87 Infectious Endocarditis
 Pathophysiology
Pathophysiology
Infectious endocarditis is a microbial infection of the endocardial surface of the heart. The process is initiated by bloodborne microorganisms that adhere directly to the endothelium or by nonbacterial thrombotic endocarditis. The most important factors facilitating nonbacterial thrombotic endocarditis are organic valvular lesions, with associated perturbation of blood flow, and prosthetic valves. Circulating microorganisms can adhere to microscopic lesions, which explains why some patients with infectious endocarditis have no previously known valvular abnormality.1
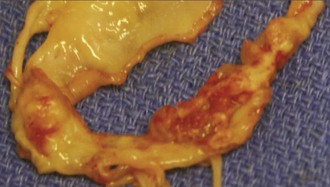
In simple infectious endocarditis, infection is limited to the valve cusps or leaflets and chordae and consists of vegetations (Figure 87-1) which are formed by pathogens, platelets, fibrin, and inflammatory cells. In advanced infectious endocarditis, deep tissue invasion results in the destruction or invasion of valvular and perivalvular structures. The infection may spread as cellulitis, with the formation of an abscess or pseudoaneurysm that can rupture to another heart chamber or even the pericardium.
In prosthetic valve endocarditis (PVE), lesions may differ according to the type of prosthesis. With biological prostheses or homografts, the infection may be limited to cusps, whereas with mechanical prostheses, involvement of the sewing ring and the valve annulus is the rule. Bacterial adherence to the prosthesis results from a complex relationship among the biomaterial, plasma proteins (e.g., fibronectin, laminin, thrombospondin, fibrinogen), and bacterial adhesion proteins. Staphylococci express numerous surface factors: clumping factors A and B, which promote their adhesion to fibrinogen and fibrin, and fibronectin-binding proteins A and B, which permit adhesion to fibronectin.2 In addition, once staphylococci have escaped the microbicidal effects of platelet peptides, they can bind to the platelet surface by a series of pathogenetic steps including direct binding to the platelet surface, up-regulation of platelet surface receptors for fibrinogen, and interaction between specific bacterial proteins and platelet surface receptors. Surface charge modifications are associated with increased in vitro resistance profiles of Staphylococcus aureus to a number of endogenous cationic antimicrobial peptides such as α-defensins.3,4
 Incidence and Classification
Incidence and Classification
The incidence of infective endocarditis ranges from one country to another within 3 to 10 episodes/100,000 person-years. This may reflect methodological differences between surveys rather than true variations.5 The overall annual incidence of infectious endocarditis in Europe and the United States is between 15 and 60 cases per million. In a study conducted in France, the crude annual incidence of infectious endocarditis was 30 (95% confidence interval [CI], 27 to 33) per million inhabitants.1 Infectious endocarditis can be classified into three groups that differ markedly in terms of incidence, clinical presentation, microbiological features, and outcome: left-sided native valve, right-sided native valve, and PVE.
Right-sided native valve infectious endocarditis is usually associated with intravenous (IV) drug use and still accounts for 10% of all infectious endocarditis episodes.6 Nosocomial cases are frequently a consequence of catheter-related infections. In most cases of pacemaker and implantable cardioverter-defibrillator infectious endocarditis, vegetations are located only on leads, but tricuspid valve involvement may also occur.7
Prosthetic valve endocarditis occurs in 1% to 6% of patients with valve prosthesis, with an incidence of 0.3% to 1.2% per patient year.4 It accounted for 21% of 2781 patients with definite infective endocarditis in the ICE Prospective Cohort Study6 (ICE-PCS). Early PVE is classically defined as occurring within 1 year of surgery, and late PVE beyond 1 year, because of significant differences between the microbiological profiles observed before (usually nosocomial origin) and after this time point (predominance of community-acquired pathogens).8 However, a recent large prospective multicenter international registry found that 37% of all PVE was associated with nosocomial infection or non-nosocomial healthcare-associated infections in outpatients with extensive healthcare contact.9
 Demographics and Etiologic Profiles
Demographics and Etiologic Profiles
Classic and Changing Patient Characteristics
The demographic characteristics of patients who develop infectious endocarditis have changed over the last few decades. Today, patients tend to be older, and their underlying diseases have changed.10,11 In ICE-PCS, 38% of all definite infectious endocarditis occurred in patients older than 65 years.11 In developing countries, rheumatic heart disease remains the most frequent underlying cardiac condition predisposing patients to infectious endocarditis. In contrast, in the United States and Western Europe, nonrheumatic heart abnormalities, including mitral valve prolapse, aortic valve calcification, aortic bicuspid valve, and hypertrophic obstructive cardiomyopathy, are the main risk factors. For patients with mitral valve prolapse, risk factors include mitral regurgitation and thickened mitral leaflet. However, results of a 1-year survey of infectious endocarditis in France showed a significantly lower incidence of known underlying heart disease between 1991 and 1999. Nowadays, congenital heart diseases are rarely involved, except bicuspid aortic valve. Other conditions including diabetes mellitus, long-term hemodialysis, and immunosuppression are associated with a higher incidence of infectious endocarditis. At Duke University Medical Center, rates of hemodialysis dependence and immunosuppression among 329 patients with infectious endocarditis rose significantly between 1993 and 1999.12 Moreover, a recent study showed that more than one third of cases of native valve endocarditis in non–injection drug users involve contact with health care. Such episodes of endocarditis maybe nosocomial if they occur in a patient hospitalized for more than 48 hours before the onset of signs or symptoms consistent with infective endocarditis. A higher proportion of non-nosocomial healthcare-associated endocarditis is now observed in patients with extensive out-of-hospital contact with healthcare interventions or systems (wound care, receipt of hemodialysis or IV chemotherapy, residence in a nursing home or long-term care facility).13
Causative Organisms
Overall Distribution
Most Frequently Isolated Pathogens
Streptococci are traditionally the most common causative agent of infectious endocarditis, but the results of the ICE-PCS show that streptococci had fallen into second place to staphylococci as the leading cause.6 However, this apparent temporal shift from predominantly streptococcal to predominantly staphylococcal infective endocarditis may be partly due to recruitment/referral bias in specialized centers, since this trend is not evident in population-based epidemiologic surveys of infective endocarditis.14 Streptococcus species (mainly Streptococcus mitis, Streptococcus sanguis, Streptococcus mutans), which abound in the mouth and nasopharynx, are associated with dental procedures and diseases. Poor dental hygiene and minor or unrecognized periodontal disease may be the source of Streptococcus viridans infectious endocarditis. Streptococcus gallolyticus (previously S. bovis) may be involved in valve infection of dental or buccal origin. In addition, the association of S. gallolyticus with carcinoma or other lesions of the colon (e.g., diverticulitis, polyps) is well known. Beta-hemolytic streptococci (groups A, B, C, and G) and Streptococcus milleri are isolated from 6% of patients with infectious endocarditis,1 with the predominant species being group B. The majority of nonpregnant patients with group B streptococcal infectious endocarditis have an underlying condition such as diabetes mellitus, breast cancer, decubitus ulcer, or cirrhosis.15
Enterococci, mainly Enterococcus faecalis and Enterococcus faecium, account for only 10% of cases of infectious endocarditis.6 These pathogens affect older patients, as demonstrated by a description of 93 episodes of enterococcal infectious endocarditis occurring in patients with a mean age of 74 years.16 The portals of entry are the gastrointestinal and urogenital tracts through a lesion or a procedure (e.g., injection sclerosis of esophageal varices, transurethral prostate resection, urethral dilatation) resulting in transient bacteremia, in which case the infection is healthcare associated.
Staphylococcus aureus is now implicated in approximately 30% of all cases of left-sided native valve infectious endocarditis,6 in 23% of PVE,9 and is the most common cause of healthcare-associated infections.15 S. aureus is also the causative agent in most acute infections, with about half of patients having no previously known heart disease. A clinically identifiable focus of infection (e.g., carbuncle, cellulitis, bursitis, ulcer, burn, osteomyelitis) may be present. However, in 50% to 60% of cases, no obvious portal of entry is detected, although the skin is probably the source in many of them. The relationship between S. aureus nasal carriage and infection has been established in specific subsets of patients, especially in IV drug users and patients with diabetes mellitus or on hemodialysis.12 Methicillin-resistant strains are isolated in healthcare-associated endocarditis, although rare cases of community-acquired methicillin-resistant endocarditis have been reported.
Coagulase-negative staphylococci (CoNS), in a recent international study, were found to cause 16% of 537 cases of definite noninjecting drug use–associated PVE. Nearly 50% of patients with CoNS PVE presented between 60 days and 365 days of valve implantation. Methicillin resistance was present in 68% of CoNS strains.17 CoNS are also a well-documented, albeit rather rare, cause of native valve infectious endocarditis. Most patients have documented valvular abnormalities, especially mitral valve prolapse. A substantial subset of CoNS infective endocarditis has been identified as being due to Staphylococcus lugdunensis, which causes destructive cardiac lesions; its differentiation from other CoNS species in the laboratory may be difficult.
Infrequent Pathogens
Enterobacteriaceae and HACEK Group. Despite the high frequency of Enterobacteriaceae bacteremia leading to severe sepsis or septic shock, infectious endocarditis caused by these pathogens is extremely uncommon, probably because gram-negative bacilli adhere less avidly to the endothelium than gram-positive cocci do. Most cases of native valve infectious endocarditis develop in patients with severe comorbidities, including cirrhosis or immunosuppression.18 Gram-negative bacilli are usually encountered in early and late PVE, but they account only for 2% of the cases. Bacteria of the HACEK group (fastidious organisms) originate from the oropharyngeal or urogenital flora and include Haemophilus aphrophilus or paraphrophilus (H), Actinobacillus actinomycetemcomitans (A), Cardiobacterium hominis (C), Eikenella corrodens (E), and Kingella species (K). These HACEK pathogens are implicated in 2% of cases of infectious endocarditis on either native or prosthetic valves.6
Streptococcus pneumoniae infectious endocarditis occurs more commonly in alcoholics, but other patients, such as those with diabetes, malignancy, or chronic obstructive pulmonary disease, may be affected. Approximately 65% to 80% of patients have no known predisposing cardiopathy. The primary infection focus is the lungs, and meningitis is present in 40% to 60% of cases.19
Fungi are a rare cause of infective endocarditis, being isolated in 2% of cases but in 4% of those patients with prosthetic valve infection. Although injection drug use was traditionally an important risk factor, a recent study showed that patients with Candida infective endocarditis were more likely to have a prosthetic valve, short-term indwelling catheters, and healthcare-associated infections.20 Other fungi such as Aspergillus spp. are even less frequently encountered. Fungi are frequently responsible for mural endocarditis.
Patients with Negative Blood Cultures
Five main points should be emphasized: (1) Abiotrophia spp. (previously classified as nutritionally variant streptococci) are the main cause of culture-negative infectious endocarditis in patients who have recently received antibiotics. (2) Only 5% to 7% of patients who have not recently taken antibiotics have negative blood cultures. Polymerase chain reaction (in blood, excised vegetation, or systemic emboli) can be used to identify the causative organism, such as Bartonella spp., Tropheryma whippelii, or Coxiella burnetii, but also streptococci or other pathogens not isolated from blood cultures.21 (3) Serologic tests are useful to diagnose infectious endocarditis caused by those organisms or by Brucella and Legionella species. (4) HACEK organisms may require prolonged incubation and subculturing. (5) Candida (but not Aspergillus) spp. are usually isolated from routine blood cultures, but in some cases, fungi are recovered only from excised vegetations or peripheral emboli.
Specific Microbiologic Characteristics of Infectious Endocarditis in ICU Patients
The microbiological characteristics of infectious endocarditis in patients who require ICU admission differ from those in the overall population. Analysis of a large series of infectious endocarditis patients hospitalized in two medical ICUs in a Parisian teaching hospital between 1994 and 2001 showed that S. aureus was the leading pathogen responsible for left-sided native valve and PVE22 (Tables 87-1 and 87-2). Those figures were confirmed by an Austrian study of 33 ICU patients with infectious endocarditis: S. aureus was isolated from 36% of them, versus 15% S. viridans and 12% enterococci.23 In a French multicenter study, S. aureus accounted for 46% of 198 critically ill patients with definite endocarditis according to Duke criteria (see later discussion).24 Clearly these findings are largely explained by S. aureus causing valve destruction, septic shock, and emboli to vital organs such as brain.
TABLE87-1 Causative Agents of Left-Sided Native Valve Infectious Endocarditis
| Microorganisms | ICE-PCE (2781 patients)6 Number (%) | Bichat-Claude Bernard ICUs (120 patients): Number* (%)22 |
|---|---|---|
| Streptococci | 810 (29) | 42 (35) |
| Staphylococcus aureus | 869 (31) | 48 (40) |
| Enterococci | 283 (10) | 4 (3) |
| CoNS | 304 (11) | 2 (1) |
| Streptococcus pneumoniae | NR | 5 (4) |
| HACEK | 44 (2) | NR |
| Fungi | 45 (2) | 4 (3) |
| Other | 121 (4) | 9 (7) |
| Negative blood cultures | 277 (10) | 10 (8) |
CoNS, coagulase-negative staphylococci; HACEK, Haemophilus aphrophilus or paraphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species; NR, not reported.
* The number of microorganisms exceeds the number of patients because some cases were polymicrobial.
TABLE87-2 Causative Agents of Prosthetic Valve Endocarditis (PVE)
| Microorganisms | Early PVE (n = 51) Number (%) |
Late PVE (n = 331) Number (%) |
|---|---|---|
| Staphylococcus aureus | 19 (37) | 61 (18) |
| Coagulase-negative staphylococci | 9 (17.5) | 66 (20) |
| Streptococcus viridans | 1 (2) | 34 (10) |
| Streptococcus bovis | 1 (2) | 22 (7) |
| Other streptococci | 0 | 11 (3) |
| Enterococci | 4 (8) | 42 (13) |
| Streptococcus pneumoniae | 0 | 3 (1) |
| Propionibacterium | 0 | 5 (1.5) |
| HACEK | 0 | 7 (2) |
| Enterobacteriaceae | 2 (4) | 3 (1) |
| Pseudomonas aeruginosa | 1 (2) | 1 (0.3) |
| Fungi | 5 (10) | 11 (3) |
| Other | 0 | 5 (1.5)* |
| Culture negative | 9 (17.5) | 41 (12) |
HACEK, Haemophilus aphrophilus or H. paraphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species.
* Other: Listeria monocytogenes, 2; Micromonas, 2; Mycobacterium spp., 1.
Adapted from Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007;297:1354-61.
 Clinical Characteristics and Diagnosis
Clinical Characteristics and Diagnosis
In 1994, a new set of diagnostic criteria for the diagnosis of infectious endocarditis, including two major and six minor criteria—known as the Duke criteria—was proposed. Modifications of these criteria were proposed in 2000 to take into account transesophageal echocardiography and to consider all S. aureus bacteremias and positive Q fever serology as major criteria.25
Clinical Characteristics
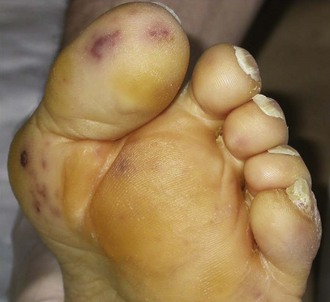
In ICU patients, the clinical presentation of infectious endocarditis often includes extracardiac manifestations or findings associated with cardiac complications. Patients are generally referred to the ICU for cardiogenic or septic shock, pulmonary edema caused by valvular or prosthetic dysfunction, neurologic events, acute renal failure, or respiratory failure in the setting of pulmonary emboli complicating right-sided infectious endocarditis. Two salient features, usually associated with high-grade fever, strongly suggest the diagnosis of infectious endocarditis: (1) a heart murmur (most commonly preexisting) or a prosthetic valve and (2) petechiae on the skin (especially the extremities; Figure 87-2) and conjunctivae. A typical ICU candidate has an acute febrile and toxic illness with heart murmur, petechiae, and meningeal signs. Cerebrospinal fluid examination finds pleocytosis and gram-positive cocci. Blood cultures yield S. aureus, and echocardiography confirms left-sided infectious endocarditis. In patients with catheter-related bacteremia, the diagnosis of infective endocarditis may be suggested by persistent positive blood cultures 3 to 5 days after the onset of antimicrobial treatment and removal of the catheter.
Echocardiography
Echocardiography has the following objectives: (1) to detect vegetations and determine their size, (2) to diagnose paravalvular extension of the infection, (3) to evaluate myocardial function, (4) to detect pericardial effusion, and (5) if cardiac surgery is being considered, to measure the valve ring to choose the appropriate prosthetic valve for replacement. Transthoracic echocardiography is rapidly obtained and noninvasive, but its overall sensitivity is only 40% to 65%. False-negative results are obtained when the examination is inadequate (especially in those with obesity or chronic obstructive pulmonary disease) or when vegetations are less than 5 mm. Transesophageal echocardiography associated with color Doppler techniques is more invasive, but its sensitivity for detecting vegetations is 90% to 100%.5 Transesophageal echocardiography is particularly useful in patients with suspected valve perforation or extension of perivalvular infectious endocarditis and in those with PVE. Its sensitivity and specificity for the detection of cardiac abscess are 80% and 95%, respectively. This technique is necessary for all patients undergoing valve surgery and may be repeated at close intervals to help the physician decide when to operate. However, transesophageal echocardiography should be used cautiously in nonintubated critically ill patients with respiratory failure. Follow-up echocardiography to monitor complications and response to treatment is mandatory.5
 Complications
Complications
Cardiac Complications and Hemodynamic Failure
Congestive heart failure (CHF) is usually caused by infection-induced valvular damage or prosthesis dysfunction. CHF is observed in 50% to 60% of cases overall and is more frequently associated with aortic than mitral disease. CHF caused by aortic failure may require urgent valve replacement. Perivalvular extension of infectious endocarditis is frequently associated with CHF, and spread into the septum may lead to heart block. Erosion of a mycotic aneurysm of the sinus of Valsalva can cause hemopericardium and tamponade or can create fistulas to the right or left ventricle. Myocardial infarction due to coronary artery embolization is a rare event. Hemodynamic failure can also be caused by septic shock, especially during the bacteremic phase of S. aureus infectious endocarditis.22 All these complications may require the administration of positive inotropes or vasoconstrictors and the use of mechanical ventilation before valve replacement.
Neurologic Complications
Frequency, Microbiology, and Timing
In most series, CNS involvement during the course of infectious endocarditis occurs in 20% to 40% of cases. Among 1329 episodes of infectious endocarditis from seven series described between 1985 and 1993, 437 (33%) were accompanied by CNS manifestations.26 In a Finnish teaching hospital, 55 of 218 infectious endocarditis (25%) were associated with neurologic complications.27 However, two other studies reported lower rates: in France, strokes occurred in 17% of 264 infectious endocarditis cases caused by staphylococci or streptococci1; in the United States, among 513 episodes of complicated, left-sided, native valve infectious endocarditis, focal neurologic signs or altered mental status were observed in 18% and 16% of cases, respectively.10 Experience from the large, contemporary, and multinational ICE-PCS study reported a similar (17%) incidence of strokes.6 The use of sensitive methods of detection such as magnetic resonance imaging (MRI) indicates that silent cerebral complications are frequent. Among 60 patients who experienced episodes of left-sided infective endocarditis, 35% had a symptomatic neurologic event, while silent cerebral complications were detected in another 30%.28 In a recently published study involving 127 patients with definite endocarditis who underwent systematic MRI, cerebral lesions were detected in 106, most being asymptomatic.29 Not surprisingly, the incidence of symptomatic neurologic complications is much higher in the subset of patients with infective endocarditis requiring admission to the ICU. A multicenter study showed a 55% incidence of symptomatic neurologic events among 198 critically ill patients with left-sided endocarditis.24 Neurologic complications are a hallmark of left-sided abnormalities of either native or prosthetic valves. When neurologic complication rates were assessed as a function of the causative agent, the frequency of CNS involvement was two to three times higher with S. aureus than with other pathogens.27
Most neurologic complications are already evident at the time of hospitalization or develop within a few days. The probability of developing these complications decreases rapidly once antimicrobial therapy has been started. In the ICE-PCS study, the crude incidence of stroke in patients receiving appropriate antimicrobial therapy was 4.82/1000 patient days in the first week of therapy and fell to 1.71/1000 patient days in the second week. This rate continued to decline with further therapy.30 Moreover, recurrent neurologic events, although possible even late, are uncommon.
Pathogenesis and Distribution
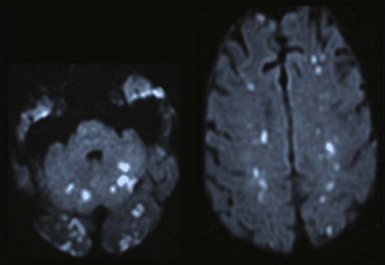
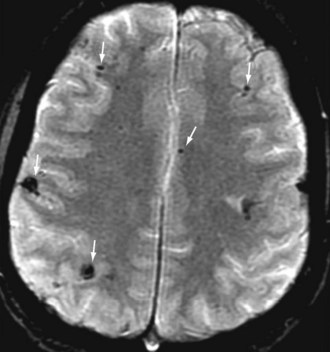
Neurologic complications of infectious endocarditis can arise through various mechanisms, but the major mechanism is cerebral embolization. Cerebral emboli (Figure 87-3) result from dislodgment or fragmentation of cardiac vegetations, followed by vessel occlusion; this results in various degrees of ischemia and infarction, depending on the vessels and the collateral blood flow. Occlusion of cerebral arteries, with either stroke or transient ischemic attack, accounts for 40% to 50% of the CNS complications of infectious endocarditis.26–27 Cerebral hemorrhage may be the consequence of different mechanisms, each of which accounts for one-third of bleeding complications: rupture of an intracranial aneurysm; septic erosion of the arterial wall, without a well-delineated aneurysm (acute necrotizing arteritis); or hemorrhagic transformation of ischemic brain infarcts, especially in anticoagulated patients. Overall, intracranial hemorrhage represents 10% of CNS complications. Brain hemorrhage is more frequent during the bacteremic phase of S. aureus infectious endocarditis and is made more likely by severe thrombopenia and anticoagulant therapy.22 A case-control study using diffusion-weighted MRI (Figure 87-4) has revealed that cerebral microbleeds were observed in 57% of patients with in infective endocarditis compared to 15% of control subjects.31 Meningitis, occurring in 5% to 40% of patients with CNS manifestations of infectious endocarditis, can be the consequence of a wide variety of mechanisms; the cerebrospinal fluid may be purulent with positive cultures, clear with moderate pleocytosis, or hemorrhagic. Brain abscesses associated with infectious endocarditis are uncommon; they account for less than 5% of CNS events, but the rate depends on the imaging technique used. In addition, many small abscesses or areas of cerebritis resolve with antibiotics alone. Finally, toxic encephalopathy, defined as mental changes or stupor without focal neurologic manifestations and without computed tomographic (CT) abnormalities, is often included among the CNS complications of infectious endocarditis. Obviously this manifestation can have different causes, such as subtle cerebral lesions, or may be present in the setting of severe sepsis.24
Specific Management
Infectious endocarditis occurring in patients receiving anticoagulant therapy poses a difficult problem. In the absence of CNS complications or in patients with nonhemorrhagic neurologic lesions, warfarin should be discontinued and replaced by heparin. However, in the presence of brain hemorrhage, anticoagulant therapy should be temporarily discontinued. CT scanning is essential for the diagnosis and management of CNS events associated with infectious endocarditis. In addition, it may be the only technique available for unstable ICU patients, especially those on mechanical ventilation. CT may show intracranial bleeding, ischemic lesions, or a pattern consistent with cerebral abscess. MRI is more sensitive for most lesions and should be performed in hemodynamically stable patients, because it can modify clinical management.29 Although conventional four-vessel angiography remains the gold standard for the evaluation of mycotic aneurysms, magnetic resonance angiography is a promising technique. In the absence of randomized trials, which are difficult (if not impossible) to organize, the respective roles of medical, endovascular, and neurosurgical treatment of intracranial aneurysms are not easily assessable. Endovascular treatment (coil embolization) seems to be a reliable and safe technique that should be considered when cerebral mycotic aneurysms are diagnosed.32
Acute Renal Failure
Acute renal failure occurs in up to 40% of complicated infectious endocarditis cases necessitating ICU admission2,23 and may result from several mechanisms. It is often the consequence of cardiogenic or septic shock (with or without multiorgan failure) leading to acute tubular necrosis. Drugs, such as the combination of a glycopeptide and an aminoglycoside, and the use of iodine contrast medium for radiologic investigations may further deteriorate renal function. In some patients with streptococcal or staphylococcal infectious endocarditis, acute renal failure is caused by severe glomerulonephritis. Acute renal failure may require the initiation of dialysis.
 Medical and Surgical Treatment
Medical and Surgical Treatment
Antibiotic Treatment
Certain general principles underlie the current guidelines5,33 for infectious endocarditis treatment. In cases of streptococcal infectious endocarditis, determination of the minimal inhibitory concentration of penicillin is necessary to choose the best regimen. Parenteral antibiotics are recommended over oral drugs because of the importance of sustained antibacterial activity, which requires high dosages (e.g., 150 to 200 mg/kg of amoxicillin for streptococcal infectious endocarditis). However, oral antibiotics may be considered for right-sided S. aureus infectious endocarditis after a few days of parenteral antibiotics when IV administration is not possible because of poor venous access. In that case, a combination of a fluoroquinolone and rifampin is an acceptable regimen. Many experts recommend using a combination of agents with activities against the cell wall (β-lactams or glycopeptides) plus an aminoglycoside (gentamicin) for most cases of infectious endocarditis, especially complicated cases such as those in ICU patients. Gentamicin can be administered in one or two daily doses, except for infective endocarditis due to enterococci, for which two doses are recommended. The use and duration of aminoglycosides depend on the pathogen and, for streptococci, their susceptibility to penicillin G and the presence of a prosthesis (Table 87-3). Although a shorter course of aminoglycosides has been proposed for enterococcal infectious endocarditis,16 no controlled study has confirmed the safety of this strategy. For staphylococcal infectious endocarditis, a triple regimen including rifampin is recommended,34 especially for patients with PVE. Short-term therapy (15 days) was shown to be effective in selected cases of uncomplicated S. aureus right-sided infectious endocarditis or left-sided native valve infectious endocarditis due to highly susceptible streptococci. However, most current recommendations emphasize prolonged antibiotic administration (4-6 weeks or even 8 weeks) for S. aureus PVE. Valve cultures, but not positive Gram staining or positive PCR, should be taken into account to decide how long to continue antimicrobial therapy after valve replacement.5
TABLE87-3 Antibiotic Treatment of Complicated Infectious Endocarditis as a Function of Valve Type, Pathogen, and Susceptibility
| Microorganism | Native Valve Infectious Endocarditis | Prosthetic Valve Endocarditis |
|---|---|---|
| Penicillin-susceptible streptococci (MIC < 0.125 mg/L) | Penicillin G, amoxicillin, or ceftriaxone for 4 wk* | Penicillin G or amoxicillin for 6 wk + gentamicin for 2 wk* |
| Relatively penicillin-resistant streptococci (MIC ≥ 0.125-2 mg/L) | Penicillin G or amoxicillin for 4 wk + gentamicin for 2 wk* | Penicillin G or amoxicillin for 4-6 wk + gentamicin for 4 wk* |
| Streptococci with penicillin G MIC > 2 mg/L, enterococci, and Abiotrophia spp. | Penicillin G or amoxicillin for 4-6 wk + gentamicin for 4 wk* | Penicillin G or amoxicillin for 6 wk + gentamicin for 6 wk* |
| MSSA | Nafcillin or oxacillin for 4-6 wk + gentamicin for 3-5 days† | Nafcillin or oxacillin + rifampin for ≥ 6 wk + gentamicin for 2 wk† |
| MRSA | Vancomycin + rifampin for 4-6 wk + gentamicin for 3-5 days | Vancomycin + rifampin for ≥ 6 wk + gentamicin for 2 wk |
| HACEK organisms | Ceftriaxone or cefotaxime for 4 wk | Ceftriaxone or cefotaxime for 6 wk |
| Enterobacteriaceae | Ceftriaxone or cefotaxime for 4 wk + gentamicin or amikacin for 1 wk‡ | Ceftriaxone or cefotaxime for 6 wk + gentamicin or amikacin for 2 wk‡ |
| Bartonella spp. | Ceftriaxone or doxicycline for 6 wk + gentamicin for 2-3 wk | Ceftriaxone or doxicycline for 6 wk + gentamicin for 2-3 wk |
| Coxiella burnetii | Doxycycline or ofloxacin + hydroxychloroquine for ≥ 18mo | Doxycycline or ofloxacin + hydroxychloroquine for ≥ 18mo |
| Candida spp. | LF AmB§ with or without 5-FC§§ or AmB with or without 5-FC or an echinocandin for 2 wk, then fluconazole for susceptible organism in stable patient with negative blood culture results for 4 wk | LF AmB§ with or without 5-FC§§ or AmB with or without 5-FC or an echinocandin for 2 wk, then fluconazole for susceptible organism in stable patient with negative blood culture results for 4 wk Lifelong suppressive therapy for prosthetic valve endocarditis if valve cannot be replaced is recommended. |
HACEK, Haemophilus aphrophilus or paraphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species; MIC, minimal inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus.
* Vancomycin or teicoplanin therapy is indicated for patients who are allergic to β-lactam antibiotics. Optimal antimicrobial therapy is not available for high-level aminoglycoside-resistant and vancomycin-resistant enterococci. Eradicating these pathogens requires consultation with an infectious disease specialist or a microbiologist.
† A first-generation cephalosporin is indicated for patients who are allergic to penicillin, except for those with immediate-type hypersensitivity reactions to β-lactam antibiotics, who should be treated with a glycopeptide.
‡ The results of susceptibility tests might indicate the need to adapt the initial regimen.
§ Lipid formulation of amphotericin B.
The role of new molecules in the treatment of infective endocarditis remains to be evaluated. Daptomycin is a bactericidal lipopeptide which can be used in methicillin-resistant S. aureus35 and vancomycin-resistant enterococci infective endocarditis36
Surgical Management
In recent series,6,9 48% to 50% of patients (up to 75% in specialized medical-surgical centers) undergo valve replacement during the acute phase of infectious endocarditis before the completion of antibiotic treatment.
Indications for and Timing of Cardiac Surgery
Absolute indications are CHF caused by valvular insufficiency, prosthesis obstruction or dehiscence, periannular abscess, or S. aureus or fungal PVE. These microorganisms cannot be eradicated without removal of the prosthesis. Development of CHF in the setting of infectious endocarditis generally requires cardiac valve replacement regardless of the number of days on antibiotics. Emergency cardiac surgery is recommended for the following situations: (1) aortic or mitral infective endocarditis with severe acute regurgitation or valve obstruction, causing refractory pulmonary edema or cardiogenic shock and (2) aortic or mitral infective endocarditis with fistula into a cardiac chamber or pericardium, causing pulmonary edema or shock.5
With regard to other potential indications, contraindications, and timing of valve replacement, the following factors should be emphasized: (1) Although the risk of systemic embolization is higher in patients with large vegetations on the mitral valve, vegetation characteristics alone rarely justify valve surgery. The decreasing risk of emboli with time, especially after the first week of effective antibiotic therapy, should be considered when deciding whether to operate.30 (2) In patients with neurologic complications, a conservative approach is to delay cardiac surgery for 2 or 3 weeks after an embolic event and for at least a month after cerebral bleeding. However, in the case of CHF, the valve can be replaced within 7 days or less after an embolic infarct, especially when it is of limited size and the patient’s good mental status prevails.5 (3) In that case, there is a high probability of complete neurologic recovery.37 True contraindications of valve surgery are rare and include uncontrolled septic shock, unhealed sternal wound infection, and severe coagulation disorders.
Coronary angiography is recommended for patients older than 40 years and those with at least one risk factor, except when emergency surgery is needed.5
Surgical Technique
Surgery includes complete removal of all infected and necrotic tissue, followed by valve reconstruction. In selected cases, good results have been achieved with conservative mitral valve valvuloplasty. In most patients, valve replacement with a mechanical or biological prosthesis or a homograft is necessary. The use of cryopreserved homografts has been suggested to reduce the risk of persistent or recurrent infection. However, mechanical prostheses and xenografts compare favorably, with improved durability.38
 Outcome and Prognostic Factors
Outcome and Prognostic Factors
The overall in-hospital mortality was 18% in the large, contemporary, and multinational ICE-PCE study.6 This figure includes all types of infectious endocarditis, however, and warrants refinement according to different categories of disease. Another recent cohort of 513 patients with complicated left-sided native valve infectious endocarditis had a 6-month mortality rate of 26%.10 Two studies found mortality rates for PVE of 33% and 22%, respectively.9,36 In the international ICE collaborative study, healthcare-associated native valve endocarditis was associated with higher in-hospital mortality (25%) compared to community-acquired endocarditis (13%).13 Survival of ICU patients with infective endocarditis is lower. Among 228 patients with infectious endocarditis referred to the two ICUs in our hospital, the in-hospital mortality rate was 45%.22 It was 42% in a multicenter study involving 198 critically ill patients with infective endocarditis.24
Prognostic factors of survival have been studied by several authors. In most cases, these reflect the site of infectious endocarditis (see earlier discussion), comorbidities, causative agent, and type of complications. CHF, septic shock, neurologic events, S. aureus PVE, increasing age, and paravalvular complications are associated with in-hospital mortality in most studies.6,10,13,23,24 The hemodynamic status of the patient at the time of valve replacement is the main determinant of perioperative mortality, with a poorer prognosis for patients with pulmonary edema or impaired left ventricular function.3,7 Neurologic events markedly increase the risk of death, which can reach 50% in patients with altered mental status.10 Among microorganisms, S. aureus is associated with higher mortality rates than streptococci for left-sided native valve and PVE.13,25,26 Finally, mounting evidence shows that for both complicated left-sided native valve infectious endocarditis and S. aureus PVE, valve replacement during active endocarditis combined with medical therapy is associated with a better outcome than medical treatment alone.6,12,13,39,40 The reoperation rate, mainly for prosthesis dehiscence or new infectious endocarditis, is 2% to 3% per year, and the 5-year survival rate is approximately 80% to 90% for native valve infectious endocarditis and 60% for PVE. A scoring system taking into account mental status, comorbidity, CHF, microbiology, and the use of surgical treatment in left-sided native valve infectious endocarditis was recently published.41
Heiro M, Nikoskelainen J, Engblom E, et al. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160:2781-2787.
Murdoch DR, Corey R, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis–Prospective Cohort Study. Arch Intern Med. 2009;169:463-473.
Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297:1354-1361.
Duval X, Iung B, Klein I, et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis. A prospective study. Ann Intern Med. 2010;152:497-504.
The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). Eur Heart J. 2009;30:2369-2413.
1 Hoen B, Alla F, Selton-Suty C, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA. 2002;288:75-81.
2 Francois P, Schrenzel J, Stoerman-Chopard C, et al. Identification of plasma proteins adsorbed on hemodialysis tubing that promote Staphylococcus aureus adhesion. J Lab Clin Med. 2000;135:35-42.
3 Weidenmaier C, Peschel A, Kempf VA, et al. DltABCD- and MprF-mediated cell envelope modifications of Staphylococcus aureus confer resistance to platelet microbicidal proteins and contribute to virulence in a rabbit endocarditis model. Infect Immun. 2005;73:8033-8038.
4 Moreillon P, Que Y. Infective endocarditis. Lancet. 2004;363:139-149.
5 Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J. 2009;30:2369-2413.
6 Murdoch DR, Corey R, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463-473.
7 Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851-1859.
8 Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-1330.
9 Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297:1354-1361.
10 Hasbun R, Vikram HR, Barakat LA, et al. Complicated left-sided native valve endocarditis in adults: Risk classification for mortality. JAMA. 2003;289:1933-1940.
11 Durante-Mangoni E, Bradley S, Selton-Sutty C, et al. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med. 2008;168:2095-2103.
12 Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med. 2002;162:90-94.
13 Benito M, Miro JM, deLazzari E, et al. Health care–associated native valve endocarditis: importance of Non-nosocomial Acquisition. Ann Intern Med. 2009;150:586-594.
14 Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293:3022-3028.
15 Lefort A, Lortholary O, Casassus P, et al. Comparison between adult endocarditis due to β-hemolytic streptococci (serogroups A, B, C, and G) and Streptococcus milleri: a multicenter study. (for the β-Hemolytic Streptococci Infective Endocarditis Study Group). Arch Intern Med. 2002;162:2450-2456.
16 Olaison L, Schadewitz K. Enterococcal endocarditis in Sweden, 1995-1999: can shorter therapy with aminoglycosides be used? (for the Swedish Society of Infectious Diseases Quality Assurance Study Group for Endocarditis). Clin Infect Dis. 2002;34:159-166.
17 Chu VH, Miro JM, Hoen B, et al. Coagulase-negative staphylococcal prosthetic valve endocarditis–a contemporary update based on the International Collaboration on Endocarditis: prospective cohort study. Heart. 2009;95:570-576.
18 Aubron C, Charpentier J, Trouillet JL, et al. Native-valve infective endocarditis caused by Enterobacteriaceae: report on 9 cases and literature review. Scand J Infect Dis. 2006;38:873-881.
19 Lefort A, Mainardi JL, Selton-Suty C, et al. Streptococcus pneumoniae endocarditis in adults: a multicenter study in France in the era of penicillin resistance (1991-1998). Medicine (Baltimore). 2000;79:327-337.
20 Baddley JW, Benjamin DK, Patelm M, et al. Candida infective endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27:519-529.
21 Marin M, Munoz P, Sanchez M, et al. Molecular diagnosis of infective endocarditis by real-time broad-range polymerase chain reaction (PCR) and sequencing directly from heart valve tissue. Medicine. 2007;86:195-202.
22 Mourvillier B, Trouillet JL, Timsit JF, et al. Infective endocarditis in the intensive care unit: clinical spectrum and prognostic factors in 228 consecutive patients. Intensive Care Med. 2004;30:2046-2052.
23 Karth GD, Koreny M, Binder T, et al. Complicated infective endocarditis necessitating ICU admission: Clinical course and prognosis. Crit Care. 2002;6:149-154.
24 Sonneville R, Mirabel M, Hagège D, et al. Complications neurologiques des endocardites infectieuses en réanimation (Etude ENDOREA). Réanimation. 2010;19(suppl 1):S16-S17.
25 Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633-638.
26 Francioli P. Complications of infective endocarditis. In: Scheld WM, Whitley RJ, Durack DT, editors. Infections of the Central Nervous System. Philadelphia: Lippincott-Raven; 1997:523-553.
27 Heiro M, Nikoskelainen J, Engblom E, et al. Neurologic manifestations of infective endocarditis: A 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160:2781-2787.
28 Snygg-Martin U, Gustafsson L, Rosengren L, et al. Cerebrovascular complications in patients with left-sided infective endocarditis are common: a prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis. 2008;47:23-30.
29 Duval X, Iung B, Klein I, et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis. Ann Intern Med. 2010;152:497-504.
30 Dickerman SA, Abrutyn E, Barsic B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE-PCS). Am Heart J. 2007;15:1086-1094.
31 Klein I, Iung B, Labreuche J, et al. Cerebral microbleeds are frequent in infective endocarditis. Stroke. 2009;40:3461-3465.
32 Chapot R, Houdard E, Saint-Maurice JP, et al. Endovascular treatment of cerebral mycotic aneurysms. Radiology. 2002;222:389-396.
33 Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394-e434.
34 Le T, Bayer AS. Combination antibiotic therapy for infective endocarditis. Clin Infect Dis. 2003;36:615-621.
35 Levine DP, Lamp KC. Daptomycin in the treatment of patients with infective endocarditis: experience from a registry. Am J Med. 2007;120(Suppl 1):S28-S33.
36 Mohr JF, Friedrich LV, Yankelev S, et al. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE). Int J Antimicrob Agents. 2009;33:543-548.
37 Ruttmann E, Willeit J, Ulmer H, et al. Neurological outcome of septic cardioembolic stroke after infective endocarditis. Stroke. 2006;37:2094-2099.
38 Akowuah EF, Davies W, Oliver S, et al. Prosthetic valve endocarditis: early and late outcome following medical or surgical treatment. Heart. 2003;89:269-272.
39 Alexiou C, Langley SM, Stafford H, et al. Surgery for active culture-positive endocarditis: Determinants of early and late outcome. Ann Thorac Surg. 2000;69:1448-1454.
40 Lalani T, Cabell CH, Benjamin DK, et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of a propensity score and instrumental variables methods to adjust for treatment-selection bias. Circulation. 2010;121:1005-1013.
41 Vickram HR, Buenconseyo Y, Hasbun R, et al. Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis; a propensity analysis. JAMA. 2003;290:3207-3214.