135 Infections of Skin, Muscle, and Soft Tissue
 Necrotizing Soft-Tissue Infections
Necrotizing Soft-Tissue Infections
NSTIs represent a spectrum of infectious processes that are extensive and rapidly progressive. Based on the depth of skin and soft-tissue involvement, NSTIs are divided into three categories: necrotizing cellulitis, necrotizing fasciitis, and myonecrosis. Table 135-1 shows the classification of NSTIs. The sine qua non of these infections is necrosis of subcutaneous tissue, fascia, and muscle, with widespread undermining of the skin. The lack of anatomic boundaries and the fact that the infection is deep to the skin helps account for the severity of the infection as well as the frequent delay in its recognition. The trunk, extremities, and perineum are the most common sites of NSTIs, but other anatomic sites may be involved. For example, intraabdominal abscess, bowel perforation, and pancreatitis can present as necrotizing infection of the abdominal wall or extend into the thigh along the psoas muscle.1,2 Similarly, cervical fasciitis due to dental or neck abscess can extend to the mediastinum.
TABLE 135-1 Classification of Necrotizing Skin, Soft-Tissue, and Muscle Infections
| Disease | Bacteriology | Comments |
|---|---|---|
| Necrotizing Cellulitis | ||
| Clostridial cellulitis | Clostridium perfringens | Local trauma, recent surgery; fascial/deep muscle spared |
| Nonclostridial cellulitis | Mixed: Escherichia coli, Enterobacter, Peptostreptococcus spp., Bacteroides fragilis | Diabetes mellitus predisposes; produces foul odor |
| Meleney’s synergistic gangrene | Staphylococcus aureus, microaerophilic streptococci | Rare infection; postoperative; slowly expanding, indolent, ulceration in superficial fascia |
| Synergistic necrotizing cellulitis | Mixed aerobic and anaerobic, including B. fragilis, Peptostreptococcus spp. | Diabetes mellitus predisposes; variant of necrotizing fasciitis type I; involves skin, muscle, fat, and fascia |
| Necrotizing Fasciitis | ||
| Type I | Mixed aerobic and anaerobic; staphylococci, B. fragilis, E. coli, group A streptococci, Peptostreptococcus spp., Prevotella, Porphyromonas spp., Clostridium spp. | Usually requires a breach in the mucous membrane layer either through surgery or penetrating injuries or from chronic medical conditions such as diabetes, peripheral vascular disease, malignancy, and anal fissures |
| Type II | Group A streptococci | Increasing in frequency and severity since 1985; very high mortality; often begins at site of nonpenetrating minor trauma such as a bruise or muscle strain but often no identified precursor |
| Predisposing factors: blunt/penetrating trauma, varicella (chickenpox), intravenous drug abuse, surgical procedures, childbirth, NSAID use | ||
| Myonecrosis | ||
| Clostridial myonecrosis | Clostridium spp. | Predisposing factors: deep/penetrating injury, bowel and biliary tract surgery, improperly performed abortion and retained placenta, prolonged rupture of the membranes, and intrauterine fetal demise or missed abortion in postpartum patients. Recurrent gas gangrene occurs at sites of previous gas gangrene. |
| Streptococcal myonecrosis | Streptococci | |
| Special Type of Necrotizing Soft-Tissue Infection | ||
| Fournier’s gangrene | Polymicrobial, with E. coli the predominant aerobe and Bacteroides the predominant anaerobe. Other microflora: Proteus, Staphylococcus, Enterococcus, aerobic and anaerobic Streptococcus, Pseudomonas, Klebsiella, and Clostridium | Necrosis of the scrotum or perineum that starts with scrotal pain and erythema and rapidly spreads onto anterior abdominal wall and gluteal muscle. It is more often seen in diabetics and can be associated with trauma. |
Pathogenesis
Host Resistance
As shown in Table 135-2, individuals who are immunocompromised or have chronic diseases are more likely to develop necrotizing skin and soft-tissue infections than those without such medical problems.
TABLE 135-2 Factors Predisposing to Necrotizing Soft-Tissue Infections
Bacterial Pathogens
There are specific bacteria that are more likely than others to cause NSTIs, as shown in Table 135-1. Although necrotizing cellulitis and fasciitis may be caused by a single bacterial pathogen such as group A Streptococcus, Vibrio spp., or zygomycetes, about 80% of necrotizing cellulitis or fasciitis results from polymicrobial infections with synergistic facultative aerobes and anaerobic gas-forming organisms. An average of 4.4 organisms are isolated from polymicrobial necrotizing infections.3 The former includes gram-positive and gram-negative aerobes such as Streptococcus pyogenes, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, or Pseudomonas aeruginosa, and the latter includes Clostridium perfringens, Bacteroides fragilis, and Peptostreptococcus.4 Certain predisposing conditions can be correlated with specific bacteria—for example, trauma with Clostridium spp., diabetes mellitus with Bacteroides spp., S. aureus, and Enterobacteriaceae, and immunosuppression with Pseudomonas spp. and Enterobacteriaceae.5
Clinical Manifestations and Diagnosis
The critical aspect of diagnosing NSTIs is maintaining a high index of suspicion, which allows for early recognition of the nonlocalized necrotizing nature of the infection and the need for surgical intervention. Although necrotizing cellulitis and fasciitis may occur after significant tissue trauma or a relatively trivial injury, up to 40% of NSTIs have no identifiable cause. NSTIs with identifiable barrier failure are more likely to be polymicrobial and are easier to diagnose than the more virulent infections caused by a single organism. In necrotizing cellulitis, gas is invariably found in the skin, but the fascia and deep muscle are spared. Early clinical findings are similar to those of common wound infections, including local edema (89%), erythema (30%), fever (71%), and local cutaneous anesthesia (27%) due to cutaneous nerve necrosis.6 These are followed by gangrenous skin changes with rapid extension beyond the borders of the original infection. Synergistic polymicrobial necrotizing fasciitis is characterized by “dishwater pus.” Patients usually have high fever, but no obvious source of clinical infection can be detected. Pain in the area of infection is usually out of proportion to the physical findings. As the infection progresses, patients develop shock and multiple organ failure. Mortality rates are high, with necrotizing fasciitis being fatal in 23.5% of cases.7
Management
Antibiotics
Although there are no data from clinical trials establishing the benefit of combined therapy in type II necrotizing fasciitis (group A streptococci), penicillin G combined with clindamycin is the antibiotic therapy of choice. Clindamycin, but not metronidazole, is recommended not for its antianaerobic properties but because of its additional activity against gram-positive organisms, including specific inhibition of toxin production.8 Cefotaxime and ceftriaxone are acceptable alternatives. For patients allergic to penicillin, vancomycin is the recommended treatment.
Surgical Intervention
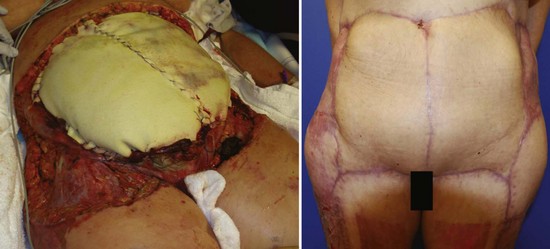
Early surgical débridement is critical in the management of NSTIs. Aggressive surgical excision of all involved tissue with a margin of normal-appearing tissue is mandatory. All necrotic tissue should be excised back to healthy bleeding margins. Additional incisions parallel to cutaneous nerves and blood vessels may be used to assess fascial viability without elevating the skin. The wound should be frequently reexamined for viability of tissue and repeat operative débridement is frequently required. Aggressive fascial débridement of abdominal surgical wounds may necessitate the use of prosthetic material to replace an abdominal wall defect, as depicted in Figure 135-1. In Fournier’s gangrene and perineal/perirectal NSTI, a colostomy for fecal diversion may be necessary to keep the wound clean. The testes generally survive because their blood supply is usually spared, but they may need to be temporarily implanted in the soft tissue of the medial thighs if the scrotum must be débrided. On rare occasion, NSTI of the extremities may require amputation.
Adjunctive Therapy
Hyperbaric Oxygen
The use of hyperbaric oxygen (HBO) in NSTIs is controversial. Although there are no randomized prospective studies of HBO in these infections, in vitro data and reviews of clinical series seem to show beneficial effects of HBO when combined with antibiotics and surgical débridement in the management of clostridial infection.9,10 Hyperbaric oxygen is toxic to clostridia and inhibits bacterial growth, blocks production of alpha toxin, and preserves marginally perfused tissue. Debate also exists about the use of HBO for nonclostridial necrotizing skin and soft-tissue infection. In one report, the addition of HBO to the surgical and antimicrobial treatment of nonclostridial necrotizing fasciitis significantly reduced mortality and the need for débridement.9
Intravenous Immunoglobulin
Intravenous immunoglobulin (IVIG) has been administered to patients with streptococcal and staphylococcal toxic shock syndrome and may be efficacious in the treatment of this toxin-mediated disorder. Some studies have demonstrated IVIG has some beneficial effect in the treatment of NSTIs, theoretically owing to its neutralization of circulating clostridial toxins and streptococcal superantigens.11 However, a large multicenter retrospective cohort study of children with streptococcal toxic shock syndrome showed no improvement in outcomes with administration of IVIG.12 There is no clear consensus at this time regarding the efficacy of IVIG.
 Important Soft-Tissue Infections of the Head and Neck
Important Soft-Tissue Infections of the Head and Neck
Ludwig’s Angina
In 1836, German physician Wilhelm Frederick von Ludwig described five patients with gangrenous induration of the connective tissues of the neck that progressed rapidly to involve the tissues covering the muscles between the larynx and the floor of the mouth.13 Ludwig’s angina is a potentially life-threatening, rapidly progressive, diffuse “woody” or brawny cellulitis of the submandibular and sublingual spaces that occurs most often in young adults with dental infections.
Pathogenesis
In adults, 50% to 80% of cases of Ludwig’s angina are caused by dental caries, and the disease has a mortality rate of 5% to 10%.14 Submandibular and sublingual spaces freely communicate, and with involvement of the deep cervical fascia, infection may spread rapidly, with grave consequences. Extension along the carotid sheath or the retropharyngeal space can cause mediastinitis.15 Infection is commonly caused by oral cavity anaerobes such as Fusobacterium, anaerobic streptococci, Bacteroides, spirochetes, and hemolytic Streptococcus organisms, although the infection may be mixed with Staphylococcus and Streptococcus, Klebsiella, or a combination of aerobic or anaerobic organisms.16 The presence of anaerobes commonly accounts for the occurrence of gas in the tissues.
Clinical Manifestations
The diagnosis of Ludwig’s angina is usually made clinically according to three criteria: (1) presence of cellulitis with little or no pus in both submandibular and sublingual spaces; (2) presence of gangrene with serosanguineous putrid fluid; and (3) rapidly spreading cellulitis in connective tissue, fascia, and muscles, without glandular tissue and lymphatic involvement.17
Management
Control of Airway
Progression from the first findings of symptoms to asphyxia may occur rapidly over several minutes to a few hours. Therefore, airway protection is a critical component of initial management. Stridor, tachypnea, dyspnea, inability to handle secretions, and agitation are all indicative of impending airway loss. In the past, the standard of care for Ludwig’s angina was early emergency intubation or tracheostomy to protect the airway. However, this practice has been gradually abandoned. Recent data show that most cases can be managed initially by close observation in a critical care unit and intravenous antibiotics.18 If an artificial airway is required, flexible fiberoptic-guided nasotracheal intubation is the preferred method of airway control. Tracheostomy, under local anesthesia and performed through the cellulitis, is still the most widely recommended means of obtaining a surgical airway.
Antibiotics and Other Pharmacotherapy
Corticosteroids have been used empirically to treat airway edema. The value of corticosteroids in the setting of Ludwig’s angina is unclear, and they probably are not indicated.19
Surgical Intervention
Surgical débridement may only moderately improve the airway. Surgical incision and drainage was the therapy of choice in the preantibiotic era. Unless antibiotic therapy is significantly delayed, it is unlikely pus will be identified, because pus collections develop relatively late. With the exception of dental extraction, surgery is reserved for those patients who do not respond to medical therapy and those with crepitus and purulent collections.20 Any patient requiring surgical intervention should have an artificial airway in place before neck exploration. The location of abscesses should be identified using CT or magnetic resonance imaging (MRI). Infection localized above the carina is usually addressed by cervical incision, but infection below the carina requires additional surgical drainage of the mediastinum.21
Acute Epiglottitis
Pathogenesis
Invading bacteria cause inflammation and edema of the epiglottis, aryepiglottic fold, and surrounding tissues. These structures then may protrude downward and over the glottic opening, causing airway obstruction. In the past, most of the cases (50%-70%) were caused by H. influenzae B (HIB).22 However, at the present time, other bacteria including group A β-hemolytic Streptococcus, S. aureus, and Streptococcus pneumoniae have become more common, and more patients present with epiglottic abscess.
Clinical Manifestations and Management
Early signs of epiglottitis include hoarseness, dysphagia, odynophagia, and a sore throat (present in 94% of patients).23 Some authors advocate direct or indirect laryngoscopy on adult patients without respiratory distress; it is safe to perform such procedures in the operating room or ICU, where both the equipment and personnel required for emergency intubation are at hand. The most common misdiagnosis is streptococcal pharyngitis. Patients who can maintain their airway and adequate oxygenation should be closely observed in an ICU where definitive airway management can be achieved in a controlled fashion. Corticosteroids, racemic epinephrine, and heliox can be considered for initial management, but their role is unresolved. Dyspnea and stridor indicate impending airway obstruction, and emergency airway control should be established. Flexible fiberoptic laryngoscopy is usually used during intubation because it provides direct visualization of the airway while serving as a guide for intubation.
 Infections of Bite Wounds
Infections of Bite Wounds
Pathogenesis
Soft-tissue infections caused by human mouth flora are usually due to a mixture of pathogens.24 It has been reported that the human mouth hosts 42 different species of bacterial flora, of which aerobes (Eikenella corrodens, Staphylococcus, Streptococcus, and Corynebacterium spp.) are the most common isolates from infected bite wounds.25 E. corrodens is a slow-growing, gram-negative bacillus frequently associated with chronic infection and abscess formation in human bites. Commonly isolated anaerobes include Bacteroides and Peptostreptococcus spp.
As in human bites, polymicrobial infections are frequently encountered in animal bites. Whereas almost any oral flora isolate is a potential pathogen, Pasteurella multocida is the most prevalent organism found in 50% of dog bite wounds and 70% of cat bite wound infections.26,27 S. aureus, α-, β-, and δ-hemolytic streptococci, gram-negative organisms, and anaerobic microorganisms that are usually part of the normal mouth flora of animals also have all been isolated.
Management
Cultures of clinically uninfected wounds are not indicated. However, it is recommended that cultures be performed in infected wounds that are not improving despite apparently adequate antibiotic treatment. In bite victims, prophylactic broad-spectrum antibiotics are recommended for patients with high-risk bites but are only of proven benefit in human bites.28 The high-risk factors for infection include human bites, wounds of the hand, foot, face, scalp, and perineum, puncture wounds, crush wounds that cannot be débrided, bites over vital structures (artery, nerve, or joint), patient age older than 50 years, or patients who are immunosuppressed.29 In most patients, amoxicillin-clavulanic acid is the preferred antibiotic. Alternatives include moxifloxacin, amoxicillin, doxycycline, and cefuroxime. In human bites, amoxicillin-clavulanic acid will cover E. corrodens as well as most other oral flora and is the recommended antibiotic. Other options include second- or third-generation cephalosporins, quinolones, or doxycycline. In patients who are allergic to penicillin, trimethoprim-sulfamethoxazole is an alternative for both dog and cat bites, whereas quinolones or erythromycin may be used for human bites.
Patients who require inpatient care for complex wounds, systemic toxicity, established infection, or suspicion of musculoskeletal, neurologic, or vascular involvement, or patients who are at very high risk of invasive infection (e.g., immunosuppression), should be treated with parenteral antibiotics, irrigation, and débridement with cultures.30 Consultation with a hand surgeon should be considered in those with hand wounds, because the risk of severe infection of bite wounds on a hand is higher than other sites.
 Infections of Burn Wounds
Infections of Burn Wounds
Burn wound infection/sepsis is one of the most common causes of death in burn patients. It is estimated that more than 100,000 of the 2.5 million burned patients in the United States require hospital admission, and 12,000 patients die per year.31 The highest risk of bacterial invasion from skin flora into the eschar occurs 5 to 7 days after burn. Mechanisms of burn wound infection include breakdown of the natural cutaneous barrier, compromised host defenses, and exposure to pathogenic and opportunistic bacteria. The surface of a burn contains a large amount of necrotic tissue and protein-rich wound exudate, so it provides an excellent growth medium for surface bacteria, leading to bacterial colonization and invasion. Burns are also associated with an immunocompromised status. The percentage of total body surface area (TBSA) burned and the duration of hospitalization correlate well with the incidence of wound infections.32 The predisposing factors for development of burn wound infection are listed in Table 135-3.
Pathogenesis
The most common organisms found in burn wound infections are bacteria, and 70% to 90% are endogenous to the patient. Bacterial organisms can also be acquired by cross-infection, principally from the hands of healthcare professionals. Before the era of penicillin, streptococci and staphylococci were the predominant pathogens. Since the 1950s, P. aeruginosa has become the most important species.33 Other important bacterial species include S. aureus, group A Streptococcus, Enterobacter cloacae, E. faecalis, Klebsiella spp., and Acinetobacter spp.34 Fungi, especially Candida albicans and Aspergillus spp., and viruses (herpesvirus) are also pathogens that can be isolated from infected burn wounds.35
Clinical Manifestations and Diagnosis
Successful treatment of burn wound infections largely depends on early detection of infection. Burn wound infection is difficult to diagnose on the basis of clinical signs and symptoms, because burn-induced inflammatory responses (e.g., fever, leukocytosis) are indistinguishable from those of infection. The local signs of infection may be absent, minimal, or late. Diagnosis is generally based on a combination of clinical signs that indicate sepsis (e.g., fever, leukocytosis, organ dysfunction, hyperdynamic state) and the results of surveillance cultures. Any of the findings listed in Table 135-4 should raise suspicion of burn wound infection.36 The practice of culturing the burn wound surface does not accurately predict progressive bacterial colonization or incipient burn wound sepsis. Qualitative and quantitative correlations are poor between flora on the surface of the burn wound and bacterial colonization and invasion of the deep layers of the eschar. It has been reported that biopsy of the wound with quantitative cultures of greater than 105 CFU per gram of tissue is an accurate indicator of invasive burn would infection.37 When bacterial invasion to viable tissue is detected, excision of the infected wound is important, and systemic antibiotics are indicated.
TABLE 135-4 Clinical Signs Suggestive of Burn Wound Infection
Management
Prevention of Burn Wound Infections
Systemic antibiotic prophylaxis is not routinely administered to burn patients admitted to the hospital, because the unexcised burn wound does not lead to significant bacteremia.38 Frequent wound dressing changes with evaluation of the burn wound and surrounding tissue allow for early detection and therapy of cellulitis. In many burn units in the United States, early excision and grafting of burn wounds has become the standard of care. Early excision is defined as the staged excision of all deep partial- and full-thickness burns by the third to seventh postburn day. The philosophy of early burn wound excision has resulted in improved survival in patients with TBSA burns greater than 30% to 40%, shorter hospital length of stay, lower costs of hospital care, and fewer painful dressing changes. If for some reason such as hemodynamic instability or severe respiratory failure, the patient cannot undergo early excision and coverage, surveillance wound cultures should be performed several times per week to diagnose burn wound infection early. In addition, strict antiseptic measures such as handwashing, barrier isolation, and equipment and room cleaning decrease the incidence of wound infection.
Topical antimicrobials are commonly used in burn patients. Their use has substantially decreased the incidence of conversion of partial-thickness to full-thickness wounds by local infection, and thereby has reduced mortality associated with burn wound infection. In addition, these agents may prolong the sterility of the full-thickness burn wound. However, they have not eliminated the need for aggressive removal of necrotic tissue and closure of the wound with autografts. The commonly used topical agents are listed in Table 135-5. According to an international survey, silver sulfadiazine is the topical agent of choice for partial- to full-thickness burn wounds.39 Nanocrystalline silver mesh dressings that adhere for a week have reduced the discomfort of dressing changes and allow more outpatient care of partial-thickness burn wounds.40
Treatment of Burn Wound Infections
Antibiotics
Systemic antibiotics are not used prophylactically in patients with burn wounds.41 Instead, they should be reserved for use in cases of known or suspected invasive infection. As long as bacterial culture results are available, antibiotics with the narrowest spectrum of activity should be used to minimize the development of resistant organisms. Recommendations for empirical therapy are based on the length of time since the burn was sustained, previous administration of antibiotics to the patient, and knowledge of likely pathogens and the local antibiogram. Combination of multiple antibiotics for a single infection is only used when bacteremia persists in the face of therapeutic doses of a single antibiotic. Inappropriate use of multiple antibiotics does not decrease mortality. Instead, it promotes overgrowth of resistant pathogens such as Candida spp., enterococci, and multiple antibiotic-resistant species.
Surgical Intervention
Invasive bacterial or fungal burn wound infections are treated with surgical excision to the level of viable tissue. Early burn wound excision significantly reduces bacterial colonization and reduces the risk of invasive burn wound infection. Patients who undergo topical treatment and delayed burn wound excision exhibit greater bacterial colonization and increased rates of infection.42 Wounds that can be excised completely should be covered with an allograft or autograft. If complete débridement is not possible, topical antimicrobials should be applied and the wound reexamined within 24 hours for possible repeat débridement.
 Infections of Pressure Ulcers
Infections of Pressure Ulcers
Pressure ulcers are caused by localized tissue necrosis and infection due to prolonged compression between a bony prominence and an external surface. Pressure ulcers in ICU patients occur primarily in patients with impaired mobility due to injury, weakness, sedation, or use of paralytic agents. Pressure ulcers result in significant morbidity in critically ill patients. Although infection of decubitus ulcers is high in the nursing home setting and in spinal cord injury patients, it is an uncommon cause of infection or sepsis in ICU patients.43 Pressure ulcers may pose a risk to other hospitalized patients by serving as a reservoir for resistant organisms such as MRSA, vancomycin-resistant enterococci, and multiply-resistant gram-negative bacilli.
Pathogenesis and Classification
Risk factors for pressure ulcers in patients in ICUs are essentially the same as for those on a general hospital floor. They include limited physical activity, impaired sensory perception, poor nutritional status, chronic disorders (e.g., diabetes mellitus, cardiovascular disease, and cerebrovascular accident), impaired circulation, low serum hemoglobin concentration, and increased blood urea nitrogen and serum creatinine concentrations.44 Also, a number of infectious complications have been implicated in the development of pressure ulcers. In order of frequency, these are local infection, cellulitis of surrounding tissue, contiguous osteomyelitis, and bacteremia.45
Several different classification systems have been developed to describe the extent of pressure ulcers. Table 135-6 shows the most commonly used system promulgated by the National Pressure Ulcer Advisory Panel.46
TABLE 135-6 National Pressure Ulcer Advisory Panel Classification of Pressure Ulcers
| Staging | Description |
|---|---|
| I | Intact skin with non-blanchable redness of a localized area usually over a bony prominence. Darkly pigmented skin may not have visible blanching; its color may differ from the surrounding area. The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue. |
| II | Partial-thickness loss of dermis presenting as a shallow open ulcer with a red pink wound bed, without slough. May also present as an intact or open/ruptured serum-filled blister. |
| III | Full-thickness skin loss. Subcutaneous fat may be visible, but bone, tendon, or muscle are not exposed. Slough may be present but does not obscure the depth of tissue loss. May include undermining and tunneling. |
| IV | Full-thickness tissue loss with exposed bone, tendon, or muscle. Slough or eschar may be present on some parts of the wound bed. Often include undermining and tunneling. |
Management
There are many different approaches to the treatment of pressure ulcers; however, none has been shown to be more effective than any other. Prevention of decubitus ulcers, including pressure relief with support surfaces and repositioning, appropriate nutrition, and skin moisturizers, is the best prophylactic treatment.47 Once the ulcer has been established and infection is present, débridement of necrotic and marginally viable tissue is absolutely necessary to obtain healing. Topical agents such as povidone-iodine, hydrogen peroxide, and others have been widely used, but there is no difference in terms of outcome among these agents. Proper use of occlusive dressings such as balsam Peru/trypsin/castor oil preparations increases patient comfort, enhances healing, decreases the possibility of infection, saves time, and reduces costs.48 Topical antimicrobial agents have not been shown to be effective. Systemic antibiotic therapy should be reserved for infected ulcers. Skin grafting of clean wounds, if the underlying cause of the pressure ulcer has been removed, is an accepted method of treatment and has been shown to be effective. However, adequate treatment frequently requires much more complex therapies, including tissue flaps and sometimes even amputation to effect wound closure. Treatment of recalcitrant wounds can be difficult and costly. Several newer therapeutic strategies include alginates, a variety of wound dressings, and growth factor therapies (e.g. platelet-derived growth factor). Cultured and tissue-engineered skin substitutes have emerged and are in varying degrees of clinical evaluation.49
Mechanical therapies aimed at healing decubitus ulcers include removal of all necrotic and undermined tissues and some strategy to relieve the pressure that caused the ulcer.50 Once dead tissue has been débrided, the ulcer may be covered with a negative-pressure wound therapy sponge, a moist dressing, or an engineered skin substitute. Studies have shown that these dressings are cost-effective in treating chronic wounds.51 However, before such local therapy is chosen, it is very important that infection be controlled and that the patient is in good nutritional balance.
Key Points
Brook I. Management of human and animal bite wound infection: an overview. Curr Infect Dis Rep. 2009;11:389-395.
Cumming J, Purdue GF, Hunt JL, et al. Objective estimates of the incidence and consequences of multiple organ dysfunction and sepsis after burn trauma. J Trauma. 2001;50:510-515.
Elliot D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg. 2000;179:361-366.
Gibbs S, van den Hoogenband HM, Kirtschig G, et al. Autologous full-thickness skin substitute for healing chronic wounds. Br J Dermatol. 2006;155:267-274.
May AK, Stafford RE, Bulger EM, et al. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt). 2009;10:467-499.
Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296:974-984.
Shah SS, Hall M, Srivastava R, et al. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis. 2009;49:1369-1376.
Wang C, Schwaitzberg S, Berliner E, et al. Hyperbaric oxygen for treating wounds: a systematic review of the literature. Arch Surg. 2003;138:272-279.
1 Pryor JP, Piotrowski E, Seltzer CW, Gracias VH. Early diagnosis of retroperitoneal necrotizing fasciitis. Crit Care Med. 2001 May;29(5):1071-1073.
2 Takakura Y, Ikeda S, Yoshimitsu M, et al. Retroperitoneal abscess complicated with necrotizing fasciitis of the thigh in a patient with sigmoid colon cancer. World J Surg Oncol. 2009;7:74.
3 Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections. Risk factors for mortality and strategies for management. Ann Surg. 1996;224:672-683.
4 Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244-269.
5 Brook I, Frazier EH. Clinical and microbiological features of necrotizing fasciitis. J Clin Microbiol. 1995;33:2382.
6 Chapnick EK, Abter EI. Infectious disease emergencies: Necrotizing soft-tissue infections. Infect Dis Clin North Am. 1996;10:835-855.
7 May AK, Stafford RE, Bulger EM, et al. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt). 2009;10:467-499.
8 Elliot D, Kufera JA, Myers RA. The microbiology of necrotizing soft tissue infections. Am J Surg. 2000;179:361-366.
9 Riseman JA, Zamboni WA, Curtis A, et al. Hyperbaric oxygen therapy for necrotizing fasciitis reduces mortality and the need for débridements. Surgery. 1990;108:847-850.
10 Wang C, Schwaitzberg S, Berliner E, et al. Hyperbaric oxygen for treating wounds: A systematic review of the literature. Arch Surg. 2003;138:272-279.
11 Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. Clin Infect Dis. 1999;28:800-807.
12 Shah SS, Hall M, Srivastava R, et al. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis. 2009;49:1369-1376.
13 Von Ludwig WF. Über eine in neuerer Zeit wiederholt hier vorgekommene Form von Halsentzündung. Medicinisches Correspondenzblatt des Württembergischen ärztlichen Vereins, Stuttgart, 6: 21-5, 1836. English translation and biographical note in Bull History Med. 1939;7:1115-1126.
14 Bansal A, Miskoff J, Lis RJ. Otolaryngologic critical care. Crit Care Clin. 2003;19:55-72.
15 Kinzer S, Pfeiffer J, Becker S, Ridder GJ. Severe deep neck space infections and mediastinitis of odontogenic origin: clinical relevance and implications for diagnosis and treatment. Acta Otolaryngol. 2009 Jan;129(1):62-70.
16 Parhiscar A, Har-El G. Deep neck abscess: a retrospective review of 210 cases. Ann Otol Rhinol Laryngol. 2001 Nov;110(11):1051-1054.
17 Spitalnic SJ, Sucov A. Ludwig’s angina: Case report and review. J Emerg Med. 1995;13:499.
18 Huang TT, Liu TC, Chen PR, et al. Deep neck infection: analysis of 185 cases. Head Neck. 2004 Oct;26(10):854-860.
19 Freund B, Timon C. Ludwig’s angina: A place for steroid therapy in its management? Oral Health. 1992;82:23.
20 Osborn TM, Assael LA, Bell RB. Deep space neck infection: principles of surgical management. Oral Maxillofac Surg Clin North Am. 2008;20:353-365.
21 Chen KC, Chen JS, Kuo SW, et al. Descending necrotizing mediastinitis: A 10-year surgical experience in a single institution. J Thorac Cardiovasc Surg. 2008;136:191-198.
22 Wurtele P. Acute epiglottitis in children: Results of a large scale anti-Haemophilus type b immunization program. J Otolaryngol. 1995;24:92-97.
23 Ng HL, Sin LM, Li MF, et al. Acute epiglottitis in adults: a retrospective review of 106 patients in Hong Kong. Emerg Med J. 2008;25:253-255.
24 Brook I. Management of human and animal bite wound infection: an overview. Curr Infect Dis Rep. 2009;11:389-395.
25 McDonough JJ, Stern PJ, Alexander JW. Management of animal and human bites and resulting human infections. In: Remington JS, Swartz MN, editors. Current clinical topics in infectious diseases. New York: McGraw-Hill; 1987:11.
26 Talan DA, Citron DM, Abrahamian FM, et al. Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340:85-92.
27 Westling K, Farra A, Cars B, et al. Cat bite wound infections: a prospective clinical and microbiological study at three emergency wards in Stockholm, Sweden. J Infect. 2006;53:403-407.
28 Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plast Reconstr Surg. 1991;88:111-114.
29 Callaham M, French SP, Tetlow P, et al. Bites and injuries inflicted by mammals. In: Auerbach PS, editor. Wilderness medicine: management of wilderness and environmental emergency. 3rd ed. St. Louis: Mosby–Year Book; 1995:943-1009.
30 Weinzweig N, Gonzalez M. Surgical infections of the hand and upper extremity: A county hospital experience. Ann Plast Surg. 2002;49:621-627.
31 American Burn Association. Burn incidence and treatment in the U.S. National health interview survey (1991-1993 data). Philadelphia, Pa: American Burn Association; 2000.
32 Cumming J, Purdue GF, Hunt JL, et al. Objective estimates of the incidence and consequences of multiple organ dysfunction and sepsis after burn trauma. J Trauma. 2001;50:510-515.
33 Keen EF, Robinson BJ, Hospenthal DR, et al. Incidence and bacteriology of burn infections at a military burn center. Burns. 2010;36:461-468. Epub 2009 Dec 31
34 Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
35 Murray CK, Loo FL, Hospenthal DR, et al. Incidence of systemic fungal infection and related mortality following severe burns. Burns. 2008;34:1108-1112.
36 Greenhalgh DG, Saffle JR, Holmes JH4th, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776-790.
37 Sjöberg T, Mzezewa S, Jönsson K, Robertson V, Salemark L. Comparison of surface swab cultures and quantitative tissue biopsy cultures to predict sepsis in burn patients: a prospective study. J Burn Care Rehabil. 2003;24:365-370.
38 Mozingo DW, McManus AT, Kim SH, et al. Incidence of bacteremia after burn wound manipulation in the early postburn period. J Trauma. 1997;42:1006-1010.
39 Hermans MH. Results of an internet survey on the treatment of partial thickness burns, full thickness burns, and donor sites. J Burn Care Res. 2007 Nov-Dec;28(6):835-847.
40 Heggers J, Goodheart RE, Washington J, et al. Therapeutic efficacy of three silver dressings in an infected animal model. J Burn Care Rehabil. 2005;26:53-56.
41 Avni T, Levcovich A, Ad-El DD, et al. Prophylactic antibiotics for burns patients: systematic review and meta-analysis. BMJ. 2010;340:c241.
42 Barret JP, Herndon DN. Effects of burn wound excision on bacterial colonization and invasion. Plast Reconstr Surg. 2003;111:744-750.
43 Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35:1390-1396.
44 Keller BP, Wille J, van Ramshorst B, et al. Pressure ulcers in intensive care patients: A review of risks and prevention. Intensive Care Med. 2002;28:1379-1388.
45 Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35:1390-1396.
46 Black J, Baharestani MM, Cuddigan J, et al. National Pressure Ulcer Advisory Panel’s updated pressure ulcer staging system. Adv Skin Wound Care. 2007 May;20(5):269-274.
47 Reddy M, Gill SS, Rochon PA. Preventing Pressure Ulcers: A Systematic Review. JAMA. 2006;296:974-984.
48 Abraham LM. Xenaderm: an essential wound care therapy. Adv Skin Wound Care. 2010;23:73-76.
49 Gibbs S, van den Hoogenband HM, Kirtschig G, Richters CD, Spiekstra SW, Breetveld M, et al. Autologous full-thickness skin substitute for healing chronic wounds. Br J Dermatol. 2006 Aug;155(2):267-274.
50 Schiffman J, Golinko MS, Yan A, et al. Operative debridement of pressure ulcers. World J Surg. 2009;33:1396-1402.
51 Langer A, Rogowski W. Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC Health Serv Res. 2009;10(9):115.