CHAPTER 276 Infections of the Spine
Infections of the spinal axis have been recognized throughout history. Evidence of tubercular disease of the spine has been found in Egyptian1 and South American mummies.2 Social changes and advances in medical technology have changed the spectrum of causative organisms and the characteristics of patients who acquire these infections. However, the uneven nature of these socioeconomic changes across the globe, combined with variations in the endemic microbial flora, has led to regional variability in the epidemiology of spinal infections. The growth of medical technologies has enhanced the ease of detection and the options for definitive management of spinal infections. Ironically, technologic advances have also promoted the development of spinal infections by increasing the number of patients with iatrogenic immunosuppression, thereby enhancing the life expectancy of patients with chronic medical illness, and by increasing the complexity of spinal procedures and thus the potential for infectious complications.3
Spontaneous Pyogenic Spine Infections
Traditionally, categorization of spinal infections has been based on the anatomic locus of the infection. The terminology used—osteomyelitis, diskitis, and epidural abscess—suggests that the infection is more or less limited to a particular anatomic structure. Advanced evaluation with magnetic resonance imaging (MRI) has made it clear that such terminology often does not accurately depict the nature of the infection. Most spinal infections spread to involve more than one of these anatomic structures and, not uncommonly, affect all of them.4–10 In addition, all three subcategories of infection share similar epidemiology, risk factors, and clinical findings and are diagnosed with the same laboratory tests and imaging studies. Furthermore, a classification based on these anatomic boundaries does not contribute much to determining the optimal management for an individual patient. Although a longer duration of antibiotic therapy is typically needed to cure osteomyelitis than an infection of the disk or the epidural space, the role of surgical intervention, the incidence of neurological deficits, and the chance of failure with maximal medical management are poorly predicted by these traditional anatomy-based descriptors. Instead, we find it more useful to categorize these infections by the neurological condition of the patient (presence or absence of deficits) and by the vertebral level affected by the infection. A classification based on these descriptors is concordant with the biology of these infections and facilitates decisions determined on the basis of the patient’s clinical condition and the radiographic findings rather than on an arbitrary anatomic label.
Epidemiology
The incidence and demographics of pyogenic spinal infections (PSIs) have been significantly influenced by social changes, advances in medical technology, and the acquired immunodeficiency syndrome (AIDS) pandemic. The incidence of spinal infections appears to be on the rise.11,12 In years past, the incidence of epidural abscess was estimated to be around 0.2 to 2 per 10,000 hospital admissions,4,7 but most recent authors have cited higher rates.13–16 The annual incidence of all PSIs is now probably between 5 and 10 cases per million individuals, with a male preponderance.11,17–19 Older series describe two age-related spikes, one during the first decade of life and the second around the fifth or sixth decade,20,21 which reflects the proclivity of the very young and the older population to acquire such infections. Infections still occur in these demographic groups, perhaps with no change in frequency,22 but are overshadowed in number by those that occur in adults with frequent bacteremia, significantly impaired host defenses, or both.13,23–26 Intravenous drug abuse and AIDS, the most common risk factors for PSI,16,27–32 have reached epidemic proportions in modern times.33,34 Medical advances have led to an increased prevalence of individuals who are susceptible to spinal infection. Patients with diabetes, end-stage renal disease, and cirrhosis live longer than in years past.35 Immunosuppression of transplant recipients,36 long-term steroid therapy for autoimmune diseases, chemotherapy, chronic indwelling catheters for venous access,37 splenectomy, genitourinary instrumentation, and other medical interventions also unfortunately enhance a patient’s risk for contracting an infection of the spine.23,28,38–40 In susceptible patients, remote pyogenic infections (e.g., skin, genitourinary tract, lungs, gastrointestinal tract) can result in bacterial seeding of the spine. Spontaneous bacteremia with the potential to inoculate the spine is probably a relatively common event that is ordinarily rendered inconsequential by competent immune mechanisms. A Danish database of all patients with staphylococcal bacteremia in that country suggests that hematogenous vertebral osteomyelitis developed in 145 patients—roughly 1% of all those who had clinically apparent bacteremia.13 In some patients with predisposing conditions for PSI, no overt source of primary infection is found. The same can also be said of patients in whom infections occur in the absence of any particular risk factors. Increased awareness of spinal infections as a cause of fevers of unknown origin and the widespread availability of MRI scanners may both have contributed to the increase in the rate of diagnosis of this condition and hence a greater reported incidence.
Pathogenic Mechanisms
Infective organisms can be carried to the spine by four routes: via the arterial blood supply, retrograde by the vertebral venous plexus,41,42 by direct inoculation (a contaminated surgical instrument/needle or a penetrating injury), or by direct extension from an adjacent nidus of infection (e.g., pulmonary abscess or sacral decubitus ulcer). In adults, the nutrient artery of the metaphyseal end plate is an end artery derived from the periosteal arteries. Infected thrombi that lodge in this nutrient artery, the metaphyseal artery, produce avascular necrosis of a portion of the metaphysis, which in turn creates a sizable nidus for infection.43 Small anastomotic arteries that do branch off from the metaphyseal arteries are unable to supplant the blood flow to an ischemic metaphysis. These connect the metaphyseal plates at opposite ends of a vertebra and are the probable pathway for spread of the infection to transequatorial metaphyses while sparing the intervening equatorial region of the vertebra.44 The equatorial region of the vertebra is supplied by multiple branches from the main segmental artery, thus making it very vascular and relatively resistant to the processes that result in infarction of the metaphysis. In addition to devitalization of the metaphyseal bone, thrombosis of the metaphyseal artery gives rise to ischemia of the intervertebral disk, which results in an infection of the disk, as well as chronic aseptic necrosis. This leads to gradual loss of disk height and, occasionally, the production of frank pus in the disk space. Purulence in the disk or the bone can result in septic thrombosis of draining veins, which in turn relay the infection to the epidural venous plexus, thereby leading to the formation of an epidural abscess.
Not all infections arrive in the spine through an arterial route—transvenous dissemination may also occur, somewhat akin to metastasis of genitourinary and gastrointestinal malignancies to the spine. Infections involving the left kidney may be more likely to spread to the spine because the left renal vein often communicates with Batson’s plexus.40 Occasionally, infection may be restricted to the epidural veins, which act as a portal to the spine and can result in the production of contiguous or heterotopic epidural abscesses over multiple spinal segments without significant involvement of the bony or cartilaginous components of the spine.6 Similar mechanisms may be involved in the infrequent occurrence of infections arising within the posterior elements of the spine.45–47 Less than 5% of spinal infections are isolated to the posterior spinal elements.6,48,49
Neurological deficits develop as a result of compression or ischemia (or both) of the spinal cord or cauda equina in 5% to 50% of all patients with PSI.27,28,48,50–52 Such compression and ischemia can be caused either by an expanding epidural abscess or, more commonly, by kyphosis of the spine as a result of the loss of bone integrity and subsequent bony compression and distortion of neural elements. The lumbar spinal canal is somewhat capacious relative to the space requirement of the cauda equina. This allows the accommodation of sizable epidural collections and retropulsed bony fragments in the lumbar spinal canal in comparison to the cervical or thoracic spinal canal, where very little “spare” room is available around the cord. For this reason, even though PSI affecting the lumbar region is relatively common,27,53,54 spinal infections that produce neurological deficits are seen predominantly with infections of the cervical and thoracic regions.6,51,55 In addition, neurological deficits are more likely to develop in immunocompromised patients as a consequence of a spinal infection.27
Microbiology
The most common organism causing PSI is Staphylococcus aureus.4,6,8,9,25,30,38,52–54,56–60 S. aureus together with other gram-positive organisms such as Staphylococcus epidermidis, Streptococcus viridans, Streptococcus pneumoniae, Streptococcus faecalis (enterococcus), Propionibacterium, and diphtheroids account for the vast majority of PSIs. Gram-negative organisms such as Escherichia coli, Pseudomonas, Salmonella, Enterobacter, Klebsiella, Haemophilus, and Proteus are less frequent and may be associated with gastrointestinal or genitourinary sources of infection.15,50,60,61 Infections in intravenous drug abusers are most likely to be caused by staphylococcal species as well,14,59,62,63 but Pseudomonas infection may be relatively more common in this patient group.27,29,55,62,64 Rarely, anaerobes such as Peptostreptococcus and Bacteroides may cause spinal infections,4,65 also with relatively greater frequency in intravenous drug abusers. Anaerobic infections are more likely than aerobic infections to be polymicrobial. Care should be taken to process all material submitted for culture, both aerobically and anaerobically.66
Clinical Findings
The diagnosis of PSI can easily be missed unless a high index of suspicion is maintained.* Given the large number of visits to emergency departments for complaints of back pain, recognizing this comparatively rare, sometimes subtle, but treatable condition is challenging. Additionally, patients with a history of substance abuse, who are especially prone to the development of such infections, can be poor historians and manifest drug-seeking behavior, thereby further confounding matters.62
Early in the course of their disease, patients typically have isolated back pain27,53 that they may relate to strenuous activity or a minor injury. Systemic signs of infection such as fever or an elevated leukocyte count may be absent. Such patients may be discharged from emergency departments or physician’s offices without a definitive diagnosis, only to return later with progressive symptoms, deformity, or neurological deficits.
Heusner described the clinical evolution of epidural abscess in four stages: pain, radiculopathy, weakness, and paralysis.7 In practice, however, such distinct progression of the infection rarely occurs. Patients can be seen anywhere along a spectrum that ranges from predominantly local manifestations of focal spinal or radicular pain at one end to systemic manifestations of infection (e.g., fevers, chills, malaise, and night sweats) at the other end. The presence of systemic manifestations may herald bacteremia and is an opportune time to obtain blood for culture. Unusual characteristics of the back pain, such as a midline location over the thoracic or upper lumbar spine that worsens with recumbency, especially at night, and the association of a thoracic radiculopathy should prompt consideration of a nondegenerative cause of the pain. Careful screening for risk factors such as intravenous drug abuse, AIDS, diabetes, recent steroid therapy, or the presence of other immunocompromised states helps detect patients who merit further evaluation. Neurological symptoms are more commonly seen with infections of the cervical and thoracic spine than with infections in the lumbar region. The rate of progression of infection and neurological deficits is variable—some infections are relatively indolent, whereas others can progress rapidly and result in profound neurological deficits in just a matter of hours.
On examination, there is usually exquisite tenderness with percussion of the affected spinal segment. When the infection has a prominent bone component, a gibbus deformity may be clinically obvious. A dermatomal level of sensory, motor, or combined deficits is common in patients with deficits. The examination is usually consistent with an acute spinal cord injury with bladder and bowel involvement. Measurement of postvoid bladder residual followed by bladder catheterization provides an objective measure of the urologic dysfunction and prevents secondary injury of the detrusor muscle. Rarely, with relatively indolent infections, chronic compression of the spinal cord may occur and long-tract signs may be present.67
Occasionally, patients may initially be seen with florid sepsis68 and an altered level of consciousness and be unable to provide any history. This is somewhat more common in immunoincompetent patients, those in whom medical attention is delayed, and those with a previous, more rostral spinal cord injury who are insensate to the pain and have no neurological function below the infected level.69 In such cases, the diagnosis of PSI may be delayed for hours or days until the patient is stable enough to undergo MRI.
Diagnosis
Laboratory Markers
The diagnosis of PSI is suggested by the clinical features described earlier. Laboratory markers of acute inflammation—leukocyte count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP)—are helpful in screening patients for further evaluation and in establishing the diagnosis in cases in which the imaging changes are nondiagnostic. Of these markers, the leukocyte count is the least commonly affected,11,27,53,70 whereas the ESR is usually affected, often in dramatic fashion.6,16,20,50,53,56,70,71 According to Carragee and colleagues, the ESR is elevated in more than 90% of patients with spinal infections.70 Measurement of the ESR is inexpensive and reasonably sensitive, although nonspecific. It is an excellent parameter to monitor in determining the response to therapy and should be measured at the initial evaluation even if the diagnosis is already clear. Given its lack of specificity, the ESR should always be interpreted in the context of the patient’s overall condition. This lack of specificity is especially an issue in the management of patients with PSI and a preexisting elevated baseline ESR—such as those with cirrhosis—in which the elevated ESR is refractory to treatment of the infection and it is hard to determine whether the infection is responding. The diagnosis of spinal infection is also strongly suggested by a persistently elevated CRP. Measurement of CRP may be useful in detecting early infections because serum levels may increase within hours of a bacterial infection.72,73 Although both the ESR and CRP will become elevated after elective spine surgery, CRP normalizes postoperatively much more quickly than the ESR does, thus making it potentially more useful in the evaluation of postoperative patients.74
Imaging
Plain radiography and CT typically show few if any changes during the very early stages of infection.26 These imaging modalities, however, are valuable in the evaluation of more advanced infections in which bone changes are apparent and in the evaluation of vertebral bony integrity and spinal stability in patients in whom surgical management is being considered. Changes that are seen on plain radiographs several weeks after onset of the infection increasingly consist of prevertebral and paravertebral soft tissue volume, loss of disk height, trabecular erosion, and, eventually, destruction of the entire vertebral end plate on either side of a disk. Vertebral collapse, loss of normal lordosis around the affected level, and the development of a kyphotic deformity occur with advanced infection.75,76
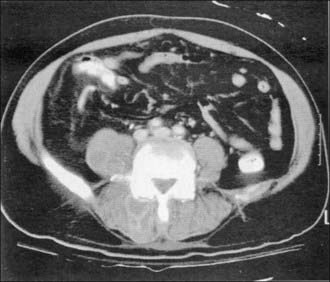
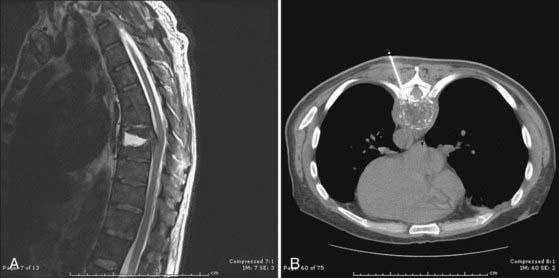
CT is generally more sensitive and specific than plain radiographs. Contrast-enhanced CT reveals inflammation of the prevertebral and paravertebral soft tissues (Fig. 276-1), visible as stranding and loss of the normal tissue planes, in infections that have been present for several days. Enhancing epidural collections may also be visible on a high-quality, contrast-enhanced CT of the spine. CT is an appropriate modality for the detection and percutaneous management of psoas abscesses and paravertebral abscesses that result from unchecked progression of the prevertebral and paravertebral components of a PSI. CT guidance is useful for percutaneous aspiration of disk spaces, paravertebral fluid collections, and necrotic bone and to provide specimens for a bacteriologic diagnosis. Myelography followed by CT provides another means of visualizing spinal cord or cauda equina compression in situations in which MRI cannot be performed.
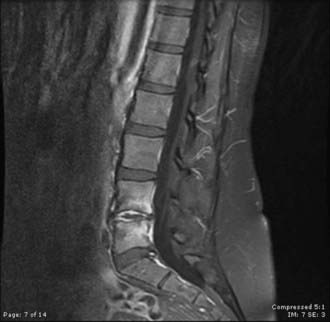
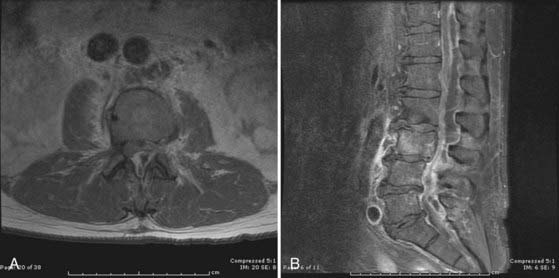
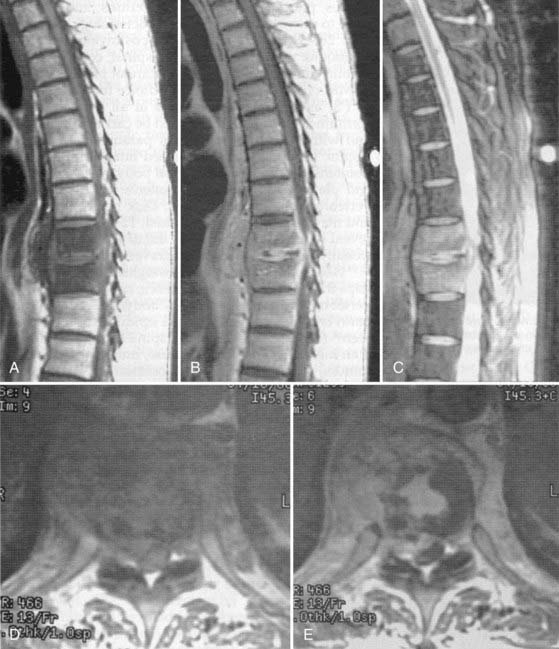
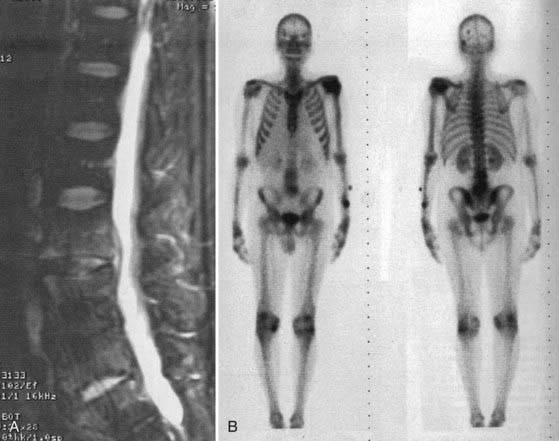
MRI is the diagnostic test of choice for the detection of PSI and should be performed in all patients unless contraindicated. Imaging should be carried out without and with the administration of paramagnetic contrast agents. Unenhanced T1-weighted images reveal a hypointense signal in the vertebral body, especially at the end plates; the normal hyperintense fat signal in the vertebral bone marrow is lost. Disk height is reduced and may be markedly diminished. T2-weighted imaging reveals high signal (edema) in the disk space and occasionally in the bone and paravertebral soft tissues.8,57,77 Gadolinium-enhanced T1-weighted imaging is perhaps the most diagnostic MRI sequence (Fig. 276-2)—enhancement of the vertebral end plates, the vertebral body, the prevertebral and paravertebral soft tissues, and the epidural space can be seen.76 The entire spine should be imaged if an infection is detected because much like metastatic tumors, spinal infections can occasionally be multifocal (Fig. 276-3 to 276-5).78,79
Radionuclide studies have a high degree of sensitivity in early infection. Gallium 67 and technetium 99 both have reasonable sensitivity for the detection of PSI. Gallium binds to iron-binding proteins at the site of inflammation, whereas technetium reflects blood flow to the bone. Gallium scans are therefore more specific than technetium scans for PSI, but both of these scanning methods are not very specific.80 Focal uptake can be seen with spondylosis, after trauma, and in tumors. In comparison, scans using white blood cells tagged with radionuclide are more specific for the detection of infection, but their sensitivity is much lower.81 Leukocytes from the buffy layer of the patient’s blood are tagged with indium 111 and reinjected into the patient (Fig. 276-6). These labeled cells localize to sites of ongoing inflammation; focal uptake in the spine strongly suggests the diagnosis of a PSI, although false-positive results can occasionally be seen with a few conditions such as tumors, especially hematogenous malignancies involving the spine. Chronic infections can occasionally lead to false-negative results with indium scanning. Fluoro-2-deoxy-D-glucose (FDG)-labeled positron emission tomography (PET) has proved to be highly sensitive for spinal infections, although it is also relatively nonspecific.82–84 Recently, scans using indium-labeled biotin have been used for the diagnosis of osseous infections.85 Biotin, a growth factor widely used by bacterial species, shows early promise as a more specific indicator of infection. Various other potential peptide markers are being investigated to aid in early diagnostic imaging.
Bacteriologic Diagnosis
The second component of the diagnostic evaluation is bacteriologic characterization of the infection. As noted earlier, the spine may be hematogenously seeded from other sites of infection such as the respiratory tract, urinary tract, or an endovascular source. Cultures of urine and sputum should be performed in patients with these potential sources. The causative organism is typically isolated from either blood or spinal tissue. Blood should be obtained for culture in all cases, and if possible, multiple sets should be collected to coincide with spikes in the patient’s temperature. If the blood cultures are positive, the causative organism is identified in 25% to 59% of cases.86 Appropriate therapy may be started without the need for further, more invasive testing. Unfortunately, blood cultures are negative in 40% to 75% of cases, possibly because some infections are indolent, although most commonly in patients who have received antibiotics before blood is drawn for culture. In such cases, biopsy of the vertebra or the disk space under CT or fluoroscopic guidance has a higher rate of success in culturing the organism. This may need to be repeated if no growth results from culturing the aspirate. A larger bore needle that obtains a core of tissue may yield microbiologic results superior to that of aspirates of fluid from bone. The use of a nucleotome for percutaneous suction-aspiration of the infected disk space has also been described.86,87 Biopsy specimens consisting of a core of tissue can also be submitted for histopathologic analysis to confirm the diagnosis. Closed biopsy techniques have reported accuracy rates ranging from 60% to 100%. If these measures fail and the imaging diagnosis is reasonably definitive for PSI, open biopsy may be necessary. This is best done in an operating suite under fluoroscopic guidance. A bacteriologic diagnosis is made in about 80% of open biopsies.86
It is difficult to establish a bacteriologic diagnosis if empirical antibiotic therapy is begun before obtaining blood for culture. It is important to exert restraint and withhold the administration of antibiotics until it is clear that an organism has been cultured, unless the patient is septic or has major systemic manifestations, in which case delaying therapy may be inappropriate.61 Even a single dose of a broad-spectrum intravenous antibiotic may significantly decrease the probability of culturing an organism. In patients with neurological deficits, antibiotics should be withheld until specimens are collected intraoperatively. If antibiotic treatment is initiated before a bacteriologic diagnosis is established in patients who are neurologically intact, it may be reasonable to terminate this empirical therapy and obtain fresh samples for culture without the influence of antimicrobials. In a small number of cases, no organism can be cultured despite multiple attempts.6,87 Mycobacterial or fungal infections should be considered in such cases. Once this possibility is reasonably excluded, empirical antibiotic therapy is the only option. Patients treated empirically with antibiotics need to be monitored closely to confirm a response to the treatment being administered.
Diagnosis of Associated Conditions
Frequently, a cutaneous pyogenic lesion that represents the index location of the infection is still present when the patient is evaluated by a neurosurgeon.88 Such lesions can often be used to obtain material for culture to make a bacteriologic diagnosis. Patients should also undergo assessment for risk factors responsible for the infection. Certain risk factors may be obvious, whereas others may be discovered only after careful evaluation. For instance, patients may be reluctant to admit to intravenous drug abuse. In some cases of indolent infection, the drug abuse may even have occurred several months previously. It is reasonable to offer intravenous drug abusers testing for human immunodeficiency virus (HIV) and type B and C viral hepatitis, as well as appropriate counseling together with such testing.
It should be emphasized that evaluation of a PSI is incomplete unless the patient is assessed for extraspinal manifestations of the infection. Infections can track along fascial planes adjacent to the infected vertebrae and result in psoas abscess, paraspinous muscle abscess, empyema, sympathetic pleural effusion, and retropharyngeal abscess.89 Infections can breach the dura spontaneously or with the unintended assistance of a biopsy or lumbar puncture needle. Changes in mental status, nuchal rigidity, or emesis in a patient with a known or suspected PSI should lead to the consideration of meningitis or parameningeal inflammation as a diagnostic possibility. The diagnosis of concomitant meningitis is best confirmed by obtaining cerebrospinal fluid (CSF) from a cisternal rather than a lumbar puncture—unless the locus of spine infection is clearly remote from the site of the puncture.62
Differential Diagnosis
Conditions that can have clinical findings similar to PSI but can be differentiated with imaging include pyogenic arthritis of the hip, septic or autoimmune sacroiliitis, pyelonephritis, primary psoas abscess, autoimmune spondylitis, spinal trauma, osteoporotic compression fractures, spinal epidural hematoma, spontaneous spinal subarachnoid hemorrhage, and leptomeningeal metastatic disease. Certain conditions may occasionally be a little harder to distinguish with imaging alone. Nonpyogenic (tubercular or fungal) spinal infections can occasionally mimic PSI rather closely, as can tumors metastatic to adjacent vertebral levels. Involvement of the vertebral body more than the disk space and the development of paravertebral abscesses rather early in the course of the infection suggest a tubercular rather than a pyogenic etiology.70 A definitive diagnosis can usually be established by needle biopsy in such cases. Tubercular infection and lymphomas should always be considered in the differential diagnosis in patients with AIDS.90 The degenerative changes seen in patients with advanced spondylosis91 can at times be confused with infections because both are preferentially localized to the vertebral end plates. They can generally be differentiated from infection; a degenerated disk is usually dehydrated and therefore hypointense, whereas an infected disk is hyperintense on T2-weighted imaging. Enhancement of the disk itself is also indicative of PSI, but enhancement of vertebral bone can be seen with either entity. The presence of gas within the disk, the vacuum disk phenomenon, is much more suggestive of degeneration than infection.76 A rare entity that may mimic PSI is avascular necrosis of the vertebral body, which is usually associated with significant collapse of the vertebral body and intravertebral vacuum clefts. Changes in the intravertebral vacuum clefts are seen as a consequence of spinal loading and unloading. T2-weighted imaging performed immediately after the patient lies supine on the scanner reveals a hypointense signal because of the presence of air, but as fluid enters the cleft, this signal becomes hyperintense.92,93
Management
Surgical Treatment
The decision to proceed with surgery should be made after consideration of the patient’s neurological status, vertebral level of involvement, extent of vertebral destruction, and findings on MRI. In the past, the decision to undertake emergency surgery was often made solely on the basis of an enhancing epidural component. The guiding principle has been that an epidural abscess constitutes a neurosurgical emergency. It has become increasingly clear that there is heterogeneity in the composition of epidural collections. Entirely liquid “abscesses” are rare, and in most cases a phlegmon with minimal, if any, liquid abscess is seen (Fig. 276-7). Such heterogeneity also applies to the clinical manifestations of epidural collections—some produce rapidly progressive neurological deficits, whereas others produce no deficits. Furthermore, as discussed in the section on the pathogenesis of PSI, neurological deficits occur more often as a result of spinal instability or deformity than as a result of compression of the cord or cauda equina by an abscess component. Thus, there is often poor correlation between an imaging diagnosis of “epidural abscess” and the development of neurological deficits. This lends further credence to the notion that management decisions in PSI are best made by taking into account multiple clinical and imaging criteria rather than a simple anatomic classification of the infection.
Surgical intervention for neurological deficits needs to address the location of the compressive lesion, such as ventral or dorsal to the spinal cord or cauda equina. Simplistic though this sounds, ignoring this principle may result in destabilization of an already compromised spine, with worsening deficits.39,49,51,94 The nature of the compressive lesion—liquid pus versus a mass of granulation tissue or retropulsed bone—is also an important consideration in determining the optimal surgical approach. Although pus may be accessed and drained by various routes, simple laminectomy does not adequately afford decompression of a solid ventrally situated extradural lesion and can exacerbate the deficits produced by a kyphotic deformity. Finally, the various anatomic regions of the spine dictate the potential approaches available and the likelihood of postoperative instability.
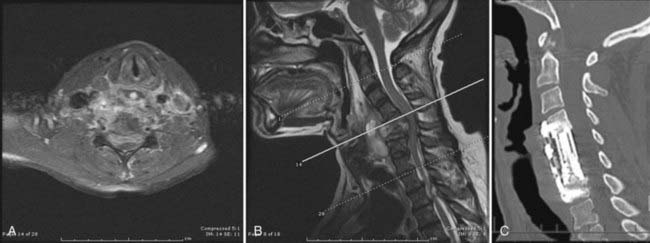
In the cervical spine, the surgical approach usually coincides with the location of the compressive lesion (i.e., an anterior approach for ventral compression and a posterior approach for dorsal compression).9,55 An exception may be cited for ventral abscesses without major bone involvement extending over more than two or three levels. In these cases, pus can usually be drained from a posterior approach without the morbidity of multilevel corpectomy and fusion. Infections of the odontoid, though rare, have been reported. If deemed to have produced instability, they are best managed by occipitocervical fusion and a transoral biopsy or decompression of the thecal sac. Stable lesions at this level can be managed medically.95,96 At the cervicothoracic junction and in the upper thoracic spine, anterior lesions may be difficult to access from a ventral approach because of the presence of the great vessels. Although partial sternotomy or manubrial resection may provide adequate access in such cases, technical challenges with débridement and reconstruction of the anterior column remain. Furthermore, a kyphotic deformity produced by the infection can make access to the apex of the deformity via an anterior approach more difficult. Transpedicular, lateral extracavitary,97–99 or periscapular100,101 approaches may be used in these cases. These approaches can be used to decompress the ventral aspect of the spinal cord, and potential or apparent segmental instability can be addressed by concurrent posterior thoracic fusion with instrumentation.39,49,94 Recent technologic advancements have increased the options available for fixation over the cervicothoracic junction.
Surgical approaches for treating PSI of the midthoracic spine are best tailored to the site of compression. Thoracotomy approaches offer excellent visualization of the ventral and ventrolateral aspects of the spinal canal. Anterior reconstruction after vertebrectomy is readily performed via this exposure. Alternatively, the lateral extracavitary approach97–99 or the retropleural approach101 can be used. The temptation to perform a laminectomy for ventral disease in the thoracic spine, other than liquid pus, should be resisted because it can result in the cord being draped over the compressive lesion along with concomitant loss of the stability offered by the posterior tension band.49,51,94 The extent of spinal instrumentation required to restore stability is a function of the number of segments involved, the degree of kyphotic deformity, the patient’s bone stock, and the integrity of the posterior tension band.
In the lower thoracic and upper lumbar spine, anterior débridement via a thoracoabdominal approach affords excellent exposure for resection of the involved vertebral bodies and reconstruction of the anterior and middle columns.22,27,56 When the posterior elements are intact, an anterior approach alone may suffice. Anterior débridement and fusion followed by posterior instrumentation and posterolateral fusion102,103 may be an option in selected patients in whom concern for appropriate placement of instrumentation from the anterior approach used for the decompression is especially high. Infections of the middle and lower lumbar spine may be approached through either a retroperitoneal or a transperitoneal approach for débridement and anterior reconstruction. Below the conus, a posterior approach can be used to decompress the neural elements; however, reconstruction of the anterior and middle columns is difficult with this approach. Transpedicular instrumentation can provide a measure of stability in such cases, but it may occasionally fail if anterior column reconstruction is not performed. After fusion and instrumentation for spinal infections, an external orthotic device appropriate for the level in question should be prescribed for approximately 3 months.
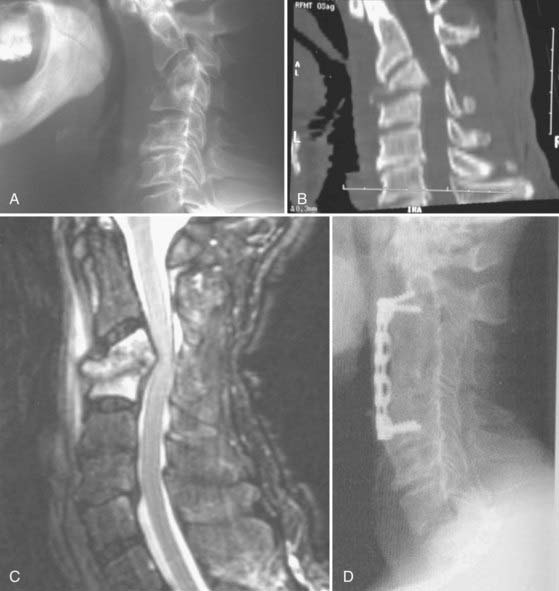
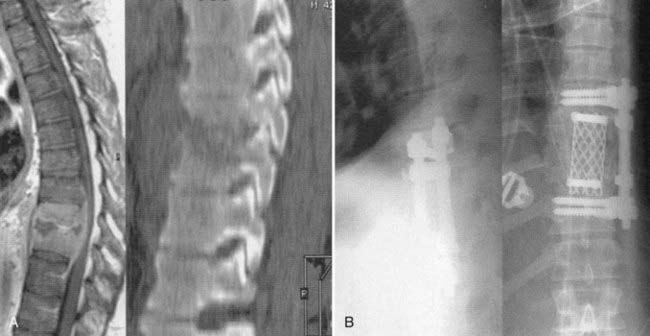
In addition to the treatment of patients with neurological deficits, surgical intervention is also indicated for the management of those who have failed medical therapy, the treatment of chronic pain after medical management, and the treatment of patients with prominent deformity or overt instability.6,32,104 Relapses of infection can be treated either with a second course of antibiotics or by surgery, depending on the clinical scenario and the patient’s preference. It has been postulated that relapses occur because of the presence of necrotic bone (sequestra) within a vertebral body that lacks blood supply and thus provides a nidus for persistent infection. Surgical treatment in these cases therefore includes débridement of the vertebral body that is infected (i.e., corpectomy), followed by reconstruction (Figs. 276-8 and 276-9). Finally, some surgeons tend have a bias toward immediate surgical management of patients with a prominent bony component of their infection because they believe that bacteriologic cure rates are then higher and thus a cure is effected earlier. This bias still remains to be validated by clinical trials. However, the fact that aggressive débridement eliminates a sizable portion of infected and necrotic bone lends credence to this viewpoint.
Timing of Surgery
Surgery needs to be performed on an emergency basis in patients with rapidly progressive neurological deficits.58 In cases in which the deficits have been slowly progressive or the patient has significant medical comorbidity (septicemia, coagulopathy, endocarditis with cardiac failure), the timing of surgical intervention should be more carefully considered. Surgery can be carried out on an elective basis in patients with no deficits, in whom the goal is to stabilize the spine or débride a necrotic focus of infection.
The role of steroids, at dose levels used for the acute management of spinal cord injury,105,106 is questionable in that there are no prospectively collected data to address the issue, nor are there likely to be any given the relatively small numbers of such cases seen at any one institution. Our experience, though anecdotal, suggests that the use of steroids at high doses in patients with a significant neurological deficit is safe in the perioperative period.
Implantation of Bone Grafts and Hardware
The surgical wound created during the operative management of PSI is heavily contaminated by the organisms responsible for the infection. Yet the spine in such cases is often unstable and some method of spinal reconstruction is essential. In years past, the implantation of devitalized bone or metal into such a field was considered contraindicated. Recent clinical experience by multiple groups has tended to refute this viewpoint. Many studies have shown that if the necrotic bone and soft tissue are adequately débrided, the implantation of bone graft (allograft or autograft) and instrumentation in patients who receive an appropriate postoperative course of antibiotics is safe and effective.6,30,32,39,68,88,107–111 Follow-up of patients over time reveals that the implantation of hardware and bone grafts is remarkably effective in producing bony union at the site of infection. This may be partly due to the enhanced vascularity of the region with the infection. Implanted hardware and bone graft seldom become secondarily infected as long as the necrotic bone is well débrided and the patient receives an appropriate course of antibiotics. Given that inadequate débridement is frequently the cause of a recrudescence of infection and that placement of hardware is safe if the débridement is adequate, thorough débridement is an important goal of surgical intervention in patients with PSI.
Management of “Epidural Abscesses”
Management of epidural abscesses merits a separate discussion given the controversies that surround it. There are no prospectively collected data that address the surgical versus medical management of these lesions. Because patients with true abscesses are at some risk for acute neurological deterioration and fatality, an observation that dates back to Dandy,112 the prevailing recommendation has been to treat all abscesses surgically7,14,35,113—”ubi pus, ibi evacua.” A review of the existing literature and our own experience in dealing with such lesions suggests that carefully selected patients have an excellent outcome with medical management alone.16,25,29,31,49,58,114,115 The clinical condition of the patient and the imaging characteristics of the epidural collection are the determining factors that direct decisions on management. Careful evaluation of the MRI characteristics of epidural lesions can determine their fluid versus formed nature and prevent unnecessary, potentially harmful intervention. A collection that enhances only peripherally, has a central nonenhancing portion, and is hyperintense on T2-weighted images is very likely to be fluid and easily drainable. Lesions that are homogeneously enhancing and isointense or hypointense on T2-weighted images probably represent a phlegmon—a collection of granulation tissue. Such collections need to be addressed surgically only if they are responsible for the production of neurological deficits. An area of controversy lies in patients with a fluid abscess but no detectable deficits. If an organism is identified from such patients and they can be monitored closely, they may be managed medically. If a bacteriologic diagnosis is not forthcoming, the symptoms persist or worsen despite medical therapy, or there is any difficulty in obtaining reliable serial neurological assessments, surgical intervention is desirable.104 Epidural abscesses of the cervical and thoracic spine are at higher risk for the sudden onset of neurological deficits than are similar lesions below the conus and therefore justify a lower threshold for surgical intervention. The presence of an epidural abscess over several segments or paralysis of more than 3 days’ duration does not present a contraindication to surgical intervention.25,115 Good outcomes can be obtained by decompression over discontinuous segments, and patients with relatively long-standing deficits may occasionally improve neurologically with surgical intervention. Finally, it cannot be overemphasized that patients with epidural abscesses who are managed conservatively need to be carefully and serially monitored with regard to their neurological status.
Medical Management
The management strategy in patients without neurological deficits or sepsis involves immobilization of the affected vertebral levels and administration of appropriate intravenous antibiotics.22 Medical management should be initiated as soon as an organism has been isolated or after cultures have been initiated in patients who are seen in septic extremis. The goal of therapy is to effect sterilization of the infected vertebral levels, prevent the occurrence of a neurological deficit, and prevent the formation of a painful deformity as the infection clears. The duration of therapy may be dependent in some measure on the extent of bone involvement seen on MRI. The duration of antibiotic therapy remains controversial, with no prospective clinical trials having addressed length of therapy. Relapses occur in 0% to 15% of patients and usually occur within 6 months of treatment.116 In patients with a minimal amount of bone infection and a competent immune system, the duration of therapy can be restricted to 6 weeks. In patients with a prominent osteomyelitis component extending over multiple segments or in immunocompromised patients (AIDS, cirrhosis, poorly controlled diabetes), an 8-week-long course of antibiotic therapy may be more effective in preventing a relapse of infection.26 The efficacy of medical management can be gauged by diminishing pain, malaise, and fevers and a decrease in the ESR. In all patients in whom there are no other unrelated coexisting causes of the persistent elevation, the ESR should be monitored closely to determine the effect of treatment. Unfortunately, however, the response of the ESR to appropriate treatment is unpredictable. Although a significant decrease in the ESR within a few weeks of treatment usually augurs a good prognosis, sustained elevations do not in themselves imply a failure of therapy.70 The ESR should be viewed in context of the patient’s overall clinical picture. If the decrement in the ESR takes far longer than expected, consideration should be given to extending the duration of antibiotic therapy. If after several weeks of therapy there is no significant alteration in the ESR and the patient continues to be symptomatic, consideration should be given to reestablishing the radiobacteriologic diagnosis.20,117
Antibiotic therapy is guided by the in vitro sensitivity of the organism that is isolated. Given that staphylococcal and streptococcal species account for the vast majority of PSI, β-lactams—penicillin and oxacillin/methicillin/nafcillin—the ureidopenicillins (ticarcillin and piperacillin), the cephalosporins, and vancomycin are the most commonly prescribed antibiotics. The addition of an aminoglycoside, trimethoprim-sulfamethoxazole, a fluoroquinolone, or rifampin as a second agent may have a synergistic effect in vivo and should be considered in patients with extensive bone involvement because vancomycin and certain cephalosporins may penetrate poorly into devascularized bone and the disk.118 The addition of fusidic acid to the treatment regimen of penicillinase-stable penicillins appears to decrease the chance of failure of medical therapy.26 Newer agents such as linezolid and daptomycin have not demonstrated superiority over standard therapies.119,120 The increasing prevalence of methicillin resistance and the decreasing susceptibility to vancomycin warrant careful assessment of the response to therapy.
Patients in whom no causative organism is isolated after multiple attempts or in whom empirical treatment was started before a full microbiologic work-up need to be monitored especially closely. Recommendations for empirical antibiotics vary somewhat with the epidemiologic factors involved. Intravenous drug abusers may tend to have a relatively greater proportion of infection with Pseudomonas.27,29,55,62 Failure of empirical therapy should prompt consideration of unusual pathogens—fungi and mycobacteria (see later). Additionally, patients need monitoring for adverse effects of the drugs used, antibiotic levels as appropriate, and management of the predisposing factors and associated complications. In addition to antibiotics, patients are prescribed about 2 weeks of bed rest and are fitted with an orthosis appropriate to the spinal level of infection to prevent the occurrence of a deformity or to help correct a mild deformity present at the time of diagnosis.
The role of oral antibiotics in treating PSI is poorly defined. The practice of initiating oral agents59,113,121 after a prolonged course of intravenous antibiotics is widespread for osteomyelitis in various locations yet finds little support from any comparative analysis of their efficacy in treating spinal infections.11 It is unlikely that any residual infection after an appropriate course of intravenous antibiotics would be cleared by oral antibiotics, which may serve simply to suppress recrudescence of the infection rather than effect sterilization of the infective nidus.
Infections isolated to the facet joints are rare.46,47 They are usually well managed by medical treatment, with surgery being reserved for patients with neurological compromise. A dorsal approach for débridement is usually adequate in these cases, and disruption of a single facet does not generally compromise stability in an otherwise intact motion segment.
Outcomes
Patients with PSI and incomplete neurological deficits of recent onset can have surprisingly good neurological recoveries, provided that timely, aggressive surgical therapy is instituted.16,39,51,55,68 The prognosis is more guarded in patients with profound deficits and in those in whom diagnosis or treatment is delayed. Nonetheless, some of these patients may show substantial improvement in neurological function over time, thus justifying an aggressive therapeutic stance even when severe deficits are encountered.
An analysis of outcome in patients with epidural abscesses suggests that older patients and those with sepsis, neurological deficits of longer than 72 hours’ duration, or significant compression of the spinal cord on imaging studies are more likely to have a poor outcome than patients without these characteristics.58,122
In patients managed medically, the prognosis depends to some extent on the patient age, the rate of decrease in the ESR, and whether the patients were substantially immunocompromised. An immunocompromised state and infections caused by virulent organisms such as S. aureus (as opposed to S. epidermidis or Propionibacter) may be associated with higher mortality rates.27 Advanced age may be an independent indicator of a bad outcome—studies have suggested that the elderly have a poor outcome,27,58 but with aggressive surgical management, they may fare rather well.22,23
Serial plain radiographs may be considered in patients with a prominent bony component of the infection and for the early detection of vertebral body collapse or the development of a deformity. Successful treatment of the infection is seen radiographically as incorporation of the bone graft (if the patient was managed surgically) or sclerosis of the vertebral end plates on plain radiographs and CT. In patients with prominent disk space or bone infection, spontaneous fusion at the affected level may occur. MRI shows resolution of the typical changes, with the vertebral body tending to have a fat-like signal quality. Serial MRI is not indicated unless a relapse of infection is suspected on clinical grounds. Radiographic findings respond very slowly to successful treatment, in contrast to the clinical response, and are therefore not immediately useful in assessing the response to therapy.57
Pyogenic Spine Infections in Children
Pyogenic infections of the spine in young children are more common than expected. Those affected usually have no risk factors for infection other than their age. Infections have been reported in neonates123 and infants124,125 and occur throughout childhood into early adolescence. The predilection for these infections appears to be a result of the frequent bacteremia that occurs in childhood. The distinct pattern of infection is thought to result from the peculiarities of the pediatric spinal vascular anatomy.
Until the age of about 7 years, profuse anastomoses exist between the intraosseous spinal arteries and thereby prevent devascularization and infarction of large portions of the metaphysis when septic emboli occlude a metaphyseal artery. This tends to limit the extent of metaphyseal and osseous infection to the cartilaginous end plate at either end of the vertebra. Hence, hematogenous spread to the pediatric spine tends to be limited more to the disk space. Additionally, the pediatric disk retains vascularity, unlike disks in adults, and occasionally blood-borne pathogens may lodge directly in the disk space in children without any involvement of the metaphyseal end plates.126
There is controversy regarding whether pediatric diskitis can exist without the presence of an infection. It has been postulated that some cases may occur secondary to partial dislocation of the epiphysis as a result of a hyperflexion injury.127 Indeed, the rate of culturing organisms from the disk in young children with radiographic changes consistent with an infection is lower than that in adults.75,128,129 Other studies, however, support the notion that these are true infections and are best treated by intravenous antibiotic therapy.10
Young children may be unable to provide an accurate history or accurately describe their symptoms, so the diagnosis should be considered whenever a child with fevers refuses to bear weight or assumes postures that avoid bending of the spine. The differential diagnosis includes the conditions that may mimic PSI in adults, but idiopathic intervertebral disk calcification, urinary tract infections, and appendicitis must also be considered in the pediatric population. The clinical and radiographic disease in children may often be milder than that seen in adults. In patients in whom no organisms are isolated, management with immobilization alone may be reasonable, but patients managed in this way should be monitored closely for clinical and radiographic evidence of deterioration. In all cases in which infection is suspected or confirmed, appropriate antibiotic therapy based on the results of culture should be initiated. Surgical intervention is only rarely indicated, usually if there is a significant epidural extension of the infection leading to neural compromise.130 Patients appear to do well with relatively short courses of antibiotic therapy and immobilization. In the long term, these patients may be at high risk for the development of block vertebrae and vertebrae magnae.124,131
Iatrogenic Infections
Postoperative Spinal Infections
Postoperative spinal infections can occasionally be catastrophic events that result in prolonged (and expensive) hospital stays and significant long-term disability.132,133 In the pre-antibiotic era, the rate of infection after spinal operations was about 0.9% to 4.6%. In series reported after the advent of Harrington instrumentation, the infection rate rose to between 1% and 12%, with an average of 6%.134 Today, however, postoperative infection rates have decreased substantially despite the increased complexity of surgical procedures.
Currently, the average rate of infection after spinal procedures is around 2%. Of course, rates vary by procedure and are affected by a variety of factors. Contamination of the surgical wound by skin commensals occurs in as many as half of all surgical cases.135 The host response to this contamination plays a significant role in determining whether an infection develops. Patient factors that predispose to infection include previous surgery, previous irradiation, preexisting neoplasm, chronic steroid therapy, diabetes, malnutrition, paraplegia, smoking, rheumatoid arthritis, nutritional state, and intercurrent infection.136–138 In addition, there are numerous technical factors that influence the risk for infection; examples include the duration of surgery, the length of the wound, the duration and force of retraction used, the presence of a CSF leak, the use of antibiotic irrigation solutions, and the implantation of instrumentation. Prophylactic administration of antibiotics in the perioperative period is probably useful in preventing colonization of the wound and, if used for appropriate durations, is not associated with the development of infection by resistant organisms.139–142 Double gloving is another practice that may reduce the risk for infection. The use of Bovie monopolar cauterization has been implicated in an increased risk for postoperative infection.143 Interestingly, the level of experience of the surgeon is not a factor; for instance, residents and fellows in training do not seem to increase the risk for infection.144 For diskectomy procedures, the risk for postoperative spondylodiskitis may be reduced by placement of a gentamicin-impregnated collagen sponge in the disk space145,146 and adding bacitracin to the irrigation fluid used during surgery.147 This seeming efficacy of locally delivered antibiotics finds support from studies that show unreliable and poorly sustained levels of antibiotics in the disk space after systemic administration.148,149 Microneurosurgical approaches for diskectomy may also be associated with a lower risk for infection than traditional laminectomy and diskectomy.150
Infections after Surgery without Instrumentation or Bone Grafts
The incidence of infection after lumbar laminectomy or diskectomy is approximately 1%.151 Postoperative diskitis occurs in about 0.1% to 5% of patients after lumbar diskectomy.145,152 The incidence is similar for posterior cervical operations but may be slightly lower when an anterior approach to the cervical spine is used. Infection usually occurs after intraoperative contamination,147 and the typical causative organisms are skin flora—commonly S. aureus and S. epidermidis. Rarely, however, gram-negative organisms and anaerobes may produce fulminant infections in the surgical bed.153 The typical scenario is recurrence of symptoms in a patient who initially experienced good relief of preoperative symptoms (e.g., radiculopathy) immediately after surgery. The surgical incision may appear to be healing uneventfully. A history of a small amount of drainage soon after surgery is occasionally obtained. The diagnosis is strongly suggested by a persistently elevated ESR145,154 or CRP and by typical changes on MRI. However, both the acute-phase reactants and the imaging changes need to be distinguished from the expected postoperative changes. The ESR generally returns to normal levels within 2 weeks after uncomplicated surgery. Measurement of CRP, which returns to normal sooner, may be useful in detecting early infections, but given the individual variability in CRP values, it is most useful when a baseline value is also available. Measurement of a specific acute-phase reactant, such as elastase–α1-proteinase inhibitor, potentially allows the detection of an infection even earlier, but it has not been shown to be relevant outside of a research setting.155 MRI with contrast enhancement almost always reveals changes suggestive of postoperative spondylodiskitis, but it may yield false-positive results152,156 and needs to be evaluated in the context of the patient’s clinical picture and laboratory data.
Depending on the number of levels operated on and the size of the postoperative fluid collection, the infection can be managed either with antibiotics alone or with surgical débridement in addition.157 Because there is little devascularized tissue or foreign material in such wounds, antibiotic penetration is usually excellent, and these infections can often be eradicated by a few weeks of intravenous therapy alone.
Infections after Surgery with Instrumentation and Bone Grafts
Modern management of spinal disorders often includes the use of instrumentation to facilitate fusion. Instrumentation provides immediate spinal stabilization, early mobilization of the patient, and a higher rate of bone fusion. However, the use of instrumentation clearly increases the risk for postoperative infectious complications. Data demonstrating the effect of instrumentation on the risk for postoperative infection first became available with the series of Harrington rod instrumentation.158 Recent estimates of infection risk in instrumented patients range from 2% to 8.5%.136,137,159,160 Infections associated with spinal instrumentation are far more common after posterior or posterolateral approaches to the spine than after anterior cervical or anterior thoracolumbar instrumentation.136 This is presumably related to an increase in necrosis of the paraspinous muscles and other soft tissues because of the devascularization that results from dissection and ischemia from prolonged retraction. Prolonged retraction is known to cause disruption of normal muscle physiology and compromise of perfusion, so the duration and extent of retraction should be minimized.161,162 In addition to frank tissue necrosis, ischemia can promote the formation of a large seroma in the wound, a fertile ground for colonization by multiple organisms.133
Infections after spinal instrumentation can become manifested in either acute or delayed fashion.163 Patients with acute infections are usually seen between 2 and 8 weeks after the initial surgery with erythema of the wound, partial wound dehiscence, and drainage of seropurulent fluid.136 Fever and leukocytosis may or may not be present, although the ESR is generally elevated. The causative organism can usually be cultured from the wound if material for culture is obtained before starting antibiotic therapy.
Distinction needs to be made between early infections that are limited to the skin and subcutaneous tissue and those that extend below the fascia. Superficial infections generally occur in obese patients with large amounts of subcutaneous fat and in those with impaired wound healing. These infections can either be closed after thorough débridement and irrigation, if there is minimal necrosis, or be managed with sterile dressing changes and delayed secondary closure if there is more substantial soft tissue loss. Early infections after instrumentation are usually caused by S. aureus, sometimes a strain resistant to the antibiotic administered perioperatively. Such infections necessitate thorough débridement of the wound, removal of suture material and necrotic tissue, and intravenous antibiotic therapy. They can generally be managed without removal of the implants.133,136 A concern in the management of deep infections after instrumentation has been the ability to deliver bactericidal concentrations of antibiotics to potentially devascularized regions. To accomplish this, some surgeons advocate the use of continuous suction-irrigation systems to deliver antibiotics to the wound.133,136,164 Others prefer to augment local bacterial control at the surgical site of grafting by implanting material that releases antibiotics locally over an extended period.165 However, Rath and colleagues stated that if the delivery vehicle used is an acrylic implant, it may allow the infective foci to persist.39 Many other surgeons, including us, prefer multiple débridements or serial open packing of the wound until all necrotic tissue is removed and delayed secondary closure can be performed. Occasionally, catastrophic mixed infections by organisms that can cause a synergistic necrotizing fasciitis or gangrene can complicate débridements, but fortunately they are rare.166
Delayed infections are manifested several months after the initial operation. Such infections are almost always attributable to indolent organisms such as Propionibacterium acnes, S. epidermidis, or Corynebacterium. If enough time has elapsed since bone grafting and rigid bone fusion is found at surgery, a case can be made for removal of the infected hardware.160,167 Dubousset and associates have argued that these delayed infections are not indeed true infections but may reflect a fretting corrosion of the metal implant168 that results in a seemingly sterile chronic inflammation surrounding the instrumentation. It has been shown, however, that if the intraoperative tissue cultures are maintained in the laboratory for at least 7 days, slow-growing organisms such as the ones listed earlier can be isolated.160,169 These infections probably result from intraoperative contamination of the instrumentation by organisms that multiply slowly.167 The instrumentation is coated with an avascular exopolysaccharide—the glycocalyx—produced by these bacteria that prevents the body’s immune mechanisms and antibiotics from eradicating them. Additionally, the glycocalyx prevents truly representative organisms from detaching in sufficient numbers to be detected by simple aspiration and culture. Because such infections usually occur late, removal of the instrumentation may not compromise the bony fusion. Hardware removal is the most effective way of eradicating the glycocalyx and thereby the nidus of the infection. This forms the basis for the recommendation by some authors for hardware removal.160,167 After hardware removal and adequate débridement and intravenous antibiotics for about 4 weeks, these infections are generally eliminated.
Infections after Diskography
Infections develop after diskography in about 0.16% to 3% of disks injected.170–173 Although the most common infectious complication is diskitis, an epidural abscess170,174 or even a subdural abscess can result after diskography. Patients usually have severe local pain a few days after the diskogram. The hematologic markers of acute inflammation175 are significantly elevated. The diagnosis is made by MRI without and with contrast enhancement. Management is chiefly medical, with antibiotics tailored to the staphylococcal species, the most common causative organisms, or to the culture results if an organism can be aspirated from the disk. The risk for spinal infection after diskography can be minimized by the administration of a broad-spectrum antibiotic either intravenously or mixed with the contrast dye.176 The use of styletted needles and a double-needle technique as recommended by Fraser and coworkers may also aid in the prevention of iatrogenic contamination of the disk space.171
Infections after Placement of Epidural Catheters
Epidural catheters used for pain control can occasionally provide a track for the entry of infection into the epidural space. The usual pathogenic organisms are S. epidermidis and S. aureus, which probably reflect contamination of the catheter by skin flora.177 The reported incidence of such infections is widely variable—from approximately 1 infection per 2000 catheter placements178 to a 12% infection rate. Much of this variability appears to originate from the duration for which epidural catheterization was used; when the incidence is expressed as the number of infections per catheter-days, there is much more homogeneity in the risk for infection. The incidence stated in this way appears to be between 0.2 and 0.77 per 1000 catheter-days.179–181 This seeming dichotomy in the incidence of infections relates to the rise in infection risk with the duration of catheterization.178 Long-term epidural anesthesia is used for the management of cancer pain, and the risk for infection “per patient” is higher.181 Given the wide use of epidural anesthesia during parturition, the large majority of reports of infection of these catheters come from the obstetric literature. Factors that appear to correlate with the occurrence of catheter infection include a prolonged time of catheter insertion, an immunocompromised state, and recent trauma.179,182,183 Early warning signs of the presence of infection include focal pain around the site of insertion of the catheter, superficial infection of the catheter entry site, and catheter dysfunction.180,181 These early signs can progress to the rapid development of neurological deficits.181 MRI with contrast enhancement provides a reliable diagnosis in most cases.181,184 Management depends on the condition of the patient at the time of evaluation. If the infection is detected early, it can usually be treated by removal of the catheter followed by appropriate antibiotic therapy. Patients in whom deficits have developed should be evaluated for urgent surgical intervention.182,184 Surgical intervention can improve the chance of neurological recovery in patients in whom deficits develop, but many patients may not improve despite aggressive intervention.183 Culture of the catheter after its removal will generally identify the causative organism, and antimicrobial therapy can be tailored accordingly. In patients with chronic pain who are debilitated, sepsis may occur after development of the epidural abscess and may result in death.181 Tunneling the catheter for a distance before exiting the skin may help in reducing the risk for infection.185
The use of 0.5% chlorhexidine in 80% ethanol rather than 10% povidone-iodine for decontamination of the skin before insertion of an epidural catheter has been promoted by some as a method of preventing catheter infections.186 It finds support in the scientific literature, where chlorhexidine solutions have been shown to be more effective than povidone-iodine in decreasing the number of bacterial colonies cultured from the skin187–189 and in preventing vascular catheter-related infections.190–193 The latter set of studies has been used by some as an analogous situation to catheterization of the epidural space because of similarities in the risk factors for colonization of the catheters. However, two prospective randomized studies comparing the use of chlorhexidine and povidone-iodine for skin preparation before the placement of epidural catheters failed to agree with each other, with one study showing a beneficial effect of using chlorhexidine in a pediatric population and the other study showing no difference in an adult population.194,195
Infected Kyphoplasty and Vertebroplasty
Percutaneous vertebroplasty and kyphoplasty have become widely accepted modalities for the treatment of refractory pain after thoracic or lumbar compression fractures. Recently, these procedures have been used for the management of pathologic fractures resulting from metastasis, myeloma, lymphoma, and hemangioma of bone. Injection of polymethyl methacrylate cement by means of either technique is associated with high rates of pain relief in otherwise refractory patients.196,197 Recent studies have reported that 90% of patients experience pain relief within 24 hours after treatment.198 Clinical series have reported low rates of complications, most of which result from cement migration.199 Infections have generally been thought to be uncommon. With broader use of these techniques, reports of osteomyelitis have begun to emerge.200–202
Walker and coauthors reported two such cases in 2004 and noted that both patients had a history of previous infection.201 They recommend careful preprocedure screening to minimize infectious complications. Findings on postvertebroplasty imaging may be atypical and infections difficult to diagnose radiographically. Intervertebral clefts may be seen adjacent to the cement. Percutaneous, CT-guided aspiration may yield an organism, although inability to culture an organism is not uncommon.202
Other Iatrogenic Infections
Spinal infections may develop after other minimally invasive procedures on the spine. The risk for infections developing after the placement of epidural spinal cord stimulators203 and after intradiscal electrothermy204 is extremely low. The use of careful sterile technique and periprocedural antibiotics minimizes these risks. Early detection and aggressive medical therapy are usually adequate for the management of infections in this setting, with surgical intervention being reserved for patients with neurological deficits or infected nonbiologic material.
Unusual Bacterial Pathogens
Actinomycosis and Nocardiosis
Actinomyces species are gram-positive, filamentous bacteria that are most commonly associated with chronic draining infections. The presence in the drainage of “sulfur granules,” discrete yellow particulate material that consists of clumps of the organism itself, is pathognomonic of Actinomyces infection. Common sites of infection include the oral cavity and paranasal sinuses with extension into the soft tissues of the face or neck. Spinal involvement is rare and generally the result of contiguous spread from adjacent sites of infection, especially the lungs and the sinuses.205,206 Vertebral destruction with deformity is uncommon with Actinomyces infection. Neurological compromise is usually due to extensive spread of the infection to the epidural space. The first-line treatment of Actinomyces osteomyelitis is intravenous penicillin G. Ciprofloxacin and rifampicin are also effective in eradicating vertebral infections.207 Surgical intervention is rarely indicated and should generally be reserved for patients with neurological compromise.208
Nocardia species are filamentous, branching, gram-positive aerobic bacteria. They are normally found in the soil and are primarily associated with pulmonary infection in immunocompromised patients. Spinal involvement is rare—about a dozen cases have been reported in the literature209–211—and it occurs through both direct extension of intrathoracic infections and hematogenous spread. Most reported infections have been caused by asteroides species. Treatment consists of sulfonamides, cephalosporins, aminoglycosides, or synthetic penicillins. Although a prolonged course of antibiotics may be required for cure of osteomyelitis, elimination of the infection occurs in most patients. Surgery is generally reserved for stabilization and correction of deformity.
Brucellosis
Osteoarticular complications of infection with Brucella species are common and occur in about 25% of cases.212 Spinal involvement, perhaps the most common bony infection, accounts for about half of the cases of osteoarticular extension. Vertebral infection occurs in about 6% to 12% of cases of brucellosis.213,214 The initial signs and symptoms of spinal brucellosis are nonspecific and similar to other forms of spinal osteomyelitis.215 The onset of symptoms of spinal brucellosis tends to be subacute, and the radiologic manifestations are nonspecific and bear some similarity to cases of tuberculosis (TB). However, the proliferative changes associated with bone repair seen in brucellosis are not found in tuberculous infection, and deformities of the spine, although common in TB, are rarely seen with brucellosis. Radionuclide bone scans are highly sensitive in demonstrating areas of involvement in patients with Brucella infections who have musculoskeletal complaints.216
Tuberculosis
TB is perhaps the most lethal infectious disease worldwide; it accounts for nearly 3 million deaths per year.217 More than 1.5 billion people either are presently infected or have had tuberculous infection in the past. The AIDS pandemic has been cited as being responsible for the recent resurgence in the reported cases of TB, especially in regions of eastern and central Africa, where the incidence of HIV infection is especially high. In the western world as well, there is evidence of an increase in cases of tuberculous infection in high-risk groups, especially those with immunosuppression or immunodeficiency syndromes, substandard nutrition and living conditions, and the extremes of age. It has been estimated that more than 2 million individuals have spinal TB worldwide.
Osseous and articular involvement is a common manifestation of well-established mycobacterial disease and occurs in approximately 3% to 5% of patients. Interestingly, the incidence of skeletal involvement in patients with concurrent HIV infection rises to 60%. The most common site of skeletal involvement is the spine, and nearly half of all cases of osseous TB are spinal. Spinal infections also typically have the most serious consequences, with severe deformity and paraplegia being the most significant. Although tuberculous involvement can be seen at any spinal level, the frequency of involvement varies widely, with the peak levels being at the thoracolumbar junction and decreasing frequencies occurring at more rostral and caudal levels. Overall, the cervical spine accounts for about 10% of cases, the thoracic spine 50%, and the lumbar spine 40% of all cases of tuberculous spondylitis.212 Although spinal involvement may result from direct extension from adjacent structures (e.g., lung and pleural cavity), it is more common for spread to the vertebral column to be through a vascular route. Work by Hodgson in the 1960s and 1970s suggested that a major mechanism for spread of infection is via Batson’s venous plexus. The strong association of spinal involvement with intra-abdominal infection such as renal TB is consistent with this hypothesis.
Fungal Infections
Candidiasis
Candida species are common opportunistic infectious agents in patients with various forms of immunocompromise, including diabetes, systemic malignancy, HIV infection, prolonged antibiotic administration, or bone marrow or organ transplantation.28 Intravenous drug abuse is also an important factor in disseminated candidiasis. Vertebral involvement as a consequence of disseminated infection is relatively uncommon; however, the increase in numbers of susceptible individuals will result in an increased number of cases. Non-albicans species such as Candida glabrata, Candida tropicalis, and Candida dubliniensis are assuming greater importance as opportunistic pathogens. This is significant because there is an increase in resistance to antifungal chemotherapy in many of these “nontraditional” species. The diagnosis of Candida osteomyelitis is supported by an elevated ESR. Positive blood cultures are obtained in about 50% of patients. Culture and histologic evaluation of vertebral biopsy specimens are both useful in confirming the correct diagnosis.
Amphotericin B is the standard form of medical therapy, although newer antifungal agents may be successful with lower rates of toxicity. According to Sugar and associates,218 fluconazole may be effective when administered on a continuous basis for prolonged periods. Variations in drug sensitivity must be considered when planning therapy. The ESR can be useful in assessing the response to treatment. As with other forms of infection, surgical débridement, fusion, and instrumentation are required for patients with advanced deformity, neurological compromise, and instability. Overall, at least 85% of patients will respond to treatment with cure of the osteomyelitis. Because of the significance of comorbid conditions, however, mortality rates in patients with Candida spinal osteomyelitis remain high.
Parasitic Infections
Parasitic infections of the spine are rare except in areas where parasitic infestation is endemic and a significant proportion of the population is infected. In these regions a relatively larger incidence of parasitic involvement of the spine is seen. Organisms that have been implicated with some regularity as being causative of spinal infection include Echinococcus granulosus (hydatid disease); Schistosoma haematobium, Schistosoma mansoni, and Schistosoma japonicum (bilharziasis); Taenia solium (cysticercosis); and Dracunculus medinensis (guinea worm infection). Of these, echinococcal219–221 and dracuncular infections222,223 can occasionally result in what are truly extra-axial lesions that compress the neural elements. Cysticercosis224–226 and schistosomiasis227,228 of the spine are usually parenchymal diseases of the spinal cord or subarachnoid in location. Given the rarity of such infections in North America, readers interested in further information on these highly unusual infections are referred to the literature just cited, most of which reflects the experience in endemic areas.
Arnold PM, Baek PN, Bernardi RJ, et al. Surgical management of nontuberculous thoracic and lumbar vertebral osteomyelitis. Surg Neurol. 1997;47:551-561.
Baker AS, Ojemann RG, Swartz MN, et al. Spinal epidural abscess. N Engl J Med. 1975;293:463-468.
Benzel EC. The lateral extracavitary approach to the spine using the three-quarter prone position. J Neurosurg. 1989;71:837-841.
Butler JS, Shelly MJ, Timlin M, et al. Nontuberculous pyogenic spinal infection in adults. A 12-year experience from a tertiary referral center. Spine. 2006;31:2695-2700.
Carragee E, Iezza A. Does acute placement of instrumentation in the treatment of vertebral osteomyelitis predispose to recurrent infection: long-term followup in immune suppressed patients. Spine. 2008;33:2089-2093.
Colmenero JD, Jimenez-Mejias ME, Sanchez-Lora FJ, et al. Pyogenic, tuberculous and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56:709-715.
Crawford AH, Kucharyzk DW, Ruda R, et al. Diskitis in children. Clin Orthop Relat Res. 1991;266:70-79.
Del Curling OJr, Gower DJ, McWhorter JM. Changing concepts in spinal epidural abscess: a report of 29 cases. Neurosurgery. 1990;27:185-192.
Dietze DD, Fessler RG, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg. 1997;86:981-989.
Gerszten PC, Gerszten E, Allison MJ. Diseases of the spine in South American mummies. Neurosurgery. 2001;48:208-213.
Hadjipavlou AG, Mader J, Necessary JT, et al. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668-1679.
Jansen BRH, Hart W, Schreuder O. Diskitis in childhood: 12-35 year follow up of 35 patients. Acta Orthop Scand. 1993;64:33-36.
Jensen AG, Espersen F, Skinhoj P, et al. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980-1990. J Infect. 1997;34:113-118.
Levi AD, Dickman CA, Sonntag VK. Management of post-operative infections after spinal instrumentation. J Neurosurg. 1997;86:975-980.
Leys D, Lesoin F, Viaud C, et al. Decreased morbidity from acute bacterial spinal epidural abscess using computed tomography and nonsurgical treatment in selected patients. Ann Neurol. 1985;17:350-355.
Mampalam TJ, Rosegay H, Andrew BT, et al. Nonoperative treatment of spinal epidural infections. J Neurosurg. 1989;71:208-210.
McCormick PC. Retropleural approach to the thoracic and thoracolumbar spine. Neurosurgery. 1995;37:908-914.
Modic MT, Steinberg PM, Ross JS. Degenerative disc disease. Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193-199.
Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg Spine. 2001;94:1-7.
Ruf M, Stoltze D, Merk HR, et al. Treatment of vertebral osteomyelitis by radical debridement and stabilization using titanium mesh cages. Spine. 2007;32:E275-E280.
Schuster JM, Avellino AM, Mann FA, et al. Use of structural allografts in spinal osteomyelitis: a review of 47 cases. J Neurosurg. 2000;93(1 suppl):8-14.
Thalgott JS, Cotler HB, Sasso RC, et al. Post-operative infections in spinal implants. Classification and analysis—a multi-center study. Spine. 1991;16:981-984.
Zeidman SM, Thompson K, Ducker TB. Complications of cervical discography: analysis of 4400 diagnostic disc injections. Neurosurgery. 1995;37:414-417.
1 Lyons AS, Pertucelli RJ. Medicine: An Illustrated History. New York: Harry N. Abrams; 1987.
2 Gerszten PC, Gerszten E, Allison MJ. Diseases of the spine in South American mummies. Neurosurgery. 2001;48:208-213.
3 Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: report of 9 cases and review of the literature. Rev Infect Dis. 1979;1:754-756.
4 Baker AS, Ojemann RG, Swartz MN, et al. Spinal epidural abscess. N Engl J Med. 1975;293:463-468.
5 Darouiche RO, Hamill RJ, Greenberg SB, et al. Bacterial spinal epidural abscess. Review of 43 cases and literature survey. Medicine (Baltimore). 1992;71:369-385.
6 Hadjipavlou AG, Mader J, Necessary JT, et al. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668-1679.
7 Heusner AP. Nontuberculous spinal epidural infections. N Engl J Med. 1948;239:845-847.
8 Kuker W, Mull M, Mayfrank L, et al. Epidural spinal infection—variability of clinical and magnetic resonance imaging findings. Spine. 1997;22:544-551.
9 Messer HD, Litvinoff J. Pyogenic cervical osteomyelitis. Arch Neurol. 1976;33:571-576.
10 Ring D, Wenger DR. Pyogenic infectious spondylitis in children. The evolution to current thought. Am J Orthop. 1996;25:342-348.
11 Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987- 97: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30:147-151.
12 Jensen AG, Espersen F, Skinhoj P, et al. Increasing frequency of vertebral osteomyelitis following Staphylococcus aureus bacteraemia in Denmark 1980-1990. J Infect. 1997;34:113-118.
13 Espersen F, Frimodt-Moller N, Thamdrup Rosdahl V, et al. Changing pattern of bone and joint infection due to Staphylococcus aureus: study of cases of bacteremia in Denmark 1959-1988. Rev Infect Dis. 1991;13:347-358.
14 Hlavin ML, Kaminski HJ, Ross JS, et al. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990;27:177-184.
15 Jones NS, Anderson DJ, Stiles PJ. A five year study showing an increase in subacute osteomyelitis. J Bone Joint Surg Br. 1987;69:779-783.
16 Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52:189-196.
17 Butler JS, Shelly MJ, Timlin M, et al. Nontuberculous pyogenic spinal infection in adults. A 12-year experience from a tertiary referral center. Spine. 2006;31:2695-2700.
18 Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87:1454-1458.
19 Grammatico L, Baron S, Rusch E, et al. Epidemiology of vertebral osteomyelitis in France: analysis of hospital discharge data. Epidemiol Infect. 2008;136:653-660.
20 Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61:47-55.
21 Waldvogel FA, Medoff G, Swartz NM. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:316-322.
22 Cahill DW, Love LC, Rechtine GR. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg. 1991;74:878-886.
23 Belzunegui J, Intxausti JJ, De Dios JR, et al. Haematogenous vertebral osteomyelitis in the elderly. Clin Rheumatol. 2000;19:344-347.
24 Broner FA, Garland DE, Zigler JE. Spinal infections in the immunocompromised host. Orthop Clin North Am. 1996;27:37-46.
25 Grieve JP, Ashwood N, O’Neill KS, et al. A retrospective study of surgical and conservative treatment for spinal extradural abscess. Eur Spine J. 2000;9:67-71.
26 Jensen AG, Espersen F, Skinhoj P, et al. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med. 1998;158:509-517.
27 Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874-880.
28 Colmenero JD, Jimenez-Mejias ME, Sanchez-Lora FJ, et al. Pyogenic, tuberculous and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56:709-715.
29 Patzakis MJ, Rao S, Wilkins J, et al. Analysis of 61 cases of vertebral osteomyelitis. Clin Orthop Relat Res. 1991;264:178-183.
30 Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg Spine. 2001;94:1-7.
31 Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23:175-204.
32 Schuster JM, Avellino AM, Mann FA, et al. Use of structural allografts in spinal osteomyelitis: a review of 47 cases. J Neurosurg. 2000;93(1 suppl):8-14.
33 Adler MW. ABC of AIDS. Development of an epidemic. BMJ. 2001;322:226-229.
34 Centers for Disease Control and Prevention. Trends in injection drug use among persons entering addiction treatment—New Jersey, 1992-1999. JAMA. 2001;285:2706-2707.
35 Del Curling OJr, Gower DJ, McWhorter JM. Changing concepts in spinal epidural abscess: a report of 29 cases. Neurosurgery. 1990;27:185-192.
36 Datta S, Hussain IR, Madden B. Spinal osteomyelitis and diskitis: a rare complication following orthotopic heart transplantation. J Heart Lung Transplant. 2001;20:1213-1216.
37 Obrador GT, Levenson DJ. Spinal epidural abscess in hemodialysis patients: report of three cases and review of the literature. Am J Kidney Dis. 1996;27:75-83.
38 Hanley EN, Phillips ED. Profiles of patients who get spine infections and the type of infections that have a predilection for the spine. Semin Spine Surg. 1990;2:257-267.
39 Rath SA, Neff U, Schneider O, et al. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926-933.
40 Siroky MB, Movlan RA, Austen G, et al. Metastatic infection secondary to genito-urinary tract sepsis. Am J Med. 1976;61:351-360.
41 Anderson R. Diodrast studies of the vertebral and cranial venous systems to show their possible role in cerebral metastases. J Neurosurg. 1951;8:411-422.
42 Batson OV. The function of vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112:138-149.
43 Wiley AM, Trueta J. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg Br. 1959;41:796-809.
44 Ratcliffe JF. Anatomic basis for the pathogenesis and radiologic features of vertebral osteomyelitis and its differentiation from childhood discitis. Acta Radiol Diagn (Stockh). 1985;26:137-143.
45 Babinchak TJ, Riley DK, Rotheram EB. Pyogenic vertebral osteomyelitis of the posterior elements. Clin Infect Dis. 1997;25:221-224.
46 Roberts WA. Pyogenic vertebral osteomyelitis of a lumbar facet joint with associated epidural abscess. A case report with a review of the literature. Spine. 1988;13:948-952.
47 Muffoletto AJ, Nader R, Westmark RM, et al. Hematogenous pyogenic facet joint infection of the subaxial cervical spine. A report of two cases and review of the literature. J Neurosurg. 2001;95(1 suppl):135-138.
48 Griffiths HED, Jones DM. Pyogenic infection of the spine: a review of 28 cases. J Bone Joint Surg Br. 1971;53:383-391.
49 Kemp HBS, Jackson JW, Jeremiah JD, et al. Anterior fusion of the spine for infective lesions in adults. J Bone Joint Surg Br. 1973;55:715-734.
50 Collert S. Osteomyelitis of the spine. Acta Orthop Scand. 1977;48:283-290.
51 Eismont FJ, Bohlman HH, Soni PL, et al. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19-29.
52 Silverthorn KG, Gillespie WJ. Pyogenic spinal osteomyelitis: a review of 61 cases. N Z Med J. 1986;99:62-65.
53 Hitchon PW, Osenbach RK, Yuh WTC, et al. Spinal infections. Clin Neurosurg. 1992;38:373-387.
54 Malawski SK, Lukawski S. Pyogenic infection of the spine. Clin Orthop Relat Res. 1991;272:58-66.
55 Stone JL, Cybulski GR, Rodroguez J, et al. Anterior cervical debridement and strut grafting for osteomyelitis of the cervical spine. J Neurosurg. 1989;70:879-883.
56 Carragee EJ. Instrumentation of the infected and unstable spine: a review of 17 cases from the thoracic and lumbar spine with pyogenic infections. J Spinal Disord. 1997;10:317-324.
57 Carragee EJ. The clinical use of magnetic resonance imaging in pyogenic vertebral osteomyelitis. Spine. 1997;22:780-785.
58 Khanna RK, Malik GM, Rock JP. Spinal epidural abscess: evaluation of factors influencing outcome. Neurosurgery. 1996;39:958-964.
59 Mampalam TJ, Rosegay H, Andrew BT, et al. Nonoperative treatment of spinal epidural infections. J Neurosurg. 1989;71:208-210.
60 Waldvogel FA, Vasev H. Osteomyelitis. The past decade. N Engl J Med. 1980;303:360-370.
61 Frederickson B, Yuan H, Olans R. Management and outcome of pyogenic vertebral osteomyelitis. Clin Orthop Relat Res. 1978;131:160-167.
62 Koppel BS, Tuchman AJ, Mangiardi JR. Epidural spinal infection in intravenous drug abusers. Arch Neurol. 1988;45:1331-1337.
63 Sapico FL. Microbiology and antimicrobial therapy of spinal infections. Orthop Clin North Am. 1996;27:9-13.
64 Lewis R, Gorbach D, Halpern M. Spinal pseudomonas chondro-osteomyelitis in heroin users. N Engl J Med. 1973;286:1303.
65 Brook I. Two cases of diskitis attributable to anaerobic bacteria in children. Pediatrics. 2001;107(2):E26.
66 Hall BB, Fitzgerald RH, Rosenblatt JE, et al. Anaerobic osteomyelitis. J Bone Joint Surg Am. 1983;65:30-35.
67 Yin KS, Wang C, Lucero Y. Myelopathy secondary to spinal epidural abscess: case reports and a review. J Spinal Cord Med. 1998;21:348-354.
68 Lifeso RM. Pyogenic spinal sepsis in adults. Spine. 1990;15:1265-1271.
69 Frisbie JH, Gore RL, Strymish JM, et al. Vertebral osteomyelitis in paraplegia: incidence, risk factors, clinical picture. J Spinal Cord Med. 2000;23:15-22.
70 Carragee EJ, Kim D, van der Vlugt T, et al. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine. 1997;22:2089-2093.
71 Garcia AJr, Grantham SA. Hematogenous pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1960;42:429-436.
72 An HS, Vaccaro AR, Dolinkas CA, et al. Differentiation between spinal tumors and infections with magnetic resonance imaging. Spine. 1991;16(suppl):S334-S338.
73 Babu NV, Titus VTK, Chittaranjan S, et al. Computed tomographically guided biopsy of the spine. Spine. 1994;19:2436-2442.
74 Takahashi J, Ebara S, Kamimura M, et al. Early-phase enhanced inflammatory reaction after spinal instrumentation surgery. Spine. 2001;26:1698-1704.
75 Crawford AH, Kucharyzk DW, Ruda R, et al. Diskitis in children. Clin Orthop Relat Res. 1991;266:70-79.
76 Rothman SLG. The diagnosis of infections of the spine by modern imaging techniques. Orthop Clin North Am. 1996;27:15-31.
77 Dagirmanjian A, Schils J, McHenry M, et al. MR Imaging of vertebral osteomyelitis revisited. AJR Am J Roentgenol. 1996;167:1539-1543.
78 Chow GH, Gebhard JS, Brown CW. Multifocal metachronous epidural abscesses of the spine. A case report. Spine. 1996;21:1094-1097.
79 Pfister HW, von Rosen F, Yousry T. MRI detection of epidural spinal abscesses at noncontiguous sites. J Neurol. 1996;243:315-317.
80 Nolla-Solé JM, Lourdes MS, Rozadilla-Sacanell A, et al. Role of technetium-99m diphosphonate and gallium-67 citrate bone scanning in the early diagnosis of infectious spondylodiscitis: A comparative study. Ann Rheum Dis. 1992;51:665-667.
81 Whalen JL, Brown ML, McLeod R, et al. Limitations of indium leukocyte imaging for the diagnosis of spine infections. Spine. 1991;16:193-197.
82 Gemmel F, Dumarey N, Palestro CJ. Radionuclide imaging of spinal infections. Eur J Nucl Med Mol Imaging. 2006;33:1226-1237.
83 Palestro CJ, Love C, Miller TT. Imaging of musculoskeletal infections. Best Pract Res Clin Rheum. 2006;20:1197-1218.
84 Bleeker-Rovers CP, Vos FJ, Corstens FH, et al. Imaging of infectious diseases using [18F] fluorodeoxyglucose PET. Q J Nucl Med Mol Imaging. 2008;52:17-29.
85 Lazzeri E, Erba P, Tascini C, et al. Scintigraphic imaging of vertebral osteomyelitis with 111In-biotin. Spine. 2008;33:E198-204.
86 Ross PM, Fleming JL. Vertebral body osteomyelitis: spectrum and natural history. A retrospective analysis of 37 cases. Clin Orthop Relat Res. 1976;118:190-198.
87 Yu WY, Siu C, Wing PC, et al. Percutaneous suction aspiration for osteomyelitis. Spine. 1991;16:198-202.
88 Liebergall M, Chaimsky G, Lowe J, et al. Pyogenic vertebral osteomyelitis with paralysis. Prognosis and treatment. Clin Orthop Relat Res. 1991;269:142-150.
89 Bass SN, Ailani RK, Shekar R, et al. Pyogenic vertebral osteomyelitis presenting as exudative pleural effusion: a series of five cases. Chest. 1998;114:642-647.
90 Thurnher MM, Post MJD, Jinkins JR. MRI of infections and neoplasms of the spine and spinal cord in 55 patients with AIDS. Neuroradiology. 2000;42:551-563.
91 Modic MT, Steinberg PM, Ross JS. Degenerative disc disease. Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193-199.
92 Malghem J, Maldague B, Labaisse M, et al. Intravertebral vacuum cleft: changes in content after supine positioning. Radiology. 1993;187:483-487.
93 Naul LG, Peet GJ, Maupin WB. Avascular necrosis of the vertebral body: MR imaging. Radiology. 1989;172:219-222.
94 Arnold PM, Baek PN, Bernardi RJ, et al. Surgical management of nontuberculous thoracic and lumbar vertebral osteomyelitis. Surg Neurol. 1997;47:551-561.
95 Young WF, Weaver M. Isolated pyogenic osteomyelitis of the odontoid process. Scand J Infect Dis. 1999;31:512-515.
96 Wiedau-Pazos M, Curio G, Grusser C. Epidural abscess of the cervical spine with osteomyelitis of the odontoid process. Spine. 1999;24:133-136.
97 Benzel EC. The lateral extracavitary approach to the spine using the three-quarter prone position. J Neurosurg. 1989;71:837-841.
98 Larson SJ, Holst RA, Hemmy DC, et al. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
99 McCormick PC. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996;38:67-74.
100 Fessler RG, Dietze DDJr, Millan MM, et al. Lateral parascapular extrapleural approach to the upper thoracic spine. J Neurosurg. 1991:7549-7555.
101 McCormick PC. Retropleural approach to the thoracic and thoracolumbar spine. Neurosurgery. 1995;37:908-914.
102 Krogel A, Kruger A, Lohscheidt K, et al. Anterior debridement, fusion and extrafocal stabilization in treatment of osteomyelitis of the spine. J Spinal Disord. 1999;12:17-26.
103 Safran O, Rand N, Kaplan L, et al. Sequential or simultaneous, same-day anterior decompression and posterior stabilization in the management of vertebral osteomyelitis of the lumbar spine. Spine. 1998;23:1885-1890.
104 Harrington P, Millner PA, Veale D. Inappropriate medical management of spinal epidural abscess. Ann Rheum Dis. 2001;60:218-222.
105 Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405-1411.
106 Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the Third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699-706.
107 Dietze DD, Fessler RG, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg. 1997;86:981-989.
108 Robinson Y, Tschoeke SK, Kayser R, et al. Reconstruction of large defects in vertebral osteomyelitis with expandable titanium cages. Int Orthop. 2009;33:745-749.
109 Carragee E, Iezza A. Does acute placement of instrumentation in the treatment of vertebral osteomyelitis predispose to recurrent infection: long-term followup in immune suppressed patients. Spine. 2008;33:2089-2093.
110 Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16:1317-1318.
111 Ruf M, Stoltze D, Merk HR, et al. Treatment of vertebral osteomyelitis by radical debridement and stabilization using titanium mesh cages. Spine. 2007;32:E275-E280.
112 Dandy WE. Abscesses and inflammatory tumors in the spinal epidural space (so called pachymeningitis externa). Arch Surg. 1926;13:477-494.
113 McGee-Collett M, Johnson IH. Spinal epidural abscess: presentation and treatment. A report of 21 cases. Med J Aust. 1991;155:14-17.
114 Bair-Merritt MH, Chung C, Collier A. Spinal epidural abscess in a young child. Pediatrics. 2000;106(3):E39.
115 Leys D, Lesoin F, Viaud C, et al. Decreased morbidity from acute bacterial spinal epidural abscess using computed tomography and nonsurgical treatment in selected patients. Ann Neurol. 1985;17:350-355.
116 Roblot F, Besnier JM, Juhel L, et al. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum. 2007;36:269-277.
117 McCain GA, Harth M, Bell DA. Septic discitis. J Rheumatol. 1981;8:100-109.
118 Eismont FJ, Wiesel SW, Brighton CT, et al. Antibiotic penetration into rabbit nucleus pulposus. Spine. 1987;12:254-256.
119 Conaughty JM, Chen J, Martinez OV, et al. Efficacy of linezolid versus vancomycin in the treatment of methicillin resistant Staphylococcus aureus discitis. Spine. 2006;31:E830-E832.
120 Lalani T, Boucher HW, Cosgrove SE, et al. Outcomes with daptomycin versus standard therapy for osteoarticular infections associated with Staphylococcus aureus bacteremia. J Antimicrob Chemother. 2008;61:177-182.
121 Mader JT, Cantrell JS, Calhoun J. Oral ciprofloxacin compared with standard parenteral antibiotic therapy for chronic osteomyelitis in adults. J Bone Joint Surg Am. 1990;72:104-110.
122 Mackenzie AR, Laing RB, Smith CC. Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65:209-212.
123 van Dalen IV, Heeg M. Neonatal infectious spondylitis of the cervical spine presenting with quadriplegia: a case report. Spine. 2000;25:1450-1452.
124 Eismont FJ, Bohlman HH, Soni PL, et al. Vertebral osteomyelitis in infants. J Bone Joint Surg Br. 1982;64:32-35.
125 Lee J, Kim J, Kim S, et al. Anterior cervical spinal epidural abscess in an infant. Childs Nerv System. 1999;15:137-139.
126 Rudert M, Tillmann B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop Scand. 1993;64:37-40.
127 Alexander CJ. The etiology of juvenile spondyloarthritis (discitis). Clin Radiol. 1970;21:178-187.
128 Brown R, Hussain M, McHugh K, et al. Discitis in young children. J Bone Joint Surg Br. 2001;83:106-111.
129 Ryoppy S, Jaaskelainen, Rapola J, et al. Nonspecific discitis in children. A non-microbial disease? Cin Orthop Relat Res. 1993;297:95-99.
130 Fischer EG, Green CS, Winston KR. Spinal epidural abscess in children. Neurosurgery. 1981;9:257-260.
131 Jansen BRH, Hart W, Schreuder O. Diskitis in childhood: 12-35 year follow up of 35 patients. Acta Orthop Scand. 1993;64:33-36.
132 Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
133 Thalgott JS, Cotler HB, Sasso RC, et al. Post-operative infections in spinal implants. Classification and analysis—a multi-center study. Spine. 1991;16:981-984.
134 Theiss SM, Lonstein JE, Winter RB. Wound infections in reconstructive spine surgery. Orthop Clin North Am. 1996;27:105-110.
135 Dietz FR, Koontz FP, Found EM, et al. The importance of positive bacterial cultures of specimens obtained during clean orthopedic procedures. J Bone Joint Surg Am. 1991;73:1200-1207.
136 Levi AD, Dickman CA, Sonntag VK. Management of post-operative infections after spinal instrumentation. J Neurosurg. 1997;86:975-980.
137 Massie JB, Heller JG, Abitbol JJ, et al. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99-108.
138 Stambough JL, Beringer D. Postoperative wound infections complicating adult spine surgery. J Spinal Disord. 1992;5:277-285.
139 Fraser RD, Osti OL, Vernon-Roberts B. Iatrogenic discitis: the role of intravenous antibiotics in prevention and treatment. An experimental study. Spine. 1989;14:1025-1032.
140 Mader JT, Cierny G. The principles of the use of preventative antibiotics. Clin Orthop Relat Res. 1984;190:72-75.
141 Ozuna RM, Delamarter RB. Pyogenic vertebral osteomyelitis and postsurgical disc space infections. Orthop Clin North Am. 1996;27:87-94.
142 Savitz M, Savitz S, Malis L. Ethical issues in the history of prophylactic antibiotic use in neurosurgery. Br J Neurosurg. 1999;13:306-311.
143 Kumar K, Crawford AH. Role of “Bovie” in spinal surgery: historical and analytical perspective. Spine. 2002;27:1000-1006.
144 Banco SP, Vaccaro AR, Blam O, et al. Spine infections: variations in incidence during the academic year. Spine. 2002;27:962-965.
145 Rohde V, Meyer B, Schaller C, et al. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine. 1998;23:615-620.
146 Zink PM, Frank MA, Trappe AE. Prophylaxis of postoperative lumbar spondylodiscitis. Neurosurg Rev. 1989;12:297-303.
147 Tronnier V, Schneider R, Kunz U, et al. Postoperative spondylodiscitis: results of a prospective study about aetiology of spondylodiscitis after operation for lumbar disc herniation. Acta Neurochir (Wien). 1992;117:149-152.
148 Rhoten RLP, Murphy MA, Kalfas IH, et al. Antibiotic penetration into cervical discs. Neurosurgery. 1995;37:418-421.
149 Luer MS, Hatton J. Appropriateness of antibiotic selection and use in laminectomy and microdiskectomy. Am J Hosp Pharm. 1993;50:667-670.
150 Dauch WA. Infection of the intervertebral space following conventional and microsurgical operation on the herniated lumbar intervertebral disc: a controlled clinical trial. Acta Neurochir (Wien). 1986;82:43-49.
151 Horwitz NH, Curtin JA. Prophylactic antibiotics and wound infections following laminectomy for lumber disc herniation. J Neurosurg. 1975;43:727-731.
152 Grane P. The postoperative lumbar spine. A radiological investigation of the lumbar spine after discectomy using MR imaging and CT. Acta Radiol Suppl. 1998;414:1-23.
153 Kristopaitis T, Jensen R, Gujrati M. Clostridium perfringens: a rare cause of postoperative spinal surgery meningitis. Surg Neurol. 1999;51:448-450.
154 Puranen J, Makela J, Lahde S. Postoperative intervertebral discitis. Acta Orthop Scand. 1984;55:461-465.
155 Huber A, Kainz C, Witzmann A, et al. Peri-operative elastase–alpha-1 proteinase inhibitor in patients with postoperative intervertebral discitis. Acta Neurochir (Wien). 1993;120:150-154.
156 Kylanpaa-Back ML, Suominen RA, Salo SA, et al. Postoperative discitis: outcome and late magnetic resonance image evaluation of ten patients. Ann Chiru Gynaecol. 1999;88:61-64.
157 Schulitz KP, Assheuer J. Discitis after procedures on the intervertebral disc. Spine. 1994;19:1172-1177.
158 Lonstein J, Winter R, Moe J. Wound infection with Harrington instrumentation and spine fusion for scoliosis. Clin Orthop Relat Res. 1973;96:222-233.
159 Abbey DM, Turner DM, Warson JS, et al. Treatment of post operative wound infections following spinal fusion with instrumentation. J Spinal Disord. 1995;8:278-283.
160 Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine. 2001;26:1990-1996.
161 Lu K, Liang C, Cho C, et al. Oxidative stress and heat shock protein response in human paraspinal muscles during retraction. J Neurosurg. 2002;97(Spine 1):75-81.
162 Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 2: histologic and histochemical analysis in humans. Spine. 1994;19:2598-2602.
163 Kowalski TJ, Huddleston PM, Steckelberg JM, et al. The management and outcome of spinal implant infections: contemporary retrospective cohort study. Clin Infect Dis. 2007;44:913-920.
164 Garrido E, Rosenwasser RH. Experience with the suction-irrigation technique in the management of spinal epidural infection. Neurosurgery. 1983;12:678-679.
165 Dietze DD, Haid RW. Antibiotic impregnated methylmethacrylate in treatment of infections with spinal instrumentation. Spine. 1992;17:981-986.
166 Kauffman CP, Bono CM, Vessa PP, et al. Postoperative synergistic gangrene after spinal fusion. Spine. 2000;25:1729-1732.
167 Viola RW, King HA, Adler SM, et al. Delayed infection after elective spinal instrumentation and fusion. A retrospective review of eight cases. Spine. 1997;22:2444-2450.
168 Dubousset J, Shufflebarger H, Wenger D. Late “infection” with CD instrumentation. Orthop Trans. 1994;18:121.
169 Clark CE, Shufflebarger HL. Late developing infection in instrumented idiopathic scoliosis. Spine. 1999;24:1909-1912.
170 Connor PM, Darden BV II. Cervical discography complications and clinical efficacy. Spine. 1993;18:2035-2038.
171 Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br. 1987;68:26-35.
172 Guyer RD, Ohnmeiss DD, Mason SL, et al. Complications of cervical discography: findings in a large series. J Spinal Disord. 1997;10:95-101.
173 Zeidman SM, Thompson K, Ducker TB. Complications of cervical discography: analysis of 4400 diagnostic disc injections. Neurosurgery. 1995;37:414-417.
174 Junila J, Niinimaki T, Tervonen O. Epidural abscess after lumbar discography: a case report. Spine. 1988;13:1352-1354.
175 Guyer RD, Collier R, Stith WJ, et al. Discitis after discography. Spine. 1988;13:1352-1354.
176 Osti OL, Fraser RD, Vernon-Roberts B. Discitis after discography. The role of prophylactic antibiotics. J Bone Joint Surg Br. 1990;72:271-274.
177 Sakuragi T, Yasunaka K, Hirata K, et al. The source of epidural infection following epidural analgesia identified by pulsed field gel electrophoresis. Anesthesiology. 1998;89:1254-1256.
178 Wang LP, Hauerberg J, Schmidt JF. Incidence of spinal epidural abscess after epidural analgesia: a national 1-year survey. Anesthesiology. 1999;91:1928-1939.
179 Byers K, Axelrod P, Michael S, et al. Infections complicating tunneled intraspinal catheter systems used to treat chronic pain. Clin Infect Dis. 1995;21:403-408.
180 Du Pen L, Peterson DG, Williams A, et al. Infection during chronic epidural catheterization: diagnosis and treatment. Anesthesiology. 1990;73:905-909.
181 Sillevis Smitt P, Tsafka A, van den Bent M, et al. Spinal epidural abscess complicating chronic epidural analgesia in 11 cancer patients: clinical findings and magnetic resonance imaging. J Neurol. 1999;246:815-820.
182 Bulow PM, Biering-Sorensen F. Paraplegia, a severe complication to epidural analgesia. Acta Anaesthesiol Scand. 1999;43:233-235.
183 Wang LP, Hauerberg J, Schmidt JF. Long-term outcome after neurosurgically treated spinal epidural abscess following epidural analgesia. Acta Anaesthesiol Scand. 2001;45:233-239.
184 Royakkers AA, Willigers H, van der Ven AJ, et al. Catheter-related epidural abscesses—don’t wait for neurological deficits. Acta Anaesthesiol Scand. 2002;46:611-615.
185 Aram L, Krane EJ, Kozloski LJ, et al. Tunneled epidural catheters for prolonged analgesia in pediatric patients. Anesth Anal. 2001;92:1432-1438.
186 Sato S, Sakuragi T, Dan K. Human skin flora a potential source of epidural abscess. Anesthesiology. 1996;85:1276-1282.
187 Mimoz O, Karim A, Mercat A, et al. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture. A randomized controlled trial. Ann Intern Med. 1999;131:834-837.
188 Pereira LJ, Lee GM, Wade KJ. An evaluation of five protocols for surgical handwashing in relation to skin condition and microbial counts. J Hosp Infect. 1997;36:49-65.
189 Peterson AF, Rosenberg A, Alatery SD. Comparative evaluation of surgical scrub preparations. Surg Gynecol Obstet. 1978;146:63-65.
190 Chaiyakunapruk N, Veenstra DL, Lipsky BA, et al. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136:792-801.
191 Goldblum SE, Ulrich JA, Goldman RS, et al. Comparison of 4% chlorhexidine in a detergent base (Hibiclens) and povidone-iodine (Betadine) for the skin preparation of hemodialysis patients and personnel. Am J Kidney Dis. 1983;2:548-552.
192 Maki DG, Ringer M, Alvarado CJ. Prospective randomized trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991;338:339-343.
193 Mimoz O, Karim A, Merkat A, et al. Chlorhexidine compared with povidone-iodine as a skin preparation before blood culture. A randomized controlled trial. Ann Intern Med. 1999;131:834-837.
194 Kasuda H, Fukuda H, Togashi H, et al. Skin disinfection before epidural catheterization: comparative study of povidone-iodine versus chlorhexidine ethanol. Dermatology. 2002;204(suppl 1):42-46.
195 Kinirons B, Mimoz O, Lafendi L, et al. Chlorhexidine versus povidone-iodine in preventing colonization of continuous epidural catheters in children: a randomized controlled trial. Anesthesiology. 2001;94:239-244.
196 Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine. 2006;31:2747-2755.
197 Muijs SP, Nieuwenhuijse MJ, Van Erkel AR, et al. Percutaneous vertebroplasty for the treatment of osteoporotic vertebral compression fractures: evaluation after 36 months. J Bone Joint Surg Br. 2009;91:379-384.
198 Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. Am J Neuroradiol. 1997;18:1897-1904.
199 Cosar M, Sasani M, Oktenoglu T, et al. The major complications of transpedicular vertebroplasty. J Neurosurg Spine. 2009;11:607-613.
200 Shin JH, Ha KY, Kim KW, et al. Surgical treatment for delayed pyogenic spondylitis after percutaneous vertebroplasty and kyphoplasty. Report of 4 cases. J Neurosurg Spine. 2008;9:265-272.
201 Walker DH, Mummaneni P, Rodts GEJr. Infected vertebroplasty: report of two cases and review of the literature. Neurosurg Focus. 2004;17(6):E6.
202 Vats HS, McKiernan FE. Infected vertebroplasty. Case report and review of literature. Spine. 2006;31:E859-E862.
203 Spincemaille GH, Klomp HM, Steyerberg EW, et al. ESES study group. Technical data and complications from spinal cord stimulation: data from a randomized trial on critical limb ischemia. Stereotact Funct Neurosurg. 2000;74:63-72.
204 Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain. Prospective study with a minimum 2-year followup. Spine. 2002;27:966-973.
205 Oruckaptan HH, Senmevsim O, Soylemezoglu F, et al. Cervical actinomycosis causing spinal cord compression and multisegmental root failure: case report and review of the literature. Neurosurgery. 1998;43:937-940.
206 Yung BC, Cheng JC, Chan TT, et al. Aggressive thoracic actinomycosis complicated by vertebral osteomyelitis and epidural abscess leading to spinal cord compression. Spine. 2000;25:745-748.
207 Voisin L, Vittecoq O, Mejjad O, et al. Spinal abscess and spondylitis due to actinomycosis. Spine. 1998;23:487-490.
208 Eftekhar B, Ketabchi E, Ghodsi M, et al. Cervical epidural actinomycosis. Case report. J Neurosurg. 2001;95(1 suppl):132-134.
209 Graat HC, Van Ooij A, Day GA, et al. Nocardia farcinica spinal osteomyelitis. Spine. 2002;27:E253-E257.
210 Harvey AL, Myslinski J, Ortiz L. A case of Nocardia epidural abscess. J Emerg Med. 1998;16:579-581.
211 Laurin JM, Resnik CS, Wheeler D, et al. Vertebral osteomyelitis caused by Nocardia asteroides: report and review of the literature. J Rheumatol. 1991;18:455-458.
212 Colmenero JD, Reguera JM, Fernandez-Nebro A, et al. Osteoarticular complications of brucellosis. Ann Rheum Dis. 1991;50:23-26.
213 Ariza J, Gudiol F, Valverde F, et al. Brucellar spondylitis. A detailed analysis based on current findings. Rev Infect Dis. 1985;7:656-664.
214 Lifeso RM, Harder E, McCorkell SJ. Spinal brucellosis. J Bone Joint Surgery Br. 1985;67:345-351.
215 Tekkok IH, Berker M, Ozcan OE, et al. Brucellosis of the spine. Neurosurgery. 1993;33:834-844.
216 Madkour MM, Sharif HS, Abed MY, et al. Osteoarticular brucellosis: results of bone scintigraphy in 140 patients. AJR Am J Roentgenol. 1988;150:1101-1105.
217 Adams JC. Technique, dangers, and safeguards in osteotomy of the spine. J Bone Joint Surg Br. 1952;334:225-232.
218 Sugar AM, Saunders C, Diamond RD. Successful treatment of Candida osteomyelitis with fluconazole. A noncomparative study of two patients. Diagn Microbiol Infect Dis. 1990;13:517-520.
219 Pamir MN, Ozduman K, Elmaci I. Spinal hydatid disease. Spinal Cord. 2002 Apr;40:153-160.
220 Govender TS, Aslam M, Parbhoo A, et al. Hydatid disease of the spine. A long-term follow-up after surgical treatment. Clin Orthop Relat Res. 2000;378:143-147.
221 Islekel S, Ersahin Y, Zileli M, et al. Spinal hydatid disease. Spinal Cord. 1998;36:166-170.
222 Legmann P, Chiras J, Launay M, et al. Epidural dracunculiasis: a rare cause of spinal cord compression. Neuroradiology. 1980;20:43-45.
223 Dinakar I, Seetharam W, Leelanaidu PS, et al. Spinal compression due to an extradural guinea worm abscess: a case report. Neurol India. 1977;25:191-192.
224 Mohanty A, Das S, Kolluri VR, et al. Spinal extradural cysticercosis: a case report. Spinal Cord. 1998;36:285-287.
225 Bandres JC, White ACJr, Samo T, et al. Extraparenchymal neurocysticercosis: report of five cases and review of management. Clin Infect Dis. 1992;15:799-811.
226 McDonald JB, Turner PT, Miller AH. Cysticercosis and spinal cord compression [letter]. Ann Neurol. 1979;6:367-368.
227 Pittella JE. The relation between involvement of the central nervous system in schistosomiasis mansoni and the clinical forms of the parasitosis. A review. J Trop Med Hyg. 1991;94:15-21.
228 Scrimgeour EM, Gajdusek DC. Involvement of the central nervous system in Schistosoma mansoni and S. haematobium infection. A review. Brain. 1985;108:1023-1038.