140 Influenza
Influenza is a zoonosis indigenous to waterfowl, with periodic introduction of the virus into humans and other mammals. The consequences of host species transfer from birds to humans can be devastating, with substantial mortality rates and rapid transmission by the respiratory route with global pandemic potential. The fate of influenza virus infection in human populations depends upon the viral virulence properties, immunologic differences from previous influenza outbreaks, fitness of the virus for replication and dissemination within humans, and status of the host immune defenses.1
In the winter months, severe disease in individual patients is usually limited to those with vulnerabilities in host defenses, including the very young, the very old, and individuals with immunodeficiency or underlying cardiopulmonary disease. The annual incidence rate varies each season depending upon the degree of antigenic “drift” (point mutations in coding regions of genes for major surface antigens) from one year to the next. However, influenza pandemics can occur following an antigenic “shift” (i.e., whole-scale reassortment of the influenza virus genome, with the expression of entirely new antigenic components), and these novel influenza hybrid viruses circulate throughout the entire susceptible global population. This set of events occurred in 2009 with the novel swine influenza virus strain where everyone, including healthy young people, became susceptible to this novel influenza infection and its complications.2
Even in a typical year between pandemics, influenza viruses account for the deaths of hundreds of thousands of people worldwide and exact billions of dollars from society in terms of morbidity and lost productivity. Recent estimates from the United States indicate that at least 610,660 life-years are lost, with 3.1 million hospital days, 31.4 million outpatient visits, and $10.4 billion in direct medical costs annually from influenza alone. The staggering amount expended for influenza care is $16.3 billion in projected lost earnings and an estimated total cost burden (including lost-life years) amounting to $87.1 billion.3 The total costs to society during a pandemic year such as 2009 are even higher and likely incalculable. The costs of intensive care services required for managing the most severely ill influenza victims alone are enormous.2
 Pathogenicity of Influenza Viruses
Pathogenicity of Influenza Viruses
Influenza virus is a single-stranded RNA virus of the family Orthomyxoviridae It affects birds and mammals and includes three genuses: influenza virus A, B, and C, based upon their matrix proteins.1,4 Influenza A virus is typically the most virulent, has pandemic potential, and leads to the most severe disease. Based upon the antibody response to two major antigenic proteins on the outside of virus, hemagglutinin (HA) and neuraminidase (NA), influenza A is subdivided into different serotypes including: H1N1 (responsible for Spanish flu in 1918, in addition to the 2009 flu pandemic); H2N2 (Asian flu of 1957); H3N2 (Hong Kong flu of 1968); H5N1 (the avian flu, often sited as the most recent pandemic threat), and a number of others currently less relevant to humans (H7N7, H1N2, H9N2, H7N2, H7N3, H10N7). The two other forms of influenza include B (which almost exclusively infects humans but is less common) and C (affecting humans, dogs, and pigs), which only rarely cause severe illness and epidemics in humans.5
A notable characteristic of influenza virus is the genomic structure consisting of eight separate single-strand segments, each encoding a single major protein to complete the synthesis of the mature virus. The RNA-based genome provides a high background mutation rate and gives the virus genetic plasticity. The multiple genome segments provide the substrate for reassortment of large sequences of RNA and permit hybrid viruses to form in hosts infected simultaneously by more than one virus strain. These events lead to whole-scale recombination of entirely novel hybrid viruses with new antigenic constituents (antigenic shift). As an example, the novel swine-origin influenza A/Mexico City/4/2009 (H1N1) outbreak strain was a quadruple-reassorted virus derived from gene segments originating from ducks, Eurasian swine, North American swine, and human-adapted influenza virus.6
Avian-adapted viruses can occasionally be transmitted to mammals, causing outbreaks in animals or giving rise to disease in human pandemics. The pig is an important “mixing vessel” host in shuttling avian influenza viruses to humans, as they can carry both avian and human influenza viruses.1 Porcine mucous membranes express a mixture of sialic acid–coated glycopeptides linked in a favorable conformation to bind both avian and human-adapted viruses. This is vitally important in the biology of influenza viruses, as the initial event in influenza infection is interaction of the hemagglutinin receptor to binding sites on host epithelial tissues. Avian species express α2,3-linked sialic acid–galactose disaccharides on their epithelial surfaces, and avian-adapted influenza preferentially binds to this linkage pattern. Human upper respiratory airways primarily express α2,6-linked sialyl-galactose surface receptors, and seasonal influenza strains in humans bind readily only to α2,6 linkages. Pigs, in contrast, normally express both α2,3- and α2,6-linked disaccharides on their mucous membranes, facilitating the opportunity for dual infections with avian- and human-adapted viruses.1,6,7
The lower airways and alveolar pneumocytes of humans actually express α2,3-linked sialylated glycopeptides, and viruses that bind efficiently to α2,3 linkages can cause severe pneumonia if deposited into the distal airways. Most seasonal influenza strains bind preferentially to α2,6-linked disaccharide hemagglutinin (HA) binding sites found in human upper airways. This usually leads to high transmission frequency by the airborne droplet nuclei deposited upon the upper airways, but a low risk of primary influenza pneumonia.8 The avian strain of H5N1 preferentially binds to α2,3 linkages and therefore is poorly transmissible from person to person, but it has the potential to cause severe pneumonia if delivered to the lower airways. Poultry workers in Asia in close proximity to infected livestock can occasionally receive enough viruses deposited into the distal airways to cause severe influenza pneumonia with a high mortality rate (60% to 70%).9,10
One of the explanations for the severity of the 1918 pandemic of H1N1 influenza was its HA that could bind with high affinity to both α2,6- and 2,3-linked sialyl-galactose moieties.11,12 The result of this unusual HA binding affinity was a highly transmissible virus with the capacity to replicate and cause severe disease in the lower airways. Disturbingly, the hemagglutinin of the 2009 outbreak strain of novel swine origin also bound with high affinity to both α2,6 and α2,3 linkages. Fortunately, influenza A Mexico City 4/2009 (H1N1) virus lacked the full complement of other known virulence factors of the influenza virus (Table 140-1), resulting an overall low case-fatality rate (<0.1 %). A further mitigating factor against mortality in older populations during the 2009 outbreak was the presence of already-existing memory cells with B-cell and T-cell epitope recognition sites in humans born before the early 1950s, induced by H1N1 viruses circulating in the first half of the 20th century.13
TABLE 140-1 Pathogenicity Traits and Virulence Factors of Influenza Viruses
| Viral Trait | Mechanism of Virulence | Comments |
|---|---|---|
| Epitope variations on HA and NA | Immune escape from recognition by pre-existing antibodies within the population from previous virus exposure | Antigenic drift (point mutations) leads to epidemics; antigenic shift (reassorted viral genomes) leads to pandemics |
| Cleavability of HA | HA undergoes proteolysis by host-derived proteases before receptor binding | Readily cleaved HA is associated with avid binding and disease severity |
| Binding preference of HA | α2,3-linked sialic acid receptor in alveoli and α2,6 linkage in upper airways | Viruses that bind to the α2,3 linkage or both α2,3 and α2,6 are more virulent |
| HA : NA ratio | NA cleaves sialic acid on glycopeptides on epithelium (binding site for HA) | Optimal ratio of NA and HA activity needed for high replication and release |
| NS-1 | This nonstructural protein inhibits host-derived interferons. | Mutation or truncated variants are associated with loss of virulence. |
| PB1-F2 | This peptide targets virus trafficking to mitochondria and induces apoptosis. | Mutations or truncated forms of PB1-F2 associated with loss of virulence |
| NA inhibitor resistance | H274Y mutation blocks NA inhibitor binding site and oseltamivir activity | Commonly seen mutation is seasonal H1N1 but rare in the 2009 outbreak strain |
| M2 inhibitor resistance | S31N mutation blocks activity of amantidine | Now commonplace in both H3N2 and H1N1 |
| PB2 temperature range | Polymerase activity at lower (mammals) and higher (avian) temperature | Broad Pol temperature range aids transfer from bird to human hosts |
H274Y, histidine substitution for tyrosine at amino acid at position 274; HA, hemagglutinin; M, matrix protein; NA, neuraminidase; NS-1, nonstructural protein; PB, polymerase basic; Pol, polymerase; S31N, serine substitution for asparagine at amino position 31.
 Clinical Manifestations and Complications of Influenza
Clinical Manifestations and Complications of Influenza
Classical seasonal influenza in adults is typified by a 4- to 5-day period of sudden-onset fever, chills, upper respiratory tract symptoms, headache, muscle pain, and weakness. Rhinitis is relatively uncommon and diarrhea is more common with influenza than with most rhinovirus upper respiratory tract infections. Severe complications and death can occur, especially in infants, the elderly, and individuals with chronic medical conditions. Among the most severe complications are primary influenza pneumonia and secondary bacterial infection leading to respiratory failure.14,15 Influenza can also cause central nervous system, cardiac, skeletal muscle, kidney, and hepatic complications.5,15 Underlying pulmonary disease is a frequent risk factor, occurring in 18% of patients, most commonly asthma (7%), followed by neurologic disease (12%), hematologic or oncologic (9.9%), and cardiac conditions (4.6%).16 However, approximately half of those hospitalized (rates ranging from 1-5/1000) for influenza are otherwise healthy.14–16
In the absence of a pandemic, 11% to 19% of patients hospitalized with laboratory-confirmed influenza require treatment in the intensive care unit (ICU).15 The mean duration of mechanical ventilation is approximately 5 days; the sickest patients require treatment with advanced techniques for the treatment of hypoxemia, such as high-frequency oscillatory ventilation (HFOV), extracorporeal membrane oxygenation (ECMO), prone positioning, and nitric oxide. These patients have an attendant increase in length of stay, duration of ventilation, and mortality.14,16,17
An estimated 50 to 100 million people died during the 1918 pandemic. Death followed from aggressive secondary bronchopneumonia, influenza-related lung disease with associated hypoxemia, and cardiac collapse.18,19 During the 1918 pandemic, there was unexplained excess influenza mortality in persons 20 to 40 years of age. This mortality increase may have been due to limited native immunity and/or a vigorous immune response directed against the virus in healthy young persons.18 Today the high mortality rate observed in the 1918 pandemic would almost certainly be reduced because of the availability of ICUs, vaccines, antibacterial agents, and antiviral medications. However, the cost would be a dramatic increase in critical care admissions and length of stay, assuming that this surge capacity is available. Long-stay ICU patients have significantly higher critical care and hospital mortality rates compared to short-stay patients, occupy a disproportionate number of critical care bed-days,4 and consume even greater resources.8 Sophisticated ICU care is often unavailable in developing countries today, and the case-fatality rates in these countries will probably be regrettably similar to the 1918 pandemic.20
 Influenza A 2009 H1N1-Related Epidemiology and Clinical Manifestations
Influenza A 2009 H1N1-Related Epidemiology and Clinical Manifestations
Since March 2009, influenza A 2009 H1N1 has spread from Mexico to virtually all countries of the world. By September 27, 2009, there were over 340,000 cases with 4100 deaths worldwide.7,21 The World Health Organization issued the first phase 6 pandemic alert of the century, anticipating substantial influenza transmission and related disease. Over the period of June to September 2009, there were dramatic spikes in H1N1-related disease in Australia, New Zealand, and South America that breached the capacity for ICU care in some regions. In Australian provinces, approximately 5% of the population developed H1N1-related illness, 0.3% of infected patients were hospitalized, and 20% of hospitalized patients required ICU care.22 In the Northern Hemisphere, an early and severe influenza outbreak occurred that was blunted in part by widespread deployment of an effective inactivated monovalent influenza vaccine program.23
The events that transpired in Canada were illustrative of the influenza situation in much of the Northern Hemisphere in 2009. Among 168 critically ill Canadian patients with influenza A 2009 H1N1, the mean age has been 32 years, with a possible predilection for more severe disease in women (67% of patients).24 Pregnant women in particular suffered from a disproportionate high level of influenza disease severity.25,26 Nosocomial transmission was the mechanism of acquisition in approximately 10% of patients. Hospital-acquired transmission to healthcare workers occurred early in the outbreak, but healthcare-related infection occurred at a low incidence rate once the pandemic was recognized and appropriate infection-control safeguards were instituted. One or more comorbidities were observed in nearly all patients, most commonly chronic lung disease such as asthma, chronic obstructive pulmonary disease, bronchopulmonary dysplasia (41%), obesity (33%, mean body mass index of 34.6 kg/m2), hypertension (24%), history of smoking (23%), and diabetes (21%). Similar clinical findings and predisposing illnesses were reported in other regions of the world during the 2009 outbreak.21,22,27,28 Serious comorbid illness was observed in only 30% of patients. Notably, aboriginal Canadians have thus far been over-represented (26% of patients). A summary of clinical risk factors and comorbidities associated with severe influenza complications is found in Table 140-2.
TABLE 140-2 Prognostic Indicators and Risk Factors for Severe Influenza Complications
| Risk Factors and Comorbidities | Comments |
|---|---|
| Age <5 years | Children < 2 years and those with chronic cardiopulmonary disease at greatest risk |
| Age >65 years | Poor vaccine response, poor host response to influenza infection |
| Chronic cardiopulmonary diseases | COPD, asthma, congestive heart failure |
| Metabolic disease and chronic liver disease | Diabetes mellitus and cirrhosis increase the risk of influenza complications. |
| Chronic neurologic illness | Neurocognitive and neuromuscular diseases associated with increased complications |
| Pregnancy | Particularly women in the third trimester |
| Obesity | BMI >35 kg/m2 increased the risk of influenza complications in the 2009 outbreak. |
| Hemoglobinopathy | Sickle cell disease patients at increased risk |
| Immunosuppression | Glucocorticoids, chemotherapy, HIV transplant recipients at increased risk |
| Children receiving salicylates | Increased risk of Reye syndrome |
| Aboriginal populations, poverty, poor access to healthcare services | Delayed treatment associated with increased risk of influenza complications |
| Secondary bacterial pneumonia | Bacterial pneumonia associated with longer ICU and hospital stays with more nosocomial complications and a greater mortality rate |
The typical clinical syndrome requiring ICU care among all age groups appeared to be a diffuse bilateral four-quadrant pneumonitis that was often rapidly progressive. This process accounted for over 80% of ICU admissions in Canada and elsewhere and often necessitated advanced ventilatory/oxygenation modalities including HFOV, inhaled nitric oxide, and/or ECMO therapy.24,29,30
Over 80% of patients with H1N1-related acute lung injury (ALI) received mechanical ventilation; very few patients were successfully managed with noninvasive ventilation strategies alone. Oxygenation support included high concentrations of inspired oxygen (mean admission PaO2/FIO2 147 mmHg), positive end-expiratory pressure (PEEP), frequent use of HFOV (12%), nitric oxide (14%), neuromuscular blockade (30%), prone ventilation (5%), and occasionally ECMO (7%). Medical therapies included neuraminidase inhibitors (90.5%), antibacterial agents (98.8%), and, despite uncertain efficacy, corticosteroids (50.6%).24
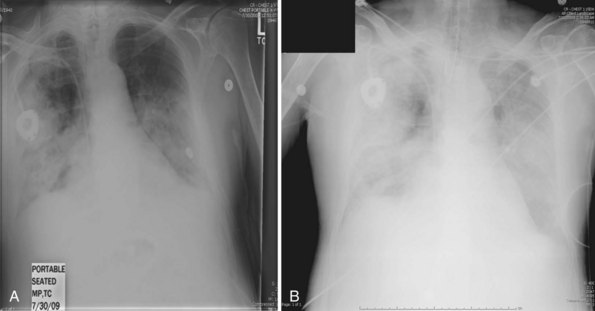
Secondary bacterial pneumonia following ICU admission was found in 24% of cases, most commonly due to S. aureus and S. pneumoniae. The frequency of secondary bacterial infection was difficult to accurately determine owing to the widespread use of empirical antibacterial therapy in influenza patients with rapidly progressive respiratory failure. Overall mortality among critically ill patients at 90 days was 17.3% (similar to that reported from Australia).22 The median duration of ventilation was 12 days. The most common cause of death was severe acute respiratory distress syndrome (ARDS) and hypoxemia, complications thereof, secondary infection, sepsis, or multiorgan dysfunction syndrome. Characteristic radiographic changes of severe primary influenza pneumonia are shown in Figure 140-1, A and B.
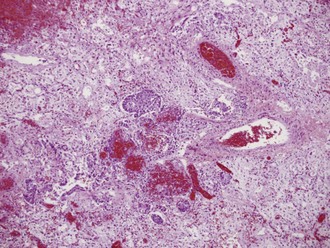
Lung pathology in fatally infected patients who underwent autopsy examination revealed a diffuse alveolar filling process, often with early hyaline membrane formation that was sometimes accompanied by focal areas of hemorrhage. The alveolar lining was usually thickened, with evidence of lymphocytic infiltrates and early organization with fibrosis. A typical lung tissue section of a patient with fatal influenza pneumonia is seen in Figure 140-2. Lung tissue in deaths occurring early in the presentation of influenza pneumonia often revealed diffuse immunohistochemical evidence of viral infection and intraalveolar hemorrhage.
In children, the median age of hospitalized patients was 5.0 years (range 1 month to 17 years); 54.4% were female, and the mean PRISM III score was 9.14–1624 One or more chronic comorbid illnesses were observed in 70.2% of patients: lung disease (44%), neurologic diseases (19%), immune suppression or immunodeficiency (16%), history of prematurity (9%), and congenital heart disease (7%). Mechanical ventilation was used in 68% of children admitted to ICU, and the median duration of ventilation was 6 days (range 0-67).
 Clinical and Laboratory Diagnosis
Clinical and Laboratory Diagnosis
Significant difficulties with definitive virologic diagnosis existed in the early phase of the 2009 influenza outbreak that were partially rectified as the pandemic unfolded. Fever and upper respiratory symptoms were present in almost all patients who progressed to critical illness. However, shortness of breath, a symptom not typical of uncomplicated influenza virus infection, was likely suggestive of severe disease. Other clinical signs noted in patients with severe disease have included hemoptysis, frothy pink sputum, and purulent sputum with diffuse lung crackles. Percutaneous oximetric assessment of oxygenation or arterial blood gas evaluation of PO2 should be performed when assessing a patient with suspected severe influenza. Relative hypoxia should trigger further assessment including a chest radiograph. Laboratory findings typically found at presentation with severe disease include normal or low-normal leukocyte counts and elevated creatine kinase22,24,28 (Figure 140-3).
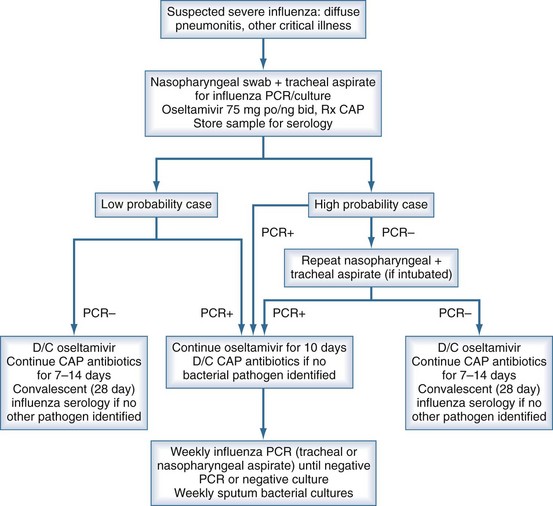
Figure 140-3 Suggested algorithm in the workup and management of suspected severe influenza pneumonia in the critical care unit.
Early laboratory diagnosis of influenza infection is greatly facilitated by the use of reverse transcriptase–polymerase chain reaction (RT-PCR) methodology. This assay should be employed when available in the evaluation of a patient with suspected severe influenza. Immunofluorescent techniques, enzyme-linked immunoassays, and other rapid diagnostic tests of clinical specimens often lack diagnostic sensitivity.31,32 Viral cultures require up to 1 week for processing. Whereas RT-PCR is the preferred definitive diagnostic technique and has very high sensitivity, the adequacy of the clinical specimen is essential. Standard nasopharyngeal swab samples are adequate but can be falsely negative. Nasopharyngeal samples should be repeated in 48 to 72 hours if diagnostic suspicion remains. Paired nasopharyngeal and tracheal aspirates are useful for RT-PCR in intubated patients and may increase the diagnostic yield in critically ill patients.
 Supportive Care
Supportive Care
Almost all patients with severe infection in the ICU setting will have deficits in oxygenation and subsequently require ventilatory support.22,24,33 Shock and renal failure can occur during therapy as a consequence of efforts to optimize oxygenation though diuresis coupled with high intrathoracic pressures and limited venous return.24,28 Other important but less frequently seen disorders at presentation may include encephalitis (with or without obtundation or seizure activity), cardiac injury (myocarditis, pericarditis, conduction defects), and rhabdomyolysis.17
Most critically ill patients with severe influenza will manifest evidence of ARDS; supportive care for severe hypoxemia with diffuse pulmonary disease and supplemental oxygenation and ventilation assistance is required.24 During pandemic periods, patients are often relatively young compared with non-pandemic years, and much greater numbers can be expected to be in need of ventilatory support than during a usual flu season.18,22,24,29,30
Primary influenza pneumonia is unusual in that patients often display a relative insensitivity to usual measures of oxygenation assistance with PEEP. Controlled ventilation with attention to a lung-protective strategy,34 in combination with appropriate sedation and judicious use of neuromuscular blockade, is appropriate. Avoidance of volume overload (and judicious diuresis) may also be associated with reduced duration of ventilation and length of stay in ICU for most patients with ALI and ARDS, and this strategy should be attempted for patients with influenza.30,35 Other ventilation measures (despite unproven benefit in other forms of ARDS) that might improve oxygenation for individual patients have included prone positioning and inhaled nitric oxide.36,37 HFOV is currently being evaluated as a rescue therapy for patients with severe ARDS in randomized controlled trials38 and might be an option in patients with influenza-related refractory hypoxemia. ECMO remains a controversial option to manage severe respiratory failure in influenza-associated ALI in adults.29,30,39 Clinicians in Australia similarly recommend consideration of ECMO for refractory hypoxemia in influenza infection.40 HFOV and ECMO might be considered as a salvage therapy in centers familiar with these modalities in desperately ill patients.
 Antiviral Therapy
Antiviral Therapy
In severely ill patients with suspected influenza, early initiation of antiviral therapy should be based upon clinical presentation and epidemiologic data and not delayed pending laboratory confirmation.22,24,41 Various influenza strains are circulating throughout the world, and susceptibility to currently available antiviral agents is strain specific. The 2009 H1N1 swine influenza variant was resistant to amantidine but sensitive to neuraminidase inhibitors including oseltamivir and zanamavir.42 Oseltamivir-resistant strains were isolated during the 2009 H1N1 influenza A pandemic but fortunately were uncommon.43 In contrast, the seasonal H1N1 influenza A strains circulating in 2008 and onwards are almost uniformly resistant to oseltamivir, yet many remain susceptible to zanamivir.44–46 At this time, only an oral form of oseltamivir and an inhaled form of zanamivir are available for use.
Initiation of antiviral therapy within 48 hours of onset of symptoms of seasonal influenza is associated with a 1-day or greater reduction in duration of symptoms in ambulatory patients.44,47 Oseltamivir therapy may reduce the risk of secondary bacterial superinfection.48 Early therapy of severe influenza A 2009 H1N1 infections requiring ICU support with neuraminidase inhibitors contributed to improved outcomes.49
Little data are available to guide the optimal dose or duration of therapy for antiviral agents. Severe influenza infections, including those caused by the 2009 H1N1 strain, can represent a systemic in addition to a pulmonary infection,50 favoring the use of a systemic rather than an inhaled antiviral agent. Despite concerns over inadequate gastrointestinal absorption of oseltamivir among critically ill patients, published studies indicate comparable blood levels in ICU patients as compared with normal volunteers.51 Available evidence suggests that an oseltamivir dose of 75 mg twice daily is adequate; higher doses might be indicated and are the current subject of ongoing clinical trials.
Viral shedding can be prolonged in hospitalized patients with seasonal or pandemic influenza. In one study, approximately one-third of patients continued to shed live virus at least 1 week after symptom onset.52 Neuraminidase-inhibitor therapy for longer than 5 days has been used in outbreak situations and in immunocompromised patients known to shed virus for prolonged periods, but formal recommendations on optimal duration are lacking.30 The intravenous neuraminidase inhibitor, peramivir, is available on a compassionate basis for emergency use in severe influenza pneumonia. The recommended dose is 600 mg of peramivir intravenously once daily for 5 days.53
 Adjunctive Pharmacologic Therapy
Adjunctive Pharmacologic Therapy
Several potential adjunctive immunomodulatory or antiviral therapies for treatment of severe influenza exist. Convalescent serum/plasma or hyperimmune globulin derived from patients who have recovered from influenza has been used for many decades. A series of studies were performed using convalescent plasma/serum during the 1918 pandemic and have recently undergone a meta-analysis showing that early, but not late, administration of such products may be associated with a significant survival benefit.54 In addition, several case series suggest the possibility that similar therapy may be of use in severe influenza A/H5N1 infection.55,56
High-dose corticosteroid therapy has been advocated for a variety of infectious and inflammatory conditions.57 Corticosteroids have been useful as adjunctive therapy to suppress inflammatory responses in certain serious infections including severe influenza pneumonia. The uncertain benefits and known risks of corticosteroids in the presence of ongoing infection warrant caution before employing this strategy in primary influenza pneumonia. The use of glucocorticoid therapy for influenza is best limited to randomized clinical trial protocols rather than uncontrolled use. Similarly, a wide variety of immunomodulator agents are commercially available and might have salutary effects in selected patients (e.g., statins, peroxisome proliferator activated receptor alpha and gamma [PPARα, PPARγ] agonists, resveratrol). Many of these agents are readily available at low cost in developing countries and should be studied in controlled clinical trials in patients with severe influenza pneumonia.58
 Secondary Bacterial Pneumonia
Secondary Bacterial Pneumonia
Available evidence indicates that the majority of deaths from the 1918 pandemic occurred as a consequence of secondary bacterial infection.18,19 Similarly, a substantial number of the deaths from the 1957 and 1968 pandemics were caused by bacterial co- or superinfection. The common pathogens in all series have been S. pneumoniae, group A streptococci, S. aureus, and Haemophilus influenzae. Given the frequency of secondary bacterial infection, clinicians should have a low threshold for considering antibiotic coverage against these commonly observed pathogens.
Secondary bacterial pneumonia as a complication of viral pneumonia takes two forms: mixed viral/bacterial pneumonia and postinfluenza pneumonia during the convalescent phase of influenza. Postinfluenza pneumonia is generally attributable to damaged airways and poor mucociliary clearance mechanisms following severe influenza pneumonia.18 The early mixed form of bacterial pneumonia during ongoing viral replication in the airways is more complex, with possible synergism between the bacterial and viral pathogens. Apoptosis of pneumocytes induced by the viral PB1-F2 protein facilitates pneumococcal growth in lung tissue.59 Pneumococci bind to epithelial surfaces more readily if sialic acids have been cleaved by neuraminidase.60 Viral neuraminidase from influenza virus has been found to promote pneumococcal adhesion in lung tissues and increase lethality in experimental pneumococcal pneumonia.61 Early institution of effective antiviral agents with neuraminidase inhibitors might serve to decrease virus replication and decrease the risk for secondary pneumonia.48
 Infection Control in the ICU
Infection Control in the ICU
Patients with suspected influenza should be managed using droplet precautions by healthcare professionals, who should wear a standard surgical tie mask. There are different recommendations as to which face mask is optimal and whether N95 masks or similar personal respirators might be preferable to surgical tie masks. A recent study found limited to no additional protection of N95 masks in comparison to surgical masks, yet many still advocate their use during cough-inducing procedures when treating patients with influenza.62 Vaccines, when available against circulating strains of influenza, should be mandatory for all healthcare workers unless specific contraindications exist. Healthcare workers should also consider appropriate gloves when likely to have contact with body fluids or to touch contaminated surfaces, and they should wear gowns during procedures and patient care activities where clothing might be contaminated. Protective eyewear is recommended when providing direct care in close proximity to the patient.63 Patients with suspected influenza should be in single patient rooms, if available, during the initial phase of hospital admission. If clinical demand exceeds the availability of such quarters, then cohorting of patients with influenza in common areas may be necessary. Influenza patients who must be transported outside of the room should wear a mask if tolerated, or when necessary, an oxygen delivery system that limits the spread of aerosols.
With respect to infection prevention and control related to mode of ventilatory assistance, there is circumstantial evidence from the SARS (severe acute respiratory syndrome) epidemic that noninvasive ventilation and HFOV may promote excess aerosolization of viral-laden particles and place surrounding patients and staff at risk. Limited evidence suggests that the process of endotracheal intubation, especially in an uncontrolled setting, may be associated with increased risk of acquiring infection; however, this risk is mitigated if adequate personal protective equipment is worn.64,65 HFOV circuits should be equipped with microbial filters and a scavenger system to the exhalation port to limit aerosol generation.
 Global Critical Care Collaboration
Global Critical Care Collaboration
A working group composed of members from the international critical care community formed the International Forum for Acute Care Trialists (InFACT) to aid with global collaborative research in critical care.66 For the 2009 H1N1 pandemic, the InFACT group focused on developing a case report form as a reference for generating an international “minimal clinical dataset” through collaboration with members of global critical care societies. This web-entry case report form system was available to clinicians around the world to contribute patient-based data in a manner that can be analyzed in real time and help inform decision makers and clinicians during the outbreak. InFACT also supports large, simple, investigator-initiated interventional studies in many countries. The impact of the InFACT initiative will only be determined over time, but this is an important global critical care attempt to more efficiently and more inclusively improve the care of critically ill patients.
Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086-5096.
Kumar A, Zyarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009;302:1872-1879.
Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Eng J Med. 2009;360:2605-2615.
Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009;360:953-956.
Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197-201.
Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004;303:1838-1842.
Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden F, et al. Seasonal influenza in adults and children–diagnosis, treatment, chemoprophylaxis and institutional outbreak management: clinical practice guidelines of the Infectious Disease Society of America. Clin Infect Dis. 2009;48:1003-1032.
1 Zimmer SM, Burke DS. Historical perspective–emergence of influenza A (H1N1) viruses. N Engl J Med. 2009;361:279-285.
2 Opal SM. Editorial commentary. Coming soon to an ICU near you: severe primary influenza pneumonia from pandemic swine H1N1 influenza. Critical Care. 2009;12:196-197.
3 Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086-5096.
4 Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324(5934):1557-1561.
5 Peltola V, Ziegler T, Ruuskanen O. Influenza A and B virus infections in children. Clin Infect Dis. 2003;36(3):299-305.
6 Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Eng J Med. 2009;360:2605-2615.
7 DuBar G, Azria E, Tesniere A, Dupont H, Le Ray C, Baugnon T, et al. French experience of 2009 A/H1N1v influenza in pregnant women. PLoS One. 5, 2010. pii e13112
8 Opal SM. Swine Flu, pandemics, and critical care. Crit Care. 2009;13:146.
9 Wang H, Feng Z, Shu Y, Hongjie Y, Zhou L, Zu R, et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008;371:1427-1434.
10 Cheung CY, Poon LLM, Lau AS, Luk W, Lau YL, Shortridge KF, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831-1837.
11 Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Therlault S, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703-707.
12 Gamblin SJ, Haire LF, Russell RJ, Stevens DJ, Xiao B, Ha Y, et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science. 2004;303(5665):1838-1842.
13 Garten RJ, Davis TD, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197-201.
14 Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The under recognized burden of influenza in young children. N Engl J Med. 2006;355(1):31-40.
15 Bhat N, Wright JG, Broder KR, Murray EL, Greenberg ME, Glover MJ, et al. Influenza-associated deaths among children in the United States, 2003-2004. N Engl J Med. 2005;353(24):2559-2567.
16 Coffin SE, Zaoutis TE, Rosenquist AB, Heydon K, Herrera G, Bridges CB, et al. Incidence, complications, and risk factors for prolonged stay in children hospitalized with community-acquired influenza. Pediatrics. 2007;119(4):740-748.
17 Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121(4):258-264.
18 Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195(7):1018-1028.
19 Chien Y-W, Klugman KP, Morens DM. Bacterial pathogens and death during the 1918 influenza pandemic. N Engl J Med. 2009;361(26):2582-2583.
20 Fedson DS. Meeting the challenge of influenza pandemic preparedness in developing countries. Emerg Infect Dis. 2009;15(3):365-367.
21 Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, et al. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA. 2009;302(17):1880-1887.
22 Webb SA, Seppelt IM. Pandemic (H1N1) 2009 influenza (“swine flu”) in Australian and New Zealand intensive care. Crit Care Resusc. 2009 September;11(3):170-172.
23 Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in infants and children: a randomized trial. JAMA. 303(1), 2010. 37–36
24 Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. 2009;302(17):1872-1879.
25 Louie JK, Acosta M, Jamieson DJ, Honein MA for the California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27-35.
26 Oluyom-Obi T, Avery L, Menticoglou S, Schneider C, Kumar A, Lapinski S, Zarychanski R. Perinatal and maternal outcomes in critically ill obstetric patients with pandemic H1N1 influenza. A. J Obstet Gynecol (Canada). May 2010. (in press)
27 Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA. 2009;302(17):1896-1902.
28 Rello J, Rodriguez A, Ibanez P, Socias L, Cebrian J, Marques A, et alSemicyuc Working Group. Intensive care adult patients with severe respiratory failure caused by influenza A (H1N1) in Spain. Crit Care. 2009 September 11;13(5):R148.
29 Ramsey CR, Miller RR, Funk D, Kumar A. Ventilator management for hypoxemic respiratory failure attributable to H1N1 novel swine origin influenza virus. Kumar A, Farmer C. Guest Editors. H1N1 novel influenza: pandemic issues for critical care practitioners. Crit Care Med. 2010;38(4):e58-e65. doi:10.1097/CCMOb013e318cde600
30 Funk D, Siddiqui F, Wiebe K, Miller RR, Bautista E, Jimenez E, et al. Practical lessons from the first outbreaks: clinical presentation, obstacles, and management strategies for severe pandemic (H1N1) 2009 influenza pneumonitis. Kumar A, Farmer C, Guest Editors. H1N1 novel influenza: pandemic issues for critical care practitioners. Crit Care Med. 2010;38(4):e30-e37. doi:10.1097/CCM.0b013e3181d10522
31 Hurt AC, Alexander R, Hibbert J, Deed N, Barr IG. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J Clin Virology. 2007;39(2):132-135.
32 Hurt AC, Baas C, Deng YM, Roberts S, Kelso A, Barr IG. Performance of influenza rapid point-of-care tests in the detection of swine lineage A(H1N1) influenza viruses. Influenza Other Respi Viruses. 2009;3(4):171-176.
33 Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, et al. Seasonal influenza in adults and children–diagnosis, treatment, chemoprophylaxis and institutional outbreak management: Clinical Practice Guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003-1032.
34 ARDSNet Investigators. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory. N Engl J Med. 2000;342:1301-1308.
35 Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc BP, et alNIH/NHLBI ARDSNet. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med.. 2006;354:2564-2575.
36 Adhikari NKJ, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334:757-758.
37 Sud S, Sud M, Friedrich JO, Adhikari NKJ. Prone ventilation improves oxygenation but not mortality in acute hypoxemic respiratory failure: systematic review and meta-analysis. CMAJ. 2008;178:1153-1161.
38 The Oscillate Trial. Available at. http://www.clinicaltrials.gov/ct2/show/NCT00474656?term=oscillate&rank=1. Accessed October 3 2009
39 Peek M, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. Early Online Publication, 16 September 2009. doi:10.1016/S0140-6736(09)61069-2.
40 Webb S, et alAustralia and New Zealand Extracorporeal Membrane Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888-1895.
41 Zarychanski R, Stuart TL, Doucette S, Elliot L, Kumar A, Kettner I, et al. Treatment delay, First Nations ethnicity and comorbidities are correlates of severe disease in patients infected with pandemic influenza A (H1N1). CMAJ. 2010;182:257-264.
42 Centers for Disease Control and Prevention. Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009-2010 Season. Available at. http://www.cdc.gov/h1n1flu/recommendations.htm. Accessed October 3, 2009
43 Chen H, Cheung CL, Tai H, Zhao P, Chan JFW, Cheng VCC, Chan K-H, Yuen K-Y. Oseltamivir-resistant influenza a pandemic (H1N1) 2009 virus, Hong Kong, China. Emerg Infect Dis. 2009;15(12):1970-1972.
44 Beigel J, Bray M. Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008;78:91-102.
45 Cheng PKC, Leung TWC, Ho ECM, Leung PCK, Ng AYY, Lai MYY, et al. Oseltamivir- and amantadine-resistant influenza viruses A (H1N1). Emerg Infect Dis. 2009;15(6):966-968.
46 Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009;360:953-956.
47 Singh S, Barghoorn J, Bagdonas A, Adler J, Treanor J, Kinnersley N, et al. Clinical benefits with oseltamivir in treating influenza in adult populations: results of a pooled and subgroup analysis. Clin Drug Invest. 2003;23(9):561-569.
48 Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163(14):1667-1672.
49 Hassan K, McGeer A, Green KA, et al. Antiviral therapy improves outcome of influenza infections in patients requiring admission to intensive care. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy 2009:V537.
50 Oughton MT, Dascal A, Laporta D, et al. Evidence of viremia in two cases of severe pandemic influenza A H1N1/09. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy 2009:V1074i.
51 Taylor WR, Thinh BN, Anh GT, Horby P, Wertheim H, Lindegardh N, et al. Oseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenza. PLoS One. 2008;3(10):e3410.
52 De Serres G, Rouleau I, Hamelin ME, et al. Shedding of novel 2009 pandemic H1N1 (nH1N1) virus at one week post illness onset. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy 2009:K1918a.
53 Birnkrant D, Cox E. The emergency use of authorization of peramivir for the treatment of 2009 H1N1 influenza. N Engl J Med. 2009. online 10.05/NEJM p09, 1047
54 Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599-609.
55 Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450-1451.
56 Kong LK, Zhou BP. Successful treatment of avian influenza with convalescent plasma [1. Hong Kong Med J. 2006;12(6):489.
57 Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et alNIH/NHLBI ARDSNet. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2007;354:1671-1684.
58 Fedson DS. Confronting the next influenza pandemic with anti-inflammatory and immunomodulatory agents: why they are needed and how they might work. Influenza Other Respi Viruses. 2009;3:129-142.
59 McAuley JL, Hornung F, Boyd KL, Smith AM, KcKeon R, Bennink J, et al. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe. 2007;2:240-249.
60 Van der Poll T, Opal SM. The molecular pathogenesis of pneumococcal pneumonia. Lancet. 2009;374:1543-1556.
61 Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia after influenza. J Infect Dis. 2005;192:249-257.
62 Loeb L, Dafoe N, Mahony J, John M, Sarabia A, Glavin V, et al. Surgical mask vs n95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009. 0.1466
63 Ontario Ministry of Health and Long-term Care. Preventing Febrile Respiratory Illnesses. Available at. http://www.health.gov.on.ca/english/providers/program/infectious/diseases/best_prac/bp_fri_080406.pdf. Accessed October 3 2009
64 Fowler RA, Guest CB, Lapinsky SE, Sibbald WJ, Louie M, Tang P, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169:1198-1202.
65 Fowler RA, Scales D, Ilan R. Evidence of airborne transmission of SARS. N Engl J Med. 2004;351:610-611.
66 Marshall J, Abraham E, Adikhari N, et al. InFACT: a global critical care research response to H1N1. Lancet. 2009. DOI:10.1016/S0140-6736(09)