Inflammatory Disorders of the Esophagus
Ana E. Bennett
John R. Goldblum
Robert D. Odze
Introduction
Inflammatory disorders of the esophagus are extremely common. Gastroesophageal reflux disease (GERD) is among the most common chronic conditions, affecting as much as 40% of people in the Western world.1 Because of the ease and frequency of use of upper endoscopy for diagnosing gastrointestinal (GI) illnesses, biopsy specimens procured from esophageal mucosa to evaluate the presence or absence of inflammatory diseases—particularly GERD and its attendant complication, Barrett’s esophagus (BE)—are commonly encountered by surgical pathologists. This chapter focuses on inflammatory conditions that affect the esophagus.
Esophagitis
Gastroesophageal Reflux Disease
Clinical Features
GERD is an extremely common chronic condition, particularly in Western countries.1 Estimates based on an assumption that reflux-like symptoms are an indicator of the disease suggest a prevalence rate of 20% to 40%.2,3 Clinically, GERD is often classified as erosive or nonerosive based on endoscopic or pathologic features. Risk factors associated with GERD include advanced age, certain lifestyle habits (e.g., alcohol consumption), body mass index, and tobacco smoking, although the clinically relevant contributions of many of these factors are not entirely clear. GERD affects people of all ages, but the risk increases with age and rises dramatically after age 40 years. The symptoms most often associated with GERD are heartburn, acid regurgitation, and dysphagia. Atypical or supraesophageal symptoms include asthma, chronic cough, chronic sore throat, pharyngitis, laryngitis, a globus sensation, and noncardiac chest pain. The clinical and pathogenetic aspects of GERD are discussed further in the section on BE.
Pathogenesis
In the traditional view, reflux esophagitis is caused by reflux of gastric or duodenal fluid into the esophagus. The injurious effect of refluxed gastric acid, bile, pepsin, and duodenal contents on the normal protective mucosal barriers causes injury to the esophageal mucosa.4–6 With ongoing reflux injury, surface esophageal cells die, triggering both an inflammatory response (infiltration of neutrophils) and a proliferative response (basal cell and papillary hyperplasia). The cause of reflux is multifactorial. Contributing factors include the presence of a hiatal hernia, a defective or weak lower esophageal sphincter (LES), impaired esophageal peristalsis with transient LES relaxation, delayed gastric emptying, decreased salivary gland secretions, increased gastric acid production, and bile reflux.2,3,7–9 Although the data have been somewhat conflicting, there is some evidence to support an association among high body mass index, the presence of hiatal hernia, and GERD.10–13 Likewise, there is evidence to suggest that Helicobacter pylori infection may actually protect against the development of GERD and its complications.14 Specifically, corpus gastritis is associated with decreased acid secretion, and GERD is less common in patients with severe corpus gastritis.7,15 When acid secretion returns to normal after the eradication of H. pylori, there is an increased risk of developing GERD.16,17
Recent studies of the pathogenesis of GERD in animal models 15,18,19 and in vitro indicate that exposing esophageal squamous epithelial cells to acids and bile salts alone can cause epithelial cells to secrete inflammatory cytokines (i.e., interleukin 8 [IL8] and IL1β) that cause inflammatory cells (T-lymphocytes and neutrophils) to migrate into the epithelium. This finding has led to the new hypothesis that it is the inflammatory response, not the direct effect of acid, that is the initial factor responsible for damaging esophageal mucosa. Histologic examination in animal models has demonstrated that infiltration of the submucosa by lymphocytes is an early manifestation of inflammation. Neutrophils involve the mucosal surface later in the course of disease progression. These findings provide further support for an alternative concept for the development of reflux esophagitis in humans.15
Pathology
On endoscopic examination, patients with erosive esophagitis reveal erosions, ulcers, strictures, or some combination of these. However, as much as 50% to 60% of all symptomatic patients with objective evidence of GERD have normal mucosa or only mild hyperemia at endoscopy.20 Furthermore, histologically inflamed esophageal mucosa (esophagitis) may appear normal endoscopically; and conversely, hyperemia does not necessarily indicate the presence of esophagitis microscopically. Because of these endoscopic/pathologic discrepancies, biopsies are always warranted in symptomatic patients to document the presence of tissue injury and to exclude other entities such as infections, BE, and other preneoplastic or inflammatory alterations. This approach is particularly important if empiric reflux therapy has failed.
Biopsies of grossly visible lesions in GERD characteristically reveal evidence of “active” esophagitis, a nonspecific injury pattern that can result from a variety of causes. Pinch biopsies obtained through standard endoscopes are often not adequate for evaluation of early histologic changes resulting from reflux because they usually do not include the entire thickness of the mucosa and are difficult to orient.4,20 Endoscopes with a large-caliber biopsy channel and jumbo biopsy forceps should be used to facilitate accurate histologic diagnosis. Use of jumbo forceps does not increase the rate of complications related to endoscopy21; rather, it greatly improves the quality of the histologic specimens.
Histologically, reflux changes are typically distributed over the distal 8 to 10 cm of the esophagus in a patchy fashion, and multiple biopsies are often necessary to consistently demonstrate histologic abnormalities. 22 However, changes are typically worse in the distal esophagus and decrease in intensity more proximally. Because biopsy specimens from the lower 1 to 2 cm of the esophagus, even in asymptomatic subjects, often reveal evidence of mild squamous hyperplasia,22,23 diagnostic biopsy specimens should be obtained usually more than 2.0 cm above the level of the gastroesophageal junction (GEJ) in order to diagnose esophagitis reliably. Furthermore, because of a high level of discordance between endoscopic and histologic findings, it is recommended that all symptomatic patients undergo biopsy, regardless of the presence or absence of endoscopic abnormalities. Esophageal biopsies are considered more useful for establishing a diagnosis of GERD in infants than in adults.24
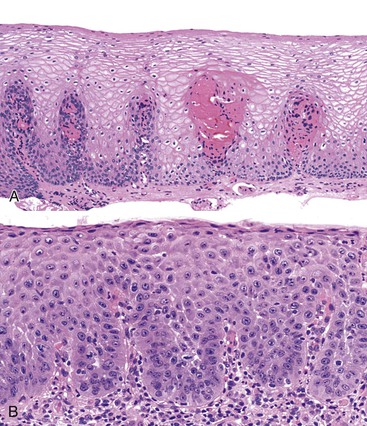
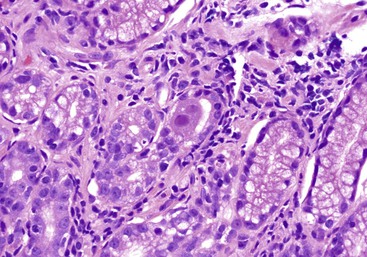
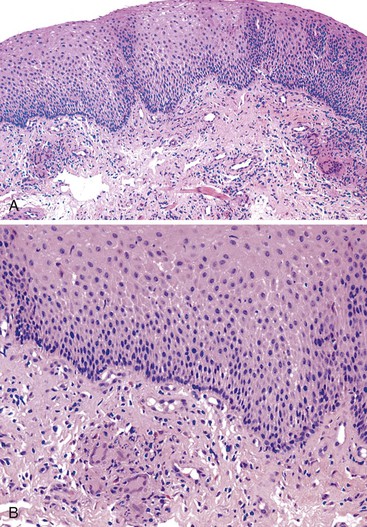
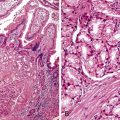
Reflux esophagitis produces a characteristic, although nonspecific, tissue injury pattern. Features of untreated active esophagitis include basal cell hyperplasia, elongation of the lamina propria papillae into more than two thirds of the thickness of the mucosa, epithelial cell necrosis, increased intraepithelial inflammation (including eosinophils, neutrophils, and lymphocytes), lack of surface maturation (nucleated cells at surface of epithelium), distended pale squamous “balloon” cells, intercellular edema (acantholysis), and, in severe cases, surface erosions or ulcerations (Figs. 14.1 to 14.3). Intercellular edema or dilated intercellular spaces reflecting increased paracellular permeability may be a useful marker of early injury in the absence of endoscopic evidence of injury.25,26
Squamous Hyperplasia
For many years, it was believed that the only true diagnostic criterion for esophagitis was the presence of intraepithelial inflammation. However, in 1970, Ismail-Beigi and co-workers found that some patients who had clinical symptoms of GERD but a normal or only minimally abnormal endoscopic appearance showed hyperplasia of the squamous epithelium, a finding that they postulated was an early histologic manifestation of reflux-induced injury.27 Squamous hyperplasia was defined by (1) lengthening of the subepithelial lamina propria to more than two thirds of the thickness of the squamous epithelium and (2) expansion of the basal zone of the squamous epithelium to more than 15% of the thickness of the epithelium (see Fig. 14.1). Subsequent studies confirmed these initial observations and also demonstrated a significant positive correlation between the severity of reflux, as measured by 24-hour pH score (a composite quantitative evaluation of acid reflux) and the length of the lamina propria papillae.28 These correlations also occur in infants and children with GERD.29 Increased mitoses, slight enlargement of basal and suprabasal nuclei, and prominent nucleoli and hyperchromatism are features often associated with true basal cell hyperplasia. True basal cell hyperplasia is best evaluated in well-oriented tissue sections that include at least three consecutive papillae. Unfortunately, this is rare in small pinch biopsy samples. Therefore, one should be cautious not to overinterpret “mild” changes as evidence in favor of esophagitis.
Inflammation
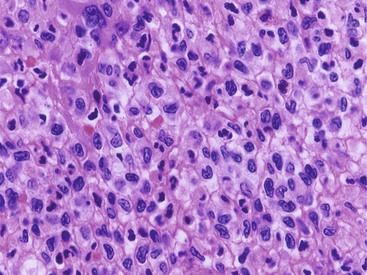
The principal inflammatory cells in patients with reflux esophagitis include neutrophils, eosinophils, and lymphocytes (see Fig. 14.2).30 However, depending on the phase of disease and the treatment status, none, one, or all of these inflammatory cells may be present in a single biopsy specimen. Therefore, a diagnosis of (reflux) esophagitis can be established in the absence of inflammation if the basal cell and lamina propria papillae changes are present, particularly in a patient who has begun treatment with antireflux agents.
Neutrophils.
Within the esophageal squamous epithelium, intraepithelial neutrophils always indicate a pathologic process. However, intraepithelial neutrophils are not a sensitive indicator of reflux esophagitis, because they are present in fewer than 30% of GERD patients with documented reflux. They also are not specific for GERD. Nevertheless, the presence of a significant number of neutrophils, particularly in association with a surface erosion or ulcer, should prompt the pathologist to search for a viral or fungal (Candida) infection.
Eosinophils.
Increased numbers of eosinophils often are present in patients with esophagitis.31 However, because rare, isolated eosinophils may be found in the mucosa of normal adults, particularly in the distal 1 to 2 cm of the esophagus, they are not considered diagnostic of esophagitis if sparse in number and not associated with other features of esophagitis, such as those discussed previously. On the other hand, the presence of even rare intraepithelial eosinophils is a valuable diagnostic aid in evaluating reflux esophagitis in children, because they are not normally present in this age group.32–34 In addition, eosinophils in the lamina propria are considered an even more sensitive indicator of GERD in infants.24
Occasionally, large numbers of eosinophils are present in esophageal biopsy specimens of adult patients with putative reflux.28,35 In this circumstance, causes of esophageal eosinophilia other than reflux, such as primary eosinophilic esophagitis (EOE), drug reaction (including Stevens-Johnson syndrome), pill-induced esophagitis, collagen vascular disease, and, very rarely, parasitic infection,36 should be excluded. Children with apparent GERD who have prominent eosinophilia may improve clinically when given an elemental diet, which suggests that certain protein sensitivities may lead to reflux-like symptoms37,38 (see Primary Eosinophilic Esophagitis).
Lymphocytes.
Lymphocytes are considered a normal intraepithelial component of the esophageal squamous mucosa.30,39,40 These are largely T lymphocytes and have either a round or an irregular nuclear contour, particularly when deformed by adjacent squamous epithelial cells. Because of their “squiggly” appearance, intraepithelial lymphocytes may resemble granulocytes. These cells have been identified as CD8+ and TIA-1+ T lymphocytes and tend to be more prominent in the peripapillary epithelium.41 Normal esophageal mucosa contains roughly 10 to 12 lymphocytes per high-power field (HPF).40,41 Although lymphocytes are present in increased numbers in patients with GERD, this finding in isolation has no independent diagnostic significance, because normal control subjects may also have increased numbers.40 In addition, other disorders, such as achalasia and Crohn’s disease, may be associated with increased numbers of intraepithelial lymphocytes.42,43
Other Microscopic Features of GERD
Dilation and congestion of lamina propria capillaries are additional characteristic features of reflux esophagitis,44 but this finding may also occur in specimens from normal controls, albeit in a mild fashion, possibly as a traumatic biopsy artefact.45 Other features that may be seen in GERD include ballooning degeneration of squamous cells, intercellular edema (acantholysis) that causes minor separation of individual squamous cells, multinucleation of squamous cells, increased mitoses, and decreased surface maturation. The presence of multinucleated (regenerative) cells may mimic herpetic esophagitis (Fig. 14.4). Multinucleated regenerative squamous cells are distinguished from virally infected cells by the lack of characteristic nuclear inclusions and by their prominence at the base of the mucosa and adjacent to ulcers, rather than in the surface cells, which is characteristic of herpes.46
Differential Diagnosis of GERD
The differential diagnosis of GERD includes infectious esophagitis; pill esophagitis; esophagitis caused by ingestion of corrosive agents such as lye; chemotherapy- and radiation-induced esophagitis; primary EOE; systemic diseases such as collagen vascular disease, Crohn’s disease, Stevens-Johnson syndrome, various bullous diseases, lichen planus, and graft-versus-host disease; and trauma. Many of these conditions share overlapping morphologic features with GERD. Therefore, an accurate diagnosis of “reflux” esophagitis requires correlation with the patient’s clinical, endoscopic, manometric, and histologic data. In the absence of clinical information, a diagnosis of reflux esophagitis cannot be established on the basis of biopsy findings alone. The histologic features are essentially nonspecific. In fact, in this scenario, a “top line” diagnosis of “active esophagitis consistent with reflux” rather than “reflux esophagitis” should be used.
Reactive Squamous Hyperplasia versus Dysplasia
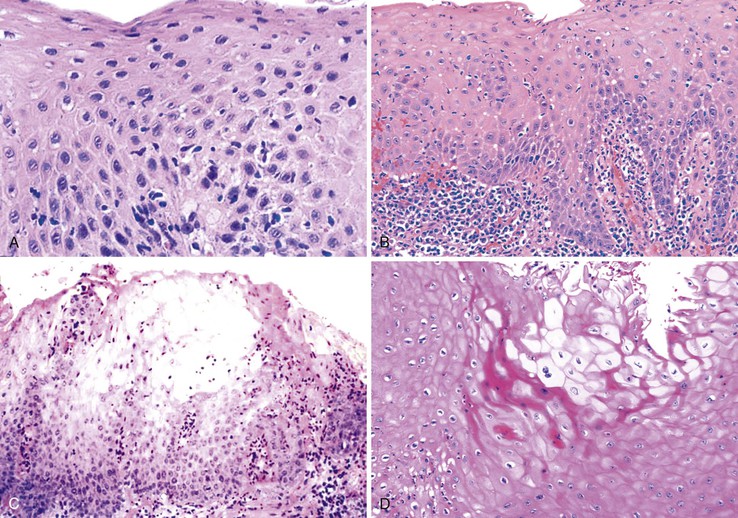
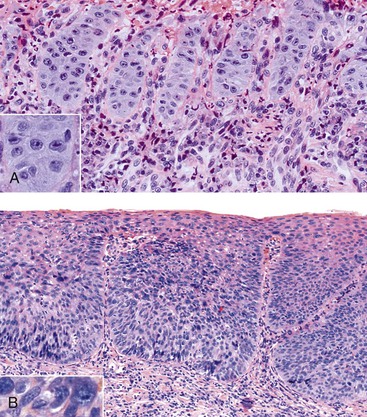
On occasion, marked reactive (pseudoepitheliomatous) hyperplasia related to reflux esophagitis may resemble squamous dysplasia histologically (Fig. 14.5). The most helpful distinguishing feature is the presence of cytoarchitectural uniformity in cases of hyperplasia, compared with cytoarchitectural pleomorphism in cases of dysplasia or carcinoma.
The mucosal architecture in hyperplastic lesions retains its uniformity, showing elongation of papillae that extend to roughly equal depths within the deep lamina propria and are usually of similar width. In contrast, dysplastic squamous epithelium typically displays architectural distortion with absent, sharply angulated, or markedly irregular papillae in terms of their length and width (Table 14.1; see also Chapter 24 for details).
Table 14.1
Differential Diagnosis of Reactive Hyperplasia versus Dysplasia of Squamous Epithelium
| Feature | Hyperplasia | Dysplasia |
| Papillae | Regular | Absent or irregular |
| Nuclear enlargement | ++ | +→+++ |
| Nuclear pleomorphism | +/− | +→+++ |
| Nuclear overlapping | − | +→+++ |
| Nuclear hyperchromasia | +/− | +→+++ |
| Nuclear membrane | Smooth | Irregular |
+, Mild degree; ++, moderate degree; +++, marked degree.
Cytologically, hyperplastic squamous epithelial cells are uniform and do not show loss of polarity or overlapping nuclei. The nuclei may be uniformly enlarged; however, they have smooth nuclear membranes, open chromatin, often prominent nucleoli, and increased mitoses but no atypical mitoses. Dysplastic squamous epithelial cells are typically more pleomorphic in size and shape, are more hyperchromatic, have irregular nuclear contours, and reveal nuclear overlapping and loss of polarity (see Fig. 14.5).
Reactive Changes in Ulcers versus Dysplasia or Carcinoma
Granulation tissue within the base of erosions or ulcers may exhibit large atypical endothelial cells and fibroblasts47,48 (Fig. 14.6). They are usually distributed in a scattered fashion within otherwise typical granulation tissue. These cells do not form solid clusters of cells and have a normal, or even decreased, nucleus-to-cytoplasm (N : C) ratio. In contrast, carcinoma usually demonstrates groups or sheets of cohesive cells with overlapping nuclei and an increased N : C ratio. In difficult cases, immunohistochemistry for cytokeratins can be helpful in differentiating true carcinoma (positive) from reactive “pseudosarcomatous” alterations of the stromal cells (negative).
Rarely, the inflammatory exudate within the ulcer or erosion may contain abundant activated and atypical lymphocytes that can simulate lymphoma (Fig. 14.7). In general, these cells are benign when confined to the surface exudate. The infiltrate should raise concern for a lymphoma if it involves the underlying tissue in a dense, confluent, and homogeneous manner.
Natural History and Treatment of GERD
The natural history of GERD in the general population remains uncertain because of the widespread use of acid inhibitors. GERD has three phenotypic presentations: nonerosive reflux disease (NERD), erosive esophagitis, and BE. Most patients with GERD (50% to 70%) have normal mucosa on endoscopy and therefore are diagnosed with NERD.49–51 Some researchers have proposed that NERD is a discrete entity because of its unique physiologic characteristics, which include a more competent antireflux barrier.52 However, most authorities believe that GERD is a progressive disease that starts with NERD and progresses with time, to erosive esophagitis or BE or both, in selected high-risk individuals. Some data suggest that progression from NERD to erosive esophagitis occurs in as much as 30% of patients annually,53–56 but it is unknown whether NERD can progress directly to BE without an erosive phase.57 Between 1% and 22% of patients with erosive esophagitis progress to a more severe form of disease, whereas regression to a less severe form of disease occurs in 6% to 42% of patients, depending on whether acid inhibitors have been used.58–60 Erosive esophagitis is a risk factor for BE.61,62 Barrett’s esophagus develops in between 1% to 13% of patients with erosive esophagitis annually.63 In a recent prospective, follow-up endoscopic study64 to evaluate the risk of BE in a Swedish general population (the Kalixanda study database),65 the incidence of BE was 9.9 per 1000 person-years, and the prevalence of BE in this GERD cohort increased from 3% to 8% during a 5-year follow-up period. In fact, progression of GERD to BE in the general population may be higher than previously recognized.55,66
The vast majority of patients with GERD have a recurrent but nonprogressive form of disease that is controlled adequately with acid inhibitor therapy.
Current medical forms of therapy, such as proton pump inhibitors (PPIs) and other acid inhibitors, are highly effective at relieving symptoms. However, progressive disease develops in some patients despite ongoing therapy.67 In one study, as much as 82% of patients who showed healing of their esophagitis with omeprazole relapsed within 6 months after cessation of therapy.67 In a 10-year follow-up study of 101 patients with reflux esophagitis, significant morbidity related to GERD developed in almost 75%, showing quality of life scores significantly lower than those of a non–GERD control population.68 Surgical treatment is considered an option for patients with chronic GERD who are not responsive to medical therapy or have a defective LES.69,70
Infectious Esophagitis
Infections remain an important cause of esophagitis and can have significant implications in the immunocompromised host. Viruses and fungi cause most forms of acute infectious esophagitis.71 Bacterial esophagitis occurs in some patients with systemic or upper respiratory infection, but this condition is rarely sampled histologically.
Viral Esophagitis—Herpes
Clinical Features
Herpes esophagitis occurs primarily in immunosuppressed patients. Common causes of immunosuppression include prior chemotherapy, solid organ and bone marrow transplantation, and acquired immunodeficiency syndrome (AIDS). Herpes esophagitis also may occur in otherwise healthy young adults with normal immune function, who usually have a self-limited infection that resolves within 1 to 2 weeks.72–74 Symptoms of herpetic esophagitis include odynophagia, dysphagia, epigastric pain, fever, and upper GI bleeding, but some patients are asymptomatic.75 Coexistent herpes labialis and oropharyngeal ulcers are seen in approximately one fourth of patients.71,74 Herpetic ulcers in the esophagus may serve as a portal of entry for other pathogens and can be associated with herpetic pneumonitis.72 Endoscopically, herpetic ulcers are typically shallow, sharply punched-out lesions and are often surrounded by relatively normal-appearing mucosa.
Pathology
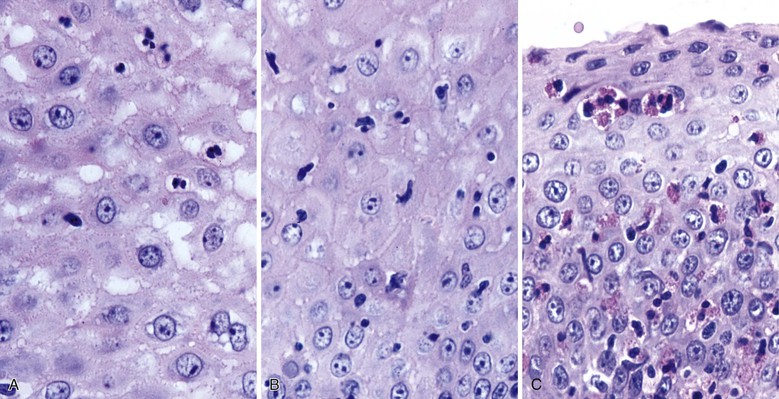
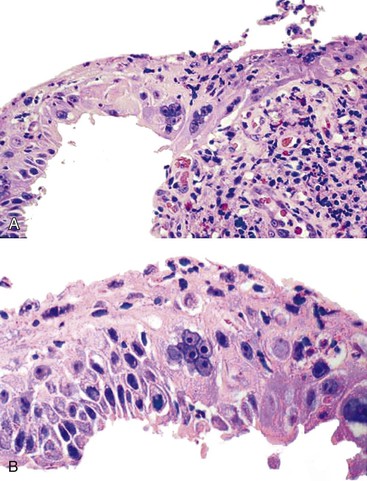
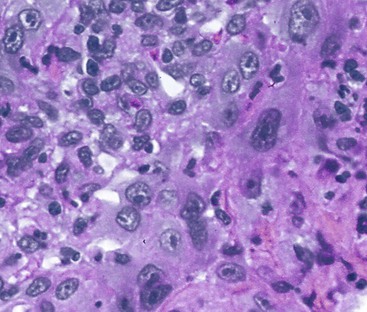
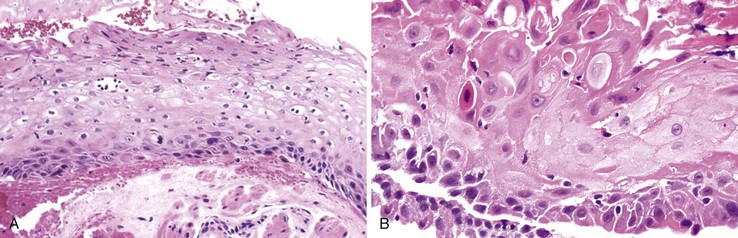
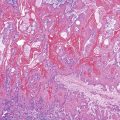
Herpes simplex or varicella-zoster virus infects esophageal squamous epithelium. Accordingly, the characteristic inclusion bodies are limited to the squamous epithelial cells, typically accentuated at the superficial lateral margin of ulcers and erosions. In fact, biopsy specimens obtained distant from the immediate edge of the ulcer lesion may not be diagnostic. Microscopic diagnostic criteria include the presence of Cowdry A intranuclear viral inclusion bodies, ground-glass nuclei, nuclear molding, multinucleated giant cells, and ballooning degeneration of infected cells (Fig. 14.8).72,73 Cowdry A inclusions are eosinophilic to amphophilic round bodies separated by a clear zone from a thickened nuclear membrane. Ground-glass nuclei have a smooth, homogeneous chromatin pattern with a pale basophilic quality. Multinucleated giant cell changes in squamous epithelial cells may occur in reflux esophagitis and should not be confused with the cytopathic effects of herpesvirus infection. Reactive cells have prominent nucleoli and perinucleolar clearing, but nuclear inclusion bodies are not present. Large numbers of mononuclear cells, primarily aggregates of macrophages with convoluted nuclei, in the surface exudate adjacent to the infected epithelium have been noted as a characteristic finding in herpetic ulcers and should make the pathologist suspect herpesvirus infection.76 However, similar cells may be observed in nonherpetic ulcers throughout the GI tract in the absence of herpesvirus infection, so this finding is not specific. Herpes simplex type I is the most common cause of herpetic esophagitis, but on morphologic grounds, this type cannot be distinguished from herpes simplex type II or varicella-zoster. Immunohistochemical staining and in situ hybridization, if clinically indicated, can help distinguish these three viral species.
Differential Diagnosis
On occasion, herpetic esophagitis may be difficult to distinguish from squamous dysplasia/carcinoma (Table 14.2). The nuclear inclusions characteristic of herpetic esophagitis may be mistaken for macronucleoli typical of malignant cells. However, in herpes esophagitis, one usually sees a halo located between the nuclear inclusion and the nuclear membrane. In addition, the ground-glass chromatin pattern that is characteristic of virally infected cells shows little structure and is pale, in contrast to the variably granulated and darkly stained chromatin typical of malignant cells. Radiation-induced esophagitis also may be mistaken for herpetic esophagitis, because radiation can result in the formation of enlarged squamous cells with multiple nuclei, nucleoli, and pale chromatin. However, careful attention to the presence or absence of characteristic Cowdry A inclusions and ground-glass nuclei allows their distinction.
Table 14.2
Differential Diagnosis of Viral Esophagitis
| Etiology | Endoscopy | Morphology |
| Herpesvirus | Superficial ulcers |
Single deep ulcers
Erosions, plaques, nodules
Treatment
Empiric treatment for herpetic lesions is often initiated on the basis of clinical suspicion, even in the absence of histologic confirmation. Acyclovir (Zovirax) can be administered orally and helps initiate healing of active disease, but it does not prevent recurrences. Other medications, including famciclovir (Famvir) and valacyclovir (Valtrex), have also been shown to be effective therapy for herpes esophagitis. Intravenous medications may be indicated for severe disease.
Viral Esophagitis—Cytomegalovirus
Clinical Features
Cytomegalovirus (CMV) is a member of the human herpesvirus family, with an estimated prevalence rate among adults ranging from 40% to 60% of all cases of infectious esophagitis in resource-rich countries. Like other herpesviruses, CMV is not normally cleared from the body but instead is kept in a state of latency by the immune system. Therefore, chronic CMV infection only rarely causes disease among immunocompetent persons, but it does represent a major cause of morbidity and mortality in immunocompromised patients(especially patients infected with the human immunodeficiency virus [HIV] whose CD4 counts are lower than 50 cells/µL), transplant recipients, patients with malignancies, and patients who have received immunosuppressive therapy.77
CMV esophagitis, although less common than herpetic esophagitis, is not infrequently found in patients with AIDS. In one study, CMV infection was found either alone or in combination with Candida and herpes in 30% of AIDS patients.78 Similar to herpes simplex or varicella-zoster esophagitis, CMV esophagitis may also rarely occur in immunocompetent individuals.79
Presenting symptoms may include odynophagia, nausea, substernal pain, and fever. Endoscopically, CMV esophagitis has a variable appearance. Most patients with CMV esophagitis have multiple, well-circumscribed ulcers,80 most often located in the middle to distal esophagus. Deep linear ulcers and shallow ulcers, erythema, diffuse erosive esophagitis, and an inflammatory exudate also may be seen in the setting of CMV esophagitis,81 but these findings lack diagnostic specificity. Viral culture is not used routinely because detection of the virus does not confirm active disease.82
Pathology
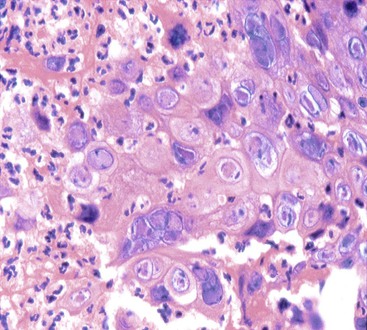
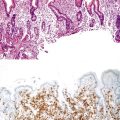
CMV-infected cells typically show marked cytomegaly and nucleomegaly with large ovoid intranuclear inclusions and thick marginated chromatin (Fig. 14.9). The inclusion bodies may be brightly eosinophilic or deeply basophilic and are usually separated from the nuclear membrane by a halo. In addition, small eosinophilic to basophilic granular inclusions may be evident in the cytoplasm of infected cells. Cytoplasmic inclusions typically appear within minute vacuoles. CMV infects mesenchymal and columnar cells, but not squamous cells. Therefore, it is important for endoscopists to biopsy or brush the base of esophageal ulcers to optimize the chance of sampling diagnostic cells.83 No single technique is 100% sensitive in establishing a diagnosis of CMV esophagitis. A combination of diagnostic modalities is often used.84 If a careful search of well-prepared, routinely stained tissue sections or cytology preparations fails to reveal CMV inclusions, or at least suspicious cells, then additional ancillary immunohistochemical or in situ hybridization tests may be helpful. Immunohistochemistry may highlight infected cells without typical CMV morphology on routine stained sections and can be more sensitive than light microscopy. As previously mentioned, CMV esophagitis may coexist with herpes and Candida infection in both transplant recipients and patients with AIDS.73
Treatment
A variety of medications, such as ganciclovir, valganciclovir, foscarnet, and cidofovir, may be effective for the treatment of CMV esophagitis. The specific choice of therapy depends on the site and severity of infection, the level of underlying immunosuppression, the patient’s ability to tolerate and adhere to the treatment regimen, and the potential drug interactions.85
Other Viruses
Symptoms related to esophageal disease are common in AIDS patients.78 In most, a specific infectious agent such as Candida, herpes simplex, varicella-zoster, or CMV can be identified.78,86 However, in some patients, no apparent cause for the esophagitis or ulcer can be found by standard techniques. Hybridization with specific DNA probes may reveal Epstein-Barr virus in some of these patients.87 In some HIV-positive patients, however, odynophagia and multiple discrete esophageal ulcers are observed in the absence of an identifiable pathogen. Ultrastructural studies in such patients have revealed the presence of viral particles consistent with retrovirus, which suggests that the esophagus may represent a primary direct target site of acute HIV infection itself.88,89
Fungal Esophagitis—Candida
Clinical Features
Fungal esophagitis is most commonly caused by Candida albicans or Candida tropicalis (Fig. 14.10). Fungal infection occurs primarily in patients with some type of underlying disease (e.g., AIDS, other immunosuppressive disorders, diabetes) and in patients who have undergone treatment with broad-spectrum antibiotics, acid-suppressive therapy,90 or inhaled corticosteroids.91 It also may be found in otherwise healthy patients.92 Clinically, the presenting symptoms are dysphagia and odynophagia, but some patients remain asymptomatic, with the infection discovered incidentally at esophagoscopy performed for other reasons, especially in elderly patients.
At endoscopy, esophageal candidiasis typically appears as white plaques of fibrinopurulent exudate, which may be focal or confluent, overlying erythematous mucosa. These plaques can be scraped away to reveal ulceration underneath.
Pathology
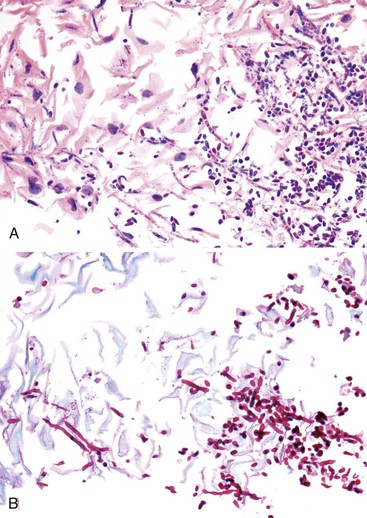
Morphologically, pseudohyphae and budding yeast forms can be demonstrated in a background of active esophagitis and are easily identified within the ulcer slough and fibrinopurulent exudate. Because Candida organisms are part of the normal flora of the GI tract, confirmation of this diagnosis requires more than simply identifying budding yeast forms; pseudohyphae should be detected within tissue to document true infection.93 Pseudohyphae have a linear or ribbon-like appearance in which small indentations, rather than true septations, are typically noted along the long axis of the organisms. Yeast forms have a slight ovoid contour and frequently manifest budding from the tips.94 In immunosuppressed patients, who occasionally reveal only minimal inflammation, special stains (silver stain, periodic acid–Schiff [PAS]) should be used to detect small numbers of invasive fungal forms within the tissue. However, special stains cannot be used to speciate the type of Candida organisms. C. tropicalis is more virulent than C. albicans because of its increased potential for tissue invasiveness.95 Candida can colonize preexisting ulcers or any damaged mucosa, and the pathologist should consider the possibility of a dual infection or pathology.96
Differential Diagnosis
On occasion, the endoscopist may identify small white plaques that, although they resemble Candida esophagitis, represent glycogenic acanthosis or ectopic sebaceous glands. Glycogenic acanthosis occurs as white mucosal plaques that measure 2 to 5 mm.97 Diffuse esophageal glycogenic acanthosis may occur as a rare manifestation of Cowden syndrome.98 Biopsy features include distention of squamous epithelial cells with glycogen, which is evident as pale-staining material that is PAS positive and diastase digestible.99 Ectopic sebaceous glands, which may also appear as small, pale yellow or white, punctate elevations of the mucosa, are rare but are easily recognized in biopsy material by their close resemblance to normal dermal sebaceous glands.100
Treatment
Fluconazole (Diflucan) is the drug of choice because it is safe and well tolerated, although other drugs, such as itraconazole and ketoconazole, are also effective.101,102 Amphotericin B is considered a second-line option and is reserved for severe cases and for patients for whom treatment with azole compounds has failed. Echinocandins (e.g., Caspofungin), a new class of antifungal agents that act on fungal cell walls, are being used in randomized trials for patients with Candida esophagitis refractory to azole compounds.103,104
Bacterial Esophagitis
Primary bacterial infections of the esophagus are exceedingly rare except in immunocompromised patients; most cases are secondary and occur in areas of ulceration. The most common infecting organisms are normal flora from the mouth and respiratory tract (Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus viridans, and Bacillus species). A diagnosis of bacterial esophagitis can be established when sheets of confluent bacteria invade the subepithelial tissue.71 The number of bacteria appears to be inversely related to the intensity of the inflammatory reaction.105 Bacterial cultures of biopsy material are not performed routinely but may be helpful to further identify organisms that are detected on routinely stained tissue sections. Actinomyces may occur in areas of previous tissue injury; the infection is characterized by the presence of sulfur granules and intertwining thin branching actinomyces filaments within the subepithelial tissue. Occasionally, one may encounter Mycobacterium tuberculosis, Mycobacterium avium-intracellulare, Histoplasma capsulatum, or Toxoplasma gondii in the esophagus.106,107
Parasitic Esophagitis—Chagas Disease
Clinical Features and Pathogenesis
The World Health Organization estimates that approximately 10 million people are infected with Trypanosoma cruzi worldwide. Approximately 100 million are at risk of contracting the infection, and these are mostly in Latin America. Humans are accidental hosts for T. cruzi. They are usually infected at night via contact with feces of blood-sucking triatomine insects. A second mechanism of transmission, responsible for as much as 10% of cases, is transfusion of whole blood or blood derivatives (except for lyophilized products).108 A third route of transmission is congenital. The likelihood of congenital transmission in children of chagasic mothers ranges from 1% to 10%.109 After 2 to 4 months, the acute clinical manifestations (inflammation of the eye, conjunctivitis, palpebral edema, periauricular satellite adenopathy, and generalized infection) disappear and the disease enters a period of clinical latency (“indeterminate” form). Studies performed in endemic areas have provided clinical and radiologic evidence of esophageal disorders in 7% to 10% of people with chronic T. cruzi infection, and megaesophagus in 3%.109–112 Dysphagia of solid and liquid food is the first and most important symptom of digestive disturbance. It may begin slowly but leads to malnutrition and severe weight loss.113–115 Other clinical symptoms include regurgitation, pyrosis, hiccups, cough, and parotid gland hypertrophy. Esophageal pain often appears with deglutition as a consequence of spasmodic contraction of a hypersensitive esophagus.109 Esophageal involvement is progressive in some patients but not in others, and it evolves independently of heart involvement.
Pathology
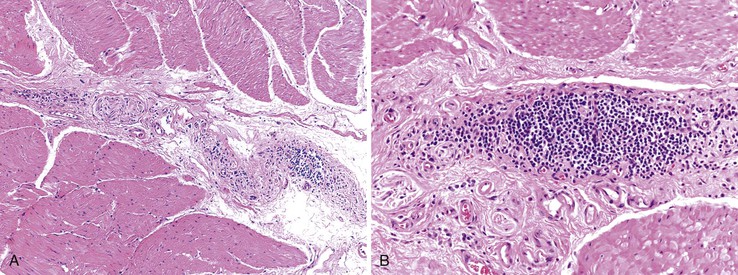
Destruction of neuron plexuses in the esophagus by the inflammatory reaction induced by T. cruzi is the main cause of dysperistalsis of the esophagus. It results in gradual narrowing of the distal esophagus with luminal enlargement of the proximal esophagus, sometimes termed chagasic megaesophagus (grade I to IV). Microscopically, distal muscular hypertrophy and mononuclear inflammatory infiltrates occur in the muscle layers. The submucosal and myenteric plexuses show an intense mononuclear infiltrate with associated neuronal injury (Fig. 14.11).36,116 A 2010 report indicates that patients with megaesophagus have a significantly decreased amount of S100- and glial fibrillary acidic protein (GFAP)-positive enteroglial cells when compared with seronegative controls and asymptomatic seropositive patients.110 With disease progression, there is intense neuronal destruction and denervation of the organ, with loss of function.
Treatment
Only two drugs are effective for the treatment of Chagas disease in the acute and chronic phases: nifurtimox and benznidazole.117–119 Both are almost 100% effective in curing the disease if treatment is begun soon after infection, at the onset of the acute phase, but their efficacy diminishes with time and duration of infection. Treatment protocols for the chronic phase of disease remain controversial.119 In the presence of megaesophagus, dilatation of the esophagogastric junction may be performed by passing a dilator or air-filled balloon through an endoscope. Repeated dilations may be needed to prevent the development of recurrent strictures. Surgery is indicated in some cases. Cardiotomy is the most frequently performed procedure. Injections of botulin toxin into the esophagus have also been used in some patients.120
Pill-, Drug,- and Toxin-Related Esophagitis
Pill Esophagitis
Clinical Features
Esophageal injury caused by prolonged direct mucosal contact with ingested, particularly large-sized, tablets or capsules occurs frequently.121–127 Commonly implicated agents include antibiotics (particularly tetracyclines and clindamycin), nonsteroidal antiinflammatory drugs (NSAIDs), potassium chloride, ascorbic acid, iron supplements, quinidine, and bisphosphonates.124,125,128 Symptoms include odynophagia, continuous retrosternal pain, and dysphagia. Elderly patients and women are affected most frequently.128,129 Affected patients often report having ingested a pill with very little or no fluid before nighttime sleep. Most affected individuals do not have an abnormality of esophageal transit. However, in some reports, patients with quinidine- or potassium chloride–induced injury were shown to have a history of external esophageal compression, such as valvular heart disease with left atrial enlargement, or esophageal entrapment by fixed mediastinal structures and adhesions after thoracic surgery.123
Pathology
Pill esophagitis often results in discrete ulcers with normal or only mildly inflamed esophageal mucosa. Ulcers may be shallow or, more commonly, deep with extension into the muscularis mucosae. They are usually located at the junction of the proximal and middle thirds of the esophagus, the area where the aortic arch compresses the esophagus and peristaltic amplitude is relatively low. Patients with left atrial enlargement are especially susceptible to pill-induced esophageal injury.
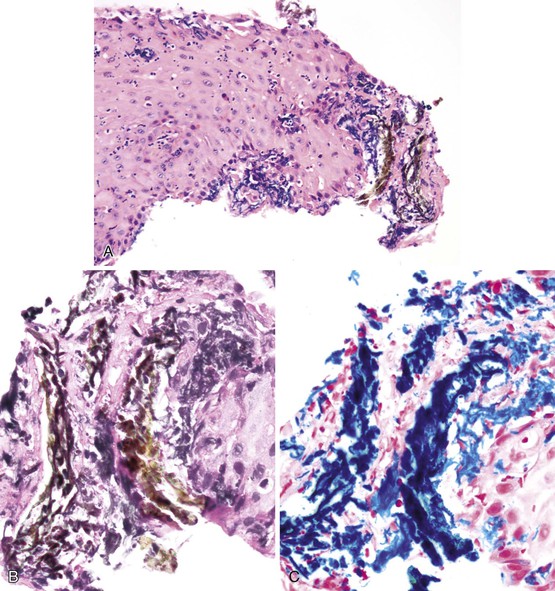
The histologic appearance of pill-induced esophagitis is typically nonspecific. However, prominent eosinophilic infiltration, spongiosis, and necrosis of squamous epithelium should always raise the possibility of pill-induced injury, particularly if located in the upper esophagus. Crystalline stainable iron may be identified in cases of ferrous sulphate–induced disease, and polarizable crystalline material may be evident in cases of alendronate-induced injury125 (Fig. 14.12). Some cases of pill esophagitis are severe and lead to perforation.
Differential Diagnosis
A variety of conditions can cause histologic features similar to those found in pill esophagitis. Herpes esophagitis is often a major consideration; a clinical history of immunosuppression and identification of characteristic viral cytopathic inclusions is useful in this differential diagnosis. Other causes of infectious esophagitis, including CMV and Candida, may mimic pill esophagitis, both clinically and pathologically. Identification of CMV cytopathic effect or fungal elements is diagnostic. GERD is more common than pill esophagitis; however, it usually is not possible to distinguish these conditions on histologic grounds in an isolated esophageal biopsy specimen and in the absence of clinical information.
Corrosive Esophagitis
Clinical Features
Corrosive or caustic esophageal injury occurs in children and adults, most commonly as a result of the ingestion of alkaline (lye) or acid (nitric).130–132 Gastroduodenal lesions also are common after ingestion of caustic agents. Coughing, crying, and vomiting after ingestion are typical presenting symptoms. Dysphagia, refusal to drink, and mouth or chest pain, with drooling and salivation, may ensue. Airway obstruction and glottic edema can result in respiratory distress and stridor.133,134
Pathology
The endoscopic appearance of the esophageal mucosa varies according to the type, physical state, concentration, and volume of the ingested substance. Typically, one encounters mucosal edema, erythema, hemorrhage, and necrosis, sometimes with the formation of circumferential ulcers and mucosal sloughing.
The pathologic features are nonspecific and are related to tissue necrosis and subsequent inflammation. Acid injury often produces coagulative necrosis, with the depth of injury limited by the eschar formation. Alkaline injury typically causes liquefactive necrosis with fat and protein digestion. Because a protective eschar does not develop in cases of alkaline injury, the depth of penetration is often greater than with acid injury,131 and perforation secondary to corrosive esophagitis develops in some patients. Initially, the esophagitis may reveal a dense neutrophilic infiltrate, vessel thrombosis, bacterial invasion, and abundant granulation tissue. Long-term complications include stricture formation, which may require endoscopic or surgical treatment, and, rarely, squamous cell carcinoma.135
Esophagitis Dissecans Superficialis
Clinical Features and Pathogenesis
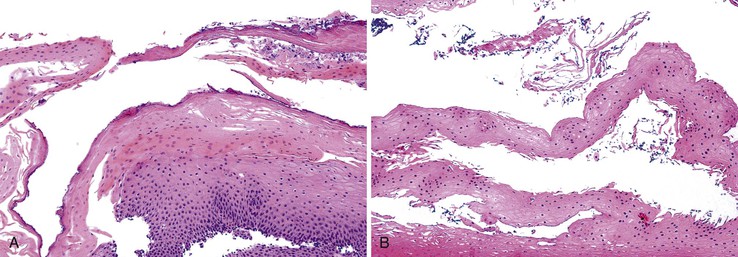
Esophagitis dissecans superficialis (EDS) is the term coined by Rosenberg in 1892 to describe a lesion characterized by extensive dissection of the squamous epithelial lining of the esophagus from its underlying corium in the form of a tubular cast. Dramatic presentations of EDS, with vomiting of esophageal casts, were documented before the endoscopic era.136 In less dramatic cases, the condition varies from sloughing of large fragments of the esophageal squamous mucosa, which may be coughed up or vomited, to a condition that is not even suspected until the endoscopist notices whitish strips or streaks (pseudomembranes) of peeling esophageal mucosa during an endoscopic examination performed for reasons that may be unrelated to the esophagus.137 The usual symptoms are dysphagia, odynophagia, and heartburn. In a series of 12 patients with documented endoscopic and histologic features of EDS, the most common symptoms or signs leading to upper endoscopy were dysphagia, occult or overt GI bleeding unrelated to EDS, weight loss, epigastric pain, and heartburn.138 Although EDS has been reported in association with several types of medications (bisphosphonates,139 NSAIDs,138 and potassium chloride), hot beverages, chemical irritants, heavy smoking, physical trauma, celiac disease,140 collagen vascular disorders, and autoimmune bullous dermatoses (pemphigus and pemphigoid),139,141 the pathogenesis remains poorly understood. A recent report of 31 patients142 described a similar pattern of injury. In that study, the term sloughing esophagitis was used to describe patients whose biopsies showed prominent parakeratosis and superficial sloughing of necrotic squamous epithelium. Endoscopy revealed plaques or membranes. The affected patients were predominantly older (range, 32 to 85 years), were chronically debilitated, and consumed multiple medications.
Pathology
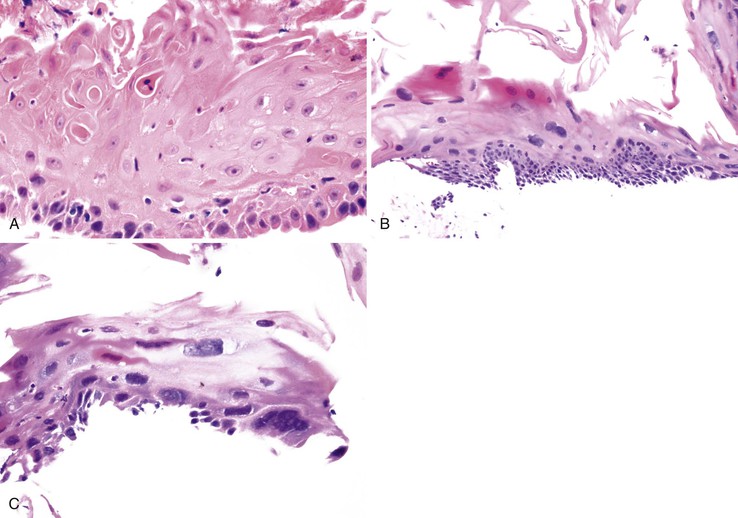
Endoscopic features of EDS may include single or multiple white patches of peeling mucosa, extending from the middle to the distal esophagus, or even diffuse sloughing of the entire esophageal mucosa.138 In addition, long linear mucosal breaks, vertical fissures, and circumferential cracks with peeling mucosa, with or without bleeding, have been described. Histologically, at low power, the biopsy samples consist of long, detached fragments of superficial squamous epithelium with some degree of intraepithelial splitting at varying levels above the basal layer of the squamous epithelium, occasionally associated with separation of the epithelial layers to form bullae.138 Other common findings include prominent parakeratosis, orthokeratosis, and fragments of necrotic epithelium with minimal or no inflammation, often associated with bacterial or fungal colonization (Fig. 14.13). Neutrophils and eosinophils may be present within the epithelium, but these inflammatory cells are not a prominent feature of this condition.
Differential Diagnosis
The differential diagnosis of EDS includes mechanical trauma related to esophagoscopy, infectious esophagitis (fungal or herpetic), and chronic bullous diseases that involve the esophagus. Repeated attempts to obtain a biopsy, combined with endoscopic trauma, causes layers of squamous epithelium to detach from the mucosa, simulating bullae. Several cases have been reported to occur after traumatic esophagoscopy.143,144 However, epithelial detachment induced by endoscopic trauma does not reveal necrosis, inflammation, or bacterial colonies on histologic examination.
Inflammation and necrosis are common features of infectious esophagitis, but prominent orthokeratosis and parakeratosis are not. Vesiculobullous dermatoses such as Stevens-Johnson syndrome, pemphigus, and pemphigoid may mimic EDS. However, chronic bullous diseases can be excluded by demonstration of an absence of complement and immunoglobulin deposits by direct immunofluorescence (DIF), lack of anti-desmoglein antibodies by enzyme-linked immunosorbent assay (ELISA),145 and lack of corresponding cutaneous and oropharyngeal lesions. Clinically, patients with EDS show a poor response to steroid therapy.
Treatment
Despite its sometimes dramatic presentation, EDS in most patients has a positive outcome. A combination of acid suppression, topical analgesics, and discontinuation of any potential precipitating medications results in healing of EDS without sequelae in most cases. Because of the superficial nature of the epithelial injury in this disease, it is presumed that stricture development is rare.
Primary Eosinophilic Esophagitis
Clinical Features
EOE is an allergic disorder that involves the esophagus. During the past 10 to 15 years, this disease has gained considerable recognition and has shown an increasing prevalence. The current definition of this disease is “chronic, immune/antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.”146,147 This condition affects both children and adults. Infants and toddlers often are brought in because of feeding difficulties, whereas school-aged children typically have nausea, vomiting, and pain. Dysphagia is the predominant symptom in young adults. Other symptoms in adult patients include solid-food dysphagia, chest pain, food impaction, and upper abdominal pain. By definition, patients have normal or near-normal pH monitoring levels and fail to respond to antireflux therapy, although a subgroup of patients do respond to PPIs, so the association with reflux is controversial. EOE tends to occur in children and young adults, with a strong male predominance (male-to-female ratio, 3 : 1), but cases are being increasingly diagnosed across the entire age spectrum.37,148–151 Many affected patients have a history of concurrent allergic diatheses (food allergy, asthma, eczema, chronic rhinitis, and environmental allergies). Between 28% and 86% of adults and between 42% and 92% of pediatric patients have been shown to have other allergic diseases.152–155 Several studies have documented peripheral blood eosinophilia in adults and pediatric patients with EOE.156–158
Pathogenesis
The pathogenesis of EOE is poorly understood and is the subject of intense ongoing research. The prevailing belief is that EOE represents a disease of type 2 (Th2) helper T cells in which genetic predisposition, environmental exposures, allergic background (including inhaled aeroallergens and ingested food allergens), and probably other factors may act as triggers.159–161 Basic murine studies and studies of human samples have proposed important roles for mast cells, lymphocytes, eotaxin 3, IL13, IL15, and thymic stromal lymphopoietin (TSLP) in the pathogenesis of EOE.162–164 TSLP is located on the 5q22 locus and is overexpressed in the mucosa of patients with EOE. TSLP is an epithelial-derived IL7-like cytokine that can activate a number of immune cells, in particular dendritic and mast cells, and plays a role in other allergic diseases such as asthma. Eotaxin–3 (now called CCL26), a gene that encodes a component of the immunologic cascade, has been shown to be highly induced in patients with EOE compared with healthy controls. Eosinophils are recruited to the esophagus in response to injurious stimuli.162 Eosinophil activation by IL5, IL13, and eotaxin 3 results in extracellular release of cytotoxic granules. The resulting inflammatory response leads to the pathologic changes observed in the mucosa, submucosal fibrosis, remodelling, and esophageal strictures.
Pathology
Gross (Endoscopic) Features
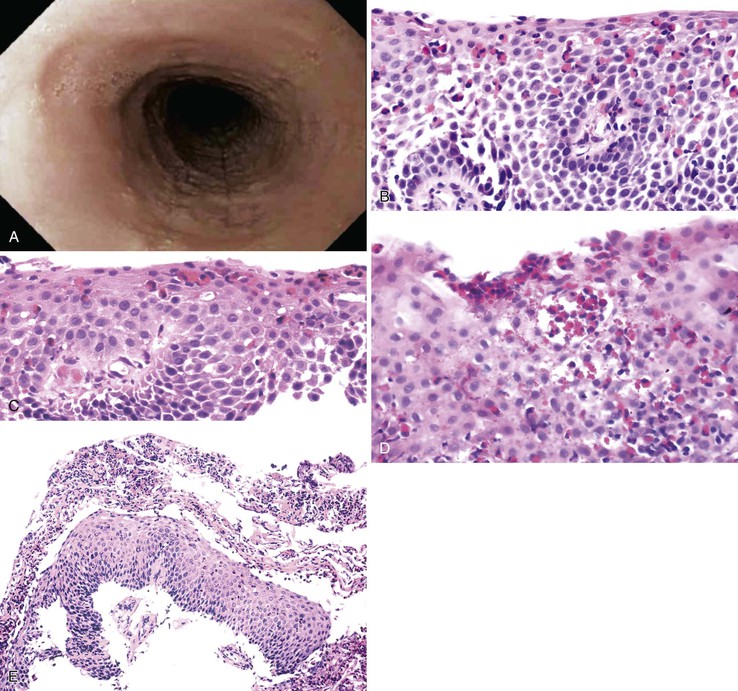
Endoscopically, patients with EOE often exhibit characteristic fixed esophageal rings, also referred to as trachealization or ringed esophagus.37,38,148,149 Other endoscopic findings include transient esophageal rings (also known as feline folds or felinization), mucosal plaques or exudates, longitudinal furrows, edema, diffuse esophageal narrowing, narrow-caliber esophagus, and mucosal tears readily induced by passage of the endoscope (Table 14.3 and Fig. 14.14, A).165,166 In some cases, the esophagus appears entirely normal, but more than 90% of affected patients display one or more of these endoscopic abnormalities.167 However, these endoscopic features have been described in other conditions and are not pathognomonic of EOE.
Table 14.3
Diagnostic Approach to Patients with Suspected Eosinophilic Esophagitis
| Clinical |
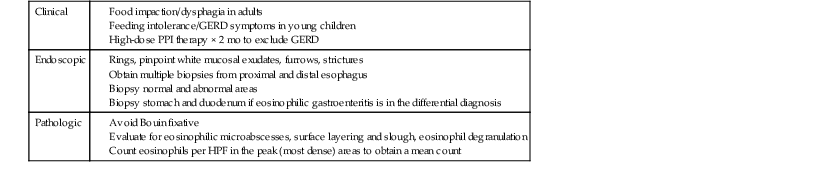
GERD, Gastroesophageal reflux disease; HPF, high-power field; PPI, proton pump inhibitor.
Data from Furuta GT. Eosinophilic esophagitis: update on clinicopathological manifestations and pathophysiology. Curr Opin Gastroenterol. 2011;27:383-388.
Microscopic Features
Histologically, most of the features of EOE overlap with those of GERD, particularly in distal esophageal biopsies. Although biopsies of patients with EOE tend to reveal more numerous intraepithelial eosinophils, typically in the range of 15 or more per high-power field, severe GERD produces pronounced eosinophilia in this range in only a minority of cases. Because intraepithelial eosinophils in both conditions may be patchy in distribution, sampling error can also affect one’s ability to establish a correct diagnosis.
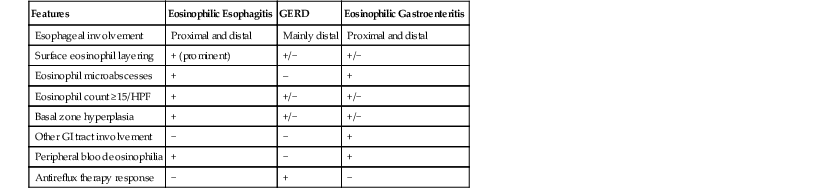
Histologic features of EOE can be divided into major and minor attributes.168 Major histologic features are considered characteristic and necessary to establish a diagnosis, but are not pathognomonic. They include (1) increased intraepithelial eosinophils (≥15/HPF) obtained from the most densely populated areas (peak density), (2) eosinophilic microabscesses (defined as a collection of four or more eosinophils within the epithelium), (3) surface layering of eosinophils (i.e., affiliation of eosinophils to occupy the outer layer of the squamous epithelium), (4) surface sloughing of squamous cells mixed with abundant eosinophils, and (5) extracellular eosinophilic granules (deposition of these proteins, including eosinophil peroxidase and eosinophil derived neurotoxin, indicates degranulation). The distribution of disease is important, because EOE often involves long segments of the esophagus, may be patchy or focal, and typically involves the proximal or middle esophagus and the distal esophagus or GEJ equally. In contrast, patients with GERD typically have higher eosinophil counts in the distal esophagus—an area where reflux affects the esophagus more severely—than in the proximal esophagus (Table 14.4; see Fig. 14.14).168–170
Table 14.4
Differential Diagnosis of Esophageal Eosinophilia
| Features | Eosinophilic Esophagitis | GERD | Eosinophilic Gastroenteritis |
| Esophageal involvement | Proximal and distal | Mainly distal | Proximal and distal |
| Surface eosinophil layering | + (prominent) | +/− | +/− |
| Eosinophil microabscesses | + | – | + |
| Eosinophil count ≥15/HPF | + | +/− | +/− |
| Basal zone hyperplasia | + | +/− | +/− |
| Other GI tract involvement | − | − | + |
| Peripheral blood eosinophilia | + | − | + |
| Antireflux therapy response | − | + | − |
GERD, Gastrointestinal reflux disease; GI, gastrointestinal; HPF, high-power field.
Minor histologic features are often helpful but are nonspecific and occur in a wider variety of disorders than the major features do. The minor features include (1) marked basal zone hyperplasia (usually >20% of the epithelial thickness), (2) lengthening of the lamina propria papillae (often greater than two thirds of the epithelial thickness), (3) increased lamina propria fibrosis and chronic inflammation, (4) increased intercellular edema, and (5) increased intraepithelial lymphocytes and mast cells. Influx of neutrophils and the development of ulcers or erosions are unusual in EOE unless complicated by another unrelated disorder such as GERD, pill-induced esophagitis, or infection.168 EOE is best evaluated in biopsy specimens obtained after 2 months of anti-GERD therapy. Ultimately, the top-line diagnosis should be nonspecific, and it is helpful to include a note as well. For example: “Active esophagitis characterized by abundant eosinophils (>15/HPF). Note that these findings are consistent with EOE in the appropriate clinical and endoscopic setting.”
Differential Diagnosis
Mucosal infiltration by eosinophils is a component of a variety of esophageal inflammatory conditions (Box 14.1), including GERD, EOE, eosinophilic gastroenteritis, Crohn’s disease, collagen vascular diseases, infectious esophagitis (secondary to herpes, Candida, or parasites), drug-induced esophagitis, and hypereosinophilic syndrome. The histologic features of GERD and EOE overlap (see Table 14.4). Biopsy sampling of the proximal, middle, and distal esophagus is important to help separate these two disorders: GERD involves the distal esophagus more severely than the proximal esophagus, and the lesions are not usually patchy or segmental in distribution. In parasitic and fungal infections, eosinophils aggregate intensively in areas of infection, even forming abscesses in the lamina propria, and are often associated with neutrophils. The plaques and exudates observed in patients with EOE may suggest Candida esophagitis endoscopically, but on microscopy, the plaques in EOE consist of sloughed squamous cells admixed with eosinophils, which are easily distinguished from the fungal elements characteristic of Candida esophagitis.
Eosinophilic gastroenteritis is characterized by tissue eosinophilia that is often patchy in distribution and may involve any portion, and any layer, of the GI tract (i.e., mucosa, muscularis propria, and serosa). Mucosal biopsies in patients with eosinophilic gastroenteritis often show numerous eosinophils, frequently degranulated, infiltrating the lamina propria, muscularis mucosae, and epithelium and usually involve one or more anatomic locations such as the esophagus, stomach, and duodenum. Therefore, obtaining biopsy specimens from the stomach and duodenum and correlating the findings with clinical symptoms such as nausea, vomiting, and diarrhea is helpful in establishing a correct diagnosis. Unlike patients with EOE, most of those with eosinophilic gastroenteritis have increased serum total and food-specific immunoglobulin E (IgE) levels and positive skin test responses to a variety of food antigens.
Esophageal involvement by Crohn’s disease can reveal nonspecific esophagitis, sometimes associated with increased numbers of eosinophils. The presence of non-necrotizing granulomas is a helpful diagnostic feature, but granulomas are relatively uncommon, observed in only 7% to 9% of patients with esophageal Crohn’s disease.171 Crohn’s disease never involves the esophagus in the absence of stomach, small intestine, or colon involvement, and other features such as erosions, sinuses, fistulas, neutrophils, and increased lymphoid tissue are often present in addition to increased eosinophils.
Drug-induced esophagitis can manifest with eosinophilia. It is helpful to establish a temporal clinicopathologic association between use of the drug, onset of symptoms, and tissue eosinophilia in determining that diagnosis. Resolution is often demonstrated when the drug is withdrawn from use.
Vasculitides, including Churg-Strauss syndrome and polyarteritis nodosa, affect the GI tract in approximately 20% to 30% of cases. Eosinophil-rich granulomas, with necrosis, involving medium to small-sized vessels, are typically found in Churg-Strauss syndrome, whereas arteritis in a background of eosinophilic inflammation is typical of polyarteritis nodosa.172
Natural History
There are limited data regarding the natural history of EOE. However, EOE often leads to persistent dysphagia if left untreated. In one study of 30 untreated patients who were observed for an average of 7.2 years, dysphagia persisted in 29 patients (97%).173 Successfully treated patients experience relapse in 25% to 40% of cases. The long-term outcome for patients with EOE who receive treatment, and the proportion of patients who require multiple courses of treatment, are unknown.174 To date, no malignant potential has been associated with this disease. Few reports have suggested an association with BE,175,176 but a recent study of a national pathology database suggests that there is a strong inverse relationship between Barrett’s metaplasia and eosinophilic infiltrates in the esophageal mucosa. In that study, the observed prevalence rate of the simultaneous occurrence of these two conditions was one third of what would be expected if they occurred independently (odds ratio, 0.29; 95% confidence interval, 0.27 to 0.33; P < .0001).177
Treatment
Several types of therapeutic modalities are available for patients with EOE. The efficacy of any particular treatment is best evaluated clinically by showing relief of symptoms, or a reduction in the degree of eosinophilic infiltration, or both. Currently, therapy for esophageal inflammation is based on antigen elimination trials, antiinflammatory medications, and physical dilatation if strictures are present. Dietary elimination is a treatment modality frequently used in children, but it is often not well tolerated by adults. Corticosteroids, either systemic or topical, have been effective in the treatment of this disorder in both children and adults.178 Systemic steroids are used for acute exacerbations, such as severe dysphagia, hospitalization, and weight loss. Topical glucocorticoids are used to provide long-term control. Glucocorticoids show a significant positive effect in reducing esophageal eosinophilia. Before 2007, swallowed fluticasone was primarily used.179,180 Since then, oral budesonide has also been shown to be effective.181,182 Treatment of EOE with cromolyn sodium, leukotriene receptor antagonists, or immunosuppressive agents is not recommended.147 Humanized antibody therapy directed to block IL5 and IL13 is currently being tested in clinical trials. Esophageal dilatation is performed for adult patients whose presenting symptoms include symptomatic esophageal narrowing resulting from fixed strictures. However, this procedure usually needs to be repeated regularly and does not reduce the degree of inflammation.183
Lymphocytic Esophagitis
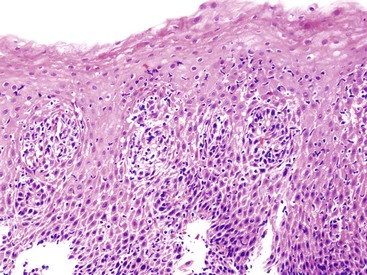
Lymphocytic esophagitis was first recognized in 2006. In the original description of 20 cases, Rubio and associates184 emphasized the occurrence of high numbers of CD3+, CD4+, and CD8+ lymphocytes in peripapillary squamous epithelium in the absence of granulocytes (neutrophils and eosinophils). Spongiosis was frequently detected as well. Eleven of the patients were children, seven of whom had Crohn’s disease. Purdy and colleagues185 further characterized this entity in 42 patients with increased intraepithelial lymphocytes in the esophagus. In their study, no significant associations with clinical features was detected. In a recent report by Haque and co-workers,186 lymphocytic esophagitis, defined histologically as the presence of increased lymphocytes in the peripapillary esophageal mucosa associated with marked spongiosis in the absence of neutrophils and eosinophils, was detected in approximately 0.1% of an endoscopic population of patients during an 18-month period (Fig. 14.15). Of the 119 study cases, more than two thirds of patients had symptoms of dysphagia or odynophagia that elicited clinical suspicion for EOE or GERD. Endoscopically, findings suggestive of EOE, including rings, furrows, and white plaques, were reported. Strictures were reported in one third of the patients. As in other studies,184,185,187,188 lymphocytic esophagitis was associated with a variety of clinical conditions, including H. pylori gastritis, celiac disease, duodenal lymphocytosis, and Crohn’s disease. Although the clinical significance of lymphocytic esophagitis remains to be defined, evidence suggests that this condition probably represents injury by a variety of potential insults, although an autoimmune etiology has been postulated in some cases.
Mechanical Causes of Esophagitis
Achalasia
Clinical Features
Achalasia is a chronic esophageal motility disorder that is characterized by an inability of the LES to relax after swallowing, which results in periodic esophageal obstruction. The symptoms of achalasia may appear gradually. Patients present initially with a history of dysphagia to solid foods, and later on with difficulty swallowing liquids. Often there is a history of weight loss, regurgitation of undigested food, and avoidance of certain solid or bulky foods. Half of the patients complain of chest pain or heartburn.189 Discomfort or fullness under the breastbone and a history of aspiration or aspiration pneumonia may be elicited as well.
As much as 95% of the patients with achalasia have a positive result on esophagography with barium contrast.190 A positive study shows characteristic tapering of the distal esophagus and the so-called “bird’s beak” sign at the level of the esophageal hiatus. Esophageal endoscopy is typically performed to evaluate for other causes of esophageal obstruction.
Pathogenesis
The pathophysiology of achalasia is linked to destruction of ganglion cells present in the esophageal wall and LES, which leads to an impairment of relaxation of the LES. Destruction of ganglion cells is associated with an inflammatory (particularly lymphocytic) response, which seems to suggest an autoimmune, viral, or chronic degenerative process.191 In a minority of patients with Allgrove syndrome, a mutation on chromosome 12 is implicated in the development of achalasia. Secondary achalasia (or pseudoachalasia) may develop as a result of obstructing lesions, such as GEJ tumors, Chagas disease, or amyloidosis, and closely mimics primary achalasia clinically. In addition, functional obstruction of the LES may be induced by a fundoplication or by gastric banding procedures.
Pathology
The primary histologic abnormality in achalasia is a marked reduction, or complete absence, of myenteric ganglion cells (Fig. 14.16).42,192 Myenteric nerve inflammation, with a predominance of T lymphocytes, and myenteric neural fibrosis are present.42,193,194 Other alterations include diffuse squamous hyperplasia, florid lymphocytic esophagitis, lymphocytic inflammation of the lamina propria and submucosa, and submucosal glandular atrophy with periductal and glandular inflammation. Mucosal changes in patients with achalasia are variable. In a series of 48 untreated patients, 10 had changes suggestive of GERD,195 whereas others had normal mucosa.196 The principal role of pretherapy biopsies is to rule out all potential causes of pseudoachalasia, including neoplastic alterations or infections.
Treatment
Management of achalasia includes several therapeutic modalities. Pharmacologic management with sublingual nitrates and calcium channel blockers has a high failure rate and is poorly tolerated.197 Injection of the LES with botulinum toxin is effective for short-term relief of dysphagia.198,199 Dilatation remains a valuable option for nonsurgical candidates. Minimally invasive Heller myotomy has low rates of morbidity and mortality; both it and laparoscopic Heller myotomy with partial fundoplication are durable, safe, and effective treatment options for patients with achalasia.200–202
Muscular Dystrophy
Some forms of muscular dystrophy (myotonic form) are associated with esophageal dysmotility and, as such, may be associated with a nonspecific esophagitis (see Chapter 7).
Mallory-Weiss Tears and Other Traumatic Esophageal Disorders
Esophageal perforations, either full thickness (Boerhaave syndrome) or partial thickness (Mallory-Weiss tears), and hematomas are manifestations of spontaneous and iatrogenic traumatic esophageal disease.203
Mallory-Weiss tears are mucosal lacerations in the distal esophagus and proximal stomach. Histologically, longitudinal tears of the mucosa extend into the submucosa but do not involve the muscularis propria. These lacerations are accompanied by acute hemorrhage with organizing fibrin, with or without accompanying neutrophilic inflammation. The prevalence of tears among patients with upper GI bleeding is approximately 5%.204 Precipitating factors include retching, vomiting, straining, coughing, blunt abdominal trauma, and cardiopulmonary resuscitation. Between 40% to 80% of patients have a history of heavy alcohol use leading to vomiting.204,205 The presence of hiatal hernia is also considered a predisposing factor and is found in 35% to 100% of patients.206 It has been proposed that, in patients with a hiatus hernia, a higher pressure gradient develops in the hernia sac compared with the rest of the stomach during retching, increasing the potential for mucosal laceration.203 Others consider a sudden increase in intraabdominal pressure to be the causative factor. The prognosis of Mallory-Weiss tears is generally good. Bleeding from these lesions stops spontaneously in 80% to 90% of cases without therapy. With conservative therapy, the lesions heal within 48 to 72 hours.
Boerhaave syndrome, or postemetic rupture of the esophagus, results from a sudden increase in intraluminal esophageal pressure due to vomiting. In the modern medical context, instrumentation of the esophagus represents the most common cause.207,208 Clinically, patients experience sudden-onset severe chest pain in the lower thorax and upper abdomen after repeated episodes of retching or vomiting. Often, they have fever or pain after forceful vomiting, esophageal instrumentation, or chest trauma. Typically, acute upper GI bleeding is not seen after esophageal rupture, which helps distinguish it from the more common Mallory-Weiss tears. The most common location of the rupture is at the posterolateral wall of the lower third of the esophagus, 2 to 3 cm proximal to the GEJ.209,210 Contrast esophagography using a water-soluble agent initially, followed by a barium study if the initial result is negative, represents the most reliable test for documenting the presence and location of the perforation.211 The initial phase of therapy includes physiologic monitoring, limiting the extent of ongoing mediastinal contamination, cessation of oral intake, and administration of broad-spectrum antibiotics. An overwhelming majority of patients require some type of surgical intervention that depends on the location, extent, and cause of the injury.
Congenital and Acquired Deformations
Esophageal Rings and Webs
Structural esophageal abnormalities include esophageal rings and webs. Although many patients are asymptomatic, some experience dysphagia, regurgitation, aspiration, or other symptoms of esophagitis.212
An esophageal ring is defined as a concentric, thin (2 to 5 mm) diaphragm of tissue located in the distal esophagus. A Schatzki ring most commonly develops at the squamocolumnar junction (SCJ) at the proximal border of a hiatal hernia, but rings may occur anywhere in the esophagus. The prevalence of esophageal rings is unknown, because most are asymptomatic. Based on data from barium studies, these lesions are present in approximately 6% to 14% of endoscopic examinations.212 Microscopically, Schatzki rings are composed of both mucosa and submucosa, with basal cell hyperplasia, hyperkeratosis, and, often, eosinophil infiltration. Cases with prominent eosinophils most likely represent a subtype of primary EOE (see earlier discussion).
Esophageal webs are defined as eccentric, thin (<2 mm) membranes of tissue in the esophagus, but they are most common in the proximal region. Because most cases are asymptomatic, the prevalence of this condition is unknown. Esophageal webs that occur in association with iron deficiency anemia, glossitis, and koilonychia are referred to as Plummer-Vinson syndrome in the United States and Paterson–Brown Kelly syndrome in the United Kingdom.213 The latter condition is most common in white women and usually responds to treatment of the patient’s underlying iron deficiency state.212,213 The cause of esophageal webs is unknown, but they are associated with an array of conditions, including cutaneous blistering disorders (e.g., epidermolysis bullosa, cicatricial pemphigoid), Zenker diverticula, esophageal duplication, and cysts.
Esophageal Diverticula
Esophageal diverticula occur most commonly in elderly men and consist of full-thickness outpouchings involving all layers of the esophageal wall, usually in the cervical esophagus, where they are termed Zenker diverticula or pharyngeal pouches214 (see Chapter 8). Therapy usually consists of myotomy rather than diverticulectomy.215 Midesophageal diverticula are termed epiphrenic diverticula and may be related to esophageal motility disorder.216
Esophageal Pseudodiverticulosis
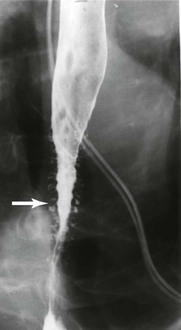
Esophageal pseudodiverticulosis is an uncommon benign condition that occurs potentially in all age groups but typically in the elderly, with a slight male predominance. The condition usually manifests with dysphagia, which is typically not severe but intermittent or slowly progressive. There is a high incidence of esophageal narrowing, usually in the upper third of the esophagus; in many cases, this may mimic carcinoma. In barium studies, multiple small (1 to 4 mm) flask- or collar stud–shaped outpouchings are present in the esophageal wall. Radiology offers the most sensitive method of diagnosis (Fig. 14.17). Endoscopy is not helpful for diagnosis because the orifices of the pseudodiverticula are difficult to recognize and most often only nonspecific mucosal inflammatory changes are seen. In addition, mucosal biopsies usually are not helpful, because the pseudodiverticula are deep in the submucosa.217
Histologically, as the name implies, outpouchings do not represent true diverticula; instead, they consist of dilated and inflamed submucosal esophageal gland ducts. Pseudodiverticula are confined to the submucosa of the esophagus and are lined by cuboidal to stratified squamous epithelium. Often, the squamous epithelium replaces the submucosal glands and duct epithelium entirely, with preservation of the normal round and smooth configuration of the glands. A prominent inflammatory infiltrate composed of lymphocytes, eosinophils, and plasma cells typically surrounds the pseudodiverticula (see Chapter 24). The lack of an infiltrative growth pattern and lack of squamous dysplasia help distinguish pseudodiverticula from invasive squamous cell carcinoma. The cause of this disorder is unclear. Postulated theories of pathogenesis include glandular secretory dysfunction, esophageal dysmotility, and chronic recurrent esophagitis.212,218
Treatment is directed at relieving esophageal obstruction, if any, and dealing with the underlying inflammatory condition. Long-term follow-up studies indicate that the condition may remain stable for long periods.219,220
Esophageal Involvement in Systemic Disease
Eosinophilic Gastroenteritis
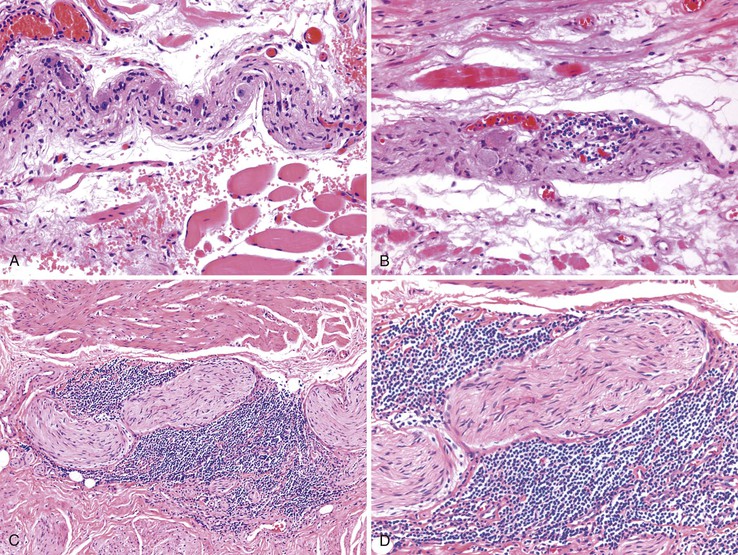
Eosinophilic gastroenteritis is a condition that is characterized by patchy or diffuse eosinophilic infiltration to variable depths (mucosa, muscularis propria, and serosa) at one or more sites in the GI tract. The mucosal subtype is the most common (25% to 100%), perhaps because of its accessibility to diagnosis by routine endoscopy and biopsies.221 The disease selectively involves the stomach (26% to 100%) and small intestine (28% to 100%),222–225 but involvement of the esophagus has been reported in some cases.222,223 Mucosal biopsy specimens from patients with eosinophilic gastroenteritis often show numerous eosinophils, frequently degranulated, infiltrating the lamina propria, muscularis mucosae, and epithelium; the lesions usually involve one or more sites of the esophagus, stomach, and duodenum. Therefore, in establishing a correct diagnosis, it is helpful to obtain biopsies from the stomach and duodenum and correlate the findings with clinical symptoms of generalized mucosal involvement, such as nausea, vomiting, diarrhea, weight loss, and protein-losing enteropathy. Unlike patients with EOE, the majority of those with eosinophilic gastroenteritis have increased serum total and food-specific IgE levels and positive skin test responses to a variety of food antigens.172,221
Esophageal Involvement in Collagen Vascular Disorders (Including Scleroderma)
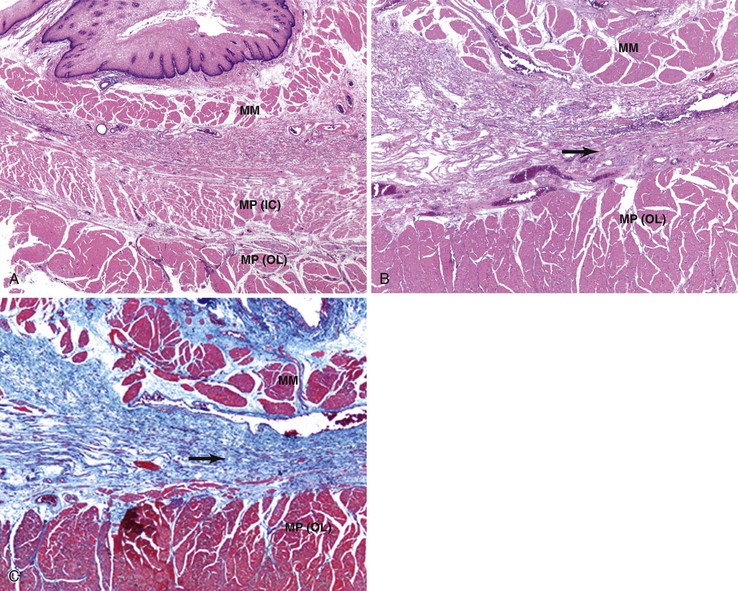
Collagen vascular disorders, including progressive systemic sclerosis (scleroderma),226 systemic lupus erythematosus,227 rheumatoid arthritis,228 mixed connective tissue disorders, polymyositis, dermatomyositis,229 and Sjögren syndrome,230 may involve the esophagus.231 Common esophageal manifestations in these conditions include myoneuroenteric dysmotility, esophagitis secondary to reflux, drug-induced esophagitis, and opportunistic infections. Esophageal pathology may be related to the underlying collagen vascular disease, the associated inflammatory conditions, or the side effects of immunosuppressive or other types of drug therapy. Scleroderma has been associated with an increased risk of BE and its neoplastic complications.232 In a series of 63 patients with scleroderma referred for upper GI symptoms at the Mayo Clinic, GERD was documented histologically in 53%, strictures in 29%, and BE in 16%.233 A similar series from the University of Pennsylvania detected BE in 37% of patients with scleroderma, including two with adenocarcinoma.234 The mucosal changes are a result of esophageal hypomotility and aperistalsis, with incompetence of the LES.235 Atrophy and fibrosis predominate in the inner circular layer of the muscularis propria in this condition (Fig. 14.18).
Esophageal Manifestations of Dermatologic Diseases
The esophagus may be affected in many different types of primary dermatologic conditions, including drug-induced diseases such as Stevens-Johnson syndrome236 and bullous diseases such as bullous pemphigoid, benign mucous membrane pemphigoid, epidermolysis bullosa acquisita, pemphigus vulgaris, and lichen planus237–239 (see Chapter 6). Esophageal disease may develop in the presence or absence of skin lesions.
Pemphigus vulgaris is the most common of the autoimmune mucocutaneous diseases characterized by bulla formation. This condition occurs predominantly in middle-aged to elderly patients of Jewish or Mediterranean descent. One report suggested that the majority of patients with pemphigus vulgaris who undergo endoscopy with biopsy show evidence of esophageal involvement.240 This disorder is caused by loss of integrity of the normal intercellular attachments within the epidermis and mucosal epithelium,241 which results in the development of flaccid bullae of various sizes. Histologically, intraepithelial blisters are caused by loss of intercellular attachments (acantholysis). For instance, the basal cells may appear separate from one another but remain attached to the basement membrane. Hence, the diagnostic hallmark of pemphigus vulgaris is acantholysis with bulla formation in the suprabasal region combined with a “row of tombstone-like” basal cells.242 A mild superficial mixed inflammatory infiltrate, which includes eosinophils both within and surrounding the bullae and in the lamina propria, often accompanies blister formation. The diagnosis is confirmed by DIF, which shows intercellular IgG and complement C3 deposits. ELISA to detect anti-desmoglein 1 and 3 antibodies may be a simpler and more quantifiable method than immunofluorescence.145
Bullous pemphigoid is a chronic autoimmune subepidermal bullous disease that affects the skin and sometimes the mucous membranes.243 IgG autoantibodies bind to the basement membrane, thereby activating complement and inflammatory mediators; this leads to the release of proteases, degradation of hemidesmosomal proteins, and blister formation. Eosinophils are characteristically identified, although their presence is not necessary to establish a definite diagnosis. DIF helps demonstrate the presence of in situ deposition of complement components (typically C3) and linear deposits of IgG at the level of the basement membrane of the esophagus.243
The term epidermolysis bullosa refers to a group of inherited disorders caused by mutations of genes that encode for structural proteins located at the dermal-epidermal junction. The disorder is characterized by the formation of blisters after minor trauma.244 Trauma from boluses of food may lead to bullae formation, ulceration, and scarring of the esophageal mucosa with the formation of webs, strictures, and stenoses, most commonly in the proximal esophagus. Esophageal lesions are similar to those in the skin, revealing subepithelial blisters formed by the separation of the esophageal squamous epithelium, with degenerated basal cells from the lamina propria in a clean plane of cleavage without significant inflammatory response. Subsequent ulceration and granulation tissue formation may eventually lead to the development of strictures and webs.
Graft-versus-Host Disease Involving the Esophagus
Esophageal graft-versus-host disease (GVHD) may manifest acutely; it can cause bullous disease and complete sloughing of the esophageal mucosa with the formation of an esophageal cast245–248 or only nonspecific esophageal ulcers, erythema, and edema. The histologic features of esophageal GVHD are similar to those in other parts of the GI tract. Apoptosis and individual cell damage, manifested in squamous mucosa as dyskeratotic keratinocytes, are typically prominent features in combination with a lichenoid interface inflammatory infiltrate (Fig. 14.19). Ulceration and submucosal fibrosis reflect long-standing disease but are not specific features of esophageal GVHD. On occasion, severe clinical disease may show only minor focal changes on mucosal biopsies. Because of the focality of the lesions, many serial tissue sections are recommended to detect the diagnostic features of esophageal GVHD if they are not evident on initial sections.249
Chemotherapy- and Radiation-Induced Esophageal Injury
Esophageal injury is a common side effect of chemotherapy and radiation therapy.250,251 Esophagitis can occur as a complication of treatment for lung, esophageal, and mediastinal tumors and lymphomas. Acute radiation toxicity typically begins 2 to 3 weeks after therapy. Patients complain of dysphagia, odynophagia, chest pain, or a combination of these symptoms. Late effects of radiation therapy manifest 3 or more months after completion of therapy and include dysphagia, strictures, ulcers, and fistula formation.252,253 The histologic features are similar to those of chemoradiation-induced injury in other parts of the GI tract. Initially (within the first 48 hours), one encounters apoptotic bodies in the mucosal basal zone. Thereafter, and within the first 4 weeks after therapy, a nonspecific type of active esophagitis develops, often with the formation of erosions and ulcers. Bizarre epithelial and stromal cell cytologic atypia may be detected in the chronic phase of disease (Fig. 14.20). Although there are macrocytic changes in epithelial and stromal cells, the N : C ratios are typically well preserved, and mitotic figures are rarely present. Nuclear and cytoplasmic degeneration and multinucleation are common. Chronic radiation-induced cytologic atypia of stromal cells may persist indefinitely. Chronic vascular alterations include the development of sclerosis, intimal foam cell arteriopathy, and obliterative vasculitis. Submucosal fibrosis, mural scarring, and strictures also may complicate deep-seated chemoradiation-induced esophageal ulcers.
Crohn’s Disease
Crohn’s disease may involve the esophagus, but this is a rare occurrence, with a variable prevalence rate of 0.2% to 11%.254,255 Mucosal biopsies in Crohn’s disease demonstrate nonspecific inflammatory infiltrates which may be predominantly eosinophilic, may show only intraepithelial lymphocytes, or may be mainly neutrophilic. Granulomatous lesions are observed in 7% to 9% of patients with esophageal Crohn’s disease.171 The superficial nature of most mucosal biopsies may account for this relatively low incidence. Usually, a definite diagnosis is established only when characteristic histologic features (strictures, deep ulcers, transmural inflammation, granulomas, mural fibrosis) are identified in the esophagus of a patient who is known to have Crohn’s disease elsewhere in the GI tract (Fig. 14.21). Although some investigators have shown an association between lymphocytic esophagitis and Crohn’s disease,184 this observation has not been confirmed in other studies.
Esophageal Amyloidosis
The esophagus may be involved in systemic amyloidosis, which, in addition to deposition of amyloid, may result in a nonspecific form of esophagitis. In one series, as much as 72% of patients with GI amyloidosis were shown to have esophageal involvement.256 Amyloid is usually present in the deeper layers of the esophageal wall, and therefore is not usually detectable in superficial biopsy specimens. However, in some cases, the lamina propria and its associated blood vessels may show involvement as well (Fig. 14.22).