137 Infections in the Immunocompromised Patient
Many immunocompromised patients are managed in intensive care units (ICUs) every year, with infection being a leading cause of ICU admission. Common examples of such infections include community-acquired pneumonia, bacteremia, and central nervous system (CNS) infections. The incidence of infections acquired by immunocompromised patients during ICU admissions is also significant.1 Mortality for certain infections in immunocompromised patients exceeds 50%.2 Early diagnosis, initiation of appropriate antimicrobial and supportive therapy, and reduction in immunosuppression where possible can improve outcome significantly.
 Commonly Encountered Immunocompromising Conditions
Commonly Encountered Immunocompromising Conditions
Immunocompromise can be broadly defined as a state in which the response of the host to a foreign antigen is subnormal. Immunocompromise could be congenital (primary) or acquired. Congenital immunodeficiencies are now much less common than acquired immunodeficiencies. In general, congenital immunodeficiency is observed more frequently in patients in pediatric ICUs than in adult ICUs. Patients with congenital immunodeficiencies usually have repeated infections, especially infections affecting the sinuses and lower respiratory tract. Congenital immunodeficiencies are usually “pure” in that the defects in host response to foreign antigens are usually specific and well defined. For example, Bruton’s X-linked agammaglobulinemia is associated with a defect in the normal maturation process of immunoglobulin-producing B cells. As a result, mature circulating B cells, plasma cells, and serum immunoglobulin are absent. The patient is susceptible to organisms normally dealt with by immunoglobulin, such as Streptococcus pneumoniae and Haemophilus influenzae. Other congenital immunodeficiency syndromes are listed in Table 137-1.
| Condition (Immunodeficiency) | Organisms with Increased Tendency to Cause Infection in This Condition |
|---|---|
| T-lymphocyte Deficiencies | |
| DiGeorge syndrome (thymic aplasia with reduced CD4 and CD3 cells) | Viruses (especially HSV and measles), sometimes Pneumocystis jirovecii, fungi, or gram-negative bacteria |
| Purine nucleoside phosphorylase deficiency (marked T-cell depletion) | P. jirovecii and viruses |
| B-lymphocyte Deficiencies | |
| Bruton’s X-linked agammaglobulinemia (absence of B cells, plasma cells, and antibody) | Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, P. jirovecii (after first 4-6 months of life when maternal antibody has been consumed) |
| Selective IgG subclass deficiencies | Variable |
| Selective IgA deficiency | S. pneumoniae, H. influenzae |
| Hyper-IgM immunodeficiency (elevated IgM but reduced IgG and IgA) | S. pneumoniae, H. influenzae, P. jirovecii (rarely) |
| Mixed T- and B-lymphocyte Deficiencies | |
| Common variable immunodeficiency (leads to various B-cell activation or differentiation defects and gradual deterioration of T-cell number and function) | S. pneumoniae, H. influenzae, CMV, VZV, P. jirovecii |
| Severe combined immunodeficiency (severe reduction in IgG and absence of T cells) | P. jirovecii, viruses, Legionella |
| Wiskott-Aldrich syndrome (decreased T-cell number and function, low IgM, occasionally low IgG) | S. pneumoniae, H. influenzae, HSV, P. jirovecii |
| Ataxia-telangiectasia (decreased T-cell number and function; IgA, IgE, IgG2, and IgG4 deficiency) | S. aureus, S. pneumoniae, H. influenzae |
| Disorders of Complement | |
| C3 deficiency (congenital absence of C3 or consumption of C3 due to deficiency of C3b inactivator) | S. pneumoniae, H. influenzae, enteric gram-negative bacilli |
| Phagocyte Defects | |
| Chronic granulomatous disease (defect in NADPH oxidase in phagocytic cells) | S. aureus, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, S. marcescens, P. aeruginosa, Aspergillus |
| Chédiak-Higashi syndrome (impaired microbicidal activity of phagocytes) | S. aureus, H. influenzae, Aspergillus |
| Kostmann syndrome, Shwachman-Diamond syndrome, cyclic neutropenia (low neutrophil count) | S. aureus, enteric gram-negative bacilli, P. aeruginosa |
CMV, Cytomegalovirus; HSV, herpes simplex virus; Ig, immunoglobulin; NADPH, nicotinamide adenine dinucleotide phosphate; VZV, varicella-zoster virus.
Hematologic Malignancies and Solid Tumors
Prolonged neutropenia from chemotherapy has a significant risk of bacterial and fungal infection. Classically, gram-negative organisms such as Pseudomonas aeruginosa and fungal organisms such as Aspergillus species have been associated with severe neutropenia. It has long been known that the severity and duration of neutropenia influence the risk of infection.3 It also has been well established that aggressive chemotherapy and radiotherapy for Hodgkin’s disease coupled with splenectomy significantly impairs humoral defense against encapsulated organisms such as S. pneumoniae, H. influenzae, and Neisseria meningitidis.4 Transplantation is associated with a risk of graft-versus-host disease (GVHD). Prophylaxis and treatment for GVHD may involve use of drugs such as cyclosporine or tacrolimus plus corticosteroids. Cyclosporine and tacrolimus inhibit calcineurin, an enzyme important in the lymphocyte activation cascade. Corticosteroids also affect lymphocyte function and depress functions of activated macrophages. As a result, patients receiving therapy for GVHD may be prone to fungal, viral, and mycobacterial infections.
Solid-Organ Transplantation
Solid-organ transplant recipients are uniquely susceptible to infection.5 They undergo significant surgery, breaching the defenses provided by the skin. They remain in ICUs for prolonged periods, requiring intravenous access and mechanical ventilation—here, cutaneous and pulmonary barriers to infection are breached. Finally, solid-organ transplant recipients receive immunosuppressive therapy to prevent graft rejection. Commonly used immunosuppressive medications are listed in Table 137-2. Immunosuppressive regimens are in a constant state of flux—more recent trends have been toward aggressive “pretreatment” immediately before transplantation, coupled with decreased immunosuppression in the posttransplant period.6
TABLE 137-2 Immunosuppressive Drugs Used in Solid-Organ Transplantation and Their Mechanisms of Activity
| Immunosuppressive | Mode of Action |
|---|---|
| Corticosteroids | Negative regulation of cytokine gene expression |
| Azathioprine | Inhibits DNA and RNA synthesis; inhibits T- and B-cell function |
| Cyclosporine | Calcineurin inhibitor; inhibits cytokine expression |
| Tacrolimus | Calcineurin inhibitor; inhibits cytokine expression |
| Sirolimus (rapamycin) | Prevents translation of mRNAs encoding cell cycle regulators |
| Mycophenolate mofetil | Blocks purine biosynthesis; inhibits T- and B-cell proliferation |
| Polyclonal antilymphocyte | Lymphocyte depletion antibodies (e.g., Atgam, Thymoglobulin) |
| Muromonab-CD3 (OKT3) | Anti-CD3 monoclonal antibody |
| Alemtuzumab (Campath) | Anti-CD52 monoclonal antibody |
| Daclizumab, basiliximab | Anti-CD25 monoclonal antibody |
In the early posttransplant period, transplant recipients are susceptible to nosocomially acquired bacterial infections such as pneumonia, catheter-related bloodstream infection associated with general ICU care, and wound and intraabdominal infections associated with surgical procedures. Opportunistic infections may be acquired from the organ graft; cytomegalovirus (CMV) is the most pertinent example,7 but a wide variety of infections (e.g., rabies, histoplasmosis, tuberculosis, West Nile virus) have been acquired from grafts. Solid-organ transplant recipients, by virtue of their iatrogenic immunosuppression, also are susceptible to reactivation of latent infection (e.g., CMV infection, tuberculosis, histoplasmosis) or to infections acquired through the hospital environment (e.g., aspergillosis, legionellosis, tuberculosis).
Rheumatoid Arthritis and Autoimmune Disorders
Therapy for rheumatoid arthritis and other autoimmune disorders may be with simple analgesics or nonsteroidal antiinflammatory drugs (NSAIDs). Drugs with the potential to cause significant immunocompromise are also frequently used. Classically, therapy has been with corticosteroids or disease-modifying antirheumatic drugs such as azathioprine, cyclosporine, penicillamine, gold salts, hydroxychloroquine, leflunomide, methotrexate, or sulfasalazine. The effects of corticosteroids, azathioprine, and cyclosporine on host defenses have been noted previously (see Table 137-2). Methotrexate reversibly inhibits dihydrofolate reductase and interferes with DNA synthesis, repair, and cellular replication. In addition to its use in rheumatoid arthritis, it also can be used as an antineoplastic agent. Methotrexate can cause significant neutropenia. Low-dose methotrexate is generally less likely to increase infection risk in patients with rheumatoid arthritis.8,9
A variety of anticytokine agents have become available for rheumatoid arthritis (Table 137-3). Use of these drugs also has been reported in treatment of Behçet’s disease, Crohn’s disease, GVHD, hairy cell leukemia, psoriasis, pyoderma gangrenosum, sarcoidosis, and ulcerative colitis. Considerable attention has been paid to the possibility of tuberculosis developing after treatment with such agents.10 The risk is sufficiently high that it is recommended that tuberculin skin testing or interferon gamma (IFN-γ) release assays be performed to detect latent tuberculosis before the initiation of anticytokine agents. Invasive infections with Histoplasma, Candida, Pneumocystis jirovecii, Aspergillus, Cryptococcus, Nocardia, Salmonella, Listeria, Brucella, Bartonella, nontuberculous mycobacteria, Leishmania, and Toxoplasma have also been reported associated with the use of these medications.11–14 As is the case with transplant-associated immunocompromise, these infections may be reactivation of latent infection or new acquisition of organisms through environmental exposure.
TABLE 137-3 Commonly Used Anticytokines for Management of Rheumatoid Arthritis
| Drug | Mechanism of Action | FDA-Approved Indications |
|---|---|---|
| Adalimumab (Humira) | Recombinant, fully human anti-TNF monoclonal antibody |
FDA, U.S. Food and Drug Administration; IL-6, interleukin 6; TNF, tumor necrosis factor.
Human Immunodeficiency Virus Infection
HIV infection remains a relatively common infection, but acquired immunodeficiency syndrome (AIDS) has become less frequently encountered in ICUs since the advent of highly active antiretroviral therapy. A decline in CD4 counts creates a predisposition to P. jirovecii pneumonia, mycobacterial infection, fungal infection (e.g., cryptococcal meningitis), and viral infection (e.g., CMV infection). Many patients with HIV infection are co-infected with hepatitis C virus, and as a result, liver failure is now a relatively common reason for ICU admission in HIV-infected patients. In some centers, liver transplantation is performed in HIV-infected patients with hepatitis virus–induced liver diseases.15,16
 General Diagnostic Approach to Immunocompromised Patients with Severe Infections
General Diagnostic Approach to Immunocompromised Patients with Severe Infections
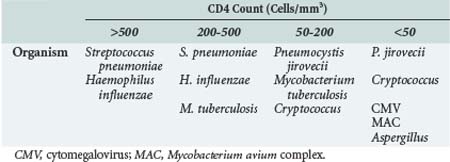
Immunocompromised patients are a heterogeneous group. The infections commonly encountered by a patient with neutropenia as a consequence of chemotherapy may be different from infections observed in a patient with rheumatoid arthritis who is receiving infliximab. Even within a particular category, different renal transplantation recipients, for example, may have a different degree of immunocompromise and a different susceptibility to infection. In solid-organ transplant recipients, the “net state of immunosuppression” (i.e., the cumulative burden of immunosuppression with a special weighting toward recent T-cell ablative therapy) influences the risk of infection. A renal transplant recipient who is receiving tacrolimus monotherapy twice per week would be less susceptible to opportunistic infection than a patient with recent acute cellular rejection treated with OKT3 or alemtuzumab. There have been more recent attempts to quantify immune function in solid-organ transplant recipients,17 although it has not yet been definitively proved that such tests predict infection risk. In contrast, with HIV infection, CD4 lymphocyte count and HIV RNA quantification (“viral load”) predict risk of infection.18 Patients with CD4 counts greater than 500 are unlikely to be infected with an opportunistic pathogen. Patients with CD4 counts of 200 to 500 may be infected with organisms such as Mycobacterium tuberculosis, but they are unlikely to be infected with opportunistic pathogens such as CMV or Mycobacterium avium complex. Patients with CD4 counts less than 200 have an increased risk of a wide variety of opportunistic infections.
Specific environmental exposures may be potentially important for immunocompromised patients. A travel history to the deserts of the southwestern United States and northern Mexico may increase the likelihood that an immunocompromised patient has coccidioidomycosis.19 Histoplasmosis is endemic in the Ohio River valley.20 Alternatively, there may be environmental risks within the ICU. Outbreaks of invasive pulmonary aspergillosis have been linked to construction activity within the hospital. Outbreaks of legionellosis may be waterborne.21 It is possible that many fungal and bacterial infections may also be waterborne.22,23 Tuberculosis transmission has been well described in ICUs caring for transplant recipients or HIV-infected patients.24 The net state of immunosuppression must be considered in the context of recent environmental exposures.
The potential for multiple diagnoses underscores the need for early invasive testing in immunocompromised patients with severe infection. Patients with unexplained severe community-acquired pneumonia may be best managed by early bronchoalveolar lavage performed before antimicrobial therapy has commenced. Bronchoalveolar lavage could be sent for Gram stain, Ziehl-Neelsen stain, modified acid-fast stain, calcofluor stain, direct fluorescent antibody tests, polymerase chain reaction (PCR), and cytologic analysis to enable rapid diagnosis of infection with bacteria, mycobacteria, Nocardia, fungi, Legionella, CMV, community-acquired respiratory viruses, and P. jirovecii. The bronchoalveolar lavage should be inoculated onto solid media, and molecular diagnostic testing should be used as appropriate. An outline of the diagnostic approach in immunocompromised patients is given in Box 137-1.
Box 137-1
Diagnostic Approach for Severe Infections in Immunocompromised Patients
History Taking and Review of Prior Records
 Major Manifestations of Infection in Immunocompromised Patients
Major Manifestations of Infection in Immunocompromised Patients
Pulmonary Infection
Infectious microorganisms usually gain access to the respiratory tract through inhalation, although hematogenous spread sometimes may occur. Mechanical defenses remove the bulk of potentially harmful agents from the lungs (Table 137-4). Inhaled particles greater than 10 µm in diameter usually become trapped in the upper airways or are removed by coughing or mucociliary clearance. Most bacteria range from 0.5 to 2 µm in size and are able to reach the terminal airways/alveoli and potentially cause infection. In the alveoli, the alveolar macrophages are the first line of defense. Subsequently an inflammatory response consisting of polymorphonuclear neutrophils is important. Finally, specific T-cell and B-cell immune responses are essential for successful defense against many pathogens.
TABLE 137-4 Host Defenses Against Respiratory Infections and How They Are Affected in Immunocompromised Patients
| Location | Host Defense | Defect |
|---|---|---|
| Upper airway | Filtration | Endotracheal intubation |
| Mucociliary apparatus | CF, cigarette smoking | |
| Cough | Impaired consciousness | |
| Lower airway (nonspecific) | Alveolar macrophages | Immunosuppressive medication, corticosteroids |
| Polymorphonuclear leukocytes | Corticosteroids, malnutrition, chemotherapy, malignancies | |
| Lower airway (specific) | B lymphocytes | Hypogammaglobulinemia, CLL, MM |
| T lymphocytes | AIDS, malignancies, immunosuppressants |
AIDS, acquired immunodeficiency syndrome; CLL, chronic lymphocytic leukemia; CF, cystic fibrosis; MM, multiple myeloma.
As noted earlier, although it may be possible to pinpoint a major immunologic deficiency, most immunocompromised individuals have an assortment of deficiencies in host defense working together. An organ transplant recipient may be intubated, have multiple intravenous lines, be diabetic, and be on corticosteroids and tacrolimus. All these factors contribute to the overall degree of immunity, each paving the way for its peculiar array of susceptibilities to pulmonary infection. In solid-organ transplant recipients, specific causes of pulmonary infection are most frequent at certain times post transplantation (Table 137-5). In a similar manner, specific causes of pulmonary infection are more frequent at different CD4 lymphocyte counts for patients with HIV infection (Table 137-6).
TABLE 137-5 Occurrence of Pulmonary Infection After Solid-Organ Transplantation Stratified by Time from Transplantation
| Time After Transplant (mo) | Organism |
|---|---|
| <1 |
Nosocomial bacteria (e.g., MRSA, ESBL-producing Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter baumannii)
|
CMV, cytomegalovirus; ESBL, extended-spectrum β-lactamase; HSV, herpes simplex virus; MRSA, methicillin-resistant Staphylococcus aureus; RSV, respiratory syncytial virus; VZV, varicella-zoster virus.
* These organisms should be considered when immune-suppression is still substantial.
† These organisms are less likely in patients on prophylactic cotrimoxazole.
‡ These viruses are less likely in patients on prophylactic ganciclovir or valganciclovir.
Central Nervous System Infections
Most infectious agents reach the CNS via hematogenous dissemination from an extraneural site. Exceptions include retrograde propagation of infected thrombi within emissary veins, spread along olfactory nerves, and spread from a contiguous focus of infection. The blood-brain barrier presents a natural and efficient barrier to hematogenous infection. The function of the blood-brain barrier in immunocompromised patients has not been well studied. It is well known, however, that when CNS infection is established, immune defenses (even in immunologically competent hosts) are inadequate to control the infection. Local opsonization is deficient within the brain. In animal models of bacterial brain abscess, corticosteroid administration led to a reduction in macrophage and glial response, with an increased number of viable bacteria in the abscess.25
Bacterial meningitis due to N. meningitidis is relatively uncommon in immunocompromised patients, except if they have undergone splenectomy. In contrast, pneumococcal meningitis seems to occur with increased frequency in patients who have undergone stem cell transplantation26–28 and in patients with HIV infection.29,30 Meningitis due to Listeria monocytogenes is classically associated with immunocompromise, reflecting the need for adequate T-cell function and IFN-γ production to kill this intercellular pathogen.31 In addition to meningitis, Listeria infection may be associated with brain abscess, particularly that occurring in the brainstem.32,33 Enteric bacteria (e.g., Escherichia coli) are rare causes of bacterial meningitis in immunocompromised patients. A classic association exists, however, between meningitis with such organisms and disseminated infection with Strongyloides stercoralis.34,35 In the presence of immunosuppression (e.g., large doses of corticosteroids), Strongyloides can migrate from the gastrointestinal (GI) tract to the CNS, carrying enteric bacterial flora into the CNS. Mortality is high without prompt recognition and treatment. Nocardia and mycobacteria must also be considered in the differential diagnosis of CNS infections in immunocompromised patients; diagnostic samples should be sent for inoculation onto appropriate media for isolation of these organisms.36–38
Fungal infection of the CNS may cause meningitis or space-occupying lesions. Cryptococcal meningitis is associated with advanced HIV infection (CD4 lymphocyte count < 100/mm3) but also can occur in transplanted patients.39 The presentation is usually subacute, although dangerous elevations in intracranial pressure sometimes are observed. Space-occupying lesions in the brain may occur with disseminated mold infections. These infections usually arise in the lung, but dissemination to the brain is part of multiorgan spread. Mortality is extremely high. Any of the pathogenic molds40,41 such as Aspergillus,2 zygomycetes,42,43 Scedosporium,44 or Fusarium45 can undergo dissemination to the brain. The dimorphic fungi (e.g., Histoplasma, Coccidioides) also may disseminate from the lung, causing infection of the CNS. Zygomycetes also may be associated with frequently fatal infection arising within the nose or sinuses (rhinocerebral mucormycosis).42,43
The most common protozoal pathogen to affect the CNS is Toxoplasma gondii. The classic association is between T. gondii infection and advanced HIV infection, although cases have been reported associated with other forms of immunocompromise.46–48 Amebic encephalitis has been reported occasionally in conjunction with advanced HIV infection or organ transplantation.49
A variety of viruses can cause CNS infections in immunocompromised patients. Perhaps as a result of the widespread use of antiherpesvirus prophylaxis in many immunocompromised populations, herpes simplex virus (HSV) encephalitis is rare.50 Some of the newer herpesviruses, such as human herpesvirus-6 (HHV-6), have been associated with neurologic infection in transplant recipients.51–53 Lack of diagnostic capabilities for these viruses may partially explain their apparent infrequency. CMV meningoencephalitis is well described in patients with advanced HIV infection54 and occasionally has been reported in transplant recipients.55 Disseminated infection with varicella-zoster virus (VZV) in immunocompromised patients also may result in CNS infection. West Nile virus may be acquired from transplanted organs or blood transfusions and is associated with a significant meningoencephalitis in transplant recipients.56,57 Table 137-7 summarizes agents capable of causing CNS infections in an immunocompromised host.
TABLE 137-7 Central Nervous System Infections in the Immunocompromised Host
| Etiologic Agent | Special Considerations |
|---|---|
| Meningitis | |
| Streptococcus pneumoniae | Especially in HIV-infected individuals |
| Listeria monocytogenes | Predilection for brainstem |
| Enteric bacteria | Associated with disseminated Strongyloides infection |
| Cryptococcus neoformans | Rapid diagnosis by cryptococcal antigen or India ink stain |
| Mycobacterium tuberculosis | Consider PCR for rapid diagnosis |
| Meningoencephalitis | |
| HSV | Rare in immunocompromised patients |
| HHV-6 | May be associated with lack of CSF pleocytosis |
| VZV | Skin lesions yield diagnosis |
| West Nile virus | Transmitted via transplanted organ or blood |
| Space-Occupying Lesions | |
| Nocardia | Pulmonary lesions usually also present |
| Toxoplasma gondii | Especially in HIV-infected individuals |
| Fungi | Pulmonary lesions usually also present |
CSF, cerebrospinal fluid; HHV-6, human herpesvirus-6; HIV, human immunodeficiency virus; HSV, herpes simplex virus; PCR, polymerase chain reaction; VZV, varicella-zoster virus.
Gastrointestinal Infections
The most commonly involved organisms in the etiology of infective esophagitis or gastritis are Candida, CMV, and HSV, although a variety of other organisms (e.g., mycobacteria, zygomycetes) occasionally are implicated. Candidal esophagitis is a common opportunistic infection in patients with AIDS. Rates of about 13.3 events of candidal esophagitis per 100 person-years occur in HIV-infected patients with CD4 counts less than 300/mm3.58 A study of renal transplant patients in the United States showed that esophageal candidiasis is the most common fungal infection in these patients, making up 22% of all fungal infections.59 Other predisposing factors for severe esophageal candidiasis include broad-spectrum antibiotic therapy, steroid therapy, cancer chemotherapy, diabetes mellitus, cutaneous burns, radiotherapy, and hematologic stem cell transplant. Although Candida albicans is the most frequently diagnosed organism, there is an increase of other species, including Candida krusei and Candida glabrata—this is notable because of the increase in resistance to fluconazole in these species. Finally, as noted previously, patients with immunocompromise may have a combination of pathogens causing infection at any one time. Upper GI endoscopy with biopsy is the gold standard for making the diagnosis.
Diarrhea is a common problem in immunocompromised patients with multifactorial etiologies. It may lead to diagnosis of immunosuppression in a previously undiagnosed patient when an opportunistic pathogen is found and appropriately investigated. Severe complications such as malabsorption leading to malnutrition, dehydration, and wasting can occur. Occasionally, intestinal perforation may result from GI infection. In an immunosuppressed patient, it is important to differentiate diarrhea due to opportunistic infections from diarrhea due to neoplasms, GVHD, drugs, and other therapeutic agents. GVHD accounts for more diarrhea in blood and bone marrow transplant patients than infective organisms.60 In these patients, organisms that cause mild self-limiting disease in the normal host may cause severe and life-threatening infections.60
Prolonged use of multiple antibiotics in high doses predisposes patients to colonization with Clostridium difficile and development of pseudomembranous colitis. Antibiotic prophylaxis to prevent P. jirovecii pneumonia or spontaneous bacterial peritonitis has been associated with C. difficile. In addition to the classic antibiotic risk factors of clindamycin or cephalosporin use, fluoroquinolones may predispose to epidemic strains of C. difficile (BI/NAP1/027 strain).61 Enteric bacterial pathogens such as Salmonella occur at increased frequency in immunocompromised patients, especially HIV-infected individuals. In some regions of Africa, nontyphoidal Salmonella infections are among the most common causes of bacteremia.62 Severe Salmonella infections may be associated with intestinal perforation. Shigella, Campylobacter jejuni, E. coli (enterotoxigenic, enteroadherent, and enteroaggregative), and Yersinia species are other bacterial causes of diarrhea, although less commonly associated with bacteremia.
Protozoal infections are seen more commonly in HIV-infected patients than other immunocompromised groups. At CD4 counts less than 200 cells/mm3, patients with HIV infection may present with unusual protozoa (e.g., Cryptosporidium and Microsporidium). Occasionally these pathogens are also seen in transplant recipients.63,64 Such pathogens are not detected on routine microscopic examination for ova, cysts, and parasites. Special stains and microbiological techniques are needed. Routine examination usually detects Giardia lamblia, Entamoeba histolytica, and other more common pathogenic protozoa.
CMV can cause significant colitis in all immunocompromised populations. CMV colitis may occur in the absence of systemic evidence of infection (i.e., PCR on peripheral blood may be negative65,66). Intestinal biopsy may be required to make the diagnosis. CMV intestinal infection may present with diarrhea but may have more profound presentations such as intestinal perforation.67,68
Finally, mycobacterial infections such as tuberculosis occasionally can be associated with colitis.69 M. avium complex can be grown readily from the feces of patients with HIV infection and CD4 counts of less than 50/mm3, but it is not always the cause of diarrhea in such patients.
 Therapeutic Difficulties in Immunocompromised Patients
Therapeutic Difficulties in Immunocompromised Patients
Empirical Therapy
Empirical antibiotic therapy in suspected bacterial infections should be tailored to the individual patient to maximize the chance that the therapy is microbiologically adequate. There is a clear link between microbiologically adequate empirical therapy and successful outcome from infections in the ICU.70 In settings such as severe pneumonia in the immunocompromised patient, empirical regimens comprising vancomycin, ciprofloxacin, meropenem, amphotericin (or voriconazole), ganciclovir, and trimethoprim/sulfamethoxazole may be necessary to cover potentially lethal infection with methicillin-resistant Staphylococcus aureus, P. aeruginosa, Legionella, fungi, CMV, and P. jirovecii. There is no established role for combination empirical therapy with antifungal agents. The decision to start empirical mycobacterial therapy is never an easy one. In general, we only advise it when there is a risk factor for tuberculosis. Empirical therapy for disseminated Strongyloides infection may have a place in immunocompromised patients coming from an endemic area and with the classic presentation of disseminated infection.
Pathogen-Directed Therapy
The importance of appropriate specimen collection is that empirical therapy can be streamlined (de-escalated) if cultures or other diagnostic tests are positive. With immunocompromised patients, antimicrobial therapy often is complicated by drug interactions or adverse reactions. Transplant recipients taking calcineurin inhibitors (e.g., cyclosporine or tacrolimus) or HIV-infected patients taking protease inhibitors are most at risk because these drugs may be metabolized by the cytochrome P450 system.71,72 Significant interactions may occur between rifampin, macrolide antibiotics, azole antifungal drugs, and the calcineurin inhibitors.72 Aggressive treatment of infections in immunocompromised hosts (e.g., with amphotericin, pentamidine, or foscarnet) may be associated with renal dysfunction, compounding the nephrotoxic effects of the calcineurin inhibitors. Antimicrobial agents such as linezolid or ganciclovir frequently cause neutropenia, potentially adding further host defense defects.
 Conclusion
Conclusion
Infection is likely to be one of the most significant problems an immunocompromised patient faces. These patients may present with severe infection or acquire infection while critically ill for other reasons. Prevention of infection in the ICU is of primary importance. Pneumonia can be readily prevented by many strategies. Ventilator-associated pneumonia may be prevented by a bundle of interventions.73 Aspiration of subglottic secretions and selective digestive tract decontamination, while supported by some trials, are still controversial. Opportunistic pneumonia with P. jirovecii can be prevented by use of prophylaxis with trimethoprim/sulfamethoxazole, dapsone, or nebulized pentamidine. Environmental exposure to Legionella and Aspergillus spp. can be prevented by ensuring water purification techniques (e.g., copper-silver ionization) and by preventing exposure of patients to construction activity. Infections due to pathogens transmitted human to human, such as M. tuberculosis, can be prevented by isolation precautions.
Many extrapulmonary infections can also be prevented. CMV infection can be prevented by universal prophylaxis with ganciclovir, valganciclovir, valacyclovir, or a preemptive approach using serial PCR of peripheral blood.74,75 A similar preemptive approach may be useful in preventing aspergillosis by monitoring peripheral blood for the galactomannan antigen, although this remains controversial.76,77 C. difficile infection is difficult to prevent because there is a clear need for antibiotic therapy for immunocompromised patients with infection. The increasing incidence, severity, and high rate of recurrence of C. difficile infection has become a significant problem.78 A recent randomized controlled study demonstrated that the addition of monoclonal antibodies against C. difficile toxins to antibiotic agents significantly reduced the recurrence of C. difficile infection, even among patients with the epidemic BI/NAP1/027 strain.79 Finally, attention to classic infection control practices such as appropriate immunizations,80–82 hand hygiene, and contact isolation is paramount in immunocompromised patients.
Key Points
Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601-2614.
Huang L, Quartin A, Jones D, Havlir DV. Intensive care of patients with HIV infection. N Engl J Med. 2006;355:173-181.
Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor-α neutralizing agent. N Engl J Med. 2001;345:1098-1104.
Kotton CN, Kumar D, Caliendo AM, et al. International consensus guidelines on the management of CMV in solid organ transplantation. Transplantation. 2010;89:779-795.
Kowalski R, Post D, Schneider MC, et al. Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003;17:77-88.
1 Appelgren P, Hellström I, Weitzberg E, Söderlund V, Bindslev L, Ransjö U. Risk factors for nosocomial intensive care infection: a long-term prospective analysis. Acta Anaesthesiol Scand. 2001;45(6):710-719.
2 Paterson DL, Singh N. Invasive aspergillosis in transplant recipients. Medicine (Baltimore). 1999;78(2):123-138.
3 Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. Feb 1966;64(2):328-340.
4 Weitzman SA, Aisenberg AC, Siber GR, Smith DH. Impaired humoral immunity in treated Hodgkin’s disease. N Engl J Med. 1977;297(5):245-248.
5 Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601-2614.
6 Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361(9368):1502-1510.
7 Kotton CN, Kumar D, Caliendo AM, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89(7):779-795.
8 Greenberg JD, Reed G, Kremer JM, et al. Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis. 2010;69(2):380-386.
9 McLean-Tooke A, Aldridge C, Waugh S, Spickett GP, Kay L. Methotrexate, rheumatoid arthritis and infection risk–what is the evidence? Rheumatology. 2009;48(8):867-871.
10 Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med. 2001;345(15):1098-1104. October 11, 2001
11 Tsiodras S, Samonis G, Boumpas DT, Kontoyiannis DP. Fungal infections complicating tumor necrosis factor α blockade therapy. Mayo Clin Proc. 2008;83(2):181-194.
12 Winthrop KL, Chang E, Yamashita S, Iademarco MF, LoBue PA. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg Infect Dis. 2009;15(10):1556-1561.
13 Koike R, Takeuchi T, Eguchi K, Miyasaka N. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol. 2007;17(6):451-458.
14 Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38(9):1261-1265.
15 Miro JM, Aguero F, Laguno M, et al. Liver transplantation in HIV/hepatitis co-infection. J HIV Ther. 2007;12(1):24-35.
16 Jean-Charles D-V, Cyrille F, Mylène S, et al. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47(2):407-417.
17 Kowalski R, Post D, Schneider MC, et al. Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003;17(2):77-88.
18 Mellors JW, Kingsley LA, Rinaldo CR, et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122(8):573-579.
19 Blair JE, Mayer AP, Currier J, Files JA, Wu Q. Coccidioidomycosis in elderly persons. Clin Infect Dis. 2008;47(12):1513-1518.
20 Freifeld AGa, Wheat LJb, Kaul DRc. Histoplasmosis in solid organ transplant recipients: early diagnosis and treatment. Curr Opin Organ Transplant. 2009;14(6):601-605.
21 Palmore TN, Stock F, White M, et al. A cluster of cases of nosocomial legionnaires disease linked to a contaminated hospital decorative water fountain. infect control hosp epidemiol. 2009;30(8):764-768.
22 Anaissie EJ, Costa SF. Nosocomial aspergillosis is waterborne. Clin Infect Dis. 2001;33(9):1546-1548.
23 Anaissie EJ, Stratton SL, Dignani MC, et al. Pathogenic Aspergillus species recovered from a hospital water system: A 3-Year Prospective Study. Clin Infect Dis. 2002;34(6):780-789.
24 Jereb JA, Burwen DR, Dooley SW, et al. Nosocomial outbreak of tuberculosis in a renal transplant unit: application of a new technique for restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolates. J Infect Dis. 1993;168(5):1219-1224.
25 Neuwelt EA, Lawrence MS, Blank NK. Effect of gentamicin and dexamethasone on the natural history of the rat Escherichia coli brain abscess model with histopathological correlation. Neurosurgery. 1984;15(4):475-483.
26 Engelhard D, Cordonnier C, Shaw PJ, et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European Bone Marrow Transplantation survey. Br J Haematol. 2002;117(2):444-450.
27 Haddad PA, Repka TL, Weisdorf DJ. Penicillin-resistant Streptococcus pneumoniae septic shock and meningitis complicating chronic graft versus host disease: a case report and review of the literature. Am J Med. 2002;113(2):152-155.
28 Winston DJ, Schiffman G, Wang DC, et al. Pneumococcal infections after human bone-marrow transplantation. Ann Intern Med. 1979;91(6):835-841.
29 Bliss SJ, O’Brien KL, Janoff EN, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis. 2008;8(1):67-80.
30 Flannery B, Heffernan RT, Harrison LH, et al. Changes in invasive pneumococcal disease among HIV-infected adults living in the era of childhood Pneumococcal Immunization. Ann Intern Med. 2006;144(1):1-9. January 3, 2006
31 Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T Cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198(10):1583-1593. November 17, 2003
32 Clauss HE, Lorber B. Central nervous system infection with Listeria monocytogenes. Curr Infect Dis Rep. 2008;10(4):300-306.
33 Hristea I, Bunnapradist S, Peng A, Puliyanda D, Vo A, Jordan SC. The onset of rapidly progressive neurologic deterioration after a brief gastrointestinal illness in a renal allograft recipient. Transpl Infect Dis. 2007;9(2):142-147.
34 Riedel DJ, Roddy KM, Sajadi MM. Abdominal pain and bacterial meningitis in a previously healthy young adult. Clin Infect Dis. 46(9), 2008. 1458–1458
35 Al-Hasan MN, McCormick M, Ribes JA. Invasive enteric infections in hospitalized patients with underlying strongyloidiasis. Am J Clin Pathol. 2007;128(4):622-627.
36 Kennedy KJ, Chung KHC, Bowden FJ, et al. A cluster of nocardial brain abscesses. Surg Neurol. 2007;68(1):43-49.
37 Lin Y-J, Yang K-Y, Ho J-T, Lee T-C, Wang H-C, Su F-W. Nocardial brain abscess. J Clin Neurosci. 2009;17(2):250-253.
38 Corti ME, Villafañe MF, Yampolsky CG, Schtirbu RB. Brain abscess due to Mycobacterium tuberculosis in a patient with AIDS: report of a case and review of the literature. Int J Infect Dis. 2005;9(4):225-227.
39 Wu G, Vilchez RA, Eidelman B, Fung J, Kormos R, Kusne S. Cryptococcal meningitis: an analysis among 5521 consecutive organ transplant recipients. Transpl Infect Dis. 2002;4(4):183-188.
40 Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised Patient. CNS Drugs. 2007;21(4):293-318.
41 Pagano L, Caira M, Falcucci P, Fianchi L. Fungal CNS infections in patients with hematologic malignancy. Expert Rev Anti Infect Ther. 2005;3(5):775-785.
42 Skiada A, Vrana L, Polychronopoulou H, et al. Disseminated zygomycosis with involvement of the central nervous system. Clin Microbiol Infect. 2009;15(Suppl 5):46-49.
43 Spellberg B, Edwards JJr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
44 Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections Caused by Scedosporium spp. Clin Microbiol Rev. 2008;21(1):157-197.
45 Kleinschmidt-Demasters BK. Disseminated Fusarium infection with brain abscesses in a lung transplant recipient. Clin Neuropathol. 2009;28(6):417-421.
46 Campbell AL, Goldberg CL, Magid MS, Gondolesi G, Rumbo C, Herold BC. First case of toxoplasmosis following small bowel transplantation and systematic review of tissue-invasive toxoplasmosis following noncardiac solid organ transplantation. Transplantation. 2006;81(3):408-417.
47 Cibickova L, Horacek J, Prasil P, et al. Cerebral toxoplasmosis in an allogeneic peripheral stem cell transplant recipient: case report and review of literature. Transpl Infect Dis. 2007;9(4):332-335.
48 Derouin F, Pelloux H. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect. Dec 2008;14(12):1089-1101.
49 MacLean RC, Hafez N, Tripathi S, Childress CG, Ghatak NR, Marciano-Cabral F. Identification of Acanthamoeba sp. in paraffin-embedded CNS tissue from an HIV+ individual by PCR. Diagn Microbiol Infect Dis. 2007;57(3):289-294.
50 Gomez E, Melon S, Aguado S, et al. Herpes simplex virus encephalitis in a renal transplant patient: diagnosis by polymerase chain reaction detection of HSV DNA. Am J Kidney Dis. 1997;30(3):423-427.
51 Nash PJ, Avery RK, Tang WH, Starling RC, Taege AJ, Yamani MH. Encephalitis owing to human herpesvirus-6 after cardiac transplant. Am J Transplant. 2004;4(7):1200-1203.
52 Paterson DL, Singh N, Gayowski T, Carrigan DR, Marino IR. Encephalopathy associated with human herpesvirus 6 in a liver transplant recipient. Liver Transpl. 1999;5(5):454-455.
53 Abdel Massih RC, Razonable RR. Human herpesvirus 6 infections after liver transplantation. World J Gastroenterol. 2009;15(21):2561-2569.
54 Mamidi A, DeSimone JA, Pomerantz RJ. Central nervous system infections in individuals with HIV-1 infection. J Neurovirol. 2002;8(3):158-167.
55 Hubacek P, Keslova P, Formankova R, et al. Cytomegalovirus encephalitis/retinitis in allogeneic haematopoietic stem cell transplant recipient treated successfully with combination of cidofovir and foscarnet. Pediatr Transplant. 2009;13(7):919-922.
56 Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348(22):2196-2203. May 29, 2003
57 Centers for Disease Control and Prevention (CDC). West Nile virus transmission via organ transplantation and blood transfusion—Louisiana, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(45):1263-1267.
58 Moore RD, Chaisson RE. Natural history of opportunistic disease in an HIV-infected urban clinical cohort. Ann Intern Med. 1996;124(7):633-642.
59 Abbott KC, Hypolite I, Poropatich RK, et al. Hospitalizations for fungal infections after renal transplantation in the United States. Transpl Infect Dis. 2001;3(4):203-211.
60 Cox GJ, Matsui SM, Lo RS, et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology. 1994;107(5):1398-1407.
61 Owens RCJr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S19-S31.
62 Gordon MA. Salmonella infections in immunocompromised adults. J Infect. 2008;56(6):413-422.
63 Arslan H, Inci EK, Azap OK, Karakayali H, Torgay A, Haberal M. Etiologic agents of diarrhea in solid organ recipients. Transpl Infect Dis. 2007;9(4):270-275.
64 Lanternier F, Boutboul D, Menotti J, et al. Microsporidiosis in solid organ transplant recipients: two Enterocytozoon bieneusi cases and review. Transpl Infect Dis. 2009;11(1):83-88.
65 Korkmaz M, Kunefeci G, Selcuk H, et al. The role of early colonoscopy in CMV colitis of transplant recipients. Transplant Proc. 2005;37(7):3059-3060.
66 Razonable RR. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World J Gastroenterol. 2008;14(31):4849-4860.
67 Camprodon RA, Jacob S, Malkawi A, Al-Ghnaniem R. CMV colitis presenting as acute abdomen requires early diagnosis and treatment to avoid mortality. Acta Chir Belg. 2007;107(4):378-381.
68 Almeida N, Romaozinho JM, Amaro P, Ferreira M, Cipriano MA, Leitao MC. Fatal mid-gastrointestinal bleeding by cytomegalovirus enteritis in an immunocompetent patient. Acta Gastroenterol Belg. 2009;72(2):245-248.
69 Kandutsch S, Feix A, Haas M, Hafner M, Sunder-Plassmann G, Soleiman A. A rare cause of anemia due to intestinal tuberculosis in a renal transplant recipient. Clin Nephrol. Aug 2004;62(2):158-161.
70 Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate Antimicrobial Treatment of Infections. Chest. 1999;115(2):462-474.
71 Huang L, Quartin A, Jones D, Havlir DV. Intensive Care of Patients with HIV Infection. N Engl J Med. 2006;355(2):173-181.
72 Kuypers DRJ. Immunotherapy in elderly transplant recipients: a guide to clinically significant drug interactions. Drugs Aging. 2009;26(9):715-737.
73 Wip C, Napolitano L. Bundles to prevent ventilator-associated pneumonia: how valuable are they? Curr Opin Infect Dis. 2009;22(2):159-166.
74 McGillicuddy JW, Weimert NA, Taber DJ, et al. Can preemptive cytomegalovirus monitoring be as effective as universal prophylaxis when implemented as the standard of care in patients at moderate risk? Transplantation. May 4, 2010.
75 Potena L, Grigioni F, Magnani G, et al. Prophylaxis versus preemptive anti-cytomegalovirus approach for prevention of allograft vasculopathy in heart transplant recipients. J Heart Lung Transplant. 2009;28(5):461-467.
76 Ben-Ami R, Lewis RE, Kontoyiannis DP. Invasive mould infections in the setting of hematopoietic cell transplantation: current trends and new challenges. Curr Opin Infect Dis. 2009;22(4):376-384.
77 Maertens J, Theunissen K, Verhoef G, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005;41(9):1242-1250.
78 Kelly CP, LaMont JT. Clostridium difficile–more difficult than ever. N Engl J Med. 2008;359(18):1932-1940.
79 Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3):197-205.
80 Centers for Disease Control and Prevention, Infectious Disease Society of America, American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep. 2000;49(RR-10):1-125. CE121-7
81 Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2004;4(Suppl 10):160-163.
82 Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(5):651-681.