Chapter 79 Inflammatory Bowel Disease
Epidemiology
The true incidence of thoracic complications in IBD patients is unknown. There have been no screening tests performed on large populations of IBD patients. The proportion of IBD patients with subjective respiratory symptoms such as cough, sputum production, wheezing, or shortness of breath has been reported to be as high as 50%. One or more pulmonary function tests (PFTs) are abnormal in approximately 40% of IBD patients, with forced expiratory volume in 1 second (FEV1), inspiratory vital capacity (IVC), or diffusion capacity (DLCO) showing a 10% to 30% reduction. Between 25% and 50% of asymptomatic IBD patients show abnormalities on high-resolution computed tomography (HRCT) scans. Often, the findings are subtle and include ground-glass opacities, mosaicism suggestive of air trapping, peripheral opacities, and cysts. At the other end of the spectrum are case reports and small series of patients with distinctive manifestations of thoracic involvement. These reports, accounting for 155 patients, were recently reviewed (see Suggested Readings). Although nothing is offered to calculate incidence or prevalence, significant symptomatic thoracic involvement by IBD probably is not a common event. However, it is common enough that physicians need to be cognizant of IBD to recognize it and prevent the potentially debilitating consequences of some of its forms.
Clinical and Pathologic Features
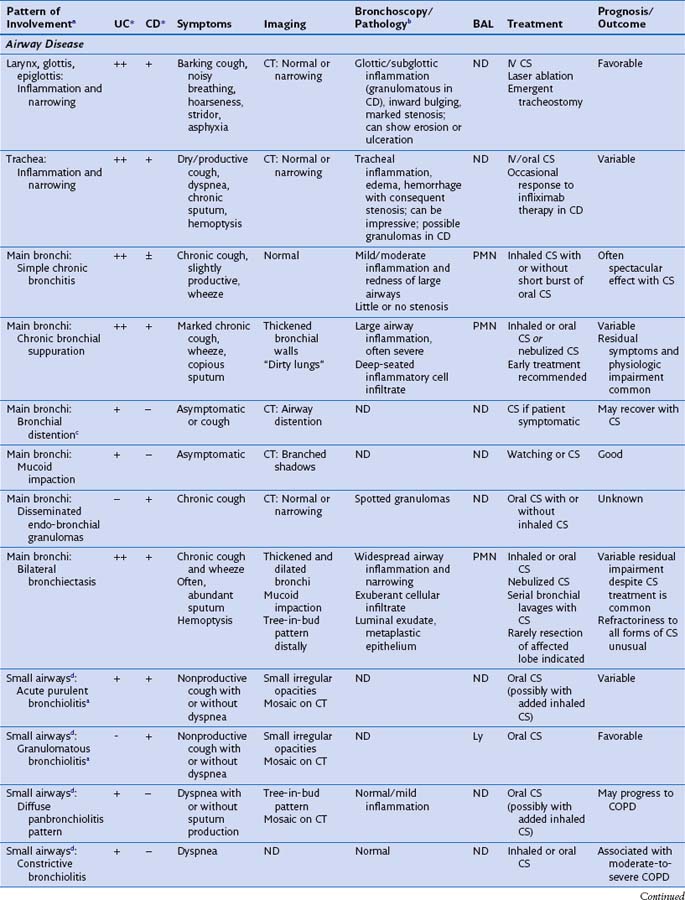
This section discusses the different compartments of the respiratory tract involved by IBD, including unique considerations of the particular location, clinical presentation, and its pathologic features (Table 79-1).
Airway Disease
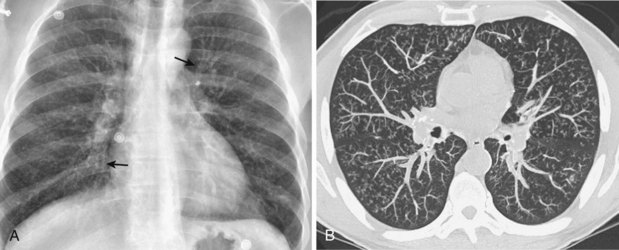
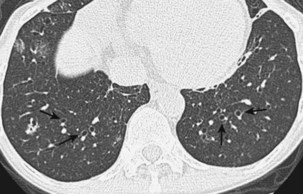
Radiographically, airway walls appear thickened with a “tram line” pattern. Larger airways show mucus plugging, smaller airways a tree-in-bud appearance. These changes tend to occur in a basilar-predominant distribution, occasionally with associated volume loss. Small airways disease can be associated with a tree-in-bud pattern, diffuse reticular shadows, or mosaic pattern due to air trapping (Figure 79-1). Bronchiectasis after long-standing disease can range from prominent thick-walled bronchi to subtle increases in airway caliber (Figure 79-2).
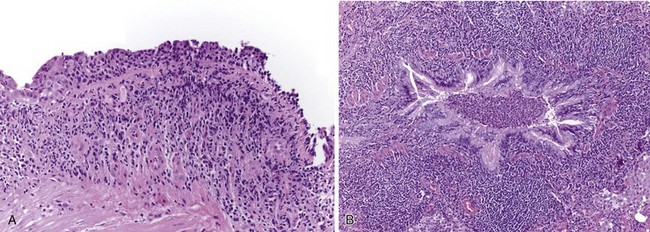
Histologically, large airways inflammation in IBD is characterized by a mucosal mononuclear inflammatory infiltrate, which is often bandlike and resembles that seen in the bowel mucosa in IBD (Figure 79-3, A). A luminal exudate containing neutrophils is common (Figure 79-3, B). Granulomas are frequently but not always seen in lung biopsies from patients with CD. On the other hand, the presence of a granuloma in a biopsy does not exclude UC as the underlying bowel disease. Small airways inflammation is similarly suppurative, with chronic inflammation of the bronchiolar wall. Occasionally, interstitial foamy macrophages are present in a centrilobular distribution, morphologically resembling diffuse panbronchiolitis. Late-stage small airways involvement may histologically present as constrictive bronchiolitis, which can be patchy throughout the lung and therefore may be missed by the biopsy sampling. An expiratory chest CT scan showing air trapping may support a clinical impression of obliterative small airways disease in these patients.
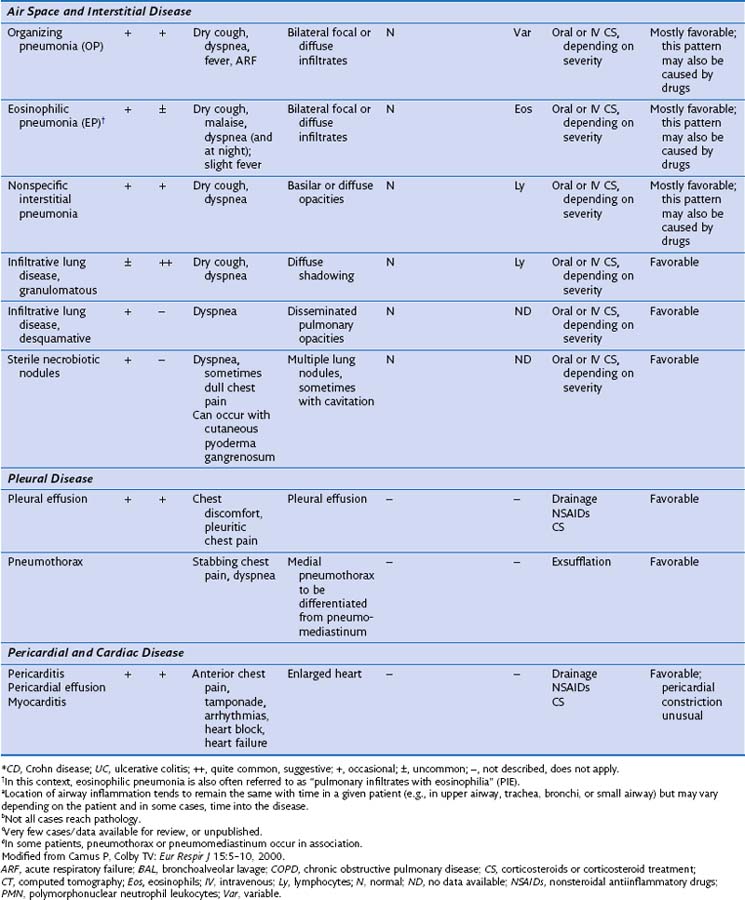
Air Space Disease
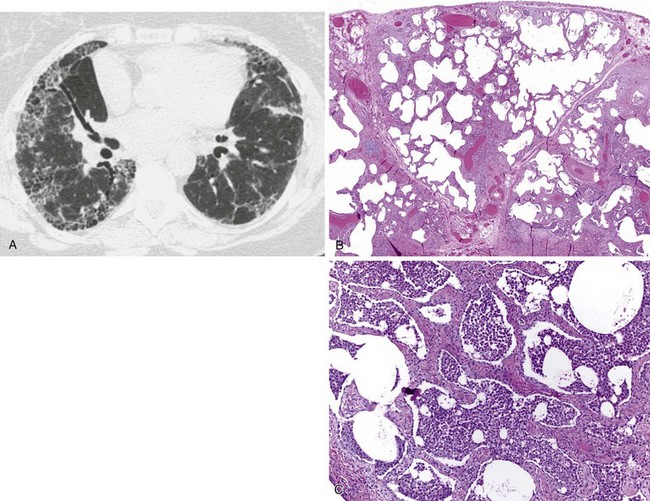
Inflammatory bowel disease can involve the distal air spaces of the lung in the form of organizing pneumonia (OP) or eosinophilic pneumonia (EP). The main difficulty with these patterns of involvement is distinguishing them from infection and drug-related lung disease. Clinically, patients present with shortness of breath ranging from moderate dyspnea to acute respiratory failure, sometimes accompanied by fever. Radiographically, OP shows bilateral, ovoid, elongated subpleural opacities, which may contain air bronchograms (Figure 79-4, A and B). EP, similar to non-IBD cases, may assume a masslike appearance or manifest as migratory opacities. Bronchoalveolar lavage (BAL) findings are often nonspecific, but an increase in neutrophils or eosinophils suggests a diagnosis of infection or EP, respectively.
Histologically, OP is characterized by loose, fibroblastic plugs in air spaces (Figure 79-4, C), whereas EP shows numerous eosinophils within the interstitium and among fibrinous air space exudates. Both patterns are nonspecific and can also be seen in infections, drug reactions, connective tissue disease, airway obstruction, or cryptogenic (idiopathic) forms of OP and EP. Biopsies may still be helpful to rule out infection or other causes of air space disease.
Interstitial Lung Disease
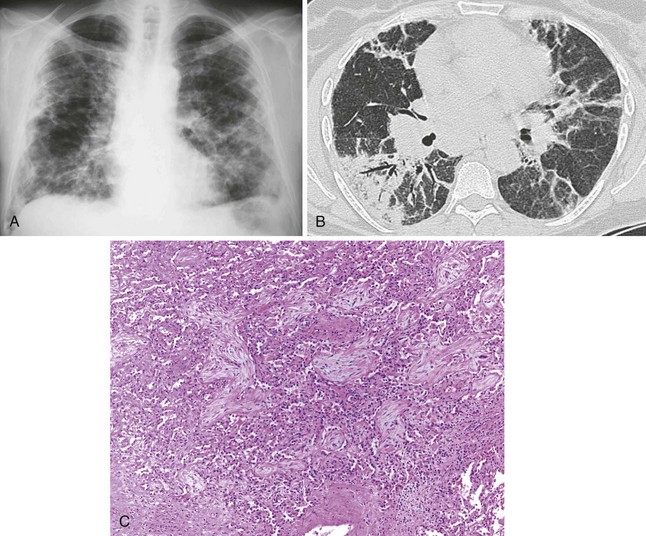
Patients with interstitial lung disease present with shortness of breath and crackles on auscultation. Radiographically, interstitial markings are increased, and often more prominent in the lower lung zones (Figure 79-5, A). Bronchoscopic examination is normal unless concomitant airway disease is present. Wedge biopsies are necessary for histologic confirmation of diffuse fibrosing lung disease, because transbronchial biopsies are usually too small for assessing the fibrosis pattern. Biopsies show interstitial collagen fibrosis with variable mononuclear inflammatory infiltrates with or without granulomas (Figure 79-5, B and C). The presence of granulomas should always prompt exclusion of infection with special histochemical stains or preferably by concomitantly submitted tissue culture.
Masses and Nodules
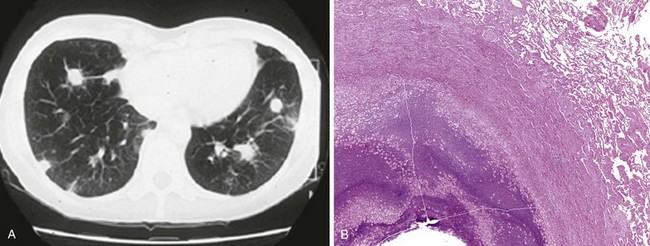
Necrobiotic nodules are an uncommon manifestation in ulcerative colitis and Crohn disease. Nodules may occur synchronously with pyoderma gangrenosum lesions in the skin, with common histologic features. One study found that necrobiotic nodules precede the diagnosis of IBD in one third of patients. Patients present with a rapid onset of fever, dyspnea, and chest pain. Imaging studies show multiple rounded lung nodules that may cavitate over time (Figure 79-6, A). Without intervention, nodules may eventually disappear, leaving a scar. The histologic picture is distinctive, showing aggregates of neutrophils and necrosis without granulomatous features or identifiable infectious organisms (Figure 79-6, B). Although drug-induced lung injury can present with lung nodules, these nodules tend not to show cavitation radiographically or sterile abscesses histologically.
Drug-Induced Lung Disease
A major difficulty when encountering patients with apparent lung involvement by IBD is excluding drug-related toxicity. A causal role of drugs in airway inflammation is unlikely for two reasons. First, airway inflammation is common among IBD patients after colectomy and no longer receiving culprit drugs such as sulfasalazine or mesalamine. Second, airway inflammation does not improve on withdrawal of these drugs in patients with no history of colectomy. Infiltrative (air space or interstitial) lung disease, on the other hand, is a pattern associated with several drugs used in IBD patients. Unfortunately, withdrawal of the drug does not always result in stabilization or reversal of the lung disease. Decisions regarding discontinuation of drug therapy must consider the relative severity of the lung disease and the IBD, because drug discontinuation may result in a flare of IBD. Table 79-2 summarizes toxicity patterns associated with common IBD drugs. An excellent resource for drug-associated pulmonary disease can be found online at Pneumotox.com.
Table 79-2 Biopsy-Proven Lung Changes Related to Drugs Used in Inflammatory Bowel Disease (Other than Infection)
| Drugs | Lung Conditions |
|---|---|
| Mesalazine Sulfasalazine Balsalazide Olsalazine (5-aminosalicylic acid derivatives) |
DAD, EP, OP, HP, chronic interstitial pneumonia |
| Azathioprine | DAD |
| 6-Mercaptopurine | ND |
| Methotrexate | DAD, OP, HP, chronic interstitial pneumonia, pulmonary edema, granulomatous inflammation |
| Infliximab Adalimumab |
DAD, EP, OP, chronic interstitial pneumonia |
| Certolizumab Natalizumab |
ND |
| Cyclosporine | Pulmonary edema |
| Mycophenolate | Chronic interstitial pneumonia |
DAD, diffuse alveolar damage (adult respiratory distress syndrome); EP, eosinophilic pneumonia; HP, hypersensitivity pneumonitis; ND, none documented; OP, organizing pneumonia.
Treatment
Emergencies
The following three thoracic conditions require immediate attention and emergency treatment:
• Acute upper airway (glottal and epiglottal) inflammation can rapidly lead to airway occlusion and asphyxiation. Treatment should be initiated immediately and should include high-dose corticosteroids and, if needed, securing the airway by intubation, tracheostomy, or laser ablation. Initial corticosteroid administration in this situation should occur intravenously with conversion to oral therapy later. The clinical and visual diagnosis can be confirmed by biopsy of the airway mucosa. A patient with Crohn disease reportedly improved on initiation of infliximab therapy.
• Diffuse infiltrative lung disease may result in acute respiratory failure requiring intubation and mechanical ventilation. The two most common differential diagnoses must be ruled out: pulmonary infection, by bronchoscopy with lavage and culture, and drug toxicity, by withdrawal of IBD drug therapy. No evidence shows that surgical lung biopsy improves outcome in these patients; however, it may help guide corticosteroid therapy and rule out infection or other underlying diseases more confidently.
• Acute pericarditis with a large pericardial effusion threatening compression or tamponade may require pericardiocentesis or surgical drainage.
Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006;6:244.
Basseri B, Enayati P, Marchevsky A, et al. Pulmonary manifestations of inflammatory bowel disease: case presentations and review. J Crohns Colitis. 2010;4:390–397.
Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–532.
Camus P, Colby TV. Respiratory manifestations in ulcerative colitis. Eur Respir Monogr. 2006;10:168–183.
Camus P, Colby TV. The lung in inflammatory bowel disease. Eur Respir J. 2000;15:5–10.
Camus P, Piard F, Ashcroft T, et al. The lung in inflammatory bowel disease. Medicine (Baltimore). 1993;72:151–183.
Casey MB, Tazelaar HD, Myers JL, et al. Noninfectious lung pathology in patients with Crohn’s disease. Am J Surg Pathol. 2003;27:213–219.
Chenivesse C, Bautin N, Wallaert B. Pulmonary manifestations in Crohn’s disease. Eur Respir Monogr. 2006;10:151–167.
Colby TV, Camus P. Pathology of pulmonary involvement in inflammatory bowel disease. Eur Respir Monogr. 2007;12:199–207.
Foucher P, Camus P. Pneumotox website, 1997. www.pneumotox.com, 2010.
Hamada S, Ito Y, Imai S, et al. Effect of inhaled corticosteroid therapy on CT scan–estimated airway dimensions in a patient with chronic bronchitis related to ulcerative colitis. Chest. 2011;139:930–932.
Higenbottam T, Cochrane GM, Clark TJH, et al. Bronchial disease in ulcerative colitis. Thorax. 1980;35:581–585.
Kraft SC, Earle RH, Roesler M, et al. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch Intern Med. 1976;136:454–459.
Mahadeva R, Walsh G, Flower CD, Shneerson JM. Clinical and radiological characteristics of lung disease in inflammatory bowel disease. Eur Respir J. 2000;15:41–48.
Marc FJ, André MFJ, Piette JC, et al. A study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86:145–161.
Pedersen N, Duricova D, Elkjaer M, et al. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010;105:1480–1487.
Satsangi J, Grootscholten C, Holt H, et al. Clinical patterns of familial inflammatory bowel disease. Gut. 1996;38:738–741.