Morganella and Providencia may be extensively resistant to antibiotics. Most isolates are resistant to ampicillin, first-generation cephalosporins, nitrofurantoin, fosfomycin, tigecycline, and the polymyxins; 40% are resistant to fluoroquinolones. Morganella and Providencia possess inducible AmpC β-lactamases; clinically significant induction or selection of stably derepressed mutants may develop during therapy. Resistance to antipseudomonal penicillins, aztreonam, gentamicin, TMP-SMX, and second- and third-generation cephalosporins is emerging but is still variably prevalent. The β-lactamase inhibitor tazobactam increases susceptibility to β-lactam agents, but sulbactam and clavulanic acid do not. Carbapenems, amikacin, and cefepime are the most active agents (>90% of isolates susceptible); however, resistance to the carbapenems, when present, is a concern because of the inherent resistance of Morganella and Providencia to the polymyxins and tigecycline. Removal of a colonized catheter or stone is critical for eradication of UTI.
EDWARDSIELLA INFECTIONS
![]() E. tarda is the only member of the genus Edwardsiella that is associated with human disease. This organism is found predominantly in freshwater and marine environments and in the associated aquatic animal species. Human acquisition occurs primarily during interaction with these reservoirs and ingestion of inadequately cooked aquatic animals. E. tarda infection is rare in the United States; recently reported cases are mostly from Southeast Asia. This pathogen shares clinical features with Salmonella species (as an intestinal pathogen; Chap. 190), Vibrio vulnificus (as an extraintestinal pathogen; Chap. 193) and Aeromonas hydrophila (as both an intestinal and extraintestinal pathogen; Chap. 183e).
E. tarda is the only member of the genus Edwardsiella that is associated with human disease. This organism is found predominantly in freshwater and marine environments and in the associated aquatic animal species. Human acquisition occurs primarily during interaction with these reservoirs and ingestion of inadequately cooked aquatic animals. E. tarda infection is rare in the United States; recently reported cases are mostly from Southeast Asia. This pathogen shares clinical features with Salmonella species (as an intestinal pathogen; Chap. 190), Vibrio vulnificus (as an extraintestinal pathogen; Chap. 193) and Aeromonas hydrophila (as both an intestinal and extraintestinal pathogen; Chap. 183e).
INFECTIOUS SYNDROMES
Gastroenteritis is the predominant infectious syndrome (50–80% of infections). Self-limiting watery diarrhea is most common, but severe colitis also occurs. The most common extraintestinal infection is wound infection due to direct inoculation, which is often associated with freshwater, marine, or snake-related injuries. Other infectious syndromes result from invasion of the gastrointestinal tract and subsequent bacteremia. Most afflicted hosts have comorbidities (e.g., hepatobiliary disease, iron overload, cancer, or diabetes mellitus). A primary bacteremic syndrome, sometimes complicated by meningitis, has a 40% case-fatality rate. Visceral (primarily hepatic) and intraperitoneal abscesses also occur. Endocarditis and empyema have been described.
DIAGNOSIS
Although E. tarda can readily be isolated and identified, most laboratories do not routinely seek to identify it in stool samples. Production of hydrogen sulfide is a characteristic biochemical property.
|
TREATMENT |
EDWARDSIELLA INFECTIONS |
E. tarda is susceptible to most antimicrobial agents appropriate for use against GNB. Gastroenteritis is generally self-limiting, but treatment with a fluoroquinolone may hasten resolution. In the setting of severe sepsis, fluoroquinolones, third- and fourth-generation cephalosporins, carbapenems, and amikacin—either alone or in combination—are the safest choices pending susceptibility data.
INFECTIONS CAUSED BY MISCELLANEOUS GENERA
Species of Hafnia, Kluyvera, Cedecea, Pantoea, Ewingella, Leclercia, and Photorhabdus are occasionally isolated from diverse clinical specimens, including blood, sputum, urine, cerebrospinal fluid, joint fluid, bile, and wounds. These organisms are rare and usually cause infection in a compromised host or in the setting of an invasive procedure or a foreign body. Cephalosporinases from Kluyvera have been implicated as the progenitors of CTX-M ESBLs.
187 |
Acinetobacter Infections |
Infections with bacteria of the genus Acinetobacter are established as a significant problem worldwide. Acinetobacter baumannii is particularly formidable because of its propensity to acquire antibiotic resistance determinants. Endemic infections caused by strains of A. baumannii resistant to multiple antibiotic classes, including carbapenems, are a serious concern in many specialized hospital units, especially intensive care units (ICUs). The foremost implication of infection with carbapenem-resistant A. baumannii is the need to use “last-line” antibiotics such as colistin, polymyxin B, or tigecycline; these options have the potential to render these bacteria resistant to all available antibiotics.
DEFINITION
Acinetobacter species are oxidase-negative, nonfermenting, short, gram-negative bacilli. They were traditionally thought of as nonmotile—a characteristic from which the genus name was derived (from the Greek akineto, meaning “nonmotile”). However, recent work has shown that Acinetobacter organisms demonstrate motility under certain growth conditions. The bacteria grow well at 37°C in aerobic conditions on a range of laboratory media (e.g., blood agar). Some species may not grow on MacConkey agar. Differentiation of Acinetobacter species is difficult with the means typically available to most clinical microbiology laboratories, including commercial semiautomated identification systems. The commonly used matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) systems are undergoing evaluation for species-level identification of Acinetobacter. DNA–DNA hybridization is a method used for speciation in reference laboratories. Naturally occurring oxacillinase genes (blaOXA) have been identified in several Acinetobacter species, and their detection by polymerase chain reaction can aid in species identification.
ETIOLOGY
Widely distributed in nature, Acinetobacter species can be found in water, in soil, and on vegetables. Acinetobacter is a component of the human skin flora and is sometimes identified as a contaminant in blood samples collected for culture. Fecal carriage can be detected in both healthy and hospitalized individuals. However, despite the ubiquity of some Acinetobacter species, the natural habitat of the most clinically important species, A. baumannii, remains to be fully defined.
EPIDEMIOLOGY
![]() A. baumannii infections have been diagnosed in patients on all inhabited continents. The vast majority of infections occur in hospitalized patients and other patients with significant health-care contact. Outbreaks of carbapenem-resistant A. baumannii are particularly problematic. A significant issue is the introduction of carbapenem-resistant A. baumannii into hospitals as a result of medical transfers, especially from hospitals where the organism is highly endemic.
A. baumannii infections have been diagnosed in patients on all inhabited continents. The vast majority of infections occur in hospitalized patients and other patients with significant health-care contact. Outbreaks of carbapenem-resistant A. baumannii are particularly problematic. A significant issue is the introduction of carbapenem-resistant A. baumannii into hospitals as a result of medical transfers, especially from hospitals where the organism is highly endemic.
The Americas In 1991 and 1992, outbreaks of carbapenem-resistant A. baumannii infection occurred in a hospital in New York City. Subsequently, numerous other hospitals in the United States and South America have had outbreaks of carbapenem-resistant A. baumannii. Infections with A. baumannii among military personnel from the United States and Canada injured in Iraq or Afghanistan were widely observed beginning in 2002. Acinetobacter was one of the most common causes of bloodstream infections and bone and soft tissue infections after war-related injury. An epidemiologic investigation revealed that A. baumannii could be grown from environmental sites in field hospitals and that the environmental strains were closely related genotypically to clinical isolates.
Europe A. baumannii infections have posed a substantial clinical challenge in many parts of Europe since the early 1980s. Three clones (European clones I, II, and III) have been the predominant causes of A. baumannii infection in hospitals in Europe. Carbapenem resistance in A. baumannii is a significant issue in many European countries, most notably the United Kingdom, Greece, Italy, Spain, and Turkey.
Asia, Australia, the Middle East, and Africa Although surveillance data are sparse from many countries in these regions, problems with carbapenem-resistant A. baumannii abound. Community-acquired infections are well described in northern Australia and some parts of Asia. These infections may be more likely in men >45 years of age who have histories of cigarette smoking, alcoholism, diabetes mellitus, or chronic obstructive airway disease. Community-acquired strains are more susceptible to antimicrobial agents than are hospital-acquired strains, but the clinical presentation of community-acquired disease is quite distinct and is characterized by overwhelming infection with severe pneumonia, septic shock, and multiorgan failure.
PATHOGENESIS
A. baumannii colonizes patients exposed to heavily contaminated hospital environments or to the hands of health care workers in these locations. Emerging data suggest that the organism can be found in the air in rooms of patients infected with Acinetobacter. Colonization of the upper airways in mechanically ventilated patients may lead to nosocomial pneumonia. Colonization of the skin may lead to central line–associated bloodstream infection, catheter-associated urinary tract infection (UTI), wound infection, or postneurosurgical meningitis. Throat carriage and microaspiration may be involved in the pathogenesis of community-acquired pneumonia due to A. baumannii.
Much less is known about the virulence mechanisms of and host responses to A. baumannii than about these aspects of other pathogenic gram-negative bacteria. Because of the emergence of multidrug-resistant strains, including those resistant to all available antibiotics, the impetus to study A. baumannii pathogenesis has grown. Novel targets for antibacterial drug development are desperately required, and drugs that have antivirulence mechanisms may provide new therapeutic options. Specific virulence mechanisms in A. baumannii include iron acquisition and transport systems; outer-membrane protein A (OmpA), which mediates mammalian cell adhesion, invasion, and cytotoxicity through mitochondrial damage and initiation of caspase-dependent apoptosis; lipopolysaccharide (LPS); and proteins important in the formation of biofilm on abiotic and biotic surfaces. Biofilm formation on abiotic surfaces is dependent on a pilus assembly system, which in turn is controlled by a traditional two-component regulatory system mediated by bfmR. Also important in biofilm formation are a gene that encodes a biofilm-associated protein (Bap); OmpA; the quorum-sensing gene abaI, which controls the secretion of 3-hydroxy-C12-homoserine lactone; and the pga locus, which is essential for the production of the polysaccharide poly-β-1,6-N-acetylglucosamine. Most recently, a global virulence regulator known as GacSA was described as important in regulating A. baumannii biofilms, motility, growth in human serum, and virulence in a mammalian infection model.
New model systems for the study of A. baumannii infection, including both nonmammalian (invertebrate) and mammalian models, have been described. Furthermore, the use of A. baumannii transposon-generated mutant libraries to screen for mutants with attenuated growth in human biologic fluids (serum and ascites fluid) has allowed the identification of new virulence mechanisms. These include phospholipase D; capsule production mediated by ptk and epsA; penicillin-binding protein 7/8 encoded by the pbpG gene; and a glycosyltransferase important for LPS biosynthesis encoded by the lpsB gene.
The LPS of A. baumannii appears to play a significant role in eliciting host responses. In studies with knockout mice, Toll-like receptor 4 and CD14 were shown to be important in host recognition, signaling, and cytokine production in response to A. baumannii. Humoral responses targeting iron-regulated outer-membrane proteins and the O-polysaccharide component of LPS also have been described.
CLINICAL MANIFESTATIONS
Pneumonia It may be difficult to distinguish between upper-airway colonization with A. baumannii and hospital-acquired pneumonia. An estimated 5–10% of cases of ventilator-associated pneumonia are due to A. baumannii, although much regional variation exists. Typically, patients with A. baumannii ventilator-associated pneumonia have had a prolonged stay in an ICU; in outbreak situations, however, patients may acquire the infection within days of arrival in an ICU.
![]() Community-acquired pneumonia due to A. baumannii has been described in tropical regions of Australia and Asia. The disease typically occurs during the “wet” season among people with a history of alcohol abuse. Infection may result in fulminant pneumonia requiring admission to an ICU, with a mortality rate of ~50%.
Community-acquired pneumonia due to A. baumannii has been described in tropical regions of Australia and Asia. The disease typically occurs during the “wet” season among people with a history of alcohol abuse. Infection may result in fulminant pneumonia requiring admission to an ICU, with a mortality rate of ~50%.
Bloodstream Infection Although A. baumannii accounts for only ~1–2% of nosocomial bloodstream infections, crude mortality rates from these infections may be as high as 40%. Sources of bloodstream infection are typically a central line or underlying pneumonia, UTI, or wound infection.
Traumatic Battlefield and Other Wounds A. baumannii is a well-known pathogen in burn units. This organism is commonly isolated from wounds of combat casualties; it was the most commonly isolated organism in one assessment of combat victims with open tibial fractures but did not appear to contribute directly to persistent nonunion or the need for amputation.
Meningitis A. baumannii may cause meningitis following neurosurgical procedures. Patients typically have an external ventricular drain in situ.
Urinary Tract Infection A. baumannii is an occasional cause of catheter-associated UTI. It is highly unusual for this organism to cause uncomplicated UTI in healthy women.
Other Clinical Manifestations A small number of case reports describe Acinetobacter prosthetic-valve endocarditis and endophthalmitis/keratitis. The latter is sometimes related to contact lens use or eye surgery.
DIAGNOSIS
Acinetobacter infection should be suspected when plump coccobacilli are seen in Gram’s-stained respiratory tract secretions, blood cultures, or cerebrospinal fluid. Sometimes the organisms are difficult to de-stain. Given their small size, they may be misidentified as either gram-negative or gram-positive cocci.
|
TREATMENT |
ACINETOBACTER INFECTION (TABLE 187-1) |
|
TREATMENT OPTIONS FOR ACINETOBACTER INFECTIONS |
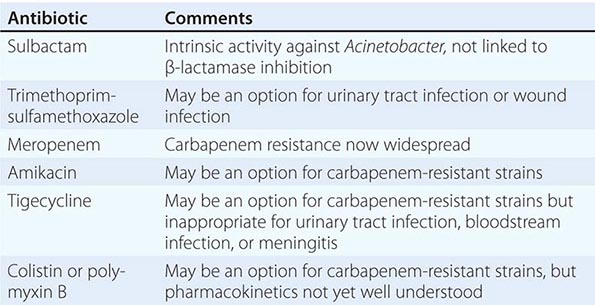
Treatment is hampered by the remarkable ability of A. baumannii to upregulate or acquire antibiotic resistance determinants. The most prominent example is that of β-lactamases, including those capable of inactivating carbapenems, cephalosporins, and penicillins. These enzymes, which include the OXA-type β-lactamases (e.g., OXA-23), the metallo-β-lactamases (e.g., NDM), and rarely KPC-type carbapenemases, are typically resistant to currently available β-lactamase inhibitors such as clavulanate or tazobactam. Plasmids that harbor genes encoding these β-lactamases may also harbor genes encoding resistance to aminoglycosides and sulfur antibiotics. The end result is that carbapenem-resistant A. baumannii may become truly multidrug resistant.
Selection of empirical antibiotic therapy when A. baumannii is suspected is challenging and must rely on a knowledge of local epidemiology. Receipt of prompt, effective antibiotic therapy is the goal. Given the diversity of resistance mechanisms in A. baumannii, definitive therapy should be based on the results of antimicrobial susceptibility testing. Carbapenems (imipenem, meropenem, and doripenem but not ertapenem) have long been thought of as the agents of choice for serious A. baumannii infections. However, the clinical utility of carbapenems is now widely jeopardized by the production of carbapenemases, as described above. Sulbactam may be an alternative to carbapenems. Unlike other β-lactamase inhibitors (e.g., clavulanic acid and tazobactam), sulbactam has intrinsic activity against Acinetobacter; this activity is mediated by the drug’s binding to penicillin-binding protein 2 rather than by its ability to inhibit β-lactamases. Sulbactam is commercially available in a combined formulation with either ampicillin or cefoperazone and may also be available as a single agent in some countries. Despite the absence of randomized clinical trials, sulbactam seems to be equivalent to carbapenems in clinical effectiveness against susceptible strains.
Therapy for carbapenem-resistant A. baumannii is particularly problematic. The only currently available choices are polymyxins (colistin and polymyxin B) and tigecycline. Neither option is perfect. Polymyxins may be nephrotoxic and neurotoxic. Definition of the optimal dose and schedule for administration of polymyxins to patients in vulnerable groups (e.g., those requiring renal replacement therapy) remains challenging, and emergence of resistance in association with monotherapy is a concern. Conventional doses of tigecycline may not result in serum concentrations adequate to treat bloodstream infections. Resistance of A. baumannii to tigecycline may develop during treatment with this drug.
As a consequence of these issues with the polymyxins and tigecycline, combination therapy is now favored for carbapenem-resistant Acinetobacter. However, in a randomized controlled trial, 30-day mortality was not reduced by the addition of rifampin to colistin. Nevertheless, a significant increase in microbiologic eradication was observed in the colistin plus rifampin arm over that attained with colistin alone. Combinations of polymyxins with a carbapenem look more promising and are being evaluated in prospective clinical trials. Fosfomycin has poor activity against Acinetobacter and should not be relied upon for treatment. Clearly, new treatment options are needed for serious Acinetobacter infections.
COMPLICATIONS AND PROGNOSIS
Given the propensity of A. baumannii to cause infections in seriously ill patients in ICUs, it is not surprising that A. baumannii infections are associated with high mortality rates. Thus a pertinent question is whether A. baumannii infections are associated with high attributable mortality rates after the severity of illness is controlled for. A number of studies have addressed this issue but have had disparate results. Whether the discrepant results can be explained purely by methodologic differences is unknown at present.
PREVENTION
Multidrug-resistant A. baumannii clearly causes outbreaks of infection and then establishes endemicity. In endemic situations, a small number of strain types predominate. In the 1991–1992 outbreaks in New York City, for example, two strain types accounted for more than 80% of carbapenem-resistant isolates. This “oligoclonality” plainly demonstrates the potential importance of infection control interventions in response to outbreaks of multidrug-resistant A. baumannii infection.
The hospital environment is an important reservoir of organisms capable of colonizing patients and causing infection. Environmental sources of A. baumannii include computer keyboards, glucometers, multidose medication vials, IV nutrition, inadequately sterilized reusable arterial pressure transducers, ventilator tubing, suction catheters, humidifiers, containers of distilled water, urine collection jugs, and moist bedding articles. Pulsatile-lavage wound treatment—a high-pressure irrigation system used to debride wounds—has been associated with an outbreak of A. baumannii infection.
Contaminated inanimate objects should be removed from the patient-care environment or subjected to enhanced environmental cleaning. Although contact-isolation procedures (use of gloves and gowns when dealing with colonized patients or their environment), accommodation of patients in single rooms, and improved hand hygiene are critical, attention to the patient-care environment may be the only measure that leads to control of outbreaks of A. baumannii infection. One study found that Acinetobacter can be cultured from the air in rooms of patients with A. baumannii infection; the infection-control implications are not yet clear.
188 |
Helicobacter pylori Infections |
DEFINITION
![]() Helicobacter pylori colonizes the stomach in ~50% of the world’s human population, essentially for life unless eradicated by antibiotic treatment. Colonization with this organism is the main risk factor for peptic ulceration (Chap. 348) as well as for gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (Chap. 109). Treatment for H. pylori has revolutionized the management of peptic ulcer disease, providing a permanent cure in most cases. Such treatment also represents first-line therapy for patients with low-grade gastric MALT lymphoma. Treatment of H. pylori is of no benefit in the treatment of gastric adenocarcinoma, but prevention of H. pylori colonization could potentially prevent gastric malignancy and peptic ulceration. In contrast, increasing evidence indicates that lifelong H. pylori colonization may offer some protection against complications of gastroesophageal reflux disease (GERD), including esophageal adenocarcinoma. Recent research has focused on whether H. pylori colonization is also a risk factor for some extragastric diseases and whether it is protective against some recently emergent medical problems, such as childhood-onset asthma and obesity.
Helicobacter pylori colonizes the stomach in ~50% of the world’s human population, essentially for life unless eradicated by antibiotic treatment. Colonization with this organism is the main risk factor for peptic ulceration (Chap. 348) as well as for gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (Chap. 109). Treatment for H. pylori has revolutionized the management of peptic ulcer disease, providing a permanent cure in most cases. Such treatment also represents first-line therapy for patients with low-grade gastric MALT lymphoma. Treatment of H. pylori is of no benefit in the treatment of gastric adenocarcinoma, but prevention of H. pylori colonization could potentially prevent gastric malignancy and peptic ulceration. In contrast, increasing evidence indicates that lifelong H. pylori colonization may offer some protection against complications of gastroesophageal reflux disease (GERD), including esophageal adenocarcinoma. Recent research has focused on whether H. pylori colonization is also a risk factor for some extragastric diseases and whether it is protective against some recently emergent medical problems, such as childhood-onset asthma and obesity.
ETIOLOGIC AGENT
Helicobacter pylori H. pylori is a gram-negative bacillus that has naturally colonized humans for at least 100,000 years, and probably throughout human evolution. It lives in gastric mucus, with a small proportion of the bacteria adherent to the mucosa and possibly a very small number of the organisms entering cells or penetrating the mucosa; the organism’s distribution is never systemic. Its spiral shape and flagella render H. pylori motile in the mucus environment. The organism has several acid-resistance mechanisms, most notably a highly expressed urease that catalyzes urea hydrolysis to produce buffering ammonia. H. pylori is microaerophilic (i.e., requires low levels of oxygen), is slow-growing, and requires complex growth media in vitro.
Other Helicobacter Species A very small proportion of gastric Helico-bacter infections are due to species other than H. pylori, possibly acquired as zoonoses. These non-pylori gastric helicobacters are associated with low-level inflammation and occasionally with disease. In immunocompromised hosts, several nongastric (intestinal) Helicobacter species can cause disease with clinical features resembling those of Campylobacter infections; these species are covered in Chap. 192.
EPIDEMIOLOGY
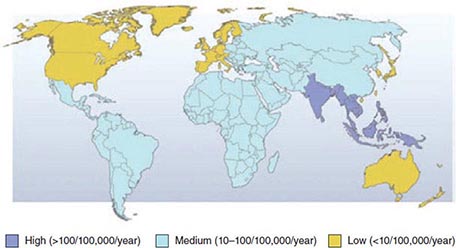
![]() Prevalence and Risk Factors The prevalence of H. pylori among adults is <30% in most parts of the United States and in other developed countries as opposed to >80% in most developing countries. In the United States, prevalence varies with age: up to 50% of 60-year-old persons, ~20% of 30-year-old persons, and fewer than 10% of children are colonized. H. pylori is usually acquired in childhood. The age association is due mostly to a birth-cohort effect whereby current 60-year-olds were more commonly colonized as children than are current children. Spontaneous acquisition or loss of H. pylori in adulthood is uncommon. Childhood acquisition explains why the main risk factors for infection are markers of crowding and social deprivation in childhood.
Prevalence and Risk Factors The prevalence of H. pylori among adults is <30% in most parts of the United States and in other developed countries as opposed to >80% in most developing countries. In the United States, prevalence varies with age: up to 50% of 60-year-old persons, ~20% of 30-year-old persons, and fewer than 10% of children are colonized. H. pylori is usually acquired in childhood. The age association is due mostly to a birth-cohort effect whereby current 60-year-olds were more commonly colonized as children than are current children. Spontaneous acquisition or loss of H. pylori in adulthood is uncommon. Childhood acquisition explains why the main risk factors for infection are markers of crowding and social deprivation in childhood.
![]() Transmission Humans are the only important reservoir of H. pylori. Children may acquire the organism from their parents (most often the primary caregiver) or from other children. The former is more common in developed countries and the latter in less developed countries. Whether transmission takes place more often by the fecal-oral or the oral-oral route is unknown, but H. pylori is easily cultured from vomitus and gastroesophageal refluxate and is less easily cultured from stool.
Transmission Humans are the only important reservoir of H. pylori. Children may acquire the organism from their parents (most often the primary caregiver) or from other children. The former is more common in developed countries and the latter in less developed countries. Whether transmission takes place more often by the fecal-oral or the oral-oral route is unknown, but H. pylori is easily cultured from vomitus and gastroesophageal refluxate and is less easily cultured from stool.
PATHOLOGY AND PATHOGENESIS
H. pylori colonization induces chronic superficial gastritis, a tissue response in the stomach that includes infiltration of the mucosa by both mononuclear and polymorphonuclear cells. (The term gastritis should be used specifically to describe histologic features; it has also been used to describe endoscopic appearances and even symptoms, but these features do not correlate with microscopic findings or even with the presence of H. pylori.) Although H. pylori is capable of numerous adaptations that prevent excessive stimulation of the immune system, colonization is accompanied by a considerable persistent local and systemic immune response, including the production of antibodies and cell-mediated responses. However, these responses are ineffective in clearing the bacterium. This inefficient clearing appears to be due in part to H. pylori’s downregulation of the immune system, which fosters its own persistence.
Most H. pylori–colonized persons do not develop clinical sequelae. That some persons develop overt disease whereas others do not is related to a combination of factors: bacterial strain differences, host susceptibility to disease, and environmental factors.
![]() Bacterial Virulence Factors Several H. pylori virulence factors are more common among strains that are associated with disease than among those that are not. The cag island is a group of genes that encodes a bacterial type IV secretion system. Through this system, an effector protein, CagA, is translocated into epithelial cells, where it may be transformed by phosphorylation and induces host cell signal transduction; proliferative, cytoskeletal, and inflammatory changes in the cell result. The protein at the tip of the secretory apparatus, CagL, binds to integrins on the cell surface, transducing further signaling. Finally, soluble components of the peptidoglycan cell wall enter the cell, mediated by the same secretory system. These components are recognized by the emergency intracellular bacterial receptor Nod1, which stimulates a proinflammatory cytokine response resulting in enhanced gastric inflammation. Carriage of cag-positive strains increases the risk of peptic ulcer or gastric adenocarcinoma. A second major virulence factor is the vacuolating cytotoxin VacA, which forms pores in cell membranes. VacA is polymorphic, and carriage of more active forms also increases the risk of disease. Other bacterial factors that are associated with increased disease risk include adhesins, such as BabA (which binds to blood group antigens on epithelial cells), and incompletely characterized factors, such as another recently described bacterial type 4 secretion system.
Bacterial Virulence Factors Several H. pylori virulence factors are more common among strains that are associated with disease than among those that are not. The cag island is a group of genes that encodes a bacterial type IV secretion system. Through this system, an effector protein, CagA, is translocated into epithelial cells, where it may be transformed by phosphorylation and induces host cell signal transduction; proliferative, cytoskeletal, and inflammatory changes in the cell result. The protein at the tip of the secretory apparatus, CagL, binds to integrins on the cell surface, transducing further signaling. Finally, soluble components of the peptidoglycan cell wall enter the cell, mediated by the same secretory system. These components are recognized by the emergency intracellular bacterial receptor Nod1, which stimulates a proinflammatory cytokine response resulting in enhanced gastric inflammation. Carriage of cag-positive strains increases the risk of peptic ulcer or gastric adenocarcinoma. A second major virulence factor is the vacuolating cytotoxin VacA, which forms pores in cell membranes. VacA is polymorphic, and carriage of more active forms also increases the risk of disease. Other bacterial factors that are associated with increased disease risk include adhesins, such as BabA (which binds to blood group antigens on epithelial cells), and incompletely characterized factors, such as another recently described bacterial type 4 secretion system.
Host Genetic and Environmental Factors The best-characterized host determinants of disease are genetic polymorphisms leading to enhanced activation of the innate immune response, including polymorphisms in cytokine genes or in genes encoding bacterial recognition proteins such as Toll-like receptors. For example, colonized people with polymorphisms in the interleukin 1 gene that increase the production of this cytokine in response to H. pylori infection are at increased risk of gastric adenocarcinoma. In addition, environmental cofactors are important in pathogenesis. Smoking increases the risks of duodenal ulcers and gastric cancer in H. pylori–positive individuals. Diets high in salt and preserved foods increase cancer risk, whereas diets high in antioxidants and vitamin C are modestly protective.
Distribution of Gastritis and Differential Disease Risk The pattern of gastric inflammation is associated with disease risk: antral-predominant gastritis is most closely linked with duodenal ulceration, whereas pan-gastritis is linked with gastric ulceration and adenocarcinoma. This difference probably explains why patients with duodenal ulceration are not at high risk of developing gastric adenocarcinoma later in life, despite being colonized by H. pylori.
PATHOGENESIS OF DUODENAL ULCERATION How gastric colonization causes duodenal ulceration is now becoming more clear. H. pylori–induced inflammation of the gastric antrum diminishes the number of somatostatin-producing D cells. Because somatostatin inhibits gastrin release, gastrin levels are higher than in H. pylori–negative persons, and these higher levels lead to increased meal-stimulated acid secretion from the relatively spared gastric corpus. How this situation increases duodenal ulcer risk remains controversial, but the increased acid secretion may contribute to the formation of the potentially protective gastric metaplasia found in the duodenum of duodenal ulcer patients. Gastric metaplasia in the duodenum may become colonized by H. pylori and subsequently inflamed and ulcerated.
PATHOGENESIS OF GASTRIC ULCERATION AND GASTRIC ADENOCARCINOMA The pathogenesis of these conditions is less well understood, although both arise in association with pan- or corpus-predominant gastritis. The hormonal changes described above still occur, but the inflammation in the gastric corpus means that it produces less acid (hypochlorhydria) despite hypergastrinemia. Gastric ulcers usually occur at the junction of antral and corpus-type mucosa, an area that is often particularly inflamed. Gastric cancer probably stems from progressive DNA damage and the survival of abnormal epithelial cell clones. The DNA damage is thought to be due principally to reactive oxygen and nitrogen species arising from inflammatory cells, perhaps in relation to other bacteria that survive in a hypochlorhydric stomach. Longitudinal analyses of gastric biopsy specimens taken years apart from the same patient show that the common intestinal type of gastric adenocarcinoma follows stepwise changes from simple gastritis to gastric atrophy, intestinal metaplasia, and dysplasia. A second, diffuse type of gastric adenocarcinoma found more commonly in younger adults may arise directly from chronic gastritis without atrophic changes.
CLINICAL MANIFESTATIONS
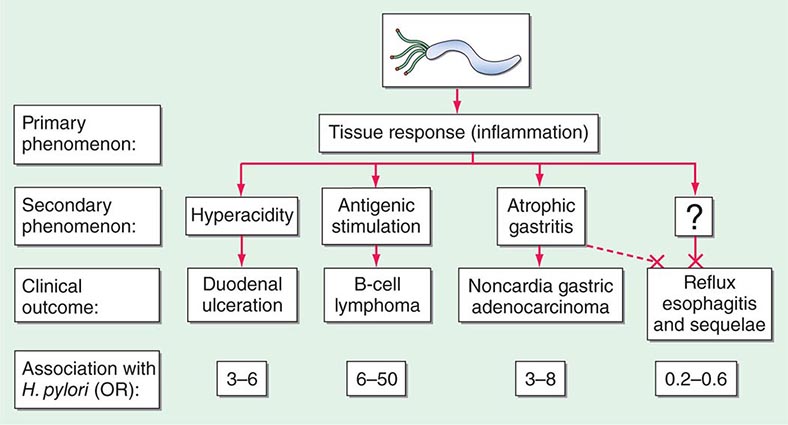
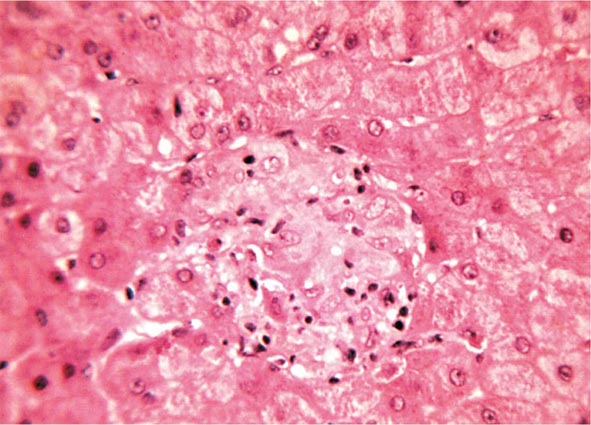
Essentially all H. pylori–colonized persons have histologic gastritis, but only ~10–15% develop associated illnesses such as peptic ulceration, gastric adenocarcinoma, or gastric lymphoma (Fig. 188-1). Rates among women are less than half of those among men for both diseases.
FIGURE 188-1 Schematic of the relationships between colonization with Helicobacter pylori and diseases of the upper gastrointestinal tract. Essentially all persons colonized with H. pylori develop a host response, which is generally termed chronic gastritis. The nature of the host’s interaction with the particular bacterial population determines the clinical outcome. H. pylori colonization increases the lifetime risk of peptic ulcer disease, noncardia gastric cancer, and B-cell non-Hodgkin’s gastric lymphoma (odds ratios [ORs] for all, >3). In contrast, a growing body of evidence indicates that H. pylori colonization (especially with cagA+ strains) protects against adenocarcinoma of the esophagus (and the sometimes related gastric cardia) and premalignant lesions such as Barrett’s esophagus (OR, <1). Although the incidences of peptic ulcer disease (cases not due to nonsteroidal anti-inflammatory drugs) and noncardia gastric cancer are declining in developed countries, the incidence of adenocarcinoma of the esophagus is increasing. (Adapted from MJ Blaser: Hypothesis: The changing relationships of Helicobacter pylori and humans: Implications for health and disease. J Infect Dis 179:1523, 1999, with permission.)
![]() Peptic Ulcer Disease Worldwide, >80% of duodenal ulcers and >60% of gastric ulcers are related to H. pylori colonization (Chap. 348). However, in particular, the proportion of gastric ulcers caused by aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) is increasing, and in many developed countries these drugs have overtaken H. pylori as a cause of gastric ulceration. The main lines of evidence supporting an ulcer-promoting role for H. pylori are that (1) the presence of the organism is a risk factor for the development of ulcers, (2) non-NSAID-induced ulcers rarely develop in the absence of H. pylori, (3) eradication of H. pylori virtually abolishes long-term ulcer relapse, and (4) experimental H. pylori infection of gerbils can cause gastric ulceration.
Peptic Ulcer Disease Worldwide, >80% of duodenal ulcers and >60% of gastric ulcers are related to H. pylori colonization (Chap. 348). However, in particular, the proportion of gastric ulcers caused by aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) is increasing, and in many developed countries these drugs have overtaken H. pylori as a cause of gastric ulceration. The main lines of evidence supporting an ulcer-promoting role for H. pylori are that (1) the presence of the organism is a risk factor for the development of ulcers, (2) non-NSAID-induced ulcers rarely develop in the absence of H. pylori, (3) eradication of H. pylori virtually abolishes long-term ulcer relapse, and (4) experimental H. pylori infection of gerbils can cause gastric ulceration.
Gastric Adenocarcinoma and Lymphoma Prospective nested case-control studies have shown that H. pylori colonization is a risk factor for adenocarcinomas of the distal (noncardia) stomach (Chap. 109). Long-term experimental infection of gerbils also may result in gastric adenocarcinoma. Moreover, H. pylori may induce primary gastric lymphoma, although this condition is much less common. Many low-grade gastric B-cell lymphomas are dependent on H. pylori for continuing growth and proliferation, and these tumors may regress either fully or partially after H. pylori eradication. However, they require careful short- and long-term monitoring, and some necessitate additional treatment with chemotherapeutic agents.
Functional Dyspepsia Many patients have upper gastrointestinal symptoms but have normal results on upper gastrointestinal endoscopy (so-called functional or nonulcer dyspepsia; Chap. 348). Because H. pylori is common, some of these patients will be colonized with the organism. H. pylori eradication leads to symptom resolution a little more commonly (from 0 to 7% in different studies) than does placebo treatment. Whether such patients have peptic ulcers in remission at the time of endoscopy or whether a small subgroup of patients with “true” functional dyspepsia respond to H. pylori treatment is unclear.
Protection Against Peptic Esophageal Disease, Including Esophageal Adenocarcinoma Much interest has focused on a protective role for H. pylori against GERD (Chap. 347), Barrett’s esophagus (Chap. 347), and adenocarcinoma of the esophagus and gastric cardia (Chap. 109). The main lines of evidence for this role are (1) that there is a temporal relationship between a falling prevalence of gastric H. pylori colonization and a rising incidence of these conditions; (2) that, in most studies, the prevalence of H. pylori colonization (especially with proinflammatory cagA+ strains) is significantly lower among patients with these esophageal diseases than among control participants; and (3) that, in prospective nested studies (see above), the presence of H. pylori is inversely related to these cancers. The mechanism underlying this protective effect is likely H. pylori–induced hypochlorhydria. Because, at the individual level, GERD symptoms may decrease, worsen, or remain unchanged after H. pylori treatment, concerns about GERD should not affect decisions about whether to treat H. pylori when an indication exists.
Other Pathologies H. pylori has an increasingly recognized role in other gastric pathologies. It may be one initial precipitant of autoimmune gastritis and pernicious anemia and also may predispose some patients to iron deficiency through occult blood loss and/or hypochlorhydria and reduced iron absorption. In addition, several extragastrointestinal pathologies have been linked with H. pylori colonization, although evidence of causality is less strong. Studies of H. pylori treatment in idiopathic thrombocytopenic purpura have consistently described improvement in or even normalization of platelet counts. Potentially important but even more controversial associations are with ischemic heart disease and cerebrovascular disease. However, the strength of the latter associations is reduced if confounding factors are taken into account, and most authorities consider the associations to be noncausal. Several studies have shown an inverse association of cagA+ H. pylori with childhood-onset asthma, hay fever, and atopic disorders. These associations have been shown to be causal in animal models, but causality in humans and the size of any effect have not been established.
DIAGNOSIS
Tests for H. pylori fall into two groups: tests that require upper gastrointestinal endoscopy and simpler tests that can be performed in the clinic (Table 188-1).
|
TESTS COMMONLY USED TO DETECT HELICOBACTER PYLORI |
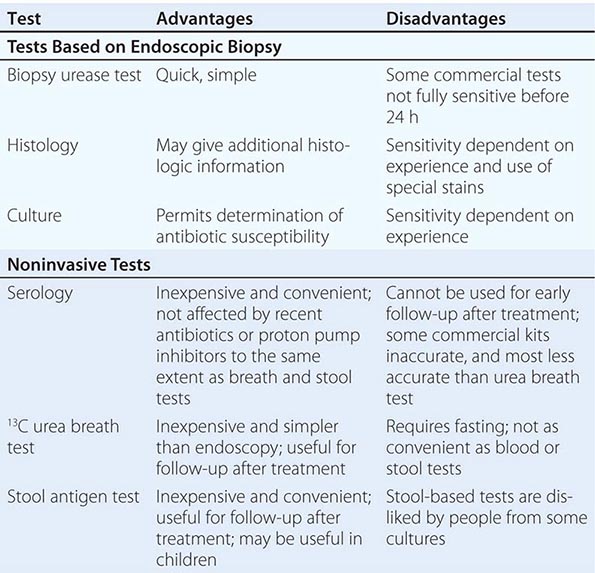
Endoscopy-Based Tests Endoscopy is usually unnecessary in the initial management of young patients with simple dyspepsia but is commonly used to exclude malignancy and make a positive diagnosis in older patients or those with “alarm” symptoms. If endoscopy is performed, the most convenient biopsy-based test is the biopsy urease test, in which one large or two small gastric biopsy specimens are placed into a gel containing urea and an indicator. The presence of H. pylori urease leads to a pH alteration and therefore to a color change, which often occurs within minutes but can require up to 24 h. Histologic examination of biopsy specimens for H. pylori also is accurate, provided that a special stain (e.g., a modified Giemsa or silver stain) permitting optimal visualization of the organism is used. If biopsy specimens are obtained from both antrum and corpus, histologic study yields additional information, including the degree and pattern of inflammation and the presence of any atrophy, metaplasia, or dysplasia. Microbiologic culture is most specific but may be insensitive because of difficulty with H. pylori isolation. Once the organism is cultured, its identity as H. pylori can be confirmed by its typical appearance on Gram’s stain and its positive reactions in oxidase, catalase, and urease tests. Moreover, the organism’s susceptibility to antibiotics can be determined, and this information can be clinically useful in difficult cases. The occasional biopsy specimens containing the less common non-pylori gastric helicobacters give only weakly positive results in the biopsy urease test. Positive identification of these bacteria requires visualization of the characteristic long, tight spirals in histologic sections; they cannot easily be cultured.
Noninvasive Tests Noninvasive H. pylori testing is the norm if gastric cancer does not need to be excluded by endoscopy. The best-established test (and a very accurate one) is the urea breath test. In this simple test, the patient drinks a solution of urea labeled with the nonradioactive isotope 13C and then blows into a tube. If H. pylori urease is present, the urea is hydrolyzed, and labeled carbon dioxide is detected in breath samples. The stool antigen test, a simple and accurate test using monoclonal antibodies specific for H. pylori antigens, is more convenient and potentially less expensive than the urea breath test, but some patients dislike sampling stool. The simplest tests for ascertaining H. pylori status are serologic assays measuring specific IgG levels in serum by enzyme-linked immunosorbent assay or immunoblot. The best of these tests are as accurate as other diagnostic methods, but many commercial tests—especially rapid office tests—do not perform well.
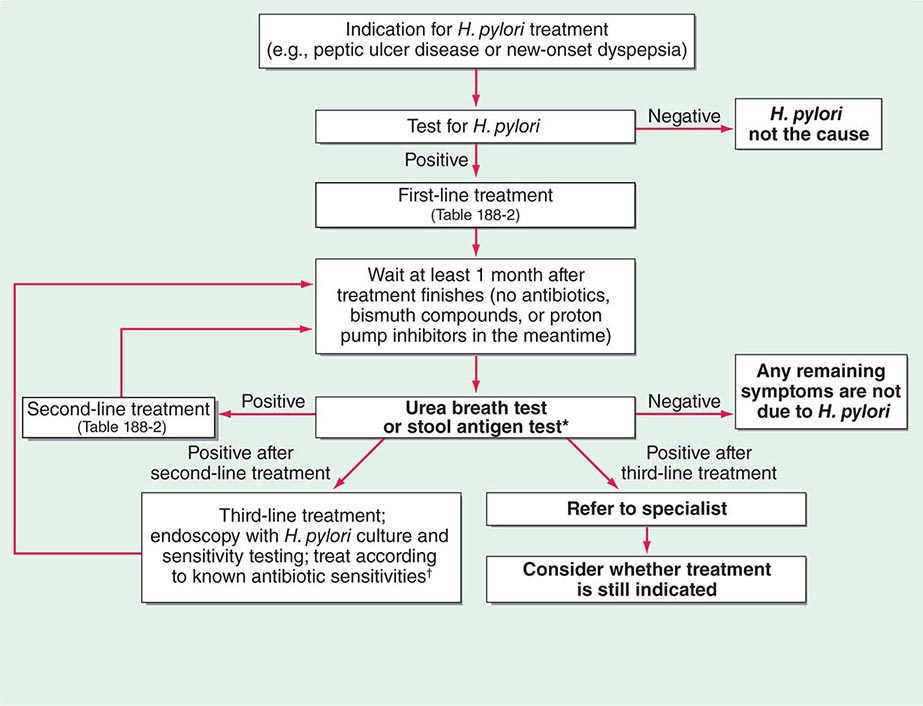
Use of Tests to Assess Treatment Success The urea breath test, the stool antigen test, and biopsy-based tests can all be used to assess the success of treatment (Fig. 188-2). However, because these tests are dependent on H. pylori load, their use <4 weeks after treatment may yield false-negative results. Furthermore, these tests are unreliable if performed within 4 weeks of intercurrent treatment with antibiotics or bismuth compounds or within 2 weeks of the discontinuation of proton pump inhibitor (PPI) treatment. In the assessment of treatment success, noninvasive tests are normally preferred; however, after gastric ulceration, endoscopy should be repeated to ensure healing and to exclude gastric carcinoma by further histologic sampling. Serologic tests are not used to monitor treatment success, as the gradual drop in titer of H. pylori–specific antibodies is too slow to be of practical use.
FIGURE 188-2 Algorithm for the management of Helicobacter pylori infection. *Note that either the urea breath test or the stool antigen test can be used in this algorithm. Occasionally, endoscopy and a biopsy-based test are used instead of either of these tests in follow-up after treatment. The main indication for these invasive tests is gastric ulceration; in this condition, as opposed to duodenal ulceration, it is important to check healing and to exclude underlying gastric adenocarcinoma. However, even in this situation, patients undergoing endoscopy may still be receiving proton pump inhibitor therapy, which precludes H. pylori testing. Thus a urea breath test or a stool antigen test is still required at a suitable interval after the end of therapy to determine whether treatment has been successful (see text). †Some authorities use empirical third-line regimens, of which several have been described.
|
TREATMENT |
HELICOBACTER PYLORI INFECTION |
INDICATIONS
The most clear-cut indications for treatment are H. pylori–related duodenal or gastric ulceration or low-grade gastric B-cell lymphoma. Whether or not the ulcers are currently active, H. pylori should be eradicated in patients with documented ulcer disease to prevent relapse (Fig. 188-2). Testing for H. pylori and treatment if the results are positive also have been advocated in uninvestigated simple dyspepsia, but only when the prevalence of H. pylori in the community is >20% are these measures more cost-effective than simply treating the dyspepsia with PPIs. Guidelines have recommended H. pylori treatment in functional dyspepsia in case the patient is one of the perhaps 0–7% who will benefit from such treatment (beyond placebo effects). Some guidelines also recommend treatment of conditions not definitively known to respond to H. pylori eradication, including idiopathic thrombocytopenic purpura, vitamin B12 deficiency, and iron-deficiency anemia (in the last instance, only when other causes have been carefully excluded). Test-and-treat has emerged as a common clinical practice in recent years despite the lack of direct evidence that it is advantageous; whether this practice will survive the scrutiny of time and further study remains to be determined. For individuals with a strong family history of gastric cancer, treatment to eradicate H. pylori in the hope of reducing their cancer risk is reasonable but of unproven value. Currently, widespread community screening for and treatment of H. pylori as primary prophylaxis for gastric cancer and peptic ulcers are not recommended in most countries, mainly because the extent of the consequent reduction in cancer risk is not known. Several studies have found a modestly reduced cancer risk after treatment, but the period of follow-up is still fairly short and the size of the effect in different populations remains unclear. Other reasons not to treat H. pylori in asymptomatic populations at present include (1) the adverse side effects (which are common and can be severe in rare cases) of the multiple-antibiotic regimens used; (2) antibiotic resistance, which may emerge in H. pylori or other incidentally carried bacteria; (3) the anxiety that may arise in otherwise healthy people, especially if treatment is unsuccessful; and (4) the existence of a subset of people who will develop GERD symptoms after treatment, although, on average, H. pylori treatment does not affect GERD symptoms or severity. Despite the absence of screening strategies, many doctors treat H. pylori if it is known to be present (particularly in children and younger adults), even when the patient is asymptomatic. The rationale is that it reduces patient concern and may reduce future gastric cancer risk and that any reduction in risk is likely to be greater in younger patients. However, such practices do not factor in any potential benefits of H. pylori colonization. Overall, despite widespread clinical activity in this area, most treatment of asymptomatic H. pylori carriage is given without a firm evidence base.
REGIMENS
Although H. pylori is susceptible to a wide range of antibiotics in vitro, monotherapy is not usually successful, probably because of inadequate antibiotic delivery to the colonization niche. Failure of monotherapy prompted the development of multidrug regimens, the most successful of which are triple and quadruple combinations. Current regimens consist of a PPI and two or three antimicrobial agents given for 7–14 days (Table 188-2). Research on optimizing drug combinations to increase efficacy continues, and guidelines are likely to change as the field develops and as countries increasingly tailor treatment to suit local antibiotic resistance patterns and economic needs.
|
COMMONLY RECOMMENDED TREATMENT REGIMENS FOR HELICOBACTER PYLORI |
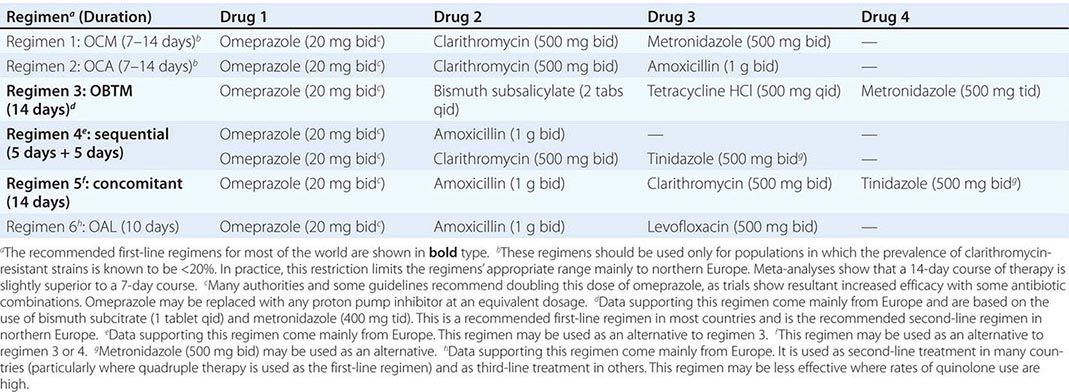
![]() The two most important factors in successful H. pylori treatment are the patient’s close compliance with the regimen and the use of drugs to which the patient’s strain of H. pylori has not acquired resistance. Treatment failure following minor lapses in compliance is common and often leads to acquired resistance to metronidazole or clarithromycin. To stress the importance of compliance, written instructions should be given to the patient, and minor side effects of the regimen should be explained. Increasing levels of H. pylori resistance to clarithromycin, quinolones, and—to a lesser extent—metronidazole are of growing concern and are thought to be responsible for the reduced efficacy of previously popular clarithromycin-based triple-therapy regimens worldwide. Treatment with these regimens is now virtually confined to certain northern European countries where the use of clarithromycin (or azithromycin) for respiratory infections has not been widespread and resistance rates in H. pylori are still low. Strains of H. pylori with some degree of in vitro resistance to metronidazole are common but still may be eradicated with metronidazole-containing regimens, which have only slightly reduced efficacy in vivo. Assessment of antibiotic susceptibilities before treatment would be optimal but is not usually undertaken because endoscopy and mucosal biopsy are necessary to obtain H. pylori for culture and because most microbiology laboratories are inexperienced in H. pylori culture. In the absence of susceptibility information, the patient’s history of (even distant) antibiotic use for other conditions should be ascertained; use of the previously administered agent(s) should then be avoided if possible, particularly in the case of clarithromycin (e.g., previous use for upper respiratory infection) and quinolones. If initial H. pylori treatment fails, the usual approach is empirical re-treatment with another drug regimen (Table 188-2). The third-line approach should ideally be endoscopy, biopsy, and culture plus treatment based on documented antibiotic sensitivities. However, empirical third-line therapies are often used.
The two most important factors in successful H. pylori treatment are the patient’s close compliance with the regimen and the use of drugs to which the patient’s strain of H. pylori has not acquired resistance. Treatment failure following minor lapses in compliance is common and often leads to acquired resistance to metronidazole or clarithromycin. To stress the importance of compliance, written instructions should be given to the patient, and minor side effects of the regimen should be explained. Increasing levels of H. pylori resistance to clarithromycin, quinolones, and—to a lesser extent—metronidazole are of growing concern and are thought to be responsible for the reduced efficacy of previously popular clarithromycin-based triple-therapy regimens worldwide. Treatment with these regimens is now virtually confined to certain northern European countries where the use of clarithromycin (or azithromycin) for respiratory infections has not been widespread and resistance rates in H. pylori are still low. Strains of H. pylori with some degree of in vitro resistance to metronidazole are common but still may be eradicated with metronidazole-containing regimens, which have only slightly reduced efficacy in vivo. Assessment of antibiotic susceptibilities before treatment would be optimal but is not usually undertaken because endoscopy and mucosal biopsy are necessary to obtain H. pylori for culture and because most microbiology laboratories are inexperienced in H. pylori culture. In the absence of susceptibility information, the patient’s history of (even distant) antibiotic use for other conditions should be ascertained; use of the previously administered agent(s) should then be avoided if possible, particularly in the case of clarithromycin (e.g., previous use for upper respiratory infection) and quinolones. If initial H. pylori treatment fails, the usual approach is empirical re-treatment with another drug regimen (Table 188-2). The third-line approach should ideally be endoscopy, biopsy, and culture plus treatment based on documented antibiotic sensitivities. However, empirical third-line therapies are often used.
Non-pylori gastric helicobacters are treated in the same way as H. pylori. However, in the absence of trials, it is unclear whether a positive outcome always represents successful treatment or whether it is sometimes due to natural clearance of the bacteria.
PREVENTION
![]() Carriage of H. pylori has considerable public health significance in developed countries, where it is associated with peptic ulcer disease and gastric adenocarcinoma, and in developing countries, where gastric adenocarcinoma may be an even more common cause of cancer death late in life. If mass prevention were contemplated, vaccination would be the most obvious method, and experimental immunization of animals has given promising results. However, given that H. pylori has co-evolved with its human host over millennia, preventing or eliminating colonization on a population basis may have biological and clinical costs. For example, lifelong absence of H. pylori is a risk factor for GERD complications, including esophageal adenocarcinoma. We have speculated that the disappearance of H. pylori may also be associated with an increased risk of other emergent diseases reflecting aspects of the current Western lifestyle, such as childhood-onset asthma and allergy.
Carriage of H. pylori has considerable public health significance in developed countries, where it is associated with peptic ulcer disease and gastric adenocarcinoma, and in developing countries, where gastric adenocarcinoma may be an even more common cause of cancer death late in life. If mass prevention were contemplated, vaccination would be the most obvious method, and experimental immunization of animals has given promising results. However, given that H. pylori has co-evolved with its human host over millennia, preventing or eliminating colonization on a population basis may have biological and clinical costs. For example, lifelong absence of H. pylori is a risk factor for GERD complications, including esophageal adenocarcinoma. We have speculated that the disappearance of H. pylori may also be associated with an increased risk of other emergent diseases reflecting aspects of the current Western lifestyle, such as childhood-onset asthma and allergy.
189 |
Infections Due to Pseudomonas Species and Related Organisms |
The pseudomonads are a heterogeneous group of gram-negative bacteria that have in common an inability to ferment lactose. Formerly classified in the genus Pseudomonas, the members of this group have been assigned to three medically important genera—Pseudomonas, Burkholderia, and Stenotrophomonas—whose biologic behaviors encompass both similarities and marked differences and whose genetic repertoires differ in many respects. The pathogenicity of most pseudomonads is based on opportunism; the exceptions are the organisms that cause melioidosis (Burkholderia pseudomallei) and glanders (Burkholderia mallei), which can be considered as primary pathogens.
Pseudomonas aeruginosa, the major pathogen of the group, is a significant cause of infections in hospitalized patients and in patients with cystic fibrosis (CF; Chap. 313). Cytotoxic chemotherapy, mechanical ventilation, and broad-spectrum antibiotic therapy probably paved the way for colonization and infection of increasing numbers of hospitalized patients by this organism. Thus most conditions predisposing to P. aeruginosa infections have involved host compromise and/or broad-spectrum antibiotic use. The other members of the genus Pseudomonas—Pseudomonas putida, Pseudomonas fluorescens, and Pseudomonas stutzeri—infect humans infrequently.
The genus Burkholderia comprises more than 40 species, of which Burkholderia cepacia is most frequently encountered in Western countries. Like P. aeruginosa, B. cepacia is both a nosocomial pathogen and a cause of infection in CF. The other medically important members of this genus are B. pseudomallei and B. mallei, the etiologic agents of melioidosis and glanders, respectively.
The genus Stenotrophomonas contains one species of medical significance, Stenotrophomonas maltophilia (previously classified in the genera Pseudomonas and Xanthomonas). This organism is strictly an opportunist that “overgrows” in the setting of potent broad-spectrum antibiotic use.
PSEUDOMONAS AERUGINOSA
EPIDEMIOLOGY
P. aeruginosa is found in most moist environments. Soil, plants, vegetables, tap water, and countertops are all potential reservoirs for this microbe, as it has simple nutritional needs. Given the ubiquity of P. aeruginosa, simple contact with the organism is not sufficient for colonization or infection. Clinical and experimental observations suggest that P. aeruginosa infection often occurs concomitantly with host defense compromise, mucosal trauma, physiologic derangement, and antibiotic-mediated suppression of normal flora. Thus, it comes as no surprise that the majority of P. aeruginosa infections occur in intensive care units (ICUs), where these factors frequently converge. The organism is initially acquired from environmental sources, but patient-to-patient spread also occurs in clinics and families.
In the past, burned patients appeared to be unusually susceptible to P. aeruginosa. For example, in 1959–1963, Pseudomonas burn-wound sepsis was the principal cause of death in 60% of burned patients dying at the U.S. Army Institute of Surgical Research. For reasons that are unclear, P. aeruginosa infection in burns is no longer the major problem that it was during the 1950s and 1960s. Similarly, in the 1960s, P. aeruginosa appeared as a common pathogen in patients receiving cytotoxic chemotherapy at many institutions in the United States, but it subsequently diminished in importance. Despite this subsidence, P. aeruginosa remains one of the most feared pathogens in this population because of its high attributable mortality rate.
![]() In some parts of Asia and Latin America, P. aeruginosa continues to be the most common cause of gram-negative bacteremia in neutropenic patients.
In some parts of Asia and Latin America, P. aeruginosa continues to be the most common cause of gram-negative bacteremia in neutropenic patients.
In contrast to the trends for burned patients and neutropenic patients in the United States, the incidence of P. aeruginosa infections among patients with CF has not changed. P. aeruginosa remains the most common contributing factor to respiratory failure in CF and is responsible for the majority of deaths among CF patients.
LABORATORY FEATURES
P. aeruginosa is a nonfastidious, motile, gram-negative rod that grows on most common laboratory media, including blood and MacConkey agars. It is easily identified in the laboratory on primary-isolation agar plates by pigment production that confers a yellow to dark green or even bluish appearance. Colonies have a shiny “gun-metal” appearance and a characteristic fruity odor. Two of the identifying biochemical characteristics of P. aeruginosa are an inability to ferment lactose on MacConkey agar and a positive reaction in the oxidase test. Most strains are identified on the basis of these readily detectable laboratory features even before extensive biochemical testing is done. Some isolates from CF patients are easily identified by their mucoid appearance, which is due to the production of large amounts of the mucoid exopolysaccharide or alginate.
PATHOGENESIS
Unraveling the mechanisms that underlie disease caused by P. aeruginosa has proved challenging. Of the common gram-negative bacteria, no other species produces such a large number of putative virulence factors (Table 189-1). Yet P. aeruginosa rarely initiates an infectious process in the absence of host injury or compromise, and few of its putative virulence factors have been shown definitively to be involved in disease in humans. Despite its metabolic versatility and possession of multiple colonizing factors, P. aeruginosa exhibits no competitive advantage over enteric bacteria in the human gut; neither is it a normal inhabitant of the human gastrointestinal tract, despite the host’s continuous environmental exposure to the organism.
|
MAIN PUTATIVE VIRULENCE FACTORS OF PSEUDOMONAS AERUGINOSA |
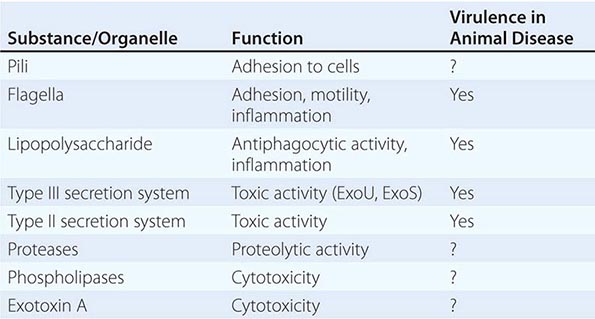
Virulence Attributes Involved in Acute P. aeruginosa Infections • MOTILITY AND COLONIZATION A general tenet of bacterial pathogenesis is that most bacteria must adhere to surfaces or colonize a host niche in order to initiate disease. Most pathogens examined thus far possess adherence factors called adhesins. P. aeruginosa is no exception. Among its many adhesins are its pili, which demonstrate adhesive properties for a variety of cells and adhere best to injured cell surfaces. In the organism’s flagellum, the flagellin molecule binds to cells, and the flagellar cap attaches to mucins through the recognition of glycan chains. Other P. aeruginosa adhesins include the outer core of the lipopolysaccharide (LPS) molecule, which binds to the cystic fibrosis transmembrane conductance regulator (CFTR) and aids in internalization of the organism, and the alginate coat of mucoid strains, which enhances adhesion to cells and mucins. In addition, membrane proteins and lectins have been proposed as colonization factors. The deletion of any given adhesin is not sufficient to abrogate the ability of P. aeruginosa to colonize surfaces. Motility is important in host invasion via mucosal surfaces in some animal models; however, nonmotile strains are not uniformly avirulent.
EVASION OF HOST DEFENSES The transition from bacterial colonization to disease requires the evasion of host defenses followed by invasion of the microorganism. P. aeruginosa appears to be well equipped for evasion. Attached bacteria inject four known toxins (ExoS or ExoU, ExoT, and ExoY) via a type III secretion system that allows the bacteria to evade phagocytic cells either by direct cytotoxicity or by inhibition of phagocytosis. Mutants with defects in this system fail to disseminate in some animal models of infection. The type II secretion system as a whole secretes toxins that can kill animals, and some of its secreted toxins, such as exotoxin A, have the potential to kill phagocytic cells. Multiple proteases secreted by this system may degrade host effector molecules, such as cytokines and chemokines, that are released in response to infection. Thus this system may also contribute to host evasion.
TISSUE INJURY Among gram-negative bacteria, P. aeruginosa probably produces the largest number of substances that are toxic to cells and thus may injure tissues. The toxins secreted by its type III secretion system are capable of tissue injury. However, their delivery requires the adherence of the organism to cells. Thus, the effects of these toxins are likely to be local or to depend on the presence of vast numbers of bacteria. On the other hand, diffusible toxins, secreted by the organism’s type II secretion system, can act freely wherever they come into contact with cells. Possible effectors include exotoxin A, four different proteases, and at least two phospholipases; in addition to these secreted toxins, rhamnolipids, pyocyanin, and hydrocyanic acid are produced by P. aeruginosa and are all capable of inducing host injury.
INFLAMMATORY COMPONENTS The inflammatory components of P. aeruginosa—e.g., the inflammatory responses to the lipid A component of LPSs and to flagellin, mediated through the Toll-like receptor (TLR) system (principally TLR4 and TLR5)—have been thought to represent important factors in disease causation. Although these inflammatory responses are required for successful defense against P. aeruginosa (i.e., in their absence, animals are defenseless against P. aeruginosa infection), florid responses are likely to result in disease. When the sepsis syndrome and septic shock develop in P. aeruginosa infection, they are probably the result of the host response to one or both of these substances, but injury to the lung by Pseudomonas toxins may also result in sepsis syndromes, possibly by causing cell death and the release of cellular components (e.g., heat-shock proteins) that may activate the TLR or another proinflammatory system.
Chronic P. aeruginosa Infections Chronic infection due to P. aeruginosa occurs mainly in the lungs in the setting of structural pulmonary diseases. The classic example is CF; others include bronchiectasis and chronic relapsing panbronchiolitis, a disease seen in Japan and some Pacific Islands. Hallmarks of these illnesses are altered mucociliary clearance leading to mucus stasis and mucus accumulation in the lungs. There is probably a common factor that selects for P. aeruginosa colonization in these lung diseases—perhaps the adhesiveness of P. aeruginosa for mucus, a phenomenon that is not noted for most other common gram-negative bacteria, and/or the ability of P. aeruginosa to evade host defenses in mucus. Furthermore, P. aeruginosa seems to evolve in ways that allow its prolonged survival in the lung without an early fatal outcome for the host. The strains found in CF patients exhibit minimal production of virulence factors. Some strains even lose the ability to produce pili and flagella, and most become complement-sensitive because of the loss of the O side chain of their LPS molecules. An example of the impact of these changes is found in the organism’s discontinuation of the production of flagellin (probably its most strongly proinflammatory molecule) when it encounters purulent mucus. This response probably dampens the host’s response, allowing the organism to survive in mucus. P. aeruginosa is also believed to lose the ability to secrete many of its injectable toxins during growth in mucus. Although the alginate coat is thought to play a role in the organism’s survival, alginate is not essential, as nonmucoid strains may also predominate for long periods. In short, virulence in chronic infections may be mediated by the chronic but attenuated host inflammatory response, which injures the lungs over decades.
CLINICAL MANIFESTATIONS
P. aeruginosa causes infections at almost all sites in the body but shows a rather strong predilection for the lungs. The infections encountered most commonly in hospitalized patients are described below.
Bacteremia Crude mortality rates exceeding 50% have been reported among patients with P. aeruginosa bacteremia. Consequently, this clinical entity has been much feared, and its management has been attempted with the use of multiple antibiotics. Recent publications report attributable mortality rates of 28–44%, with the precise figure depending on the adequacy of treatment and the seriousness of the underlying disease. In the past, the patient with P. aeruginosa bacteremia classically was neutropenic or had a burn injury. Today, however, a minority of such patients have bacteremic P. aeruginosa infections. Rather, P. aeruginosa bacteremia is seen most often in patients in ICUs.
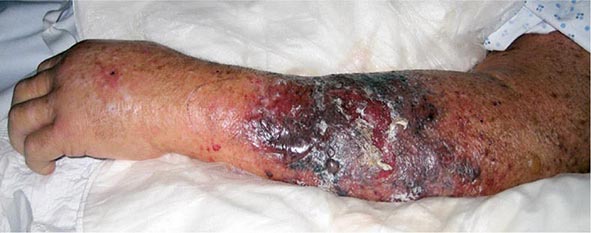
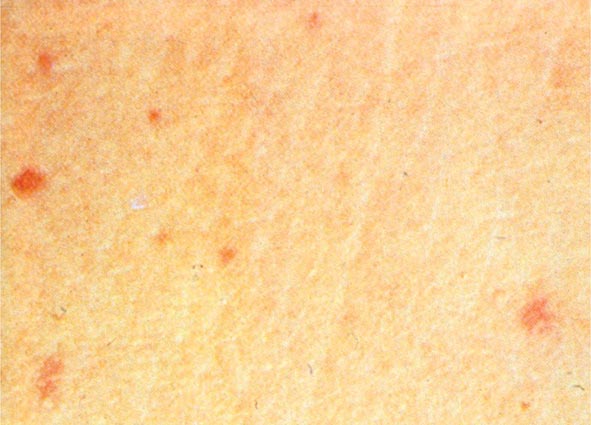
The clinical presentation of P. aeruginosa bacteremia rarely differs from that of sepsis in general (Chap. 324). Patients are usually febrile, but those who are most severely ill may be in shock or even hypothermic. The only point differentiating this entity from gram-negative sepsis of other causes may be the distinctive skin lesions (ecthyma gangrenosum) of Pseudomonas infection, which occur almost exclusively in markedly neutropenic patients and patients with AIDS. These small or large, painful, reddish, maculopapular lesions have a geographic margin; they are initially pink, then darken to purple, and finally become black and necrotic (Fig. 189-1). Histopathologic studies indicate that the lesions are due to vascular invasion and are teeming with bacteria. Although similar lesions may occur in aspergillosis and mucormycosis, their presence suggests P. aeruginosa bacteremia as the most likely diagnosis.
FIGURE 189-1 Ecthyma gangrenosum in a neutropenic patient 3 days after onset.
|
TREATMENT |
P. AERUGINOSA BACTEREMIA |
(Table 189-2) Antimicrobial treatment of P. aeruginosa bacteremia has been controversial. Before 1971, the outcome of Pseudomonas bacteremia in febrile neutropenic patients treated with the available agents—gentamicin and the polymyxins—was dismal. However, treatment with carbenicillin, with or without an aminoglycoside, significantly improved outcomes. Concurrently, several retrospective analyses suggested that the use of two agents that were synergistic against gram-negative pathogens in vitro resulted in better outcomes in neutropenic patients. Thus, combination therapy became the standard of care—first for P. aeruginosa bacteremia in febrile neutropenic patients and then for all P. aeruginosa infections in neutropenic or nonneutropenic patients.
|
ANTIBIOTIC TREATMENT OF INFECTIONS DUE TO PSEUDOMONAS AERUGINOSA AND RELATED SPECIES |
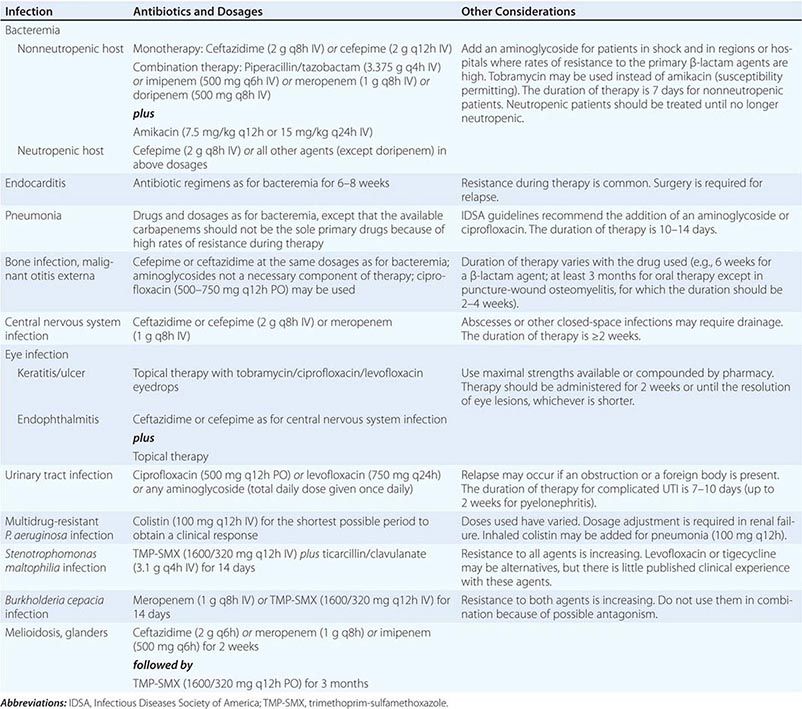
With the introduction of newer antipseudomonal drugs, a number of studies have revisited the choice between combination treatment and monotherapy for Pseudomonas bacteremia. Although the majority of experts still favor combination therapy, most of these observational studies indicate that a single modern antipseudomonal β-lactam agent to which the isolate is sensitive is as efficacious as a combination. Even in patients at greatest risk of early death from P. aeruginosa bacteremia (i.e., those with fever and neutropenia), empirical antipseudomonal monotherapy is deemed to be as efficacious as empirical combination therapy by the practice guidelines of the Infectious Diseases Society of America. One firm conclusion is that monotherapy with an aminoglycoside is not optimal.
![]() There are, of course, institutions and countries where rates of susceptibility of P. aeruginosa to first-line antibiotics are <80%. Thus, when a septic patient with a high probability of P. aeruginosa infection is encountered in such settings, empirical combination therapy should be administered until the pathogen is identified and susceptibility data become available. Thereafter, whether one or two agents should be continued remains a matter of individual preference. Recent studies suggest that extended infusions of β-lactams such as cefepime or piperacillin-tazobactam may result in better outcomes of Pseudomonas bacteremia and possibly Pseudomonas pneumonia.
There are, of course, institutions and countries where rates of susceptibility of P. aeruginosa to first-line antibiotics are <80%. Thus, when a septic patient with a high probability of P. aeruginosa infection is encountered in such settings, empirical combination therapy should be administered until the pathogen is identified and susceptibility data become available. Thereafter, whether one or two agents should be continued remains a matter of individual preference. Recent studies suggest that extended infusions of β-lactams such as cefepime or piperacillin-tazobactam may result in better outcomes of Pseudomonas bacteremia and possibly Pseudomonas pneumonia.
Acute Pneumonia Respiratory infections are the most common of all infections caused by P. aeruginosa. This organism appears first or second among the causes of ventilator-associated pneumonia (VAP). However, much debate centers on the actual role of P. aeruginosa in VAP. Many of the relevant data are based on cultures of sputum or endotracheal tube aspirates and may represent nonpathogenic colonization of the tracheobronchial tree, biofilms on the endotracheal tube, or simple tracheobronchitis.
Older reports of P. aeruginosa pneumonia described patients with an acute clinical syndrome of fever, chills, cough, and necrotizing pneumonia indistinguishable from other gram-negative bacterial pneumonias. The traditional accounts described a fulminant infection. Chest radiographs demonstrated bilateral pneumonia, often with nodular densities with or without cavities. This picture is now remarkably rare. Today, the typical patient is on a ventilator, has a slowly progressive infiltrate, and has been colonized with P. aeruginosa for days. While some cases may progress rapidly over 48–72 h, they are the exceptions. Nodular densities are not commonly seen. However, infiltrates may go on to necrosis. Necrotizing pneumonia has also been seen in the community (e.g., after inhalation of hot-tub water contaminated with P. aeruginosa). The typical patient has fever, leukocytosis, and purulent sputum, and the chest radiograph shows a new infiltrate or the expansion of a preexisting infiltrate. Chest examination generally detects rales or dullness. Of course, such findings are quite common among ventilated patients in the ICU. A sputum Gram’s stain showing mainly polymorphonuclear leukocytes (PMNs) in conjunction with a culture positive for P. aeruginosa in this setting suggests a diagnosis of acute P. aeruginosa pneumonia. There is no consensus about whether an invasive procedure (e.g., bronchoalveolar lavage or protected-brush sampling of the distal airways) is superior to tracheal aspiration to obtain samples for lung cultures in order to substantiate the occurrence of P. aeruginosa pneumonia and prevent antibiotic overuse.
|
TREATMENT |
ACUTE PNEUMONIA |
(Table 189-2) Therapy for P. aeruginosa pneumonia has been unsatisfactory. Reports suggest mortality rates of 40–80%, but how many of these deaths are attributable to underlying disease remains unknown. The drugs of choice for P. aeruginosa pneumonia are similar to those given for bacteremia. A potent antipseudomonal β-lactam drug is the mainstay of therapy. Failure rates were high when aminoglycosides were used as single agents, possibly because of their poor penetration into the airways and their binding to airway secretions. Thus a strong case cannot be made for the inclusion of the aminoglycoside component in regimens used against fully susceptible organisms, especially given the evidence that aminoglycosides are not optimally active in the lungs at concentrations normally reached after IV administration. Nonetheless, aminoglycosides are commonly used in clinical practice. Some experts suggest the combination of a β-lactam agent and an antipseudomonal fluoroquinolone instead when combination therapy is desired.
![]() Chronic Respiratory Tract Infections P. aeruginosa is responsible for chronic infections of the airways associated with a number of underlying or predisposing conditions—most commonly CF (Chap. 313). A state of chronic colonization beginning early in childhood is seen in some Asian populations with chronic or diffuse panbronchiolitis, a disease of unknown etiology. P. aeruginosa is one of the organisms that colonizes damaged bronchi in bronchiectasis, a disease secondary to multiple causes in which profound structural abnormalities of the airways result in mucus stasis.
Chronic Respiratory Tract Infections P. aeruginosa is responsible for chronic infections of the airways associated with a number of underlying or predisposing conditions—most commonly CF (Chap. 313). A state of chronic colonization beginning early in childhood is seen in some Asian populations with chronic or diffuse panbronchiolitis, a disease of unknown etiology. P. aeruginosa is one of the organisms that colonizes damaged bronchi in bronchiectasis, a disease secondary to multiple causes in which profound structural abnormalities of the airways result in mucus stasis.
|
TREATMENT |
CHRONIC RESPIRATORY TRACT INFECTIONS |
Optimal management of chronic P. aeruginosa lung infection has not been determined. Patients respond clinically to antipseudomonal therapy, but the organism is rarely eradicated. Because eradication is unlikely, the aim of treatment for chronic infection is to quell exacerbations of inflammation. The regimens used are similar to those used for pneumonia, but an aminoglycoside is almost always added because resistance is common in chronic disease. However, it may be appropriate to use an inhaled aminoglycoside preparation in order to maximize airway drug levels.
Endovascular Infections Infective endocarditis due to P. aeruginosa is a disease of IV drug users whose native valves are involved. This organism has also been reported to cause prosthetic valve endocarditis. Sites of prior native-valve injury due to the injection of foreign material such as talc or fibers probably serve as niduses for bacterial attachment to the heart valve. The manifestations of P. aeruginosa endocarditis resemble those of other forms of endocarditis in IV drug users except that the disease is more indolent than Staphylococcus aureus endocarditis. While most disease involves the right side of the heart, left-sided involvement is not rare and multivalvular disease is common. Fever is a common manifestation, as is pulmonary involvement (due to septic emboli to the lungs). Hence, patients may also experience chest pain and hemoptysis. Involvement of the left side of the heart may lead to signs of cardiac failure, systemic emboli, and local cardiac involvement with sinus of Valsalva abscesses and conduction defects. Skin manifestations are rare in this disease, and ecthyma gangrenosum is not seen. The diagnosis is based on positive blood cultures along with clinical signs of endocarditis.
|
TREATMENT |
ENDOVASCULAR INFECTIONS |
(Table 189-2) It has been customary to use synergistic antibiotic combinations in treating P. aeruginosa endocarditis because of the development of resistance during therapy with a single antipseudomonal β-lactam agent. Which combination therapy is preferable is unclear, as all combinations have failed. Cases of P. aeruginosa endocarditis that relapse during or fail to respond to therapy are often caused by resistant organisms and may require surgical therapy. Other considerations for valve replacement are similar to those in other forms of endocarditis (Chap. 155).
Bone and Joint Infections P. aeruginosa is an infrequent cause of bone and joint infections. However, Pseudomonas bacteremia or infective endocarditis caused by the injection of contaminated illicit drugs has been documented to result in vertebral osteomyelitis and sternoclavicular joint arthritis. The clinical presentation of vertebral P. aeruginosa osteomyelitis is more indolent than that of staphylococcal osteomyelitis. The duration of symptoms in IV drug users with vertebral osteomyelitis due to P. aeruginosa varies from weeks to months. Fever is not uniformly present; when present, it tends to be low grade. There may be mild tenderness at the site of involvement. Blood cultures are usually negative unless there is concomitant endocarditis. The erythrocyte sedimentation rate (ESR) is generally elevated. Vertebral osteomyelitis due to P. aeruginosa has also been reported in the elderly, in whom it originates from urinary tract infections (UTIs). The infection generally involves the lumbosacral area because of a shared venous drainage (Batson’s plexus) between the lumbosacral spine and the pelvis. Sternoclavicular septic arthritis due to P. aeruginosa is seen almost exclusively in IV drug users. This disease may occur with or without endocarditis, and a primary site of infection often is not found. Plain radiographs show joint or bone involvement. Treatment of these forms of disease is generally successful.
Pseudomonas osteomyelitis of the foot most often follows puncture wounds through sneakers and mostly affects children. The main manifestation is pain in the foot, sometimes with superficial cellulitis around the puncture wound and tenderness on deep palpation of the wound. Multiple joints or bones of the foot may be involved. Systemic symptoms are generally absent, and blood cultures are usually negative. Radiographs may or may not be abnormal, but the bone scan is usually positive, as are magnetic resonance imaging (MRI) studies. Needle aspiration usually yields a diagnosis. Prompt surgery, with exploration of the nail puncture tract and debridement of the involved bones and cartilage, is generally recommended in addition to antibiotic therapy.
Central Nervous System (CNS) Infections CNS infections due to P. aeruginosa are relatively rare. Involvement of the CNS is almost always secondary to a surgical procedure or head trauma. The entity seen most often is postoperative or posttraumatic meningitis. Subdural or epidural infection occasionally results from contamination of these areas. Embolic disease arising from endocarditis in IV drug users and leading to brain abscesses has also been described. The cerebrospinal fluid (CSF) profile of P. aeruginosa meningitis is no different from that of pyogenic meningitis of any other etiology.
|
TREATMENT |
CENTRAL NERVOUS SYSTEM INFECTIONS |
(Table 189-2) Treatment of Pseudomonas meningitis is difficult; little information has been published, and no controlled trials in humans have been undertaken. However, the general principles involved in the treatment of meningitis apply, including the need for high doses of bactericidal antibiotics to attain high drug levels in the CSF. The agent with which there is the most published experience in P. aeruginosa meningitis is ceftazidime, but other antipseudomonal β-lactam drugs that reach high CSF concentrations, such as cefepime and meropenem, have also been used successfully. Other forms of P. aeruginosa CNS infection, such as brain abscesses and epidural and subdural empyema, generally require surgical drainage in addition to antibiotic therapy.
Eye Infections Eye infections due to P. aeruginosa occur mainly as a result of direct inoculation into the tissue during trauma or surface injury by contact lenses. Keratitis and corneal ulcers are the most common types of eye disease and are often associated with contact lenses (especially the extended-wear variety). Keratitis can be slowly or rapidly progressive, but the classic description is disease progressing over 48 h to involve the entire cornea, with opacification and sometimes perforation. P. aeruginosa keratitis should be considered a medical emergency because of the rapidity with which it can progress to loss of sight. P. aeruginosa endophthalmitis secondary to bacteremia is the most devastating of P. aeruginosa eye infections. The disease is fulminant, with severe pain, chemosis, decreased visual acuity, anterior uveitis, vitreous involvement, and panophthalmitis.
|
TREATMENT |
EYE INFECTIONS |
(Table 189-2) The usual therapy for keratitis is the administration of topical antibiotics. Therapy for endophthalmitis includes the use of high-dose local and systemic antibiotics (to achieve higher drug concentrations in the eye) and vitrectomy.
Ear Infections P. aeruginosa infections of the ears vary from mild swimmer’s ear to serious life-threatening infections with neurologic sequelae. Swimmer’s ear is common among children and results from infection of moist macerated skin of the external ear canal. Most cases resolve with treatment, but some patients develop chronic drainage. Swimmer’s ear is managed with topical antibiotic agents (otic solutions). The most serious form of Pseudomonas infection involving the ear has been given various names: two of these designations, malignant otitis externa and necrotizing otitis externa, are now used for the same entity. This disease was originally described in elderly diabetic patients, in whom the majority of cases still occur. However, it has also been described in patients with AIDS and in elderly patients without underlying diabetes or immunocompromise. The usual presenting symptoms are decreased hearing and ear pain, which may be severe and lancinating. The pinna is usually painful, and the external canal may be tender. The ear canal almost always shows signs of inflammation, with granulation tissue and exudate. Tenderness anterior to the tragus may extend as far as the temporomandibular joint and mastoid process. A small minority of patients have systemic symptoms. Patients in whom the diagnosis is made late may present with cranial nerve palsies or even with cavernous venous sinus thrombosis. The ESR is invariably elevated (≥100 mm/h). The diagnosis is made on clinical grounds in severe cases; however, the “gold standard” is a positive technetium-99 bone scan in a patient with otitis externa due to P. aeruginosa. In diabetic patients, a positive bone scan constitutes presumptive evidence for this diagnosis and should prompt biopsy or empirical therapy.
|
TREATMENT |
EAR INFECTIONS |
(Table 189-2) Given the infection of the ear cartilage, sometimes with mastoid or petrous ridge involvement, patients with malignant (necrotizing) otitis externa are treated as for osteomyelitis.
Urinary Tract Infections UTIs due to P. aeruginosa generally occur as a complication of a foreign body in the urinary tract, an obstruction in the genitourinary system, or urinary tract instrumentation or surgery. However, UTIs caused by P. aeruginosa have been described in pediatric outpatients without stones or evident obstruction.
|
TREATMENT |
URINARY TRACT INFECTIONS |
(Table 189-2) Most P. aeruginosa UTIs are considered complicated infections that must be treated longer than uncomplicated cystitis. In general, a 7- to 10-day course of treatment suffices, with up to 2 weeks of therapy in cases of pyelonephritis. Urinary catheters, stents, or stones should be removed to prevent relapse, which is common and may be due not to resistance but rather to factors such as a foreign body that has been left in place or an ongoing obstruction.
Skin and Soft Tissue Infections Besides pyoderma gangrenosum in neutropenic patients, folliculitis and other papular or vesicular lesions due to P. aeruginosa have been extensively described and are collectively referred to as dermatitis. Multiple outbreaks have been linked to whirlpools, spas, and swimming pools. To prevent such outbreaks, the growth of P. aeruginosa in the home and in recreational environments must be controlled by proper chlorination of water. Most cases of hot-tub folliculitis are self-limited, requiring only the avoidance of exposure to the contaminated source of water.
![]() Toe-web infections occur especially often in the tropics, and the “green nail syndrome” is caused by P. aeruginosa paronychia, which results from frequent submersion of the hands in water. In the latter entity, the green discoloration results from diffusion of pyocyanin into the nail bed. P. aeruginosa remains a prominent cause of burn wound infections in some parts of the world. The management of these infections is best left to specialists in burn wound care.
Toe-web infections occur especially often in the tropics, and the “green nail syndrome” is caused by P. aeruginosa paronychia, which results from frequent submersion of the hands in water. In the latter entity, the green discoloration results from diffusion of pyocyanin into the nail bed. P. aeruginosa remains a prominent cause of burn wound infections in some parts of the world. The management of these infections is best left to specialists in burn wound care.
![]() Infections in Febrile Neutropenic Patients In febrile neutropenia, P. aeruginosa has historically been the organism against which empirical coverage is always essential. Although in Western countries these infections are now less common, their importance has not diminished because of persistently high mortality rates. In other parts of the world as well, P. aeruginosa continues to be a significant problem in febrile neutropenia, causing a larger proportion of infections in febrile neutropenic patients than any other single organism. For example, P. aeruginosa was responsible for 28% of documented infections in 499 febrile neutropenic patients in one study from the Indian subcontinent and for 31% of such infections in another. In a large study of infections in leukemia patients from Japan, P. aeruginosa was the most frequently documented cause of bacterial infection. In studies performed in North America, northern Europe, and Australia, the incidence of P. aeruginosa bacteremia in febrile neutropenia was quite variable. In a review of 97 reports published in 1987–1994, the incidence was reported to be 1–2.5% among febrile neutropenic patients given empirical therapy and 5–12% among microbiologically documented infections. The most common clinical syndromes encountered were bacteremia, pneumonia, and soft tissue infections manifesting mainly as ecthyma gangrenosum.
Infections in Febrile Neutropenic Patients In febrile neutropenia, P. aeruginosa has historically been the organism against which empirical coverage is always essential. Although in Western countries these infections are now less common, their importance has not diminished because of persistently high mortality rates. In other parts of the world as well, P. aeruginosa continues to be a significant problem in febrile neutropenia, causing a larger proportion of infections in febrile neutropenic patients than any other single organism. For example, P. aeruginosa was responsible for 28% of documented infections in 499 febrile neutropenic patients in one study from the Indian subcontinent and for 31% of such infections in another. In a large study of infections in leukemia patients from Japan, P. aeruginosa was the most frequently documented cause of bacterial infection. In studies performed in North America, northern Europe, and Australia, the incidence of P. aeruginosa bacteremia in febrile neutropenia was quite variable. In a review of 97 reports published in 1987–1994, the incidence was reported to be 1–2.5% among febrile neutropenic patients given empirical therapy and 5–12% among microbiologically documented infections. The most common clinical syndromes encountered were bacteremia, pneumonia, and soft tissue infections manifesting mainly as ecthyma gangrenosum.
|
TREATMENT |
INFECTIONS IN FEBRILE NEUTROPENIC PATIENTS |
(Table 189-2) Compared with rates three decades ago, improved rates of response to antibiotic therapy have been reported in many studies. A study of 127 patients demonstrated a reduction in the mortality rate from 71% to 25% with the introduction of ceftazidime and imipenem. Because neutrophils—the normal host defenses against this organism—are absent in febrile neutropenic patients, maximal doses of antipseudomonal β-lactam antibiotics should be used for the management of P. aeruginosa bacteremia in this setting.
Infections in Patients with AIDS Both community- and hospital-acquired P. aeruginosa infections were documented in patients with AIDS before the advent of antiretroviral therapy. Since the introduction of protease inhibitors, P. aeruginosa infections in AIDS patients have been seen less frequently but still occur, particularly in the form of sinusitis. The clinical presentation of Pseudomonas infection (especially pneumonia and bacteremia) in AIDS patients is remarkable in that, although the illness may appear not to be severe, the infection may nonetheless be fatal. Patients with bacteremia may have only a low-grade fever and may present with ecthyma gangrenosum. Pneumonia, with or without bacteremia, is perhaps the most common type of P. aeruginosa infection in AIDS patients. Patients with AIDS and P. aeruginosa pneumonia exhibit the classic clinical signs and symptoms of pneumonia, such as fever, productive cough, and chest pain. The infection may be lobar or multilobar and shows no predisposition for any particular location. The most striking feature is the high frequency of cavitary disease.
|
TREATMENT |
INFECTIONS IN PATIENTS WITH AIDS |
Therapy for any of these conditions in AIDS patients is no different from that in other patients. However, relapse is the rule unless the patient’s CD4+ T cell count rises to >50/μL or suppressive antibiotic therapy is given. In attempts to achieve cures and prevent relapses, therapy tends to be more prolonged than in the case of an immunocompetent patient.
![]() Multidrug-Resistant Infections (Table 189-2) P. aeruginosa has a notorious propensity to develop antibiotic resistance. During three decades, the impact of resistance was minimized by the rapid development of potent antipseudomonal agents. However, the situation has recently changed, with the worldwide selection of strains carrying determinants that mediate resistance to β-lactams, fluoroquinolones, and aminoglycosides. This situation has been compounded by the lack of development of new classes of antipseudomonal drugs for nearly two decades. Physicians now resort to drugs such as colistin and polymyxin, which were discarded decades ago. These alternative approaches to the management of multiresistant P. aeruginosa infections were first used some time ago in CF patients, who receive colistin (polymyxin E) IV and by aerosol despite its renal toxicity. Colistin is rapidly becoming the last-resort agent of choice, even in non-CF patients infected with multiresistant P. aeruginosa.
Multidrug-Resistant Infections (Table 189-2) P. aeruginosa has a notorious propensity to develop antibiotic resistance. During three decades, the impact of resistance was minimized by the rapid development of potent antipseudomonal agents. However, the situation has recently changed, with the worldwide selection of strains carrying determinants that mediate resistance to β-lactams, fluoroquinolones, and aminoglycosides. This situation has been compounded by the lack of development of new classes of antipseudomonal drugs for nearly two decades. Physicians now resort to drugs such as colistin and polymyxin, which were discarded decades ago. These alternative approaches to the management of multiresistant P. aeruginosa infections were first used some time ago in CF patients, who receive colistin (polymyxin E) IV and by aerosol despite its renal toxicity. Colistin is rapidly becoming the last-resort agent of choice, even in non-CF patients infected with multiresistant P. aeruginosa.
The clinical outcome of multidrug-resistant P. aeruginosa infections treated with colistin is difficult to judge from case reports, especially given the many drugs used in the complicated management of these patients. Although earlier reports described marginal efficacy and serious nephrotoxicity and neurotoxicity, recent reports have been more encouraging. Because colistin shows synergy with other antimicrobial agents in vitro, it may be possible to reduce the dosage—and thus the toxicity—of this drug when it is combined with drugs such as rifampin and β-lactams; however, no studies in humans or animals support this approach at this time.
OTHER PSEUDOMONADS
STENOTROPHOMONAS MALTOPHILIA
S. maltophilia is the only potential human pathogen among a genus of ubiquitous organisms found in the rhizosphere (i.e., the soil that surrounds the roots of plants). The organism is an opportunist that is acquired from the environment but is even more limited than P. aeruginosa in its ability to colonize patients or cause infections. Immunocompromise is not sufficient to permit these events; rather, major perturbations of the human flora are usually necessary for the establishment of S. maltophilia. Accordingly, most cases of human infection occur in the setting of very broad-spectrum antibiotic therapy with agents such as advanced cephalosporins and carbapenems, which eradicate the normal flora and other pathogens. The remarkable ability of S. maltophilia to resist virtually all classes of antibiotics is attributable to the possession of antibiotic efflux pumps and of two β-lactamases (L1 and L2) that mediate β-lactam resistance, including that to carbapenems. It is fortunate that the virulence of S. maltophilia appears to be limited. Although a serine protease is present in some strains, virulence is probably a result of the host’s inflammatory response to components of the organism such as LPS and flagellin. S. maltophilia is most commonly found in the respiratory tract of ventilated patients, where the distinction between its roles as a colonizer and as a pathogen is often difficult to make. However, S. maltophilia does cause pneumonia and bacteremia in such patients, and these infections have led to septic shock. Also common is central venous line–associated infection (with or without bacteremia), which has been reported most often in patients with cancer. S. maltophilia is a rare cause of ecthyma gangrenosum in neutropenic patients. It has been isolated from ~5% of CF patients but is not believed to be a significant pathogen in this setting.
|
TREATMENT |
S. MALTOPHILIA INFECTIONS |
The intrinsic resistance of S. maltophilia to most antibiotics renders infection difficult to treat. The antibiotics to which it is most often (although not uniformly) susceptible are trimethoprim-sulfamethoxazole (TMP-SMX), ticarcillin/clavulanate, levofloxacin, and tigecycline (Table 189-2). Consequently, a combination of TMP-SMX and ticarcillin/clavulanate is recommended for initial therapy. Catheters must be removed in the treatment of bacteremia to hasten cure and prevent relapses. The treatment of VAP due to S. maltophilia is much more difficult than that of bacteremia, with the frequent development of resistance during therapy.
BURKHOLDERIA CEPACIA
B. cepacia gained notoriety as the cause of a rapidly fatal syndrome of respiratory distress and septicemia (the “cepacia syndrome”) in CF patients. Previously, it had been recognized as an antibiotic-resistant nosocomial pathogen (then designated P. cepacia) in ICU patients. Patients with chronic granulomatous disease are also predisposed to B. cepacia lung disease. The organism has been reclassified into nine subgroups, only some of which are common in CF. B. cepacia is an environmental organism that inhabits moist environments and is found in the rhizosphere. This organism possesses multiple virulence factors that may play roles in disease as well as colonizing factors that are capable of binding to lung mucus—an ability that may explain the predilection of B. cepacia for the lungs in CF. B. cepacia secretes elastase and possesses components of an injectable toxin-secretion system like that of P. aeruginosa; its LPS is among the most potent of all LPSs in stimulating an inflammatory response in the lungs. Inflammation may be the major cause of the lung disease seen in the cepacia syndrome. The organism can penetrate epithelial surfaces by virtue of motility and inhibition of host innate immune defenses. Besides infecting the lungs in CF, B. cepacia appears as an airway colonizer during broad-spectrum antibiotic therapy and is a cause of VAP, catheter-associated infections, and wound infections.
|
TREATMENT |
B. CEPACIA INFECTIONS |
B. cepacia is intrinsically resistant to many antibiotics. Therefore, treatment must be tailored according to sensitivities. TMP-SMX, meropenem, and doxycycline are the most effective agents in vitro and may be started as first-line agents (Table 189-2). Some strains are susceptible to third-generation cephalosporins and fluoroquinolones, and these agents may be used against isolates known to be susceptible. Combination therapy for serious pulmonary infection (e.g., in CF) is suggested when multidrug-resistant strains are implicated; the combination of meropenem and TMP-SMX may be antagonistic, however. Resistance to all agents used has been reported during therapy.
BURKHOLDERIA PSEUDOMALLEI
![]() B. pseudomallei is the causative agent of melioidosis, a disease of humans and animals that is geographically restricted to Southeast Asia and northern Australia, with occasional cases in countries such as India and China. This organism may be isolated from individuals returning directly from these endemic regions and from military personnel who have served in endemic regions and then returned home after stops in Europe. Symptoms of this illness may develop only at a later date because of the organism’s ability to cause latent infections. B. pseudomallei is found in soil and water. Humans and animals are infected by inoculation, inhalation, or ingestion; only rarely is the organism transmitted from person to person. Humans are not colonized without being infected. Among the pseudomonads, B. pseudomallei is perhaps the most virulent. Host compromise is not an essential prerequisite for disease, although many patients have common underlying medical diseases (e.g., diabetes or renal failure). B. pseudomallei is a facultative intracellular organism whose replication in PMNs and macrophages may be aided by the possession of a polysaccharide capsule. The organism also possesses elements of a type III secretion system that plays a role in its intracellular survival. During infection, there is a florid inflammatory response whose role in disease is unclear.
B. pseudomallei is the causative agent of melioidosis, a disease of humans and animals that is geographically restricted to Southeast Asia and northern Australia, with occasional cases in countries such as India and China. This organism may be isolated from individuals returning directly from these endemic regions and from military personnel who have served in endemic regions and then returned home after stops in Europe. Symptoms of this illness may develop only at a later date because of the organism’s ability to cause latent infections. B. pseudomallei is found in soil and water. Humans and animals are infected by inoculation, inhalation, or ingestion; only rarely is the organism transmitted from person to person. Humans are not colonized without being infected. Among the pseudomonads, B. pseudomallei is perhaps the most virulent. Host compromise is not an essential prerequisite for disease, although many patients have common underlying medical diseases (e.g., diabetes or renal failure). B. pseudomallei is a facultative intracellular organism whose replication in PMNs and macrophages may be aided by the possession of a polysaccharide capsule. The organism also possesses elements of a type III secretion system that plays a role in its intracellular survival. During infection, there is a florid inflammatory response whose role in disease is unclear.
B. pseudomallei causes a wide spectrum of disease, ranging from asymptomatic infection to abscesses, pneumonia, and disseminated disease. It is a significant cause of fatal community-acquired pneumonia and septicemia in endemic areas, with mortality rates as high as 44% reported in Thailand. Acute pulmonary infection is the most commonly diagnosed form of melioidosis. Pneumonia may be asymptomatic (with routine chest radiographs showing mainly upper-lobe infiltrates) or may present as severe necrotizing disease. B. pseudomallei also causes chronic pulmonary infections with systemic manifestations that mimic those of tuberculosis, including chronic cough, fever, hemoptysis, night sweats, and cavitary lung disease. Besides pneumonia, the other principal form of B. pseudomallei disease is skin ulceration with associated lymphangitis and regional lymphadenopathy. Spread from the lungs or skin, which is most often documented in debilitated individuals, gives rise to septicemic forms of melioidosis that carry a high mortality rate.
|
TREATMENT |
B. PSEUDOMALLEI INFECTIONS |
B. pseudomallei is susceptible to advanced penicillins and cephalosporins and to carbapenems (Table 189-2). Treatment is divided into two stages: an intensive 2-week phase of therapy with ceftazidime or a carbapenem followed by at least 12 weeks of oral TMP-SMX to eradicate the organism and prevent relapse. The recognition of this bacterium as a potential agent of biologic warfare has stimulated interest in the development of a vaccine.
BURKHOLDERIA MALLEI
![]() B. mallei causes the equine disease glanders in Africa, Asia, and South America. The organism was eradicated from Europe and North America decades ago. The last case seen in the United States occurred in 2001 in a laboratory worker; before that, B. mallei had last been seen in this country in 1949. In contrast to the other organisms discussed in this chapter, B. mallei is not an environmental organism and does not persist outside its equine hosts. Consequently, B. mallei infection is an occupational risk for handlers of horses, equine butchers, and veterinarians in areas of the world where it still exists. The polysaccharide capsule is a critical virulence determinant; diabetics are thought to be especially susceptible to infection by this organism. The organism is transmitted from animals to humans by inoculation into the skin, where it causes local infection with nodules and lymphadenitis. Regional lymphadenopathy is common. Respiratory secretions from infected horses are extremely infectious. Inhalation results in clinical signs of typical pneumonia but may also cause an acute febrile illness with ulceration of the trachea. The organism may disseminate from the skin or lungs to cause septicemia with signs of sepsis. The septicemic form is frequently associated with shock and a high mortality rate. The infection may also enter a chronic phase and present as disseminated abscesses. B. mallei infection may present as early as 1–2 days after inhalation or (in cutaneous disease) may not become evident for months.
B. mallei causes the equine disease glanders in Africa, Asia, and South America. The organism was eradicated from Europe and North America decades ago. The last case seen in the United States occurred in 2001 in a laboratory worker; before that, B. mallei had last been seen in this country in 1949. In contrast to the other organisms discussed in this chapter, B. mallei is not an environmental organism and does not persist outside its equine hosts. Consequently, B. mallei infection is an occupational risk for handlers of horses, equine butchers, and veterinarians in areas of the world where it still exists. The polysaccharide capsule is a critical virulence determinant; diabetics are thought to be especially susceptible to infection by this organism. The organism is transmitted from animals to humans by inoculation into the skin, where it causes local infection with nodules and lymphadenitis. Regional lymphadenopathy is common. Respiratory secretions from infected horses are extremely infectious. Inhalation results in clinical signs of typical pneumonia but may also cause an acute febrile illness with ulceration of the trachea. The organism may disseminate from the skin or lungs to cause septicemia with signs of sepsis. The septicemic form is frequently associated with shock and a high mortality rate. The infection may also enter a chronic phase and present as disseminated abscesses. B. mallei infection may present as early as 1–2 days after inhalation or (in cutaneous disease) may not become evident for months.
|
TREATMENT |
B. MALLEI INFECTIONS |
The antibiotic susceptibility pattern of B. mallei is similar to that of B. pseudomallei; in addition, the organism is susceptible to the newer macrolides azithromycin and clarithromycin. B. mallei infection should be treated with the same drugs and for the same duration as melioidosis.
190 |
Salmonellosis |
Bacteria of the genus Salmonella are highly adapted for growth in both humans and animals and cause a wide spectrum of disease. The growth of serotypes Salmonella typhi and Salmonella paratyphi is restricted to human hosts, in whom these organisms cause enteric (typhoid) fever. The remaining serotypes (nontyphoidal Salmonella, or NTS) can colonize the gastrointestinal tracts of a broad range of animals, including mammals, reptiles, birds, and insects. More than 200 serotypes of Salmonella are pathogenic to humans, in whom they often cause gastroenteritis and can be associated with localized infections and/or bacteremia.
ETIOLOGY
This large genus of gram-negative bacilli within the family Enterobacteriaceae consists of two species: Salmonella enterica, which contains six subspecies, and Salmonella bongori. S. enterica subspecies I includes almost all the serotypes pathogenic for humans. Members of the seven Salmonella subspecies are classified into >2500 serotypes (serovars); for simplicity, Salmonella serotypes (most of which are named for the city where they were identified) are often used as the species designation. For example, the full taxonomic designation S. enterica subspecies enterica serotype Typhimurium can be shortened to Salmonella serotype Typhimurium or simply S. typhimurium. Serotyping is based on the somatic O antigen (lipopolysaccharide cell-wall components), the surface Vi antigen (restricted to S. typhi and S. paratyphi C), and the flagellar H antigen.
Salmonellae are gram-negative, non-spore-forming, facultatively anaerobic bacilli that measure 2–3 μm by 0.4–0.6 μm. The initial identification of salmonellae in the clinical microbiology laboratory is based on growth characteristics. Salmonellae, like other Enterobacteriaceae, produce acid on glucose fermentation, reduce nitrates, and do not produce cytochrome oxidase. In addition, all salmonellae except Salmonella gallinarum–pullorum are motile by means of peritrichous flagella, and all but S. typhi produce gas (H2S) on sugar fermentation. Notably, only 1% of clinical isolates ferment lactose; a high level of suspicion must be maintained to detect these rare clinical lactose-fermenting isolates.
Although serotyping of all surface antigens can be used for formal identification, most laboratories perform a few simple agglutination reactions that define specific O-antigen serogroups, designated A, B, C1, C2, D, and E. Strains in these six serogroups cause ~99% of Salmonella infections in humans and other warm-blooded animals. Molecular typing methods, including pulsed-field gel electrophoresis, polymerase chain reaction fingerprinting, and genomic DNA microarray analysis, are used in epidemiologic investigations to differentiate Salmonella strains of a common serotype.
PATHOGENESIS
All Salmonella infections begin with ingestion of organisms, most commonly in contaminated food or water. The infectious dose ranges from 200 colony-forming units (CFU) to 106 CFU, and the ingested dose is an important determinant of incubation period and disease severity. Conditions that decrease either stomach acidity (an age of <1 year, antacid ingestion, or achlorhydric disease) or intestinal integrity (inflammatory bowel disease, prior gastrointestinal surgery, or alteration of the intestinal flora by antibiotic administration) increase susceptibility to Salmonella infection.
Once S. typhi and S. paratyphi reach the small intestine, they penetrate the mucus layer of the gut and traverse the intestinal layer through phagocytic microfold (M) cells that reside within Peyer’s patches. Salmonellae can trigger the formation of membrane ruffles in normally nonphagocytic epithelial cells. These ruffles reach out and enclose adherent bacteria within large vesicles by bacterial-mediated endocytosis. This process is dependent on the direct delivery of Salmonella proteins into the cytoplasm of epithelial cells by the specialized bacterial type III secretion system. These bacterial proteins mediate alterations in the actin cytoskeleton that are required for Salmonella uptake.
After crossing the epithelial layer of the small intestine, S. typhi and S. paratyphi, which cause enteric (typhoid) fever, are phagocytosed by macrophages. These salmonellae survive the antimicrobial environment of the macrophage by sensing environmental signals that trigger alterations in regulatory systems of the phagocytosed bacteria. For example, PhoP/PhoQ (the best-characterized regulatory system) triggers the expression of outer-membrane proteins and mediates modifications in lipopolysaccharide so that the altered bacterial surface can resist microbicidal activities and potentially alter host cell signaling. In addition, salmonellae encode a second type III secretion system that directly delivers bacterial proteins across the phagosome membrane into the macrophage cytoplasm. This secretion system functions to remodel the Salmonella-containing vacuole, promoting bacterial survival and replication.
Once phagocytosed, typhoidal salmonellae disseminate throughout the body in macrophages via the lymphatics and colonize reticuloendothelial tissues (liver, spleen, lymph nodes, and bone marrow). Patients have relatively few or no signs and symptoms during this initial incubation stage. Signs and symptoms, including fever and abdominal pain, probably result from secretion of cytokines by macrophages and epithelial cells in response to bacterial products that are recognized by innate immune receptors when a critical number of organisms have replicated. Over time, the development of hepatosplenomegaly is likely to be related to the recruitment of mononuclear cells and the development of a specific acquired cell-mediated immune response to S. typhi colonization. The recruitment of additional mononuclear cells and lymphocytes to Peyer’s patches during the several weeks after initial colonization/infection can result in marked enlargement and necrosis of the Peyer’s patches, which may be mediated by bacterial products that promote cell death as well as the inflammatory response.
In contrast to enteric fever, which is characterized by an infiltration of mononuclear cells into the small-bowel mucosa, NTS gastroenteritis is characterized by massive polymorphonuclear leukocyte infiltration into both the large- and small-bowel mucosa. This response appears to depend on the induction of interleukin 8, a strong neutrophil chemotactic factor, which is secreted by intestinal cells as a result of Salmonella colonization and translocation of bacterial proteins into host cell cytoplasm. The degranulation and release of toxic substances by neutrophils may result in damage to the intestinal mucosa, causing the inflammatory diarrhea observed with nontyphoidal gastroenteritis. An additional important factor in the persistence of nontyphoidal salmonellae in the intestinal tract and the organisms’ capacity to compete with endogenous flora is the ability to utilize the sulfur-containing compound tetrathionate for metabolism in a microaerophilic environment. In the presence of intestinal inflammation, tetrathionate is generated from thiosulfate produced by epithelial cells through inflammatory cell production of reactive oxygen species.
ENTERIC (TYPHOID) FEVER
Enteric (typhoid) fever is a systemic disease characterized by fever and abdominal pain and caused by dissemination of S. typhi or S. paratyphi. The disease was initially called typhoid fever because of its clinical similarity to typhus. In the early 1800s, typhoid fever was clearly defined pathologically as a unique illness on the basis of its association with enlarged Peyer’s patches and mesenteric lymph nodes. In 1869, given the anatomic site of infection, the term enteric fever was proposed as an alternative designation to distinguish typhoid fever from typhus. However, to this day, the two designations are used interchangeably.
EPIDEMIOLOGY
![]() In contrast to other Salmonella serotypes, the etiologic agents of enteric fever—S. typhi and S. paratyphi serotypes A, B, and C—have no known hosts other than humans. Most commonly, food-borne or waterborne transmission results from fecal contamination by ill or asymptomatic chronic carriers. Sexual transmission between male partners has been described. Health care workers occasionally acquire enteric fever after exposure to infected patients or during processing of clinical specimens and cultures.
In contrast to other Salmonella serotypes, the etiologic agents of enteric fever—S. typhi and S. paratyphi serotypes A, B, and C—have no known hosts other than humans. Most commonly, food-borne or waterborne transmission results from fecal contamination by ill or asymptomatic chronic carriers. Sexual transmission between male partners has been described. Health care workers occasionally acquire enteric fever after exposure to infected patients or during processing of clinical specimens and cultures.
With improvements in food handling and water/sewage treatment, enteric fever has become rare in developed nations. Worldwide, however, there are an estimated 27 million cases of enteric fever, with 200,000–600,000 deaths annually. The annual incidence is highest (>100 cases/100,000 population) in south-central and Southeast Asia; medium (10–100 cases/100,000) in the rest of Asia, Africa, Latin America, and Oceania (excluding Australia and New Zealand); and low in other parts of the world (Fig. 190-1). A high incidence of enteric fever correlates with poor sanitation and lack of access to clean drinking water. In endemic regions, enteric fever is more common in urban than rural areas and among young children and adolescents than among other age groups. Risk factors include contaminated water or ice, flooding, food and drinks purchased from street vendors, raw fruits and vegetables grown in fields fertilized with sewage, ill household contacts, lack of hand washing and toilet access, and evidence of prior Helicobacter pylori infection (an association probably related to chronically reduced gastric acidity). It is estimated that there is one case of paratyphoid fever for every four cases of typhoid fever, but the incidence of infection associated with S. paratyphi A appears to be increasing, especially in India; this increase may be a result of vaccination for S. typhi.
FIGURE 190-1 Annual incidence of typhoid fever per 100,000 population. (Adapted from JA Crump et al: The global burden of typhoid fever. Bull World Health Organ 82:346, 2004.)
Multidrug-resistant (MDR) strains of S. typhi emerged in the 1980s in China and Southeast Asia and have since disseminated widely. These strains contain plasmids encoding resistance to chloramphenicol, ampicillin, and trimethoprim—antibiotics long used to treat enteric fever. With the increased use of fluoroquinolones to treat MDR enteric fever in the 1990s, strains of S. typhi and S. paratyphi with decreased ciprofloxacin susceptibility (DCS; minimal inhibitory concentration [MIC], 0.125–0.5 μg/mL) or ciprofloxacin resistance (MIC, ≥1 μg/mL) have emerged on the Indian subcontinent, in southern Asia, and (most recently) in sub-Saharan Africa and have been associated with clinical treatment failure. Testing of isolates for resistance to the first-generation quinolone nalidixic acid detects many but not all strains with reduced susceptibility to ciprofloxacin and is no longer recommended. Strains of S. typhi and S. paratyphi producing extended-spectrum β-lactamases have emerged recently, primarily in India and Nepal.
Approximately 300 cases of typhoid and 150 cases of paratyphoid fever are reported annually in the United States. Of 1902 cases of S. typhi–associated enteric fever reported to the Centers for Disease Control and Prevention in 1999–2006, 79% were associated with recent international travel, most commonly to India (47%), Pakistan (10%), Bangladesh (10%), Mexico (7%), and the Philippines (4%). Only 5% of travelers diagnosed with enteric fever had received S. typhi vaccine. Overall, 13% of S. typhi isolates in the United States were resistant to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole (TMP-SMX), and the proportion of DCS isolates increased from 19% in 1999 to 58% in 2006. Infection with DCS S. typhi was associated with travel to the Indian subcontinent. Of the 25–30% of reported cases of enteric fever in the United States that are domestically acquired, the majority are sporadic, but outbreaks linked to contaminated food products and previously unrecognized chronic carriers continue to occur.
CLINICAL COURSE
Enteric fever is a misnomer, in that the hallmark features of this disease—fever and abdominal pain—are variable. While fever is documented at presentation in >75% of cases, abdominal pain is reported in only 30–40%. Thus, a high index of suspicion for this potentially fatal systemic illness is necessary when a person presents with fever and a history of recent travel to a developing country.
The incubation period for S. typhi averages 10–14 days but ranges from 5 to 21 days, depending on the inoculum size and the host’s health and immune status. The most prominent symptom is prolonged fever (38.8°–40.5°C; 101.8°–104.9°F), which can continue for up to 4 weeks if untreated. S. paratyphi A is thought to cause milder disease than S. typhi, with predominantly gastrointestinal symptoms. However, a prospective study of 669 consecutive cases of enteric fever in Kathmandu, Nepal, found that the infections caused by these organisms were clinically indistinguishable. In this series, symptoms reported on initial medical evaluation included headache (80%), chills (35–45%), cough (30%), sweating (20–25%), myalgias (20%), malaise (10%), and arthralgia (2–4%). Gastrointestinal manifestations included anorexia (55%), abdominal pain (30–40%), nausea (18–24%), vomiting (18%), and diarrhea (22–28%) more commonly than constipation (13–16%). Physical findings included coated tongue (51–56%), splenomegaly (5–6%), and abdominal tenderness (4–5%).
Early physical findings of enteric fever include rash (“rose spots”; 30%), hepatosplenomegaly (3–6%), epistaxis, and relative bradycardia at the peak of high fever (<50%). Rose spots (Fig. 190-2; see also Fig. 25e-9) make up a faint, salmon-colored, blanching, maculopapular rash located primarily on the trunk and chest. The rash is evident in ~30% of patients at the end of the first week and resolves without a trace after 2–5 days. Patients can have two or three crops of lesions, and Salmonella can be cultured from punch biopsies of these lesions. The faintness of the rash makes it difficult to detect in highly pigmented patients.
FIGURE 190-2 “Rose spots,” the rash of enteric fever due to Salmonella typhi or Salmonella paratyphi.
The development of severe disease (which occurs in ~10–15% of patients) depends on host factors (immunosuppression, antacid therapy, previous exposure, and vaccination), strain virulence and inoculum, and choice of antibiotic therapy. Gastrointestinal bleeding (10–20%) and intestinal perforation (1–3%) most commonly occur in the third and fourth weeks of illness and result from hyperplasia, ulceration, and necrosis of the ileocecal Peyer’s patches at the initial site of Salmonella infiltration (Fig. 190-3). Both complications are life-threatening and require immediate fluid resuscitation and surgical intervention, with broadened antibiotic coverage for polymicrobial peritonitis (Chap. 159) and treatment of gastrointestinal hemorrhages, including bowel resection. Neurologic manifestations occur in 2–40% of patients and include meningitis, Guillain-Barré syndrome, neuritis, and neuropsychiatric symptoms (described as “muttering delirium” or “coma vigil”), with picking at bedclothes or imaginary objects.
FIGURE 190-3 Typical ileal perforation associated with Salmonella typhi infection. (From JM Saxe, R Cropsey: Is operative management effective in treatment of perforated typhoid? Am J Surg 189:342, 2005.)
Rare complications whose incidences are reduced by prompt antibiotic treatment include disseminated intravascular coagulation, hematophagocytic syndrome, pancreatitis, hepatic and splenic abscesses and granulomas, endocarditis, pericarditis, myocarditis, orchitis, hepatitis, glomerulonephritis, pyelonephritis and hemolytic-uremic syndrome, severe pneumonia, arthritis, osteomyelitis, endophthalmitis, and parotitis. Up to 10% of patients develop mild relapse, usually within 2–3 weeks of fever resolution and in association with the same strain type and susceptibility profile.
Up to 10% of untreated patients with typhoid fever excrete S. typhi in the feces for up to 3 months, and 1–4% develop chronic asymptomatic carriage, shedding S. typhi in either urine or stool for >1 year. Chronic carriage is more common among women, infants, and persons who have biliary abnormalities or concurrent bladder infection with Schistosoma haematobium. The anatomic abnormalities associated with the latter conditions presumably allow prolonged colonization.
DIAGNOSIS
Because the clinical presentation of enteric fever is relatively nonspecific, the diagnosis needs to be considered in any febrile traveler returning from a developing region, especially the Indian subcontinent, the Philippines, or Latin America. Other diagnoses that should be considered in these travelers include malaria, hepatitis, bacterial enteritis, dengue fever, rickettsial infections, leptospirosis, amebic liver abscesses, and acute HIV infection (Chap. 149). Other than a positive culture, no specific laboratory test is diagnostic for enteric fever. In 15–25% of cases, leukopenia and neutropenia are detectable. Leukocytosis is more common among children, during the first 10 days of illness, and in cases complicated by intestinal perforation or secondary infection. Other nonspecific laboratory findings include moderately elevated values in liver function tests and muscle enzyme levels.
The definitive diagnosis of enteric fever requires the isolation of S. typhi or S. paratyphi from blood, bone marrow, other sterile sites, rose spots, stool, or intestinal secretions. The sensitivity of blood culture is only 40–80%, probably because of high rates of antibiotic use in endemic areas and the small number of S. typhi organisms (i.e., <15/mL) typically present in the blood. Because almost all S. typhi organisms in blood are associated with the mononuclear cell/platelet fraction, centrifugation of blood and culture of the buffy coat can substantially reduce the time to isolation of the organism but do not increase sensitivity.
Bone marrow culture is 55–90% sensitive, and, unlike that of blood culture, its yield is not reduced by up to 5 days of prior antibiotic therapy. Culture of intestinal secretions (best obtained by a noninvasive duodenal string test) can be positive despite a negative bone marrow culture. If blood, bone marrow, and intestinal secretions are all cultured, the yield is >90%. Stool cultures, although negative in 60–70% of cases during the first week, can become positive during the third week of infection in untreated patients.
![]() Serologic tests, including the classic Widal test for “febrile agglutinins,” and rapid tests to detect antibodies to outer-membrane proteins or O:9 antigen are available for detection of S. typhi in developing countries but have lower positive predictive values than blood culture. More sensitive antigen and nucleic acid amplification tests have been developed to detect S. typhi and S. paratyphi in blood but are not yet commercially available and remain impractical in many areas where enteric fever is endemic.
Serologic tests, including the classic Widal test for “febrile agglutinins,” and rapid tests to detect antibodies to outer-membrane proteins or O:9 antigen are available for detection of S. typhi in developing countries but have lower positive predictive values than blood culture. More sensitive antigen and nucleic acid amplification tests have been developed to detect S. typhi and S. paratyphi in blood but are not yet commercially available and remain impractical in many areas where enteric fever is endemic.
|
TREATMENT |
ENTERIC (TYPHOID) FEVER |
![]() Prompt administration of appropriate antibiotic therapy prevents severe complications of enteric fever and results in a case-fatality rate of <1%. The initial choice of antibiotics depends on the susceptibility of the S. typhi and S. paratyphi strains in the area of residence or travel (Table 190-1). For treatment of drug-susceptible typhoid fever, fluoroquinolones are the most effective class of agents, with cure rates of ~98% and relapse and fecal carriage rates of <2%. Experience is most extensive with ciprofloxacin. Short-course ofloxacin therapy is similarly successful against infection caused by quinolone-susceptible strains. However, the increased incidence of DCS S. typhi in Asia, which is probably related to the widespread availability of fluoroquinolones over the counter, is now limiting the use of this drug class for empirical therapy. Patients infected with DCS S. typhi strains should be treated with ceftriaxone, azithromycin, or high-dose ciprofloxacin. A 7-day course of high-dose fluoroquinolone therapy for DCS enteric fever has been associated with delayed resolution of fever and high rates of fecal carriage during convalescence. Thus, for DCS strains, a 10- to 14-day course of high-dose ciprofloxacin is preferred.
Prompt administration of appropriate antibiotic therapy prevents severe complications of enteric fever and results in a case-fatality rate of <1%. The initial choice of antibiotics depends on the susceptibility of the S. typhi and S. paratyphi strains in the area of residence or travel (Table 190-1). For treatment of drug-susceptible typhoid fever, fluoroquinolones are the most effective class of agents, with cure rates of ~98% and relapse and fecal carriage rates of <2%. Experience is most extensive with ciprofloxacin. Short-course ofloxacin therapy is similarly successful against infection caused by quinolone-susceptible strains. However, the increased incidence of DCS S. typhi in Asia, which is probably related to the widespread availability of fluoroquinolones over the counter, is now limiting the use of this drug class for empirical therapy. Patients infected with DCS S. typhi strains should be treated with ceftriaxone, azithromycin, or high-dose ciprofloxacin. A 7-day course of high-dose fluoroquinolone therapy for DCS enteric fever has been associated with delayed resolution of fever and high rates of fecal carriage during convalescence. Thus, for DCS strains, a 10- to 14-day course of high-dose ciprofloxacin is preferred.
|
ANTIBIOTIC THERAPY FOR ENTERIC FEVER IN ADULTS |
Ceftriaxone, cefotaxime, and (oral) cefixime are effective for treatment of MDR enteric fever, including that caused by DCS and fluoroquinolone-resistant strains. These agents clear fever in ~1 week, with failure rates of ~5–10%, fecal carriage rates of <3%, and relapse rates of 3–6%. Oral azithromycin results in defervescence in 4–6 days, with rates of relapse and convalescent stool carriage of <3%. Against DCS strains, azithromycin is associated with lower rates of treatment failure and shorter durations of hospitalization than are fluoroquinolones. Despite efficient in vitro killing of Salmonella, first- and second-generation cephalosporins as well as aminoglycosides are ineffective in the treatment of clinical infections.
Most patients with uncomplicated enteric fever can be managed at home with oral antibiotics and antipyretics. Patients with persistent vomiting, diarrhea, and/or abdominal distension should be hospitalized and given supportive therapy as well as a parenteral third-generation cephalosporin or fluoroquinolone, depending on the susceptibility profile. Therapy should be administered for at least 10 days or for 5 days after fever resolution.
![]() In a randomized, prospective, double-blind study of critically ill patients with enteric fever (i.e., those with shock and obtundation) in Indonesia in the early 1980s, the administration of dexamethasone (an initial dose of 3 mg/kg followed by eight doses of 1 mg/kg every 6 h) with chloramphenicol was associated with a substantially lower mortality rate than was treatment with chloramphenicol alone (10% vs 55%). Although this study has not been repeated in the “post-chloramphenicol era,” severe enteric fever remains one of the few indications for glucocorticoid treatment of an acute bacterial infection.
In a randomized, prospective, double-blind study of critically ill patients with enteric fever (i.e., those with shock and obtundation) in Indonesia in the early 1980s, the administration of dexamethasone (an initial dose of 3 mg/kg followed by eight doses of 1 mg/kg every 6 h) with chloramphenicol was associated with a substantially lower mortality rate than was treatment with chloramphenicol alone (10% vs 55%). Although this study has not been repeated in the “post-chloramphenicol era,” severe enteric fever remains one of the few indications for glucocorticoid treatment of an acute bacterial infection.
The 1–5% of patients who develop chronic carriage of Salmonella can be treated for 4–6 weeks with an appropriate oral antibiotic. Treatment with oral amoxicillin, TMP-SMX, ciprofloxacin, or norfloxacin is ~80% effective in eradicating chronic carriage of susceptible organisms. However, in cases of anatomic abnormality (e.g., biliary or kidney stones), eradication often requires both antibiotic therapy and surgical correction.
PREVENTION AND CONTROL
![]() Theoretically, it is possible to eliminate the salmonellae that cause enteric fever because they survive only in human hosts and are spread by contaminated food and water. However, given the high prevalence of the disease in developing countries that lack adequate sewage disposal and water treatment, this goal is currently unrealistic. Thus, travelers to developing countries should be advised to monitor their food and water intake carefully and to strongly consider immunization against S. typhi.
Theoretically, it is possible to eliminate the salmonellae that cause enteric fever because they survive only in human hosts and are spread by contaminated food and water. However, given the high prevalence of the disease in developing countries that lack adequate sewage disposal and water treatment, this goal is currently unrealistic. Thus, travelers to developing countries should be advised to monitor their food and water intake carefully and to strongly consider immunization against S. typhi.
Two typhoid vaccines are commercially available: (1) Ty21a, an oral live attenuated S. typhi vaccine (given on days 1, 3, 5, and 7, with a booster every 5 years); and (2) Vi CPS, a parenteral vaccine consisting of purified Vi polysaccharide from the bacterial capsule (given in a single dose, with a booster every 2 years). The old parenteral whole-cell typhoid/paratyphoid A and B vaccine is no longer licensed, largely because of significant side effects, especially fever. An acetone-killed whole-cell vaccine is available only for use by the U.S. military. The minimal age for vaccination is 6 years for Ty21a and 2 years for Vi CPS. In a recent meta-analysis of vaccines for preventing typhoid fever in populations in endemic areas, the cumulative efficacy was 48% for Ty21a at 2.5–3.5 years and 55% for Vi CPS at 3 years. Although data on typhoid vaccines in travelers are limited, some evidence suggests that efficacy rates may be substantially lower than those for local populations in endemic areas. Currently, there is no licensed vaccine for paratyphoid fever.
Vi CPS typhoid vaccine is poorly immunogenic in children <5 years of age because of T cell–independent properties. In the more recently developed Vi-rEPA vaccine, Vi is bound to a nontoxic recombinant protein that is identical to Pseudomonas aeruginosa exotoxin A. In 2- to 4-year-olds, two injections of Vi-rEPA induced higher T cell responses and higher levels of serum IgG antibody to Vi than did Vi CPS in 5- to 14-year-olds. In a two-dose trial in 2- to 5-year-old children in Vietnam, Vi-rEPA provided 91% efficacy at 27 months and 89% efficacy at 46 months and was very well tolerated. This vaccine is not yet commercially available in the United States. Efforts to improve the immunogenicity and reduce the number of doses of live attenuated oral vaccines are ongoing.
Typhoid vaccine is not required for international travel, but it is recommended for travelers to areas where there is a moderate to high risk of exposure to S. typhi, especially those who are traveling to southern Asia and other developing regions of Asia, Africa, the Caribbean, and Central and South America and who will be exposed to potentially contaminated food and drink. Typhoid vaccine should be considered even for persons planning <2 weeks of travel to high-risk areas. In addition, laboratory workers who deal with S. typhi and household contacts of known S. typhi carriers should be vaccinated. Because the protective efficacy of vaccine can be overcome by the high inocula that are commonly encountered in food-borne exposures, immunization is an adjunct and not a substitute for the avoidance of high-risk foods and beverages. Immunization is not recommended for adults residing in typhoid-endemic areas or for the management of persons who may have been exposed in a common-source outbreak.
Enteric fever is a notifiable disease in the United States. Individual health departments have their own guidelines for allowing ill or colonized food handlers or health care workers to return to their jobs. The reporting system enables public health departments to identify potential source patients and to treat chronic carriers in order to prevent further outbreaks. In addition, because 1–4% of patients with S. typhi infection become chronic carriers, it is important to monitor patients (especially child-care providers and food handlers) for chronic carriage and to treat this condition if indicated.
NONTYPHOIDAL SALMONELLOSIS
EPIDEMIOLOGY
In the United States, NTS causes ~12 million illnesses annually, and the incidence has remained relatively unchanged during the past two decades. In 2011, the incidence of NTS infection in this country was 16.5/100,000 persons—the highest rate among the 10 food-borne enteric pathogens under active surveillance. Five serotypes accounted for more than half of U.S. infections during the period 1996–2006: typhimurium (23%), enteritidis (16%), newport (10%), heidelberg (6%), and javiana (5%).
The incidence of nontyphoidal salmonellosis is highest during the rainy season in tropical climates and during the warmer months in temperate climates—a pattern coinciding with the peak in food-borne outbreaks. Rates of morbidity and mortality associated with NTS are highest among the elderly, infants, and immunocompromised individuals, including those with hemoglobinopathies, HIV infection, or infections that cause blockade of the reticuloendothelial system (e.g., bartonellosis, malaria, schistosomiasis, histoplasmosis).
Unlike S. typhi and S. paratyphi, whose only reservoir is humans, NTS can be acquired from multiple animal reservoirs. Transmission is most commonly associated with food products of animal origin (especially eggs, poultry, undercooked ground meat, and dairy products), fresh produce contaminated with animal waste, and contact with animals or their environments.
S. enteritidis infection associated with chicken eggs emerged as a major cause of food-borne disease during the 1980s and 1990s. S. enteritidis infection of the ovaries and upper oviduct tissue of hens results in contamination of egg contents before shell deposition. Infection is spread to egg-laying hens from breeding flocks and through contact with rodents and manure. The percentage of Salmonella outbreaks attributed to eggs has declined significantly in the United States, from 33% during 1998–1999 to 15% during 2006–2008. This decrease probably reflects the impact of the coordinated public health response to S. enteritidis infection attributed to eggs, including improved on-farm control measures, refrigeration, and education of consumers and food-service workers. Transmission via contaminated eggs can be prevented by cooking eggs until the yolk is solidified and pasteurizing egg products. Despite these control efforts, outbreaks of S. enteritidis infection associated with shell eggs continue to occur. In 2010, a national outbreak of S. enteritidis infection resulted in more than 1900 reported illnesses and the recall of 500 million eggs.
Centralization of food processing and widespread food distribution have contributed to the increased incidence of NTS in developed countries. Manufactured foods to which recent Salmonella outbreaks have been traced include peanut butter; milk products, including infant formula; and various processed foods, including packaged breakfast cereal, salsa, frozen prepared meals, and snack foods. Large outbreaks have also been linked to fresh produce, including alfalfa sprouts, cantaloupe, mangoes, papayas, and tomatoes; these items become contaminated by manure or water at a single site and then are widely distributed.
An estimated 6% of sporadic Salmonella infections in the United States are attributed to contact with reptiles or amphibians, especially iguanas, snakes, turtles, and lizards. Reptile-associated Salmonella infection more commonly leads to hospitalization and more frequently involves children, including infants, than do other Salmonella infections. Other pets, including African hedgehogs, birds, rodents, baby chicks, ducklings, dogs, and cats, are also potential sources of NTS.
![]() Increasing antibiotic resistance in NTS species is a global problem and has been linked to the widespread use of antimicrobial agents in food animals and especially in animal feed. In the early 1990s, S. typhimurium definitive phage type 104 (DT104), characterized by resistance to at least five antibiotics (ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracyclines; R-type ACSSuT), emerged worldwide. In 2010, resistance to at least ACSSuT was reported in 4.3% of NTS isolates, including 18.6% of S. typhimurium isolates. Acquisition is associated with exposure to ill farm animals and to various meat products, including uncooked or undercooked ground beef. Although probably no more virulent than susceptible S. typhimurium strains, DT104 strains are associated with an increased risk of bloodstream infection and hospitalization. DCS and trimethoprim-resistant DT104 strains are emerging, especially in the United Kingdom.
Increasing antibiotic resistance in NTS species is a global problem and has been linked to the widespread use of antimicrobial agents in food animals and especially in animal feed. In the early 1990s, S. typhimurium definitive phage type 104 (DT104), characterized by resistance to at least five antibiotics (ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracyclines; R-type ACSSuT), emerged worldwide. In 2010, resistance to at least ACSSuT was reported in 4.3% of NTS isolates, including 18.6% of S. typhimurium isolates. Acquisition is associated with exposure to ill farm animals and to various meat products, including uncooked or undercooked ground beef. Although probably no more virulent than susceptible S. typhimurium strains, DT104 strains are associated with an increased risk of bloodstream infection and hospitalization. DCS and trimethoprim-resistant DT104 strains are emerging, especially in the United Kingdom.
Because of increased resistance to conventional antibiotics such as ampicillin and TMP-SMX, extended-spectrum cephalosporins and fluoroquinolones have emerged as the agents of choice for the treatment of MDR NTS infections. In 2010, 2.8% of all NTS strains were resistant to ceftriaxone. Most ceftriaxone-resistant isolates were from children <18 years of age, in whom ceftriaxone is the antibiotic of choice for treatment of invasive NTS infection. These strains contained plasmid-encoded AmpC β-lactamases that were probably acquired by horizontal genetic transfer from Escherichia coli strains in food-producing animals—an event linked to the widespread use of the veterinary cephalosporin ceftiofur.
Over the last decade, strains of DCS NTS (MIC, 0.125–1 μg/mL) have emerged and have been associated with delayed response and treatment failure. In 2009, 2.4% of NTS isolates in the United States were DCS or resistant to ciprofloxacin. These strains have diverse resistance mechanisms, including single and multiple mutations in the DNA gyrase genes gyrA and gyrB and plasmid-encoded quinolone resistance determinants that may not be reliably detected by nalidixic acid susceptibility testing. In 2012, the U.S. Clinical Laboratory Standards Institute proposed a lower ciprofloxacin susceptibility breakpoint (≥0.06 μg/mL) for all Salmonella species to address this issue. Currently, because commercial test systems do not contain ciprofloxacin concentrations low enough to allow use of these breakpoints, laboratories need to determine the ciprofloxacin MIC by Etest or another alternative method.
CLINICAL MANIFESTATIONS
Gastroenteritis Infection with NTS most often results in gastroenteritis indistinguishable from that caused by other enteric pathogens. Nausea, vomiting, and diarrhea occur 6–48 h after the ingestion of contaminated food or water. Patients often experience abdominal cramping and fever (38–39°C; 100.5–102.2°F). Diarrheal stools are usually loose, nonbloody, and of moderate volume. However, large-volume watery stools, bloody stools, or symptoms of dysentery may occur. Rarely, NTS causes pseudoappendicitis or an illness that mimics inflammatory bowel disease.
Gastroenteritis caused by NTS is usually self-limited. Diarrhea resolves within 3–7 days and fever within 72 h. Stool cultures remain positive for 4–5 weeks after infection and—in rare cases of chronic carriage (<1%)—for >1 year. Antibiotic treatment usually is not recommended and may prolong fecal carriage. Neonates, the elderly, and immunosuppressed patients (e.g., transplant recipients, HIV-infected persons) with NTS gastroenteritis are especially susceptible to dehydration and dissemination and may require hospitalization and antibiotic therapy. Acute NTS gastroenteritis was associated with a threefold increased risk of dyspepsia and irritable bowel syndrome at 1 year in a study from Spain.
Bacteremia and Endovascular Infections Up to 8% of patients with NTS gastroenteritis develop bacteremia; of these, 5–10% develop localized infections. Bacteremia and metastatic infection are most common with Salmonella choleraesuis and Salmonella dublin and among infants, the elderly, and immunocompromised patients, especially those with HIV infection. NTS endovascular infection should be suspected in high-grade or persistent bacteremia, especially with preexisting valvular heart disease, atherosclerotic vascular disease, prosthetic vascular graft, or aortic aneurysm. Arteritis should be suspected in elderly patients with prolonged fever and back, chest, or abdominal pain developing after an episode of gastroenteritis. Endocarditis and arteritis are rare (<1% of cases) but are associated with potentially fatal complications, including valve perforation, endomyocardial abscess, infected mural thrombus, pericarditis, mycotic aneurysms, aneurysm rupture, aortoenteric fistula, and vertebral osteomyelitis.
![]() In some areas of sub-Saharan Africa, NTS may be among the most common causes—or even the most common cause—of bacteremia in children. NTS bacteremia among these children is not associated with diarrhea and has been associated with nutritional status and HIV infection.
In some areas of sub-Saharan Africa, NTS may be among the most common causes—or even the most common cause—of bacteremia in children. NTS bacteremia among these children is not associated with diarrhea and has been associated with nutritional status and HIV infection.
Localized Infections • INTRAABDOMINAL INFECTIONS Intraabdominal infections due to NTS are rare and usually manifest as hepatic or splenic abscesses or as cholecystitis. Risk factors include hepatobiliary anatomic abnormalities (e.g., gallstones), abdominal malignancy, and sickle cell disease (especially with splenic abscesses). Eradication of the infection often requires surgical correction of abnormalities and percutaneous drainage of abscesses.
CENTRAL NERVOUS SYSTEM INFECTIONS NTS meningitis most commonly develops in infants 1–4 months of age. It often results in severe sequelae (including seizures, hydrocephalus, brain infarction, and mental retardation), with death in up to 60% of cases. Other rare central nervous system infections include ventriculitis, subdural empyema, and brain abscesses.
PULMONARY INFECTIONS NTS pulmonary infections usually present as lobar pneumonia, and complications include lung abscess, empyema, and bronchopleural fistula formation. The majority of cases occur in patients with lung cancer, structural lung disease, sickle cell disease, or glucocorticoid use.
URINARY AND GENITAL TRACT INFECTIONS Urinary tract infections caused by NTS present as either cystitis or pyelonephritis. Risk factors include malignancy, urolithiasis, structural abnormalities, HIV infection, and renal transplantation. NTS genital infections are rare and include ovarian and testicular abscesses, prostatitis, and epididymitis. Like other focal infections, both genital and urinary tract infections can be complicated by abscess formation.
BONE, JOINT, AND SOFT TISSUE INFECTIONS Salmonella osteomyelitis most commonly affects the femur, tibia, humerus, or lumbar vertebrae and is most often seen in association with sickle cell disease, hemoglobinopathies, or preexisting bone disease (e.g., fractures). Prolonged antibiotic treatment is recommended to decrease the risk of relapse and chronic osteomyelitis. Septic arthritis occurs in the same patient population as osteomyelitis and usually involves the knee, hip, or shoulder joints. Reactive arthritis can follow NTS gastroenteritis and is seen most frequently in persons with the HLA-B27 histocompatibility antigen. NTS rarely can cause soft tissue infections, usually at sites of local trauma in immunosuppressed patients.
DIAGNOSIS
The diagnosis of NTS infection is based on isolation of the organism from freshly passed stool or from blood or another ordinarily sterile body fluid. All salmonellae isolated in clinical laboratories should be sent to local public health departments for serotyping. Blood cultures should be done whenever a patient has prolonged or recurrent fever. Endovascular infection should be suspected if there is high-grade bacteremia (>50% of three or more positive blood cultures). Echocardiography, computed tomography (CT), and indium-labeled white cell scanning are used to identify localized infection. When another localized infection is suspected, joint fluid, abscess drainage, or cerebrospinal fluid should be cultured, as clinically indicated.
|
TREATMENT |
NONTYPHOIDAL SALMONELLOSIS |
Antibiotics should not be used routinely to treat uncomplicated NTS gastroenteritis. The symptoms are usually self-limited, and the duration of fever and diarrhea is not significantly decreased by antibiotic therapy. In addition, antibiotic treatment has been associated with increased rates of relapse, prolonged gastrointestinal carriage, and adverse drug reactions. Dehydration secondary to diarrhea should be treated with fluid and electrolyte replacement.
Preemptive antibiotic treatment (Table 190-2) should be considered for patients at increased risk for invasive NTS infection, including neonates (probably up to 3 months of age); persons >50 years of age with suspected atherosclerosis; and patients with immunosuppression, cardiac valvular or endovascular abnormalities, or significant joint disease. Treatment should consist of an oral or IV antibiotic administered for 48–72 h or until the patient becomes afebrile. Immunocompromised persons may require up to 7–14 days of therapy. The <1% of persons who develop chronic carriage of NTS should receive a prolonged antibiotic course, as described above for chronic carriage of S. typhi.
|
ANTIBIOTIC THERAPY FOR NONTYPHOIDAL SALMONELLA INFECTION IN ADULTS |
Because of the increasing prevalence of antibiotic resistance, empirical therapy for life-threatening NTS bacteremia or focal NTS infection should include a third-generation cephalosporin or a fluoroquinolone (Table 190-2). If the bacteremia is low-grade (<50% of positive blood cultures), the patient should be treated for 7–14 days. Patients with HIV/AIDS and NTS bacteremia should receive 1–2 weeks of IV antibiotic therapy followed by 4 weeks of oral therapy with a fluoroquinolone. Patients whose infections relapse after this regimen should receive long-term suppressive therapy with a fluoroquinolone or TMP-SMX, as indicated by bacterial sensitivities.
If the patient has endocarditis or arteritis, treatment for 6 weeks with an IV β-lactam antibiotic (such as ceftriaxone or ampicillin) is indicated. IV ciprofloxacin followed by prolonged oral therapy is an option, but published experience is limited. Early surgical resection of infected aneurysms or other infected endovascular sites is recommended. Patients with infected prosthetic vascular grafts that cannot be resected have been maintained successfully on chronic suppressive oral therapy. For extraintestinal nonvascular infections, a 2- to 4-week course of antibiotic therapy (depending on the infection site) is usually recommended. In chronic osteomyelitis, abscess, or urinary or hepatobiliary infection associated with anatomic abnormalities, surgical resection or drainage may be required in addition to prolonged antibiotic therapy for eradication of infection.
PREVENTION AND CONTROL
Despite widespread efforts to prevent or reduce bacterial contamination of animal-derived food products and to improve food-safety education and training, recent declines in the incidence of NTS in the United States have been modest compared with those of other food-borne pathogens. This observation probably reflects the complex epidemiology of NTS. Identifying effective risk-reduction strategies requires monitoring of every step of food production, from handling of raw animal or plant products to preparation of finished foods. Contaminated food can be made safe for consumption by pasteurization, irradiation, or proper cooking. All cases of NTS infection should be reported to local public health departments because tracking and monitoring of these cases can identify the source(s) of infection and help authorities anticipate large outbreaks. Lastly, the prudent use of antimicrobial agents in both humans and animals is needed to limit the emergence of MDR Salmonella.
191 |
Shigellosis |
The discovery of Shigella as the etiologic agent of dysentery—a clinical syndrome of fever, intestinal cramps, and frequent passage of small, bloody, mucopurulent stools—is attributed to the Japanese microbiologist Kiyoshi Shiga, who isolated the Shiga bacillus (now known as Shigella dysenteriae type 1) from patients’ stools in 1897 during a large and devastating dysentery epidemic. Shigella cannot be distinguished from Escherichia coli by DNA hybridization and remains a separate species only on historical and clinical grounds.
DEFINITION
Shigella is a non-spore-forming, gram-negative bacterium that, unlike E. coli, is nonmotile and does not produce gas from sugars, decarboxylate lysine, or hydrolyze arginine. Some serovars produce indole, and occasional strains utilize sodium acetate. Shigella dysenteriae, Shigella flexneri, Shigella boydii, and Shigella sonnei (serogroups A, B, C, and D, respectively) can be differentiated on the basis of biochemical and serologic characteristics. Genome sequencing of E. coli K12, S. flexneri 2a, S. sonnei, S. dysenteriae type 1, and S. boydii has revealed that these species have ~93% of genes in common. The three major genomic “signatures” of Shigella are (1) a 215-kb virulence plasmid that carries most of the genes required for pathogenicity (particularly invasive capacity); (2) the lack or alteration of genetic sequences encoding products (e.g., lysine decarboxylase) that, if expressed, would attenuate pathogenicity; and (3) in S. dysenteriae type 1, the presence of genes encoding Shiga toxin, a potent cytotoxin.
EPIDEMIOLOGY
![]() The human intestinal tract represents the major reservoir of Shigella, which is also found (albeit rarely) in the higher primates. Because excretion of shigellae is greatest in the acute phase of disease, the bacteria are transmitted most efficiently by the fecal-oral route via hand carriage; however, some outbreaks reflect foodborne or waterborne transmission. In impoverished areas, Shigella can be transmitted by flies. The high-level infectivity of Shigella is reflected by the very small inoculum required for experimental infection of volunteers (100 colony-forming units [CFU]), by the very high attack rates during outbreaks in day-care centers (33–73%), and by the high rates of secondary cases among family members of sick children (26–33%). Shigellosis can also be transmitted sexually.
The human intestinal tract represents the major reservoir of Shigella, which is also found (albeit rarely) in the higher primates. Because excretion of shigellae is greatest in the acute phase of disease, the bacteria are transmitted most efficiently by the fecal-oral route via hand carriage; however, some outbreaks reflect foodborne or waterborne transmission. In impoverished areas, Shigella can be transmitted by flies. The high-level infectivity of Shigella is reflected by the very small inoculum required for experimental infection of volunteers (100 colony-forming units [CFU]), by the very high attack rates during outbreaks in day-care centers (33–73%), and by the high rates of secondary cases among family members of sick children (26–33%). Shigellosis can also be transmitted sexually.
Throughout history, Shigella epidemics have often occurred in settings of human crowding under conditions of poor hygiene—e.g., among soldiers in campaigning armies, inhabitants of besieged cities, groups on pilgrimages, and refugees in camps. Epidemics follow a cyclical pattern in areas such as the Indian subcontinent and sub-Saharan Africa. These devastating epidemics, which are most often caused by S. dysenteriae type 1, are characterized by high attack and mortality rates. In Bangladesh, for instance, an epidemic caused by S. dysenteriae type 1 was associated with a 42% increase in mortality rate among children 1–4 years of age. Apart from these epidemics, shigellosis is mostly an endemic disease, with 99% of cases occurring in the developing world and the highest prevalences in the most impoverished areas, where personal and general hygiene is below standard. S. flexneri isolates predominate in the least developed areas, whereas S. sonnei is more prevalent in economically emerging countries and in the industrialized world.
Prevalence in the Developing World In a review published under the auspices of the World Health Organization (WHO), the total annual number of cases in 1966–1997 was estimated at 165 million, and 69% of these cases occurred in children <5 years of age. In this review, the annual number of deaths was calculated to range between 500,000 and 1.1 million. More recent data (2000–2004) from six Asian countries indicate that, even though the incidence of shigellosis remains stable, mortality rates associated with this disease may have decreased significantly, possibly as a result of improved nutritional status. However, extensive and essentially uncontrolled use of antibiotics, which may also account for declining mortality rates, has increased the rate of emergence of multidrug-resistant Shigella strains. A 2013 prospective matched case-control study of children <5 years of age emphasizes the importance of Shigella in the burden and etiology of diarrheal diseases in developing countries. Shigella is one of the top four pathogens associated with moderate to severe diarrhea and is now ranked first among children 12–59 months of age. These moderate to severe cases account for an 8.5-fold increase in mortality incidence over the average diarrheal disease–related mortality. The study’s authors conclude that Shigella remains a major pathogen to be targeted by health care programs.
An often-overlooked complication of shigellosis is the short- and long-term impairment of the nutritional status of infected children in endemic areas. Combined with anorexia, the exudative enteropathy resulting from mucosal abrasions contributes to rapid deterioration of the patient’s nutritional status. Shigellosis is thus a major contributor to stunted growth among children in developing countries.
Peaking in incidence in the pediatric population, endemic shigellosis is rare among young and middle-aged adults, probably because of naturally acquired immunity. Incidence then increases again in the elderly population.
Prevalence in the Industrialized World In pediatric populations, local outbreaks occur when proper and adapted hygiene policies are not implemented in group facilities like day-care centers and institutions for the mentally retarded. In adults, as in children, sporadic cases occur among travelers returning from endemic areas, and rare outbreaks of varying size can follow waterborne or food-borne infections.
PATHOGENESIS AND PATHOLOGY
Shigella infection occurs essentially through oral contamination via direct fecal-oral transmission, the organism being poorly adapted to survive in the environment. Resistance to low-pH conditions allows shigellae to survive passage through the gastric barrier, an ability that may explain in part why a small inoculum (as few as 100 CFU) is sufficient to cause infection.
The watery diarrhea that usually precedes the dysenteric syndrome is attributable to active secretion and abnormal water reabsorption—a secretory effect at the jejunal level described in experimentally infected rhesus monkeys. This initial purge is probably due to the combined action of an enterotoxin (ShET-1) and mucosal inflammation. The dysenteric syndrome, manifested by bloody and mucopurulent stools, reflects invasion of the mucosa.
![]() The pathogenesis of Shigella is essentially determined by a large virulence plasmid of 214 kb comprising ~100 genes, of which 25 encode a type III secretion system that inserts into the membrane of the host cell to allow effectors to transit from the bacterial cytoplasm to the host cell cytoplasm (Fig. 191-1). Bacteria are thereby able to invade intestinal epithelial cells by inducing their own uptake after the initial crossing of the epithelial barrier through M cells (the specialized translocating epithelial cells in the follicle-associated epithelium that covers mucosal lymphoid nodules). The organisms induce apoptosis of subepithelial resident macrophages. Once inside the cytoplasm of intestinal epithelial cells, Shigella effectors trigger the cytoskeletal rearrangements necessary to direct uptake of the organism into the epithelial cell. The Shigella-containing vacuole is then quickly lysed, releasing bacteria into the cytosol.
The pathogenesis of Shigella is essentially determined by a large virulence plasmid of 214 kb comprising ~100 genes, of which 25 encode a type III secretion system that inserts into the membrane of the host cell to allow effectors to transit from the bacterial cytoplasm to the host cell cytoplasm (Fig. 191-1). Bacteria are thereby able to invade intestinal epithelial cells by inducing their own uptake after the initial crossing of the epithelial barrier through M cells (the specialized translocating epithelial cells in the follicle-associated epithelium that covers mucosal lymphoid nodules). The organisms induce apoptosis of subepithelial resident macrophages. Once inside the cytoplasm of intestinal epithelial cells, Shigella effectors trigger the cytoskeletal rearrangements necessary to direct uptake of the organism into the epithelial cell. The Shigella-containing vacuole is then quickly lysed, releasing bacteria into the cytosol.
FIGURE 191-1 Invasive strategy of Shigella flexneri. IL, interleukin; NF-κB, nuclear factor κB; NLR, NOD-like receptor; PMN, polymorphonuclear leukocyte.
Intracellular shigellae next use cytoskeletal components to propel themselves inside the infected cell; when the moving organism and the host cell membrane come into contact, cellular protrusions form and are engulfed by neighboring cells. This series of events permits bacterial cell-to-cell spread.
Cytokines released by a growing number of infected intestinal epithelial cells attract increased numbers of immune cells (particularly polymorphonuclear leukocytes [PMNs]) to the infected site, thus further destabilizing the epithelial barrier, exacerbating inflammation, and leading to the acute colitis that characterizes shigellosis. Evidence indicates that some type III secretion system–injected effectors can control the extent of inflammation, thus facilitating bacterial survival.
Shiga toxin produced by S. dysenteriae type 1 increases disease severity. This toxin belongs to a group of A1-B5 protein toxins whose B subunit binds to the receptor globotriaosylceramide on the target cell surface and whose catalytic A subunit is internalized by receptor-mediated endocytosis and interacts with the subcellular machinery to inhibit protein synthesis by expressing RNA N-glycosidase activity on 28S ribosomal RNA. This process leads to inhibition of binding of the amino-acyl-tRNA to the 60S ribosomal subunit and thus to a general shutoff of cell protein biosynthesis. Shiga toxins are translocated from the bowel into the circulation. After binding of the toxins to target cells in the kidney, pathophysiologic alterations may result in hemolytic-uremic syndrome (HUS; see below).
CLINICAL MANIFESTATIONS
The presentation and severity of shigellosis depend to some extent on the infecting serotype but even more on the age and the immunologic and nutritional status of the host. Poverty and poor standards of hygiene are strongly related to the number and severity of diarrheal episodes, especially in children <5 years old who have been weaned.
Shigellosis typically evolves through four phases: incubation, watery diarrhea, dysentery, and the postinfectious phase. The incubation period usually lasts 1–4 days but may be as long as 8 days. Typical initial manifestations are transient fever, limited watery diarrhea, malaise, and anorexia. Signs and symptoms may range from mild abdominal discomfort to severe cramps, diarrhea, fever, vomiting, and tenesmus. The manifestations are usually exacerbated in children, with temperatures up to 40°–41°C (104.0°–105.8°F) and more severe anorexia and watery diarrhea. This initial phase may represent the only clinical manifestation of shigellosis, especially in developed countries. Otherwise, dysentery follows within hours or days and is characterized by uninterrupted excretion of small volumes of bloody mucopurulent stools with increased tenesmus and abdominal cramps. At this stage, Shigella produces acute colitis involving mainly the distal colon and the rectum. Unlike most diarrheal syndromes, dysenteric syndromes rarely present with dehydration as a major feature. Endoscopy shows an edematous and hemorrhagic mucosa, with ulcerations and possibly overlying exudates resembling pseudomembranes. The extent of the lesions correlates with the number and frequency of stools and with the degree of protein loss by exudative mechanisms. Most episodes are self-limited and resolve without treatment in 1 week. With appropriate treatment, recovery takes place within a few days to a week, with no sequelae.
Acute life-threatening complications are seen most often in children <5 years of age (particularly those who are malnourished) and in elderly patients. Risk factors for death in a clinically severe case include nonbloody diarrhea, moderate to severe dehydration, bacteremia, absence of fever, abdominal tenderness, and rectal prolapse. Major complications are predominantly intestinal (e.g., toxic megacolon, intestinal perforations, rectal prolapse) or metabolic (e.g., hypoglycemia, hyponatremia, dehydration). Bacteremia is rare and is reported most frequently in severely malnourished and HIV-infected patients. Alterations of consciousness, including seizures, delirium, and coma, may occur, especially in children <5 years old, and are associated with a poor prognosis; fever and severe metabolic alterations are more often the major causes of altered consciousness than is meningitis or the Ekiri syndrome (toxic encephalopathy associated with bizarre posturing, cerebral edema, and fatty degeneration of viscera), which has been reported mostly in Japanese children. Pneumonia, vaginitis, and keratoconjunctivitis due to Shigella are rarely reported. In the absence of serious malnutrition, severe and very unusual clinical manifestations, such as meningitis, may be linked to genetic defects in innate immune functions (i.e., deficiency in interleukin 1 receptor–associated kinase 4 [IRAK-4]) and may require genetic investigation.
Two complications of particular importance are toxic megacolon and HUS. Toxic megacolon is a consequence of severe inflammation extending to the colonic smooth-muscle layer and causing paralysis and dilation. The patient presents with abdominal distention and tenderness, with or without signs of localized or generalized peritonitis. The abdominal x-ray characteristically shows marked dilation of the transverse colon (with the greatest distention in the ascending and descending segments); thumbprinting caused by mucosal inflammatory edema; and loss of the normal haustral pattern associated with pseudopolyps, often extending into the lumen. Pneumatosis coli is an occasional finding. If perforation occurs, radiographic signs of pneumoperitoneum may be apparent. Predisposing factors (e.g., hypokalemia and use of opioids, anticholinergics, loperamide, psyllium seeds, and antidepressants) should be investigated.
![]() Shiga toxin produced by S. dysenteriae type 1 has been linked to HUS in developing countries but rarely in industrialized countries, where enterohemorrhagic E. coli (EHEC) predominates as the etiologic agent of this syndrome. HUS is an early complication that most often develops after several days of diarrhea. Clinical examination shows pallor, asthenia, and irritability and, in some cases, bleeding of the nose and gums, oliguria, and increasing edema. HUS is a nonimmune (Coombs test–negative) hemolytic anemia defined by a diagnostic triad: microangiopathic hemolytic anemia (hemoglobin level typically <80 g/L [<8 g/dL]), thrombocytopenia (mild to moderate in severity; typically <60,000 platelets/μL), and acute renal failure due to thrombosis of the glomerular capillaries (with markedly elevated creatinine levels). Anemia is severe, with fragmented red blood cells (schizocytes) in the peripheral smear, high serum concentrations of lactate dehydrogenase and free circulating hemoglobin, and elevated reticulocyte counts. Acute renal failure occurs in 55–70% of cases; however, renal function recovers in most of these cases (up to 70% in various series). Leukemoid reactions, with leukocyte counts of 50,000/μL, are sometimes noted in association with HUS.
Shiga toxin produced by S. dysenteriae type 1 has been linked to HUS in developing countries but rarely in industrialized countries, where enterohemorrhagic E. coli (EHEC) predominates as the etiologic agent of this syndrome. HUS is an early complication that most often develops after several days of diarrhea. Clinical examination shows pallor, asthenia, and irritability and, in some cases, bleeding of the nose and gums, oliguria, and increasing edema. HUS is a nonimmune (Coombs test–negative) hemolytic anemia defined by a diagnostic triad: microangiopathic hemolytic anemia (hemoglobin level typically <80 g/L [<8 g/dL]), thrombocytopenia (mild to moderate in severity; typically <60,000 platelets/μL), and acute renal failure due to thrombosis of the glomerular capillaries (with markedly elevated creatinine levels). Anemia is severe, with fragmented red blood cells (schizocytes) in the peripheral smear, high serum concentrations of lactate dehydrogenase and free circulating hemoglobin, and elevated reticulocyte counts. Acute renal failure occurs in 55–70% of cases; however, renal function recovers in most of these cases (up to 70% in various series). Leukemoid reactions, with leukocyte counts of 50,000/μL, are sometimes noted in association with HUS.
The postinfectious immunologic complication known as reactive arthritis can develop weeks or months after shigellosis, especially in patients expressing the histocompatibility antigen HLA-B27. About 3% of patients infected with S. flexneri later develop this syndrome, with arthritis, ocular inflammation, and urethritis—a condition that can last for months or years and can progress to difficult-to-treat chronic arthritis. Postinfectious arthropathy occurs only after infection with S. flexneri and not after infection with the other Shigella serotypes.
LABORATORY DIAGNOSIS
![]() The differential diagnosis in patients with a dysenteric syndrome depends on the clinical and environmental context. In developing areas, infectious diarrhea caused by other invasive pathogenic bacteria (Salmonella, Campylobacter jejuni, Clostridium difficile, Yersinia enterocolitica) or parasites (Entamoeba histolytica) should be considered. Only bacteriologic and parasitologic examinations of stool can truly differentiate among these pathogens. A first flare of inflammatory bowel disease, such as Crohn’s disease or ulcerative colitis (Chap. 351), should be considered in patients in industrialized countries. Despite the similarity in symptoms, anamnesis discriminates between shigellosis, which usually follows recent travel in an endemic zone, and these other conditions.
The differential diagnosis in patients with a dysenteric syndrome depends on the clinical and environmental context. In developing areas, infectious diarrhea caused by other invasive pathogenic bacteria (Salmonella, Campylobacter jejuni, Clostridium difficile, Yersinia enterocolitica) or parasites (Entamoeba histolytica) should be considered. Only bacteriologic and parasitologic examinations of stool can truly differentiate among these pathogens. A first flare of inflammatory bowel disease, such as Crohn’s disease or ulcerative colitis (Chap. 351), should be considered in patients in industrialized countries. Despite the similarity in symptoms, anamnesis discriminates between shigellosis, which usually follows recent travel in an endemic zone, and these other conditions.
Microscopic examination of stool smears shows erythrophagocytic trophozoites with very few PMNs in E. histolytica infection, whereas bacterial enteroinvasive infections (particularly shigellosis) are characterized by high PMN counts in each microscopic field. However, because shigellosis often manifests only as watery diarrhea, systematic attempts to isolate Shigella are necessary.
The “gold standard” for the diagnosis of Shigella infection remains the isolation and identification of the pathogen from fecal material. One major difficulty, particularly in endemic areas where laboratory facilities are not immediately available, is the fragility of Shigella and its common disappearance during transport, especially with rapid changes in temperature and pH. In the absence of a reliable enrichment medium, buffered glycerol saline or Cary-Blair medium can be used as a holding medium, but prompt inoculation onto isolation medium is essential. The probability of isolation is higher if the portion of stools that contains bloody and/or mucopurulent material is directly sampled. Rectal swabs can be used, as they offer the highest rate of successful isolation during the acute phase of disease. Blood cultures are positive in fewer than 5% of cases but should be done when a patient presents with a clinical picture of severe sepsis.
In addition to quick processing, the use of several media increases the likelihood of successful isolation: a nonselective medium such as bromocresol-purple agar lactose; a low-selectivity medium such as MacConkey or eosin-methylene blue; and a high-selectivity medium such as Hektoen, Salmonella–Shigella, or xylose-lysine-deoxycholate agar. After incubation on these media for 12–18 h at 37°C (98.6°F), shigellae appear as non-lactose-fermenting colonies that measure 0.5–1 mm in diameter and have a convex, translucent, smooth surface. Suspected colonies on nonselective or low-selectivity medium can be subcultured on a high-selectivity medium before being specifically identified or can be identified directly by standard commercial systems on the basis of four major characteristics: glucose positivity (usually without production of gas), lactose negativity, H2S negativity, and lack of motility. The four Shigella serogroups (A–D) can then be differentiated by additional characteristics. This approach adds time and difficulty to the identification process; however, after presumptive diagnosis, the use of serologic methods (e.g., slide agglutination, with group- and then type-specific antisera) should be considered. Group-specific antisera are widely available; in contrast, because of the large number of serotypes and subserotypes, type-specific antisera are rare and more expensive and thus are often restricted to reference laboratories.
|
TREATMENT |
SHIGELLOSIS |
ANTIBIOTIC SUSCEPTIBILITY OF SHIGELLA
![]() As an enteroinvasive disease, shigellosis requires antibiotic treatment. Since the mid-1960s, however, increasing resistance to multiple drugs has been a dominant factor in treatment decisions. Resistance rates are highly dependent on the geographic area. Clonal spread of particular strains and horizontal transfer of resistance determinants, particularly via plasmids and transposons, contribute to multidrug resistance. The current global status—i.e., high rates of resistance to classic first-line antibiotics such as amoxicillin—has led to a rapid switch to quinolones such as nalidixic acid. However, resistance to such early-generation quinolones has also emerged and spread quickly as a result of chromosomal mutations affecting DNA gyrase and topoisomerase IV; this resistance has necessitated the use of later-generation quinolones as first-line antibiotics in many areas. For instance, a review of the antibiotic resistance history of Shigella in India found that, after their introduction in the late 1980s, the second-generation quinolones norfloxacin, ciprofloxacin, and ofloxacin were highly effective in the treatment of shigellosis, including cases caused by multidrug-resistant strains of S. dysenteriae type 1. However, investigations of subsequent outbreaks in India and Bangladesh detected resistance to norfloxacin, ciprofloxacin, and ofloxacin in 5% of isolates. The incidence of multidrug resistance parallels the widespread, uncontrolled use of antibiotics and calls for the rational use of effective drugs.
As an enteroinvasive disease, shigellosis requires antibiotic treatment. Since the mid-1960s, however, increasing resistance to multiple drugs has been a dominant factor in treatment decisions. Resistance rates are highly dependent on the geographic area. Clonal spread of particular strains and horizontal transfer of resistance determinants, particularly via plasmids and transposons, contribute to multidrug resistance. The current global status—i.e., high rates of resistance to classic first-line antibiotics such as amoxicillin—has led to a rapid switch to quinolones such as nalidixic acid. However, resistance to such early-generation quinolones has also emerged and spread quickly as a result of chromosomal mutations affecting DNA gyrase and topoisomerase IV; this resistance has necessitated the use of later-generation quinolones as first-line antibiotics in many areas. For instance, a review of the antibiotic resistance history of Shigella in India found that, after their introduction in the late 1980s, the second-generation quinolones norfloxacin, ciprofloxacin, and ofloxacin were highly effective in the treatment of shigellosis, including cases caused by multidrug-resistant strains of S. dysenteriae type 1. However, investigations of subsequent outbreaks in India and Bangladesh detected resistance to norfloxacin, ciprofloxacin, and ofloxacin in 5% of isolates. The incidence of multidrug resistance parallels the widespread, uncontrolled use of antibiotics and calls for the rational use of effective drugs.
ANTIBIOTIC TREATMENT OF SHIGELLOSIS (TABLE 191-1)
|
RECOMMENDED ANTIMICROBIAL THERAPY FOR SHIGELLOSIS |
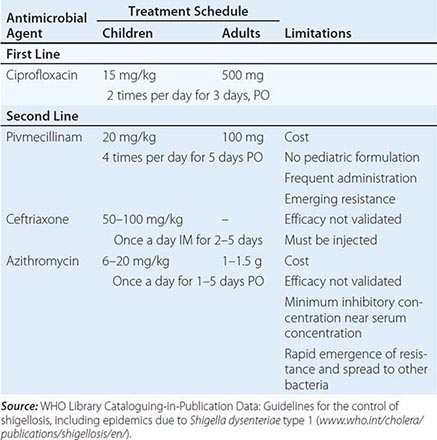
Because of the ready transmissibility of Shigella, current public health recommendations in the United States are that every case be treated with antibiotics. Ciprofloxacin is recommended as first-line treatment. A number of other drugs have been tested and shown to be effective, including ceftriaxone, azithromycin, pivmecillinam, and some fifth-generation quinolones. Whereas infections caused by non-dysenteriae Shigella in immunocompetent individuals are routinely treated with a 3-day course of antibiotics, it is recommended that S. dysenteriae type 1 infections be treated for 5 days and that Shigella infections in immunocompromised patients be treated for 7–10 days.
![]() Treatment for shigellosis must be adapted to the clinical context, with the recognition that the most fragile patients are children <5 years old, who represent two-thirds of all cases worldwide. There are few data on the use of quinolones in children, but Shigella-induced dysentery is a well-recognized indication for their use. The half-life of ciprofloxacin is longer in infants than in older individuals. The ciprofloxacin dose generally recommended for children is 30 mg/kg per day in two divided doses. Adults living in areas with high standards of hygiene are likely to develop milder, shorter-duration disease, whereas infants in endemic areas can develop severe, sometimes fatal, dysentery. In the former setting, treatment will remain minimal and bacteriologic proof of infection will often come after symptoms have resolved; in the latter setting, antibiotic treatment and more aggressive measures, possibly including resuscitation, are often required.
Treatment for shigellosis must be adapted to the clinical context, with the recognition that the most fragile patients are children <5 years old, who represent two-thirds of all cases worldwide. There are few data on the use of quinolones in children, but Shigella-induced dysentery is a well-recognized indication for their use. The half-life of ciprofloxacin is longer in infants than in older individuals. The ciprofloxacin dose generally recommended for children is 30 mg/kg per day in two divided doses. Adults living in areas with high standards of hygiene are likely to develop milder, shorter-duration disease, whereas infants in endemic areas can develop severe, sometimes fatal, dysentery. In the former setting, treatment will remain minimal and bacteriologic proof of infection will often come after symptoms have resolved; in the latter setting, antibiotic treatment and more aggressive measures, possibly including resuscitation, are often required.
REHYDRATION AND NUTRITION
![]() Shigella infection rarely causes significant dehydration. Cases requiring aggressive rehydration (particularly in industrialized countries) are uncommon. In developing countries, malnutrition remains the primary indicator for diarrhea-related death, highlighting the importance of nutrition in early management. Rehydration should be oral unless the patient is comatose or presents in shock. Because of the improved effectiveness of reduced-osmolarity oral rehydration solution (especially for children with acute noncholera diarrhea), the WHO and UNICEF now recommend a standard solution of 245 mOsm/L (sodium, 75 mmol/L; chloride, 65 mmol/L; glucose [anhydrous], 75 mmol/L; potassium, 20 mmol/L; citrate, 10 mmol/L). In shigellosis, the coupled transport of sodium to glucose may be variably affected, but oral rehydration therapy remains the easiest and most efficient form of rehydration, especially in severe cases.
Shigella infection rarely causes significant dehydration. Cases requiring aggressive rehydration (particularly in industrialized countries) are uncommon. In developing countries, malnutrition remains the primary indicator for diarrhea-related death, highlighting the importance of nutrition in early management. Rehydration should be oral unless the patient is comatose or presents in shock. Because of the improved effectiveness of reduced-osmolarity oral rehydration solution (especially for children with acute noncholera diarrhea), the WHO and UNICEF now recommend a standard solution of 245 mOsm/L (sodium, 75 mmol/L; chloride, 65 mmol/L; glucose [anhydrous], 75 mmol/L; potassium, 20 mmol/L; citrate, 10 mmol/L). In shigellosis, the coupled transport of sodium to glucose may be variably affected, but oral rehydration therapy remains the easiest and most efficient form of rehydration, especially in severe cases.
Nutrition should be started as soon as possible after completion of initial rehydration. Early refeeding is safe, well tolerated, and clinically beneficial. Because breast-feeding reduces diarrheal losses and the need for oral rehydration in infants, it should be maintained in the absence of contraindications (e.g., maternal HIV infection).
NONSPECIFIC, SYMPTOM-BASED THERAPY
Antimotility agents have been implicated in prolonged fever in volunteers with shigellosis. These agents are suspected of increasing the risk of toxic megacolon and are thought to have been responsible for HUS in children infected by EHEC strains. For safety reasons, it is better to avoid antimotility agents in bloody diarrhea.
TREATMENT OF COMPLICATIONS
There is no consensus regarding the best treatment for toxic megacolon. The patient should be assessed frequently by both medical and surgical teams. Anemia, dehydration, and electrolyte deficits (particularly hypokalemia) may aggravate colonic atony and should be actively treated. Nasogastric aspiration helps to deflate the colon. Parenteral nutrition has not been proven to be beneficial. Fever persisting beyond 48–72 h raises the possibility of local perforation or abscess. Most studies recommend colectomy if, after 48–72 h, colonic distention persists. However, some physicians recommend continuation of medical therapy for up to 7 days if the patient seems to be improving clinically despite persistent megacolon without free perforation. Intestinal perforation, either isolated or complicating toxic megacolon, requires surgical treatment and intensive medical support.
Rectal prolapse must be treated as soon as possible. With the health care provider using surgical gloves or a soft warm wet cloth and the patient in the knee-chest position, the prolapsed rectum is gently pushed back into place. If edema of the rectal mucosa is evident (rendering reintegration difficult), it can be osmotically reduced by the application of gauze impregnated with a warm solution of saturated magnesium sulfate. Rectal prolapse often relapses but usually resolves along with the resolution of dysentery.
HUS must be treated by water restriction, including discontinuation of oral rehydration solution and potassium-rich alimentation. Hemofiltration is usually required.
PREVENTION
Hand washing after defecation or handling of children’s feces and before handling of food is recommended. Stool decontamination (e.g., with sodium hypochlorite), together with a cleaning protocol for medical staff as well as for patients, has proven useful in limiting the spread of infection during Shigella outbreaks. Ideally, patients should have a negative stool culture before their infection is considered cured. Recurrences are rare if therapeutic and preventive measures are correctly implemented.
Although several live attenuated oral and subunit parenteral vaccine candidates have been produced and are undergoing clinical trials, no vaccine against shigellosis is currently available. Especially given the rapid progression of antibiotic resistance in Shigella, a vaccine is urgently needed.
192 |
Infections Due to Campylobacter and Related Organisms |
DEFINITION
Bacteria of the genus Campylobacter and of the related genera Arcobacter and Helicobacter (Chap. 188) cause a variety of inflammatory conditions. Although acute diarrheal illnesses are most common, these organisms may cause infections in virtually all parts of the body, especially in compromised hosts, and these infections may have late nonsuppurative sequelae. The designation Campylobacter comes from the Greek for “curved rod” and refers to the organism’s vibrio-like morphology.
ETIOLOGY
Campylobacters are motile, non-spore-forming, curved, gram-negative rods. Originally known as Vibrio fetus, these bacilli were reclassified as a new genus in 1973 after their dissimilarity to other vibrios was recognized. More than 15 species have since been identified. These species are currently divided into three genera: Campylobacter, Arcobacter, and Helicobacter. Not all of the species are pathogens of humans. The human pathogens fall into two major groups: those that primarily cause diarrheal disease and those that cause extraintestinal infection. The principal diarrheal pathogen is Campylobacter jejuni, which accounts for 80–90% of all cases of recognized illness due to campylobacters and related genera. Other organisms that cause diarrheal disease include Campylobacter coli, Campylobacter upsaliensis, Campylobacter lari, Campylobacter hyointestinalis, Campylobacter fetus, Arcobacter butzleri, Arcobacter cryaerophilus, Helicobacter cinaedi, and Helicobacter fennelliae. The two Helicobacter species causing diarrheal disease, H. cinaedi and H. fennelliae, are intestinal rather than gastric organisms; in terms of the clinical features of the illnesses they cause, these species most closely resemble Campylobacter rather than Helicobacter pylori (Chap. 188) and thus are considered in this chapter. The pathogenic roles of Campylobacter concisus, Campylobacter ureolyticus, Campylobacter troglodytis, and Campylobacter pyloridis are uncertain. A new subspecies—C. fetus subspecies testudinum—has been described, chiefly in Asian patients; its close resemblance to strains isolated from reptiles suggests a food source.
The major species causing extraintestinal illnesses is C. fetus. However, any of the diarrheal agents listed above may cause systemic or localized infection as well, especially in compromised hosts. Neither aerobes nor strict anaerobes, these microaerophilic organisms are adapted for survival in the gastrointestinal mucous layer. This chapter focuses on C. jejuni and C. fetus as the major pathogens in and prototypes for their groups. The key features of infection are listed by species (excluding C. jejuni, described in detail in the text below) in Table 192-1.
|
CLINICAL FEATURES ASSOCIATED WITH INFECTION DUE TO “ATYPICAL” CAMPYLOBACTER AND RELATED SPECIES IMPLICATED AS CAUSES OF HUMAN ILLNESS |
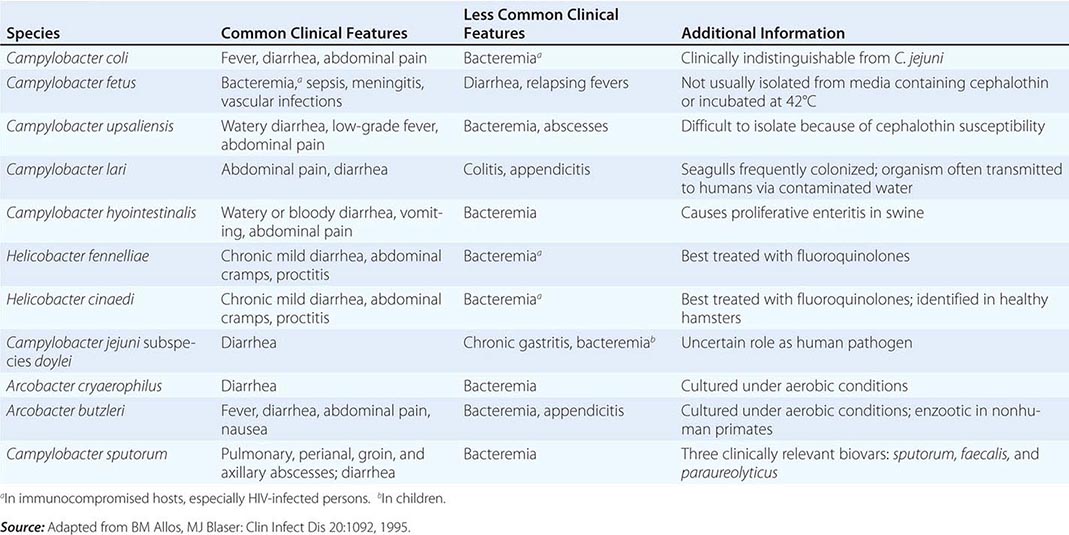
EPIDEMIOLOGY
![]() Campylobacters are found in the gastrointestinal tract of many animals used for food (including poultry, cattle, sheep, and swine) and many household pets (including birds, dogs, and cats). These microorganisms usually do not cause illness in their animal hosts. In most cases, campylobacters are transmitted to humans in raw or undercooked food products or through direct contact with infected animals. In the United States and other developed countries, ingestion of contaminated poultry that has not been sufficiently cooked is the most common mode of acquisition (30–70% of cases). Other modes include ingestion of raw (unpasteurized) milk or untreated water, contact with infected household pets, travel to developing countries (campylobacters being among the leading causes of traveler’s diarrhea; Chaps. 149 and 160), oral-anal sexual contact, and (occasionally) contact with an index case who is incontinent of stool (e.g., a baby).
Campylobacters are found in the gastrointestinal tract of many animals used for food (including poultry, cattle, sheep, and swine) and many household pets (including birds, dogs, and cats). These microorganisms usually do not cause illness in their animal hosts. In most cases, campylobacters are transmitted to humans in raw or undercooked food products or through direct contact with infected animals. In the United States and other developed countries, ingestion of contaminated poultry that has not been sufficiently cooked is the most common mode of acquisition (30–70% of cases). Other modes include ingestion of raw (unpasteurized) milk or untreated water, contact with infected household pets, travel to developing countries (campylobacters being among the leading causes of traveler’s diarrhea; Chaps. 149 and 160), oral-anal sexual contact, and (occasionally) contact with an index case who is incontinent of stool (e.g., a baby).
Campylobacter infections are common. Several studies indicate that, in the United States, diarrheal disease due to campylobacters is more common than that due to Salmonella and Shigella combined. Infections occur throughout the year, but their incidence peaks during summer and early autumn. Persons of all ages are affected; however, attack rates for C. jejuni are highest among young children and young adults, whereas those for C. fetus are highest at the extremes of age. Systemic infections due to C. fetus (and to other Campylobacter and related species) are most common among compromised hosts. Persons at increased risk include those with AIDS, hypogammaglobulinemia, neoplasia, liver disease, diabetes mellitus, and generalized atherosclerosis as well as neonates and pregnant women. However, apparently healthy nonpregnant persons occasionally develop transient Campylobacter bacteremia as part of a gastrointestinal illness.
In contrast, in many developing countries, C. jejuni infections are hyperendemic, with the highest rates among children <2 years old. Infection rates fall with age, as does the illness-to-infection ratio. These observations suggest that frequent exposure to C. jejuni leads to the acquisition of immunity.
PATHOLOGY AND PATHOGENESIS
![]() C. jejuni infections may be subclinical, especially in hosts in developing countries who have had multiple prior infections and thus are partially immune. Symptomatic infections mostly occur within 2–4 days (range, 1–7 days) of exposure to the organism in food or water. The sites of tissue injury include the jejunum, ileum, and colon. Biopsies show an acute nonspecific inflammatory reaction, with neutrophils, monocytes, and eosinophils in the lamina propria, as well as damage to the epithelium, including loss of mucus, glandular degeneration, and crypt abscesses. Biopsy findings may be consistent with Crohn’s disease or ulcerative colitis, but these “idiopathic” chronic inflammatory diseases should not be diagnosed unless infectious colitis, specifically including that due to infection with Campylobacter species and related organisms, has been ruled out.
C. jejuni infections may be subclinical, especially in hosts in developing countries who have had multiple prior infections and thus are partially immune. Symptomatic infections mostly occur within 2–4 days (range, 1–7 days) of exposure to the organism in food or water. The sites of tissue injury include the jejunum, ileum, and colon. Biopsies show an acute nonspecific inflammatory reaction, with neutrophils, monocytes, and eosinophils in the lamina propria, as well as damage to the epithelium, including loss of mucus, glandular degeneration, and crypt abscesses. Biopsy findings may be consistent with Crohn’s disease or ulcerative colitis, but these “idiopathic” chronic inflammatory diseases should not be diagnosed unless infectious colitis, specifically including that due to infection with Campylobacter species and related organisms, has been ruled out.
The high frequency of C. jejuni infections and their severity and recurrence among hypogammaglobulinemic patients suggest that antibodies are important in protective immunity. The pathogenesis of infection is uncertain. Both the motility of the strain and its capacity to adhere to host tissues appear to favor disease, but classic enterotoxins and cytotoxins (although they have been described and include cytolethal distending toxin, or CDT) appear not to play substantial roles in tissue injury or disease production. The organisms have been visualized within the epithelium, albeit in low numbers. The documentation of a significant tissue response and occasionally of C. jejuni bacteremia further suggests that tissue invasion is clinically significant, and in vitro studies are consistent with this pathogenetic feature.
The pathogenesis of C. fetus infections is better defined. Virtually all clinical isolates of C. fetus possess a proteinaceous capsule-like structure (an S-layer) that renders the organisms resistant to complement-mediated killing and opsonization. As a result, C. fetus can cause bacteremia and can seed sites beyond the intestinal tract. The ability of the organism to switch the S-layer proteins expressed—a phenomenon that results in antigenic variability—may contribute to the chronicity and high rate of recurrence of C. fetus infections in compromised hosts.
CLINICAL MANIFESTATIONS
The clinical features of infections due to Campylobacter and the related Arcobacter and intestinal Helicobacter species causing enteric disease appear to be highly similar. C. jejuni can be considered the prototype, in part because it is by far the most common enteric pathogen in the group. A prodrome of fever, headache, myalgia, and/or malaise often occurs 12–48 h before the onset of diarrheal symptoms. The most common signs and symptoms of the intestinal phase are diarrhea, abdominal pain, and fever. The degree of diarrhea varies from several loose stools to grossly bloody stools; most patients presenting for medical attention have ≥10 bowel movements on the worst day of illness. Abdominal pain usually consists of cramping and may be the most prominent symptom. Pain is usually generalized but may become localized; C. jejuni infection may cause pseudoappendicitis. Fever may be the only initial manifestation of C. jejuni infection, a situation mimicking the early stages of typhoid fever. Febrile young children may develop convulsions. Campylobacter enteritis is generally self-limited; however, symptoms persist for >1 week in 10–20% of patients seeking medical attention, and clinical relapses occur in 5–10% of such untreated patients. Studies of common-source epidemics indicate that milder illnesses or asymptomatic infections may commonly occur.
C. fetus may cause a diarrheal illness similar to that due to C. jejuni, especially in normal hosts. This organism also may cause either intermittent diarrhea or nonspecific abdominal pain without localizing signs. Sequelae are uncommon, and the outcome is benign. C. fetus may also cause a prolonged relapsing systemic illness (with fever, chills, and myalgias) that has no obvious primary source; this manifestation is especially common among compromised hosts. Secondary seeding of an organ (e.g., meninges, brain, bone, urinary tract, or soft tissue) complicates the course, which may be fulminant. C. fetus infections have a tropism for vascular sites: endocarditis, mycotic aneurysm, and septic thrombophlebitis may all occur. Infection during pregnancy often leads to fetal death. A variety of Campylobacter species and H. cinaedi can cause recurrent cellulitis with fever and bacteremia in immunocompromised hosts.
COMPLICATIONS
Except in infection with C. fetus, bacteremia is uncommon, developing most often in immunocompromised hosts and at the extremes of age. Three patterns of extraintestinal infection have been noted: (1) transient bacteremia in a normal host with enteritis (benign course, no specific treatment needed); (2) sustained bacteremia or focal infection in a normal host (bacteremia originating from enteritis, with patients responding well to antimicrobial therapy); and (3) sustained bacteremia or focal infection in a compromised host. Enteritis may not be clinically apparent. Antimicrobial therapy, possibly prolonged, is necessary for suppression or cure of the infection.
Campylobacter, Arcobacter, and intestinal Helicobacter infections in patients with AIDS or hypogammaglobulinemia may be severe, persistent, and extraintestinal; relapse after cessation of therapy is common. Hypogammaglobulinemic patients also may develop osteomyelitis and an erysipelas-like rash or cellulitis.
Local suppurative complications of infection include cholecystitis, pancreatitis, and cystitis; distant complications include meningitis, endocarditis, arthritis, peritonitis, cellulitis, and septic abortion. All these complications are rare, except in immunocompromised hosts. Hepatitis, interstitial nephritis, and the hemolytic-uremic syndrome occasionally complicate acute infection. Reactive arthritis and other rheumatologic complaints may develop several weeks after infection, especially in persons with the HLA-B27 phenotype. Guillain-Barré syndrome or its Miller Fisher (cranial polyneuropathy) variant follows Campylobacter infections uncommonly—i.e., in 1 of every 1000–2000 cases or, for certain C. jejuni serotypes (such as O19), in 1 of every 100–200 cases. Despite the low frequency of this complication, it is now estimated that Campylobacter infections, because of their high incidence, may trigger 20–40% of all cases of Guillain-Barré syndrome. The presence of sialylated lipopolysaccharides on C. jejuni strains is a form of molecular mimicry that promotes autoimmune recognition of sialylated cell surface molecules on axons. Asymptomatic Campylobacter infection also may trigger Guillain-Barré syndrome. Immunoproliferative small-intestinal disease (alpha chain disease), a form of lymphoma that originates in small-intestinal mucosa-associated lymphoid tissue, has been associated with C. jejuni; antimicrobial therapy has led to marked clinical improvement.
DIAGNOSIS
In patients with Campylobacter enteritis, peripheral leukocyte counts reflect the severity of the inflammatory process. However, stools from nearly all Campylobacter-infected patients presenting for medical attention in the United States contain leukocytes or erythrocytes. Gram- or Wright-stained fecal smears should be examined in all suspected cases. When the diagnosis of Campylobacter enteritis is suspected on the basis of findings indicating inflammatory diarrhea (fever, fecal leukocytes), clinicians can ask the microbiology laboratory to attempt the visualization of organisms with characteristic vibrioid morphology by direct microscopic examination of stools with Gram’s staining or to use phase-contrast or dark-field microscopy to identify the organisms’ characteristic “darting” motility. Confirmation of the diagnosis of Campylobacter infection is based on identification of an isolate from cultures of stool, blood, or another site. Campylobacter-specific media should be used to culture stools from all patients with inflammatory or bloody diarrhea. Because all Campylobacter species are fastidious, they will not be isolated unless selective media or other selective techniques are used. Not all media are equally useful for isolation of the broad array of campylobacters; therefore, failure to isolate campylobacters from stool does not entirely rule out their presence. Species-specific polymerase chain reaction techniques have been developed to facilitate exact diagnoses. The detection of the organisms in stool almost always implies infection; there is a brief period of postconvalescent fecal carriage and no obvious commensalism in humans. In contrast, Campylobacter sputorum and related organisms found in the oral cavity are commensals that only rarely have pathogenic significance. Because of the low levels of metabolic activity of Campylobacter species in standard blood culture media, Campylobacter bacteremia may be difficult to detect unless laboratorians check for low-positive results in quantitative assays.
DIFFERENTIAL DIAGNOSIS
The symptoms of Campylobacter enteritis are not sufficiently unusual to distinguish this illness from that due to Salmonella, Shigella, Yersinia, and other pathogens. The combination of fever and fecal leukocytes or erythrocytes is indicative of inflammatory diarrhea, and definitive diagnosis is based on culture or demonstration of the characteristic organisms on stained fecal smears. Similarly, extrain-testinal Campylobacter illness is diagnosed by culture. Infection due to Campylobacter should be suspected in the setting of septic abortion, and that due to C. fetus should be suspected specifically in the setting of septic thrombophlebitis. It is important to reiterate that (1) the presentation of Campylobacter enteritis may mimic that of ulcerative colitis or Crohn’s disease, (2) Campylobacter enteritis is much more common than either of the latter (especially among young adults), and (3) biopsy may not distinguish among these entities. Thus a diagnosis of inflammatory bowel disease should not be made until Campylobacter infection has been ruled out, especially in persons with a history of foreign travel, significant animal contact, immunodeficiency, or exposure incurring a high risk of transmission.
|
TREATMENT |
CAMPYLOBACTER INFECTION |
Fluid and electrolyte replacement is central to the treatment of diarrheal illnesses (Chap. 160). Even among patients presenting for medical attention with Campylobacter enteritis, not all clearly benefit from specific antimicrobial therapy. Indications for therapy include high fever, bloody diarrhea, severe diarrhea, persistence for >1 week, and worsening of symptoms. A 5- to 7-day course of erythromycin (250 mg orally four times daily or—for children—30–50 mg/kg per day, in divided doses) is the regimen of choice. Both clinical trials and in vitro susceptibility testing indicate that other macrolides, including azithromycin (a 1- or 3-day regimen), also are useful therapeutic agents. An alternative regimen for adults is ciprofloxacin (500 mg orally twice daily) or another fluoroquinolone for 5–7 days, but resistance to this class of agents as well as to tetracyclines is substantial; ~22% of U.S. isolates in 2010 were resistant to ciprofloxacin. Because macrolide resistance usually is much less common (<10%), these drugs are the empirical agents of choice. Patients infected with antibiotic-resistant strains are at increased risk of adverse outcomes. Use of antimotility agents, which may prolong the duration of symptoms and have been associated with toxic megacolon and with death, is not recommended.
For systemic infections, treatment with gentamicin (1.7 mg/kg IV every 8 h after a loading dose of 2 mg/kg), imipenem (500 mg IV every 6 h), or chloramphenicol (50 mg/kg IV each day in three or four divided doses) should be started empirically, but susceptibility testing should then be performed. Ciprofloxacin and amoxicillin-clavulanate are alternative agents for susceptible strains. In the absence of immunocompromise or endovascular infections, therapy should be administered for 14 days. For immunocompromised patients with systemic infections due to C. fetus and for patients with endovascular infections, prolonged therapy (for up to 4 weeks) is usually necessary. For recurrent infections in immunocompromised hosts, lifelong therapy/prophylaxis is sometimes necessary.
PROGNOSIS
Nearly all patients recover fully from Campylobacter enteritis, either spontaneously or after antimicrobial therapy. Volume depletion probably contributes to the few deaths that are reported. As stated above, occasional patients develop reactive arthritis or Guillain-Barré syndrome or its variants. Systemic infection with C. fetus is much more often fatal than that due to related species; this higher mortality rate reflects in part the population affected. Prognosis depends on the rapidity with which appropriate therapy is begun. Otherwise healthy hosts usually survive C. fetus infections without sequelae. Compromised hosts often have recurrent and/or life-threatening infections due to a variety of Campylobacter species.
193 |
Cholera and Other Vibrioses |
Members of the genus Vibrio cause a number of important infectious syndromes. Classic among them is cholera, a devastating diarrheal disease caused by Vibrio cholerae that has been responsible for seven global pandemics and much suffering over the past two centuries. Epidemic cholera remains a significant public health concern in the developing world today. Other vibrioses caused by other Vibrio species include syndromes of diarrhea, soft tissue infection, or primary sepsis. All Vibrio species are highly motile, facultatively anaerobic, curved gram-negative rods with one or more flagella. In nature, vibrios most commonly reside in tidal rivers and bays under conditions of moderate salinity. They proliferate in the summer months when water temperatures exceed 20°C. As might be expected, the illnesses they cause also increase in frequency during the warm months.
CHOLERA
DEFINITION
Cholera is an acute diarrheal disease that can, in a matter of hours, result in profound, rapidly progressive dehydration and death. Accordingly, cholera gravis (the severe form) is a much-feared disease, particularly in its epidemic presentation. Fortunately, prompt aggressive fluid repletion and supportive care can obviate the high mortality that is historically associated with cholera. Although the term cholera has occasionally been applied to any severely dehydrating secretory diarrheal illness, whether infectious in etiology or not, it now refers to disease caused by V. cholerae serogroup O1 or O139—i.e., the serogroups with epidemic potential.
MICROBIOLOGY AND EPIDEMIOLOGY
The species V. cholerae is classified into more than 200 serogroups based on the carbohydrate determinants of their lipopolysaccharide (LPS) O antigens. Although some non-O1 V. cholerae serogroups (strains that do not agglutinate in antisera to the O1 group antigen) have occasionally caused sporadic outbreaks of diarrhea, serogroup O1 was, until the emergence of serogroup O139 in 1992, the exclusive cause of epidemic cholera. Two biotypes of V. cholerae O1, classical and El Tor, are distinguished. Each biotype is further subdivided into two serotypes, termed Inaba and Ogawa.
The natural habitat of V. cholerae is coastal salt water and brackish estuaries, where the organism lives in close relation to plankton. V. cholerae can also exist in freshwater in the presence of adequate nutrients and warmth. Humans become infected incidentally but, once infected, can act as vehicles for spread. Ingestion of water contaminated by human feces is the most common means of acquisition of V. cholerae. Consumption of contaminated food also can contribute to spread. There is no known animal reservoir. Although the infectious dose is relatively high, it is markedly reduced in hypochlorhydric persons, in those using antacids, and when gastric acidity is buffered by a meal. Cholera is predominantly a pediatric disease in endemic areas, but it affects adults and children equally when newly introduced into a population. In endemic areas, the burden of disease is often greatest during “cholera seasons” associated with high temperatures, heavy rainfall, and flooding, but cholera can occur year-round. For unexplained reasons, susceptibility to cholera is significantly influenced by ABO blood group status; persons with type O blood are at greatest risk of severe disease if infected, whereas those with type AB are at least risk.
![]() Cholera is native to the Ganges delta in the Indian subcontinent. Since 1817, seven global pandemics have occurred. The current (seventh) pandemic—the first due to the El Tor biotype—began in Indonesia in 1961 and spread in serial waves throughout Asia as V. cholerae El Tor displaced the endemic classical biotype, which is thought to have caused the previous six pandemics. In the early 1970s, El Tor cholera erupted in Africa, causing major epidemics before becoming a persistent endemic problem. Currently, >90% of cholera cases reported annually to the World Health Organization (WHO) are from Africa (Fig. 193-1), but the true burden in Africa as well as in Asia is unknown because diagnosis is often syndromic and because many countries with endemic cholera do not report cholera to the WHO. It is possible that >3 million cases of cholera occur yearly (of which only ~200,000 are reported to the WHO), resulting in >100,000 deaths annually (of which <5000 are reported to the WHO).
Cholera is native to the Ganges delta in the Indian subcontinent. Since 1817, seven global pandemics have occurred. The current (seventh) pandemic—the first due to the El Tor biotype—began in Indonesia in 1961 and spread in serial waves throughout Asia as V. cholerae El Tor displaced the endemic classical biotype, which is thought to have caused the previous six pandemics. In the early 1970s, El Tor cholera erupted in Africa, causing major epidemics before becoming a persistent endemic problem. Currently, >90% of cholera cases reported annually to the World Health Organization (WHO) are from Africa (Fig. 193-1), but the true burden in Africa as well as in Asia is unknown because diagnosis is often syndromic and because many countries with endemic cholera do not report cholera to the WHO. It is possible that >3 million cases of cholera occur yearly (of which only ~200,000 are reported to the WHO), resulting in >100,000 deaths annually (of which <5000 are reported to the WHO).
FIGURE 193-1 World distribution of cholera in 2010–2012. WHO, World Health Organization. (Courtesy of Drs. M. and R. Piarroux, Université de la Méditerranée, France; with permission.)
After a century without cholera in Latin America, the current cholera pandemic reached Central and South America in 1991. Following an initial explosive spread that affected millions, the burden of disease has markedly decreased in Latin America. In 2010, a severe cholera outbreak began in Haiti, a country with no recorded history of this disease. Several lines of evidence indicate that cholera was likely introduced into Haiti by United Nations security forces from Asia, raising the possibility that asymptomatic carriers of V. cholerae play an important role in transmitting cholera over long distances. To date, the outbreak has involved more than 700,000 individuals, resulting in thousands of deaths. The recent history of cholera has been punctuated by such severe outbreaks, especially among impoverished or displaced persons. These outbreaks are often precipitated by war or other circumstances that lead to the breakdown of public health measures. Such was the case in the camps for Rwandan refugees set up in 1994 around Goma, Zaire, and in 2008–2009 in Zimbabwe.
Sporadic endemic infections due to V. cholerae O1 strains related to the seventh-pandemic strain have been recognized along the U.S. Gulf Coast of Louisiana and Texas. These infections are typically associated with the consumption of contaminated, locally harvested shellfish. Occasionally, cases in U.S. locations remote from the Gulf Coast have been linked to shipped-in Gulf Coast seafood.
In October 1992, a large-scale outbreak of clinical cholera caused by a new serogroup, O139, occurred in southeastern India. The organism appears to be a derivative of El Tor O1 but has a distinct LPS and an immunologically related O-antigen polysaccharide capsule. (O1 organisms are not encapsulated.) After an initial spread across 11 Asian countries, V. cholerae O139 has once again been almost entirely replaced by O1 strains. The clinical manifestations of disease caused by V. cholerae O139 are indistinguishable from those of O1 cholera. Immunity to one, however, is not protective against the other.
PATHOGENESIS
In the final analysis, cholera is a toxin-mediated disease. The watery diarrhea characteristic of cholera is due to the action of cholera toxin, a potent protein enterotoxin elaborated by the organism in the small intestine. The toxin-coregulated pilus (TCP), so named because its synthesis is regulated in parallel with that of cholera toxin, is essential for V. cholerae to survive and multiply in (colonize) the small intestine. Cholera toxin, TCP, and several other virulence factors are coordinately regulated by ToxR. This protein modulates the expression of genes coding for virulence factors in response to environmental signals via a cascade of regulatory proteins. Additional regulatory processes, including bacterial responses to the density of the bacterial population (in a phenomenon known as quorum sensing), modulate the virulence ofV. cholerae.
Once established in the human small bowel, the organism produces cholera toxin, which consists of a monomeric enzymatic moiety (the A subunit) and a pentameric binding moiety (the B subunit). The B pentamer binds to GM1 ganglioside, a glycolipid on the surface of epithelial cells that serves as the toxin receptor and makes possible the delivery of the A subunit to its cytosolic target. The activated A subunit (A1) irreversibly transfers ADP-ribose from nicotinamide adenine dinucleotide to its specific target protein, the GTP-binding regulatory component of adenylate cyclase. The ADP-ribosylated G protein upregulates the activity of adenylate cyclase; the result is the intracellular accumulation of high levels of cyclic AMP. In intestinal epithelial cells, cyclic AMP inhibits the absorptive sodium transport system in villus cells and activates the secretory chloride transport system in crypt cells, and these events lead to the accumulation of sodium chloride in the intestinal lumen. Because water moves passively to maintain osmolality, isotonic fluid accumulates in the lumen. When the volume of that fluid exceeds the capacity of the rest of the gut to resorb it, watery diarrhea results. Unless the wasted fluid and electrolytes are adequately replaced, shock (due to profound dehydration) and acidosis (due to loss of bicarbonate) follow. Although perturbation of the adenylate cyclase pathway is the primary mechanism by which cholera toxin causes excess fluid secretion, cholera toxin also enhances intestinal secretion via prostaglandins and/or neural histamine receptors.
![]() The V. cholerae genome comprises two circular chromosomes. Lateral gene transfer has played a key role in the evolution of epidemic V. cholerae. The genes encoding cholera toxin (ctxAB) are part of the genome of a bacteriophage, CTXФ. The receptor for this phage on the V. cholerae surface is the intestinal colonization factor TCP. Because ctxAB is part of a mobile genetic element (CTXФ), horizontal transfer of this bacteriophage may account for the emergence of new toxigenic V. cholerae serogroups. Many of the other genes important for V. cholerae pathogenicity, including the genes encoding the biosynthesis of TCP, those encoding accessory colonization factors, and those regulating virulence gene expression, are clustered together in the V. cholerae pathogenicity island. Similar clustering of virulence genes is found in other bacterial pathogens. It is believed that pathogenicity islands are acquired by horizontal gene transfer. V. cholerae O139 is probably derived from an El Tor O1 strain that acquired the genes for O139 O-antigen synthesis by horizontal gene transfer.
The V. cholerae genome comprises two circular chromosomes. Lateral gene transfer has played a key role in the evolution of epidemic V. cholerae. The genes encoding cholera toxin (ctxAB) are part of the genome of a bacteriophage, CTXФ. The receptor for this phage on the V. cholerae surface is the intestinal colonization factor TCP. Because ctxAB is part of a mobile genetic element (CTXФ), horizontal transfer of this bacteriophage may account for the emergence of new toxigenic V. cholerae serogroups. Many of the other genes important for V. cholerae pathogenicity, including the genes encoding the biosynthesis of TCP, those encoding accessory colonization factors, and those regulating virulence gene expression, are clustered together in the V. cholerae pathogenicity island. Similar clustering of virulence genes is found in other bacterial pathogens. It is believed that pathogenicity islands are acquired by horizontal gene transfer. V. cholerae O139 is probably derived from an El Tor O1 strain that acquired the genes for O139 O-antigen synthesis by horizontal gene transfer.
CLINICAL MANIFESTATIONS
Individuals infected with V. cholerae O1 or O139 exhibit a range of clinical manifestations. Some individuals are asymptomatic or have only mild diarrhea; others present with the sudden onset of explosive and life-threatening diarrhea (cholera gravis). The reasons for the range in signs and symptoms of disease are incompletely understood but include the level of preexisting immunity, blood type, and nutritional status. In a nonimmune individual, after a 24- to 48-h incubation period, cholera characteristically begins with the sudden onset of painless watery diarrhea that may quickly become voluminous. Patients often vomit. In severe cases, volume loss can exceed 250 mL/kg in the first 24 h. If fluids and electrolytes are not replaced, hypovolemic shock and death may ensue. Fever is usually absent. Muscle cramps due to electrolyte disturbances are common. The stool has a characteristic appearance: a nonbilious, gray, slightly cloudy fluid with flecks of mucus, no blood, and a somewhat fishy, inoffensive odor. It has been called “rice-water” stool because of its resemblance to the water in which rice has been washed (Fig. 193-2). Clinical symptoms parallel volume contraction: at losses of <5% of normal body weight, thirst develops; at 5–10%, postural hypotension, weakness, tachycardia, and decreased skin turgor are documented; and at >10%, oliguria, weak or absent pulses, sunken eyes (and, in infants, sunken fontanelles), wrinkled (“washerwoman”) skin, somnolence, and coma are characteristic. Complications derive exclusively from the effects of volume and electrolyte depletion and include renal failure due to acute tubular necrosis. Thus, if the patient is adequately treated with fluid and electrolytes, complications are averted and the process is self-limited, resolving in a few days.
FIGURE 193-2 Rice water cholera stool. Note floating mucus and gray watery appearance. (Courtesy of Dr. A. S. G. Faruque, International Centre for Diarrhoeal Disease Research, Dhaka; with permission.)
Laboratory data usually reveal an elevated hematocrit (due to hemoconcentration) in nonanemic patients; mild neutrophilic leukocytosis; elevated levels of blood urea nitrogen and creatinine consistent with prerenal azotemia; normal sodium, potassium, and chloride levels; a markedly reduced bicarbonate level (<15 mmol/L); and an elevated anion gap (due to increases in serum lactate, protein, and phosphate). Arterial pH is usually low (~7.2).
DIAGNOSIS
Cholera should be suspected when a patient ≥2 years of age develops acute watery diarrhea in an area known to have cholera or when a patient ≥5 years of age develops severe dehydration or dies from acute watery diarrhea, even in an area where cholera is not known to be present. The clinical suspicion of cholera can be confirmed by the identification of V. cholerae in stool; however, the organism must be specifically sought. With experience, it can be detected directly by dark-field microscopy on a wet mount of fresh stool, and its serotype can be discerned by immobilization with specific antiserum. Laboratory isolation of the organism requires the use of a selective medium such as taurocholate-tellurite-gelatin (TTG) agar or thiosulfate–citrate–bile salts–sucrose (TCBS) agar. If a delay in sample processing is expected, Carey-Blair transport medium and/or alkaline-peptone water-enrichment medium may be used as well. In endemic areas, there is little need for biochemical confirmation and characterization, although these tasks may be worthwhile in places where V. cholerae is an uncommon isolate. Standard microbiologic biochemical testing for Enterobacteriaceae will suffice for identification of V. cholerae. All vibrios are oxidase-positive. A point-of-care antigen-detection cholera dipstick assay is now commercially available for use in the field or where laboratory facilities are lacking.
|
TREATMENT |
CHOLERA |
Death from cholera is due to hypovolemic shock; thus treatment of individuals with cholera first and foremost requires fluid resuscitation and management. In light of the level of dehydration (Table 193-1) and the patient’s age and weight, euvolemia should first be rapidly restored, and adequate hydration should then be maintained to replace ongoing fluid losses (Table 193-2). Administration of oral rehydration solution (ORS) takes advantage of the hexose-Na+ co-transport mechanism to move Na+ across the gut mucosa together with an actively transported molecule such as glucose (or galactose). Cl– and water follow. This transport mechanism remains intact even when cholera toxin is active. ORS may be made by adding safe water to prepackaged sachets containing salts and sugar or by adding 0.5 teaspoon of table salt and 6 teaspoons of table sugar to 1 L of safe water. Potassium intake in bananas or green coconut water should be encouraged. A number of ORS formulations are available, and the WHO now recommends “low-osmolarity” ORS for treatment of individuals with dehydrating diarrhea of any cause (Table 193-3). If available, rice-based ORS is considered superior to standard ORS in the treatment of cholera. ORS can be administered via a nasogastric tube to individuals who cannot ingest fluid; however, optimal management of individuals with severe dehydration includes the administration of IV fluid and electrolytes. Because profound acidosis (pH <7.2) is common in this group, Ringer’s lactate is the best choice among commercial products (Table 193-4); it must be used with additional potassium supplements, preferably given by mouth. The total fluid deficit in severely dehydrated patients (>10% of body weight) can be replaced safely within the first 3–4 h of therapy, half within the first hour. Transient muscle cramps and tetany are common. Thereafter, oral therapy can usually be initiated, with the goal of maintaining fluid intake equal to fluid output. However, patients with continued large-volume diarrhea may require prolonged IV treatment to match gastrointestinal fluid losses. Severe hypokalemia can develop but will respond to potassium given either IV or orally. In the absence of adequate staff to monitor the patient’s progress, the oral route of rehydration and potassium replacement is safer than the IV route.
|
ASSESSING THE DEGRE OF DEHYDRATION IN PATIENTS WITH CHOLERA |
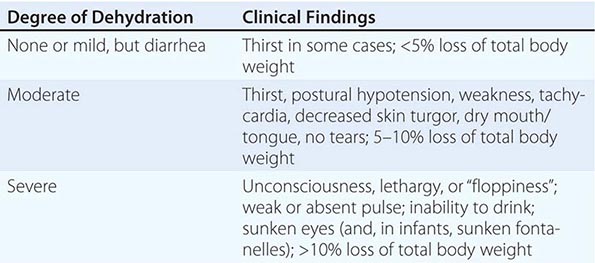
|
TREATMENT OF CHOLERA, BASED ON DEGREE OF DEHYDRATIONa |
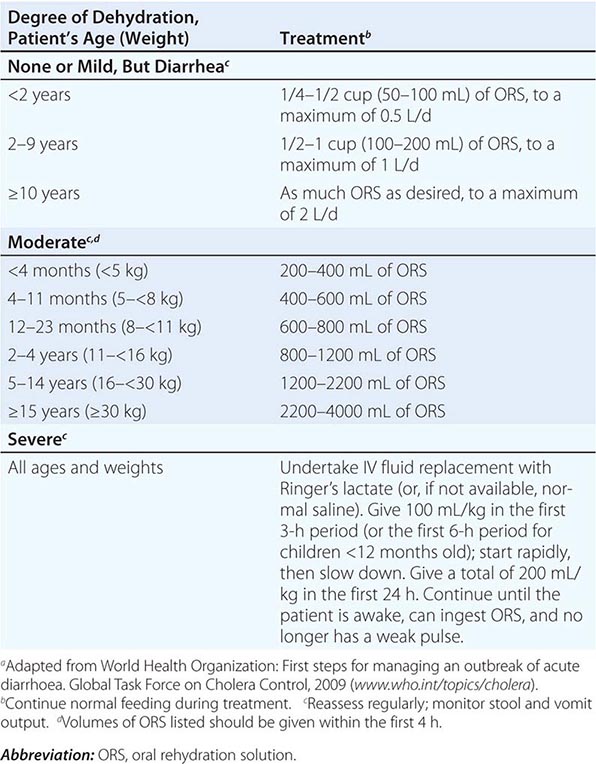
|
COMPOSITION OF WORLD HEALTH ORGANIZATION REDUCED-OSMOLARITY ORAL REHYDRATION SOLUTION (ORS)a,b |
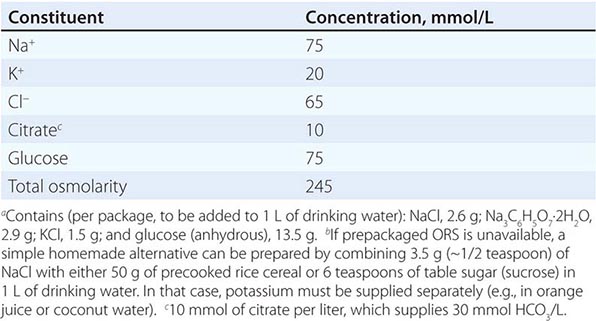
|
ELECTROLYTE COMPOSITION OF CHOLERA STOOL AND OF INTRAVENOUS REHYDRATION SOLUTION |
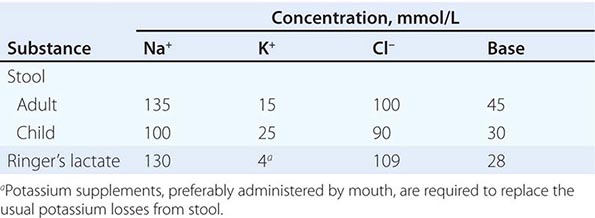
Although not necessary for cure, the use of an antibiotic to which the organism is susceptible diminishes the duration and volume of fluid loss and hastens clearance of the organism from the stool. Adjunctive antibiotics should therefore be administered to patients with moderate or severe dehydration due to cholera. In many areas, macrolides such as erythromycin (adults, 250 mg orally four times a day for 3 days; children, 12.5 mg/kg per dose four times a day for 3 days) or azithromycin (adults, a single 1-g dose; children, a single 20-mg/kg dose) are the agents of choice. Increasing resistance to tetracyclines is widespread; however, in areas with confirmed susceptibility, tetracycline (nonpregnant adults, 500 mg orally four times a day for 3 days; children >8 years old, 12.5 mg/kg per dose four times a day for 3 days) or doxycycline (nonpregnant adults, a 300-mg single dose; children >8 years old, a single dose of 4–6 mg/kg) may be used. Similarly, increasing resistance to fluoroquinolones is being reported, but in areas with confirmed susceptibility, a fluoroquinolone such as ciprofloxacin may be used (adults, 500 mg twice a day for 3 days; children, 15 mg/kg twice a day for 3 days).
PREVENTION
Provision of safe water and of facilities for sanitary disposal of feces, improved nutrition, and attention to food preparation and storage in the household can significantly reduce the incidence of cholera. In addition, precautions should be taken to prevent the spread of cholera via infected and potentially asymptomatic persons from endemic to nonendemic regions of the world (as was probably the case in the ongoing outbreak in Haiti; see “Microbiology and Epidemiology,” above).
Much effort has been devoted to the development of an effective cholera vaccine over the past few decades, with a particular focus on oral vaccine strains. In an attempt to maximize mucosal responses, two types of oral cholera vaccine have been developed: oral killed vaccines and live attenuated vaccines. Currently, two oral killed cholera vaccines have been prequalified by the WHO and are available internationally. WC-rBS (Dukoral®; Crucell, Stockholm, Sweden) contains several biotypes and serotypes of V. cholerae O1 supplemented with 1 mg of recombinant cholera toxin B subunit per dose. BivWC (Shanchol™; Shantha Biotechnics–Sanofi Pasteur, Mumbai, India) contains several biotypes and serotypes of V. cholerae O1 and V. cholerae O139 without supplemental cholera toxin B subunit. The vaccines are administered as a two- or three-dose regimen, with doses usually separated by 14 days. They provide ~60–85% protection for the first few months. Booster immunizations of WC-rBS are recommended after 2 years for individuals ≥6 years of age and after 6 months for children 2–5 years of age. For BivWC, which was developed more recently, no formal recommendation regarding booster immunizations exists. However, BivWC was associated with ~60% protection over 5 years among recipients of all ages in a study in Kolkata, India; the rate of protection among children ≤5 years of age approximated 40%. Models predict significant herd immunity when vaccination coverage rates exceed 50%. The killed vaccines have been safely administered among populations with high rates of HIV.
Oral live attenuated vaccines for V. cholerae are also in development. These strains have in common the fact that they lack the genes encoding cholera toxin. One such vaccine, CVD 103-HgR, was safe and immunogenic in phase 1 and 2 studies but afforded minimal protection in a large field trial in Indonesia. Other live attenuated vaccine candidate strains have been prepared from El Tor and O139 V. cholerae and have been tested in studies of volunteers. A possible advantage of live attenuated cholera vaccines is that they may induce protection after a single oral dose. Conjugate and subunit cholera vaccines are also being developed. Recognizing that it may be decades before safe water and adequate sanitation become a reality for those most at risk of cholera, the WHO has now recommended incorporation of cholera vaccination into comprehensive control strategies and has established an international stockpile of oral killed cholera vaccine to assist in outbreak responses. No cholera vaccine is commercially available in the United States.
OTHER VIBRIO SPECIES
![]() The genus Vibrio includes several human pathogens that do not cause cholera. Abundant in coastal waters throughout the world, noncholera vibrios can reach high concentrations in the tissues of filter-feeding mollusks. As a result, human infection commonly follows the ingestion of seawater or of raw or undercooked shellfish (Table 193-5). Most noncholera vibrios can be cultured on blood or MacConkey agar, which contains enough salt to support the growth of these halophilic species. In the microbiology laboratory, the species of noncholera vibrios are distinguished by standard biochemical tests. The most important of these organisms are Vibrio parahaemolyticus and Vibrio vulnificus.
The genus Vibrio includes several human pathogens that do not cause cholera. Abundant in coastal waters throughout the world, noncholera vibrios can reach high concentrations in the tissues of filter-feeding mollusks. As a result, human infection commonly follows the ingestion of seawater or of raw or undercooked shellfish (Table 193-5). Most noncholera vibrios can be cultured on blood or MacConkey agar, which contains enough salt to support the growth of these halophilic species. In the microbiology laboratory, the species of noncholera vibrios are distinguished by standard biochemical tests. The most important of these organisms are Vibrio parahaemolyticus and Vibrio vulnificus.
|
FEATURES OF SELECTED NONCHOLERA VIBRIOSES |
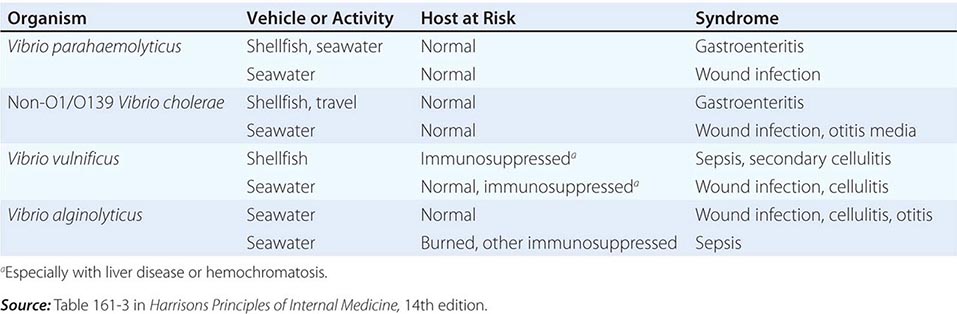
The two major types of syndromes for which these species are responsible are gastrointestinal illness (due to V. parahaemolyticus, non-O1/O139 V. cholerae, Vibrio mimicus, Vibrio fluvialis, Vibrio hollisae, and Vibrio furnissii) and soft tissue infections (due to V. vulnificus, Vibrio alginolyticus, and Vibrio damselae). V. vulnificus is also a cause of primary sepsis in some compromised individuals.
SPECIES ASSOCIATED PRIMARILY WITH GASTROINTESTINAL ILLNESS
![]() V. parahaemolyticus Widespread in marine environments, the halophilic V. parahaemolyticus causes food-borne enteritis worldwide. This species was originally implicated in enteritis in Japan in 1953, accounting for 24% of reported cases in one study—a rate that presumably was due to the common practice of eating raw seafood in that country. In the United States, common-source outbreaks of diarrhea caused by this organism have been linked to the consumption of undercooked or improperly handled seafood or of other foods contaminated by seawater. Since the mid-1990s, the incidence of V. parahaemolyticus infections has increased in several countries, including the United States. Serotypes O3:K6, O4:K68, and O1:K-untypable, which are genetically related to one another, account in part for this increase. Serotypes O4:K12 and O4:KUT were initially unique to the Pacific Northwest but caused recent outbreaks in the eastern United States and Spain. The enteropathogenicity of V. parahaemolyticus is linked to its ability to cause hemolysis on Wagatsuma agar (i.e., the Kanagawa phenomenon). Although the mechanisms by which the organism causes diarrhea are not fully defined, the genome sequence of V. parahaemolyticus contains two type III secretion systems, which directly inject toxic bacterial proteins into host cells. The activity of one of these secretion systems is required for intestinal colonization and virulence in animal models. V. parahaemolyticus should be considered a possible etiologic agent in all cases of diarrhea that can be linked epidemiologically to seafood consumption or to the sea itself.
V. parahaemolyticus Widespread in marine environments, the halophilic V. parahaemolyticus causes food-borne enteritis worldwide. This species was originally implicated in enteritis in Japan in 1953, accounting for 24% of reported cases in one study—a rate that presumably was due to the common practice of eating raw seafood in that country. In the United States, common-source outbreaks of diarrhea caused by this organism have been linked to the consumption of undercooked or improperly handled seafood or of other foods contaminated by seawater. Since the mid-1990s, the incidence of V. parahaemolyticus infections has increased in several countries, including the United States. Serotypes O3:K6, O4:K68, and O1:K-untypable, which are genetically related to one another, account in part for this increase. Serotypes O4:K12 and O4:KUT were initially unique to the Pacific Northwest but caused recent outbreaks in the eastern United States and Spain. The enteropathogenicity of V. parahaemolyticus is linked to its ability to cause hemolysis on Wagatsuma agar (i.e., the Kanagawa phenomenon). Although the mechanisms by which the organism causes diarrhea are not fully defined, the genome sequence of V. parahaemolyticus contains two type III secretion systems, which directly inject toxic bacterial proteins into host cells. The activity of one of these secretion systems is required for intestinal colonization and virulence in animal models. V. parahaemolyticus should be considered a possible etiologic agent in all cases of diarrhea that can be linked epidemiologically to seafood consumption or to the sea itself.
Infections with V. parahaemolyticus can result in two distinct gastrointestinal presentations. The more common of the two presentations (including nearly all cases in North America) is characterized by watery diarrhea, usually occurring in conjunction with abdominal cramps, nausea, and vomiting and accompanied in ~25% of cases by fever and chills. After an incubation period of 4 h to 4 days, symptoms develop and persist for a median of 3 days. Dysentery, the less common presentation, is characterized by severe abdominal cramps, nausea, vomiting, and bloody or mucoid stools. V. parahaemolyticus also causes rare cases of wound infection and otitis and very rare cases of sepsis.
Most cases of V. parahaemolyticus–associated gastrointestinal illness, regardless of the presentation, are self-limited. Fluid replacement should be stressed. The role of antimicrobials is uncertain, but they may be of benefit in moderate or severe disease. Doxycycline, fluoroquinolones, or macrolides are usually used. Deaths are extremely rare among immunocompetent individuals. Severe infections are associated with underlying diseases, including diabetes, preexisting liver disease, iron-overload states, or immunosuppression.
Non-O1/O139 (Noncholera) V. cholerae The heterogeneous non-O1/O139 V. cholerae organisms cannot be distinguished from V. cholerae O1 or O139 by routine biochemical tests but do not agglutinate in O1 or O139 antiserum. Non-O1/O139 strains have caused several well-studied food-borne outbreaks of gastroenteritis and have also been responsible for sporadic cases of otitis media, wound infection, and bacteremia; although gastroenteritis outbreaks can occur, non-O1/O139 V. cholerae strains do not cause epidemics of cholera. Like other vibrios, non-O1/O139 V. cholerae organisms are widely distributed in marine environments. In most instances, recognized cases in the United States have been associated with the consumption of raw oysters or with recent travel. The broad clinical spectrum of diarrheal illness caused by these organisms is probably due to the group’s heterogeneous virulence attributes.
In the United States, about half of all non-O1/O139 V. cholerae isolates are from stool samples. The typical incubation period for gastroenteritis due to these organisms is <2 days, and the illness lasts for ~2–7 days. Patients’ stools may be copious and watery or may be partly formed, less voluminous, and bloody or mucoid. Diarrhea can result in severe dehydration. Many cases include abdominal cramps, nausea, vomiting, and fever. Like those with cholera, patients who are seriously dehydrated should receive oral or IV fluids; the value of antibiotics is not clear.
Extraintestinal infections due to non-O1/O139 V. cholerae commonly follow occupational or recreational exposure to seawater. Around 10% of non-O1/O139 V. cholerae isolates come from cases of wound infection, 10% from cases of otitis media, and 20% from cases of bacteremia (which is particularly likely to develop in patients with liver disease). Extraintestinal infections should be treated with antibiotics. Information to guide antibiotic selection and dosing is limited, but most strains are sensitive in vitro to tetracycline, ciprofloxacin, and third-generation cephalosporins.
SPECIES ASSOCIATED PRIMARILY WITH SOFT TISSUE INFECTION OR BACTEREMIA
(See also Chap. 156)
V. vulnificus Infection with V. vulnificus is rare, but this organism is the most common cause of severe Vibrio infections in the United States. Like most vibrios, V. vulnificus proliferates in the warm summer months and requires a saline environment for growth. In the United States, infections in humans typically occur in coastal states between May and October and most commonly affect men >40 years of age. V. vulnificus has been linked to two distinct syndromes: primary sepsis, which usually occurs in patients with underlying liver disease, and primary wound infection, which generally affects people without underlying disease. (Vulnificus is Latin for “wound maker.”) Some authors have suggested that V. vulnificus also causes gastroenteritis independent of other clinical manifestations. V. vulnificus is endowed with a number of virulence attributes, including a capsule that confers resistance to phagocytosis and to the bactericidal activity of human serum as well as a cytolysin. Measured as the 50% lethal dose in mice, the organism’s virulence is considerably increased under conditions of iron overload; this observation is consistent with the propensity of V. vulnificus to infect patients who have hemochromatosis.
Primary sepsis most often develops in patients who have cirrhosis or hemochromatosis. However, V. vulnificus bacteremia can also affect individuals who have hematopoietic disorders or chronic renal insufficiency, those who are using immunosuppressive medications or alcohol, or (in rare instances) those who have no known underlying disease. After a median incubation period of 16 h, the patient develops malaise, chills, fever, and prostration. One-third of patients develop hypotension, which is often apparent at admission. Cutaneous manifestations develop in most cases (usually within 36 h of onset) and characteristically involve the extremities (the lower more often than the upper). In a common sequence, erythematous patches are followed by ecchymoses, vesicles, and bullae. In fact, sepsis and hemorrhagic bullous skin lesions suggest the diagnosis in appropriate settings. Necrosis and sloughing may also be evident. Laboratory studies reveal leukopenia more often than leukocytosis, thrombocytopenia, or elevated levels of fibrin split products. V. vulnificus can be cultured from blood or cutaneous lesions. The mortality rate approaches 50%, with most deaths due to uncontrolled sepsis (Chap. 325). Accordingly, prompt treatment is critical and should include empirical antibiotic administration, aggressive debridement, and general supportive care. V. vulnificus is sensitive in vitro to a number of antibiotics, including tetracycline, fluoroquinolones, and third-generation cephalosporins. Data from animal models suggest that either a fluoroquinolone or the combination of a tetracycline and a third-generation cephalosporin should be used in the treatment of V. vulnificus septicemia.
V. vulnificus–associated soft tissue infection can complicate either a fresh or an old wound that comes into contact with seawater; the patient may or may not have underlying disease. After a short incubation period (4 h to 4 days; mean, 12 h), the disease begins with swelling, erythema, and (in many cases) intense pain around the wound. These signs and symptoms are followed by cellulitis, which spreads rapidly and is sometimes accompanied by vesicular, bullous, or necrotic lesions. Metastatic events are uncommon. Most patients have fever and leukocytosis. V. vulnificus can be cultured from skin lesions and occasionally from the blood. Prompt antibiotic therapy and debridement are usually curative.
V. alginolyticus First identified as a pathogen of humans in 1973, V. alginolyticus occasionally causes eye, ear, and wound infections. This species is the most salt-tolerant of the vibrios and can grow in salt concentrations of >10%. Most clinical isolates come from superinfected wounds that presumably become contaminated at the beach. Although its severity varies, V. alginolyticus infection tends not to be serious and generally responds well to antibiotic therapy and drainage. A few cases of otitis externa, otitis media, and conjunctivitis due to this pathogen have been described. Tetracycline treatment usually results in cure. V. alginolyticus is a rare cause of bacteremia in immunocompromised hosts.
ACKNOWLEDGMENT
The authors gratefully acknowledge the valuable contributions of Drs. Robert Deresiewicz and Gerald T. Keusch, coauthors of this chapter for previous editions.
194e |
Brucellosis |
DEFINITION
Brucellosis is a bacterial zoonosis transmitted directly or indirectly to humans from infected animals, predominantly domesticated ruminants and swine. The disease is known colloquially as undulant fever because of its remittent character. Although brucellosis commonly presents as an acute febrile illness, its clinical manifestations vary widely, and definitive signs indicative of the diagnosis may be lacking. Thus the clinical diagnosis usually must be supported by the results of bacteriologic and/or serologic tests.
ETIOLOGIC AGENTS
Human brucellosis is caused by strains of Brucella, a bacterial genus that was previously suggested, on genetic grounds, to comprise a single species, B. melitensis, with a number of biologic variants exhibiting particular host preferences. This view was challenged on the basis of detailed differences in chromosomal structure and host preference. The traditional classification into nomen species is now favored both because of these differences and because this classification scheme closely reflects the epidemiologic patterns of the infection. The nomen system recognizes B. melitensis, which is the most common cause of symptomatic disease in humans and for which the main sources are sheep, goats, and camels; B. abortus, which is usually acquired from cattle or buffalo; B. suis, which generally is acquired from swine but has one variant enzootic in reindeer and caribou and another in rodents; and B. canis, which is acquired most often from dogs. B. ovis, which causes reproductive disease in sheep, and B. neotomae, which is specific for desert rodents, have not been clearly implicated in human disease. Two new species, B. ceti and B. pinnipedialis, have recently been identified in marine mammals, including seals and dolphins. At least one case of laboratory-acquired human disease due to one of these species has been described, and apparent cases of natural human infection have been reported. As infections in marine mammals appear to be widespread, more cases of zoonotic infection in humans may be identified. Other newly reported species include B. microti (isolated from field voles) and B. inopinata (isolated from a patient with a breast implant). Additional novel strains have been described from diverse species, including baboons, foxes, frogs, and various rodents, and the genus likely will expand further in forthcoming years. Moreover, it has become apparent that Brucella is closely related to the genus Ochrobactrum, which includes environmental bacteria sometimes associated with opportunistic infections. Genomics-based studies are beginning to elucidate the pathway of evolution from free-living soil bacteria to highly successful intracellular pathogens.
All brucellae are small, gram-negative, unencapsulated, nonsporulating, nonmotile rods or coccobacilli. They grow aerobically on peptone-based medium incubated at 37°C; the growth of some types is improved by supplementary CO2. In vivo, brucellae behave as facultative intracellular parasites. The organisms are sensitive to sunlight, ionizing radiation, and moderate heat; they are killed by boiling and pasteurization but are resistant to freezing and drying. Their resistance to drying renders brucellae stable in aerosol form, facilitating airborne transmission. The organisms can survive for up to 2 months in soft cheeses made from goat’s or sheep’s milk; for at least 6 weeks in dry soil contaminated with infected urine, vaginal discharge, or placental or fetal tissues; and for at least 6 months in damp soil or liquid manure kept under cool dark conditions. Brucellae are easily killed by a wide range of common disinfectants used under optimal conditions but are likely to be much more resistant at low temperatures or in the presence of heavy organic contamination.
EPIDEMIOLOGY
![]() Brucellosis is a zoonosis whose occurrence and control are closely related to its prevalence in domesticated animals. Its distribution is worldwide apart from the few countries where it has been eradicated from the animal reservoir. The true global prevalence of human brucellosis is unknown because of the imprecision of diagnosis and the inadequacy of reporting and surveillance systems in many countries. Recently, there has been increased recognition of the high incidence of brucellosis in India and parts of China and of importations to countries in Oceania, such as Fiji. In Europe, the incidence of brucellosis in a country is inversely related to gross domestic product, and, in both developed and less well-resourced settings, human brucellosis is related to rural poverty and inadequate access to medical care. Failure of veterinary control programs due to conflict or for economic reasons contributes further to the emergence and re-emergence of disease, as seen currently in some eastern Mediterranean countries.
Brucellosis is a zoonosis whose occurrence and control are closely related to its prevalence in domesticated animals. Its distribution is worldwide apart from the few countries where it has been eradicated from the animal reservoir. The true global prevalence of human brucellosis is unknown because of the imprecision of diagnosis and the inadequacy of reporting and surveillance systems in many countries. Recently, there has been increased recognition of the high incidence of brucellosis in India and parts of China and of importations to countries in Oceania, such as Fiji. In Europe, the incidence of brucellosis in a country is inversely related to gross domestic product, and, in both developed and less well-resourced settings, human brucellosis is related to rural poverty and inadequate access to medical care. Failure of veterinary control programs due to conflict or for economic reasons contributes further to the emergence and re-emergence of disease, as seen currently in some eastern Mediterranean countries.
Even in well-resourced settings, the true incidence of brucellosis in domesticated animals may be 10–20 times higher than the reported figures. Bovine brucellosis has been the target of control programs in many parts of the world and has been eradicated from the cattle populations of Australia, New Zealand, Bulgaria, Canada, Cyprus, Great Britain (including the Channel Islands), Japan, Luxembourg, Romania, the Scandinavian countries, Switzerland, and the Czech and Slovak Republics, among other nations. Its incidence has been reduced to a low level in the United States and most Western European countries, with a varied picture in other parts of the world. Efforts to eradicate B. melitensis infection from sheep and goat populations have been much less successful. These efforts have relied heavily on vaccination programs, which have tended to fluctuate with changing economic and political conditions. In some countries (e.g., Israel), B. melitensis has caused serious outbreaks in cattle. Infections with B. melitensis still pose a major public health problem in Mediterranean countries; in western, central, and southern Asia; and in parts of Africa and South and Central America.
Human brucellosis is usually associated with occupational or domestic exposure to infected animals or their products. Farmers, shepherds, goatherds, veterinarians, and employees in slaughterhouses and meat-processing plants in endemic areas are occupationally exposed to infection. Family members of individuals involved in animal husbandry may be at risk, although it is often difficult to differentiate food-borne infection from environmental contamination under these circumstances. Laboratory workers who handle cultures or infected samples also are at risk. Travelers and urban residents usually acquire the infection through consumption of contaminated foods. In countries that have eradicated the disease, new cases are most commonly acquired abroad. Dairy products, especially soft cheeses, unpasteurized milk, and ice cream, are the most frequently implicated sources of infection; raw meat and bone marrow may be sources under exceptional circumstances. Infections acquired through cosmetic treatments using materials of fetal origin have been reported. Person-to-person transmission is extremely rare, as is transfer of infection by blood or tissue donation. Although brucellosis is a chronic intracellular infection, there is no evidence for increased prevalence or severity among individuals with HIV infection or with immunodeficiency or immunosuppression of other etiologies.
Brucellosis may be acquired by ingestion, inhalation, or mucosal or percutaneous exposure. Accidental injection of the live vaccine strains of B. abortus (S19 and RB51) and B. melitensis (Rev 1) can cause disease. B. melitensis and B. suis have historically been developed as biological weapons by several countries and could be exploited for bioterrorism (Chap. 261e). This possibility should be borne in mind in the event of sudden unexplained outbreaks.
IMMUNITY AND PATHOGENESIS
Exposure to brucellosis elicits both humoral and cell-mediated immune responses. The mechanisms of protective immunity against human brucellosis are presumed to be similar to those documented in laboratory animals, but such generalizations must be interpreted with caution. The response to infection and its outcome are influenced by the virulence, phase, and species of the infecting strain. Differences have been reported between B. abortus and B. suis in modes of cellular entry and subsequent compartmentalization and processing. Antibodies promote clearance of extracellular brucellae by bactericidal action and by facilitation of phagocytosis by polymorphonuclear and mononuclear phagocytes; however, antibodies alone cannot eradicate infection. Organisms taken up by macrophages and other cells can establish persistent intracellular infections. The key target cell is the macrophage, and bacterial mechanisms for suppressing intracellular killing and apoptosis result in very large intracellular populations. Opsonized bacteria are actively phagocytosed by neutrophilic granulocytes and by monocytes. In these and other cells, initial attachment takes place via specific receptors, including Fc, C3, fibronectin, and mannose-binding proteins. Opsonized—but not unopsonized—bacteria trigger an oxidative burst inside phagocytes. Unopsonized bacteria are internalized via similar receptors but at much lower efficiency. Smooth strains enter host cells via lipid rafts. Smooth lipopolysaccharide (LPS), β-cyclic glucan, and possibly an invasion-attachment protein (IalB) are involved in this process. Tumor necrosis factor α (TNF-α) produced early in the course of infection stimulates cytotoxic lymphocytes and activates macrophages, which can kill intracellular brucellae (probably mainly through production of reactive oxygen and nitrogen intermediates) and may clear infection. However, virulent Brucella cells can suppress the TNF-α response, and control of infection in this situation depends on macrophage activation and interferon γ (IFN-γ) responses. Cytokines such as interleukin (IL) 12 promote production of IFN-γ, which drives TH1-type responses and stimulates macrophage activation. Inflammatory cytokines, including IL-4, IL-6, and IL-10, downregulate the protective response. As in other types of intracellular infection, it is assumed that initial replication of brucellae takes place within cells of the lymph nodes draining the point of entry. Subsequent hematogenous spread may result in chronic localizing infection at almost any site, although the reticuloendothelial system, musculoskeletal tissues, and genitourinary system are most frequently targeted. Both acute and chronic inflammatory responses develop in brucellosis, and the local tissue response may include granuloma formation with or without necrosis and caseation. Abscesses may also develop, especially in chronic localized infection.
The determinants of pathogenicity of Brucella have not been fully characterized, and the mechanisms underlying the manifestations of brucellosis are incompletely understood. The organism is a “stealth” pathogen whose survival strategy is centered on several processes that avoid triggering innate immune responses and that permit survival within monocytic cells. The smooth Brucella LPS, which has an unusual O-chain and core-lipid composition, has relatively low endotoxin activity and plays a key role in pyrogenicity and in resistance to phagocytosis and serum killing in the nonimmune host. In addition, LPS is believed to play a role in suppressing phagosome–lysosome fusion and diverting the internalized bacteria into vacuoles located in endoplasmic reticulum, where intracellular replication takes place. Specific exotoxins have not been isolated, but a type IV secretion system (VirB) that regulates intracellular survival and trafficking has been identified. In B. abortus this system can be activated extracellularly, but in B. suis it is activated (by low pH) only during intracellular growth. Brucellae then produce acid-stable proteins that facilitate the organisms’ survival in phagosomes and may enhance their resistance to reactive oxygen intermediates. A type III secretion system based on modified flagellar structures also has been inferred, although not yet confirmed. Virulent brucellae are resistant to defensins and produce a Cu-Zn superoxide dismutase that increases their resistance to reactive oxygen intermediates. A hemolysin-like protein may trigger the release of brucellae from infected cells.
CLINICAL FEATURES
Brucellosis almost invariably causes fever, which may be associated with profuse sweats, especially at night. In endemic areas, brucellosis may be difficult to distinguish from the many other causes of fever. However, two features recognized in the nineteenth century distinguish brucellosis from other tropical fevers, such as typhoid and malaria: (1) Left untreated, the fever of brucellosis shows an undulating pattern that persists for weeks before the commencement of an afebrile period that may be followed by relapse. (2) The fever of brucellosis is associated with musculoskeletal symptoms and signs in about one-half of all patients.
The clinical syndromes caused by the different nomen species are similar, although B. melitensis tends to be associated with a more acute and aggressive presentation and B. suis with focal abscess induction. B. abortus infections may be more insidious in onset and more likely to become chronic. B. canis infections are reported to present frequently with acute gastrointestinal symptoms.
The incubation period varies from 1 week to several months, and the onset of fever and other symptoms may be abrupt or insidious. In addition to experiencing fever and sweats, patients become increasingly apathetic and fatigued; lose appetite and weight; and have nonspecific myalgia, headache, and chills. Overall, the presentation of brucellosis often fits one of three patterns: febrile illness that resembles typhoid but is less severe; fever and acute monoarthritis, typically of the hip or knee, in a young child; and long-lasting fever, misery, and low-back or hip pain in an older man. In an endemic area (e.g., much of the Middle East), a patient with fever and difficulty walking into the clinic would be regarded as having brucellosis until it was proven otherwise.
Diagnostic clues in the patient’s history include travel to an endemic area, employment in a diagnostic microbiology laboratory, consumption of unpasteurized milk products (including soft cheeses), contact with animals, accidental inoculation with veterinary Brucella vaccines, and—in an endemic setting—a history of similar illness in the family (documented in almost 50% of cases). Focal features are present in the majority of patients. The most common are musculoskeletal pain and physical findings in the peripheral and axial skeleton (~40% of cases). Osteomyelitis more commonly involves the lumbar and low thoracic vertebrae than the cervical and high thoracic spine. Individual joints that are most commonly affected by septic arthritis are the knee, hip, sacroiliac, shoulder, and sternoclavicular joints; the pattern may be one of monoarthritis or polyarthritis. Osteomyelitis may also accompany septic arthritis.
In addition to the usual causes of vertebral osteomyelitis or septic arthritis, the most important disease in the differential diagnosis is tuberculosis. This point influences the therapeutic approach as well as the prognosis, given that several antimicrobial agents used to treat brucellosis are also used to treat tuberculosis. Septic arthritis in brucellosis progresses slowly, starting with small pericapsular erosions. In the vertebrae, anterior erosions of the superior end plate are typically the first features to become evident, with eventual involvement and sclerosis of the whole vertebra. Anterior osteophytes eventually develop, but vertebral destruction or impingement on the spinal cord is rare and usually suggests tuberculosis (Table 194e-1).
|
RADIOLOGY OF THE SPINE: DIFFERENTIAT ION OF BRUCELLOSIS FROM TUBERCULOSIS |
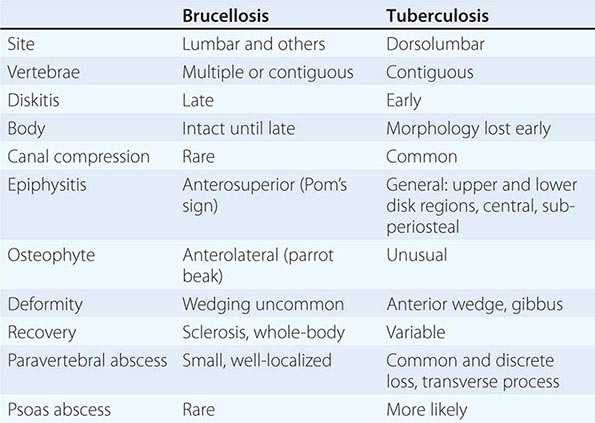
Other systems may be involved in a manner that resembles typhoid. About one-quarter of patients have a dry cough, usually with few changes visible on the chest x-ray, although pneumonia, empyema, intrathoracic adenopathy, or lung abscess can occur. Sputum or pleural effusion cultures are rarely positive in such cases, which respond well to standard brucellosis treatment. One-quarter of patients have hepatosplenomegaly, and 10–20% have significant lymphadenopathy; the differential diagnosis includes glandular fever–like illness such as that caused by Epstein-Barr virus, Toxoplasma, cytomegalovirus, HIV, or Mycobacterium tuberculosis. Up to 10% of men have acute epididymoorchitis, which must be distinguished from mumps and from surgical problems such as torsion. Prostatitis, inflammation of the seminal vesicles, salpingitis, and pyelonephritis all occur. There is an increased incidence of fetal loss among infected pregnant women, although teratogenicity has not been described and the tendency toward abortions is much less pronounced in humans than in farm animals.
Neurologic involvement is common, with depression and lethargy whose severity may not be fully appreciated by either the patient or the physician until after treatment. A small proportion of patients develop lymphocytic meningoencephalitis that mimics neurotuberculosis, atypical leptospirosis, or noninfectious conditions and that may be complicated by intracerebral abscess, a variety of cranial nerve deficits, or ruptured mycotic aneurysms.
Endocarditis occurs in ~1% of cases, most often affecting the aortic valve (natural or prosthetic). Any site in the body may be involved in metastatic abscess formation or inflammation; the female breast and the thyroid gland are affected particularly often. Nonspecific maculopapular rashes and other skin manifestations are uncommon and are rarely noticed by the patient even if they develop.
DIAGNOSIS
Because the clinical picture of brucellosis is not distinctive, the diagnosis must be based on a history of potential exposure, a presentation consistent with the disease, and supporting laboratory findings. Results of routine biochemical assays are usually within normal limits, although serum levels of hepatic enzymes and bilirubin may be elevated. Peripheral leukocyte counts are usually normal or low, with relative lymphocytosis. Mild anemia may be documented. Thrombocytopenia and disseminated intravascular coagulation with raised levels of fibrinogen degradation products can develop. The erythrocyte sedimentation rate and C-reactive protein levels are often normal but may be raised.
In body fluids such as cerebrospinal fluid (CSF) or joint fluid, lymphocytosis and low glucose levels are the norm. Elevated CSF levels of adenosine deaminase cannot be used to distinguish tubercular meningitis, as they may also be found in brucellosis. Biopsied samples of tissues such as lymph node or liver may show noncaseating granulomas (Fig. 194e-1) without acid/alcohol-fast bacilli. The radiologic features of bony disease develop late and are much more subtle than those of tuberculosis or septic arthritis of other etiologies, with less bone and joint destruction. Isotope scanning is more sensitive than plain x-ray and continues to give positive results long after successful treatment.
FIGURE 194e-1 Liver biopsy specimen from a patient with brucellosis shows a noncaseating granuloma. (From Mandell’s Atlas of Infectious Diseases, Vol II, in DL Stevens [ed]: Skin, Soft Tissue, Bone and Joint Infections, Fig. 5-9; with permission.)
Isolation of brucellae from blood, CSF, bone marrow, or joint fluid or from a tissue aspirate or biopsy sample is definitive, and attempts at isolation are usually successful in 50–70% of cases. Duplicate cultures should be incubated for up to 6 weeks (in air and 10% CO2, respectively). Concentration and lysis of buffy coat cells before culture may increase the isolation rate. Cultures in modern nonradiometric or similar signaling systems (e.g., Bactec) usually become positive within 7–10 days but should be maintained for at least 3 weeks before the results are declared negative. All cultures should be handled under containment conditions appropriate for dangerous pathogens. Brucella species may be misidentified as Agrobacterium, Ochrobactrum, or Psychrobacter (Moraxella) phenylpyruvicus by the gallery identification strips commonly used in the diagnostic laboratory. In recent years, matrix-assisted laser desorption ionization time-of-flight spectrometry (MALDI-TOF MS) has emerged as a powerful tool in bacterial identification. The relative homogeneity of classical Brucella species makes identification beyond the genus level by routine approaches challenging, although further improvements may facilitate discrimination at the species level. The place of this technique in routine diagnostic practice will depend on such refinements. Meanwhile, the authors are aware of cases in which blood culture isolates have been identified incorrectly using MALDI-TOF MS.
The peripheral blood–based polymerase chain reaction has enormous potential to detect bacteremia, to predict relapse, and to exclude “chronic brucellosis.” This method is probably more sensitive and is certainly quicker than blood culture, and it does not carry the attendant biohazard risk posed by culture. Nucleic acid amplification techniques are now quite widely used, although no single standardized procedure has been adopted. Primers for the spacer region between the genes encoding the 16S and 23S ribosomal RNAs (rrs-rrl), various outer-membrane protein–encoding genes, the insertion sequence IS711, and the protein BCSP31 are sensitive and specific. Blood and other tissues are the most suitable samples for analysis.
Serologic examination often provides the only positive laboratory findings in brucellosis. In acute infection, IgM antibodies appear early and are followed by IgG and IgA. All these antibodies are active in agglutination tests, whether performed by tube, plate, or microagglutination methods. The majority of patients have detectable agglutinins at this stage. As the disease progresses, IgM levels decline, and the avidity and subclass distribution of IgG and IgA change. The result is reduced or undetectable agglutinin titers. However, the antibodies are detectable by alternative tests, including the complement fixation test, Coomb’s antiglobulin test, and enzyme-linked immunosorbent assay. There is no clear cutoff value for a diagnostic titer. Rather, serology results must be interpreted in the context of exposure history and clinical presentation. In endemic areas or in settings of potential occupational exposure, agglutinin titers of 1:320–1:640 or higher are considered diagnostic; in nonendemic areas, a titer of ≥1:160 is considered significant. Repetition of tests after 2–4 weeks may demonstrate a rising titer.
In most centers, the standard agglutination test is still the mainstay of serologic diagnosis, although some investigators rely on the rose bengal test, which has not been fully validated for human diagnostic use. Dipstick assays for anti-Brucella IgM are useful for the diagnosis of acute infection but are less sensitive for infection with symptoms of several months’ duration. In an endemic setting, more than 90% of patients with acute bacteremia have standard agglutination titers of at least 1:320. Other screening tests are used in some centers.
Antibody to the Brucella LPS O chain—the dominant antigen—is detected by all the conventional tests that employ smooth B. abortus cells as antigen. Since B. abortus cross-reacts with B. melitensis and B. suis, there is no advantage in replicating the tests with these antigens. Cross-reactions also occur with the O chains of some other gram-negative bacteria, including Yersinia enterocolitica O:9, Escherichia coli O157, Francisella tularensis, Salmonella enterica group N, Stenotrophomonas maltophilia, and Vibrio cholerae. Cross-reactions do not occur with the cell-surface antigens of rough Brucella strains such as B. canis or B. ovis; serologic tests for these nomen species must employ an antigen prepared from either one. The live B. abortus vaccine strain RB51 does not elicit antibody responses in serologic tests that use smooth antigens, and this fact must be taken into account if serologic tests are employed in attempts to identify or follow the course of infections in persons accidentally exposed to the vaccine.
|
TREATMENT |
BRUCELLOSIS |
The broad aims of antimicrobial therapy are to treat and relieve the symptoms of current infection and to prevent relapse. Focal disease presentations may require specific intervention in addition to more prolonged and tailored antibiotic therapy. In addition, tuberculosis must always be excluded, or—to prevent the emergence of resistance—therapy must be tailored to specifically exclude drugs active against tuberculosis (e.g., rifampin used alone) or to include a full antituberculous regimen.
Early experience with streptomycin monotherapy showed that relapse was common; thus dual therapy with tetracyclines became the norm. This is still the most effective combination, but alternatives may be used, with the options depending on local or national policy about the use of rifampin for the treatment of nonmycobacterial infection. For the several antimicrobial agents that are active in vivo, efficacy can usually be predicted by in vitro testing. However, numerous Brucella strains show in vitro sensitivity to a whole range of antimicrobials that are therapeutically ineffective, including assorted β-lactams. Moreover, the use of fluoroquinolones remains controversial despite the good in vitro activity and white-cell penetration of most agents of this class. Low intravacuolar pH is probably a factor in the poor performance of these drugs.
For adults with acute nonfocal brucellosis (duration, <1 month), a 6-week course of therapy incorporating at least two antimicrobial agents is required. Complex or focal disease necessitates ≥3 months of therapy. Adherence to the therapeutic regimen is very important, and poor adherence underlies almost all cases of apparent treatment failure; such failure is rarely due to the emergence of drug resistance, although increasing resistance to trimethoprim-sulfamethoxazole (TMP-SMX) has been reported at one center. There is good retrospective evidence that a 3-week course of two agents is as effective as a 6-week course for treatment and prevention of relapse in children, but this point has not yet been proven in prospective studies.
The gold standard for the treatment of brucellosis in adults is IM streptomycin (0.75–1 g daily for 14–21 days) together with doxycycline (100 mg twice daily for 6 weeks). In both clinical trials and observational studies, relapse follows such treatment in 5–10% of cases. The usual alternative regimen (and the current World Health Organization recommendation) is rifampin (600–900 mg/d) plus doxycycline (100 mg twice daily) for 6 weeks. The relapse/failure rate is ~10% in trial conditions but rises to >20% in many non-trial situations, possibly because doxycycline levels are reduced and clearance rates increased by concomitant rifampin administration. Patients who cannot tolerate or receive tetracyclines (children, pregnant women) can be given high-dose TMP-SMX instead (two or three standard-strength tablets twice daily for adults, depending on weight).
Increasing evidence supports the use of an aminoglycoside such as gentamicin (5–6 mg/kg per day for at least 2 weeks) instead of streptomycin, although this regimen is not approved by the U.S. Food and Drug Administration. Shorter courses have been associated with high failure rates in adults. A 5- to 7-day course of therapy with gentamicin and a 3-week course of TMP-SMX may be adequate for children with uncomplicated disease, but prospective trials are still needed to support this recommendation. Early experience with fluoroquinolone monotherapy was disappointing, although it was suggested that ofloxacin or ciprofloxacin, given together with rifampin for 6 weeks, might be an acceptable alternative to the other 6-week regimens for adults. A substantial meta-analysis did not support the use of fluoroquinolones in first-line treatment regimens, and these drugs are not recommended by an expert consensus group (Ioannina) except in the context of well-designed clinical trials. However, a more recent meta-analysis is more supportive of the efficacy of these drugs, and an adequately powered prospective study will be needed to resolve their role in standard combination therapy. A triple-drug regimen—doxycycline and rifampin combined with an initial course of an aminoglycoside—was superior to double-drug regimens in a meta-analysis. The triple-drug regimen should be considered for all patients with complicated disease and for those for whom treatment adherence is likely to be a problem.
Significant neurologic disease due to Brucella species requires prolonged treatment (i.e., for 3–6 months), usually with ceftriaxone supplementation of a standard regimen. Brucella endocarditis is treated with at least three drugs (an aminoglycoside, a tetracycline, and rifampin), and many experts add ceftriaxone and/or a fluoroquinolone to reduce the need for valve replacement. Treatment is usually given for at least 6 months, and clinical endpoints for its discontinuation are often difficult to define. Surgery is still required for the majority of cases of infection of prosthetic heart valves and prosthetic joints.
There is no evidence base to guide prophylaxis after exposure to Brucella organisms (e.g., in the laboratory), inadvertent immunization with live vaccine intended for use in animals, or exposure to deliberately released brucellae. Most authorities have recommended the administration of rifampin plus doxycycline for 3 weeks after a low-risk exposure (e.g., an unspecified laboratory accident) and for 6 weeks after a major exposure to aerosol or injected material. However, such regimens are poorly tolerated, and doxycycline monotherapy of the same duration may be substituted. (Monotherapy is now the standard recommendation in the United Kingdom but not in the United States.) Rifampin should be omitted after exposure to vaccine strain RB51, which is resistant to rifampin but sensitive to doxycycline. After significant brucellosis exposure, expert consultation is advised for women who are (or may be) pregnant.
PROGNOSIS AND FOLLOW-UP
Relapse occurs in up to 30% of poorly compliant patients. Thus patients should ideally be followed clinically for up to 2 years to detect relapse, which responds to a prolonged course of the same therapy used originally. The general well-being and the body weight of the patient are more useful guides than serology to lack of relapse. IgG antibody levels detected by the standard agglutination test and its variants can remain in the diagnostic range for >2 years after successful treatment. Complement fixation titers usually fall to normal within 1 year of cure. Immunity is not solid; patients can be reinfected after repeated exposures. Fewer than 1% of patients die of brucellosis. When the outcome is fatal, death is usually a consequence of cardiac involvement; more rarely, it results from severe neurologic disease. Despite the low mortality rate, recovery from brucellosis is slow, and the illness can cause prolonged inactivity, with domestic and economic consequences.