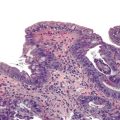
Infectious Disorders of the Gastrointestinal Tract
Laura W. Lamps
Introduction
Gastrointestinal (GI) infections are a major cause of morbidity and mortality worldwide. As the number of transplant patients and those with other immunocompromising conditions increases, and as global urbanization and transcontinental travel become more frequent, the surgical pathologist must be familiar with infectious diseases that were once limited to tropical regions of the world or the realm of esoterica.
The goal of the surgical pathologist in evaluating GI specimens for infectious colitis is twofold. First, acute self-limited processes and infectious processes must be differentiated from chronic idiopathic inflammatory bowel disease (IBD)—ulcerative colitis or Crohn’s disease. Second, dedicated attempts must be made to identify the specific infecting organisms.1 In recent years, the surgical pathologist’s ability to diagnose infectious processes in tissue sections has grown exponentially with the advent of new histochemical stains, immunohistochemistry, and molecular analysis. As these techniques have evolved, our knowledge of the specific histologic patterns of inflammation related to various organisms has also increased.
Most enteric infections are self-limited. Patients who undergo endoscopic biopsies usually have chronic or debilitating diarrhea or systemic symptoms, or they are immunocompromised. A discussion with the gastroenterologist regarding symptomatology and colonoscopic findings, as well as knowledge of the patient’s travel history, food intake (e.g., sushi, poorly cooked beef), sexual practices, and immune status can aid immeasurably in the evaluation of biopsies for infectious diseases.
Viral Infections of the GI Tract
The type of viral infection and the manifestations of disease vary with the site of infection and the immune status of the patient.
Cytomegalovirus
Clinical Features
Primary cytomegalovirus (CMV) infections in immunocompetent persons are usually asymptomatic. CMV infection may develop anywhere in the GI tract, from mouth to anus, in both immunocompromised and immunocompetent persons. CMV is best known as an opportunistic pathogen in patients with a suppressed immune system, most commonly in those with the acquired immunodeficiency syndrome (AIDS) and in patients who have undergone solid organ or bone marrow transplantation.2 In fact, CMV remains the most common GI pathogen overall in patients with AIDS.
Primary infections in healthy persons are typically asymptomatic. When symptomatic disease does occur, it is usually self-limited and may manifest as a mononucleosis-like syndrome. In most of these cases, GI involvement is subclinical. Symptomatic GI infection has been reported occasionally in immunocompetent patients,3,4 although many of these were elderly or were eventually found to have underlying malignancies or hematologic disorders and therefore may not have had entirely competent immune systems.
Symptoms vary with site of infection. The most common clinical symptoms are diarrhea (either bloody or watery), abdominal pain, fever, and weight loss.2 Patients with esophageal infection often have dysphagia and odynophagia.5 A rare, but important, entity associated with pediatric CMV infection is hypertrophic gastropathy and protein-losing enteropathy resembling Ménétrier disease.6,7
In addition, secondary CMV infection may be superimposed on chronic GI diseases such as ulcerative colitis and Crohn’s disease. In such cases, CMV superinfection is associated with exacerbations of the underlying disease, steroid-refractory disease, toxic megacolon, and a higher mortality rate. Some authorities recommend immunohistochemical evaluation for CMV as part of the routine evaluation of biopsies in patients with steroid-refractory ulcerative colitis.8,9
Pathologic Features
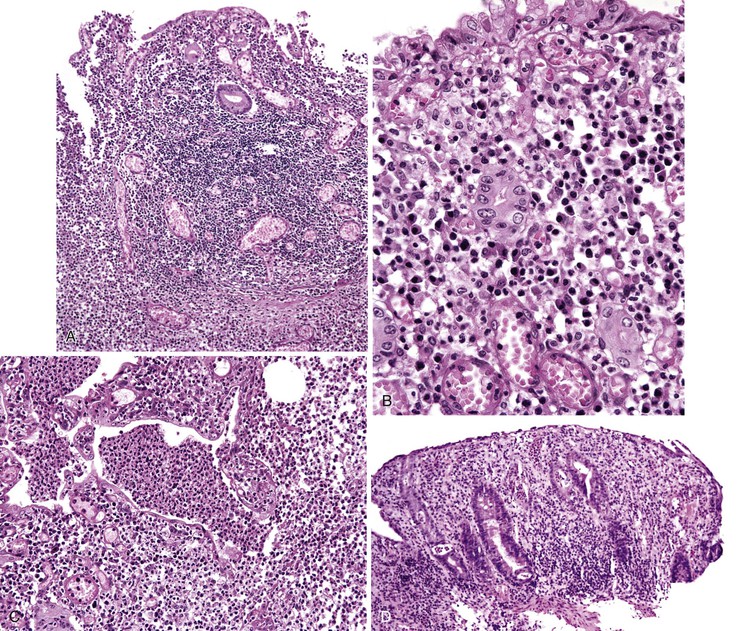
CMV causes a remarkable variety of gross lesions.2,10 Ulceration is the most common; the ulcers may be single or multiple, and superficial or deep. They can be quite large (>10 cm), and often have a well-circumscribed, “punched-out” appearance. Segmental ulcerative lesions and linear ulcers may mimic Crohn’s disease. Other gross lesions include mucosal hemorrhage, pseudomembranes, and inflammatory polyps or masses.11,12
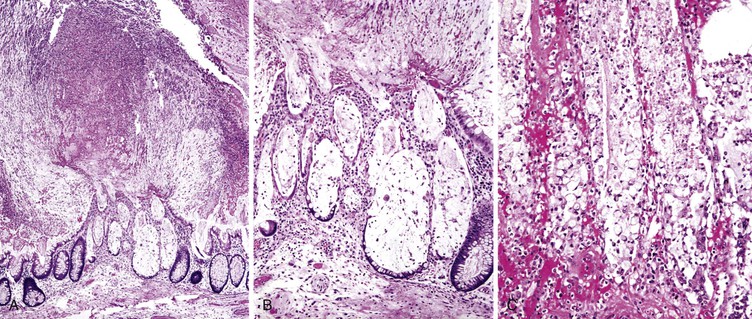
The histologic spectrum of CMV infection is extremely variable, ranging from minimal inflammation to deep ulcers with prominent granulation tissue and necrosis. Frequently observed histologic features include mucosal ulceration, a neutrophil-rich mixed inflammatory infiltrate, and cryptitis of glandular epithelium.2 Crypt abscesses, crypt atrophy and loss, and numerous apoptotic enterocytes may be seen as well.10 CMV may also cause a vasculitis featuring numerous endothelial viral inclusions with associated inflammation, necrosis, and thrombosis of the affected vessel and resultant mucosal ischemia.13
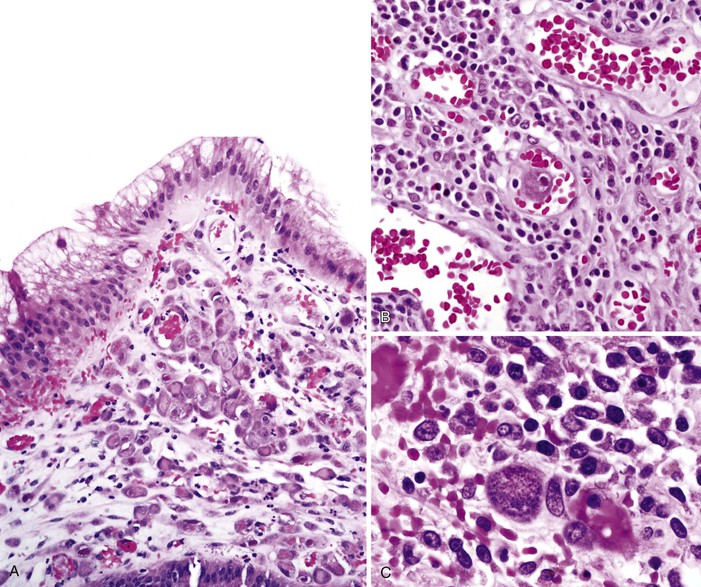
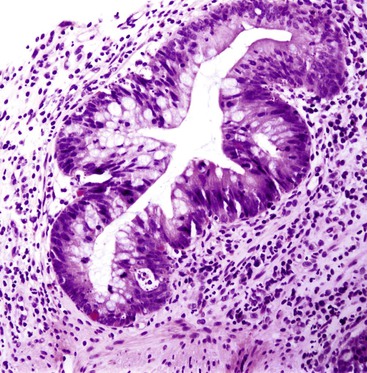
Infected cells show both nuclear and cytoplasmic enlargement (hence the name, “cytomegalovirus”) (Fig. 4.1, A). Characteristic “owl’s-eye” intranuclear viral inclusions and basophilic granular intracytoplasmic inclusions may be seen on routine hematoxylin and eosin (H&E) preparations (see Fig. 4.1, B and C). Inclusions are preferentially found in endothelial cells, stromal cells, and macrophages, and, rarely, in glandular epithelial cells. In contrast to adenovirus or herpes, CMV inclusions are often found deep within ulcer bases rather than at the edges of ulcers or in the superficial mucosa. Adjacent nuclei may be enlarged, appear smudged, or have a “ground-glass” appearance, but they lack typical inclusions. Characteristic inclusions with virtually no associated inflammatory reaction may occur in severely immunocompromised patients.
In biopsy specimens, the diagnosis are easily missed when only rare inclusions are present. Examination of multiple levels and use of immunohistochemistry can be invaluable in detecting the rare cells containing an inclusion. Other diagnostic aids include viral culture, polymerase chain reaction (PCR) assays, in situ hybridization, serologic studies, and antigen tests. Serologic studies often have limited usefulness because of the persistence of latent CMV infection. In addition, isolation of CMV in culture does not imply active infection, because the virus can be excreted for months to years after a primary infection.2
Differential Diagnosis
The differential diagnosis of CMV includes primarily other viral infections (Table 4.1), particularly adenovirus.5 Adenovirus inclusions are usually crescent shaped, located in surface epithelium, and only intranuclear in location. CMV inclusions typically have an owl’s-eye morphology, are typically located in endothelial or stromal cells, and exist in either the nucleus or cytoplasm. The ballooning degeneration phase of adenovirus infection, just before cell lysis, most closely resembles CMV.
Table 4.1
Light Microscopic Comparison of CMV, Adenovirus, and HSV Infection
| CMV | Adenovirus | HSV | |
| Cell involved | Stromal and endothelial cells; macrophages; rarely epithelial cells | Epithelial only—either surface epithelial cells or goblet cells in colon | Epithelial cells, usually squamous |
| Location of inclusion | Nucleus and cytoplasm | Exclusively intranuclear | Intranuclear |
| Characteristics of inclusion | “Owl’s-eye” morphology in nucleus; basophilic and granular in cytoplasm | Basophilic “smudge cell” filling entire nucleus most common; rarely, acidophilic inclusions with halos (Cowdry type A) | Homogeneous with “ground-glass” appearance or acidophilic with clear halo and peripheral chromatin margination (Cowdry type A) |
| Associated changes | Cellular enlargement Apoptosis Mixed inflammatory infiltrate Vasculitis |
Surface cell disorder, loss of orientation, apoptosis; cells not enlarged | Sloughing of epithelial cells, neutrophilic infiltrate; multinucleated cells common |
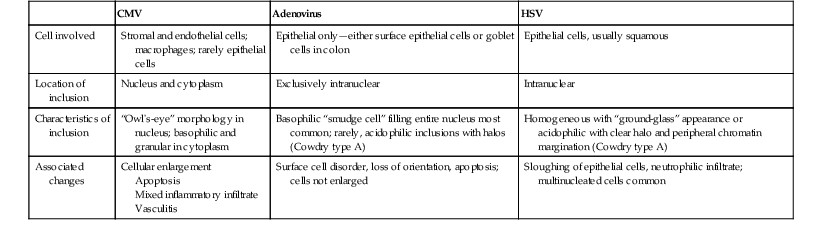
CMV, Cytomegalovirus; HSV, herpes simplex virus.
The distinction between CMV infection and graft-versus-host disease in bone marrow transplant recipients can be particularly difficult because the clinical and histologic features are similar. Immunohistochemistry or in situ hybridization studies should be used to rule out CMV infection in this setting, because failure to identify CMV infection could result in delay of antiviral therapy.10 Furthermore, these conditions may coexist. Graft-versus-host disease is favored when there is abundant apoptosis associated with crypt necrosis and dropout in the setting of minimal inflammation. The presence of viable nests of endocrine cells favors graft-versus-host disease.
Herpesvirus
Clinical Features
Herpetic infection can occur throughout the GI tract but is most common in the esophagus and anorectum. Although herpes infection of the gut is often seen in immunocompromised patients and remains one of the most common infections in patients with human immunodeficiency virus (HIV), it is not limited to this group. In immunocompetent patients, infection is often self-limited.14 Immunocompromised patients, however, are at risk for disseminated infection and life-threatening illness.
Patients with herpetic esophagitis are seen with odynophagia, dysphagia, chest pain, nausea, vomiting, fever, and GI bleeding. Many have disseminated herpes infection at the time of diagnosis.15 Herpetic proctitis is the most common cause of nongonococcal proctitis in homosexual men. Patients typically are seen with severe anorectal pain, tenesmus, constipation, discharge, hematochezia, and fever. Concomitant neurologic symptoms (difficulty in urination and paresthesias of the buttocks and upper thighs) are also well described, as is inguinal lymphadenopathy.15
Pathologic Features
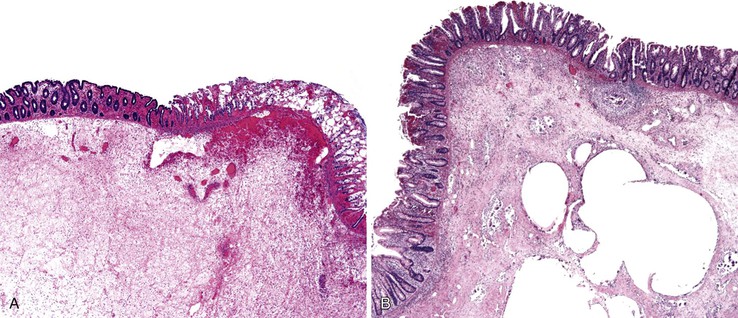
Ulcers are the most common gross finding in the esophagus, and these are usually associated with an exudate. The ulcers are often shallow and well-circumscribed. Some patients have vesicles surrounding the ulcers. Many patients have a nonspecific erosive esophagitis without discrete ulcers, however. In herpetic proctitis, the presence of perianal vesicles is common, often in association with pustules or shallow ulcers. Proctoscopic findings include ulceration and mucosal friability. Vesicles may extend into the rectum or anal canal.15,16
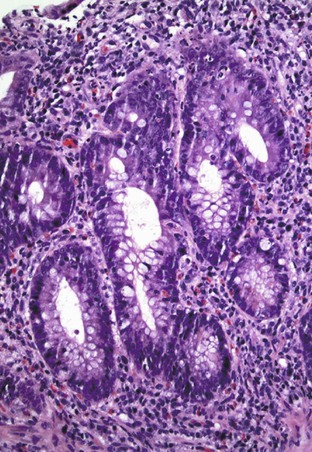
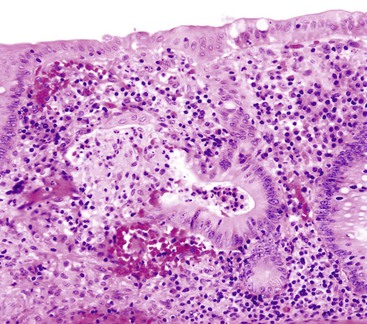
Typical histologic findings, regardless of site, include ulceration, neutrophils in the lamina propria, and an inflammatory exudate that often contains sloughed epithelial cells (Fig. 4.2, A). Prominent aggregates of macrophages may also be present.17 In the anorectum, perivascular lymphocytic cuffing and crypt abscesses may be seen as well.
Characteristic viral inclusions and multinucleate giant cells are present in only a minority of biopsy specimens (see Fig. 4.2, B and C).7 The best place to search for viral inclusions is in the squamous epithelium at the edges of ulcers and in sloughed cells in the exudate. Two types of nuclear inclusions may be found: the homogeneous ground-glass inclusions and the acidophilic inclusions with a surrounding clear halo and peripheral chromatin margination (Cowdry type A inclusions). Inclusions may be single or multinucleate. The histologic findings for herpes simplex virus 1 (HSV-1) and HSV-2 are indistinguishable.
Differential Diagnosis
The differential diagnosis predominantly contains other viral infections, including CMV and varicella-zoster, that may infect the GI tract (see Table 4.1).18 Varicella produces histologic findings identical to those of HSV, but patients often have a rash.18 Mixed infections are common in many situations in which herpetic infection is found. Immunohistochemistry and in situ hybridization are very useful, and viral culture is a valuable diagnostic aid as well. Serologic studies may be useful if there is a very high or rising antibody titer, but serologic testing has limited use because latent infections can persist for years.
Adenovirus
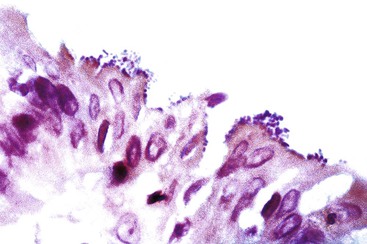
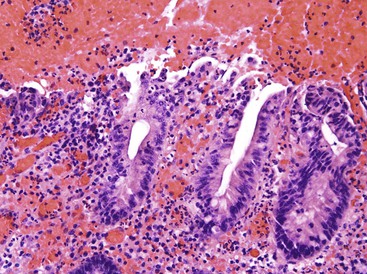
Adenovirus infection is second only to rotavirus as a cause of childhood diarrhea, and it is associated with a broad spectrum of diseases in both children and adults. It has gained more attention in recent years as a cause of diarrhea in immunocompromised patients, especially those with AIDS and those who have received bone marrow or solid organ transplants.19–21 Virtually all patients are seen with diarrhea, sometimes accompanied by fever, weight loss, and abdominal pain. Characteristic inclusions may be seen, especially in immunocompromised patients, in the nuclei of surface epithelial cells (particularly goblet cells); these are often accompanied by apoptotic epithelial cells and epithelial degenerative changes.19,24 Adenovirus is also one of the associated with ileal and cecal intussusception in children.22–24 The characteristic nuclear inclusions are known as “smudge cells” because of their enlarged, homogeneous, basophilic characteristics (Fig. 4.3; see Table 4.1). Useful aids to help in the diagnosis of adenovirus infection include immunohistochemistry, stool examination by electron microscopy, molecular studies, and viral culture. This entity is discussed further and illustrated in Chapter 5.
Other Enteric Viruses
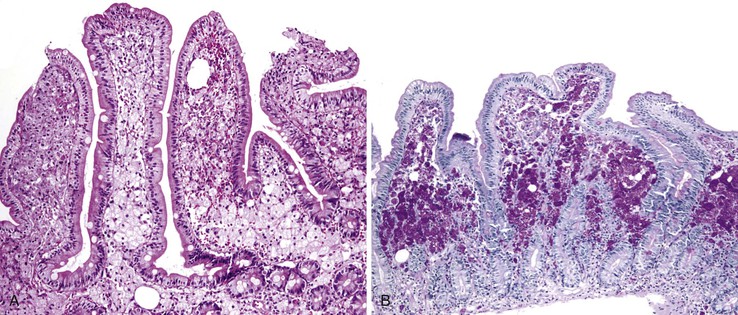
Acute viral gastroenteritis is one of the most common causes of illness worldwide. Although most infections are self-limited, viral gastroenteritis can cause severe dehydration (particularly when caused by rotavirus), as well as chronic diarrhea in children with immunodeficiency syndromes such as severe combined immunodeficiency. Enteric viral infections are a significant cause of diarrhea in patients with AIDS. Rotavirus and enterovirus, like adenovirus, are associated with intussusception in children. Many enteric viruses do not cause disease in humans; others seldom cross the stage of the surgical pathologist because they are detected in stool samples rather than biopsy specimens. Common enteric viruses known to cause diarrhea in humans include, but are not limited to, adenovirus, rotavirus, coronavirus, astrovirus, Norwalk virus and other enteric caliciviruses, and echovirus and other enteroviruses.25–30 Enteric involvement was documented in the coronavirus-associated severe acute respiratory syndrome (SARS), and diarrhea was a common presenting symptom in that outbreak.25
Small bowel biopsy findings include villous fusion, broadening, and blunting; crypt hypertrophy; and an increased mononuclear cell infiltrate within the lamina propria with variably present neutrophils (Fig. 4.4). There may also be an increase in intraepithelial lymphocytes. Reactive and degenerative epithelial changes are usually present, particularly at the surface, including epithelial cell disarray and loss of nuclear polarity.31,32 Increased apoptosis may be seen in surface and glandular epithelium. In the limited number of human studies available, the severity of the histologic lesion does not appear to correlate with clinical severity of illness. Most human studies of the histopathology associated with enteric viral infection are limited to the duodenum. The rare reports that have evaluated the large bowel have documented histologic findings ranging from normal to focal cryptitis with increased apoptosis. With the exception of adenovirus infection, inclusions are not seen on light microscopy.
Other viruses that affect the GI tract include measles (rubeola),33,34 human herpesvirus 8 (HHV-8; also known as Kaposi sarcoma–associated herpesvirus),35 HHV-6,36,37 and Epstein-Barr virus (EBV), which is associated with a wide variety of lymphoproliferative disorders.
Human Papillomaviruses
Human papillomavirus (HPV) has been implicated in the pathogenesis of esophageal papillomas, esophageal squamous cell carcinomas, anal condylomas, and anal squamous cell carcinomas. These entities are discussed in detail in Chapters 19, 24, and 32.
Human Immunodeficiency Virus
GI disease is an important cause of morbidity and mortality in patients affected by HIV and in those with AIDS. Although opportunistic pathogens are often found in these patients, there is a subgroup in whom no pathogens are found despite extensive clinical and pathologic evaluation. The two major disease entities associated with HIV in the absence of other demonstrable pathogens are chronic idiopathic esophageal ulcers and AIDS enterocolopathy.
Chronic idiopathic esophageal ulcers reportedly cause 40% to 50% of ulcers found in HIV-infected patients.38,39 The ability of HIV to directly cause these ulcers remains controversial, although evidence of HIV within the ulcerative lesions has been demonstrated by molecular analysis, immunohistochemical studies, and enzyme-linked immunosorbent assays (ELISA).40 Patients are seen with severe odynophagia, independent of food intake; chest pain; and weight loss. The middle esophagus is the most common location, followed by the distal esophagus. Endoscopically, the ulcers consist of one or more well-circumscribed lesions of variable depth that can mimic ulcers caused by other infectious agents, particularly viral pathogens. They can be quite large (>3.0 cm in greatest dimension) and deep, with irregular margins and overhanging, edematous edges. Mucosal bridges and sinus tract formation may occur. Histologically, the ulcers contain granulation tissue with a mixed acute and chronic inflammatory infiltrate that often contains eosinophils. By definition, special histochemical stains and immunohistochemical stains for identifiable pathogens must be negative. This finding is especially important because these ulcers are sometimes treated with steroids.41 This entity is also discussed in Chapter 5.
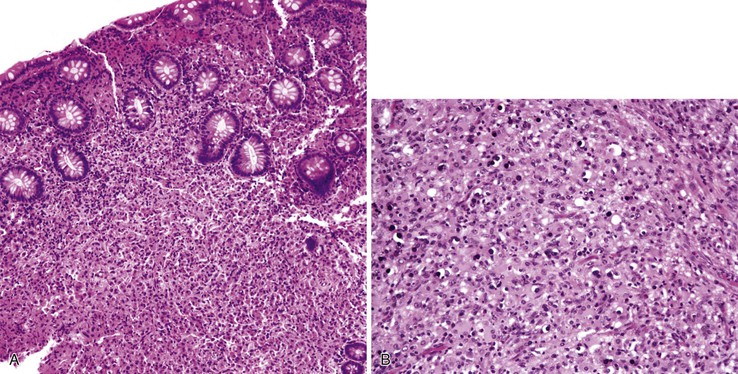
HIV/AIDS enteropathy/colopathy is a somewhat controversial entity that has been loosely defined as the morphologic changes seen in the gut of patients with HIV/AIDS and chronic diarrhea for which no other infectious cause has been identified. The controversy arises because asymptomatic patients may have similar morphologic findings on biopsy and, conversely, severely symptomatic patients may have normal biopsies.42 In addition, there is always the added concern that a causative pathogen simply has been missed. Because patients with HIV/AIDS do have severe impairments of GI function including diarrhea, malabsoprtion, and weight loss, even in the absence of any demonstrable pathogens, many authors support use of the term AIDS enteropathy (or colopathy) to describe the morphologic findings, provided that the bowel has been adequately sampled and all other infectious causes have been excluded.43–45 However, other authorities believe that this is a poorly understood term that does not clearly represent a specific disease entity and therefore should be avoided.
Endoscopy and colonoscopy findings are usually normal. In the small bowel, the histologic features include villous blunting and atrophy, crypt hypertrophy, increased intraepithelial lymphocytes, variably increased mononuclear cells in the lamina propria, increased mitoses within glandular epithelial cells, and increased numbers of apoptotic enterocytes at the surface and in the glands. In the colon, inflammatory changes are similar, but the apoptotic epithelial cells in the glandular epithelium are often very prominent (Fig. 4.5). The changes resemble those seen in mild graft-versus-host disease and chemotherapy-related mucosal injury.43–45 Other pathogens, particularly other viruses such as CMV and adenovirus that can produce similar histologic features, must be rigorously excluded.
Bacterial Infections of the Gastrointestinal Tract
Bacterial diarrhea is a worldwide health problem. Many bacterial infections of the gut are related to ingestion of contaminated water or food or to foreign travel, especially from an area of good sanitation to one of poor sanitation. Although bacterial pathogens are often recovered by microbial culture, surgical pathologists may play a valuable role in diagnosis. Despite the dizzying array of bacterial infections that may affect the GI tract, many produce a similar spectrum of histologic features and may be generally categorized as follows (Table 4.2):
Table 4.2
Classification of Bacterial Infections of the Gastrointestinal Tract by Histologic Pattern
| Minimal or No Inflammatory Change | Acute Self-Limited Colitis Pattern | Pseudomembranous Pattern | Predominantly Granulomatous | Diffuse Histiocytic | Predominantly Lymphohistiocytic | Architectural Distortion | Ischemic Pattern |
| Toxogenic Vibrio cholerae (O1 strain) | Shigella | Enterohemorrhagic E. coli | Yersinia | Rhodococcus equi | Lymphogranuloma venereum | Salmonella typhimurium | Enterohemorrhagic E. coli |
| Enteropathogenic E. coli | Campylobacter | Clostridium difficile | Mycobacterium tuberculosis | Whipple disease | Salmonella typhimurium | Shigella | Clostridium difficile |
| Enteroadherent E. coli | Aeromonas | Occasionally Shigella | Actinomycosis | MAI (immunocompromised patients) | Focal or mild: Aeromonas, Campylobacter | ||
| Spirochetosis | Occasionally Salmonella (especially other Vibrio species non-typhoid) | MAI (immunocompetent patients) | |||||
| Neisseria spp. | Occasionally Clostridium difficile | ||||||
| Syphilis (± increased plasma cells) |
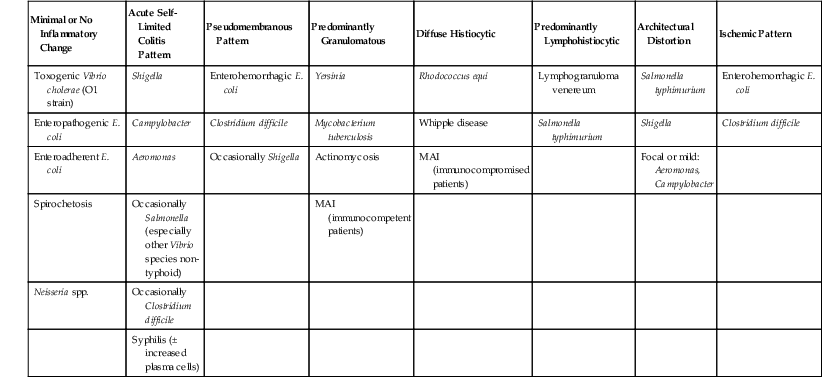
E. coli, Escherichia coli; MAI, Mycobacterium avium-intracellulare.
Acute Self-Limited Colitis
The ASLC pattern is the most common pattern in enteric infections. Typical histologic features include neutrophils in the lamina propria, with or without crypt abscesses and cryptitis; preservation of crypt architecture; and lack of basal plasmacytosis.1,46–51 The acute inflammatory component is often most prominent in the middle to upper levels of the crypts. Lack of crypt distortion, Paneth cell metaplasia, and basal lymphoplasmacytosis help to distinguish ASLC from IBD.1,51 The changes may be focal, as in focal active colitis, or diffuse.
Surgical pathologists should be aware of the infections that are most likely to mimic other IBD, particularly Crohn’s disease, ulcerative colitis, and ischemic colitis (see Table 4.2). Because most patients do not present for endoscopy until several weeks after the onset of symptoms, pathologists usually are not exposed to the classic histologic features of acute infectious colitis. This is important because the resolving phase of infectious colitis is more challenging to diagnose. At this stage, only occasional foci of neutrophilic cryptitis and only patchy increases in lamina propria inflammation may be found, and these may, in fact, contain abundant plasma cells and increased intraepithelial lymphocytes, features that are also seen in Crohn’s disease or even lymphocytic colitis. It is important to be aware of the patient’s symptoms (particularly acute versus chronic onset) and, ideally, the culture results, because the exact diagnosis may be difficult to resolve on histologic grounds alone. The pathologic details of specific bacterial infections are discussed in the following sections.
Major Causes of Bacterial Enterocolitis
Vibrio cholerae and Related Species
V. cholerae (specifically the toxigenic O1 strain) is the causative agent of cholera, an important worldwide cause of watery diarrhea and dysentery that may lead to significant dehydration, electrolyte imbalance, and death within hours.52,53 Most infections result from consumption of raw or undercooked seafood, especially shellfish. Other vibrios, including non-O1 strains of V. cholerae, Vibrio hollisae, and Vibrio parahaemolyticus, also can cause severe gastroenteritis.54 Symptoms of cholera include the abrupt onset of diarrhea, usually profusely watery and rarely bloody, accompanied by abdominal pain, vomiting, muscle cramps, and fever. Disseminated infection is a particularly important risk with immunocompromised patients; patients with underlying liver disease, partial or total gastrectomy, and diseases of iron metabolism are also at risk for more serious Vibrio infections.
Despite the severity of the illness, V. cholerae O1 is a noninvasive toxin-producing organism that cause minimal or no histologic change to the intestinal mucosa. Nonspecific findings such as small bowel mucin depletion, degenerative surface epithelial changes, and a mild increase in lamina propria mononuclear cells have been rarely reported.55,56 Non-toxigenic O1 and other non-cholerae Vibrio species may show an erosive enterocolitis with active neutrophilic inflammation and associated hemorrhage. Useful ancillary diagnostic tests include culture, PCR, and serologic studies.57 A history of travel to or emigration from an endemic or epidemic area also can be invaluable.
Aeromonas and Related Species
Initially believed to be nonpathogenic gram-negative bacteria, Aeromonas and related species are increasingly recognized as causes of gastroenteritis in both children and adults.58–61 Aeromonas hydrophila and Aeromonas sobria most often cause GI disease in humans. Infection usually is caused by exposure to untreated water but also may result from consumption of contaminated foods such as produce, meat, and dairy products. Infections most frequently occur in the late spring, summer, and early fall, and children are most commonly affected. A mild, self-limited diarrheal illness is most common, sometimes accompanied by nausea, vomiting, and cramping abdominal pain. A more severe, dysentery-like illness occurs in 15% to 25% of patients, featuring bloody or mucoid diarrhea and fecal leukocytes. This variant is most likely to mimic chronic idiopathic IBD endoscopically.
Endoscopically, findings include mucosal edema, friability, erosions, exudates, and loss of vascular pattern. The distribution is often segmental, either right- or left-sided, and may mimic ischemic colitis, Crohn’s disease, or ulcerative colitis macroscopically.62,63 A severe pancolitis mimicking fulminant ulcerative colitis has also been described. The histologic features are usually those of ASLC, including cryptitis, crypt abscesses, and a neutrophilic infiltrate in the lamina propria. However, ulceration and focal architectural distortion may be seen in some cases (Fig. 4.6).
The differential diagnosis includes other infectious colitides, ischemic colitis, and chronic idiopathic IBD. When architectural distortion is present in a patient with more chronic symptoms or macroscopic features mimicking chronic idiopathic IBD, it may be difficult to resolve the issue of Aeromonas infection versus Crohn’s disease or ulcerative colitis. Although there are no histologic features specific for Aeromonas infection (as with many infections of the GI tract), it is important for the surgical pathologist to realize that this is one of the bacteria that can most closely mimic chronic idiopathic IBD. Stool cultures are critical to diagnosis, and certain selective media may be required.
Escherichia coli
Escherichia coli is the most common gram-negative human pathogen. The diarrheogenic E. coli are classified into five groups, based primarily on serotyping.64,65 If pathogenic E. coli is suspected, the clinical laboratory should be notified to search for it specifically, because it may be missed on routine culture. In addition, because pathogenic E. coli strains are often cleared rapidly from stool (often within 4 to 7 days), cultures should be taken as early as possible.
Enterotoxigenic and Enteropathogenic E. coli
These noninvasive organisms cause nonbloody diarrhea. Enterotoxigenic E. coli (ETEC) is a major cause of traveler’s diarrhea and of outbreaks within industrialized nations.66–68 Enteropathogenic E. coli (EPEC) is predominantly an infection of infants and neonates. The gross and microscopic pathology of ETEC and EPEC have not been well described in humans.
Enteroinvasive E. coli
The pathology of enteroinvasive E. coli (EIEC) has not been well described in humans. These organisms are very similar to Shigella genetically and in their clinical presentation and pathogenesis; therefore, the pathology could be expected to be similar as well.69 Symptoms include diarrhea (typically mucoid and watery but nonbloody), tenesmus, fever, malaise, and abdominal cramps. EIEC is transmitted via contaminated cheese, water, and person-to-person contact; it is also a cause of traveler’s diarrhea. The organisms produce a severe, dysentery-like illness as well as bacteremia; this can be a particular problem in AIDS patients.70,71
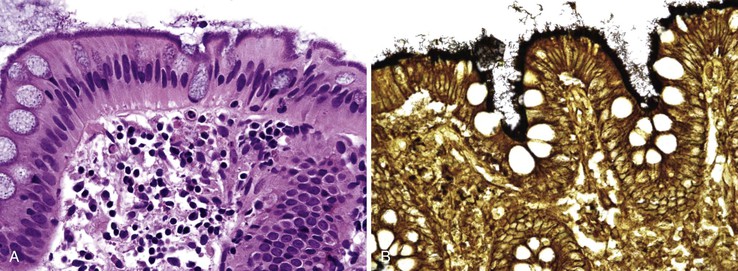
Enteroadherent E. coli
This noninvasive strain of enteroadherent E. coli (EAEC) is similar to EPEC. Both have been increasingly recognized as causes of chronic diarrhea and wasting in AIDS patients.72–74 Although endoscopic findings are usually unremarkable, right colon biopsies more often yield pathologic findings. Histologic examination shows degenerated surface epithelial cells with associated intraepithelial inflammatory cells. A coating of adherent bacteria at the surface epithelium is the most prominent feature (Fig. 4.7).74 The bacteria may be tightly or loosely adherent. The histologic findings can be patchy and can resemble an exaggerated brush border; they may easily be missed at low power. In addition, specimens from infected patients may show no associated inflammatory reaction whatsoever. The main entities in the differential diagnosis are normal mucosa and spirochetosis; the bacteria in EPEC and EAEC are not spirillar, in contrast to spirochetosis, and in general, they stain gram negative.
Enterohemorrhagic E. coli
The most common strain of enterohemorrhagic E. coli (EHEC) is O157 : H7.75,76 This pathogen gained national attention in 1993 when a massive outbreak in the western United States was linked to contaminated ground beef. Although contaminated meat is the most frequent mode of transmission, infection may also occur through contaminated water, milk, and produce and through person-to-person contact. EHEC produces a cytotoxin similar to that of Shigella dysenteriae, but there is no invasion. Hemolytic-uremic syndrome or thrombotic thrombocytopenic purpura may develop in affected persons, and children and the elderly are at particular risk for grave illness. In some studies, the use of antibiotics to treat EHEC appears to increase the risk for hemolytic-uremic syndrome. Symptoms usually consist of bloody diarrhea with severe abdominal cramps and mild or no fever. Nonbloody, watery diarrhea may occur, however. Only one third of patients have fecal leukocytes.
Endoscopically, patients typically have severe mural edema with associated hemorrhage. The mucosa is eroded and ulcerated, and ulcers often have an overlying purulent exudate. The edema may be so marked as to cause obstruction, and surgical resection may be required to relieve this or to control bleeding. The right colon is usually most severely affected. The histologic features closely resemble ischemic colitis of other causes, including marked edema and hemorrhage in the lamina propria and submucosa with associated mucosal acute inflammation, crypt withering, and lamina propria hyalinization (Fig. 4.8). Microthrombi may be present within small-caliber blood vessels, and pseudomembranes resembling antibiotic-associated pseudomembranous colitis (PMC) are occasionally present as well. Mucosal necrosis is frequently seen, often involving the upper portion of the mucosa but sparing the deeper crypts.77,78
The differential diagnosis primarily includes Clostridium difficile–related PMC and ischemic colitis of other causes, from which EHEC may be histologically indistinguishable. The clinical history, including the possibility of consumption of contaminated food, the age of the patient, and macroscopic findings may aid in distinguishing ischemia from E. coli infection. The C. difficile antigen test or PCR assays, or both, may be very helpful in distinguishing C. difficile–related colitis from EHEC.
An antibiotic-associated hemorrhagic colitis has been reported in association with Klebsiella oxytoca.79 This colitis is hemorrhagic, segmental, most common in the right and transverse colon, and lacks pseudomembranes. A history of antibiotic usage, especially penicillins, may help distinguish this infection from EHEC. Stool culture is invaluable in making the diagnosis; however, routine stool cultures cannot distinguish O157 : H7 from normal intestinal flora, because microbiologic diagnosis requires screening on selective agar. An immunohistochemical stain for the EHEC organism has been described,80 and molecular assays exist but are not widely available.
Salmonella
Salmonellae are gram-negative bacilli that are transmitted through food and water and are prevalent where sanitation is poor. They are an important cause of both food poisoning and traveler’s diarrhea. The majority of medically important salmonellae are in the species Salmonella enterica. They are differentiated by their serotyping, but the nomenclature has changed. For example, the bacterium Salmonella enteritidis is now designated Salmonella enterica ser. Enteritidis, or simply S. enteritidis.
Discussion of Salmonella infection can generally be divided into infection by typhoid and nontyphoid species.81 Enteric (typhoid) fever is usually caused by the serovar S. Typhi but may also be caused by S. paratyphi; the most common nontyphoid species include S. enteritidis, S. typhimurium, S. muenchen, S. anatum, S. paratyphi., and S. give. Although historically enteric fever was considered a much more severe disease and nontyphoid salmonellosis a milder one, more recent literature suggests a greater degree of overlap (both clinically and pathologically) than previously thought. Patients with low gastric acidity are at increased risk of salmonellosis, and patients with AIDS have a greater risk of Salmonella infection as well as a greater likelihood of severe infection and septicemia.
Typhoid (Enteric) Fever
Typhoid fever typically manifests with abdominal pain, headache, an elevation in fever over several days, and occasionally constipation. An abdominal rash and leukopenia are often seen. Diarrhea, which begins in the second or third week of infection, is initially watery but may progress to severe GI bleeding.81,82 Perforation and toxic megacolon may complicate typhoid fever.82–85
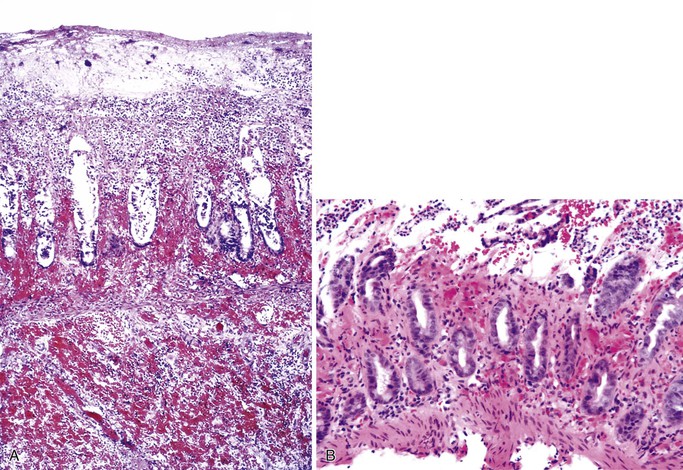
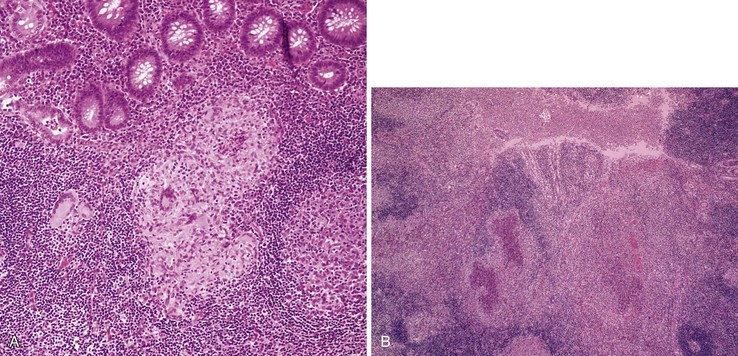
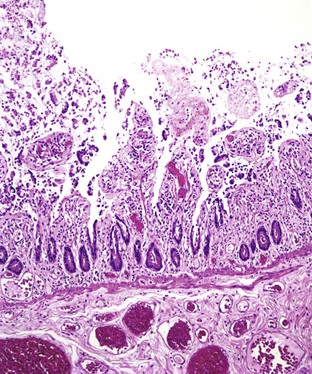
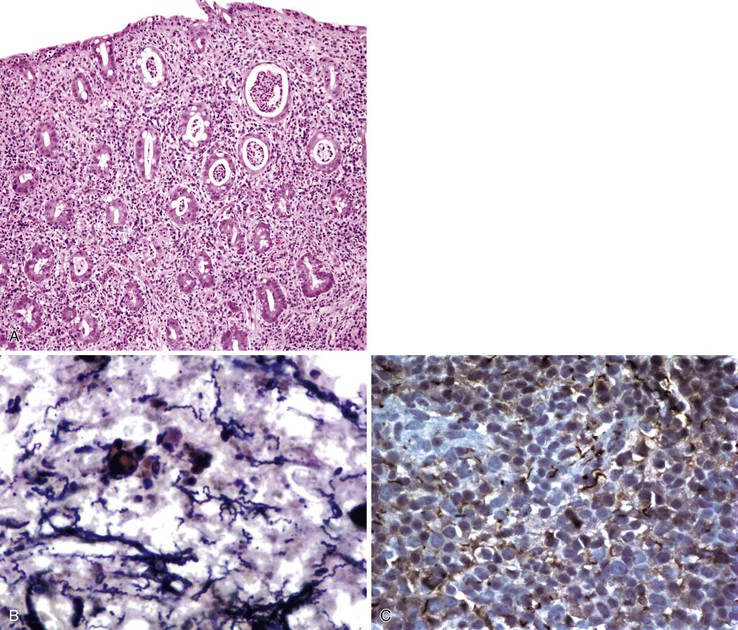
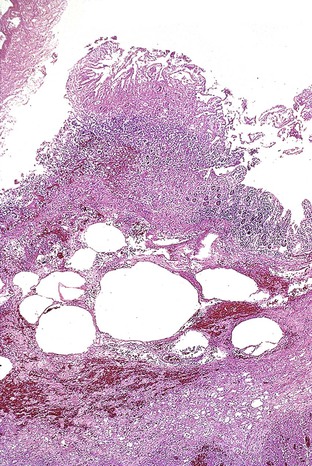
Any level of the alimentary tract may be involved, but the characteristic pathology is associated with lymphoid aggregates and Peyer patches and therefore is most prominent in the ileum, appendix, and colon. Grossly, the bowel wall is thickened, and raised nodules may be seen corresponding to hyperplastic lymphoid tissue. Aphthous ulcers overlying lymphoid aggregates, linear ulcers, discoid ulcers, and full-thickness ulceration and necrosis are common as the disease progresses. Associated suppurative mesenteric lymphadenitis may occur. Occasionally, the mucosa is grossly normal or only mildly inflamed and edematous.82–85
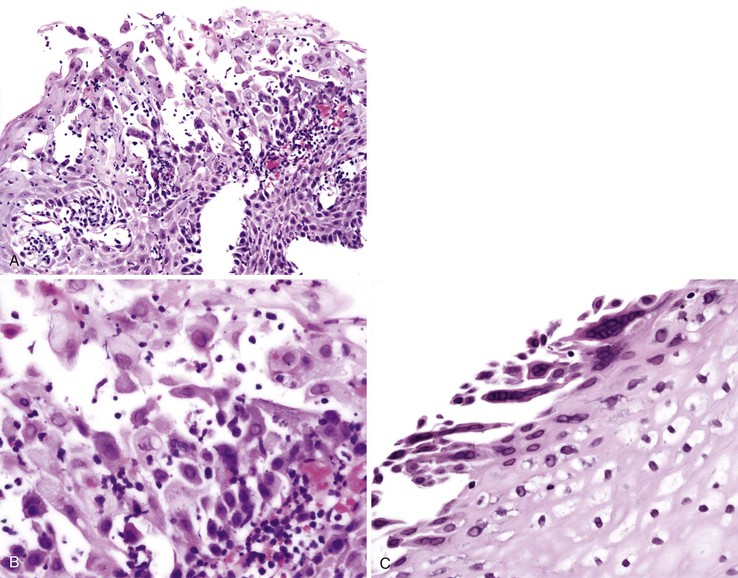
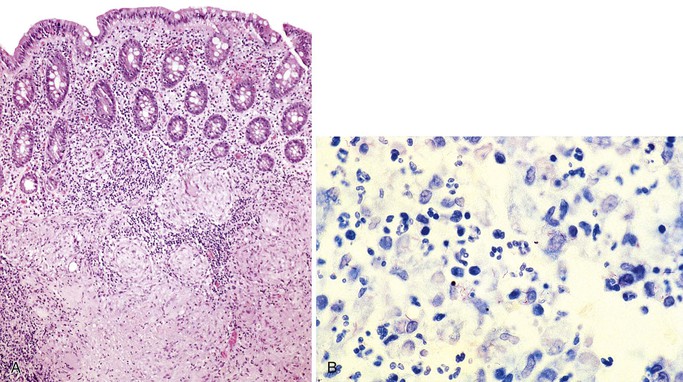
The histiocyte is the predominant inflammatory cell. Hyperplasia of Peyer patches leads to acute inflammation of the overlying epithelium (Fig. 4.9, A and B). Eventually, macrophages, mixed with occasional lymphocytes and plasma cells, infiltrate and obliterate the lymphoid follicles; neutrophils are not prominent.82–85 Necrosis then begins in the Peyer patch and spreads to the surrounding mucosa, which eventually ulcerates. Ulcers are typically very deep. Architectural distortion that may mimic ulcerative colitis or Crohn’s disease is well described (see Fig. 4.9, C). Typhoid fever occasionally shows features more consistent with ASLC, including prominent neutrophils, cryptitis, crypt abscesses, and overlying fibrinous exudate.82–85 Granulomas are occasionally seen.
Nontyphoid Salmonella Species
Nontyphoid Salmonella infection typically manifests as a self-limited gastroenteritis. Endoscopic findings include mucosal redness, ulceration, and exudates; pathologic features are those of nonspecific ASLC. Occasionally, significant crypt distortion may be seen (see Fig. 4.9, D).82,83,86,87
The differential diagnosis of typhoid fever includes other infections as well as chronic idiopathic IBD (Table 4.3), and there may be significant histologic overlap among these entities.82–85 A prominence of neutrophils and granulomas is rare in typhoid fever, and although significant crypt distortion has been reported in some cases of salmonellosis, it is usually more pronounced in chronic idiopathic IBD. The differential diagnosis of nontyphoid Salmonella infection includes other causes of infectious ASLC, and occasionally chronic idiopathic IBD as well. Clinical presentation and blood and stool cultures can be invaluable to the pathologist in sorting out this differential diagnosis, and bone marrow biopsy culture may be useful in establishing the diagnosis of typhoid fever. Of note, Salmonella infection may complicate preexisting idiopathic IBD.
Table 4.3
Enteric Bacterial Infectious Mimics of Chronic Idiopathic Inflammatory Bowel Disease and Ischemic Colitis
| Mimics of Crohn’s Disease | Mimics of Ulcerative Colitis | Mimics of Ischemic Colitis |
| Salmonella typhimurium | Shigella spp. | Enterohemorrhagic Escherichia coli |
| Shigella spp. | Nontyphoid Salmonella spp. | Clostridium difficile (pseudomembranous colitis) |
| Yersinia | Aeromonas spp. | Clostridium perfringens |
| Mycobacterium tuberculosis | Campylobacter (rarely) | |
| Aeromonas spp. | Lymphogranuloma venereum | |
| Campylobacter (rarely) | ||
| Lymphogranuloma venereum |
Shigella
Shigella species are virulent, invasive, gram-negative bacilli that cause severe watery and bloody diarrhea and are a major cause of infectious diarrhea worldwide.52 Infection is transmitted by water contaminated with feces, and person-to-person transmission is also possible. It has the highest infectivity rate of the enteric gram-negative bacteria; symptoms may result from ingestion of a very low number of organisms. Children younger than 6 years of age are commonly affected, as are homosexual men and malnourished or debilitated patients. Constitutional symptoms are the earliest manifestation and include abdominal pain, malaise, and fever. The diarrhea is often watery initially; this is followed by the onset of bloody diarrhea containing mucus or pus and accompanied by tenesmus. Complications include severe dehydration, sepsis, perforation, toxic megacolon, reactive arthritis, Reiter syndrome, and hemolytic-uremic syndrome. Chronic disease is rare.
Grossly, the large bowel is typically affected (the left side usually more severely), but the ileum may be involved. The mucosa is hemorrhagic, with exudates that may form pseudomembranes. Ulcerations are variably present.88–90 Histologically, early disease has the features of ASLC with cryptitis, crypt abscesses (often superficial), and ulceration (Fig. 4.10). Pseudomembranes similar to those of C. difficile infection may be seen,90 as may aphthous ulcers similar to those seen in Crohn’s disease. As the disease continues, increased mucosal destruction occurs with many neutrophils and other inflammatory cells in the lamina propria. Marked architectural distortion mimicking idiopathic IBD is well described.87,88,91
The differential diagnosis of early shigellosis is primarily that of other infections, particularly EIEC and C. difficile. Shigellosis, especially later in the disease course, can be extremely difficult to distinguish from Crohn’s disease or ulcerative colitis, both endoscopically and histologically. Stool cultures and clinical presentation may be helpful in this instance. Multiple stool cultures may be necessary, because the organism dies rapidly and prolonged transit time may affect culture yield.
Campylobacter
Campylobacter (the name is derived from the Greek for “curved rod”) is a genus of gram-negative bacteria that are major causes of diarrhea worldwide and are the most common stool isolate in the United States.92–94 Infection is most commonly associated with consumption of undercooked poultry, raw milk, or untreated water. Campylobacter jejuni is more frequently associated with food-borne gastroenteritis93; Campylobacter fetus and the other, less common species are more often seen in immunosuppressed patients and homosexual men. Campylobacter infects patients of all ages, but infants, children, and young adults are most often affected. The incidence in HIV-positive patients is higher than in the general population, and severe, chronic, recurrent, or disseminated infections are more common in this group. Campylobacter infection is associated with the subsequent development of several autoimmune disorders, including Guillain-Barré syndrome, Henoch-Schönlein purpura, and reactive arthropathy.92,94 Campylobacter infection may also cause exacerbations of underlying chronic idiopathic IBD. Patients typically have fever, malaise, abdominal pain (often severe), and watery diarrhea that may contain blood and fecal leukocytes.94,95 Nausea, vomiting, tenesmus, myalgias, and headache are variably present. Symptoms typically resolve within 1 to 2 weeks, but relapse is common.
Endoscopic findings include friable colonic mucosa with associated erythema and hemorrhage. Histologic examination shows features of ASLC.94,96,97 Mild crypt distortion may occasionally be seen, although the architecture overall is preserved (Fig. 4.11). The differential diagnosis primarily includes other forms of infectious enterocolitis that produce the acute infectious-type colitis or focal active colitis pattern. Occasionally, when crypt distortion is seen, Campylobacter colitis can mimic chronic idiopathic IBD.98 Cultures are useful in resolving the differential diagnosis.
Yersinia
Yersinia are among the most common agents of bacterial enteritis in western and northern Europe, and the incidence is rising in both Europe and the United States. These gram-negative coccobacilli may cause appendicitis, ileitis, colitis, and mesenteric lymphadenitis.99–101 Although yersiniosis is usually a self-limited process, chronic infections (including chronic colitis) have been well documented. Immunocompromised and debilitated patients, as well as patients who are taking desferrioxamine or have iron overload, are at risk for serious disease. Yersinia enterocolitica and Yersinia pseudotuberculosis are the species that cause human GI disease.102,103
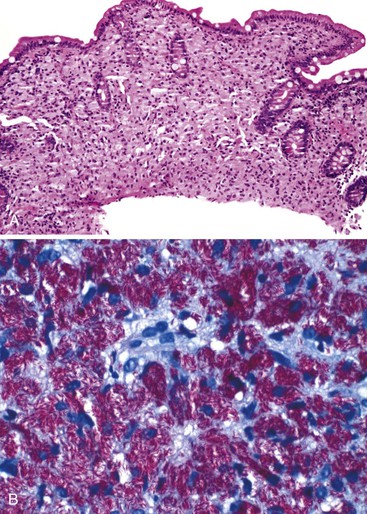
Yersinia organisms preferentially involve the ileum, right colon, and appendix and may cause a pseudoappendicular syndrome. In addition, they are responsible for many cases of isolated granulomatous appendicitis.104 Grossly, the involved bowel has a thickened, edematous wall with nodular inflammatory masses centered on Peyer patches.105–107 Aphthous and linear ulcers may be seen. Involved appendices are enlarged and hyperemic, as in suppurative appendicitis; perforation is often seen. Involved lymph nodes may show gross foci of necrosis.
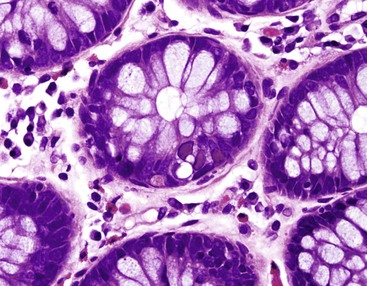
Both suppurative and granulomatous patterns of inflammation are common, and these are often mixed.106 Significant overlap is seen between the histologic features of infection with either species, and either may show epithelioid granulomas with prominent lymphoid cuffing (Fig. 4.12, A), lymphoid hyperplasia, transmural lymphoid aggregates, mucosal ulceration, and lymph node involvement.105 GI infection with Y. pseudotuberculosis has characteristically been described as a granulomatous process with central microabscesses, almost always accompanied by mesenteric adenopathy (see Fig. 4.12, B).107 Gram stains are usually not helpful, but cultures, serologic studies, and PCR assays may be useful in confirming the diagnosis.
The major differential diagnosis includes other infectious processes, particularly with mycobacteria and Salmonella species. Acid-fast stains and culture results should help to distinguish mycobacterial infection; clinical features and the presence of greater numbers of neutrophils, microabscesses, and granulomas may help to distinguish yersiniosis from salmonellosis.
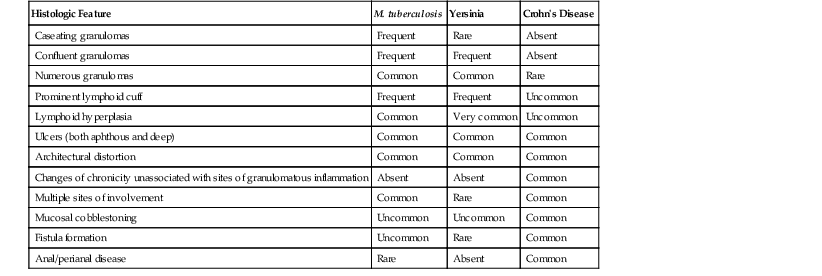
Crohn’s disease and yersiniosis may be difficult to distinguish from one another, and they have a long and complicated relationship (Table 4.4). They may show similar histologic features, including transmural lymphoid aggregates, skip lesions, and fissuring ulcers. In fact, isolated granulomatous appendicitis has in the past frequently been interpreted as primary Crohn’s disease of the appendix. However, generalized IBD rarely develops in patients with granulomatous inflammation confined to the appendix.104 Features that favor a diagnosis of Crohn’s disease include cobblestoning of mucosa and creeping fat, grossly, and microscopic changes of chronicity, including crypt distortion, thickening of the muscularis mucosae, and prominent neural hyperplasia. However, some cases are indistinguishable on histologic grounds alone.
Table 4.4
Comparison of Histologic Features Useful in the Differential Diagnosis of Mycobacterium tuberculosis, Yersinia, and Crohn’s disease
| Histologic Feature | M. tuberculosis | Yersinia | Crohn’s Disease |
| Caseating granulomas | Frequent | Rare | Absent |
| Confluent granulomas | Frequent | Frequent | Absent |
| Numerous granulomas | Common | Common | Rare |
| Prominent lymphoid cuff | Frequent | Frequent | Uncommon |
| Lymphoid hyperplasia | Common | Very common | Uncommon |
| Ulcers (both aphthous and deep) | Common | Common | Common |
| Architectural distortion | Common | Common | Common |
| Changes of chronicity unassociated with sites of granulomatous inflammation | Absent | Absent | Common |
| Multiple sites of involvement | Common | Rare | Common |
| Mucosal cobblestoning | Uncommon | Uncommon | Common |
| Fistula formation | Uncommon | Rare | Common |
| Anal/perianal disease | Rare | Absent | Common |
Clostridial Diseases of the Gut
Clostridial organisms are some of the most potent toxigenic bacteria in existence. Members of this group of bacteria are responsible for PMC/antibiotic-associated colitis (usually C. difficile); necrotizing jejunitis (usually Clostridium perfringens [formerly Clostridium welchii]); neutropenic enterocolitis or typhlitis (often Clostridium septicum), a serious complication of both chemotherapy-associated and primary neutropenia; and botulism (Clostridium botulinum).108
Clostridium difficile–Related Colitis
C. difficile infection is most commonly related to prior antibiotic exposure (especially orally administered antibiotics), because the organism cannot establish infection within the gut in the presence of normal flora. It is the most common nosocomial GI pathogen. The majority of patients are elderly, although infection is certainly not limited to this group. Recurrent disease is seen in as many as one half of cases, despite successful treatment. Furthermore, the incidence of severe or life-threatening C. difficile colitis in North America has increased because of the hypervirulent BI/NAP1 strain.109,110 The range of clinical disease is highly variable, from mild diarrhea to fully developed PMC to fulminant disease with perforation or toxic megacolon.108,111,112 Watery diarrhea is almost always present initially and may be accompanied by abdominal pain, cramping, fever, and leukocytosis. Bloody diarrhea is sometimes seen. Symptoms can occur as long as several weeks after discontinuation of antibiotic therapy.
The entire colon is often involved, but the disease may be patchy or segmental, and any segment of the bowel may be affected, including the small bowel and the appendix. Classic PMC shows yellow-white pseudomembranes, most commonly in the left side of the colon, that bleed when scraped. The distribution is often patchy, and the rectum may be spared.111 Atypical findings include mucosal erythema and friability without pseudomembranes, and typical histologic findings may be seen in the absence of macroscopically evident pseudomembranes.
Histologically, classic PMC features “volcano” or “mushroom” lesions with intercrypt necrosis and ballooned crypts, giving rise to the laminated pseudomembrane composed of fibrin, mucin, and neutrophils (Fig. 4.13, A and B). Ballooned glands are filled with neutrophils and mucin, and the superficial epithelial cells are often lost. Degenerated goblet cells may slough and spill into the lumen of degenerated and necrotic crypts (see Fig. 4.13, C); it is important to be aware of this reactive morphologic change in the context of PMC, because it can mimic signet ring cell adenocarcinoma. Severe and prolonged PMC may lead to full-thickness mucosal necrosis. Less characteristic nonspecific lesions, usually focal active colitis with occasional crypt abscesses but lacking the pseudomembranous feature, have been well described in association with a positive C. difficile toxin assay.112
It is important to remember that the term “pseudomembranous colitis” is descriptive, not a specific diagnosis. Although most cases of PMC are related to C. difficile, other infectious entities (especially Shigella and EHEC), as well as ischemic colitis, can have a similar appearance. A hyalinized lamina propria favors the diagnosis of ischemia; other features, such as crypt withering, pseudomembranes, and mucosal necrosis, may be seen in either entity.113 Endoscopically, pseudomembrane formation is more frequent in PMC, although it can be seen in ischemia.
A history of antibiotic use may be important in making the diagnosis. C. difficile PCR assays are replacing the C. difficile enzyme-linked immunosorbent assays (ELISA) that detect toxins in many centers, owing to their superior sensitivity and specificity. Another approach is to screen stools by using enzyme immunoassays (EIA) that detect glutamate dehydrogenase derived from C. difficile. Unlike those that detect toxin, this immunoassay is highly sensitive, although positive results require confirmation (usually by PCR).114 Studies comparing the sensitivity and specificity of all of these ancillary diagnostic modalities are ongoing.
C. perfringens causes a segmental necrotizing enteritis (also termed enteritis necroticans or pigbel) related to food poisoning. This bacteria also causes diarrhea unrelated to food poisoning, which is often associated with antibiotic use and hospitalization in elderly patients.115,116 Food-related infection is most common in Southeast Asia and New Guinea. The onset of disease usually follows a meal rich in infected meat and occurs in persons with malnutrition and those in endemic areas who routinely eat very-low-protein diets and foods that are high in protease inhibitors.117 Similar cases have been described after eating binges in Western countries. Infection also has been associated with diabetes,118 possibly because of the delayed gastric emptying and reduced intestinal motility seen in these patients. Symptoms include abdominal pain, bloody diarrhea, and vomiting, often with abdominal distention. Complications include perforation, obstruction, bowel gangrene, and septicemia with shock and rapid death. Mild or subacute forms have also been described. Involvement is predominantly seen in the jejunum but is not limited to that site. The bowel is often dusky gray-green, as in ischemia. Necrotic areas may be segmental and quite focal, with intervening areas of normal mucosa. The mucosal exudate can be similar to that of PMC, but inflammation and necrosis often become transmural and lead to perforation. Histologically, the mucosa is necrotic and ulcerated, with a heavy acute inflammatory infiltrate at the edges of ulcers (Fig. 4.14, A). Small-vessel vasculitis and microthrombi may be seen.115,116 Pneumatosis may be present in severe cases, particularly in the mucosa and submucosa (see Fig. 4.14, B). Gram-positive bacilli typical of Clostridium can be found in the necrotic exudate. The major entities in the differential diagnosis of necrotizing enteritis include ischemia and other infections that cause an ischemic-type injury, such as EHEC. The clinical history of consumption of large quantities of meat (especially pork or pork products) may be helpful, along with exclusion of other possible causes of ischemia. Cultures can help to distinguish C. perfringens from other bacteria.
Clostridium septicum is associated with neutropenic enterocolitis (typhlitis), a serious complication of both chemotherapy-related and primary neutropenia.119 Most patients have received chemotherapy within the month before the onset of colitis. Although C. septicum has been frequently reported as a causative agent of typhlitis, especially in adults, other commonly implicated bacteria include other clostridial species, E. coli, Pseudomonas species, and enterococci.120 C. septicum infection is also associated with malignancies (particularly adenocarcinoma) in the colon and distal ileum, and infection may be the first indication of such a tumor.121 Patients usually are seen with the abrupt onset of GI hemorrhage, accompanied by fever, abdominal pain and distention, and diarrhea. The pain often initially localizes to the right lower quadrant but quickly progresses to peritonitis, shock, and sepsis. Perforation is a well-described complication, and infection is often fatal.
The right colon (especially the cecum) is preferentially involved, although the ileum and other sites in the colon may be affected. Gross findings include diffuse dilatation and edema of the bowel, with varying severity of ulceration and hemorrhage.120 Exudates and pseudomembranes resembling C. difficile colitis are common. Microscopically, changes range from mild hemorrhage to prominent submucosal edema, ulceration, marked hemorrhage, and focal necrosis, often with a striking absence of neutrophils (Fig. 4.15). However, neutrophils may sometimes be found despite peripheral neutropenia. Sometimes organisms can be detected in the wall of the bowel on Gram staining. The differential diagnosis includes ischemic colitis and PMC. The appropriate clinical setting and dearth of inflammatory cells should favor a diagnosis of neutropenic enterocolitis. Culture is the most helpful technique for confirming the diagnosis of C. septicum infection, and isolation of the organism from blood cultures may be helpful in septic patients.
Mycobacterial Infections of the GI Tract
Mycobacterium tuberculosis
There has been a remarkable resurgence of tuberculosis in Western countries, due in large part to AIDS, but also to institutional overcrowding and immigrant populations. Tuberculosis remains common in developing countries as well. GI tuberculosis may be acquired through several mechanisms, including swallowing infected sputum in pulmonary tuberculosis; ingestion of contaminated milk (rare where pasteurization is common); hematogenous spread from pulmonary or military disease; and direct extension from adjacent organs.
GI symptoms (rather than pulmonary) may be the initial presentation of disease, and extrapulmonary manifestations of tuberculosis are more common in AIDS patients than in immunocompetent persons. In addition, primary GI tuberculosis in the absence of pulmonary infection has been well documented. Symptoms and signs of GI tuberculosis vary with the site or sites of involvement. The ileocecal and jejunoileal areas are most commonly involved, probably because of the abundance of lymphoid tissue at those locations.122–124 Associated mesenteric adenopathy is very common. Involvement of the ascending colon, duodenum, or rectum is less frequent. Gastroesophageal, appendiceal, or anal/perianal involvement is rare but is well documented in the literature.125–127 Peritoneal tuberculosis is slightly more common than GI tuberculosis and may cause ascites as well as clinically significant adhesions.
Regardless of the site of involvement, patients often have nonspecific symptoms including weight loss, fever, abdominal pain, diarrhea, or a palpable abdominal mass.122–124 Other symptoms include night sweats, malaise, anorexia, GI bleeding, and signs of malabsorption. Symptoms have often been present for months. Laboratory abnormalities include an elevated erythrocyte sedimentation rate and mild anemia.
Strictures and ulcers (often occurring together) are the most common endoscopic findings, along with thickened mucosal folds and inflammatory nodules.122 The ulcers are often circumferential and transverse. Multiple and segmental lesions with skip areas are common and may mimic those of Crohn’s disease (see Table 4.4). Large inflammatory masses (tuberculomas), usually involving the ileocecum, may be seen, and well-described complications include obstruction, perforation, and hemorrhage. The wall of the bowel is often thickened and edematous, with transmural inflammation, lymphoid hyperplasia, and fibrosis in later stages of the disease. Ulcers may be superficial or deep and may overlie hyperplastic Peyer patches (aphthoid ulcers). The characteristic histologic lesion consists of caseating, often confluent, granulomas (Fig. 4.16, A) that may be present at any level of the gut wall but most commonly appear in the submucosa. A rim of lymphocytes is often present at the periphery of the granulomas, and giant cells are variably present. Older lesions are frequently hyalinized and calcified, and well-formed granulomas may be rare and difficult to find. Inflammation of submucosal vessels is common, and architectural distortion that mimics chronic idiopathic IBD is frequently seen overlying areas of granulomatous inflammation. Characteristic granulomas are often present within involved lymph nodes, with confluence and caseation.
Acid-fast stains sometimes demonstrate organisms (see Fig. 4.16, B), preferentially within necrotic areas or macrophages, but culture is usually required for definitive diagnosis. The acid-fast bacilli of Mycobacterium tuberculosis are typically rod shaped and have a “beaded” morphology. Organisms may be abundant in immunocompromised patients yet rare and difficult to detect in immunocompetent persons, and the number of organisms may vary with the age of the lesion and previous antituberculosis therapy. PCR assays are becoming more widely available, but sensitivity also suffers with this methodology if the number of organisms is very low. The purified protein derivative (PPD) tests may be helpful but are unreliable in immunocompromised or debilitated patients. Some atypical mycobacteria, such as Mycobacterium kansasii and Mycobacterium bovis, may cause similar pathologic findings.
The differential diagnosis includes other granulomatous infectious processes, especially yersiniosis and fungal disease, as well as noninfectious processes such as sarcoidosis, Behçet disease, reaction to foreign material, and granulomatous changes secondary to a delayed or interval appendectomy. The granulomas of yersiniosis are typically noncaseating with striking lymphoid cuffs, but there may be considerable histologic overlap. Crohn’s disease may be difficult to distinguish from tuberculosis128; features favoring Crohn’s disease are linear rather than circumferential ulcers, transmural lymphoid aggregates, and deep fistulas and fissures. Tuberculosis also commonly lacks mucosal cobblestoning. A further confounding factor is that tuberculosis has recently been associated with the use of infliximab, a tumor necrosis factor-α neutralizing agent used in treating Crohn’s disease and rheumatoid arthritis. The pattern of involvement in these patients is somewhat unusual, with most exhibiting extrapulmonary tuberculosis. The emergence of infection is often associated with initiation of treatment.129
Mycobacterium avium-intracellulare Complex
Mycobacterium avium-intracellulare complex is the most common mycobacterium isolated from the GI tract. It is typically found in patients with AIDS and other immunocompromising conditions, although it is occasionally seen in immunocompetent persons.130,131 Symptoms include diarrhea, abdominal pain, fever, and weight loss and often reflect systemic infection. Endoscopy findings are usually normal, although white nodules, small ulcers, or hemorrhages may be seen.132 The small bowel is preferentially involved, but colonic and gastroesophageal involvement may be present, as may mesenteric adenopathy.133,134
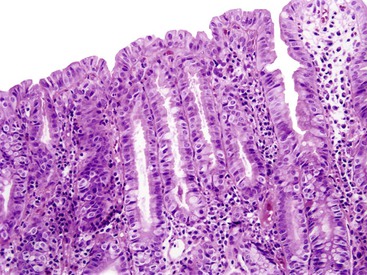
Histologic manifestations vary with the site and the immune status of the patient. Immunocompetent patients usually have a well-formed, often epithelioid granulomatous response, either with or without necrosis. Small bowel biopsies from immunocompromised patients typically show villi distended by a diffuse infiltration of histiocytes containing bacilli (Fig. 4.17, A), with little inflammatory response other than occasional poorly formed granulomas. The small bowel is most often involved, but similar histiocytic infiltrates may be seen in the colon and other areas of the GI tract. Bacilli stain with acid-fast stains (see Fig. 4.17, B), as well as periodic acid–Schiff (PAS) and Gomori’s methenamine silver (GMS) stains. Mycobacteria (including M. tuberculosis and Mycobacterium leprae) may also stain for desmin, actin, and keratin.135 Culture and PCR assays can be extremely helpful in diagnosis. Organisms are usually abundant in the immunocompromised host but may be harder to detect in healthy patients. The differential diagnosis includes Whipple disease and other infectious processes.
Spirochetal Infections of the GI Tract
Syphilis
GI syphilis (Treponema pallidum infection) predominantly involves the anorectum, although other sites may be involved, particularly the stomach.136 Homosexual men are at particularly high risk of infection, and many authorities believe that syphilis, particularly anorectal syphilis, is markedly underdiagnosed because of the variability of the clinical findings. Patients are often asymptomatic,137 but pain (often with defecation), constipation, bleeding, and discharge may be present in syphilitic proctitis. Luetic gastritis commonly manifests with upper GI bleeding, which can occur early or late in the course of the disease. Melena and coffee-ground emesis may be present, along with nausea, fever, malaise, anorexia, early satiety, and epigastric pain.
Gross findings in primary syphilis include anal chancres (indurated, circular lesions as large as 2 cm in diameter that may be single or multiple, with variably present tenderness) that may be associated with a mild proctitis. Signs of secondary syphilis typically manifest 6 to 8 weeks later and include masses, a mucocutaneous rash, or condyloma lata (raised, moist, smooth warts that secrete mucus and are associated with itching and a foul odor).138,139 Inguinal adenopathy is typical. Gross signs of primary and secondary infection sometimes coexist. The mass lesions of secondary syphilis may mimic malignancy, and surgical removal without a prior biopsy should be avoided. Gastric involvement may be either an early or a late manifestation of syphilis. The most common presenting sign is upper GI bleeding, and patients typically have antral erosions, ulcers, or features of gastritis endoscopically.136 Ulcers may have irregular, heaped edges that mimic malignancy.140
Histologically, anorectal syphilis features a dense mononuclear cell infiltrate with prominent plasma cells (Fig. 4.18, A). Cryptitis and crypt abscesses are often present, along with gland destruction and reactive epithelial changes. Granulomas have been reported rarely; occasionally, prominent proliferative capillary endothelial cells may be observed (proliferative endarterteriologitis).139,141 Syphilitic proctitis may be very nonspecific, showing features of ASLC or focal active colitis with or without an increase in plasma cells. Syphilitic gastritis often features a dense plasmacytic infiltrate.136 The glands may be relatively spared by inflammation. Fibrosis may be prominent as the disease progresses.
The gross differential diagnosis of chancre includes anal fissures, fistulas, and traumatic lesions. In general, condyloma acuminata are more dry and more keratinized than condyloma lata. As mentioned earlier, both anorectal and gastric syphilis can mimic malignancy. The histologic differential diagnosis primarily includes other infectious processes, such as H. pylori infection in the stomach. If the plasma cell infiltrate is prominent and monomorphic and effaces the normal architecture, a hematopoietic neoplasm with plasmacytic differentiation should be considered. T. pallidum staining with silver impregnation stains such as Warthin-Starry (see Fig. 4.18, B), Steiner, and Dieterle stains is available, as is immunohistochemistry (see Fig. 4.18, C). Darkfield examination of anorectal discharge may show organisms, although care must be taken in interpretation, because spirochetes are also present in the normal gut flora as well as in intestinal spirochetosis. Serologic studies such as the rapid plasma reagin (RPR), venereal disease research laboratory (VDRL), and fluorescent treponemal antibody absorption (FTA-ABS) tests are extremely helpful in confirming the diagnosis.
Intestinal Spirochetosis
Intestinal spirochetosis is a condition characterized by the presence of spirochetal microorganisms on the luminal surface of the large bowel mucosa.142 The prevalence of spirochetosis ranges from 2% to 16% in Western nations but is significantly higher in developing countries and among homosexual and HIV-infected patients, in whom the prevalence is reportedly as high as 50% based on both biopsy findings and stool culture. It has also been described in association with a wide variety of conditions including diverticular disease, chronic idiopathic IBD, hyperplastic polyps, and adenomatous polyps. Spirochetosis represents infection by a heterogeneous group of related organisms, most importantly Brachyspira aalborgi and Brachyspira pilosicoli, which are genetically unrelated to T. pallidum. Patients with spirochetosis may harbor one or both of these species.142
Although patients with this histologic finding often have symptoms such as diarrhea or anal pain and discharge, it is not clear that spirochetosis causes these symptoms.143,144 Many patients have other infections (especially gonorrhea) complicating the clinical picture. Any level of the colon may be involved, as may the appendix. Typically, endoscopic abnormalities are mild or absent.
On H&E staining, spirochetosis resembles a fuzzy, “fringed” blue line at the luminal border of the colonic mucosa (Fig. 4.19, A). Invasion is not seen, and the changes can be focal. Most have no associated inflammatory infiltrate, although occasionally an associated cryptitis is seen. The organisms stain intensely with Warthin-Starry, Dieterle, or similar silver stains (see Fig. 4.19, B). They also stain with Alcian blue (pH 2.5) and PAS.142,145 Currently there is an excellent immunohistochemical stain available, and many authorities argue that the immunostain is superior, because the quality of silver impregnation stains varies widely depending on the freshness of the reagents and the ability of the technician.146,147 PCR and in situ hybridization assays also exist for identification of the organisms. The differential diagnosis consists primarily of a prominent glycocalyx, which should not stain with silver impregnation stains. Occasionally, EAEC can give a similar appearance, but E. coli should stain strongly gram negative and lack spirillar morphology.
Other Causes of Sexually Transmitted Bacterial Proctocolitis
Other causes of sexually transmitted bacterial proctocolitis include Neisseria gonorrhoeae, Calymmatobacterium granulomatis, and Chlamydia species. Patients typically are seen with anal discharge, pain, diarrhea, constipation, bloody stools, and tenesmus. Proctoscopic findings range from normal to mucosal friability, erosions, and erythema.138,139,148
Chlamydia trachomatis serotypes L1, L2, and L3 cause lymphogranuloma venereum (LGV). Anal pain is usually severe and accompanied by bloody discharge and tenesmus.139,149 The anorectum is the most common site, but LGV has been described in the ileum and colon as well.149 The inflammatory infiltrate is variable; most patients have a lymphoplasmacytic infiltrate in the mucosa and submucosa, but neutrophils may be prominent. Granulomatous inflammation is sometimes present. Histologic features mimicking chronic idiopathic IBD have been described, including a striking “follicular” proctitis and significant architectural distortion that can mimic ulcerative colitis.149–151 Culture, direct immunofluorescence studies, immunohistochemistry, and molecular tests may serve as valuable diagnostic aids. Granuloma inguinale, caused by C. granulomatis, features anal and perianal disease that can appear similar to LGV, although extension into the rectum favors LGV.148 Warthin-Starry or Giemsa stains may aid in visualization of the Donovan bodies typical of granuloma inguinale. Anorectal gonococcal infection is reportedly present in more than 40% of both women and men with uncomplicated gonorrhea. Proctoscopic examination is usually unremarkable. Most biopsy findings in rectal gonorrhea are normal; some reveal a mild increase in neutrophils and mononuclear cells or focal cryptitis.152 Gram-negative cocci can occasionally be seen on Gram staining of anal discharge, and culture can be a valuable diagnostic aid. Neisseria meningitidis has also been isolated from the anorectums of homosexual men, but it remains unclear whether this represents colonization or an actual pathogen in this location.
Miscellaneous Bacterial Infections
Bacterial Esophagitis
Bacterial esophagitis is rare and usually is found in immunocompromised or debilitated patients. Implicated bacteria include Staphylococcus aureus, Lactobacillus acidophilus, and Klebsiella pneumoniae. Endoscopic findings include ulceration, pseudomembrane formation, and hemorrhage. Histologic findings include acute inflammation and necrosis with bacteria demonstrable invading the wall of the esophagus (Fig. 4.20).153
Phlegmonous Gastritis and Enteritis
Phlegmonous enteritis, gastritis, and esophagitis have all been well documented. This is a suppurative, primarily submucosal inflammatory process characterized by marked edema. The causative organisms vary and include E. coli, clostridia, Proteus species, staphylococci, and group A streptococci.154,155 Most patients are debilitated, and many have cirrhosis or alcoholic liver disease.155 Affected patients may have nonspecific GI or systemic symptoms, or phlegmonous disease may be found incidentally at autopsy. Patients typically develop an acute abdomen, sometimes complicated by hematemesis or vomiting of purulent material. Any portion of the alimentary tract may be involved. Typically, the gut wall is markedly thick and edematous. Occasionally, gas-producing organisms such as C. perfringens may lead to the formation of gas bubbles in the submucosa (“emphysematous” changes) (Fig. 4.21). Although the mucosa may be red and friable, discrete ulceration is rarely present. Histologically, there is intense edema and acute inflammation located predominantly in the submucosa, and there may be transmural involvement as well.155 The mucosa may be spared or sloughed entirely, especially in the stomach. Venous thrombosis may complicate the picture, causing ischemic changes. Gram staining may show organisms in the bowel wall, a finding that is diagnostic.
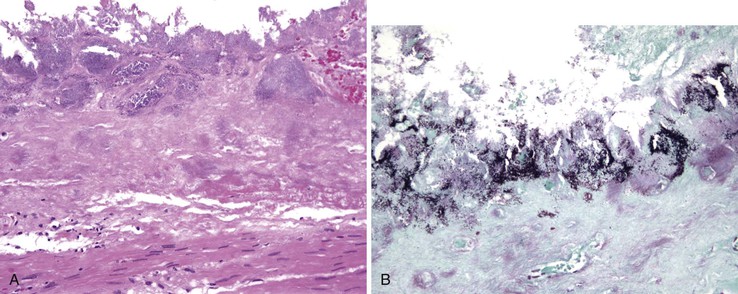
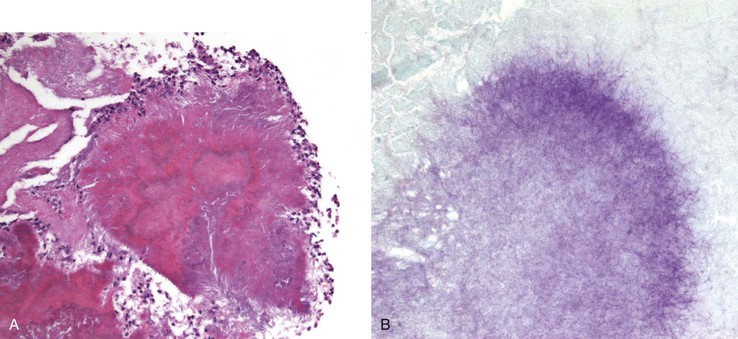
Actinomycosis
The filamentous, anaerobic, gram-positive bacterium Actinomyces israelii is a normal inhabitant of the oral cavity and the upper GI tract. Rarely, it produces a chronic, nonopportunistic GI infection.156–158 Infection is usually in a solitary site, and it may occur at any level of the GI tract. Symptoms include fever, weight loss, abdominal pain, and, occasionally, a palpable mass. Perianal fistulas and chronic (often granulomatous) appendicitis have been described. Actinomycosis is sometimes associated with diverticular disease. Grossly, inflammation may produce a large, solitary mass, with or without ulceration, and infiltration into surrounding structures.157 The organism typically produces actinomycotic (“sulfur”) granules, consisting of irregular round clusters of bacteria rimmed by eosinophilic, clublike projections (Splendore-Hoeppli material). The inflammatory reaction is predominantly neutrophilic, with occasional abscess formation (Fig. 4.22, A). Palisading histiocytes and giant cells, as well as frank granulomas, often surround the neutrophilic inflammation. There may be an associated fibrotic response. Gram staining reveals the filamentous, gram-positive organisms (see Fig. 4.22, B). GMS and Warthin-Starry stains are also used to show these organisms. Commensal actinomyces may be present at the luminal surface, and these do not necessarily imply invasive infection, particularly if there is no inflammatory response. Invasive actinomycosis requires several weeks of intravenous antibiotic therapy; therefore, a definite diagnosis of invasive actinomycosis (rather than the presence of commensals) is important and requires demonstration of the organisms within the wall of the bowel with an associated inflammatory response. This may require multiple levels of lesional tissue sections.
The macroscopic differential diagnosis includes peptic ulcer, lymphoma, and carcinoma. The histologic differential diagnosis includes primarily other infectious agents, particularly Nocardia. Nocardia are partially acid-fast and do not form the typical sulfur granules of actinomycosis; however, cultures may be required to distinguish these two filamentous organisms. Even though actinomyces are GMS positive, they have a more slender morphology than fungi and do not bud or produce hyphae. Care should be taken not to confuse actinomycosis with other bacteria that form clusters and chains but are not truly filamentous, such as Pseudomonas and E. coli. Occasionally, the transmural inflammation, fibrosis, and granulomatous inflammation produced by actinomycotic infection may mimic Crohn’s disease.
Whipple Disease
Whipple disease typically occurs in middle-aged white men with chronic weight loss, arthritis, malabsorption, and lymphadenopathy. Many patients also have significant neuropsychiatric manifestations.159 The small bowel is most often affected, although colonic and appendiceal involvement may be seen as well. Endoscopically, mucosal folds are thickened and coated with yellow-white plaques, often with surrounding erythema and friability. Histologically, the characteristic lesion results from massive infiltration of the lamina propria and submucosa with foamy macrophages (Fig. 4.23, A). The infiltrate often blunts and distends villi. Involvement may be diffuse or patchy. There is usually no associated mononuclear inflammatory infiltrate, but varying numbers of neutrophils are present. The lamina propria may contain small foci of fat, and overlying vacuolization of enterocytes may occur as well.160 Whipple bacillus was identified as Tropheryma whippelii, an actinobacterium, 84 years after Whipple initially reported the disease. This bacillus is strongly PAS positive (see Fig. 4.23, B); electron microscopy and PCR assays may be diagnostic as well. The differential diagnosis includes predominantly M. avium-intracellulare infection. Infection by other intracellular organisms such as Histoplasma or Rhodococcus may rarely simulate Whipple disease.
Rhodococcus equi
The gram-positive coccobacillus, Rhodococcus equi, may occasionally infect humans, particularly the immunocompromised. GI infection manifests as chronic (often bloody) diarrhea and is usually a manifestation of systemic involvement. R. equi produces inflammatory polyps, sometimes with associated mesenteric adenitis. Histologically, polyps consist of organism-laden macrophages that pack the mucosa and submucosa, often with an associated granulomatous response. Organisms stain with PAS and Gram stains, and they may be partially acid-fast. The histologic features may mimic infection with M. avium-intracellulare or Whipple disease.161
Rocky Mountain Spotted Fever
Rocky Mountain spotted fever is caused by Rickettsia rickettsii, which is transmitted by the bite of the common wood or dog tick. Many patients have significant GI findings, including nausea, vomiting, diarrhea, pain, and GI bleeding. These manifestations may precede the rash. Involvement of every portion of the GI tract has been documented.162 Typical histologic findings include vasculitis, often with accompanying nonocclusive microthrombi, and hemorrhage. The inflammatory infiltrate is composed of mononuclear cells with occasional lymphocytes, macrophages, and neutrophils. Immunofluorescence staining demonstrates the organism, and serologic studies may also be of use.
Malakoplakia
Malakoplakia is a rare disorder that can affect any portion of the GI tract.163 It consists of soft, yellow plaques containing a dense histiocytic infiltrate with characteristic Michaelis-Gutmann bodies (Fig. 4.24). Most cases are associated with colorectal adenocarcinoma or some other immunocompromising condition. Numerous bacteria have been associated with GI malakoplakia, including E. coli, Klebsiella, Yersinia, mycobacterial organisms, and R. equi.
Bacillary Angiomatosis
Bacillary angiomatosis comprises pyogenic granuloma-like lesions that occur in immunocompromised patients and mimic Kaposi sarcoma. They are usually associated with Bartonella quintana.
Helicobacter pylori and Helicobacter heilmannii
These bacteria are discussed in detail in Chapter 15.