Chapter 4 Infectious diseases, tropical medicine and sexually transmitted infections
Infection and infectious disease
In the developing world successes such as the eradication of smallpox have been balanced or outweighed by the new plagues. Infectious diseases cause nearly 25% of all human deaths (Table 4.1), rising to more than 50% in low income countries. Two billion people – one-third of the world’s population – are infected with tuberculosis (TB), up to 400 million people catch malaria every year and 200 million are infected with schistosomiasis. Some 500 million people are chronically infected with a hepatitis virus (either HBV or HCV) and 34 million people are living with HIV/AIDS, with 2.6 million new HIV infections in 2008 (65% in sub-Saharan Africa). Infections are often multiple and there is synergy both between different infections and between infection and other factors such as malnutrition. Many of the infectious diseases affecting developing countries are preventable or treatable, but continue to thrive owing to lack of money and political will.
| Disease | Estimated deaths (annual) |
|---|---|
|
Acute lower respiratory infection |
3.5 million |
|
HIV/AIDS |
2 million |
|
Tuberculosis |
2 million |
|
Diarrhoeal disease |
1.8 million |
|
Malaria |
1 million |
|
Measles |
350 000 |
|
Whooping cough |
301 000 |
|
Tetanus |
292 000 |
|
Meningitis |
175 000 |
|
Leishmaniasis |
51 000 |
|
Trypanosomiasis |
10 000 |
Infectious agents
The causative agents of infectious diseases can be divided into four groups:
Other higher classes, notably the insects and the arachnids, also contain species which can parasitize man and cause disease: these are discussed in more detail on page 160.
Host–organism interactions
The symptoms and signs of infection are a result of the interaction between host and pathogen. In some cases, such as the early stages of influenza, symptoms are almost entirely due to killing of host cells by the invading organism. Usually, however, the harmful effects of infection are due to a combination of direct microbial pathogenicity and the body’s response to infection. In meningococcal septicaemia, for example, much of the tissue damage is caused by cytokines released in an attempt to fight the bacteria. The molecular mechanisms underlying host-pathogen interactions are discussed in more detail on page 78.
Sources of infection
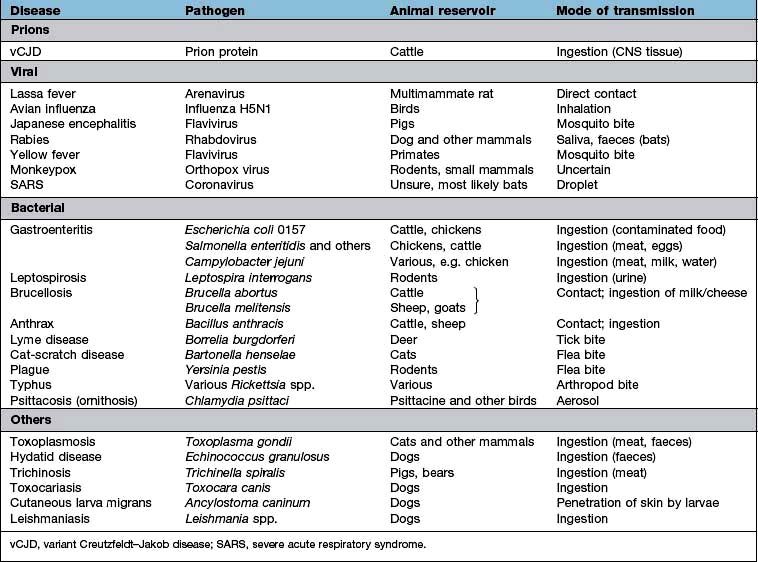
Zoonoses are infections that can be transmitted from wild or domestic animals to man. Infection can be acquired in a number of ways: direct contact with the animal, ingestion of meat or animal products, contact with animal urine or faeces, aerosol inhalation, via an arthropod vector or by inoculation of saliva in a bite wound. Many zoonoses can also be transmitted from person to person. Some zoonoses are listed in Table 4.2.
Most microorganisms do not have a vertebrate or arthropod host but are free-living in the environment. The vast majority of these environmental organisms are non-pathogenic, but a few can cause human disease (Table 4.3). Person-to-person transmission of these infections is rare. Some parasites may have a stage of their life cycle which is environmental (e.g. the free-living larval stage of Strongyloides stercoralis and the hookworms), even though the adult worm requires a vertebrate host. Other pathogens can survive for periods in water or soil and be transmitted from host to host via this route (see below): these should not be confused with true environmental organisms.
Table 4.3 Environmental organisms which can cause human infection
| Organism | Disease (most common presentations) |
|---|---|
|
Bacteria |
|
|
Burkholderia pseudomallei |
Melioidosis |
|
Burkholderia cepacia |
Lung infection in cystic fibrosis |
|
Pseudomonas spp. |
Various |
|
Legionella pneumophila |
Legionnaires’ disease (pneumonia) |
|
Bacillus cereus |
Gastroenteritis |
|
Listeria monocytogenes |
Various |
|
Clostridium tetani |
Tetanus |
|
Clostridium perfringens |
Gangrene, septicaemia |
|
Mycobacteria other than tuberculosis (MOTT) |
Pulmonary infections |
|
Fungi |
|
|
Candida spp. |
Local and disseminated infection |
|
Cryptococcus neoformans |
Meningitis, pulmonary infection |
|
Histoplasma capsulatum |
Pulmonary infection |
|
Coccidioides immitis |
Pulmonary infection |
|
Mucor spp. |
Mucormycosis (rhinocerebral, cutaneous) |
|
Sporothrix schenckii |
Lymphocutaneous sporotrichosis |
|
Blastomyces dermatitidis |
Pulmonary infection |
|
Aspergillus fumigatus |
Pulmonary infections |
Routes of transmission
Many tropical infections, including malaria, are spread from person to person or from animal to person by an arthropod vector. Vector-borne diseases are also found in temperate climates, but are relatively uncommon. In most cases part of the parasite life cycle takes place within the body of the arthropod and each parasite species requires a specific vector. Simple mechanical transfer of infective organisms from one host to another can occur, but is rare. Some vector-borne diseases are shown in Table 4.4.
| Vector | Disease | Microorganism |
|---|---|---|
|
Mosquito |
Malaria |
Plasmodium spp. |
|
Lymphatic filariasis |
Wuchereria bancrofti, Brugia malayi |
|
|
Yellow fever |
Flavivirus |
|
|
West Nile fever |
Flavivirus |
|
|
Dengue |
Flavivirus |
|
|
Sandfly |
Leishmaniasis |
Leishmania spp. |
|
Blackfly |
Onchocerciasis |
Onchocerca volvulus |
|
Tsetse fly |
Sleeping sickness |
Trypanosoma brucei |
|
Flea |
Plague |
Yersinia pestis |
|
Endemic typhus |
Rickettsia typhi |
|
|
Carrion’s disease |
Bartonella bacilliformis |
|
|
Reduviid bug |
Chagas’ disease |
Trypanosoma cruzi |
|
Louse |
Epidemic typhus |
Rickettsia prowazekii |
|
Louse-borne relapsing fever |
Borrelia recurrentis |
|
|
Hard tick |
Lyme disease |
Borrelia burgdorferi |
|
Typhus (spotted fever group) |
Rickettsia spp. |
|
|
Babesiosis |
Babesia spp. |
|
|
Tick-borne relapsing fever |
Borrelia duttonii |
|
|
Tick-borne encephalitis |
Flavivirus |
|
|
Congo-Crimean haemorrhagic fever |
Nairovirus (Bunyavirus) |
Direct person-to-person spread
Organisms can be passed on directly in a number of ways. Sexually transmitted infections are dealt with on page 160. Skin infections such as ringworm, and ectoparasites such as scabies and head lice, can be spread by simple skin-to-skin contact. Other organisms are passed on by blood- (or occasionally other body fluid) to-blood transmission. Blood-to-blood transmission can occur during sexual contact, from mother to infant either transplacentally or in the peripartum, between intravenous drug users sharing any part of their injecting equipment, when infected medical or other (e.g. tattoo needles) equipment is reused, if contaminated blood or blood products are transfused, or in any sporting or accidental contact when blood is spilled. Ingestion of infected breast milk is another route of person-to-person spread for some infections (e.g. HIV).
Prevention and control
 Infection control measures. Poor infection control practice in hospitals and other healthcare environments can cause the transfer of infection from person to person. This may be air-borne, via fomites or a direct contact route. It is essential that all healthcare workers wash or clean their hands before and after patient contact and whenever necessary they should wear gloves, aprons and other protective equipment. This is particularly necessary when performing invasive procedures, or manipulating indwelling devices such as cannulae.
Infection control measures. Poor infection control practice in hospitals and other healthcare environments can cause the transfer of infection from person to person. This may be air-borne, via fomites or a direct contact route. It is essential that all healthcare workers wash or clean their hands before and after patient contact and whenever necessary they should wear gloves, aprons and other protective equipment. This is particularly necessary when performing invasive procedures, or manipulating indwelling devices such as cannulae.
 Eradication of reservoir. In a few diseases, for which man is the only natural reservoir of infection, it may be possible to eliminate disease by an intensive programme of case finding, treatment and immunization. This has been achieved in the case of smallpox. If there is an animal or environmental reservoir, complete eradication is unlikely, but local control methods may decrease the risk of human infection (e.g. killing of rodents to control plague, leptospirosis and other diseases).
Eradication of reservoir. In a few diseases, for which man is the only natural reservoir of infection, it may be possible to eliminate disease by an intensive programme of case finding, treatment and immunization. This has been achieved in the case of smallpox. If there is an animal or environmental reservoir, complete eradication is unlikely, but local control methods may decrease the risk of human infection (e.g. killing of rodents to control plague, leptospirosis and other diseases).
Classification of outbreaks
 Person to person where infection is passed from one infected individual to another and outbreaks of infection are separated by the incubation period.
Person to person where infection is passed from one infected individual to another and outbreaks of infection are separated by the incubation period.
 ‘Point source’ is where there is a single source of infection, e.g. food eaten at a social function. All those infected will develop symptoms at the same time, around the expected incubation period.
‘Point source’ is where there is a single source of infection, e.g. food eaten at a social function. All those infected will develop symptoms at the same time, around the expected incubation period.
 Common source where there is a single source of infection but over a period of time, e.g. a symptomatic carrier of infection working with food preparation. Many people will be exposed over a long period of time.
Common source where there is a single source of infection but over a period of time, e.g. a symptomatic carrier of infection working with food preparation. Many people will be exposed over a long period of time.
 Epidemic. An increased unusual widespread infection in the community, causing waves of infection. These spread through communities and affect all people who have no active immunity to that infection.
Epidemic. An increased unusual widespread infection in the community, causing waves of infection. These spread through communities and affect all people who have no active immunity to that infection.
Cases of some infectious diseases should be notified to the public health authorities so that they are aware of cases and outbreaks. Diseases that are notifiable in England and Wales are listed in Table 4.5.
Table 4.5 Diseases notifiable (to Local Authority Proper Officers) in England and Wales, under the Health Protection (Notification) Regulations 2010
FURTHER READING
Cardo D, Dennehy PH, Halverson P et al. Moving toward elimination of healthcare-associated infections: a call to action. Am J Infect Control 2010; 38:671–675.
Chomel B, Belotto A, Meslin FX. Wildlife, exotic pets and emerging zoonoses. Emerg Infect Dis 2007; 13:6–11.
Horton R, Das P. Indian health: the path from crisis to progress. Lancet 2011; 377:181–183.
Relman DA. Microbial genomics and infectious diseases. N Engl J Med 2011; 365: 347–357.
Shurman EK. Global climate change and infectious disease. N Engl J Med 2010; 282:1061–1063.
Principles and basic mechanisms
Specificity
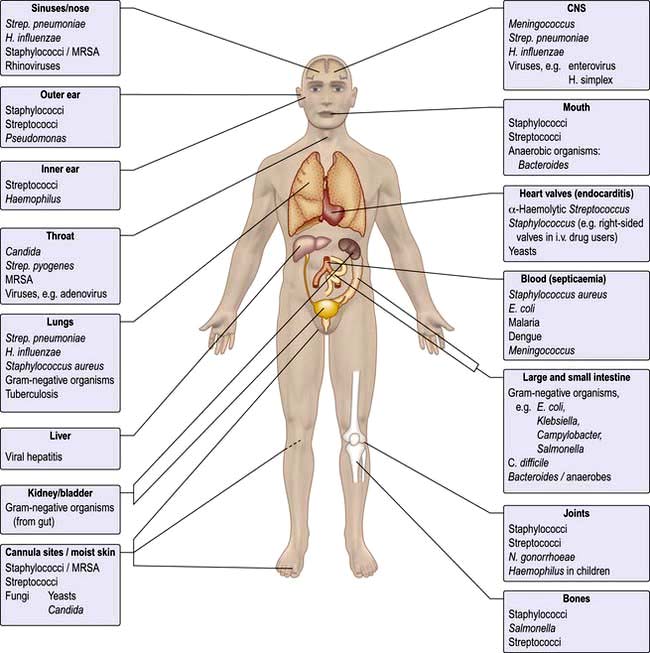
Microorganisms are often highly specific with respect to the organ or tissue they infect (Fig. 4.1). For example, a number of viruses are hepatotropic, such as those responsible for hepatitis A, B, C and E and yellow fever. This predilection for specific sites in the body relates partly to the presence of appropriate receptors on different cell types and partly to the immediate environment in which the organism finds itself; e.g. anaerobic organisms colonize the anaerobic colon, whereas aerobic organisms are generally found in the mouth, pharynx and proximal intestinal tract. Other organisms that show selectivity include:
Even within a species of bacterium such as E. coli, there are clear differences between strains with regard to their ability to cause gastrointestinal disease (see p. 110), which in turn differ from uropathogenic E. coli responsible for urinary tract infection.
Within an organ a pathogen may show selectivity for a particular cell type. In the intestine, for example, rotavirus predominantly invades and destroys intestinal epithelial cells on the upper portion of the villus, whereas reovirus selectively enters the body through the specialized epithelial cells, known as M cells that cover the Peyer’s patches (see p. 262).
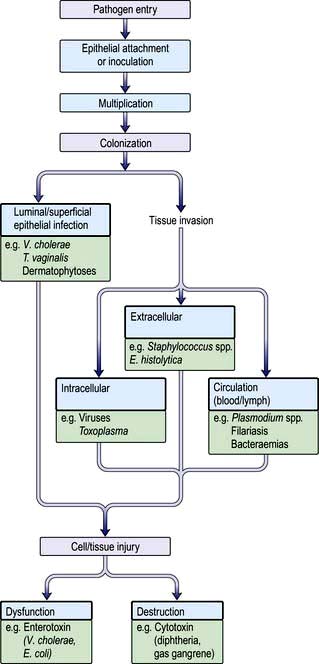
Pathogenesis
Figure 4.2 summarizes some of the steps that occur during the pathogenesis of infection. In addition, pathogens have developed a variety of mechanisms to evade host defences. For example, some pathogens produce toxins directed at phagocytes: Staphylococcus aureus (α-toxin), Streptococcus pyogenes (streptolysin) and Clostridium perfringens (α-toxin), while others such as Salmonella spp. and Listeria monocytogenes can survive within macrophages. Several pathogens possess a capsule that protects against complement activation (e.g. Strep. pneumoniae). Antigenic variation is an additional mechanism for evading host defences that is recognized in viruses (antigenic shift and drift in influenza), bacteria (flagella of salmonella and gonococcal pili) and protozoa (surface glycoprotein changes in Trypanosoma).
Epithelial attachment
Many bacteria attach to the epithelial substratum by specific organelles called pili (or fimbriae) that contain a surface lectin(s) – a protein or glycoprotein that recognizes specific sugar residues on the host cell. This family of molecules is known as adhesins (see p. 23). Following attachment, some bacteria, such as species of coagulase-negative staphylococci, produce an extracellular slime layer and recruit additional bacteria, which cluster together to form a biofilm. These biofilms can be difficult to eradicate and are a frequent cause of medical device-associated infections which affect prosthetic joints and heart valves as well as indwelling catheters. Many viruses and protozoa (e.g. Plasmodium spp., Entamoeba histolytica) attach to specific epithelial target-cell receptors. Other parasites such as hookworm have specific attachment organs (buccal plates) that firmly grip the intestinal epithelium.
Colonization
 an intracellular location for the pathogen (e.g. viruses, Mycobacterium spp., Toxoplasma gondii, Plasmodium spp.)
an intracellular location for the pathogen (e.g. viruses, Mycobacterium spp., Toxoplasma gondii, Plasmodium spp.)
 an extracellular location for the pathogen (e.g. pneumococci, E. coli, Entamoeba histolytica)
an extracellular location for the pathogen (e.g. pneumococci, E. coli, Entamoeba histolytica)
 invasion directly into the blood or lymph circulation (e.g. schistosome schistosomula and trypanosomes).
invasion directly into the blood or lymph circulation (e.g. schistosome schistosomula and trypanosomes).
Tissue dysfunction or damage
Microorganisms produce disease by a number of well-defined mechanisms:
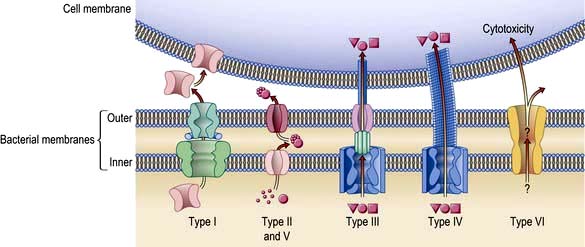
 Exotoxins have many diverse activities including inhibition of protein synthesis (diphtheria toxin), neurotoxicity (Clostridium tetani and C. botulinum) and enterotoxicity, which results in intestinal secretion of water and electrolytes (E. coli, Vibrio cholerae). Colonization and secretion in many classical diarrhoeal diseases is the result of virulence-associated genes which encode protein secretion systems (Fig. 4.3).
Exotoxins have many diverse activities including inhibition of protein synthesis (diphtheria toxin), neurotoxicity (Clostridium tetani and C. botulinum) and enterotoxicity, which results in intestinal secretion of water and electrolytes (E. coli, Vibrio cholerae). Colonization and secretion in many classical diarrhoeal diseases is the result of virulence-associated genes which encode protein secretion systems (Fig. 4.3).
 Endotoxin is a lipopolysaccharide (LPS) in the cell wall of Gram-negative bacteria. It is responsible for many of the features of septic shock (see p. 881), namely hypotension, fever, intravascular coagulation and, at high doses, death. The effects of endotoxin are mediated predominantly by release of tumour necrosis factor.
Endotoxin is a lipopolysaccharide (LPS) in the cell wall of Gram-negative bacteria. It is responsible for many of the features of septic shock (see p. 881), namely hypotension, fever, intravascular coagulation and, at high doses, death. The effects of endotoxin are mediated predominantly by release of tumour necrosis factor.
Staphylococcus aureus presents an excellent example of the repertoire of microbial virulence. The clinical expression of disease varies according to site, invasion and toxin production and is summarized in Table 4.6. Furthermore, host susceptibility to infection may be linked to genetic or acquired defects in host immunity that may complicate intercurrent infection, injury, ageing and metabolic disturbances (Table 4.7).
Table 4.6 Clinical conditions produced by Staphylococcus aureus
|
|
Table 4.7 Examples of host factors that increase susceptibility to staphylococcal infections (predominantly Staphylococcus aureus)
|
|
Host response to infection
Antibody and cell-mediated immune mechanisms play a vital role in combating infection. All organisms can initiate secondary immunological mechanisms, such as complement activation, immune complex formation and antibody-mediated cytolysis of cells. The immunological response to infection is described in Chapter 3.
Metabolic and immunological consequences of infection
Body temperature is controlled by the thermoregulatory centre in the anterior hypothalamus in the floor of the third ventricle. Body temperature is maintained at 36.8°C in health, with a diurnal variation of ±0.5°C. Gram-negative bacteria contain lipopolysaccharide (LPS) and peptidoglycan, which is also a component of Gram-positive bacterial cell walls. Toll-like receptors (TLR, see p. 55) on monocytes and dendritic cells recognize these lipopolysaccharides and generate signals leading to formation of inflammatory cytokines, e.g. IL-1, -6, -12, TNF-α and many others. These cytokines act on the thermoregulatory centre by increasing prostaglandin (PGE2) synthesis. The antipyretic effect of salicylates is brought about, at least in part, through its inhibitory effects on prostaglandin synthase.
The biological behaviour of the pathogen and the consequent host response are responsible for the clinical expression of disease that often allows clinical recognition. The incubation period following exposure can be helpful (e.g. chickenpox 14–21 days). The site and distribution of a rash may be diagnostic (e.g. shingles), while symptoms of cough, sputum and pleuritic pain point to lobar pneumonia. Fever and meningismus characterize classical meningitis. Infection may remain localized or become disseminated and give rise to the sepsis syndrome and disturbances of protein metabolism and acid–base balance (see Ch. 16). Many infections are self-limiting and immune and non-immune host defence mechanisms will eventually clear the pathogens. This is generally followed by tissue repair, which may result in complete resolution or leave residual damage.
FURTHER READING
Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med 2011; 364:656–665.
Fey PD, Olson ME. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol 2010; 5:917–933.
Lemichez E, Lecuit M, Nassif X et al. Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat Rev Microbiol 2010; 8:93–104.
Yahr TL. A critical new pathway for toxin secretion? N Engl J Med 2006; 355:1171–1172.
Approach to the patient with a suspected infection
History
 Travel history: some diseases are more prevalent in certain geographical locations and many infections common in the tropics are seen rarely, if at all, in the UK.
Travel history: some diseases are more prevalent in certain geographical locations and many infections common in the tropics are seen rarely, if at all, in the UK.
 Food and water history: systemic as well as gastroenteric infections can be caught via this route.
Food and water history: systemic as well as gastroenteric infections can be caught via this route.
 Animal contact: domestic, farm and wild animals can all be responsible for zoonotic infection.
Animal contact: domestic, farm and wild animals can all be responsible for zoonotic infection.
 Sexual activity: as well as the traditional sexually transmitted diseases, HIV, hepatitis B and occasionally other blood-borne infections can be transmitted sexually. Some enteric infections are more common among men who have sex with men.
Sexual activity: as well as the traditional sexually transmitted diseases, HIV, hepatitis B and occasionally other blood-borne infections can be transmitted sexually. Some enteric infections are more common among men who have sex with men.
 Intravenous drug use: as well as blood-borne viruses, drug injectors are susceptible to a variety of bacterial and fungal infections due to inoculation. Other needle exposures, such as tattooing and body piercing and receipt of blood products (especially outside the UK), are also risk factors for blood-borne viruses.
Intravenous drug use: as well as blood-borne viruses, drug injectors are susceptible to a variety of bacterial and fungal infections due to inoculation. Other needle exposures, such as tattooing and body piercing and receipt of blood products (especially outside the UK), are also risk factors for blood-borne viruses.
 Leisure activities: certain pastimes may predispose to water-borne infections or zoonoses.
Leisure activities: certain pastimes may predispose to water-borne infections or zoonoses.
Clinical examination
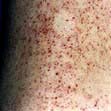
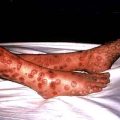
A thorough examination covering all systems is required. Skin rashes and lymphadenopathy are common features of infectious diseases and the ears, eyes, mouth and throat should also be inspected. Infections commonly associated with a rash are listed in Box 4.1. Rectal, vaginal and penile examination is required in sexually transmitted infections. The fever pattern may occasionally be helpful, e.g. the tertian fever of falciparum malaria, but too much weight should not be placed on the pattern or degree.
Investigations
General investigations (to assess health and identify organ(s) involved)
These will vary depending on circumstances:
 Blood tests. Routine blood count, ESR and C-reactive protein (CRP), biochemical profile, urea and electrolytes are performed in the majority of cases. CRP is a nonspecific marker of inflammation and is raised in many different infections: it is more useful in monitoring response to treatment than in making a diagnosis. Procalcitonin may be a more specific marker of bacterial infection, but this remains to be confirmed (Box 4.2).
Blood tests. Routine blood count, ESR and C-reactive protein (CRP), biochemical profile, urea and electrolytes are performed in the majority of cases. CRP is a nonspecific marker of inflammation and is raised in many different infections: it is more useful in monitoring response to treatment than in making a diagnosis. Procalcitonin may be a more specific marker of bacterial infection, but this remains to be confirmed (Box 4.2).
 Imaging. X-ray, ultrasound, echocardiography, CT and MR scanning are used to identify and localize infections. Positron emission tomography (PET) and single photon emission tomography (SPECT) have proved useful in localizing infection, especially when combined with CT scanning. However, the sensitivity and specificity of these tests in diagnosing infection has yet to be determined and their use remains limited. Biopsy or aspiration of tissue for microbiological examination may also be facilitated by ultrasound or CT guidance.
Imaging. X-ray, ultrasound, echocardiography, CT and MR scanning are used to identify and localize infections. Positron emission tomography (PET) and single photon emission tomography (SPECT) have proved useful in localizing infection, especially when combined with CT scanning. However, the sensitivity and specificity of these tests in diagnosing infection has yet to be determined and their use remains limited. Biopsy or aspiration of tissue for microbiological examination may also be facilitated by ultrasound or CT guidance.
 Radionuclide scanning after injection of indium- or technetium-labelled white cells (previously harvested from the patient) may occasionally help to localize infection. It is most effective when the peripheral white cell count is raised and is of particular value in localizing occult abscesses.
Radionuclide scanning after injection of indium- or technetium-labelled white cells (previously harvested from the patient) may occasionally help to localize infection. It is most effective when the peripheral white cell count is raised and is of particular value in localizing occult abscesses.
![]() Box 4.2
Box 4.2
General investigations for a patient with suspected infection
| Investigation | Possible cause |
|---|---|
|
Full blood count |
|
|
Neutrophilia |
Bacterial infection |
|
Neutropenia |
Viral infection |
|
|
Brucellosis |
|
|
Typhoid |
|
|
Typhus |
|
|
Overwhelming sepsis |
|
Lymphocytosis |
Viral infection |
|
Lymphopenia |
HIV infection (not specific) |
|
Atypical lymphocytes |
Infectious mononucleosis |
|
Eosinophilia |
Invasive parasitic infection |
|
Thrombocytopenia |
Overwhelming sepsis |
|
|
Malaria |
|
ESR or C-reactive protein |
Elevated in many infections |
|
Urea and electrolytes |
Potentially deranged in severe illness from any cause |
|
Procalcitonin |
Elevated particularly in bacterial infection |
|
Liver enzymes |
|
|
Minor elevation of transferases |
Nonspecific feature of many infections |
|
|
Mild viral hepatitis |
|
High transferases, elevated bilirubin |
Viral hepatitis (usually A, B or E) |
|
Coagulation |
May be deranged in hepatitis and in overwhelming infection of any type |
Microbiological investigations (to identify causative organism)
Culture
Specimens to be sent for microscopy and culture (Box 4.3):
 Blood and urine should routinely be sent for bacterial culture if infection is suspected, regardless of whether fever is present at the time.
Blood and urine should routinely be sent for bacterial culture if infection is suspected, regardless of whether fever is present at the time.
 Cerebrospinal fluid, sputum and biopsy specimens are sent if clinically indicated.
Cerebrospinal fluid, sputum and biopsy specimens are sent if clinically indicated.
 Special culture techniques are required for fungi, mycobacteria and some other bacteria such as Brucella spp., and the laboratory must be informed if these are suspected.
Special culture techniques are required for fungi, mycobacteria and some other bacteria such as Brucella spp., and the laboratory must be informed if these are suspected.
 Faecal culture for viruses is not helpful in the investigation of gastroenteritis – the viruses responsible for this do not grow in routine tissue culture. Antigen or nucleic acid detection techniques (see below) are more appropriate, especially in the investigation of an outbreak of diarrhoea and vomiting. Protozoa should be considered as a cause of diarrhoea in returning travellers, immunocompromised patients, toddlers, men having sex with men, farm workers and in any cases of prolonged unexplained diarrhoea. Detection of a specific clostridial toxin is a more reliable test for diarrhoea caused by Clostridium difficile than culture of the organism itself. Stool culture is a costly routine test and is often requested unnecessarily.
Faecal culture for viruses is not helpful in the investigation of gastroenteritis – the viruses responsible for this do not grow in routine tissue culture. Antigen or nucleic acid detection techniques (see below) are more appropriate, especially in the investigation of an outbreak of diarrhoea and vomiting. Protozoa should be considered as a cause of diarrhoea in returning travellers, immunocompromised patients, toddlers, men having sex with men, farm workers and in any cases of prolonged unexplained diarrhoea. Detection of a specific clostridial toxin is a more reliable test for diarrhoea caused by Clostridium difficile than culture of the organism itself. Stool culture is a costly routine test and is often requested unnecessarily.
![]() Box 4.3
Box 4.3
Specimens and indications for microscopy, culture and other microbiological tests
| Specimen | Investigation | Indication |
|---|---|---|
|
Blood |
Giemsa stain for malaria |
Any symptomatic traveller returning from a malarious area |
|
Malaria antigen detection test |
||
|
Stains for other parasites |
Specific tropical infections |
|
|
Culture |
All suspected bacterial infections |
|
|
Urine |
Microscopy and culture |
All suspected bacterial infections |
|
Tuberculosis (TB) culture |
Suspected TB |
|
|
|
Unexplained leucocytes in urine |
|
|
NAAT |
STIs |
|
|
Faeces |
Microscopy ± iodine stain |
Suspected protozoal diarrhoea |
|
Culture |
All unexplained diarrhoea |
|
|
PCR/antigen detection (not usually necessary to do both) |
Suspected viral diarrhoea in children |
|
|
Clostridium difficile toxin |
Diarrhoea following hospital stay or antibiotic treatment |
|
|
Throat swabs |
Culture |
Suspected bacterial tonsillitis and pharyngitis |
|
PCR |
Viral meningitis |
|
|
PCR/antigen detection |
Viral respiratory infections where urgent diagnosis is considered necessary |
|
|
Sputum |
Microscopy and culture |
Unusual chest infections; pneumonia |
|
Auramine stain/TB culture (liquid culture, see p. 120) |
Suspected TB |
|
|
Other special stains/cultures |
Immunocompromised patients |
|
|
Cerebrospinal fluid |
Microscopy and culture |
Suspected meningitis |
|
Auramine stain/TB culture |
Suspected TB, meningitis |
|
|
Other special stains/cultures |
Immunocompromised patients |
|
|
Suspected fungal infections |
||
|
PCR |
Suspected encephalitis or viral or bacterial meningitis |
|
|
Rash aspirate: |
|
|
|
Petechial |
Microscopy and culture |
Meningococcal disease |
|
Vesicular |
PCR/antigen detection/viral culture |
Herpes simplex/zoster |
Treatment
Serious infections may require supportive therapy in addition to antibiotics. It is always preferable to have a definite microbial diagnosis before starting treatment, so that an antibiotic with the most appropriate spectrum of activity and site of action can be used. However, some patients are too unwell to wait for results (which in the case of culture may take days). In diseases such as meningitis or septicaemia delay in treatment may be fatal and therapy must be started on an empirical basis. Appropriate samples for culture should be taken before the first dose of antibiotic and an antibiotic regimen chosen on the basis of the most likely causative organisms. Usually patients are less unwell and specific therapy can be deferred pending results. (Antibiotic therapy is discussed in more detail on page 85.)
Returning travellers. A detailed travel itinerary, including any flight stopovers, should be taken from anyone who is unwell after arriving from another country. Previous travel should also be covered as some infections may be chronic or recurrent. It is necessary to find out not just which countries were visited but also the type of environment: a stay in a remote jungle village carries different health risks from a holiday in an air-conditioned coastal holiday resort. Food and water consumption, bathing and swimming habits, animal and insect contact and contact with human illness all need to be established. Enquiry should be made about sexual contacts, drug use and medical treatment (especially parenteral) while abroad. In some parts of the world, over 90% of professional sex workers are HIV-positive and hepatitis B and C are very common in parts of Africa and Asia. In addition to the investigations described in the previous section, special tests may be needed depending on the epidemiological risks and clinical signs and malaria films are mandatory in anyone who is unwell after being in a malarious area. Some of the more common causes of a febrile illness in returning travellers are listed in Table 4.8.
Table 4.8 Causes of febrile illness in travellers returning from the tropics and worldwide
|
WHO advises that fever occurring in a traveller 1 week or more after entering a malaria risk area and up to 3 months after departure is a medical emergency |
|
|
Developing countries |
Specific geographical areas (see text) |
|
Malaria |
Histoplasmosis |
|
Schistosomiasis |
Brucellosis |
|
Dengue |
Worldwide |
|
Tick typhus |
Influenza |
|
Typhoid |
Pneumonia |
|
Tuberculosis |
URTI |
|
Dysentery |
UTI |
|
Hepatitis A |
Traveller’s diarrhoea |
|
Amoebiasis |
Viral infection |
URTI, upper respiratory tract infection; UTI, urinary tract infection.
Immunocompromised patients. Advances in medical treatment over the past three decades have led to a huge increase in the number of patients living with immunodeficiency states. Cancer chemotherapy, the use of immunosuppressive drugs and the worldwide AIDS epidemic have all contributed to this. The presentation may be very atypical in the immunocompromised patient with few, if any, localizing signs or symptoms. Infection can be due to organisms which are not usually pathogenic, including environmental bacteria and fungi. The normal physiological responses to infection (e.g. fever, neutrophilia) may be diminished or absent. The onset of symptoms may be sudden and the course of the illness fulminant. A high index of suspicion for infections in people who are known to be immunosuppressed is required. These patients may need early and aggressive antibiotic therapy without waiting for the results of investigations. Samples for culture should be sent before starting treatment, but therapy should not be delayed if this proves difficult. The choice of antibiotics should be guided by the likely causative organisms: these are shown in Box 4.4.
![]() Box 4.4
Box 4.4
Common causes of infection in immunocompromised patients
| Deficiency | Causes | Organisms |
|---|---|---|
|
Neutropenia |
Chemotherapy |
Escherichia coli |
|
|
Myeloablative therapy |
Klebsiella pneumoniae |
|
|
Immunosuppressant drugs |
Staph. aureus |
|
|
|
Staph. epidermidis |
|
|
|
Aspergillus spp. |
|
|
|
Candida spp. |
|
Cellular immune defects |
HIV infection |
Respiratory syncytial virus |
|
|
Lymphoma |
Cytomegalovirus |
|
|
Myeloablative therapy |
Epstein–Barr virus |
|
|
Congenital syndromes |
Herpes simplex and zoster |
|
|
|
Salmonella spp. |
|
|
|
Mycobacterium spp. (esp. M. avium-intracellulare) |
|
|
|
Cryptococcus neoformans |
|
|
|
Candida spp. |
|
|
|
Cryptosporidium parvum |
|
|
|
Pneumocystis jiroveci |
|
|
|
Toxoplasma gondii |
|
Humoral immune deficiencies |
Congenital syndromes |
Haemophilus influenzae |
|
|
Chronic lymphocytic leukaemia |
Streptococcus pneumoniae |
|
|
Corticosteroids |
Enteroviruses |
|
Terminal complement deficiencies (C5–C9) |
Congenital syndromes |
Neisseria meningitidis |
|
|
|
N. gonorrhoeae |
|
Splenectomy |
Surgery |
Strep. pneumoniae |
|
|
Trauma |
N. meningitidis |
|
|
|
H. influenzae |
|
|
|
Malaria |
Pyrexia of unknown origin
Successful diagnosis of the cause of PUO depends on knowledge of the likely and possible aetiologies. These have been documented in a number of studies and are summarized in Box 4.5.
Improvements in imaging techniques have diminished the need for invasive investigations in PUO and scanning has superseded the blind diagnostic laparotomy. Ultrasound, echocardiography, CT, MRI, PET and labelled white cell scanning can all help in establishing a diagnosis if used appropriately: the temptation to scan all patients with PUO from head to toe as a first measure should be avoided. Biopsy of bone marrow (and less frequently liver) may be useful even in the absence of obvious abnormalities and temporal artery biopsy should be considered in the elderly (see p. 543). Bronchoscopy can be used to obtain samples for microbiological and histological examination if sputum specimens are not adequate. Molecular and serological tests have greatly improved the diagnosis of infectious causes of PUO, but these tests should only be ordered and interpreted in the context of the clinical findings and epidemiology.
Antimicrobial chemotherapy
Principles of use
FURTHER READING
McDonald LC. Trends in antimicrobial resistance in healthcare associated pathogens and effect on treatment. Clin Infect Dis 2006; 42: S65–S71.
Moellering RC Jr. NDM-1 – a cause for worldwide concern. N Engl J Med 2010; 363:2377–2379.
Robicsek A, Jacoby GA, Hooper DC. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis 2006; 6:629–640.
Antibiotic chemoprophylaxis
The value of antibiotic chemoprophylaxis has been questioned as there are few controlled trials to prove efficacy (see p. 708). The evidence for chemoprophylaxis against infective endocarditis (IE) is an example. New English guidelines recognize that procedures can cause bacteraemia but without significant risk of infective endocarditis. Even patients at ‘high risk’, e.g. previous IE, prosthetic heart valves and surgical shunts, do not always require prophylaxis (Table 4.9). However, there are a number of indications for which the prophylactic use of antibiotics is still advised. These include surgical procedures where the risk of infection is high (colon surgery) or the consequences of infection are serious (post-splenectomy sepsis). The choice of agent(s) is determined by the likely infectious risk and the established efficacy and safety of the regimen.
|
(a) General |
||
|
Clinical problem |
Aim |
Drug regimena |
|
Splenectomy/spleen malfunction |
To prevent serious pneumococcal sepsis |
Phenoxymethylpenicillin 500 mg 12-hourly |
|
Rheumatic fever |
To prevent recurrence and further cardiac damage |
Phenoxymethylpenicillin 250 mg × 2 daily or sulfadiazine 1 g if allergic to penicillin |
|
Meningitis: |
|
|
|
Due to meningococci |
To prevent infection in close contacts |
Adults: rifampicin 600 mg twice-daily for 2 days |
|
Due to H. influenzae type b |
To reduce nasopharyngeal carriage and prevent infection in close contacts |
Adults: rifampicin 600 mg daily for 4 days |
|
Tuberculosis |
To prevent infection in exposed (close contacts) tuberculin-negative individuals, infants of infected mothers and immunosuppressed patients |
Oral isoniazid 300 mg daily for 6 months (Children: 5–10 mg/kg daily) |
|
(b) Endocarditis (NICE guidelines for adults and children undergoing interventional procedures March 2008) |
||
|
Antibacterial prophylaxis and chlorhexidine mouthwash are not recommended for the prevention of endocarditis in patients undergoing dental procedures. |
||
|
Antibacterial prophylaxis is not recommended for the prevention of endocarditis in patients undergoing procedures of the: |
||
|
Upper and lower respiratory tract (including ear, nose and throat procedures and bronchoscopy) |
||
|
Genitourinary tract (including urological, gynaecological and obstetric procedures) |
||
|
Upper and lower gastrointestinal tract. |
||
|
Any infection in patients at risk of endocarditis should be investigated promptly and treated appropriately to reduce the risk of endocarditis. |
||
|
If patients at risk of endocarditis are undergoing a gastrointestinal or genitourinary tract procedure at a site where infection is suspected, they should receive appropriate antibacterial therapy that includes cover against organisms that cause endocarditis. |
||
|
Patients at risk of endocarditis should be: |
||
|
Advised to maintain good oral hygiene |
||
|
Told how to recognize signs of infective endocarditis and advised when to seek expert advice. |
||
a Unless stated, doses are those recommended in adults. For surgical procedure, see individual procedures in text.
Adapted from Joint Formulary Committee British National Formulary, 63rd edn. London: BMJ Group and RPS Publishing; 2012.
Mechanisms of action and resistance to antimicrobial agents
Resistance to an antibiotic can be the result of:
 Impaired or altered permeability of the bacterial cell envelope, e.g. penicillins in Gram-negative bacteria
Impaired or altered permeability of the bacterial cell envelope, e.g. penicillins in Gram-negative bacteria
 Active expulsion from the cell by membrane efflux systems
Active expulsion from the cell by membrane efflux systems
 Alteration of the target site (e.g. single point mutations in E. coli or a penicillin-binding protein in Strep. pneumoniae leading to acquired resistance, see below)
Alteration of the target site (e.g. single point mutations in E. coli or a penicillin-binding protein in Strep. pneumoniae leading to acquired resistance, see below)
 Specific enzymes which inactivate the drug before or after cell entry (e.g. β-lactamases)
Specific enzymes which inactivate the drug before or after cell entry (e.g. β-lactamases)
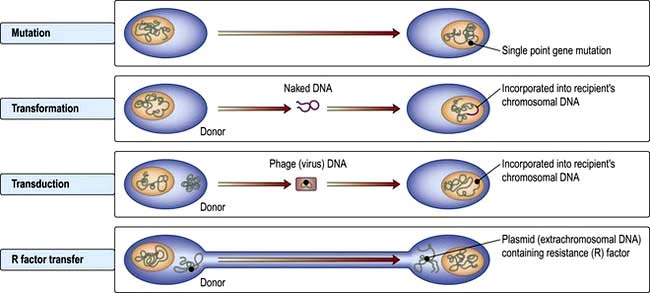
The development or acquisition of resistance to an antibiotic by bacteria involves either a mutation at a single point in a gene or transfer of genetic material from another organism (Fig. 4.4).
Transformation is probably the least clinically relevant mechanism, whereas transduction and R factor transfer are usually responsible for the sudden emergence of multiple antibiotic resistance in a single bacterium. Increasing resistance to many antibiotics has developed (Table 4.10).
Table 4.10 Some bacteria that have developed resistance to common antibiotics
| Pathogen | Previously fully sensitive to: |
|---|---|
|
Enterobacteria |
Amoxicillin, trimethoprim, ciprofloxacin, gentamicin, glycopeptide (GRE), vancomycin (VRE) |
|
Helicobacter pylori |
Metronidazole, clarithromycin |
|
Haemophilus influenzae |
Amoxicillin, chloramphenicol |
|
E. coli |
Quinolones |
|
Neisseria gonorrhoeae |
Penicillin, ciprofloxacin |
|
Pseudomonas aeruginosa |
Gentamicin |
|
Salmonella spp. |
Amoxicillin, sulphonamides, ciprofloxacin |
|
Shigella spp. |
Amoxicillin, trimethoprim, tetracycline |
|
Staphylococcus aureus |
Penicillin, meticillin (MRSA), vancomycin (VRSA), ciprofloxacin |
|
Streptococcus pneumoniae |
Penicillin, erythromycin, cefotaxime |
|
Streptococcus pyogenes |
Erythromycin, tetracycline |
|
Vibrio cholerae |
Quinolones, azithromycin |
Antibacterial drugs
β-Lactams (penicillins, cephalosporins and monobactams)
Penicillins
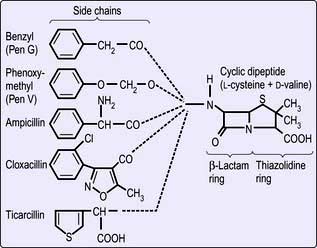
Structure. The β-lactams share a common ring structure (Fig. 4.5). Changes to the side-chain of benzylpenicillin (penicillin G) render the phenoxymethyl derivative (penicillin V) acid resistant and allow it to be orally absorbed. The presence of an amino group in the phenyl radical of benzylpenicillin increases its antimicrobial spectrum to include many Gram-negative and Gram-positive organisms. More extensive modification of the side-chain (e.g. as in flucloxacillin) renders the drug insensitive to bacterial penicillinase. This is useful in treating infections caused by penicillinase (β-lactamase)-producing staphylococci.
Mechanisms of action. β-lactams block bacterial cell wall mucopeptide formation by binding to and inactivating specific penicillin-binding proteins (PBPs), which are peptidases involved in the final stages of cell wall assembly and division. Meticillin-resistant Staph. aureus (MRSA) (see p. 115) produce a low-affinity PBP which retains its peptidase activity even in the presence of high concentrations of meticillin. Many bacteria have developed the ability to produce penicillinases and beta-lactamases, which inactivate antibiotics of this class. Recent years have seen the emergence of Gram-negative organisms producing extended-spectrum beta-lactamases (ESBLs), rendering the bacteria potentially resistant to all β-lactam antibiotics.
Interactions. Penicillins inactivate aminoglycosides when mixed in the same solution.
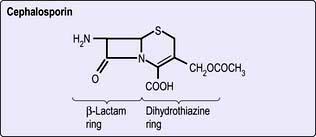
Cephalosporins
The cephalosporins (Fig. 4.6) have an advantage over the penicillins in that they are resistant to staphylococcal penicillinases (but are still inactive against meticillin-resistant staphylococci) and have a broader range of activity that includes both Gram-negative and Gram-positive organisms, but excludes enterococci and anaerobic bacteria. Ceftazidime and cefpirome are active against Pseudomonas aeruginosa.
Indications for use (Table 4.11). These potent broad-spectrum antibiotics are useful for the treatment of serious systemic infections, particularly when the precise nature of the infection is unknown. They may be used for serious sepsis in postoperative and immunocompromised patients, as well as for meningitis and intra-abdominal sepsis, but are increasingly being replaced by other agents because of their link with Clostridium difficile associated diarrhea (CDAD).
| Activity | Use | |
|---|---|---|
|
First generation |
Gram-positive cocci and Gram-negative organisms |
Urinary tract infections |
|
Cefalexin (oral) |
|
Penicillin allergy |
|
Cefradine (oral) |
|
|
|
Cefadroxil (oral) |
|
|
|
Second generation |
Extended spectrum |
Prophylaxis and treatment of Gram-negative infections and mixed aerobic-anaerobic infections |
|
Cefuroxime |
More effective than first generation against E. coli, Klebsiella spp. and Proteus mirabilis, but less effective against Gram-positive organisms |
|
|
Cefoxitina |
Includes Bacteroides fragilis |
Peritonitis |
|
Cefaclor (oral) |
|
|
|
Cefuroxime (oral) |
|
|
|
Cefprozil (oral)a |
|
|
|
Third generation |
|
|
|
Cefotaxime |
Broad-spectrum |
Especially severe infection with Enterobacteriaceae, Pseudomonas aeruginosa (ceftazidime, cefpirome) and Neisseria gonorrhoeae, N. meningitidis, Lyme disease (ceftriaxone) |
|
Ceftazidime |
More potent against aerobic Gram-negative bacteria than first or second generation |
|
|
Cefpiromea |
|
Urinary tract infections and infections with neutropenia |
|
Ceftriaxone |
|
Once daily. Septicaemia, pneumonia, meningitis |
|
Cefpodoxime (oral) |
|
|
|
Cefixime (oral) |
|
|
|
Fourth generation |
Aerobic Gram-negative bacteria including P. aeruginosa |
Febrile, neutropenic patients |
|
Cefepimea |
|
|
|
Fifth generation |
Similar to third generation but active against MRSA |
Not yet in widespread use |
|
Ceftarolinea |
|
|
|
Ceftobiprolea |
|
|
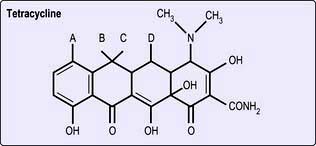
Tetracyclines and glycylcyclines
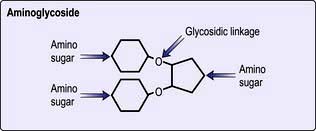
Structure. These are bacteriostatic drugs possessing a four-ring hydronaphthacene nucleus (Fig. 4.8). Included among the tetracyclines are tetracycline, oxytetracycline, demeclocycline, lymecycline, doxycycline and minocycline. Tigecycline is an injectable glycylcycline, which is structurally related to the tetracyclines.
Macrolides
Erythromycin and clarithromycin
Mechanism of action. Macrolides inhibit protein synthesis by interrupting ribosomal function.
Other macrolides
These include azithromycin and telithromycin. They have a broad spectrum of activity that includes selective Gram-negative organisms, mycobacteria and Toxoplasma gondii. Compared with erythromycin, they have superior pharmacokinetic properties with enhanced tissue and intracellular penetration and longer half-life that allows once daily dosage. Azithromycin is also used for trachoma and cholera (see p. 136). Fidaxomicin is useful in C. difficile.
Chloramphenicol
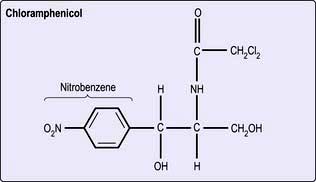
Structure. Chloramphenicol is the only naturally occurring antibiotic containing nitrobenzene (Fig. 4.9). This structure probably accounts for its toxicity in humans and for its activity against bacteria.
Sodium fusidate
Structure. Sodium fusidate has a structure resembling that of bile salts (see p. 78).
Sulphonamides and trimethoprim
Mechanism of action. Sulphonamides block thymidine and purine synthesis by inhibiting microbial folic acid synthesis. Trimethoprim prevents the reduction of dihydrofolate to tetrahydrofolate (see Fig. 8.12).
Interactions. Sulphonamides potentiate oral anticoagulants and some hypoglycaemic agents.
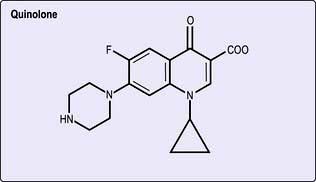
Quinolones
The quinolone antibiotics, such as ciprofloxacin, norfloxacin, ofloxacin and levofloxacin, are useful oral broad-spectrum antibiotics, related structurally to nalidixic acid. The latter achieves only low serum concentrations after oral administration and its use is limited to the urinary tract where it is concentrated. Newer quinolones, including moxifloxacin, gemifloxacin and gatifloxacin, have greater activity against Gram-positive pathogens. The structure is shown in Figure 4.10.
Indications for use. The extended-spectrum quinolones such as ciprofloxacin have activity against Gram-negative bacteria, including some Pseudomonas aeruginosa and some Gram-positive bacteria (e.g. anthrax, p. 132). They are useful in Gram-negative septicaemia, skin and bone infections, urinary and respiratory tract infections, meningococcal carriage, in some sexually transmitted diseases such as gonorrhoea and nonspecific urethritis due to Chlamydia trachomatis and in severe cases of travellers’ diarrhoea (see p. 122). The newer oral quinolones provide an alternative to β-lactams in the treatment of community-acquired lower respiratory tract infections.
Interactions. Ciprofloxacin can induce toxic concentrations of theophylline.
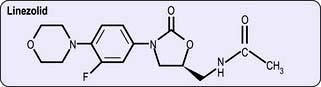
Oxazolidinones
Structure. The oxazolidinones are a novel class of antibacterial agents, of which linezolid (Fig. 4.11) is the first to be available.
Glycopeptides
Vancomycin
Vancomycin is given intravenously for meticillin-resistant Staph. aureus and other multiresistant Gram-positive organisms. It is also used for treatment and prophylaxis against Gram-positive infections in penicillin-allergic patients. It is used for Strep. pneumoniae meningitis when caused by penicillin-resistant strains. By mouth, it is an alternative to metronidazole for CDAD. Glycopeptide-resistant enterococci (GRE) are now seen, as well as vancomycin-resistant Staphylococcus (see p. 116).
Antituberculosis drugs
These are described on page 842. Rifampicin is also used in other infections apart from tuberculosis.
Antiviral drugs
Drugs for HIV infection are discussed on page 182.
Nucleoside analogues
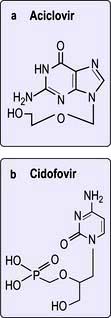
Aciclovir. Aciclovir (also known as acycloguanosine) is an acyclic nucleoside analogue which acts as a chain terminator of herpesvirus DNA synthesis. This drug is converted to aciclovir monophosphate by a virus-encoded thymidine kinase produced by alpha herpesviruses, herpes simplex types 1 and 2 and varicella zoster virus (Table 4.13). Conversion to the triphosphate is then achieved by cellular enzymes. Aciclovir triphosphate acts as a potent inhibitor of viral (but not cellular) DNA polymerase and also competes with deoxyguanine triphosphate for incorporation into the growing chains of herpesvirus DNA, thereby resulting in chain synthesis termination due to its acyclic structure. This highly specific mode of activity, targeted only to virus-infected cells, means that aciclovir has very low toxicity. Crystallization in the renal tubules is a well-recognized adverse effect. Intravenous, oral and topical preparations are available for the treatment of herpes simplex and varicella zoster virus infections (Table 4.13). Treatment does not eliminate the virus so relapses do occur.
Table 4.13 Antiviral agents (for drugs used in HIV, see Table 4.53)
| Drug | Use |
|---|---|
|
Nucleoside analogues |
|
|
Aciclovir |
Topical – HSV infection |
|
Oral and intravenous – VZV and HSV |
|
|
Famciclovir |
Oral – VZV and HSV |
|
Valaciclovir |
Oral – VZV and HSV |
|
Ganciclovir |
Intravenous – CMV |
|
Valganciclovir |
Oral – CMV |
|
Lamivudine |
Oral – HBV infection |
|
Emtricitabine |
Oral – HBV infection |
|
Telbivudine |
Oral – HBV infection |
|
Entecavir |
Oral – HBV |
|
Nucleotide analogues |
|
|
Tenofovir |
Oral – HBV infection |
|
Adefovir |
Oral – HBV infection |
|
Cidofovir |
Intravenous – CMV |
|
Pyrophosphate analogues |
|
|
Foscarnet |
Intravenous – CMV |
|
Adamantanes |
|
|
Amantadine |
Oral – influenza A |
|
Neuraminidase inhibitors |
|
|
Zanamivir |
Topical (inhalation) – influenza A and B |
|
Oseltamivir |
Oral – influenza A and B |
|
Other drugs |
|
|
Ribavirin |
Topical (inhalation) – RSV |
|
Oral – Lassa fever, hepatitis C |
|
|
Pegylated INF-α |
HBV, HCV, some malignancies (e.g. renal cell carcinoma) (Given once weekly) |
|
Palivizumab |
Prevention in RSV |
|
Protease inhibitors |
|
|
Telaprevir |
Oral – HCV |
|
Boceprevir |
Oral – HCV |
Lamivudine is a reverse transcriptase inhibitor active against both HIV and hepatitis B. Drug resistance is a problem (see pp. 81, 184).
Nucleotide analogues
Adefovir dipivoxil has activity against hepatitis B virus DNA polymerase. However, tenofovir disoproxil is much more potent, has a much higher barrier to resistance and has therefore largely superseded adefovir in use (see p. 322).
Protease inhibitors
Two new protease inhibitors, bocepravir and telapravir, have recently become available for treating chronic hepatitis C infection (in combination with ribavirin and interferon) (see p. 304).
Other drugs
Ribavirin. This synthetic purine nucleoside derivative which interferes with 5′-capping of messenger RNA, is active against several RNA and DNA viruses, at least in vitro. Its major use is in the treatment of chronic hepatitis C infection in combination with pegylated interferon-α, although it has no effect when given alone (see p. 324). It is administered orally. Haemolytic anaemia is the most frequent adverse reaction. It is also administered by a small-particle aerosol generator (SPAG) to infants with acute respiratory syncytial virus (RSV) infection. Another indication is in the treatment of Lassa fever virus infection.
Immunization against infectious diseases
Immunization, immunoprophylaxis and immunotherapy
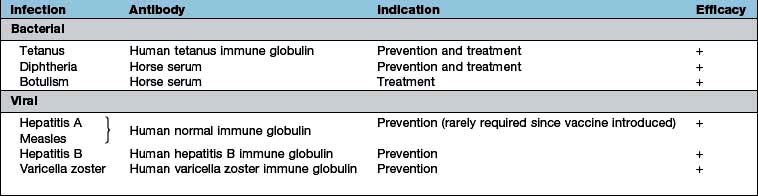
Immunization has changed the course and natural history of many infectious diseases. Passive immunization by administering preformed antibody, either in the form of immune serum or purified normal immunoglobulin, provides short-term immunity and has been effective in both the prevention (immunoprophylaxis) and treatment (immunotherapy) of a number of bacterial and viral diseases (Table 4.14). The active immunization schedule currently recommended is summarized in Box 4.6. Long-lasting immunity is achieved only by active immunization with a live attenuated or an inactivated organism or a subunit thereof (Table 4.15). Active immunization may also be performed with microbial toxin (either native or modified) – that is, a toxoid. Immunization should be kept up to date with booster doses throughout life. Travellers to developing countries, especially if visiting rural areas, should in addition enquire about further specific immunizations.
![]() Box 4.6
Box 4.6
Recommended immunization schedules: (i) in the UK; (ii) WHO model schedule for developing countries
| Time of immunization | Vaccine |
|---|---|
|
(i) UK |
|
|
2 months |
DTaP, IPV, Hib + PCV (BCGa) |
|
3 months |
DTaP, IPV, Hib + MenC |
|
4 months |
DTaP, IPV, Hib + MenC + PCV |
|
12 months |
Hib, MenC |
|
13 months |
MMR + PCV |
|
3 years 4 months – 5 years |
DTaP, IPV, MMR |
|
12–13 years |
HPV |
|
13–18 years |
Td, IPV |
|
(ii) Developing countriesb |
|
|
Birth (or first contact) |
PV, BCG |
|
6 weeks |
DPT, PV, HBV |
|
10 weeks |
DPT, PV, HBV |
|
14 weeks |
DPT, PV, HBV |
|
9 months |
Measles, YFc |
DTaP, adsorbed diphtheria, tetanus triple vaccine, acellular pertussis; DPT, adsorbed diphtheria, whole cell pertussis, tetanus triple vaccine; Hib, Haemophilus influenzae type b vaccine; IPV, inactivated polio vaccine; PV, polio vaccine; MenC, meningococcus group C conjugate vaccine; aP, acellular pertussis; MMR, measles, mumps, rubella triple vaccine; T, tetanus; d, adsorbed low-dose diphtheria; HBV, hepatitis B vaccine; HPV, human papillomavirus vaccine; YF, yellow fever vaccine; PCV, pneumococcal conjugate vaccine.
Mumps vaccine is given in many developing countries.
a Children at high risk of contact with tuberculosis. For more detailed advice about childhood BCG immunization, see The Green Book (see Significant Websites for details).
b Model scheme, adapted locally depending on need and availability of vaccines.
Table 4.15 Preparations available for active
BCG, bacille Calmette–Guérin.
a Individual vaccines for measles, mumps and rubella are not licensed in the UK.
In 1974, the World Health Organization introduced the Expanded Programme on Immunization (EPI). By 1994, more than 80% of the world’s children had been immunized against tuberculosis, diphtheria, tetanus, pertussis, polio and measles. Poliomyelitis should shortly be eradicated worldwide, which will match the past success of global smallpox eradication. Introduction of conjugate vaccines against Haemophilus influenzae type b (Hib) has proved highly effective in controlling invasive H. influenzae infection, notably meningitis (see p. 1130).
Immunization e.g. tetanus, HBV, should be kept up to date in adults.
FURTHER READING
Chaves SS, Gargiullo P, Zhang JX et al. Loss of vaccine-induced immunity to varicella over time. N Engl J Med 2007; 356:1121–1129.
Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet 2006; 367:1926–1936.
Gardner P. Prevention of meningococcal disease. N Engl J Med 2006; 355:1466–1473.
Pallansch MA, Sandhu HS. The eradication of polio – progress and challenges. N Engl J Med 2006; 355:2508–2511.