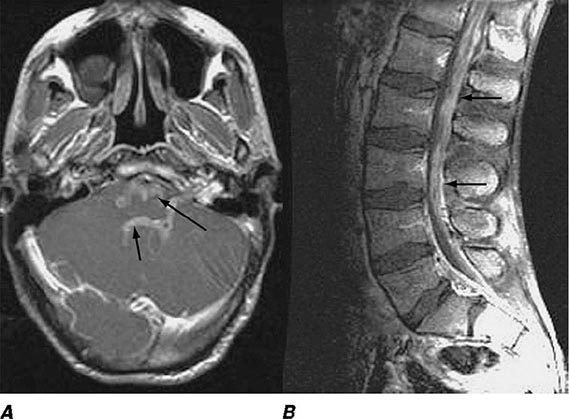
FIGURE 164-3 Coronal fluid-attenuated inversion recovery (FLAIR) magnetic resonance image from a patient with herpes simplex encephalitis. Note the area of increased signal in the right temporal lobe (left side of image) confined predominantly to the gray matter. This patient had predominantly unilateral disease; bilateral lesions are more common but may be quite asymmetric in their intensity.
Significant MRI abnormalities are found in only approximately two-thirds of patients with WNV encephalitis, a frequency less than that with HSV encephalitis. When present, abnormalities often involve deep brain structures, including the thalamus, basal ganglia, and brainstem, rather than the cortex and may only be apparent on FLAIR images. EEGs in patients with WNV encephalitis typically show generalized slowing that may be more anteriorly prominent rather than the temporally predominant pattern of sharp or periodic discharges more characteristic of HSV encephalitis. Patients with VZV encephalitis may show multifocal areas of hemorrhagic and ischemic infarction, reflecting the tendency of this virus to produce a CNS vasculopathy rather than a true encephalitis. Immunocompromised adult patients with CMV often have enlarged ventricles with areas of increased T2 signal on MRI outlining the ventricles and subependymal enhancement on T1-weighted postcontrast images. Table 164-5 highlights specific diagnostic test results in encephalitis that can be useful in clinical decision making.
|
USE OF DIAGNOSTIC TESTS IN ENCEPHALITIS |
Abbreviations: CNS, central nervous system; CSF, cerebrospinal fluid; DWI, diffusion-weighted imaging; EA, early antigen; EBNA, EBV-associated nuclear antigen; EBV, Epstein-Barr virus; FLAIR, fluid-attenuated inversion recovery; HSV, herpes simplex virus; IgM, immunoglobulin M; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; VCA, viral capsid antibody; VZV, varicella-zoster virus; WNV, West Nile virus.
Brain Biopsy Brain biopsy is now generally reserved for patients in whom CSF PCR studies fail to lead to a specific diagnosis, who have focal abnormalities on MRI, and who continue to show progressive clinical deterioration despite treatment with acyclovir and supportive therapy.
DIFFERENTIAL DIAGNOSIS
Infection by a variety of other organisms can mimic viral encephalitis. In studies of biopsy-proven HSV encephalitis, common infectious mimics of focal viral encephalitis included mycobacteria, fungi, rickettsiae, Listeria, Mycoplasma, and other bacteria (including Bartonella sp.). Autoimmune causes of encephalitis, including those associated with antibodies against N-methyl-D-aspartate (NMDA) receptor, voltage-gated potassium channels (VGKC), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and γ-aminobutyric acid (GABA) receptors, and GAD-65, have been increasingly recognized as causes of encephalitis that can mimic that caused by viral infection. In most cases, diagnosis is made by detection of the specific autoantibodies in serum and/or CSF. NMDA receptor antibodies have recently been reported in some patients with HSE encephalitis, and their presence should not exclude appropriate testing and treatment for HSV encephalitis. Autoimmune encephalitis may also be associated with specific cancers (paraneoplastic) and onconeuronal antibodies (e.g., anti-Hu, Yo, Ma2, amphiphysin, CRMP5, CV2) (Chap. 122). Subacute or chronic forms of encephalitis may occur in association with autoantibodies against thyroglobulin and thyroperoxidase (Hashimoto’s encephalopathy) and with prion diseases.
Infection caused by the ameba Naegleria fowleri can also cause acute meningoencephalitis (primary amebic meningoencephalitis), whereas that caused by Acanthamoeba and Balamuthia more typically produces subacute or chronic granulomatous amebic meningoencephalitis. Naegleria thrive in warm, iron-rich pools of water, including those found in drains, canals, and both natural and human-made outdoor pools. Infection has typically occurred in immunocompetent children with a history of swimming in potentially infected water. The CSF, in contrast to the typical profile seen in viral encephalitis, often resembles that of bacterial meningitis with a neutrophilic pleocytosis and hypoglycorrhachia. Motile trophozoites can be seen in a wet mount of warm, fresh CSF. There have been an increasing number of cases of Balamuthia mandrillaris amebic encephalitis mimicking acute viral encephalitis in children and immunocompetent adults. This organism has also been associated with encephalitis in recipients of transplanted organs from a donor with unrecognized infection. No effective treatment has been identified, and mortality approaches 100%.
Encephalitis can be caused by the raccoon pinworm Baylisascaris procyonis. Clues to the diagnosis include a history of raccoon exposure, especially of playing in or eating dirt potentially contaminated with raccoon feces. Most patients are children, and many have an associated eosinophilia.
Once nonviral causes of encephalitis have been excluded, the major diagnostic challenge is to distinguish HSV from other viruses that cause encephalitis. This distinction is particularly important because in virtually every other instance the therapy is supportive, whereas specific and effective antiviral therapy is available for HSV, and its efficacy is enhanced when it is instituted early in the course of infection. HSV encephalitis should be considered when clinical features suggesting involvement of the inferomedial frontotemporal regions of the brain are present, including prominent olfactory or gustatory hallucinations, anosmia, unusual or bizarre behavior or personality alterations, or memory disturbance. HSV encephalitis should always be suspected in patients with signs and symptoms consistent with acute encephalitis with focal findings on clinical examination, neuroimaging studies, or EEG. The diagnostic procedure of choice in these patients is CSF PCR analysis for HSV. A positive CSF PCR establishes the diagnosis, and a negative test dramatically reduces the likelihood of HSV encephalitis (see above).
![]() The anatomic distribution of lesions may provide an additional clue to diagnosis. Patients with rapidly progressive encephalitis and prominent brainstem signs, symptoms, or neuroimaging abnormalities may be infected by flaviviruses (WNV, St. Louis encephalitis virus, Japanese encephalitis virus), HSV, rabies, or L. monocytogenes. Significant involvement of deep gray matter structures, including the basal ganglia and thalamus, should also suggest possible flavivirus infection. These patients may present clinically with prominent movement disorders (tremor, myoclonus) or parkinsonian features. Patients with WNV infection can also present with a poliomyelitis-like acute flaccid paralysis, as can patients infected with EV71 and, less commonly, other enteroviruses. Acute flaccid paralysis is characterized by the acute onset of a lower motor neuron type of weakness with flaccid tone, reduced or absent reflexes, and relatively preserved sensation. The complete eradication of polio remains an ongoing challenge despite a continuing World Health Organization poliovirus elimination campaign. Three hundred forty-one cases of polio (almost all due to serotype 1) have been reported in 2013 from eight countries (Somalia 183 cases, Pakistan 63, Nigeria 51, Kenya 14, Syria 13, Afghanistan 9, Ethiopia 6, and Cameroon 2). There have been small outbreaks of poliomyelitis associated with vaccine strains of virus that have reverted to virulence through mutation or recombination with circulating wild-type enteroviruses in Hispaniola, China, the Philippines, Indonesia, Nigeria, and Madagascar.
The anatomic distribution of lesions may provide an additional clue to diagnosis. Patients with rapidly progressive encephalitis and prominent brainstem signs, symptoms, or neuroimaging abnormalities may be infected by flaviviruses (WNV, St. Louis encephalitis virus, Japanese encephalitis virus), HSV, rabies, or L. monocytogenes. Significant involvement of deep gray matter structures, including the basal ganglia and thalamus, should also suggest possible flavivirus infection. These patients may present clinically with prominent movement disorders (tremor, myoclonus) or parkinsonian features. Patients with WNV infection can also present with a poliomyelitis-like acute flaccid paralysis, as can patients infected with EV71 and, less commonly, other enteroviruses. Acute flaccid paralysis is characterized by the acute onset of a lower motor neuron type of weakness with flaccid tone, reduced or absent reflexes, and relatively preserved sensation. The complete eradication of polio remains an ongoing challenge despite a continuing World Health Organization poliovirus elimination campaign. Three hundred forty-one cases of polio (almost all due to serotype 1) have been reported in 2013 from eight countries (Somalia 183 cases, Pakistan 63, Nigeria 51, Kenya 14, Syria 13, Afghanistan 9, Ethiopia 6, and Cameroon 2). There have been small outbreaks of poliomyelitis associated with vaccine strains of virus that have reverted to virulence through mutation or recombination with circulating wild-type enteroviruses in Hispaniola, China, the Philippines, Indonesia, Nigeria, and Madagascar.
Epidemiologic factors may provide important clues to the diagnosis of viral meningitis or encephalitis. Particular attention should be paid to the season of the year; the geographic location and travel history; and possible exposure to animal bites or scratches, rodents, and ticks. Although transmission from the bite of an infected dog remains the most common cause of rabies worldwide, in the United States very few cases of dog rabies occur, and the most common risk factor is exposure to bats—although a clear history of a bite or scratch is often lacking. The classic clinical presentation of encephalitic (furious) rabies is fever, fluctuating consciousness, and autonomic hyperactivity. Phobic spasms of the larynx, pharynx, neck muscles, and diaphragm can be triggered by attempts to swallow water (hydrophobia) or by inspiration (aerophobia). Patients may also present with paralytic (dumb) rabies characterized by acute ascending paralysis. Rabies due to the bite of a bat has a different clinical presentation than classic rabies due to a dog or wolf bite. Patients present with focal neurologic deficits, myoclonus, seizures, and hallucinations; phobic spasms are not a typical feature. Patients with rabies have a CSF lymphocytic pleocytosis and may show areas of increased T2 signal abnormality in the brainstem, hippocampus, and hypothalamus. Diagnosis can be made by finding rabies virus antigen in brain tissue or in the neural innervation of hair follicles at the nape of the neck. PCR amplification of viral nucleic acid from CSF and saliva or tears may also enable diagnosis. Serology is frequently negative in both serum and CSF in the first week after onset of infection, which limits its acute diagnostic utility. No specific therapy is available, and cases are almost invariably fatal, with isolated survivors having devastating neurologic sequelae.
State public health authorities provide a valuable resource concerning isolation of particular agents in individual regions. Regular updates concerning the number, type, and distribution of cases of arboviral encephalitis can be found on the CDC and U.S. Geological Survey (USGS) websites (http://www.cdc.gov and http://diseasemaps.usgs.gov).
|
TREATMENT |
VIRAL ENCEPHALITIS |
Specific antiviral therapy should be initiated when appropriate. Vital functions, including respiration and blood pressure, should be monitored continuously and supported as required. In the initial stages of encephalitis, many patients will require care in an intensive care unit. Basic management and supportive therapy should include careful monitoring of ICP, fluid restriction, avoidance of hypotonic intravenous solutions, and suppression of fever. Seizures should be treated with standard anticonvulsant regimens, and prophylactic therapy should be considered in view of the high frequency of seizures in severe cases of encephalitis. As with all seriously ill, immobilized patients with altered levels of consciousness, encephalitis patients are at risk for aspiration pneumonia, stasis ulcers and decubiti, contractures, deep venous thrombosis and its complications, and infections of indwelling lines and catheters.
Acyclovir is of benefit in the treatment of HSV and should be started empirically in patients with suspected viral encephalitis, especially if focal features are present, while awaiting viral diagnostic studies. Treatment should be discontinued in patients found not to have HSV encephalitis, with the possible exception of patients with severe encephalitis due to VZV or EBV. HSV, VZV, and EBV all encode an enzyme, deoxypyrimidine (thymidine) kinase, that phosphorylates acyclovir to produce acyclovir-5’-monophosphate. Host cell enzymes then phosphorylate this compound to form a triphosphate derivative. It is the triphosphate that acts as an antiviral agent by inhibiting viral DNA polymerase and by causing premature termination of nascent viral DNA chains. The specificity of action depends on the fact that uninfected cells do not phosphorylate significant amounts of acyclovir to acyclovir-5’-monophosphate. A second level of specificity is provided by the fact that the acyclovir triphosphate is a more potent inhibitor of viral DNA polymerase than of the analogous host cell enzymes.
Adults should receive a dose of 10 mg/kg of acyclovir intravenously every 8 h (30 mg/kg per day total dose) for 14–21 days. CSF PCR can be repeated at the completion of this course, with PCR-positive patients receiving additional treatment, followed by a repeat CSF PCR test. Neonatal HSV CNS infection is less responsive to acyclovir therapy than HSV encephalitis in adults; it is recommended that neonates with HSV encephalitis receive 20 mg/kg of acyclovir every 8 h (60 mg/kg per day total dose) for a minimum of 21 days.
Prior to intravenous administration, acyclovir should be diluted to a concentration ≤7 mg/mL. (A 70-kg person would receive a dose of 700 mg, which would be diluted in a volume of 100 mL.) Each dose should be infused slowly over 1 h, rather than by rapid or bolus infusion, to minimize the risk of renal dysfunction. Care should be taken to avoid extravasation or intramuscular or subcutaneous administration. The alkaline pH of acyclovir can cause local inflammation and phlebitis (9%). Dose adjustment is required in patients with impaired renal glomerular filtration. Penetration into CSF is excellent, with average drug levels ~50% of serum levels. Complications of therapy include elevations in blood urea nitrogen and creatinine levels (5%), thrombocytopenia (6%), gastrointestinal toxicity (nausea, vomiting, diarrhea) (7%), and neurotoxicity (lethargy or obtundation, disorientation, confusion, agitation, hallucinations, tremors, seizures) (1%). Acyclovir resistance may be mediated by changes in either the viral deoxypyrimidine kinase or DNA polymerase. To date, acyclovir-resistant isolates have not been a significant clinical problem in immunocompetent individuals. However, there have been reports of clinically virulent acyclovir-resistant HSV isolates from sites outside the CNS in immunocompromised individuals, including those with AIDS.
Oral antiviral drugs with efficacy against HSV, VZV, and EBV, including acyclovir, famciclovir, and valacyclovir, have not been evaluated in the treatment of encephalitis either as primary therapy or as supplemental therapy following completion of a course of parenteral acyclovir. A recently completed National Institute of Allergy and Infectious Disease (NIAID)/National Institute of Neurological Disorders and Stroke–sponsored phase III trial of supplemental oral valacyclovir therapy (2 g tid for 3 months) following the initial 14- to 21-day course of therapy with parenteral acyclovir (www.clinicaltrials.gov, identifier NCT00031486) was terminated early due to low enrollment. Although analysis was compromised due to low numbers, no differences were seen in the 12-month endpoints including dementia rating scale, mini-mental state exam, and Glasgow coma score in patients receiving valacyclovir versus placebo. The role of adjunctive intravenous glucocorticoids in treatment of HSV and VZV infection remains unclear, with most guidelines considering the existing supportive evidence weak and recommendation for possible use based on expert opinion only.
Ganciclovir and foscarnet, either alone or in combination, are often used in the treatment of CMV-related CNS infections, although their efficacy remains unproven. Cidofovir (see below) may provide an alternative in patients who fail to respond to ganciclovir and foscarnet, although data concerning its use in CMV CNS infections are extremely limited.
Ganciclovir is a synthetic nucleoside analogue of 2’-deoxyguanosine. The drug is preferentially phosphorylated by virus-induced cellular kinases. Ganciclovir triphosphate acts as a competitive inhibitor of the CMV DNA polymerase, and its incorporation into nascent viral DNA results in premature chain termination. Following intravenous administration, CSF concentrations of ganciclovir are 25–70% of coincident plasma levels. The usual dose for treatment of severe neurologic illnesses is 5 mg/kg every 12 h given intravenously at a constant rate over 1 h. Induction therapy is followed by maintenance therapy of 5 mg/kg every day for an indefinite period. Induction therapy should be continued until patients show a decline in CSF pleocytosis and a reduction in CSF CMV DNA copy number on quantitative PCR testing (where available). Doses should be adjusted in patients with renal insufficiency. Treatment is often limited by the development of granulocytopenia and thrombocytopenia (20–25%), which may require reduction in or discontinuation of therapy. Gastrointestinal side effects, including nausea, vomiting, diarrhea, and abdominal pain, occur in ~20% of patients. Some patients treated with ganciclovir for CMV retinitis have developed retinal detachment, but the causal relationship to ganciclovir treatment is unclear. Valganciclovir is an orally bioavailable prodrug that can generate high serum levels of ganciclovir, although studies of its efficacy in treating CMV CNS infections are limited.
Foscarnet is a pyrophosphate analogue that inhibits viral DNA polymerases by binding to the pyrophosphate-binding site. Following intravenous infusion, CSF concentrations range from 15 to 100% of coincident plasma levels. The usual dose for serious CMV-related neurologic illness is 60 mg/kg every 8 h administered by constant infusion over 1 h. Induction therapy for 14–21 days is followed by maintenance therapy (60–120 mg/kg per day). Induction therapy may need to be extended in patients who fail to show a decline in CSF pleocytosis and a reduction in CSF CMV DNA copy number on quantitative PCR tests (where available). Approximately one-third of patients develop renal impairment during treatment, which is reversible following discontinuation of therapy in most, but not all, cases. This is often associated with elevations in serum creatinine and proteinuria and is less frequent in patients who are adequately hydrated. Many patients experience fatigue and nausea. Reductions in serum calcium, magnesium, and potassium occur in ~15% of patients and may be associated with tetany, cardiac rhythm disturbances, or seizures.
Cidofovir is a nucleotide analogue that is effective in treating CMV retinitis and equivalent to or better than ganciclovir in some experimental models of murine CMV encephalitis, although data concerning its efficacy in human CMV CNS disease are limited. The usual dose is 5 mg/kg intravenously once weekly for 2 weeks, then biweekly for two or more additional doses, depending on clinical response. Patients must be prehydrated with normal saline (e.g., 1 L over 1–2 h) prior to each dose and treated with probenecid (e.g., 1 g 3 h before cidofovir and 1 g 2 and 8 h after cidofovir). Nephrotoxicity is common; the dose should be reduced if renal function deteriorates.
Intravenous ribavirin (15–25 mg/kg per day in divided doses given every 8 h) has been reported to be of benefit in isolated cases of severe encephalitis due to California encephalitis (La Crosse) virus. Ribavirin might be of benefit for the rare patients, typically infants or young children, with severe adenovirus or rotavirus encephalitis and in patients with encephalitis due to LCMV or other arenaviruses. However, clinical trials are lacking. Hemolysis, with resulting anemia, has been the major side effect limiting therapy.
No specific antiviral therapy of proven efficacy is currently available for treatment of WNV encephalitis. Patients have been treated with a-interferon, ribavirin, an Israeli IVIg preparation that contains high-titer anti-WNV antibody (Omr-IgG-am) (www.clinicaltrials.gov, identifier NCT00069316 and 0068055), and humanized monoclonal antibodies directed against the viral envelope glycoprotein (www.clinicaltrials.gov, identifier NCT00927953 and 00515385). WNV chimeric vaccines, in which WNV envelope and premembrane proteins are inserted into the background of another flavivirus, are already undergoing human clinical testing and have been found to be both safe and immunogenic in healthy adults but have not yet been tested for disease prevention in humans (www.clinicaltrials.gov, identifier NCT00746798, 00442169, 00094718, and 00537147). Both chimeric and killed inactivated WNV vaccines have been found to be safe and effective in preventing equine WNV infection, and several effective flavivirus vaccines are already in human use, creating optimism that a safe and effective human WNV vaccine can also be developed.
SEQUELAE
There is considerable variation in the incidence and severity of sequelae in patients surviving viral encephalitis. In the case of EEE virus infection, nearly 80% of survivors have severe neurologic sequelae. At the other extreme are infections due to EBV, California encephalitis virus, and Venezuelan equine encephalitis virus, where severe sequelae are unusual. For example, approximately 5–15% of children infected with La Crosse virus have a residual seizure disorder, and 1% have persistent hemiparesis. Detailed information about sequelae in patients with HSV encephalitis treated with acyclovir is available from the NIAID-Collaborative Antiviral Study Group (CASG) trials. Of 32 acyclovir-treated patients, 26 survived (81%). Of the 26 survivors, 12 (46%) had no or only minor sequelae, 3 (12%) were moderately impaired (gainfully employed but not functioning at their previous level), and 11 (42%) were severely impaired (requiring continuous supportive care). The incidence and severity of sequelae were directly related to the age of the patient and the level of consciousness at the time of initiation of therapy. Patients with severe neurologic impairment (Glasgow coma score 6) at initiation of therapy either died or survived with severe sequelae. Young patients (<30 years) with good neurologic function at initiation of therapy did substantially better (100% survival, 62% with no or mild sequelae) compared with their older counterparts (>30 years; 64% survival, 57% no or mild sequelae). Some recent studies using quantitative HSV CSF PCR tests indicate that clinical outcome following treatment also correlates with the amount of HSV DNA present in CSF at the time of presentation. Many patients with WNV infection have sequelae, including cognitive impairment; weakness; and hyper- or hypokinetic movement disorders, including tremor, myoclonus, and parkinsonism. In a large longitudinal study of prognosis in 156 patients with WNV infection, the mean time to achieve recovery (defined as 95% of maximal predicted score on specific validated tests) was 112–148 days for fatigue, 121–175 days for physical function, 131–139 days for mood, and 302–455 days for mental function (the longer interval in each case representing patients with neuroinvasive disease).
SUBACUTE MENINGITIS
CLINICAL MANIFESTATIONS
Patients with subacute meningitis typically have an unrelenting headache, stiff neck, low-grade fever, and lethargy for days to several weeks before they present for evaluation. Cranial nerve abnormalities and night sweats may be present. This syndrome overlaps that of chronic meningitis, discussed in detail in Chap. 165.
ETIOLOGY
Common causative organisms include M. tuberculosis, C. neoformans, H. capsulatum, C. immitis, and T. pallidum. Initial infection with M. tuberculosis is acquired by inhalation of aerosolized droplet nuclei. Tuberculous meningitis in adults does not develop acutely from hematogenous spread of tubercle bacilli to the meninges. Rather, millet seed–sized (miliary) tubercles form in the parenchyma of the brain during hematogenous dissemination of tubercle bacilli in the course of primary infection. These tubercles enlarge and are usually caseating. The propensity for a caseous lesion to produce meningitis is determined by its proximity to the subarachnoid space (SAS) and the rate at which fibrous encapsulation develops. Subependymal caseous foci cause meningitis via discharge of bacilli and tuberculous antigens into the SAS. Mycobacterial antigens produce an intense inflammatory reaction that leads to the production of a thick exudate that fills the basilar cisterns and surrounds the cranial nerves and major blood vessels at the base of the brain.
![]() Fungal infections are typically acquired by the inhalation of airborne fungal spores. The initial pulmonary infection may be asymptomatic or present with fever, cough, sputum production, and chest pain. The pulmonary infection is often self-limited. A localized pulmonary fungal infection can then remain dormant in the lungs until there is an abnormality in cell-mediated immunity that allows the fungus to reactivate and disseminate to the CNS. The most common pathogen causing fungal meningitis is C. neoformans. This fungus is found worldwide in soil and bird excreta. H. capsulatum is endemic to the Ohio and Mississippi River valleys of the central United States and to parts of Central and South America. C. immitis is endemic to the desert areas of the southwest United States, northern Mexico, and Argentina.
Fungal infections are typically acquired by the inhalation of airborne fungal spores. The initial pulmonary infection may be asymptomatic or present with fever, cough, sputum production, and chest pain. The pulmonary infection is often self-limited. A localized pulmonary fungal infection can then remain dormant in the lungs until there is an abnormality in cell-mediated immunity that allows the fungus to reactivate and disseminate to the CNS. The most common pathogen causing fungal meningitis is C. neoformans. This fungus is found worldwide in soil and bird excreta. H. capsulatum is endemic to the Ohio and Mississippi River valleys of the central United States and to parts of Central and South America. C. immitis is endemic to the desert areas of the southwest United States, northern Mexico, and Argentina.
Syphilis is a sexually transmitted disease that is manifest by the appearance of a painless chancre at the site of inoculation. T. pallidum invades the CNS early in the course of syphilis. Cranial nerves VII and VIII are most frequently involved.
LABORATORY DIAGNOSIS
The classic CSF abnormalities in tuberculous meningitis are as follows: (1) elevated opening pressure, (2) lymphocytic pleocytosis (10–500 cells/μL), (3) elevated protein concentration in the range of 1–5 g/L, and (4) decreased glucose concentration in the range of 1.1–2.2 mmol/L (20–40 mg/dL). The combination of unrelenting headache, stiff neck, fatigue, night sweats, and fever with a CSF lymphocytic pleocytosis and a mildly decreased glucose concentration is highly suspicious for tuberculous meningitis. The last tube of fluid collected at LP is the best tube to send for a smear for acid-fast bacilli (AFB). If there is a pellicle in the CSF or a cobweb-like clot on the surface of the fluid, AFB can best be demonstrated in a smear of the clot or pellicle. Positive smears are typically reported in only 10–40% of cases of tuberculous meningitis in adults. Cultures of CSF take 4–8 weeks to identify the organism and are positive in ~50% of adults. Culture remains the gold standard to make the diagnosis of tuberculous meningitis. PCR for the detection of M. tuberculosis DNA should be sent on CSF if available, but the sensitivity and specificity on CSF have not been defined. The CDC recommends the use of nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis.
The characteristic CSF abnormalities in fungal meningitis are a mononuclear or lymphocytic pleocytosis, an increased protein concentration, and a decreased glucose concentration. There may be eosinophils in the CSF in C. immitis meningitis. Large volumes of CSF are often required to demonstrate the organism on India ink smear or grow the organism in culture. If spinal fluid examined by LP on two separate occasions fails to yield an organism, CSF should be obtained by high-cervical or cisternal puncture.
The cryptococcal polysaccharide antigen test is a highly sensitive and specific test for cryptococcal meningitis. A reactive CSF cryptococcal antigen test establishes the diagnosis. The detection of the Histoplasma polysaccharide antigen in CSF establishes the diagnosis of a fungal meningitis but is not specific for meningitis due to H. capsulatum. It may be falsely positive in coccidioidal meningitis. The CSF complement fixation antibody test is reported to have a specificity of 100% and a sensitivity of 75% for coccidioidal meningitis.
The diagnosis of syphilitic meningitis is made when a reactive serum treponemal test (fluorescent treponemal antibody absorption test [FTA-ABS] or microhemagglutination assay–T. pallidum [MHA-TP]) is associated with a CSF lymphocytic or mononuclear pleocytosis and an elevated protein concentration, or when the CSF Venereal Disease Research Laboratory (VDRL) test is positive. A reactive CSF FTA-ABS is not definitive evidence of neurosyphilis. The CSF FTA-ABS can be falsely positive from blood contamination. A negative CSF VDRL does not rule out neurosyphilis. A negative CSF FTA-ABS or MHA-TP rules out neurosyphilis.
|
TREATMENT |
SUBACUTE MENINGITIS |
Empirical therapy of tuberculous meningitis is often initiated on the basis of a high index of suspicion without adequate laboratory support. Initial therapy is a combination of isoniazid (300 mg/d), rifampin (10 mg/kg per day), pyrazinamide (30 mg/kg per day in divided doses), ethambutol (15–25 mg/kg per day in divided doses), and pyridoxine (50 mg/d). When the antimicrobial sensitivity of the M. tuberculosis isolate is known, ethambutol can be discontinued. If the clinical response is good, pyrazinamide can be discontinued after 8 weeks and isoniazid and rifampin continued alone for the next 6–12 months. A 6-month course of therapy is acceptable, but therapy should be prolonged for 9–12 months in patients who have an inadequate resolution of symptoms of meningitis or who have positive mycobacterial cultures of CSF during the course of therapy. Dexamethasone therapy is recommended for HIV-negative patients with tuberculous meningitis. The dose is 12–16 mg/d for 3 weeks, and then tapered over 3 weeks.
Meningitis due to C. neoformans in non-HIV, nontransplant patients is treated with induction therapy with amphotericin B (AmB) (0.7 mg/kg IV per day) plus flucytosine (100 mg/kg per day in four divided doses) for at least 4 weeks if CSF culture results are negative after 2 weeks of treatment. Therapy should be extended for a total of 6 weeks in the patient with neurologic complications. Induction therapy is followed by consolidation therapy with fluconazole 400 mg/d for 8 weeks. Organ transplant recipients are treated with liposomal AmB (3–4 mg/kg per day) or AmB lipid complex (ABLC) 5 mg/kg per day plus flucytosine (100 mg/kg per day in four divided doses) for at least 2 weeks or until CSF culture is sterile. Follow CSF yeast cultures for sterilization rather than the cryptococcal antigen titer. This treatment is followed by an 8- to 10-week course of fluconazole (400–800 mg/d [6–12 mg/kg] PO). If the CSF culture is sterile after 10 weeks of acute therapy, the dose of fluconazole is decreased to 200 mg/d for 6 months to a year. Patients with HIV infection are treated with AmB or a lipid formulation plus flucytosine for at least 2 weeks, followed by fluconazole for a minimum of 8 weeks. HIV-infected patients may require indefinite maintenance therapy with fluconazole 200 mg/d. Meningitis due to H. capsulatum is treated with AmB (0.7–1.0 mg/kg per day) for 4–12 weeks. A total dose of 30 mg/kg is recommended. Therapy with AmB is not discontinued until fungal cultures are sterile. After completing a course of AmB, maintenance therapy with itraconazole 200 mg two or three times daily is initiated and continued for at least 9 months to a year. C. immitis meningitis is treated with either high-dose fluconazole (1000 mg daily) as monotherapy or intravenous AmB (0.5–0.7 mg/kg per day) for >4 weeks. Intrathecal AmB (0.25–0.75 mg/d three times weekly) may be required to eradicate the infection. Lifelong therapy with fluconazole (200–400 mg daily) is recommended to prevent relapse. AmBisome (5 mg/kg per day) or AmB lipid complex (5 mg/kg per day) can be substituted for AmB in patients who have or who develop significant renal dysfunction. The most common complication of fungal meningitis is hydrocephalus. Patients who develop hydrocephalus should receive a CSF diversion device. A ventriculostomy can be used until CSF fungal cultures are sterile, at which time the ventriculostomy is replaced by a ventriculoperitoneal shunt.
Syphilitic meningitis is treated with aqueous penicillin G in a dose of 3–4 million units intravenously every 4 h for 10–14 days. An alternative regimen is 2.4 million units of procaine penicillin G intramuscularly daily with 500 mg of oral probenecid four times daily for 10–14 days. Either regimen is followed with 2.4 million units of benzathine penicillin G intramuscularly once a week for 3 weeks. The standard criterion for treatment success is reexamination of the CSF. The CSF should be reexamined at 6-month intervals for 2 years. The cell count is expected to normalize within 12 months, and the VDRL titer to decrease by two dilutions or revert to nonreactive within 2 years of completion of therapy. Failure of the CSF pleocytosis to resolve or an increase in the CSF VDRL titer by two or more dilutions requires retreatment.
CHRONIC ENCEPHALITIS
PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
Clinical Features and Pathology Progressive multifocal leukoencephalopathy (PML) is characterized pathologically by multifocal areas of demyelination of varying size distributed throughout the brain but sparing the spinal cord and optic nerves. In addition to demyelination, there are characteristic cytologic alterations in both astrocytes and oligodendrocytes. Astrocytes are enlarged and contain hyperchromatic, deformed, and bizarre nuclei and frequent mitotic figures. Oligodendrocytes have enlarged, densely staining nuclei that contain viral inclusions formed by crystalline arrays of JC virus (JCV) particles. Patients often present with visual deficits (45%), typically a homonymous hemianopia; mental impairment (38%) (dementia, confusion, personality change); weakness, including hemi- or monoparesis; and ataxia. Seizures occur in ~20% of patients, predominantly in those with lesions abutting the cortex.
Almost all patients have an underlying immunosuppressive disorder or are receiving immunomodulatory therapy. In recent series, the most common associated conditions were AIDS (80%), hematologic malignancies (13%), transplant recipients (5%), and chronic inflammatory diseases (2%). It has been estimated that up to 5% of AIDS patients will develop PML. There have been over 400 reported cases of PML occurring in patients being treated for multiple sclerosis and inflammatory bowel disease with natalizumab, a humanized monoclonal antibody that inhibits lymphocyte trafficking into CNS and bowel mucosa by binding to α4 integrins. Overall risk in these patients has been estimated at ~3.4 PML cases per 1000 treated patients, but the risk depends on a variety of factors including anti-JCV antibody serostatus, prior immunosuppressive therapy use, and duration of natalizumab therapy. Patients who lack detectable JCV antibody have a risk of developing PML of <0.1 case/1000 patients, whereas those who are JCV seropositive and have been exposed to prior immunosuppressive therapy and have received >24 months of natalizumab therapy have a risk of >1 case/100 treated patients. PML cases have also been reported in patients receiving other humanized monoclonal antibodies with immunomodulatory activity including efalizumab and rituximab, although the relative risks have not been clearly established. The basic clinical and diagnostic features appear to be similar in HIV-associated PML and PML associated with immunomodulatory drugs with the exception of an increased likelihood of peripheral enhancement in MRIs of PML lesions in immunomodulatory cases. In natalizumab-associated PML, patients will also almost invariably develop clinical and radiographic worsening of lesions with discontinuation of therapy, attributed to development of immune reconstitution inflammatory syndrome (IRIS).
Diagnostic Studies The diagnosis of PML is frequently suggested by MRI. MRI reveals multifocal asymmetric, coalescing white matter lesions located periventricularly, in the centrum semiovale, in the parietal-occipital region, and in the cerebellum. These lesions have increased signal on T2 and FLAIR images and decreased signal on T1-weighted images. HIV-PML lesions are classically nonenhancing (90%), but patients with immunomodulatory drug associated PML may have peripheral ring enhancement. PML lesions are not typically associated with edema or mass effect. CT scans, which are less sensitive than MRI for the diagnosis of PML, often show hypodense nonenhancing white matter lesions.
The CSF is typically normal, although mild elevation in protein and/or IgG may be found. Pleocytosis occurs in <25% of cases, is predominantly mononuclear, and rarely exceeds 25 cells/μL. PCR amplification of JCV DNA from CSF has become an important diagnostic tool. The presence of a positive CSF PCR for JCV DNA in association with typical MRI lesions in the appropriate clinical setting is diagnostic of PML, reflecting the assay’s relatively high specificity (92–100%); however, sensitivity is variable, and a negative CSF PCR does not exclude the diagnosis. In HIV-negative patients and HIV-positive patients not receiving highly active antiviral therapy (HAART), sensitivity is likely 70–90%. In HAART-treated patients, sensitivity may be closer to 60%, reflecting the lower JCV CSF viral load in this relatively more immunocompetent group. Studies with quantitative JCV CSF PCR indicate that patients with low JCV loads (<100 copies/μL) have a generally better prognosis than those with higher viral loads. Patients with negative CSF PCR studies may require brain biopsy for definitive diagnosis. In biopsy or necropsy specimens of brain, JCV antigen and nucleic acid can be detected by immunocytochemistry, in situ hybridization, or PCR amplification.
Serologic studies are of no utility in diagnosis due to high basal seroprevalence level, but may contribute to risk stratification in patients contemplating therapy with immunomodulatory drugs such as natalizumab.
|
TREATMENT |
PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY |
No effective therapy for PML is available. There are case reports of potential beneficial effects of the 5-HT2a receptor antagonist mirtazapine, which may inhibit binding of JCV to its receptor on oligodendrocytes. Retrospective noncontrolled studies have also suggested a possible beneficial effect of treatment with interferon-α. Neither of these agents has been tested in randomized controlled clinical trials. A prospective multicenter clinical trial to evaluate the efficacy of the antimalarial drug mefloquine failed to show benefit. Intravenous and/or intrathecal cytarabine were not shown to be of benefit in a randomized controlled trial in HIV-associated PML, although some experts suggest that cytarabine may have therapeutic efficacy in situations where breakdown of the blood-brain barrier allows sufficient CSF penetration. A randomized controlled trial of cidofovir in HIV-associated PML also failed to show significant benefit. Because PML almost invariably occurs in immunocompromised individuals, any therapeutic interventions designed to enhance or restore immunocompetence should be considered. Perhaps the most dramatic demonstration of this is disease stabilization and, in rare cases, improvement associated with the improvement in the immune status of HIV-positive patients with AIDS following institution of HAART. In HIV-positive PML patients treated with HAART, 1-year survival is ~50%, although up to 80% of survivors may have significant neurologic sequelae. HIV-positive PML patients with higher CD4 counts (>300/μL) and low or nondetectable HIV viral loads have a better prognosis than those with lower CD4 counts and higher viral loads. Although institution of HAART enhances survival in HIV-positive PML patients, the associated immune reconstitution in patients with an underlying opportunistic infection such as PML may also result in a severe CNS inflammatory syndrome (IRIS) associated with clinical worsening, CSF pleocytosis, and the appearance of new enhancing MRI lesions. Patients receiving natalizumab or other immunomodulatory antibodies, who are suspected of having PML, should have therapy immediately halted and circulating antibodies removed by plasma exchange. Patients should be closely monitored for development of IRIS, which is generally treated with intravenous glucocorticoids, although controlled clinical trials of efficacy remain lacking.
SUBACUTE SCLEROSING PANENCEPHALITIS (SSPE)
SSPE is a rare chronic, progressive demyelinating disease of the CNS associated with a chronic nonpermissive infection of brain tissue with measles virus. The frequency has been estimated at 1 in 100,000–500,000 measles cases. An average of five cases per year are reported in the United States. The incidence has declined dramatically since the introduction of a measles vaccine. Most patients give a history of primary measles infection at an early age (2 years), which is followed after a latent interval of 6–8 years by the development of a progressive neurologic disorder. Some 85% of patients are between 5 and 15 years old at diagnosis. Initial manifestations include poor school performance and mood and personality changes. Typical signs of a CNS viral infection, including fever and headache, do not occur. As the disease progresses, patients develop progressive intellectual deterioration, focal and/or generalized seizures, myoclonus, ataxia, and visual disturbances. In the late stage of the illness, patients are unresponsive, quadriparetic, and spastic, with hyperactive tendon reflexes and extensor plantar responses.
Diagnostic Studies MRI is often normal early, although areas of increased T2 signal develop in the white matter of the brain and brainstem as disease progresses. The EEG may initially show only nonspecific slowing, but with disease progression, patients develop a characteristic periodic pattern with bursts of high-voltage, sharp, slow waves every 3–8 s, followed by periods of attenuated (“flat”) background. The CSF is acellular with a normal or mildly elevated protein concentration and a markedly elevated gamma globulin level (>20% of total CSF protein). CSF antimeasles antibody levels are invariably elevated, and oligoclonal antimeasles antibodies are often present. Measles virus can be cultured from brain tissue using special cocultivation techniques. Viral antigen can be identified immunocytochemically, and viral genome can be detected by in situ hybridization or PCR amplification.
|
TREATMENT |
SUBACUTE SCLEROSING PANENCEPHALITIS |
No definitive therapy for SSPE is available. Treatment with isoprinosine (Inosiplex, 100 mg/kg per day), alone or in combination with intrathecal or intraventricular interferon-α, has been reported to prolong survival and produce clinical improvement in some patients but has never been subjected to a controlled clinical trial.
PROGRESSIVE RUBELLA PANENCEPHALITIS
This is an extremely rare disorder that primarily affects males with congenital rubella syndrome, although isolated cases have been reported following childhood rubella. After a latent period of 8–19 years, patients develop progressive neurologic deterioration. The manifestations are similar to those seen in SSPE. CSF shows a mild lymphocytic pleocytosis, slightly elevated protein concentration, markedly increased gamma globulin, and rubella virus–specific oligoclonal bands. No therapy is available. Universal prevention of both congenital and childhood rubella through the use of the available live attenuated rubella vaccine would be expected to eliminate the disease.
BRAIN ABSCESS
DEFINITION
A brain abscess is a focal, suppurative infection within the brain parenchyma, typically surrounded by a vascularized capsule. The term cerebritis is often employed to describe a nonencapsulated brain abscess.
EPIDEMIOLOGY
![]() A bacterial brain abscess is a relatively uncommon intracranial infection, with an incidence of ~0.3–1.3:100,000 persons per year. Predisposing conditions include otitis media and mastoiditis, paranasal sinusitis, pyogenic infections in the chest or other body sites, penetrating head trauma or neurosurgical procedures, and dental infections. In immunocompetent individuals the most important pathogens are Streptococcus spp. (anaerobic, aerobic, and viridans [40%]), Enterobacteriaceae (Proteus spp., E. coli sp., Klebsiella spp. [25%]), anaerobes (e.g., Bacteroides spp., Fusobacterium spp. [30%]), and staphylococci (10%). In immunocompromised hosts with underlying HIV infection, organ transplantation, cancer, or immunosuppressive therapy, most brain abscesses are caused by Nocardia spp., Toxoplasma gondii, Aspergillus spp., Candida spp., and C. neoformans. In Latin America and in immigrants from Latin America, the most common cause of brain abscess is Taenia solium (neurocysticercosis). In India and East Asia, mycobacterial infection (tuberculoma) remains a major cause of focal CNS mass lesions.
A bacterial brain abscess is a relatively uncommon intracranial infection, with an incidence of ~0.3–1.3:100,000 persons per year. Predisposing conditions include otitis media and mastoiditis, paranasal sinusitis, pyogenic infections in the chest or other body sites, penetrating head trauma or neurosurgical procedures, and dental infections. In immunocompetent individuals the most important pathogens are Streptococcus spp. (anaerobic, aerobic, and viridans [40%]), Enterobacteriaceae (Proteus spp., E. coli sp., Klebsiella spp. [25%]), anaerobes (e.g., Bacteroides spp., Fusobacterium spp. [30%]), and staphylococci (10%). In immunocompromised hosts with underlying HIV infection, organ transplantation, cancer, or immunosuppressive therapy, most brain abscesses are caused by Nocardia spp., Toxoplasma gondii, Aspergillus spp., Candida spp., and C. neoformans. In Latin America and in immigrants from Latin America, the most common cause of brain abscess is Taenia solium (neurocysticercosis). In India and East Asia, mycobacterial infection (tuberculoma) remains a major cause of focal CNS mass lesions.
ETIOLOGY
A brain abscess may develop (1) by direct spread from a contiguous cranial site of infection, such as paranasal sinusitis, otitis media, mastoiditis, or dental infection; (2) following head trauma or a neurosurgical procedure; or (3) as a result of hematogenous spread from a remote site of infection. In up to 25% of cases, no obvious primary source of infection is apparent (cryptogenic brain abscess).
Approximately one-third of brain abscesses are associated with otitis media and mastoiditis, often with an associated cholesteatoma. Otogenic abscesses occur predominantly in the temporal lobe (55–75%) and cerebellum (20–30%). In some series, up to 90% of cerebellar abscesses are otogenic. Common organisms include streptococci, Bacteroides spp., Pseudomonas spp., Haemophilus spp., and Enterobacteriaceae. Abscesses that develop as a result of direct spread of infection from the frontal, ethmoidal, or sphenoidal sinuses and those that occur due to dental infections are usually located in the frontal lobes. Approximately 10% of brain abscesses are associated with paranasal sinusitis, and this association is particularly strong in young males in their second and third decades of life. The most common pathogens in brain abscesses associated with paranasal sinusitis are streptococci (especially Streptococcus milleri), Haemophilus spp., Bacteroides spp., Pseudomonas spp., and S. aureus. Dental infections are associated with ~2% of brain abscesses, although it is often suggested that many “cryptogenic” abscesses are in fact due to dental infections. The most common pathogens in this setting are streptococci, staphylococci, Bacteroides spp., and Fusobacterium spp.
Hematogenous abscesses account for ~25% of brain abscesses. Hematogenous abscesses are often multiple, and multiple abscesses often (50%) have a hematogenous origin. These abscesses show a predilection for the territory of the middle cerebral artery (i.e., posterior frontal or parietal lobes). Hematogenous abscesses are often located at the junction of the gray and white matter and are often poorly encapsulated. The microbiology of hematogenous abscesses is dependent on the primary source of infection. For example, brain abscesses that develop as a complication of infective endocarditis are often due to viridans streptococci or S. aureus. Abscesses associated with pyogenic lung infections such as lung abscess or bronchiectasis are often due to streptococci, staphylococci, Bacteroides spp., Fusobacterium spp., or Enterobacteriaceae. Abscesses that follow penetrating head trauma or neurosurgical procedures are frequently due to methicillin-resistant S. aureus (MRSA), S. epidermidis, Enterobacteriaceae, Pseudomonas spp., and Clostridium spp. Enterobacteriaceae and P. aeruginosa are important causes of abscesses associated with urinary sepsis. Congenital cardiac malformations that produce a right-to-left shunt, such as tetralogy of Fallot, patent ductus arteriosus, and atrial and ventricular septal defects, allow bloodborne bacteria to bypass the pulmonary capillary bed and reach the brain. Similar phenomena can occur with pulmonary arteriovenous malformations. The decreased arterial oxygenation and saturation from the right-to-left shunt and polycythemia may cause focal areas of cerebral ischemia, thus providing a nidus for microorganisms that bypassed the pulmonary circulation to multiply and form an abscess. Streptococci are the most common pathogens in this setting.
PATHOGENESIS AND HISTOPATHOLOGY
Results of experimental models of brain abscess formation suggest that for bacterial invasion of brain parenchyma to occur, there must be preexisting or concomitant areas of ischemia, necrosis, or hypoxemia in brain tissue. The intact brain parenchyma is relatively resistant to infection. Once bacteria have established infection, brain abscess frequently evolves through a series of stages, influenced by the nature of the infecting organism and by the immunocompetence of the host. The early cerebritis stage (days 1–3) is characterized by a perivascular infiltration of inflammatory cells, which surround a central core of coagulative necrosis. Marked edema surrounds the lesion at this stage. In the late cerebritis stage (days 4–9), pus formation leads to enlargement of the necrotic center, which is surrounded at its border by an inflammatory infiltrate of macrophages and fibroblasts. A thin capsule of fibroblasts and reticular fibers gradually develops, and the surrounding area of cerebral edema becomes more distinct than in the previous stage. The third stage, early capsule formation (days 10–13), is characterized by the formation of a capsule that is better developed on the cortical than on the ventricular side of the lesion. This stage correlates with the appearance of a ring-enhancing capsule on neuroimaging studies. The final stage, late capsule formation (day 14 and beyond), is defined by a well-formed necrotic center surrounded by a dense collagenous capsule. The surrounding area of cerebral edema has regressed, but marked gliosis with large numbers of reactive astrocytes has developed outside the capsule. This gliotic process may contribute to the development of seizures as a sequela of brain abscess.
CLINICAL PRESENTATION
A brain abscess typically presents as an expanding intracranial mass lesion rather than as an infectious process. Although the evolution of signs and symptoms is extremely variable, ranging from hours to weeks or even months, most patients present to the hospital 11–12 days following onset of symptoms. The classic clinical triad of headache, fever, and a focal neurologic deficit is present in <50% of cases. The most common symptom in patients with a brain abscess is headache, occurring in >75% of patients. The headache is often characterized as a constant, dull, aching sensation, either hemicranial or generalized, and it becomes progressively more severe and refractory to therapy. Fever is present in only 50% of patients at the time of diagnosis, and its absence should not exclude the diagnosis. The new onset of focal or generalized seizure activity is a presenting sign in 15–35% of patients. Focal neurologic deficits including hemiparesis, aphasia, or visual field defects are part of the initial presentation in >60% of patients.
The clinical presentation of a brain abscess depends on its location, the nature of the primary infection if present, and the level of the ICP. Hemiparesis is the most common localizing sign of a frontal lobe abscess. A temporal lobe abscess may present with a disturbance of language (dysphasia) or an upper homonymous quadrantanopia. Nystagmus and ataxia are signs of a cerebellar abscess. Signs of raised ICP—papilledema, nausea and vomiting, and drowsiness or confusion—can be the dominant presentation of some abscesses, particularly those in the cerebellum. Meningismus is not present unless the abscess has ruptured into the ventricle or the infection has spread to the subarachnoid space.
DIAGNOSIS
Diagnosis is made by neuroimaging studies. MRI (Fig. 164-4) is better than CT for demonstrating abscesses in the early (cerebritis) stages and is superior to CT for identifying abscesses in the posterior fossa. Cerebritis appears on MRI as an area of low-signal intensity on T1-weighted images with irregular postgadolinium enhancement and as an area of increased signal intensity on T2-weighted images. Cerebritis is often not visualized by CT scan, but when present, appears as an area of hypodensity. On a contrast-enhanced CT scan, a mature brain abscess appears as a focal area of hypodensity surrounded by ring enhancement with surrounding edema (hypodensity). On contrast-enhanced T1-weighted MRI, a mature brain abscess has a capsule that enhances surrounding a hypodense center and surrounded by a hypodense area of edema. On T2-weighted MRI, there is a hyperintense central area of pus surrounded by a well-defined hypointense capsule and a hyperintense surrounding area of edema. It is important to recognize that the CT and MRI appearance, particularly of the capsule, may be altered by treatment with glucocorticoids. The distinction between a brain abscess and other focal CNS lesions such as primary or metastatic tumors may be facilitated by the use of diffusion-weighted imaging sequences on which a brain abscess typically shows increased signal due to restricted diffusion of the abscess cavity with corresponding low signal on apparent diffusion coefficient images.
FIGURE 164-4 Pneumococcal brain abscess. Note that the abscess wall has hyperintense signal on the axial T1-weighted magnetic resonance imaging (MRI) (A, black arrow), hypointense signal on the axial proton density images (B, black arrow), and enhances prominently after gadolinium administration on the coronal T1-weighted image (C). The abscess is surrounded by a large amount of vasogenic edema and has a small “daughter” abscess (C, white arrow). (Courtesy of Joseph Lurito, MD; with permission.)
Microbiologic diagnosis of the etiologic agent is most accurately determined by Gram’s stain and culture of abscess material obtained by CT-guided stereotactic needle aspiration. Aerobic and anaerobic bacterial cultures and mycobacterial and fungal cultures should be obtained. Up to 10% of patients will also have positive blood cultures. LP should not be performed in patients with known or suspected focal intracranial infections such as abscess or empyema; CSF analysis contributes nothing to diagnosis or therapy, and LP increases the risk of herniation.
Additional laboratory studies may provide clues to the diagnosis of brain abscess in patients with a CNS mass lesion. About 50% of patients have a peripheral leukocytosis, 60% an elevated ESR, and 80% an elevated C-reactive protein. Blood cultures are positive in ~10% of cases overall but may be positive in >85% of patients with abscesses due to Listeria.
DIFFERENTIAL DIAGNOSIS
Conditions that can cause headache, fever, focal neurologic signs, and seizure activity include brain abscess, subdural empyema, bacterial meningitis, viral meningoencephalitis, superior sagittal sinus thrombosis, and acute disseminated encephalomyelitis. When fever is absent, primary and metastatic brain tumors become the major differential diagnosis. Less commonly, cerebral infarction or hematoma can have an MRI or CT appearance resembling brain abscess.
|
TREATMENT |
BRAIN ABSCESS |
Optimal therapy of brain abscesses involves a combination of high-dose parenteral antibiotics and neurosurgical drainage. Empirical therapy of community-acquired brain abscess in an immunocompetent patient typically includes a third- or fourth-generation cephalosporin (e.g., cefotaxime, ceftriaxone, or cefepime) and metronidazole (see Table 164-1 for antibiotic dosages). In patients with penetrating head trauma or recent neurosurgical procedures, treatment should include ceftazidime as the third-generation cephalosporin to enhance coverage of Pseudomonas spp. and vancomycin for coverage of staphylococci. Meropenem plus vancomycin also provides good coverage in this setting.
Aspiration and drainage of the abscess under stereotactic guidance are beneficial for both diagnosis and therapy. Empirical antibiotic coverage should be modified based on the results of Gram’s stain and culture of the abscess contents. Complete excision of a bacterial abscess via craniotomy or craniectomy is generally reserved for multiloculated abscesses or those in which stereotactic aspiration is unsuccessful.
Medical therapy alone is not optimal for treatment of brain abscess and should be reserved for patients whose abscesses are neurosurgically inaccessible, for patients with small (<2–3 cm) or nonencapsulated abscesses (cerebritis), and for patients whose condition is too tenuous to allow performance of a neurosurgical procedure. All patients should receive a minimum of 6–8 weeks of parenteral antibiotic therapy. The role, if any, of supplemental oral antibiotic therapy following completion of a standard course of parenteral therapy has never been adequately studied.
In addition to surgical drainage and antibiotic therapy, patients should receive prophylactic anticonvulsant therapy because of the high risk (~35%) of focal or generalized seizures. Anticonvulsant therapy is continued for at least 3 months after resolution of the abscess, and decisions regarding withdrawal are then based on the EEG. If the EEG is abnormal, anticonvulsant therapy should be continued. If the EEG is normal, anticonvulsant therapy can be slowly withdrawn, with close follow-up and repeat EEG after the medication has been discontinued.
Glucocorticoids should not be given routinely to patients with brain abscesses. Intravenous dexamethasone therapy (10 mg every 6 h) is usually reserved for patients with substantial periabscess edema and associated mass effect and increased ICP. Dexamethasone should be tapered as rapidly as possible to avoid delaying the natural process of encapsulation of the abscess.
Serial MRI or CT scans should be obtained on a monthly or twice-monthly basis to document resolution of the abscess. More frequent studies (e.g., weekly) are probably warranted in the subset of patients who are receiving antibiotic therapy alone. A small amount of enhancement may remain for months after the abscess has been successfully treated.
PROGNOSIS
The mortality rate of brain abscess has declined in parallel with the development of enhanced neuroimaging techniques, improved neurosurgical procedures for stereotactic aspiration, and improved antibiotics. In modern series, the mortality rate is typically <15%. Significant sequelae, including seizures, persisting weakness, aphasia, or mental impairment, occur in ≥20% of survivors.
NONBACTERIAL CAUSES OF INFECTIOUS FOCAL CNS LESIONS
ETIOLOGY
Neurocysticercosis is the most common parasitic disease of the CNS worldwide. Humans acquire cysticercosis by the ingestion of food contaminated with the eggs of the parasite T. solium. Toxoplasmosis is a parasitic disease caused by T. gondii and acquired from the ingestion of undercooked meat and from handling cat feces.
CLINICAL PRESENTATION
The most common manifestation of neurocysticercosis is new-onset partial seizures with or without secondary generalization. Cysticerci may develop in the brain parenchyma and cause seizures or focal neurologic deficits. When present in the subarachnoid or ventricular spaces, cysticerci can produce increased ICP by interference with CSF flow. Spinal cysticerci can mimic the presentation of intraspinal tumors. When the cysticerci first lodge in the brain, they frequently cause little in the way of an inflammatory response. As the cysticercal cyst degenerates, it elicits an inflammatory response that may present clinically as a seizure. Eventually the cyst dies, a process that may take several years and is typically associated with resolution of the inflammatory response and, often, abatement of seizures.
Primary Toxoplasma infection is often asymptomatic. However, during this phase parasites may spread to the CNS, where they become latent. Reactivation of CNS infection is almost exclusively associated with immunocompromised hosts, particularly those with HIV infection. During this phase patients present with headache, fever, seizures, and focal neurologic deficits.
DIAGNOSIS
The lesions of neurocysticercosis are readily visualized by MRI or CT scans. Lesions with viable parasites appear as cystic lesions. The scolex can often be visualized on MRI. Lesions may appear as contrast-enhancing lesions surrounded by edema. A very early sign of cyst death is hypointensity of the vesicular fluid on T2-weighted images when compared with CSF. Parenchymal brain calcifications are the most common finding and evidence that the parasite is no longer viable. MRI findings of toxoplasmosis consist of multiple lesions in the deep white matter, the thalamus, and basal ganglia and at the gray-white junction in the cerebral hemispheres. With contrast administration, the majority of the lesions enhance in a ringed, nodular, or homogeneous pattern and are surrounded by edema. In the presence of the characteristic neuroimaging abnormalities of T. gondii infection, serum IgG antibody to T. gondii should be obtained and, when positive, the patient should be treated.
|
TREATMENT |
INFECTIOUS FOCAL CNS LESIONS |
Anticonvulsant therapy is initiated when the patient with neurocysticercosis presents with a seizure. There is controversy about whether or not anthelmintic therapy should be given to all patients, and recommendations are based on the stage of the lesion. Cysticerci appearing as cystic lesions in the brain parenchyma with or without pericystic edema or in the subarachnoid space at the convexity of the cerebral hemispheres should be treated with anticysticidal therapy. Cysticidal drugs accelerate the destruction of the parasites, resulting in a faster resolution of the infection. Albendazole and praziquantel are used in the treatment of neurocysticercosis. Approximately 85% of parenchymal cysts are destroyed by a single course of albendazole, and ~75% are destroyed by a single course of praziquantel. The dose of albendazole is 15 mg/kg per day in two doses for 8 days. The dose of praziquantel is 50 mg/kg per day for 15 days, although a number of other dosage regimens are also frequently cited. Prednisone or dexamethasone is given with anticysticidal therapy to reduce the host inflammatory response to degenerating parasites. Many, but not all, experts recommend anticysticidal therapy for lesions that are surrounded by a contrast-enhancing ring. There is universal agreement that calcified lesions do not need to be treated with anticysticidal therapy. Antiepileptic therapy can be stopped once the follow-up CT scan shows resolution of the lesion. Long-term antiepileptic therapy is recommended when seizures occur after resolution of edema and resorption or calcification of the degenerating cyst.
CNS toxoplasmosis is treated with a combination of sulfadiazine, 1.5–2.0 g orally qid, plus pyrimethamine, 100 mg orally to load, then 75–100 mg orally qd, plus folinic acid, 10–15 mg orally qd. Folinic acid is added to the regimen to prevent megaloblastic anemia. Therapy is continued until there is no evidence of active disease on neuroimaging studies, which typically takes at least 6 weeks, and then the dose of sulfadiazine is reduced to 2–4 g/d and pyrimethamine to 50 mg/d. Clindamycin plus pyrimethamine is an alternative therapy for patients who cannot tolerate sulfadiazine, but the combination of pyrimethamine and sulfadiazine is more effective.
SUBDURAL EMPYEMA
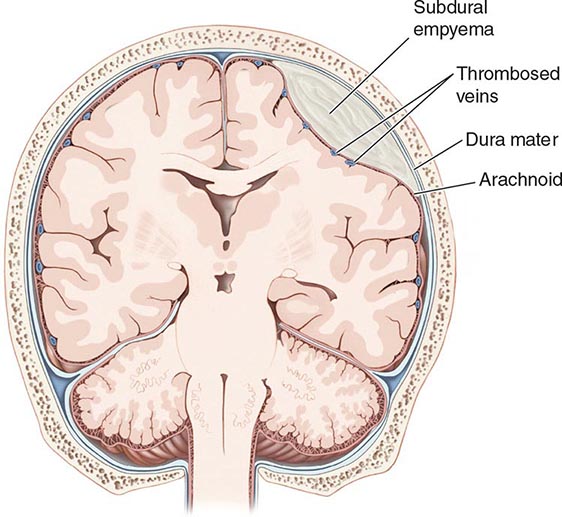
A subdural empyema (SDE) is a collection of pus between the dura and arachnoid membranes (Fig. 164-5).
FIGURE 164-5 Subdural empyema.
EPIDEMIOLOGY
SDE is a rare disorder that accounts for 15–25% of focal suppurative CNS infections. Sinusitis is the most common predisposing condition and typically involves the frontal sinuses, either alone or in combination with the ethmoid and maxillary sinuses. Sinusitis-associated empyema has a striking predilection for young males, possibly reflecting sex-related differences in sinus anatomy and development. It has been suggested that SDE may complicate 1–2% of cases of frontal sinusitis severe enough to require hospitalization. As a consequence of this epidemiology, SDE shows an ~3:1 male/female predominance, with 70% of cases occurring in the second and third decades of life. SDE may also develop as a complication of head trauma or neurosurgery. Secondary infection of a subdural effusion may also result in empyema, although secondary infection of hematomas, in the absence of a prior neurosurgical procedure, is rare.
ETIOLOGY
Aerobic and anaerobic streptococci, staphylococci, Enterobacteriaceae, and anaerobic bacteria are the most common causative organisms of sinusitis-associated SDE. Staphylococci and gram-negative bacilli are often the etiologic organisms when SDE follows neurosurgical procedures or head trauma. Up to one-third of cases are culture-negative, possibly reflecting difficulty in obtaining adequate anaerobic cultures.
PATHOPHYSIOLOGY
Sinusitis-associated SDE develops as a result of either retrograde spread of infection from septic thrombophlebitis of the mucosal veins draining the sinuses or contiguous spread of infection to the brain from osteomyelitis in the posterior wall of the frontal or other sinuses. SDE may also develop from direct introduction of bacteria into the subdural space as a complication of a neurosurgical procedure. The evolution of SDE can be extremely rapid because the subdural space is a large compartment that offers few mechanical barriers to the spread of infection. In patients with sinusitis-associated SDE, suppuration typically begins in the upper and anterior portions of one cerebral hemisphere and then extends posteriorly. SDE is often associated with other intracranial infections, including epidural empyema (40%), cortical thrombophlebitis (35%), and intracranial abscess or cerebritis (>25%). Cortical venous infarction produces necrosis of underlying cerebral cortex and subcortical white matter, with focal neurologic deficits and seizures (see below).
CLINICAL PRESENTATION
A patient with SDE typically presents with fever and a progressively worsening headache. The diagnosis of SDE should always be suspected in a patient with known sinusitis who presents with new CNS signs or symptoms. Patients with underlying sinusitis frequently have symptoms related to this infection. As the infection progresses, focal neurologic deficits, seizures, nuchal rigidity, and signs of increased ICP commonly occur. Headache is the most common complaint at the time of presentation; initially it is localized to the side of the subdural infection, but then it becomes more severe and generalized. Contralateral hemiparesis or hemiplegia is the most common focal neurologic deficit and can occur from the direct effects of the SDE on the cortex or as a consequence of venous infarction. Seizures begin as partial motor seizures that then become secondarily generalized. Seizures may be due to the direct irritative effect of the SDE on the underlying cortex or result from cortical venous infarction (see above). In untreated SDE, the increasing mass effect and increase in ICP cause progressive deterioration in consciousness, leading ultimately to coma.
DIAGNOSIS
MRI (Fig. 164-6) is superior to CT in identifying SDE and any associated intracranial infections. The administration of gadolinium greatly improves diagnosis by enhancing the rim of the empyema and allowing the empyema to be clearly delineated from the underlying brain parenchyma. Cranial MRI is also extremely valuable in identifying sinusitis, other focal CNS infections, cortical venous infarction, cerebral edema, and cerebritis. CT may show a crescent-shaped hypodense lesion over one or both hemispheres or in the interhemispheric fissure. Frequently the degree of mass effect, exemplified by midline shift, ventricular compression, and sulcal effacement, is far out of proportion to the mass of the SDE.
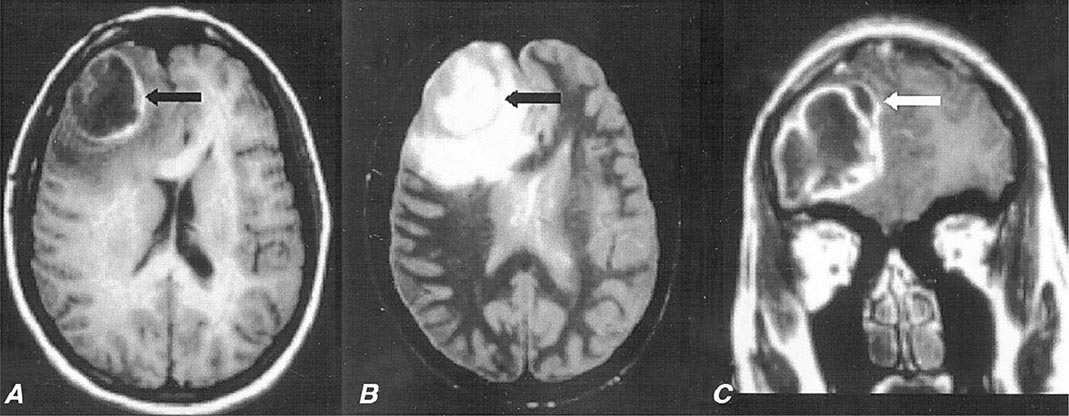
FIGURE 164-6 Subdural empyema. There is marked enhancement of the dura and leptomeninges (A, B, straight arrows) along the left medial hemisphere. The pus is hypointense on T1-weighted images (A, B) but markedly hyperintense on the proton density–weighted (C, curved arrow) image. (Courtesy of Joseph Lurito, MD; with permission.)
CSF examination should be avoided in patients with known or suspected SDE because it adds no useful information and is associated with the risk of cerebral herniation.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of the combination of headache, fever, focal neurologic signs, and seizure activity that progresses rapidly to an altered level of consciousness includes subdural hematoma, bacterial meningitis, viral encephalitis, brain abscess, superior sagittal sinus thrombosis, and acute disseminated encephalomyelitis. The presence of nuchal rigidity is unusual with brain abscess or epidural empyema and should suggest the possibility of SDE when associated with significant focal neurologic signs and fever. Patients with bacterial meningitis also have nuchal rigidity but do not typically have focal deficits of the severity seen with SDE.
|
TREATMENT |
SUBDURAL EMPYEMA |
SDE is a medical emergency. Emergent neurosurgical evacuation of the empyema, either through craniotomy, craniectomy, or burr-hole drainage, is the definitive step in the management of this infection. Empirical antimicrobial therapy for community-acquired SDE should include a combination of a third-generation cephalosporin (e.g., cefotaxime or ceftriaxone), vancomycin, and metronidazole (see Table 164-1 for dosages). Patients with hospital-acquired SDE may have infections due to Pseudomonas spp. or MRSA and should receive coverage with a carbapenem (e.g., meropenem) and vancomycin. Metronidazole is not necessary for antianaerobic therapy when meropenem is being used. Parenteral antibiotic therapy should be continued for a minimum of 3–4 weeks after SDE drainage. Patients with associated cranial osteomyelitis may require longer therapy. Specific diagnosis of the etiologic organisms is made based on Gram’s stain and culture of fluid obtained via either burr holes or craniotomy; the initial empirical antibiotic coverage can be modified accordingly.
PROGNOSIS
Prognosis is influenced by the level of consciousness of the patient at the time of hospital presentation, the size of the empyema, and the speed with which therapy is instituted. Long-term neurologic sequelae, which include seizures and hemiparesis, occur in up to 50% of cases.
CRANIAL EPIDURAL ABSCESS
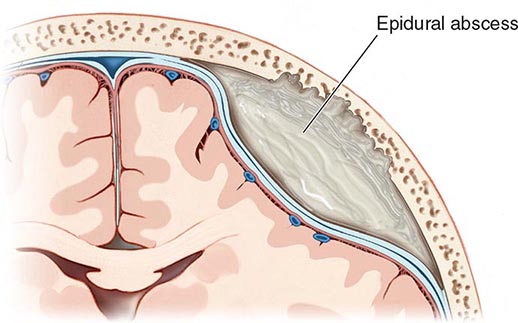
Cranial epidural abscess is a suppurative infection occurring in the potential space between the inner skull table and dura (Fig. 164-7).
FIGURE 164-7 Cranial epidural abscess is a collection of pus between the dura and the inner table of the skull.
ETIOLOGY AND PATHOPHYSIOLOGY
Cranial epidural abscess is less common than either brain abscess or SDE and accounts for <2% of focal suppurative CNS infections. A cranial epidural abscess develops as a complication of a craniotomy or compound skull fracture or as a result of spread of infection from the frontal sinuses, middle ear, mastoid, or orbit. An epidural abscess may develop contiguous to an area of osteomyelitis, when craniotomy is complicated by infection of the wound or bone flap, or as a result of direct infection of the epidural space. Infection in the frontal sinus, middle ear, mastoid, or orbit can reach the epidural space through retrograde spread of infection from septic thrombophlebitis in the emissary veins that drain these areas or by way of direct spread of infection through areas of osteomyelitis. Unlike the subdural space, the epidural space is really a potential rather than an actual compartment. The dura is normally tightly adherent to the inner skull table, and infection must dissect the dura away from the skull table as it spreads. As a result, epidural abscesses are often smaller than SDEs. Cranial epidural abscesses, unlike brain abscesses, only rarely result from hematogenous spread of infection from extracranial primary sites. The bacteriology of a cranial epidural abscess is similar to that of SDE (see above). The etiologic organisms of an epidural abscess that arises from frontal sinusitis, middle-ear infections, or mastoiditis are usually streptococci or anaerobic organisms. Staphylococci or gram-negative organisms are the usual cause of an epidural abscess that develops as a complication of craniotomy or compound skull fracture.
CLINICAL PRESENTATION
Patients present with fever (60%), headache (40%), nuchal rigidity (35%), seizures (10%), and focal deficits (5%). Development of symptoms may be insidious, as the empyema usually enlarges slowly in the confined anatomic space between the dura and the inner table of the skull. Periorbital edema and Pott’s puffy tumor, reflecting underlying associated frontal bone osteomyelitis, are present in ~40%. In patients with a recent neurosurgical procedure, wound infection is invariably present, but other symptoms may be subtle and can include altered mental status (45%), fever (35%), and headache (20%). The diagnosis should be considered when fever and headache follow recent head trauma or occur in the setting of frontal sinusitis, mastoiditis, or otitis media.
DIAGNOSIS
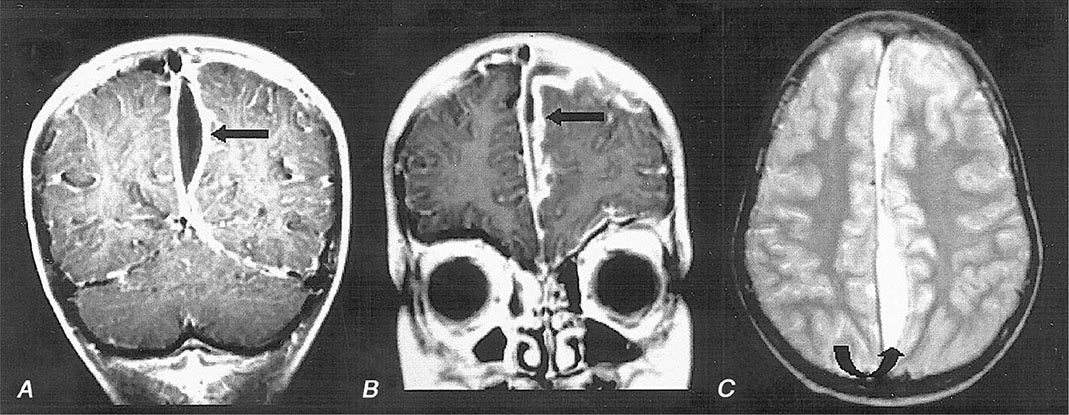
Cranial MRI with gadolinium enhancement is the procedure of choice to demonstrate a cranial epidural abscess. The sensitivity of CT is limited by the presence of signal artifacts arising from the bone of the inner skull table. The CT appearance of an epidural empyema is that of a lens or crescent-shaped hypodense extraaxial lesion. On MRI, an epidural empyema appears as a lentiform or crescent-shaped fluid collection that is hyperintense compared to CSF on T2-weighted images. On T1-weighted images, the fluid collection may be either isointense or hypointense compared to brain. Following the administration of gadolinium, there is linear enhancement of the dura on T1-weighted images. In distinction to subdural empyema, signs of mass effect or other parenchymal abnormalities are uncommon.
|
TREATMENT |
EPIDURAL ABSCESS |
Immediate neurosurgical drainage is indicated. Empirical antimicrobial therapy, pending the results of Gram’s stain and culture of the purulent material obtained at surgery, should include a combination of a third-generation cephalosporin, vancomycin, and metronidazole (Table 164-1). Ceftazidime or meropenem should be substituted for ceftriaxone or cefotaxime in neurosurgical patients. Metronidazole is not necessary for antianaerobic coverage in patients receiving meropenem. When the organism has been identified, antimicrobial therapy can be modified accordingly. Antibiotics should be continued for 3–6 weeks after surgical drainage. Patients with associated osteomyelitis may require additional therapy.
PROGNOSIS
The mortality rate is <5% in modern series, and full recovery is the rule in most survivors.
SUPPURATIVE THROMBOPHLEBITIS
DEFINITION
Suppurative intracranial thrombophlebitis is septic venous thrombosis of cortical veins and sinuses. This may occur as a complication of bacterial meningitis; SDE; epidural abscess; or infection in the skin of the face, paranasal sinuses, middle ear, or mastoid.
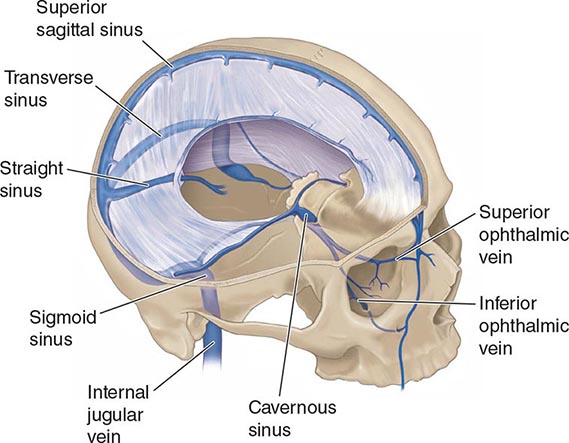
ANATOMY AND PATHOPHYSIOLOGY
The cerebral veins and venous sinuses have no valves; therefore, blood within them can flow in either direction. The superior sagittal sinus is the largest of the venous sinuses (Fig. 164-8). It receives blood from the frontal, parietal, and occipital superior cerebral veins and the diploic veins, which communicate with the meningeal veins. Bacterial meningitis is a common predisposing condition for septic thrombosis of the superior sagittal sinus. The diploic veins, which drain into the superior sagittal sinus, provide a route for the spread of infection from the meninges, especially in cases where there is purulent exudate near areas of the superior sagittal sinus. Infection can also spread to the superior sagittal sinus from nearby SDE or epidural abscess. Dehydration from vomiting, hypercoagulable states, and immunologic abnormalities, including the presence of circulating antiphospholipid antibodies, also contribute to cerebral venous sinus thrombosis. Thrombosis may extend from one sinus to another, and at autopsy, thrombi of different histologic ages can often be detected in several sinuses. Thrombosis of the superior sagittal sinus is often associated with thrombosis of superior cortical veins and small parenchymal hemorrhages.
FIGURE 164-8 Anatomy of the cerebral venous sinuses.
The superior sagittal sinus drains into the transverse sinuses (Fig. 164-8). The transverse sinuses also receive venous drainage from small veins from both the middle ear and mastoid cells. The transverse sinus becomes the sigmoid sinus before draining into the internal jugular vein. Septic transverse/sigmoid sinus thrombosis can be a complication of acute and chronic otitis media or mastoiditis. Infection spreads from the mastoid air cells to the transverse sinus via the emissary veins or by direct invasion. The cavernous sinuses are inferior to the superior sagittal sinus at the base of the skull. The cavernous sinuses receive blood from the facial veins via the superior and inferior ophthalmic veins. Bacteria in the facial veins enter the cavernous sinus via these veins. Bacteria in the sphenoid and ethmoid sinuses can spread to the cavernous sinuses via the small emissary veins. The sphenoid and ethmoid sinuses are the most common sites of primary infection resulting in septic cavernous sinus thrombosis.
CLINICAL MANIFESTATIONS
Septic thrombosis of the superior sagittal sinus presents with headache, fever, nausea and vomiting, confusion, and focal or generalized seizures. There may be a rapid development of stupor and coma. Weakness of the lower extremities with bilateral Babinski’s signs or hemiparesis is often present. When superior sagittal sinus thrombosis occurs as a complication of bacterial meningitis, nuchal rigidity and Kernig’s and Brudzinski’s signs may be present.
The oculomotor nerve, the trochlear nerve, the abducens nerve, the ophthalmic and maxillary branches of the trigeminal nerve, and the internal carotid artery all pass through the cavernous sinus (see Fig. 455-4). The symptoms of septic cavernous sinus thrombosis are fever, headache, frontal and retroorbital pain, and diplopia. The classic signs are ptosis, proptosis, chemosis, and extraocular dysmotility due to deficits of cranial nerves III, IV, and VI; hyperesthesia of the ophthalmic and maxillary divisions of the fifth cranial nerve and a decreased corneal reflex may be detected. There may be evidence of dilated, tortuous retinal veins and papilledema.
Headache and earache are the most frequent symptoms of transverse sinus thrombosis. A transverse sinus thrombosis may also present with otitis media, sixth nerve palsy, and retroorbital or facial pain (Gradenigo’s syndrome). Sigmoid sinus and internal jugular vein thrombosis may present with neck pain.
DIAGNOSIS
The diagnosis of septic venous sinus thrombosis is suggested by an absent flow void within the affected venous sinus on MRI and confirmed by magnetic resonance venography, CT angiogram, or the venous phase of cerebral angiography. The diagnosis of thrombophlebitis of intracerebral and meningeal veins is suggested by the presence of intracerebral hemorrhage but requires cerebral angiography for definitive diagnosis.
|
TREATMENT |
SUPPURATIVE THROMBOPHLEBITIS |
Septic venous sinus thrombosis is treated with antibiotics, hydration, and removal of infected tissue and thrombus in septic lateral or cavernous sinus thrombosis. The choice of antimicrobial therapy is based on the bacteria responsible for the predisposing or associated condition. Optimal duration of therapy is unknown, but antibiotics are usually continued for 6 weeks or until there is radiographic evidence of resolution of thrombosis. Anticoagulation with dose-adjusted intravenous heparin is recommended for aseptic venous sinus thrombosis and in the treatment of septic venous sinus thrombosis complicating bacterial meningitis in patients who have progressive neurologic deterioration despite antimicrobial therapy and intravenous fluids. The presence of a small intracerebral hemorrhage from septic thrombophlebitis is not an absolute contraindication to heparin therapy. Successful management of aseptic venous sinus thrombosis has been reported with surgical thrombectomy, catheter-directed urokinase therapy, and a combination of intrathrombus recombinant tissue plasminogen activator (rtPA) and intravenous heparin, but there are not enough data to recommend these therapies in septic venous sinus thrombosis.
165 |
Chronic and Recurrent Meningitis |
Chronic inflammation of the meninges (pia, arachnoid, and dura) can produce profound neurologic disability and may be fatal if not successfully treated. Chronic meningitis is diagnosed when a characteristic neurologic syndrome exists for >4 weeks and is associated with a persistent inflammatory response in the cerebrospinal fluid (CSF) (white blood cell count >5/μL). The causes are varied, and appropriate treatment depends on identification of the etiology. Five categories of disease account for most cases of chronic meningitis: (1) meningeal infections, (2) malignancy, (3) autoimmune inflammatory disorders, (4) chemical meningitis, and (5) parameningeal infections.
CLINICAL PATHOPHYSIOLOGY
Neurologic manifestations of chronic meningitis (Table 165-1) are determined by the anatomic location of the inflammation and its consequences. Persistent headache with or without a stiff neck, hydrocephalus, cranial neuropathies, radiculopathies, and cognitive or personality changes are the cardinal features. These can occur alone or in combination. When they appear in combination, widespread dissemination of the inflammatory process along CSF pathways has occurred. In some cases, the presence of an underlying systemic illness points to a specific agent or class of agents as the probable cause. The diagnosis of chronic meningitis is usually made when the clinical presentation prompts the physician to examine the CSF for signs of inflammation. CSF is produced by the choroid plexus of the cerebral ventricles, exits through narrow foramina into the subarachnoid space surrounding the brain and spinal cord, circulates around the base of the brain and over the cerebral hemispheres, and is resorbed by arachnoid villi projecting into the superior sagittal sinus. CSF flow provides a pathway for rapid spread of infectious and other infiltrative processes over the brain, spinal cord, and cranial and spinal nerve roots. Spread from the subarachnoid space into brain parenchyma may occur via the arachnoid cuffs that surround blood vessels that penetrate brain tissue (Virchow-Robin spaces).
|
SYMPTOMS AND SIGNS OF CHRONIC MENINGITIS |
Intracranial Meningitis Nociceptive nerve fibers of the meninges are stimulated by the inflammatory process, resulting in headache, neck pain, or back pain. Obstruction of CSF pathways at the foramina or arachnoid villi may produce hydrocephalus and symptoms of raised intracranial pressure (ICP), including headache, vomiting, apathy or drowsiness, gait instability, papilledema, visual loss, impaired upgaze, or palsy of the sixth cranial nerve (CN) (Chap. 455). Cognitive and behavioral changes during the course of chronic meningitis may also result from vascular damage, which may similarly produce seizures, stroke, or myelopathy. Inflammatory deposits seeded via the CSF circulation are often prominent around the brainstem and cranial nerves and along the undersurface of the frontal and temporal lobes. Such cases, termed basal meningitis, often present as multiple cranial neuropathies, with decreased vision (CN II), facial weakness (CN VII), decreased hearing (CN VIII), diplopia (CNs III, IV, and VI), sensory or motor abnormalities of the oropharynx (CNs IX, X, and XII), decreased olfaction (CN I), or decreased facial sensation and masseter weakness (CN V).
Spinal Meningitis Injury may occur to motor and sensory roots as they traverse the subarachnoid space and penetrate the meninges. These cases present as multiple radiculopathies with combinations of radicular pain, sensory loss, motor weakness, and urinary or fecal incontinence. Meningeal inflammation can encircle the cord, resulting in a myelopathy. Patients with slowly progressive involvement of multiple cranial nerves and/or spinal nerve roots are likely to have chronic meningitis. Electrophysiologic testing (electromyography, nerve conduction studies, and evoked response testing) may be helpful in determining whether there is involvement of cranial and spinal nerve roots.
Systemic Manifestations In some patients, evidence of systemic disease provides clues to the underlying cause of chronic meningitis. A complete history of travel, sexual practice, and exposure to infectious agents should be sought. Infectious causes are often associated with fever, malaise, anorexia, and signs of localized or disseminated infection outside the nervous system. Infectious causes are of major concern in the immunosuppressed patient, especially in patients with AIDS, in whom chronic meningitis may present without headache or fever. Noninfectious inflammatory disorders often produce systemic manifestations, but meningitis may be the initial manifestation. Carcinomatous meningitis may or may not be accompanied by clinical evidence of the primary neoplasm.
|
INFECTIOUS CAUSES OF CHRONIC MENINGITIS |
|
NONINFECTIOUS CAUSES OF CHRONIC MENINGITIS |
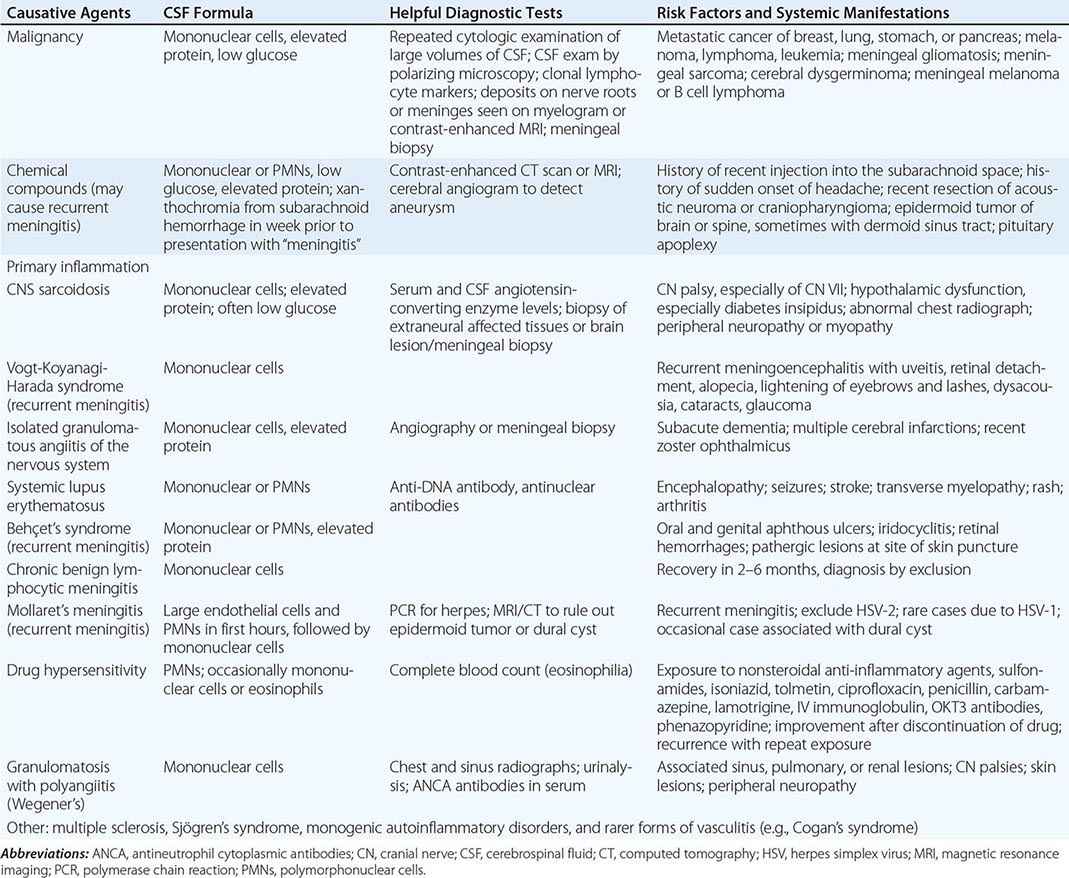
FIGURE 165-1 Primary central nervous system lymphoma. A 24-year-old man, immunosuppressed due to intestinal lymphangiectasia, developed multiple cranial neuropathies. CSF findings consisted of 100 lymphocytes/μL and a protein of 2.5 g/L (250 mg/dL); cytology and cultures were negative. Gadolinium-enhanced T1 MRI revealed diffuse, multifocal meningeal enhancement surrounding the brainstem (A), spinal cord, and cauda equina (B).
Angiographic studies can identify evidence of cerebral arteritis in patients with chronic meningitis and stroke.
THE IMMUNOSUPPRESSED PATIENT
Chronic meningitis is not uncommon in the course of HIV infection. Pleocytosis and mild meningeal signs often occur at the onset of HIV infection, and occasionally low-grade meningitis persists. Toxoplasmosis commonly presents as intracranial abscesses and also may be associated with meningitis. Other important causes of chronic meningitis in AIDS include infection with Cryptococcus, Nocardia, Candida, or other fungi; syphilis; and lymphoma (Fig. 165-1). Toxoplasmosis, cryptococcosis, nocardiosis, and other fungal infections are important etiologic considerations in individuals with immunodeficiency states other than AIDS, including those due to immunosuppressive medications. Because of the increased risk of chronic meningitis and the attenuation of clinical signs of meningeal irritation in immunosuppressed individuals, CSF examination should be performed for any persistent headache or unexplained change in mental state.
ACKNOWLEDGMENT
Morton N. Swartz contributed to earlier editions of this chapter.
166e |
Infectious Complications of Burns |
The skin is an essential component of immunity, protecting the host from potential pathogens in the environment. Breaches in this protective barrier thus represent a form of immunocompromise that predisposes the patient to infection. Thermal burns may cause massive destruction of the integument as well as derangements in humoral and cellular immunity, permitting the development of infection caused by environmental opportunists and components of the host’s skin flora.
EPIDEMIOLOGY
Over the past decade, the estimated incidence of burn injuries in the United States has steadily declined; still, however, >1 million burn injuries are brought to medical attention each year. While many burn injuries are minor and require little or no intervention, 183,000 cases were reported between 2002 and 2011 to the National Burn Repository from specialized burn care facilities; of the 45,000 persons hospitalized for these injuries, 60% required intensive care and 20,000 had major burns involving at least 25% of the total body surface area. The majority of burn patients are men. Children under the age of 5 account for ~20% of all reported cases. Scalds, structural fires, and flammable liquids and gases are the major causes of burns, but electrical, chemical, and smoking-related sources also are important. Burns predispose to infection by damaging the protective barrier function of the skin, thus facilitating the entry of pathogenic microorganisms, and by inducing systemic immunosuppression. It is therefore not surprising that multiorgan failure and infectious complications are the major causes of morbidity and death in serious burn injury. More than 3000 patients in the United States die of burn-related infections each year, and 6 of the top 10 complications recently identified by the American Burn Association’s 10-year review are infectious: pneumonia (4.6%), septicemia (2.7%), cellulitis/traumatic injury (2.6%), respiratory failure (2.5%), wound infection (2.2%), another infection (2.0%), renal failure (1.5%), line infection (1.4%), acute respiratory distress syndrome (1.2%), and arrhythmia (1.0%).
PATHOPHYSIOLOGY
Loss of the cutaneous barrier facilitates entry of the patient’s own flora and of organisms from the hospital environment into the burn wound. Initially, the wound is colonized with gram-positive bacteria from the surrounding tissue, but the number of bacteria grows rapidly beneath the burn eschar, reaching ~8.4 × 103 cfu/g on day 4 after the burn. The avascularity of the eschar, along with the impairment of local immune responses, favors further bacterial colonization and proliferation. By day 7, the wound is colonized with other microbes, including gram-positive bacteria, gram-negative bacteria, and yeasts derived from the gastrointestinal and upper respiratory flora. Invasive infection—localized and/or systemic—occurs when these bacteria penetrate viable tissue. In addition, a role for biofilms has been recognized in experimental animal models of burn-wound infection. (Biofilms are surface-associated communities of bacteria, often embedded in a matrix, that allow the microbes to persist and to resist the effects of host immunity and antimicrobial agents.)
Streptococci and staphylococci were the predominant causes of burn-wound infection in the preantibiotic era and remain important pathogens at present. With the advent of antimicrobial agents, Pseudomonas aeruginosa became a major problem in burn-wound management. In animal models of cutaneous thermal injury and wound infection with Pseudomonas, there is an early, steady increase of neutrophils in the skin and bacterial dissemination to lungs and spleen within 72 h. Less common anaerobic bacteria are typically found in infections of electrical burns or when open wound dressings are used. The widespread use of topical and more effective antimicrobial agents has resulted in a decline in bacterial wound infections and the emergence of fungi (particularly Candida albicans, Aspergillus species, and the agents of mucormycosis) as increasingly important pathogens in burn-wound patients. Herpes simplex virus also has been found in burn wounds, especially those on the neck and face and those associated with inhalation injury. Cytomegalovirus viremia has been described in up to 71% of seropositive burn patients in prospective studies, and high levels (>1000 copies/mL) have been associated with increased duration of mechanical ventilation and longer stay in the intensive care unit (ICU).
Autopsy reports from patients with severe thermal burns over the last decade have identified P. aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus in association with mortality, independent of the percentage of total body surface area burned, the percentage of full-thickness burn, inhalation injury, and day of death after the burn. Indeed, burn trauma patients who acquire secondary P. aeruginosa infection have a fourfold greater mortality rate than those without P. aeruginosa. Historically, mortality rates among burn patients infected with P. aeruginosa have been as high as 77% over a 25-year period. In addition, Acinetobacter calcoaceticus-baumannii is among the top pathogens in some burn centers.
The cascade of events that follow a severe burn injury and that lead to multiorgan system failure and death is thought to represent a two-step process. The burn injury itself, with ensuing hypovolemia and tissue hypoxia, is followed by invasive infection arising from large amounts of devitalized tissue. The frequency of infection parallels the extent and severity of the burn injury. Severe burn injuries cause a state of immunosuppression that affects innate and adaptive immune responses. The substantial impact of immunocompromise on infection is due to effects on both the cellular and the humoral arms of the immune system. For example, decreases in the number and activity of circulating helper T cells, increases in suppressor T cells, decreases in production and release of monocytes and macrophages, and diminution in levels of immunoglobulin follow major burns. Neutrophil and complement functions also are impaired after burns. The increased levels of multiple cytokines detected in burn patients are compatible with the widely held belief that the inflammatory response becomes dysregulated in these individuals; bacterial cell products play a potent role in inducing proinflammatory mediators that contribute to this uncontrolled systemic inflammatory response. Increased permeability of the gut wall to bacteria and their components (e.g., endotoxin) also contributes to immune dysregulation and sepsis. Thus, the burn patient is predisposed to infection at remote sites (see below) as well as at the sites of burn injury. Another contributor to secondary immunosuppression after burn injuries is the endocrine system; increasing levels of vasopressin, aldosterone, cortisol, glucagon, growth hormone, catecholamines, and other hormones that directly affect lymphocyte proliferation, secretion of proinflammatory cytokines, natural killer cell activity, and suppressive T cells are seen.
CLINICAL MANIFESTATIONS AND DIAGNOSIS
Since clinical indications of wound infection are difficult to interpret, wounds must be monitored carefully for changes that may reflect infection. A margin of erythema frequently surrounds the sites of burns and by itself is not usually indicative of infection. Signs of infection include the conversion of a partial-thickness to a full-thickness burn, color changes (e.g., the appearance of a dark brown or black discoloration of the wound), the new appearance of erythema or violaceous edema in normal tissue at the wound margins, the sudden separation of the eschar from subcutaneous tissues, and the degeneration of the wound with the appearance of a new eschar.
Early surgical excision of devitalized tissue is now widely used, and burn-wound infections can be classified in relation to the excision site as (1) burn-wound impetigo (infection characterized by loss of epithelium from a previously re-epithelialized surface, as seen in a partial-thickness burn that is allowed to close by secondary intention, a grafted burn, or a healed skin donor site); (2) burn-related surgical-wound infection (purulent infection of excised burn and donor sites that have not yet epithelialized, accompanied by positive cultures); (3) burn-wound cellulitis (extension of infection to surrounding healthy tissue; Fig. 166e-1); and (4) invasive infection in unexcised burn wounds (infection that is secondary to a partial- or full-thickness burn wound and is manifested by separation of the eschar or by violaceous, dark brown, or black discoloration of the eschar; Fig. 166e-2). The appearance of a green discoloration of the wound or subcutaneous fat (Fig. 166e-3) or the development of ecthyma gangrenosum (see Fig. 25e-35) at a remote site points to a diagnosis of invasive P. aeruginosa infection.
FIGURE 166e-1 Cellulitis complicating a burn wound of the arm, with extension of the infection to adjacent healthy tissue. (Courtesy of Dr. Robert L. Sheridan, Massachusetts General Hospital, Boston; with permission.)
FIGURE 166e-2 A severe upper-extremity burn infected with Pseudomonas aeruginosa. The wound requires additional debridement. Note the dark brown to black discoloration of the eschar. (Courtesy of Dr. Robert L. Sheridan, Massachusetts General Hospital, Boston; with permission.)
FIGURE 166e-3 A burn wound infected with Pseudomonas aeruginosa, with liquefaction of tissue. Note the green discoloration at the wound margins, which is suggestive of Pseudomonas infection. (Courtesy of Dr. Robert L. Sheridan, Massachusetts General Hospital, Boston; with permission.)
Changes in body temperature, hypotension, tachycardia, altered mentation, neutropenia or neutrophilia, thrombocytopenia, and renal failure may result from invasive burn wounds and sepsis. However, because profound alterations in homeostasis occur as a consequence of burns per se and because inflammation without infection is a normal component of these injuries, the assessment of these changes is complicated. Alterations in body temperature, for example, are attributable to thermoregulatory dysfunction; tachycardia and hyperventilation accompany the metabolic changes induced by extensive burn injury and are not necessarily indicative of bacterial sepsis.
Given the difficulty of evaluating burn wounds solely on the basis of clinical observation and laboratory data, wound biopsies are necessary for definitive diagnosis of infection. The timing of these biopsies can be guided by clinical changes, but in some centers burn wounds are routinely biopsied at regular intervals. The biopsy specimen is examined for histologic evidence of bacterial invasion, and quantitative microbiologic cultures are performed. The presence of >105 viable bacteria per gram of tissue is highly suggestive of invasive infection and of a dramatically increased risk of sepsis. Histopathologic evidence of the invasion of viable tissue and the presence of microorganisms in unburned blood vessels and lymphatics is a more definitive indicator of infection. A blood culture positive for the same organism seen in large quantities in biopsied tissue is a reliable indicator of burn sepsis. Surface cultures may provide some indication of the microorganisms present in the hospital environment but are not indicative of the etiology of infection. This noninvasive technique may be of use in determining the flora present in excised burn areas or in areas where the skin is too thin for biopsy (e.g., over the ears, eyes, or digits). Rapid identification of organisms and institution of appropriate therapy are critical to the survival of patients with severe burn injury; polymerase chain reaction (PCR) is now being used for rapid identification of specific pathogens, sometimes in <6 h, to allow earlier treatment interventions.
In addition to infection of the burn wound itself, a number of other infections due to the immunosuppression caused by extensive burns and the manipulations necessary for clinical care put burn patients at risk. Pneumonia, now the most common infectious complication among hospitalized burn patients, is most often acquired nosocomially via the respiratory route. The incidence of ventilator-associated pneumonia among burn patients is 22–30 cases per 1000 ventilator-days—more than double that among surgical or medical ICU cohorts; this infection usually results from colonization of the lower respiratory tract and parenchyma because of sustained microaspiration. Among the risk factors associated with secondary pneumonia are inhalation injury, intubation, full-thickness chest wall burns, cutaneous thermal injuries, immobility, blood transfusions, and uncontrolled wound sepsis with hematogenous spread. Septic pulmonary emboli also may occur. Suppurative thrombophlebitis may complicate the vascular catheterization necessary for fluid and nutritional support in burns. Endocarditis, urinary tract infection, bacterial chondritis (particularly in patients with burned ears), and intraabdominal infection also complicate serious burn injury. Staphylococcal scalded skin syndrome due to burn-wound infection with S. aureus has been described as a rare complication. Finally, burn surgical-wound infections contribute to morbidity and have been found in up to 39% of patients; these infections often result in repeat skin grafting and prolonged hospitalization.
|
BURN-WOUND INFECTIONS |
The ultimate goal of burn-wound management is closure and healing of the wound. Early surgical excision of burned tissue, with extensive debridement of necrotic tissue and grafting of skin or skin substitutes, greatly decreases mortality rates associated with severe burns. In addition, the four widely used topical antimicrobial agents—silver sulfadiazine cream, mafenide acetate cream, silver nitrate cream, and nanocrystalline silver dressings—dramatically decrease the bacterial burden of burn wounds and reduce the incidence of burn-wound infection; these agents are routinely applied to partial- and full-thickness burns. The bactericidal properties of silver are related to its effect on respiratory enzymes on bacterial cell walls; its interaction with structural proteins causes keratinocyte and fibroblast toxicity that can delay wound healing if silver-based compounds are used indiscriminately. All four agents are broadly active against many bacteria and some fungi and are useful before bacterial colonization is established. Silver sulfadiazine is often used initially, but its value can be limited by bacterial resistance, poor wound penetration, or toxicity (leukopenia). Mafenide acetate has broader activity against gram-negative bacteria. The cream penetrates eschars and thus can prevent or treat infection beneath them; its use without dressings allows regular examination of the wound area. The foremost disadvantages of mafenide acetate are that it can inhibit carbonic anhydrase, resulting in metabolic acidosis, and that it elicits hypersensitivity reactions in up to 7% of patients. This agent is most often used when gram-negative bacteria invade the burn wound and when treatment with silver sulfadiazine fails. The activity of mafenide acetate against gram-positive bacteria is limited. Nanocrystalline silver dressings provide broader antimicrobial coverage than any other available topical preparation, exhibiting activity against methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococci (VRE), moderate ability to penetrate eschars, and limited toxicity. In addition, this approach provides controlled and prolonged release of nanocrystalline silver into the wound, limiting the number of dressing changes and therefore reducing the risk of nosocomial infections as well as the cost of treatment. Mupirocin, a topical antimicrobial agent used to eradicate nasal colonization with MRSA, is increasingly being used in burn units where MRSA is prevalent. The efficacy of mupirocin in reducing burn-wound bacterial counts and preventing systemic infections is comparable to that of silver sulfadiazine.
In recent years, rates of fungal infection have increased in burn patients. When superficial fungal infection occurs, nystatin may be mixed with silver sulfadiazine or mafenide acetate as topical therapy. A small study found that nystatin powder (6 million units/g) was effective for treatment of superficial and deep burn-wound infections caused by Aspergillus or Fusarium species. In addition to these products, moisture-retention ointments with antimicrobial properties can promote rapid autolysis, debridement, and moist healing of partial-thickness wounds.
When invasive wound infection is diagnosed, topical therapy should be changed to mafenide acetate. Subeschar clysis (the direct instillation of an antibiotic, often piperacillin, into wound tissues under the eschar) is a useful adjunct to surgical and systemic antimicrobial therapy. Systemic treatment with antibiotics active against the pathogens present in the wound should be instituted. In the absence of culture data, treatment should be broad in spectrum, covering organisms commonly encountered in that particular burn unit. Such coverage is usually achieved with an antibiotic active against gram-positive pathogens (e.g., oxacillin, 2 g IV every 4 h) and with a drug active against P. aeruginosa and other gram-negative rods (e.g., mezlocillin, 3 g IV every 4 h; gentamicin, 5 mg/kg IV per day). In penicillin-allergic patients, vancomycin (1 g IV every 12 h) may be substituted for oxacillin (and is efficacious against MRSA), and ciprofloxacin (400 mg IV every 12 h) may be substituted for mezlocillin. Oxazolidinone antibiotics like linezolid have demonstrated efficacy in reducing bacterial growth and toxic shock syndrome toxin 1 levels in animal models of MRSA burn-wound infections.
Patients with burn wounds frequently have alterations in metabolism and renal clearance mechanisms that mandate the monitoring of serum antibiotic levels. The levels achieved with standard doses are often subtherapeutic.
Treatment of infections caused by emerging resistant pathogens remains a challenge in the care of burn patients. MRSA, resistant enterococci, multidrug-resistant gram-negative rods, and Enterobacteriaceae producing extended-spectrum β-lactamases have been associated with burn-wound infections and identified in burn-unit outbreaks. Strict infection-control practices (including microbiologic surveillance in burn units) and appropriate antimicrobial therapy remain important measures in reducing rates of infection due to resistant organisms.
In general, prophylactic systemic antibiotics have no role in the management of burn wounds and can, in fact, lead to colonization with resistant microorganisms. In some studies, antibiotic prophylaxis has been associated with increases in secondary infections of the upper and lower respiratory tract and the urinary tract as well as with prolonged hospitalization. An exception involves cases requiring burn-wound manipulation. Since procedures such as debridement, excision, or grafting frequently result in bacteremia, prophylactic systemic antibiotics are administered at the time of wound manipulation; the specific agents used should be chosen on the basis of data obtained by wound culture or data on the hospital’s resident flora.
The use of oral antibiotics for selective digestive decontamination (SDD) to decrease bacterial colonization and the risk of burn-wound infection is controversial and has not been widely adopted. In a randomized, double-blind, placebo-controlled trial in patients with burns involving >20% of the total body surface area, SDD was associated with reduced mortality rates in the burn ICU and in the hospital and also with a reduced incidence of pneumonia. The effects of SDD on the normal anaerobic bowel flora must be taken into consideration before this approach is used.
Strategies to reduce or limit systemic spread of wound infections, particularly to the lung, may be useful adjuncts to therapy. Some of these strategies are aimed at reducing neutrophilic inflammation at the site of injury, which can accelerate biofilm formation, particularly by P. aeruginosa. For example, in animal models of cutaneous burns with P. aeruginosa wound inoculation, a single dose of azithromycin administered early reduces rates of Pseudomonas infection and systemic spread to lung and spleen and appears to have effects similar to those of classic anti-Pseudomonas agents, such as tobramycin. The extent to which azithromycin can be administered early in humans to prevent dissemination remains to be studied.
All burn-injury patients should undergo tetanus booster immunization if they have completed primary immunization but have not received a booster dose in the past 5 years. Patients without prior immunization should receive tetanus immune globulin and undergo primary immunization.
167e |
Infectious Complications of Bites |
The skin is an essential component of nonspecific immunity, protecting the host from potential pathogens in the environment. Breaches in this protective barrier thus represent a form of immunocompromise that predisposes the patient to infection. Bites and scratches from animals and humans allow the inoculation of microorganisms past the skin’s protective barrier into deeper, susceptible host tissues.
Each year in the United States, millions of animal-bite wounds are sustained. The vast majority are inflicted by pet dogs and cats, which number >100 million; the annual incidence of dog and cat bites has been reported as 300 bites per 100,000 population. Other bite wounds are a consequence of encounters with animals in the wild or in occupational settings. While many of these wounds require minimal or no therapy, a significant number result in infection, which may be life-threatening. The microbiology of bite-wound infections in general reflects the oropharyngeal flora of the biting animal, although organisms from the soil, the skin of the animal and the victim, and the animal’s feces may also be involved.
DOG BITES
In the United States, dogs bite >4.7 million people each year and are responsible for 80% of all animal-bite wounds, an estimated 15–20% of which become infected. Each year, 800,000 Americans seek medical attention for dog bites; of those injured, 386,000 require treatment in an emergency department, with >1000 emergency department visits each day and about a dozen deaths per year. Most dog bites are provoked and are inflicted by the victim’s pet or by a dog known to the victim. These bites are frequently sustained during efforts to break up a dogfight. Children are more likely than adults to sustain canine bites, with the highest incidence of 6 bites/1000 population among boys 5–9 years old. Victims are more often male than female, and bites most often involve an upper extremity. Among children <4 years old, two-thirds of all these injuries involve the head or neck. Infection typically manifests 8–24 h after the bite as pain at the site of injury with cellulitis accompanied by purulent, sometimes foul-smelling discharge. Septic arthritis and osteomyelitis may develop if a canine tooth penetrates synovium or bone. Systemic manifestations (e.g., fever, lymphadenopathy, and lymphangitis) also may occur. The microbiology of dog-bite wound infections is usually mixed and includes β-hemolytic streptococci, Pasteurella species, Staphylococcus species (including methicillin-resistant Staphylococcus aureus [MRSA]), Eikenella corrodens, and Capnocytophaga canimorsus. Many wounds also include anaerobic bacteria such as Actinomyces, Fusobacterium, Prevotella, and Porphyromonas species.
While most infections resulting from dog-bite injuries are localized to the area of injury, many of the microorganisms involved are capable of causing systemic infection, including bacteremia, meningitis, brain abscess, endocarditis, and chorioamnionitis. These infections are particularly likely in hosts with edema or compromised lymphatic drainage in the involved extremity (e.g., after a bite on the arm in a woman who has undergone mastectomy) and in patients who are immunocompromised by medication or disease (e.g., glucocorticoid use, systemic lupus erythematosus, acute leukemia, or hepatic cirrhosis). In addition, dog bites and scratches may result in systemic illnesses such as rabies (Chap. 232) and tetanus (Chap. 177).
Infection with C. canimorsus following dog-bite wounds may result in fulminant sepsis, disseminated intravascular coagulation, and renal failure, particularly in hosts who have impaired hepatic function, who have undergone splenectomy, or who are immunosuppressed. This thin gram-negative rod is difficult to culture on most solid media but grows in a variety of liquid media. The bacteria are occasionally seen within polymorphonuclear leukocytes on Wright-stained smears of peripheral blood from septic patients. Tularemia (Chap. 195) also has been reported to follow dog bites.
CAT BITES
Although less common than dog bites, cat bites and scratches result in infection in more than half of all cases. Because the cat’s narrow, sharp canine teeth penetrate deeply into tissue, cat bites are more likely than dog bites to cause septic arthritis and osteomyelitis; the development of these conditions is particularly likely when punctures are located over or near a joint, especially in the hand. Women sustain cat bites more frequently than do men. These bites most often involve the hands and arms. Both bites and scratches from cats are prone to infection from organisms in the cat’s oropharynx. Pasteurella multocida, a normal component of the feline oral flora, is a small gram-negative coccobacillus implicated in the majority of cat-bite wound infections. Like that of dog-bite wound infections, however, the microflora of cat-bite wound infections is usually mixed. Other microorganisms causing infection after cat bites are similar to those causing dog-bite wound infections.
The same risk factors for systemic infection following dog-bite wounds apply to cat-bite wounds. Pasteurella infections tend to advance rapidly, often within hours, causing severe inflammation accompanied by purulent drainage; Pasteurella may also be spread by respiratory droplets from animals, resulting in pneumonia or bacteremia. Like dog-bite wounds, cat-bite wounds may result in the transmission of rabies or in the development of tetanus. Infection with Bartonella henselae causes cat-scratch disease (Chap. 197) and is an important late consequence of cat bites and scratches. Tularemia (Chap. 195) also has been reported to follow cat bites.
OTHER ANIMAL BITES
Infections have been attributed to bites from many animal species. Often these bites are sustained as a consequence of occupational exposure (farmers, laboratory workers, veterinarians) or recreational exposure (hunters and trappers, wilderness campers, owners of exotic pets). Generally, the microflora of bite wounds reflects the oral flora of the biting animal. Most members of the cat family, including feral cats, harbor P. multocida. Bite wounds from aquatic animals such as alligators or piranhas may contain Aeromonas hydrophila. Venomous snakebites (Chap. 474) result in severe inflammatory responses and tissue necrosis—conditions that render these injuries prone to infection. The snake’s oral flora includes many species of aerobes and anaerobes, such as Pseudomonas aeruginosa, Serratia marcescens, Proteus species, Staphylococcus epidermidis, Bacteroides fragilis, and Clostridium species. Bites from nonhuman primates are highly susceptible to infection with pathogens similar to those isolated from human bites (see below). Bites from Old World monkeys (Macaca) may also result in the transmission of B virus (Herpesvirus simiae, cercopithecine herpesvirus), a cause of serious infection of the human central nervous system. Bites of seals, walruses, and polar bears may cause a chronic suppurative infection known as seal finger, which is probably due to one or more species of Mycoplasma colonizing these animals.
![]() Small rodents, including rats, mice, and gerbils, as well as animals that prey on rodents may transmit Streptobacillus moniliformis (a microaerophilic, pleomorphic gram-negative rod) or Spirillum minor (a spirochete); these organisms cause a clinical illness known as rat-bite fever. The vast majority of cases in the United States are streptobacillary, whereas Spirillum infection occurs mainly in Asia.
Small rodents, including rats, mice, and gerbils, as well as animals that prey on rodents may transmit Streptobacillus moniliformis (a microaerophilic, pleomorphic gram-negative rod) or Spirillum minor (a spirochete); these organisms cause a clinical illness known as rat-bite fever. The vast majority of cases in the United States are streptobacillary, whereas Spirillum infection occurs mainly in Asia.
In the United States, the risk of rodent bites is usually greatest among laboratory workers or inhabitants of rodent-infested dwellings (particularly children). Rat-bite fever is distinguished from acute bite-wound infection by its typical manifestation after the initial wound has healed. Streptobacillary disease follows an incubation period of 3–10 days. Fever, chills, myalgias, headache, and severe migratory arthralgias are usually followed by a maculopapular rash, which characteristically involves the palms and soles and may become confluent or purpuric. Complications include endocarditis, myocarditis, meningitis, pneumonia, and abscesses in many organs. Haverhill fever is an S. moniliformis infection acquired from contaminated milk or drinking water and has similar manifestations. Streptobacillary rat-bite fever was frequently fatal in the preantibiotic era. The differential diagnosis includes Rocky Mountain spotted fever, Lyme disease, leptospirosis, and secondary syphilis. The diagnosis is made by direct observation of the causative organisms in tissue or blood, by culture of the organisms on enriched media, or by serologic testing with specific agglutinins.
Spirillum infection (referred to in Japan as sodoku) causes pain and purple swelling at the site of the initial bite, with associated lymphangitis and regional lymphadenopathy, after an incubation period of 1–4 weeks. The systemic illness includes fever, chills, and headache. The original lesion may eventually progress to an eschar. The infection is diagnosed by direct visualization of the spirochetes in blood or tissue or by animal inoculation.
Finally, NO-1 (CDC nonoxidizer group 1) is a bacterium associated with dog- and cat-bite wounds. Infections in which NO-1 has been isolated have tended to manifest locally (i.e., as abscess and cellulitis). These infections have occurred in healthy persons with no underlying illness and in some instances have progressed from localized to systemic illnesses. The phenotypic characteristics of NO-1 are similar to those of asaccharolytic Acinetobacter species; i.e., NO-1 is oxidase-, indole-, and urease-negative. To date, all strains identified have been shown to be susceptible to aminoglycosides, β-lactam antibiotics, tetracyclines, quinolones, and sulfonamides.
HUMAN BITES
Human bites may be self-inflicted; may be sustained by medical personnel caring for patients; or may take place during fights, domestic abuse, or sexual activity. Human-bite wounds become infected more frequently (~10–15% of the time) than do bites inflicted by other animals. These infections reflect the diverse oral microflora of humans, which includes multiple species of aerobic and anaerobic bacteria. Common aerobic isolates include viridans streptococci, S. aureus, E. corrodens (which is particularly common in clenched-fist injury; see below), and Haemophilus influenzae. Anaerobic species, including Fusobacterium nucleatum and Prevotella, Porphyromonas, and Peptostreptococcus species, are isolated from 50% of wound infections due to human bites; many of these isolates produce β-lactamases. The oral flora of hospitalized and debilitated patients often includes Enterobacteriaceae in addition to the usual organisms. Hepatitis B, hepatitis C, herpes simplex virus infection, syphilis, tuberculosis, actinomycosis, and tetanus have been reported to be transmitted by human bites; it is biologically possible to transmit HIV through human bites, although this event is quite unlikely.
Human bites are categorized as either occlusional injuries, which are inflicted by actual biting, or clenched-fist injuries, which are sustained when the fist of one individual strikes the teeth of another, causing traumatic laceration of the hand. For several reasons, clenched-fist injuries, which are sometimes referred to as “fight bite” and which are more common than occlusional injuries, result in particularly serious infections. The deep spaces of the hand, including the bones, joints, and tendons, are frequently inoculated with organisms in the course of such injuries. The clenched position of the fist during injury, followed by extension of the hand, may further promote the introduction of bacteria as contaminated tendons retract beneath the skin’s surface. Moreover, medical attention is often sought only after frank infection develops.
|
TREATMENT |
BITE-WOUND INFECTIONS |
WOUND MANAGEMENT
Wound closure is controversial in bite injuries. Many authorities prefer not to attempt primary closure of wounds that are or may become infected, choosing instead to irrigate these wounds copiously, debride devitalized tissue, remove foreign bodies, and approximate the wound edges. Delayed primary closure may be undertaken after the risk of infection is over. Small uninfected wounds may be allowed to close by secondary intention. Puncture wounds due to cat bites should be left unsutured because of the high rate at which they become infected. Facial wounds are usually sutured after thorough cleaning and irrigation because of the importance of a good cosmetic result in this area and because anatomic factors such as an excellent blood supply and the absence of dependent edema lessen the risk of infection.
ANTIBIOTIC THERAPY
Established Infection Antibiotics should be administered for all established bite-wound infections and should be chosen in light of the most likely potential pathogens, as indicated by the biting species and by Gram’s stain and culture results (Table 167e-1). For dog and cat bites, antibiotics should be effective against S. aureus, Pasteurella species, C. canimorsus, streptococci, and oral anaerobes. For human bites, agents with activity against S. aureus, H. influenzae, and β-lactamase-positive oral anaerobes should be used. The combination of an extended-spectrum penicillin with a β-lactamase inhibitor (amoxicillin/clavulanic acid, ticarcillin/clavulanic acid, ampicillin/sulbactam) appears to offer the most reliable coverage for these pathogens. Second-generation cephalosporins (cefuroxime, cefoxitin) also offer substantial coverage. The choice of antibiotics for penicillin-allergic patients (particularly those in whom immediate-type hypersensitivity makes the use of cephalosporins hazardous) is more difficult and is based primarily on in vitro sensitivity since data on clinical efficacy are inadequate. The combination of an antibiotic active against gram-positive cocci and anaerobes (such as clindamycin) with trimethoprim-sulfamethoxazole or a fluoroquinolone, which is active against many of the other potential pathogens, would appear reasonable. In vitro data suggest that azithromycin alone provides coverage against most commonly isolated bite-wound pathogens. As MRSA becomes more common in the community and evidence of its transmission between humans and their animal contacts increases, empirical use of agents active against MRSA should be considered in high-risk situations while culture results are awaited.
|
MANAGEMENT OF WOUND INFECTIONS FOLLOWING ANIMAL AND HUMAN BITES |
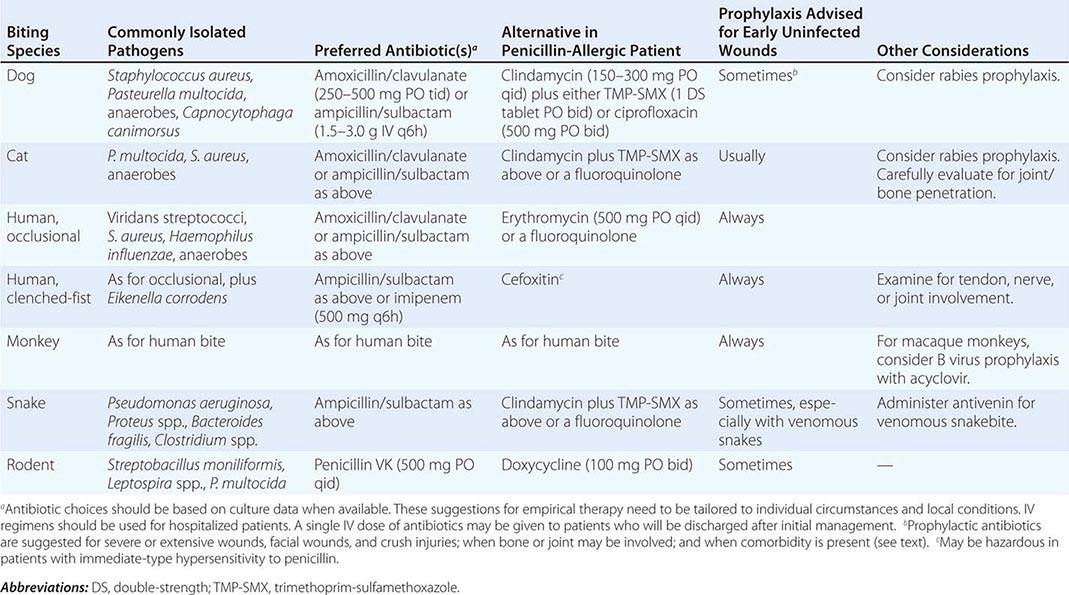
Antibiotics are generally given for 10–14 days, but the response to therapy must be carefully monitored. Failure to respond should prompt a consideration of diagnostic alternatives and surgical evaluation for possible drainage or debridement. Complications such as osteomyelitis or septic arthritis mandate a longer duration of therapy.
Management of C. canimorsus sepsis requires a 2-week course of IV penicillin G (2 million units IV every 4 h) and supportive measures. Alternative agents for the treatment of C. canimorsus infection include cephalosporins and fluoroquinolones. Serious infection with P. multocida (e.g., pneumonia, sepsis, or meningitis) also should be treated with IV penicillin G. Alternative agents include second- or third-generation cephalosporins or ciprofloxacin.
Bites by venomous snakes (Chap. 474) may not require antibiotic treatment. Because it is often difficult to distinguish signs of infection from tissue damage caused by the envenomation, many authorities continue to recommend treatment directed against the snake’s oral flora—i.e., the administration of broadly active agents such as ceftriaxone (1–2 g IV every 12–24 h) or ampicillin/sulbactam (1.5–3.0 g IV every 6 h).
Seal finger appears to respond to doxycycline (100 mg twice daily for a duration guided by the response to therapy).
Presumptive or Prophylactic Therapy The use of antibiotics for patients presenting early (within 8 h) after bite injury is controversial. Although symptomatic infection frequently will not yet have manifested at this point, many early wounds will harbor pathogens, and many will become infected. Studies of antibiotic prophylaxis for wound infections are limited and have often included only small numbers of cases in which various types of wounds have been managed according to various protocols. A meta-analysis of eight randomized trials of prophylactic antibiotics in patients with dog-bite wounds demonstrated a reduction in the rate of infection by 50% with prophylaxis. However, in the absence of sound clinical trials, many clinicians base the decision to treat bite wounds with empirical antibiotics on the species of the biting animal; the location, severity, and extent of the bite wound; and the existence of comorbid conditions in the host. All human- and monkey-bite wounds should be treated presumptively because of the high rate of infection. Most cat-bite wounds, particularly those involving the hand, should be treated. Other factors favoring treatment for bite wounds include severe injury, as in crush wounds; potential bone or joint involvement; involvement of the hands or genital region; host immunocompromise, including that due to liver disease or splenectomy; and prior mastectomy on the side of an involved upper extremity. When prophylactic antibiotics are administered, they are usually given for 3–5 days.
Rabies and Tetanus Prophylaxis Rabies prophylaxis, consisting of both passive administration of rabies immune globulin (with as much of the dose as possible infiltrated into and around the wound) and active immunization with rabies vaccine, should be given in consultation with local and regional public health authorities for some animal bites and scratches as well as for certain nonbite exposures (Chap. 232). Rabies is endemic in a variety of animals, including dogs and cats in many areas of the world. Many local health authorities require the reporting of all animal bites. A tetanus booster immunization should be given if the patient has undergone primary immunization but has not received a booster dose in the past 5 years. Patients who have not previously completed primary immunization should be immunized and should also receive tetanus immune globulin. Elevation of the site of injury is an important adjunct to antimicrobial therapy. Immobilization of the infected area, especially the hand, also is beneficial.
SECTION 3 |
CLINICAL SYNDROMES: HEALTH CARE–ASSOCIATED INFECTIONS |
168 |
Infections Acquired in Health Care Facilities |
The costs of hospital-acquired (nosocomial) and other health care–associated infections are great. These infections have affected as many as 1.7 million patients at a cost of ~$28–33 billion and 99,000 lives in U.S. hospitals annually. Although efforts to lower infection risks have been challenged by the numbers of immunocompromised patients, antibiotic-resistant bacteria, fungal and viral superinfections, and invasive devices and procedures, a prevailing viewpoint—often termed “zero tolerance”—is that almost all health care–associated infections should be avoidable with strict application of evidence-based prevention guidelines (Table 168-1). In fact, rates of device-related infections—historically, the largest drivers of risk—have fallen steadily over the past few years. Unfortunately, at the same time, antimicrobial-resistant pathogens have risen in number and are estimated to contribute to ~23,000 deaths in and outside of hospitals annually. This chapter reviews health care–associated and device-related infections as well as basic surveillance, prevention, control, and treatment activities.
|
SOURCES OF INFECTION CONTROL GUIDELINES AND OVERSIGHT |
ORGANIZATION, RESPONSIBILITIES, AND INCREASING SCRUTINY OF HEALTH CARE–ASSOCIATED INFECTION PROGRAMS
The standards of the Joint Commission require all accredited hospitals to have active programs for surveillance, prevention, and control of nosocomial infections. Education of physicians in infection control and health care epidemiology is required in infectious disease fellowship programs and is available in online courses. Concerns over “patient safety” have led to federal legislation that prevents U.S. hospitals from upgrading Medicare charges to pay for hospital costs resulting from at least 14 specific nosocomial events (Table 168-2) and have prompted national efforts to publicly report on processes of patient care (e.g., timely administration and appropriateness of perioperative antibiotic prophylaxis) and patient outcomes (e.g., surgical wound infection rates). Neither the carrot (pay-for-performance) nor the stick (nonpayment for preventable infections) appears to have impacted infection rates. The effect of public attention may be more positive; in 2009, the U.S. Department of Health and Human Services released a major interagency Action Plan to Prevent Healthcare-Associated Infections, including a list of 5-year national prevention targets that are mostly on track (Table 168-3).
aBased on the U.S. Federal Deficit Reduction Act of 2005. As of October 2012, Medicare stopped paying additional money to hospitals for these 14 health care–acquired conditions. See www.cms.gov/HospitalAcqCond/ (last accessed November 13, 2014).
|
SUMMARY OF PROGRESS TOWARD THE NINE NATIONAL TARGETS FOR ELIMINATION OF HEALTH CARE–ASSOCIATED INFECTIONS, U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES: MIDPOINT EVALUATION |
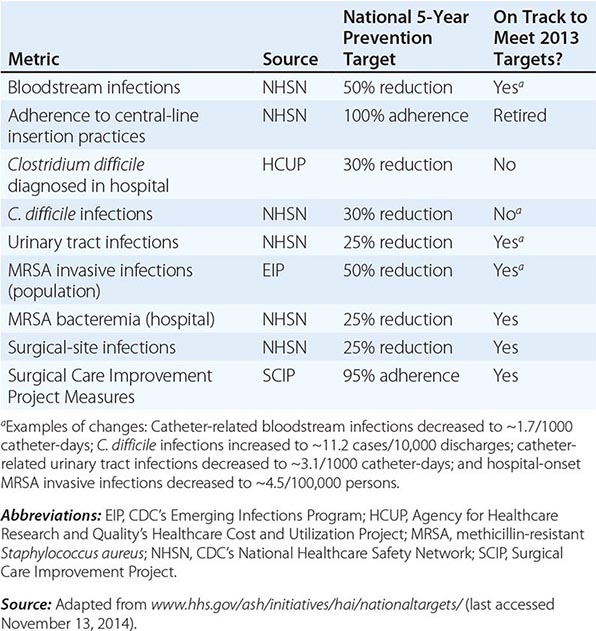
SURVEILLANCE
Traditionally, infection preventionists have surveyed inpatients for infections acquired in hospitals (defined as those neither present nor incubating at the time of admission). Surveillance most often requires review of microbiology laboratory results, “shoe-leather” epidemiology on nursing wards, and application of standardized definitions of infection. Progressively more infection-control programs use computerized hospital databases for algorithm-driven electronic surveillance (e.g., of vascular catheter and surgical wound infections) that removes observer bias and, by so doing, provides data that are more reliable for interfacility comparisons. Although infection surveillance in nursing homes and some long-term acute-care hospitals (LTACHs) is still in its formative stage, the role of these facilities in the transmission of antimicrobial-resistant pathogens will require their increased attention to infection surveillance and control.
Most hospitals aim surveillance at infections associated with high-level morbidity or expense. Quality-improvement activities in infection control have led to increased surveillance of personnel compliance with infection control policies (e.g., adherence to influenza vaccination recommendations). In the spirit of “what is measured improves,” the majority of states now require public reporting of processes for prevention of health care–associated infection and/or patient outcomes. As a result, in some locales, the surveillance pendulum is swinging back to use of “house-wide” surveillance, and many states now require that hospitals use the Centers for Disease Control and Prevention’s (CDC’s) National Healthcare Safety Network (NHSN) reporting system to provide uniform definitions and to facilitate transmission of data. Increasing reliance on the NHSN by states to facilitate public reporting has led to participation by more than 12,000 facilities (~4700 of the ~5700 acute-care hospitals in the United States, ~540 LTACHs, ~270 inpatient rehabilitation facilities, ~6000 outpatient dialysis facilities, ~300 ambulatory surgery centers, ~150 long-term-care facilities). This level of participation provides a nationwide view of health care–associated infections and represents a watershed in potential access to national rates of antimicrobial use and resistance.
Results of surveillance are expressed as rates. In general, for example, 5–10% of patients develop nosocomial infections. However, such broad statistics have little value unless qualified by duration of risk, by site of infection, by patient population, and by exposure to risk factors. To account for some of these variables, the CDC now uses a Standardized Infection Ratio (SIR; www.cdc.gov/hai/national-annual-sir/) as part of NHSN rate reporting. Meaningful denominators for infection rates include the number of patients exposed to a specific risk (e.g., patients using mechanical ventilators) or the number of intervention days (e.g., 1000 patient-days on a ventilator). As use of invasive devices such as indwelling bladder catheters has purposely been decreased, the denominators have become smaller, but the fact that patients who still require such devices may be those at intrinsically higher risk (potential numerators) may paradoxically increase rates when device-days account for the denominator. Temporal trends in rates should be reviewed, and rates should be compared with regional and national benchmarks that incorporate the SIR. Interhospital comparisons still may be misleading because of the wide range in risk factors and severity of underlying illnesses. Process measures (e.g., adherence to hand hygiene) do not usually require risk adjustment, and outcome measures (e.g., cardiac surgery wound-infection rates) can identify hospitals with outlier infection rates (e.g., in the top deciles) for further evaluation. Moreover, temporal analysis of a hospital’s infection rates can help to determine whether control measures are succeeding and where increased efforts should be focused.
EPIDEMIOLOGIC BASIS AND GENERAL MEASURES FOR PREVENTION AND CONTROL
Nosocomial infections follow basic epidemiologic patterns that can help to direct prevention and control measures. Nosocomial pathogens have reservoirs, are transmitted by largely predictable routes, and require susceptible hosts. Reservoirs and sources exist in the inanimate environment (e.g., residual Clostridium difficile spores on frequently touched surfaces in patients’ rooms) and in the animate environment (e.g., infected or colonized health care workers, patients, and hospital visitors). The mode of transmission usually is either cross-infection (e.g., indirect spread of pathogens from one patient to another on the inadequately cleaned hands of hospital personnel) or autoinoculation (e.g., aspiration of oropharyngeal flora into the lungs along an endotracheal tube). Occasionally, pathogens (e.g., group A streptococci and many respiratory viruses) are spread from person to person via large infectious droplets released by coughing or sneezing. Much less common—but often devastating in terms of epidemic risk—is true airborne spread of small or droplet nuclei (as in nosocomial chickenpox) or common-source spread (e.g., by contaminated IV fluids). Factors that increase host susceptibility include underlying conditions, abnormalities of innate defense (e.g., due to genetic polymorphisms; see Chap. 82), and medical-surgical interventions and procedures that compromise host defenses.
Hospitals’ infection-control programs must determine general and specific control measures. Given the prominence of cross-infection, hand hygiene is cited traditionally as the most important preventive measure. Health care workers’ rates of adherence to hand-hygiene recommendations are abysmally low (often <50%). Reasons cited include inconvenience, time pressures, and skin damage from frequent washing. Sinkless alcohol rubs are quick and highly effective and actually improve hand condition since they contain emollients and allow the retention of natural protective oils that would be removed with repeated rinsing. Use of alcohol hand rubs between patient contacts is recommended for all health care workers except when hands are visibly soiled or after care of a patient who is part of a health care facility outbreak of infection with C. difficile, whose spores resist killing by alcohol and require mechanical removal. In these cases, washing with soap and running water is recommended. A number of innovative systems have been developed to track hand-hygiene adherence in real time and to provide feedback; although this approach is exciting, sustained improvements in rates remain to be seen.
NOSOCOMIAL AND DEVICE-RELATED INFECTIONS
The fact that >25–50% of nosocomial infections are due to the combined effect of the patient’s own flora and invasive devices highlights the importance of improvements in the use and design of such devices. Intensive education, “bundling” of evidence-based interventions (Table 168-4), and use of checklists to facilitate adherence have reduced infection rates (Table 168-3) through improved asepsis in handling and earlier removal of invasive devices. It is especially noteworthy that turnover or shortages of trained personnel jeopardize safe and effective patient care and have been associated with increased infection rates.
|
EXAMPLES OF EVIDENCE-BASED “BUNDLED INTERVENTIONS” TO PREVENT COMMON HEALTH CARE–ASSOCIATED INFECTIONS AND OTHER ADVERSE EVENTS |
aThese components of care are supported by clinical trials and experimental evidence in the specified populations; they may prove valuable for other surgical patients as well.
Source: Adapted from information presented at the following websites: www.cdc.gov/hicpac/pubs.html; www.cdc.gov/HAI/prevent/prevention.html; www.ihi.org.
URINARY TRACT INFECTIONS
Urinary tract infections (UTIs) account for ~30–40% of nosocomial infections; up to 3% of bacteriuric patients develop bacteremia. Although UTIs contribute at most 15% to prolongation of hospital stay and may have an attributable cost in the range of only $1300, these infections are reservoirs and sources for spread of antibiotic-resistant bacteria. Most nosocomial UTIs are associated with preceding instrumentation or indwelling bladder catheters, which create a 3–7% risk of infection each day. UTIs generally are caused by pathogens that spread up the periurethral space from the patient’s perineum or gastrointestinal tract—the most common pathogenesis in women—or via intraluminal contamination of urinary catheters, usually due to cross-infection by caregivers who are irrigating catheters or emptying drainage bags. Pathogens come occasionally from inadequately disinfected urologic equipment and rarely from contaminated supplies.
Hospitals should monitor essential performance measures for preventing nosocomial UTIs (Table 168-4). Prompts to clinicians to assess a patient’s need for continued use of an indwelling bladder catheter can improve removal rates and lessen the risk of UTI. Guidelines for managing postoperative urinary retention (e.g., with bladder scanners) also may limit the use or duration of catheterization. Other approaches to the prevention of UTIs have included the use of topical meatal antimicrobial agents, drainage bag disinfectants, and anti-infective catheters. None of the latter three measures is considered routine.
Administration of systemic antimicrobial agents for other purposes decreases the risk of UTI during the first 4 days of catheterization, after which resistant bacteria or yeasts emerge as pathogens. Prophylactic antibiotic administration at the time of catheter removal has been reported to decrease the risk of UTI. Selective decontamination of the gut also is associated with a reduced risk. Again, however, none of these approaches is routine.
Irrigation of catheters, with or without antimicrobial agents, may actually increase the risk of infection. A condom catheter for men without bladder obstruction may be more acceptable than an indwelling catheter and may lessen the risk of UTI if maintained carefully. The role of suprapubic catheters in preventing infection is not well defined.
Treatment of UTIs is based on the results of quantitative urine cultures (Chap. 162). The most common pathogens are Escherichia coli, nosocomial gram-negative bacilli, enterococci, and Candida. Several caveats apply in the treatment of institutionally acquired infection. First, in patients with chronic indwelling bladder catheters, especially those in long-term-care facilities, “catheter flora”—microorganisms living on encrustations within the catheter lumen—may differ from actual urinary tract pathogens. Therefore, for suspected UTI in the setting of chronic catheterization (especially in women), it is useful to replace the bladder catheter and to obtain a freshly voided urine specimen. Second, as in all nosocomial infections, at the time treatment is initiated on the basis of a positive culture, it is useful to repeat the culture to verify the persistence of infection. Third, the frequency with which UTIs occur may lead to the erroneous assumption that the urinary tract alone is the source of infection in a febrile hospitalized patient. Fourth, recovery of Staphylococcus aureus from urine cultures may result from hematogenous seeding and may indicate an occult systemic infection. Finally, although Candida is now the most common pathogen in nosocomial UTIs among patients on intensive care units (ICUs), treatment of candiduria is often unsuccessful and is recommended only when there is upper-pole or bladder-wall invasion, obstruction, neutropenia, or immunosuppression.
PNEUMONIA
Historically, pneumonia has accounted for ~10–15% of nosocomial infections; ventilator-associated pneumonia (VAP) occurred in 1 to >4 patients per 1000 ventilator-days, and these infections were reported as responsible for a mean of 10 extra hospital days and $23,000 in extra costs per episode. Most cases of bacterial nosocomial pneumonia are caused by aspiration of endogenous or hospital-acquired oropharyngeal (and occasionally gastric) flora. Nosocomial pneumonias are associated with more deaths than are infections at any other body site. However, attributable mortality rates suggest that the risk of dying from nosocomial pneumonia is affected greatly by other factors, including comorbidities, inadequate antibiotic treatment, and the involvement of specific pathogens (particularly Pseudomonas aeruginosa or Acinetobacter). Surveillance and accurate diagnosis of pneumonia have been problematic in hospitals because many patients, especially those in the ICU, have abnormal chest roentgenographs, fever, and leukocytosis potentially attributable to multiple causes. This diagnostic uncertainty has led to a refocus from VAP to “ventilator-associated events” (VAEs), conditions, and complications, for which worsening physiologic parameters, such as oxygenation, are key metrics. Early data suggest that ~5–10% of patients using mechanical ventilators develop VAEs. Viral pneumonias, which are particularly important in pediatric and immunocompromised patients, are discussed in the virology section and in Chap. 153.
Risk factors for nosocomial pneumonia include those events that increase colonization by potential pathogens (e.g., prior antimicrobial therapy, contaminated ventilator circuits or equipment, or decreased gastric acidity); those that facilitate aspiration of oropharyngeal contents into the lower respiratory tract (e.g., intubation, decreased levels of consciousness, or presence of a nasogastric tube); and those that reduce host defense mechanisms in the lung and permit overgrowth of aspirated pathogens (e.g., chronic obstructive pulmonary disease, extremes of age, or upper abdominal surgery).
Control measures for pneumonia (Table 168-4) are aimed at frequent testing of readiness for extubation, remediation of risk factors in patient care (e.g., minimizing aspiration-prone supine positioning), and aseptic care of respirator equipment (e.g., disinfecting or sterilizing all inline reusable components such as nebulizers, replacing tubing/breathing circuits only if required because of malfunction or visible soiling—rather than on the basis of duration of use—to lessen the number of breaks in the system, and teaching aseptic technique for suctioning). Although the benefits of selective decontamination of the oropharynx and gut with nonabsorbable antimicrobial agents and/or use of short-course postintubation systemic antibiotics have been controversial, a randomized multicenter trial demonstrated lowered ICU mortality rates among patients on mechanical ventilation who underwent oropharyngeal decontamination.
Among the logical preventive measures that require further investigation are placement of endotracheal tubes that provide channels for subglottic drainage of secretions, which has been associated with reduced infection risks during short-term postoperative use, and noninvasive mechanical ventilation whenever feasible. Use of silver-coated endotracheal tubes may lessen risk of VAP but is not considered routine. It is noteworthy that reducing the rate of VAP often has not reduced overall ICU mortality; this fact suggests that this infection is a marker for patients with an otherwise-heightened risk of death.
The most likely pathogens for nosocomial pneumonia and treatment options are discussed in Chap. 153. Several considerations regarding diagnosis and treatment are worth emphasizing. First, clinical criteria for diagnosis (e.g., fever, leukocytosis, development of purulent secretions, new or changing radiographic infiltrates, changes in oxygen requirement or ventilator settings) have high sensitivity but relatively low specificity. These criteria are most useful for selecting patients for bronchoscopic or nonbronchoscopic procedures that yield lower respiratory tract samples protected from upper-tract contamination; quantitative cultures of such specimens have diagnostic sensitivities in the range of 80%. Second, early-onset nosocomial pneumonia, which manifests within the first 4 days of hospitalization, is most often caused by community-acquired pathogens such as Streptococcus pneumoniae and Haemophilus species, although some studies have challenged this view. Late-onset pneumonias most commonly are due to S. aureus, P. aeruginosa, Enterobacter species, Klebsiella pneumoniae, or Acinetobacter. When invasive techniques are used to diagnose VAP, the proportion of isolates accounted for by gram-negative bacilli decreases from 50–70% to 35–45%. Infection is polymicrobial in as many as 20–40% of cases. The role of anaerobic bacteria in VAP is not well defined. Third, one multicenter study suggested that 8 days is an appropriate duration of therapy for nosocomial pneumonia, with a longer duration (15 days in that study) when the pathogen is Acinetobacter or P. aeruginosa. Finally, in febrile patients (particularly those who have endotracheal or gastric tubes inserted through the nares), occult respiratory tract infections, especially bacterial sinusitis and otitis media, should be considered.
SURGICAL WOUND INFECTIONS
Wound infections occur in ~500,000 patients each year, account for ~15–20% of nosocomial infections, contribute up to 7–10 extra postoperative hospital days, and result in $3000 to $29,000 in extra costs, depending on the operative procedure and pathogen(s). The average wound infection has an incubation period of 5–7 days—longer than many postoperative stays. For this reason and because many procedures are now performed on an outpatient basis, the incidence of wound infections has become more difficult to assess. These infections usually are caused by the patient’s endogenous or hospital-acquired skin and mucosal flora and occasionally are due to airborne spread of skin squames that may be shed into the wound from members of the operating-room team. True airborne spread of infection through droplet nuclei is rare in operating rooms unless there is a “disseminator” (e.g., of group A streptococci or staphylococci) among the staff. In general, the common risks for postoperative wound infection are related to the surgeon’s technical skill, the patient’s underlying conditions (e.g., diabetes mellitus, obesity) or advanced age, and inappropriate timing of antibiotic prophylaxis. Additional risks include the presence of drains, prolonged preoperative hospital stays, shaving of operative sites by razor the day before surgery, long duration of surgery, and infection at remote sites (e.g., untreated UTI).
The substantial literature related to risk factors for surgical-site infections and the recognized morbidity and cost of these infections have led to national prevention efforts and to recommendations for “bundling” preventive measures (Table 168-4). Additional measures include attention to technical surgical issues (e.g., avoiding open or prophylactic drains), operating-room asepsis, and preoperative therapy for active infection. Reporting surveillance results to surgeons has been associated with reductions in infection rates. Preoperative administration of intranasal mupirocin to patients colonized with S. aureus, preoperative antiseptic bathing, and intra- and postoperative oxygen supplementation have been controversial because of conflicting study results, but evidence seems mostly to favor these interventions.
The process of diagnosing and treating wound infections begins with a careful assessment of the surgical site in the febrile postoperative patient. Diagnosis of deeper organ-space infections or subphrenic abscesses requires a high index of suspicion and the use of CT or MRI. Diagnosis of infections of prosthetic devices, such as orthopedic implants, may be particularly difficult and often requires the use of interventional radiographic techniques to obtain periprosthetic specimens for culture. Cultures of periprosthetic joint tissue obtained at surgery may miss pathogens that are cloistered in prosthesis-adherent biofilms; cultures of sonicates from explanted prosthetic joints have been more sensitive, particularly for patients who have received antimicrobial agents within 2 weeks of surgery.
The most common pathogens in postoperative wound infections are S. aureus, coagulase-negative staphylococci, and enteric and anaerobic bacteria. In rapidly progressing postoperative infections manifesting within 24–48 h of a surgical procedure, the level of suspicion regarding group A streptococcal or clostridial infection (Chaps. 173 and 179) should be high. Treatment of postoperative wound infections requires drainage or surgical excision of infected or necrotic material and antibiotic therapy aimed at the most likely or laboratory-confirmed pathogens.
INFECTIONS RELATED TO VASCULAR ACCESS AND MONITORING
Intravascular device–related bacteremias cause ~10–15% of nosocomial infections; central vascular catheters (CVCs) account for most of these bloodstream infections. Past national estimates indicated that as many as 200,000 bloodstream infections associated with CVCs occurred each year in the United States, with attributable mortality rates of 12–25%, an excess mean length of hospital stay of 12 days, and an estimated cost of $3700 to $29,000 per episode; one-third to one-half of these episodes occurred in ICUs. However, infection rates have dropped steadily (Table 168-3) since the publication of guidelines by the Healthcare Infection Control Practices Advisory Committee (HICPAC) in 2002. With increasing care of seriously ill patients in the community, vascular catheter–associated bloodstream infections acquired in outpatient settings are becoming more frequent. Broader surveillance for infections—outside ICUs and even outside hospitals—will be needed.
Catheter-related bloodstream infections derive largely from the cutaneous microflora of the insertion site, with pathogens migrating extraluminally to the catheter tip, usually during the first week after insertion. In addition, contamination of the hubs of CVCs or of the ports of “needle-less” systems may lead to intraluminal infection over longer periods, particularly with surgically implanted or cuffed catheters. Intrinsic (during the manufacturing process) or extrinsic (on-site in a health care facility) contamination of infusate, although rare, is the most common cause of epidemic device-related bloodstream infection; extrinsic contamination may cause up to half of endemic bacteremias related to arterial infusions used for hemodynamic monitoring. The most common pathogens isolated from vascular device–associated bacteremias include coagulase-negative staphylococci, S. aureus (with ≥50% of isolates in the United States resistant to methicillin), enterococci, nosocomial gram-negative bacilli, and Candida. Many pathogens, especially staphylococci, produce extracellular polysaccharide biofilms that facilitate attachment to catheters and provide sanctuary from antimicrobial agents. “Quorum-sensing” proteins, a target for future interventions, help bacterial cells communicate during biofilm development.
Evidence-based bundles of control measures (Table 168-4) have been strikingly effective, eliminating almost all CVC-associated infections in one ICU study. Additional control measures for infections associated with vascular access include use of a chlorhexidine-impregnated patch at the skin-catheter junction; daily bathing of ICU patients with chlorhexidine; application of semitransparent access-site dressings (for ease of bathing and site inspection and protection of the site from secretions); avoidance of the femoral site for catheterization because of a higher risk of infection (most likely related to the density of the skin flora); rotation of peripheral catheters to a new site at specified intervals (e.g., every 72–96 h), which may be facilitated by use of an IV therapy team; and application of aseptic technique when accessing pressure transducers or other vascular ports.
Unresolved issues include the role of gut translocation rather than vascular access sites as a cause of primary bacteremia in immunocompromised patients and the implications for surveillance definitions; the best frequency for rotation of CVC sites (given that guidewire-assisted catheter changes at the same site do not lessen and can even increase infection risk); the appropriate role of mupirocin ointment, a topical antibiotic with excellent antistaphylococcal activity, in site care; the relative degrees of risk posed by peripherally inserted central catheters (PICC lines); and the risk-benefit of prophylactic use of heparin (to avoid catheter thrombi, which may be associated with increased risk of infection) or of vancomycin or alcohol (as catheter flushes or “locks”—i.e., concentrated anti-infective solutions instilled into the catheter lumen) for high-risk patients.
Vascular device–related infection is suspected on the basis of the appearance of the catheter site or the presence of fever or bacteremia without another source in patients with vascular catheters. The diagnosis is confirmed by the recovery of the same species of microorganism from peripheral-blood cultures (preferably two samples drawn from peripheral veins by separate venipunctures) and from semiquantitative or quantitative cultures of the vascular catheter tip. Less commonly used diagnostic measures include (1) differential (faster) time to positivity (>2 h) for blood drawn through the vascular access device than for a sample from a peripheral vein and (2) differences in quantitative cultures (a threefold or greater “step-up”) for blood samples drawn simultaneously from a peripheral vein and from a CVC, which should show the step-up if infected. When infusion-related sepsis is considered (e.g., because of the abrupt onset of fever or shock temporally related to infusion therapy), a sample of the infusate or blood product should be retained for culture.
Therapy for vascular access–related infection is directed at the pathogen recovered from the blood and/or infected site. Important considerations in treatment are the need for an echocardiogram (to evaluate the patient for endocarditis), the duration of therapy, and the need to remove potentially infected catheters. In one report, approximately one-fourth of patients with intravascular catheter–associated S. aureus bacteremia who were studied by transesophageal echocardiography had evidence of endocarditis; this test may be useful in determining the appropriate duration of treatment.
Detailed consensus guidelines for the management of intravascular catheter–related infections have been published and recommend catheter removal in most cases of bacteremia or fungemia due to nontunneled CVCs. When attempting to salvage a potentially infected catheter, some clinicians use the “antibiotic lock” technique, which may facilitate penetration of infected biofilms, in addition to systemic antimicrobial therapy (see www.idsociety.org/Other_Guidelines/).
The authors of the consensus treatment guidelines advise that the decision to remove a tunneled catheter or implanted device suspected of being the source of bacteremia or fungemia should be based on the severity of the patient’s illness, the strength of evidence that the device is infected, the presence of local or systemic complications, an assessment of the specific pathogens, and the patient’s response to antimicrobial therapy if the catheter or device is initially retained. For patients with track-site infection, successful therapy without catheter removal is unusual. For patients with suppurative venous thrombophlebitis, excision of affected veins is usually required.
ISOLATION TECHNIQUES
Written policies for the isolation of infectious patients are a standard component of infection control programs. To replace its prior pathogen-specific guidelines, the CDC published recommendations in 2006 for the control of multidrug-resistant organisms in health care settings; in 2007, the CDC published a revised edition of its basic isolation guidelines to provide updated recommendations for all components of health care, including acute-care hospitals and long-term, ambulatory, and home-care settings (see www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf).
Standard precautions are designed for the care of all patients in hospitals and aim to reduce the risk of transmission of microorganisms from both recognized and unrecognized sources. These precautions include gloving as well as hand cleansing for potential contact with (1) blood; (2) all other body fluids, secretions, and excretions, whether or not they contain visible blood; (3) nonintact skin; and (4) mucous membranes. Depending on exposure risks, standard precautions also include use of masks, eye protection, and gowns.
Precautions for the care of patients with potentially contagious clinical syndromes (e.g., acute diarrhea) or with suspected or diagnosed colonization or infection by transmissible pathogens are based on probable routes of transmission: airborne, droplet, or contact, for which personnel don, at a minimum, N95 respirators, surgical face masks, or glove and gown, respectively. Sets of precautions may be combined for diseases that have more than one route of transmission (e.g., contact and airborne isolation for varicella).
Some prevalent antibiotic-resistant pathogens, particularly those that colonize the gastrointestinal tract (e.g., vancomycin-resistant enterococci [VRE] and even multidrug-resistant gram-negative bacilli such as carbapenemase-producing strains of K. pneumoniae [KPCs]), may be present on intact skin of patients in hospitals (the “fecal patina”). This issue has led some experts to recommend gloving for all contact with patients who are acutely ill and/or in high-risk units, such as ICUs or LTACHs. Wearing gloves does not replace the need for hand hygiene because hands sometimes (in up to 20% of interactions) become contaminated during wearing or removal of gloves.
EPIDEMIC AND EMERGING PROBLEMS
Outbreaks are always big news but probably account for <5% of nosocomial infections. The investigation and control of nosocomial epidemics require that infection control personnel (1) develop a case definition, (2) confirm that an outbreak really exists (since apparent epidemics may actually be pseudo-outbreaks due to surveillance or laboratory artifacts), (3) review aseptic practices and disinfectant use, (4) determine the extent of the outbreak, (5) perform an epidemiologic investigation to determine modes of transmission, (6) work closely with microbiology personnel to culture for common sources or personnel carriers as appropriate and to type epidemiologically important isolates, and (7) heighten surveillance to judge the effect of control measures. Control measures generally include reinforcing routine aseptic practices and hand hygiene, ensuring appropriate isolation of cases (and instituting cohort isolation and nursing if needed), and implementing further controls on the basis of the investigation’s findings. Examples of some emerging and potential epidemic problems follow.
VIRAL RESPIRATORY INFECTIONS: PANDEMIC INFLUENZA
![]() Infections caused by the severe acute respiratory syndrome (SARS)–associated coronavirus challenged health care systems globally in 2003 (Chap. 223), and in 2012 Middle East respiratory syndrome coronavirus (MERS-CoV) emerged as a more geographically localized problem (Chap. 223). For SARS, basic infection-control measures helped to keep the worldwide case and death counts at ~8000 and ~800, respectively, although the virus was unforgiving of lapses in protocol adherence or laboratory biosafety. The epidemiology of SARS—spread largely in households once patients were ill or in hospitals—contrasts markedly with that of influenza (Chap. 224), which is often contagious a day before symptom onset; can spread rapidly in the community among nonimmune persons; and, even in its seasonal variety, kills as many as 35,000 persons each year in the United States.
Infections caused by the severe acute respiratory syndrome (SARS)–associated coronavirus challenged health care systems globally in 2003 (Chap. 223), and in 2012 Middle East respiratory syndrome coronavirus (MERS-CoV) emerged as a more geographically localized problem (Chap. 223). For SARS, basic infection-control measures helped to keep the worldwide case and death counts at ~8000 and ~800, respectively, although the virus was unforgiving of lapses in protocol adherence or laboratory biosafety. The epidemiology of SARS—spread largely in households once patients were ill or in hospitals—contrasts markedly with that of influenza (Chap. 224), which is often contagious a day before symptom onset; can spread rapidly in the community among nonimmune persons; and, even in its seasonal variety, kills as many as 35,000 persons each year in the United States.
Control of seasonal influenza has depended on (1) the use of effective vaccines, with increasingly broad evidence-based recommendations for vaccination of children, the general public, and health care workers; (2) the use of antiviral medications for early treatment and for prophylaxis as part of outbreak control, especially for high-risk patients and in high-risk settings like nursing homes or hospitals; and (3) infection control (surveillance and droplet precautions) for symptomatic patients. Controversial infection-control issues have been the questionable role of airborne spread of influenza and the need to mandate influenza vaccination of health care workers because of the embarrassingly low rates of vaccination in this high-risk group.
With the occurrence of localized outbreaks of avian (H5N1) influenza in Asia over the past few years, concerns about potential pandemic influenza led to (1) recommendations for universal “respiratory hygiene and cough etiquette” (basically, “cover your cough”), as described and promoted in the CDC’s 2007 Guideline for Isolation Precautions, and for “source containment” (e.g., use of face masks and spatial separation) for outpatients with potentially infectious respiratory illnesses; (2) re-examinations of the value in the 1918–1919 influenza pandemic of nonpharmacologic interventions, such as “social distancing” (e.g., closing of schools and community venues); and (3) debate about the level of respiratory protection required for health care workers (i.e., whether to use the higher-efficiency N95 respirators recommended for airborne isolation rather than the surgical masks used for droplet precautions).
In the spring of 2009, a novel strain of influenza virus—H1N1 or “swine flu” virus—caused the first influenza pandemic in four decades. Recombinant events that create new strains (e.g., H7N9) continue to challenge global efforts at infection control and vaccine development (Chap. 224).
NOSOCOMIAL DIARRHEA
A new, more virulent strain of C. difficile—NAP1/BI/027—emerged in North America, and overall rates of C. difficile–associated diarrhea (Chap. 161) have increased, especially among older patients, in U.S. hospitals during the past few years. C. difficile control measures include judicious use of all antibiotics, especially fluoroquinolone antibiotics that have been implicated in driving these changes; heightened suspicion for “atypical” presentations (e.g., toxic megacolon or leukemoid reaction without diarrhea); and early diagnosis, treatment, and contact precautions. To improve diagnosis, use of more sensitive polymerase chain reaction–based rather than enzyme immunoassay–based testing of diarrheal stool is now recommended, with resultant artificial doubling of infection rates in some hospitals. Preliminary data suggest a role for probiotics in the prevention of C. difficile– associated diarrhea in patients in whom systemic antibiotic therapy is being initiated. Fecal transplantation has had dramatic results in the treatment of relapsing cases of C. difficile–associated diarrhea (Chap. 161). Successes with fecal transplants and probiotics have called attention to the potential role of manipulation of the intestinal microbiome as a broader infection-control strategy.
Outbreaks of norovirus infection (Chap. 227) in U.S. and European health care facilities appear to continue to increase in frequency or at least in reporting, with the virus often introduced by ill visitors or staff. This pathogen should be suspected when nausea and vomiting are prominent aspects of bacterial culture–negative diarrheal syndromes. Contact precautions may need to be augmented by aggressive environmental cleaning (given the persistence of norovirus on inanimate objects), prevention of secondary cases in cleaning staff through an emphasis on the use of personal protective equipment and hand hygiene, and active exclusion of ill staff and visitors.
CHICKENPOX
Infection control practitioners institute a varicella exposure investigation and control plan whenever health care workers have been exposed to chickenpox (Chap. 217) or have worked while having or during the 24 h before developing chickenpox. The names of exposed workers and patients are obtained; medical histories are reviewed, and (if necessary) serologic tests for immunity are conducted; physicians are notified of susceptible exposed patients; postexposure prophylaxis with a preparation of varicella-zoster immune globulin (VZIG) is considered for immunocompromised or pregnant contacts, with administration as soon as possible (but as long as 10 days after exposure) (Table 217-1); varicella vaccine is recommended or preemptive use of acyclovir is considered as an alternative strategy in other susceptible persons; and susceptible exposed employees are furloughed during the at-risk period for disease (8–21 days or, if VZIG has been administered, 28 days). Routine varicella vaccination of children and susceptible employees has made nosocomial spread less common and less problematic.
TUBERCULOSIS
Important measures for the control of tuberculosis (Chap. 202) include prompt recognition, isolation, and treatment of cases; recognition of atypical presentations (e.g., lower-lobe infiltrates without cavitation); use of negative-pressure, 100% exhaust, private isolation rooms with closed doors and at least 6–12 air changes per hour; use of N95 respirators by caregivers entering isolation rooms; possible use of high-efficiency particulate air filter units and/or ultraviolet lights for disinfecting air when other engineering controls are not feasible or reliable; and follow-up testing of susceptible personnel who have been exposed to infectious patients before isolation. The use of serologic tests, rather than skin tests, in the diagnosis of latent tuberculosis for infection control purposes has become common, mostly for logistic reasons. As tuberculosis once again is on the decline in the United States, we need to remember that the price of freedom—in this instance, from a communicable disease—is eternal vigilance.
GROUP A STREPTOCOCCAL INFECTIONS
The potential for an outbreak of group A streptococcal infection (Chap. 173) should be considered when even one or two nosocomial cases occur. Most outbreaks involve surgical wounds and are due to the presence of an asymptomatic carrier in the operating room. Investigation can be confounded by carriage at extrapharyngeal sites such as the rectum and vagina. Health care workers in whom carriage has been linked to nosocomial transmission of group A streptococci are removed from the patient-care setting and are not permitted to return until carriage has been eliminated by antimicrobial therapy.
FUNGAL INFECTIONS
Fungal spores are common in the environment, particularly on dusty surfaces. When dusty areas are disturbed during hospital repairs or renovation, the spores become airborne. Inhalation of spores by immunosuppressed (especially neutropenic) patients creates a risk of pulmonary and/or paranasal sinus infection and disseminated aspergillosis (Chap. 241). Routine surveillance among neutropenic patients for infections with filamentous fungi, such as Aspergillus and Fusarium, helps hospitals to determine whether they are facing environmental risks. As a matter of routine, hospitals should inspect and clean air-handling equipment, review all planned renovations with infection control personnel and subsequently construct appropriate barriers, remove immunosuppressed patients from renovation sites, and consider the use of high-efficiency particulate air intake filters for rooms housing immunosuppressed patients.
A major multistate iatrogenic outbreak of meningitis, localized spinal or paraspinal infection, and arthritis due to Exserohilum rostratum was recognized in 2012 and traced to contamination of an injectable preservative-free steroid product produced by a single compounding pharmacy (Chap. 241).
LEGIONELLOSIS
Nosocomial Legionella pneumonia (Chap. 184) is most often due to contamination of potable water and predominantly affects immunosuppressed patients, particularly those receiving glucocorticoid medications. The risk varies greatly within and among geographic regions, depending on the extent of hospital water contamination and on specific hospital practices (e.g., inappropriate use of nonsterile water in respiratory therapy equipment). Laboratory-based surveillance for nosocomial Legionella should be performed, and a diagnosis of legionellosis should probably be considered more often than it is. If nosocomial cases are detected, environmental samples (e.g., tap water) should be cultured. If cultures yield Legionella and if typing of clinical and environmental isolates reveals a correlation, eradication measures should be pursued. An alternative approach is to periodically culture tap water in wards housing high-risk patients. If Legionella is found, a concerted effort should be made to culture samples from all patients with nosocomial pneumonia for Legionella.
ANTIBIOTIC-RESISTANT BACTERIA
Emerging multidrug-resistant bacteria like KPCs are harbingers of a potential “postantibiotic” era. Control of antibiotic resistance depends on close laboratory surveillance, with early detection of problems; on aggressive reinforcement of routine asepsis; on implementation of barrier precautions for all colonized and/or infected patients; on use of patient-surveillance cultures to more fully ascertain the extent of patient colonization; on antimicrobial stewardship to lessen ecologic pressures; and on timely initiation of an epidemiologic investigation when rates increase. Molecular typing (e.g., pulsed-field gel electrophoresis and, most recently, whole-genome sequencing) can help differentiate an outbreak due to a single strain (which necessitates an emphasis on hand hygiene and an evaluation of potential common-source exposures) from a polyclonal outbreak (which requires an emphasis on antibiotic prudence and device bundles; Table 168-4). Continuing emergence of multidrug-resistant organisms suggests that control efforts have been insufficient and that regional or broader (national and global) strategies and interventions are urgently needed (see www.cdc.gov/drugresistance/threat-report-2013/ and www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018/).
Currently, several antibiotic resistance problems are of particular concern. First, over the past decade or so, the emergence of community-associated methicillin-resistant S. aureus (CA-MRSA) has been dramatic in many countries, with as many as 50% of community-acquired “staph infections” in some U.S. cities now caused by strains resistant to β-lactam antibiotics (Chap. 172). The incursion of CA-MRSA into hospitals is well documented and has impacted surveillance and control of nosocomial MRSA infections.
Second, in the ongoing global reemergence of nosocomial multidrug-resistant gram-negative bacilli, new problems include plasmid-mediated resistance to fluoroquinolones, metallo-β-lactamase-mediated resistance to carbapenems, KPCs, and panresistant strains of Acinetobacter. The problematic New Delhi metallo-β-lactamase (NDM) is plasmid-mediated, has been highly successful in inter-genus transmission, and has quickly become a global threat (see wwwnc.cdc.gov/eid/article/17/10/11-0655_article.htm). For several years, KPCs were a very focal problem in the United States (predominantly in Brooklyn, NY), but more recently these strains have become a national threat. Many multidrug-resistant gram-negative bacilli are susceptible only to colistin, a drug that is consequently being “rediscovered,” or to no available agents.
Third, there has been renewed recognition of the role of nursing homes, and now LTACHs, in the spread of resistant gram-negative bacilli such as KPCs. In some LTACHs, as many as 30–50% of patients may be colonized with KPCs.
Fourth, there has been increasing community-based spread of E. coli strains harboring an enzyme, CTX-M, that renders them broadly resistant to β-lactam antibiotics. Given the community focus of spread, these strains may be seen as a gram-negative version of CA-MRSA.
Finally, clinical infections with MRSA strains exhibiting high-level vancomycin resistance due to VRE-derived plasmids have been reported in a few patients—almost all in the United States and most in Michigan—in the setting of prolonged or repeated treatment with vancomycin and/or VRE colonization. Much more common is vancomycin “MIC creep”: an increasing prevalence of MRSA strains that exhibit upper-limit susceptibility to vancomycin.
Colonized personnel who are implicated in nosocomial transmission of multidrug-resistant pathogens and patients who pose a threat can be decontaminated, depending on the pathogen. In a few ICUs, nonabsorbed antimicrobial agents for gastrointestinal decontamination of patients have been used successfully as a temporary emergency control measure for outbreaks of infection due to gram-negative bacilli. Potentially, manipulation of patients’ intestinal microbiome could be a more durable strategy to control outbreaks of multidrug-resistant pathogens that have a gastrointestinal reservoir.
![]() In several trials over the past 10 years, source control—i.e., removal of patients’ fecal patina—by daily bathing with chlorhexidine has reduced the risk of bacteremia in ICU patients. “Search-and-destroy” methods—i.e., active surveillance cultures to detect and isolate the “resistance iceberg” of patients colonized with MRSA—in nonoutbreak settings are credited with elimination of nosocomial MRSA in the Netherlands and Denmark. In a recent multicenter trial in the United States, universal source control with chlorhexidine and nasal mupirocin was significantly more effective for controlling MRSA than was a search-and-destroy approach and led to control of other pathogens as well, providing a broad (“horizontal”) rather than a narrower (“vertical”) intervention (see www.ahrq.gov/professionals/systems/hospital/universal_icu_decolonization/). For some pathogens, such as VRE, enforcement of environmental cleaning also reduces cross-transmission risk.
In several trials over the past 10 years, source control—i.e., removal of patients’ fecal patina—by daily bathing with chlorhexidine has reduced the risk of bacteremia in ICU patients. “Search-and-destroy” methods—i.e., active surveillance cultures to detect and isolate the “resistance iceberg” of patients colonized with MRSA—in nonoutbreak settings are credited with elimination of nosocomial MRSA in the Netherlands and Denmark. In a recent multicenter trial in the United States, universal source control with chlorhexidine and nasal mupirocin was significantly more effective for controlling MRSA than was a search-and-destroy approach and led to control of other pathogens as well, providing a broad (“horizontal”) rather than a narrower (“vertical”) intervention (see www.ahrq.gov/professionals/systems/hospital/universal_icu_decolonization/). For some pathogens, such as VRE, enforcement of environmental cleaning also reduces cross-transmission risk.
Because the excessive use of broad-spectrum antibiotics underlies many resistance problems, “antibiotic stewardship” has been promulgated actively. The main tenets are to restrict the use of particular agents to narrowly defined indications in order to limit selective pressure on the nosocomial flora and, when broad-spectrum therapy is begun empirically in critically ill patients, to “de-escalate” treatment as soon as possible on the basis of the results of culture and susceptibility tests.
BIOTERRORISM AND OTHER “SURGE-EVENT” PREPAREDNESS
![]() The horrific attack on the World Trade Center in New York City on September 11, 2001; the subsequent mailings of anthrax spores in the United States; the Boston Marathon bombing in 2013; and ongoing revelations of terrorist plans and activities in many other countries as well as the United States have made bioterrorism a prominent source of concern to hospital infection-control programs. The essentials for hospital preparedness entail education, internal and external communication, and risk assessment. Up-to-date information is available from the CDC (see www.bt.cdc.gov).
The horrific attack on the World Trade Center in New York City on September 11, 2001; the subsequent mailings of anthrax spores in the United States; the Boston Marathon bombing in 2013; and ongoing revelations of terrorist plans and activities in many other countries as well as the United States have made bioterrorism a prominent source of concern to hospital infection-control programs. The essentials for hospital preparedness entail education, internal and external communication, and risk assessment. Up-to-date information is available from the CDC (see www.bt.cdc.gov).
EMPLOYEE HEALTH SERVICE ISSUES
An institution’s employee health service is a critical component of its infection control efforts. New employees should be processed through the service, where a contagious-disease history can be taken; evidence of immunity to a variety of diseases, such as hepatitis B, chickenpox, measles, mumps, and rubella, can be sought; immunizations for hepatitis B, measles, mumps, rubella, varicella, and pertussis (the only vaccine-preventable childhood disease that is on the rise again in the United States) can be given as needed; baseline tuberculosis testing can be performed; and education about personal responsibility for infection control can be initiated. Evaluations of employees should be codified to meet the requirements of accrediting and regulatory agencies.
The employee health service must have protocols for dealing with workers exposed to contagious diseases (e.g., influenza) and those percutaneously or mucosally exposed to the blood of patients infected with HIV or hepatitis B or C virus. For example, postexposure HIV prophylaxis (PEP) with combination antiretroviral agents is recommended, as indicated; free consultation is available from the CDC-funded PEPLine (888-HIV-4911). Protocols are also needed for dealing with caregivers who have common contagious diseases (such as chickenpox, group A streptococcal infection, influenza or another respiratory infection, or infectious diarrhea) and for those who have less common but high-visibility public health problems (such as chronic hepatitis B or C or HIV infection) for which exposure-control guidelines have been published by the CDC and by the Society for Healthcare Epidemiology of America.
169 |
Infections in Transplant Recipients |
This chapter considers aspects of infection unique to patients receiving transplanted tissue. The evaluation of infections in transplant recipients involves consideration of both the donor and the recipient of the transplanted cells or organ. Two central issues are of paramount importance: (1) infectious agents (particularly viruses, but also bacteria, fungi, and parasites) can be introduced into the recipient by the donor; and (2) treatment of the recipient with medicine to prevent rejection can suppress normal immune responses, greatly increasing susceptibility to infection. Thus, what might have been a latent or asymptomatic infection in an immunocompetent donor or in the recipient prior to therapy can become a life-threatening problem when the recipient becomes immunosuppressed. The pretransplantation evaluation of each patient should be guided by an analysis of both (1) what infections the recipient is currently harboring, since organisms that exist in a state of latency or dormancy before the procedure may cause fatal disease when the patient receives immunosuppressive treatment; and (2) what organisms are likely to be transmitted by the donor, particularly those to which the recipient may be naïve.
PRETRANSPLANTATION EVALUATION
The Donor A variety of organisms have been transmitted by organ transplantation. Transmission of infections that may have been latent or not clinically apparent in the donor has resulted in the development of specific donor-screening protocols. Results from routine blood bank studies, including those for antibodies to Treponema pallidum (syphilis), Trypanosoma cruzi, hepatitis B and C viruses, HIV-1 and -2, human T-lymphotropic virus types 1 and 2 (HTLV-1 and -2), and West Nile virus (WNV), should be documented. Serologic studies should be ordered to identify latent infection with viruses such as herpes simplex virus types 1 and 2 (HSV-1, HSV-2), varicella-zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), Kaposi’s sarcoma–associated herpesvirus (KSHV); acute infection with hepatitis A virus; and infection with the common parasite Toxoplasma gondii. Donors should be screened, when relevant, for viruses such as rabies virus and lymphocytic choriomeningitis virus as well as for parasites such as Strongyloides stercoralis and Schistosoma species. Clinicians caring for prospective organ donors should examine chest radiographs for evidence of granulomatous disease (e.g., caused by mycobacteria or fungi) and should perform skin testing or obtain blood for immune cell–based assays that detect active or latent Mycobacterium tuberculosis infection. An investigation of the donor’s dietary habits (e.g., consumption of raw meat or fish or of unpasteurized dairy products), occupations or avocations (e.g., gardening or spelunking), and travel history (e.g., travel to areas with endemic fungi) also is indicated and may mandate additional testing. Creutzfeldt-Jakob disease has been transmitted through corneal transplants. Whether it can be transmitted by transfused blood is not known. Variant Creutzfeldt-Jakob disease can be transmitted with transfused non-leukodepleted blood, posing a theoretical risk to transplant recipients.
The Recipient It is expected that the recipient will have been even more comprehensively assessed than the donor. Additional studies recommended for the recipient include evaluation for acute respiratory viruses and gastrointestinal pathogens in the immediate pretransplantation period. An important caveat is that, because of immune dysfunction resulting from chemotherapy or underlying chronic disease, serologic testing of the recipient may prove less reliable than usual.
The Donor Cells/Organ Careful attention to the sterility of the medium used to process the donor organ, combined with meticulous microbiologic evaluation, reduces rates of transmission of bacteria (or, rarely, yeasts) that may be present or grow in the organ culture medium. From 2% to >20% of donor kidneys are estimated to be contaminated with bacteria—in most cases, with the organisms that colonize the skin or grow in the tissue culture medium used to bathe the donor organ while it awaits implantation. The reported rate of bacterial contamination of transplanted stem cells (bone marrow, peripheral blood, cord blood) is as high as 17% but most commonly is ~1%. The use of enrichment columns and monoclonal antibody depletion procedures results in a higher incidence of contamination. In one series of patients receiving contaminated stem cells, 14% had fever or bacteremia, but none died. Results of cultures performed at the time of cryopreservation and at the time of thawing were helpful in guiding therapy for the recipient.
INFECTIONS IN HEMATOPOIETIC STEM CELL TRANSPLANT RECIPIENTS
Transplantation of hematopoietic stem cells (HSCs) from bone marrow or from peripheral or cord blood for cancer, immunodeficiency, or autoimmune disease most often results in a transient state of complete immunologic incompetence. Immediately after myeloablative chemotherapy and transplantation, both innate immune cells (phagocytes, dendritic cells, natural killer cells) and adaptive immune cells (T and B cells) are absent, and the host is extremely susceptible to infection. The reconstitution that follows transplantation has been likened to maturation of the immune system in neonates. The analogy does not entirely predict infections seen in HSC transplant recipients, however, because the stem cells mature in an old host who has several latent infections already. The choice among the current variety of methods for obtaining stem cells is determined by availability and by the need to optimize the chances of a cure for an individual recipient. One strategy is autologous HSC transplantation, in which the donor and the recipient are the same. After chemotherapy, stem cells are collected and are purged (ex vivo) of residual neoplastic populations. Allogeneic HSC transplantation has the advantage of providing a graft-versus-tumor effect. In this case, the recipient is matched to varying degrees for human leukocyte antigens (HLAs) with a donor who may be related or unrelated. In some individuals, nonmyeloablative therapy (mini-allo transplantation) is used and permits recipient cells to persist for some time after transplantation while preserving the graft-versus-tumor effect and sparing the recipient myeloablative therapy. Cord-blood transplantation is increasingly utilized in adults; two independent cord-blood units are typically required for suitable neutrophil engraftment early after transplantation, even though only one of the units is likely to provide long-term engraftment. In each circumstance, a different balance is struck among the toxicity of conditioning therapy, the need for a maximal graft-versus-target effect, short-term and long-term infectious complications, and the risk of graft-versus-host disease (GVHD; acute versus chronic). The various approaches differ in terms of reconstitution speed, cell lineages introduced, and likelihood of GVHD—all factors that can produce distinct effects on the risk of infection after transplantation (Table 169-1). Despite these caveats, most infections occur in a predictable time frame after transplantation (Table 169-2).
|
RISK OF INFECTION, BY TYPE OF HEMATOPOIETIC STEM CELL TRANSPLANT |
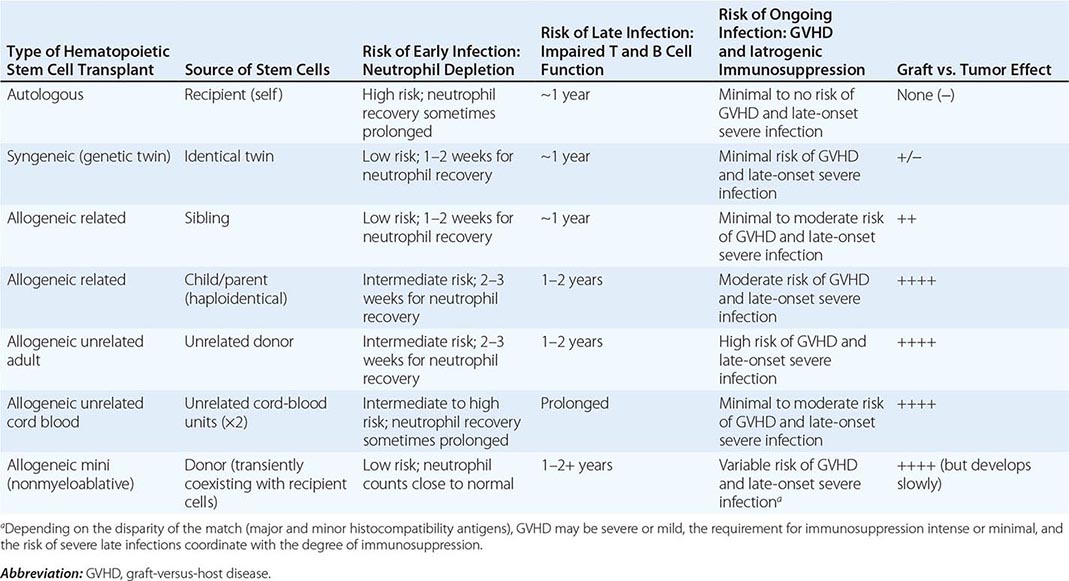
|
COMMON SOURCES OF INFECTIONS AFTER HEMATOPOIETIC STEM CELL TRANSPLANTATION |
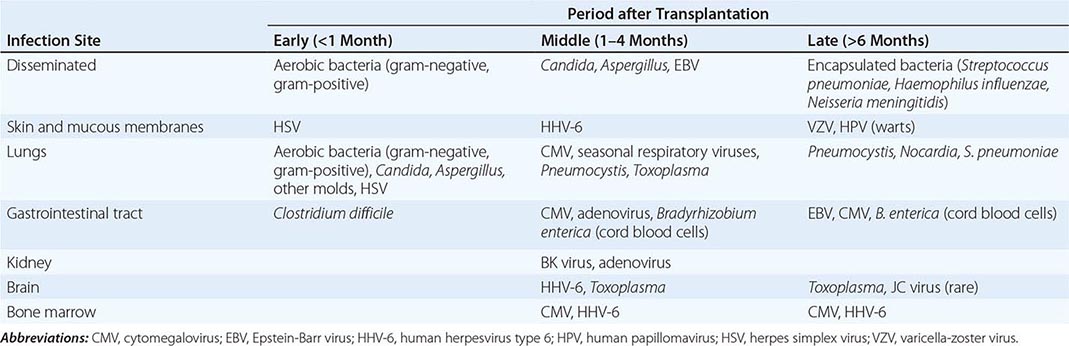
BACTERIAL INFECTIONS
In the first month after HSC transplantation, infectious complications are similar to those in granulocytopenic patients receiving chemotherapy for acute leukemia (Chap. 104). Because of the anticipated 1- to 4-week duration of neutropenia and the high rate of bacterial infection in this population, many centers give prophylactic antibiotics to patients upon initiation of myeloablative therapy. Quinolones decrease the incidence of gram-negative bacteremia among these patients. Bacterial infections are common in the first few days after HSC transplantation. The organisms involved are predominantly those found on skin, mucosa, or IV catheters (Staphylococcus aureus, coagulase-negative staphylococci, streptococci) or aerobic bacteria that colonize the bowel (Escherichia coli, Klebsiella, Pseudomonas). Bacillus cereus, although rare, has emerged as a pathogen early after transplantation and can cause meningitis, which is unusual in these patients. Chemotherapy, use of broad-spectrum antibiotics, and delayed reconstitution of humoral immunity place HSC transplant patients at risk for diarrhea and colitis caused by Clostridium difficile overgrowth and toxin production.
Beyond the first few days of neutropenia, infections with nosocomial pathogens (e.g., vancomycin-resistant enterococci, Stenotrophomonas maltophilia, Acinetobacter species, and extended-spectrum β-lactamase–producing gram-negative bacteria) as well as with filamentous bacteria (e.g., Nocardia species) become more common. Vigilance is indicated, particularly for patients with a history of active or known latent tuberculosis, even when they have been appropriately pretreated. A form of bacterial colitis among cord-blood recipients has occurred 90–300 days after transplantation, responds to antimicrobial agents such as metronidazole, and—as determined by polymerase chain reaction (PCR) of biopsy specimens—may be attributed to the bacterium Bradyrhizobium enterica (related to B. japonicum). Episodes of bacteremia due to encapsulated organisms mark the late posttransplantation period (>6 months after HSC reconstitution); patients who have undergone splenectomy and those with persistent hypogammaglobulinemia are at particular risk.
FUNGAL INFECTIONS
Beyond the first week after transplantation, fungal infections become increasingly common, particularly among patients who have received broad-spectrum antibiotics. As in most granulocytopenic patients, Candida infections are most commonly seen in this setting. However, with increased use of prophylactic fluconazole, infections with resistant fungi—in particular, Aspergillus and other non-Aspergillus molds (Rhizopus, Fusarium, Scedosporium, Penicillium)—have become more common, prompting some centers to replace fluconazole with agents such as micafungin, voriconazole, or posaconazole. The role of antifungal prophylaxis with these different agents, in contrast to empirical treatment for suspected infection that is based on a positive β-D-glucan assay or galactomannan antigen test, remains controversial (Chap. 104). Documented infection should be aggressively treated, ideally with agents of proven activity. In patients with GVHD who require prolonged or indefinite courses of glucocorticoids and other immunosuppressive agents (e.g., cyclosporine, tacrolimus [FK 506, Prograf], mycophenolate mofetil [Cellcept], rapamycin [sirolimus, Rapamune], antithymocyte globulin, or anti-CD52 antibody [alemtuzumab, Campath—an antilymphocyte and antimonocyte monoclonal antibody]), there is a high risk of fungal infection (usually with Candida or Aspergillus) even after engraftment and resolution of neutropenia. These patients are also at high risk for reactivation of latent fungal infection (histoplasmosis, coccidioidomycosis, or blastomycosis) in areas where endemic fungi reside and after involvement in activities such as gardening or caving. Prolonged use of central venous catheters for parenteral nutrition (lipids) increases the risk of fungemia with Malassezia. Some centers administer prophylactic antifungal agents to these patients. Because of the high and prolonged risk of Pneumocystis jirovecii pneumonia (especially among patients being treated for hematologic malignancies), most patients receive maintenance prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX) starting 1 month after engraftment and continuing for at least 1 year.
PARASITIC INFECTIONS
The regimen just described for the fungal pathogen Pneumocystis may also protect patients seropositive for the parasite T. gondii, which can cause pneumonia, visceral disease (occasionally), and central nervous system (CNS) lesions (more commonly). The advantages of maintaining HSC transplant recipients on daily TMP-SMX for 1 year after transplantation include some protection against Listeria monocytogenes and nocardial disease as well as late infections with Streptococcus pneumoniae and Haemophilus influenzae, which stem from the inability of the immature immune system to respond to polysaccharide antigens.
With increasing international travel, parasitic diseases typically restricted to particular environmental niches may pose a risk of reactivation in certain patients after HSC transplantation. Thus, in recipients with an appropriate history who were not screened and/or treated before transplantation or in patients with recent exposures, evaluation for infection with Strongyloides, Leishmania, schistosomes, trypanosomes, or various parasitic causes of diarrheal illness (Giardia, Entamoeba, Cryptosporidium, microsporidia) may be warranted.
VIRAL INFECTIONS
HSC transplant recipients are susceptible to infection with a variety of viruses, including primary and reactivation syndromes caused by most human herpesviruses (Table 169-3) and acute infections caused by viruses that circulate in the community.
|
HERPESVIRUS SYNDROMES OF TRANSPLANT RECIPIENTS |
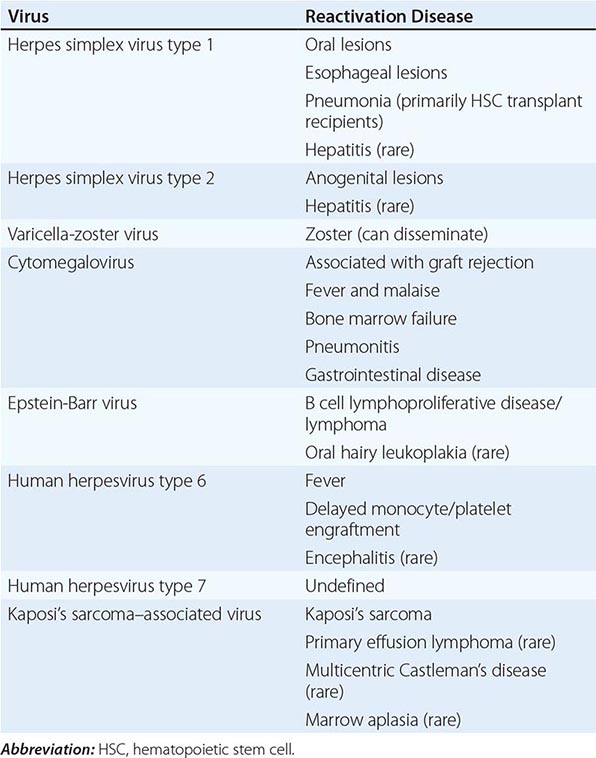
Herpes Simplex Virus Within the first 2 weeks after transplantation, most patients who are seropositive for HSV-1 excrete the virus from the oropharynx. The ability to isolate HSV declines with time. Administration of prophylactic acyclovir (or valacyclovir) to seropositive HSC transplant recipients has been shown to reduce mucositis and prevent HSV pneumonia (a rare condition reported almost exclusively in allogeneic HSC transplant recipients). Both esophagitis (usually due to HSV-1) and anogenital disease (commonly caused by HSV-2) may be prevented with acyclovir prophylaxis. For further discussion, see Chap. 216.
Varicella-Zoster Virus Reactivation of VZV manifests as herpes zoster and may occur within the first month but more commonly occurs several months after transplantation. Reactivation rates are ∼40% for allogeneic HSC transplant recipients and 25% for autologous recipients. Localized zoster can spread rapidly in an immunosuppressed patient. Fortunately, disseminated disease can usually be controlled with high doses of acyclovir. Because of frequent dissemination among patients with skin lesions, acyclovir is given prophylactically in some centers to prevent severe disease. Low doses of acyclovir appear to be effective in preventing reactivation of VZV. However, acyclovir can also suppress the development of VZV-specific immunity. Thus, its administration for only 6 months after transplantation does not prevent zoster from occurring when treatment is stopped. Administration of low doses of acyclovir for an entire year after transplantation is effective and may eliminate most cases of posttransplantation zoster, even among cord-blood recipients. For further discussion, see Chap. 217.
Cytomegalovirus The onset of CMV disease (interstitial pneumonia, bone marrow suppression, graft failure, hepatitis/colitis) usually begins 30–90 days after HSC transplantation, when the granulocyte count is adequate but immunologic reconstitution has not occurred. CMV disease rarely develops earlier than 14 days after transplantation and may become evident as late as 4 months after the procedure. It is of greatest concern in the second month after transplantation, particularly in allogeneic HSC transplant recipients. In cases in which the donor marrow is depleted of T cells (to prevent GVHD or eliminate a T cell tumor) and in cord-blood recipients, the disease may manifest earlier. The use of alemtuzumab to prevent GVHD in nonmyeloablative transplantation has been associated with an increase in CMV disease. Patients who receive ganciclovir for prophylaxis, preemptive treatment, or treatment (see below) may develop recurrent CMV infection even later than 4 months after transplantation, as treatment appears to delay the development of the normal immune response to CMV infection. Although CMV disease may present as isolated fever, granulocytopenia, thrombocytopenia, or gastrointestinal disease, the foremost cause of death from CMV infection in the setting of HSC transplantation is pneumonia.
With the standard use of CMV-negative or filtered blood products, CMV infection should be a major risk in allogeneic transplantation only when the recipient is CMV-seropositive and the donor is CMV-seronegative. This situation is the reverse of that in solid organ transplant recipients. CMV reactivates from latent reservoirs present in the recipient at a time when donor T cells (especially cord-blood T cells) are too immature to control CMV replication. If the T cells from the donor have never encountered CMV and the recipient carries the virus, the patient is at maximal risk of severe disease. Reactivation disease or superinfection with another strain from the donor also can occur in CMV-positive recipients, but clinical manifestations are typically less severe, presumably because of CMV-specific memory in transplanted donor T cells. Most patients infected with CMV who undergo HSC transplantation excrete virus, with or without clinical findings. Serious CMV disease is much more common among allogeneic than autologous recipients and is often associated with GVHD. In addition to pneumonia and marrow suppression (and, less often, graft failure), manifestations of CMV disease in HSC transplant recipients include fever with or without arthralgias, myalgias, hepatitis, and esophagitis. CMV ulcerations occur in both the lower and the upper gastrointestinal tract, and it may be difficult to distinguish diarrhea due to GVHD from that due to CMV infection. The finding of CMV in the liver of a patient with GVHD does not necessarily mean that CMV is responsible for hepatic enzyme abnormalities. It is interesting that the ocular and neurologic manifestations of CMV infections, which are common in patients with AIDS, are uncommon in patients who develop disease after transplantation.
Management of CMV disease in HSC transplant recipients includes strategies directed at prophylaxis, preemptive therapy (suppression of silent replication), and treatment of disease. Prophylaxis results in a lower incidence of disease at the cost of treating many patients who otherwise would not require therapy. Because of the high fatality rate associated with CMV pneumonia in these patients and the difficulty of early diagnosis of CMV infection, prophylactic IV ganciclovir (or oral valganciclovir) has been used in some centers and has been shown to prevent CMV disease during the period of maximal vulnerability (from engraftment to day 120 after transplantation). Ganciclovir also prevents HSV reactivation and reduces the risk of VZV reactivation; thus acyclovir prophylaxis should be discontinued when ganciclovir is administered. The foremost problem with the administration of ganciclovir relates to adverse effects, which include dose-related bone marrow suppression (thrombocytopenia, leukopenia, anemia, and pancytopenia). Because the frequency of CMV pneumonia is lower among autologous HSC transplant recipients (2–7%) than among allogeneic HSC transplant recipients (10–40%), prophylaxis in the former group will not become the rule until a less toxic oral antiviral agent becomes available. Several are under study.
Preemptive treatment of CMV—that is, initiation of therapy with drugs only after CMV is detected in blood by a nucleic acid amplification test (NAAT)—is used at most centers. To limit variability between tests, the World Health Organization (WHO) has developed an international reference standard for measurement of CMV load by NAAT-based assays. Because of toxic drug side effects (e.g., neutropenia and bone marrow suppression), the preemptive approach has supplanted prophylactic therapy; it has also replaced treatment of all seropositive (recipient and/or donor) HSC transplants with an antiviral agent (typically ganciclovir). A positive test (or increasing viral load) prompts the initiation of preemptive therapy with ganciclovir. Preemptive approaches that target patients who have quantitative NAAT evidence of CMV infection can still lead to unnecessary treatment of many individuals with drugs that have adverse effects on the basis of a laboratory test that is not highly predictive of disease; however, invasive disease, particularly in the form of pulmonary infection, is difficult to treat and is associated with high mortality rates. When prophylaxis or preemptive therapy is stopped, late manifestations of CMV replication may occur, although by then the HSC transplant patient is often equipped with improved graft function and is better able to combat disease. Cord-blood transplant recipients are especially vulnerable to disease caused by members of the human herpesvirus family, including CMV. Implementation of the WHO standard for CMV load measurement will facilitate large-scale comparative studies and thus the establishment of optimal guidelines for distinct patient subsets.
CMV pneumonia in HSC transplant recipients (unlike that in other clinical settings) is often treated with both IV immunoglobulin (IVIg) and ganciclovir. In patients who cannot tolerate ganciclovir, foscarnet is a useful alternative, although it may produce nephrotoxicity and electrolyte imbalance. When neither ganciclovir nor foscarnet is clinically tolerated, cidofovir can be used; however, its efficacy is less well established, and its side effects include nephrotoxicity. A lipid-conjugate form of cidofovir and an oral antiviral agent, maribavir, are in clinical trials. Case reports have suggested that the immunosuppressive agent leflunomide may be active in this setting, but controlled studies are lacking. Transfusion of CMV-specific T cells from the donor has decreased viral load in a small series of patients; this result suggests that immunotherapy (e.g., banked T cells) may play a role in the management of this disease in the future. For further discussion, see Chap. 219.
Human Herpesviruses 6 and 7 Human herpesvirus type 6 (HHV-6), the cause of roseola in children, is a ubiquitous herpesvirus that is reactivated (as determined by quantitative plasma PCR) in ∼50% of HSC transplant recipients 2–4 weeks after transplantation. Reactivation is more common among patients requiring glucocorticoids for GVHD and among those receiving second transplants. Reactivation of HHV-6, primarily type B, may be associated with delayed monocyte and platelet engraftment. Limbic encephalitis developing after transplantation has been associated with HHV-6 in cerebrospinal fluid (CSF). The causality of the association is not well defined; in several cases, plasma viremia was detected long before the onset of encephalitis. Nevertheless, most patients with encephalitis had very high viral loads in plasma at the time of CNS illness, and viral antigen has been detected in hippocampal astrocytes. HHV-6 DNA is sometimes found in lung samples after transplantation. However, its role in pneumonitis is unclear, as co-pathogens are frequently present. While HHV-6 is susceptible to foscarnet or cidofovir (and possibly to ganciclovir) in vitro, the efficacy of antiviral treatment has not been well studied. Little is known about the related herpesvirus HHV-7 or its role in posttransplantation infection. For further discussion, see Chap. 219.
Epstein-Barr Virus Primary EBV infection can be fatal to HSC transplant recipients; EBV reactivation can cause EBV–B cell lymphoproliferative disease (EBV-LPD), which may also be fatal to patients taking immunosuppressive drugs. Latent EBV infection of B cells leads to several interesting phenomena in HSC transplant recipients. The marrow ablation that occurs as part of the HSC transplantation procedure may sometimes eliminate latent EBV from the host. Infection can then be reacquired immediately after transplantation by transfer of infected donor B cells. Rarely, transplantation from a seronegative donor may result in a cure. The recipient is then at risk for a second primary infection.
EBV-LPD can develop in the recipient’s B cells (if any survive marrow ablation) but is more likely to be a consequence of outgrowth of infected donor cells. Both lytic replication and latent replication of EBV are more likely during immunosuppression (e.g., they are associated with GVHD and the use of antibodies to T cells). Although less likely in autologous transplantation, reactivation can occur in T cell–depleted autologous recipients (e.g., patients being given antibodies to T cells for the treatment of a T cell lymphoma with marrow depletion). EBV-LPD, which can become apparent as early as 1–3 months after engraftment, can cause high fevers and cervical adenopathy resembling the symptoms of infectious mononucleosis but more commonly presents as an extranodal mass. The incidence of EBV-LPD among allogeneic HSC transplant recipients is 0.6–1%, which contrasts with figures of ∼5% for renal transplant recipients and up to 20% for cardiac transplant patients. In all cases, EBV-LPD is more likely to occur with high-dose, prolonged immunosuppression, especially that caused by the use of antibodies to T cells, glucocorticoids, and calcineurin inhibitors (e.g., cyclosporine, tacrolimus). Cord-blood recipients constitute another high-risk group because of delayed T cell function. Ganciclovir, administered to preempt CMV disease, may reduce EBV lytic replication and thereby diminish the pool of B cells that can become newly infected and give rise to LPD. Increasing evidence indicates that replacement of calcineurin inhibitors with mTor inhibitors (e.g., rapamycin) exerts an antiproliferative effect on EBV-infected B cells that decreases the likelihood of development of LPD or unrelated proliferative disorders associated with transplant-related immunosuppression.
PCR can be used to monitor EBV production after HSC transplantation. High or increasing viral loads predict an enhanced likelihood of EBV-LPD development and should prompt rapid reduction of immunosuppression and a search for nodal or extranodal disease. If reduction of immunosuppression does not have the desired effect, administration of a monoclonal antibody to CD20 (e.g., rituximab) for the treatment of B cell lymphomas that express this surface protein has elicited dramatic responses and currently constitutes first-line therapy for CD20-positive EBV-LPD. However, long-term suppression of new antibody responses accompanies therapy, and recurrences are not infrequent. Additional B cell–directed antibodies, including anti-CD22, are under study. The role of antiviral drugs is uncertain because no available agents have been documented to have activity against the different forms of latent EBV infection. Diminishing lytic replication and virion production in these patients would theoretically produce a statistical decrease in the frequency of latent disease by decreasing the number of virions available to cause additional infection. In case reports and animal studies, ganciclovir and/or high-dose zidovudine, together with other agents, has been used to eradicate EBV-LPD and CNS lymphomas, another EBV-associated complication of transplantation. Both interferon and retinoic acid have been employed in the treatment of EBV-LPD, as has IVIg, but no large-scale prospective studies have assessed the efficacy of any of these agents. Several additional drugs are undergoing preclinical evaluation. Standard chemotherapeutic regimens are used if disease persists after reduction of immunosuppressive agents and administration of antibodies. EBV-specific T cells generated from the donor have been used experimentally to prevent and treat EBV-LPD in allogeneic recipients, and efforts are under way to increase the activity and specificity of ex vivo–generated T cells. For further discussion, see Chap. 218.
Human Herpesvirus 8 (KSHV) The EBV-related gammaherpesvirus KSHV, which is causally associated with Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease, has rarely resulted in disease in HSC transplant recipients, although some cases of virus-associated marrow aplasia have been reported in the peritransplantation period. The relatively low seroprevalence of KSHV in the population and the limited duration of profound T cell suppression after HSC transplantation provide a plausible explanation for the currently low incidence of KSHV disease compared with that in recipients of solid organ transplants and patients with HIV infection. For further discussion, see Chap. 219.
Other (Non-Herpes) Viruses The diagnosis of pneumonia in HSC transplant recipients poses special problems. Because patients have undergone treatment with multiple chemotherapeutic agents and sometimes irradiation, their differential diagnosis should include—in addition to bacterial and fungal pneumonia—CMV pneumonitis, pneumonia of other viral etiologies, parasitic pneumonia, diffuse alveolar hemorrhage, and chemical- or radiation-associated pneumonitis. Since fungi and viruses (e.g., influenza A and B viruses, respiratory syncytial virus [RSV], parainfluenza virus [types 1–4], adenovirus, enterovirus, bocavirus, human metapneumovirus, coronavirus, and rhinovirus [increasingly detected by multiplex PCR]) also can cause pneumonia in this setting, it is important to obtain a specific diagnosis. Diagnostic modalities include Gram’s stain, microbiologic culture, antigen testing, and—increasingly—multipathogen PCR and mass spectrometry assays. M. tuberculosis has been an uncommon cause of pneumonia among HSC transplant recipients in Western countries (accounting for <0.1–0.2% of cases) but is common in Hong Kong (5.5%) and in countries where the prevalence of tuberculosis is high. The recipient’s exposure history is clearly critical in an assessment of posttransplantation infections.
Both RSV and parainfluenza viruses, particularly type 3, can cause severe or even fatal pneumonia in HSC transplant recipients. Infections with both of these agents sometimes occur as disastrous nosocomial epidemics. Therapy with palivizumab or ribavirin for RSV infection remains controversial. New agents, some host-directed, are under study. Influenza also occurs in HSC transplant recipients and generally mirrors the presence of infection in the community. Progression to pneumonia is more common when infection occurs early after transplantation and when the recipient is lymphopenic. The neuraminidase inhibitors oseltamivir (oral) and zanamivir (aerosolized) are active against both influenza A virus and influenza B virus and are a reasonable treatment option. Parenteral forms of neuraminidase inhibitors such as peramivir (intravenous) and several new oral agents remain in trial status. An important preventive measure is immunization of household members, hospital staff members, and other frequent contacts. Adenoviruses can be isolated from HSC transplant recipients at rates varying from 5% to ≥18%. Like CMV infection, adenovirus infection usually occurs in the first to third month after transplantation and is often asymptomatic, although pneumonia, hemorrhagic cystitis/nephritis, severe gastroenteritis with hemorrhage, and fatal disseminated infection have been reported and may be strain-specific. A role for cidofovir therapy has been suggested, but the efficacy of this agent in adenovirus infection remains to be determined. Banked virus-specific T cell therapy is under study for adenovirus infection (as well as for CMV and EBV infections).
Although diverse respiratory viruses can sometimes cause severe pneumonia and respiratory failure in HSC transplant recipients, mild or even asymptomatic infection may be more common. For example, rhinoviruses and coronaviruses are frequent co-pathogens in HSC transplant recipients; however, whether they independently contribute to significant pulmonary infection is not known. At present, the overall contribution of these viral respiratory pathogens to the burden of lower respiratory tract disease in HSC transplant recipients requires further study. Infections with parvovirus B19 (presenting as anemia or occasionally as pancytopenia) and disseminated enteroviruses (sometimes fatal) can occur. Parvovirus B19 infection can be treated with IVIg (Chap. 221).
Rotaviruses are a cause of gastroenteritis in HSC transplant recipients, more frequently in children. Norovirus is a common cause of vomiting and diarrhea, and symptoms can be prolonged in HSC recipients. The polyomavirus BK virus is found at high titers in the urine of patients who are profoundly immunosuppressed. BK viruria may be associated with hemorrhagic cystitis in these patients. In contrast to its incidence among patients with impaired T cell function due to AIDS (4–5%), progressive multifocal leukoencephalopathy caused by the related JC virus is relatively rare among HSC transplant recipients (Chap. 164). When transmitted by mosquitoes or by blood transfusion, WNV can cause encephalitis and death after HSC transplantation.
INFECTIONS IN SOLID ORGAN TRANSPLANT RECIPIENTS
Rates of morbidity and mortality among recipients of solid organ transplants (SOTs) are reduced by the use of effective antibiotics. The organisms that cause acute infections in recipients of SOTs are different from those that infect HSC transplant recipients because SOT recipients do not go through a period of neutropenia. As the transplantation procedure involves major surgery, however, SOT recipients are subject to infections at anastomotic sites and to wound infections. Compared with HSC transplant recipients, SOT patients are immunosuppressed for longer periods (often permanently). Thus they are susceptible to many of the same organisms as patients with chronically impaired T cell immunity (Chap. 104, especially Table 104-1). Moreover, the persistent HLA mismatch between recipient immune cells (e.g., effector T cells) and the donor organ (allograft) places the organ at permanently increased risk of infection.
During the early period (<1 month after transplantation; Table 169-4), infections are most commonly caused by extracellular bacteria (staphylococci, streptococci, enterococci, and E. coli and other gram-negative organisms, including nosocomial organisms with broad antibiotic resistance), which often originate in surgical wound or anastomotic sites. The type of transplant largely determines the spectrum of infection. In subsequent weeks, the consequences of the administration of agents that suppress cell-mediated immunity become apparent, and acquisition—or, more commonly, reactivation—of viruses, mycobacteria, endemic fungi, and parasites (from the recipient or from the transplanted organ) can occur. CMV infection is often a problem, particularly in the first 6 months after transplantation, and may present as severe systemic disease or as infection of the transplanted organ. HHV-6 reactivation (assessed by plasma PCR) occurs within the first 2–4 weeks after transplantation and may be associated with fever, leukopenia, and very rare cases of encephalitis. Data suggest that replication of HHV-6 and HHV-7 may exacerbate CMV-induced disease. CMV is associated not only with generalized immunosuppression but also with organ-specific, rejection-related syndromes: glomerulopathy in kidney transplant recipients, bronchiolitis obliterans in lung transplant recipients, vasculopathy in heart transplant recipients, and the vanishing bile duct syndrome in liver transplant recipients. A complex interplay between increased CMV replication and enhanced graft rejection is well established: elevated immunosuppression leads to increased CMV replication, which is associated with graft rejection. For this reason, considerable attention has been focused on the diagnosis, prophylaxis, and treatment of CMV infection in SOT recipients. Early transmission of WNV to transplant recipients from a donated organ or transfused blood has been reported; however, the risk of WNV acquisition has been reduced by implementation of screening procedures. In rare instances, rabies virus and lymphocytic choriomeningitis virus also have been acutely transmitted in this setting; although accompanied by distinct clinical syndromes, both viral infections have resulted in fatal encephalitis. As screening for unusual viruses is not routine, only vigilant assessment of the prospective donor is likely to prevent the use of an infected organ.
|
COMMON INFECTIONS AFTER SOLID ORGAN TRANSPLANTATION, BY SITE OF INFECTION |
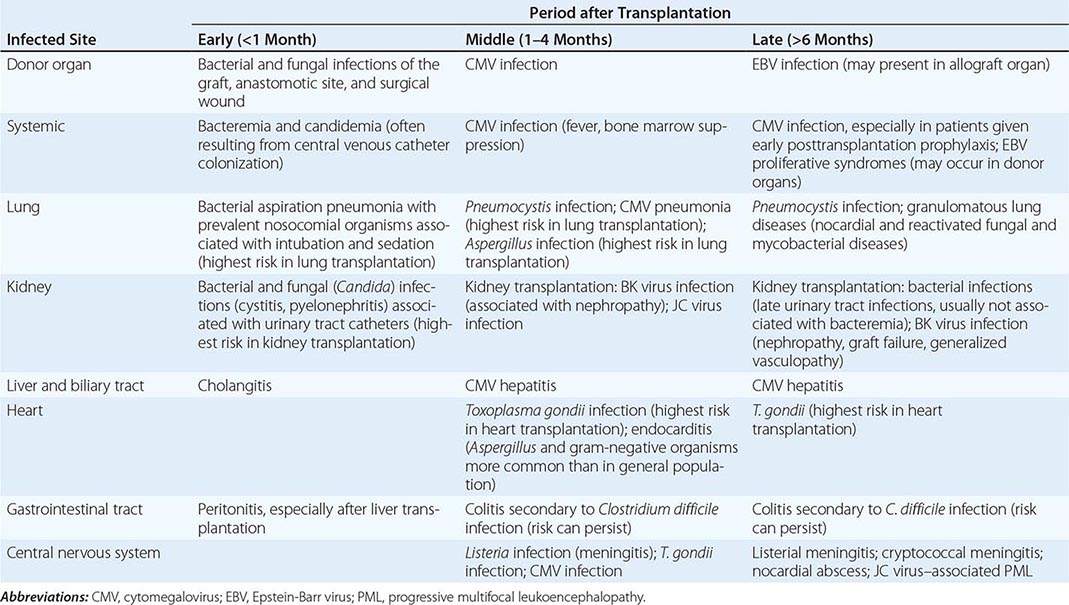
Beyond 6 months after transplantation, infections characteristic of patients with defects in cell-mediated immunity—e.g., infections with Listeria, Nocardia, Rhodococcus, mycobacteria, various fungi, and other intracellular pathogens—may be a problem. International patients and global travelers may experience reactivation of dormant infections with trypanosomes, Leishmania, Plasmodium, Strongyloides, and other parasites. Reactivation of latent M. tuberculosis infection, while rare in Western nations, is far more common among persons from developing countries. The recipient is typically the source, although reactivation and spread from the donor organ can occur. While pulmonary disease remains most common, atypical sites can be involved and mortality rates can be high (up to 30%). Vigilance, prophylaxis/preemptive therapy (when indicated), and rapid diagnosis and treatment of infections can be lifesaving in SOT recipients, who, unlike most HSC transplant recipients, continue to be immunosuppressed.
SOT recipients are susceptible to EBV-LPD from as early as 2 months to many years after transplantation. The prevalence of this complication is increased by potent and prolonged use of T cell–suppressive drugs. Decreasing the degree of immunosuppression may in some cases reverse the condition. Among SOT patients, those with heart and lung transplants—who receive the most intensive immunosuppressive regimens—are most likely to develop EBV-LPD, particularly in the lungs. Although the disease usually originates in recipient B cells, several cases of donor origin, particularly in the transplanted organ, have been noted. High organ-specific content of B lymphoid tissues (e.g., bronchus-associated lymphoid tissue in the lung), anatomic factors (e.g., lack of access of host T cells to the transplanted organ because of disturbed lymphatics), and differences in major histocompatibility loci between the host T cells and the organ (e.g., lack of cell migration or lack of effective T cell/macrophage/dendritic cell cooperation) may result in defective elimination of EBV-infected B cells. SOT recipients are also highly susceptible to the development of Kaposi’s sarcoma and, less frequently, to the B cell–proliferative disorders associated with KSHV, such as primary effusion lymphoma and multicentric Castleman’s disease. Kaposi’s sarcoma is 550–1000 times more common among SOT recipients than in the general population, can develop very rapidly after transplantation, and can also occur in the allograft. However, because the seroprevalence of KSHV is very low in Western countries, Kaposi’s sarcoma is not common. Recipients (or donors) from Iceland, the Middle East, Mediterranean countries, and Africa are at highest risk of disease. Data suggest that a switch of immunosuppressive agents—from calcineurin inhibitors (cyclosporine, tacrolimus) to mTor pathway–active agents (sirolimus, everolimus)—after adequate wound healing may significantly reduce the likelihood of development of Kaposi’s sarcoma and perhaps of EBV-LPD and certain other posttransplantation malignancies.
KIDNEY TRANSPLANTATION
See Table 169-4.
Early Infections Bacteria often cause infections that develop in the period immediately after kidney transplantation. There is a role for perioperative antibiotic prophylaxis, and many centers give cephalosporins to decrease the risk of postoperative complications. Urinary tract infections developing soon after transplantation are usually related to anatomic alterations resulting from surgery. Such early infections may require prolonged treatment (e.g., 6 weeks of antibiotic administration for pyelonephritis). Urinary tract infections that occur >6 months after transplantation may be treated for shorter periods because they do not seem to be associated with the high rate of pyelonephritis or relapse seen with infections that occur during the first 3 months.
Prophylaxis with TMP-SMX for the first 4–6 months after transplantation decreases the incidence of early and middle-period infections (see below, Table 169-4, and Table 169-5).
|
PROPHYLACTIC REGIMENS COMMONLY USED TO DECREASE RISK OF INFECTION IN TRANSPLANT RECIPIENTSa |
Middle-Period Infections Because of continuing immunosuppression, kidney transplant recipients are predisposed to lung infections characteristic of those in patients with T cell deficiency (i.e., infections with intracellular bacteria, mycobacteria, nocardiae, fungi, viruses, and parasites). A high mortality rate associated with Legionella pneumophila infection (Chap. 184) led to the closing of renal transplant units in hospitals with endemic legionellosis.
About 50% of all renal transplant recipients presenting with fever 1–4 months after transplantation have evidence of CMV disease; CMV itself accounts for the fever in more than two-thirds of cases and thus is the predominant pathogen during this period. CMV infection (Chap. 219) may also present as arthralgias, myalgias, or organ-specific symptoms. During this period, this infection may represent primary disease (in the case of a seronegative recipient of a kidney from a seropositive donor) or may represent reactivation disease or superinfection. Patients may have atypical lymphocytosis. Unlike immunocompetent patients, however, they rarely have lymphadenopathy or splenomegaly. Therefore, clinical suspicion and laboratory confirmation are necessary for diagnosis. The clinical syndrome may be accompanied by bone marrow suppression (particularly leukopenia). CMV also causes glomerulopathy and is associated with an increased incidence of other opportunistic infections. Because of the frequency and severity of disease, a considerable effort has been made to prevent and treat CMV infection in renal transplant recipients. An immune globulin preparation enriched with antibodies to CMV was used by many centers in the past in an effort to protect the group at highest risk for severe infection (seronegative recipients of seropositive kidneys). However, with the development of effective oral antiviral agents, CMV immune globulin is no longer used. Ganciclovir (or valganciclovir) is beneficial for prophylaxis (when indicated) and for the treatment of serious CMV disease. The availability of valganciclovir has allowed most centers to move to oral prophylaxis for transplant recipients. Infection with the other herpesviruses may become evident within 6 months after transplantation or later. Early after transplantation, HSV may cause either oral or anogenital lesions that are usually responsive to acyclovir. Large ulcerating lesions in the anogenital area may lead to bladder and rectal dysfunction and may predispose the patient to bacterial infection. VZV may cause fatal disseminated infection in nonimmune kidney transplant recipients, but in immune patients reactivation zoster usually does not disseminate outside the dermatome; thus disseminated VZV infection is a less fearsome complication in kidney transplantation than in HSC transplantation. HHV-6 reactivation may take place and (although usually asymptomatic) may be associated with fever, rash, marrow suppression, or rare instances of renal impairment, hepatitis, colitis, or encephalitis.
EBV disease is more serious; it may present as an extranodal proliferation of B cells that invade the CNS, nasopharynx, liver, small bowel, heart, and other organs, including the transplanted kidney. The disease is diagnosed by the finding of a mass of proliferating EBV-positive B cells. The incidence of EBV-LPD is elevated among patients who acquire EBV infection from the donor and among patients given high doses of cyclosporine, tacrolimus, glucocorticoids, and anti–T cell antibodies. Disease may regress once immunocompetence is restored. KSHV infection can be transmitted with the donor kidney and result in development of Kaposi’s sarcoma, although it more often represents reactivation of latent infection of the recipient. Kaposi’s sarcoma often appears within 1 year after transplantation, although the time of onset ranges widely (1 month to ∼20 years). Avoidance of immunosuppressive agents that inhibit calcineurin has been associated with less Kaposi’s sarcoma, less EBV disease, and even less CMV replication. The use of rapamycin (sirolimus) has independently led to regression of Kaposi’s sarcoma.
The papovaviruses BK virus and JC virus (polyomavirus hominis types 1 and 2) have been cultured from the urine of kidney transplant recipients (as they have from that of HSC transplant recipients) in the setting of profound immunosuppression. High levels of BK virus replication detected by PCR in urine and blood are predictive of pathology, especially in the setting of renal transplantation. JC virus may rarely cause similar disease in kidney transplantation. Urinary excretion of BK virus and BK viremia are associated with the development of ureteral strictures, polyomavirus-associated nephropathy (1–10% of renal transplant recipients), and (less commonly) generalized vasculopathy. Timely detection and early reduction of immunosuppression are critical and can reduce rates of graft loss related to polyomavirus-associated nephropathy from 90% to 10–30%. Therapeutic responses to IVIg, quinolones, leflunomide, and cidofovir have been reported, but the efficacy of these agents has not been substantiated through adequate clinical study. Most centers approach the problem by reducing immunosuppression in an effort to enhance host immunity and decrease viral titers. JC virus is associated with rare cases of progressive multifocal leukoencephalopathy. Adenoviruses may persist and cause hemorrhagic nephritis/cystitis with continued immunosuppression in these patients, but disseminated disease like that seen in HSC transplant recipients is much less common.
Kidney transplant recipients are also subject to infections with other intracellular organisms. These patients may develop pulmonary infections with Mycobacterium, Aspergillus, and Mucor species as well as infections with other pathogens in which the T cell/macrophage axis plays an important role. L. monocytogenes is a common cause of bacteremia ≥1 month after renal transplantation and should be seriously considered in renal transplant recipients presenting with fever and headache. Kidney transplant recipients may develop Salmonella bacteremia, which can lead to endovascular infections and require prolonged therapy. Pulmonary infections with Pneumocystis are common unless the patient is maintained on TMP-SMX prophylaxis. Acute interstitial nephritis caused by TMP-SMX is rare. However, because transient increases in creatinine (artifactual) and hyperkalemia (manageable) can occur, early discontinuation of prophylaxis, especially after kidney transplantation, is recommended by some groups. Although additional monitoring is indicated, the benefits of TMP-SMX in kidney transplant recipients may outweigh the risks; otherwise, second-line prophylactic agents should be used. Nocardia infection (Chap. 199) may present in the skin, bones, and lungs or in the CNS, where it usually takes the form of single or multiple brain abscesses. Nocardiosis generally occurs ≥1 month after transplantation and may follow immunosuppressive treatment for an episode of rejection. Pulmonary manifestations most commonly consist of localized disease with or without cavities, but the disease may be disseminated. The diagnosis is made by culture of the organism from sputum or from the involved nodule. As it is for P. jirovecii infection, prophylaxis with TMP-SMX is often efficacious in the prevention of nocardiosis.
Toxoplasmosis can occur in seropositive patients but is less common than in other transplantation settings, usually developing in the first few months after kidney transplantation. Again, TMP-SMX is helpful in prevention. In endemic areas, histoplasmosis, coccidioidomycosis, and blastomycosis may cause pulmonary infiltrates or disseminated disease.
Late Infections Late infections (>6 months after kidney transplantation) may involve the CNS and include CMV retinitis as well as other CNS manifestations of CMV disease. Patients (particularly those whose immunosuppression has been increased) are at risk for subacute meningitis due to Cryptococcus neoformans. Cryptococcal disease may present in an insidious manner (sometimes as a skin infection before the development of clear CNS findings). Listeria meningitis may have an acute presentation and requires prompt therapy to avoid a fatal outcome. TMP-SMX prophylaxis may reduce the frequency of Listeria infections.
Patients who continue to take glucocorticoids are predisposed to ongoing infection. “Transplant elbow,” a recurrent bacterial infection in and around the elbow that is thought to result from a combination of poor tensile strength of the skin of steroid-treated patients and steroid-induced proximal myopathy, requires patients to push themselves up with their elbows to get out of chairs. Bouts of cellulitis (usually caused by S. aureus) recur until patients are provided with elbow protection.
Kidney transplant recipients are susceptible to invasive fungal infections, including those due to Aspergillus and Rhizopus, which may present as superficial lesions before dissemination. Mycobacterial infection (particularly that with Mycobacterium marinum) can be diagnosed by skin examination. Infection with Prototheca wickerhamii (an achlorophyllic alga) has been diagnosed by skin biopsy. Warts caused by human papillomaviruses (HPVs) are a late consequence of persistent immunosuppression; imiquimod or other forms of local therapy are usually satisfactory. Merkel cell carcinoma, a rare and aggressive neuroendocrine skin tumor whose frequency is increased fivefold in elderly SOT (especially kidney) recipients, is causally linked to a novel polyomavirus, Merkel cell polyomavirus.
Notably, although BK virus replication and virus-associated disease can be detected far earlier, polyomavirus-associated nephropathy is clinically diagnosed in a median of ∼300 days and thus qualifies as a late-onset disease. With the establishment of better screening procedures (e.g., urine cytology, urine nucleic acid load, plasma PCR), disease onset is being detected earlier (see “Middle-Period Infections,” above) and preemptive strategies (decrease or modification of immunosuppression) are being instituted more promptly, as the efficacy of antiviral therapy is not well established.
HEART TRANSPLANTATION
Early Infections Sternal wound infection and mediastinitis are early complications of heart transplantation. An indolent course is common, with fever or a mildly elevated white blood cell count preceding the development of site tenderness or drainage. Clinical suspicion based on evidence of sternal instability and failure to heal may lead to the diagnosis. Common microbial residents of the skin (e.g., S. aureus, including methicillin-resistant strains, and Staphylococcus epidermidis) as well as gram-negative organisms (e.g., Pseudomonas aeruginosa) and fungi (e.g., Candida) are often involved. In rare cases, mediastinitis in heart transplant recipients can also be due to Mycoplasma hominis (Chap. 212); since this organism requires an anaerobic environment for growth and may be difficult to see on conventional medium, the laboratory should be alerted that its involvement is suspected. M. hominis mediastinitis has been cured with a combination of surgical debridement (sometimes requiring muscle-flap placement) and the administration of clindamycin and tetracycline. Organisms associated with mediastinitis may sometimes be cultured from pericardial fluid.