Chapter 22 Infections of the Luminal Digestive Tract
Introduction
Epidemiology
The prevalence, incidence, and etiology of luminal GI tract infections are influenced by many factors (Box 22.1). In the normal host, luminal GI infections generally occur randomly after exposure to a pathogen and are self-limited. Exposure may take many forms, such as occupational (day care centers), dietary, and environmental (contaminated water). Coexisting host factors may promote the clinical expression of infection and either attenuate or exacerbate the disease. For immunosuppressed patients, the incidence and severity of infection are linked to the cause and degree of the immunodeficiency state. Patients undergoing solid organ transplantation are at the highest risk of infection early on after transplantation because of profound medication-induced immunodeficiency. Latent infections become manifest during this period, and susceptibility to infections is greatest. Over time, however, as drug-induced immunosuppression is tapered, the incidence of infections decreases. For patients infected with human immunodeficiency virus (HIV), the incidence of GI infections increases markedly as immune function deteriorates, and the infection risk can be accurately stratified by the absolute CD4 lymphocyte count and HIV-1 RNA levels.1,2
Rarely, opportunistic infections have been observed in the apparently normal host, but in contrast to an immunodeficient patient, these infections are typically self-limited.3,4 Generally, the more severe the immunodeficiency state required for development of an opportunistic infection, the less likely the pathogen will be observed in the normal host. Although herpes simplex virus (HSV) is a well-recognized pathogen in otherwise healthy people,3 HSV esophagitis occurs most often in patients with some predisposing factor. In contrast, until the advent of transplantation, cytomegalovirus (CMV) was a rare pathogen, and its identification in any patient suggests some type of immune dysfunction.4,5 CMV is regarded as one of the most common opportunistic infections. This frequency of infection relates to the high prevalence of prior exposure to CMV, as reflected by seropositivity rates of more than 90% in developed countries,6 and to the fact that CMV disease generally occurs from recrudescence of latent infection during periods of profound immunosuppression.
Overall, the frequency of GI infections has been decreasing in immunosuppressed patients. Extensive research has defined the time course and spectrum of infections that complicate immunodeficiency states.7 Based on these observations, targeted preemptive antimicrobial prophylaxis has become the standard of care against many of these infections during periods of greatest vulnerability. Different classes of agents are used at varying time points depending on the infection risk and pathogens associated with the level of immune impairment. However, antimicrobial prophylaxis is associated with an increased risk for other infections and the development of drug resistance. The use of prophylaxis for Pneumocystis carinii infection in AIDS is associated with an increase in the prevalence of other opportunistic infections, such as viral disease, because patients are now living longer.8 The implementation of Candida prophylaxis in selected patients undergoing transplantation and the widespread use of oral antifungal therapies in AIDS has reduced the incidence of fungal infections in these settings but has been linked with drug resistance to Candida species.9
The use of highly active antiretroviral therapy for HIV-infected patients has drastically reduced the frequency of GI complications, including infections and neoplasms.10–12 More selective immunosuppressive therapy, such as with cyclosporine, has been beneficial in reducing the incidence of infections after transplantation.13 Methods for prophylaxis other than use of antimicrobials have also played a beneficial role in the reduction of opportunistic infections. In high-risk transplant patients, the use of CMV-seronegative organs and blood products for seronegative recipients, the use of leukocyte-depleted platelets for patients after bone marrow transplantation, and the administration of preemptive antiviral therapy all have reduced the incidence of CMV disease.14,15
With the increase in international travel for both business and pleasure, geography plays an increasingly important role in the prevalence of some GI infections. Pathogens are endemic in certain portions of the world and in specific regions within countries. In the United States, histoplasmosis is endemic in the Midwest and Mississippi Valley, and coccidioidomycosis is endemic in the Southwest. Mycobacterium tuberculosis (TB) is endemic in Third World countries and involvement of the GI tract is well recognized. Penicillium marneffei has been described as a pathogen more recently and appears to be limited geographically to Southeast Asia.16 Traveler’s diarrhea, usually caused by enteropathogenic Escherichia coli, is characteristically seen with travel to Mexico and other developing countries.17
Pathogenesis
The pathogenic mechanisms of luminal GI infections are (1) exposure to a pathogen, (2) reactivation of prior infection (recrudescence), (3) overgrowth of a commensal organism, and (4) local spread or dissemination. The specific organ involved with any infectious process, other than local spread, is dictated by the organ-specific tropism of the infecting pathogen. Candida and HSV almost exclusively infect squamous epithelium, whereas Campylobacter jejuni and Shigella species are colonic pathogens. Inherent to any discussion of pathogenesis is the issue of host-related factors. Numerous nonspecific and immune-based defense mechanisms both prevent and attenuate GI infections.18 These defenses may be altered by disease, by medications, or as a part of the aging process. Saliva provides an effective physical barrier because of its physical properties, and immunoglobulins that are present in saliva and in intestinal secretions provide an important early line of defense. Gastric acid is a barrier to enteropathogens, and hypochlorhydria has been shown to be a risk factor for the development of cholera and other GI infections.19 GI motility moves ingested pathogens through the gut and prevents stasis, which can lead to bacterial overgrowth. Inherent antibacterial proteins secreted by Paneth cells, termed defensins, seem to play a key role in the host response to bacterial infections of the gut.20
The mucosal immune system is composed of inflammatory cells, most notably T cells.18 After exposure to a foreign antigen, these cells differentiate into helper or cytotoxic cells depending on whether the cells express the CD4 or CD8 receptor. The release of cytokines by these cells plays a key role in limiting infection but can also result in tissue damage. The critical role of the mucosal immune system in preventing and controlling infections is best shown by the array and severity of luminal GI infections that occur in AIDS, in which a progressive loss of CD4 lymphocytes from both the systemic circulation and the mucosal-based immune system occurs.21 Loss of these cells predisposes to small intestinal infections by opportunistic infections such as cryptosporidiosis and microsporidiosis. Likewise, lymphocyte dysfunction, either medication-induced or as part of an immunodeficiency state, predisposes to symptomatic primary infection or recrudescence (e.g., CMV).
Bacterial esophagitis is a polymicrobial infection consisting of oral flora, particularly gram-positive organisms, including Streptococcus viridans, staphylococci, and other bacilli. This rare cause of esophagitis occurs under conditions of absolute granulocytopenia or severely impaired granulocyte function in which these commensal bacteria invade mucosa that has been damaged from reflux disease, from radiation therapy, or from chemotherapy, leading to an active local infection and potential dissemination.22 GI infections may result secondarily from active disease in adjacent organs. Esophageal disease may be caused by contiguously infected mediastinal lymph nodes or pulmonary parenchymal infection23 and by spread of infection via a draining fistula or obstructed lymphatics, resulting in tracheoesophageal fistula.24 Widespread lymphohematogenous dissemination of opportunistic infections causes either diffuse or focal disease anywhere in the gut; this process is generally limited to only the most severely immunocompromised patients.25,26
Most intestinal infections result in tissue inflammation of varying degrees. Local upregulation of cytokines plays a central role in the local immune response to the pathogen but may also cause tissue injury. CMV esophagitis is associated with high mucosal concentrations of the proinflammatory cytokine tumor necrosis factor.27 Toxin production and virulence factors play a key role in the clinical expression and tissue damage caused by many GI infections, especially bacterial infections.28
Clinical and Endoscopic Features
Esophagus
Clinical Features
The most prevalent cause of esophageal infection in both the normal host and the immunosuppressed patient is Candida followed by the herpesviruses.5,29,30 CMV occurs more commonly in patients with AIDS, whereas HSV is more often observed in the normal host and non–HIV-infected immunosuppressed patients. Odynophagia is the characteristic symptom of esophageal infection, and infections resulting in esophageal ulceration almost uniformly cause odynophagia.5,31 Although less common, dysphagia may be observed with esophageal infections, especially Candida esophagitis, or may represent esophageal obstruction or dysmotility from the infection or its sequelae. Bleeding is generally observed only when there is ulceration, and although generally mild, it can be severe if there is an associated coagulopathy. Pulmonary symptoms may predominate when there is fistula formation to the tracheobronchial tree or coexistent pulmonary involvement. Patients with AIDS often have multiple coexisting esophageal disorders, which complicate management further.5,32
Physical examination, particularly of the oropharynx, may be helpful in suggesting the diagnosis of esophageal infection. Approximately two-thirds of patients with AIDS and esophageal candidiasis have oral candidiasis (thrush).33 In other immunocompromised patients, oropharyngeal candidiasis is also commonly associated with esophageal candidiasis.5 Thrush may be absent, however, if antifungal therapy, such as nystatin, is currently administered. The presence of oropharyngeal candidiasis does not prove that Candida is the only cause of symptoms, and the absence of oropharyngeal candidiasis does not exclude Candida esophagitis. Patients with chronic mucocutaneous candidiasis may have fungal involvement of various mucous membranes, hair, nails, and skin and have a history of adrenal or parathyroid dysfunction. Coexistent oropharyngeal ulceration is common in patients with HSV esophagitis but is infrequent in patients with CMV esophagitis or other systemic infections.34,35
After Candida species, herpesviruses are the most frequent infectious agents that cause esophagitis. After transplantation, HSV and CMV occur with equal frequency as causes of esophagitis,5 whereas in patients with AIDS, HSV esophagitis is uncommon and far less frequent than CMV. In a study of 100 HIV-infected patients with esophageal ulcer, HSV was found in only 9 (in 4, it was a copathogen with CMV).30 HSV esophageal infection commonly manifests with the sudden onset of severe odynophagia, heartburn, or chest pain.3,34 Autopsy studies suggest that esophageal symptoms may be absent. Herpes labialis (i.e., cold sores) and oropharyngeal ulcers may coexist, antedate, or develop during the esophageal infection, whereas skin infection is rare.5 Numerous systemic manifestations, including low-grade fever or upper respiratory symptoms, may precede the onset of esophageal symptoms. In untreated immunocompetent persons, spontaneous resolution of HSV esophageal infection occurs within 2 weeks of the onset of symptoms. Rarely, bleeding is the initial presentation and may be observed in the absence of esophageal complaints.
Odynophagia is almost uniformly present and is characteristically severe with CMV esophagitis. Chest pain, weight loss, and fever may be reported. The onset of symptoms is often more subacute than the acute presentation of HSV. A prior or coexistent diagnosis of CMV infection in other organs (e.g., retinitis or colitis) is frequent. Although rare in transplant patients, retinitis may be observed in approximately 15% of AIDS patients at the time of diagnosis of GI disease.35 The frequency of esophageal involvement with other pathogens is rare. Bacterial esophagitis has been observed in patients with severe neutropenia, usually patients with hematologic malignancies, but occasionally it is observed after bone marrow transplantation,36 diabetic ketoacidosis,37 or steroid therapy. The presentation is similar to the presentation with other infection agents.
Bacteria reported to involve the esophagus include Brucella,38 Actinomyces,39 Nocardia,40 and Bartonella henselae.41 The symptoms of esophageal TB depend on the degree and type of involvement.24,42,43 Systemic symptoms of fever and weight loss are common. Pulmonary complaints often predominate because of a fistula to the trachea, bronchus, or pleural space. Dysphagia may be prominent with the formation of long strictures or traction diverticula resulting from the fibrotic response. Upper GI hemorrhage caused by esophageal ulcers or tuberculous arterioesophageal fistulas may be the primary manifestation. Bleeding caused by extensive mucosal disease has been described in an AIDS patient with esophageal Mycobacterium avium complex (MAC).44,45 Fungi other than Candida species and parasitic diseases have rarely been reported to involve the esophagus.46–51
Barium radiography plays a minor role in the diagnosis of esophageal infection. In any patient, the presence of severe odynophagia limits the ability to drink barium, hampering the adequacy of the examination. Although specific barium esophagogram findings may be more typical for certain disorders,52 given the potential overlap, many of the findings are nonspecific, and endoscopy with biopsy is generally indicated. The wide spectrum of causes coupled with the specific antimicrobial regimens that are required necessitates a definitive diagnosis rather than empiric antimicrobial therapy. Nevertheless, a sinus tract or fistulous connection to the bronchial tree or mediastinum at the level of the hilum is highly suggestive of TB, although it also may be the result of malignancy. An esophageal neoplasm may be mimicked by an ulcerated tuberculous granulomatous mass or CMV ulcer.42,53 Chest radiography or computed tomography (CT) scan of the chest may support the diagnosis of TB.
Endoscopic Features
The characteristics of the esophageal lesions provide very important diagnostic clues. The location, size, and appearance of all endoscopic abnormalities should be documented because these features form the basis of the differential diagnosis and are useful for comparison on follow-up endoscopic examinations. The differential diagnosis of the lesion dictates how lesions should be sampled and what recommendations for diagnostic testing should be made on the biopsy or cytologic specimens (discussed later). Serologic testing plays no significant role in the diagnosis of acute infectious esophagitis. Endoscopic examination of the esophagus is the most sensitive and specific method for diagnosing esophageal candidiasis (Table 22.1).
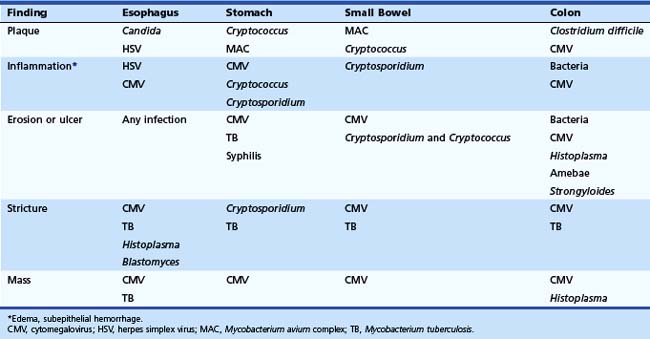
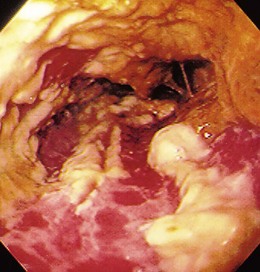
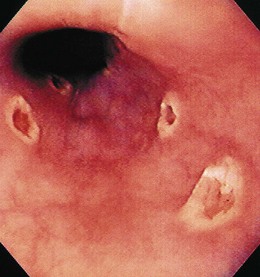
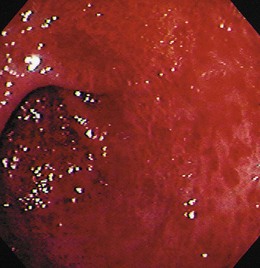
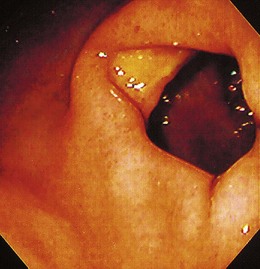
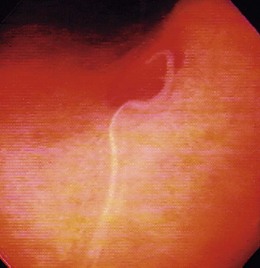
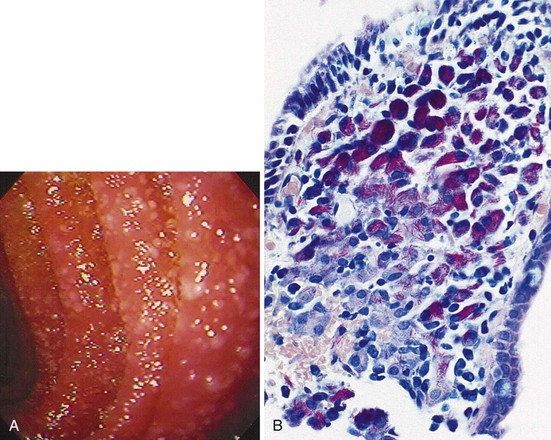
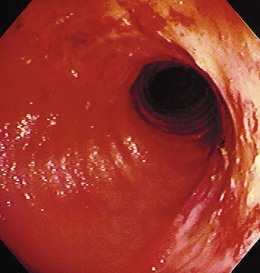
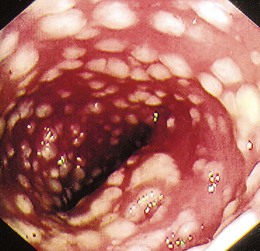
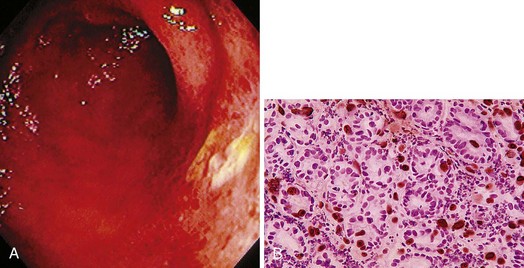
The gross endoscopic appearance of Candida esophagitis is pathognomonic (Fig. 22.1) and may be graded according to published criteria.54 A large, well-circumscribed ulceration should not be attributed to Candida. The endoscopic characteristics of HSV esophagitis reflect the pathologic changes. HSV esophagitis appears as discrete, usually small (<1 cm), well-circumscribed shallow ulcers; a diffuse erosive esophagitis; or rarely vesicles (Fig. 22.2).5,55 Small, scattered lesions covered with exudate mimic esophageal candidiasis. Deep ulcers, as seen with CMV, are very rare. CMV esophagitis is characteristically associated with one or more ulcerations that can be quite striking in patients with AIDS. Nevertheless, as with other infections, variability has been reported with appearances ranging from multiple shallow ulcers, to solitary giant ulcers, to a diffuse superficial esophagitis (Fig. 22.3).56 Although serologic testing is not helpful because of the high rate of prior exposure to CMV, the absence of CMV DNA or antigenemia in the blood would suggest an alternative diagnosis. Esophageal TB can manifest with a fistula to the tracheobronchial tree easily visualized endoscopically and rarely as an ulcer or mass lesion resembling a neoplasm. In normal hosts from endemic areas in South America, Trypanosoma cruzi may involve the myenteric plexus of the esophagus resulting in Chagas’ disease and an appearance that is indistinguishable clinically, radiographically, manometrically, and endoscopically from idiopathic achalasia.57 This diagnosis may be established by antibody testing. The endoscopic appearances of other rare infections have been described in case reports and resemble other infections.
Stomach
Clinical Features
Symptomatic gastric infections are much less prevalent than infections of the esophagus. The primary gastric pathogens are Helicobacter pylori and CMV; parasites and mycobacteria are also reported but are uncommon.58 Because of the relative infrequency of gastric infections, understanding of the presentation and endoscopic findings of most of these infections is based on case reports or small series. With the exception of H. pylori, most infections of the stomach occur in the setting of an immunodeficiency state. Gastric infections are typically manifested by upper abdominal pain that is generally steady and may radiate to the back. Associated symptoms may include nausea with or without vomiting; vomiting may be prominent when mucosal infection is severe. Infrequently, nausea alone in the absence of abdominal pain may be observed. Fever and weight loss are variable. Diarrhea may be the prominent symptom if the infecting pathogen also involves the small bowel (e.g., Cryptosporidium). Bleeding, both occult and overt, is usually a marker of mucosal ulceration. However, because most gastric infections are superficial, severe bleeding is unusual unless there is an associated coagulopathy.
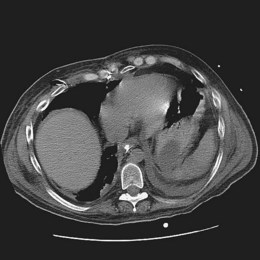
As in the esophagus, the primary symptom is dictated by the infecting pathogen. CMV gastric infection typically produces ulceration; abdominal pain with or without bleeding is the most frequent presentation. Mucosal infections that result in gastritis without ulceration such as cryptosporidiosis more commonly manifest with nausea without pain or may be asymptomatic. H. pylori infection of the stomach is generally considered an asymptomatic infection in most people regardless of immune status. Physical examination is generally unrevealing. Mild abdominal pain may be elicited on palpation of the epigastrium. A Hemoccult-positive stool is nonspecific. Radiologic studies may suggest the presence of gastric infection. Although abnormalities can often be identified, the findings are typically nonspecific, and further investigation with endoscopy is often required. Barium findings of gastric infection may include fold thickening or ulceration, whereas the most common CT finding is wall thickening, usually diffuse, and focal lesions mimicking a mass lesion (Fig. 22.4).
Endoscopic Features
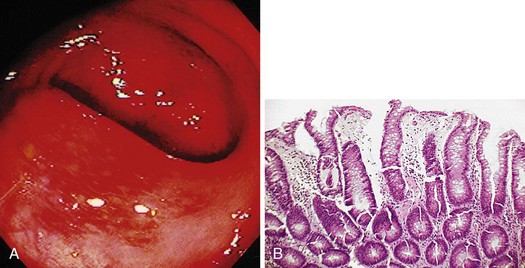
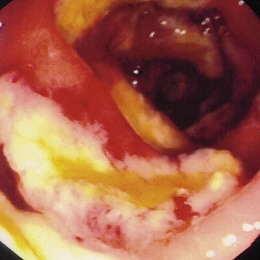
Similar to all GI infections, the primary endoscopic abnormality is dictated by the infecting pathogen, and the severity of disease clinically and endoscopically depends on the presence and degree of immunodeficiency. CMV, the most common opportunistic gastric pathogen, generally manifests with a diffuse gastritis characteristically with a hemorrhagic component (Fig. 22.5). Mucosal breaks are typical with focal or diffuse erosions or frank ulcerations (Fig. 22.6) that may be large, are usually well circumscribed, and may mimic a malignancy.53,59 Ulcerations have also been described with fungi, secondary to syphilis.60–63 The endoscopic features of gastric cryptosporidiosis and mycobacterial infection may appear as inflammation, polyps, or antral narrowing.64–66 In the normal host, gastric anisakiasis has been associated with ingestion of raw fish, and the Anisakis larvae may be visualized and removed at the time of endoscopy (Fig. 22.7).67
Small Intestine
Clinical Features
Parasitic disorders are the predominant cause of small bowel infection. In the normal host, Giardia infection predominates. With the exception of CMV, opportunistic small bowel infections are uncommon in transplant patients, but they are a hallmark of AIDS, where Cryptosporidium and microsporidia are frequent pathogens. Although typically a pathogen complicating immunodeficiency syndromes, self-limited cryptosporidiosis has been observed in the normal host usually during single-source outbreaks and may be a more common cause of acute diarrhea than previously thought.68 MAC, a pathogen principally restricted to patients with AIDS, is a common small bowel pathogen that is widely disseminated at the time of diagnosis (Fig. 22.8). A localized proximal small bowel infection can occur with CMV, and distal ileitis has been reported with CMV, bacteria, mycobacteria, fungi, and parasites.26,69–75
Diarrhea is the hallmark of small bowel infections; the severity and chronicity depend on the etiology and the host. Severe watery diarrhea causing dehydration is characteristic of intestinal cryptosporidiosis, whereas less severe diarrhea is observed with most other pathogens. Although crampy abdominal pain may be seen with any diarrheal disorder, more constant discomfort would be most typical for CMV enteritis and MAC.44 Symptoms of malabsorption may be prominent when the infection is diffuse and severe, although overt steatorrhea suggests pancreatic rather than small intestinal disease. Significant borborygmi may occur when the diarrhea is more voluminous. Because CMV generally causes focal mucosal ulceration sparing the intervening mucosa, abdominal pain and overt bleeding can be observed in the absence of diarrhea.
Weight loss may be profound with some infections. As noted, a distal ileitis, as reported from CMV, bacteria, TB, MAC, and parasites including Isospora, may result in a right lower quadrant pain syndrome. An acute abdomen can result from intestinal perforation and is most commonly due to CMV.76 The physical examination is variable from normal to cachexia and dehydration. Dehydration and electrolyte abnormalities would suggest a more severe process, such as cryptosporidiosis. Abdominal tenderness may be elicited and would suggest CMV or perhaps MAC. Occult blood in the stool is nonspecific. Extraintestinal signs and symptoms may be associated with infection by Yersinia species.75 Radiologic studies are of limited use in the setting of suspected small bowel infection. Small bowel barium studies may obscure stool studies and should be avoided. CT can be helpful if thickened small bowel segments are visualized, although the differential diagnosis is broad.73,76,77
Endoscopic Features
The endoscopic findings of small bowel infections vary from normal to widespread hemorrhage and ulceration. Focal erosions and ulcerations are typical for a viral infection with CMV, whereas minimal mucosal changes, if any, are common with parasitic diseases. Ulcers have also been reported with Toxoplasma.72 Small bowel atrophy is associated with some of these infections and endoscopically mimics celiac sprue.78 MAC infection has a characteristic appearance of small to confluent nodular lesions often with a yellow color resembling Whipple’s disease (see Fig. 22.8). Disseminated fungal infections can also manifest with small nodular lesions.79 Rarely, obstructive symptoms may predominate if there is an obstructive process. Stricture and ulceration of the ileum is typical for TB and rare for CMV (Fig. 22.9); these are best characterized by radiographic rather than endoscopic examination.80,81 Ileitis can also be observed with some bacterial infections, including Yersinia species and Salmonella species.74,82 The endoscopic and radiographic features of any severe ileitis may mimic Crohn’s disease. The diffuse nature of most small bowel infections highlights the importance of ileal examination with mucosal biopsy if colonoscopy is performed. Capsule endoscopy may also help characterize small bowel infections, although mucosal biopsy would require double-balloon enteroscopy.
Colon
Clinical Features
In contrast to the upper GI tract, bacteria are the most common colonic pathogens. Campylobacter is the most prevalent isolate in most series of acute infectious diarrhea and colitis.28 Depending on the clinical setting, CMV is the most frequent opportunistic pathogen, whereas Clostridium difficile remains an important pathogen in all patients regardless of immune status. Diarrhea and abdominal pain are the cardinal manifestations of colonic infection. Acute diarrhea with urgency, tenesmus, and small-volume bleeding is typical for bacterial colitis in any patient regardless of immune status. Although colonic infection is typically acute, especially in the normal host, chronic or recurrent diarrhea may be observed in immunodeficient patients. If the proximal colon is preferentially involved, right-sided abdominal pain may predominate. Bleeding is uniformly present with E. coli O157H7 enteritis, and this infection should not be considered in its absence.83 Parasitic diseases can involve the colon and manifest either acutely (amebic disease) or with a chronic watery diarrhea (cryptosporidiosis); concomitant small bowel disease is seen with cryptosporidiosis. Microsporidia do not infect the colon.84 Colonic CMV infection characteristically manifests as a chronic watery diarrhea; pain is often a prominent feature, and occult and overt bleeding may occur. Fever is common in bacterial infection, less so with CMV, and absent in most parasitic disorders. MAC can involve the colon; although rare in developed countries, TB involvement of the colon is well recognized.80 Toxic megacolon and perforation may complicate severe infection with either bacteria or viruses.85
The physical examination is generally dictated by the infecting pathogen. With acute bacterial colitis, the patient may appear toxic and have significant abdominal tenderness suggesting an acute abdomen, and the pain may predominate over the diarrhea. Fever and abdominal pain may also be the main features of severe C. difficile colitis. Laboratory studies may show a leukocytosis with left shift, which may approach a leukemoid reaction, but are otherwise nonspecific. The most common radiographic study used in colonic infection is CT scanning. CT scans are often performed because of the marked pain that is observed. Colonic wall thickening, which may be dramatic, is the typical finding for any colitis and may be found on routine abdominal radiographs (Fig. 22.10). Additional findings on CT may include small bowel thickening or lymphadenopathy.85,86 Depending on the infectious cause, radiographic abnormalities may be either focal or diffuse. Barium enema examination, if indicated, should not be performed in patients with suspected colonic infection until all stool studies are collected.
Endoscopic Features
The endoscopic findings in colonic infection range from normal to severe pancolonic edema and ulceration typical for a fulminant ulcerative colitis. Campylobacter, Shigella, and Salmonella infections may appear similar endoscopically with mucosal edema, subepithelial hemorrhage, erosions, and ulcers of varying size (Fig. 22.11). Distal disease is typical for Campylobacter and Shigella infections, whereas infections with Salmonella and Yersinia preferentially involve the right colon and ileum.87–89 Salmonella typhi infection results in lymphoid hyperplasia leading to ulceration at the site of Peyer’s patches; this may explain the geographic location in the bowel.90
With any bacterial colitis, the colitis may be patchy, segmental, or diffuse. C. difficile colitis has a well-recognized appearance of plaquelike lesions that are typically confluent and are generally present in the distal colorectum (Fig. 22.12). When the disease is severe, mucosal edema is prominent. Subepithelial hemorrhage is characteristic of CMV infection, as is ulceration of variable distribution (Fig. 22.13). An appearance of inflammatory bowel disease, either ulcerative colitis or Crohn’s disease, has been described with bacteria and CMV.91 HSV can rarely involve the colon, but generally only the distal rectum and anus are involved given the tropism of HSV for squamous mucosa. Amebic colitis may resemble a fulminant colitis or more commonly cause multiple ulcers that can be mistaken for idiopathic inflammatory bowel disease (Fig. 22.14).92 The colonoscopic findings of cryptosporidiosis may be minimal edema or normal-appearing colon. TB may manifest with a mass lesion or serpiginous ulceration and nodularity.80 Fungi have rarely been reported to involve the colon with histoplasmosis noted to cause ulceration or mass lesions resembling carcinoma.26,93 Helminthic and other pathogens of the colon have also been described.94,95 Abdominal CT may be helpful when evaluating for complications such as toxic megacolon.
Pathology
The pathologic features of GI infections depend on the infecting pathogen, and tissue tropism dictates the organs of involvement. The gross pathologic appearance of esophageal candidiasis ranges from a few white or yellow plaques on the mucosal surface to a dense, thick plaque coating the mucosa and encroaching on the esophageal lumen. Although potentially misinterpreted as “ulcer,” this plaque material is composed of desquamated squamous epithelial cells, admixed with fungal organisms, inflammatory cells, and bacteria.54 True ulceration (granulation tissue) is rarely caused by Candida alone and has been documented most commonly in patients with profound granulocytopenia or when Candida is a coinfection with another cause of ulceration.32 More deep-seated submucosal infections can occur with some fungi, and disseminated fungal infections can lead to ulceration.
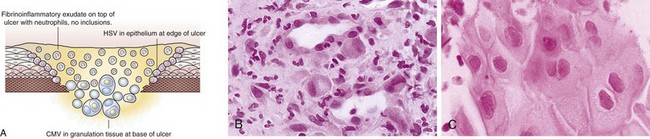
The histologic hallmark of CMV esophagitis is mucosal ulceration. Although variable, deep ulcers are very characteristic for disease in patients with AIDS, whereas in other immunocompromised patients, lesions tend to remain more superficial. Despite the depth of the lesions, perforation is rare. In contrast to HSV, the viral cytopathic effect of CMV is located in endothelial and mesenchymal cells in the granulation tissue of the ulcer base rather than in squamous cells. Inclusions are large (cytomegalic) and often have an eosinophilic appearance that may be located either in the nucleus or in the cytoplasm.96 The inclusions can assume an atypical appearance especially in patients with AIDS97; immunohistochemical stains play a valuable role in selected patients to confirm the presence of CMV, and they often highlight more infected cells than are appreciated by routine hematoxylin and eosin staining (see Fig. 22.13B).98 CMV may coexist with HSV or Candida or other pathogens in patients with AIDS. The gross pathologic appearance of bacterial esophagitis depends on the etiologic pathogen and ranges from diffuse, shallow ulcerations to ulcers associated with erythema, plaques, pseudomembranes, nodules, or hemorrhage.
Microscopic examination reveals pseudomembranes and bacterial invasion that may be superficial and limited to squamous epithelium or may be invasive and transmural with infiltration of blood vessels (i.e., phlegmonous esophagitis). Esophageal actinomycosis is characterized by ulceration and sinuses leading from abscess cavities with sulfur granules and filamentous gram-positive branching bacteria seen on tissue biopsy specimens.39 In the one reported case, B. henselae esophagitis resulted in multiple nodules resulting from a lobulated proliferation of capillary vessels lined by plump endothelial cells.41 Bacterial infection of the stomach is generally limited to H. pylori infection, which has the characteristic active chronic gastritis often with lymphoid aggregates.99 Phlegmonous gastritis, whose pathogenesis is not well understood, may involve gram-positive and gram-negative bacilli.100 Acute bacterial colitis is pathologically shown by crypt abscess with a preponderance of polymorphonuclear leukocytes and preservation of the mucosal architecture. These features help distinguish these processes from idiopathic inflammatory bowel disease.101
TB generally results in disease secondarily from paraesophageal infected nodes in the midesophagus at the level of the carina or tracheoesophageal fistula. In other parts of the gut, primary mucosal infection can occur. Histologically, granulomas are often present in ulcer tissue, with mycobacteria identifiable by mycobacterial staining. MAC, in contrast to TB, may not result in well-formed granulomas. Staining for MAC often yields an abundance of organisms (see Fig. 22.8B), whereas tuberculous bacilli may be few in number even in patients with AIDS.
Although generally an excellent stain, hematoxylin and eosin staining may not identify all pathogens. Various pathogen-specific stains are available to aid in the identification of nearly all common GI infections (with the exception of non-herpesviruses). These stains highlight infecting pathogens and make identification easier. Immunohistochemical stains for viral antigens are very helpful when examining for herpesviruses.102 Because a battery of stains is not routinely performed on all biopsy specimens, communication with the pathologist is essential to ensure appropriate pathologic evaluation.
Differential Diagnosis
Diagnostic considerations are determined by the clinical presentation, risk group and severity of immunodeficiency, and specific endoscopic findings. Although overlap is broad, some endoscopic abnormalities are typical for a specific pathogen and may be organ-specific (see Table 22.1). In the esophagus, CMV esophagitis and the idiopathic esophageal ulcer of AIDS are difficult to differentiate.35,103 These two processes generally result in one or more large ulcers. Pill-induced esophagitis must be excluded by history because the pathologic findings of esophageal biopsy specimens are similar. Likewise, distal esophageal ulcer may suggest gastroesophageal reflux disease, and the histopathologic features cannot distinguish idiopathic esophageal ulcer from gastroesophageal reflux disease. The clinical history is different, however, and the endoscopic appearance helps suggest gastroesophageal reflux. Small esophageal ulcers can be observed in the acute phase of HIV infection, which can mimic viral or pill-induced esophagitis.104 Esophageal strictures can result from opportunistic infections.105 The history coupled with mucosal biopsy helps differentiate infection from gastroesophageal reflux disease.
Treatment
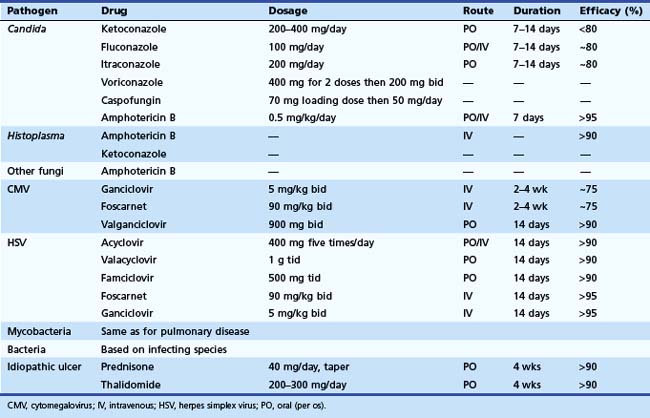
Effective antimicrobial therapies are available for most GI infections (Table 22.2). The pathogen rather than the organ of involvement dictates the treatment regimen. Systemic therapy should be provided for patients with severe disease and associated immunodeficiency. In the normal host, many infections are acute and self-limited and require no specific therapy. Antimicrobial therapy may be contraindicated and potentially harmful with some infections, such as E. coli O157H7, in which antibiotic therapy may be associated with hemolytic uremic syndrome in children.106 For HIV-infected patients, treatment of the underlying immunodeficiency with highly active antiretroviral drugs is quite effective and is fundamental to the treatment of any opportunistic infection in this setting. Antiretroviral therapy alone can result in remission of some opportunistic infections and AIDS-associated neoplasms, which underscores the importance of immune reconstitution in any setting.107,108 Likewise, in a transplant patient, reducing the dosage of immunosuppressive drugs, when possible, plays an important adjunctive role in the management of any opportunistic infection.
Preoperative History and Considerations
In patients with AIDS and thrush, the presence of dysphagia or odynophagia usually indicates Candida esophagitis. In a symptomatic patient with associated thrush, an empiric trial of antifungal therapy should be instituted, reserving endoscopy for patients who fail to respond. Further evaluation should be delayed no longer than 1 week for patients with severe persistent symptoms because the response to antifungal therapy is rapid, with clinical improvement occurring in most patients within days.109,110 If patients fail to improve with empiric antifungal therapy, endoscopy should be performed because disorders other than Candida are identified in most patients.111 This empiric strategy has not been critically studied in the transplant setting; however, clinical experience suggests it is effective. Empiric therapy for acute diarrhea in other immunosuppressed patients is commonly practiced, but few studies exist.
Description of Techniques
Viral culture of biopsy specimens may increase the diagnostic yield, although false-positive and false-negative results occur, and viral cultures are less sensitive than multiple biopsy specimens. Use of shell vial techniques improves the turnaround time for CMV culture to 48 hours. Bacterial culture of colonic biopsy specimens has been found in some series to enhance the diagnostic yield. Cytologic brushings and endoscopic mucosal biopsy specimens should be taken from the ulcer edge when HSV disease is suspected because the viral cytopathic effect is best identified in epithelial cells rather than in granulation tissue in the ulcer bed.112 In contrast, a biopsy specimen of the ulcer base must be obtained when viral infection is suspected with CMV (Fig. 22.15). Multiple biopsy specimens (up to 10) may be required to establish the diagnosis in patients with AIDS and should be taken from the base of the ulcer.113 Overall, mucosal biopsy for histologic diagnosis is the preferred method to distinguish the cause of ulcer.114 Culture of an aliquot of stool obtained at colonoscopy and bacterial culture of mucosal biopsy specimens may increase the diagnostic yield.115
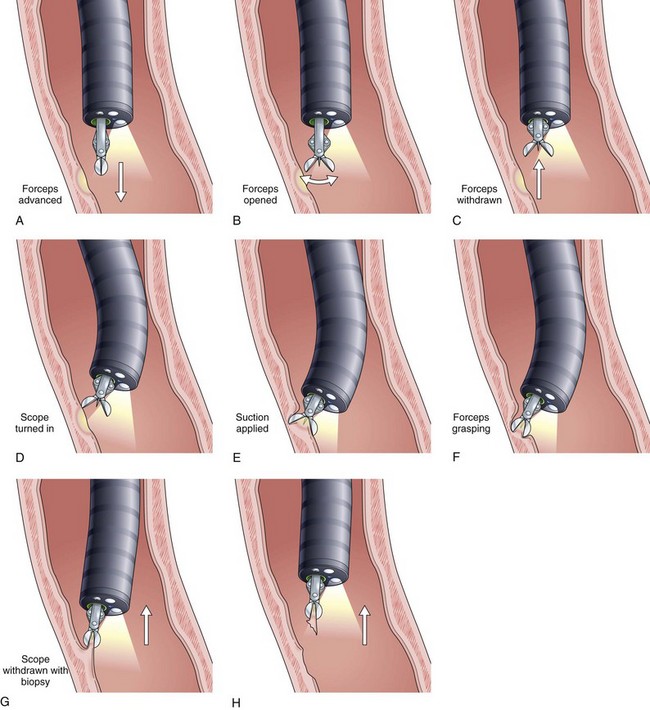
As mentioned previously, many histologic stains are available to identify pathogens. Immunohistochemical staining on biopsy samples using specific monoclonal antibodies to viruses such as HSV and CMV helps confirm the diagnosis when the viral cytopathic effect is difficult to appreciate. We generally rely on histology for the diagnosis of viral GI infections and use brushings and viral culture selectively. A technique first described for gastric biopsies, whereby the forceps are tilted into the lesion and the tissue is avulsed, is especially useful for taking samples from esophageal lesions (Fig. 22.16). Lastly, as noted previously, communication with the pathologist is essential so that appropriate attention can be drawn to specific pathogens and so that special stains can be performed.
Complications
Complications from endoscopy in immunocompromised patients are similar to complications in the normal host. There have been rare reports of bacteremia after endoscopic examination in patients with neutropenia. In this setting, antibiotic therapy before endoscopy is appropriate. Bleeding is generally self-limited but may occur in anticoagulated patients. Vigorous biopsy of esophageal ulceration is safe with no reported cases of biopsy-induced perforation.113
1 Podlekareva D, Mocroft A, Dragsted UB, et al. Factors associated with the development of opportunistic infections in HIV-1-infected adults with high CD4+ cell counts: a EuroSIDA study. J Infect Dis. 2006;194:633-641.
2 Wilcox CM, Saag MS. Gastrointestinal complications of HIV infection: changing priorities in the HAART era. Gut. 2008;57:861-870.
3 Ramanathan J, Rammouni M, Baran JJr, et al. Herpes simplex virus esophagitis in the immunocompetent host: An overview. Am J Gastroenterol. 2000;95:2171-2176.
4 Venkataramani A, Schlueter AJ, Spech TJ, et al. Cytomegalovirus esophagitis in an immunocompetent host. Gastrointest Endosc. 1994;40:392-393.
5 Baehr PH, McDonald GB. Esophageal infections: Risk factors, presentation, diagnosis, and treatment. Gastroenterology. 1994;106:509-532.
6 Kothari A, Ramachandran VG, Gupta P, et al. Seroprevalence of cytomegalovirus among voluntary blood donors in Delhi, India. J Health Popul Nutr. 2002;20:348-351.
7 Rubin RR. Infections in the liver and renal transplant patient. In: Rubin RH, Young LS, editors. Clinical approach to infection in the compromised host. ed 2. New York: Plenum Publishing; 1988:561.
8 Porter K, Fairley CK, Wall PG, et al. AIDS defining diseases in the UK: The impact of PCP prophylaxis and twelve years of change. Int J STD AIDS. 1996;7:252-257.
9 Fichtenbaum CJ, Koletar S, Yiannoutsos C, et al. Refractory mucosal candidiasis in advanced human immunodeficiency virus infection. Clin Infect Dis. 2000;30:749-756.
10 Monkemuller KE, Call SA, Lazenby AJ, et al. Declining prevalence of opportunistic gastrointestinal disease in the era of combination antiretroviral therapy. Am J Gastroenterol. 2000;95:457-462.
11 Ives NJ, Gazzard BG, Easterbrook PJ. The changing pattern of AIDS-defining illnesses with the introduction of highly active antiretroviral therapy (HAART) in a London clinic. J Infect. 2001;42:134-139.
12 Dore GJ, Li Y, McDonald A, et al. Impact of highly active antiretroviral therapy on individual AIDS-defining illness incidence and survival in Australia. J Acquir Immune Defic Syndr. 2002;29:388-395.
13 Hofflin JM, Potasman I, Baldwin JC, et al. Infectious complications in heart transplant recipients receiving cyclosporine and corticosteroids. Ann Intern Med. 1987;106:209-216.
14 Gerna G, Lilleri D, Callegaro A, et al. Prophylaxis followed by preemptive therapy versus preemptive therapy for prevention of human cytomegalovirus disease in pediatric patients undergoing liver transplantation. Transplantation. 2008;86:163-166.
15 Boeckh M, Ljungman P. How we treat CMV in hematopoietic cell transplant recipients. Blood. 2009;113:5711-5719.
16 Ranjana KH, Priyokumar K, Singh TJ, et al. Disseminated Penicillium marneffei infection among HIV-infected patients in Manipur state. Indian J Infect. 2002;45:268-271.
17 De Las Casas C, Adachi J, Dupont H. Travellers’ diarrhoea. Aliment Pharmacol Ther. 1999;13:1373-1378.
18 James SP. Cellular immune mechanisms of defense in the gastrointestinal tract. In: Blaser MJ, Smith PD, Ravdin JI, et al, editors. Infections of the gastrointestinal tract. New York: Raven Press; 1995:213-236.
19 Evans CA, Gilman RH, Rabbani GH, et al. Gastric acid secretion and enteric infection in Bangladesh. Trans R Soc Trop Med Hyg. 1997;91:681-685.
20 Salzman NH, Ghosh D, Huttners KM, et al. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522-526.
21 Kotler DP. HIV infection and the gastrointestinal tract. AIDS. 2005;19:107-117.
22 Walsh TJ, Belitsos NJ, Hamilton SR. Bacterial esophagitis in immunocompromised patients. Arch Intern Med. 1986;146:1345-1348.
23 Marshall JB, Singh R, Demmy TL, et al. Mediastinal histoplasmosis presenting with esophageal involvement and dysphagia: Case study. Dysphagia. 1995;10:53-58.
24 Devarbhavi HC, Alvares JF, Radhikidevi M. Esophageal tuberculosis associated with esophagotracheal or esophagomediastinal fistula: Report of 10 cases. Gastrointest Endosc. 2003;57:588-592.
25 Suzuki K, Sugawara Y, Sekine T, et al. Breakthrough disseminated zygomycosis induced massive gastrointestinal bleeding in a patient with acute myeloid leukemia receiving micafungin. J Infect Chemother. 2009;15:42-45.
26 Assi M, McKinsey DS, Driks MR, et al. Gastrointestinal histoplasmosis in the acquired immunodeficiency syndrome: report of 18 cases and literature review. Diagn Microbiol Infect Dis. 2006;55:195-201.
27 Wilcox CM, Harris PR, Redman TK, et al. High mucosal levels of tumor necrosis factor alpha messenger RNA in AIDS-associated cytomegalovirus-induced esophagitis. Gastroenterology. 1998;114:77-82.
28 Ina K, Kusugami K, Ohta M. Bacterial hemorrhagic enterocolitis. J Gastroenterol. 2003;38:111-120.
29 Bonacini M, Young T, Laine L. The causes of esophageal symptoms in human immunodeficiency virus infection. Arch Intern Med. 1991;151:1567-1572.
30 Wilcox CM, Schwartz DA, Clark WS. Esophageal ulceration in human immunodeficiency virus infection: Causes, diagnosis, and management. Ann Intern Med. 1995;123:143-149.
31 Bashir RM, Wilcox CM. Symptom-specific use of upper gastrointestinal endoscopy in human immunodeficiency virus-infected patients yields high dividends. J Clin Gastroenterol. 1996;23:292-298.
32 Wilcox CM. Evaluation of a technique to evaluate the underlying mucosa in patients with AIDS and severe Candida esophagitis. Gastrointest Endosc. 1995;42:360-363.
33 Wilcox CM, Straub RF, Clark WS. Prospective evaluation of oropharyngeal findings in human immunodeficiency virus-infected patients with esophageal ulceration. Am J Gastroenterol. 1995;90:1938-1941.
34 Genereau T, Lortholary O, Bouchaud O, et al. Herpes simplex esophagitis in patients with AIDS: Report of 34 cases. Clin Infect Dis. 1996;22:926-931.
35 Wilcox CM, Straub RF, Schwartz DA. Cytomegalovirus esophagitis in AIDS: A prospective study of clinical response to ganciclovir therapy, relapse rate, and long-term outcome. Am J Med. 1995;98:169-176.
36 McDonald GB, Sharma P, Hackman RC, et al. Esophageal infections in immunosuppressed patients after marrow transplantation. Gastroenterology. 1985;88:1111-1117.
37 Ezzell JHJr, Bremer J, Adamec TA. Bacterial esophagitis: An often forgotten cause of odynophagia. Am J Gastroenterol. 1990;85:296-298.
38 Laso FJ, Cordero M, Giarcia-Sanchez. Esophageal brucellosis: A new location of Brucella infection. Clin Invest. 1994;72:393-395.
39 Abdalla J, Myers J, Moorman J. Actinomycotic infection of the oesophagus. J Infect. 2005;51:E39-E43.
40 Kim J, Minamoto GY, Grieco MH. Nocardial infection as a complication of AIDS: Report of six cases and review. Rev Infect Dis. 1991;13:624-629.
41 Chang AD, Drachenberg CI, James SP. Bacillary angiomatosis associated with extensive esophageal polyposis: A new mucocutaneous manifestation of acquired immunodeficiency disease (AIDS). Am J Gastroenterol. 1996;91:2220-2223.
42 Musoğlu A, Ozütemiz O, Tekin F, et al. Esophageal tuberculosis mimicking esophageal carcinoma. Turk J Gastroenterol. 2005;16:105-107.
43 Seivewright N, Feehally J, Wicks AC. Primary tuberculosis of the esophagus. Am J Gastroenterol. 1984;79:842-843.
44 Gray JR, Rabeneck L. Atypical mycobacterial infection of the gastrointestinal tract in AIDS patients. Am J Gastroenterol. 1989;89:1521-1524.
45 Cappell MS, Gupta A. Gastrointestinal hemorrhage due to gastrointestinal Mycobacterium avium-intracellulare of esophageal candidiasis in patients with the acquired immunodeficiency syndrome. Am J Gastroenterol. 1992;87:224-229.
46 Margolis PS, Epstein A. Mucormycosis esophagitis in a patient with the acquired immunodeficiency syndrome. Am J Gastroenterol. 1994;89:1900-1902.
47 Choi JH, Yoo JH, Chung IJ, et al. Esophageal aspergillosis after bone marrow transplant. Bone Marrow Transplant. 1997;19:293-294.
48 Ng FH, Wong SY, Chang CM, et al. Esophageal actinomycosis: A case report. Endoscopy. 1997;29:133.
49 Khandekar A, Moser D, Fidler WJ. Blastomycosis of the esophagus. Ann Thorac Surg. 1980;30:76-79.
50 Kazlow PG, Shah K, Benkov KJ, et al. Esophageal cryptosporidiosis in a child with acquired immune deficiency syndrome. Gastroenterology. 1986;91:1301-1303.
51 Laguna F, Garcia-Samaniegh J, Soriano V, et al. Gastrointestinal leishmaniasis in human immunodeficiency virus-infected patients: Report of five cases and review. Clin Infect Dis. 1994;19:48-53.
52 Levine MS, Rubesin SE, Laufer I. Barium esophagography: A study for all seasons. Clin Gastroenterol Hepatol. 2008;6:11-25.
53 Laguna F, Garcia-Samaniego J, Alonso MJ, et al. Pseudotumoral appearance of cytomegalovirus esophagitis and gastritis in AIDS patients. Am J Gastroenterol. 1993;88:1108-1111.
54 Wilcox CM, Schwartz DA. Endoscopic-pathologic correlates of Candida esophagitis in acquired immunodeficiency syndrome. Dig Dis Sci. 1996;41:1337-1345.
55 McBane RD, Gross JRJr. Herpes esophagitis: Clinical syndrome, endoscopic appearance, and diagnosis in 23 patients. Gastrointest Endosc. 1991;37:600-603.
56 Wilcox CM, Straub RA, Schwartz DA. Prospective endoscopic characterization of cytomegalovirus esophagitis in patients with AIDS. Gastrointest Endosc. 1994;40:481-484.
57 Lages-Silva E, Crema E, Ramirez LE, et al. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: Blood and tissue parasitism. Am J Trop Med Hyg. 2001;65:435-441.
58 Pensa E, Borum ML. Opportunistic infections: Gastric infections in HIV/AIDS. AIDS Read. 2000;10:347-349.
59 Lladó L, Castellote J, Fabregat J, et al. Antral mass due to cytomegalovirus infection requiring gastrectomy in a liver transplant recipient. Transpl Infect Dis. 2008;10:354-357.
60 Sheu BS, Lee PC, Yang HB. A giant gastric ulcer caused by mucormycosis infection in a patient with renal transplantation. Endoscopy. 1998;30:S60-S61.
61 Greenstein DB, Wilcox CM, Schwartz DA. Gastric syphilis: Report of seven cases and review of the literature. J Clin Gastroenterol. 1994;18:4-9.
62 Merzianu M, Gorelick SM, Paje V, et al. Gastric toxoplasmosis as the presentation of acquired immunodeficiency syndrome. Arch Pathol Lab Med. 2005;129:e87-e90.
63 Coppola F, Recchia S, Ferrari A, et al. Visceral leishmaniasis in AIDS with gastric involvement. Gastrointest Endosc. 1992;38:76-78.
64 Cersosimo E, Wilkowske CJ, Rosenblatt JE, et al. Isolated antral narrowing associated with gastrointestinal cryptosporidiosis in acquired immunodeficiency syndrome. Mayo Clin Proc. 1992;67:553-556.
65 Chalasani N, Lazenby AJ, Wilcox CM. Unusual endoscopic features of gastric and duodenal cryptosporidiosis in AIDS. Gastrointest Endosc. 1997;45:525-527.
66 Tromba JL, Inglese R, Rieders B, et al. Primary gastric tuberculosis presenting as pyloric outlet obstruction. Am J Gastroenterol. 1991;86:1820-1822.
67 Lopez-Serrano MC, Gomez AA, Daschner A, et al. Gastroallergic anisakiasis: Findings in 22 patients. J Gastroenterol Hepatol. 2000;15:503-506.
68 Leav BA, Mackay M, Ward HD. Cryptosporidium species: New insights and old challenges. Clin Infect Dis. 2003;36:903-908.
69 Kotler DP, Baer JW, Scholes JV. Isolated ileitis due to cytomegalovirus in a patient with AIDS. Gastrointest Endosc. 1991;37:571-574.
70 Schneebaum CW, Nivick DM, Chabon AB, et al. Terminal ileitis associated with Mycobacterium avium-intracellulare infection in a homosexual man with acquired immune deficiency syndrome. Gastroenterology. 1987;92:1127-1132.
71 Gompels MM, Todd J, Peters BS, et al. Disseminated strongyloidiasis in AIDS: Uncommon but important. AIDS. 1991;5:329-332.
72 Bertoli F, Espino M, Arosemena JR5th, et al. A spectrum in the pathology of toxoplasmosis in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1995;119:214-224.
73 Balthazar EJ, Charles HW, Megibow AJ. Salmonella- and Shigella-induced ileitis: CT findings in four patients. J Comput Assist Tomogr. 1996;20:375-378.
74 Puylaert JB, Van der Zant FM, Mutsaers JA. Infectious ileocecitis caused by Yersinia, Campylobacter and Salmonella: Clinical, radiological and US findings. Eur Radiol. 1997;7:3-9.
75 Saebo A, Lessen J. Acute and chronic gastrointestinal manifestations associated with Yersinia enterocolitica infection: A Norwegian 10-year follow-up study on 458 hospitalized patients. Ann Surg. 1992;215:250-255.
76 Meza AD, Bin-Sagheer S, Zuckerman MJ, et al. Ileal perforation due to cytomegalovirus infection. J Natl Med Assoc. 1994;86:145-148.
77 Wisser J, Zingerman B, Wasik M, et al. Cytomegalovirus pseudotumor presenting as bowel obstruction in a patient with acquired immunodeficiency syndrome. Am J Gastroenterol. 1992;87:771-774.
78 Shah VH, Rotterdam H, Kotler DP, et al. All that scallops is not celiac disease. Gastrointest Endosc. 2000;51:717-720.
79 Chalasani N, Wilcox CM, Hunter HT, Schwartz DA. Endoscopic features of gastroduodenal cryptococcosis in AIDS. Gastrointest Endosc. 1997;45:315-317.
80 Muneef MA, Memish Z, Mahmoud SA, et al. Tuberculosis in the belly: A review of forty-six cases involving the gastrointestinal tract and peritoneum. Scand J Gastroenterol. 2001;36:528-532.
81 Knollmann FD, Grumewald T, Adler A, et al. Intestinal disease in acquired immunodeficiency syndrome: Evaluation by CT. Eur Radiol. 1997;7:1419-1429.
82 Stolk-Engelaar VM, Hoogkamp-Korstanje JA. Clinical presentation and diagnosis of gastrointestinal infections by Yersinia enterocolitica in 261 Dutch patients. Scand J Infect Dis. 1996;28:571-575.
83 Griffin PM, Ostroff SM, Tauxe RV, et al. Illnesses associated with Escherichia coli O157:H7 infections: A broad clinical spectrum. Ann Intern Med. 1988;109:705-712.
84 Blanshard C, Francis N, Gazzard BG. Investigation of chronic diarrhoea in acquired immunodeficiency syndrome: A prospective study of 155 patients. Gut. 1996;39:824-832.
85 Kirkpatrick ID, Greenberg HM. Gastrointestinal complications in the neutropenic patient: Characterization and differentiation with abdominal CT. Radiology. 2003;226:668-674.
86 Suri S, Gupta S, Suri R. Computed tomography in abdominal tuberculosis. Br J Radiol. 1999;72:92-98.
87 Shigeno T, Akamatsu T, Fujimori K, et al. The clinical significance of colonoscopy in hemorrhagic colitis due to enterohemorrhagic Escherichia coli O157:H7 infection. Endoscopy. 2002;34:311-314.
88 Rutggerts P, Geboes K, Ponette E, et al. Acute infective colitis caused by endemic pathogens in western Europe: Endoscopic features. Endoscopy. 1982;14:212-219.
89 Khuroo MS, Mahajan R, Zargar SA, et al. The colon in shigellosis: Serial colonoscopic appearance in Shigella dysenteriae I. Endoscopy. 1990;22:35-38.
90 Hepps K, Sutton FM, Goodgame RW. Multiple left-sided colon ulcers due to typhoid fever. Gastrointest Endosc. 1991;37:479-480.
91 Wilcox CM, Chalasani N, Lazenby A, et al. Cytomegalovirus colitis in acquired immunodeficiency syndrome: A clinical and endoscopic study. Gastrointest Endosc. 1998;48:39-43.
92 Takahashi T, Gamboa-Dominguez A, Gomez-Mendez TJ, et al. Fulminant amebic colitis: Analysis of 55 cases. Dis Colon Rectum. 1997;40:1362-1367.
93 Garcia RA, Jagirdar J. Colonic histoplasmosis in acquired immunodeficiency syndrome mimicking carcinoma. Ann Diagn Pathol. 2003;7:14-19.
94 Linder JD, Monkemuller KE, Lazenby AJ, et al. Streptococcus bovis bacteremia associated with Strongyloides stercoralis colitis. Gastrointest Endosc. 2000;52:796-798.
95 Bellomo AR, Perlman DC, Kaminsky DL, et al. Pneumocystis colitis in a patient with the acquired immunodeficiency syndrome. Am J Gastroenterol. 1992;87:759-761.
96 Beaugerie L, Cywiner-Golenzer C, Monfort L, et al. Definition and diagnosis of cytomegalovirus colitis in patients infected by human immunodeficiency virus. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:423-429.
97 Schwartz DA, Wilcox CM. Atypical cytomegalovirus inclusions in gastrointestinal biopsy specimens from patients with the acquired immunodeficiency syndrome: Diagnostic role of in situ nucleic acid hybridization. Hum Pathol. 1992;23:1019-1026.
98 Orenstein JM, Dieterich DT. The histopathology of 103 consecutive colonoscopy biopsies from 82 symptomatic patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 2001;125:1042-1046.
99 El-Zimaity HM, Segura AM, Genta RM, et al. Histologic assessment of Helicobacter pylori status after therapy: Comparison of Giemsa, Diff-Quik, and Genta stains. Mod Pathol. 1998;11:288-291.
100 Schultz MJ, van der Hulst RW, Tytgat GN. Acute phlegmonous gastritis. Gastrointest Endosc. 1996;44:80-83.
101 Abreu MT, Harpaz N. Diagnosis of colitis: Making the initial diagnosis. Clin Gastroenterol Hepatol. 2007;5:295-301.
102 Monkemuller KE, Bussian AH, Lazenby AJ, et al. Special histologic stains are rarely beneficial for the evaluation of HIV-related gastrointestinal infections. Am J Clin Pathol. 2000;114:387-394.
103 Wilcox CM, Schwartz DA. Endoscopic characterization of idiopathic esophageal ulceration associated with human immunodeficiency virus infection. J Clin Gastroenterol. 1993;16:251-256.
104 Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
105 Wilcox CM. Esophageal strictures complicating ulcerative esophagitis in patients with AIDS. Am J Gastroenterol. 1999;94:339-343.
106 Safdar N, Said A, Gangnon RE, et al. Risk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 enteritis: A meta-analysis. JAMA. 2002;288:996-1001.
107 Carr A, Marriott D, Field A, et al. Treatment of HIV-1 associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256-261.
108 Murdaca G, Campelli A, Setti M, et al. Complete remission of AIDS/Kaposi’s sarcoma after treatment with a combination of two nucleoside reverse transcriptase inhibitors and one non-nucleoside reverse transcriptase inhibitor. AIDS. 2002;16:304-305.
109 Wilcox CM, Alexander LN, Clark WS, et al. Fluconazole compared with endoscopy for human immunodeficiency virus-infected patients with esophageal symptoms. Gastroenterology. 1996;110:1803-1809.
110 Lai YP, Wu MS, Chen MY, et al. Timing and necessity of endoscopy in AIDS patients with dysphagia or odynophagia. Hepatogastroenterology. 1998;45:186-189.
111 Wilcox CM, Straub RF, Alexander LN, et al. Etiology of esophageal disease in human immunodeficiency virus-infected patients who fail antifungal therapy. Am J Med. 1996;101:599-604.
112 Wilcox CM. Approach to esophageal disease in AIDS: A primer for the endoscopist. Tech Gastrointest Endosc. 2002;4:59-65.
113 Wilcox CM, Straub RF, Schwartz DA. A prospective evaluation of biopsy number for the diagnosis of viral esophagitis in patients with HIV infection and esophageal ulcer. Gastrointest Endosc. 1996;44:587-593.
114 Wilcox CM, Rodgers W, Lazenby A. Prospective comparison of brush cytology, viral culture, and histology for the diagnosis of ulcerative esophagitis in AIDS. Clin Gastroenterol Hepatol. 2004;2:564-567.
115 Barbut F, Beaugerie L, Delas N, et al. Comparative value of colonic biopsy and intraluminal fluid culture for diagnosis of bacterial acute colitis in immunocompetent patients. Infectious Colitis Study Group. Clin Infect Dis. 1999;29:356-360.