15 Inflammatory bowel disease
Case
A 28-year-old female presents with a 3-month history of diarrhoea, weight loss, fatigue, and right lower quadrant abdominal pain. She complains of six to eight non-bloody bowel movements daily with at least one at night that awakens her from sleep. She has lost 10 kg since her symptom onset. She has fevers to 38.5°C without chills. She also complains of perianal pain with purulent drainage. She has generalised joint aches with intermittent swelling of the knees and mouth sores. Her past medical history is otherwise unremarkable and she does not take any medications. She denies taking any non-steroidal anti-inflammatory drugs. She is an active smoker of one pack a day for the last 5 years. She has no family history of inflammatory bowel disease.
Introduction
Inflammatory bowel disease (IBD) is traditionally divided into ulcerative colitis (UC) and Crohn’s disease (CD). Unlike infectious colitis that is typically self-limited, IBD is a chronic illness that is punctuated by disease exacerbations and remissions. While some similarities exist, UC and CD are very different diseases with regard to their clinical presentation, pattern of bowel involvement, response to therapy and prognosis (Table 15.1 and Box 15.1). With current methods, proper diagnosis of CD or UC can be made, although 10–15% of patients are labelled indeterminate colitis. Incidence and prevalence rates for UC and CD vary throughout the world but are highest in developed countries of northern Europe and North America.
Table 15.1 Pathological features in inflammatory bowel disease
| Ulcerative colitis | Crohn’s disease |
|---|---|
| Distribution | |
∗ Preservation of crypt architecture suggests acute self-limited colitis.
† Granulomas may occur in tuberculosis, schistosomiasis and syphilis.
Ulcerative Colitis
Clinical presentation
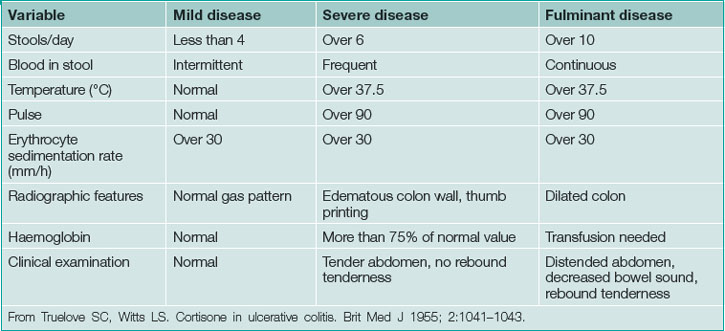
UC typically presents in the third or fourth decade of life. Clinical presentation is based on disease location and severity. Severity is classified as mild, severe or fulminant with moderate disease between mild and severe (Table 15.2). Diarrhoea, usually bloody with cramping typically awakening the patient from sleep, signifies mucosal inflammation. Mild disease may be accompanied by frequent diarrhoea but is not associated with significant life impairment. Moderate disease, between mild and severe, typically leads to significant symptoms including profound diarrhoea (usually bloody), dehydration, fatigue and weight loss. This may progress to severe disease, which may require hospitalisation. Fulminant disease is most severe often requiring intensive care or surgery.
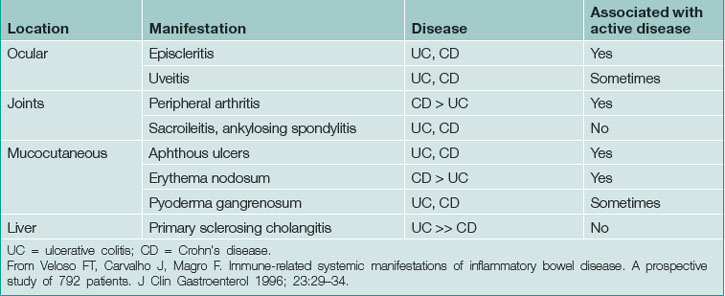
UC may be associated with extraintestinal manifestations in some patients (Table 15.3) which may be associated with disease activity (Fig 15.1). Those not associated with disease activity may run a clinical course independent of the disease and persist after colectomy.
Disease extent and location
Frequent low-volume bowel movements, urgency, rectal bleeding and tenesmus alone suggest proctitis. However, patients with or without these symptoms may also present with prostration, fever, tachycardia, dehydration and complications of blood loss, suggesting more severe disease or more extensive bowel involvement. Advanced cases may present with massive abdominal distention because of megacolon. In fulminant colitis, severe diarrhoea and high C-reactive protein despite intensive treatment predict eventual colectomy. Fortunately, the presentation of fulminant colitis is relatively uncommon.
Diagnosis
Among patients without a prior history of ulcerative colitis, presenting features are non-specific so that additional diagnostic testing is required. Diagnosis is confirmed by clinical features, endoscopic and histological findings as well as stool examination to exclude acute colitis. Infection, ischaemia, non-steroidal anti-inflammatory medication-induced colitis, diverticular colitis and Crohn’s disease are important considerations.
Radiology
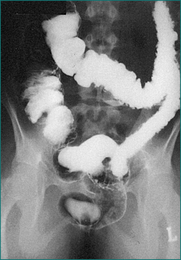
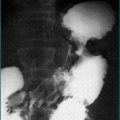
Plain abdominal x-rays should be performed to exclude toxic megacolon and perforation among severely ill patients. Computed tomography (CT) scanning may be considered if there is concern of other aetiologies such as Crohn’s disease. CT of the abdomen may reveal colonic thickening and provide additional information as to the extent of the disease. Barium enema is rarely performed because of the widespread use of sigmoidoscopy and colonoscopy (Fig 15.2).
Sigmoidoscopy, colonoscopy and biopsy
Endoscopic visualisation with biopsy is essential for all patients with suspected UC and is often necessary to document disease activity for a suspected flare. Disease activity, if present, should be visible in the rectum and may extend more proximally. Rectal sparing or segmental colitis raises the possibility of infection, ischaemia, Crohn’s disease or other aetiology. Biopsies confirm the presence of chronic colitis and exclude acute colitis as seen with infection. Among patients with colitis refractory to therapy, especially if they are receiving corticosteroid or immunosuppressive therapy, cytomegalovirus should be excluded by biopsy with special studies. Severely ill patients may undergo an unprepped flexible sigmoidoscopy whereas patients with mild to moderate disease are often prepped for colonoscopy. There is usually little value in performing a complete colonoscopy on patients with active UC and it may be dangerous. However, prepping for colonoscopy, if possible, will allow for better visualisation above the sigmoid colon, especially if findings are not typical for UC or it is important to determine extent of disease for therapy. Flexible sigmoidoscopy with biopsy is often sufficient for those with established disease to confirm disease activity. Among patients with long-standing quiescent disease proximal to the rectum, colonoscopy with biopsies is routinely performed for dysplasia surveillance.
Mild to moderate disease
Oral mesalamine
The best option for patients with mild to moderate UC is mesalamine (5-aminosalicylate), orally or rectally. Response rates vary because of differences in dose, disease activity and definitions of outcomes but about two-thirds will respond. Sulfasalazine, the first of this class, is both an inexpensive and effective medication. It remains essentially unchanged until it reaches the colon where it is cleaved into the active moiety 5-aminosalicylate and sulfapyridine by bacteria. Sulfapyridine (a sulfa antibiotic) is responsible for most of the observed toxicity that includes allergic reaction or renal toxicity (Box 15.2). The remaining mesalamine formulations differ on their mechanism and site of drug delivery. Newer preparations release in the colon, require fewer tablets and can be dosed once or twice daily to improve compliance. These medications are well tolerated. Headache is the most common adverse effect and, rarely, renal toxicity occurs. A baseline serum creatinine should be determined prior to starting therapy. Pancreatitis has also been described with sulfasalazine due to its sulfa moiety but also with mesalamine, although the likelihood with mesalamine is extremely low.
Box 15.2 Side effects of sulfasalazine∗
∗ Start at one 500 mg table, twice daily on the first day after meals, then increase by two tablets every second day until the patient is taking the full amount (typically, 1 g four times a day in acute disease; and 1 g twice a day for prophylaxis). Usually efficacious within 2–4 weeks (in 40–80% of patients) A blood count and urinalysis should be performed before starting therapy and then regularly during the first 3 months of treatment, and thereafter every 6 months. Supplement with folate, 1 mg/day orally. The drug may also be of specific benefit for the arthropathy in ankylosis spondylitis associated with inflammatory bowel disease.
Mesalamine is not useful in moderate to severe UC and in mild to moderate disease is often administered at too low a dose. In appropriate doses all are probably equally effective. Sulfasalazine is relatively inexpensive and once or twice daily preparations will enhance compliance. Olsalazine should be avoided if possible, because it can cause secretory diarrhoea. Common preparations with the usual doses to induce and maintain remission are outlined in Table 15.4.
Table 15.4 Oral mesalamine preparations for induction and maintenance of remission in mild to moderate ulcerative colitis
| Optimal daily dose for remission (g) | ||
|---|---|---|
| Drug | Induction | Maintenance |
| Oral mesalamine | ||
| Sulfasalazine | 4–6 | 2–4 |
| Eudragit S coated | 3.6–4.8 | 2.4–3.6 |
| Ethylcellulose coated | 3–4 | 2–3 |
| Balsalazide | 6.75 | 3–4.5 |
| Topical mesalamine | ||
| Mesalamine suppository | 0.5–1 twice daily | 1 daily |
| Mesalamine enema | 4 nightly | 4 nightly or every other night |
From Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults. Am J Gastroenterol 2010; 105:501–523.
Moderate to severe colitis
In cases of moderate to severe UC systemic corticosteroids or infliximab are the best options.
Infliximab
Infliximab, an antibody to tumour necrosis factor alpha (TNF-α), is an important option for the both the induction and maintenance of remission among patients with moderately to severely active ulcerative colitis. It is typically reserved for patients whose disease is refractory to corticosteroids, and resistant to corticosteroid tapering for those who have had significant steroid related side effects. Skin testing for tuberculosis and chest x-ray should be obtained prior to starting therapy because this agent has been associated with the reactivation of tuberculosis. It is also rarely associated with reactivation of histoplasmosis or hepatitis B and specific testing of at-risk groups should be performed.
Maintenance of remission
Mesalamine
Mesalamine is the best treatment to maintain remission in cases of mild to moderate colitis where remission was induced by one of these medications. It may also maintain remission on selected patients whose remission was induced by corticosteroids and who were not taking mesalamine or were taking too low a dose of these medications. Maintenance dosing for various preparations in outlined in Table 15.4.
Azathioprine or 6-mercaptopurine
Azathioprine (2–2.5mg/kg) and 6–mercaptopurine (1–1.5mg/kg) are equally effective in 60–70% of patients in maintaining remission and allowing corticosteroid tapering but are slow to act, requiring 3–4 months or more to take effect. Clinical and haematological monitoring by experienced clinicians is necessary. Pancreatitis, hepatitis and severe leukopenia are important adverse events. Pancreatitis and hepatitis are idiosyncratic whereas leukopenia is dose dependent (Box 15.3).
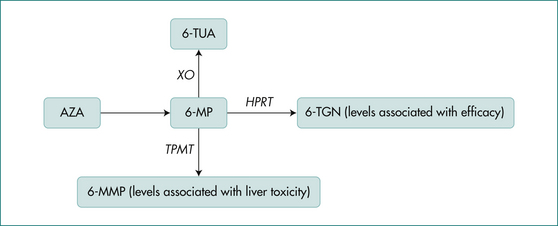
Once ingested, azathioprine is rapidly converted to 6-mercaptopurine. The main competing pathways for the metabolism of 6-mercaptopurine are conversion to 6-thioguanine (6-TG), the active therapeutic moiety, and 6-methylmercaptopurine(6-MMP), an inactive excretion product associated with liver toxicity (Fig 15.3). Elevated 6-TG results in immunosuppression and leukopenia and levels may correlate with efficacy, but this is controversial. The enzyme responsible for conversion to 6-MMP is thiopurine methyltransferase (TPMT). Its activity is genetically determined and can be normal, intermediate or absent. Patients with normal TPMT (88%) can start either medication at the target dose, 2–2.5 mg/kg azathioprine or 1–1.5 mg/kg 6-mercaptopurine. Those with an intermediate TPMT level (11%) are usually started on half this dose while those unable to metabolise the drug (0.3%) should not receive it. Diminished capacity of TPMT results in elevated 6-TG and subsequent toxicity manifested by profound leukopenia. Assays for TPMT, 6-TG and 6-MMP assist in the proper use of these medications.
Colorectal neoplasia
The methods to avoid development of colorectal cancer include prophylactic colectomy and surveillance colonoscopy. Surveillance colonoscopy is preferred but colectomy should be considered for patients with longstanding disease especially if their disease is refractory or requires immunosuppressive therapy. Decisions must be individualised based on patient preference with understanding of the risks and benefits of each strategy. Patients with pancolitis and disease longer than 8–10 years should have surveillance colonoscopy yearly or every other year. Patients with left-sided disease follow a similar schedule but some begin surveillance later. In patients with UC who have primary sclerosing cholangitis, surveillance should begin at diagnosis, given the risk of earlier cancer.
Surgical treatment
UC can be cured by colectomy and in many cases the patient’s overall quality of life improved. Colectomy is indicated for treatment failures, development of acute complications from active disease, chronic complications such as dysplasia, carcinoma and recurrent haemorrhage, or growth retardation in children (Box 15.4). The surgical procedure of choice is the ileal pouch anal canal anastomosis (IPAA). First the colon is completely removed and a ‘J’-shaped ileal pouch constructed with anastomosis to the anal canal. A diverting loop ileostomy is created to allow the anastomosis to heal. The ileostomy is closed 2 months later. Although this surgery is becoming more common, results are still dependent on the surgeon’s experience. Pouch failure rates vary but are generally under 10%. Most patients can expect to have about five bowel movements a day and one nocturnal bowel movement after IPAA. This can be much higher when the patient has refractory pre-existing diarrhoea-predominant irritable bowel syndrome (IBS). These patients might be better off with a standard ileostomy.
Crohn’s Disease
Aetiology
Crohn’s disease (CD) is a chronic idiopathic inflammatory bowel disease affecting primarily young adults. While, like UC, family history is the most important risk factor, only about 10% of patients overall have an affected family member. It tends to occur within families and is more common in individuals of Jewish ethnicity. Unlike UC, where cigarette smoking is protective, in CD it results in an earlier age at diagnosis and a worse disease course.
Clinical presentation
Crohn’s disease is a heterogeneous disorder with clinical presentation, prognosis and response to therapy is based on age at diagnosis, site of involvement and behaviour (Table 15.4). Younger age at diagnosis is associated with a worse prognosis. CD may be associated with extraintestinal manifestations in some patients (Table 15.5), which may be associated with disease activity. Those not associated with disease activity may run a clinical course independent of the disease and persist after treatment.
| Age at diagnosis | A1 below 16 years |
| A2 between 17 and 40 years | |
| A3 above 40 years | |
| Location | L1 ileal |
| L2 colonic | |
| L3 ileocolonic | |
| L4 isolated upper disease∗ | |
| Behaviour | B1 non-stricturing, non-penetrating |
| B2 stricturing | |
| B3 penetrating | |
| Perianal disease | Present or absent |
∗ Isolated upper disease is a modifier that can be added to other locations when concomitant upper disease is present.
From Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus and implications. Gut 2006; 55:749–753.
Disease location and behaviour
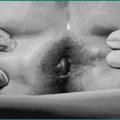
Most patients (40%) present with disease involving their terminal ileum and caecum, 30% have isolated small bowel, 25% have isolated colonic and 5% have oesophageal, gastric or duodenal involvement. While the location of disease tends to remain constant, behaviour may change. Inflammation may be confined to bowel wall (non-stricturing, non-penetrating) initially, only to progress to fistula, abscess or perforation (penetrating) or result in fibrosis causing bowel obstruction (stricturing). Non-stricturing, non-penetrating disease leads to fever, diarrhoea, rectal bleeding or temporary obstructive narrowing. Penetrating disease can present as high fever, abdominal pain or peritonitis. Fistula to the bladder results in persistent urinary tract infection or pneumaturia, to the vagina causes vaginal discharge, and to the skin results in external drainage. Perianal fistulae are also a common manifestation of Crohn’s disease (see Ch 22 on perianal disease). Stricturing disease leads to repeated episodes dominated by obstructive signs or symptoms.
Diagnosis
Stool biomarkers
As discussed in the UC section, data on biomarkers, while encouraging, are limited. They can not be recommended for routine use in practice but may be of benefit in the future.
Radiology
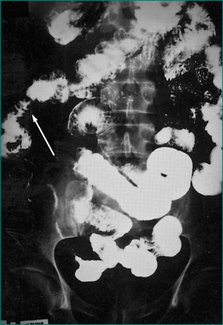
For patients with severe abdominal pain or findings suspicious for perforation, plain abdominal x-rays should be performed. Contrast enemas are rarely used because of the wide availability of colonoscopy. Contrast enemas usually employ a water-soluble agent and may be performed to assess colonic strictures that cannot be traversed by the endoscope. Contrast CT scanning of the abdomen is useful to evaluate for the presence of fluid collections such as an abscess and for a general assessment of the bowel wall thickening. Barium small bowel series can identify areas of inflammation, stricturing and the presence of bowel dilation (Fig 15.4). CT enterography is fast replacing small bowel series as the preferred method for assessing the small bowel. It is more accurate in assessment of disease activity and complications of stricture and abscess. It is common for patients with CD to have perianal fistula or penetrating complication in the pelvis. CT scanning is inaccurate in the assessment of these complications and magnetic resonance imaging (MRI), endoscopic ultrasound or examination under anaesthesia are the procedures of choice.
CT scanning has become pervasive among non-IBD patients for many disorders. Recent studies have led to significant concerns regarding radiation exposure because of the associated higher rates of malignancy and death compared to the general population. This risk is particularly important among patients with CD where CT is commonly and frequently performed. MRI enterography is attractive in the assessment of the small bowel disease since it avoids radiation exposure and may replace CT enterography in the future.
Moderate to severe disease
Enteric-release budesonide
Enteric-release budesonide is an important corticosteroid agent that is very effective among patients with active CD involving the terminal ileum or ascending colon. Its benefit rests in its high first pass metabolism in the liver, resulting in decreased systemic side effects. A dose of 9 mg/day is dramatically better than mesalamine and nearly equivalent to prednisone. At a dose of 9 mg/day some adrenal suppression and steroid-related side effects do occur, but are less than those of prednisolone. It has now become a first-line therapy to induce remission among patients with CD involving the terminal ileum or ascending colon, but it does not maintain remission.
TNF-α antibodies
Infliximab is a parenterally administered chimeric antibody (part-mouse and part-human) to TNF-α. TNF-α is important cytokine in the inflammatory response resulting in Crohn’s disease. After administration of infliximab, the majority of patients will improve and half will go into remission with onset of effect at about 2 weeks after the infusion.In order to maintain response and minimize infusion reactions patients continue on this medication indefinitely. Adalumimab and certiluzimab are two additional agents that work through the same mechanism, have similar efficacy and are also continued indefinitely to maintain response. The use of additional immunosuppressive medications with these agents is controversial. Improved prognosis has led some to advocate earlier use in patients to alter the natural history of their disease. However, a significant number of patients may become intolerant or lose response to these medications over time. Contraindications are listed in Box 15.5.
Azathioprine and 6-mercaptopurine
Azathioprine (2–2.5 mg/kg) and 6-mercaptopurine (1–1.5 mg/kg) are equally effective in two-thirds of cases in maintaining remission and allowing corticosteroid tapering. As with UC, they are slow to act, requiring 3–4 months or more to take effect; this limits their use in active disease. Clinical and haematological monitoring by experienced clinicians is necessary. Pancreatitis, hepatitis and severe leukopenia are important adverse events. The metabolism of these agents was previously discussed in the UC section and the mechanism of action in treating inflammation in CD appears to be the same. TPMT should be checked prior to starting therapy (Fig 15.1).
Perforating (fistulising) Crohn’s disease
The treatment of perforating (fistulising) Crohn’s disease is problematic. Mesalamine, corticosteroids and methotrexate do not heal or close fistulae. Metronidazole in doses of 1–2 g/day decreases fistula drainage and in some cases results in healing but must be maintained indefinitely to maintain its effect. Ciprofloxacin in doses of 1–1.5 g/day has also shown a similar benefit in small studies. Azathioprine is effective in fistulizing Crohn’s disease with about one-third of fistulas closing. Slow time to onset in addition to low closure rates remain significant problems. This response may be improved at the higher doses currently used today.
Health maintenance in IBD patients
Clinicians must be sure that patient vaccination schedules are up to date and vaccinations given prior to the institution of immunosuppressive therapy if possible.
A summary of the key management issues is presented in Box 15.6.
Box 15.6 Key management issues in Crohn’s disease
Treatment approach
Key Points
Farmer R.G., Easley K.A., Rankin G.B. Clinical patterns, natural history and progression of ulcerative colitis—a long term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137-1146.
Kornbluth A, Sachar DB Ulcerative colitis practice guidelines in adults
Lichtenstein G.R., Hanauer S.B., Sandborn W.J., et al. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465-483.
Mac Kalski B.A., Bernstein C.N. New diagnostic imaging tools for inflammatory bowel disease. Gut. 2006;55:733-741.
Mahadevan U. Fertility and pregnancy in the patient with inflammatory bowel disease. Gut. 2006;55:1198-1206.
Moscandrew M., Mahadevan U., Kane S. General health maintenance in IBD. Inflamm Bowel Dis. 2009;15:1399-1409.
Satsangi J., Silverberg M.S., Vermeire S., et al. The Montreal classification of inflammatory bowel disease: controversies, consensus and implications. Gut. 2006;55:749-753.
Swaminath A., Kornbluth A. Optimizing drug therapy in inflammatory bowel disease. Current Gastrenterol Rep. 2007;9:513-520.