49 Infectious Disease Considerations for the Operating Room
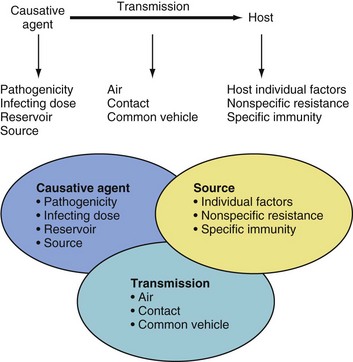
THE TRANSMISSION OF INFECTION depends on the presence of three interconnected elements: a causative agent, a source, and a mode of transmission (Fig. 49-1). Understanding the characteristics of each element provides the practicing anesthesiologist with a methodologic aid to protect susceptible patients and health care workers and to avoid spreading infection.
There has always been concern about the transmission of infectious agents both to the patient from the anesthesiologist as well as from the anesthesiologist to the patient.1 In addition, there are many sites within the hospital environment where moist or desiccated organic material with the ability to host potentially pathogenic microbes may survive for extended periods of time (Table 49-1)2,3; some may even resist the usual cleaning and disinfection techniques.4 Their transmission from the source to the host may occur via indirect nonapparent mechanisms (e.g., most commonly through hand contact).
TABLE 49-1 Nosocomial Pathogens and Environmental Contamination
| Pathogen | Types of Environmental Contamination | Organism Survival Time |
|---|---|---|
| Influenza virus | Aerosolization after cleaning; fomites | 24-48 hours on nonporous surfaces |
| Parainfluenza virus | Clothes and nonporous surfaces | 10 hours on nonporous surfaces; 6 hours on clothes |
| Norovirus | Extensive environmental contamination, possible aerosolization | ≤14 days on fecal specimens, ≤12 days on carpets |
| Hepatitis B virus | Environmental contamination with blood | 7 days |
| Coronavirus-SARS | Possible results from emergency department specimens; super-spreading events | 24-72 hours on fomites and fecal specimens |
| Candida | Fomite contamination | 3 days for Candida albicans and 14 days for Candida parapsilosis |
| Clostridium difficile | Extensive environmental contamination | 5 months on hospital floors |
| Pseudomonas aeruginosa | Drain sink contamination | 7 hours on glass slides |
| Acinetobacter baumannii | Extensive environmental contamination | 33 hours on laminated plastic surfaces |
| MRSA | Extensively contaminated burn units | ≤9 weeks after drying; 2 days on laminated plastic surfaces |
| VRE | Extensive environmental contamination | ≤58 days on working surfaces |
MRSA, Methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Modified from Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis 2004;39:1182-9.
Host
 Nonspecific defense mechanisms include the skin, mucous membranes, secretions, excretions, enzymes, inflammatory responses, genetic factors, hormonal responses, nutritional status, behavior patterns, and the presence of other diseases.
Nonspecific defense mechanisms include the skin, mucous membranes, secretions, excretions, enzymes, inflammatory responses, genetic factors, hormonal responses, nutritional status, behavior patterns, and the presence of other diseases.
 Specific defense mechanisms or immunity may occur as a result of exposure to an infectious agent (antibody formation) or through placental transfer of antibodies; artificial defenses may be acquired through vaccines, toxoids, or exogenously administered immunoglobulins.
Specific defense mechanisms or immunity may occur as a result of exposure to an infectious agent (antibody formation) or through placental transfer of antibodies; artificial defenses may be acquired through vaccines, toxoids, or exogenously administered immunoglobulins.
Methods of Transmission
Air Transmission
Droplets
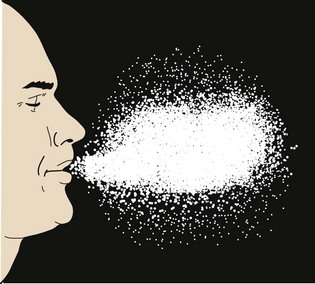
Droplet contamination is considered a direct transmission of organisms because there is a direct transfer of microorganisms from the colonized or infected person to the host. This generally occurs with particles whose diameters are greater than 5 µm that are expelled from an individual’s mouth or nose, mainly during sneezing, coughing, talking or during procedures such as suction, laryngoscopy, and bronchoscopy (Fig. 49-2). Transmission occurs when the microorganism-containing droplets, expelled or shed by the infected person (source), are propelled a short distance (usually not exceeding 60 cm or about 2 feet through the air) and deposited on the host’s conjunctivae or oral or nasal mucous membranes. When a person coughs, the exhaled air may reach a speed of up to 965 km/hr (600 mph).5 However, because the droplets are relatively large, they tend to descend quickly and remain suspended in the air for a very brief period, thus obviating the need for special handling procedures for the OR air. Examples of droplet-borne diseases include influenza, respiratory syncytial virus, severe acute respiratory syndrome (SARS), and others commonly found in droplets from the respiratory tract.
Droplet Nuclei
Droplet nuclei result from the evaporation of droplets while suspended in the air. Unlike droplets, the nuclei have an outer layer of desiccated organic material and a very small diameter (1 to 5 µm) and remain suspended in air indefinitely. The microorganisms contained within these nuclei may be spread by air drafts over great distances, depending on the environmental conditions (dry and cold atmosphere, with limited or no exposure to sunlight favoring the spread).6 In contrast to droplets, which are deposited on mucous membranes, droplet nuclei may enter the susceptible host by inhalation; examples of droplet nuclei–borne diseases include tuberculosis, varicella, and measles.
Contact Transmission
Direct Contact
This type of disease transmission involves direct physical contact between two individuals. The physical transfer of microorganisms from an infected or colonized person to a susceptible host may occur from child to health care provider or from health care provider to child during professional practice (e.g., venous cannulation, laryngoscopy, burn care, or suction of secretions). Health care providers working in the OR may be exposed to skin contamination by body fluids. This is an issue of grave concern because of the potential exposure of health care providers to patients with unrecognized infections, especially hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV). Hepatitis B is a highly infectious virus that requires a small amount of blood (10−7 to 10−9 mL) to transmit the disease. The incidence of skin contamination of anesthesiologists and related personnel by blood and saliva is substantial. One study examined 270 anesthetic procedures during 7 consecutive days. The blood of 35 patients (14%) contaminated the skin of 65 anesthesiologists in 46 incidents. Of these contamination events, 28 (61%) occurred during venous cannulation. Of anesthesiologists who had been contaminated by blood, 5 of 65 (8%) had cuts in the skin of their hands.7 The importance of this observation is that seroconversion of health care providers has been reported after skin contamination by infected blood from HIV carriers8 and HBV infection after blood splashing into health care workers’ eyes.9 Scabies, pediculosis, and herpes simplex are among the diseases most frequently transmitted by direct contact.10–17 These studies explain why meticulous hand washing and routine use of barriers such as gloves and eye protection are such an important part of protecting ourselves even during routine procedures such as starting an IV line or performing laryngoscopy.18
Indirect Contact
Indirect contact involves the transmission of microorganisms from a source (animate or inanimate) to a susceptible host by means of a vehicle (e.g., an intermediary object) contaminated by body fluids. Tables 49-2 and 49-3 provide examples of diseases associated with bodily fluids to which health care workers may be exposed. The vehicle for transmission may be the hands of a health care provider who is not wearing gloves or a provider who fails to wash his or her hands after providing care to a child.19–22 This type of contact can also come from health care providers who touch (with or without gloves) contaminated monitoring or other patient care devices (e.g., blood pressure cuffs, stethoscopes, electrocardiographic cables, or ventilation systems [respirators, corrugated tubes, Y pieces, valves]), which are used with several children without proper cleaning or disinfection between each use.23–25
| Body Fluid | Disease Transmitted |
|---|---|
| Blood | HBV, HIV, HCV, CMV, EBV, NANBH |
| Seminal fluid | HIV, HBV, CMV |
| Vaginal discharge | HIV, HBV, CMV |
| Saliva and sputum | HSV, TB, CMV, respiratory diseases |
| Cerebrospinal fluid | Encephalopathic organisms (see Table 49-5), HIV |
| Breast milk | HIV, HBV, CMV |
| Urine | CMV, EBV, HBV |
| Feces and intestinal fluid | HAV, gastrointestinal diseases (see Table 49-5) |
CMV, Cytomegalovirus; EBV, Epstein-Barr virus; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HSV, herpes simplex types I and II; NANBH, non-A, non-B hepatitis; TB, tuberculosis.
Modified with permission from Browne RA, Chenesky MA. Infectious diseases and the anaesthetist. Can J Anesth 1988;35:655-65.
TABLE 49-3 Infectious Agents That May Be Found in the Operating Room
Viruses: hepatitis A virus, rotavirus, adenovirus, enterovirus
Bacteria: Giardia,* Cryptosporidium, Isospora*
Viruses: Human immunodeficiency virus,* herpes simplex virus,* Epstein-Barr virus*
*Opportunistic infections in immunocompromised patients, especially those with acquired immunodeficiency.
Modified with permission from Browne RA, Chenesky MA. Infectious diseases and the anaesthetist. Can J Anesth 1988;35:655-65.
There are also reports of equipment, fomites, and drugs (mainly propofol) that have resulted in hospital-acquired infections.14,26–44 However, many of the following situations could potentially cause an infection:
 Up to 40% of the anesthetic equipment in the OR that was in direct or indirect contact with the child (blood pressure cuffs, cables, oximeters, laryngoscopes, monitors, respirator settings, and horizontal and vertical surfaces) may be contaminated with blood because of inadequate cleansing procedures between uses.2,3,23,45,46
Up to 40% of the anesthetic equipment in the OR that was in direct or indirect contact with the child (blood pressure cuffs, cables, oximeters, laryngoscopes, monitors, respirator settings, and horizontal and vertical surfaces) may be contaminated with blood because of inadequate cleansing procedures between uses.2,3,23,45,46
 In some institutions, up to 8% of the Bain circuits that were reused without previous sterilization were contaminated.47
In some institutions, up to 8% of the Bain circuits that were reused without previous sterilization were contaminated.47
 Contamination of syringe contents has occurred with glass particles during ampule opening, which in turn may compromise the sterility of the contents, presumably because of the passage of bacteria contained on glass particles into the solution.48–50
Contamination of syringe contents has occurred with glass particles during ampule opening, which in turn may compromise the sterility of the contents, presumably because of the passage of bacteria contained on glass particles into the solution.48–50
 IV tubing has a significant blood contamination rate as well as contamination by blood from syringes used to inject medications. This can occur with the absence of visible blood reflux in the tubing or syringe. Simply replacing the needle on a syringe that will be reused is ineffective in preventing cross-infection. The only certain strategy to prevent infection is to not use the same syringe in multiple patients.51
IV tubing has a significant blood contamination rate as well as contamination by blood from syringes used to inject medications. This can occur with the absence of visible blood reflux in the tubing or syringe. Simply replacing the needle on a syringe that will be reused is ineffective in preventing cross-infection. The only certain strategy to prevent infection is to not use the same syringe in multiple patients.51
 Refilling both glass and plastic syringes several times has been shown to result in contamination of the contents; single use is therefore recommended.51,52
Refilling both glass and plastic syringes several times has been shown to result in contamination of the contents; single use is therefore recommended.51,52
 Some drug formulations, especially propofol, can sustain bacterial growth under certain conditions. Thus great care should be given to aseptic technique when transferring drugs from the vial to a syringe and to use the contents of the syringe within 4 hours.53–57
Some drug formulations, especially propofol, can sustain bacterial growth under certain conditions. Thus great care should be given to aseptic technique when transferring drugs from the vial to a syringe and to use the contents of the syringe within 4 hours.53–57
 Needles that had been used for spinal or epidural anesthesia were found to be contaminated with coagulase-negative staphylococci (15.7%), yeasts (1.5%), enterococci (0.8%), pneumococci (0.8%), and micrococci (0.8%), suggesting that despite standard skin preparation and cleansing there may be a significant rate of needle contamination.58 It is unclear whether these skin organisms can be transmitted and cause an infection during administration of a neuraxial block.
Needles that had been used for spinal or epidural anesthesia were found to be contaminated with coagulase-negative staphylococci (15.7%), yeasts (1.5%), enterococci (0.8%), pneumococci (0.8%), and micrococci (0.8%), suggesting that despite standard skin preparation and cleansing there may be a significant rate of needle contamination.58 It is unclear whether these skin organisms can be transmitted and cause an infection during administration of a neuraxial block.
 Blood and saliva frequently contaminate the skin of anesthetic personnel during routine anesthetic practice.7
Blood and saliva frequently contaminate the skin of anesthetic personnel during routine anesthetic practice.7
 Violations of contemporary guidelines for preventing infections (e.g., hand-washing, wearing gloves, surgical masks, ocular protection, scrubs, or syringe reuse) by anesthesiologists are frequent.18 Anesthesia staff are aware that they work in a potentially infectious environment, but whether they adopt the protective measures to prevent infections in both themselves and their patients is quite variable (11% to 99%).23,59–61
Violations of contemporary guidelines for preventing infections (e.g., hand-washing, wearing gloves, surgical masks, ocular protection, scrubs, or syringe reuse) by anesthesiologists are frequent.18 Anesthesia staff are aware that they work in a potentially infectious environment, but whether they adopt the protective measures to prevent infections in both themselves and their patients is quite variable (11% to 99%).23,59–61
Accidents with Cutting or Piercing Devices
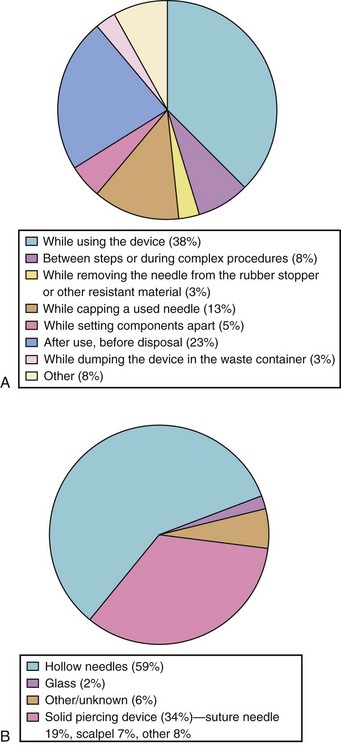
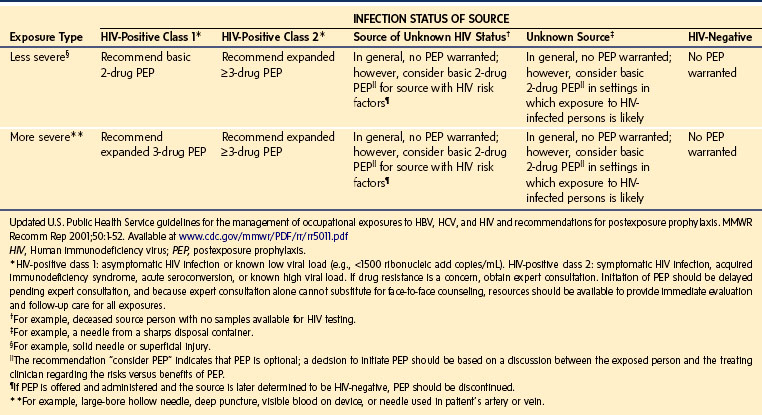
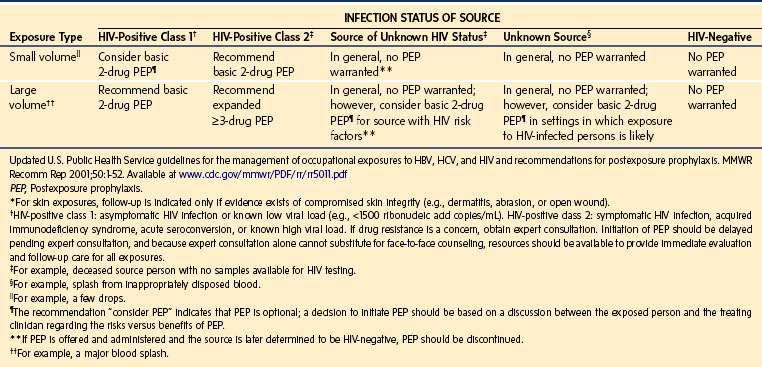
Percutaneous contamination as a result of a cutting or piercing accident is the most effective means to transmit bloodborne pathogens. Evidence suggests that this is the main route of HIV, HBV, and HCV infection,62–64 especially if the injury is caused by hollow-bore needles that were used to draw blood or establish IV access.65,66 Over 20 other bloodborne pathogens have been transmitted by this means, including those causing herpes, malaria, and tuberculosis.67 The infectious risk after a percutaneous exposure to blood or body fluids from an HIV positive person is approximately 0.3%. Among health care providers lacking protective antibodies, the risk of HBV infection after an injury with a cutting or piercing device contaminated with hepatitis B antigen is approximately 37%; in the case of HCV it is approximately 1.8% (range 0% to 7%). Anesthesia staff lacking HBV protective antibodies are at great risk for acquiring the disease.68,69 These infection rates underscore the need for the use of “safe” needles and the need to advocate the use of “needleless” systems even though they are significantly more expensive. This also emphasizes the need for meticulous handling and disposal of needles and other sharp instruments as well as the use of special “sharps boxes” designed to minimize accidental needle sticks (e.g., “mail box” type boxes that do not allow the hand to enter the disposal area).70–85 The U.S. Centers for Disease Control and Prevention (CDC) has estimated that in the United States there are approximately 385,000 cutting and piercing accidents annually among health care providers in hospitals; 25% of these occur in the OR.67 However, the actual prevalence is thought to be much greater, because many of these events are unreported. The distribution of these accidents among anesthesiologists is shown in Figure 49-3, A; the distribution of the items most frequently associated with cutting and piercing injuries in health care providers is shown in Figure 49-3, B. Should such an accident occur (e.g., needle puncture, exposure to nonintact skin, or mucous membrane exposure) there are now specific recommendations regarding immediate assessment of risk, assessment of the exposure source (chart review, inform the patient that an accident has occurred and ask permission to determine HBV, HCV, and HIV serologic status) and initiation of appropriate treatment of the health care worker. It is advised to obtain as much information regarding the patient as possible, if the patient is known, to obtain a sample of blood from the patient for determination of potential carrier state (Table 49-4), and to report to the health service for immediate institution of prophylaxis and follow-up (Table 49-5), especially for HIV exposure (Tables 49-6 and 49-7).
TABLE 49-4 Recommendations for the Contents of the Occupational Exposure Report
• Details of the procedure being performed, including where and how the exposure occurred; if related to a sharp device, the type and brand of device; and how and when in the course of handling the device the exposure occurred
• Details of the exposure, including the type and amount of fluid or material and the severity of the exposure; for example, for a percutaneous exposure, depth of injury and whether fluid was injected and for a skin or mucous membrane exposure, the estimated volume of material and the condition of the skin (e.g., chapped, abraded, intact)
• Details about the exposure source (e.g., whether the source material contained hepatitis B virus, hepatitis C virus, or human immunodeficiency virus; if the source is infected with human immunodeficiency virus, the stage of disease, history of antiretroviral therapy, viral load, and antiretroviral resistance information, if known)
• Details about the exposed person (e.g., hepatitis B vaccination and vaccine-response status)
• Details about counseling, postexposure management, and follow-up
Modified with permission from Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001;50:1-52. Available at www.cdc.gov/mmwr/PDF/rr/rr5011.pdf
TABLE 49-5 Factors to Consider in Assessing the Need for Follow-up of Occupational Exposures
HBsAg, Hepatitis B virus surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
From Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2001;50:1-52. Available at www.cdc.gov/mmwr/PDF/rr/rr5011.pdf
Strategy for Preventing Infection Transmission in Health Care Institutions
Institutional administrative measures aimed at developing, implementing, and monitoring specifically designed accident prevention policies and procedures are key factors in reducing and preventing transmission of infectious agents in health care centers. To this end, the center must take the following actions67,86,87:
 Include infection control as a major goal in the organizational mission statement and implement safety programs, both for patients and health care workers.
Include infection control as a major goal in the organizational mission statement and implement safety programs, both for patients and health care workers.
 Provide sufficient administrative and financial support to carry out this mission.
Provide sufficient administrative and financial support to carry out this mission.
 Provide sufficient administrative and financial support for the microbiology laboratory and implement an infection surveillance plan, especially for postsurgical infections.
Provide sufficient administrative and financial support for the microbiology laboratory and implement an infection surveillance plan, especially for postsurgical infections.
 Establish a multi-discipline cross-functional team (e.g., a team manager, an epidemiologist, a representative from industrial health, and a person trained in quality control) to identify health and safety issues within the institution, analyze trends, assess outcomes, implement interventions, and make recommendations to other members of the organization.
Establish a multi-discipline cross-functional team (e.g., a team manager, an epidemiologist, a representative from industrial health, and a person trained in quality control) to identify health and safety issues within the institution, analyze trends, assess outcomes, implement interventions, and make recommendations to other members of the organization.
 Provide sufficient administrative and financial support to develop and implement education programs for health care providers, patients, and their families. One positive example of such education is that anesthesiologists who have read the CDC’s Universal Precaution Guidelines for the Prevention of Occupational Transmission of HIV and HBV develop better hygienic practices.59
Provide sufficient administrative and financial support to develop and implement education programs for health care providers, patients, and their families. One positive example of such education is that anesthesiologists who have read the CDC’s Universal Precaution Guidelines for the Prevention of Occupational Transmission of HIV and HBV develop better hygienic practices.59
 Provide health care workers with hepatitis B vaccine and document that an appropriate immunologic response was achieved. Provide hepatitis B immune globulin (HBIG) for those exposed who do not have established immunity.4
Provide health care workers with hepatitis B vaccine and document that an appropriate immunologic response was achieved. Provide hepatitis B immune globulin (HBIG) for those exposed who do not have established immunity.4
 Provide a health care service for employees for counseling and postexposure prophylaxis should an exposure to HIV occur.88
Provide a health care service for employees for counseling and postexposure prophylaxis should an exposure to HIV occur.88
 Provide regular surveillance of health care workers to determine established immunity to infectious diseases such as tuberculosis, measles, mumps, rubella, and chickenpox. Lack of immunity may require immunization; several studies have demonstrated the cost-effectiveness of immunization (for prevention of disease) versus the cost of replacement of health care workers who have become infected.63,89–94
Provide regular surveillance of health care workers to determine established immunity to infectious diseases such as tuberculosis, measles, mumps, rubella, and chickenpox. Lack of immunity may require immunization; several studies have demonstrated the cost-effectiveness of immunization (for prevention of disease) versus the cost of replacement of health care workers who have become infected.63,89–94
Measures for Prevention of Infection Transmission in the Operating Room
Prevention of Airborne Pathogen Transmission
Airborne pathogens may be transmitted through the OR heating, ventilation, and air conditioning systems. Thus it is vital to have in place proper systems to (1) remove contaminated air, (2) facilitate air management requirements to protect susceptible health care providers and children against hospital-related airborne pathogens, and (3) minimize the risk of airborne pathogens being transmitted by children. Table 49-8 shows the 2003 HICPAC’s (Healthcare Infection Control Practices Advisory Committee) and CDC’s general recommendations for ventilation system specifications for the OR.95 Children with tuberculosis require special consideration because of the high risk of occupational transmission of Mycobacterium tuberculosis,96,97 especially after the emergence of multidrug-resistant strains (Table 49-9). An easy preventive measure is to screen all children before coming to the OR to determine recent exposure to infectious disease such as measles, mumps, rubella, and chickenpox because these infections can pose a significant risk to health care providers and patients, especially those who are immunocompromised.63,93 Another potential source for airborne spread of pathogens is through the anesthesia circuit; this may be reduced by the use of circuit filters. However, at present there are no regulatory requirements to use such devices, and performance characteristics vary widely.24,25,98–102
TABLE 49-8 Ventilation System Specifications for the Operating Room
• Minimize the circulation of people during surgeries. It has been proved that the level of microbes in the operating room air is directly proportional to the number of people moving inside the room.
• Maintain humidity under 68% and temperature control to prevent environmental conditions that favor the development of germs.
• Maintain positive pressure compared with corridors and surrounding areas to prevent microorganisms from entering the operating room.
• Provide at least 15 air changes per hour in the operating room, 20% of which should be fresh air. Air should be recirculated through a high-efficiency particulate air (HEPA) filter.
• Air should be introduced at ceiling level and disposed of at ground level.
From Guidelines for environmental infection control in healthcare facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). 2003. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5210a1.htm
TABLE 49-9 Summary of Recommended Tuberculosis Control Guidelines
Availability and access to diagnostic tests
Improved tests and reporting of results
Containment of infectious nuclei from coughs and sneezes
Negative-pressure patient environments*
Minimum of 6 air changes per hour
High-efficiency particulate air (HEPA) filters
Sterilization and disinfection of equipment
*Negative-pressure rooms are to prevent the escape of contaminated air to the outside.
From Tait A. Occupational transmission of tuberculosis: implications for anesthesiologists. Anesth Analg 1997; 85:444-51.
Standard Precautions
Standard precautions103 assume that any person or patient is potentially infected or colonized by microorganisms that could be transmitted and cause an infectious process. Standard precautions must be implemented with all patients and include:
 Universal precautions—blood and body fluid precautions, developed to reduce bloodborne pathogen transmission
Universal precautions—blood and body fluid precautions, developed to reduce bloodborne pathogen transmission
 Body substance isolation, designed to reduce the risk of pathogen transmission by moist body substances
Body substance isolation, designed to reduce the risk of pathogen transmission by moist body substances
Standard precautions are used to reduce the transmission of all infectious agents from one person to another, thus protecting health care providers and children against exposure to the most common microorganisms. Standard precautions are implemented for any contact with blood and body fluids, secretions, and excretions (except sweat), whether or not they contain visible blood, as well as for any contact with nonintact skin, mucous membranes, and intact skin that is visibly soiled with blood and/or body fluids. Summaries of standard precautions, droplet precautions, airborne precautions, and contact precautions are available on line.87,104–107
Hand Washing
Hand washing is considered the most important and cost-effective individual intervention in the prevention of hospital-acquired infections in children and health care providers.108 Its significance in medical practice had not been universally accepted, despite the pioneering work by Oliver Wendell Holmes109 (1843) and Ignaz Semmelweis110 (1846), who separately recognized that the contaminated hands of physicians performing autopsies were the vectors responsible for the spread of puerperal fever caused by streptococci, and how by washing their hands before delivering a baby, they could reduce the risk of infectious transmission and maternal mortality, the latter by 90%! Unfortunately, the scientific basis for hand washing was not established until the introduction of the germ theory of disease by Louis Pasteur111 and the discovery of the microorganism that caused anthrax (Bacillus anthracis) by Robert Koch112 in the late 19th century. More than one-and-a-half centuries later, and with strong evidence that health care providers are a leading source of hospital acquired infections,19,20,113 health care providers’ compliance with hand hygiene protocols in the hospital environment is generally poor (5%-48%) and difficult to change,114–120 especially in intensive care areas, ORs, and postanesthesia care units. The risk of pathogen transmission via the hands is proportional to the power of the number of times a child is touched.121 Table 49-10 presents a summary of the indications for hand washing and antisepsis. Compared with soap and water, alcohol-based hand rubs are more effective in reducing microbial colonization of hands.122,123 The use of alcohol-based hand rubs prompted some authors to change the term hand washing to hand hygiene. An important addition to the 2002 CDC Hand Washing Guide120 is to use alcohol-based hand rubs, because they work more rapidly (10 to 20 seconds compared with 90 to 120 seconds for hand washing) and can be used while ambulating. These advantages preclude the usual objections of health care workers to hand washing that include a lack of time, absence of sinks, and skin damage.18,124 Furthermore, the scarcity of water in developing countries no longer needs to be a constraint against hand hygiene.
TABLE 49-10 Indications for Hand Washing and Antisepsis
1. When hands are visibly dirty or contaminated with proteinaceous material or are visibly soiled with blood or other body fluids, wash hands with either a non-antimicrobial soap and water or with an antimicrobial soap and water.
2. If hands are not visibly soiled, use an alcohol-based hand rub for routinely decontaminating hands in all other clinical situations described in items 3 to 10. Alternatively, wash hands with an antimicrobial soap and water in all clinical situations described in items 3 to 10.
3. Decontaminate hands before having direct contact with patients.
4. Decontaminate hands before donning sterile gloves when inserting a central intravascular catheter.
5. Decontaminate hands before inserting indwelling urinary catheters, peripheral vascular catheters, or other invasive devices that do not require a surgical procedure.
6. Decontaminate hands after contact with a patient’s intact skin (e.g., when taking a pulse or blood pressure and lifting a patient).
7. Decontaminate hands after contact with body fluids or excretions, mucous membranes, nonintact skin, and wound dressings if hands are not visibly soiled.
8. Decontaminate hands if moving from a contaminated body site to a clean body site during patient care. Decontaminate hands after contact with inanimate objects (including medical equipment) in the immediate vicinity of the patient.
9. Decontaminate hands after removing gloves.
10. Before eating and after using a rest room, wash hands with a non-antimicrobial soap and water or with an antimicrobial soap and water.
11. Antimicrobial agent–impregnated wipes (i.e., towelettes) may be considered as an alternative to washing hands with non-antimicrobial soap and water. Because they are not as effective as alcohol-based hand rubs or washing hands with an antimicrobial soap and water for reducing bacterial counts on the hands of health care workers, they are not a substitute for using an alcohol-based hand rub or antimicrobial soap.
12. Wash hands with non-antimicrobial soap and water or with antimicrobial soap and water if exposure to Bacillus anthracis is suspected or proven. The physical action of washing and rinsing hands under such circumstances is recommended because alcohols, chlorhexidine, iodophors, and other antiseptic agents have poor activity against spores.
Modified from Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control 2002;30:S1-46.
After hand washing, it is very important to dry the hands properly with appropriate paper towels, hot air flow, or both, because the level of pathogen transmission from a health care worker’s hands to a patient is greatly increased if the hands are wet.125 Transmission may also occur from patients’ wet sites, such as groins or armpits, or when a health care worker gets his or her hands wet when opening parenteral solutions. It is critical for health institutions to establish written procedures and protocols to support adherence to the recommended hand hygiene practices.
Gloves
Recommendations for the use of gloves include126–128:
 Wear gloves in case of contact with blood or any other potentially infecting body fluid such as excretions, secretions (except sweat), mucous membranes, and nonintact skin.
Wear gloves in case of contact with blood or any other potentially infecting body fluid such as excretions, secretions (except sweat), mucous membranes, and nonintact skin.
 Remove the gloves immediately after providing care to a child. Staff should not wear the same pair of gloves to take care of more than one child, nor should they touch the surfaces of any equipment, monitoring devices, or even light switches. Contaminated gloves can pass blood or other body fluids to working surfaces and are vectors for hepatitis transmission.62
Remove the gloves immediately after providing care to a child. Staff should not wear the same pair of gloves to take care of more than one child, nor should they touch the surfaces of any equipment, monitoring devices, or even light switches. Contaminated gloves can pass blood or other body fluids to working surfaces and are vectors for hepatitis transmission.62
 Change gloves when taking care of a child if you must move from a contaminated to a clean body site.
Change gloves when taking care of a child if you must move from a contaminated to a clean body site.
 Apply hand hygiene measures immediately after removing the gloves because, despite the use of gloves, hands may get contaminated through small (microscopic) holes in the gloves.113,129,130 Microbial contamination of hands and possible infection transmission have been reported even with the use of gloves.131
Apply hand hygiene measures immediately after removing the gloves because, despite the use of gloves, hands may get contaminated through small (microscopic) holes in the gloves.113,129,130 Microbial contamination of hands and possible infection transmission have been reported even with the use of gloves.131
 Remove the gloves by using an appropriate technique (so as not to contaminate your hands with the contaminated surface of the gloves).
Remove the gloves by using an appropriate technique (so as not to contaminate your hands with the contaminated surface of the gloves).
 Alcohol-based hand rub dispensers and clean glove boxes (at least two sizes) should be in place near every patient care site (e.g., on top of every anesthesia cart, medication cart, or in the nursing station).
Alcohol-based hand rub dispensers and clean glove boxes (at least two sizes) should be in place near every patient care site (e.g., on top of every anesthesia cart, medication cart, or in the nursing station).
 Disposable gloves should not be washed, re-sterilized, or disinfected. If gloves are reused, appropriate reprocessing methods should be in place to ensure the physical integrity of the gloves and their full decontamination.
Disposable gloves should not be washed, re-sterilized, or disinfected. If gloves are reused, appropriate reprocessing methods should be in place to ensure the physical integrity of the gloves and their full decontamination.
 Sterile gloves are much more expensive than clean, disposable gloves and should be used only for certain procedures, such as when hands are in contact with normally sterile body areas or when inserting intravascular or urinary catheters. Clean gloves should be used during any other procedure, including wound dressing.
Sterile gloves are much more expensive than clean, disposable gloves and should be used only for certain procedures, such as when hands are in contact with normally sterile body areas or when inserting intravascular or urinary catheters. Clean gloves should be used during any other procedure, including wound dressing.
 Latex-free gloves should be worn when caring for children at risk for latex allergy.
Latex-free gloves should be worn when caring for children at risk for latex allergy.
Antimicrobial Prophylaxis
Surgical antimicrobial prophylaxis is an essential tool to reduce the risk of postoperative infections, and the anesthesia team plays a central role in ensuring the proper timing of drug administration.132,133 The aim of the perioperative administration of antibiotics is to obtain plasma and tissue drug concentrations exceeding the minimal inhibitory concentration of those organisms most likely to cause an infection. This will reduce the microbial load of the intraoperative contamination to a level not exceeding the host defenses; it is not the intent to cover all possible pathogens, because this can lead to the selection of drug-resistant bacteria.
Selection of the Antimicrobial Agent
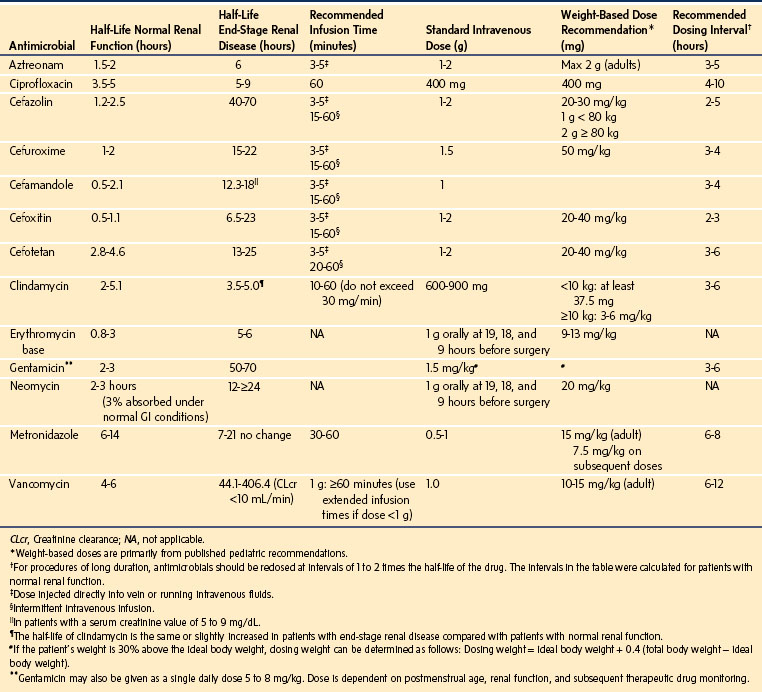
Several antimicrobial prophylaxis guidelines have been published (Table 49-11). For most surgical procedures that do not involve chronically colonized organs, the most common pathogens are the skin flora, Streptococcus and Staphylococcus. A first-generation cephalosporin (i.e., cefazolin) can provide cost-effective coverage for these organisms. Surgical procedures that involve contamination from the bowel require antibiotic treatment against gram-negative and anaerobic pathogens. For these procedures, cefoxitin, cefotetan, or a second-generation cephalosporin is appropriate.134 The selection of antibiotics requires consideration of resistance patterns as determined by local microbiology or health center infectious disease departments. The newer-generation broad-spectrum antibiotics should not be used for routine antibiotic prophylaxis but should be reserved for the treatment of resistant organisms. Moreover, the dose of antibiotic selected should be based on the child’s weight or body mass index; administration should be repeated intraoperatively if surgery exceeds more than two half-lives after the first antibiotic administration (see Table 49-11), if the duration of surgery exceeds 4 to 8 hours, if blood loss is extreme, or if the drug has a particularly short half-life (e.g., penicillin or cefoxitin) to ensure appropriate tissue concentrations of antibiotic until wound closure.135
The Timing of Antibiotic Prophylaxis
A key element in the prevention of surgical site infection is the timely administration of prophylactic antibiotics. For most surgical procedures, a single prophylactic dose of antibiotics should be administered 30 to 60 minutes before the skin incision. This should provide appropriate plasma concentrations of the antibiotic.136,137 However, in the case of children, IV access is often established after induction of anesthesia. With a brief time interval between establishing IV access and skin incision, it is important to administer the antibiotics as soon as possible after IV access is established and before surgical incision. If vancomycin must be used for prophylaxis, it should be infused slowly over 60 minutes (to minimize the risk of severe hypotension, [“red man” syndrome]) beginning within 2 hours of skin incision. If a tourniquet is required, the full antibiotic dose should be administered before the tourniquet is pressurized.138 Postsurgical prophylactic antibiotics are not necessary for most procedures and should generally be stopped within 24 hours after the surgical procedure.138
Allergy to β-Lactams
Several studies have shown that the true incidence of allergy to antibiotics is less than that reflected in medical charts.139 For surgical procedures where cephalosporins are the prophylaxis of choice, alternative antibiotics should be administered to those children at risk of anaphylaxis to β-lactams, based on their history or diagnostic tests (e.g., skin testing). However, the incidence of severe allergic reactions to first-generation cephalosporins in children with reported allergy to penicillin is rare (but not zero)140,141; furthermore, skin testing does not reliably predict the likelihood of adverse reactions to cephalosporins in those with reported allergy to penicillin.142–144 There is no evidence of any risk of cross-reactivity between penicillin and second- and third-generation cephalosporins. For the most part, “allergies” to oral antibiotics that appear on children’s charts (rash, vomiting, gastrointestinal disturbances) are most likely reactions to the additives in the antibiotic formulation, including food dyes, fillers, and other compounds, or a manifestation of the underlying infection. IV administration of small test doses of the pure antibiotics in a fully monitored (and anesthetized) child with a so-called allergy will determine whether the child is at risk for an allergic reaction to the antibiotic. In the case of surgical procedures where antibiotic prophylaxis is mainly directed at gram-positive cocci, children who are truly allergic to β-lactams (cephalosporins) should receive either vancomycin or clindamycin.134
Indications for Prophylactic Antibiotics
Surgical wounds are classified in four categories (Table 49-12). The use of antibiotic prophylaxis for postoperative infections is well established for clean-contaminated procedures. Within the clean category, prophylaxis has been traditionally reserved for surgical procedures involving a foreign body implantation or for any surgical procedure where a surgical site infection would be catastrophic (e.g., cardiac surgery or neurosurgical procedures). However, there is evidence that postoperative infections resulting from procedures not involving prosthetic elements are underreported; estimates show that over 50% of all complications occur after the patient is discharged and are thus unrecognized by the surgical team. Therefore, antibiotic prophylaxis is also recommended for certain procedures, such as herniorrhaphy.145,146 The direct and indirect costs of these complications may not affect the hospital budget; however, they represent a substantial cost for the community at large. In the case of contaminated or dirty procedures, bacterial contamination or infection is established before the procedure begins. Accordingly, the perioperative administration of antibiotics is a therapeutic, not a prophylactic, measure. The use of antibiotics in children has implications not only for the response to the current treatment, but also to future treatments. Thus all medical professionals are jointly responsible for the rational use of antibiotics.
| Wound Category | Description |
|---|---|
| Class I/clean | Uninfected wound with no inflammation and the respiratory, alimentary, genital, or uninfected urinary tract is not entered. Clean wounds primarily are closed and drained, when necessary, with closed drainage. Operative wounds after blunt trauma may be included in this category if they meet criteria. |
| Class II/clean contaminated | Operative wound in which the respiratory, alimentary, genital, or urinary tract is entered under controlled conditions and without unusual contamination. Specifically, operations involving the biliary tract, appendix, vagina, and oropharynx are included in the category, provided no evidence of infection or major break in technique is encountered. |
| Class III/contaminated | Open, fresh, accidental wounds; operations with major breaks in sterile technique (e.g., open cardiac massage) or gross spillage from the gastrointestinal tract; and incisions in which acute, nonpurulent inflammation is encountered |
| Class IV/dirty-infected | Old traumatic wounds with retained devitalized tissue and those that involve existing clinical infection or perforated viscera, suggesting that the organisms causing postoperative infection were present in the operative field before operation. |
From Neville HL, Lally KP. Pediatric surgical wound infections. Semin Pediatr Infect Dis 2001;12:124-9.
Protocols, although effective, require continuous feedback on their acceptance and surgical-site infection results.147 No surgical protocol can replace the judgment of the medical professional; clinical reasoning must be tailored to the individual circumstances. Finally, children with congenital heart disease and a subgroup of those with repaired congenital heart disease may require bacterial endocarditis prophylaxis (see also Tables 14-2 and 14-3).148
Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39:1182–1189.
Rizzo M. Striving to eliminate catheter-related bloodstream infections: a literature review of evidence-based strategies. Semin Anesth Perioper Med Pain. 2005;24:214–225.
Sagoe-Moses CH, Pearson R, Perry J, Jagger J. Risks to health care workers in developing countries. N Engl J Med. 2001;345:538–541.
1 Skinner T. Anaesthetics and inhalers. Br Med J. 1873;1:353–354.
2 Hall JR. Blood contamination of anesthesia equipment and monitoring equipment. Anesth Analg. 1994;78:1136–1139.
3 Ben-David B, Gaitini L. The routine wearing of gloves: impact on the frequency of needlestick and percutaneous injury and on surface contamination in the operating room. Anesth Analg. 1996;83:623–628.
4 Herwaldt LA, Smith SD, Carter CD. Infection control in the outpatient setting. Infect Control Hosp Epidemiol. 1998;19:41–74.
5 Sandhu NS, Schaffer S, Capan LM, Gill JS. Prevention of airborne exposure during endotracheal intubation. Anesth Analg. 1999;89:1067–1068.
6 Sehulster L, Chinn RY. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52:1–42.
7 Harrison CA, Rogers DW, Rosen M. Blood contamination of anaesthetic and related staff. Anaesthesia. 1990;45:831–833.
8 Centers for Disease Control and Prevention. Update: human immunodeficiency virus infections in health-care workers exposed to blood of infected patients. Morb Mortal Wkly Rep. 1987;36:285–289.
9 Cobo JC, Harley DP. The eyes as a portal of entry for hepatitis and other infectious diseases. Surg Gynecol Obstet. 1985;161:71.
10 Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
11 Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47–54.
12 Larrosa A, Cortes-Blanco M, Martinez S, et al. Nosocomial outbreak of scabies in a hospital in Spain. Euro Surveill. 2003;8:199–203.
13 Obasanjo OO, Wu P, Conlon M, et al. An outbreak of scabies in a teaching hospital: lessons learned. Infect Control Hosp Epidemiol. 2001;22:13–18.
14 DeYoung GG, Harrison AW, Shapley JM. Herpes simplex cross infection in the operating room. Can Anaesth Soc J. 1968;15:394–396.
15 Avitzur Y, Amir J. Herpetic whitlow infection in a general pediatrician—an occupational hazard. Infection. 2002;30:234–236.
16 Gunbay T, Gunbay S, Kandemir S. Herpetic whitlow. Quintessence Int. 1993;24:363–364.
17 Jones JG. Herpetic whitlow: an infectious occupational hazard. J Occup Med. 1985;27:725–728.
18 Koff MD, Loftus RW, Burchman CC, et al. Reduction in intraoperative bacterial contamination of peripheral intravenous tubing through the use of a novel device. Anesthesiology. 2009;110:978–985.
19 Loftus RW, Muffly MK, Brown JR, et al. Hand contamination of anesthesia providers is an important risk factor for intraoperative bacterial transmission. Anesth Analg. 2011;112:98–105.
20 Loftus RW, Koff MD, Burchman CC, et al. Transmission of pathogenic bacterial organisms in the anesthesia work area. Anesthesiology. 2008;109:399–407.
21 Hopf HW, Rollins MD. Reducing perioperative infection is as simple as washing your hands. Anesthesiology. 2009;110:959–960.
22 Tschudin-Sutter S, Pargger H, Widmer AF. Hand hygiene in the intensive care unit. Crit Care Med. 2010;38:S299–S305.
23 el Mikatti N, Dillon P, Healy TE. Hygienic practices of consultant anaesthetists: a survey in the northwest region of the UK. Anaesthesia. 1999;54:13–18.
24 Wilkes AR. Preventing the transmission of pathogenic microbes during anesthesia. Expert Rev Med Devices. 2005;2:319–326.
25 Peng PW, Wong DT, Bevan D, Gardam M. Infection control and anesthesia: lessons learned from the Toronto SARS outbreak. Can J Anaesth. 2003;50:989–997.
26 Ostrowsky BE, Whitener C, Bredenberg HK, et al. Serratia marcescens bacteremia traced to an infused narcotic. N Engl J Med. 2002;346:1529–1537.
27 Bennett SN, McNeil MM, Bland LA, et al. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N Engl J Med. 1995;333:147–154.
28 Kuehnert MJ, Webb RM, Jochimsen EM, et al. Staphylococcus aureus bloodstream infections among patients undergoing electroconvulsive therapy traced to breaks in infection control and possible extrinsic contamination by propofol. Anesth Analg. 1997;85:420–425.
29 McNeil MM, Lasker BA, Lott TJ, Jarvis WR. Postsurgical Candida albicans infections associated with an extrinsically contaminated intravenous anesthetic agent. J Clin Microbiol. 1999;37:1398–1403.
30 Sepkowitz KA. Occupationally acquired infections in health care workers. Part II. Ann Intern Med. 1996;125:917–928.
31 Sepkowitz KA. Occupationally acquired infections in health care workers. Part I. Ann Intern Med. 1996;125:826–834.
32 Esteban JI, Gomez J, Martell M, et al. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med. 1996;334:555–560.
33 Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993;118:117–128.
34 Pearson ML, Jereb JA, Frieden TR, et al. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis. A risk to patients and health care workers. Ann Intern Med. 1992;117:191–196.
35 Joseph JM. Disease transmission by inefficiently sanitized anesthetizing apparatus. JAMA. 1952;149:1196–1198.
36 Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39:1182–1189.
37 Olds JW, Kisch AL, Eberle BJ, Wilson JN. Pseudomonas aeruginosa respiratory tract infection acquired from a contaminated anesthesia machine. Am Rev Respir Dis. 1972;105:629–632.
38 Phillips I, Spencer G. Pseudomonas aeruginosa cross-infection due to contaminated respiratory apparatus. Lancet. 1965;2:1325–1327.
39 Chant K, Lowe D, Rubin G, et al. Patient-to-patient transmission of HIV in private surgical consulting rooms. Lancet. 1993;342:1548–1549.
40 Alter MJ, Ahtone J, Maynard JE. Hepatitis B virus transmission associated with a multiple-dose vial in a hemodialysis unit. Ann Intern Med. 1983;99:330–333.
41 Grieble HG, Colton FR, Bird TJ, Toigo A, Griffith LG. Fine-particle humidifiers. Source of Pseudomonas aeruginosa infections in a respiratory-disease unit. N Engl J Med. 1970;282:531–535.
42 Fierer J, Taylor PM, Gezon HM. Pseudomonas aeruginosa epidemic traced to delivery-room resuscitators. N Engl J Med. 1967;276:991–996.
43 Berry AJ, Nolte FS. An alternative strategy for infection control of anesthesia breathing circuits: a laboratory assessment of the Pall HME Filter. Anesth Analg. 1991;72:651–655.
44 Call TR, Auerbach FJ, Riddell SW, et al. Nosocomial contamination of laryngoscope handles: challenging current guidelines. Anesth Analg. 2009;109:479–483.
45 Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. Kogenate Previously Untreated Patient Study Group. N Engl J Med. 1993;328:453–459.
46 Tobin MJ, Stevenson GW, Hall SC. A simple, cost-effective method of preventing laryngoscope handle contamination. Anesthesiology. 1995;82:790.
47 Enright AC, Moore RL, Parney FL. Contamination and resterilization of the Bain circuit. Can Anaesth Soc J. 1976;23:545–549.
48 Sabon RL, Jr., Cheng EY, Stommel KA, Hennen CR. Glass particle contamination: influence of aspiration methods and ampule types. Anesthesiology. 1989;70:859–862.
49 Carbone-Traber KB, Shanks CA. Glass particle contamination in single-dose ampules. Anesth Analg. 1986;65:1361–1363.
50 Zacher AN, Zornow MH, Evans G. Drug contamination from opening glass ampules. Anesthesiology. 1991;75:893–895.
51 Lessard MR, Trepanier CA, Gourdeau M, Denault PH. A microbiological study of the contamination of the syringes used in anaesthesia practice. Can J Anaesth. 1988;35:567–569.
52 Heseltine P. Anesthesiologists should not give IV medications with common syringe. Hosp Infect Control. 1986;13:84–85.
53 Batai I, Kerenyi M, Falvai J, Szabo G. Bacterial growth in ropivacaine hydrochloride. Anesth Analg. 2002;94:729–731.
54 Kampe S, Poetter C, Buzello S, et al. Ropivacaine 0.1% with sufentanil 1 microg/mL inhibits in vitro growth of Pseudomonas aeruginosa and does not promote multiplication of Staphylococcus aureus. Anesth Analg. 2003;97:409–411. table
55 Sosis MB, Braverman B. Growth of Staphylococcus aureus in four intravenous anesthetics. Anesth Analg. 1993;77:766–768.
56 Sosis MB, Braverman B, Villaflor E. Propofol, but not thiopental, supports the growth of Candida albicans. Anesth Analg. 1995;81:132–134.
57 Crowther J, Hrazdil J, Jolly DT, et al. Growth of microorganisms in propofol, thiopental, and a 1:1 mixture of propofol and thiopental. Anesth Analg. 1996;82:475–478.
58 Raedler C, Lass-Florl C, Puhringer F, et al. Bacterial contamination of needles used for spinal and epidural anaesthesia. Br J Anaesth.. 1999;83:657–658.
59 Tait AR, Tuttle DB. Preventing perioperative transmission of infection: a survey of anesthesiology practice. Anesth Analg. 1995;80:764–769.
60 Rosenberg AD, Bernstein DB, Bernstein RL, et al. Accidental needlesticks: do anesthesiologists practice proper infection control precautions? Am J Anesthesiol. 1995;22:125–132.
61 Kempen PM, Learned DW. Anesthesia practice—a vector of infection? Anesthesiology. 1989:71. A948
62 Favero MS, Maynard JE, Petersen NJ, et al. Letter: Hepatitis-B antigen on environmental surfaces. Lancet. 1973;2:1455.
63 Fedeli U, Zanetti C, Saia B. Susceptibility of healthcare workers to measles, mumps rubella and varicella. J Hosp Infect. 2002;51:133–135.
64 Weber DJ, Rutala WA, Schaffner W. Lessons learned: protection of healthcare workers from infectious disease risks. Crit Care Med. 2010;38:S306–S314.
65 Centers for Disease Control and Prevention. Guidelines for prevention of transmission of human immunodeficiency virus and hepatitis B virus to health-care and public-safety workers. Morb Mortal Wkly Rep. 1989;38(Suppl 6):1–37.
66 Joint Working Party of the Hospital Infection Society and the Surgical Infection Study Group. Risks to surgeons and patients from HIV and hepatitis: guidelines on precautions and management of exposure to blood or body fluids. BMJ. 1992;305:1337–1343.
67 Workbook for Designing, Implementing and Evaluating a Sharps Injury Prevention Program. 2008. Available at http://www.cdc.gov/sharpssafety/pdf/sharpsworkbook_2008.pdf (accessed Oct. 20, 2012).
68 Berry AJ, Greene ES. The risk of needlestick injuries and needlestick-transmitted diseases in the practice of anesthesiology. Anesthesiology. 1992;77:1007–1021.
69 Greene ES, Berry AJ, Arnold WP, III., Jagger J. Percutaneous injuries in anesthesia personnel. Anesth Analg. 1996;83:273–278.
70 Asai T, Matsumoto S, Matsumoto H, Yamamoto K, Shingu K. Prevention of needle-stick injury. Efficacy of a safeguarded intravenous cannula. Anaesthesia. 1999;54:258–261.
71 Berguer R, Heller PJ. Strategies for preventing sharps injuries in the operating room. Surg Clin North Am. 2005;85:1299–1305.
72 Culver J. Preventing transmission of blood-borne pathogens: a compelling argument for effective device-selection strategies. Am J Infect Control. 1997;25:430–433.
73 Dale JC, Pruett SK, Maker MD. Accidental needlesticks in the phlebotomy service of the Department of Laboratory Medicine and Pathology at Mayo Clinic Rochester. Mayo Clin Proc. 1998;73:611–615.
74 Fisher J, Wilburn S. Don’t get stuck with unsafe needles. Instead, get involved in needle device selection. Am J Nurs. 2000;100:139.
75 Foley M. Update on needlestick and sharps injuries: the Needle Stick Safety and Prevention Act of 2000. Am J Nurs. 2004;104:96.
76 Greene ES, Berry AJ, Jagger J, et al. Multicenter study of contaminated percutaneous injuries in anesthesia personnel. Anesthesiology. 1998;89:1362–1372.
77 Melzer SM, Vermund SH, Shelov SP. Needle injuries among pediatric housestaff physicians in New York City. Pediatrics. 1989;84:211–214.
78 Patel N, Tignor GH. Device-specific sharps injury and usage rates: an analysis by hospital department. Am J Infect Control. 1997;25:77–84.
79 Porta C, Handelman E, McGovern P. Needlestick injuries among health care workers. A literature review. AAOHN J. 1999;47:237–244.
80 Shiao JS, McLaws ML, Huang KY, Guo YL. Sharps injuries among hospital support personnel. J Hosp Infect. 2001;49:262–267.
81 Sinclair RC, Maxfield A, Marks EL, Thompson DR, Gershon RR. Prevalence of safer needle devices and factors associated with their adoption: results of a national hospital survey. Public Health Rep. 2002;117:340–349.
82 Sohn S, Eagan J, Sepkowitz KA, Zuccotti G. Effect of implementing safety-engineered devices on percutaneous injury epidemiology. Infect Control Hosp Epidemiol. 2004;25:536–542.
83 Trim JC, Elliott TS. A review of sharps injuries and preventative strategies. J Hosp Infect. 2003;53:237–242.
84 Wilburn SQ, Eijkemans G. Preventing needlestick injuries among healthcare workers: a WHO-ICN collaboration. Int J Occup Environ Health. 2004;10:451–456.
85 Zimmers T. Sharps disposal in the ED: simple techniques and equipment. Am J Emerg Med. 1999;17:53–54.
86 Jaichenco A, Fraire C, Barrios F. Monografía-Manual para el Desarrollo de un Programa de Prevención de Infecciones de Sitio Quirúrgico. 2005. Escuela de Salud Pública, Universidad de Buenos Aires. [Dissertation]
87 Sociedad Argentina de Infectología, Instituto Nacional de Epidemiología Documento de Consenso. Prevención de Infección de Sitio Quirúrgico y seguridad del paciente en pre, intra y postquirúrgico. 2009. Available at http://www.sadi.org.ar/files/CONSENSOISQSADIINE%202009.PDF (accessed Oct. 20, 2012).
88 U.S. Public Health Service. Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis. MMWR Recomm Rep. 2001;50:1–52.
89 O’Neill J, Buttery J. Varicella and paediatric staff: current practice and vaccine cost-effectiveness. J Hosp Infect. 2003;53:117–119.
90 Brunell PA, Wood D. Varicella serological status of healthcare workers as a guide to whom to test or immunize. Infect Control Hosp Epidemiol. 1999;20:355–357.
91 Nettleman MD, Schmid M. Controlling varicella in the healthcare setting: the cost effectiveness of using varicella vaccine in healthcare workers. Infect Control Hosp Epidemiol. 1997;18:504–508.
92 Tennenberg AM, Brassard JE, Van Lieu J, Drusin LM. Varicella vaccination for healthcare workers at a university hospital: an analysis of costs and benefits. Infect Control Hosp Epidemiol. 1997;18:405–411.
93 Lane NE, Paul RI, Bratcher DF, Stover BH. A survey of policies at children’s hospitals regarding immunity of healthcare workers: are physicians protected? Infect Control Hosp Epidemiol. 1997;18:400–404.
94 Weber DJ, Rutala WA, Hamilton H. Prevention and control of varicella-zoster infections in healthcare facilities. Infect Control Hosp Epidemiol. 1996;17:694–705.
95 Sehulster L, Chinn RY, CDCHICPAC. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-10):1–42.
96 Tait AR. Occupational transmission of tuberculosis: implications for anesthesiologists. Anesth Analg. 1997;85:444–451.
97 Alonso-Echanove J, Granich RM, Laszlo A, et al. Occupational transmission of Mycobacterium tuberculosis to health care workers in a university hospital in Lima, Peru. Clin Infect Dis. 2001;33:589–596.
98 Wilkes AR, Benbough JE, Speight SE, Harmer M. The bacterial and viral filtration performance of breathing system filters. Anaesthesia. 2000;55:458–465.
99 Cochs J, Casals P, Villalonga R, et al. [Prevention of cross contamination, patient to anesthesia apparatus to patient, using filters]. Rev Esp Anestesiol Reanim. 1994;41:322–327.
100 Wilkes AR. Heat and moisture exchangers and breathing system filters: their use in anaesthesia and intensive care. Part 1. History, principles and efficiency. Anaesthesia. 2011;66:31–39.
101 Wilkes AR. Heat and moisture exchangers and breathing system filters: their use in anaesthesia and intensive care. Part 2. Practical use, including problems, and their use with paediatric patients. Anaesthesia. 2011;66:40–51.
102 Lawes EG. Hidden hazards and dangers associated with the use of HME/filters in breathing circuits. Their effect on toxic metabolite production, pulse oximetry and airway resistance. Br J Anaesth. 2003;91:249–264.
103 Centers for Disease Control and Prevention: Standard Precautions. 2007. Available at http://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf
104 Standard Precautions. Centers for Disease Control and Prevention. 2011. Available at http://www.cdc.gov/HAI/pdfs/guidelines/standatds-of-ambulatory-care-7-2011.pdf
105 Contact Precautions. Centers for Disease Control and Prevention. 2006. Available at http://www.cdc.gov/mrsa/pdf/mdroGuideline2006.pdf
106 Airborne Precautions. Centers for Disease Control and Prevention. 2011. Available at http://www.cdc.gov/HAI/settings/outpatient/basic-infection-control-prevention-plan-2011/transmission-based-precautions.html
107 World Health Organization. Practical guidelines for infection control in health care facilities. SEARO Regional Publication No. 41. http://www.searo.who.int/LinkFiles/Publications_PracticalguidelinSEAROpub-41.pdf, 2004. Available at
108 Burke JP. Infection control—a problem for patient safety. N Engl J Med. 2003;348:651–656.
109 Holmes OW. Classic pages in obstetrics and gynecology. Oliver Wendell Holmes. The contagiousness of puerperal fever. The New England Quarterly Journal of Medicine and Surgery, vol. 1, pp. 503-530, 1842-3. Am J Obstet Gynecol. 1974;119:852.
110 Semmelweis I. The etiology, concept, and prophylaxis of childbed fever. Madison, Wis.: University of Wisconsin Press; 1983.
111 Pasteur L. Puerperal sepsis. Bull Am Acad Med (Paris). 1879;8:256–260.
112 Katz JD. Hand washing and hand disinfection: more than your mother taught you. Anesthesiol Clin North America. 2004;22:457–471.
113 Tenorio AR, Badri SM, Sahgal NB, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis. 2001;32:826–829.
114 Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23:251–269.
115 Pittet D, Mourouga P, Perneger TV. Compliance with handwashing in a teaching hospital. Infection Control Program. Ann Intern Med. 1999;130:126–130.
116 Pittet D. Improving compliance with hand hygiene in hospitals. Infect Control Hosp Epidemiol. 2000;21:381–386.
117 Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356:1307–1312.
118 Pittet D. Hand hygiene: improved standards and practice for hospital care. Curr Opin Infect Dis. 2003;16:327–335.
119 Pittet D. Improving adherence to hand hygiene practice: a multidisciplinary approach. Emerg Infect Dis. 2001;7:234–240.
120 Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1–46.
121 Cooper BS, Medley GF, Scott GM. Preliminary analysis of the transmission dynamics of nosocomial infections: stochastic and management effects. J Hosp Infect. 1999;43:131–147.
122 Girou E, Loyeau S, Legrand P, Oppein F, Brun-Buisson C. Efficacy of handrubbing with alcohol based solution versus standard handwashing with antiseptic soap: randomised clinical trial. BMJ. 2002;325:362.
123 Dugani S, Kumar A, Wilkes AR. Influence of patient factors on the efficacy of breathing system filters at preventing contamination of breathing systems. Anaesthesia. 2010;65:468–472.
124 Teare L, Cookson B, Stone S. Hand hygiene. BMJ. 2001;323:411–412.
125 Merry AF, Miller TE, Findon G, Webster CS, Neff SP. Touch contamination levels during anaesthetic procedures and their relationship to hand hygiene procedures: a clinical audit. Br J Anaesth. 2001;87:291–294.
126 WHO Guidelines on Hand Hygiene in Health Care 2009. Available at http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf
127 Garner JS. Guideline for isolation precautions in hospitals. The Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1996;17:53–80.
128 Institute for Healthcare Improvement. How-to guide: improving hand hygiene. A guide for improving practices among health care workers. www.ihi.org, 2006. Available at
129 Pittet D, Dharan S, Touveneau S, Sauvan V, Perneger TV. Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med. 1999;159:821–826.
130 Albin MS, Bunegin L, Duke ES, Ritter RR, Page CP. Anatomy of a defective barrier: sequential glove leak detection in a surgical and dental environment. Crit Care Med. 1992;20:170–184.
131 Olsen RJ, Lynch P, Coyle MB, et al. Examination gloves as barriers to hand contamination in clinical practice. JAMA. 1993;270:350–353.
132 Gnass SA, Barboza L, Bilicich D, et al. Prevention of central venous catheter-related bloodstream infections using non-technologic strategies. Infect Control Hosp Epidemiol. 2004;25:675–677.
133 Jarvis WR. Benchmarking for prevention: the Centers for Disease Control and Prevention’s National Nosocomial Infections Surveillance (NNIS) system experience. Infection. 2003;31(Suppl 2):44–48.
134 Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38:1706–1715.
135 Zanetti G, Giardina R, Platt R. Intraoperative redosing of cefazolin and risk for surgical site infection in cardiac surgery. Emerg Infect Dis. 2001;7:828–831.
136 Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–286.
137 Burke JP. Maximizing appropriate antibiotic prophylaxis for surgical patients: an update from LDS Hospital, Salt Lake City. Clin Infect Dis. 2001;33:S78–S83.
138 Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395–404.
139 Hung OR, Bands C, Laney G, et al. Drug allergies in the surgical population. Can J Anaesth. 1994;41:1149–1155.
140 Apter AJ, Kinman JL, Bilker WB, et al. Is there cross-reactivity between penicillins and cephalosporins? Am J Med. 2006;119:354–359.
141 Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2005;115:1048–1057.
142 Robinson JL, Hameed T, Carr S. Practical aspects of choosing an antibiotic for patients with a reported allergy to an antibiotic. Clin Infect Dis. 2002;35:26–31.
143 Solensky R. Hypersensitivity reactions to beta-lactam antibiotics. Clin Rev Allergy Immunol. 2003;24:201–220.
144 Prosser DP, Gompels M. Anaphylactic shock due to cefuroxime in a patient taking penicillin prophylaxis. Paediatr Anaesth. 2002;12:73–75.
145 Manian FA, Meyer L. Comprehensive surveillance of surgical wound infections in outpatient and inpatient surgery. Infect Control Hosp Epidemiol. 1990;11:515–520.
146 Bailey IS, Karran SE, Toyn K, et al. Community surveillance of complications after hernia surgery. BMJ. 1992;304:469–471.
147 Varughese AM, Hagerman NS, Kurth CD. Quality in pediatric anesthesia. Paediatr Anaesth. 2010;20:684–696.
148 Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA. 1997;277:1794–1801.