CHAPTER 86 Inflammatory Disorders of the Salivary Glands
Bacterial Infection of the Salivary Glands
Acute Suppurative Sialadenitis
Acute sialadenitis is a bacterial inflammation of the salivary glands. Although these infections occur in all major salivary glands, the parotid gland is most commonly involved. Frequently associated with medically debilitated and postoperative patients, the incidence of acute sialadenitis is approximately 0.02% of hospital admissions. It occurs between 1 in 1000 and 1 in 2000 operative procedures.1 Patients undergoing major abdominal and hip repair surgery have been identified as being at increased risk for acute suppurative sialadenitis. The disease is most commonly reported to occur within the first 2 postoperative weeks.2 Of the medically ill patients affected by sialadenitis, approximately 25% have a malignant lesion and another 50% have a preexisting infection elsewhere than the head and neck. Most affected patients are between 50 and 60 years of age, with an equal incidence among men and women.3
In contrast to the mainly serous saliva produced by the parotid gland, the saliva made by the submandibular and sublingual glands is primarily mucoid. Serous saliva, unlike mucinous saliva, is deficient in lysosomes, IgA antibodies, and sialic acid, which have antimicrobial properties. In addition, the saliva from the submandibular and sublingual glands contains high-molecular-weight glycoproteins that competitively inhibit bacterial attachment to the epithelial cells of the salivary ducts.4 Several predisposing factors might lead to acute sialadenitis. These include diseases such as diabetes mellitus, periodontal disease, hypothyroidism, renal failure, and Sjögren’s syndrome. Medications might produce reduced salivary flow through multiple mechanisms (Fig. 86-1).
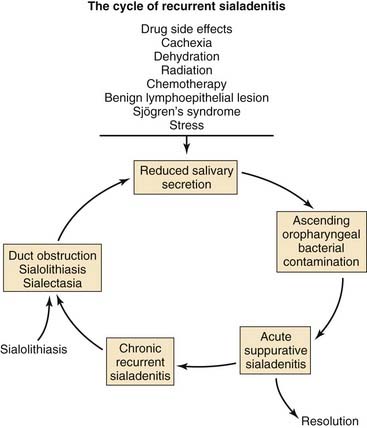
Figure 86-1. Pathophysiology and predisposing factors for the development of acute and chronic suppurative sialadenitis.
Mechanical impairment of salivary flow also predisposes to acute infection. Stenosis of the salivary ducts secondary to trauma or a foreign body has been reported to result in acute sialadenitis. Sialolithiasis, more frequent in the ducts of the submandibular and sublingual glands, may also contribute to acute infection but more commonly produces chronic salivary gland infection.5 The most commonly cultured organism among hospitalized patients with acute sialadenitis is penicillin-resistant Staphylococcus aureus, which is responsible for 50% to 90% of cases. In addition, Streptococcus species including Streptococcus pyogenes, Streptococcus viridans, and Streptococcus pneumoniae, as well as Hemophilus influenzae, can be isolated in community-acquired cases. More recently, the importance of anaerobic and gram-negative bacteria in acute suppurative parotitis has been established. In one study, anaerobic bacteria were isolated from 64% of cases of acute suppurative sialadenitis. The anaerobic bacteria most frequently cultured were Peptostreptococcus, bacteroides species, Prevotella species, and fusobacterium. In Southeast Asia, Burkholderia pseudomallei is a common cause of parotitis. This apparent shift in microbiology might be the result of improved anaerobic bacterial culture technique rather than a true change in the flora of acute salivary gland infections.3,6,7
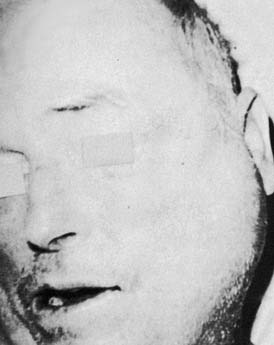
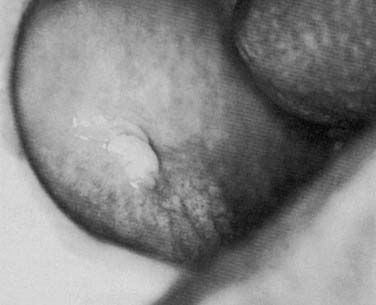
Acute suppurative sialadenitis presents with local symptoms consisting of rapid onset of pain and swelling over the affected salivary gland (Fig. 86-2). Systemic manifestations include fever, chills, and malaise. Importantly, one should review the medical and surgical history for the previously noted risk factors that would make a patient more susceptible to acute salivary gland infection. On physical examination the patient might demonstrate signs of systemic dehydration with dry mucous membranes. Local findings include tenderness to palpation, with warmth and induration of the overlying skin. Bimanual palpation of the gland results in suppurative discharge from the duct orifice in approximately three fourths of cases (Fig. 86-3).2 Although typically limited to one gland, multiple glands might be affected, with a reported incidence of bilateral involvement of up to 25% of cases.8
Cultures of purulent drainage from the duct orifice should be obtained. Recent reports advocate the use of percutaneous needle aspiration of the involved duct. This technique limits the amount of contamination encountered by means of an intraoral culture.6,9
Although the diagnosis of acute parotitis is usually apparent, nonparotid swelling can mimic parotitis. Such conditions include lymphoma, lymphangitis external otitis, Bezold’s abscess, cervical adenitis, dental abscesses presenting as buccal or masseteric space abscesses, and infected branchial cleft or sebaceous cysts.10
Antimicrobial therapy is an essential part of the management of acute salivary gland infections. Antimicrobial therapy is initiated empirically toward gram-positive and anaerobic bacteria. However, the recovery of β-lactamase–producing bacteria in 75% of patients requires the use of augmented penicillin and antistaphylococcal penicillin or a first-generation cephalosporin.6,11 The use of clindamycin or the addition of metronidazole to the first-line agents to broaden anaerobic coverage has been advocated by some authors.6 In patients from nursing home environments or cases of nosocomial infection, empiric vancomycin has been advocated given the prevalence of methicillin-resistant S. aureus. Aminoglycosides have also been proposed by some in the treatment of critically ill patients. Culture results should be used to further direct antimicrobial treatment. Response to antimicrobial therapy is seen within 48 to 72 hours of initiating treatment and should continue for 1 week after resolution of symptoms.1
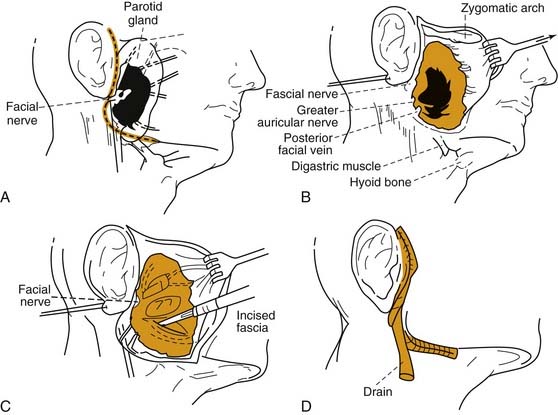
Rarely, conservative measures fail to eradicate the infection, and surgical drainage of a loculated abscess is necessary. The surgical approach involves elevation of an anterior-based facial flap with abscess drainage by way of radial incisions in the parotid fascia parallel to the facial nerve branches. A drain should be placed, and the wound edges should be loosely approximated, with the central aspect left to heal by secondary intention (Fig. 86-4).
Complications of acute suppurative parotitis are unusual. Suppuration confined to the duct lumen might eventually erode through the epithelium into the interstices of the parenchyma, creating multiple small abscesses that might coalesce into larger collections that can infiltrate a number of potential spaces of the neck. Osteomyelitis of the mandible and temporomandibular joint, thrombophlebitis of the jugular vein, septicemia, respiratory obstruction, and death are potential sequelae of suppurative parotitis. Rupture through the floor of the external auditory canal, spontaneous drainage through the cheek, and extension to the face, neck, and mediastinum have also been reported (Fig. 86-5).12,13 Facial nerve paralysis, whether complete or incomplete, is uncommon. The etiology of paralysis is unknown but is thought to be due to the virulence of the offending organisms, perineuritis, or acute nerve compression. Because facial nerve paralysis is highly suggestive of parotid malignancy, all patients with paralysis should be followed until the inflammatory process has resolved and facial nerve function returned.14 Acute suppurative sialadenitis is associated with 20% to 40% reported mortality; however, the prognosis is primarily related to the medical condition of the patient.2
Neonatal Suppurative Parotitis
Infections of the salivary glands are uncommon in the neonate. Neonatal suppurative parotitis (NSP) is more common in preterm and male neonates and those with dehydration. S. aureus is the most common pathogen, and Escherichia coli, Pseudomonas aeruginosa, and group B streptococci have also been implicated in NSP.15 The oral cavity serves as the portal of entry in most patients.16 However, transient bacteremia, usually from gram-negative bacteria, may also be responsible.17
Diagnosis of NSP is made on the basis of clinical findings and Gram’s stain, as well as culture of pus from the duct or from fine-needle aspiration (FNA) of the gland. Therapy consists of parenteral antibiotics directed at S. aureus and gram-negative bacilli until culture and sensitivity results are available. Drainage procedures should be used only when prompt clinical improvement does not occur or fluctuance of the gland increases.15
Recurrent Parotitis of Childhood
Recurrent parotitis of childhood (RPC) is an uncommon inflammatory condition of the parotid gland characterized by periodic episodes of swelling and pain. It can be distinguished from suppurative parotitis by the inability to express pus from the parotid duct. The age of onset ranges from 8 months to 16 years, with a reported peak incidence between the ages of 3 and 6 years.17,18 Boys are more frequently affected than girls.
The cause of recurrent parotitis of childhood remains unknown, and multiple etiologies have been proposed. Several authors advocate that congenital ectasia of portions of the secondary ductal system predisposes children for bacterial colonization leading to recurrent parotitis.17 S. aureus and S. viridans are the most commonly isolated organisms from the parotid ducts of patients. A familial form of RPC has been described with autosomal inheritance.19 Immunologic abnormalities with isolated IgG3 and IgA deficiencies have been associated with recurrent parotitis. In addition, parotid swelling might be the sole manifestation of juvenile-onset primary Sjögren’s syndrome. Several viruses might also play a role in RPC. A history of mumps parotitis often exists. Repeated multiplication of the Epstein-Barr virus in the parotid gland might be responsible for establishing repeated parotid gland inflammation, and RPC has a reported incidence of 20% in HIV-infected children.20,21
The histologic appearance of affected parotid glands demonstrates massive periductal lymphocytic infiltration. The intraglandular ducts are dilated and measure approximately 1 to 2 mm in diameter.20
Clinically, patients are seen with recurrent episodes of acute or subacute parotid gland swelling along with fever, malaise, and pain frequently after a meal. Episodes of RPC are commonly unilateral, but bilateral involvement has been reported in up to 44% of cases. Afflicted patients tend to experience exacerbations every 3 to 4 months; however, in one series with 53 patients, mean frequency was eight episodes per year. Each might last days to weeks.17 Diagnosis is usually delayed more than 1 year.
Several imaging modalities may be used to study the ductal system of patients with RPC. The characteristic finding is sialectasis, which on conventional sialography appears as numerous scattered punctate pools of contrast. US of the parotid gland reveals an enlarged gland with multiple small hypoechoic areas. These areas represent both sialectasis of the ducts and the surrounding lymphocytic infiltration. Recently, MR sialography has been reported to be useful in the evaluation of RPC.20 It is a noninvasive study, not requiring contrast, and may be used during acute episodes.
Chronic Sialadenitis
Chronic sialadenitis is a localized condition of the salivary gland characterized by repeated episodes of pain and inflammation. The parotid gland is the most frequently affected site.22 The initial inciting factor is believed to be salivary duct obstruction, with resulting salivary stasis. The most common cause of ductal obstruction is sialolithiasis. Stricture of the salivary duct, extrinsic compression by tumor, stenosis secondary to scar in adjacent tissue, congenital dilation, and foreign body are additional causes of duct obstruction.23 Salivary stasis from obstruction predisposes patients to episodes of infection and inflammation. Recurrent inflammatory reactions result in progressive acinar destruction with fibrous replacement and sialectasis (Fig. 86-6).
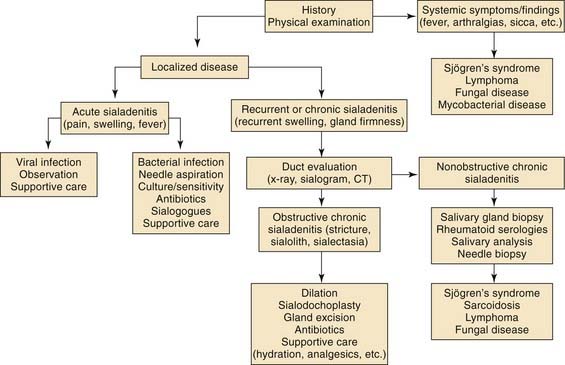
Figure 86-6. A simplified algorithm for the diagnosis and management of chronic salivary gland inflammation.
Treatment of chronic sialadenitis can be frustrating, and no treatments are consistently successful. Tympanic neurectomy and parotid duct ligation are procedures advocated for chronic parotitis to induce atrophy of the gland, but these have failed to show reliable benefit. Surgical removal of the gland when conservative management has failed to control symptoms has been shown to be safe and effective.24
Possible complications of chronic sialadenitis include the development of a benign lymphoepithelial lesion, Kuttner’s tumor, and ductal carcinoma.25–27 A benign lymphoepithelial lesion is characterized by a lymphoreticular infiltrate with acinar atrophy, irregularly placed nuclei, and ductal metaplasia. The metaplasia results in the development of epimyoepithelial islands. Women in the fifth to sixth decade of life are more commonly affected. It has been associated with Sjögren’s syndrome and has also been termed Mikulicz’s disease. A benign lymphoepithelial lesion typically presents as an asymptomatic mass. FNA may be used for diagnosis and allow for a nonsurgical approach to management.27 However, patients must be followed because there are reports of the development of malignancy from these lesions.26
Kuttner’s tumor is a benign inflammatory process similar to a benign lymphoepithelial lesion. However, the lesion occurs almost exclusively in the submandibular gland. Kuttner’s tumors are most common in middle-aged adults and present as a painless mass. Histologically, there is a heavy lymphoid infiltrate among discrete tubular structures with regularly aligned nuclei, which differentiates them from a benign lymphoepithelial lesion. Kuttner’s tumor might also be followed clinically. Again, careful and routine assessment of patients with chronic sialadenitis is underscored by reports of the development of malignancies including salivary ductal carcinoma.26
Sialolithiasis
Sialolithiasis is the formation of calculi in the ductal system of the salivary glands. The submandibular gland is most commonly affected, with 80% to 90% of stones developing in Wharton’s duct. Approximately 10% to 20% of calculi form in the parotid (Stensen’s) duct and 1% in the sublingual duct.28 Patients in their fifth to eighth decade of life represent most cases. Sialolithiasis in children is rare; however, if afflicted, children most commonly are seen at 10 years of age.6 Men tend to develop calculi much more frequently than women.
Salivary stones are composed predominantly of calcium phosphate and carbonate in combination with an organic matrix of glycoproteins and mucopolysaccharides. Small amounts of other salts such as magnesium, potassium, and ammonium are also involved in stone formation.29
The exact etiology of sialolithiasis is uncertain. There does not seem to be a relationship between their formation and serum calcium or phosphorous levels.30 Salivary stasis and ductal inflammation and injury are important factors contributing to stone formation. It is believed that intermittent salivary stasis results in an alteration of the mucoid elements of saliva forming an organic gel. This gel becomes the framework for the deposition of salts, which leads to the development of calculi. The relationship between sialolithiasis and chronic sialadenitis might differ between salivary glands.28 In the submandibular gland, development of a sialolith might be the primary event that results in stagnation of saliva and inflammation, encouraging bacterial migration and resultant sialadenitis. In contrast, inflammation and ductal injury in the parotid gland from chronic sialadenitis are believed to be the inciting processes for sialolithiasis.
Several factors might account for the propensity of salivary stones to form in the submandibular gland. Wharton’s duct is longer, has a larger caliber, and is angulated against gravity as it courses around the mylohyoid muscle, all of which results in slower salivary flow rates. Also, the saliva produced by the gland itself is more viscous and has a higher calcium and phosphorous concentration.28,31
In the parotid gland, stones are most commonly located at the hilum or parenchyma. Sialolithiasis in the submandibular gland tends to develop in the duct.32
Patients with sialolithiasis are seen with recurrent episodes of postprandial salivary colic with pain and swelling. The patient might also have a history of multiple cases of acute suppurative sialadenitis (Fig. 86-7). On physical examination, bimanual palpation will reveal the presence of a palpable stone in most cases involving the submandibular duct. Parotid stones might be noted at the orifice of Stensen’s duct or along the course of the duct.
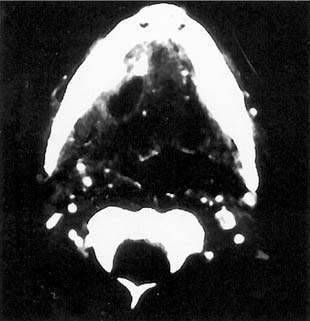
Figure 86-7. Development of acute suppurative sialadenitis and abscess secondary to submandibular gland sialolithiasis.
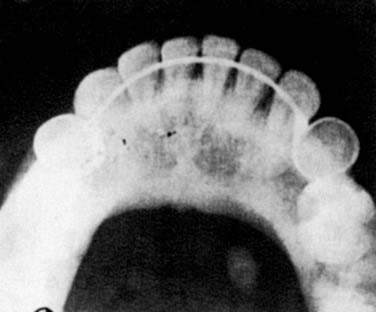
Imaging of the salivary glands for sialolithiasis may be accomplished with plain radiographs, conventional and digital sialography, US, CT, and MRI. Plain radiographs that use intraoral or occlusal views might be used to identify radiopaque stones (Fig. 86-8). These stones are much more common in the submandibular gland than in the parotid gland, where up to 80% of stones are radiolucent. However, other calcifications in the area such as phleboliths, arterial atherosclerosis of the lingual artery, or calcified cervical lymphadenopathy make the interpretation of plain radiographs more difficult. US has proven value in the diagnosis of sialolithiasis, with the ability to detect 90% of stones greater than 2 mm.33 CT scanning with fine cuts is extremely accurate at detecting salivary stones. Digital subtraction sialography, which lessens the interference of surrounding bony structures, has been the diagnostic test of choice for sialolithiasis. This can detect radiolucent stones and has a reported sensitivity of 95% to 100%.27 Several disadvantages are inherent to sialography. It is an invasive technique with the possible side effects of contrast material. Sialography is also contraindicated for stones located in the oral portion of Wharton’s duct and in cases of active infection. MR sialography is a relatively new and noninvasive technique being used to evaluate the salivary duct system. Studies indicate that MR sialography of the submandibular duct with evoked salivation has accuracy similar to digital sialography and superior to US.33 This imaging modality becomes a superior alternative in patients with contraindications to digital sialography. FNA might be indicated in patients with suspected sialolithiasis when no stone is detected and the possibility of malignancy exists. However, pathologic diagnosis in this setting is difficult, and the ductal metaplasia of sialolithiasis secondary to stone formation might be misinterpreted with mucoepidermoid carcinoma.30
Initial nonsurgical management of patients with sialolithiasis consists of the use of sialogogues, local heat, hydration, and massaging of the involved gland. If salivary gland infection is suspected, prompt antibiotic coverage should be started.28
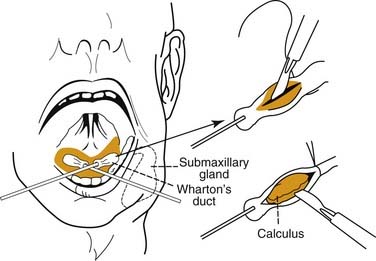
Surgical intervention for sialolithiasis depends on the anatomic location of the stone. Submandibular stones that are palpable in the mouth and are no more than 2 cm from the duct orifice may be removed using an intraoral approach. This may simply involve milking the stone through the duct opening manually or incising the duct directly over the stone without the need for closure of Wharton’s duct after the procedure (Fig. 86-9). Submandibular stones located in a more posterior location will require sialadenectomy,28 which may be performed through a transcervical or a transoral approach.34
Relatively recent treatment alternatives for sialolithiasis are extracorporeal shock wave lithotripsy and the use of sialoendoscopy. Lithotripsy reduces calculi to small fragments that are then flushed out of the duct with spontaneous salivation or the use of a secretagogue.35 Sialoendoscopy involves the use of rigid endoscopes to visualize and remove salivary duct stones. Several researchers have reported on the efficacy of this technique using a combination of baskets, graspers, and intracorporeal lithotripsy to treat sialolithiasis in both the parotid and submandibular glands.36–38
Viral Infections of the Salivary Glands
Mumps
Mumps classically defines an acute nonsuppurative viral parotitis caused by the paramyxovirus. The term “mumps” is derived from the Danish “mompen,” which means mumbling (like an old man), and describes the difficulty with speech because of inflammation and trismus.5 Mumps is the most common cause of nonsuppurative acute sialadenitis, and 85% of cases occur in children younger than the age of 15.11 The disease is highly contagious and occurs worldwide, with a peak incidence in the spring in temperate climates and with little variation in the tropics.39
The paramyxovirus is a ribonucleic acid virus that is endemic in the community and is disseminated by means of airborne droplets from salivary, nasal, and urinary secretions. Interestingly, no known animal reservoir, insect vector, or human carrier has been identified. The disease is maintained by spread from acute cases.2 Viruses other than paramyxovirus have been implicated as causes of acute viral parotitis: Coxsackie viruses A and B, enteric cytopathic human orphan virus, cytomegalovirus, and lymphocytic choriomeningitis virus.40
The virus enters through the upper respiratory tract and has an incubation period of 2 to 3 weeks. During this incubation period, the virus multiplies in the upper respiratory tract epithelium and parotid gland. The virus then localizes to biologically active glandular and central nervous system tissue. Patients will typically experience a viral prodrome consisting of low-grade fever, headache, myalgia, anorexia, arthralgia, and malaise just before parotid gland symptoms. The parotitis is characterized by localized pain and edema of the gland along with otalgia, trismus, and dysphagia. Eating or chewing exacerbates the pain. In 75% of cases, there will be bilateral parotid gland swelling that causes displacement of the pinna.2 Commonly, the gland on one side will swell first, followed by enlargement of the other gland in 1 to 5 days. The submandibular gland might be affected in rare cases. Physical examination of the involved gland will demonstrate nonpitting edema that is tense and firm. The overlying skin will be stretched with a glazed appearance, but there should be no erythema or warmth.
The diagnosis of viral parotitis is confirmed through viral serology. Complement-fixing soluble (S) antibodies against the nucleoprotein core of the virus are the earliest antibodies to appear. Their levels peak at 10 days to 2 weeks and disappear within 8 to 9 months. Therefore these S antibodies are associated with active infection. Complement-fixing viral (V) antibodies against outer surface hemagglutinin appear later than S antibodies but persist at low levels for years. If serology for paramyxovirus is negative, antibody titers for other viral agents that might also result in parotitis may be obtained. A fourfold increase in antibody titer is diagnostic for acute infection.5 The mumps skin test has no diagnostic value in the setting of acute infection because dermal hypersensitivity does not develop until 3 to 4 weeks after exposure to the virus. A leukocyte count will occasionally be remarkable for leukopenia, and there is also an elevation in the serum salivary-type amylase.
Management of acute viral parotitis involves supportive measures that include bedrest, oral hygiene, hydration, and dietary modifications to minimize glandular secretory activity. Fever will usually subside before the resolution of glandular edema, which requires several weeks. Complications of mumps virus include orchitis, aseptic meningitis, pancreatitis, nephritis, and sensorineural hearing loss.2
The prevention of mumps is by means of vaccination with the live attenuated Jerry Lynn vaccine. The vaccine is administered subcutaneously, usually in combination with measles and rubella vaccines after 12 months of age. The antibodies produced persist for at least 5 years. The vaccine is contraindicated in pregnancy, immunocompromised states, allergies to neomycin, and pregnancy.3
HIV
HIV is associated with several pathologic processes involving the salivary glands. These include neoplasms such as Kaposi’s sarcoma and lymphoma and lymphoproliferative and cystic enlargement of the major salivary glands with accompanying salivary dysfunction (Fig. 86-10). HIV-associated salivary gland disease (HIV-SGD) is a term used to describe this diffuse enlargement of the salivary glands. HIV-SGD may affect patients throughout all stages of the disease and may be the initial manifestation of HIV infection.41 The parotid gland is the most commonly affected of the salivary glands. Salivary secretions have been shown to contain low concentrations of the virus; however, studies have failed to show infection in the epithelial cells of the salivary gland. No other viruses, such as the cytomegalovirus or Epstein-Barr virus, have been isolated in association with HIV-SGD.
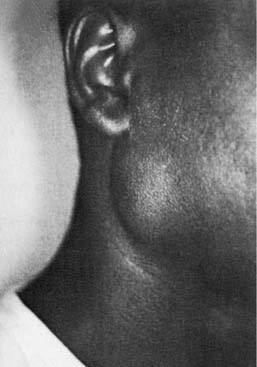
Figure 86-10. Clinical appearance of human immunodeficiency virus–related enlargement of the parotid gland.
(From Debo RF, Davidson M, Petrow CA. Pathologic quiz case 2. Benign lymphoepithelial cyst of the parotid gland associated with HIV infection. Arch Otolaryngol Head Neck Surg. 1990;116:487.)
The histologic findings of the salivary glands affected by HIV-SGD are varied. The enlarged intraparotid and periparotid lymph nodes demonstrate a uniform follicular hyperplasia characteristic of the generalized lymphadenopathy associated with HIV infection elsewhere in the body. In addition, there are parotid nodes containing salivary epithelial structures and epithelial-lined cysts in addition to the follicular hyperplasia. In some cases, there is diffuse infiltration of the gland by lymphoid elements with cystic dilation of the salivary ducts. It is believed that the surrounding lymphoid hyperplasia results in incomplete ductal obstruction, leading to the growth of epithelial cysts (Fig. 86-11).42
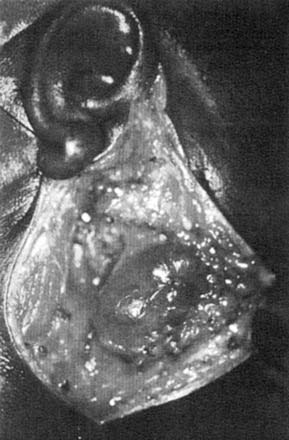
Figure 86-11. Intraoperative appearance of a parotid gland cyst occurring in a human immunodeficiency virus–seropositive patient.
(From Debo RF, Davidson M, Petrow CA. Pathologic quiz case 2. Benign lymphoepithelial cyst of the parotid gland associated with HIV infection. Arch Otolaryngol Head Neck Surg. 1990;116:487).
Patients with HIV-SGD report a history of gradual, nontender enlargement of one or more of the salivary glands. The glandular swellings might fluctuate but are generally stable and long standing. Decreased salivary gland function results in xerostomia and sicca symptoms. This sicca symptom complex clinically mimics Sjögren’s syndrome and has resulted in the classification of another HIV-related salivary gland pathologic process known as the diffuse infiltrative lymphocytosis syndrome (DILS).43
The radiographic evaluation of patients with HIV-SGD involves the use of CT and MRI. On CT, the parotid gland generally demonstrates multiple cysts that appear as low-attenuation, thin-walled masses and diffuse lymphadenopathy. The MRI will show homogeneous masses of intermediate signal intensity on proton density and T2-weighted images.41
Medical treatment of HIV-SGD involves the use of zidovudine, maintenance of good oral hygiene, and the use of sialogogues. Patients with DILS and progressive visceral lymphocytic infiltration may be treated with corticosteroids and immunosuppressive therapy.44 Some controversy exists regarding HIV-SGD and the need for surgical excision and pathologic examination of an enlarged salivary gland. In general, in patients with documented HIV-SGD, it has been demonstrated that the findings of needle aspiration and CT or MRI are sufficiently typical to provide a presumptive diagnosis, justifying conservative clinical observation.45
Granulomatous Infections of the Salivary Glands
Tuberculous Mycobacterial Disease
Tuberculous salivary gland infection is most common in older children and adults. The disease is spread by close person-to-person contact. Primary salivary gland infection by M. tuberculosis is believed to evolve from a focus in the tonsils or gingival sulcus ascending to the glands by way of their ducts. This may then spread to the cervical nodes through the lymphatic drainage.46 This localized, extrapulmonary type of tuberculous infection most frequently affects the parotid gland. Secondary infection of the salivary glands occurs by way of hematogenous or lymphatic spread from the lungs. Mycobacteria are encapsulated in the intraglandular lymph nodes and might be reactivated many years after the acute pulmonary infection. The submandibular gland is the more commonly involved gland after systemic tuberculous infection.47
Clinically, tuberculous salivary gland infection presents in two different forms. The first is an acute inflammatory lesion with diffuse glandular edema that may be confused with an acute sialadenitis or abscess. A second chronic tumorous lesion is seen as a discrete slow-growing mass that mimics a neoplasm.27 Constitutional signs including fever, night sweats, and weight loss might be absent. Involvement of the facial nerve is rare.
The chest x-ray is commonly negative but might show evidence of healed granulomatous disease. The CT images of tuberculous infection in the head and neck are described as having three patterns. The first, early in the course of disease, demonstrates involved lymph nodes with nonspecific homogeneous enhancement. In the second pattern, there is a nodal mass with central lucency and thick rims of enhancement and minimally effaced fascial planes. The third is described as fibrocalcified nodes, usually seen in patients previously treated for tuberculosis.30
In the setting of a tuberculous infection, the purified protein derivative (PPD) skin test should be positive. FNA biopsy may be used for diagnosis with a lower risk of producing a draining fistula than incisional biopsy. The FNA sample is analyzed for characteristic cytologic features: granulomatous inflammation and epithelioid histiocytes. In addition, material may be sent for culture and acid-fast smears.48
Once diagnosed, M. tuberculosis infection may be treated with triple-drug therapy. In cases in which the diagnosis is uncertain or the lesion is resistant to medical therapy, complete surgical excision is both diagnostic and curative.27
Nontuberculous Mycobacterial Disease
Nontuberculous mycobacteria (NTM) have become an increasingly important disease of childhood. In fact, more than 92% of mycobacterial cervicofacial infections in children are a result of NTM.49 The disease mainly affects children younger than 5 years of age, with most cases occurring between 16 and 32 months of age. The specific organisms most commonly classified as NTM are Myocbacterium kansasii, Myocbacterium scrofulaceum, and Myocbacterium avium–intracellulare. Infection by Mycobacterium bovis has decreased dramatically with the institution of milk pasteurization. These organisms are commonly found in soil, water, domestic and wild animals, milk, and other food items.50 The portal of entry is believed to be through the mouth, and the tonsils have been implicated in particular.
The typical clinical presentation is of a rapidly enlarging and persistent neck mass that has failed to respond to antibiotic therapy in a pediatric patient. The skin becomes adherent to the surrounding tissues and develops a characteristic violaceous discoloration (Fig. 86-12). The infection might progress to fluctuation and the development of a draining sinus. These lesions generally produce few systemic symptoms. Associated cervical lymphadenopathy is more commonly unilateral and located in the high jugular nodes or preauricular areas.49 The differential diagnosis should include all diseases specific to the salivary glands, as well as other granulomatous diseases, acute bacterial or viral infection, and malignancy. Chest x-ray findings are typically absent. Contrast-enhanced CT images show asymmetric cervical lymphadenopathy with contiguous low-density, necrotic, ring-enhancing masses involving the subcutaneous fat and skin. In addition, inflammatory stranding of the subcutaneous fat characteristic of bacterial inflammation is minimal or absent with NTM infection.51
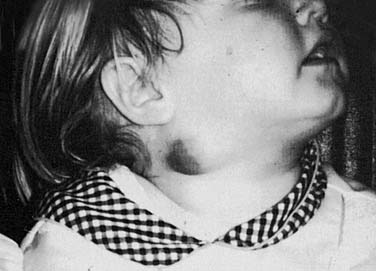
Figure 86-12. A 4-year-old girl with nontuberculous mycobacterial involvement of periparotid lymph nodes.
Recently, there have been numerous approaches described to diagnose an NTM infection. FNA biopsy is one method, but it does carry the risk of fistula formation. PPD skin testing might be negative; however, NTM-specific antigens have been developed and are reported to be extremely successful for diagnosis. Newer methods that use polymerase chain reaction techniques to detect mycobacterial RNA in tissue and Mycobacterium avium deoxyribonucleic acid in gastric aspirates are still not widely available.50 Traditional culture of NTM might take up to 6 weeks and is frequently negative.
Medical treatment of NTM with prolonged courses of antimicrobials, such as clarithromycin, has been advocated but is not well established. The treatment of choice is complete surgical excision of the involved salivary gland and nodes.49,50
Other Granulomatous Diseases
Actinomycosis
Actinomycosis is an infectious disease caused by the actinomyces species, a gram-positive, anaerobic, non–acid-fast bacillus. The actinomyces have a branching, filamentous appearance and are similar in appearance and pathogenicity to mycobacteria and fungi. They are a normal commensal organism found in high concentrations in the tonsils and carious teeth. Actinomycosis israelii is the most commonly encountered species, with the balance of cases attributable to Actinomycosis bovis and Actinomycosis naeslundii. The three major clinical infections caused by actinomyces are cervicofacial (55%), abdominopelvic (20%), and pulmonothoracic (15%).52
In most cases, poor oral hygiene combined with trauma to the mucosa permits invasion of the organism, leading to a slowly progressive inflammatory reaction. Diabetes, immune suppression, long-term steroid use, and malnutrition have also been implicated as predisposing factors. Isolated salivary gland involvement probably occurs by means of retrograde ductal migration and primarily occurs in the parotid gland.53 Involvement of the salivary glands also can occur as part of direct spread of an invasive cervicofacial infection.
Patients typically have a painless, indurated enlargement of the involved gland that might suggest a neoplasm. A chronic purulent drainage might occur with granulomatous involvement and spread to adjacent tissue. The periphery of the lesion is densely fibrotic and avascular. The development of multiple draining cutaneous fistulas is quite common.52 Facial nerve involvement has not been described. A history of recent dental disease and manipulation is common. Constitutional symptoms, malaise, leukocytosis, and lymphadenopathy are typically absent. CT scans typically demonstrate obliteration of the normal tissue planes and extensive soft-tissue destruction.49
Anaerobic cultures are obtained for species identification and to confirm the diagnosis; however, the recovery rate in culture is less than 50%.52 Diagnosis is assisted by needle aspiration of the mass or a fistula swab with smears and stains to examine for the presence of sulfur granules and the organism. Sulfur granules have also been described for nocardiosis, but their identification in the presence of filamentous gram-positive rods is diagnostic for actinomycosis.54 Biopsy specimens show firm fibrous encasement of multiloculated abscesses containing whitish yellow purulent discharge.
Antimicrobial therapy should consist of a 6-week parenteral course followed by an additional 6 months of oral management to completely eradicate the organism. The antimicrobial of choice remains penicillin because Actinomyces species are not known to be resistant to penicillin. Other acceptable alternatives include clindamycin, doxycycline, or erythromycin.52 Surgical excision is necessary to remove extensive fibrosis and sinus tracts, in cases with a poor response to antibiotics, and for diagnosis. Response to management is generally favorable, with cure rates approaching 90% despite a delayed diagnosis in most instances.55
Cat-Scratch Disease
Cat-scratch disease (CSD) is a granulomatous lymphadenitis that most commonly results from cutaneous inoculation caused by scratch trauma from a domestic cat. The causative organism is classified as Bartonella henselae, a gram-negative bacillus. Approximately 90% of patients who have CSD report a history of exposure to cats, and 75% of these patients have experienced a cat scratch or bite. Dogs have been implicated in 5% of CSD cases. CSD affects approximately 22,000 persons and results in hospitalization of approximately 2000 persons each year in the United States. The head and neck is the second most common site for CSD after the upper extremity.56 The reservoir for B. henselae has been shown to be kittens. The major vector by which cats themselves are believed to become infected is the cat flea.57
The typical history is of a papule or pustule at a scratch or bite site followed in 1 to 2 weeks by the development of lymphadenopathy in the region of inoculation. The nodes will slowly enlarge over a period of 1 to 2 weeks and might not resolve for 2 to 3 months. Erythema and fluctuance of the involved nodes with spontaneous suppuration will occur in a reported 10% to 30% of patients.57,58 Fever and mild systemic symptoms might occur in up to one third of patients. In the head and neck, this most commonly occurs in the submandibular and cervical areas. However, preauricular adenopathy might be confused with a parotid neoplasm.
The diagnosis of CSD has changed with advances in serologic and molecular biology techniques. These methods have replaced the need for cat-scratch skin testing. Testing for the presence of antibodies to B. henselae is now the most commonly used test to confirm the diagnosis.57 The two methods used for antibody detection are the indirect fluorescent antibody (IFA) and the enzyme immunoassay (EIA). The test with greatest sensitivity is a Bartonella polymerase chain reaction (PCR) hybridization assay with an aspirate or biopsy specimen. This test might not be as easily available as the antibody detection techniques.59 If tissue is removed for diagnosis, histologic examination might demonstrate cat-scratch bacilli with the use of Warthin-Starry silver staining. Lymph node involvement shows reticular cell hyperplasia, granuloma formation, and widening of arteriolar walls. In more advanced stages, stellate areas of necrosis coalesce to form multiple microabscesses. Bartonella is a slow-growing organism, and culture requires a 6-week incubation period.
Atypical presentations of CSD in the head and neck have been reported. Parinaud’s oculoglandular syndrome is a unilateral granulomatous conjunctivitis associated with a preauricular or submandibular lymph node on the affected side. In addition, a report of diffuse parotid enlargement with facial nerve palsy secondary to CSD has been described.58 Other atypical presentations include vertebral osteomyelitis encephalitis, granulomatous hepatitis, and optic neuritis. In immunocompromised patients, systemic B. henselae infection might result in cutaneous proliferative vascular lesions known as bacillary angiomatosis.
In most cases no active therapy is required. The patient should be reassured that the lymphadenopathy is self-limited and usually will resolve spontaneously in 2 to 4 months. However, in patients who are systemically ill or highly symptomatic, antibiotic therapy is recommended. The β-lactam antibiotics are ineffective in the treatment of CSD. The antibiotics reported to be most effective are rifampin, erythromycin, gentamycin, azithromycin, and ciprofloxacin.57
Toxoplasmosis
Both disseminated and lymphadenopathic forms of the disease have been described. Immunocompromised individuals are most at risk for the disseminated form of the disease, which features myalgia, lethargy, and anorexia combined with hepatosplenomegaly, pericarditis, and myocarditis. Alternately, the lymphadenopathic variety occurs much more commonly, with most patients being seen with isolated cervical lymphadenopathy.60
Definitive diagnosis can only rarely be provided by isolation of the organism. However, there are characteristic histopathologic findings in affected lymph nodes. The lymph node architecture is preserved, with hyperplastic follicles and germinal centers showing abundant mitoses and necrotic nuclear debris. Epithelioid cells with abundant, pale eosinophilic cytoplasm, which occur singly or in groups, are found in cortical and paracortical zones and sinuses.61 Confirmation of a presumptive histologic diagnosis is made by acute and convalescent serologic testing. Chemotherapy is generally reserved for obviously progressive infections or those involving pregnant or immunocompromised individuals and consists of the combined administration of pyrimethamine and trisulfapyrimidines.
Noninfectious Inflammatory Diseases of the Salivary Glands
Sjögren’s Syndrome
Sjögren’s syndrome is a chronic autoimmune disorder of the exocrine glands. The disease might be systemic and involve multiple glands; however, the salivary and lacrimal glands are primarily affected. The disease is characterized by a lymphocytic infiltration, with resulting glandular hypofunction leading to dryness of the mouth and eyes.62
Sjögren’s syndrome might have a variable clinical presentation. When Sjögren’s syndrome is confined to the exocrine glands, it is defined as primary Sjögren’s syndrome. Secondary Sjögren’s syndrome refers to patients who have the characteristic signs and symptoms of primary Sjögren’s syndrome associated with another autoimmune disease. The disease might even evolve into a malignant lymphoid process. The estimated prevalence of Sjögren’s syndrome is believed to be 1% to 3%. The disease is most commonly seen in patients during their fourth to fifth decades of life. More than 90% of patients are women.63
The etiology of Sjögren’s syndrome is uncertain but is believed to involve an interaction among a patient’s genetics, immune system, and environmental exposures. Genetic factors involving the major histocompatibility complex and a certain group of its alleles, such as HLA-B8 and HLA-DR3, are believed to create a predisposition for the development of Sjögren’s syndrome.62 These patients are susceptible to an initial, inciting environmental event. This event is most likely a viral infection that results in an aberrant autoimmune reaction. This autoimmune reaction leads to a dense lymphocytic infiltration of the exocrine glands and the production of multiple autoantibodies. Two distinct autoantibodies found in Sjögren’s syndrome are two ribonuclear proteins known as Ro (or SS-A) and La (or SS-B). The presence of these antibodies is used to assist in establishing the diagnosis of Sjögren’s syndrome.63
The physical examination of patients with Sjögren’s syndrome will demonstrate dry, sticky oral mucosal surfaces. Multiple dental caries and the absence of pooled saliva in the floor of the mouth may be visualized. The tongue is typically smooth with fissures and atrophy of the filiform papillae occurs. Patients with Sjögren’s syndrome will also commonly have an intraoral fungal overgrowth with Candida albicans. The examination of the salivary glands results in the expression of scant or cloudy saliva from the ducts. Salivary gland enlargement, most commonly in the parotid glands, occurs in 25% to 66% of patients.64 This might begin unilaterally, but most patients eventually develop bilateral enlargement, which may be recurrent and episodic or chronic and fixed. Objective evaluation of salivary flow rate can be performed with Lashley cups that fit over the opening of Stensen’s duct and collect saliva. Sialography demonstrates sialectasis in 85% to 97% of patients with Sjögren’s syndrome.62
The ocular findings in patients with Sjögren’s syndrome include dilation of the bulbar conjunctival vessels, pericorneal injection, irregularity of the corneal image, and occasionally enlargement of the lacrimal gland. The tear secretion rate may be assessed by means of Schirmer’s test. In addition, staining of damaged corneal and conjunctival epithelia by rose bengal dye is reported to be more specific for keratoconjunctivitis sicca.64
The systemic nature of Sjögren’s syndrome might result in a wide variety of findings other than dryness of the mouth and eyes. Systemic manifestations include generalized malaise, low-grade fever, and myalgia and arthralgia. Dryness of the pharynx and esophagus results in dysphagia, and involvement of the tracheobronchial system might lead to bronchitis or pneumonia. The renal system might be affected, resulting in renal tubular acidosis. Vasculitis is reported to occur in 20% to 30% of patients.63 Most commonly, the vasculitis manifests itself in the skin in the form of Raynaud’s phenomenon and recurrent urticaria-like lesions. Central nervous system involvement is reported to occur in Sjögren’s syndrome. The neurologic involvement includes peripheral sensory and motor polyneuropathies that may mimic multiple sclerosis.
In addition to the clinical examination, establishing the presence of an autoimmune process is mandated for the diagnosis of Sjögren’s syndrome, which can be done by detecting the presence of autoantibodies and minor salivary gland biopsy. Testing for autoantibodies to the ribonuclear proteins Ro (SS-A) and La (SS-B) is done by use of enzyme-linked immunosorbent assay (ELISA). The biopsy of labial accessory salivary glands demonstrating salivary gland disease is one of the most consistent features of primary Sjögren’s syndrome. The biopsy should include several glandular lobes obtained from areas with normal overlying mucosa to exclude nonspecific inflammatory findings.62 The histopathologic lesion is a lymphocytic infiltrate producing a chronic focal sialadenitis. More specifically, the lesion consists of multiple focal mononuclear aggregates that are adjacent to and replace the normal acini. Several histologic classification methods that score the number of inflammatory foci seen on salivary gland biopsy have been devised.
Several sets of diagnostic criteria, which encompass findings on history, physical examination, and laboratory testing, have been proposed for the diagnosis of Sjögren’s syndrome. In general, the diagnosis consists of establishing the presence of keratoconjunctivitis sicca and xerostomia by means of clinical examination and objective testing. This testing should include objective measurements of decreased salivary and tear flow along with a minor salivary gland biopsy. In addition, laboratory evidence suggesting a systemic autoimmune disease, specifically against SS-A and SS-B ribonuclear proteins, is necessary for the diagnosis of Sjögren’s syndrome.63 The presence of another autoimmune disorder, such as rheumatoid arthritis or systemic lupus erythematosus, would mandate a diagnosis of secondary Sjögren’s syndrome. Patients who have objective signs of sicca complex but no evidence of an autoimmune process should be evaluated for other causes.
Multiple factors must be considered in the differential diagnosis of patients being evaluated for Sjögren’s syndrome. A patient’s general state of hydration and the presence of systemic conditions such as diabetes and cystic fibrosis should be assessed. One of the most common causes of xerostomia is medications. Sedatives, antipsychotics, antidepressants, antihistamines, and diuretics are the classes of drugs most often associated with oral dryness. Salivary gland exposure to therapeutic irradiation greater than 4000 cGy will result in severe and permanent secretory hypofunction.64
The salivary gland enlargement frequently found in Sjögren’s syndrome may also be caused by several other conditions. Unilateral salivary gland enlargement despite an established diagnosis of Sjögren’s syndrome should raise the suspicion of tumor.62 Bacterial infection, chronic sialadenitis, and ductal obstruction are all causes of unilateral salivary gland enlargement. Bilateral salivary gland enlargement might be the result of a viral infection, malnutrition, or granulomatous diseases such as sarcoidosis.
The treatment of Sjögren’s syndrome involves symptomatic treatment and prevention of irreversible damage to the teeth and eyes. The management of the oral component of Sjögren’s syndrome involves increasing the secretion rate of remaining salivary glands, using saliva substitutes, treating and preventing dental caries, and eradicating fungal overgrowth.64 Stimulation of residual salivary gland function might be accomplished by local methods such as chewing sugarless gum or candies. The muscarinic-cholinergic agonist pilocarpine is the most commonly used systemic sialogogue. The drug is administered in 5-mg doses three to four times daily; however, side effects such as sweating, flushing, and increased urination are common. Dental treatment with fluoride is used to prevent and control dental caries. The treatment for keratoconjunctivitis consists of the use of commercially available eye lubricants and eye patching if corneal ulceration develops. The use of systemic corticosteroids or cytotoxic drugs is reserved for the severe extraglandular complications such as glomerulonephritis or necrotizing vasculitis.62
Brook I. Aerobic and anaerobic microbiology of suppurative sialadenitis. Clin Microbiol. 2002;51:526.
Brook I. Diagnosis and management of parotitis. Arch Otolaryngol Head Neck Surg. 1992;118:469.
Cheuk W, Chan JKC. Kuttner tumor of the submandibular gland. Am J Clin Pathol. 2002;117:103.
Coban A, Ince Z, Uçsel R, et al. Neonatal suppurative parotitis: a vanishing disease? Eur J Pediatr. 1993;52:1004.
Jain V, Moni NB, Singh M, et al. Juvenile recurrent parotitis. Indian Pediatr. 2000;37:1126.
Kedjanyi WK, Gupta D. Shock wave lithotripsy of a parotid duct calculus. J Laryngo Otol. 2002;116:61.
Lary B. Postoperative suppurative parotitis. Arch Surg. 1963;89:653.
Manoussakis M, Moutsopoulos M. Sjögren’s syndrome. Otolaryngol Clin North Am. 1999;32:843.
O’Brien CJ, Murrant NJ. Surgical management of chronic parotitis. Head Neck. 1993;15:445.
Roveda S. Cervicofacial actinomycosis: report of two cases involving major salivary glands. Aust Dent J. 1973;18:7.
Schiodt M, Dodd C, Greenspan D, et al. Natural history of HIV-associated salivary gland disease. Oral Surg Oral Med Oral Pathol. 1992;74:327.
Stanley RB, Fernandez JA, Peppard SB. Cervicofacial mycobacterial infections presenting as major salivary gland disease. Laryngoscope. 1983;93:1271.
Williams MF. Sialolithiasis. Otolaryngol Clin North Am. 1999;32:819.
1. Perry RS. Recognition and management of acute suppurative parotitis. Clin Pharm. 1985;4:566.
2. McAnally T. Parotitis: clinical presentation and management. Postgrad Med. 1982;71:87.
3. Raad II, Sabbagh MF, Caransos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990;12:591.
4. Tabak LA, Levine MJ, Mandel ID. Role of salivary mucins in the protection of oral cavity. Oral Pathol. 1982;11:1.
5. McQuone SJ. Acute viral and bacterial infections of the salivary glands. Otolaryngol Clin North Am. 1999;32:793.
6. Brook I, Frazier EH, Thompson DH. Aerobic and anaerobic microbiology of acute suppurative parotitis. Laryngoscope. 1991;101:170.
7. Lewis MAO, Lamey PJ, Gibson J. Quantitative bacteriology of a case of acute parotitis. Oral Surg Oral Med Oral Pathol. 1989;68:571.
8. Work WP, Hecht DW. Inflammatory diseases of the major salivary glands. In: Paparella MM, Shumrick DA, editors. Otolaryngology. Philadelphia: WB Saunders, 1980.
9. Lundgren A, Kyle P, Odkvist LM. Nosocomial parotitis. Acta Otolaryngol (Stockh). 1976;82:275.
10. Brook I. Diagnosis and management of parotitis. Arch Otolaryngol Head Neck Surg. 1992;118:469.
11. Chiu AG, Hecht DA, Prendiville SA, et al. Atypical presentations of cat scratch disease in the head and neck. Otolaryngol Head Neck Surg. 2001;125:414.
12. Loughran DH, Smith LG. Infectious disorders of the parotid gland. N Engl J Med. 1988;85:311.
13. Spratt J. Etiology and therapy of acute pyogenic parotitis. Surg Gynecol Obstet. 1961;112:391.
14. Andrews JC, Abemayor E, Alessi DM, et al. Parotitis and facial nerve dysfunction. Arch Otolaryngol Head Neck Surg. 1989;115:240.
15. Marcy SM, Klein JO. Focal bacterial infections. In: Remington JS, Klein JO, editors. Infectious Disease of the Fetus and Newborn Infant. Philadelphia: WB Saunders, 1990.
16. Coban A. Neonatal suppurative parotitis: a vanishing disease? Eur J Pediatr. 1993;52:1004.
17. Leake D, Leake R. Neonatal suppurative parotitis. Pediatrics. 1970;46:203.
18. Jain V, Moni NB, Singh M, et al. Juvenile recurrent parotitis. Ind Pediatr. 2000;37:1126.
19. Reid E, Douglas F, Crow Y, et al. Autosomal dominant juvenile recurrent parotitis. J Med Genet. 1998;35:417.
20. Marsman WA, Sukhai RN. Recurrent parotitis and isolated IGg3 deficiency. Eur J Pediatr. 1999;158:684.
21. Ostuni PA, Ianiello A, Sfriso P, et al. Juvenile onset primary Sjögren’s syndrome: report of ten cases. Clin Exp Rheum. 1996;14:689.
22. Saunders JR, Hirata RM, Jacques DA. Salivary glands. Surg Clin North Am. 1986;66:59.
23. Zhao-Ju Z. Chronic obstructive parotitis: report of 92 cases. Oral Surg Oral Med Oral Pathol. 1992;73:434.
24. O’Brien CJ, Murrant NJ. Surgical management of chronic parotitis. Head Neck. 1993;15:445.
25. Cheuk W, Chan JKC. Kuttner tumor of the submandibular gland. Am J Clin Pathol. 2002;117:103.
26. Hogg RP, Ayshford C, Watkinson JC. Parotid duct carcinoma arising in bilateral chronic sialadenitis. J Laryngol Otol. 1999;113:686.
27. Rice DH. Chronic inflammatory disorders of the salivary glands. Otolaryngol Clinics North America. 1999;32(5):813.
28. Williams MF. Sialolithiasis. Otolaryngol Clin North Am. 1999;32:819.
29. Hiraide F, Nomura Y. Fine surface structure and composition of salivary calculi. Laryngoscope. 1980;90:152.
30. Stanley MW, Bardales RH, Beneke J, et al. Sialolithiasis. Differential diagnostic problems in fine-needle aspiration cytology. Am J Clin Pathol. 1996:229-233.
31. Brodner L, Azaz B. Submandibular sialolithiasis in children. J Oral Maxillofac Surg. 1982;40:551.
32. Lustmann J, Regev E, Melamed Y. Sialolithiasis: a survey on 245 patients and a review of the literature. Int J Oral Maxillofac Surg. 1990;19:135.
33. Jager L, Menauer F, Holznecht N, et al. Sialolithiasis: MR sialography of the submandibular duct—an alternative to conventional sialography and US. Radiology. 2000;216:665.
34. Hong KH, Young KK. Intraoral removal of the submandibular gland: a new surgical approach. Otolaryngol Head Neck Surg. 2000;132:798.
35. Kedjanyi WK, Gupta D. Shock wave lithotripsy of a parotid duct calculus. J Laryngo Otol. 2002;116:61.
36. Baurmash H. Sialoendoscopy: three years’ experience as a diagnostic and treatment modality. J Oral Maxillofac Surg. 1997;56:919.
37. Konigsberger R, Feyh J. Endoscopically controlled electrohydraulic intracorporeal shock wave lithotripsy of salivary glands. J Otolaryngol. 1993;22:12.
38. Nahlieli O, Baruchin A. Long term experience with endoscopic diagnosis and treatment of salivary gland inflammatory diseases. Laryngoscope. 2000;110:988.
39. Lary B. Postoperative suppurative parotitis. Arch Surg. 1963;89:653.
40. Buckley JM, Poche P, McIntosh K. Parotitis and parainfluenza 3 virus. Am J Dis Child. 1972;124:789.
41. Schiødt M, Dodd C, Greenspan D, et al. Natural history of HIV-associated salivary gland disease. Oral Surg Oral Med Oral Pathol. 1992;74:327.
42. Shugar J, Som PM, Jacobson AL, et al. Multicentric parotid cysts and cervical adenopathy in AIDS patients. A newly recognized entity: CT and MR manifestations. J Laryngol Otolaryngol. 1988;28:772.
43. Williams F, Cohen P, Jumshyd J. Prevalence of the diffuse infiltrative lymphocytosis syndrome among human immunodeficiency virus type1-positive outpatients. Arthritis Rheum. 1998;41:863.
44. Itescu S. Diffuse infiltrative lymphocytosis syndrome in human immunodeficiency virus infection—a Sjögren’s-like disease. AIDS Rheum Dis. 1991;17:99.
45. Falloon J. Human immunodeficiency virus infection in children. J Pediatr. 1989;114:1.
46. Tomblin J, Roberts F. Tuberculous cervical lymphadenitis. CMA J. 1979;121:324.
47. Stanley RB, Fernandez JA, Peppard SB. Cervicofacial mycobacterial infections presenting as major salivary gland disease. Laryngoscope. 1983;93:1271.
48. Tunkel D, Baroody F, Sherman M. Fine-needle aspiration biopsy of cervicofacial masses in children. Arch Otolaryngol Head Neck Surg. 1995;121:533.
49. Roveda S. Cervicofacial actinomycosis: report of two cases involving major salivary glands. Aust Dent J. 1973;18:7.
50. Olson NR. Nontuberculous mycobacterial infections of the face and neck: practical consideration. Laryngoscope. 1981;91:1714.
51. Robson C. Radiologic evaluation of the neck: imaging of granulomatous lesions of the neck in children. Radiol Clin North Am. 2000;38:5.
52. Burns BV, Ayubi A, Ray J, et al. Actinomycosis of the posterior triangle: a case report and review of the literature. J Laryngol Otol. 1997;111:1082.
53. Bartels LJ, Vrabec DP. Cervicofacial actinomycosis: a variable disorder. Arch Otolaryngol. 1978;4:705.
54. Harris LF, Kakani PR, Selah CE. Actinomycosis: surgical aspects. Am Surg. 1985;51:262.
55. Weese WC, Smith IM. A study of 57 cases of actinomycosis over a 36 year period: a diagnostic “failure” with good prognosis after treatment. Arch Intern Med. 1972;135:1562.
56. Regnery RL, Olson JG, Perkins BA, et al. Serologic response to “Rochalimaea henselae” antigen in suspected cat scratch disease. Lancet. 1992;339:1443.
57. Schutze G. Diagnosis and treatment of Bartonella henselae infections. Pediatr Infect Dis. 2000;19:1185.
58. Chiu CH, Lin TY. Clinical and microbiological analysis of six children with acute suppurative parotitis. Acta Pediatr. 1996;85:106.
59. Bergmans AM, Peeters MF, Schellekens JF, et al. Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J Clin Microbiol. 1997;35:1931.
60. Rafaty FM. Cervical adenopathy secondary to toxoplasmosis. Arch Otolaryngol Head Neck Surg. 1977;103:547.
61. Dobie R. Toxoplasmosis lymphadenitis occurring in a parotid gland. Otolaryngol Head Neck Surg. 1986;94:237.
62. Manoussakis M, Moutsopoulos M. Sjögren’s syndrome. Otolaryngol Clin North Am. 1999;32:843.
63. Talal N. Sjögren’s syndrome: historical overview and clinical spectrum of disease. Rheum Dis Clin North Am. 1992;18:507.
64. Daniels T, Fox PC. Salivary and oral components of Sjögren’s syndrome. Rheum Dis Clin North Am. 1992;18:571.