Chapter 180 Infertility, Male
 Diagnostic Summary
Diagnostic Summary
• Inability to conceive a child after 12 months of regular unprotected intercourse (at least twice weekly) with the same female partner and in the absence of female causes.
• Sperm abnormalities confirmed by two properly performed semen analyses of sperm count, morphology, motility, or other aspects.
 General Considerations
General Considerations
Infertility affects about 7.3 million women and their partners in the United States, which equates to approximately 12% of the reproductive-age population.1 One out of seven couples will experience difficulty conceiving. Infertility affects men and women equally, with approximately one third of cases being due to male factors, one third to female factors, and the remaining one third to joint issues. In the United States, it is estimated that about 6% of men between the ages of 15 and 50 years are infertile.2
Throughout the world there has been a recent and dramatic decline in fertility that appears to be unrelated to the socioeconomic status of any given country; however, deferred childbearing and improved contraception are undoubtedly major factors. Population growth is below the replacement rate in several countries—such as Sri Lanka, Denmark, and Spain—where there have been no obvious increases in abortion rates or contraceptive use. This loss of fertility has affected Denmark to the point where approximately 7% of all newborn babies are now being conceived by assisted methods.3 In the United States, some patients will require assisted reproductive technologies (ARTs) in order to conceive. Statistics indicate that in vitro fertilization (IVF) and similar treatments account for less than 3% of infertility services and approximately 0.07% of U.S. health care costs.
Fertility is a reflection of general health and well-being and can also indicate latent or undiagnosed genetic abnormalities or other etiologic considerations. These considerations are many and varied; thus, a comprehensive, holistic review is essential. An overview of the causes of male infertility is given in Table 180-1.
| Aspermia | An absence of semen despite male orgasm |
| Azoospermia | A complete absence of sperm (spermatozoa) in the semen. |
| Oligozoospermia | Reduced number of normal motile sperm cells (spermatozoa) in the ejaculate (compared with azoospermia, which means no sperm in the ejaculate). It includes laborious terms such as asthenozoospermia, teratozoospermia, and oligoasthenoteratozoospermia |
| Teratozoospermia | Sperm with abnormal morphology |
| Necrospermia | Death of sperm |
| Oligoasthenoteratozoospermia | An unnecessarily long name that indicates low count, weak motility, and abnormal morphology |
If a sperm deviates from normal, it is defined by the terminology given in Table 180-1.
 Diagnostic Considerations
Diagnostic Considerations
Andrology Assessments
Semen Analysis
An individual’s semen quality can vary considerably between samples, even in males with normal semen parameters. In interpreting the assessment, it is imperative that the clinician acknowledge the important fact that a diagnosis is not achieved until an abnormality is confirmed by two separate investigations. As a result, at least two and occasionally three semen analyses are needed, each several weeks apart, in order to gain an accurate picture of an individual’s average semen quality. It is well recognized that sperm count can be adversely affected by illness, especially fevers, which may temporarily suppress sperm count in normal males for several months. In this case, the semen analysis should of course be delayed. Additionally, general recommendations such as in-clinic collection (versus at-home collection), careful consideration of laboratory guidelines (abstinence timing, lubricant usage), and the standard of the andrology laboratory facility must be considered in reviewing results. Table 180-2 outlines the World Health Organization’s (WHO’s) guidelines for assessing a semen analysis.
| SEMEN PARAMETER | LOWEST REFERENCE (REFERENCE RANGE) | INTERPRETATION AND TREATMENT OBJECTIVE |
|---|---|---|
| Standard Components of a Semen Analysis | ||
| Abstinence | Parameters are defined based on abstinence of 3 days. | It is essential to ensure that males ejaculate and then count the required 3 days’ abstinence. |
| Collection method | Optimal collection is via masturbation; however, specialized condoms can be provided for males with religious restrictions. Additionally, standard lubricants can interfere with the accuracy of the reading and must be avoided. Andrology laboratories are able to supply alternatives. | |
| Specimen | Semen sample must be complete. | Incomplete samples are frequent and will distort readings. Assessment can be determined only by reviewing a full sample owing to variations in prostatic secretion vs. epididymal involvement. |
| Analysis time | Sample must be analyzed within 60 min and is best collected in the clinic environment to prevent complications. | |
| Appearance | Nil debris, nil clumping, or viscosity changes, liquefaction complete. | Debris, clumping, viscosity, or liquefaction issues can suggest systemic congestion, poor hydration, poor elimination, or immune reactions (clumping especially). It can also indicate poor ejaculation frequency. |
| pH | >7.2 (7.2-7.8) | pH control is essential for sperm survival. An abnormally high or low semen pH can kill sperm or affect their ability to move or to penetrate an egg. The pH of the sample will be affected if there was a delay between sample collection and analysis. If the pH is <7.0 and the sample is azoospermic, there may be an obstruction of the ejaculatory ducts or bilateral congenital absence of the vas deferens (CBAVD). pH irregularities can relate to dietary intake and hydration level. Very acidic samples can indicate obstruction and require referral. |
| Volume | >1.5 mL (1.4-1.7 mL) | Volume can be affected by the period of abstinence (3 days are recommended), incomplete ejaculation, and retrograde ejaculation. It generally indicates dehydration; treatment should consist of hydration calculation based on weight and energy expenditure. |
| Concentration | ||
| Sperm concentration | > 15 million/mL (12-16 million/mL) | Concentration can be affected by a number of factors, including:
The finding of no sperm in the ejaculate suggests either an absence of sperm production or obstruction to sperm outflow. It is most important that an azoospermic semen sample be spun down to carefully examine whether the ejaculate contains even a few sperm. |
(>25% rapid, >40% progressive, >50% motile)
There are other important conditions that predominantly affect sperm motility, such as sperm autoimmunity, a condition that accounts for about 6% of male infertility. No movement (immotile sperm) may be due to structural problems in the sperm’s tail or to death of sperm.
The percentage of sperm that are alive (sperm vitality) is noted because this declines in association with genital tract infections and disorders of sperm transport through the genital tract. The proportion of live sperm is assessed if total motility is <50%. Low motility and high vitality could indicate a disturbance of the motility apparatus. If >75% of sperm are dead, immobilizing antisperm antibodies might be present and testing is encouraged.
Poor motility can often indicate autoimmune processes; infection; lack of mitochondrial energy to propel the sperm; or medication, alcohol or other toxins that affect semen quality.
Note: A trial wash can provide specificities of morphologic abnormalities (i.e., head, neck, tail).
Morphology is often a direct reflection of generalized toxicity, because semen is a by-product of the body and is a major eliminatory channel. Detoxification, avoidance of environmental toxins, and immaculate dietary practices are essential. Key nutrients in sperm structure must be considered, including protein, essential fatty acids (DHA), all antioxidants including coenzyme Q10, zinc, vitamins C and E, and selenium.
Ascertaining the type of infection is the primary objective, with subsequent targeted treatment to eradicate it. It is essential to assess the female partner to prevent cross-infection.
Abstinence or a barrier method until the immune system is regulated is essential, along with concurrent autoimmune treatment with herbal medicines, dietary modifications, lifestyle modifications, and nutritional supplementation.
>50% pathologically significant (except tail tip binding)
High green stain (HG):
DNA fragmentation can be attributed to various pathologic conditions including cryptorchidism, cancer, varicocele, fever, age, infection, leukocytospermia, and others. Many environmental conditions can also affect DNA fragmentation, such as chemotherapy, radiation, prescribed medicines, air pollution, smoking, pesticides, chemicals, heat and ART preparation protocols.
Research indicates that sperm with high levels of DNA fragmentation have a lower probability of producing a successful pregnancy. Samples with a DNA fragmentation level greater than 29% are likely to have significantly reduced fertility potential, including a significant reduction in term pregnancies and an increased miscarriage rate. Sperm that appears to be normal by traditional semen analysis parameters may have extensive DNA fragmentation.
It is normal for up to 1:5 sperm (20%) to have some DNA fragmentation. Mature sperm are protected from damage, because 85% of the chromosome are bound by protamines into a condensed, compact structure. If more than 20% of sperm have DNA damage, there is an increased risk of infertility, poor oocyte fertilization, defective/impaired embryo development, increased probability of implantation failure, miscarriage and recurrent miscarriage (up to 3-4 times higher) and genetic disease or childhood cancer in the next generation.
Once male germ cells have completed meiosis, they lose their capacity for DNA repair, discard their cytoplasm (containing the defensive enzymes that protect most cell types from oxidative stress), and eventually become separated from the Sertoli cells that have nursed and protected them throughout their differentiation into spermatozoa. In this isolated state spermatozoa must spend a week or so journeying through the male reproductive tract and, uniquely in our species, a further period (up to 3 or 4 days) in the female tract waiting for an egg. During this period of isolation, sperm DNA is vulnerable to damage by both xenobiotics and electromagnetic radiation. Such DNA damage is associated with male infertility; its aberrant repair in the fertilized egg may result in mutations in the embryo with the potential to either induce abortion or impair the health and fertility of the offspring.134,135
Treatment consists of environmental review and modification as well as exceptionally high doses of antioxidant prescription.
Data from World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed. Geneva, Switzerland: 2010, cited in Hechtman L. Clinical Naturopathic Medicine. Chatswood, Australia: Elsevier Australia.
A semen analysis can be conducted by a number of assessments, including the following:
• General semen analysis (SA): To assess motility, morphology, concentration, volume, appearance, and so forth.
• Sperm chromatin integrity test: To determine the level of DNA damage to sperm
• Immunobead test: To assess for antibodies against sperm
• Semen culture: To assess for infection
• Retrograde ejaculatory testing: To assess for retrograde semen flow or obstruction if semen analysis yields an extremely low count
• Trial wash: To assess sperm factors and determine whether IVF or intracytoplasmic sperm injection (ICSI) is more appropriate, conducted prior to IVF and/or ICSI procedures
• MESA/TESE/PESE (microsurgical epididymal sperm aspiration/testicular sperm extraction/percutaneous epididymal sperm aspiration): To extract sperm from the testicles by a specialized procedure when sperm count is low/absent or if ejaculation is not possible
Male Reproductive Assessment
A thorough assessment of the male patient is crucial to accurately determine his general and fertility health. Some of these assessments may require referral to a fertility specialist, urologist, or endocrinologist; however, thorough questioning should be conducted by the naturopath to elucidate a full history and assess causative or contributing factors. Tables 180-3 through 180-6 highlight the most relevant assessments required in a fertility workup.
| ASSESSMENT | ELABORATION AND EXPLANATION |
|---|---|
| Age | What ages are the couple? |
| Fertility history | How long have they been trying to conceive, and have they ever conceived previously (together/separately)? Do they have any idea why they have not been able to conceive? |
| Sexual history | STI screen: Potential sexually transmitted disease exposure, symptoms of genital inflammation (e.g., urethral discharge, dysuria) |
| Medication history | Such as sulfasalazine (Azulfidine), methotrexate, colchicine, cimetidine (Tagamet), spironolactone (Aldactone) |
| Surgical history | Such as previous genitourinary surgery |
| Contraception | When it was ceased and the likely speed of its reversibility |
| Fertile times | Whether the couple engage in regular intercourse during fertile times |
| Lifestyle factors | Diet, exercise, alcohol, smoking cessation, recreational drug use, environmental toxin screen |
| Prior paternity | Previous fertility |
| Psychosexual issues (erectile, ejaculatory) | Interference with conception |
| Pubertal development | Poor progression suggests underlying reproductive issue |
| A history of undescended testes | Risk factor for infertility and testicular cancer |
| Previous genital infection (STI) or trauma | Risk of testis damage or obstructive azoospermia |
| Symptoms of androgen deficiency | Indicative of hypogonadism |
| Previous inguinal, genital, or pelvic surgery | Testicular vascular impairments, damage to vasa, ejaculatory ducts, ejaculation mechanisms |
| Medications, drug use | Transient or permanent damage to spermatogenesis |
| General health (diet, exercise, smoking) | General health screen |
Modified from Hechtman L. Clinical Naturopathic Medicine. Chatswood, Australia: Elsevier Australia, 2011.
TABLE 180-4 Physical Examination
| ASSESSMENT | ELABORATION AND EXPLANATION |
|---|---|
| General examination | Acute/chronic illness, nutritional status |
| Genital examination | Assess for varicocele, testicular size, and other genital factors Testes: Small testes suggest spermatogenic failure Presence of vas deferens: may be congenitally absent Epididymides: thickening or cysts may suggest previous infection and resultant obstructive problems Varicoceles: detected when standing, coughing, or performing Vaslsalva maneuver Penis: assessed for abnormalities (e.g., Peyronie’s disease) that may interfere with intercourse |
| Degree of virilization assessment | Assess for signs of virility Signs of androgen deficiency (e.g., increased body fat, decreased muscle mass, decreased facial and body hair, small testes, Tanner stage <5) |
| Prostate examination | Assess if history suggests prostatitis or a sexually transmitted infection |
Modified from Hechtman L. Clinical Naturopathic Medicine. Chatswood, Australia: Elsevier Australia, 2011.
TABLE 180-5 Endocrinologic Assessments
| ASSESSMENT | JUSTIFICATION FOR ASSESSMENT |
|---|---|
| Follicle stimulating hormone (FSH) | Assessment to ensure that hormonal status is optimal to eliminate hormonal abnormalities
Testosterone Testosterone is often normal (8-27 nmol/L) even in men with significant spermatogenic defects. Some men with severe testicular problems display a fall in testosterone levels and rise in serum LH. These men should undergo evaluation for androgen deficiency. The finding of low serum testosterone and low LH suggests a hypothalamic–pituitary problem (e.g., prolactinoma; serum prolactin levels required). Elevated levels are seen when spermatogenesis is poor (primary in testicular failure); in normal men, the upper reference value is approximately 8 IU/L. In azoospermic men, 14 IU/L strongly suggests spermatogenic failure, 5 IU/L suggests obstructive azoospermia; a testis biopsy may be required to confirm that diagnosis. |
| Progesterone (P4) | |
| Prolactin (PRL) | |
| Luteinizing hormone (LH) | |
| Total testosterone, free testosterone | |
| Sex hormone–binding globulin (SHBG) | Evaluates if concentration of SHBG is affecting the amount of testosterone available to body tissues. |
| DHEA-S, cortisol | Additional hormone levels should be reviewed on an individual basis, including a full adrenal profile if the impact of stress is considered relevant. |
Modified from Hechtman L. Clinical Naturopathic Medicine. Chatswood, Australia: Elsevier Australia, 2011.
| ASSESSMENT | JUSTIFICATION |
|---|---|
| General Health Assessments | |
| FBC, blood type Standard blood chemistries 25[OH]D3 Fasting glucose Cholesterol profile General sexually transmitted infection (STI) screen |
General health assessments to eliminate other abnormalities |
| TSH and urinary iodine (24-hour or morning spot) | Query thyroid function and iodine status |
| Urinalysis/swab | |
| Infection screen | General urinalysis to eliminate underlying infection or abnormality. Urogenital infections have been found to play a part in the genesis of miscarriage136,137 and infertility.138 Most patients are unaware of their presence owing to the asymptomatic nature of these infections. The most common infections that require screening include Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, and Neisseria gonorrhoeae. |
| Advanced Fertility Assessments | |
| Karyotyping and subsequent genetic testing | Advanced fertility assessments if previous results show no abnormality or if infertility remains unexplained. WHO guidelines suggest that peripheral blood karyotyping analysis can be diagnostically helpful. Abnormal genotype may be present in up to 12% of azoospermic men and 4% of oligospermic men. Cystic fibrosis screening is recommended for azoospermia if due to CBAVD. Optional screening for Y-chromosome microdeletion if sperm count is <5 million/mL. |
| Scrotal (testicular) ultrasonography | History of undescended testes or concern regarding testicular cancer. |
| Transrectal ultrasonography | If ejaculatory duct obstruction suspected. |
| MTHFR C677T MTHFR 1298C Prothrombin G20210A Factor V Lieden Selenium assay Fasting homocysteine |
Indicated in instances of miscarriage, unexplained infertility, marked sperm abnormalities, or implantation issues. |
| Other Considerations | |
| Nutrient and toxic element screening | Assessments of toxic elements, including aluminum, arsenic, cadmium, lead, and mercury are crucial so as to eliminate them as causative or contributing factors. It is widely accepted that excessive exposure to heavy metals has a detrimental effects on fertility139,140 and must therefore be assessed and remedied during the preconception period. |
| Environmental impact | Other environmental assessments, including those that assess porphyrins, PCBs, chlorinated pesticides, volatile solvents, phthalates, parabens, and other toxins. These should additionally be considered due to their deranging effects on reproductive function, endocrinology, gamete development, and thus embryologic potential. |
Modified from Hechtman L. Clinical Naturopathic Medicine. Chatswood, Australia: Elsevier Australia, 2011.
 Therapeutic Considerations
Therapeutic Considerations
• Treatable conditions: One in eight infertile men has a treatable condition that can be overcome, so that natural conception may become possible.
• Untreatable subfertility: Three quarters of infertile men have sperm present in the semen, but in lower numbers than normal. In these cases the problem cannot be solved without intervention. These men are often defined as subfertile, since pregnancies may occur but at lower rates than usual. On average, more months of trying are needed to achieve conception, or conception may simply not occur. Following naturopathic intervention, it may be possible to address infertility factors or, alternatively, offer a referral for ARTs/IVF.
• Untreatable male sterility: About one in nine infertile men has no sperm in his semen or testes and cannot be treated. Sperm-producing cells in the testes either have not developed or have been irreversibly destroyed. Adoption or donor insemination are the only possibilities for couples in this group who wish to have families.
Of importance, acknowledging and addressing nutritional status throughout this developmental stage can significantly influence the prospective outcome. The concept of nutrient repletion is highly applicable within a fertility context. Optimal fertility is best achieved when prime health is realized. The repletion model indicates that prescriptions are required for a minimum of 3 months to properly address any deficiencies present and to ensure that all nutrients are used within each required pathway in the body. For example, zinc is involved in hundreds of pathways, including those that affect reproductive health and function. To rectify all possible deficiencies, sufficient zinc supplementation at a repletion dose (typically higher than in general prescriptions) for an extended time (at least 3 months) can enable optimal correction. At lower doses or doses of insufficient duration, zinc treatment may address only those health concerns that have the highest priority (i.e., immune function vs. sperm production).
Improving Sperm-Controlling and Sperm-Damaging Factors
Scrotal Temperature
Infertile men should wear boxer-type underwear and periodically apply a cold shower or ice to the scrotum. They can also choose to use a device called a testicular hypothermia device or “testicle cooler” to reduce scrotal temperatures. The testicle cooler looks like a jock strap from which long, thin tubes have been extended. The tubes are attached to a small fluid reservoir filled with cold water that attaches to a belt around the waist. The fluid reservoir is also a pump that causes the water to circulate. When the water reaches the surface of the scrotum, it evaporates and keeps the scrotum cool. Because of evaporation, the reservoir must be filled every 6 hours or so. It is recommended that the testicle cooler be worn daily during waking hours. Most users claim that it is fairly comfortable and easy to conceal.15
Estrogen and Xenoestrogen Exposure
According to experts on the impact of the environment and diet on fetal development, we now live in an environment that can be viewed as “a virtual sea of estrogens.”16,17 Increased exposure to environmental estrogens and other environmental pollutants during fetal development as well as during the reproductive years is suggested to be a major cause of the tremendous rise in the incidence of disorders of development and function of the male sexual system18 (see Box 180-1).
BOX 180-1 Compounds that Exert Estrogenic Activity
• Alkylphenols (intermediate chemicals used in the manufacture of other chemicals)
• 4-Methylbenzylidene camphor (4-MBC) (sunscreen lotions)
• Butylated hydroxyanisole, BHA (food preservative)
• Bisphenol A (monomer for polycarbonate plastic and epoxy resin; antioxidant in plasticizers)
• Dichlorodiphenyldichloroethylene (one of the breakdown products of DDT)
• Dieldrin (banned insecticide)
• Endosulfan (widely banned insecticide)
• Ethinylestradiol (released into the environment as a xenoestrogen)
• Heptachlor (restricted insecticide)
• Lindane, hexachlorocyclohexane (restricted insecticide)
• Metalloestrogens (a class of inorganic xenoestrogens)
• Methoxychlor (banned insecticide)
• Nonylphenol and derivatives (industrial surfactants; emulsifiers for emulsion polymerization; laboratory detergents; pesticides)
• Pentachlorophenol (restricted general biocide and wood preservative)
• Polychlorinated biphenyls, PCBs (banned; formerly used in electrical oils, lubricants, adhesives, paints)
• Phenosulfothiazine (a red dye)
• Propyl gallate (used to protect oils and fats in products from oxidation)
One can best view the relationship between estrogens and male sexual development by examining the effects of the synthetic estrogen diethylstilbestrol (DES). Between 1945 and 1971, several million women were treated with DES. By 1970, the side effects of DES became better known. DES is now recognized to have led to substantial increases in the number of men suffering from developmental problems of the reproductive tract as well as decreased semen volume and sperm count.16 Apart from having been used in humans, DES and other synthetic estrogens were used for 20 to 30 years in the livestock industry to fatten the animals and make them grow faster.
There are reports that estrogens have been detected in drinking water.17,19 Presumably they are recycled from excreted synthetic estrogens (birth control pills) at water treatment plants. These estrogens may be harmful to male sexual vitality because they are more potent—they do not bind to sex hormone–binding globulin (SHBG). Purified or spring water may be a suitable option to prevent exposure. It is also important to ensure that bottled water is avoided owing to the bisphenol A content of plastic bottles.
All of the estrogenic factors previously discussed are thought to have their greatest impact during fetal development. On the basis of animal studies, these estrogens inhibit multiplication of the Sertoli cells. The number of Sertoli cells is directly proportional to the number of sperm that can be produced, because each Sertoli cell can support only a fixed number of germ cells that will develop into sperm. Sertoli cell multiplication occurs primarily during fetal life and before puberty and is controlled by follicle-stimulating hormone (FSH). In animal studies, estrogens administered early in life have been found to inhibit FSH secretion, resulting in a reduced number of Sertoli cells and, in adult life, diminished sperm counts.
One example of the impairment of male sexual development by environmental estrogens is the ability of vinclozolin, a fungicide used in the wine industry, to disrupt the fertility of male rats.20 Alarmingly, just one exposure of a pregnant female rat to this fungicide was found to disrupt spermatogenesis in more than 90% of the male offspring for at least four generations via an effect exclusively transmitted through the male germ line.
Environmental toxins are also linked to increasing testicular cancer rates, testicular dysgenesis syndrome (TDS), cryptorchidism, and hypospadias.3,21 Whether the outcome is impaired spermatogenesis, TDS, testicular cancer, or any other disturbance may depend on the timing and nature of the xenobiotic attack and the genetic background on which these factors are acting. As such, a determination of the outcome will have to take into account the patient’s polymorphism profile for proteins involved in detoxification, such as the cytochrome P450s and glutathione-S-transferases. The bottom line is that the environmental impact on spermatogenesis cannot be underestimated. Industrial growth since the end of World War II has introduced many complex chemicals into the environment that are novel to biological detoxification systems. Some of these molecules are reproductive toxicants, capable of impairing fertility and inducing developmental abnormalities in the embryo, including errors in normal sexual differentiation.
The power of reproductive toxicants that target the germ line lies in their capacity to generate damage that can be passed down the generations via genetic or epigenetic means. A prime example is the effect of paternal smoking. Men who smoke heavily generate spermatozoa that may have high levels of DNA damage, largely as a result of oxidative stress. One of the consequences of this DNA damage is that the children of such men exhibit an increased incidence of childhood cancer.22 Although we have traditionally focused on the ability of cigarette smoke to induce lung cancer, a far more sinister effect is its ability to induce DNA damage in the germ line and thereby influence the health and well-being of future generations.
Exposure Route
1. Women may be exposed to xenobiotics during pregnancy, thereby disrupting the normal differentiation of the germ line in the fetus.
2. Women exposed to toxicants may transmit xenobiotics to their offspring via breast milk.
3. Because of acquired toxicity, men may contribute adverse effects on the integrity of DNA in the male germ line. Toxicologic studies in animal models reporting infertility, abortion, and birth defects as a result of male exposure to xenobiotics demonstrate that such associations are possible.9 Epidemiologic studies suggest that they are clinically significant.3,21
For a full discussion of potential environmental effects, see Chapter 35, “Environmental Medicine.”
Heavy Metals
Sperm are also particularly susceptible to the damaging effects of heavy metals such as lead, cadmium, arsenic, and mercury.18A hair mineral analysis for heavy metals should be performed on all men with reduced sperm counts to rule out heavy metals as a cause.
Radiation
Cell phones operate at 400- and 2000-MHz frequency bands and emit radiofrequency electromagnetic waves (EMWs).23 Cordless phones must be considered in this context as well; these use the 900-MHz, 1.9-GHz, 2.4-GHz, and 5.8-GHz bands. Reports of potential adverse effects of radiofrequency EMWs on the brain, heart, endocrine system, and DNA in humans and animals are commonly found in the literature. Specifically from a fertility context, they have also been implicated in DNA strand breaks.24 The relationship between cell phone use and male infertility remains unclear. Harmful EMWs emitted from cell phones may interfere with normal spermatogenesis and result in a significant decrease in sperm quality. Specific findings pertaining to sperm motility in humans have also been noted.25,26
In one observational study,27 the use of cell phones decreased semen quality by decreasing the sperm count as well as the sperms’ motility, viability, and normal morphology. This decrease in sperm parameters was dependent on the duration of daily exposure to cell phones and independent of initial semen quality. Of greatest importance was that sperm count, viability, and morphology declined as cell phone use increased. Specifically, use of the cell phone for more than 4 hours a day caused a 25% drop in the number of sperm produced, and only 20% of these looked normal.
It is therefore prudent to discourage cell phone use and recommend that male patients refrain as much as possible from storing cell phones in their pockets, owing to increased genital exposure to the frequency bands.
Cigarettes, Alcohol, and Illicit Drugs
Cigarette Smoking
A common source of oxidants is cigarette smoking, which is associated with decreased sperm counts and sperm motility as well as a higher frequency of abnormal sperm.28 Cigarette smoking, as well as the increase in environmental pollution, is thought to be a major contributor to the diminution in sperm counts seen in many industrialized nations over the past few decades.
Although passive and active smoking is known to lead to a number of health concerns, male patients are often complacent about its impact. However, heavy smokers have been shown to produce more than 20% less sperm. Cigarette smoking accelerates DNA damage of the sperm, and smoking cessation is the quickest treatment strategy to reverse such damage and thus affect sperm morphology parameters positively. When the sperm enters the oocyte during conception, the mRNA within the oocyte attempts to defragment the DNA and provide antioxidant support so as to improve sperm quality. It is important to note that the improvements to sperm occur prior to any oocyte maintenance. It is only once the sperm has been positively addressed that the mRNA and any antioxidant potential within the oocyte can address any oocyte deficiency, oxidative process, or DNA (or chromosomal) abnormality. When the male partner is a heavy smoker, it often means that there are simply insufficient reserves to repair the damage for both gametes; as a result, the oocyte fails, causing either fertilization deficits, a lack of implantation, or abnormalities in the embryo, thus initiating an early miscarriage or negative effects on the long-term health and fertility of the subsequent child.29 The female’s surveillance does not typically allow the compromised embryo to grow beyond early miscarriage, because the chance of a healthy birth is then unlikely. Therefore, this highly pertinent clinical discussion can motivate most male patients to take responsibility for their oxidative processes and commit to smoking cessation.
A recent systematic Cochrane review found that in both fertile and infertile populations, active and passive smoking is associated with reduced fertility and a decreased chance of producing a healthy, live infant. In males, cigarette smoking has been observed to impair sperm respiration, thus affecting the sperms’ mitochondrial function30 as well as causing a reduction in sperm motility and semen quality.31 It is therefore strongly recommended that all males cease both passive and active smoking for at least the preconception period and ideally beyond it.
Marijuana and Other Recreational Drugs
The effects of marijuana and other recreational drugs are difficult to determine because their use is illegal. Nevertheless, the use of such drugs generally should be discouraged, particularly because they have well-documented harmful effects on the developing fetus.32 A known fertility toxicant, marijuana contains constituents known as cannabinoids, which have been shown to impair signaling pathways, alter hormonal regulation, and complicate timing issues during embryo implantation.33 In males, cannabinoids have been found to inhibit the mitochondrial respiration of sperm,34 reduce testosterone production,33 decrease sperm motility, compromise sperm morphology, and decrease sperm function, specifically capacitation and acrosome reactions.35
Obesity
Obese men are known to have lower sperm counts (up to 50%), reduced sperm motility, reduced spermatogenesis, increased DNA fragmentation in sperm, and increased levels of erectile dysfunction. Additionally, extra-abdominal weight can increase scrotal temperature. Hormonal changes are primarily responsible for such changes in obese men. The levels of total and free testosterone are reduced in obese men in proportion to the level of obesity. Estrogen is increased owing to the peripheral aromatization of androgens in adipose tissue. The estrogens produced have a negative feedback effect on gonadotropin production, thereby reducing FSH. This reduction in FSH further reduces testosterone production and spermatogenesis. Additionally, increased body fat and a sedentary lifestyle are associated with raised testicular temperature, which further adversely affects spermatogenesis.36
Infections and Infertility
Infections in the male genitourinary tract—including infections of the epididymis, seminal vesicles, prostate, bladder, and urethra—are thought to play a major role in many cases of infertility.37 The exact extent of this role is largely unknown because of the lack of suitable diagnostic criteria coupled with the asymptomatic nature of many infections. The presence of antisperm antibodies or high levels of debris in the semen sample are considered to be good indicators of chronic infection in the absence of other clinical findings.
A wide number of bacteria, viruses, and other organisms can infect the male genitourinary system. Table 180-6 offers a list of the more common potential causative agents.
 Nutritional Considerations
Nutritional Considerations
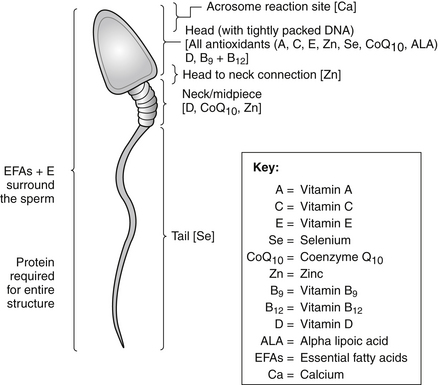
A single sperm comprises the key nutritional ingredients (see Figure 180-1).
Diet
The dietary guidelines in Chapter 44 provide sound guidance for improving fertility. In particular, it is important to eat the right types of fats. Surrounding the entire sperm is a “shield” of essential fatty acids protecting its structure, enabling continuity of movement, and safeguarding the integrity of the precious genetic material within. The male’s dietary intake must be assessed so as to ensure that all trans, rancid, and oxidized fats and excessive saturated fats are avoided. It is recommended to prescribe sufficient essential fatty acids from both supplemental and dietary sources. The presence of essential fatty acids ensures that sperm are kept fluid and flexible, which regulates the acrosome reaction, sperm-oocyte fusion, and sperm-oocyte fertilization.38
Because of the effects of fats and oils on agglutination and cell membrane dynamics, certain fats are best avoided in infertile men and consumption of others should be increased. Hydrogenated oils, trans fatty acids, and cotton oils should be avoided. Cottonseed is especially problematic since it may contain toxic residues because of the heavy spraying of cotton and its high levels of gossypol, a substance known to inhibit sperm function. In fact, gossypol is being investigated for possible use in a “male birth control pill.” Its potential as an antifertility agent became known after studies demonstrated that men who had used crude cottonseed oil for cooking had low sperm counts followed by total testicular failure.39 Excessive consumption of long-chain saturated fats from meat should also be avoided; these, combined with inadequate intake of essential fatty acids, can change the fatty acid composition of the sperm membranes, thus reducing fluidity and interfering with sperm motility. The patient must be taught to read food labels carefully and avoid all sources of cottonseed oil and other damaging oils.
To promote proper sperm membrane function, it is important to supplement with the long-chain omega-3 fatty acids from fish oil, because studies have shown that sperm motility strongly correlates with levels of omega-3 fatty acids, in particular docosahexaenoic acid (DHA), in the sperm membrane.40 One paper noted that excessive omega-6 compared with omega-3 fatty acids in seminal fluid decreased sperm concentration, sperm motility, and sperm morphology among patients with idiopathic oligoasthenoteratozoospermia.41 It is therefore advisable to encourage omega-3 sources as a preference and to restrict the use of popular omega-6 cooking oils, such as soy, corn, and safflower. Supplemental goals should be to prescribe an optimal dose of 1000 to 2000 mg of EPA+DHA daily, with additional dietary recommendations such as raw nuts and seeds; cold-pressed monounsaturated oils like olive, canola, and macadamia nut oils; avocados; and wild and sustainably farmed high EFA containing fish.
 Nutritional Supplements
Nutritional Supplements
Antioxidants
A recent Cochrane review42 assessed the impact of antioxidants on male subfertility by reviewing 34 trials and 2876 couples. Of importance, the authors concluded that there is sufficient evidence to prove that antioxidant supplementation in subfertile males improves the outcomes of live births and pregnancy rates for subfertile couples undergoing ART cycles. In greater detail, important points in this report included the following:
• Antioxidant use was associated with a statistically significant increased pregnancy rate compared with control (pooled OR 4.18, 95%; CI 2.65 to 6.59; P <0.00001).
• No studies reported evidence of harmful side effects of the antioxidant therapy used.
• One man in 20 will be affected by subfertility and 3% to 80% of male-factor subfertility is believed to be due to oxidative stress.43
• Subfertile men are confirmed to have lower levels of antioxidants in their semen than fertile men.43
• Levels of reactive oxygen species (ROS) are significantly higher in infertile sperm samples compared with others from healthy controls.
Free radical or oxidative damage to sperm is thought to be responsible for many cases of male infertility, high levels of free radicals having been found in the semen of approximately 40% of infertile men.44–46 The following three factors combine to render sperm particularly susceptible to damage by free radicals:
• A high membrane concentration of polyunsaturated fatty acids (PFAs)
All of these factors combine to make the health of the sperm critically dependent on antioxidants. Although most free radicals are produced during normal metabolic processes, the environment contributes greatly to the free radical load. Men exposed to higher levels of free radicals are much more likely to have abnormal sperm and sperm counts.44–46 Antioxidants are required to protect sperm against oxidative damage (which may alter DNA) as well as to instigate cellular repair of damage caused by environmental factors or aging. In the healthy male, the seminal plasma is naturally rich in antioxidants, since sperm are highly susceptible to the effects of ROS.
Vitamin C
Vitamin C improves all semen parameters. A marginal deficiency causes oxidative damage to sperm, resulting in reduced sperm motility and viability. Supplementation leads to improvement in both viability and motility, reduced numbers of abnormal sperm, and reduced sperm agglutination.47,48
Vitamin C (ascorbic acid), a major antioxidant present in extracellular fluid, is present at a high concentration in seminal fluid compared with blood plasma (364 vs. 40 mM) and is present in detectable amounts in sperm,49 where it prevents sperm agglutination and oxidative damage. In infertile men, vitamin C has been found in reduced quantity in the seminal plasma.50,51 Males with inadequate seminal vitamin C have also been observed to suffer from sperm DNA damage,50 suggesting that a defect or inadequate intake of vitamin C may prompt ROS to cause breakage and oxidation of sperm DNA.
When dietary vitamin C was reduced from 250 to 5 mg/day in healthy human subjects, the ascorbic acid content in seminal fluid decreased by 50% and the number of sperm with damage to their DNA rose by 91%.52 These results indicate that dietary vitamin C plays a critical role in protecting against sperm damage and that low dietary vitamin C levels were likely to lead to infertility.
It is now well documented that cigarette smoking greatly reduces vitamin C levels throughout the body, and it is proved that smokers require at least twice as much vitamin C as nonsmokers. In one study, men who smoked one pack of cigarettes a day received either 0, 200, or 1000 mg of vitamin C. After 1 month, sperm quality improved in proportion to the level of vitamin C supplementation.53 Nonsmokers appear to benefit from vitamin C as much as do smokers. In one study, 30 infertile but otherwise healthy men received either 200 or 1000 mg of vitamin C or placebo daily.54 Sperm count, viability, motility, agglutination, abnormalities, and immaturity were measured weekly. After 1 week, the 1000-mg group demonstrated a 140% increase in sperm count, the 200-mg group a 112% increase, and the placebo group no change. After 3 weeks, both vitamin C groups continued to improve, with the 200-mg group catching up to the improvement of the 1000-mg group. One of the key improvements was observed in the number of agglutinated sperm. Sperm become agglutinated when antibodies produced by the immune system bind to them. Antibodies to sperm are often associated with chronic genitourinary tract or prostatic infection. When more than 25% of the sperm are agglutinated, fertility is very unlikely. At the beginning of the study all three groups had more than 25% agglutinated sperm. After 3 weeks, the proportion of agglutinated sperm in the vitamin C groups dropped to 11%. Although this result is significant, the most impressive result of the study was that at the end of 60 days, several men in both of the vitamin C groups had impregnated their wives, compared with none in the placebo group. Therefore, vitamin C supplementation can be very effective in treating male infertility, particularly if it is due to antibodies against sperm.
Vitamin E
Vitamin E supplementation appears to be especially warranted because this vitamin is the main antioxidant in various cell membranes, including those surrounding sperm. Free radicals, if left alone, lead to the peroxidation of phospholipids in the mitochondria of the sperm, making the sperm immotile. Vitamin E has been shown to play an essential role in inhibiting free radical damage to the unsaturated fatty acids of the sperm membrane55 and in enhancing the ability of sperm to fertilize an egg in an IVF setting. Additionally, it has been shown to protect DNA within the sperm from damage.56
In one study, supplementation with vitamin E was found to decrease malondialdehyde concentration in sperm pellet suspensions and to improve sperm motility. Even more important, however, 11 of 52 (21%) treated infertile men impregnated their spouses, whereas none in the placebo group did so. Following completion of the study, 26 of the placebo patients were switched to vitamin E; soon thereafter, 4 were able to successfully impregnate their spouses.57 In another study, vitamin E (400 IU) and selenium (225 mcg) significantly improved sperm quality.58 Supplementation appears to be indicated on the basis of its physiologic effects alone.
Supplementation with vitamin E may also be useful for couples undergoing IVF. Vitamin E (200 mg/day for at least 3 months) was found to improve the in vitro fertilization rate of fertile normospermic males with low fertilization rates after 1 month of treatment, possibly by reducing the lipid peroxidation potential.59 Beneficial results have also been observed in patients undergoing ICSI, where vitamin E helps to prevent DNA fragmentation, thus improving ICSI outcomes.60
Vitamin A, Beta-Carotene, and Lycopene
Vitamin A is an antioxidant required for cellular growth and differentiation, gene expression and cellular differentiation, immunity, regulatory functions, and epithelial tissue integrity. It is necessary for the health of the testes and for sperm production. Low concentrations of vitamin A are associated with abnormal semen parameters in men,61 and deprivation of vitamin A in animals has been shown to lead to a loss of spermatogenesis due to degeneration of the germ cells, which is restored once vitamin A is reintroduced.62
Beta-carotene levels are significantly reduced in immune-infertile men. Intake is associated positively with a higher sperm concentration as well as higher quantities of motile sperm.63 Lycopene may be even more useful than beta-carotene. Lycopene is found in high concentrations in the testes and seminal plasma, and reduced levels have been demonstrated in men with infertility. In one clinical trial, 30 men with idiopathic nonobstructive oligo/astheno/teratozoospermia were administered 2 mg of lycopene twice a day for 3 months. Twenty patients (66%) showed an improvement in sperm concentration, 16 (53%) had improved motility, and 14 (46%) showed improvement in sperm morphology. In patients showing an improvement, the median changes were 22 million/mL in concentration, 25% in motility, and 10% in morphology.58
Selenium
Selenium is a critical antioxidant that is essential for male fertility because of its role in testosterone synthesis, normal sperm maturation, and motility64; moreover, clinical trials reveal that selenium has the ability to increase sperm motility and assist in the production of healthy spermatozoa.65 Selenium is also required structurally, because the sperm’s capsular selenoprotein is involved in the stability and motility of the mature sperm and also forms part of the glutathione peroxidase antioxidant system, which is vital for spermatogenesis and protects the sperm against the effects of ROS.66 In animal studies, depletion of mitochondrial glutathione peroxidase has been found to cause impaired sperm quality and severe structural abnormalities in the midpiece of spermatozoa, leading to infertility.67 The tail of the sperm relies on adequate selenium status to maintain its “whip-like” action. Without sufficient selenium, sperm are unable to swim in the right direction or may display marked immotility, thus preventing oocyte location and fusion.
The effects of selenium on sperm motility are highlighted in a study involving a subgroup of individuals with poorly motile sperm and subsequent subfertility.68 Over a 3-month period, the administration of selenium (either on it its own or as a combination of antioxidants, including vitamins A, C, and E) to males led to increased sperm motility when compared with placebo. Five men (11%) achieved paternity in the treatment group, in contrast to none in the placebo group. This small study highlights the efficacy of selenium supplementation in subfertile men and suggests that improved selenium status can, in turn, improve sperm motility and the possibility of successful conception. Although this number may be seen as small, it was highly meaningful to those who were successful in conceiving—and all the more significant in terms of the cost and convenience of supplementation as compared with IVF or ICSI.
More recently selenium (200 mcg/day) in combination with the antioxidant n-acetyl-cysteine (600 mg/day) was found to improve semen parameters in idiopathic oligo-asthenoterato-spermiaplasma males in a double-blind placebo-controlled randomized study undertaken over 6.5 months.69 Improvements in sperm count, motility, and morphology were all observed; however, once supplementation stopped, the parameters reverted back to their readings at baseline in two spermatogenesis cycles. This study did not include pregnancy rate.
Zinc
Zinc is perhaps the most important trace mineral for male sexual function and is found in high concentrations within the prostate and testes; particularly high amounts are also found in the semen (approximately 2.5 mg of zinc is lost per ejaculate). It is involved in virtually every aspect of male reproduction, including hormone metabolism, spermatogenesis, and sperm motility.70
Zinc plays an important role in all human living cells; it is involved in the transcription of RNA, the replication of DNA, and the synthesis of protein, all of which are crucial for reproduction and fertility. Additionally, it protects against free radical damage and ROS, which can impair sperm.71 Deficiency of zinc in males can lead to gonadal dysfunction70 and has been observed to be associated with idiopathic male infertility71 and impotence.
Zinc levels are typically much lower in infertile men with low sperm counts, indicating that a low zinc status may be the contributing factor to infertility.72,73 It has also been shown that zinc status directly correlates with an increase in sperm count as well as improvements in morphology and motility.74 In considering sperm structure, zinc has been shown to influence motility and the head-neck connection of the sperm75; it is also important in the stabilization of cell membranes and sperm chromatin.76 Finally, it has been shown to exert an antimicrobial effect on the seminal plasma, which is helpful if sperm antibodies or underlying genitourinary infection is present.77
Several studies have evaluated the effect of zinc supplementation on sperm counts and motility.78–81 The results of all of the studies support the use of zinc supplementation in the treatment of oligospermia, especially in the presence of low testosterone levels. The effectiveness of zinc is best illustrated by a study in 37 men with infertility of greater than 5 years’ duration whose sperm counts were less than 25 million/mL.27 Blood testosterone levels were also measured. The men received a supplement of zinc sulfate (60 mg elemental zinc daily) for 45 to 50 days. In the 22 patients with initially low testosterone levels, the mean sperm count rose significantly from 8 to 20 million/mL. Testosterone levels also increased, and 9 of the 22 wives became pregnant during the study. This result is quite impressive, given the long-term nature of the infertility and the rapidity of the results. In contrast, in the 15 men who had normal testosterone levels before the study, sperm counts increased slightly, but there was no change in testosterone levels and no pregnancies occurred.
B Vitamins (Especially Folic Acid and vitamin B12)
Folic acid and vitamin B12 are concentrated within the head of the sperm and provide vital nutritional potential in sperm generation and survival. Folic acid and B12 are required for healthy DNA and RNA synthesis, normal protein synthesis, and the regulation of gene expression.80 These nutrients are required to ensure that the DNA within the head of the sperm is structured appropriately and that each sperm (and the DNA within it) replicates identically.
Both folic acid and B12 facilitate spermatogenesis,82 which is reliant on DNA synthesis80 for germ cell growth and the rapid division of cells. Multiple studies have found that low levels of folic acid in seminal plasma are associated with increased sperm DNA damage,83 whereas B12 deficiency is strongly associated with reduced sperm motility and count.84 Because the human body has a high turnover of B12 and requires a continuous supply on a daily basis, supplementation is advisable for all men experiencing infertility regardless of proved deficiency state and especially for men who have sperm counts below 20 million/mL or a motility rate of less than 50%. In one study, 27% of men with sperm counts less than 20 million/mL who were given 1000 mcg/day of B12 were able to achieve a total sperm count in excess of 100 million/mL.81 In another study, 57% of men with low sperm counts who took 6000 mcg/day demonstrated improvements.85
Carnitine
Carnitine is derived from the amino acids lysine and/or methionine and plays a vital role in fatty acid metabolism. It works synergistically with coenzyme Q10 (CoQ10), highlighting the importance of coprescription for optimal benefit. It is essential in the transport of fatty acids into the mitochondria, and deficiency results in a decrease in fatty acid concentrations in the mitochondria and reduced energy production. It is believed to have protective antioxidant effects and provides energy to the testicles and spermatozoa specifically. Several studies comparing fertile with infertile men found that fertile men had a statistically significant larger amount of carnitine in their seminal sample than the infertile men, and that low levels of L-carnitine in the seminal plasma may be a potent marker for infertility.86
Carnitine concentrations are extremely high in the epididymis and sperm, suggesting a role in male reproductive function. The epididymis derives the majority of its energy requirements from fatty acids, as do the sperm, during transport through the epididymis. After ejaculation, the motility of sperm correlates directly with carnitine content; the higher the carnitine content, the more motile the sperm. Conversely, when carnitine levels are low, sperm development, function, and motility are drastically reduced.44
Several clinical studies have shown that carnitine supplementation can produce dramatic improvements in sperm counts and sperm motility. In the Italian Study Group on Carnitine and Male Infertility, 100 subjects were given 3000 mg of L-carnitine daily for 4 months.87 Carnitine was able to increase sperm counts and sperm motility in both a qualitative and a quantitative manner, as follows:
• The number of ejaculated sperm per milliliter increased from 142 billion to 163 billion.
• The percentage of motile sperm increased from 26.9% to 37.7%.
• The percentage of sperm with rapid linear progression increased from 10.8% to 18%.
These results are even more impressive if results for only the patients with the poorest sperm motility are examined. This subgroup saw even more significant gains on all parameters. For example, the percentage of motile sperm increased from 19.3% to 40.9%, and the percentage of sperm with rapid linear progression increased from 3.1% to 20.3%. These results have been confirmed in several double-blind studies.88–92
Alpha Lipoic Acid
Alpha lipoic acid is a powerful antioxidant indicated for its lipid and water solubility and because it assists in the chelation of heavy metals regardless of their storage site in the body. It is especially useful owing to its ability to regenerate other antioxidants, including vitamins C and E, CoQ10, and glutathione.93
Alpha lipoic acid exhibits marked antioxidant activity to sperm in animal studies.94–96 On review of research, it appears to act as a shield for the sperm, forming a protective barrier around the midpiece (aqueous layer) and within the structure itself (lipid layer). This protection is crucial, because it has been identified as one of the first places at which free radicals attack.96 It is therefore useful to consider in patients with high DNA fragmentation levels. Additionally, animal studies reveal that alpha lipoic acid improves sperm motility and viability, minimizes DNA damage,96 and protects against bacterial lipopolysaccharides, which can induce acute inflammation97; it and may also assist with energy supply to the sperm.96
Coenzyme Q10
CoQ10 is concentrated in the head and midpiece (neck) of the sperm. It is considered to be the most crucial and powerful antioxidant in sperm structure because of its role in mitochondrial energy release. It is believed to promote motility, foster sperm survival, and provide optimal energy to assist the sperm’s travel on its journey to the oocyte.
As a fat-soluble antioxidant and free radical scavenger, CoQ10 is required for the maintenance of cell membrane integrity and cell functioning. It is also specific for the health of all new cells, especially spermatozoa. CoQ10 in the seminal fluid and sperm98 helps to maintain optimal sperm motility.99,100 Decreased levels have been found in the seminal plasma and spermatozoa of males with idiopathic and varicocele-associated asthenospermia.101
L-Arginine
The amino acid arginine is required for the replication of cells, making it essential in sperm formation. Nitric oxide synthase uses L-arginine to synthesize nitric oxide, which can protect spermatozoa from lipid peroxidase damage. Via its role as a precursor to nitric oxide synthesis, arginine is required for angiogenesis, spermatogenesis, and hormone secretion.102
Some studies have shown that L-arginine can improve sperm count and motility.84,103 Stress, in particular, has been found to decrease the levels of arginine in sperm production pathways. Arginine supplementation is often but not always an effective treatment for male infertility. The critical determinant appears to be the level of oligospermia. If sperm counts are less than 20 million/mL, arginine supplementation is less likely to be of benefit. In order to be effective, the dose of L-arginine must be at least 4 g/day for 3 months. In perhaps the most favorable study, 74% of 178 men with low sperm counts had significant improvements in sperm counts and motility after arginine therapy.104
One double-blind randomized placebo-controlled crossover clinical trial examined the effects of Prelox, a combination of 80 mg/day of pycnogenol and 3 g/day of L-arginine aspartate.105 The results showed an improvement in semen parameters in 50 males with idiopathic infertility over a treatment period of 4 weeks. Also observed were significant increases in ejaculate volume, concentration, and number of spermatozoa as well as the percentage of vital spermatozoa as compared wih placebo. The percentage of spermatozoa with good progressing motility also increased significantly, whereas the percentage of immotile spermatozoa decreased. These results appear to be due to a combination of the antioxidant activity of Pycnogenol and/or the activity of L-arginine in stimulating the activity of endothelial NOS, leading to enhanced motility of spermatozoa. In a small pilot study, Pycnogenol (200 mg daily for 90 days) alone was shown to improve sperm morphology by 38% and the mannose receptor binding assay scores by 19%.106
Botanical Medicines
Panax ginseng (Korean ginseng)
Current scientific investigation suggests that Panax ginseng (Chinese or Korean ginseng) may be supportive in the treatment of male infertility. Both botanicals have a long history of use as male “tonics.” In studies with animals, Panax ginseng has been shown to improve the growth of the testes, raise sperm formation and testosterone levels, and increase sexual activity and mating behavior. It is classed as an energy tonic, indicated when there is lowered vitality and therefore impaired physical performance and sexual function. The active constituents (ginsenosides) have been shown to enhance nitric oxide production, which is useful in regulating the capacitating process of spermatozoa and the acrosome reaction, thus improving fertilization sperm motility.107,108 Additionally, ginsenosodes have been shown to affect different levels of the hypothalamic-pituitary-testicular axis,109 which can assist in modulating stress-induced infertility or lowered testosterone from insufficient DHEA synthesis. In clinical trials, Panax ginseng has been shown to increase testosterone levels as well as, sperm counts and motility in patients with oligospermia, some of whom had varicocele,109 and to improve erection and libido.110 For additional information and references, see Chapter 110.
Pygeum africanum (Pygeum)
Pygeum africanum may be effective in improving fertility in cases where diminished prostatic secretion plays a significant role. Pygeum has been shown to increase prostatic secretions and improve the composition of the seminal fluid.111–113 Specifically, Pygeum administration to men with decreased prostatic secretion has led to increased levels of total seminal fluid plus increases in alkaline phosphatase and protein content.
Pygeum appears to be most effective in men in whom alkaline phosphatase activity is reduced (i.e., <400 IU/cm3) and there is no evidence of inflammation or infection (i.e., absence of white blood cells and immunoglobulin A[IgA]). The lack of IgA in the semen is a good indicator of clinical success. In one study, the patients with no IgA in their semen demonstrated an increase in alkaline phosphatase from 265 to 485 IU/cm3.113 In contrast, those with IgA showed only a modest increase, from 213 to 281 IU/cm3.
Pygeum extract has also shown an ability, in a double-blind clinical trial, to improve the capacity of patients with benign prostatic hypertrophy (BPH) or prostatitis to achieve an erection, as determined by nocturnal penile tumescence.114 BPH and prostatitis are often associated with erectile dysfunction and other sexual disturbances. Presumably by alleviating the underlying condition, Pygeum can improve sexual function.
Tribulus terrestris
In animal studies, Tribulus has been shown to increase the levels of certain sex hormones including testosterone and also to improve nitric oxide synthesis115; however, these same results have not been observed in some human studies.116 One possible explanation is that there were differences in the extract and plant parts of the Tribulus used as well as the fact that many of the studies have comprised healthy males with normal testosterone levels rather than males with testosterone deficiency.
Tribulus appears to enhance male fertility through its ability to increase sperm count, viability, and libido; however, the published material is unclear, reporting studies that were poorly designed and and results that are not sufficiently definitive. A number of papers to support the extract product Tribestan highlight significant efficacy; however, owing to poor study design and lack of reliability, it is difficult to rely on these findings, which include increased ejaculate volume, sperm concentration, and motile sperm117 and improved conception rates.118
Astragalus membranaceous
In experimental studies, Astragalus membranaceus has been observed to increase the motility of sperm in semen.119 Studies show that Astragalus increases the motility of sperm in semen, but it also increases the motility of washed sperm, which is of special relevance to those seeking ART treatment.120 Additionally, it has been shown to increase sperm motility and progression.121
Turnera diffusa (Damiana)
The traditional application of damiana was for “its positive aphrodisiac effects, acting energetically on the genito urinary organs of both genders where it was highly indicated for sexual weakness and debility,”122 and “its ability to act as a stimulant tonic of the sexual apparatus especially if there is enfeeblement of the central nervous system.”123 Modern clinicians continue to prescribe damiana in this context and find it is especially beneficial when there is sexual debility, erectile difficulty, and depression. Human studies are lacking; however, a number of animal studies show promising supportive research. Turnera diffusa has been shown to facilitate the sexual behavior of male rats with sexual dysfunction, to reduce ejaculation latency,124 to produce a restorative effect in sexually exhausted male rats, and to hasten their recovery.125 It has also been observed to suppress aromatase activity, leading to the hypothesis that it may increase levels of testosterone.126
Mucuna pruriens (Velvet Bean)
Velvet bean has been used in Ayurvedic medicine for endurance against stress, general resistance against infection, retardation of the aging process, and eventual improvement of male sexual function; it has been known to alleviate disorders including psychogenic impotence and unexplained infertility.127 One paper showed that M. puriens seed powder produced dramatic improvements in 70% of study participants and helped fight stress-mediated poor semen quality; it has also acted as a restorative and invigorating tonic/aphrodisiac in infertile subjects.128 The same researchers reporting these positive effects determined that they were achieved through the regulation of steroidogenesis and a resulting improvements in semen quality.129 Specifically, this herbal treatment significantly improved testosterone, luteinizing hormone (LH), dopamine, adrenaline, and noradrenaline levels in infertile men and reduced levels of follicle stimulating hormone (FSH) and prolactin. Sperm count and motility were also significantly improved in infertile men.
Withania somnifera (Withania)
Withania has shown considerable antistress and adaptogenic effects. To investigate the effects of Withania on male fertility, 75 normal healthy fertile men (control subjects) and 75 men undergoing infertility screening participated in a 3-month clinical trial. The men in the Withania group received 5 g of the powdered root daily. Before and after the treatment, semen analysis, antioxidant vitamins, and serum sex hormone levels were determined. Results showed that Withania inhibited lipid peroxidation and improved sperm count and motility. Treatment also significantly increased serum testosterone and luteinizing hormone (LH) and reduced the levels of follicle stimulating hormone (FSH) and prolactin (PRL), all beneficial effects in infertile men.130
 Therapeutic Approach
Therapeutic Approach
General Measures
• Maintain scrotal temperature between 94° F and 96.8° F.
• Avoid exposure to free radicals.
• Identify and eliminate environmental pollutants.
• Stop or reduce all drugs, especially antihypertensives, antineoplastics such as cyclophosphamide, and antiinflammatory drugs such as sulfasalazine.
• Use effective stress-reduction techniques and employ psychological counseling if needed.
• Avoid cigarette smoking and the use of recreational drugs.
Diet
• Avoid dietary sources of free radicals, saturated fats, hydrogenated oils, trans fatty acids, and cottonseed oil.
• Increase consumption of legumes, especially soy (high in phytoestrogens and phytosterols), good dietary sources of antioxidant vitamins, carotenes, flavonoids (dark vegetables and fruits), as well as EFAs and zinc (nuts and seeds).
• Consume 8 to 12 servings of vegetables and 1 to 2 servings of fresh fruits daily.
• Optimize protein intake from both vegetarian and organic animal sources.
• Ensure that hydration requirements are met, using a 30-mL/2.2-lb (1 kg) rule.
• Eliminate caffeine, alcohol, sugar, and artificial substances (preservatives, colorings, additives).
• Ensure sufficient EFA intake several times a week in the form of half a cup of raw nuts or seeds, cold-pressed oils (from organic sources), and sustainably farmed fish.
Nutritional Supplements
• High-potency multivitamin/mineral supplement
• Fish oils: 1000 to 2000 mg EPA+DHA
• Vitamin C: 500 to1000 mg three times a day
• Vitamin E: 200 to 400 IU/day
• Beta-carotene: 15,000 to 30,000 IU/day (preferably as mixed carotenoids)
• Vitamin B12 (methylcobalamin): 1000 mcg/day
• Selenium: 200 to 400 mcg/day
Botanical Medicines
Choose one or more of the following:
Panax ginseng (Korean ginseng)
Astragalus membranaceous (Astragalus)
Dosages to be administered one to three times a day are as follows:
Withania somnifera (Withania)
1. National Survey of Family Growth. Washington, DC: CDC; 2002.
2. Brugh V.M., III., Lipshultz L.I. Male factor infertility: evaluation and management. Med Clin North Am. 2004;88:367–385.
3. Skakkebaek N.E., Jørgensen N., Main N.E., et al. Is human fecundity declining? Int J Androl. 2006;29:2–12.
4. ASRM, Sperm Shape (Morphology): Does It Affect Fertility? ASRM Fact Sheet
5. Hechtman L. Clinical Naturopathic Medicine. Australia: Elsevier; 2011.
6. Jose-Miller A., Boyden J.W., Frey K.A. Infertility. Am Fam Physician. 2007;75:849–856. 857-8
7. World Health Organisation. WHO laboratory manual for the examination and processing of human semen, 5th ed. Switzerland, 2010.
8. Aitken R.J., Koopman P., Lewis S.E. Seeds of concern. Nature. 2004;432:48–52.
9. Lewis S.E., Aitken R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005;322:33–41.
10. Penney G.C. Preventing infective sequelae of abortion. Hum Reprod. 1997 Nov;12(suppl 11):107–112.
11. Hay P.E., Lamont R.F., Taylor-Robinson D., et al. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ. 1994 Jan 29;308(6924):295–298.
12. Gaudoin M., Rekha P., Morris A., et al. Bacterial vaginosis and past chlamydial infection are strongly and independently associated with tubal infertility but do not affect in vitro fertilization success rates. Fertil Steril. 1999 Oct;72(4):730–732.
13. Kasperczyk A., Kasperczyk S., Horak S., et al. Assessment of semen function and lipid peroxidation among lead exposed men. Toxicol Appl Pharmacol. 2008 May 1;228(3):378–384. Epub 2008 Jan 3
14. Wu H.M., Lin-Tan D.T., Wang M.L., et al. Cadmium level in seminal plasma may affect the pregnancy rate for patients undergoing infertility evaluation and treatment. Reprod Toxicol. 2008 Aug;25(4):481–484. Epub 2008 May 3
15. Zorgniotti A.W., Cohen M.S., Sealfon A.I. Chronic scrotal hypothermia: results in 90 infertile couples. J Urol. 1986;135:944–947.
16. Sharpe R.M., Skakkebaek N.E. Are oestrogens involved in falling sperm counts and disorders of the male reproduction tract? Lancet. 1993;341:1392–1395.
17. Field B., Selub M., Hughes C.L. Reproductive effects of environmental agents. Semin Reprod Endocrinol. 1990;8:44–54.
18. Joffe M. Infertility and environmental pollutants. Br Med Bull. 2003;68:47–70.
19. Sharpe R.M., Skakkebaek N.E. Are oestrogens involved in falling sperm counts and disorders of the male reproduction tract? Lancet. 1993;341:1392–1395.
20. Anway M.D., Cupp A.S., Uzumcu M., et al. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469.
21. Boisen K., Chellakooty M., Schmidt I.M., et al. Hypospadias in a cohort of 1072 Danish newborn boys: prevalence and relationship to placental weight, anthropometrical measurements at birth, and reproductive hormone levels at 3 months of age. J Clin Endocrinol Metab. 2005;90:4041–4046.
22. Ji B.T., Shu X.O., Linet M.S., et al. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J Natl Cancer Inst. 1997;89:238–244.
23. British Medical Association Board of Science and Education. Mobile phones and health: an interim report. BMA Policy Report. 2001:1–15.
24. Lai H., Singh N.P. Single- and double-strand DNA breaks in rat brain cells after acute exposure to radiofrequency electromagnetic radiation. Int J Radiat Biol. 1996;69:513–521.
25. Fejes I., Zavaczki Z., Szollosi J., et al. Is there a relationship between cell phone use and semen quality? Arch Androl. 2005;51:385–393.
26. Davoudi M., Brossner C., Kuber W. The influence of electromagnetic waves on sperm motility. Urol Urogynaecol. 2002;19:18–22.
27. Agarwal A., Deepinder F., Sahrma R.K., et al. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Ster. 2008;1(89):124–128.
28. Saleh R.A., Agarwal A., Sharma R.K., et al. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril. 2002 Sep;78:491–499.
29. Fuentes A., Muñoz A., Barnhart K., et al. Recent cigarette smoking and assisted reproductive technologies outcome. Fertil Steril. 2010 Jan;93(1):89–95.
30. Chohan K.R., Badawy S.Z. Cigarette smoking impairs sperm bioenergetics. Int Braz J Urol. 2010 Jan-Feb;36(1):60–65.
31. Gaur D.S., Talekar M.S., Pathak V.P. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010 Jan-Mar;53(1):35–40.
32. Addis A., Moretti M.E., Ahmed Syed F., et al. Fetal effects of cocaine: an updated meta-analysis. Reprod Toxicol. 2001;15:341–369.
33. Battista N., Pasquariello N., Di Tommaso M., et al. Interplay between endocannabinoids, steroids and cytokines in the control of human reproduction. J Neuroendocrinol. 2008 May;20(suppl. 1):82–89.
34. Badawy Z.S., Chohan K.R., Whyte D.A., et al. Cannabinoids inhibit the respiration of human sperm. Fertil Steril. 2009 Jun;91(6):2471–2476. Epub 2008 Jun 18
35. Rossato M. Endocannabinoids, sperm functions and energy metabolism. Mol Cell Endocrinol. 2008 Apr 16;286(1-2 suppl. 1):S31–S35. Epub 2008 Feb 29
36. Lighten A. A weighty issue: managing reproductive problems in the obese. Conceptions, Sydney IVF. June 2009:9.
37. Purvis K., Christiansen E. Review: infection in the male reproductive tract: impact, diagnosis and treatment in relation to male infertility. Int J Androl. 1993;16:1–13.
38. Lenzi A., Gandini L., Maresca V., et al. Fatty acid composition of spermatozoa and immature germ cells. Mol Hum Reprod. 2000;6(3):226–231.
39. Weller D.P., Zaneveld J.D., Farnsworth N.R. Gossypol. Pharmacology and current status as a male contraceptive. Econ Med Plant Res. 1985;1:87–112.
40. Gulaya N.M., Margitich V.M., Govseeva N.M., et al. Phospholipid composition of human sperm and seminal plasma in relation to sperm fertility. Arch Androl. 2001;46(3):169–175.
41. Safarinejad M.R., Hosseini S.Y., Dadkhah F., et al. Relationship of omega-3 and omega-6 fatty acids with semen characteristics, and anti-oxidant status of seminal plasma: a comparison between fertile and infertile men. Clin Nutr. 2010 Feb;29(1):100–105.
42. Showell M.G., Brown J., Tazdani A., et al. Antioxidants for male, subfertility (Review)The Cochrane Library,, Wiley Publishers, 2011;Issue 2.
43. Tremellen K., Miari G., Froilan D., et al. A randomized control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust and New Zea J Ob & Gyn. 2007;47:216–221.
44. Agarwal A., Nallella K.P., Allamaneni S.S., et al. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–627.
45. Zini A., de Lamirande E., Gagnon C. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl. 1993;16:183–188.
46. Pasqualotto F.F., Sharma R.K., Nelson D.R., et al. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril. 2000;73:459–464.
47. Akmal M., Qadri J.Q., Al-Waili N.S., et al. Improvement in human semen quality after oral supplementation of vitamin C. J Med Food. 2006;9(3):440–442. Fall
48. Colagar A.H., Marzony E.T. Ascorbic acid in human seminal plasma: determination and its relationship to sperm quality. J Clin Biochem Nutr. 2009 Sep;45(2):144–149. Epub 2009 Aug 28
49. Patel S.R., Sigman M. Antioxidant therapy in male infertility. Urol Clin N Am. 2008;35:319–330.
50. Song G.J., Norkus E.P., Lewis V. Relationship between seminal ascorbic acid and sperm DNA integrity in infertile men. Int J Androl. 2006 Dec;29(6):569–575.
51. Kao S.H., Chao H.T., Chen H.W., et al. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008 May;89(5):1183–1190. Epub 2007 Jul 31
52. Fraga C.G., Motchnik P.A., Shigenaga M.K., et al. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991;88:11003–11006.
53. Dawson E., Harris W., Powell L. Effect of vitamin C supplementation on sperm quality of heavy smokers. FASEB J. 1991;5:A915.
54. Dawson E.B., Harris W.A., Rankin W.E., et al. Effect of ascorbic acid on male fertility. Ann NY Acad Sci. 1987;498:312–323.
55. Aitken R.J., Clarkson J.S., Hargreave T.B., et al. Analysis of the relationship between defective sperm function and the generation of reactive oxygen species in cases of oligozoospermia. J Androl. 1989;10:214–220.
56. Greco E., et al. Journal of Andrology. 2005;26(3):349–353.
57. Suleiman S.A., Ali M.E., Zaki Z.M., et al. Lipid peroxidation and human sperm mobility: protective role of vitamin E. J Androl. 1996;17:530–537.
58. Gupta N.P., Kumar R. Lycopene therapy in idiopathic male infertility: a preliminary report. Int Urol Nephrol. 2002;34:369–372.
59. Geva E., Bartoov B., Zabludovsky N., et al. The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil Steril. 1996 Sep;66(3):430–434.
60. Greco E., Iacobelli M., Rienzi L., et al. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005 May-Jun;26(3):349–353.
61. Al-Azemi M.K., Omu A.E., Fatinikun T., et al. Factors contributing to gender differences in serum retinol and alpha-tocopherol in infertile couples. Reprod Biomed Online. 2009 Oct;19(4):583–590.
62. Morales A., Cavicchia J.C. Spermatogenesis and blood-testis barrier in rats after long-term vitamin A deprivation. Tissue Cell. 2002 Oct;34(5):349–355.
63. Eskenazi B., et al. Antioxidant intake is associated with semen quality in healthy men. Human Reproduction. 2005;20(4):1006–1012.
64. Ursini F., Heim S., Kiess M., et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999; 285:1393. selenium on sperm motility in healthy men. J Androl. 2001;22:764.
65. Vézina D., Mauffette F., Roberts K.D., et al. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and micronutrient levels and distribution. Biol Trace Elem Res. 1996 Summer;53(1-3):65–83.
66. Rayman M.P., Rayman M.P. The argument for increasing selenium intake. Proc Nutr Soc. 2002;61:203–215. 71
67. Schneider M., Förster H., Boersma A., et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009 Sep;23(9):3233–3242. Epub 2009 May 5
68. Scott R., MacPherson A., Yates R.W., et al. The effect of oral selenium supplementation on human sperm motility. Br J Urol. 1998 Jul;82(1):76–80.
69. Safarinejad M.R., Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol. 2009 Feb;181(2):741–751.
70. Wong W.Y., Thomas C.M., Merkus J.M., et al. Male factor subfertility: possible causes and the impact of nutritional factors. Fertil Steril. 2000;73:435–442.
71. Colagar A.H., Marzony E.T., Chaichi M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. 2009 Feb;29(2):82–88.
72. Prasad A.S. Zinc in growth and development and spectrum of human zinc deficiency. J Am Coll Nutr. 1988;7:377–384.
73. El-Tawil A.M. Zinc deficiency in men with Crohn’s disease may contribute to poor sperm function and male infertility. Andrologia. 2003;35:337–341.
74. Chia S.E., Ong C., Chua L., et al. Comparison of zinc concentration in blood and seminal plasma and various sperm parameters between fertile and infertile men. J Androl. 2000;21:53–57.
75. Bjorndahl L., Kvist U. Importance of zinc for human sperm head-tail connection. Acta Physiol Scand. 1982;126:51–55. as cited Seibel, M. The role of nutrition and nutritional supplements in women’s health Fertility and Sterility, Volume 72, Issue 4, October 1999, pp 579-591
76. Caldamone A.A., Freytag M.K., Cockett A.T. Seminal zinc and male infertility. Urology. 13, 1979. 280–28
77. Carreras A., Mendosa C. Zinc levels in seminal plasma of infertile and fertile men. Andrologia. 1990;22:279–283.
78. Takihara H., Cosentino M.J., Cockett A.T. Zinc sulfate therapy for infertile males with or without varicocelectomy. Urology. 1987;29:638–641.
79. Netter A., Hartoma R., Nakoul K. Effect of zinc administration on plasma testosterone, dihydrotestosterone and sperm count. Arch Androl. 1981;7:69–73.
80. Wong W.Y., Merkus H.M., Thomas C.M., et al. Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002;77:491–498.
81. Sandler B., Faragher B. Treatment of oligospermia with vitamin B12. Infertility. 1984;7:133–138.
82. Boxmeer J.C., Smit M., Weber R.F., et al. Seminal plasma cobalamin significantly correlates with sperm concentration in men undergoing IVF or ICSI procedures. J Androl. 2007 Jul-Aug;28(4):521–527. Epub 2007 Feb 7
83. Boxmeer J.C., Smit M., Utomo E., et al. Low folate in seminal plasma is associated with increased sperm DNA damage. Fertil Steril. 2009 Aug;92(2):548–556.
84. Sinclair S. Male infertility: nutritional and environmental considerations. Altern Med Rev. 2000 Feb;5(1):28–38.
85. Kumamoto Y., Maruta H., Ishigami J., et al. Clinical efficacy of mecobalamin in treatment of oligozoospermia: results of a double-blind comparative clinical study. Acta Urol Japan. 1988;34:1109–1132.
86. Ng C.M., Blackman M.R., Wang C., et al. The role of carnitine in the male reproductive system. Ann N Y Acad Sci. 2004 Nov;1033:177–178.
87. Costa M., Canale D., Filicori M., et al. L-Carnitine in idiopathic asthenozoospermia: a multicenter study. Italian Study Group on Carnitine and Male Infertility. Andrologia. 1994;26:155–159.
88. Vitali G., Parente R., Melotti C. Carnitine supplementation in human idiopathic asthenospermia: clinical results. Drugs Exp Clin Res. 1995;21:157–159.
89. Vicari E., La Vignera S., Calogero A.E. Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti-inflammatory compounds. Fertil Steril. 2002;78:1203–1208.
90. Lenzi A., Sgrò P., Salacone P., et al. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil Steril. 2004;81:1578–1584.
91. Lenzi A., Lombardo F., Sgro P., et al. Use of carnitine therapy in selected cases of male factor infertility: a double-blind crossover trial. Fertil Steril. 2003;79:292–300.
92. Balercia G., Regoli F., Armeni T., et al. Placebo-controlled, double-blind, randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic astheno zoospermia. Fertil Steril. 2005;84:662–671.
93. Bilska A., Włodek L. Lipoic acid: the drug of the future? Pharmacol Rep. 2005 Sep-Oct;57(5):570–577.
94. Selvakumar E., Prahalathan C., Sudharsan P.T., et al. Chemoprotective effect of lipoic acid against cyclophosphamide-induced changes in the rat sperm. Toxicology. 2006 Jan 5;217(1):71–78. Epub 2005 Oct 3
95. Prahalathan C., Selvakumar E., Varalakshmi P. Modulatory role of lipoic acid on adriamycin-induced testicular injury. Chem Biol Interact. 2006 Mar 25;160(2):108–114. Epub 2006 Jan 24
96. Ibrahim S.F., Osman K., Das S., et al. A study of the antioxidant effect of alpha lipoic acids on sperm quality. Clinics (Sao Paulo). 2008 Aug;63(4):545–550.
97. Aly H.A., Lightfoot D.A., El-Shemy H.A. Modulatory role of lipoic acid on lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Chem Biol Interact. 2009 Dec 10;182(2-3):112–118. Epub 2009 Aug 21
98. Mancini A., De Marinis L., Oradei A., et al. Coenzyme Q10 concentration in normal and pathological human seminal fluid. J Androl. 15, 1994. 591–59
99. Balercia G., Mosca F., Mantero F., et al. Coenzyme Q10 supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril. 2004;81:93–98.
100. Balercia G., Buldreghini E., Vignini A., et al. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril. 2009 May;91(5):1785–1792. Epub 2008 Apr 8
101. Balercia G., Arnaldi G., Fazioli F., et al. Coenzyme Q10 levels in idiopathic and varicocele-associated asthenozoospermia. Andrologia. 2002;34:107–111.
102. Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009 May;37(1):1–17.
103. Scibona M., Meschini P., Capparelli S., et al. L-arginine and male infertility. Minerva Urol Nefrol. 1994 Dec;46(4):251–253.
104. Schacter A., Goldman J.A., Zukerman Z. Treatment of oligospermia with the amino acid arginine. J Urol. 1973;110:311–313.
105. Stanislavov R., Nikolova V., Rohdewald P. Improvement of seminal parameters with Prelox: a randomized, double-blind, placebo-controlled, cross-over trial. Phytother Res. 2009 Mar;23(3):297–302.
106. Roseff S.J. Improvement in sperm quality and function with French maritime pine tree bark extract. J Reprod Med. 2002;47:821–824.
107. Zhang H., Zhou Q.M., Li X.D., et al. Ginsenoside R(e) increases fertile and asthenozoospermic infertile human sperm motility by induction of nitric oxide synthase. Arch Pharm Res. 2006 Feb;29(2):145–151.
108. Zhang H., Zhou Q., Li X., et al. Ginsenoside Re promotes human sperm capacitation through nitric oxide-dependent pathway. Mol Reprod Dev. 2007 Apr;74(4):497–501.
109. Salvati G., Genovesi G., Marcellini L., et al. Effects of Panax Ginseng C.A. Meyer saponins on male fertility. Panminerva Med. 1996 Dec;38(4):249–254.
110. Choi H.K., Seong D.H., Rha K.H. Clinical efficacy of Korean red ginseng for erectile dysfunction. Int J Impot Res. 1995;7(3):181–187.
111. Lucchetta G., Weill A., Becker N., et al. Reactivation of the secretion from the prostatic gland in cases of reduced fertility: biological study of seminal fluid modifications. Urol Int. 1984;39:222–224.
112. Menchini-Fabris G.F., Giorgi P., Reini F., et al. New perspectives of treatment of prostato-vesicular pathologies with Pygeum africanum. Arch Int Urol. 1988;60:313–322.
113. Clavert A., Cranz C., Riffaud J.P., et al. Effects of an extract of the bark of Pygeum africanum on prostatic secretions in the rat and man. Ann Urol. 1986;20:341–343.
114. Carani C., Salvioli C., Scuteri A., et al. Urological and sexual evaluation of treatment of benign prostatic disease using Pygeum africanum at high dose. Arch Ital Urol Nefrol Androl. 1991;63:341–345.
115. Gauthaman K., Ganesan A.P. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction: an evaluation using primates, rabbit and rat. Phytomedicine. 2008 Jan;15(1-2):44–54.
116. Neychev V.K., Mitev V.I. The aphrodisiac herb Tribulus terrestris does not influence the androgen production in young men. J Ethnopharmacol. 2005 Oct 3;101(1-3):319–323.
117. Protich M., Tsvetkov D., Nalbanski B., et al. Clinical trial of a tribestan preparation in infertile men. Akush.Ginekol. (Sofiia). 1983;22(4):326–329. as cited in National Standard monogrph
118. Adimoelja A. Phytochemicals and the breakthrough of traditional herbs in the management of sexual dysfunctions. Int Androl. 4 Jan 2002;23(S2):82–84. Published online
119. Hong C.Y., Ku J., Wu P. Astragalus membranaceus stimulates human sperm motility in vitro. Am J Chin Med. 1992;20(3-4):289–294.
120. Liu J., Liang ., Yin C. Effects of several Chinese herbal aqueous extracts on human sperm motility in vitro. Andrologia. 2004;36(2):78–83.
121. Dinchev D., Janda B., Evstatieva L., et al. Distribution of steroidal saponins in Tribulus terrestris from different geographical regions. Phytochemistry. 2008;69(1):176–186.
122. Felter H.W., Lloyd J.U.. Kings American Dispensatory Eclectic Materia Medica publications, 1905.
123. Ellingwood F. The American Materia Medica, Therapeutics and Pharmacognosy. Eclectic Materia Medica Publications; 1919.
124. Arletti R., Benelli A., Cavazzuti E., et al. Stimulating property of Turnera diffusa and Pffafia paniculata extracts on the sexual behavior of male rats. Psychopharmacology. 1999;144:15–19.
125. Estrada-Reyes R., Ortiz-López P., Gutiérrez-Ortíz J., et al. Turnera diffusa Wild (Turneraceae) recovers sexual behavior in sexually exhausted males. J Ethnopharmacol. 2009 Jun 25;123(3):423–429. Epub 2009 Mar 31
126. Zhao J., Dasmahapatra A., Khan S., et al. Anti-aromatase activity of the constituents from damiana (Turnera diffusa). J Ethnopharmacol. 2008;120(3):387–393.
127. Tripathi Y.B., Upadhyay A.K. Antioxidant property of Mucuna pruriens. Curr Sci. 2001;80:1377–1378.
128. Ahmad M.K., Mahdi A.A., Shukla K.K., et al. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008;90:627–635.
129. Ahmad M.K., Mahdi A.A., Shukla K.K., et al. Mucuna pruriens improves male fertility by its action on the hypothalamus–pituitary–gonadal axis. Fertil Steril. 2009;92:1934–1940.
130. Ahmad M.K., Mahdi A.A., Shukla K.K., et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril. 2010 Aug;94(3):989–996.