the whole of the temporal fossa up to the inferior temporal line – except the part formed by the zygomatic bone – and from the deep surface of the temporal fascia. Its fibres converge and descend into a tendon which passes through the gap between the zygomatic arch and the side of the skull. A plane exists beneath the temporal fascia, which is attached to the superior surface of the zygomatic arch, and the muscle, which passes beneath the arch. An elevator introduced into this plane through an incision above the hairline may therefore be placed beneath a fractured zygomatic arch or bone in order to reduce the fracture (Gillies approach). Temporalis is attached to the medial surface, apex, anterior and posterior borders of the coronoid process and to the anterior border of the mandibular ramus almost up to the third molar tooth. Its anterior fibres are orientated vertically, the most posterior fibres almost horizontally, and the intervening fibres with intermediate degrees of obliquity, in the manner of a fan. Fibres of temporalis may occasionally gain attachment to the articular disc.
Lateral pterygoid
Lateral pterygoid (Fig. 31.12) is a short, thick muscle consisting of two parts. The upper head arises from the infratemporal surface and infratemporal crest of the greater wing of the sphenoid bone. The lower head arises from the lateral surface of the lateral pterygoid plate. From the two origins, the fibres converge, and pass backwards and laterally, to be inserted into a depression on the front of the neck of the mandible (the pterygoid fovea). A part of the upper head may be attached to the capsule of the temporomandibular joint and to the anterior and medial borders of its articular disc. Unlike the other muscles of mastication, lateral pterygoid is not pennate, nor does it have a significant number of Golgi tendon organs associated with its attachments.
The mandibular ramus and masseter, the maxillary artery – which crosses either deep or superficial to the muscle – and the superficial head of medial pterygoid and the tendon of temporalis, are all superficial relations. Deep to the muscle are the deep head of medial pterygoid, the sphenomandibular ligament, the middle meningeal artery, and the mandibular nerve. The upper border is related to the temporal and masseteric branches of the mandibular nerve and the lower border is related to the lingual and inferior alveolar nerves. A deeply placed posterior superior alveolar nerve block has been known to also anaesthetize the lingual nerve. The buccal nerve and the maxillary artery pass between the two heads of the muscles. In temporomandibular joint dysfunction syndrome, spasm of lateral pterygoid can give rise to tenderness when palpating behind the maxillary tuberosity high in the buccal sulcus (the pterygoid sign).
When left and right muscles contract together the condyle is pulled forward and slightly downward. This protrusive movement alone has little or no function except to assist opening the jaw. Digastric and geniohyoid are the main jaw opening muscles: unlike lateral pterygoid, when acting alone they rotate the jaw open, provided other muscles attached to the hyoid prevent if from being pulled forward. If only one lateral pterygoid contracts, the jaw rotates about a vertical axis passing roughly through the opposite condyle and is pulled medially toward the opposite side. This contraction together with that of the adjacent medial pterygoid (both attached to the lateral pterygoid plate) provides most of the strong medially directed component of the force used when grinding food between teeth of the same side. It is arguably the most important function of the inferior head of lateral pterygoid. It is often stated that the upper head is used to pull the articular disc forward when the jaw is opened. But electromyography studies have proved that the upper head is inactive during jaw opening and most active when the jaws are clenched. An explanation for this surprising activity is as follows (Osborn 1995a). Most of the power of a clenching force is due to contractions of masseter and temporalis. The associated backward pull of temporalis is greater than the associated forward pull of (superficial) masseter, and so their combined jaw closing action potentially pulls the condyle backward. This is prevented by the simultaneous contraction of the upper head of lateral pterygoid.
Medial pterygoid
Medial pterygoid (Fig. 31.12) is a thick, quadrilateral muscle with two heads of origin. The major component is the deep head which arises from the medial surface of the lateral pterygoid plate of the sphenoid bone and is therefore deep to the lower head of lateral pterygoid. The small, superficial head arises from the maxillary tuberosity and the pyramidal process of the palatine bone, and therefore lies on the lower head of lateral pterygoid. The fibres of medial pterygoid descend posterolaterally and are attached by a strong tendinous lamina to the posteroinferior part of the medial surface of the ramus and angle of the mandible, as high as the mandibular foramen and almost as far forwards as the mylohyoid groove. This area of attachment is often ridged. Inferior alveolar nerve block injection can occasionally cause haemorrhage into the muscle, which may give rise to painful trismus.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Maxillary artery
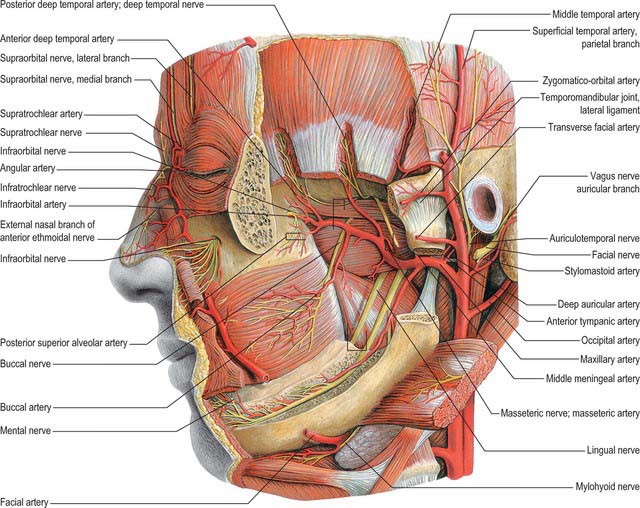
The maxillary artery, the larger terminal branch of the external carotid artery, arises behind the neck of the mandible, and is at first embedded in the parotid gland (see Ch. 29). It then crosses the infratemporal fossa to enter the pterygopalatine fossa through the pterygomaxillary fissure. The artery is widely distributed to the mandible, maxilla, teeth, muscles of mastication, palate, nose and cranial dura mater (Figs 31.12, 31.13). It will be described in three parts, mandibular, pterygoid and pterygopalatine.
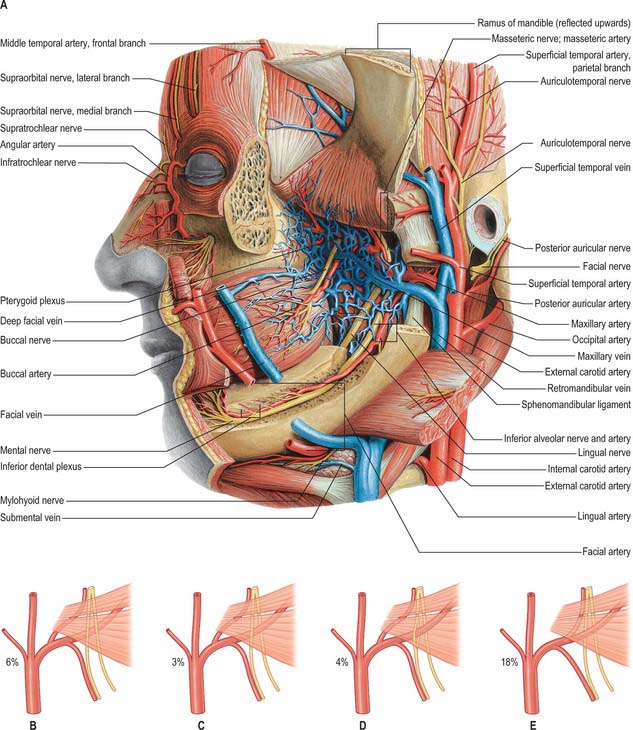
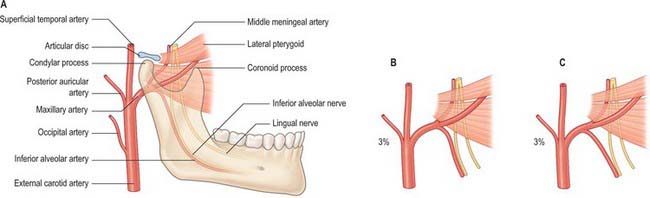
Fig. 31.13 A, Vessels and nerves of the head, exposed at a level deeper than that shown in Fig. 31.12 Note the pterygoid venous plexus. B–E, Variations in the course of the maxillary artery. In B, the maxillary artery passes medial to lateral pterygoid, and to the lingual and inferior alveolar nerves; in C, the artery passes between the lingual and inferior alveolar nerves; in D, the artery passes through a loop formed by the inferior alveolar nerve; in E, the middle meningeal artery branches off distal to the inferior alveolar artery.
(From Sobotta 2006.)
The mandibular part of the maxillary artery has five branches which all enter bone, namely, deep auricular, anterior tympanic, middle meningeal, accessory meningeal and inferior alveolar arteries. The pterygoid part of the maxillary artery has five branches that do not enter bone, but supply muscle, and include deep temporal, pterygoid, masseteric and buccal arteries. The branches of the pterygopalatine part of the artery accompany similarly named branches of the maxillary nerve (including those associated with the pterygopalatine ganglion) and are described in Chapter 32.
Middle meningeal artery
The middle meningeal artery is the main source of blood to the bones of the vault of the skull (Fig. 31.14). It may arise either directly from the first part of the maxillary artery or from a common trunk with the inferior alveolar artery. When the maxillary artery lies superficial to lateral pterygoid, the middle meningeal artery is usually the first branch of the maxillary artery. However, when the maxillary artery takes a deep course in relation to the muscle this is not usually the case. The middle meningeal artery ascends between the sphenomandibular ligament and lateral pterygoid, passes between the two roots of the auriculotemporal nerve and leaves the infratemporal fossa through the foramen spinosum to enter the cranial cavity medial to the midpoint of the zygomatic bone. Its further course is described in Chapter 27.
Maxillary veins and the pterygoid venous plexus
Maxillary vein
The maxillary vein is a short trunk which accompanies the first part of the maxillary artery (Fig. 31.13A). It is formed from the confluence of veins from the pterygoid plexus and passes back between the sphenomandibular ligament and the neck of the mandible, to enter the parotid gland. It unites within the substance of the gland with the superficial temporal vein to form the retromandibular vein.
INNERVATION
The infratemporal fossa contains the major subdivisions of the mandibular branch of the trigeminal nerve, together with the chorda tympani, which enters the fossa and joins the lingual nerve, and the otic ganglion, which is functionally related to the parotid gland. The main sensory branches of the mandibular nerve extend beyond the infratemporal fossa and their distribution to the face is described in Chapter 29.
Mandibular nerve
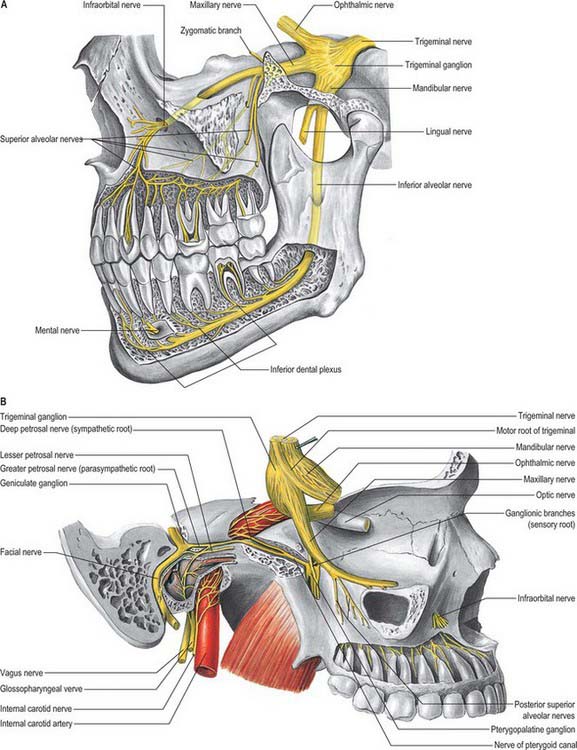
The mandibular nerve is the largest trigeminal division and is a mixed nerve. Its sensory branches supply the teeth and gums of the mandible, the skin in the temporal region, part of the auricle – including the external meatus and tympanic membrane – and the lower lip, the lower part of the face and the mucosa of the anterior two-thirds (presulcal part) of the tongue and the floor of the oral cavity (Figs 31.12, 31.13A, 31.15). The motor branches innervate the muscles of mastication. The large sensory root emerges from the lateral part of the trigeminal ganglion and exits the cranial cavity through the foramen ovale. The small motor root passes under the ganglion and through the foramen ovale to unite with the sensory root just outside the skull. As it descends from the foramen ovale, the nerve is usually around 4 cm from the surface and a little anterior to the neck of the mandible. The mandibular nerve immediately passes between tensor veli palatini, which is medial, and lateral pterygoid, which is lateral, and gives off a meningeal branch and the nerve to medial pterygoid from its medial side. The nerve then divides into a small anterior and large posterior trunk. The anterior division gives off branches to the four main muscles of mastication and a buccal branch which is sensory to the cheek. The posterior division gives off three main sensory branches, the auriculotemporal, lingual and inferior alveolar nerves, and motor fibres to supply mylohyoid and the anterior belly of digastric.
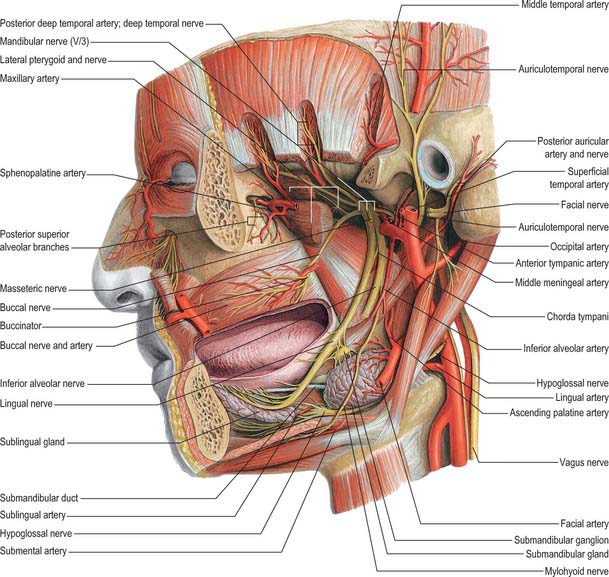
Fig. 31.15 Arteries and nerves of the head, deepest lateral regions. Compare with Figs 31.12 and 31.13A.
(From Sobotta 2006.)
Auriculotemporal nerve
The auriculotemporal nerve usually has two roots which encircle the middle meningeal artery. It runs back under lateral pterygoid on the surface of tensor veli palatini, passes between the sphenomandibular ligament and the neck of the mandible, and then runs laterally behind the temporomandibular joint related to the upper part of the parotid gland. Emerging from behind the joint, it ascends over the posterior root of the zygoma, posterior to the superficial temporal vessels, and divides into superficial temporal branches. It communicates with the facial nerve and otic ganglion. The rami to the facial nerve, usually two, pass anterolaterally behind the neck of the mandible to join the facial nerve at the posterior border of masseter. Filaments from the otic ganglion join the roots of the auriculotemporal nerve close to their origin. The sensory distribution of the auriculotemporal nerve on the face is described in Chapter 29.
Lingual nerve
The lingual nerve is sensory to the mucosa of the anterior two-thirds of the tongue, the floor of the mouth and the mandibular lingual gingivae. It arises from the posterior trunk of the mandibular nerve and at first runs beneath lateral pterygoid and superficial to tensor veli palatini, where it is joined by the chorda tympani branch of the facial nerve, and often by a branch of the inferior alveolar nerve. Emerging from under cover of lateral pterygoid, the lingual nerve then runs downwards and forwards on the surface of medial pterygoid, and is thus carried progressively closer to the medial surface of the mandibular ramus. It becomes intimately related to the bone a few millimetres below and behind the junction of the vertical ramus and horizontal body of the mandible. Here it lies anterior to, and slightly deeper than, the inferior alveolar (dental) nerve. It next passes below the mandibular attachment of the superior pharyngeal constrictor and pterygomandibular raphe, closely applied to the periosteum of the medial surface of the mandible, until it lies opposite the posterior root of the third molar tooth, where it is covered only by the gingival mucoperiosteum. At this point it usually lies 2–3 mm below the alveolar crest and 0.6 mm from the bone, however in 5% of cases it lies above the alveolar crest. It next passes medial to the mandibular origin of mylohyoid, and this carries it progressively away from the mandible, and separates it from the alveolar bone covering the mesial root of the third molar tooth. The rest of the nerve is described with the mouth and oral cavity in Chapter 30.
Inferior alveolar (dental) nerve
The inferior alveolar nerve descends behind lateral pterygoid. At the lower border of the muscle the nerve passes between the sphenomandibular ligament and the mandibular ramus and enters the mandibular canal via the mandibular foramen. Below lateral pterygoid it is accompanied by the inferior alveolar artery, a branch of the first part of the maxillary artery, which also enters the canal with associated veins. The subsequent course of the inferior alveolar nerve is described in Chapter 30.
Chorda tympani
The chorda tympani nerve enters the infratemporal fossa region by passing through the medial end of the petrotympanic fissure behind the capsule of the temporomandibular joint. The nerve descends medial to the spine of the sphenoid bone – which it sometimes grooves – lying posterolateral to tensor veli palatini. It is crossed medially by the middle meningeal artery, the roots of the auriculotemporal nerve and by the inferior alveolar nerve (Fig. 31.15). The chorda tympani joins the posterior aspect of the lingual nerve at an acute angle. It carries taste fibres for the anterior two-thirds of the tongue and efferent preganglionic parasympathetic (secretomotor) fibres destined for the submandibular ganglion in the floor of the mouth.
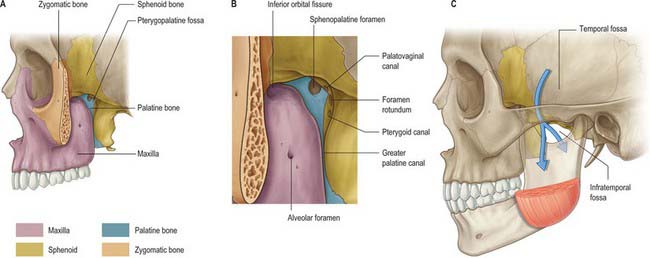
PTERYGOPALATINE FOSSA
The pterygopalatine fossa is a small pyramidal space below the apex of the orbit on the lateral side of the skull. The posterior boundary is the root of the pterygoid process and adjoining anterior surface of the greater wing of the sphenoid, and the anterior boundary is the superomedial part of the infratemporal surface of the maxilla. The perpendicular plate of the palatine bone, with its orbital and sphenoidal processes forms the medial boundary, and the pterygomaxillary fissure is the lateral boundary. The fossa communicates with the nasal cavity via the sphenopalatine foramen, with the orbit via the medial end of the inferior orbital fissure, and with the infratemporal fossa via the pterygomaxillary fissure, which lies between the back of the maxilla and the pterygoid process of the sphenoid and transmits the maxillary artery. It also communicates with the oral cavity via the greater palatine canal, which opens in the posterolateral aspect of the hard palate. There are two openings in the posterior wall of the pterygopalatine fossa, the foramen rotundum, which transmits the maxillary nerve, and the pterygoid canal, which transmits the nerve of the pterygoid canal (Vidian nerve). When the anterior aspect of the pterygoid plate is examined in a disarticulated sphenoid, the foramen rotundum is seen to lie above and lateral to the pterygoid canal (see Fig. 31.2).
Maxillary artery
Posterior superior alveolar artery
The posterior superior alveolar artery arises from the maxillary artery within the pterygopalatine fossa and runs through the pterygomaxillary fissure onto the maxillary tuberosity. It gives off branches which penetrate the bone here to supply the maxillary molar and premolar teeth and the maxillary air sinus, and other branches that supply the buccal mucosa. Occasionally the posterior superior alveolar artery arises from the infraorbital artery.
Maxillary nerve
The maxillary division of the trigeminal nerve is wholly sensory (Fig. 31.16). It leaves the skull via the foramen rotundum, which leads directly into the posterior wall of the pterygopalatine fossa. Crossing the upper part of the pterygopalatine fossa, the nerve gives off two large ganglionic branches which contain fibres destined for the nose, palate and pharynx, and these pass through the pterygopalatine ganglion without synapsing. It then inclines sharply laterally on the posterior surface of the orbital process of the palatine bone and on the upper part of the posterior surface of the maxilla in the inferior orbital fissure (which is continuous posteriorly with the pterygopalatine fossa): it lies outside the orbital periosteum, and gives off its zygomatic, and then posterior superior alveolar branches. About halfway between the orbital apex and the orbital rim the maxillary nerve turns medially to enter the infraorbital canal as the infraorbital nerve. The subsequent course of the maxillary nerve is described in Chapter 29.
Zygomatic nerve
The zygomatic branch of the maxillary nerve leaves the pterygopalatine fossa through the inferior orbital fissure together with the maxillary nerve. Its subsequent course is described in Chapter 29.
Pterygopalatine ganglion
The pterygopalatine ganglion is the largest of the peripheral parasympathetic ganglia. It is placed deeply in the pterygopalatine fossa, near the sphenopalatine foramen, and anterior to the pterygoid canal and foramen rotundum (Fig. 31.16B). It is flattened, reddish-grey in colour, and lies just below the maxillary nerve as it crosses the pterygopalatine fossa. The majority of the ‘branches’ of the ganglion are connected with it morphologically, but not functionally, because they are primarily sensory branches of the maxillary nerve. Thus they pass through the ganglion without synapsing, and enter the maxillary nerve through its ganglionic branches, but they convey some parasympathetic fibres to the palatine, pharyngeal and nasal mucous glands.
Orbital branches
Fine orbital branches enter the orbit through the inferior orbital fissure and supply orbital periosteum. Some fibres also pass through the posterior ethmoidal foramen to supply the sphenoidal and ethmoidal sinuses. The orbital branches probably join branches of the internal carotid nerve to form a ‘retro-orbital’ plexus from which orbital structures such as the lacrimal gland and orbitalis receive an autonomic innervation.
Palatine nerves (greater and lesser)
The greater and lesser palatine nerves pass downwards from the pterygopalatine ganglion through the greater palatine canal. The greater palatine nerve descends through the greater palatine canal, emerges on the hard palate from the greater palatine foramen and runs forwards in a groove on the inferior surface of the bony palate almost to the incisor teeth. It supplies the gingivae, mucosa and glands of the hard palate and also communicates with the terminal filaments of the nasopalatine nerve. In the greater palatine canal it gives off posterior inferior nasal branches that emerge through the perpendicular plate of the palatine bone and ramify over the inferior nasal concha and walls of the middle and inferior meatuses. As it leaves the greater palatine canal, it gives off branches which are distributed to both surfaces of the adjacent part of the soft palate.
Barker BCW, Davies PL. The applied anatomy of the pterygomandibular space. Br J Surg. 1972;10:43-55.
Bertilsson O, Strom D. A literature survey of a hundred years of anatomic and functional lateral pterygoid muscle research. J Orofac Pain. 1995;9:17-23.
De Bont LGM, Boering G, Havinga P, Liem RSB. Spacial arrangement of collagen fibrils in the articular cartilage of the mandibular condyle. J Oral Maxillofac Surg. 1984;42:306-313.
Hansson T, Oberg T, Carlsson GE, Kopp S. Thickness of the soft tissue layers and the articular disc in the temporomandibular joint. Acta Odont Scand. 1977;35:77-83.
Hollingshead WH. The Head and Neck. 2nd edn. Anatomy for Surgeons. vol. 1. New York: Harper & Row; 1968.
Lang J. Clinical Anatomy of the Masticatory Apparatus and Peripharyngeal Spaces. New York: Thième Medical Publishers, 1995.
Langdon JD, Patel MF. Operative Maxillofacial Surgery. London: Chapman and Hall, 1998.
Langdon JD, Berkovitz BKB, Moxham BJ. The Surgical Anatomy of the Infratemporal Fossa. London: Dunitz, 2002.
Orchardson R, Cadden W. Mastication. Linden RWA, editor. The Scientific Basis of Eating. Taste and Smell, Salivation. Mastication and Swallowing and their Dysfunctions. Frontiers of Oral Biology Series Vol 9. Basel: Karger; 1998:76-121
Osborn JW. Internal derangement and the accessory ligaments around the temporomandibular joint. J Oral Rehab. 1995;22:731-740.
Pogrel MA, Renaut A, Schmidt B, Ammar A. The relationship of the lingual nerve to the mandibular third molar region. J Oral Maxillofac Surg. 1995;53:1178-1181.
Robinson PD. Articular cartilage of the temporomandibular joint – can it regenerate? Ann R Coll Surg Engl. 1993;75:231-236.
Toller PA. Temporomandibular arthropathy. Proc Roy Soc Med. 1974;67:153-159.
Turker KS. Reflex control of human jaw muscles. Crit Rev Oral Biol Med. 2002;13:85-104.