Inflammatory Disorders of the Appendix
Leona A. Doyle
Robert D. Odze
Normal Histology
In humans, the appendix is a vestigial organ. It has no proven significant physiologic function.
The vermiform appendix arises from the medial aspect of the cecum, inferior and posterior to the ileocecal orifice. It is approximately 8 cm long (range 2 to 20 cm) and 0.7 cm in diameter.1 The anatomic location of the appendix varies among individuals. It may be located behind the cecum and ascending colon (most common location), behind the ileum and mesentery, along the pericolic gutter, in the subhepatic region, or in the lesser pelvis. The appendix is maintained in its position by a fold of peritoneum that invests mesoappendiceal fat throughout the length of the appendix. The appendiceal artery is derived from the ileocolic artery, which is derived from the superior mesenteric artery, and it is located at the free edge of the peritoneal fold.
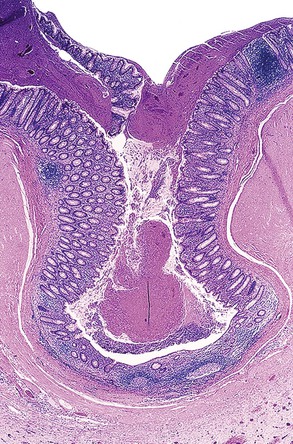
The layers of the appendix are similar to those in other portions of the large bowel, consisting of mucosa, submucosa, muscularis propria, and serosa. However, the mucosa has abundant, organized lymphoid tissue circumferentially arranged. It closely resembles mucosa from the terminal ileum, particularly in young individuals. The epithelium of the appendix contains goblet cells, absorptive cells, neuroendocrine cells (predominantly Kulchitsky type and basally located), and scattered Paneth cells.2 Unlike the colon, in which crypts are uniformly aligned, appendiceal crypts tend to be irregularly spaced and can be entirely absent in areas of mucosa adjacent to lymphoid aggregates.
In addition to lymphoid tissue, abundant immunoglobulin A (IgA)–secreting plasma cells are normally present in the lamina propria. The muscularis propria consists of an inner circular and outer longitudinal layer of smooth muscle, similar to other parts of the gastrointestinal (GI) tract. The appendix is enveloped by serosa up to the point of attachment of the mesoappendix, where the serosa envelops the mesoappendiceal fat up to the peritoneal fold. Under normal circumstances, neutrophils and eosinophils are absent from the mucosa and wall of the appendix.
Congenital, Developmental, and Acquired Anatomic Abnormalities
The appendix may exhibit a variety of anatomic abnormalities, including an atypical location,3 duplication,4,5 congenital absence,6 and luminal septal formation.7 An abnormally long appendix (>7 to 10 cm) has been linked to the development of torsion, although this complication has also been reported for appendices of normal length.8
Abnormal Location
The anatomic location of the appendix varies among individuals. The position of the appendix is determined mainly by changes in the position and shape of the cecum that occur during organ development, growth, and rotation. If the cecum does not descend fully, the appendix becomes located retroperitoneally in an ascending retrocecal position anterior to the right kidney. The frequency of a retrocecal location ranges from 26% to 65%.9,10 If the appendix is in a retrocecal position, it may be positioned intraperitoneally in a paracecal pouch of peritoneum or retroperitoneally with or without a paracecal fossa formed by the peritoneum.11
The clinical manifestations of acute appendicitis depend on the location of the appendix in the abdomen. If the appendix is located retrocecally, it may give rise to an abscess in the pararenal space, or infection may spread along the right paracolic gutter up to the right posterior subhepatic and right subphrenic spaces.12,13 More than 50% of patients with ascending retrocecal appendicitis have an atypical clinical presentation. They may have right upper quadrant pain or nonlocalizing abdominal pain instead of the more common presentation of central periumbilical pain followed by localization to the right lower quadrant, signs that are more often seen in cases of appendicitis when the appendix is in its normal anatomic location.14,15 Because of the atypical clinical presentation, retrocecal appendicitis is more likely to be diagnosed later in its course, resulting in a higher incidence of perforation and more serious complications.14 However, some studies have not shown an association between retrocecal location and perforation at the time of presentation.16
Duplication
Duplication of the vermiform appendix is rare. It occurs in approximately 1 of 25,000 patients (0.004%) who have undergone surgery for acute appendicitis.17 The clinical presentation depends on the location of the appendices in the colon.17,18 Cave and Wallbridge classified appendiceal septal formation as three types.19,20 Type A has incomplete duplication, with both appendices having a common base. Type B has complete duplication, with the first appendix arising from its usual location at the confluence of the teniae coli and the second located at various sites along the colon. Type C has complete duplication of the cecum, with each part having its own appendix.19,20
In one report, duplication of the appendix was associated with duplication of the colon in a 28-year-old man who was evaluated for an abdominal mass.21 He also had hydronephrosis and atrophy of the right kidney resulting from pressure atrophy from an inflammatory cecal mass.21
Absence and Atresia
Agenesis and atresia of the vermiform appendix are rare, occurring at an estimated frequency of 1 case per 100,000 resected appendices.6,22 Congenital absence should be diagnosed only after thorough examination of the entire ileocecal region to exclude the possibility of an abnormally located appendix.
Atresia of the appendix may be associated with atresia of the entire ileocecal region23 or with atresia of other sites in the small intestine.24 Congenital absence of the appendix has been associated with other congenital malformations, such as congenital diaphragmatic hernia.25 Acute appendicitis may occur in an atretic appendix, but the diagnosis of atresia is usually made only after pathologic examination of the resection specimen, because radiologic findings are often nonspecific and may be obscured by periappendiceal inflammation.26
Appendiceal Septa
The appendix can have complete or incomplete septa formation within the appendiceal lumen, a finding that is principally seen in children and young adults, who usually have acute appendicitis at clinical presentation.7 Possible contributing factors to the formation of septa include congenital abnormality, postinflammatory fusion of mucosal folds, and ischemia caused by thrombosed vessels.
Diverticular Disease of the Appendix
Diverticular disease of the appendix is rare. It has a reported incidence of 1 case (0.77% to 2%) per 50 to 130 appendectomies, or 1 case (0.004%) per 25,000 for true congenital diverticula.22,27 Diverticula may be congenital, in which case the muscularis propria represents a component of the diverticular wall, or it may be acquired, in which case the diverticulum results from increased intraluminal pressure and subsequent mucosal herniation through a defect or weak area of the muscularis propria, often at the site of a penetrating artery.
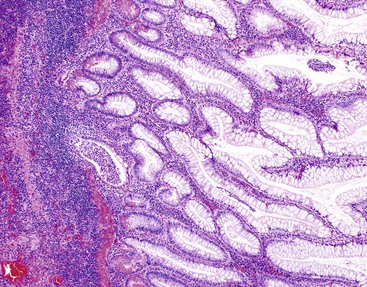
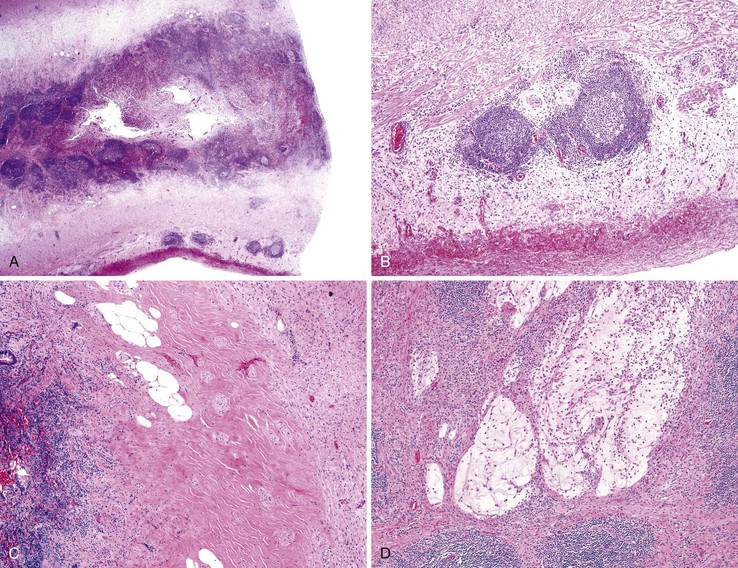
Acquired diverticula are more common than congenital diverticula. They occur most often in older adult males. Acquired diverticula are usually located in the distal third of the appendix (60%) on the mesenteric border and usually have a diameter of less than 5 mm.27 The incidence of acquired diverticula is greatly increased among patients with cystic fibrosis in whom mucosal secretions are markedly thickened. In this patient population, diverticular disease may be identified in as many as 22% of appendectomy specimens (Fig. 18.1).28
The disease may be asymptomatic or may manifest with acute or chronic pain, mimicking acute appendicitis.27,29 Computed tomography (CT) may help to visualize inflamed diverticula, which may appear as small, cystic outpouchings, but there is a high rate of false positives with this method of imaging.30 During the acute phase of illness, the incidence of perforation is approximately three times higher than that for patients with classic appendicitis (33% versus 10%).31 Because acquired diverticula lack a muscular wall, perforation is not surprising. The morbidity and mortality rates are greater than those for patients with classic acute appendicitis.27
Pathologically, the appendix may appear edematous, but diverticula may not necessarily be easily appreciated. If perforation has occurred, the serosa may appear dusky and have a yellow-tan exudate. Histologic findings include an outpouching of appendiceal epithelium through the muscularis propria of the appendix, with or without associated acute suppurative appendicitis, and chronic changes such as muscular hypertrophy and transmural, periappendiceal fibrosis, and lymphoid atrophy.29,32 In some cases, fibrous obliteration of the lumen may be seen.
Chronic changes suggest prior rupture or inflammation of a diverticulum. A ruptured appendiceal diverticulum can mimic a low-grade appendiceal mucinous neoplasm (LAMN) (see Chapter 28),29 as mucin extravasation onto the serosal surface or into periappendiceal tissue mimics mucin dissection of LAMN. Less commonly, detached fragments of benign-appearing epithelium may be associated with mucin as a result of perforated diverticula, mimicking localized pseudomyxoma peritonei. Occasionally, identification of diverticula is made grossly, but in most cases it is apparent only on microscopic examination of the tissue. In some instances, communication between the diverticulum and the appendiceal lumen is not seen in the original histologic plane of section, but it may become apparent after deep tissue sectioning.
Differentiation of hyperplastic or regenerative epithelial changes associated with diverticular disease from LAMN may be difficult. Mucosal regeneration shows architectural changes that are more pronounced in the superficial than in the basal portion of the mucosa. Gland serration, crypt disarray, and increased mucin-producing cells are seen in the upper half of the mucosa. The crypts are usually separated by lamina propria and show little or no crowding. In contrast, LAMN shows back-to-back crypts with minimal intervening lamina propria and slender, elongated, and filiform villi.29 In some cases, this distinction can be extremely difficult, and examination of the entire appendix is recommended. Preserved mucosal architecture, multiple diverticula, mucosal neuromas, and other chronic changes are clues to the diagnosis of diverticular disease rather than LAMN (Table 18.1).
Table 18.1
Features Differentiating Diverticular Disease from Low-Grade Mucinous Neoplasms of the Appendix
| Feature | Diverticular Disease | Low-Grade Mucinous Appendiceal Neoplasm |
| Extraappendiceal mucin | May be present Almost always acellular |
Frequently present May be cellular or acellular |
| Crypt architecture | Preserved | Crowded |
| Epithelial morphology | Bland, reactive hyperplastic changes | Dysplastic, usually low-grade |
| Filiform growth pattern | No | Yes |
| Acute inflammation | Usually present | May be present or absent |
| Mural fibrosis | Usually present | May be present (usually mild) or absent |
| Fibrous obliteration | Often present | May be present or absent |
| Muscular hypertrophy | Usually present | Not usually present |
| Diffuse pseudomyxoma peritonei | No | May be present or absent |
Fibrous Obliteration of the Appendiceal Lumen
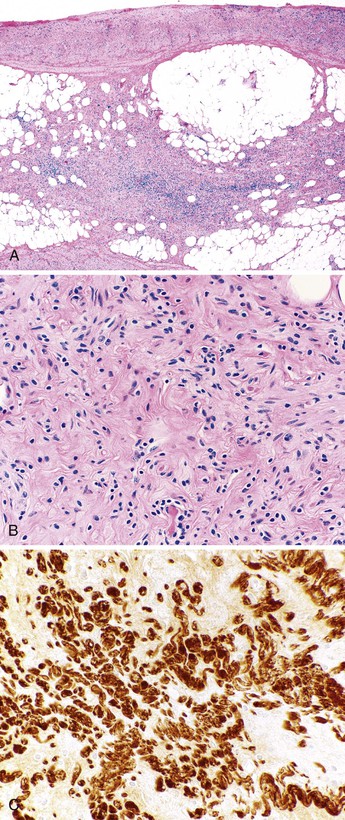
Obliteration of the appendiceal lumen (also known as neuroma or neural hyperplasia) by spindle cells located within a collagenous and myxoid background is seen in approximately one third of excised appendices. It is usually an incidental finding. The frequency of occurrence increases with patient age.
The tip of the appendix is usually affected, but the whole appendix may be progressively involved. Grossly, the appendix may appear narrow and white in areas of obliteration compared with adjacent normal appendix. Lesional cells include fibroblasts, Schwann cells, and axons. Admixed mast cells, eosinophils, and scattered endocrine cells may also be present. The infiltrate may be confined to the mucosa, but more commonly, it replaces the entire lumen and underlying crypts (Fig. 18.2).
Immunohistochemical staining shows a mixed population of S100 protein–reactive and neuron-specific enolase–reactive spindle cells corresponding to intermingled Schwann cells and axons, respectively. Admixed fibroblasts may be positive for CD34. The finding of lesions with a predominantly neural composition has led to the alternative designation of appendiceal neuroma.33 This phenomenon is thought to be a reactive process, either as a normal part of aging or as a response to prior acute appendicitis, with progressive phases of growth, involution, and fibrosis.33,34 The common occurrence of fibrous obliteration and neuromatous changes in appendices with ruptured diverticula supports the hypothesis that this phenomenon is a reactive process.29
Acute Appendicitis and Associated Disorders
Clinical Features and Pathogenesis
Acute appendicitis is predominantly a disease of children and young adults. It occurs in children and adolescents between 5 and 15 years old, although no age group is exempt from this condition.35,36 One crude estimate of the incidence of acute appendicitis in the United States is 11 cases per 10,000 population.37 Acute appendicitis is more common in Western countries than in Asia or Africa.
The pathogenesis of acute appendicitis is thought by some to reflect an initial insult to the mucosa resulting from luminal obstruction by a fecalith, a fragment of undigested food, lymphoid hyperplasia, or a tumor, followed by bacterial infection that progressively spreads outward from the mucosa and into and through the wall of the organ. However, the evidence for this mechanism is circumstantial at best. Some authorities believe that acute appendicitis represents one manifestation of a range of injuries that include hypersensitivity reactions, infections, and ischemic lesions. The potential causes of acute appendicitis are listed in Box 18.1.
The classic symptom triad of acute appendicitis consists of periumbilical pain, which eventually localizes to the right lower quadrant of the abdomen, accompanied by anorexia and nausea. Mild fever, leukocytosis, elevated C-reactive protein level, and right lower quadrant tenderness are usually present. If perforation has occurred, signs of peritonitis may be present. Common clinical mimics of acute appendicitis include mesenteric lymphadenitis (particularly in children), ovarian cysts, appendiceal or colonic diverticulitis, and Meckel diverticulitis.
Imaging methods, particularly CT, used to detect acute appendicitis have improved.38,39 Laparoscopic appendectomy has emerged as a relatively safe technique.40 CT findings suggesting acute appendicitis include distention, wall thickening and enhancement, periappendiceal fat stranding, cecal thickening, and free peritoneal fluid. Approximately 70% of patients suspected of having appendicitis by clinical or imaging methods are found to have acute appendicitis at the time of surgery.41,42 Some authorities believe that all appendices, even when grossly normal, should be removed when the indication for surgery was suspected acute appendicitis, because almost 20% of grossly normal-appearing appendices may contain acute inflammation on microscopic examination of the tissue.41,42 One possible exception is for patients who may require urologic surgery in the future, because their appendices may prove useful as a urinary conduit.43 Patients with appendicitis in the setting of human immunodeficiency virus (HIV) infection have a similar clinical presentation, although sometimes with a less striking elevation in the peripheral white blood cell count. In one surgical series of appendicitis in patients with HIV infection, delay before operation increased the likelihood of perforation.44
Pathologic Features
The appendix may appear grossly normal when inflammation is limited to the mucosa and submucosa. However, when inflammation extends into the muscularis propria, the appendix frequently becomes swollen and erythematous due to dilation of the serosal vessels. When the serosa is affected, the peritoneum is initially dull and gray, and a purulent exudate then develops. In approximately one third of cases, a fecalith is identified. Perforation from mural necrosis (i.e., gangrenous appendicitis) can follow, which may lead to abscess formation. Sometimes, an appendix resected in the clinical setting of acute appendicitis is grossly and histologically normal, even after submission of the complete specimen for histologic examination. In these cases, a specific cause is rarely found. Possible causes are listed in Box 18.2.
On microscopic examination, early lesions may reveal mucosal erosions and a neutrophilic infiltrate resulting in cryptitis and crypt abscesses (Fig. 18.3). Later, the inflammation extends into the submucosa and muscularis propria. Collections of neutrophils may be seen in the lumen. However, luminal neutrophils alone are not sufficient for a diagnosis of acute appendicitis. When inflammation extensively damages the muscularis propria, mural necrosis can lead to perforation (see Fig. 18.3). Thrombosed vessels may be seen. When periappendiceal inflammation occurs in the absence of mural involvement, other causes of peritonitis should be sought clinically (see “Periappendicitis”). Anaerobic bacteria are detected in approximately 50% of cases, but they may represent secondary colonization rather than being the primary cause of appendicitis.45
The morphologic differential diagnosis, at least for very early lesions, includes infectious gastroenteritis and trauma from fecaliths, both of which may result in mild superficial neutrophilic inflammation. If inflammation is limited to the mucosa, additional sections may reveal mural inflammation, supporting a diagnosis of acute appendicitis. The differential diagnosis also includes inflammatory bowel disease involving the appendix (discussed later).
Natural History and Outcomes
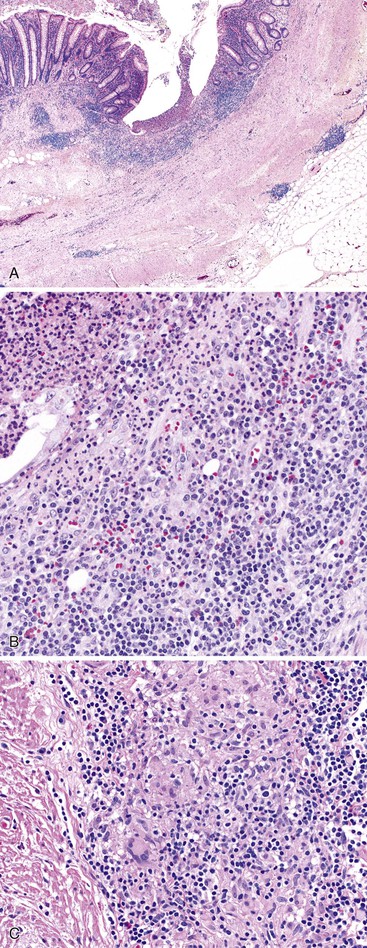
Two basic patterns of progression may occur in patients with acute appendicitis.46,47 In the first and more common pattern, there is a mixed inflammatory infiltrate ranging from patchy and mild, to diffuse and transmural. In some appendices, there may be intramural or serosal foreign body–type giant cells surrounded by granulation tissue, suggesting prior rupture. Serositis, fibrous adhesions, and prominent submucosal fibrosis can occur. Mucin extravasation is often seen. A second pattern, which has been called xanthogranulomatous appendicitis, consists of an infiltrate of foam cells and multinucleate histiocytes, with hemosiderin deposition, luminal obliteration, and sparing of lymphoid follicles (Fig. 18.4). This reaction pattern shares features with Crohn’s disease (CD) but lacks epithelioid granulomas, has fewer lymphoid aggregates, and has less subserosal fibrosis. However, in one study, a patient exhibiting this pattern of inflammation was found to have CD on follow-up.48 Careful clinical correlation is important in such cases.
The most common complications of acute appendicitis are perforation with the development of peritonitis and abscess formation. Young children and elderly adults have the highest risk of perforation. For some patients in whom the appendix has already ruptured at the time of presentation, the surgeon may elect to treat the condition initially with antibiotics and drainage, followed by an appendectomy 4 to 8 weeks later. This approach is called an interval appendectomy (see Chronic Appendicitis).
Periappendiceal abscesses may result in an inflammatory mass that may mimic a neoplasm clinically. If not surgically treated, an abscess may fistulize into the small intestine or colon or onto the skin surface. In women, obstruction of an adjacent fallopian tube may lead to infertility. Inflammation of adjacent blood vessels may result in pylephlebitis. Early diagnosis and surgical intervention (i.e., appendectomy) combined with antibiotic therapy has drastically reduced the mortality rate associated with acute appendicitis since the early 1900s. The overall mortality rate for acute appendicitis is now less than 0.5%.
Stump Appendicitis
Stump appendicitis is an uncommon late complication of appendectomy. It is defined as residual or progressive acute inflammation in the remaining stump of the appendix after surgery.49–51 A diagnosis of stump appendicitis is often delayed because of its relatively infrequent occurrence, but a high level of suspicion should be maintained for patients who have signs and symptoms of appendicitis and have had a prior appendicectomy.52–54 A history of colicky central abdominal pain that localizes to the right lower quadrant does not often occur in patients with stump appendicitis. Other signs and symptoms include generalized abdominal pain and tenderness, nausea, vomiting, fever, and peritonitis. Abdominal CT findings that reveal a distended appendicular stump, fecalith, pericecal fat stranding, or abscess help to confirm the diagnosis.49 The consequences of delayed diagnosis include stump necrosis, gangrene, and perforation, which occur in as many as 40% of patients.49
Complete pathologic features of stump appendicitis have not been described. However, in most cases, findings are similar to those of classic acute appendicitis, consisting of neutrophils, cryptitis, and some degree of transmural inflammation. Transmural necrosis and perforation can occur in patients with late-stage disease. Granulomatous inflammation has been described in one patient with stump appendicitis.55
Management usually requires surgical resection of the inflamed residual appendix and antibiotic therapy. It is unclear whether the increasing use of laparoscopic instruments for appendectomy is associated with an increase in the incidence of stump appendicitis.
Periappendicitis
Preoperative mechanical manipulation of the appendix may cause mild, diffuse neutrophilic infiltration of the periappendiceal serosa.56 However, when inflammation in the serosa is accompanied by fibrin deposition or adhesions, it is a potentially significant clinical finding. Periappendicitis without mucosal or mural involvement occurs in 1% to 5% of appendices resected for clinically suspected acute appendicitis. Most cases are caused by salpingitis.57
A clinical review of cases of periappendicitis showed that there were significant clinical differences, including longer duration of pain, localization less often in the right lower quadrant, and fewer peritoneal signs, compared with patients with classic acute appendicitis.58 In two large series, suspected “periappendicitis” was attributable to a variety of processes: gonococcal and chlamydial salpingitis, yersiniosis, Meckel diverticulitis and associated intraperitoneal abscess, urologic disorders, colonic neoplasms, infectious colitis, abdominal aortic aneurysm, bacterial peritonitis, and GI perforation.57,59
Grossly, the serosal surface of the appendix and mesoappendix may appear dull and coated with a fibrinous exudate. Microscopically, a neutrophilic infiltrate is seen within the serosa. The inflammation may extend into the subserosa and rarely into the muscularis propria. Fibrinous adhesions may also be identified. By definition, the mucosa of the appendix is uninvolved. Because the serosal findings are common in patients with acute appendicitis, examination of the entire appendix is recommended to exclude this diagnosis completely. The management of periappendicitis depends on the underlying cause.
Chronic Appendicitis
The criteria and definition of chronic appendicitis as clinical or pathologic diagnostic entities are controversial. For instance, the term chronic appendicitis has been used to describe fibrous replacement of the appendiceal wall after severe or recurrent bouts of acute appendicitis. This process also has been referred to clinically as subacute appendicitis. The term has also been used for patients who have had an interval appendectomy, when resection of a perforated appendix was delayed as a result of initial conservative management with antibiotics and drainage. Chronic appendicitis is an umbrella diagnosis that encompasses any type of potentially chronic inflammatory condition of the appendix other than classic acute appendicitis.
Recurrent episodes of acute appendicitis (i.e., subacute appendicitis), ulcerative colitis (UC) and CD of the appendix, granulomatous appendicitis (and its many causes), interval appendicitis, and cystic fibrosis are potential causes of chronic appendicitis. The causes of chronic appendicitis are summarized in Box 18.3 and discussed in more detail later.
Ulcerative Colitis
The appendix plays an interesting but poorly defined role in patients with UC or CD. For instance, appendectomy is a protective factor in UC. The prevalence of prior appendectomy is lower among patients with UC compared with the general population.60–62
Ulcerative appendicitis, which represents appendiceal involvement in patients with UC, is typically seen in patients with pancolitis,63 but it may also occur as a skip lesion in patients with subtotal, left-sided, or rectal-only disease. It has an overall incidence of 50% among patients with UC.63–65
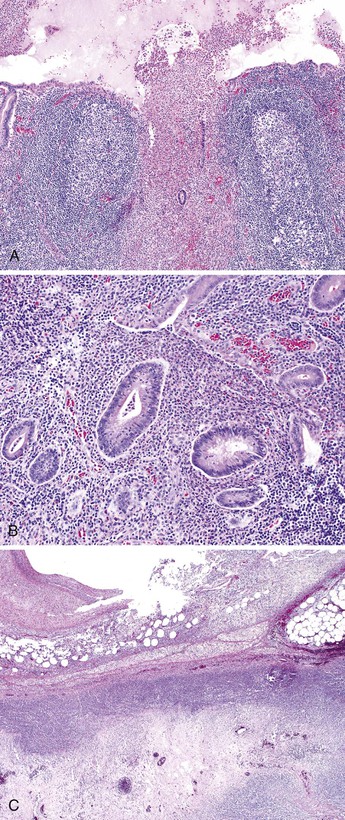
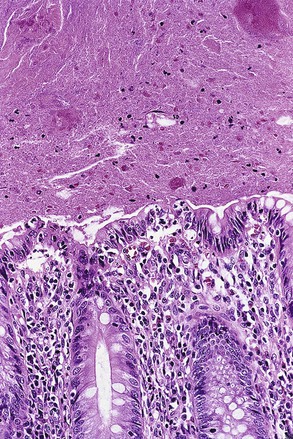
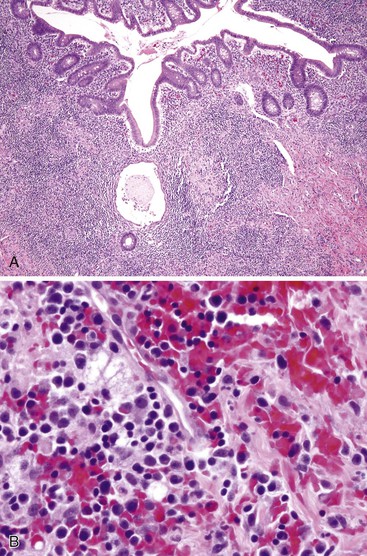
Grossly, erythema and ulceration at the appendiceal orifice may be seen endoscopically. Ulcerative appendicitis shows histologic features similar to those of the colon in UC (see Chapter 17). Active mucosal inflammation is characterized by cryptitis, crypt abscesses, and suppurative luminal exudate. Chronic changes include lamina propria lymphoplasmacytosis, basal lymphoid aggregates, crypt architectural distortion, and Paneth cell hyperplasia (Fig. 18.5). Early acute appendicitis in non-UC patients exhibits less crypt distortion and plasmacytosis than in UC-associated appendicitis. In UC, immunostains may show prominent S100 protein–reactive and MAC387-positive dendritic cells, which are not present in non-UC cases of acute appendicitis.66 The prognostic implication of appendiceal involvement in UC is dictated by the degree and extent of colonic involvement, but there is little outcome data in this regard.
Crohn’s Disease
Unlike UC, the relationship between appendectomy and CD is not well established. However, one study did find a protective effect of appendectomy on the occurrence of disease after the bias of appendectomy at the time of diagnosis of CD was removed from the analysis.61 Most appendices removed from patients with CD of the small and large intestine are histologically normal. Patients with appendiceal involvement in CD usually have extensive ileocolonic involvement. Many cases labeled granulomatous appendicitis in the past were thought to represent CD, but many of these cases instead represent a variety of different disorders, all of which feature granulomatous inflammation in the appendix. A crude estimate of the incidence of appendiceal involvement in CD from the recent literature is approximately 20%.67
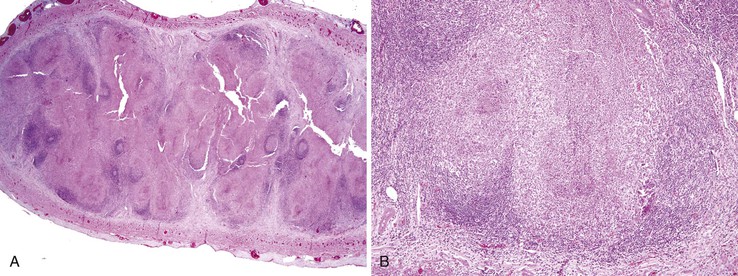
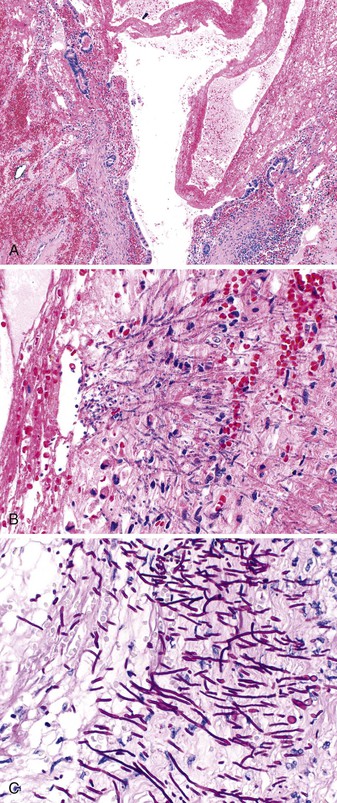
In patients with appendiceal involvement, the histologic features are similar to those seen in other sites of the GI tract. Mucosal ulceration, active inflammation with cryptitis and crypt abscesses, fissures, transmural inflammation, transmural lymphoid aggregates, fissures, and fistulas are characteristic features. Paneth cell hyperplasia may also occur. Scattered non-necrotizing granulomas are identified in approximately 50% to 80% of cases (Fig. 18.6).46,68 Occasionally, focal necrosis may be seen within the granulomas. Many of these findings may also be seen in patients with idiopathic granulomatous appendicitis (discussed later), and at initial presentation, the distinction between the two entities may be impossible in the absence of a history of CD. Patients with appendiceal CD usually have extensive ileocolic involvement, and the prognosis depends on the degree of extraappendiceal involvement.
Granulomatous Appendicitis
Granulomatous appendicitis is an uncommon finding in appendectomy specimens. It is identified in less than 1% of cases.69 Historically, most cases of granulomatous appendicitis were thought to result from involvement of the appendix by CD, but later data suggest that this is true in only 5% to 10% of cases. Other potential causes of granulomatous appendicitis are summarized in Box 18.4. They include primary (i.e., idiopathic granulomatous appendicitis) and secondary causes such as interval appendicitis, sarcoidosis, foreign body reactions, and infectious disorders (e.g., Yersinia, tuberculosis, actinomycosis, schistosomiasis, strongyloidiasis, Candida, Histoplasma).70
Clinically, patients with granulomatous appendicitis may have symptoms that mimic acute suppurative appendicitis or symptoms related to the underlying disease. In some cases, it may be entirely asymptomatic, in which case the finding of granulomatous appendicitis is made in appendectomy specimens that were removed for non–appendix-related disorders.
Depending on the cause, the size of the appendix may be normal or enlarged. There also may be serosal exudates or fibrous adhesions. Granulomas, which are composed of histiocytes surrounded by a cuff of lymphocytes, may contain multinucleate giant cells, and they may be necrotizing or non-necrotizing. Necrosis is most commonly seen in infectious disorders, but occasionally granulomas in sarcoidosis and CD may have focal necrosis. Granulomas may involve any layer of the appendiceal wall. The crypt architecture may be distorted. Cryptitis or crypt abscesses may also occur. Yersinia and tuberculosis-associated cases are also associated with transmural inflammation and lymphoid hyperplasia and therefore mimic CD.
Management of granulomatous appendicitis depends on the underlying cause. Idiopathic granulomatous appendicitis, sarcoidosis, and infectious causes of granulomatous appendicitis are discussed in later sections. Distinguishing features of the principal causes of granulomatous appendicitis are summarized in Table 18.2.
Table 18.2
Distinguishing Features in the Differential Diagnoses of Granulomatous Appendicitis
| Feature | Crohn’s Disease | Idiopathic Granulomatous Appendicitis | Interval Appendicitis | Sarcoidosis | Tuberculosis | Yersinia |
| Relevant clinical history | With or without known Crohn’s disease | None | Acute appendicitis | With or without known sarcoidosis | Compromised immune system | Young age |
| Granulomas | Occasional | Numerous | Occasional (variable) | Numerous | Numerous | Numerous |
| Necrosis | Uncommon | Uncommon | Uncommon | Uncommon | Common | Common |
| Active inflammation | Yes | Yes | Variable | Uncommon (mild) | Yes | Variable |
| Fissures, fistulas | Yes | Occasional | Rarely | No | No | No |
| Transmural lymphoid aggregates | Yes | Yes | Occasionally | Uncommon | No | No |
| Fibrosis | Yes | Yes | Yes | Yes | Mild, variable | Mild, variable |
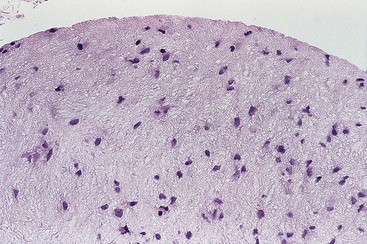
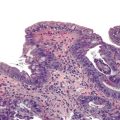
Idiopathic Granulomatous Appendicitis
Idiopathic granulomatous appendicitis is a primary granulomatous inflammatory disorder of the appendix of unknown origin. It occurs in patients without a history of CD. Idiopathic granulomatous appendicitis typically arises in young adults at an average age of 29 years and shows no gender predilection.68 Patients usually have acute right lower quadrant abdominal pain, but some have subacute pain or are entirely asymptomatic.
Idiopathic granulomatous appendicitis is characterized by multiple granulomas, usually non-necrotizing, involving any or all layers of the appendiceal wall. Associated histologic findings include acute changes such as neutrophilic infiltration resulting in cryptitis, crypt abscesses, mucosal erosion, and ulceration. Chronic changes include fissures, transmural lymphoid aggregates, and mural fibrosis. This form of appendicitis may be histologically indistinguishable from CD based solely on evaluation of the appendix.
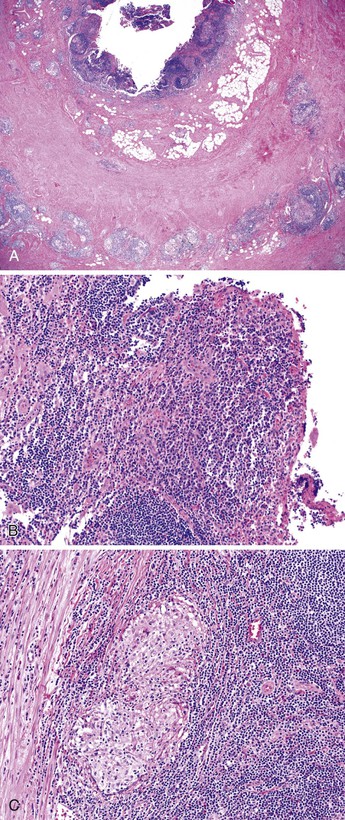
Because fissures, transmural lymphoid aggregates, and fibrosis may be seen in idiopathic granulomatous appendicitis (Fig. 18.7) at first presentation, distinction between this disorder and CD may be impossible in the absence of a clinical history of CD. However, most patients who have isolated granulomatous appendiceal disease do not develop CD elsewhere in the GI tract on follow-up.68
Differentiation of Crohn’s Disease from Idiopathic Granulomatous Appendicitis
Isolated CD of the appendix can be distinguished from idiopathic granulomatous appendicitis on the basis of combined clinical and histologic features. For instance, patients with idiopathic granulomatous appendicitis lack a history of CD, and the disease is always limited to the appendix.68,71–73 In contrast, most patients with appendiceal CD have a history of CD or have extensive ileocolonic disease at the time of presentation with appendiceal disease.
The disorders have overlapping histologic features. However, two histologic findings may be helpful in distinguishing the two entities. Fistulization is more common in CD,68 and the degree of granulomatous inflammation is greater in primary granulomatous appendicitis. Granulomas are not always seen in CD of the appendix; they occur in 50% to 80% of cases. Dudley and Dean studied the number of granulomas per cross section of the appendix in appendices resected from patients with typical CD, compared with cases of isolated idiopathic granulomatous appendicitis.68 They found that appendicitis associated with clinical CD had 0.3 granulomas per tissue section, in contrast to approximately 20 granulomas per tissue section in patients with idiopathic granulomatous appendicitis. In another series, none of nine patients (identified among 1133 consecutive appendectomy specimens) with idiopathic granulomatous appendicitis developed CD within a mean follow-up period of more than 7 years.74 In contrast, one patient reported in a series by Huang and Appelman who had 21 granulomas per cross section developed CD elsewhere in the gut on follow-up. The number of granulomas per cross section is not entirely reliable in separating these entities.48
Sarcoidosis
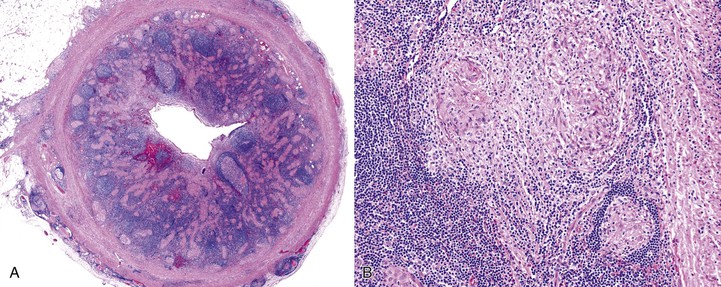
Sarcoidosis of the appendix is rare. It can manifest as acute appendicitis or chronic abdominal pain, or it may be asymptomatic.69 For this reason, the true incidence of appendiceal sarcoidosis is unknown. The main histologic finding is multiple epithelioid granulomas, which are usually non-necrotizing and involve any or all layers of the appendix (Fig. 18.8).75–77 Associated mucosal findings include neutrophilic cryptitis. A diagnosis of sarcoidosis is based on exclusion of other causes of granulomatous appendicitis. The differential diagnosis of sarcoidosis is listed in Box 18.4. Ultimately, the prognosis of patients with sarcoidosis depends on the extent of involvement of other organs, such as the heart, lungs, and brain.
Interval (Delayed) Appendicitis
Some patients in whom the appendix has already ruptured at the time of clinical presentation may be treated initially with antibiotics and drainage rather than appendectomy. After a delay of typically 4 to 8 weeks, an appendectomy is performed.
The “interval” appendectomy specimen may show a variety of changes, such as cryptitis, crypt abscesses, mucosal crypt distortion, mural fibrosis, and transmural chronic inflammation with lymphoid aggregates (Fig. 18.9). Interval appendicitis is also a cause of granulomatous appendicitis.69 In a study by Guo and Greenson, 13 (59.1%) of 22 interval appendectomy specimens showed granulomatous inflammation.47 Multinucleate giant cells were identified in almost 50% of cases.47 Other histologic findings of interval appendicitis may mimic CD, including cryptitis, crypt distortion, mural fibrosis, and transmural chronic inflammation with lymphoid aggregates. However, in the same study, none of the patients showed clinical signs of CD on follow-up.47 Xanthogranulomatous inflammation was identified in 8 (36.4%) of 22 patients in that series. In the control group of patients who underwent immediate appendectomy for acute appendicitis, only 3 of 44 patients had granulomatous inflammation. All three patients with granulomas had periappendiceal adhesions or a clinical history of abdominal pain 4 days to 1 month before surgery.47 Another smaller case series showed similar results: Five (83%) of six patients who had a protracted clinical course consisting of abdominal pain that lasted more than 1 week had granulomatous appendicitis pathologically.78
Cystic Fibrosis
Cystic fibrosis is an inherited, multisystem, autosomal recessive disorder that results in defective chloride transport mechanisms. Mutations occur in the gene that codes for the cystic fibrosis transmembrane conductance protein (CFTR), encoded by a chloride ion channel gene located on chromosome 7q31. Seventy percent of patients have a mutation (i.e., deletion of the three-nucleotide codon for phenylalanine) at position ΔF508. Mutations result in defective transcellular salt and water transport and thick secretions of mucus. Cystic fibrosis is the most common type of inherited disease among white North Americans and Europeans. It has an incidence of 1 case per 2500 live births. The clinical features vary and result from the effects of thickened mucous secretions.
The most readily identifiable abnormality is thick, inspissated mucin in the lumen of the appendix (Fig. 18.10). Accumulation of mucin may result in marked enlargement of the appendix. In some cases, this alone can cause clinical symptoms of appendicitis.79 The most common histologic finding in patients with cystic fibrosis is enlargement and distention of goblet cells, which contain normal-appearing mucin. Inspissated eosinophilic mucin within dilated glands may be seen, but this is a nonspecific finding. Overall, patients with cystic fibrosis have a lower frequency of acute appendicitis (1.5%) compared with the general population (approximately 10%) for unknown reasons.79 However, the incidence of acquired diverticula is markedly increased (see Fig. 18.1).28
Treatment of cystic fibrosis includes pancreatic enzyme replacement, ursodeoxycholic acid to stimulate secretion of bile, airway clearance, and organ transplantation. Experimental therapeutic approaches include gene therapy and exogenous administration of nucleoside triphosphate. Insulin-dependent diabetes mellitus develops in approximately 25% of adult patients. The median survival of patients with cystic fibrosis now exceeds 30 years. The most common causes of death include liver disease, cardiorespiratory complications, and complications of organ transplantation.
Eosinophilic Appendicitis
Eosinophilic appendicitis is rare. In a review of 5262 appendectomy specimens, only two cases showed eosinophilic appendicitis.80 In most cases, eosinophilic appendicitis is associated with parasitic infection, particularly Strongyloides stercoralis81,82 and Schistosoma japonicum.83 Examination of these cases showed an acute suppurative appendicitis with a predominance of eosinophils. Variable numbers of neutrophils may be seen. Increased eosinophils in the lamina propria are rarely found in patients with Enterobius vermicularis (formerly Oxyuris vermicularis) infection.84
Infectious Causes of Acute and Chronic Appendicitis
In most patients with classic acute appendicitis, no organisms are identified by histologic analysis, although cultures often reveal mixed aerobic and anaerobic bacteria. In one study of 41 children with acute appendicitis, an average of 14 bacterial isolates per specimen was detected.85 Bacterial isolates are usually composed of normal organisms85–87 and are thought to play a secondary role after mucosal injury. Bacteria belonging to the Bacteroides fragilis group are the most frequently isolated anaerobes, whereas Escherichia coli is the most frequently isolated aerobe.85–87 Bacteria belonging to the Streptococcus milleri group are also common aerobes. These organisms may be of greater significance because they have been linked to a sevenfold higher risk of abscess formation.88
Bacterial Infections
Yersinia
In a study in which specific cultures were obtained, Yersinia enterocolitica was identified in approximately 4% of cases of acute appendicitis.89 In a study by Lamps and colleagues in 2001, 10 (25%) of 40 patients with granulomatous appendicitis had evidence of pathogenic Yersinia species detected by polymerase chain reaction (PCR).90 Yersinia causes acute enteritis in young children and terminal ileitis and mesenteric adenitis in older children and young adults.
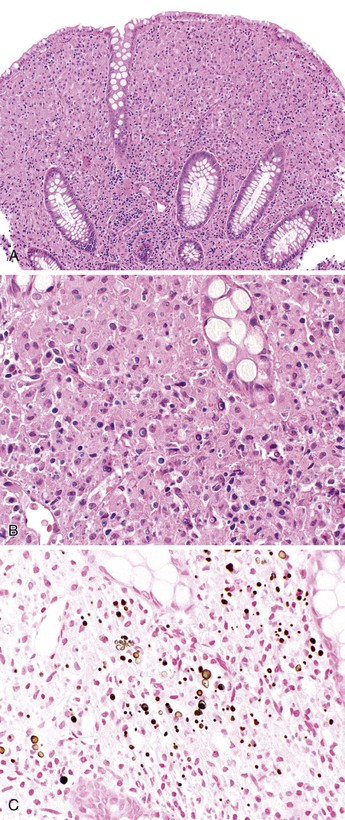
In most cases, appendices are grossly normal. Histologically, the characteristic finding is suppurative granulomatous inflammation, but in many cases the appendix may be normal or show only mild superficial acute inflammation (Fig. 18.11).89 Both Yersinia enterocolitica and Yersinia pseudotuberculosis can cause granulomatous inflammation with the formation of large, epithelioid, usually non-necrotizing granulomas surrounded by a prominent lymphoid cuff.90 In these cases, acute inflammation is common, including central microabscesses within granulomas. Lymphoid hyperplasia is typically present. Regional lymph nodes may also show evidence of granulomatous inflammation with or without suppuration.90,91
Mycobacterium tuberculosis
Tuberculosis of the appendix is almost always accompanied by GI or pulmonary tuberculosis, although isolated infections of the appendix have been reported.92,93 The histologic findings (e.g., necrotizing granulomatous inflammation) are identical to those seen elsewhere in the body. Acid-fast stain can help to detect bacilli, and PCR analysis can be used to subtype the organism.
Actinomyces
Actinomyces israelii is an uncommon cause of appendicitis. Rarely, A. israelii can lead to infections of the small intestine, appendix, or colon. Histologically, long, filamentous organisms stain dark blue on routine hematoxylin and eosin preparations and are particularly easy to recognize when associated with characteristic sulfur granules. Active inflammation is typically seen in the mucosal wall. Fistula formation, rupture of the appendix, and abscess formation may complicate the clinical course and mimic CD.94 Actinomyces turicensis also has been implicated in causing appendicitis. It is frequently accompanied by aerobic bacterial isolates of the Streptococcus anginosus group.95
Campylobacter
Campylobacter jejuni is an uncommon cause of bacterial appendicitis. In one study, Campylobacter was detected by immunohistochemical methods in 3 (2.6%) of 116 cases of acute appendicitis.96 Most patients were young children who had grossly normal appendices. Histologically, active inflammatory changes were limited to the mucosa and consisted of cryptitis and surface erosions with focal aggregates of histiocytes that occasionally resulted in granuloma formation.96 One study that used more sensitive PCR techniques found C. jejuni DNA in 11 (22%) of 50 cases of acute appendicitis. In that study, all 20 incidental appendectomy specimens (controls) were found to be negative. Whether this organism is a significant cause of acute appendicitis, an innocent bystander, or a cause of superinfection remains unknown.97
Clostridium difficile and Shigella
Clostridium difficile and Shigella infections that involve the appendix are usually associated with generalized colonic disease, although acute appendicitis may rarely represent the initial manifestation. Overall, the histologic findings are identical to those seen in the colon. Features include pseudomembrane formation, ulceration, cryptitis, crypt abscesses, and lamina propria hemorrhage.
Malakoplakia
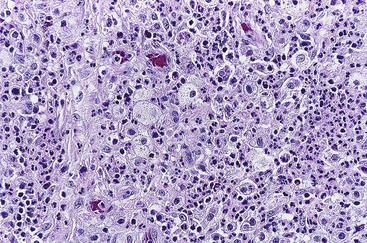
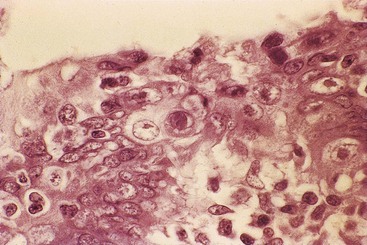
Malakoplakia is most frequently encountered in the urinary tract, but it can also be found in many other organs, including the appendix. In one case report, it was associated with ova of Taenia species.98 Malakoplakia results from an abnormal immune response in which bacteria are incompletely digested and accumulate in histiocytes.99 Typical histologic findings include diffuse or nodular thickening of the mucosa resulting from an accumulation of macrophages that contain eosinophilic cytoplasm. Scattered lymphocytes and plasma cells are always seen. Michaelis-Gutmann bodies, which are round, laminated structures with a targetoid appearance, are the most characteristic feature (Fig. 18.12). These structures can be highlighted with an iron or calcium stain.
Spirochetosis
Spirochetosis, most often caused by Brachyspira aalborgi, may rarely occur in the appendix. In one study, spirochetosis was detected in 1.9% of incidentally removed appendices, in 0.7% of appendices in patients with histologically proven acute appendicitis, and in 12.3% of patients with clinically suspected acute appendicitis but histologically normal appendices.100 These results suggest that spirochetosis may contribute to clinical symptoms in patients with otherwise normal appendices. Spirochetosis of the small intestine and colon is more commonly found in HIV-infected patients than in non–HIV-infected patients, but little information is available on whether this is also true for the appendix.
Overall, adults are more commonly infected than children.101 Histologically, spirochetosis is characterized by the presence of a hazy hematoxylin-positive band of organisms, which is approximately 3 µm thick, situated on the surface of the epithelium, and can be highlighted by silver stains. Typically, there is no associated inflammatory response. Electron microscopy reveals organisms within epithelial cells and macrophages.101
Parasitic Infections
Enterobius vermicularis
Enterobius vermicularis (i.e., pinworm) is one of the most common parasites encountered in the appendix, especially in temperate climates. The overall frequency ranges from 2.4% in Iran104 to 8.7% in Czechoslovakia.105 At the Johns Hopkins Hospital in Baltimore, Maryland, only 4 (0.25%) of 1584 appendices removed during a 17-year period from patients 15 years of age or younger had proven Enterobius infection of the appendix. Another study at Children’s Hospital in Columbus, Ohio, showed that 21 (1.4%) of 1549 patients had intraluminal pinworms of the appendix.106 Children in late childhood and early adolescence (5 to 15 years old) have the highest incidence of infection,105,107 which is as high as 24% in some studies.
Most patients are asymptomatic. The worm is commonly found in the lumen of the appendix without any significant inflammatory response (Fig. 18.13). However, large worms may obstruct the appendiceal lumen, resulting in development of a mucocele. Eggs and worms in the stool may cause pruritus ani.
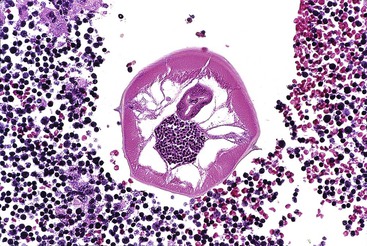
Grossly, the white worms may be visible to the naked eye and usually measure between 2 to 5 mm in greatest diameter. They can also colonize the ileum and proximal colon. Microscopically, the worms have a thick outer cuticle, from which lateral ala project. Internal organs or eggs may be visible in the internal aspect of the worm. In one study in which almost 22,000 appendices were evaluated, granulomatous inflammation and increased eosinophils in the lamina propria were rare, occurring in less than 2% of infections.84 Rarely, worms may invade surrounding tissues, resulting in ulceration and a marked inflammatory response composed of neutrophils and eosinophils. In most cases, there is minimal tissue response to the worm in surrounding tissues. An inverse relationship between active mucosal inflammation and pinworm infection has been observed in several studies.107–109 Mucosal inflammation has been more strongly linked to the presence of parasite ova.110 Treatment requires antiparasitic medication such as mebendazole or albendazole.
Strongyloides stercoralis and Eosinophilic Appendicitis
Eosinophilic appendicitis has been associated with Strongyloides stercoralis infection.81 Morphologic features consist of a diffuse infiltrate of eosinophils that is often associated with abscess formation and necrosis. Granulomas may also be seen. Identification of the organism in stool specimens is a more sensitive method of detecting infection than analysis of appendiceal tissues.81,111
Viral Infections
Viral gastroenteritis does not typically result in appendectomy, although some cases of appendicitis are no doubt caused by viral agents. Viral enteritis may lead to surgical excision of the appendix when the infection causes ileocecal intussusception. In such cases, a segment of small intestine, with or without a segment of the right colon, is also resected. Intussusception is commonly attributed to lymphoid hyperplasia in the terminal ileum, which forms the leading edge of the intussuscepted segment. It classically occurs in infants and young children. Viral agents that are most commonly implicated in this setting are rotavirus, echovirus, and adenovirus.
Adenovirus
Adenovirus is the most frequently detected virus in the appendix.114,115 In appendices infected with adenovirus, lymphoid hyperplasia is prominent, and erosions may be identified. Viral inclusions are typically found in intact mucosal epithelial cells. Zones in which inclusions may be identified are best selected by scanning at low magnification for areas of mucosa that have a ragged appearance to the epithelium. In these areas, the epithelium shows a loss of nuclear polarity and loss of goblet cell mucin, which imparts an eosinophilic appearance to the cells. These zones are frequently associated with reactive lymphoid follicles. Intranuclear adenovirus inclusions are typically Cowdry type B bodies, showing prominent nuclear smudging. Cowdry type A inclusions, which show sharply demarcated intranuclear inclusions surrounded by a clear zone or halo, are found in a minority of cases (Fig. 18.14).
Cytomegalovirus
Cytomegalovirus (CMV) may be found in the appendices of immunocompromised hosts, where the histologic features are similar to those of other infected sites of the GI tract. Viral cytopathic effects are most often detected in endothelial cells or stromal fibroblasts rather than in epithelial cells. CMV infection results in cytoplasmic inclusions with or without intranuclear eosinophilic inclusions. Other findings include epithelial cell apoptosis and neutrophilic cryptitis. CMV infection occasionally is responsible for the development of acute appendicitis.44,116
Epstein-Barr Virus
Epstein-Barr virus has been detected in a small subset of individuals with acute appendicitis. It is found in appendices with acute suppurative appendicitis and those with marked lymphoid hyperplasia as the only major histologic abnormality.117–119 In some reports, patients with infectious mononucleosis developed superimposed acute suppurative appendicitis that required surgery.117 Lymphoid hyperplasia associated with Epstein-Barr virus infection exhibits multiple transformed lymphocytes and immunoblasts (Fig. 18.15). These cells may raise the possibility of lymphoma if the polymorphous nature of the lymphoid proliferation is not appreciated.
Measles Virus
The incidence of measles infection has declined drastically in the past 50 years, mainly because of implementation of vaccination programs. However, small outbreaks still occur. Acute appendicitis may occur in the prodromal or fulminant phases of measles virus infection. In the prodromal phase, the histologic appearance is that of marked lymphoid hyperplasia with multinucleate giant cells (i.e., Warthin-Finkeldey cells) with or without other classic features of acute appendicitis.120–122 In the late stages of infection, acute suppurative appendicitis may develop, which may be the result of luminal obstruction caused by lymphoid hyperplasia.123
Varicella-Zoster Virus
Varicella-zoster virus (VZV) associated acute appendicitis has been reported in a 1-year-old boy with active chickenpox. The appendectomy specimen showed an edematous retrocecal appendix with transmural acute inflammation and multiple intranuclear viral inclusions surrounded by a clear halo (i.e., Cowdry type A inclusions). PCR analysis of peripheral blood and appendiceal tissue demonstrated VZV DNA.124
Fungal Infections
Fungal infections such as aspergillosis, mucormycosis, and candidiasis can involve the appendix,125,126 but they usually are part of a systemic infection. Patients are typically immunosuppressed because of organ transplantation or chemotherapy. Granulomatous inflammation associated with invasive candidiasis involving the appendix has been reported,127 but most cases show suppurative inflammation without granulomas. However, because these patients are usually immunosuppressed, the inflammatory infiltrate may be quite mild despite a high volume of organisms. Accompanying epithelial changes, such as denudation and degeneration, are common (Fig. 18.16).
Miscellaneous Disorders of the Appendix
Intussusception of the Appendix
Intussusception of the appendix is frequently associated with other abnormalities, such as endometriosis or polyps, with the latter located mainly in the base of the appendix.128–130 The causes of appendiceal intussusception include anatomic factors, such as a mobile mesoappendix and wide proximal appendicular lumen, and intrinsic abnormalities, such as polyps, tumors, parasites, endometriosis, and lymphoid hyperplasia.
The clinical presentation of affected patients varies. Symptoms include recurrent, crampy right lower quadrant abdominal pain that mimics acute appendicitis. Intussusception may also act as a lead point for ileocolic intussusception. Histologically, affected mucosa may show prolapse-type changes, with crypt architectural distortion, ingrowth of smooth muscle into the lamina propria, and fibrosis. Treatment is usually surgical, consisting of appendectomy with or without ileocolic resection.
Endometriosis and Deciduosis
Endometriosis
Endometriosis affects the GI tract in as many as 40% of patients with pelvic endometriosis. The sigmoid colon is the most common site of GI involvement, but the appendix may be involved in as many as 15% of cases.131 The actual incidence of appendiceal endometriosis is between 0.4% and 2.8%.132 Although appendiceal endometriosis can masquerade clinically as acute appendicitis,131,133 patients typically have a nonspecific clinical presentation.131,133–135 Pain may wax and wane with the menstrual cycle. Occasionally, endometriosis acts as the leading edge for appendiceal intussusception.129
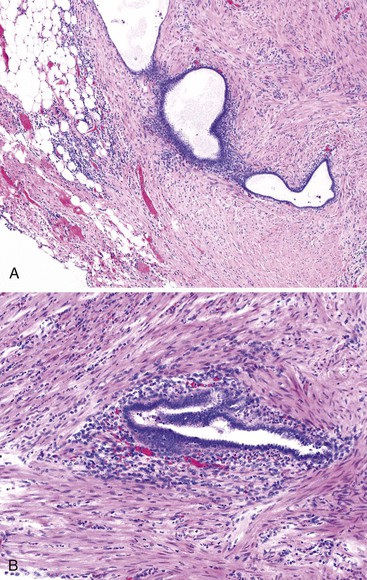
Most cases involve the serosa or muscularis propria and are accompanied by abundant fibrosis and adhesions, although exclusively submucosal cases also occur.133–135 Cysts filled with hemorrhagic debris may be identified. Histologically, endometriosis of the appendix consists of endometrial-type glands and stroma (Fig. 18.17) associated with hemosiderin deposition and a fibroblastic reaction. The endometrial epithelium undergoes changes in response to the menstrual cycle. For instance, a stromal decidual reaction may be detected in endometriotic foci in pregnant patients.134 Most of the morbidity associated with endometriosis results from adhesions, which can lead to infertility, intestinal obstruction, and chronic pain.
Management of endometriosis often requires a multimodality approach that includes hormonal therapy and surgical intervention. Rarely, clear cell carcinoma or other primary müllerian carcinomas may arise in endometriotic foci. A similar phenomenon, called deciduosis (i.e., ectopic decidua), has been found in the appendices of pregnant women.
Deciduosis
Deciduosis results from the effects of progesterone on extrauterine mesenchymal cells during pregnancy. It is most often seen in ovarian tissues. Deciduosis, in contrast to endometriosis, is more often associated with signs and symptoms of acute appendicitis.136–138 It usually appears as white plaques or small nodules on the serosa. Deciduosis differs from endometriosis histologically in that it lacks glands. It consists of large polyhedral cells with abundant granular eosinophilic cytoplasm and small, round nuclei with distinct nucleoli arranged in sheets in the serosa or outer muscularis propria. Rarely, deciduosis occurs in the mucosa of the GI tract.139 Decidual cells can sometimes be mistaken for malignant (often epithelial) tumors, but immunohistochemical stains for cytokeratins, carcinoembryonic antigen, epithelial membrane antigen, and S100 protein are typically negative. Decidualized cells may show desmin or muscle actin positivity.136
Gliomatosis and Heterotopias
Gliomatosis peritonei is found in patients with ovarian teratomas and consists of mature glial tissue on the serosal surfaces.140 Pelvic gliomatosis can affect the serosal aspect of the appendix (Fig. 18.18). It has also been reported as a rare complication of ventricular shunts.141 Malignant transformation has been recorded.142,143 Heterotopic gastric and esophageal tissue have also been described in the appendix.144
Pigments, Foreign Material, and Processing Artifacts
Melanosis
Melanosis involves the appendix in approximately 7% of individuals older than 40 years. It is histologically identical to that seen in the colon.145 Pigmented macrophages contain cytoplasmic material (e.g., lipofuscin) that ranges from pale green to dark brown. The pigment lipofuscin accumulates due to ingestion of apoptotic surface epithelial cells by macrophages.146 The most common cause is laxative use in patients with chronic constipation. Melanosis of the appendix is also relatively common in pediatric patients, and it is thought to represent a response to infection rather than to laxatives.147
Barium
Barium can be present in the lumen of the appendix with no associated mucosal response. However, occasionally barium extravasates into the mucosa, where it is phagocytosed by macrophages and induces a granulomatous reaction. Barium is a nonpolarizable, crystalline material that is typically light green.
Foreign Body Appendicitis
Foreign body appendicitis is rare. However, it has generated considerable interest and has led to a large number of case reports, suggesting it as a potential cause of acute appendicitis. Foreign bodies include dog hair, toothpicks, and lead. A 100-year review of the literature revealed 256 reported cases, with the highest risk associated with stiff or pointed objects.148 Not all foreign body–associated appendicitis occurs after initial ingestion. Appendicitis may develop years after the offending agent was consumed.149
Other foreign materials that can be seen in the appendix include vegetable fragments, which usually do not elicit a mucosal inflammatory response. However, in some cases, vegetable material may obstruct the appendiceal lumen and lead to acute appendicitis. Foreign heterotopic bone formation in the appendix is a rare finding that has been associated with some mucin-producing tumors.150 Rarely foreign bodies in the peritoneum, such as dialysis catheters, entrap the appendix and lead to acute appendicitis.151
Rosai-Dorfman Disease
Rosai-Dorfman disease, also known as sinus histiocytosis with massive lymphadenopathy, is a histiocytic, proliferative disorder of unknown origin. It frequently occurs in extranodal locations, but appendiceal involvement is rare. It has occurred in the appendices of two patients who also had nodal involvement.152 One patient had vague abdominal symptoms 5 years after the initial diagnosis of nodal Rosai-Dorfman disease, and the other had a right lower quadrant mass that became evident weeks after the development of cervical lymphadenopathy.
The characteristic appearance is sheets of S100 protein–positive histiocytes with abundant, pale cytoplasm that engulf inflammatory cells (i.e., emperipolesis). Within the GI tract, the proliferation involves submucosal tissue, but it may also focally involve the lamina propria.152 The background stroma is often collagenous and may contain a scattered, mixed inflammatory infiltrate.
The clinical course varies. Although many patients have an indolent clinical course, some die of disease. Chemotherapy has been used with various results. The two patients described earlier were alive with disease 11 and 12 years after the initial diagnoses.
Acute Necrotizing Arteritis of Appendiceal Vessels
Acute necrotizing vasculitis of the arterial vessels of the appendix is rare.127,153 In most cases, vasculitis is limited to the appendix, but in one study, widespread vasculitis in a pattern similar to polyarteritis nodosum developed in as much as one third of patients.127