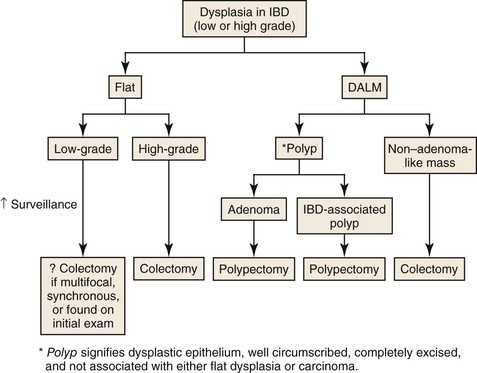
Inflammatory Disorders of the Large Intestine
Deepa T. Patil
Joel K. Greenson
Robert D. Odze
Approach to Evaluating Colitis
Pathologists are asked to evaluate colorectal biopsy specimens for a variety of reasons, but often only a pattern of injury can be identified, at best. This evaluation is performed with the hope that a specific diagnosis can be rendered once appropriate clinical, radiologic, and laboratory information is obtained. However, some forms of colitis, such as lymphocytic colitis, collagenous colitis, and ischemic colitis, do have specific histologic features, and a diagnosis can be rendered in the absence of clinical information. Many histologic features are characteristic of chronic inflammatory bowel disease (IBD). However, it is often difficult or impossible to distinguish ulcerative colitis (UC) from Crohn’s disease (CD) on the basis of colorectal biopsy specimens only, particularly after the patient has been treated medically, in which case the features of these two disorders overlap considerably.
Normal Versus Abnormal
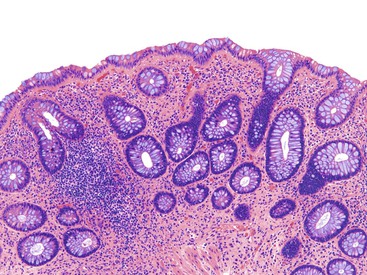
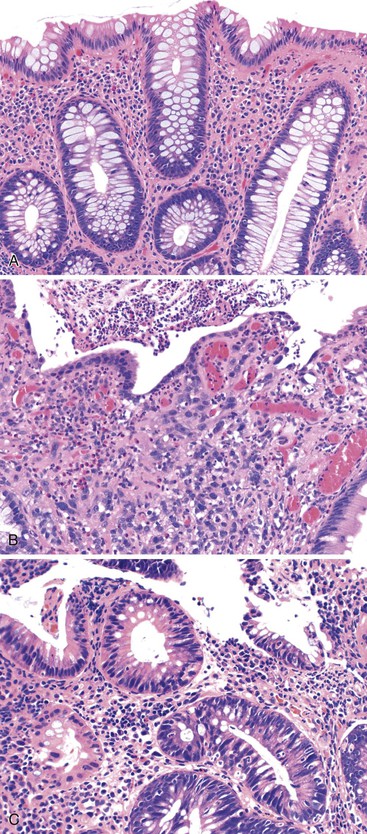
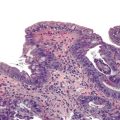
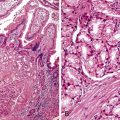
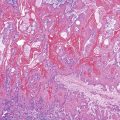
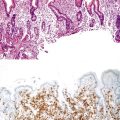
Perhaps the most important aspect of evaluating colorectal biopsy specimens is to determine normal from abnormal, which can be difficult because of the presence of bowel preparation and biopsy procedure artifacts. These artifacts include surface epithelial degeneration, edema, hemorrhage and congestion (Figs. 17.1 and 17.2), aggregation of inflammatory cells, pseudolipomatosis (intramucosal air; Fig. 17.3), mucin depletion, and even neutrophilic cryptitis in extreme cases. Pathologists should not be afraid to render a diagnosis of “normal colon.” After all, that is the most common diagnosis in the general population. Terms such as “nonspecific (or increased) chronic inflammation,” “nonspecific colitis,” and “increased acute and chronic inflammation” are inappropriate pathologic diagnoses that often cause confusion for clinicians.
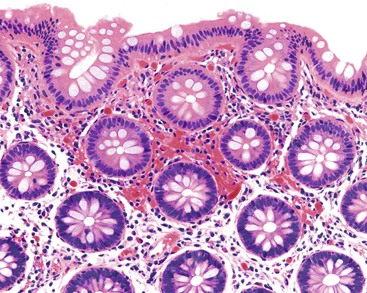
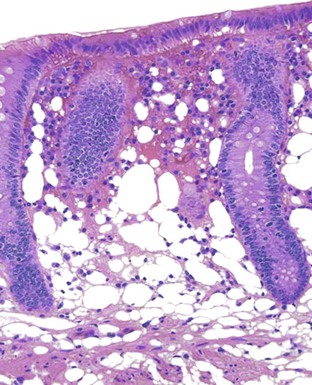
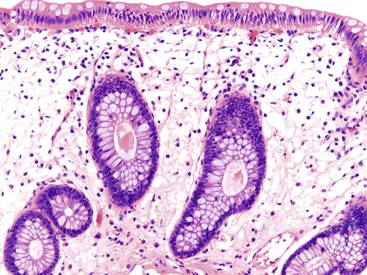
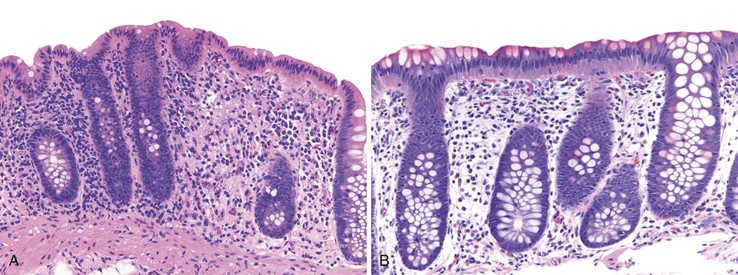
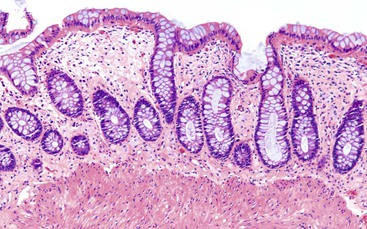
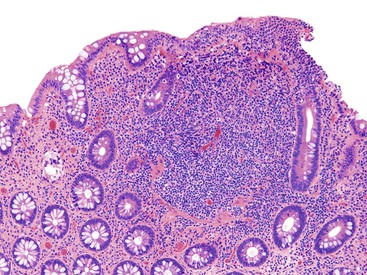
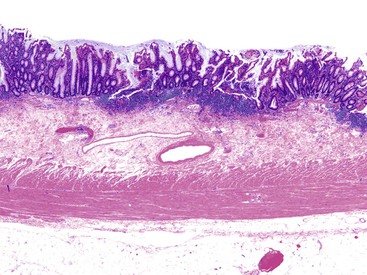
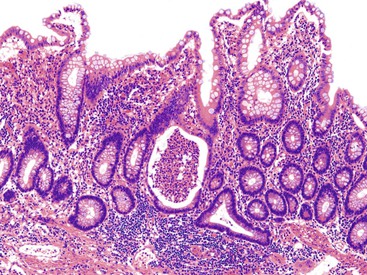
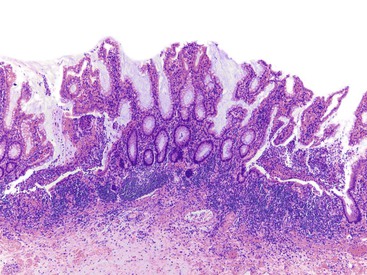
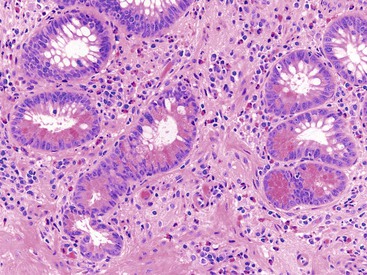
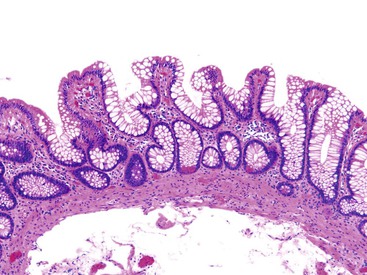
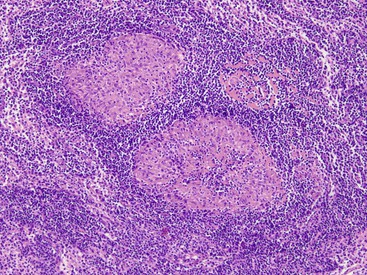
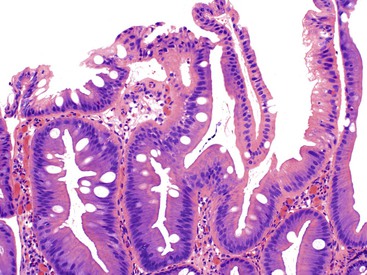
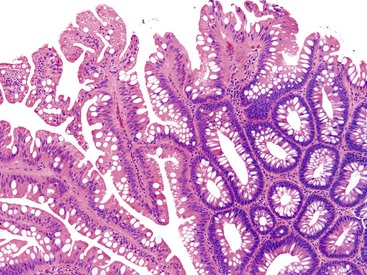
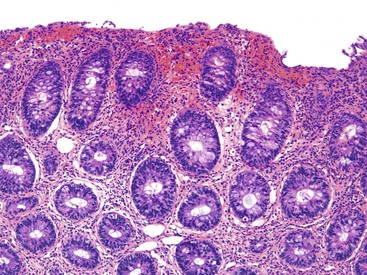
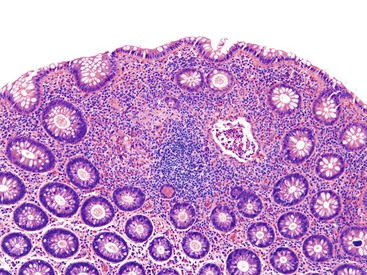
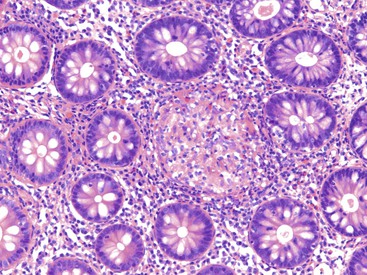
Pathologists should be aware of several important points when evaluating colorectal biopsy specimens, particularly with regard to histologic findings that are considered normal. For example, lymphocytes and plasma cells are always present in the lamina propria of colorectal mucosa, regardless of the anatomic location. However, the density of lamina propria inflammatory cells varies among the different anatomic locations. In general, the cecum and right colon are more cellular than other segments of the colon. A progressive decrease in the cellular constituents of the lamina propria is normal from the right to the left colon (Fig. 17.4). In addition, although the colonic crypts are arranged in a straight and tubular configuration, show an even distribution, and typically extend directly up to the muscularis mucosae, the distal rectum shows variation in crypt architecture under normal circumstances (Fig. 17.5). Lymphocytes are normally present in the surface epithelium of the colorectal epithelium and number approximately 5 per 100 epithelial cells.1 Surface intraepithelial lymphocytes are generally more prominent in the cecum and right colon than in the remainder of the distal colon. In addition, intraepithelial lymphocytes are more numerous in areas of mucosa overlying lymphoid follicles (Fig. 17.6).2,3 Eosinophil counts also vary substantially in different portions of the colon (more in the right colon than in the left), and “normal” numbers depend on other factors, such as the geographic location and the latitude of patient’s principal habitat.4,5 Individuals who live in the southern states of North America or closer to the equator have a higher number of lamina propria eosinophils compared with individuals who live in more northern states. Knowledge of the anatomic location of the colonic biopsy specimens is important but has become increasingly difficult because gastroenterologists have a tendency to place biopsy specimens from different sites into one specimen container.
Acute Versus Chronic Colitis
Evaluation of patterns of injury in colorectal biopsy specimens is often best performed at low magnification (see Chapter 13). For example, lamina propria cellularity and crypt architecture are easier to evaluate at low magnification than at high magnification. In addition, it is easier to compare histologic changes among different fragments of tissue within the same specimen block under low-power examination.
After a biopsy is determined to be “abnormal,” distinction of acute from chronic changes is important clinically. The most consistent and reliable markers of chronic injury (colitis) are crypt architectural distortion, basally located lymphoid aggregates, basal plasmacytosis, diffuse mixed inflammation, Paneth cell (or pyloric gland) metaplasia (or hyperplasia in biopsy specimens from the right colon), and lamina propria fibrosis (Table 17.1). Many types of colitides, including acute infectious colitis, may result in an expansion of the lamina propria by plasma cells; however, basal plasmacytosis, wherein plasma cells fill the space between the bases of the crypts and the muscularis mucosae, is an excellent histologic marker of chronic colitis.6 This feature is also helpful to differentiate acute infectious colitis from acute-onset IBD: In IBD, features of chronicity are almost always present in the cecum, right colon, and proximal portion of the transverse colon,7 even in these portions of the colon, an increase in the number and a change in the distribution of Paneth cells helps to indicate and confirm the presence of chronic injury.
Table 17.1
Microscopic Features of Acute Colitis versus Chronic Colitis
| Feature | Acute Colitis | Chronic Colitis |
| Crypt architecture | Preserved | Often distorted |
| Expansion of lamina propria | Usually superficial, predominantly neutrophils ± eosinophils | Diffuse (superficial and deep), mixed lymphocytes and plasma cells |
| Basal lymphoid aggregates | Usually absent | Often present |
| Basal plasmacytosis | Usually absent | Almost always present |
| Granulomas | Usually absent | Present in Crohn’s disease; in ulcerative colitis they are related to crypt rupture |
| Cryptitis and crypt abscesses | Present, superficial | Present, superficial and deep |
| Pyloric or Paneth cell metaplasia | Absent | Often present |
| Lamina propria fibrosis | Absent | May be present |
Features of “active injury” in the colon include neutrophil- or eosinophil-mediated epithelial injury in the form of cryptitis, crypt abscesses, mucosal erosions and ulceration, and epithelial degenerative changes. These changes may be superimposed on a background of chronic colitis, in which case they are termed chronic active colitis.
Ulcerative Colitis
Epidemiology
UC is a chronic, episodic inflammatory disease of the colon. It has a propensity to develop in adolescents and young adults, although there is a second incidence peak among middle-aged men. The incidence and prevalence rates of UC are highest in North America, England, northern Europe, and Australia. Estimates of the annual incidence of UC in North America and Europe range from 1.5 to 20.3 cases per 100,000 individuals.8 The incidence of UC appears to have stabilized during the past 25 years and is no longer increasing, unlike that of CD, which seems to be increasing in incidence. It has been estimated that UC will develop in approximately 1% of the U.S. and European population during their lifetime. There is marked ethnic variation in the incidence of UC, with a high incidence in the Jewish population. In the United States, the annual incidence of UC among Jews is 13 per 100,000 person-years, compared with 3.8 per 100,000 among non-Jewish whites.9 UC is more common in industrialized countries compared with less-developed countries, and in urban compared with rural populations. The incidence rate of UC among immigrants who have moved to high-risk geographic regions is higher than that of the same ethnic groups in their native countries.
Clinical Features
The clinical symptoms of UC vary depending on the phase and extent of disease. They include urgency, passage of mucus, tenesmus and rectal bleeding in patients with proctitis, and diarrhea (mainly bloody), rectal bleeding, abdominal pain, fever, and weight loss in patients with extensive colitis. Among patients who are seen with fulminant colitis, the symptoms include fever, generalized abdominal pain, rectal bleeding, and abdominal distention. Patients may also complain of symptoms related to anemia and hypoalbuminemia, such as fatigue, dyspnea, and peripheral edema. In general, the clinical symptoms correlate with the severity of disease. However, on occasion, there may be evidence of histologically or endoscopically active disease in asymptomatic patients. The onset of symptoms is typically slow and insidious. In most cases, patients are symptomatic for weeks or months before seeking medical attention. Some patients with UC are seen more acutely and show symptoms that mimic acute infectious colitis. In some instances, infection such as with Salmonella or Clostridium difficile precedes an initial episode of UC (see Acute Self-Limited [Infectious] Colitis).
Extraintestinal manifestations of UC can affect any organ system but are most common in the skin, eyes, mouth, joints, and liver. Cutaneous hypersensitivity, photosensitivity, and urticarial rashes may occur in response to medical therapy (especially sulfasalazine) rather than the underlying disease itself. Erythema nodosum occurs in 2% to 4% of patients with UC. It manifests as single or multiple, tender, erythematous nodules on the extensor surfaces of extremities. Pyoderma gangrenosum is less common, occurring in 1% to 2% of patients with UC. The lesions may be single or multiple and may occur on the trunk, extremities, face, breast, and stoma sites. Less common skin manifestations include Sweet syndrome and oral aphthous ulcers.
The two most common ocular manifestations of UC are episcleritis and uveitis; these occur in 5% and 8% of patients, respectively. Seronegative arthropathy (type 1-pauciarticular or type 2- polyarticular) occurs in 5% to 20% of individuals with UC and is more common than axial arthropathy; the latter manifests as sacroiliitis and ankylosing spondylitis. In terms of liver involvement, most patients with UC have mild elevations of serum aminotransferase and alkaline phosphatase levels. The most important complication is primary sclerosing cholangitis (PSC), which occurs in almost 3% of patients with UC. Unlike all of the complications listed previously, which typically follow the colonic disease activity, PSC may follow an independent progressive course, even when UC has been stable or inactive for years.
Patients with mild or moderately severe disease usually exhibit minimal signs on physical examination. The affected portion of the colon may be tender on abdominal palpation, but abdominal rigidity or guarding is highly unusual. Severe (fulminant) colitis is usually associated with generalized abdominal tenderness, with either normal or hyperactive bowel sounds, which decrease with disease progression. Distention of the abdomen with absent bowel sounds is an ominous sign that suggests peritoneal irritation in cases of fulminant colitis. Other signs that may be associated with UC include aphthous ulceration of oral mucosa, clubbing of fingernails (typically in long-standing UC), peripheral edema, and mild perianal disease. Digital rectal examination is often normal but may occasionally reveal velvety and edematous mucosa. In addition to anemia that results from acute or chronic gastrointestinal (GI) blood loss, patients with UC are predisposed to hypercoagulability and its complications, such as deep vein thrombosis, pulmonary embolism, renal artery thrombosis, cerebrovascular accidents, mesenteric vein thrombosis (and consequent ischemic colitis), and coronary thrombosis.10
Laboratory findings in UC depend on disease activity. Anemia, leukocytosis, thrombocytosis, increased erythrocyte sedimentation rate (ESR), elevated C-reactive protein level, and hypoalbuminemia are typically associated with active disease. Stool cultures for organisms such as C. difficile, Campylobacter species, and Escherichia coli are usually performed to exclude an infectious cause or complication. Perinuclear antineutrophil cytoplasmic antibodies (pANCA) are positive in 60% to 80% of patients with UC.11 Immunoglobulin A (IgA) anti–Saccharomyces cerevisiae antibody (ASCA) is found in fewer than 1% of patients with UC, whereas IgG ASCA may be seen in as many as 20% of patients (see Ancillary [Serologic] Diagnostic Tests for IBD).
Radiologic studies help to provide a general assessment of the extent of disease and complications associated with UC. Plain radiographs are indicated in cases of severe UC to assess for the presence of intraperitoneal air. The finding of marked colonic dilatation suggests fulminant colitis. Because the transverse colon is the least dependent part of the colon, a diameter larger than 5 cm is highly suggestive of toxic megacolon. In the earliest stage of UC, a double-contrast barium enema may show a fine granular appearance of the colon. With advanced disease, deep submucosal ulcers result in characteristic “collar-button” ulcers. Diffuse absence of mucosal haustrations, thumbprinting, and narrowing or shortening of the colon, are some of the features associated with pancolitis. Computed tomography (CT) is not very helpful in detecting mucosal changes in early disease. However, in advanced UC, the hallmark finding is the presence of mural thickening. In almost 70% of patients with UC, CT with contrast reveals the classic target or double halo sign caused by inhomogeneous enhancement of the thickened bowel wall. Rectal narrowing and widening of the presacral space are typical findings of long-standing UC.12
Assessment of disease activity and prognosis is based on clinical, endoscopic, or histologic findings or a combination of these indices. Although it is not standardized, a widely accepted clinical classification is that of Truelove and Witts.13 Frequency of bowel movements, rectal bleeding, fever, tachycardia, anemia, and elevated ESR are used to classify disease activity as mild, moderate, or severe. Because this classification does not correlate with disease status in patients with limited colitis, a numerical disease activity score, known as the Sutherland index or UC disease activity index, is now more commonly used, especially in clinical trials. It combines scores from four components (stool frequency, rectal bleeding, sigmoidoscopic findings, and physician’s global assessment).14
Risk Factors and Pathogenesis
The exact etiology of UC still remains unknown. However, its pathogenesis is related to a combination of three major elements: genetic susceptibility of the host, immunity, and environmental factors. Although specific agents may incite an inflammatory response in a susceptible host, such agents have not yet been identified. However, studies have shown that luminal microorganisms, their metabolic byproducts, and interactions with normal epithelial structures play key roles in stimulating a host immune response in UC.15
Genetic Factors
The observation that 10% to 20% of patients have at least one other affected family member lends support to the role of genetic factors in the development of UC.16 The strongest evidence of a genetic influence is derived from three European studies wherein 6% to 16% of monozygotic twin pairs had concordant UC, compared with 0% to 5% of dizygotic twin pairs.17–19 The lifetime risk of developing disease is higher among first-degree relatives of a patient of Jewish descent and among relatives of patients with early-onset disease.20
A wide array of genes are responsible for conferring genetic susceptibility, disease specificity, and phenotype in patients with UC.21 Linkage analyses have demonstrated that chromosomes 1, 2, 3, 5, 6, 7, 10, 12, and 17 harbor susceptibility genes for UC.22 Specifically, the IBD2 locus on chromosome 12 has a strong association among families with UC.23 Additionally, the C3435T polymorphism of the human multidrug resistance 1 (MDR1) gene is also linked to susceptibility to UC.24
Besides susceptibility genes, human leukocyte antigen (HLA) alleles also influence disease behavior in UC. A significantly increased frequency of HLA-A11 and HLA-A7 has been observed to occur in patients with UC. Specifically, HLA-DR1 (DRB1*0103) has been associated with severe colitis.25 HLA-DR2 (DRB1*1502) has been associated with UC in Japanese and Jewish populations.26,27
Environmental Factors
It is now widely accepted that continuous antigenic stimulation by commensal bacteria, fungi, or viruses leads to chronic inflammation in individuals who have defects in immunoregulation, mucosal barrier function, and microbial killing. The distal terminal ileum and colon contain the highest concentrations of bacteria (almost 1012 organisms per gram of luminal content), and they are a source of constant antigenic stimulus to the host immune system. Animal studies have shown rapid development of colitis when germ-free HLA-B27 transgenic rats28 and interleukin 10 (IL10)-deficient mice29 are populated with normal specific pathogen-free bacteria. Administration of antibiotics effectively prevents and reduces the severity of colitis in these animal models.30 There are several postulated mechanisms by which gut flora may initiate, or contribute to, the development of colitis (see Immune Factors). By virtue of their ability to adhere to or invade the surface epithelium or to produce enterotoxins, microbial organisms stimulate production of inflammatory cytokines. Alteration of the balance between protective and harmful bacteria (e.g., Bacteroides species) reduces the concentration of short-chain fatty acids, which provide nourishment to colonocytes. An impaired mucosal barrier and inability to kill microbes because of impaired host defense mechanisms also contribute to hyperresponsiveness and production of high levels of inflammatory cytokines. Abnormal antigen processing, loss of tolerance, autoimmunity, and an abnormally excessive T-cell response are some other mechanisms that influence the severity of inflammation.
A T-cell α-chain receptor knockout mouse model of colitis has demonstrated lack of development of inflammation after appendectomy in animals at 3 to 5 weeks of age.31 Subsequent case-control studies on humans have also suggested that appendectomy may have a beneficial effect on the disease course.32–34 However, there have been no prospective studies confirming a possible protective effect.
The best-characterized environmental factor associated with UC is cigarette smoking. UC is more common among nonsmokers than current smokers.35 In fact, the second incidence peak in middle-aged men may, in part, be linked to patients who have stopped smoking later in life. A recent prospective study of a cohort of 229,111 women who were followed over a period of 32 years (Nurses Health I and II cohort) showed that the risk of UC is highest during the first 2 to 5 years after cessation of smoking but remains elevated for more than 20 years.36 The postulated mechanisms for the protective effect of smoking include modulation of cellular and humoral immunity, increased generation of oxygen free radicals, and alteration of cytokine levels.
Immune Factors
Both humoral and cell-mediated immunologic mechanisms play major roles in the pathogenesis of UC. UC is associated with an increase in the synthesis of IgG, notably the IgG1 and IgG3 subclasses. Most patients have circulating antibodies to a variety of dietary, bacterial, and self-antigens that are of the IgG1 subclass, and these are polyclonal in nature. Because serum antibody titers usually do not correlate with disease activity or course, it has been postulated that cross-reactivity between antibodies to bacterial antigens and colonocyte epithelial epitopes may help trigger an immunologic response that leads to mucosal inflammation.37
UC is associated with several autoimmune diseases such as diabetes mellitus, pernicious anemia, and thyroid disease. The possibility that UC is an autoimmune disease is supported by the fact that patients with UC have serum antibodies directed against lymphocytes, ribonucleic acid, smooth muscle, gastric parietal cells, and thyroid tissue. Patients also have antibodies to epithelial cell–associated components, notably an autoantibody against a 40-kDa epithelial antigen found in normal colonic epithelium. This IgG autoantibody was eluted specifically from colonic mucosa of patients with UC and was not found in patients with CD or other colonic inflammatory conditions.38 This antigen also shares epitopes with antigens found in the bile ducts, skin, eyes, and joints, sites that are commonly associated with extraintestinal manifestations of UC. The other autoantibody associated with UC is pANCA. It is found in 60% to 80% of UC patients and belongs to the IgG3 subclass. The exact antigen to which pANCA is directed is unknown. There is some evidence that the antigen is a 50-kDa nuclear envelope protein that is specific to myeloid cells.39 The pathogenic relevance of pANCA is unclear. It appears to be associated with an aggressive disease course and development of pouchitis.40
Bacterial antigens also trigger innate immunity by activation of pattern-recognition receptors, which include Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). Activation of TLRs and NLRs results in downstream activation of nuclear factor κB, which further stimulates production of various proinflammatory cytokines and chemokines. Defects in any of these pathways can result in abnormal bacterial processing and possibly IBD.41
Colonic epithelial cells express class II major histocompatibility complex (MHC) antigens and can initiate an inflammatory response by acting as antigen-presenting cells.42 Increased turnover of colonic epithelium, reduced metabolism of short-chain fatty acids, abnormal membrane permeability, and altered composition of mucus layers contribute to the pathogenesis of UC.43,44 Animal models of colitis produced by disruption of colonic epithelium further support the role of epithelial cells in the pathogenesis of IBD.45
Release of various cytokines from the T-cell inflammatory pathways may also lead to increased epithelial cell permeability and alteration of the endothelium, contributing to diarrhea and localized ischemia, respectively.
Pathologic Features
Gross Features
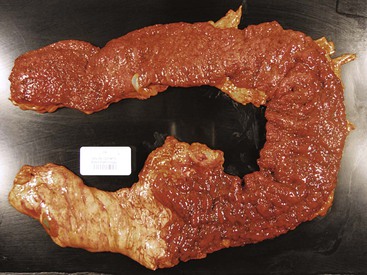
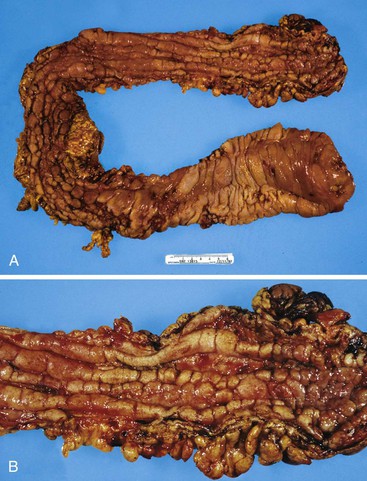
In untreated cases, the extent of colonic involvement depends on the clinical severity of disease. UC classically involves the rectum with variable, but continuous, involvement of the colon more proximally (Fig. 17.7). According to the Montreal classification, the extent of UC is divided into ulcerative proctitis (involvement limited to the rectum), left-sided or distal UC (involvement limited to a portion of the colorectum distal to the splenic flexure), and extensive UC or pancolitis (involvement of the colon proximal to the splenic flexure).46 At the initial onset of disease, pancolitis occurs in approximately 20% of patients, left-sided colitis in 50% to 60%, and proctitis or rectosigmoiditis in approximately 45% of patients.47 Skip lesions, in the form of appendiceal, periappendiceal, or ascending colon/cecal involvement, have been observed in as many as 80% of patients with subtotal UC (see Unusual Morphologic Variants of Ulcerative Colitis).47 Based on a long-term follow-up study by Farmer and colleagues, pancolitis eventually develops in almost half (46%) of patients with proctitis or rectosigmoiditis and in more than 70% of those with left-sided colitis.48
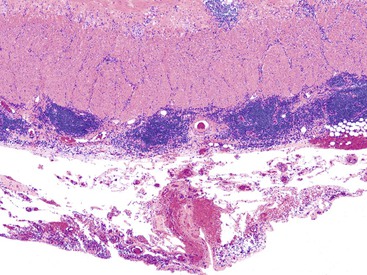
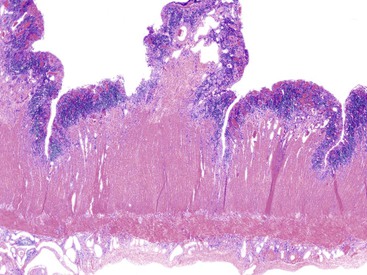
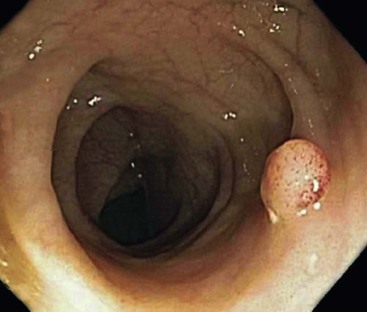
In the active phase of disease, the mucosa usually appears diffusely congested, granular, and edematous. Ulcers, when present, are usually small and oriented longitudinally in relation to the teniae coli. They often appear to undermine adjacent areas of mucosa, which leads to the formation of polypoid mucosal folds or inflammatory pseudopolyps. In such cases, the mucosa may have a cobblestone appearance, similar to that observed in CD. Rarely, an exaggerated form of pseudopolyp formation, known as filiform polyposis, may be present (Fig. 17.8). It is characterized by the presence of elongated, slender, villiform, worm like, polypoid mucosal projections and usually spares the rectum. In severe cases, ulcers may be extensive, involve large segments of bowel, and lead to near-total or total mucosal loss.
In cases of toxic megacolon, the bowel wall appears extremely thin, dilated, and congested. The serosal surface usually demonstrates fibrinous or fibrinopurulent exudate. Rarely, there is evidence of perforation. The mucosal surface in these cases is extensively denuded, hemorrhagic, ulcerated, and, often, covered with purulent exudates.
In the quiescent (inactive) phase of UC, the mucosa may appear completely normal, or it may show diffuse granularity, either with or without inflammatory pseudopolyps. In some cases of long-standing UC, the bowel wall is thickened and contracted (“colonic foreshortening”), and the mucosal surface may appear atrophic.
In treated UC, especially when patients have been given steroid enemas, the rectal mucosa may show minimal or no abnormalities on gross examination. Similarly, in patients who have received medical treatment before surgical resection, there may be focal, diffuse, or even widespread areas of grossly normal-appearing bowel between areas of affected bowel.
Microscopic Findings
Depending on the phase of disease and the degree of inflammatory activity, UC-related colitis is categorized as chronic inactive, chronic active, or active (without features of chronicity) for the purpose of sign-out. Chronic colitis (regardless of “activity”) is defined by the presence of histologic features of chronicity (Box 17.1), such as crypt architectural distortion, crypt atrophy, diffuse mixed lamina propria inflammation, basal plasmacytosis, basally located lymphoid aggregates, and Paneth cell metaplasia (in the left colon). Other changes of chronicity include lamina propria fibrosis, pyloric gland metaplasia, and Paneth cell hyperplasia in the right colon. Common changes of “activity” include neutrophilic or eosinophilic cryptitis, crypt abscesses, regenerative or degenerative epithelial changes, hemorrhage, necrosis, erosions, and ulceration. The degree of activity is graded as mild if less than 50% of the mucosa shows evidence of activity, moderate if more than 50% shows these features, and severe if surface erosion or ulceration is present.
Chronic Active Colitis.
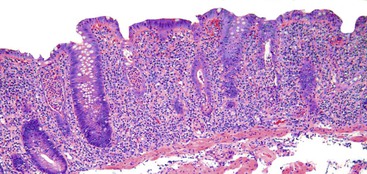
Histologically, previously untreated UC involves regions of colon in a diffuse and continuous manner, always beginning at the distalmost portion of rectum and extending proximally to the point at which inflammation stops, which in most cases is rather abrupt (Table 17.2). Typically, specimens from involved regions of colon have a similar appearance. Usually, each biopsy fragment shows a homogeneous and diffuse pattern of injury, although the severity of inflammation may vary from region to region in the bowel (usually worse distally) or between individual biopsy fragments from one area of colon.
Table 17.2
Microscopic Features of Untreated Ulcerative Colitis and Crohn’s Disease
| Ulcerative Colitis | Crohn’s Disease |
| Diffuse, continuous disease | Segmental disease |
| Rectal involvement | Variable rectal involvement |
| Disease worse distally | Variable disease severity |
| No fissures | Fissures, sinuses, fistulous tracts |
| No transmural aggregates | Transmural lymphoid aggregates |
| No ileal involvement (except in backwash ileitis) | Ileal involvement |
| Upper GI tract involvement less common | Upper GI tract involvement common |
| Crypt-rupture (mucin) granulomas | Epithelioid granulomas unrelated to ruptured crypts |
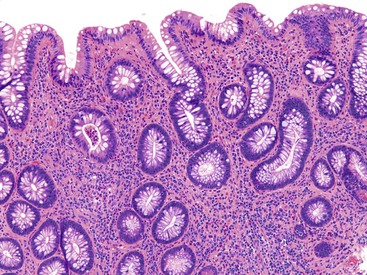
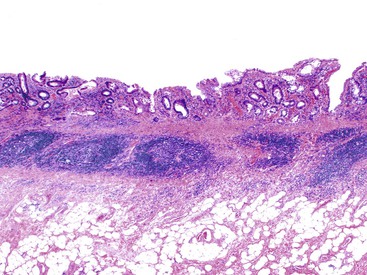
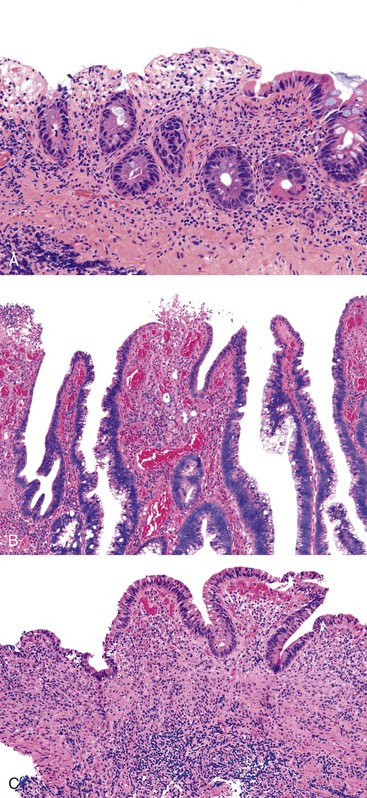
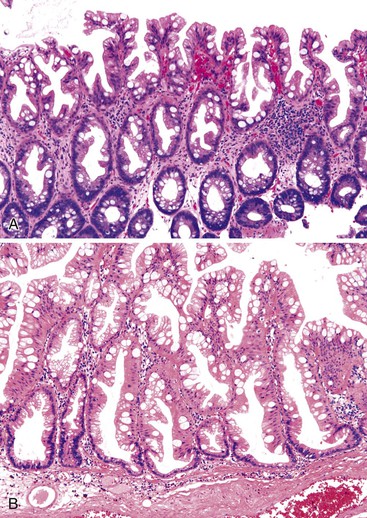
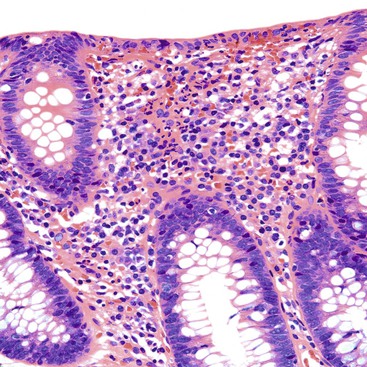
UC characteristically involves the mucosa and sometimes the superficial submucosa (Fig. 17.9). The histologic findings vary depending on the clinical phase of disease. Ultimately, in periods of clinical activity, UC is predominantly a lymphoplasmacytic inflammatory process with superimposed neutrophils, hemorrhage, and epithelial degeneration. A dense, homogeneous lymphoplasmacytic infiltrate typically expands the lamina propria (Fig. 17.10).49–51 The density of plasma cells is usually greatest in the basal region of the lamina propria (termed “basal plasmacytosis”). Basally located lymphoid aggregates (situated between the bases of the crypts and the muscularis mucosae) are also common. They may show germinal centers. Expansion of the lamina propria and the presence of basal lymphoid aggregates contribute to irregular spacing of the crypts.
One characteristic and frequent morphologic feature of UC is crypt architectural distortion, which is characterized by irregularly arranged, branched, dilated, and/or shortened crypts. In some circumstances, branching and shortening of crypts represent morphologic manifestations of crypt regeneration. Most patients with new-onset (pretreatment) UC have experienced several weeks to months of subclinical or minimal inflammation, during which time the lamina propria has been inflamed, plasma cells have congregated in the basilar region of the lamina propria, and significant crypt injury with regeneration has occurred. Crypt architectural distortion is considered a hallmark of chronic injury. However, it may result from any inflammatory disease of the colon that results in repeated bouts of injury and repair. Crypt distortion therefore is not a specific feature of UC (or CD). It occurs in many other types of disorders, such as chronic recurrent ischemia, persistent or recurrent infections (e.g., C. difficile), radiation colitis, drug-induced colitis, graft-versus-host disease (GVHD), and even microscopic colitis. Furthermore, not all patients with UC, even in an active phase of disease, reveal crypt distortion in every portion of the colonic mucosa. For this reason, absence of crypt distortion does not necessarily rule out a diagnosis of UC.
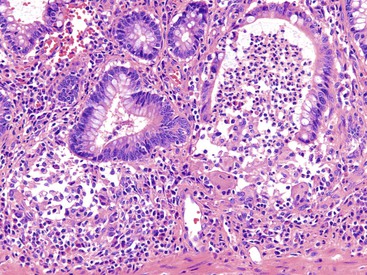
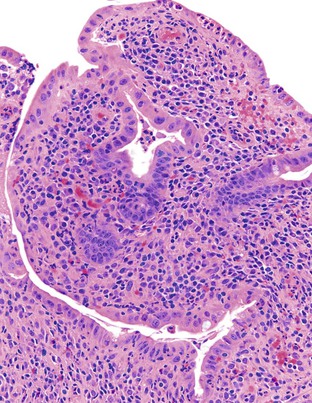
Depending on the severity of active disease, a neutrophilic inflammatory cell infiltrate (with or without eosinophils) may be seen within the lamina propria and surface and crypt epithelium (cryptitis); it may be minimal, focal and patchy, or diffuse and severe. Similarly, aggregates of neutrophils within the crypt lumina (crypt abscesses) may be focal or diffuse (Figs. 17.11 and 17.12). Rupture of crypts because of inflammation can lead to the development of aggregates of histiocytes, foreign body giant cells, and even well-developed granulomas, as a response to extravasated mucin (Fig. 17.13). So-called mucin granulomas (crypt rupture–associated granulomas) are usually present in the deeper portions of the mucosa, where crypts tend to rupture. Often, deeper cuts though tissue blocks are necessary to determine whether a mucosa-based granuloma is related to a ruptured crypt. Some patients show a marked foreign body giant cell response, or even a fully developed granulomatous response, which can be seen at the base of the mucosa. Distinguishing granulomas in UC from those in CD can be challenging, but the latter are more often randomly located in the mucosa and are more often superficial in location (see Crohn’s Colitis). When a mucosal granuloma is identified, serial sections should be evaluated to determine whether it is located immediately adjacent to a ruptured crypt. The presence of eosinophils within a granuloma is often a clue that one is dealing with a mucin granuloma.
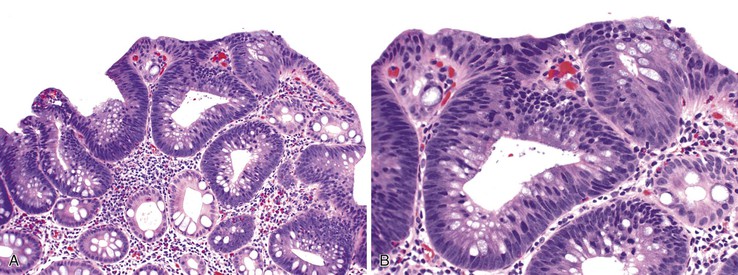
In areas of activity, the surface and crypt epithelium often shows regenerative and degenerative changes. Regenerative changes include loss of mucin, enlarged and variably sized nuclei with or without nuclear stratification, hyperchromasia, prominent nucleoli, and increased mitotic activity. In addition, the crypts may show increased apoptotic activity. In areas adjacent to erosions and ulcers, cells may acquire a syncytial appearance, with abundant eosinophilic cytoplasm (Fig. 17.14). In some instances, the syncytial epithelium overlies stroma that is devoid of crypts and contains actively inflamed granulation tissue. Later, regenerating surface epithelial cells, which are cuboidal initially and then columnar with maturation, acquire a slightly more basophilic cytoplasm. On occasion, the surface epithelium may be villiform in appearance, resembling small intestinal mucosa. Regenerative changes may mimic dysplasia (see Dysplasia in Inflammatory Bowel Disease). Mucin reappears slowly, first in cuboidal cells and later in goblet cells. With time, goblet cells may become numerous.
Paneth cell metaplasia in the left colon and pyloric gland metaplasia are reliable histologic indicators of chronic injury. In active disease, even the ascending colon, cecum, and transverse colon may show irregularity in the distribution and hyperplasia of Paneth cells (Fig. 17.15). Pyloric gland metaplasia is less common in UC than in CD. It is more commonly observed in samples from the proximal colon and is often seen in close proximity to ulcerated mucosa.
Occasionally, the rectum appears grossly free of disease (absolute rectal sparing) or has less activity than the proximal colon (relative rectal sparing). This is usually a result of prior medical treatment, either orally, or more commonly, with steroid enemas.
Grossly normal-appearing colonic mucosa proximal to regions of active colitis may also display a spectrum of abnormalities. Most commonly, there is a mild lymphoplasmacytic infiltrate within the lamina propria. However, the crypts are usually more evenly arranged and lack distortion. A few neutrophils may be present in the lamina propria, or occasionally within a crypt, but neutrophilic crypt abscesses are rare. Eosinophils may also be increased and may produce small eosinophilic crypt abscesses on occasion. In the context of a patient with classic UC in the more distal colon, these changes in the proximal bowel are a reflection of the underlying inflammatory disorder.
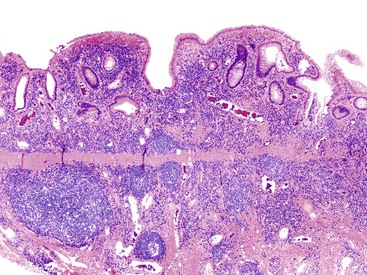
Most patients with UC eventually enter a resolving, or healing, phase of disease, characterized by decreasing activity (and symptoms) after an active colitis episode. This phase of disease is characterized morphologically by less activity and less crypt injury, but higher levels of crypt regeneration and remodeling (Fig. 17.16). Injured crypts typically heal from the base of the mucosa progressively upward toward the luminal surface. Neutrophils and other active components of crypt injury decrease first, followed by a reduction in lamina propria lymphocytes and plasma cells. During this initial healing phase, there is often much variability in the type and degree of mucosal inflammatory changes within biopsy fragments from different regions of the colon and even within individual fragments of mucosa from a single site. Neuroendocrine cell hyperplasia occurs in some patients, whereas others develop prominent lymphoid follicles that are more common in the distal colon and rectum (follicular proctitis). Follicular proctitis appears to identify a subgroup of patients who have a less favorable response to medical therapy.52,53
Chronic Inactive (Quiescent) Colitis.
The resolution period of UC, characterized by decreasing activity and increasing repair, may last for several weeks or months. Thereafter, UC patients may be symptom-free for variable but often long periods. This is the inactive or quiescent period of disease. During this time, one may see either completely normal mucosa or chronic inactive disease (mainly crypt distortion), with or without mild patchy activity (Fig. 17.17). Architecturally distorted crypts remain a biomarker of previous bouts of colitis. However, the colon may heal completely and appear normal histologically. The rate at which this occurs is variable among patients. Patients with only mild active colitis of short duration can show complete restitution of architecturally normal mucosa within several months after the initial active episode. However, the pace of crypt remodeling is usually slow in most patients; such remodeling occurs typically over many months.
Effects of Prior Treatment on the Histology of Ulcerative Colitis.
In patients who have received medical therapy (oral or enema), mucosal histologic changes can vary considerably. Portions of mucosa may heal completely, whereas others may still be active. Healing occurs in a segmental or patchy fashion. This pattern gives an impression of segmental or patchy disease (skip lesions), which may be mistaken for CD. In this circumstance, exceptions to the classic principles of UC pathology may lead to diagnostic confusion.
Classic teaching emphasizes that UC is characterized morphologically by the presence of diffuse fixed architectural or cellular mucosal changes (or both) that categorize the process as chronic. However, in 1993, Odze and colleagues prospectively evaluated 123 rectal mucosal biopsy specimens from 14 patients with pathologically confirmed UC treated with either 5-aminosalicylic acid (5-ASA) or placebo enemas.54 During the course of treatment, 29% of rectal biopsies from 64% of patients were histologically normal, showing no evidence of chronic or active disease. Patients treated with 5-ASA showed a significantly higher percentage of normal biopsy specimens (obtained from areas of mucosa previously shown to be involved with chronic active disease) than did the placebo group. This was the first report to demonstrate that “fixed” chronic features in UC may revert to normal in the natural course of the patient’s illness, and that this phenomenon may be enhanced by topical therapy. Subsequent studies by Kleer and Appelman,55 Bernstein and colleagues,56 and Kim and colleagues,57 all of whom evaluated patchiness of disease and patterns of involvement in UC colorectal biopsy specimens with time, confirmed and expanded the initial findings of Odze’s group.
In these studies, 30% to 59% of patients, some of whom were treated with oral sulfasalazine or steroids (or both), showed either patchiness of disease or rectal sparing on follow-up surveillance biopsies. Awareness of these data should prevent misinterpretation of the findings of a normal rectal biopsy specimen or patchiness of disease in medically treated patients with UC as evidence against this diagnosis or as representing skip areas characteristic of CD. In addition, patients with low-grade indolent disease, particularly those in clinical and pathologic remission, may show minimal architectural features of chronicity or perhaps even a completely normal-appearing biopsy specimen, during the natural waxing and waning course of their illness. However, it must be emphasized that these data relate primarily to biopsy material from treated patients. They do not apply to patients whose UC has not yet been treated or in whom a diagnosis is being considered on the basis of the evaluation of a resection specimen. Evaluation of disease “continuity” by analysis of mucosal biopsies is not useful to distinguish UC from CD of the colon in previously treated IBD patients. In contrast, large portions of mucosa from a resection specimen with a normal histologic appearance are an indication of true segmental disease and normally provide reliable evidence in support of an alternative diagnosis such as CD.
Unusual Morphologic Variants of Ulcerative Colitis
A summary of the causes of unusual morphologic patterns of disease in UC is provided in Box 17.2. It is important that pathologists recognize these variants so they can avoid falling into diagnostic traps and misdiagnosing UC as CD. This section provides a summary of many of the causes of Crohn’s-like changes in UC, which include segmental or patchy involvement of the colon, rectal sparing, skip lesions, ileal or upper GI inflammation, aphthous ulceration, and granulomas.
Ascending Colon, Cecum, and Appendiceal Involvement as Skip Lesions in Ulcerative Colitis
Some patients with either subtotal or left-sided colitis show patchy, mild, chronic, or active inflammation in the cecum or ascending colon that may be falsely interpreted as CD because of the impression of segmental involvement.58–60 As many as 65% of patients with UC have limited left-sided involvement initially, but eventual extension to more proximal portions of the colon occurs in 29% to 58% of such patients.58,61,62 In one study by D’Haens and colleagues of 20 patients with established left-sided UC, 6 showed a sharp demarcation between affected and unaffected portions of colon, whereas 14 showed a more gradual transition.58 The area of transition in such cases may appear somewhat patchy, giving a false impression of skip lesions. Seventy-five percent of this latter group of patients showed an area of inflammation in the cecum, primarily in the periappendiceal mucosa, that was separate from the distal inflamed segment. In a study by Mutinga and co-workers, 14 patients with both left-sided UC and pathologically confirmed patchy right-sided chronic inflammation were compared with 35 control patients who had limited left-sided UC only.47 The two groups had similar demographic features, extraintestinal manifestations, severity of disease, prevalence of extension to pancolitis, and natural history, which suggests that patchy right-sided inflammation in patients with left-sided colitis has little clinical significance. This fact should be recognized by pathologists so that a false diagnosis of CD can be prevented.
In a prospective study of 271 patients with UC, including 63 with inactive left-sided or subtotal colitis, skip areas of periappendiceal cecal mucosal involvement were identified in 32% of patients. Similarly, since the original description by Davison and Dixon in 1990 of “discontinuous involvement” wherein the appendix was found to be inflamed in 21% of 62 cases of distal UC,63 several other studies have shown that the appendix may be involved as a “skip lesion” in this disease,64,65 although at least one other study failed to confirm this finding.66 In another study by Groisman and colleagues, ulcerative appendicitis was present in 86% and 87% of patients with “nonuniversal” and “universal” UC, respectively.64 Their study included two cases with limited left-sided involvement combined with appendiceal involvement. Overall, the role of the appendix in UC is poorly understood. Patients with prior appendectomy have been shown to have a lower risk for UC.67,68 In one study, the severity of appendiceal inflammation (ulceration) in patients with UC was a strong predictor of the development of pouchitis after a total proctocolectomy and ileoanal pouch procedure.69 In summary, involvement of both the appendix and the cecal or ascending colon can occur in patients with subtotal colitis. This phenomenon should be recognized by pathologists as an acceptable potential skip lesion in UC.
Initial Presentation of Ulcerative Colitis in Pediatric Patients
Several recent studies have shown that pediatric patients with untreated UC at presentation may show evidence of relative, or even complete, rectal sparing or patchy colonic disease.70–74 Markowitz and co-workers reported on 12 pediatric patients with untreated UC, 5 (42%) of whom showed patchy, mild active inflammation and mild crypt changes in rectal, compared with proximal, colonic biopsy specimens.70 One patient had a completely normal rectal biopsy specimen. A study by Glickman and associates compared the rectal mucosal biopsy appearance of 70 pediatric patients who had UC with that of 44 adult patients, all at initial presentation before treatment.71 Compared with adults, pediatric patients showed significantly fewer cases of chronic active disease and a greater number of patients with microscopic skip areas and relative rectal sparing. Two of the 70 pediatric patients had completely normal rectal biopsy specimens at initial presentation, in contrast to none of the adult patients. Therefore, an absence of features of chronicity or the presence of mild active disease and microscopic skip areas at initial presentation in pediatric patients does not exclude a diagnosis of UC. In adults, relative (but not absolute) rectal sparing (i.e., less severe inflammation in the rectum compared with the proximal colon) may be seen, rarely, at initial presentation before treatment.75,76 In one study, a 31% prevalence rate of relative rectal sparing was noted in a series of 46 adult patients with UC at initial presentation, but even in those cases, histologic features of chronicity were almost always present in rectal mucosa at the time of initial diagnosis.76
Ileitis in Ulcerative Colitis
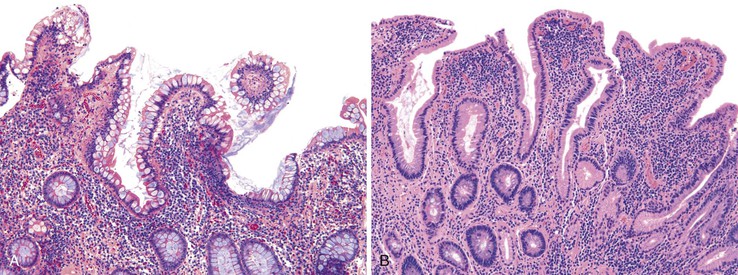
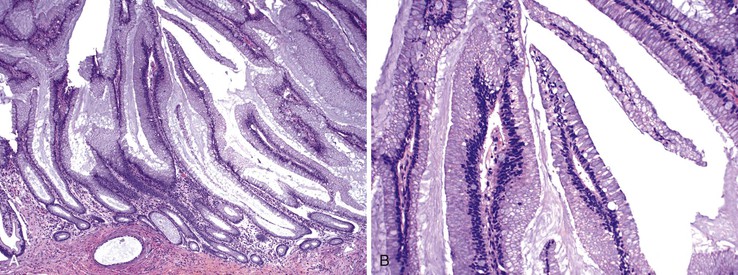
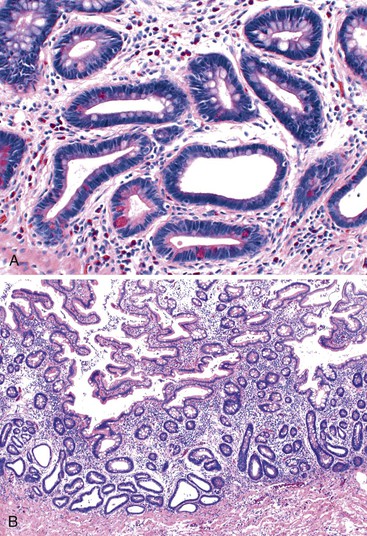
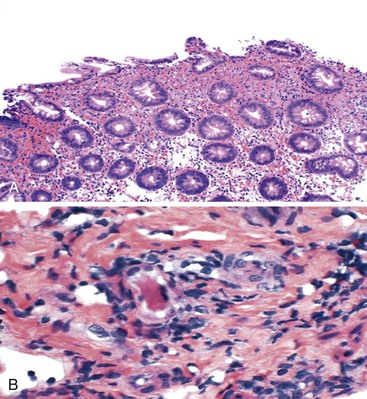
Patients with severe pancolitis, including involvement of the cecum and ileocecal valve, may show a mild degree of active inflammation in the distal few centimeters of terminal ileum; this is presumably related to inflammation-induced incompetence of the ileocecal valve and subsequent reflux of colonic contents,77,78 termed backwash ileitis. Pathologic criteria for backwash ileitis were defined in 2005.79 Haskell and colleagues reviewed 200 UC resection specimens and found active ileitis in 17%, confined in most cases to the distal 1 cm of ileum.79 Ninety percent of the cases consisted of mild, patchy neutrophilic inflammation in the lamina propria, focal cryptitis or crypt abscesses, and patchy villous atrophy and regenerative changes (Fig. 17.18, A). In rare instances, surface ulceration or even pyloric (mucous gland) metaplasia was present (see Fig 17.18, B). In their study, a backwash mechanism for ileitis in UC was questioned in some cases that showed lack of cecal involvement (or only mild inflammation in the cecum) and patchy or discontinuous ileal involvement. These cases may have resulted from a drug effect or bowel preparation effect. Nevertheless, ileal findings such as crypt architectural distortion, fissuring ulceration, submucosal inflammation, granulomas, or greater lengths of ileal involvement (>5 cm) should raise a strong suspicion for CD, particularly if the patient does not have severe disease up to and involving the proximal cecum and ileocecal valve. More recently, in a large case-control study, Arrossi and associates evaluated pouch outcome in patients with UC and IBD of indeterminate type and reported that the presence of backwash ileitis was not a significant risk factor for the development of pouchitis.80
Rarely, premalignant dysplastic changes and even adenocarcinoma have been shown to develop in the setting of backwash ileitis.81 Heuschen and colleagues found a strong association between backwash ileitis and the development of colorectal cancer in patients with UC who had undergone proctocolectomy.82 However, discrete pathologic criteria for backwash ileitis were not defined, and it is likely that most of the patients in whom cancer developed had CD. Neither Haskell’s nor Arrossi’s group found an increased risk for dysplasia or cancer in patients who had UC with ileitis, compared with those without ileitis.79,80
Other factors may also lead to the development of active inflammation in the distal ileum (active ileitis) in UC, including infections, drugs (e.g., nonsteroidal antiinflammatory drugs [NSAIDs]), and, most commonly, the effects of certain bowel preparatory agents.83 It has been speculated that the ileum may be involved by UC in some cases, but this is controversial.83–85 In a study consisting of 50 patients with active UC, 16% of patients had ileal inflammation but without involvement of the cecum, indicating that backwash was not the cause of the ileitis (“non-backwash ileitis”).86 These patients had higher levels of ileal inflammatory cytokines (IL6, IL8, and tumor necrosis factor-α [TNF-α]) and, more commonly, had extraintestinal manifestations of UC (e.g., arthritis, pyoderma gangrenosum) at presentation. This condition should not be confused with CD of the terminal ileum. In CD, the distal ileum typically shows longer lengths of involvement (often >5 cm) and is associated with histologic features of chronicity, such as pyloric gland metaplasia, ulceration, and established radiologic abnormalities.78
Upper GI Involvement in Ulcerative Colitis
Gastric or duodenal involvement, or both, has been rarely reported in association with clinically and pathologically confirmed UC.87–92 A study by Valdez and co-workers described four patients with chronic active inflammation in the duodenum similar in appearance to the patients’ colonic disease.87 In another study, five patients had chronic active gastritis and four had chronic active duodenitis; in these cases, the upper GI findings resolved after colectomy.93 Several similar cases have been reported in the Japanese literature.94 In a recent study by Lin and colleagues, esophageal, gastric, and duodenal biopsies from 69 patients with proven UC were compared with those of 97 control subjects.95 The most common pattern of inflammation in the upper GI tract was “focal gastritis,” followed by mixed inflammation in the basal aspect of gastric mucosa and superficial plasmacytosis. Diffuse chronic duodenitis was observed in 10% of UC patients, even after colectomy. The authors did not find specific mucosal changes in esophageal biopsy specimens from patients with UC. Until such cases have been followed for longer periods, it is difficult to know whether upper GI involvement in UC represents a manifestation of the primary inflammatory disorder or simply an unidentified, but unrelated, inflammatory disorder. Precise characterization of these cases with long-term follow-up is needed to help establish specific criteria for upper GI involvement in patients with UC.
Crohn’s-Like Aphthous Ulcers in Ulcerative Colitis
Although aphthous ulcers are characteristic of CD, they may occur in other types of colitides and even in UC. They have been reported in infectious colitis, diverticular disease, and diversion colitis, among other conditions.83 In one study, aphthous-type ulcers were present in 17% of UC resection specimens.69 In that study, manifestations of CD did not develop in any of the patients, nor was the presence of aphthous ulcers associated with the subsequent development of pouchitis.
Granulomas in Ulcerative Colitis
Some cases of active UC may show granulomas in association with ruptured crypts (see Fig. 17.13). This finding may be prominent and may involve many crypts at the base of the mucosa. Granulomas may also develop in UC from unrelated causes, such as degenerated collagen, particulate matter, superimposed infections, or as a result of drug reaction.96–100 In equivocal cases, mucin granulomas related to ruptured crypts may be distinguished from granulomas in CD by use of histochemical stains or evaluation of multiple deep tissue sections. In general, granulomas present in the superficial mucosa (and certainly in the submucosa) are more likely to be CD related than basal mucosal granulomas, which are often crypt related.
Natural History and Treatment
Current therapeutic strategies for UC are separated into those that treat active disease (induction therapy) and those that prevent recurrence of disease once remission has been achieved (maintenance therapy). Medical therapy focuses on agents that alter the host’s immune response in an effort to decrease mucosal inflammation. First-line therapy consists of oral 5-ASA preparations such as sulfasalazine, Pentasa, Asacol, and Balsalazide. Sulphasalazine induces remission in 39% to 62% of patients with mild to moderate UC.101 Topical 5-ASA enemas can be used to treat disease located as much as 20 cm from the anal verge. Moderate to severe flares of UC are treated with systemic glucocorticosteroids. Azathioprine and 6-mercaptopurine are two purine analogue immunomodulators that interfere with nucleic acid metabolism and exert a cytotoxic effect on lymphocytes. Cyclosporine A, another potent inhibitor of cell-mediated immunity, is primarily indicated in patients with severe steroid-refractory disease.
Surgical therapy is indicated in cases of medically refractory disease, recurrent systemic complications, unacceptable side effects of medical therapy, colonic perforation, colonic dysplasia, or carcinoma. Surgical choices include subtotal colectomy with ileostomy, colectomy with ileorectal anastomosis, and proctocolectomy with ileal pouch–anal anastomosis (IPAA).
The typical course of UC consists of periods of remission interrupted by flares of activity. In approximately 5% of patients, the course is complicated by toxic megacolon, defined as acute colonic dilatation (with a transverse colon diameter >6 cm radiologically), with loss of haustration, in a patient with a severe attack of colitis. It is usually encountered early in the course of disease and in some cases may be the initial presentation of UC. Approximately 50% of patients respond to medical therapy alone. Perforation is the most important predictor of mortality. Surgery is recommended for patients with perforation or clinical deterioration after 48 to 72 hours of medical therapy.
Colonic strictures develop in almost 5% of UC patients. Their presence should always prompt a high index of suspicion for malignancy, especially if the stricture is located proximal to the splenic flexure. In a retrospective study comprising 1156 patients, 24% of strictures were found to be malignant.102 Most of the malignant strictures were located proximal to the splenic flexure, were clinically symptomatic, and developed late in the course of disease (after 20 years). Cancers associated with strictures tend to be more advanced than those not associated with a stricture.
Patients with UC have an increased risk for colorectal cancer, and the primary risk factor is the duration and extent of the disease (see Dysplasia in Inflammatory Bowel Disease).
Crohn’s Colitis
Clinical Features
CD is a chronic inflammatory condition that can affect any part of the GI tract but has a propensity to involve the distal small and proximal large intestine. Descriptions of CD date back to more than three centuries, when it was termed “terminal ileitis,” “regional enteritis,” and “granulomatous enterocolitis.” Crohn, Ginzburg, and Oppenheimer are credited with the first modern description of CD in 1932.103
The clinical classification of CD, known as the Montreal classification, is based on age at disease onset, principal anatomic location, and clinical behavior (Box 17.3). It distinguishes disease of the ileum from that of the colon or both ileum and colon. Approximately 30% to 40% of patients have small bowel involvement only, and 30% to 40% have ileocolonic involvement; only 10% to 20% have exclusive involvement of the colon (Crohn’s colitis). In patients with ileal disease, colonic lesions develop in fewer than 20% of patients during a period of 10 years.104 Similarly, ileal involvement occurs in 20% of patients with colonic disease.105 In a retrospective analysis of 84 patients with Crohn’s colitis, 52% had right-sided colitis, 40% had left-sided colitis, and 6% had pancolonic involvement. Small bowel involvement was more frequently associated with right-sided disease, whereas proctitis and perianal lesions were more frequent in patients with left-sided disease.106
Accurate assessment of the incidence and prevalence of CD worldwide is limited because of inconsistencies in diagnostic criteria, lack of thorough clinical evaluation with the use of modern radiologic techniques, and, in some cases, an inability to differentiate UC from CD pathologically. Despite these limitations, reproducible epidemiologic trends have been discerned. The age-adjusted annual incidence rate was reported to be 9 per 100,000 persons in Olmsted County, Minnesota. More recently, the prevalence of CD in the United States was estimated at 201 per 100,000 adults or 43 per 100,000 people younger than 20 years of age.8 There is a higher incidence of CD in northern latitudes (e.g., Denmark, 9/100,000; Nova Scotia, 20/100,000) than in southern Europe(e.g., Spain, 0.9/100,000; Italy, 3.4/100,000). In Asian, South American, and most African countries, the incidence is very low.8 Although all ethnic groups may be affected, CD is more prevalent among white North Americans, northern Europeans, Ashkenazi Jews, Scandinavians, and the Welsh.
Women are slightly more commonly affected than men (female-to-male ratio, 1.3 : 1). Most patients with CD are diagnosed during the second to fourth decades of life, although there is a smaller peak between the fifth and seventh decades. There is no relationship between pathologic findings and age at onset, but some studies have identified a greater proportion of colonic and distal colonic disease among older patients and a predominance of ileocolonic disease in younger patients.107,108 In one recent study that evaluated 118 patients with either isolated colonic or ileocolonic CD, those with isolated colonic CD were significantly older at disease onset and had a shorter interval from initial diagnosis to surgery. Compared with patients with ileocolonic disease, those with isolated colonic CD more often had subtotal or total colitis and were more likely to have left-sided colitis.109
The clinical presentation of CD varies substantially depending on the principal location of disease, the intensity of inflammation, and the presence or absence of specific intestinal and extraintestinal complications. In some cases, weight loss and fever may be the only presenting features, especially in children. Crohn’s colitis often manifests with diarrhea, either with or without blood. Depending on the extent of colonic involvement and the severity of inflammation, patients may have a range of initial findings, ranging from minimally altered bowel habits to fulminant colitis. Intermittent and colicky abdominal pain is a common presenting symptom. Although most patients have either relative or complete sparing of the rectum, proctitis may be the initial, or even the only, area of involvement at presentation in some cases. Perianal skin tags, anal fissures, or ulcers are often present at the time of diagnosis as well. In a subset of patients (almost 24%), perianal disease precedes intestinal symptoms by a mean period of as long as 4 years.110
The clinical pattern of disease is typically divided into aggressive fistulizing, fibrostenosing, and cicatrizing disease. Fistulas are a common finding in CD and may involve different segments of the bowel; more rarely, they may involve adjacent organs as well (coloduodenal, cologastric, or rectovaginal fistula). Symptoms of intestinal obstruction or jaundice, or both, are more common in the fibrostenotic form of disease.
Extraintestinal manifestations of CD occur in 6% to 25% of cases and are more common among patients with colonic involvement.111,112 They include musculoskeletal disorders such as pauciarticular arthropathy (6%), polyarticular arthropathy (4%), and peripheral arthralgias (16% to 20%). Axial arthropathies, granulomatous vasculitis, periostitis, and amyloidosis are other rare rheumatologic complications.113–115 Mucocutaneous lesions include pyoderma gangrenosum, erythema nodosum, and oral aphthous ulcers. Episcleritis and uveitis tend to occur in association with active intestinal disease and occur in as many as 6% of patients. Among hepatobiliary manifestations, more than 25% of cases manifest with symptomatic cholelithiasis. Although it is more commonly associated with UC, PSC may develop in as many as 4% of patients who have colonic CD.116 Hyperoxaluria with calcium oxalate stone formation, interstitial nephritis, and cardiomyopathy, have associations with CD as well.
Patients with CD have a prothrombic tendency, and therefore may present with venous thromboembolism or, less commonly, arterial thrombosis. In more than 50% of patients, a predisposing factor cannot be identified.117,118
As in UC, there is no specific clinical or laboratory test that establishes a definite diagnosis of CD. The diagnosis is usually established based on a compilation of clinical findings combined with radiologic, endoscopic, and pathologic findings. Laboratory tests may be completely normal. In some patients, the white blood cell count is elevated, which suggests a pyogenic complication. The presence of anemia, elevated ESR, and elevated C-reactive protein in a patient with abdominal pain is not specific for CD but should always prompt a work-up for IBD. Stool studies, including culture, examination for ova and parasites, C. difficile toxin assay, and serologic testing for Entamoeba histolytica, are usually performed to exclude an infectious cause. Serologic testing shows elevated ASCA levels in 41% to 76% of patients.119,120
Barium studies are a popular method of investigation for patients with suspected CD. They are especially helpful in delineating late transmural complications of CD. Aphthous ulcers, thickened mucosal folds, submucosal edema, fistulas, sinus tracts, and fixed strictures are some of the findings that may be detected by barium studies. Currently, CT or magnetic resonance enterography is preferred over barium studies. Radiologic findings that correlate with disease activity include mural enhancement and increased density of pericolonic fat.121,122
Common endoscopic findings of CD include aphthous ulcers, edema, cobblestoning, and luminal narrowing. Segmental involvement is characteristically present in early-stage disease. Rectal sparing is often present in untreated cases.
Once a diagnosis of CD has been established, clinical disease activity is usually monitored with the use of a composite scoring system. The Crohn’s Disease Activity Index (CDAI) is a commonly used scoring system that evaluates eight variables (stool count, abdominal pain, general well-being, features of extraintestinal disease, opiate intake for diarrhea, presence of abdominal mass, hematocrit value, and body weight).123
Risk Factors and Pathogenesis
The cause of CD is unknown; similar to UC, it probably involves a combination of environmental factors (e.g., luminal bacteria, infectious agents), abnormalities in immune regulation, and genetic predisposition for development of disease.
Many infectious agents, including Chlamydia, Listeria monocytogenes, Pseudomonas species, paramyxovirus, and Mycobacterium paratuberculosis, have been etiologically linked to CD as a cause of granulomatous vasculitis and bowel injury. Molecular techniques have detected M. paratuberculosis in tissues of some patients with CD.124 One study by Lamps and colleagues found Yersinia species DNA in 31% of Crohn’s resection specimens.124 There are many histologic similarities between these infections and CD, and the possibility that one or more of them triggers the development of CD is an appealing hypothesis. However, thus far, there is no conclusive evidence to implicate any one specific organism in the pathogenesis of CD.
Genetic Factors
The relative risk of development of IBD among first-degree relatives of patients with CD is 14 to 15 times higher than in the general population.125 Ethnicity also appears to play a significant role. Eastern European (Ashkenazi) Jews have a twofold to fourfold higher risk for CD than non-Jews from the same geographic location. Further support for a genetic predisposition is provided by data on monozygotic and dizygotic twins.19,126 The concordance rate for CD is 67% among monozygotic twins and 8% among dizygotic twins, suggesting a strong genetic influence. Although results are inconsistent and vary with the population being studied, numerous studies have found both positive and negative associations between HLA antigens and the development of CD.127,128
Genome-wide association studies and computerized meta-analyses have identified 71 susceptibility loci for CD on 17 chromosomes thus far.129 Three important pathways have been highlighted by these studies. The first susceptibility locus was identified in 2001 as nucleotide-binding oligodimerization domain 2 (NOD2) or caspase-recruitment domain 15 (CARD15).130–132 A homozygous carrier of disease-specific allelic variants has a 17.1-fold increased risk for CD, whereas the odds ratio for a heterozygous carrier is 2.5.133 Genetic polymorphisms in NOD2/CARD15 are present in 20% to 30% of patients with CD, and the abnormalities correlate with younger age at onset, ileal location of disease, and an increased likelihood of stricture formation.134,135 NOD2/CARD15 mutations are more common in white patients with CD but are rare in Asians and Africans.136 The NOD2/CARD15 gene product binds to muramyl dipeptide, a component of bacterial peptidoglycan found in both gram-positive and gram-negative bacteria.137,138 NOD2/CARD1 is expressed in Paneth cells139 that produce endogenous antimicrobial peptides known as defensins. NOD2/CARD15 gene variants interfere with binding to muramyl peptide, resulting in decreased antibacterial defense.
The second important pathway implicated in CD pathogenesis is related to autophagy, a unique process by which cytoplasmic constituents are isolated within a membrane-bound vesicle and then delivered to lysosomes for elimination. Misfolded or misaggregated proteins are eliminated via this pathway without inciting an inflammatory or autoimmune response. Variants in at least two autophagy-related genes have been associated with CD—namely, autophagy-related 16-like 1 (ATG16L1) and immunity-related guanosine triphosphatase family member M (IRGM) gene on chromosome 5.140–143
The third pathway associated with CD is related to IL23.144 IL23 is a cytokine produced by dendritic cells and macrophages in response to various antigenic signals. In response to IL-6 and transforming growth factor-β (TGF-β), naïve CD4+ T cells upregulate IL23 receptor (IL23R), which results in autocrine generation of effector T cells that produce IL17. Although most common single nucleotide polymorphisms (SNPs) in the IL23R gene are associated with an increased risk for CD and UC, rare variants appear to be protective against the development of CD.145,146
Environmental Factors
The gradually rising incidence of CD has also been attributed to a variety of environmental factors. Higher socioeconomic status, use of oral contraceptives, NSAIDs use, increased intake of refined sugars, and decreased intake of dietary fiber have all been implicated as risk factors for CD. Zinc deficiency is associated with immunologic dysfunction in patients with CD,147 and some data suggest that an elemental diet may improve CD by reducing intestinal permeability.148
In contrast to UC, CD is more prevalent among smokers. Smoking is an independent risk factor for clinical, surgical, and endoscopic recurrences in CD and also appears to influence disease activity after surgery. Although the pathogenesis is unclear, it is believed that smoking causes alteration of intestinal permeability, induces cytokine production, and promotes production of microvascular thrombi.149,150
Immune Factors
Interaction of effector T cells and antigen-presenting cells is vital to the pathogenesis of CD. Processing of luminal antigens by dendritic cells located within the lamina propria and the subsequent interaction between MHC class II molecules and T-cell receptors leads to activation and differentiation of T cells. The helper T-cell Th1 and Th17 responses that characterize CD are influenced by cytokines IL23, IL6, and TGF-β. Within mononuclear cells, NF-κB plays a key role in regulating transcription of IL1, IL6, IL8, TNF, and other peptides that generate an inflammatory response. TNF is not only essential in formation of granulomas but also causes neutrophil activation and, along with interferon-γ, induces expression of MHC class II on intestinal epithelial cells.
TNF and other proinflammatory cytokines also promote expression of adhesion molecules on endothelial cells, which leads to trafficking of inflammatory cells into mucosa. Integrins α4β7 and αEβ7 have ligands (mucosal addressin cellular adhesion molecule and E-cadherin, respectively) that are specific to the intestinal environment.151 Antibodies to the α4 subunit of integrin have a therapeutic role in the management of CD.152
Tissue destruction (especially penetrating ulcers, fistulas, and sinuses) ultimately results when proinflammatory substances such as prostaglandins and matrix metalloproteinases are elaborated by mononuclear cells and granulocytes. Mural fibrosis, another characteristic finding of CD, is a result of TGF-β that is released in the presence of inflammation. It stimulates production of type III collagen, which not only promotes healing of ulcers but also contributes to the formation of strictures.153 Fat wrapping (“creeping fat”) is an indicator of transmural disease that is often identified intraoperatively. It results from upregulation of peroxisome proliferator–activated receptor-γ (PPAR-γ), which regulates homeostasis of adipose tissue.154 Histologically, the finding of pyloric gland metaplasia in the lower GI tract indicates chronic mucosal injury. Pyloric glands are a form of ulcer-associated cell lineage (UACL); they represent bud-like glandular structures that develop from the base of intestinal crypts at sites of chronic ulceration. The UACL expresses a variety of peptides implicated in the repair of damaged GI mucosa, notably epidermal growth factor and members of the trefoil peptide family, which restores epithelium in areas of mucosal ulceration.155
The pathophysiology of diarrhea in CD is related to multiple factors. Increased mucosal permeability because of inflammation and production of prostaglandins, biogenic amines, neuropeptides, and reactive oxygen metabolites results in exudation of proteins and fluids. Bacterial overgrowth and altered colonic mobility in areas of strictured bowel also contribute to diarrhea.
Pathologic Features
Gross Features
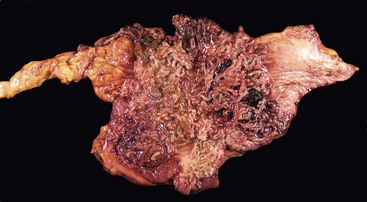
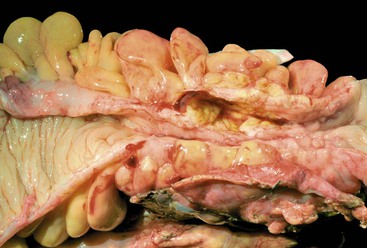
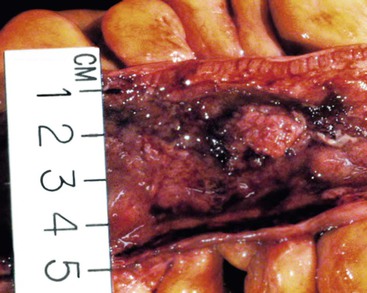
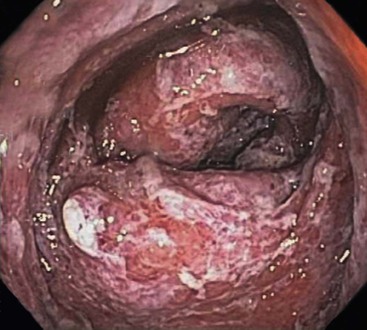
CD is characterized by segmental involvement of the affected areas of bowel, although pancolitis may occur in a small proportion of cases (Fig. 17.19, A). The serosal surface often appears congested and may be covered with a fibrinous exudate. Fat wrapping (creeping fat) along the antimesenteric border is a common finding in CD. In contrast to UC, the bowel wall in CD is typically thickened. It does not lie flat on opening. Mucosal aphthous ulcers overlying lymphoid aggregates are an early feature of CD. A zone of hyperemia often surrounds larger ulcers. As the disease progresses, ulcers enlarge to form discontinuous, serpiginous, or bearclaw-type longitudinal furrows (see Fig. 17.19, B). Areas of edematous, mildly inflamed, or even normal mucosa are located between areas of longitudinal ulcers; this results in the development of a cobblestone appearance of the mucosa. Inflammatory pseudopolyps are most commonly encountered in the transverse colon and splenic flexure. In segments of bowel involved by disease, the wall of the bowel is usually thick and fibrotic. Fissures, sinus and fistulous tracts, and mural or pericolonic abscesses may be present in complicated cases.
Strictures are more common with long-standing disease. Depending on the degree of obstruction, the bowel wall proximal to the stricture may be secondarily dilated and congested (Fig. 17.20). Perforations are uncommon in CD, occurring only in 1% to 3% of cases.156,157 They occur as a result of superimposed ischemia or infection or as a complication of fissures, sinus tracts, or fistulas. Fibrous adhesions may seal off sites of perforation, so they may not be visible upon gross examination of the bowel.
Microscopic Features
General Comments.
Histologically, colonic CD (whether isolated or combined with ileal disease) classically shows skip areas of involvement, both grossly and microscopically. Areas of involvement alternating with areas of normal mucosa are characteristic (“segmental” colitis). However, as mentioned earlier, pancolitis occurs in a small proportion of cases. Overall, CD is characterized by the presence of a wide variety of mucosal and mural changes (Box 17.4). Other major pathologic features include aphthous and fissuring ulcers, sinuses and fistulas, transmural lymphoid aggregates, and non-necrotizing granulomas. All of these features may affect the bowel wall in a patchy and segmental distribution. Other, less characteristic but common features include submucosal fibrosis, neural hypertrophy, muscularis mucosae and muscularis propria hypertrophy, neural plexitis, perivascular lymphoid aggregates, serositis, and pyloric gland metaplasia.
Mucosal Changes.
Similar to UC, and depending on the phase of disease, the mucosa may show a wide spectrum of changes ranging from completely normal to diffuse and severe chronic active inflammation with ulceration. In biopsies, mucosal disease is categorized similar to UC: chronic inactive, chronic active, or active (see earlier discussion). Histologic features of chronicity include crypt architectural distortion, crypt atrophy, diffuse mixed lamina propria inflammation, basal plasmacytosis, basally located lymphoid aggregates, pyloric gland metaplasia, and Paneth cell metaplasia (in the left colon). Other changes of chronicity include lamina propria fibrosis and Paneth cell hyperplasia in the right colon. “Activity” is characterized by the presence of neutrophilic or eosinophilic cryptitis, crypt abscesses, regenerative or degenerative epithelial changes, necrosis, erosions, and ulceration. The degree of activity is graded as mild if less than 50% of the mucosa shows evidence of activity, moderate if more than 50% of the mucosa shows these features, and severe if surface erosion or ulceration is present.
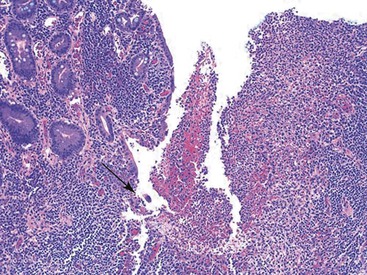
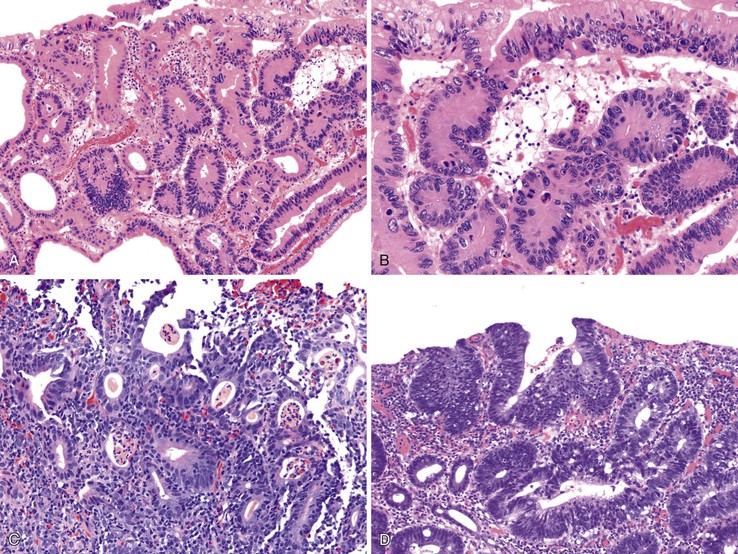
In active CD, the disease may be patchy, with foci of injured and inflamed crypts situated adjacent to completely normal crypts. Two types of ulcers are characteristic of active CD—aphthous and fissuring ulcers. Aphthous ulcers arise in focal, mildly active CD. They are well-delineated, small, and superficial lesions that overlie lymphoid aggregates. They usually involve a length of mucosa occupied by two to four crypts. The earliest stage is a mild neutrophilic infiltrate in the superficial half of the lymphoid aggregate. Neutrophils infiltrate the crypts and form small basilar crypt abscesses, producing epithelial necrosis and an intraluminal exudate (Fig. 17.21). Concomitant neutrophilic infiltration and erosion of the superficial epithelium develops into a small microabscess that covers the lymphoid aggregate as the ulcer expands. Irregularly shaped crypts with regenerative epithelial changes are typically found at the edges of older (healing) ulcers. Aphthous ulcers can continue to expand and connect to form serpiginous or longitudinally oriented ulcers.
Crypt disarray is a feature of mucosal involvement in CD, similar to UC. There is significant variation in the size and shape of crypts. Features such as crypt branching and shortening are most easily appreciated at medium or low magnification (Fig. 17.22). Heterogeneity in the density and distribution of lymphoplasmacytic inflammation within the lamina propria is a common finding in CD, as it is in UC. Well-circumscribed, focal collections of lymphocytes that surround several crypts (lymphoid aggregates) simulate normal lymphoid follicles. However, lymphoid aggregates in CD may reveal crypts within their center, whereas in normal lymphoid follicles, the crypts are pushed toward the periphery.
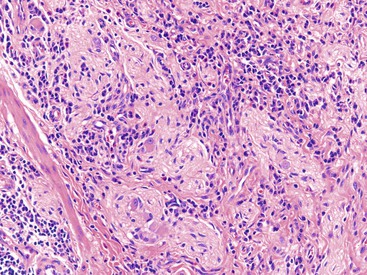
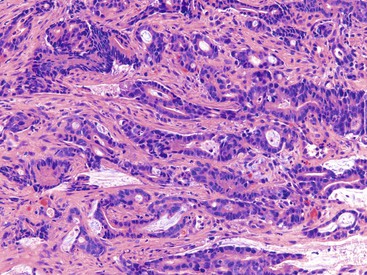
Although non-necrotizing epithelioid granulomas are characteristic of CD, they are neither specific nor sensitive for this diagnosis.99,158,159 The prevalence of granulomas in endoscopic biopsy samples ranges from 13% to 50%; in resections, it ranges from 40% to 60%.160 Granulomas are more frequently encountered early in the course of disease. They are typically sarcoid-like, composed of aggregates of epithelioid histiocytes admixed with lymphocytes and neutrophils (Fig. 17.23). Occasionally, giant cells are present. In some cases, they may be very sparse and poorly formed, consisting only of small, pericryptal collections of closely arranged histiocytes, referred to as pericryptal microgranulomas.161 Serial step sections enhance the likelihood of detecting pericryptal microgranulomas.162–164 They may be present in involved as well as in uninvolved segments of colon. They may be present in any layer of the bowel wall or within pericolonic lymph nodes (Fig. 17.24).
Granulomas in CD should be distinguished from mucin granulomas that form around ruptured crypts, which is common in UC. Macrophages within mucin granulomas usually have a greater amount of bubbly or clear cytoplasm, which is caused by phagocytosis of mucin and crypt contents. Foreign body giant cells usually are not a component of CD-related granulomas, but they may be found in mucin granulomas. Mucin stains are not helpful, because a small amount of mucin may also be present in the cytoplasm of macrophages in CD-related granulomas. Thick-walled capillaries, pericryptal fibroblastic sheaths, and tangential sections of germinal centers may mimic the well-formed granulomas of CD. The presence of necrotizing granulomas or a large number of granulomas should prompt a work-up for an infectious etiology such as tuberculosis, histoplasmosis, yersiniosis, or sarcoidosis.
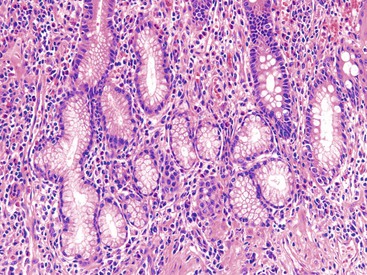
Pyloric gland metaplasia is far more common in CD than in UC, and it is frequently present in the small bowel. In colonic CD, pyloric gland metaplasia is more common in the cecum and right colon (Fig. 17.25).
Mural Changes.
Among the common mural changes of CD are knife like fissuring ulcers and sinus tracts, which typically occur at right angles to the longitudinal axis of the bowel. They may extend through the bowel wall and may develop into a fistula or result in pericolonic abscess formation (Fig. 17.26). They are usually lined by acute inflammatory cells, necrotic and granulation tissue, and loose aggregates of epithelioid histiocytes resembling early granulomas. Multinucleate giant cells may be present as well. Dense lymphoid aggregates, with or without germinal centers, are usually found at the mucosal-submucosal junction. However, transmural lymphoid aggregates that are located in the deeper aspects of the bowel wall, including the subserosal adipose tissue (“Crohn’s rosary”), are characteristic of CD (Figs. 17.27 and 17.28). These are defined as collections of predominantly small, mature-appearing lymphocytes of variable size that are distributed throughout the bowel wall. Some cases of CD show prominent perivascular lymphoid aggregates, which may or may not be associated with granulomas, particularly in the submucosa. This feature has led some authorities to postulate that CD may represent a vascular disorder.
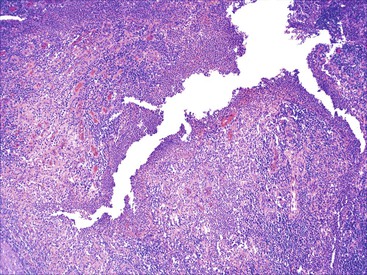
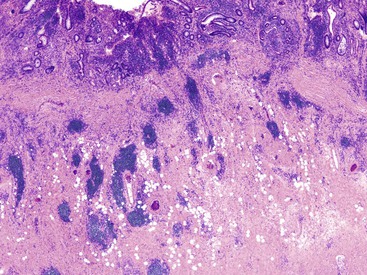
Abnormalities in the smooth muscle, such as splaying and hypertrophy of muscularis mucosae and distortion (and hypertrophy) of the muscularis propria, are common in long-standing CD. Proliferation of smooth muscle that obliterates the submucosa (“muscularization of submucosa”) occurs more commonly in stricturing CD.165 CD also affects the neural tissue. There is both hypertrophy of nerve trunks and an increase in the number of ganglion cells.166,167 Large, abnormal fusiform nerve bundles may be seen throughout the affected bowel wall. Additionally, the myenteric plexuses may show infiltration by lymphocytes (“myenteric plexitis”) (Fig. 17.29). Some studies have shown that myenteric plexitis in the proximal margins of an ileocolonic resection is more likely to be present in resection specimens of patients with a previous surgery for CD and of those with a shorter duration of disease before surgery.168 The severity of inflammation also appears to correlate with severity of endoscopic recurrence.169
Some studies suggest that colonic CD less frequently shows major pathologic features than does ileal CD. A recent study by Soucy and colleagues showed that patients with isolated CD had significantly fewer major pathologic features of CD, such as strictures/stenosis, pericolonic adhesions, segmental disease, the finding of proximal disease worse than distal disease, perivascular lymphoid aggregates, and pyloric gland metaplasia, compared with those with ileal CD.109 A small proportion of patients from both groups showed inflammatory changes limited to the mucosa, similar to what is seen in UC (see later discussion).
Superficial (Ulcerative Colitis-Like) Crohn’s Colitis
As mentioned previously, the colon is involved in almost 50% of CD cases, either alone or in combination with the small intestine. As many as 20% of CD patients have disease limited to the colon. Although some cases of Crohn’s colitis show classic features of CD (e.g., fissuring ulcers, sinus tracts, transmural lymphoid aggregates, submucosal fibrosis, granulomas), others show few or none of these features and resemble UC, both clinically and histologically. These cases have been termed “superficial Crohn’s disease” or “UC-like Crohn’s disease” because the inflammatory changes are limited to the mucosa and superficial submucosa, similar to UC (Fig. 17.30).170–173 In these cases, a diagnosis of CD may be established by noting the presence of typical CD in other regions of the GI tract, such as the distal small intestine or the perianal region, or by showing other cardinal features of CD such as granulomas unrelated to ruptured crypts, segmental involvement of the colon with skip areas unrelated to treatment effect, or absolute rectal sparing from initial onset of disease. In addition, CD also should be suspected in patients who have UC-like features in the colon and severe anal or perianal disease (e.g., fissures, fistulas). UC-like CD is rare. In one of the earlier studies that evaluated 10 patients with “superficial” CD, the diagnosis of CD was rendered based on the association with classic CD in other segments of the same resection specimens, a previous history of resection for CD, small bowel involvement, segmental or irregular disease distribution, and presence of granulomas.170
In a recent, comprehensive case-control study by Soucy and associates comprising 73 patients with isolated colonic CD and 45 patients with ileocolonic CD, all of whom had detailed pathologic evaluation of a wide variety of UC and CD features, the incidence of UC-like CD was 14% and 13%, respectively, in the two groups.109 Patients with UC-like CD were younger than those who had classic CD with mural involvement. Fifty percent of patients with superficial UC-like CD had only left-sided colitis, whereas 19%, 13%, and 6% showed right-sided, total, and subtotal colitis, respectively. Granulomas were observed in 44% of cases. In this study, of the four UC-like CD patients who underwent an IPAA procedure, none showed an anastomosis breakdown. When the UC-like colonic CD cohort was compared with patients with non–UC-like colonic CD, the mean age at diagnosis was significantly younger in the former group (25 versus 35 years; P = .02). However, there were no differences in the pathologic (nonmural) features or in overall outcome between these two groups.
In another study consisting of 21 CD patients with UC-like pancolitis at presentation who underwent a pouch procedure, only 14% had pouch complications that necessitated pouch resection.173 Furthermore, recurrent CD in the small intestine developed in only 5%. Therefore, it appears that many patients with UC-like colonic CD have good success after a pouch procedure, and this approach may be a viable option in affected patients who are either resistant to permanent ileostomy or prefer a pouch procedure.
Natural History and Treatment
The natural history of CD is quite variable, and the type of therapy administered also affects the disease course. Two population-based studies that evaluated patient outcome—from Olmsted County, Minnesota (225 patients), and Copenhagen County, Denmark (373 patients)—found that a relapse or exacerbation of disease developed in 10% to 30% of CD patients, respectively, after the first year of diagnosis, 15% to 25% experienced low disease activity, and 50% to 65% were in remission. On long-term follow-up (>10 years), 10% to 13% of patients remained in remission, 67% to 73% experienced a chronic intermittent course, and a chronic course with continuous activity developed in 13% to 20%.174,175 Penetrating/fistulizing CD, young age at presentation, short duration of disease before first surgery, and ileocolonic disease are high risk factors for CD recurrence after surgery.175 Based on the outcome data from a detailed clinicopathologic study consisting of 118 patients with isolated and ileocolonic CD, colonic recurrence will develop in as many as 28% of patients after surgery, noncolonic recurrence will develop in 10%, and recurrent disease at both sites will develop in 6%, during a mean follow-up interval of more than 5 years.109
The goal of therapy is to induce and maintain clinical remission. Aminosalicylates are used in the treatment of mild to moderate CD. Antibiotics, especially metronidazole and ciprofloxacin, are used to treat perianal disease, fistulas, and active luminal CD. Moderately severe CD is treated with tapered doses of prednisone. The thiopurine analogues, azathioprine and 6-mercaptopurine, inhibit cell-mediated immunity and therefore play a role in management of CD. Methotrexate induces remission in patients who do not tolerate thiopurine analogues. Infliximab and adalimumab are monoclonal anti-TNF antibodies that benefit approximately 60% of patients with luminal disease. Forty percent of these patients maintain a good response after 1 year. Among newer therapeutic agents is natalizumab, a humanized monoclonal antibody against α4-integrin that inhibits leukocyte adhesion and migration into inflamed tissue. It has been found to be an effective induction and maintenance agent for treatment of moderately to severely active CD.176
Surgery is most often indicated for patients with intraabdominal abscess, medically refractory disease, intestinal obstruction, toxic megacolon, hemorrhage, dysplasia, or cancer. Postoperative disease recurrence has been associated with the presence of perianal disease, ileorectal anastomosis, and segmental resection.177 In a study by Morpurgo and colleagues, patients with either granulomas or segmental colitis at presentation showed a higher recurrence rate when compared with patients without granulomas and patients with pancolitis.173 In a recent study by Soucy and colleagues, which specifically evaluated patients with colonic CD at initial presentation, there were no significant differences in the development of adverse outcomes between patients with isolated colonic CD versus those with ileocolonic CD.109
Patients with CD are at risk for small bowel and colorectal adenocarcinoma. The risk is higher in patients with a family history of sporadic colorectal cancer, uncontrolled inflammation, shortened colon, or multiple inflammatory pseudopolyps.178 The range of cumulative prevalence of dysplasia in colonic CD reported in earlier studies was between 2% to 5%.179,180 More recently, the estimated 25-year risk of dysplasia in colonic CD has ranged from 12% to 25%181,182 (see Dysplasia in Inflammatory Bowel Disease).
Chronic Inflammatory Bowel Disease, Unknown Type (Indeterminate Colitis)
In a small proportion of IBD cases (1% to 10%, depending on the study), a definite diagnosis of UC or CD cannot be established with absolute certainty after evaluation of the patient’s resection specimen. This occurs most commonly because of insufficient clinical, radiologic, endoscopic, or pathologic data or because of overlapping features between these two disorders, which occurs most commonly in patients with fulminant colitis.183–187 In 1998, Swan and colleagues evaluated 95 cases of “fulminant colitis” with the aim of identifying features that could help separate UC from CD.188 After all pathologic material and clinical follow-up information had been reviewed, microscopic examination correctly diagnosed UC or CD in 91% of cases. Granulomas and transmural lymphoid aggregates away from areas of ulceration were the most specific indicators of CD. In these circumstances, the term indeterminate colitis (IC) has been used. However, this is a grossly overused term in IBD pathology. IC is not a specific disease entity and therefore has no diagnostic criteria. Rather, it represents a provisional term used by pathologists only when a definite diagnosis of UC, CD, or any other cause of chronic colitis cannot be established with complete certainty given the clinical and pathologic tissue available at the time of sign-out of the patient’s resection specimen. In as many as 80% of cases, the true nature of the patient’s underlying IBD becomes apparent within several years of follow-up or when all of the patient’s clinical, endoscopic, and previous pathology material (including all prior mucosal biopsies) is obtained and reviewed in detail.189
Historical Perspective
The IC designation was originally applied to cases of fulminant pancolitis (i.e., severe colitis with systemic toxicity often associated with colonic dilation), a disease in which the classic features of UC may be obscured by the presence of severe, diffuse ulceration with early superficial fissuring ulceration, transmural lymphoid aggregates (in areas of deep ulceration), and relative rectal sparing—features that are normally associated with CD.188 However, it is now recognized that these “CD-like” features may occur in patients with fulminant UC, so most if not all of the types of specimens originally interpreted as indeterminate colitis can now, more conclusively, be diagnosed as fulminant UC. This is based on data suggesting that with time patients with fulminant UC who have one or more CD-like features do not develop CD in other portions of the GI tract and have a successful rate of ileoanal pouch anal anastomosis (IPAA) procedure. For instance, in one study of 21 patients with fulminant pancolitis and superficial fissuring ulcers, the incidence of CD-like complications after total colectomy and IPAA was very low and was statistically similar to that of a control group of patients without fissuring ulcers in their resection specimen.190 Therefore, the presence of these ulcers in patients with fulminant colitis is not considered necessarily indicative of CD and should not be used as evidence to deny the patient an opportunity for an IPAA procedure (Fig. 17.31).
More recently, the term indeterminate colitis has been broadened to include any IBD case in which a definite diagnosis cannot be established pathologically in a resection specimen. Naturally, the ability to establish a precise diagnosis of either UC or CD is highly dependent on the level of awareness of the pathologist regarding the range of morphologic features seen in these disorders and the unusual patterns of injury in each disorder that can mimic the other. In a study by Farmer and associates, 84 IBD colectomy specimens were reviewed by 24 university pathologists, whose diagnostic accuracy was compared with that of a single GI pathologist who had a particular interest in IBD.186 The GI pathologist rendered a diagnosis that was different from that of the others in 45% of the specimens; in most cases, this decision resulted in a change of diagnosis from UC to CD. Therefore, it is highly recommended that pathologists seek the opinion of an expert GI pathologist regarding any case that is ruled indeterminate from their point of view before final sign-out.
Pathologic Features
The most common (clinical and pathologic) reasons for establishing an interim diagnosis of IC are listed in Box 17.5. In addition to the reasons described in the table, pathologists often diagnose IC when a definite diagnosis of UC or CD cannot be made by evaluation of biopsy specimens. This practice is strongly discouraged because the cardinal features of CD cannot be seen in biopsies, and the pattern of inflammation in both UC and CD becomes altered and more similar to each other after medical treatment (see earlier discussion). Therefore, IC is a diagnosis that should never be made on biopsy material. In many instances, a diagnosis of IC is made because of a lack of awareness of the many types of unusual variants of UC that can mimic CD and the subtypes of CD that mimic UC (i.e., UC-like CD). Finally, in some cases, a diagnosis of IC is made because of the unwillingness of pathologists to accept a particular finding, such as true segmental disease with skip lesions in an untreated patient, granulomatous inflammation unrelated to ruptured crypts, transmural lymphoid aggregates in patients without fulminant colitis, or deep fissuring ulceration as a definite diagnostic criterion for CD in colonic resection specimens. In reality, any one of these features should be considered as supportive evidence for a diagnosis of CD in the appropriate clinical setting.
In most studies of IC, the majority of cases diagnosed as IC represent UC; a small proportion (10% to 40%) are, in fact, CD.184 However, in some circumstances, other pathologic mimics of IBD, such as NSAID-related colitis, diverticular disease–associated colitis (DAC), radiation or ischemic colitis, and infectious colitis, may also show histologic features in resection specimens that mimic either UC or CD (Box 17.6). These other disorders should always be considered when pathologists are confronted with an IC resection specimen.
Natural History and Treatment
As mentioned earlier, there is a strong clinical need to classify IBD as CD or UC (or some other disorder), because the IPAA (“pouch”) procedure is usually contraindicated for patients with CD. In studies of IPAA in CD, there was a high risk for morbidity related to pouchitis, fistulas, incontinence, or anastomotic leaks.191,192 Many studies have evaluated the pathologic features, natural history, and outcome of ileoanal pouches in patients with IC.185–187,189,193–197 Results vary considerably, because most of these studies were retrospective, used varying and poorly defined criteria for IC, and lacked sufficient follow-up information. Nevertheless, in general, approximately 20% of IC patients experienced severe pouch complications, a frequency that is intermediate between that seen in UC (8% to 10%) and in CD (30% to 40%).187,191,193,195 This finding is not unexpected, because in most studies, the IC study group consists of a mixture of true UC and CD patients. In a study by Yu and co-workers of 82 cases of IC and 1437 cases of UC, all of which involved an IPAA operation, patients with IC had higher incidences of pelvic sepsis, pouch fistulas, and pouch failure than patients with UC.185 However, 15% of the patients with IC ultimately had their diagnosis changed to CD, and when newly diagnosed CD patients were removed from the analysis, the rates of pouch complications in IC and UC patients were statistically similar.
In 1995, McIntyre and colleagues compared 71 patients with IC and 1232 patients with UC for frequency of bowel movements, incontinence, and prevalence of pouchitis and pouch failure after an IPAA procedure.193 Although the failure rate in IC was higher than in UC (19% versus 8%), IC and UC patients had similar overall outcomes, once again suggesting that most patients with IC probably have UC as the cause of the colonic inflammatory disorder. Although a substantial proportion of CD patients who undergo an IPAA operation experience pouch failure (30% to 45%), some studies have suggested that CD patients whose pouches can be retained in situ have acceptable pouch function.191,198
Ancillary (Serologic) Diagnostic Tests for IBD
In certain circumstances, serologic testing for ANCAs and ASCAs may be helpful in classifying IC cases as UC or CD.11,199 In addition to these antibodies, a widely used serologic profile by Prometheus Laboratories tests for antibodies against outer membrane porin C (OmpC), a protein belonging to E. coli that regulates metabolite and toxin transport, and CBir1, a flagellin that presumably induces colitis through adaptive immune response.200 A typical profile for CD includes detectable ASCA IgA and anti-CBir1 IgG antibodies and an absence of anti-OmpC IgA and pANCA. Elevated serum pANCA and low levels (below the reference range) of ASCA IgA, anti-OmpC, and anti-CBir1 IgA are usually associated with UC.
ANCAs are detected in the serum of 60% to 70% of patients with UC but in only 10% to 40% of CD patients. Patients with UC who have a high level of pANCA are at an increased risk of pouchitis after IPAA.201 Of the CD patients who are positive for ANCAs, most have left-sided colitis with clinical, endoscopic, or histologic features of UC.202 ASCAs are present in 50% to 60% of CD patients. As a serum marker for CD, ASCA has a sensitivity of 67% and a specificity of 92%. In a meta-analysis designed to evaluate the diagnostic precision of ANCA and ASCA in IBD, these serologic tests were shown to be quite specific but not very sensitive in distinguishing CD from UC. In that study, an ASCA+/ANCA test result offered the best sensitivity and specificity for CD (55% and 43%, respectively).11 In fact, these tests showed even higher rates in the pediatric IBD population. In a study consisting of 135 pediatric patients (81 with CD and 54 with UC), an antibody panel consisting of pANCA, ASCA, OmpC, and CBir1 identified 65% of children with CD and 76% of those with UC, with a specificity of 94%.203 In CD, OmpC has been correlated with increased risk of fibrostenosing disease, internal penetrating disease, and the need for small bowel surgery.204 CBir1 has been shown to identify cases of CD not detected with other markers.200 Approximately 55% of CD patients are positive for anti-CBir1, and this marker independently helps identify a subset of CD patients (particularly those with fibrostenosing disease) who may also be ANCA positive.
Use of serologic testing has not been extensively studied for IC. In a prospective European study of 97 patients with a diagnosis of IC, only a small minority showed characteristic serologic patterns that helped establish a diagnosis of ASCA+/ANCA− CD or ASCA−/ANCA+ UC. In most patients, there was insufficient information to accurately classify IC patients as having either CD or UC.205 Therefore, the clinical utility of these markers in IC appears limited.
Differential Diagnosis of Ulcerative Colitis versus Crohn’s Disease
Resection Specimens
UC characteristically involves the mucosa in a diffuse and continuous manner. It almost always affects the rectum. The terminal ileum may be affected to a lesser degree and exclusively in patients with pancolitis (i.e., backwash ileitis). Infections, drug or medication-related injury (e.g., NSAIDs), and bowel preparation agents can also result in inflammation of the terminal ileum. In contrast, CD shows segmental or patchy involvement of the bowel and often affects longer lengths of terminal ileum, both actively as well as chronically. In a resection specimen, a diagnosis of UC is usually established by the presence of diffuse chronic active colitis or chronic inactive colitis involving the mucosa in the absence of mural changes typical of CD, such as fissuring ulcers, transmural lymphoid aggregates, non–mucin-related granulomas, mural fibrosis, and fistulas or sinus tracts. Neural hyperplasia, hyperplasia of muscularis mucosae, and submucosal fibrosis are also less common in UC than in CD. In addition, involvement of other regions of the GI tract, especially perianal disease, is a sign of CD. Upper GI involvement in UC has been described, but it is unclear whether these changes are specific to UC or represent a nonspecific systemic immune response to a generalized inflammatory condition. A summary of the classic microscopic features of UC and CD is provided in Table 17.2.
Because most resections are performed for medically refractory disease or disease-related complications and therefore have been treated medically, both UC and CD may show patchy, or segmental, histologic changes. One may also see a reduction in the amount of transmural inflammation in CD. Granulomas are also far less common after treatment.
Biopsy Specimens—Untreated
UC and CD demonstrate considerable overlap in morphologic features in biopsy samples with chronic and/or active disease. Furthermore, because the characteristic features of CD are typically found deeper in the bowel wall, it is difficult or impossible to distinguish UC from CD based solely on analysis of mucosal biopsy specimens. However, in untreated cases, features in favor of CD are granulomas not associated with crypt rupture, longer segments of small bowel involvement, upper GI involvement, rectal sparing, fistulas, and perianal disease.
Biopsy Specimens—Treated
After therapy, uneven healing and therapeutic effects of drugs used for the disease cause the histologic features of UC to become patchy, mimicking CD (see Unusual Morphologic Variants of Ulcerative Colitis). In this setting, one cannot consider patchiness of disease or rectal sparing as a feature indicative of CD. In treated patients, unless there is evidence of anal or perianal IBD, granulomas unrelated to ruptured crypts, or definite chronic active ileal or upper GI involvement confirmed radiologically, it is almost impossible to distinguish UC from CD.
Dysplasia in Inflammatory Bowel Disease
Fewer than 1% of all colorectal cancers in the United States are associated with IBD. Colorectal carcinoma arising in UC was first documented in 1945 by Crohn and Rosenberg.206 Warren and Sommers were the first to document colorectal cancer in a patient with CD.207 Both UC and CD patients are at increased risk for the development of dysplasia and carcinoma. However, estimation of risk is influenced by numerous factors, such as differences in sampling protocols, recognition and classification of disease, treatment regimens, and methods used to detect neoplasia. Evidence from two population-based studies—from Manitoba, Canada (relative risk in CD, 2.64, and in UC, 2.75),208 and from Olmstead County, Minnesota (standardized incidence ratio in CD, 1.9, and in UC, 2.4)209—indicated that the risk of cancer is roughly equivalent in UC and CD. Another study, by Choi and colleagues, found no significant differences in age at diagnosis, duration of IBD, multiplicity and distribution of cancer, presence of dysplasia, or 5-year survival rate in patients with CD-associated versus UC-associated colorectal cancer.210
Ulcerative Colitis: Incidence and Risk Factors of Dysplasia/Cancer
The overall incidence of colorectal cancer after 25 to 35 years of UC ranges from 3% to 43%.211,212 In two recent population-based cohort studies, UC-associated colorectal cancer accounted for only 0.15% to 0.4% of all cases of colorectal cancer diagnosed in the general population.213–215 The change in incidence rate, especially during the first 1 to 10 years after diagnosis, is likely the result of improved surveillance, early use of colectomy for medically refractory disease, and significant improvements in medical therapy.
A meta-analysis of 116 studies estimated the risk of cancer in patients with UC at 2% after 10 years, 8.5% after 20 years, and 17.8% after 30 years of disease.216 Five years later, data from a 30-year surveillance program at St. Mark’s Hospital in the United Kingdom calculated the risk of cancer and dysplasia as 2.5% at 20 years, 7.6% at 30 years, and 10.8% at 40 years.217 Subsequent population-based studies also suggested that the risk of developing dysplasia/cancer has decreased with time. In a recent study that included 32,911 Danish patients diagnosed with UC, the relative risk of colorectal cancer (1.07; 95% confidence interval, 0.95 to 1.21) was comparable to that of the general population.218
Features associated with an increased risk of dysplasia and carcinoma in patients with UC include increased duration of disease, anatomic extent of disease, PSC, family history of sporadic cancer, young age at onset, and severity of endoscopic and histologic inflammation. The relative risk for dysplasia or cancer significantly increases after 8 to 10 years of disease duration. It is therefore recommended that regular colonoscopic surveillance should begin after 8 years.
Most cases of dysplasia/cancer arise in a background of pancolitis or subtotal colitis. Left-sided colitis carries an intermediate risk, whereas proctitis or proctosigmoiditis carries little or no risk of cancer.219,220 Compared with relative risks of 1.7 and 2.8 for patients with proctitis and left-sided colitis, respectively, patients with pancolitis have a relative risk of 14.8 for neoplasia.219
PSC develops in approximately 2% to 5% of UC patients develop PSC. In a meta-analysis that included 22 studies, patients with PSC and UC were found to have a fourfold increased risk of dysplasia/cancer.221 UC patients who have undergone liver transplantation for PSC also have a higher risk of dysplasia/cancer, estimated at 1% per year.222–224 For these reasons, it is highly recommended that patients undergo annual colonoscopy from the onset of PSC.
A positive family history of sporadic colon cancer is a well-documented predisposing factor for colon cancer, with a more than twofold associated risk.225 Younger age at onset of colitis has been associated with an 8.6-fold increased risk of dysplasia/cancer.209,226 However, this estimate needs to be interpreted with caution because of the lower rates of colorectal cancer observed in the general population used for comparison in this age group. Nevertheless, this increased risk likely reflects the high proportion of pediatric IBD patients who have extensive disease.218,227
Two previous studies from the United Kingdom demonstrated that the severity of endoscopic and histologic inflammation is related to colon cancer risk.228,229 In a recent meta-analysis, a diagnosis of extensive colitis was associated with a 4.8-fold increased risk of dysplasia/colon cancer.215
Crohn’s Disease: Incidence and Risk Factors of Dysplasia/Cancer
There is a wide variability in the reported risk of dysplasia/colorectal cancer in CD, primarily because of inconsistencies in documentation of the duration and location of CD (colonic versus isolated small bowel CD).230,231 In CD, there is also an increased risk for dysplasia and adenocarcinoma in excluded segments of bowel and in the small intestine.232
In a large, population-based survey of 1655 patients with long-standing and extensive CD, the reported relative risk of colorectal cancer was 2.5 (95% CI, 1.3 to 4.3).233 A meta-analysis of 12 studies showed a higher relative risk (4.5) for patients with colonic disease.234 In a recent Danish study, the relative risk of dysplasia/colon cancer among 14,463 patients diagnosed with CD did not differ significantly from that in the background population, nor did it change over a period of 30 years.218
As in UC, patients with long-standing CD and a family history of sporadic colon cancer are at increased risk for dysplasia/cancer.225,231,235 There is a convincing association between degree of inflammation and dysplasia/cancer in CD as well.233 A population-based study from Sweden showed that the relative risk for colon cancer was 5.6 in CD patients with disease restricted to the colon.233 The relative risk was even greater in patients who were younger than 30 years of age at the time of diagnosis. Therefore, extent of colitis, duration of disease, family history of sporadic colon cancer, and young age at presentation are risk factors for development of dysplasia and cancer in colonic CD. In contrast to UC, PSC does not seem to increase the risk of colon cancer in patients with colonic CD. This was recently demonstrated in a case-control study consisting of 114 patients with colonic CD.236
Pathologic Features of IBD Dysplasia
Dysplasia is defined as unequivocal neoplastic epithelium confined to the basement membrane. At present, it is the best and most reproducible marker of malignancy risk in patients with IBD.237 It is present in as many as 90% of patients with UC and as many as 87% of patients with CD who have carcinoma both adjacent to and distant from the primary tumor. Dysplasia may occur in any portion of the colon but often parallels the location of cancer; it may occur as an isolated focus but more often is multifocal. Rarely, it is diffuse.
Gross Features and Anatomic Distribution
Grossly, dysplasia is classified as either flat or elevated; the latter is known as dysplasia-associated lesion or mass (DALM). This classification is important because the treatment is different for these two types of dysplasia.238–241 There is inconsistency in the literature with regard to the criteria used to define raised, endoscopically visible, dysplastic lesions as DALMs. For instance, the term “flat dysplasia” has been used to describe endoscopically detectable, but slightly raised, lesions in some studies but not in others.
Raised dysplastic lesions in UC (and CD) are broadly separated into those that resemble non–IBD-related sporadic adenomas (“adenoma-like”) and those that do not resemble adenomas, which are termed “non–adenoma-like.”241–243 Adenoma-like dysplastic lesions (also known as adenoma-like polyp, adenoma-like dysplastic polyp, adenoma-like low-grade or high-grade dysplasia, polypoid dysplasia, or adenoma-like mass) are typically well-circumscribed, smooth or papillary, non-necrotic, sessile or pedunculated polyps (Fig. 17.32). These are endoscopically resectable lesions.239,242 Non–adenoma-like dysplastic lesions include velvety patches, plaques, irregular nodules, wart-like thickenings, carpet-like lesions, stricturing lesions, and broad-based masses that cannot be resected by endoscopic methods and therefore require surgery (Fig. 17.33).
Retrospective studies have shown that most dysplastic lesions in IBD are, in fact, elevated or polypoid rather than flat.228,244 For instance, in a large retrospective review of 2204 surveillance colonoscopies in UC patients, 77% of the 104 dysplastic lesions found were elevated or polypoid (or both).228
Regarding the anatomic distribution of dysplasia in UC, low-grade dysplasia was found in one study to be more commonly localized to the distal colorectum (68%).245 Similarly, in a pooled analysis, Choi and co-workers reported that 52% (range, 47% to 62%) of colitis-related colorectal cancers occurred in the rectosigmoid region.246 Additionally, a 20-year prospective study and a 30-year analysis of surveillance colonoscopy showed that 44% and 58.6% of dysplastic lesions occurred within the rectosigmoid, respectively.217,247 A recent retrospective study of flat dysplasia by Goldstone and colleagues confirmed that in patients undergoing surveillance for long-standing UC, 74.6% of neoplasia (flat low-grade dysplasia, flat high-grade dysplasia, or carcinoma) occurred in the distal colon (64.4% in the rectosigmoid and 10.2% in the descending colon), whereas only 25.4% occurred in the ascending colon, cecum, or transverse colon.248
In CD, dysplasia also occurs more often in areas close to, rather than distant from, the primary tumor. Dysplasia distant from cancer has been identified in 38% to 41% of specimens with colonic CD.232,249 Regardless of the location of the primary tumor, dysplasia/cancer always develops in areas of colitis, as in UC. In a study by Sigel and associates, dysplasia was found adjacent to carcinoma in 87% of cases and away from carcinoma in 41% of cases.232 In their study of 30 cases of CD-related adenocarcinoma, 27% occurred in the small intestine and 73% occurred in the colon. In a recent study that evaluated 50 cases of Crohn’s colitis-associated dysplasia, 44% of patients were found to have multifocal dysplasia/cancer in segments not affected by colitis.250
Microscopic Features
Morphologic Types of Dysplasia
Morphologic subtypes of dysplasia include intestinal (“adenomatous”), hypermucinous/villous, and serrated dysplasia. Some patients show a mixture of subtypes in the same patient (“mixed”). In one study of IBD cancers, 52% of dysplastic lesions were villous, 29% were serrated, 5% were tubular (adenomatous), and 13% showed mixed features.251 Only the intestinal type of dysplasia has been well characterized both histologically and clinically, however, and the following discussion applies mainly to that type.
Grading of Dysplasia
According to the grading system proposed by Riddell and colleagues in 1983, dysplastic changes in UC and CD are separated into three distinct categories: negative for dysplasia, indefinite for dysplasia, and positive for dysplasia (low and high grade).237 The Vienna grading system, which denotes five diagnostic categories, is used by many pathologists in Japan and Europe.252 A comparison of the two systems is outlined in Table 17.3. Both adenoma-like and non–adenoma-like lesions are graded in the same manner as flat dysplasia.
Table 17.3
Comparison of Vienna and Riddell’s Classifications of Dysplasia in Inflammatory Bowel Disease
| Vienna | Riddell |
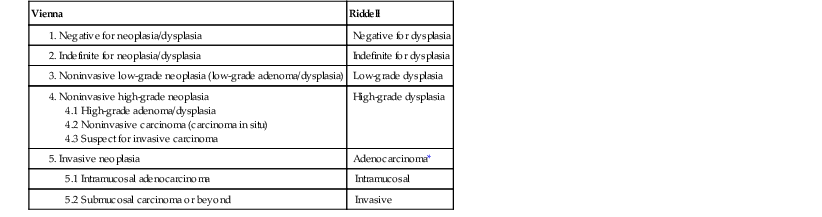
Grade of dysplasia is determined by evaluating a combination of cytologic and architectural alterations of the epithelium. A diagnosis of “negative” is reserved for nondysplastic, regenerating epithelium (Fig. 17.34).
Cases are considered indefinite for dysplasia when cytologic features of atypical epithelium approach or meet the criteria for low-grade dysplasia but, because of other factors such as inflammation, ulceration, or technical artifacts, that diagnosis cannot be established with certainty (Fig. 17.35). Inflammation and ulceration make interpretation of atypical changes difficult (i.e., reactive versus neoplastic). Regenerating epithelium often shows enlarged and variably sized nuclei, nuclear stratification, prominent nucleoli, and mitoses. These changes overlap with those observed in true dysplasia. In some cases, tangential sectioning of the tissue or severe cautery or processing artifact may render an atypical focus difficult to interpret. In general, surface maturation is usually indicative of a regenerative process. This feature should evoke caution in diagnosing dysplasia (Table 17.4).
Table 17.4
Regenerative Epithelial Changes versus Dysplasia
| Feature | Regenerative Changes | Dysplasia |
| Nuclear enlargement | Usually minimal | Always present |
| Nuclear hyperchromasia | May be present | Always present |
| Nuclear stratification | May be present near healing ulcers | Present in adenomatous (intestinal)-type dysplasia |
| Nuclear pleomorphism | Usually minimal | Always present |
| Irregular nuclear contour | Usually minimal | Always present |
| Loss of nuclear polarity | Absent | Present (in high-grade dysplasia) |
| Prominent nucleoli | May be present | Present in high-grade dysplasia |
| Vesicular chromatin | Usually absent | Present (especially in high-grade dysplasia) |
| Loss of surface maturation | Absent | Present (except in crypt dysplasia) |
| Increased mitoses | May be present | Usually present |
| Atypical miotic figures | Usually absent | Present |
| Increased nucleus-to-cytoplasm ratio | May be present | Always present |
| Syncytial appearance of cells with abundant eosinophilic cytoplasm | Present | Usually absent |
| Villous configuration | May be present | Present |
| Mucin loss | Present | Present |
| Epithelial “atypia” overlying inflamed stroma that lacks glands | Present | Usually absent |
| Intraepithelial neutrophils | Usually present | May be present |
| Complex glandular architecture | Absent | Present in high-grade dysplasia |
In low-grade dysplasia, crypts show a tubular and/or villous configuration and may also demonstrate mild crypt budding and crowding. Cytologically, dysplastic crypts are lined by cells that show nuclear enlargement, elongation (“pencillate”), increased nucleus–to-cytoplasm (N : C) ratio, hyperchromasia, stratification, clumped chromatin pattern, single or multiple nucleoli, and hypereosinophilic, mucin-depleted cytoplasm (Fig. 17.36). Dysplastic epithelium normally involves both the crypt and surface epithelium, but early cases may show involvement of crypts only (“crypt dysplasia”; see later discussion). Mitotic figures are often plentiful, but atypical mitotic figures are less common. In low-grade dysplasia, the nuclei are usually limited to the basal half of the cell cytoplasm, and there are no significant architectural abnormalities such as glandular crowding, cribriforming, or a back-to-back pattern. Other, less common features of low-grade dysplasia include dystrophic goblet cells and endocrine and Paneth cell metaplasia.
With progression from low-grade to high-grade dysplasia, the cells acquire more cytologic atypia, characterized by markedly enlarged, pleomorphic, hyperchromatic nuclei and loss of nuclear polarity (Fig. 17.37). The nuclei typically show a more “open” chromatin pattern with prominent nucleoli. Full-thickness nuclear stratification is usually present. Architecturally, back-to-back glands or cribriforming may be present. Mitotic figures, both typical and atypical, are more frequent and are usually present in both the upper and lower portions of the crypts and in the surface epithelium. In general, the features of the most atypical portion of dysplastic mucosa determine the overall grade of dysplasia in any particular biopsy sample. However, there is no uniform agreement regarding the proportion of high-grade dysplastic crypts that is necessary to upgrade a biopsy from low to high grade. A simple rule of thumb is that there should be more than one or two (i.e., more than rare) high-grade crypts to designate a biopsy as high grade, but this number varies among expert pathologists.
Intramucosal carcinoma is defined by the presence of cells or glands that have penetrated into but not beyond the lamina propria or muscularis mucosae. Common patterns of early invasion include single-cell or small-gland infiltration, glands with irregular jagged contours, and extensive cribriforming with pushing borders. Desmoplasia is absent when the carcinoma is limited to the mucosa, and it’s presence is diagnostic of submucosal invasion (Fig. 17.38).
Nonintestinal Types of Dysplasia
Mucinous Dysplasia.
Rarely, IBD-associated dysplasia shows predominantly mucinous epithelium that may be difficult to recognize as unequivocally neoplastic. This type of dysplasia often exhibits a villiform growth pattern. The epithelium shows small, hyperchromatic, basally oriented nuclei with little cytologic atypia.253,254 This form of dysplasia has been referred to by a variety of names, such as “villous dysplasia,” or “villous hypermucinous mucosa,” and it may be associated with either UC or CD (Fig. 17.39). Purely mucinous epithelium is rare in UC or CD. Therefore, the presence of mucinous epithelium, particularly if it is villiform in contour, should raise a strong suspicion for dysplasia. The natural history and biologic characteristics of mucinous dysplasia are currently unknown.
Serrated Dysplasia and Other Serrated Lesions.
In some studies of UC, serrated dysplasia has represented the most common type of dysplasia.251 It is characterized by the presence of epithelium with a serrated or saw-toothed appearance. It usually shows a predominance of nongoblet cells. The epithelial cells show nuclear enlargement, stratification, irregular nuclear membranes, hypereosinophilic cytoplasm, and prominent nucleoli. The cytologic and architectural features are reminiscent of traditional serrated adenomas or, in some cases, a sessile serrated adenoma/polyp (SSA/P) (Figs. 17.40 and 17.41). High-grade cytologic changes are uncommon in this type of dysplasia.
A spectrum of serrated lesions develops in the colon in some patients, ranging from conventional hyperplastic polyps (Fig. 17.42, A) to SSA/Ps (see Fig. 17.42, B) and traditional serrated adenomas (TSAs). The morphologic features of these lesions in IBD are similar to those that occur in non-IBD patients (see Chapter 22 for details).
Little is known regarding the biologic characteristics and natural history of serrated lesions in IBD. In a large clinicopathologic and outcome study published in abstract form, 188 polyps from 161 IBD patients with at least one serrated polyp were analyzed. Most were hyperplastic polyps (97%) that were detected microscopically, not endoscopically. Adenocarcinoma, adenoma, or flat or elevated dysplasia did not develop in any of the patients, but additional hyperplastic or inflammatory polyps developed in 33% of the patients.255 In a retrospective study consisting of 94 patients with hyperplastic polyp-like changes in biopsies of flat mucosa obtained during routine surveillance (“flat serrated change”), the authors found that although 12.8% of these patients had subsequent dysplasia (DALM, low-grade dysplasia, or high-grade dysplasia) on follow-up, a multivariate analysis did not show a statistically significant difference in the risk between patients with and without these lesions. Based on these results, the authors suggested that there should be no change in routine surveillance protocols for patients with flat serrated change.256 One recent study, published only in abstract form,257 evaluated serrated lesions in 56 UC and 19 CD patients and classified them as SSA/P-like (negative for dysplasia), TSA-like (positive for low-grade dysplasia), and cytologically atypical hyperplasia-like (indefinite for dysplasia). The authors found that SSA/P-like polyps in IBD occurred preferentially in females and in the right colon, similar to sporadic polyps. However, they were not associated with synchronous or metachronous neoplasia over a follow-up period of 91 months. In contrast, TSA-like and cytologically atypical hyperplasia-like polyps were significantly associated with synchronous or metachronous neoplasia (low-grade dysplasia in 21, high-grade dysplasia in 7, and invasive adenocarcinoma in 5).
Finally, IBD patients may rarely show a spectrum of hyperplastic and serrated lesions in their colon, reminiscent of serrated polyposis syndrome. In one study by Srivastava and colleagues, three patients with IBD (UC in 2, CD in 1) developed a spectrum of serrated lesions ranging from hyperplastic polyps to sessile serrated adenomas/polyps and TSAs in large numbers throughout the colon.258 Two of the three patients had adenocarcinoma. These findings suggest that the presence of multiple, large, serrated lesions in a patient with IBD should prompt close surveillance for synchronous or metachronous carcinoma.
Crypt Dysplasia.
Although better characterized in patient with Barrett’s esophagus, rarely patients with IBD may develop early dysplastic changes limited to the bases of the crypts without involvement of the surface epithelium (i.e., surface maturation is present).259 This is known as crypt dysplasia (Fig. 17.43). In our experience, most patients with crypt dysplasia reveal conventional dysplasia (involving both crypt and surface epithelium) elsewhere in the colon at the time of discovery of crypt dysplasia. Because crypt regeneration also shows surface maturation, a diagnosis of crypt dysplasia should not be made in biopsy specimens exhibiting inflammation or ulceration, nor in those that are not well oriented. At this time, the biologic characteristics and natural history of crypt dysplasia in IBD are unknown.
Natural History and Treatment of Elevated (Raised) Dysplasia (DALMs)
As discussed earlier, DALMs are categorized as adenoma-like (endoscopically resectable) or non–adenoma-like (not endoscopically resectable) lesions and are graded in the same manner as flat dysplasia. Detailed discussions of the pathologic, immunohistochemical, and molecular features and the treatment of DALMs are presented in Chapters 2 and 22.
Historically, distinction between adenoma-like DALM and sporadic adenoma was considered important because the former was usually treated by colectomy, whereas the latter was treated by polypectomy.238 However, more recent data suggest that patients with UC and an adenoma-like lesion may be treated adequately by polypectomy and continued surveillance, regardless of the pathogenesis of the lesion.241–243 In a study consisting of 24 UC patients with an adenoma-like DALM within areas of colitis, 10 UC patients with an adenoma-like DALM outside areas of colitis, and 49 non-IBD (control) patients with sporadic adenomas,241 62.5% patients in the first group developed an additional adenoma-like DALM, only one patient (4%) developed a single focus of flat dysplasia, and one patient (4%) developed a poorly differentiated adenocarcinoma of the cecum by 7.5 years after polypectomy.242 The patient with adenocarcinoma also had a history of PSC and orthotopic liver transplantation. Among the patients with adenoma-like DALMs outside areas of colitis, 50% developed additional adenoma-like DALMs proximal to areas of colitis, but none developed flat dysplasia or carcinoma. This group was not significantly different from the control group, 49% of whom developed further adenomas during the follow-up interval. More recently, Kisiel and associates, provided follow-up data on 77 UC patients with an adenoma-like DALM located within (57%) or outside (43%) areas of colitis.260 In this study, 36% of patients developed another adenoma-like DALM, 5% developed flat low-grade dysplasia, and only one patient (1%) developed carcinoma after a median follow-up interval of 20.1 months. None of the patients developed high-grade dysplasia.
The presence of high-grade dysplasia within an adenoma-like DALM is not considered a contraindication to polypectomy. In the study by Blonski and colleagues, flat dysplasia or carcinoma did not develop in any of 9 UC patients with such lesions during a follow-up period of 76.5 months.261 All adenoma-like DALMs should be removed with negative margins. Incomplete removal of the lesion has been associated with a high rate of neoplastic progression.262
Biopsy specimens from non–adenoma-like DALMs often show only dysplastic epithelium, which usually represents surface sampling of a carcinoma. Therefore, IBD patients who have non–adenoma-like DALMs should be treated with colectomy because of its high association with adenocarcinoma. In the 1981 study by Blackstone and colleagues, 58% of patients with chronic UC who had non–adenoma-like DALM had adenocarcinoma underlying the site of their lesional biopsy.263 Since then, many other studies have detected a strong association between non–adenoma-like DALMs and cancer at colectomy. Given the high risk of synchronous or metachronous adenocarcinoma, the presence of a non–adenoma-like DALM is a strong indication for colectomy.178,264 Nevertheless, the most important element in the management decision process for DALM is the endoscopic appearance and resectability of the lesion (Fig. 17.44).
Regarding DALMs in patients with CD, a recent study by Quinn suggested that adenoma-like DALMs, regardless of their location, can be treated safely with polypectomy and continued surveillance, similar to the situation in UC patients with the same type of lesion.265
Natural History and Treatment of Flat Dysplasia
Most of the data regarding the natural history and treatment of dysplasia in IBD apply mainly to UC. It has been reported that the 5-year rate of development of either high-grade dysplasia or carcinoma in patients with low-grade dysplasia is 54%.212 As a result, many institutions recommend colectomy for UC patients with flat low-grade dysplasia, particularly if it is found on initial colonoscopy, is multifocal, or develops in a metachronous fashion.212,264,266 However, some institutions prefer to place patients with flat low-grade dysplasia under surveillance (see later discussion). Ullman and colleagues showed that 27% of patients with UC who underwent a colectomy within 6 months after a biopsy diagnosis of flat low-grade dysplasia had unexpected high-grade dysplasia or cancer in their resection specimen.267 In contrast, flat high-grade dysplasia is associated with a higher probability of detecting cancer at colectomy (40% to 67%) and of progression to carcinoma (5-year predictive value, 40% to 90%).178,212,268 For these reasons, patients with flat high-grade dysplasia, even if it is unifocal, are referred for colectomy.
Patients with biopsy specimens considered indefinite for dysplasia should be treated for their inflammation and have a repeat endoscopy within 6 months, preferably after the inflammation has subsided.178,264 In all cases of dysplasia assessment, it is important and highly recommended by the American Gastroenterology Association and the American College of Gastroenterology that the presence or absence of dysplasia be confirmed by at least one pathologist who is skilled in GI pathology or who has broad experience with dysplasia in UC.178,264
A recent study by Kiran and colleagues suggests that instead of segmental colectomy, total proctocolectomy should be strongly considered for CD patients with a preoperative diagnosis of dysplasia, because as many as 44% show multifocal dysplasia.250 Similarly, patients with high-grade dysplasia have a 45% risk of having synchronous cancer.
Surveillance of Dysplasia
The optimal surveillance strategy for patients with UC remains controversial269 (see Chapter 2). Much debate centers on the sensitivity of the detection system, the predictive value of dysplasia for assessing risk for colorectal cancer, and the cost.270–272 Data supporting the effectiveness of surveillance in patients with UC are not uniform but do suggest a reduction in mortality from carcinoma in those who are willing to undergo prophylactic colectomy should dysplasia be detected. As a result, the overall balance of evidence supports surveillance for dysplasia in UC.
Widespread agreement on appropriate surveillance strategies in patients with UC has not been established.268,273 The American Gastroenterology Association recommends that surveillance should begin after 8 years of disease in patients with pancolitis and after 15 years in patients with colitis involving the left colon only. Colonoscopy should be repeated every 1 to 2 years.178,264 Four-quadrant biopsy specimens should be obtained from every 10 cm of mucosa between the cecum to the sigmoid colon and from every 5 cm more distally. In addition, any suspicious lesions or masses should be biopsied. In a study by Rubin and associates, 33 biopsy specimens were needed to detect dysplasia with 90% probability, and at least 64 biopsy specimens were needed to reach a 95% probability of detecting dysplasia.274 The finding of dysplasia, when confirmed by an expert pathologist, is usually an indication for colectomy. For patients for whom a colectomy is not feasible or is unacceptable, frequent surveillance (e.g., every 3 to 6 months) is considered an acceptable alternative. At present, surveillance is not indicated for patients with ulcerative proctitis.
In 2% to 16% of CD patients, dysplasia/cancer is detected during the course of the illness. Overall, the survival benefit of endoscopic surveillance in CD is controversial, primarily because of the lack of prospective studies.233,275,276 However, there is a growing trend toward endoscopic surveillance in patients with long-standing CD.276 The Crohn’s and Colitis Foundation of America consensus conference recommendations for surveillance in patients with colonic CD includes screening colonoscopy for those who have major colonic disease (involving at least one third of the colon) with a disease duration of at least 8 to 10 years.178,264 However, surveillance is often difficult to perform because of the presence of strictures that often require the use of a pediatric endoscope. Although most strictures are a manifestation of transmural disease, as many as 12% of CD-related strictures are malignant from the onset.277
Sampling Error and Adjunctive Markers
Sampling error and interobserver variability are common problems encountered in the interpretation of dysplasia in IBD.237 Since the original 1983 study by Riddell and co-workers, several investigations have shown only moderate levels of interobserver agreement.237,278–280 In general, levels of agreement among pathologists are highest for the category of high-grade dysplasia and negative for dysplasia; they are lowest for biopsy specimens showing low-grade dysplasia or indefinite for dysplasia. Recent studies have focused on finding other, more reproducible, adjunctive methods of assessing malignancy risk in UC. These include a variety of histochemical (e.g., mucins, sialosyl-Tn), immunohistochemical (e.g., proliferation markers, TP53), and molecular (APC, p27, p16, aneuploidy) techniques.274,281–285 α-Methylacyl-CoA racemase has been shown to be sensitive and highly specific for dysplasia in IBD.286 In one study, 90% of low-grade dysplasia, 80% of high-grade dysplasia, and 71% of adenocarcinomas in IBD were positive, in contrast to none of the foci of nondysplastic regenerating epithelium. Several studies have shown increased expression of TP53 in the progression of neoplasia in IBD,287,288 but a small proportion of nondysplastic cases may also be positive. Furthermore, TP53 immunostaining is fraught with a high false-positive rate, which makes TP53 less useful for differentiating regeneration and true dysplasia in IBD.
Molecular Features of IBD Neoplasia
Tissue-based studies have demonstrated that IBD-related neoplasia is associated with aberrant methylation in EYA4,289 ER, CDKN2A, MYOD, CDH1, RUNX3, MINT1, and COX-2.290–294 A tissue-based study showed that methylation of genes in the WNT signaling pathway is an early event in patients with IBD colitis, and there is a progressive increase in methylation of the WNT signaling genes APC1A, APC2, SFRP1, and SFRP2 during development of IBD-associated neoplasia.295 There is growing evidence that various soluble factors produced by epithelial cells and immune cells play pathogenic roles in colonic carcinogenesis. One such marker, chitinase 3-like 1 (CHI3L1), seems to be one of the soluble factors that play pivotal roles in protecting cancer cells from undergoing apoptosis and in promoting tissue remodeling (by interacting with the extracellular matrix and by stimulating angiogenesis). Increased CHI3L1 expression was found in nondysplastic mucosa of UC patients with dysplasia or adenocarcinoma elsewhere in the colon when compared with healthy controls and UC patients without dysplasia.296 In another study, downregulation and loss of the tumor suppressor gene, nuclear programmed cell death 4 (PDCD4), was found to be associated with biopsies from patients with active IBD and IBD-associated dysplasia.297 (See Chapter 23 for more details on the molecular basis of IBD neoplasia.)
A stool assay of exfoliated markers represents an alternative, noninvasive approach that could serve as an adjunct to colonoscopic surveillance in estimating risk of neoplasia in IBD.298,299 One recent study investigated the feasibility of the use of stool assay of exfoliated DNA markers to detect IBD-related neoplasia.300 Several candidate genes that are methylated in colorectal cancer, including BMP3, VIM (vimentin), EYA4, and NDRG4, showed high discrimination for cancer. At 89% specificity, the combination of BMP3 and mNDRG4 detected all cases of cancer and 80% of dysplasia, including all cases of high-grade dysplasia and 67% of cases with low-grade dysplasia. Although these markers show some promise as adjunctive biomarkers, additional studies need to be performed before any of this knowledge can be translated into clinical management of patients with long-standing IBD.
Other Colitides
Acute Self-Limited (Infectious) Colitis
Clinical Features
Acute self-limited colitis (ASLC) is defined as a transient, presumably infectious, acute inflammatory disorder of the colon. Patients typically come to medical attention because of sudden-onset fever, abdominal tenderness, and bloody diarrhea.6,301 The inflammatory process usually resolves completely within 2 to 4 weeks. A wide variety of pathogens, most commonly bacterial organisms such as Campylobacter jejuni, Salmonella, Shigella species, E. coli, and Yersinia enterocolitica, result in this pattern of colonic injury. In immunocompromised patients, and very rarely in immunocompetent individuals, viral infections such as enteric adenoviruses (serotypes 40 and 41) and infections by parasitic organisms like Entamoeba histolytica and Cryptosporidium species may also cause a mild, self-limited form of infectious colitis. A detailed description of infectious disorders of the GI tract is provided in Chapter 4. Here, we discuss the clinicopathologic features of the most common organisms that cause ASLC (Table 17.5).
Table 17.5
Clinicopathologic Features of the Most Common Pathogens Causing Acute Self-Limited Colitis
| Organism | Clinical Features | Laboratory Findings | Colonoscopic Findings | Location of Disease | Microscopic Findings | Complications |
| Campylobacter jejuni | Diarrhea, fever, abdominal pain | Fecal blood and leukocytes, positive stool culture | Erythema, edema, multiple superficial ulcers as large as 1 cm | Any part of the colon may be involved | Neutrophilic cryptitis, histiocyte aggregates | GI hemorrhage, toxic megacolon, pancreatitis, cholecystitis, reactive arthritis, Guillain-Barré syndrome, HUS |
| Salmonella spp. (S. enteritidis and S. typhimurium) | Nausea, diarrhea, fever, and bloody diarrhea within 48 hr after exposure | Fecal blood and leukocytes, positive stool culture | Mucosal congestion, granularity, friability, and ulceration | Any part of the colon may be involved | Mucosal edema, congestion, cryptitis, crypt abscesses, thrombi in small venules | Toxic megacolon, bleeding, sepsis |
| Shigella spp. | Abdominal cramping, fever, and diarrhea | Fecal blood and leukocytes, positive stool culture | Small, ragged ulcers with normal intervening mucosa; severe cases with diffuse mucosal congestion and erosions | Any part of the colon may be involved | Aphthous ulcers, cellular lamina propria with neutrophils, cryptitis, and crypt abscesses | Appendicitis, HUS, myocarditis, pneumonitis, reactive arthritis, toxic megacolon |
| Escherichia coli (enteroinvasive E. coli and enterohemorrhagic E. coli [EHEC]) | Watery to bloody mucoid diarrhea, tenesmus, fever, abdominal cramps | Fecal blood and leukocytes, positive stool culture (for EHEC) | Segmental involvement with marked mucosal congestion, friability, edema, and diffuse ulcers, rarely pseudomembranes | Right colon is most commonly involved | Neutrophilic cryptitis, crypt abscesses with erosions, ischemic pattern of injury | HUS |
| Yersinia enterocolitica | Fever, abdominal cramps, diarrhea within 1-3 wk after infection | Fecal blood and leukocytes, stool culture, serology | Focal or diffuse mucosal edema, erythema, and ulcers | Proximal colon more commonly involved than rectosigmoid colon | Mucosal edema, cryptitis, and crypt abscesses (necrotizing granulomas are found only in patients with prolonged disease course) | Septicemia, chronic relapsing fever, appendicitis |
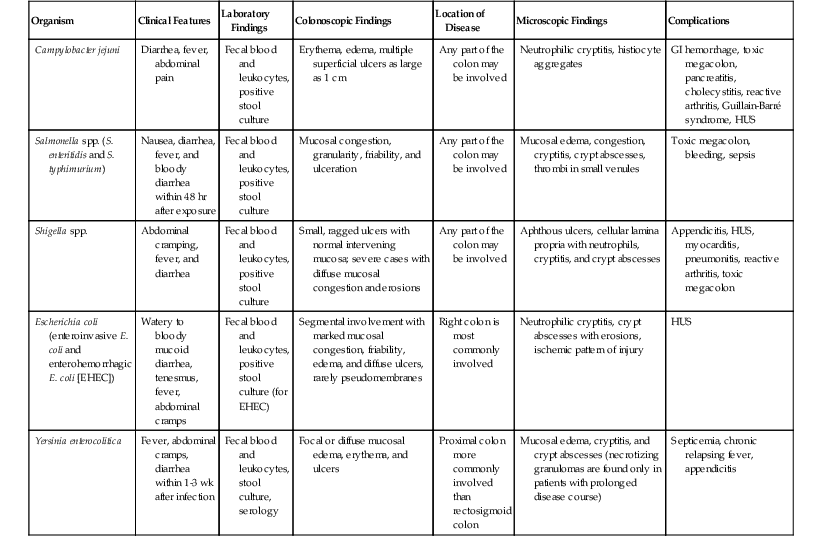
HUS, Hemolytic-uremic syndrome.
The clinical presentation of patients with ASLC includes rapid-onset abdominal pain, diarrhea, and fever. Typical laboratory findings include peripheral blood and fecal leukocytosis. In ASLC related to bacterial infection, stool cultures are positive in only 40% to 60% of cases. Therefore, a negative result does not entirely exclude an infectious etiology.6,302 In fact, in most instances, the exact cause of the patient’s colitis is never actually determined. Imaging studies may reveal nonspecific thickening of the bowel wall. However, because of the acute onset, they are seldom performed.301
Specific Infectious Organisms
Campylobacter jenuni
C. jejuni is one of the most frequently isolated stool pathogens in patients with ASLC. In immunocompromised patients, Campylobacter fetus is more frequently isolated. The incidence of C. jejuni–related diarrhea is 4% to 11% in the United States. Bacterial transmission usually occurs via contaminated food.303 In developing countries, the infection is hyperendemic in children younger than 2 years of age. The most common presenting symptoms are diarrhea (90%), fever (90%), and abdominal pain (70%). These may be accompanied by headache, myalgia, and vomiting. Stool examination typically reveals red blood cells and leukocytes. Campylobacter DNA may be isolated in as many as 19% of affected patients with acute-onset diarrhea.304
Salmonella Species
At least 2000 serotypes of non-typhoid Salmonella species have been identified as potential causative agents of gastroenteritis (nontyphoid salmonellosis). Almost 45,000 cases of salmonella gastroenteritis are reported annually.305 In the United States, Salmonella enteritidis and Salmonella typhimurium are the most commonly isolated species. Infection is most likely to develop in children younger than 1 year of age. In addition, patients with achlorhydria, hemolytic anemia, immunosuppression, or malignancy (e.g., disseminated carcinoma, hematolymphoid malignancies) are predisposed to Salmonella infection. Gastroenteritis develops in 75% of patients infected with this organism. Patients usually present with nausea, vomiting, fever, and bloody diarrhea.
Shigella Species
Shigella infection is the most common cause of bacillary dysentery. The first documentation of shigellosis-related GI illness dates back to the American Civil War period. Currently, the incidence of shigellosis worldwide is reported to be 10% to 20% of infectious diarrhea. Although it is more common in tropical countries and temperate zones, its prevalence in the United States and Europe is increasing. In fact, the incidence of Shigella sonnei (which is more common in the United States than Shigella flexneri) is 60% to 80% in patients with diarrhea.306 The infection is common in children aged 6 months to 5 years. Microbiology laboratory workers are also at risk. The clinical presentation consists of lower abdominal pain, rectal burning, bloody diarrhea, and fever.307 A biphasic illness is characteristic. The initial phase of infection is characterized by fever, abdominal pain, and watery diarrhea, which, after 3 to 5 days, is usually followed by tenesmus and small-volume bloody diarrhea. Very rarely, patients have an acute abdomen secondary to perforation. Presence of red blood cells and leukocytes in stool is a common finding. Stool cultures are often positive during the first few days of infection.
Escherichia coli
Although six major strains of E. coli have been implicated in human diarrheal disease, only two specific strains, enteroinvasive E. coli (EIEC) and enterohemorrhagic E. coli (EHEC), invade the colonic tissue and cause ASLC. In the United States, EHEC O157:H7 is the pathogenic strain most commonly isolated from stools of patients with bloody diarrhea.308 Consumption of infected hamburger meat is a common mode of disease transmission. The disease is most common in Northern climates such as Minnesota, Massachusetts, and the U.S. Pacific Northwest. Clinical symptoms for both EIEC and EHEC begin with watery diarrhea and fever, which is usually followed by 3 to 8 days of bloody diarrhea. Stool examination shows red and white blood cells. Peripheral blood examination often reveals leukocytosis with an increased number of immature white blood cells. Radiologic examination during the first week of illness may reveal a thumbprinting sign, which is the result of subepithelial edema and hemorrhage. Stool cultures are very helpful in establishing a definite diagnosis. Occasionally, polymerase chain reaction (PCR) and EIA testing for Shiga-like toxins may be necessary to confirm the diagnosis.
Yersinia enterocolitica
Y. enterocolitica causes a spectrum of clinical illnesses that range from mild gastroenteritis to invasive ileitis and colitis.309 In comparison to the United States, Y. enterocolitica gastroenteritis is far more common in Scandinavian and European countries. The serotypes prevalent in these countries also differ. Strain O:8 is more common in the United States, whereas O:3 and O:9 are more prevalent in Scandinavian and European countries.310 Almost two thirds of infections involve both the small bowel and colon (enterocolitis). Children younger than 5 years of age are commonly affected. Clinical symptoms last for 1 to 3 weeks and include fever, abdominal pain, and bloody diarrhea. As with other causes of ASLC, stool analysis often reveals the presence of blood and leukocytes. Stool culture and serologic studies may be helpful to establish a diagnosis. Radiologic findings in cases of mild gastroenteritis are nonspecific. In severe cases, the radiologic changes may mimic CD.
Pathogenesis
The clinical manifestation of ASLC is related to the ability of microorganisms to invade mucosa or produce enterotoxins. Although toxigenic organisms usually target the small bowel (and rarely the colon), tissue-invasive organisms typically cause injury to the terminal ileum and colon.
Tissue-Invasive Organisms
C. jejuni, Salmonella and Shigella species, E. coli, and Y. enterocolitica are organisms that invade the tissue and cause epithelial injury and death. Tissue invasion also results in the production of inflammatory cytokines that trigger an acute inflammatory reaction. In addition to tissue invasion, Shigella species undergo intracellular multiplication that allows for transmission of the infection into adjacent epithelial cells. In cases of Salmonella or Y. enterocolitica infection, organisms invade the lamina propria and submucosa and disseminate to extraintestinal sites via the bloodstream. Increased intracellular calcium and release of prostaglandin and leukotriene metabolites, serotonin, substance P, and reactive oxygen metabolites contribute to increased fluid output from epithelial cells.
Toxigenic Organisms
Enterotoxins are polypeptides secreted by bacteria that alter the transport of electrolytes and water through colonocytes without causing cellular injury. Shigella, enterotoxigenic E. coli (ETEC), Staphylococcus aureus, Clostridium perfringens, and Vibrio cholera are most associated with toxin production. Binding of enterotoxins to specific mucosal receptors causes decreased influx of sodium and chloride ions into the cells and active secretion of chloride ions from cells into the intestinal lumen. Thus, enterotoxins are responsible for the acute phase of illness. Clinical symptoms are a result of fluid and electrolyte disturbances.311
Cytotoxins are polypeptides that cause tissue injury by inhibiting protein synthesis, disrupting actin and tight junction integrity, damaging mitochondria, and depleting adenosine triphosphate (ATP) within cells. Organisms that elaborate cytotoxins include C. difficile, enteropathogenic E. coli (EPEC), EHEC, and Shigella. In addition, EHEC also produces an enterohemolysin that causes endothelial damage. Endothelial injury results in initiation of the coagulation cascade that ultimately leads to an ischemic colitis–pattern of tissue injury.
Pathologic Features
Gross Findings
Grossly, the colonic mucosa may show minimal changes such as patchy edema, erythema, loss of the normal vascular pattern, and superficial erosions or ulcers. In severe cases, large segments of colon may show mucosal friability and ulceration. Some organisms reveal characteristic endoscopic findings. For instance, C. jejuni can affect any part of the colon; typically, the mucosa shows numerous, superficial ulcers that range from 2 mm to 1 cm in size. Salmonella species may produce hemorrhagic mucosa with granularity, friability, and ulceration; very rarely, toxic megacolon may develop. The colon is the major site of involvement in cases of Shigella infection; in most cases, the colonic mucosa shows patchy involvement by small, ragged ulcers with normal intervening mucosa. Aphthous ulcers may also be present. If only the rectosigmoid region is involved, mucosal involvement is usually continuous and consists of diffuse areas of ulceration and granularity. EHEC O157:H7 infection is usually segmental; infection results in friable, erythematous, and edematous mucosa with superficial ulcers. The right colon is more commonly involved than the transverse or the left colon. Pseudomembranes may develop in severe cases (Fig. 17.45). Y. enterocolitica tends to affect the proximal more that the distal colon. The mucosa may appear edematous with patchy or diffuse areas of superficial erosions. Pseudomembranes and perforation are uncommon in the early phase of infection.
Microscopic Findings
In the early phase of ASLC, the mucosa typically shows intact crypt architecture, edema, and prominent neutrophilic infiltration of the lamina propria, crypt, and surface epithelium (Fig. 17.46). Crypt abscesses and crypt rupture–associated granulomas are not uncommon. The epithelium may appear flattened, with reactive changes and mucin loss. The lamina propria shows increased lymphocytes, histiocytes, and neutrophils, often with a lesser component of plasma cells (Fig. 17.47). Basal plasma cells are not typical of ASLC because this feature develops only in patients with chronic disease.
Unless biopsies are obtained within the first few days of disease, the classic histologic phenotype of ASLC just described is uncommon. Biopsies are more commonly obtained several weeks after disease onset, in which case they show partial histologic resolution characterized by sparse neutrophilic inflammation, reactive epithelial changes, and mucosal edema. After 2 to 3 weeks of infection, the mucosa may be completely normal or may show only residual, patchy, mild inflammation (often referred to “focal active colitis”; see later discussion) and a mildly hypercellular lamina propria. In some cases, resolving ASLC reveals persistent residual surface epithelial damage and increased intraepithelial lymphocytes, reminiscent of lymphocytic colitis.
Some organisms show characteristic microscopic findings. For instance, C. jejuni infection may be associated with histiocytic aggregates that resemble granulomas. A Warthin-Starry silver stain performed in the early phase of infection highlights curved rods within crypt and surface epithelium.
Salmonella infection is associated with mild and focal changes. Mucosal edema and congestion of capillaries within the lamina propria are usually prominent in mild cases. In severe cases, the mucosa may show numerous crypt abscesses (Fig. 17.48), ulceration, and microthrombi within small venules of the lamina propria.
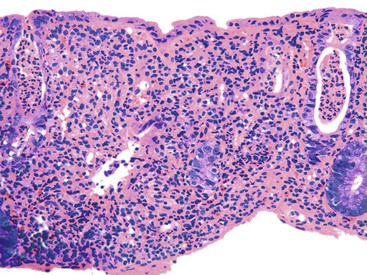
Early Shigella infection is characterized by the presence of aphthous ulcers with patchy foci of cryptitis and reactive epithelial changes in adjacent epithelium. With ongoing injury, the lamina propria typically shows a prominent mixed inflammatory infiltrate composed of neutrophils, lymphocytes, and plasma cells (Fig. 17.49).
EHEC O157:H7 infection may cause marked epithelial and endothelial damage. Neutrophilic cryptitis and occasional crypt abscesses are accompanied by superficial erosions and prominent hemorrhage and edema (“hemorrhagic colitis”). Damage to the endothelium may result in an ischemic pattern of injury associated with mucosal necrosis, withering crypts, and hyalinization of the lamina propria, with or without pseudomembrane formation (Fig. 17.50).
Differential Diagnosis
The most important (clinical) differential diagnosis of ASLC is IBD. Several studies have shown that histopathology can help differentiate these two disorders.6,302,312 In ASLC, endoscopic examination usually shows patchy mucosal involvement. In UC, the mucosal changes are diffuse and continuous. Discrete, serpiginous ulcers characteristic of CD and are seldom, if ever, encountered in ASLC. During the early phase of illness, the presence of diffuse, regional, or focal active colitis without crypt architectural distortion or other features of chronic mucosal injury (e.g., basal plasmacytosis, Paneth cell metaplasia) and with prominent neutrophilic cryptitis favors a diagnosis of ASLC over IBD.302,313,314 In a seminal study by Surawicz and colleagues312 that compared clinicopathologic features of 37 cases of ASLC with 14 cases of IBD, the histologic features that were significantly associated with IBD included crypt architectural distortion (93% in ASLC versus 14% in IBD), expansion of the lamina propria by a mixed inflammatory infiltrate (86% versus 37%), basal plasmacytosis (71% versus 0%), and basal lymphoid aggregates (43% versus 0%). Giant cells and even granulomas may be present, but in ASLC they are usually present in the basal half of the mucosa in association with ruptured crypts.
In some infectious cases, the disease is prolonged (typically more than 3 to 4 weeks), leading to the development of histologic features of chronicity. For instance, Shigella infection has been associated with crypt distortion mimicking IBD.313 However, in the “chronic” phase of bacterial infection, lamina propria cellularity typically diminishes with time. In contrast, increased cellularity of the lamina propria with basal lymphoplasmacytosis supports a diagnosis of IBD.
During the resolution phase of ASLC, the disease may be patchy and mimic Crohn’s colitis. Epithelioid granulomas, when present, should raise suspicion of CD. Clinical resolution of symptoms with supportive therapy and lack of involvement of the rest of the GI tract are additional features that help distinguish ASLC from Crohn’s colitis.
Natural History and Treatment
Most cases of ASLC resolve within 2 to 3 weeks with supportive therapy. In most bacterial infections, treatment does not provide a significant benefit over conservative management. Antibiotics are usually prescribed for complications, such as sepsis or disseminated infection. Some infections (e.g., C. jejuni) may result in the development of pancreatitis, cholecystitis, reactive arthritis, and Guillain-Barré syndrome. Prolonged infection by Shigella has been associated with appendicitis, myocarditis, pneumonitis, reactive arthritis, and hemolytic-uremic syndrome. EHEC infection may be complicated by hemolytic-uremic syndrome, end-stage renal disease, seizures, or cerebral edema.
Classic IBD develops in a small percentage of patients after an infectious type of illness. In one Spanish study, the incidence rate of IBD after an episode of infectious gastroenteritis was 68 per 100,000 person-years.315 The risk of developing IBD is highest during the first year after an episode of ASLC. The pathogenesis is unknown. One possibility is that the initial infection serves as an initiator, or catalyst, for the development of IBD in a genetically and immunologically susceptible host. However, in some cases, the initial diarrheal disorder is simply misdiagnosed as infectious colitis.
Focal Active Colitis
Focal active colitis is defined as a pattern of colonic injury characterized by the presence of focal neutrophilic infiltrates within the colonic epithelium (and/or lamina propria) in the absence of any other significant microscopic abnormality.316 The changes may involve a single crypt, a few adjacent crypts, or small regions of mucosa across a series of multiple colorectal biopsy specimens (Fig. 17.51).
Clinical Features
Based on one prospective study317 and three retrospective studies316,318,319 that evaluated, in total, 163 adult patients and 31 pediatric patients, a focal active colitis pattern of injury is more common in adult women than in adult men but shows an equal gender distribution in children. The most common causes of focal active colitis in adults are resolving infectious colitis (19% to 55%), drugs (most notably NSAIDs; 24% to 45%), and irritable bowel syndrome (IBS; 14% to 33%) (Table 17.6). Although CD is less common, it accounts for 13% of cases in adults. The presenting symptoms are usually sudden-onset diarrhea, abdominal tenderness, and fever. C. jejuni is the most frequently isolated causative organism. C. jejuni DNA is isolated in up to 19% of patients.304 This pattern of injury may also be observed incidentally (8% to 29%) during colonoscopic assessment for cancer surveillance or in patients with guaiac-positive stools.
Table 17.6
Prevalence of Conditions Associated with a Focal Active Colitis Pattern of Injury
| Diagnosis | Adults316 (%) | Adults318 (%) | Adults (%)317 | Children319 (%) |
| Infectious | 55 | 48 | 19 | 31 |
| Incidental | 40 | 29 | 8 | 26.6 |
| Ischemia | 5 | 10 | 0 | 0 |
| Crohn’s disease | 0 | 13 | 11 | 27.6 |
| Ulcerative colitis | 0 | 0 | 2.2* | 3.45 |
| Drugs | 39 | 0 | 24 | 0 |
| Irritable bowel syndrome | 14 | 0 | 33 | 0 |
| Allergic | 0 | 0 | 0 | 6.9 |
| Hirschsprung disease | 0 | 0 | 0 | 3.45 |
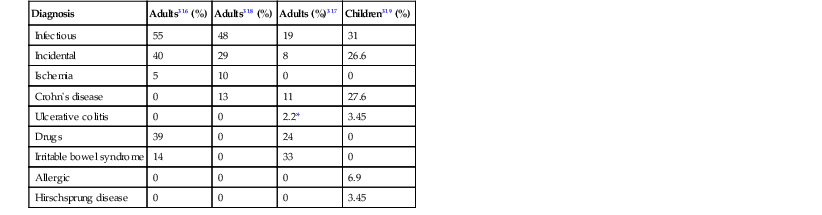
* Excludes >2 cases of indeterminate colitis.
In the pediatric population, focal active colitis is most often associated with infectious colitis (31%), CD (28%), allergic colitis (7%), UC (3%), and Hirschsprung disease (3%). As in adults, this pattern of inflammation is observed as an incidental finding in approximately 28% of cases.319 Occasionally, bowel preparation agents, especially oral sodium phosphate solutions, cause focal active colitis.
Pathologic Features
Depending on the cause, endoscopic findings range from completely normal to patchy mucosal erythema with scattered aphthous ulcers. Focal active colitis is characterized by the presence of neutrophilic cryptitis that may involve a single crypt or a small collection of crypts (see Fig. 17.51). The lamina propria may be mildly expanded by an inflammatory infiltrate that is rich in neutrophils or eosinophils. The finding of a florid neutrophilic inflammatory infiltrate at the crypt bases associated with prominent crypt apoptosis (so-called basal focal active colitis) is often associated with drug therapy, especially NSAIDs, antibiotics, proton pump inhibitors (PPIs), and steroids.317 Oral sodium phosphate bowel preparations cause cryptitis, often centered on lymphoid follicles (i.e., aphthous lesions), that is identical to that seen in some types of infectious colitis and in CD.320
Natural History and Treatment
Between 0% and 16% of adult patients and about 25% of pediatric patients with focal active colitis develop IBD. These patients develop CD more commonly than UC (see Table 17.6).