Chapter 20 Infections and immunity
With contribution from Dr Lily Tomas
Introduction
The immune system is a complex of tissues, cells and molecules with specialised roles in defence against infection. There are 2 fundamentally different types of responses to invading microbes — innate (natural) immunity responses that occur to the same extent however many times the infectious agent is encountered, whereas acquired (adaptive) immune responses improve on repeated exposure to a given infection or antigen.
Of significant importance is that humans represent a scaffold on which diverse microbial ecosystems are established. Immediately after birth, all mammals are initiated into a lifelong process of colonisation by foreign microorganisms that inhabit all mucosal surfaces as well as the skin. Fashioned by millennia of evolutionary inputs, some host–bacterial associations have developed into beneficial relationships, creating an environment for mutual benefit and endowing the human immune system with advantageous signalling capacities that can control pathogenic insults throughout a lifetime of interactions. This then raises the possibility that, rather than the mammalian immune system being designed to control microorganisms, it is in fact controlled by microorganisms.1
Lifestyle factors
For example, recurring infections such as recurrent ear, urinary and sinus infections are a common presentation to medical practitioners. These can be quite distressing for families to deal with, not only in its treatment but also its impact physically, psychologically and socially, such as in time away from work and school, and dependency on others such as grandparents to care for sick children.
Table 20.1 summarises the lifestyle factors that may impact adversely on the immune system. These factors will be discussed throughout the chapter. Prevention is the best method to help maintain a healthy immune system.
| Stress — chronic |
Mind–body medicine
Humans as well as all other organisms have a requisite to maintain a complex dynamic equilibrium (homeostasis), which is constantly challenged by internal or external adverse stressor events. The brain’s stressor-handling system that constitutes the limbic-hypothalamic-pituitary-adrenal axis is one of the most thoroughly studied circuitry systems of the central nervous system. As a result of stressor–axis activation, different behavioural and physical changes can develop which allow the organism to adapt. These are the domains of psychoneuroimmunology (PNI) and psychoneuroendocrinology (PNE).2, 3
Psychoneuroimmunology (PNI)
The emerging specialty fields of PNI and PNE are justification enough that stress and/or adverse stressors have a profound impact upon every system within the body.2, 3, 4
There is a wealth of evidence demonstrating that psychological stress can adversely affect the development and progression of almost every known disease. Both acute and chronic stressful states produce documentable changes in the innate and adaptive immune responses, which are predominantly mediated via neuroendocrine mediators from the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal axis.5–13
Indeed, this is an elaborate multi-directional communication system which continually and simultaneously relays multiple messages between the immune, gastrointestinal, neurological, endocrinological, dermatological and cardiovascular systems in an ongoing attempt to restore and maintain homeostasis.14–24
Neurotransmitters, hormones and neuropeptides all regulate the cells of the immune system, subsequently communicating with all other systems through the secretion of a wide variety of different cytokines.14 It is beyond the scope of this chapter to discuss such complex and intricate interactions, however, there is sufficient literature available today specifically dealing with PNI.
Acute stress has been shown to have a stimulating effect on the immune system whereas chronic stress down-regulates the immune system.12, 16 Chronic stress has been associated with increased susceptibility of the patient to infectious diseases and cancer.6, 7, 9 It is also linked with worse outcomes in many immune-related disorders, including cancer, inflammatory and infectious diseases, indicating that the effects of mental states on our immune system are directly and clinically relevant to disease expression.10, 16, 18
Psychological interventions
There is certainly considerable variability in each individual’s immune response to stress. Encouraging particular activities that increase that person’s ability to cope with stress may therefore have a significantly beneficial effect on immune function with subsequent modification in the development and progression of many different diseases.12, 14, 21, 23
It is also important to note that stress during fetal and neonatal development can alter the programming of the neuro-endocrine-immune axis, influencing stress, immune-responsiveness and even disease resistance in later life.16 Therefore identification and treatment of suboptimal moods in pregnant women is imperative.
Various behavioural strategies, psychological and psychopharmacotherapeutic interventions that enhance effective coping and reduce affective distress show beneficial effects in many disease, including cancer.15, 25
A recent Australian survey of women with breast cancer indicates that 87.5% of surveyed women had used complementary therapies in order to improve their physical health (86.3%), emotional wellbeing (83.2%) and to boost their immune system (68.8%). Support groups and meditation were commonly used therapies.26
Mindfulness-based stress reduction (MBSR)
There have been many recent studies, including a systematic review, which demonstrate the efficacious potential of MBSR in the management of cancer, particularly breast and prostate, and Human Immunodeficiency Virus (HIV).27–35
Those with breast and prostate cancer not only showed improvements in mood but also improved cytokine parameters with a reduction in levels of pro-inflammatory cytokines.28, 29, 31 In comparison with controls, MBSR practised by those with HIV showed an increase in natural killer (NK) cells and stable CD4+ lymphocyte counts.33, 34
Hypnosis, relaxation and guided imagery
These therapies have been shown to be effective in improving immunity in cases of breast cancer, viral illnesses including chronic herpes simplex and the common cold, and in 1 case of the auto-immune condition dermatomyositis when combined with meditation.36, 37, 38
Six weeks of training was found to almost halve the recurrence of herpes simplex outbreaks as well as reduce levels of anxiety and depression. Immune functions were up-regulated, notably functional NK-cell activity to HSV-1.39
In those with breast cancer, significant effects have been found with respect to NK-cell activity, mixed lymphocyte responsiveness and the number of peripheral blood lymphocytes when compared with controls.40, 41, 42 Thus, there appears to be a role for hypnotic guided imagery as an adjuvant therapy to breast cancer.
Cognitive behavioural therapy (CBT)
There have been several studies demonstrating the effectiveness of CBT with regard to immune parameters in HIV positive men. Significantly greater numbers of T-cytotoxic/suppressor lymphocytes, reduced urinary cortisol output and significantly reduced HSV-2 IgG titres in HIV-positive men with concomitant herpes simplex virus have all been documented.43, 44, 45
Autogenic training and group psychotherapy for women with breast cancer have also resulted in improved immune parameters.46, 47
A Cochrane systematic review of 15 studies, inclusive of 1043 chronic fatigue syndrome (CFS) sufferers found CBT is effective in reducing the symptoms of fatigue at post-treatment compared with usual care, and may be more effective in reducing fatigue symptoms compared with other psychological therapies.48
Environment
Developmental immuno-toxicology (DIT) and/or Early Life Immune Insult (ELII)
Many chronic diseases of increasing incidence are now recognised to have immune dysregulation as an important underlying component of the disease process.49 These include many childhood illnesses such as asthma, allergic disease, leukaemia, auto-immunity and certain infections.50
The developing immune system is extremely sensitive to environmental toxins, such as infectious agents, allergens, maternal smoking, maternally administered drugs, exposure to xenobiotics, diesel exhaust and traffic-related particles, antibiotics, environmental oestrogens, heavy metals, chemicals and other prenatal/neonatal stressors.51–58 Dysfunctional immune responses to infections in childhood have been postulated to play a role in childhood leukaemia.56, 57 Furthermore, many prenatal and postnatal neurological lesions are now also being recognised as being linked to prenatal immune insult and inflammatory dysregulation.57 Evidence for an association between environmentally associated childhood immune dysfunction and autistic spectrum disorders also suggests that ELII and DIT may contribute to these conditions.53, 54, 56, 57
Indeed, ELII have been proposed to be pivotal in producing chronic symptoms in later life. In particular, the period from mid-gestation until 2 years seems to be one of particular concern, with this critical maturational window displaying a heightened sensitivity to chemical disruption with the outcome of persistent immune dysfunction and/or misregulation.57 It is also important to note that the same toxin may result in different immune maturational processes according to the dose and timing of the insult.57 The important emerging field of epigenetics (combined environmental and genetic history) is also relevant in this situation.
T Helper lymphocytes
Available data indicates that ELII results in a shift from T-helper (Th) lymphocytes 1 towards Th2 predominance, alterations in regulatory T-cell function and problematic regulation of inflammatory cell function leading to hyper-inflammatory responses and perturbation of cytokine networks. The resulting health risks may extend far beyond infectious diseases, cancer, allergy and auto-immunity to pathologies in the neurological, cardiovascular, endocrinological, respiratory and reproductive systems.53–55, 57
Environmental syndromes
There is also strong evidence indicating that complex syndromes such as Multiple Chemical Sensitivity, Gulf War Syndrome, Sick Building Syndrome and Chronic Fatigue Syndrome, that often have no clear underlying medical explanation, may have an environmental component to their aetiology.59
Electromagnetic radiation
There is currently much debate regarding the potential health risks from extremely low frequency electromagnetic fields (ELF) and radiofrequency/microwave radiation emissions from wireless communications (RF). In addition to immune system dysregulation, other risks may include childhood leukaemia, brain tumours, genotoxic effects, neurodegenerative diseases, allergic and inflammatory responses, breast cancer, miscarriage and some cardiovascular effects.60, 61, 62
Specific reports on immunological dysfunction are scarce, however, 1 earlier study demonstrated that people who worked in close proximity to transformers and high tension cables full-time for 1–5 years experienced a significant decrease in total lymphocytes and CD2, CD3 and CD4 lymphocytes as well as an increase in NK-cells. Leukopaenia and neutropaenia were observed in 2 people who were permanently exposed to 1.2–6.6microT.63
The recent Bioinitiative Report has concluded that a ‘reasonable suspicion of risk exists based on clear evidence of bioeffects at environmentally relevant levels which, with prolonged exposures, may reasonably be presumed to result in health impacts’.64
Multiple chemical sensitivity (MCS)
Another topic of debate, MCS is characterised by various signs including neurological disorders, allergy and immune dysregulation. MCS is now becoming well recognised as a disease state affecting a number of people worldwide (see: www.nicnas.gov.au/currentissues/mcs.asp). Exposure may occur through a major event, such as a chemical spill, or from chronic exposure to chemicals at low levels. Animal studies have demonstrated immune changes and allergic reactions to different chemicals including the well-known Th2 type sensitisers TMA (trimetallic anhydride) and TDI (toluene diisocyanate) and the Th1 sensitiser DNCB (2, 4-dinitrochlorobenzene).65, 66, 67
Sick Building Syndrome
This is another poorly understood syndrome whereby immunological dysfunction, neurotoxicity and allergies may arise from exposure to bioaerosols, especially moulds, in the indoor environment of water-damaged buildings.68 Epidemiological and toxicological studies have demonstrated an increase in auto-antibodies (IgA, IgM, IgG) to neural-specific antigens with resulting neuro-physiological abnormalities, including peripheral neuropathy, in exposed individuals.69 Mould exposure has also been shown to initiate inflammatory and allergic (IgE) processes with significant alterations in B- and T-lymphocyte counts as well as NK-cells.51, 52, 70, 71
Infections and vitamin D
Tuberculosis still kills more people than any other pathogen-associated disease, with approximately one-third of the world’s population being infected. Among these, however, only 10% will actually develop the disease. Recently identified genetic polymorphisms in the vitamin D receptor and the vitamin D-binding protein are believed to generate either susceptibility or resistance to M. Tuberculosis infection.70
Current investigations are also focused on the role of vitamin D for the prevention of upper respiratory tract infections. In 1 study, 162 adults were randomly given 2000 IU D3 daily for 3 months. No benefits were seen in decreasing the incidence or severity of upper respiratory tract infections during winter.71 Results of trials with higher doses of vitamin D3 are currently underway.
Maternal vitamin D supplementation is also extremely important as low prenatal vitamin D levels may also increase susceptibility to the same diseases later in life.72
Auto-immune diseases and vitamin D
There are a multitude of studies associating vitamin D deficiency with the development and progression of auto-immune diseases such as multiple sclerosis (MS), rheumatoid arthritis, insulin dependent diabetes mellitus (IDDM) and inflammatory bowel disease (IBD). The immune-regulatory role of vitamin D affects both the innate and the adaptive immune systems.73
The discoveries that activated macrophages produce active vitamin D and immune system cells express the vitamin D receptor, both initially suggested how the vitamin D endocrine system influenced immune system function.74 Auto-immune diseases occur because of an inappropriate immune-mediated attack against self-tissue. Without vitamin D, auto-reactive T-cells develop whereas in the presence of vitamin D, the enhanced activity of immune cells is suppressed, balance in the T-cell response is restored and the process of autoimmunity is subsequently avoided.75, 76
Experimental animal studies have demonstrated that vitamin D deficiency accelerates the development and progression of both auto-immune diseases and cancers.77
Recent evidence also strongly suggests that supplementation with vitamin D may be beneficial, especially for Th1-mediated auto-immune disorders. By decreasing the Th1-immune driven response, the severity of symptoms is decreased. Some reports indicate that vitamin D may even be preventative in such disorders as MS and type 1 diabetes mellitus (T1DM).49, 78–82
Sleep
Good sleep is essential for physical and mental health.83 There is strong evidence demonstrating that inadequate sleep is associated with a multitude of health problems, including cognitive impairment, mood disorders, parasitical infections, cardiovascular diseases and compromised immunity.84–87 Unfortunately, frequently disrupted and restricted sleep is a common problem in today’s society with more than 50% adults over 65 years reporting at least 1 chronic complaint.83, 85 Many younger adults also suffer chronic sleep deprivation secondary to occupational hazards such as shift work or mental disorders such as anxiety.88
Both animal and human studies have revealed that sleep restriction/deprivation can result in mild temporary increases in the activity of the major neuroendocrine stress systems — the autonomic sympatho-adrenal system and the HPA axis. Chronic sleep deprivation may also affect the reactivity of these systems to future stresses and challenges, such as physical and mental illness.85, 89 Chronic sleep deprivation tends to cause a gradual and persistent desensitisation of the 5-HT 1A receptor system, thus altering serotonergic neurotransmission.90 As expected, these changes in neurotransmitter receptor systems and neuroendocrine reactivity are extremely similar to those seen with chronic stress/depression.86
Poor sleep quality has recently been confirmed to increase susceptibility to the common cold.91 Atypical time schedules such as shift-work has also been associated with breast cancer, due to a circadian disruption and to a nocturnal suppression in melatonin production.88
Melatonin is our natural sleep hormone and it is known to decrease with increasing age. Recent studies have shown that melatonin, itself, has an immune-modulating effect, stimulating the production of NK-cells and CD4+ cells and inhibiting CD8+ cells. It also stimulates the production of granulocytes and macrophages, as well as the release of various cytokines from NK-cells and T-helper lymphocytes. Thus, enhancement of the production of melatonin, or melatonin itself, has the potential therapeutic value to enhance immune function.92
The recognition and treatment of sleep dysfunctions can therefore be an important part of management of many health-related conditions.88
Physical activity
Exercise
There is a wealth of evidence supporting the beneficial effects of exercise upon the immune system. In particular, exercise has beneficial effects on many chronic diseases. It is known to have an anti-inflammatory effect, reducing body fat percentage and macrophage accumulation in adipose tissue as well as muscle-released IL-6 inhibition of TNF-alpha and the cholinergic anti-inflammatory pathway.93, 94 In particular, exercise training improves macrophage innate immune function in both a beta(2) adrenergic receptor dependent and independent manner.95
NK-cells have been found to be the most responsive immune cell to acute exercise. Their sensitivity to physiological stress combined with their important role in innate immune defences indicate that these cells are 1 link between regular physical activity and general health status.96
Anaerobic exercise in animal studies has been shown to increase both innate and adaptive immune function, decreasing tumour growth and cancer cachexia.97 Secretory IgA, which is the predominant immunoglobulin in mucosal secretions providing first-line of defence against pathogens and antigens presented at the mucosa, have also been shown to be increased after exercise in elderly people over 75 years.98, 99
It is important to note, however, that exercise needs to be performed in moderation. Multiple effects of over-training resulting in impaired immune response have been documented in the literature.100–104 Unlike moderate exercise, intense habitual exercise can cause chronic suppression of mucosal immune parameters, especially salivary IgA and IgM.105, 106 This can result in increased susceptibility to respiratory infections.95, 105
Vigorous exercise or activity may exacerbate chronic conditions such as chronic fatigue syndrome by promoting immune dysfunction, which in turn increases symptoms. It is vital that patients with chronic fatigue syndrome are provided with a well designed individualised graded exercise program to cater for individual’s physical capabilities and should take into account the fluctuating nature of symptoms. Patients should be encouraged to pace their activities and respect their physical and mental limitations with the ultimate aim of improving their everyday functioning.106
A Cochrane systematic review of the literature identified 9 studies but only 5 randomised control studies were included in the review.107
An interesting recent study has shown that heavy exercise in early post-partum months may be associated with elevated pro-inflammatory cytokines in breast milk. More studies are required to confirm these findings.103
Yoga
There have been limited studies on the efficacy of yoga practice on the immune system. Most have been focused on the breathing disciplines within yoga, namely Pranayama and Sudarshan Kriya, that are both rhythmic breathing processes traditionally used to reduce stress and improve the immune system.108
Studies on healthy individuals practicing the above techniques have shown a better anti-oxidant status (increased glutathione peroxidise, SOD; reduced lactic acid) at the enzyme and RNA level accompanied by improved immune status secondary to the prolonged lifespan of lymphocytes by up-regulation of anti-apoptotic genes and pro-survival genes in the subjects.109, 110
Cancer patients who were either undergoing or had completed their conventional therapy also showed a significant increase in NK-cells at 12 and 24 weeks after practicing the above yogic breathing techniques compared with controls.111, 112
Qigong
Qigong is an ancient Chinese psychosomatic exercise that integrates movement, meditation and breathing into a single exercise. All studies have been on healthy people and most demonstrate that after 1 month, there are significant changes in immune parameters.113 Neutrophil phagocytosis and lifespan was significantly increased whilst the inflammatory neutrophils displayed accelerated apoptosis. The changes in gene expression compared with controls were characterised by enhanced immunity, down-regulation of cellular metabolism and alteration of apoptotic genes in favour of rapid inflammation resolution.114 There is also evidence in some, but not all, studies of reduced cortisol and changes in the number of cytokine-secreting cells (increased IFN-gamma and reduced IL-10).115, 116
It is possible that qigong may regulate immunity, metabolic rate and apoptosis, perhaps at the transcriptional level.114 Further studies are required to validate these findings.
Obesity
The prevalence of obesity has reached epidemic levels in many parts of the world and therefore represents a major public health problem.117 The accumulation of visceral fat has well-established links with chronic low-grade systemic inflammation (metaflammation), cellular metabolic imbalances and impaired immunity.118–122
Through different biochemical cascades leading to immune cell senescence, obesity can promote a multitude of chronic diseases.123–125 Amongst many others, these include metabolic syndrome, inflammatory diseases, bronchial asthma, Type II diabetes and insulin resistance, depression, cardiovascular disease (CVD), osteoarthritis, fatty liver disease and cancer.2, 9, 126, 127 Leptin is increased in states of obesity and can influence mediators of innate immunity, such as IL-6. Leptin-resistance can therefore injure numerous tissues, including the liver, pancreas, platelets, vasculature and myocardium.
White adipose tissue actively participates in many physiological and pathological processes, including immunity and inflammation, playing a major role in the development of leptin, adrenaline and insulin resistance.There are, indeed, complex links between the metabolic and immune systems, with multiple neuroendocrine peptides, cytokines and chemokines interacting in order to integrate energy balance with immune function. Such interactions are heightened in obesity and lessened with caloric restriction (CR).126
Ghrelin and leptin are 2 important hormones and cytokines that both regulate energy balance and influence immune function.13 Ghrelin regulates immune function by reducing pro-inflammatory cytokines and promoting thymopoiesis during ageing and is found to be reduced in states of obesity. Thus, this provides 1 mechanism by which obesity can be associated with a state of immunodeficiency and chronic inflammation which can contribute to an increased risk of premature death.
In stark contrast, CR, which increases ghrelin and reduces leptin, can reduce oxidative stress and is a potentially immune-enhancing state which has prolonged a healthy lifespan in all species studied to date.10, 16, 127, 128
Nutrition
Dietary modulation
Pro-inflammatory foods
There is strong evidence regarding the pro-inflammatory effects of fast–foods that contain high amounts of saturated fatty and trans-fatty acids, refined carbohydrates with a high glycaemic index.122, 123, 129–142
The presence of these substances, in particular trans-fatty acids, fructose, glucose (sugars) in the diet negatively impacts on immunity and induces inflammation, creating a pro-inflammatory state, which contributes to many diseases.143–158
Protective foods
Likewise, there is a wealth of evidence concerning the anti-inflammatory and immune-enhancing properties of foods such as fish, fruits, vegetables, nuts, seeds, cocoa / dark chocolate, low GI foods, white button, maitake, oyster and shiitake mushrooms, high fibre intake, dairy calcium, green tea, and lean game meats.158–177
Spices, herbs, garlic and ginger
Spices, garlic, ginger and herbs have traditionally been highly regarded in cooking for many centuries by many cultures who believe they play an important role in health enhancement and protecting the immune system.178–183
For instance, curcumin and capsaicin are known to protect the immune system and re-regulate the systemic inflammatory responses.179, 184, 187
Grape polyphenols
Polyphenols have diverse biological effects.188 Polyphenolic compounds found in red wine are a complex mixture of flavonoids (predominantly anthocyanins and flavan-3-ols) and non-flavonoids such as resveratrol and gallic acid. Flavan-3-ols are the most abundant, with oligomeric and polymeric procyanidins often representing 25–50% of the total phenolic constituents.189 Inflammation is the process by which the immune system deals with infections or injury due to pathogenic bacteria, viruses and other pathogens. Recently it has been reported that the daily moderate consumption of alcohol and of red wine for 2 weeks at doses which inversely correlate with CVD risk had no adverse effects on human immune cell functions.190 Polyphenol-rich beverages such as red grape juice and de-alcoholised red wine did not suppress immune responses in healthy men.
Cocoa and/or dark chocolate
Cacao liquor polyphenols have been reported to affect human immune system cells in vitro. The results demonstrated that at least in vitro, treatment of normal peripheral blood lymphocytes inhibited mitogen-induced proliferation of T-cells and polyclonal Ig production by B-cells in a dose-dependent manner. Also the treatment inhibited both IL-2 mRNA expression of and IL-2 secretion by T-cells. These results suggest that cacao derived polyphenolic compounds have immuno-regulatory effects.191 The effects of polyphenolic compounds from chocolate may be due to the modulation of cellular metabolic functions (re-regulation of inflammatory responses) that are synergistic with immunological functions.192 A number of phenolic compounds can be found in a variety of food sources (see Table 20.2).
Table 20.2 Flavonoid types, representative flavonoids in food and beverages sources
| Class | Representative flavonoids | Food and beverage sources |
|---|---|---|
| Flavonols |
Dairy calcium
A recent study provides significant in vivo evidence that dietary calcium and dairy may regulate metabolic processes associated with inflammatory responses in a mouse model of diet-induced obesity and redox metabolism aberration as well as in obese adult humans. Dietary calcium-induced suppression of circulating 1alpha, 25-dihydroxycholecalciferol may be responsible for calcium-induced suppression of inflammatory responses, although further effects of dairy foods on oxidative stress appear to be mediated by additional mechanisms.193
Lactoferrin from milk
Lactoferrin is an iron-binding glycoprotein of the transferrin family with a molecular mass of about 80 kilodalton (kDa). It is a component of milk, saliva, tears, airway mucus and secondary granules of neutrophils.194, 195 It is also a major component of mammals’ innate immune system. Lactoferrin has a range of protective effects from direct antimicrobial activities — in relation to bacteria, viruses, fungi, and parasites — and anti-inflammatory and anti-cancer activities.196 In addition, lactoferrin has demonstrated activities that are immuno-modulatory/anti-inflammatory (down-regulating cytokines)197 and that regulate cell proliferation198 and intestinal iron absorption.199
Similar to human lactoferrin, bovine lactoferrin has been shown to induce proliferation and differentiation of human enterocytes and to modulate their cytokine production.200 The beneficial effects of orally administered bovine lactoferrin on infections and iron status have also been recently demonstrated in clinical trials in human adults and infants.201–206 The protective effect of lactoferrin towards microbial and viral infections has been widely demonstrated in a large number of in vitro studies, although the number of clinical trials so far completed is not extensive. Nevertheless, lactoferrin can be considered not only a primary defence factor against mucosal infections but also a polyvalent regulator, which interacts with several microbial, viral and host components involved in infectious processes. The capability of lactoferrin to exert antiviral activity through its binding to host-cells and/or viral particles strengthens the hypothesis that this glycoprotein constitutes a significant barrier in the mucosal wall of the GI tract, which has been demonstrated to be effective against both microbial and viral insults.
Essential fatty acids; omega-3 fish oils
Inflammation is part of the body’s immediate response to infection or injury. It is typified by redness, swelling, heat and pain. These occur as a result of increased blood flow, increased permeability across blood capillaries, which allows large molecules (e.g. complement, antibodies, pro-inflammatory cytokines) to leave the bloodstream and cross the endothelial wall, and increased movement of leukocytes from the bloodstream into the surrounding tissue. Inflammation functions to begin the immunological process of elimination of pathogenic and toxin insults and to repair damaged tissue. The key link between fatty acids and inflammation is that the eicosanoid family of inflammatory mediators is generated from 20 carbon polyunsaturated fatty acids (PUFAs) liberated from cell membrane phospholipids. The membrane phospholipids of inflammatory cells taken from humans consuming typical Western diets usually contain approximately 20% of fatty acids as the n-6 PUFA arachidonic acid.207, 208 The proportions of other 20 carbon PUFAs such as the n-6 PUFA linolenic acid and the n-3 PUFA eicosapentaenoic acid are typically about 2% and approximately 1% of fatty acids, respectively. Therefore arachidonic acid is usually the dominant substrate for eicosanoid synthesis promoting and sustaining the inflammatory response. Addition of n-3 PUFAs to the diet adds potentially useful anti-inflammatory agents that can regulate the inflammatory response by directly replacing arachidonic acid as an eicosanoid substrate, by inhibiting arachidonic acid metabolism, and by giving rise to anti-inflammatory resolvins, and indirectly, by altering the expression of inflammatory genes through effects on transcription factor activation.209, 210
Mediterranean diet, portfolio diet, healthy diet
Different dietary regimes have been reported to be useful in influencing immune function.180, 211–217
Recently it was demonstrated that adherence to a Mediterranean type diet with addition of virgin olive oil resulted in down-regulation of cellular and circulating inflammatory biomarkers.218 Additional dietary strategies that focus on healthy food consumptions, such as the portfolio diet, reduced low-density lipoprotein cholesterol by approximately 30% and produced clinically significant reductions in CHD risk.219 Consequently it would be expected that pro-inflammatory mediators would be reduced and re-regulated to normal levels.
Nuts
The consumption of nuts (i.e. almonds, brazil nuts, cashews, chestnuts, hazelnuts, macadamias, pecans, pine nuts, pistachios, walnuts) in the diet can significantly down regulate inflammatory responses of the immune system.220–222 Nuts are an excellent source of phytochemicals (phyotsterols, phenolic acids, flavonoids, stilbenes, and carotenoids). The total phenolic constituents probably contribute to the overall metabolic regulation of immune function anti-inflammatory responses.220
Soy protein
The intestines are an important organ responsible for nutrient absorption, metabolism and recognition of food signals.223 Soy proteins have been reported to modulate immune function pro-inflammatory activity.224, 225 Recently it was demonstrated that soy milk and supplemental isoflavones modulated B-cell populations and appeared to be protective against DNA damage in post-menopausal women.226
Teas
Green tea
EPG (Epigallocatechin-3-gallate), present in green tea, is well-known for its ability to reduce the risk of a variety of immunodeficiency disorders.227 Green tea possesses anti-oxidant, anti-inflammatory, anti-carcinogenic and immune enhancing properties. They have also been found to be photo-protective in nature, having the potential to be used for the prevention of photo-ageing, melanoma and non-melanoma skin cancers.228 In a recent randomised, double-blind, placebo-controlled trial, healthy adults were given green tea capsules or placebo twice daily for 3 months. Those taking the active formulation had enhanced gammadelta T-cell function with 32% fewer subjects experiencing symptoms of cold and flu. Those who did become unwell experienced a significantly shorter duration of symptoms.229–232
Black tea
Habitual consumption of tea has been associated with improved immune system function.233–235
A recent review has concluded that the current scientific evidence supports the concept that dietary intervention with tea or L-theanine leads to T-cell priming. Such priming is beneficial because it is associated with an enhancement of the magnitude and the breadth of early responses to microbial and neoplastic disease. Small clinical trials in normal volunteers have demonstrated that increased intake of tea or a supplement containing the bioactive tea components L-theanine and epigalenocatechins affect T-cell activity and the latter was associated with a decrease in cold and flu symptoms.236
Food intolerance
It is important to realise that any food, however, may be pro-inflammatory for an individual who is intolerant to that food. Adverse reactions to foods can have a significant impact on the immune system and general wellbeing of an individual’s life. Immune-mediated adverse reactions may be roughly divided into IgE-mediated and non IgE-mediated. Non IgE-mediated food reactions are not well understood and their negative effects on wellbeing and immune efficiency may be highly underestimated. In the first few years of life, humans gradually develop an intricate balance between tolerance and immune reactivity in the gut mucosa along with a tremendous expansion of the gut-associated lymphoid tissue (GALT), which is profoundly affected by changes in commensal flora.237
The simplest test to determine which foods contribute to gastrointestinal or other symptoms is to perform a food elimination diet (FED), with initial avoidance then separate re-introduction of individual foods (see: www.integrative-medicine.com.au for details). Some of the most common dietary intolerances are due to wheat, dairy and soy. In such cases, immune reactivity may be associated with apparent dietary protein intolerance (gliadin, cow’s milk protein, soy) and gastrointestinal inflammation that may be partly associated with an aberrant innate immune response against endotoxin, a product of specific gut bacteria.238
Alcohol
Alcohol in light-moderate amounts (1 glass for women, 2 glasses for men, every second day) has been shown to be particularly beneficial for the immune system when compared with both non-drinkers and heavy drinkers.239 Resveratrol, a polyphenol from red wine, is able to stimulate both innate and adaptive immune responses; in particular, the release of cytokines such as IL-12, IL-10 and IFN-gamma and immunoglobulins that may be important for host protection in different immune-related disorders.240, 241 Its effects may be also related to cytokine production by both CD4+ and CD8+ T-cells.242
Nutritional supplementation
Nutrition is a critical determinant of immunity and malnutrition is the most common cause of immunodeficiency worldwide.243 Nutrients either enhance or depress immune function depending on the nutrient and level of its intake.244 Indeed, both insufficient and excess nutrient intakes can have negative consequences on immune status and susceptibility to a variety of pathogens.245 Deficiency of nutrients may suppress immunity by affecting innate, T-cell mediated and adaptive antibody responses, leading to dysregulation of the host response. This can subsequently lead to increased susceptibility to infections which can then lead to further nutrient deficiency and so on.246
Available data indicates that vitamins A, B6, B12, C, D, E, folic acid and the trace elements Fe, Zn, Cu and Se all work synergistically to support the protective activities of the immune cells. With the exception of iron and vitamin C, they are all also intricately involved in antibody production.245, 246 Anti-oxidant vitamins and trace elements (vitamins C, E, Se, Cu, Zn) counteract damage to tissues secondary to reactive oxygen species whilst simultaneously modulating immune cell function by affecting the production of cytokines and prostaglandins. Adequate intakes of vitamin B6, B12, C, E, folic acid and trace elements Se, Zn, Cu and Fe all support a Th1 cytokine-mediated immune response with sufficient production of pro-inflammatory cytokines. This maintains an effective immune response,246 avoiding a shift to an anti-inflammatory Th2 immune state and an increased risk of extracellular infections. Supplementation with these nutrients reverses the Th2 cell-mediated immune response to Th1, thereby enhancing innate immunity.247–249
Probiotics
The gastrointestinal mucosa is the major contact area between the host and the external world of microflora. Over 400 square metres in size, it is colonised by an immense number of bacteria that are in constant communication with our immune cells.250 In fact, it is the GALT itself that houses the largest number of immune cells in the body.251
Although the intestinal immune system is fully developed after birth, the actual protective function of the gut requires the microbial stimulation of bacterial colonisation. Breast milk naturally contains prebiotic oligosaccharides, designed to feed and proliferate specific resident bacteria with important protective functions (probiotics), primarily Lactobacillus and Bifidobacterium, in the infant’s gut.251 However, the nature and species of microflora is also determined by many other factors, including external environment microflora, use of antibiotics and immuno-modulatory agents and early introduction of cow’s milk.252
Until recently, the gut bacteria were regarded as residents without any specific functions.251 However, it now appears that altered mucosal microflora in early childhood alters signalling reactions which determine T-helper cell differentiation and/or the induction of tolerance.253–254 Thus, the nature of mucosal microflora acquired in early infancy determines the outcome of mucosal inflammation and the subsequent development of mucosal disease, autoimmunity and allergic diseases later in life.254
Probiotics are recognised for their roles in nutrient absorption, mucosal barrier function, angiogenesis, morphogenesis and postnatal maturation of intestinal cell lineages, intestinal motility and, most importantly, the maturation of the GALT.256
An important adjustment of the immune system to bacterial colonisation of the gut is the production of secretory immunoglobulin A (sIgA) by B-cells in the GALT.248, 255, 256 Probiotics stimulate both the production and secretion of polymeric IgA, the antibody that coats and protects mucosal surfaces against harmful bacterial invasion.253 Secretory IgA also promotes an anti-inflammatory environment by neutralising immune stimulatory antigens.250 Thus, sIgA plays a significant role in the regulation of bacterial communities and maintenance of immune homeostasis.255
In addition, appropriate colonisation with probiotics helps to produce a balanced T-helper cell response and prevent an imbalance which can contribute in part to clinical disease. For example, Th2 imbalance may contribute to atopic disease and Th1 imbalance may contribute to Crohn’s disease and Helicobacter pylori-induced gastritis.253 Th1>Th2 cytokine expression in the respiratory tract associated with increased allergic disease has been correlated with reduced exposure to microbial agents associated with Th1 responses. In contrast, reduced exposure to helminths in the gut associated with reduced Th2 expression appears to correlate well with dominant Th1 cytokine expression and IBD.249, 254, 255
Pre and probiotics are certainly attractive options for maintaining the steady nutritional state of the host with defective gut barrier functions. Prebiotics (inulin from chicory root, fructooligosaccharides, arabinogalactans) resist enzymatic digestion in the upper GI tract and therefore reach the colon virtually intact where they undergo bacterial fermentation. The consumption of prebiotics favours the growth of probiotics and impedes growth of pathogenic organisms, thereby modulating immune parameters in the GALT, secondary lymphoid tissues and peripheral circulation.257 The change in gut microflora may decrease intestinal permeability, consequently influencing both intestinal and systemic body functions.258
Adverse reactions to foods can have a significant impact on an individual’s life. In the first few years of life, humans gradually develop an intricate balance between tolerance and immune reactivity in the gut mucosa along with a tremendous expansion of the GALT, which is profoundly affected by changes in commensal flora.237
Probiotics modify the structure of potentially harmful food antigens and thereby alter their immunogenicity.258 Both IgE and non IgE-mediated food allergy are frequently seen in the early years of life, with cow’s milk and soy proteins being the most common causative dietary proteins for non IgE-mediated food allergy.237 Certain probiotics have recently been found to release low-molecular-weight peptides into milk products using bacterial-derived proteases that degrade milk casein, and thereby generate peptides, triggering immune responses. Thus the intestinal microbial communities contribute to the processing of food antigens in the gut.259
Abnormalities in Th1 function may not only play a role in some patients with non IgE-mediated food allergy in whom decreased Th1 function is seen, but also in patients with coeliac disease in whom an increased Th1 is seen. Lymphonodular hyperplasia may also be a hallmark histologic lesion in patients with non IgE-mediated food allergy.260 In such patients, a localised IgE-mediated response rather than a systemic food-specific IgE response may be responsible for these gastrointestinal symptoms.261
The pathogenesis of IBD involves an interaction between genetically determined host susceptibility, dysregulated immune response and the enteric microbiota.262, 263 Inappropriate secretion of quorum sensing molecules by certain gut bacteria may alter the GALT and thereby deregulate the immune tolerance normally present.264, 265 Thus manipulation of the luminal contents with antibiotics, prebiotics or probiotics represents a potentially effective therapeutic option.266 Both inulin and oligofructose stimulate the colonic production of short chain fatty acids and favour the growth of lactobacilli and/or bifidobacteria, which are associated with reduced mucosal inflammation, particularly in relapsing pouchitis.267 Clinical trials using specific probiotics to treat IBDs have demonstrated that the multi-agent mixture VSL3# is particularly beneficial for ulcerative colitis and pouchitis whereas Escherichia coli Nissle 1917 is effective in the prevention of recurrence of ulcerative colitis.268, 269 Thus far, probiotics seem to be less effective in patients with Crohn’s disease.270 Lactobacillus rhamnosus GG is another strain that has been effectively used for the prevention and treatment of rotavirus and antibiotic-associated diarrhoea, the prevention of cow’s milk-induced food allergy and pouchitis.271–275
Some gluten peptides are able to induce an innate immune response in intestinal mucosa and gluten intake is linked to the production of pro-inflammatory cytokines IL-15, IL-18 and IL-21. The failure to control this inflammatory response may also be one of the factors underlying gluten intolerance in individuals with coeliac disease.274
Zinc
Zinc is an essential trace element that is critical for cellular function and structural integrity.276 Normal zinc homeostasis is required for a functional immune system (both innate and adaptive), metabolic homeostasis (energy utilisation and hormone turnover), anti-oxidant activity, glucose homeostasis and wound healing.276, 277 Zinc is known to regulate the immune system systemically as well as having direct T-cellular effects resulting in the regulation of gene expression, bioenergetics, metabolic pathways, signal transduction and cell invasion, proliferation and apoptosis.278
Furthermore, zinc is an essential cofactor for the structure and function of a wide range of cellular proteins including enzymes, structural proteins, transcription and replication factors. It is now known that nearly 2000 of these transcription factors require zinc for their structural integrity.279–282 Zinc also affects entire functional networks of genes that are related to pro-inflammatory cytokines and cellular survival.282, 283 Thus an individual’s zinc status has a significant impact on their immune system, with zinc deficiency having the ability to profoundly modulate immune function and zinc supplementation, the ability to prevent and treat many acute and chronic diseases.284–288
Even a mild deficiency of zinc in humans results in immune dysfunction by decreasing serum thymulin activity, which is required for the maturation of T-helper cells. In particular, Th1 cytokines are decreased whilst Th2 cytokines remain relatively unaffected.287, 288 This shift of Th1 to Th2 function results in cell-mediated immune dysfunction. Decreased Th1 results in decreased IL-2 production, which leads to decreased activities of NK-cells and T-cytolytic cells, in turn enhancing susceptibility to malignancies and infections with viruses and bacteria.289 Ageing is associated with the same Th1/Th2 imbalance and moderate zinc supplementation has been shown to alter these proportions.290–292
Recent research has shown that zinc can either activate or inhibit several signalling pathways that interact with the signal transduction of pathogen-sensing receptors, the so-called toll-like receptors (TLR), which, upon activation, lead to secretion of pro-inflammatory cytokines. Thus zinc can play a major regulatory role in the immune system.293
Zinc also directly influences GALT, contributing to host defence by maintaining the integrity of the gut mucosal barrier and thereby controlling inflammatory cell infiltration.294
Oxidative stress is known to be an important contributing factor in many chronic diseases and zinc deficiency is constantly observed in states of chronic inflammation.295 Zinc supplementation has been shown to decrease the gene expression and production of both pro-inflammatory cytokines and oxidative stress markers.296 Metallothionein increase in ageing and chronic inflammation allows a continuous sequestration of intracellular zinc with subsequent low zinc ion availability against stressor agents and inflammation. This phenomenon influences NF-kappaB and the inhibitory protein A20, leading to an impaired inflammatory/immune response.295 Zinc deficiency also induces vascular pro-inflammatory parameters associated with NF-kappaB and PPAR signalling, markedly modulating mechanisms of the pathology of inflammatory diseases such as atherosclerosis.297 As such, zinc may prove to be a useful chemo-preventative agent for many chronic diseases, including neurodegenerative disorders, rheumatoid arthritis, macular degeneration, IBD and cancer.298
Many studies have demonstrated the beneficial effects of zinc supplementation in the management of the common cold, cold-sores, influenza, acute and chronic diarrhoea and acute respiratory infections. Average daily doses were 15–30mg elemental zinc for adults, 7.5–20mg for children and 10mg for infants < 6 months.297–301 Zinc gluconate lozenges, in particular, have been shown to significantly decrease common cold duration and number of antibiotics required whereas prophylactic use significantly decreased cold frequency.302–306 The formulation of the lozenge also appears to be important because the addition of citric or tartaric acid may reduce the efficacy due to chelation of the zinc ions.307, 308 Current evidence also shows that zinc (and selenium) improve humoral immunity in elderly subjects after an influenza vaccination.
Serum levels of zinc are significantly lower in HIV+ individuals and an imbalance between Th1 and Th2 responses in these patients has been implicated in the immune dysregulation. Researchers have proposed that resistance to infection and/or progression to AIDS is dependent on a Th1>Th2 dominance.309, 310
Several recent animal studies have also demonstrated a link between zinc deficiency and several auto-immune diseases, including systemic lupus erythematosus (SLE) and type 1 diabetes. Egr-2 is a zinc-finger transcription factor which controls the self-tolerance of T-cells preventing the development of auto-immunity.311 Another zinc-finger transcription factor, Gfi1, is also emerging as a novel master regulator restricting B-cell mediated autoimmunity.312 The zinc transporter ZnT8 is targeted by 60–80% new-onset type 1 diabetics compared with <2% controls and <3% Type 2 diabetics. It is also targeted in up to 30% patients with other auto-immune diseases associated with T1DM.313–316
Vitamin A
Vitamin A has received particular attention in recent years. It is now recognised that it modulates a wide range of immune functions, such as lymphocyte activation and proliferation, T-helper cell differentiation, tissue-specific lymphocyte homing, the production of specific antibody isotypes and regulation of the immune response.317
Retinoic acid is produced naturally from intestinal dendritic cells.318 The presence of high levels of retinoic acid in the intestine and GALT can promote B-cell class switching to IgA, hence boosting the production of IgA in the intestinal mucosa.318 When B- and T-cells are activated in the GALT, gut-homing receptors are induced on these cells via the actions of retinoic acid. These gut-homing B- and T-cells play essential roles in protecting the digestive tract from pathogens i.e. the development of ‘oral tolerance’. Furthermore, retinoic acid is also318 required for the maturation of phagocytes in the bone marrow.318, 319
Superfluous activation of natural immune system cascades is now thought to be one of the underlying mechanisms in psoriasis. vitamin A derivatives are currently being investigated in the treatment of psoriasis.320
Vitamin A deficiency is also a risk factor for low antibody production. In countries where vitamin A deficiency is endemic, many children are receiving retinol as an adjunct in their vaccinations, especially polio, DPT and measles. This is because vitamin A appears to promote the vaccine antibody response. In particular, the oral polio seroconversion rate is increased by vitamin A in those children who are deficient in this nutrient.321
Current evidence does not support vitamin A supplementation to pregnant HIV+ women to reduce transmission to their child, however, there is an indication that supplementation improves birth weight.322 Periodic vitamin A supplementation of HIV-infected infants and children is recommended as it has been shown to be beneficial in reducing all-cause mortality and morbidity.323, 324
Current recommendations for vitamin A intake are based simply on the maintenance of normal vision. However, it has been realised that higher levels may in fact be necessary in order to optimise innate immune function.325 Vitamin A supplementation above dietary requirements has been shown to enhance inflammatory responses with decreased Th1 and increased mucosal responses in animals.326
Selenium
Selenium is a potent nutritional antioxidant that influences immune responses through its incorporation into selenoproteins. Given that these selenoproteins play crucial roles in regulating reactive oxygen species in nearly every tissue in the body, it is hardly surprising that selenium significantly influences inflammatory and immune responses.327–329
Evidence is accumulating that selenium levels lower than previously thought can cause adverse health effects, such that the RDA levels have recently been increased to 150ug/day. Furthermore, it has also been demonstrated that higher levels of selenium may give additional protection from many diseases by significantly enhancing immune responses.330 Perhaps these higher levels are required for the full expression of protective selenoproteins.331, 332
Selenium is a key nutrient in the protection from certain viral infections, including the Coxsackie virus and Influenza virus. Selenium plus zinc has been found to improve humoral immunity in elderly subjects after the influenza vaccination. Many individuals with HIV are deficient in selenium, and it is in the selenium deficient host, that HIV infection progresses more rapidly to AIDS.333, 334
A recent clinical study investigated a high selenium yeast supplement (200mcg/day) in a double-blind, randomised, placebo-controlled trial. Intention-to-treat analyses assessed the effect on HIV-1 viral load and CD4 count after 9 months of treatment. This study concluded that a daily selenium supplement suppressed the progression of HIV-1 viral burden and provided indirect improvement of CD4 T lymphocyte count. Hence the results support the use of selenium as a simple, inexpensive, and safe adjunct therapy in HIV spectrum disease.335
Just as an inadequate status of selenium is linked to an increased risk of cancer, there is also growing evidence that an elevated selenium intake may be associated with a reduced risk of cancer. 336–339 Interventions with selenium (at least 200mcg/day) have shown benefit in reducing both the incidence of cancer and the mortality in all cancers combined. This has been shown to be particularly relevant in liver, prostate, colorectal, lung, oesophageal and stomach cancers, the effect of which is most pronounced in those that are most deficient in selenium. There is also some new evidence that selenium may also affect the risk of cancer progression and metastasis.337 It should be noted that the dose and form of selenium are critical factors in cancer prevention.338
The protective roles of selenium are still not entirely clear. Selenium is essential for the proper functioning of neutrophils, macrophages, NK-cells and T lymphocytes.318 Other proposed mechanisms include the protective role of selenoproteins/ selenoenzymes, the induction of apoptosis, immune system effects, detoxification of antagonistic metals, inactivation of nuclear transcription factor, regulation of lipoxygenases, reduction of oxidative stress, induction of Phase II enzymes, androgen receptor down-regulation, inhibition of DNA adduct formation and cell cycle arrest.335, 337, 338
Vitamin C
The human body is not able to synthesize vitamin C, hence we are entirely dependent upon dietary sources and/or nutritional supplementation to maintain adequate levels of this important water-soluble anti-oxidant.340, 341 It has long been known that vitamin C concentrations in the plasma and leukocytes rapidly decline during infections and stress, subsequently resulting in a reduced resistance to pathogens.342, 343 For this reason, vitamin C has traditionally been used as a cure for the common cold.
Supplementation of vitamin C has, indeed, been found to improve components of the immune system such as antimicrobial and NK-cell activities, lymphocyte proliferation, chemotaxis and delayed-type hypersensitivity. It also contributes to the maintenance of the redox activity of cells, thereby protecting them from reactive oxygen species generated during the inflammatory response.342
There has been a large number of RCTs that indicate vitamin C supplementation (up to 1g/day) with zinc (up to 30mg/day) can ameliorate the symptoms and shorten the duration of respiratory infections when used prophylactically. However, there has been no reduced incidence of the common cold.340, 342 It is important to note that optimal dosing is critical to intervention studies using vitamin C.340
When combined with zinc, vitamin C has also been found to reduce the incidence and improve the outcome of pneumonia, malaria and diarrhoeal infections, especially in children in developing countries.340
A recent Cochrane review has concluded that supplementation of vitamin C is only effective in preventing the common cold in cases of excess physical activity or in cold environments.343 Intense exercise is known to increase lipid peroxidation and cause muscle damage. Supplementation with anti-oxidant vitamins, including vitamin C, reduces these without blocking the cellular adaptation to exercise.344, 345
Smoking-induced oxidative stress impairs the function of peripheral mononuclear cells. Results are mixed as to the efficacy of vitamin C in reducing the oxidative stress and ameliorating the impaired migratory activity of these mononuclear cells.346–348
Whilst the efficacy of vitamin C supplementation is still controversial, it is argued by some authors that to achieve an optimal daily allowance of vitamin C, we require 1g daily supplementation accompanied by a diet high in fruits and vegetables.342
Vitamin D
Vitamin D has been rediscovered in recent years and there is a now a plethora of studies regarding the serious health consequences due to deficiency of vitamin D. Very few foods naturally contain vitamin D, so sun exposure is the primary source of vitamin D.349
Indeed, vitamin D deficiency is now a recognised global pandemic, in both developing and developed countries.350–352 As such, there are a growing number of diseases which are associated with vitamin D deficiency.353 Originally, vitamin D deficiency was only regarded to be important for bone health, however, it is now understood that this ‘vitamin’ is actually a complex hormone that is intricately involved in the integrity of the innate immune system.354
The deficiency of vitamin D is strongly associated with an increased risk of common cancers, auto-immune diseases (e.g. MS), rheumatoid arthritis (RA), IDDM, IBD, SLE, infectious diseases (e.g. TB), mental health disorders, cardiovascular disease (hypertension), skin disorders (psoriasis) and bone disorders (osteoporosis, osteomalacia, rickets).355–361 In the third National Health and Nutrition Survey linked mortality files, the lowest quartile of 25(OH)D level (<17.8ng/mL) was found to be independently associated with all-cause mortality in the general population.362
Most tissues, including the breast, colon and prostate, not only have a vitamin D receptor, but also have the ability to make 1,25-dihydroxyvitamin D(3). This active form of vitamin D influences the modulation of the immune system, acting in an autocrine fashion to regulate cell growth and decrease the risk of cells becoming malignant. It is believed that the local production of 1,25-dihydroxyvitamin D(3) may be responsible for the anti-carcinogenic benefits of vitamin D. It enhances innate immunity by inducing the cathelicidin antimicrobial peptide (hCAP).355, 363–369 Furthermore, 1,25-dihydroxyvitamin D is now known to either directly or indirectly regulate more than 200 different genes in the human body that are responsible for a wide range of biological processes.367, 368
Cancer
Vitamin D deficiency has now been linked to 17 different types of cancer.368, 370 It has been estimated that there is a 30–50% reduction in the risk of developing breast, colorectal and prostate cancer by increasing one’s vitamin D intake to at least 1000IU/day. Women who are vitamin D deficient are estimated to have a 253% increased risk for developing colorectal cancer. Furthermore, women who consume 1500mg/day calcium and 1100IU/day vitamin D3 for 4 years, reduce their risk of developing cancer by more than 60%.361
In a recent study, patients with head and neck squamous cell carcinoma were treated with vitamin D3 for 3 weeks before surgery. This resulted in reduced levels of immune inhibitory CD34+ cells with increased maturation of dendritic cells, demonstrating intra-tumoural immune competence.369
Inflammatory bowel disease (IBD)
Crohn’s disease is associated with a higher Th1 cytokine expression, whereas ulcerative colitis appears to express a modified type 2 response. Studies have demonstrated that calcitriol, the hormonally active form of vitamin D, exerts immuno-regulatory effects such as modulation of the Th1/Th2 cytokine balance on these conditions.371
Both vitamin D deficiency and vitamin D receptor deficiency result in acceleration of IBD. Dietary calcium is an important additional factor that determines the effect of vitamin D status on immune function. Animal studies show that treatment with vitamin D3 to animals with high calcium levels improves symptoms of IBD more than the same treatment administered to animals on low-calcium diets.372
Herbal Medicines
Astragalus membranaceus
Astragalus membranaceus (astragalus) is a common traditional Chinese medicinal plant that has been widely used to enhance the body’s natural defence mechanisms for centuries.373, 374 It is a chemically complex herb which contains over 60 components, including polysaccharides, B-sitosterol, glycosides, saponins, plants acids, choline, betaine, isoflavones, amino acids and various micro-elements.375
There have been numerous studies in recent years demonstrating the immuno-modulating and immuno-restorative effects, both in vitro and in vivo, of the roots of astragalus. 373–378 It appears that the immuno-potentiating effect of astragalus is primarily due to the polysaccharide fraction (APS), as it increases both cellular and humoral immune responses.377 Studies have shown that astragalus can stimulate immune cells within 24 hours of ingestion and continue for at least 7 days.378
Astragalus can reverse the Th2 dominant status of many common illnesses.379 For instance, Th1 cell subset dysfunction may exist in children with recurrent tonsillitis at the remission stage, suggesting that this may play an important role in its pathogenesis. In vitro studies demonstrate that astragalus can improve Th1 status, thereby displaying an important significance in the treatment of recurrent tonsillitis.376 Although astragalus has been used traditionally for respiratory tract infections, there is a paucity of good quality studies demonstrating its effectiveness for this particular condition.
In contrast to this, however, there are many studies showing that astragalus has the potential to reverse the Th2 predominant status in patients with asthma. It appears to do this by increasing the expression of T-bet mRNA and Th1 cytokines such as IFNgamma.381, 382, 383
Th2 cytokines are also predominant in cancer patients and have been found to be associated with tumour progression. In vitro studies have shown that astragalus is able to enhance gene expression levels of Th1 cytokines (IFNgamma and IL-2) and reverse the predominance of Th2 cytokines and their up-stream transcript factors in lung cancer patients, making it a possible CAM therapy for the future.379
Levels of Th1 cytokines (IFNgamma, IL-2) are significantly lower and Th2 cytokines (IL-4, IL-10) are significantly higher in patients with herpes simplex keratitis (HSK). Astragalus is able to modulate this imbalance state of Th1/Th2 in such patients, improving their immune system dysfunction, again suggesting that it may be an effective treatment for treating HSK.384
There have also been multiple animal studies on the effects of astragalus as an effective preventative and treatment for T1DM. This is a chronic auto-immune disease that is also related to the disequilibrium state of Th1 and Th2 subgroups of T-helper lymphocytes and their cytokines. Here it has been shown that astragalus can correct the imbalance between the Th1/Th2 cytokines with a lower incidence rate of developing T1DM in those animals treated with astragalus.385 Astragalus has also been shown to down-regulate the Th1/Th2 cytokine ratio in mice that already have T1DM.386 Astragalus ameliorates both the clinical and histological parameters of these mice in a long-lasting fashion, demonstrating that it can both attenuate insulitis and preserve beta cells from apoptosis. This is most likely through its immuno-regulatory actions on the Th1/Th2 ratio, which is strongly associated with PPAR gamma gene expression in spleens.387, 388
Astragalus has also been shown to be capable of restoring the impaired T-cell functions in cancer patients. It exhibits anti-tumour effects both in vitro and in vivo, which appear to be achieved through activating the host’s anti-tumour immune mechanisms.389 Thus patients may use astragalus to help inhibit tumour growth or to boost resistance to infections.390 In 1 recent trial children with acute leukaemia were given conventional chemotherapy alone or with 90g astragalus daily for 1 month. It was found that large doses of astragalus increased the dendritic cell induction of peripheral mononuclear cells and enhanced the antigen-presenting ability of dendritic cells in children with acute leukaemia.391
A recent Cochrane review has concluded that astragalus can stimulate immuno-competent T-cells and significantly reduce side-effects such as nausea and vomiting in patients treated with chemotherapy.392 There was no evidence of harm arising from the use of astragalus.393 Another meta-analysis has also demonstrated that astragalus may increase the effectiveness of platinum-based chemotherapy drugs used for treating non-small-cell lung cancer. Furthermore, high dose astragalus injection used together with cyclophosphamide (CTX) is more effective than CTX alone in decreasing infection rate and urine protein and improving immune function for patients with lupus nephritis.393
Studies have revealed that astragalus also has diuretic, anti-oxidant and anti-inflammatory activities.394It has tissue generating effects and improves the inflammatory reaction in wound healing.395 It may also suppress the development of atopic dermatitis by reducing IFN-gamma production and inhibit the enzyme 5-lipoxygenase which is important in the treatment of psoriasis.396, 397 Furthermore, astragalus polysaccharides and astragalosides have strong promoting effects on the phagocytosis of mycobacterium tuberculosis by macrophages and the secretion of IL-1beta, IL-6 and TNF alpha by activated macrophages.398
Cranberry
The proanthocyanidins in cranberry juice (vaccinium macrocarpon) are thought to reduce susceptibility to urinary tract infections (UTIs) by preventing bacteria from attaching to uroepithelial cells.399 Recent in vitro studies of the non-dialysed material of cranberry indicate the possibility that cranberry acts as an anti-adhesive agent upon bacteria through decreasing the secretion of extracellular fructosyltransferase (FTF), an extracellular enzyme associated with the pathogenesis of oral bacteria.400 In the same study cranberry was also noted to decrease the genetic expression of FTF in a dose dependent manner. The resulting effect may explain the demonstrated disruption of bacterial ligand-uroepithelial cell binding that has been observed to occur in the presence of cranberry juice.401
Indeed investigations of the ability of the uropathic bacteria, Esherichia coli and Enterococcus fecalis, to adhere to biomaterials in the presence proanthocyanidins extracted from cranberry402 demonstrates that is does reduce the binding capacity of this bacteria. Similar in vitro examinations identified that diluted cranberry juice inhibited the growth of Staphylococcus aureus, Escherichia coli (up to a 20:1 dilution), Salmonella spp, and Pseudomonus aureus, however having a less inhibitory influence on Pseudomona aeruginosa.403 This decrease in bacterial adherence has been noted to occur in a dose dependent relationship in which Escherichia coli loses its adherence the higher the dosage of cranberry given to a subject (36mg vs 108mg of cranberry capsule).404
The clinical efficacy of the use of cranberry juice for UTIs has been reviewed by the Cochrane Database (2008) with a supportive conclusion that use of cranberry over a 12-month period may decrease the incidence of recurrent infections amongst women.401 Earlier reviews have clarified that the use of cranberry juice in other groups susceptible to urinary tract infections (children, elderly men and women and patients that require catheterisation) remained presently unsubstantiated.406, 407 However, continuing studies indicate the potential for cranberry to be effective in these groups, notable amongst older women as evidenced by a 2009 RCT in which the use of trimethoprim (100mg) was only found to have very limited advantage when compared to 500mg of cranberry extract in instances in which the women had experienced 2 or more antibiotic treated UTIs in the previous 12 months.406 Notably, there was almost double the rate of withdrawal from the trimethoprim group (16%) compared to the cranberry group (9%) suggesting cranberry may be better tolerated.406 While multiple daily cranberry juice cocktails may be effective in reducing UTIs in pregnancy, as demonstrated in a 2008 randomised pilot study of only low statistical weighting, concern might be raised by the 38.8% of active participants who withdrew from the trial, many due to gastrointestinal upset.408
Echinacea purpurea
Clinical evaluation of the herb Echinacea and its influences upon the immune system are problematic due to the disparate use of different preparations across studies. Unfortunately only 1 extract, using the aerial components of Echinacea purpurea, has been evaluated in more than a single study preventing the pooling of data on specific extracts, 409 which may in itself explain the varied results achieved from Echinacea use. There is, however, preliminary evidence that the use of the aerial component of Echinacea purpurea may be beneficial in the early treatment of the common cold in adults, as concluded by a Cochrane review in 2006.410
Interestingly, in vitro studies of different extracts and forms of Echinacea consumed orally suggest that root components have an increased effect on phagocytosis when compared with aerial.411
In regards to safety, whilst concern has been raised regarding the long term use of Echinacea in depressing the immune system, a 2001 study of immune parameters demonstrated that 6 months oral use of Echinacea did not alter these measures. Furthermore, a critical evaluation of drug interaction with Echinacea (angustifolia, pallida and purpurea extracts) concluded that the use of Echinacea did not presently appear of risk to the consumer.412 There have been allergic reactions to Echinacea reported in susceptable individuals, although this is rare.
Medicinal foods
Manuka honey
The use of specific honey forms as derived from the tree origins, Leptospermum scoparium (New Zealand manuka) and Leptospermum polygalifolium (Australian jelly bush) are suggested to have therapeutic effects on wound and ulcer healing when applied with dressings.414, 415 These forms of honey would appear to have antibacterial qualities as demonstrated by in vitro studies in which it has been demonstrated that manuka honey can inhibit several oral bacterial pathogens including Escherichia coli, Salmonella typhimurium, Shigella sonnei, Listeria monocytogenes, Staphylococcus aureus (including wound-associated methicillin resistance strains),416 Bacillus cereus, and Streptococcus mutans.415, 417, 418
Due to this antibacterial quality, clinical evidence as reported by a 2008 Cochrane review indicates honey may reduce wound healing times as compared to conventional dressings in partial thickness burns, 419 however, it does not appear to provide additional benefit to compression bandages for leg ulcers when measured over a 12-week period.420 Recent studies continue to report the benefits of medicinal honeys. An RCT of wounds healing by secondary intention identified that, when compared to conventional dressings, honey was effective in reducing healing times (100 v 140 days).421
Manuka honey (Woundcare 18+) appears to have benefit in reducing MRSA infections in patients with sloughy venous leg ulcers when compared to hydrogel (IntraSite Gel) over a 4-week period but may be less effective in wounds with Pseudomonas aeruginosa, although the results of this RCT are derived from a small population (n = 10 for honey; n = 6 for hydrogel).422 Manuka honey was also reported to decrease mean slough area, wound size and infection rates when compared to hydrogel.423 An examination of wound pH indicates Manuka honey dressings have the potential to lower pH in association with improved wound healing for chronic wounds.424
It must be noted that honey used in medical practice should be of ‘medical grade’ — that is, it has been sterilised by gamma irradiation and has standardised antibacterial activity.425
Physical therapies
Massage
There are mixed results as to the benefits of massage for immune enhancement. Two earlier studies have noted increases in dopamine, serotonin, NK-cells and lymphocytes in women with breast cancer after thrice weekly massage for 5 weeks. Depression, anxiety and anger were also significantly reduced.426, 427 However, a more recent randomised control trial (RCT) showed that effleurage massage had no significant effect on immune and neuroendocrine parameters.428
Acupuncture
Acupuncture has been used for centuries to prevent and treat various conditions and to simply maintain good health.429 In addition to its known effects on the nervous system, emerging evidence suggests that it may also effectively modulate the innate immune system which plays important roles in inflammation, pain, metabolism, cell proliferation and apoptosis.430, 431 There is now experimental evidence that the electrical stimulation of the vagus nerve inhibits macrophage activation and the production of pro-inflammatory cytokines including TNF, IL-1 beta, IL-6 and IL-18, indicating a possible underlying neuro-immune basis to acupuncture.432
A small study involving healthy volunteers has shown a statistically significant increase in the number of CD2+, CD4+, CD8+, CD11b+, CD16+, CD19+, CD56+ cells in addition to IL-4, IL-1beta and IFN-gamma levels after acupuncture stimulation of meridian points. Another small study on HIV+ individuals demonstrated an increase in the total lymphocyte count after moxibustion at specific points in the treatment group compared with controls. Such observations suggest that acupuncture may regulate the immune system by promoting both humoral and cellular immunity as well as NK-cell activity.433, 434
Conclusion
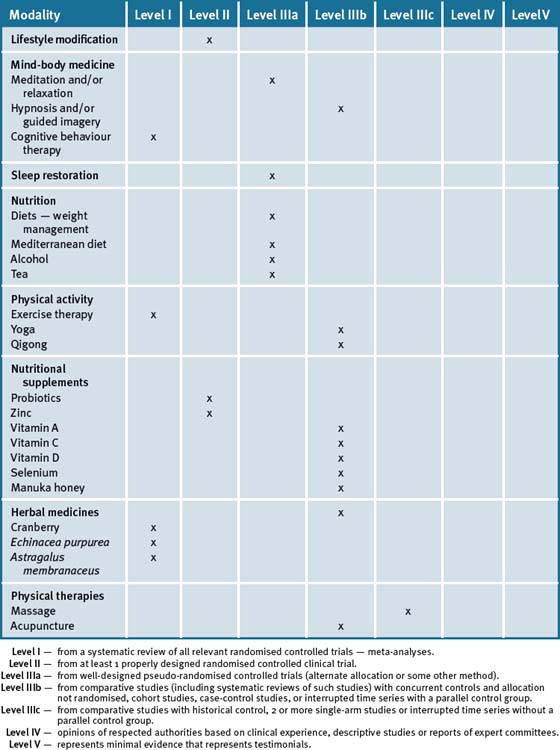
Table 20.3 summarises the level of evidence for some CAM therapies for the management of immune deficiencies and infections.
Table 20.3 Levels of evidence for lifestyle and complementary medicines/therapies in the management of immune deficiencies and infections
Note: Parts of this chapter were extracted with permission from the article ‘Immunity’ by Dr Lily Tomas in The Journal of Complementary Medicine 2009;8(3):12–8.
Clinical tips handout for patients — infections and immunity
1 Lifestyle advice
Sunshine
2 Physical activity/exercise
3 Mind-body medicine
4 Environment
5 Dietary changes
6 Physical therapies
7 Supplementation
Vitamin A
Vitamin C
Vitamin D3 (cholecalciferol)
Zinc
Herbs
Immune support
Astragalus herbs (A. membranaceus)
Probiotics
Throat infection and wound healing
Urinary tract infection and/or Helicobactor pylori
Cranberry (v. Macrocarpon)
1 Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313-323.
2 Irwen M.R. Human Psychoneuro–immunology: 20 years of discovery. Brain Behav Immun. 2008;22(2):129-139.
3 Ziemssen T., Kern S. Psychoneuro–immunology-cross-talk between the immune and nervous systems. J Neurol. 2007;254(Suppl 2):II8-II11.
4 Tausk F., Elenkov I., Moynihan J. Psychoneuro–immunology. Dermatol Ther. 2008;21(1):22-31.
5 Kemeny M.E., Schedlowski M. Understanding the interaction between psychological stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21(8):1009-1018.
6 Reiche E.M., Nunes S.O., Morimoto H.K. Stress, depression, the immune system and cancer. Lancet Oncol. 2004;5(10):617-625.
7 Reiche E.M., Morimoto H.K., Nunes S.M. Stress and depression-induced immune dysfunction: implications of the development and progression of cancer. Int Rev Psych. 2005;17(6):515-527.
8 Alves G.J. Palermo-Neto J. Neuroimmunomodulation: the cross-talk between nervous and immune systems. s.l. Rev Bras Psiquiatr. 2007;29(4):363-369.
9 Leonard B. Stress, depression and the activation of the immune system. World J Biol Psychiatry. 2000;1(1):17-25.
10 Mawdsley J.E., Rampton D.S. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):1481-1491.
11 Gareau M.G., Silva M.A., Perdue M.H. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008;8(4):274-281.
12 Olff M. Stress, depression and immunity: the role of defense and coping styles. Psychiatry Res. 1999;85(1):7-15.
13 Miller A.H. Neuroendocrine and immune system interactions in stress and depression. Psychiatr Clin North Am. 1998;21(2):443-463.
14 Tausk F., Elenkov I., Moynihan J. Psychoneuro–immunology. Dermatol Ther. 2008;21(1):22-31.
15 Karrow N.A. Activation of the HPAA and ANS during inflammation and altered programming of the neuroendocrine-immune axis during foetal and neonatal devt: lessons learned from the model inflammagen, lipopolysaccharide. Brain Behav Immun. 2006;20(2):144-158.
16 Raison C.L., Miller A.H. The neuroimmunolgy of stress and depression. Semin Clin Neuropsychiatry. 2001;6(4):277-294.
17 Stasi C., Orlandelli E. Role of the brain-gut axis in the pathophysiology of Crohns disease. Dig Dis. 2008;26(2):156-166.
18 Maunder R.G., Levelstein S. The role of stress in the devt and clinical course of IBD: epidemiological evidence. Curr Mol Med. 2008;8(4):247-252.
19 Maunder R.G. Evidence that stress contributes to IBD: evaluation, sntheis and future directions. IBD. 2005;11(6):600-608.
20 Boye B., Jahnson J., Mokleby K., et al. The INSPIRE study: are different personality traits related to disease-specific QOL in distressed patients with UC and Crohns disease? IBD. 2008;14:680-686.
21 Grippo A.J., Johnson A.K. Stress, depression and CV dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12(1):1-21.
22 Grippo A.J. Mechanisms underlying altered mood and CV dysfunction: trhe value of neurobiological and behavioural research with animal models. Neurosci Biobehav Rev. 2009;33(2):171-180.
23 Gold S.M., Irwin M.R. Depression and immunity: inflammation and depressive symptoms in MS. Neurol Clin. 2006;24(3):507-519.
24 Mawdsley J.E., Rampton D.S. The role of psychological stress in IBD. s.l.:. Neuroimmunomodulation. 2006;13(5–6):327-336.
25 McGregor B.A., Antoni M.H. Psych intervention and health noutcomes among women treated for breast cancer: a review of stress pathways and biological mediatore. Brain Behav Immun. 2009;23(2):159-166.
26 Kremser T., Evans A., Moore A., et al. Use of CAM by Australian women with breast cancer. Breast. 2008;17(4):387-394.
27 Smith J.E., Richardson J., Hoffman C., et al. MBSR as supportive therapy in cancer care: a systematic review. J Adv Nurs. 2005;52(3):315-327.
28 Witek-Janusek L., Albuquerque K., Chroniak K.R., et al. Effect of MBSR on immune function, QOL and coping in women newly-diagnosed with early stage breast cancer. Brain Beh Immun. 2008;22:969-981.
29 Carlson L.E., Speca M., Faris P., et al. 1 year pre-post intervention follow-up of psychological, immune, endocrine and BP outcomes of MBSR in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21(8):1038-1049.
30 Carlson L.E., Speca M., Patel K.D., et al. MBSR in relation to QOL, mood, symptoms of stress and levels of cortisol, DHEA and melatonin in breast and prostate cancer outpots. Psychoneuroendocrinology. 2004;29(4):448-474.
31 Carlson L.E., Speca M., Patel K.D., et al. MBSR in relation to QOL, mood, symptoms of stress and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003;65(4):571-581.
32 Ott M.J., Norris R.L., Bauer-Wu S.M. Mindfulness meditation for oncology pts: a discussion and critical review. Integr Cancer Ther. 2006;5(2):98-108.
33 Creswell J.D., Myers H.F., Cole S.W., et al. Mindfulness meditation training effects on CD4+ lymphocytes in HIV+ adults: a small RCT. Brain behav Immun. 2009;23(2):184-188.
34 Robinson F.P., Mathews H.L., Witek-Janusek L. Psycho-endocrine-immune response to MBSR in individulals infected with HIV: a quasiexperimental study. J Altern Comp Med. 2003;9(5):683-689.
35 Taylor D.N. Effects of a behavioural stress management program on anxiety, mood, self-esteem and T count in HIV positive men. s.l. Psychol Rep. 1995;76(2):451-457.
36 Collins M.P., Dunn L.F. The effects of meditation and visual imagery on an immune system disorder: dermatomyositis. J Alt Comp Med. 2005;11(2):275-284.
37 Gruzelier J., Smith F., Nagy A. Cellular and humoral immunity, mood and exam stress: the influences of self-hypnosis and personality predictors. Int J Psychphysiol. 2001;42(1):55-71.
38 Whitehouse W.G., Dinges D.F., Orne E.C., et al. Psychosocial and immune effects of self-hypnosis training for stress management throughout the first semester of medical school. Psychsom Med. 1996;58(3):249-263.
39 Gruzelier J.H. A review of the impact of hypnosis, relaxation, guided imagery and individual differences on aspects of immunity and health. Stress. 2002;5(2):147-163.
40 Gruber B.L., Hersh S.P., Hall N.R., et al. Immunological responses of breast cancer patients to behavioual interventions. Biofeedback Self Regul. 1993;18(1):1-22.
41 Lengacher C.A., Bennett M.P., Gonzalez L., et al. Immune reponses to guided imagery during breast cancer treatment. Biol Res Nurs. 2008;9(3):205-214.
42 Bakke A.C., Purtzer M.Z., Newton P. The effect of hypnotic guided imagery on psychological wellbeing and immune function in patients with prior breast cancer. J Psychosom Res. 2002;53(6):1131-1137.
43 Cruess S., Antoni M., Cruess D., et al. Reductions in HSV-2 antibody titres after CBT and relationships with neuroendocrine function, relaxation skills and social support in HIV positive men. Psychosom Med. 2000;62(6):828-837.
44 Antoni M.H., Cruess S., Cruess D.G., et al. CBT reduces distress and 24 hr urinary cortisol output among symptomatic HIV positive gay men. Ann Behav Med. 2000;22(1):29-37.
45 Antoni M.H., Cruess Dg, Cruess S., et al. CBT effects on anxiety, 24 hour urinary norepinephrine output and T-cytotoxic/suppressor cells over time among symptomatic HIV positive gay men. J Consult Clin Psychol. 2000;68(1):35-45.
46 Hidderley M., Holt M. A pilot RCT assessing the effects of autogenic training in early stage cancer pts in relation to psychological status and immune system responses. Eur J Oncol Nurs. 2004;8:61-65.
47 van der Pompe G., Antoni M.H., Duivenvoorden H.J., et al. An exploratory study into the effects of group psychotherapy on cardiovascular and immunoreactivity to acute stress in breast cancer patients. Psychther Psychosom. 2001;70(6):307-318.
48 Price J.R., Mitchell E., Tidy E., et al. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database of Systematic Reviews. (Issue 3):2008. Art. No.: CD001027. DOI: 10.1002/14651858.CD001027.pub2
49 Dietert R.R. DIT: focus on health risks. Chem Res Toxicol. 2009;22(1):17-23.
50 Dietert R.R. DIT in drug safety testing: matching DIT testing to adverse outcomes and childhood disease risk. Curr Drug Saf. 2008;3(3):216-226.
51 Kilburn H.K. Summary of the 5th Int Conf on Bioaerosols, Fungi, Bacteria, Mycotoxins and Human Health. Arch Environ Health. 2003;58(8):538-542.
52 Edmondson D.A., Nordness M.E., Zacharisen M.C., et al. Allergy and toxic mould syndrome. Ann Allergy Asthma Immun ol. 2005;94(2):234-239.
53 Dietert R.R., Zelikoff J.T. Early-life environment, DIT and the risk of paediatric allergic disease including asthma. Birth Defects Res B Dev Reprod Toxicol. 2008;83(6):547-560.
54 Dietert R.R., Dietert J.M. Possible role for ELII inc DIT in CFS or ME. Toxicology. 2008;247:61-72.
55 Dietert R.R., Dierert J.M. ELII and DIT-associated diseases: potential of herbal and fungal derived medicinals. Curr Med Chem. 2007;14(10):075-085.
56 Dietert R.R. DIT, postnatal immune dysfunction and childhood leukaemia. Blood Cells Mol Dis. 2009;42(2):108-112.
57 Dietert R.R., Dietart J.M. Potential for ELII inc DIT in autism and ASD: focus on critical windows of immune vulnerability. J Toxicol Environ Health B Crit Rev. 2008;11(8):660-680.
58 Kipen H.M., Fiedler N. Envt’al factors in medically unexplained symptoms and related syndromes: the evidence and the challenge. Environ Health Perapect. 2002;110(Suppl 4):597-599.
59 Krewski D., Glickman B.W., Habash R.W., et al. Recent advances in research on RF fields and health: 2001–2203. J Toxicol Environ Health B Crit Rev. 2007;10(4):287-318.
60 Valberg P.A., van Deventer T.E., Repacholi M.H. Worgroup report: base stations and wireless networks RF exposures and health consequences. Environ Health Perspect. 2007;115(3):416-424.
61 Knave B. EMF and health outcomes. Ann Acad Med Singapore. 2001;30(5):489-493.
62 Bonhomme-Fivre L., Marion S., Bezie Y., et al. Study of human neurovegetative and haematological effects of envt’al low frez (50Hz) EMF produced by transformers. Arch Env Health. 1998;53:87-92.
63 Hardell L., Sage C. Biological effects from EMF exposure and public exposure standards. Biomed Pharmacother. 2008;62(2):104-109.
64 Fukuyama T., Ueda H., Hayashi K., et al. Detection of low-level envt’al chemical allergy by a long-term sensitisation method. Toxicol Lett. 2008;180(1):1-8.
65 Dietert R.R., Hedge A. Chemical sensitivity and the immune system: a paradigm to approach potential immune involvement. Neurotoxicology. 1998;19(2):253-257.
66 Bernstein D.I. MCS: state of the art symposium. the role of chemical allergens. Regul Toxicol Pharmacol. 1996;24(1 Pt 2):S28-S31.
67 Laumbach R.J., Kipen H.M. Bioaerosols and SBS:particles, inflammation and allergy. Curr Opin Allergy Clin Immunol. 2005;5(2):135-139.
68 Campbell A.W., Thrasher J.D., Madison R.A., et al. Neural auto-antibodies and neurophysiological abnormalities in patients exposed to mould in water-damaged buildings. Arch Environ Health. 2003;58(8):464-474.
69 Gray M.R., Thrasher J.D., Crago R., et al. Mixed mould mycotoxicosis: immunological changes in humans following exposure in water-damaged buildings. Arch Environ Health. 2003;58(7):410-420.
70 Lander F., Meyer H.W., Norn S. Serum IgE specific to indoor moulds, measured by basophil histamine release, is associated with building-related symptoms in damp buildings. Infl amm Res. 2001;50(4):227-231.
71 Li-Ng M., Aloia J.F., Pollack S., et al. A RCT of D3 supplements for the prevention of symptomatic URTIs. Epidemiol Infect. 2009 Mar 19:1-9.
72 Lucas R.M., Ponsonby A.L., Pasco J.A., et al. Future health implications of prenatal and early-life vitamin D status. Nutr Rev. 2008;66(12):710-720.
73 Szodoray P., Nakken B., Gaal J., et al. The complex role of Vit D in auto-immune disease. Scand J Immunol. 2008;68(3):261-269.
74 Hayes C.E., Nashold F.E., Spach K.M., et al. The immunological functions of the vitamin D endocrine system. Cell Mol Biol. 2003;29(2):277-300.
75 Cantorna M.T. vitamin D and its role in immunology: MS and IBD. Prog Biophys Mol Biol. 2006;92(1):60-64.
76 Lips P. Vit D Physiology. Prog Biophys Mol Biol. 2006;92(1):4-8.
77 Kurylowicz A., Bednarczuk T., Nauman J. The influence of Vit D deficiency on cancers and auto-immune disease development. Endokrynol Pol. 2007;58(2):140-152.
78 Cantorna M.T. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92(1):60-64.
79 Arnson Y., Amital H., Shoenfeld Y. vitamin D and Autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66(9):1137-1142.
80 Cantorna M.T., Mahon B.D. Mounting evidence for Vit D as an environmental factor affecting auto-immune disease prevalence. Exp Biol Med. 2004;229(11):1136-1142.
81 Du T., Zhou Z.G., You S., et al. Regulation by Vit D3 on altered TLRs expression and response to ligands of monocyte from AI diabetes. Clin Chim Acta. 2009;402(1–2):133-138.
82 Smolders J., Damoiseaux J., Menheere P., et al. Fok-1 Vit D receptor gene polymorphism and Vit D metabolism in MS. J Neuroimmunol. 2009;207(1–2):117-121.
83 Cantorna M.T. Vit D and Autoimmunity: is Vit D status an environmental factor affecting auto-immune disease prevalence? Proc Soc Exp Biol Med. 2000;223(3):230-233.
84 Imeri L., Opp M.R. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10(3):199-210.
85 Smyth C.A. Evaluating sleep quality in older adults: the Pittsburgh Sleep Quality Index can be used to detect sleep disturbances or deficits. Am J Nurs. 2008;108(5):42-50.
86 Meerlo P., Sgoifo A., Suchecki D. Restricted and disrupted sleep: effects on ANS function, neuroendocrine stress systems and stress responsitivity. Sleep Med Rev. 2008;12(3):197-210.
87 Novati A., Roman V., Cetin T., et al. Chronically restricted sleep leads to depression-like changes in NT receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31(11):1579-1585.
88 Preston B.T., Capellini I., McNamara P., et al. Parasite resistance and the adaptive significance of sleep. BMC Evol Biol. 2009;9:7.
89 Spaggiari M.C. Sleep medicine in occupational health. G Ital Med Lav Ergon. 2008;30(3):276-279.
90 Bentivoglio M., Kristensson K. Neural-immune interactions in disorders of sleep-wakefulness organisation. Trends Neurosci. 2007;30(12):645-652.
91 Roman V., Walastra I., Luiten P.G., et al. Too little sleep gradually desensitises the serotonin 1A receptor system. Sleep. 2005;28(12):1505-1510.
92 Cohen S., Doyle W.J., Alper C.M., et al. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169(1):62-67.
93 Cardinali D.P., Esquifino A.I., Srinivasan V., et al. Melatonin and the IS in ageing. Neuroimmunomodulation. 2008;15(4–6):272-278.
94 Woods J.A., Vieira V.J., Keylock K.T. Exercise, inflammation and innate immunity. Immunol Allergy Clin North Am. 2009;29(2):381-393.
95 Schedlowski M., Schmidt R.E. Stress and the immune system. s.l. Naturwissenschaften. 1996;83(5):214-220.
96 Kisaki T., Takemasa T., Sakurai T., et al. Adaptation of macrophages to exercise training improves innate immunity. Biochem Biophys Res Commun. 2008;372(1):152-156.
97 Timmons B.W., Cieslak T. Human NK-cell subsets and acute exercise: a brief review. Exerc Immunol Rev. 2008;14:8-23.
98 de Lima C., Alves L.E., Iagher F., et al. Anaerobic exercise reduces tumour growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumour-bearing rats. s.l. Eur J Appl Physiol. 2008;104(6):957-964.
99 Sakamoto Y., Ueki S., Kasai T., et al. Effect of exercise, ageing and functional capacity on acute sIgA response in elderly people over 75 years of age. Geriatr Gerontol Int. 2009;9(1):81-88.
100 Bishop N.C., Gleeson M. Acute and chronic effects of exercise on markers of mucosal immunity. Front Biosci. 2009;14:4444-4456.
101 Matsuzawa-Nagata N., Takamura T., Ando H., et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57(8):1071-1077.
102 Gleeson M., Pyne D.B. Special feature for the Olympics: effects of exercise on the immune system: exercise effects on mucosal immunity. Immunol Cell Biol. 2000;78(5):536-544.
103 Nijs J., Paul L., Wallman K. Chronic fatigue syndrome: an approach combining self-management with graded exercise to avoid exacerbations. J Rehabil Med. 2008;40(4):241-247.
104 West N.P., Pyne D.B., Kyd J.M., et al. The effect of exercise on innate mucosal immunity. Br J Sports Med. 2008 May 22.
105 Nieman D.C., Henson D.A., McMahon M., et al. Beta-glucan, immune function and URTI in athletes. Med Sci Sports Exerc. 2008;40(8):1463-1471.
106 Close P., Thielen V., Bury T. Mucosal immunity in elite athletes. Rev Med Liege. 2003;58:548-553.
107 Groer M.W., Shelton M.M. Exercise is associated with elevated proinflammatory cytokines in human milk. J Obstet Gynaec Neonatal Nurs. 2009;38(1):35-41.
108 Edmonds M., McGuire H., Price J. Exercise therapy for chronic fatigue syndrome. Cochrane Database of Systematic Reviews. (Issue 3):2004. Art. No.: CD003200. DOI: 10.1002/14651858.CD003200.pub2
109 Kjellgren A., Bood S.A., Axelsson K., et al. Wellness through a comprehensive yogic breathing program- a controlled pilot trial. BMC Comp Altern Med. 2007;7:43.
110 Sharma H., Datta P., Singh A., et al. Gene expression profiling in practitioners of SK. J Psychosom Res. 2008;64(2):213-218.
111 Sharma H., Sen S., Singh A. SK practitioners exhibit better antioxidant status and lower blood lactate levels. Biol Psychol. 2003;63(3):281-291.
112 Kochupillai V., Kumar P., Singh D., et al. Effect of rhythmic breathing (SK and P) on immune functions and tobacco addiction. Ann N Y Acad Sci. 2005;1056:242-252.
113 Rao R.M., Tellas S., Nagendra H.R., et al. Effects of yoga on NK-cell counts in early breast cancer pts undergoing conventional treatment. Med Sci Monit. 2008;14(2):LE3-LE4.
114 Manzaneque J.M., Vera F.M., Maldonado E.F., et al. Assessment of immunological parameters following a Q training program. Med Sci Monit. 2004;10(6):CR264-CR270.
115 Li Q.Z., Li P., Garcia G.E., et al. Genomic profiling of neutrophil transcripts in Asian Qigong practitioners: a pilot study in gene regulation by MB interaction. J Altern Complement Med. 2005;11(1):29-39.
116 Jones B.M. Changes in cytokine production in healthy subjects practicing Guolin Qigong: a pilot study. BMC Comp Altern Med. 2001;1:8.
117 Manzanaque J.M., Vera F.M., Rodriguez F.M., et al. Serum cytokines, mood and sleep after a Qigong program: Is Qigong an effective psychobiological tool? J Health Psychol. 2009;14(1):60-67.
118 Devaraj S., Wang-Polagruto J., Polagruto J., et al. High-fat, energy-dense, fast-food-style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism. 2008 Jun;57(6):867-870.
119 Okamatsu Y., Matsuda K., Hiramoto I., et al. Ghrelin and leptin modulate immunity and liver function in overweight children. Pediatr. 2009;51(1):9-13.
120 Aeberli I., Beljean N., Lehmann R., L’allemand D., Spinas G.A., Zimmermann M.B. The increase of fatty acid-binding protein aP2 in overweight and obese children: interactions with dietary fat and impact on measures of subclinical inflammation. Int J Obes (Lond). 2008 Aug 5. [Epub ahead of print]
121 Moreira A., Arsati F., Cury P.R., et al. The impact of a 17 day training period for an international championship on mucosal immune parameters in top-level basketball players and staff members. Eur J Oral Sci. 2008;116(5):431-437.
122 Fung T.T., Rimm E.B., Spiegelman D., Rifai N., Tofler G.H., Willett W.C., Hu F.B. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61-67.
123 Matsuzawa-Nagata N., Takamura T., Ando H., et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism. 2008;57(8):1071-1077.
124 Basdevant A., Ciangura C. [Obesity, a disease] Bull Acad Natl Med. 2010;194(1):13-20. discussion 20–4
125 Bulló M., Casas-Agustench P., Amigó-Correig P., Aranceta J., Salas-Salvadó J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. 2007;10(10A):1164-1172.
126 Epel E.S. Psychological and metabolic stress: a recipe for accelerated cellular ageing? Hormones (Athens). 2009;8(1):7-22.
127 Miranda-Garduno L.M., Reza-Albarran A. Obesity, inflammation and diabetes. Gac Med Mex. 2008;144(1):39-46.
128 Hofer T., Fontana L., Anton S.D., et al. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in WBCs and urine in humans. Rejuvenation Res. 2008;11(4):793-799.
129 Fontana L., Villareal D.T., Weiss E.P., et al. Caloric restriction or exercise: effects on coronary heart disease RFs. A RCT. Am J Physiol Endocrinol Metab. 2007;293(1):E197-E202.
130 Crujeiras A.B., Parra D., Milagro F.I., et al. Differential expression of ox stress and indflammation related genes in peripheral MNCs on response to a low-calorie diet: a nutrigenomics study. OMICS. 2008;12(4):251-261.
131 Aljada A., Mohanty P., Ghanim H., Abdo T., Tripathy D., Chaudhuri A., Dandona P. Increase in intranuclear nuclear factor B and decrease in inhibitor B in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. AJCN. 2004;79:682-690.
132 Devaraj S., Wang-Polagruto J., Polagruto J., et al. High-fat, energy-dense, fast-food-style breakfast results in an increase in oxidative stress in metabolic syndrome. Metabolism. 2008 Jun;57(6):867-870.
133 Esmaillzadeh A., Kimiagar M., Mehrabi Y., Azadbakht L., Hu F.B., Willett W.C. Dietary Patterns and Markers of Systemic Inflammation among Iranian Women. J Nutr. 2007;137:992-998.
134 Kechagias S., Ernersson A., Dahlqvist-Lundberg P., et al. Fast food based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut. 0, 2008. 200713179
135 Lopez-Garcia E., Schulze M.B., Fung T.T., Meigs J.B., Rifai N., Manson J.E., Hu F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029-1035.
136 Delgado-Lista J., Lopez-Miranda J., Cortes B., Perez-Martinez P., Lozano A., Gomez-Luna R., Gomez P., Gomez M.J., Criado J., Fuentes F., Perez-Jiminez F. Chronic dietary fat intake modifies the postprandial response of hemostatic markers to a single fatty test meal. AJCN. 2008;87:317-322.
137 Erridge C., Attina T., Spickett C.M., Webb D.J. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. AJCN. 2007;86(5):1286-1292.
138 Håversen L., Danielsson K.N., Fogelstrand L., Wiklund O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis. 2008 May 28. [Epub ahead of print]
139 Jakulj F., Zernicke K., Bacon S., et al. A high fat meal increases cardiovascular reactivity to psychological stress in healthy young adults. J Nutr. 2007;137:935-939.
140 Mohanty P., G.H., Hamouda W. Both lipid and protein intakes stimulated increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr. 75(767–72), 2002.
141 O’Keefe J., Bell D. The post-prandial hyperglycemia/hyperlipemia hypothesis: a hidden cardiovascular risk factor? Am J Cardiol. 2007;100:899-904.
142 Petersson H., Lind L., Hulthe J., Elmgren A., Cederholm T., Risérus U. Relationships between serum fatty acid composition and multiple markers of inflammation and endothelial function in an elderly population. Atherosclerosis. 2008 Jul 1. [Epub ahead of print]
143 Tschop M., Thomas G. Fat fuels insulin resistance through toll-like receptors. Nature. 2006;12(12):1359-1361.
144 Velloso L.A., Araújo E.P., de Souza C.T. Diet-Induced Inflammation of the Hypothalamus in Obesity. Neuroimmunomodulation. 2008 Sep 9;15(3):189-193.
145 Han N.S., Leka L.S., Lichtenstein A.H., Ausman L.M., Schaefer E.J., Meydani S.N. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hyper-cholesterolemia. J Lipid Res. 2002;43:445-452.
146 Mozaffarian D, Katan MB, Ascherio A, et al. Trans Fatty Acids and Cardiovascular Disease. NEJM, Volume 354:1601–1613
147 Parks E.J., Skokan L.E., Timlin M.T., Dingfelder C.S. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008;138(6):1039-1046.
148 Galgani J., Aguirre C., Diaz E. Acute effect of meal glycaemic index and glycaemic load on blood glucose and insulin response in humans. Nutr J. 2006;5:22-28.
149 Kallio P., Kolhmainen M., Laaksonen D.E., Pulkkinenen L., Atalay M., Mykkanen H. Uusitupa m, Poutanen K, Niskanen L. Inflammation markers are modulated by responses to diets differing in postprandial responses in individuals with the metabolic syndrome. AJCN. 2008;87:1497-1503.
150 Qi L., Hu F.B. Dietary glycaemic load, whole grains and systemic inflammation in diabetes: the epidemiological evidence. Curr Opin Lipidol. 2007;18(3):3-8.
151 Rayssiguier Y., Gueux E., Nowacki W., Rock E., Mazur A. High fructose consumption combined with low dietary magnesium intake may increase the incidence of the metabolic syndrome by inducing inflammation. Magnes Res. 2006;19(4):237-243.
152 Rebolledo O.R., Marra C.A., Raschia A., et al. Abdominal Adipose Tissue: Early Metabolic Dysfunction Associated to Insulin Resistance and Oxidative Stress Induced by an Unbalanced Diet. Horm Metab Res. 2008 Jul 11. [Epub ahead of print]
153 Vinayagamoorthi R., Bobby Z. Sridharmg. Antioxidants preserve redox balance and inhibit c-Jun-N-terminal kinase pathway while improving insulin signaling in fat-fed rats: evidence for the role of oxidative stress on IRS-1 serine phosphorylation and insulin resistance. J Endocrin. 2008;197:287-296.
154 Dickinson S., Hancock D.P., Petocz P., Ceriello A., Brand-Miller J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. AJCN. 2008;87(5):1188-1193.
155 Kallio P., Kolhmainen M., Laaksonen D.E., et al. Inflammation markers are modulated by responses to diets differing in postprandial responses in individuals with the metabolic syndrome. AJCN. 2008;87:1497-1503.
156 Nareika A., Im Y.B., Game B.A., et al. High glucose enhances lipopolysaccharide-stimulated CD14 expression in U937 mononuclear cells by increasing nuclear factor kappaB and AP-1 activities. J Endocrinol. 2008;196:45-55.
157 Beulens J.W., de Bruijne L.M., Stolk R.P., et al. High dietary glycaemic load and glycaemic index increase risk of cardiovascular disease among middle-aged women. J Am Coll Cardiol. 2007;50:14-21.
158 Galgani J., Aguirre C., Diaz E. Acute effect of meal glycaemic index and glycaemic load on blood glucose and insulin response in humans. Nutr J. 2006;5:22-28.
159 Ye X, Franco OH, Yu Z, et al. Associations of inflammatory factors with glycaemic status among middle-aged and older Chinese people. Clin Endocrinol (Oxf). Sep 2. [Epub ahead of print]
160 Yu S., Weaver V., Martin K., et al. The Effects of whole mushrooms during inflammation. BMC Immunol. 2009;10:12.
161 Florentino R.F. Symposium on diet, nutrition and immunity. Asia Pac J Clin Nutr. 2009;18(1):137-142.
162 Chun O.K., Chung S.-J., Claycombe K.J., Song W.O. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008;138:753-760.
163 Ock K.C., Chung S.-J., Claycombe K.J., Song W.O. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008;138:753-760.
164 Murakami A., Ohigashi H., Targeting N.O.X. INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121(11):2357-2363.
165 Middleton E.Jr., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673-751.
166 Calder P.C. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008 May 26. [Epub ahead of print
167 Yokoyama M., Origasa H., Matsuzaki M., et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090-1098.
168 Bo S., Ciccone G., Guidi S., Gambino R., Gentile L., Cassader M., Cavallo-Perin P., Pagano G. Diet or exercise: what is more effective in preventing or reducing metabolic alterations? Eur J Endocrinol. 2008 Sep 4. [Epub ahead of print]
169 Esposito K., Nappo F., Giugliano F., et al. Meal modulation of circulating interleukin 18 and Adiponectin concentrations in healthy subjects and in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2003;78:1135-1140.
170 King D.E., Egan B.M., Woolson R.F., Mainous A.G.3rd, Al-Solaiman Y., Yesri A. Effect of a high fibre diet vs a fiber supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502-506.
171 Ma Y., Griffith J., Chasan-Taber L., et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760-766.
172 Ma Y., Hébert J.R., Li W., Bertone-Johnson E.R., Olendzki B., Pagoto S.L., Tinker L., Rosal M.C., Ockene I.S., Ockene J.K., Griffith J.A., Liu S. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition. 2008 Jun 16.
173 Oi L., Meigs J.B., Liu S., Manson J.E., Mantzoros C., Hu F.B. Dietary fibers and glycaemic load, obesity, and plasma Adiponectin levels in women with type 2 diabetes. Diabetes Care. 2006;29:1501-1505.
174 Lichtenstein A.H., Appel L.J., Brands M., et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol. 2006;26(10):2186-2191.
175 Puglisi MJ, Vaishnav U, Shrestha S, Torres-Gonzalez, M, Wood, RJ, Volek, JS, Fernandez, ML Raisins and additional walking have distinct effects on plasma lipids and inflammatory cytokines. Lipids in Health and Disease. 7:14 doi:10.1186/1476–511X-7–14
176 Zern T.L., Fernandez M.L. Cardioprotective effects of dietary polyphenols. J Nutr. 2005;135:2291-2294.
177 Zern T.L., Wood R.J., Greene C., et al. Grape polyphenols exert a cardioproteictive effect in pre and post-menopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135:1911-1917.
178 Hodgson J.M., Ward N.C., Burke V., Beilin L., Puddy I.B. Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. J Nutr. 2007;137(2):363-367.
179 Hickling S., Hung J., Knuiman M., Divitini M., Beilby J. Are the associations between diet and C-reactive protein independent of obesity? Prev Med. 2008. doi:10.1016/j.ypmed.2008.02.007
180 Franco O.H., Bonneux L., de Laet C., Peeters A., Steyerberg E.W., Mackenbach J.P. The Polymeal: a more natural, safer, and probably tastier (than the Polypill) strategy to reduce cardiovascular disease by more than 25%. BMJ. 2004;329:1447-1450.
181 Krishnaswamy K. Traditional Indian spices and their health significance. Asia Pac J Clin Nutr. 2008;17(Suppl 1):265-268.
182 Tapsell L.C., Hemphill I., Cobiac L., et al. Health benefits of herbs and spices: the past, the present, the future. Med J Aust. 2006 Aug 21;185(Suppl 4):S4-S24.
183 Shukla Y., Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683-690.
184 Surh Y.J. Anti-tumor promoting potential of selected spice ingredients with anti-oxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40:1091-1097.
185 Gonzales A.M., Orlando R.A. Curcumin and resveratrol inhibit Nuclear Factor-kappaB-mediated cytokine expression in adipocytes. Nutr Metab (Lond). 2008;5(1):17.
186 Anand P., Sundaram C., Jhurani S., et al. Curcumin and cancer: an ‘old-age’ disease with an ‘age-old’ solution. Cancer Lett. 2008;267(1):133-164.
187 Kang J.-H., Kim C.-S., Han I.-S., Kawada T., Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages FEBS Letters. 2007;581:4389-4396.
188 Yoon J.H., Baek S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med J. 2005;46(5):585-596.
189 Waterhouse A.L. Wine phenolics. Ann N Y Acad Sci. 2002;957:21-36.
190 Watzl B., Bub A., Pretzer G., et al. Daily moderate amounts of red wine or alcohol have no effect on the immune system of healthy men. Eur J Clin Nutr. 2004;58(1):40-45.
191 Sanbongi C., Suzuki N., Sakane T. Polyphenols in chocolate, which have antioxidant activity, modulate immune functions in humans in vitro. Cell Immunol. 1997;177(2):129-136.
192 Faridi Z., Njike V.Y., Dutta S., Ali A., Katz D.L. Acute dark chocolate and cocoa ingestion and endothelial function: a randomised controlled crossover trial. AJCN. 2008;88:58-63.
193 Zemel M.B., Sun X. Dietary Calcium and Dairy Products Modulate Oxidative and Inflammatory Stress. J Nutr. 2008;138:1047-1052.
194 Lönnerdal B., Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr. 1995;15:93-110.
195 Yamauchi K., Wakabayashi H., Shin K., Takase M. Bovine lactoferrin: benefits and mechanism of action against infections. Biochem Cell Biol. 2006;84(3):291-296.
196 Shin K., Yamauchi K., Teraguchi S., et al. Antibacterial activity of bovine lactoferrin and its peptides against enterohaemorrhagic Escherichia coli O157:H7. Lett Appl Microbiol. 1998;26(6):407-411.
197 Shin K., Wakabayashi H., Yamauchi K., et al. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J Med Microbiol. 2005;54(Pt 8):717-723.
198 Buccigrossi V., de Marco G., Bruzzese E., et al. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr Res. 2007;61(4):410-414.
199 Suzuki Y.A., Shin K., Lönnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40(51):15771-15779.
200 Berlutti F., Schippa S., Morea C., et al. Lactoferrin downregulates pro-inflammatory cytokines upexpressed in intestinal epithelial cells infected with invasive or noninvasive Escherichia coli strains. Biochem Cell Biol. 2006;84(3):351-357.
201 Di Mario F., Aragona G., Dal Bò N., et al. Use of bovine lactoferrin for Helicobacter pylori eradication. Dig Liver Dis. 2003;35(10):706-710.
202 Paesano R., Torcia F., Berlutti F., et al. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Bioch Cell Biol. 2006;84(3):377-380.
203 Trümpler U., Straub P.W., Rosenmund A. Antibacterial prophylaxis with lactoferrin in neutropenic patients. Eur J Clin Microbiol Infect Dis. 1989;8(4):310-313.
204 Tanaka K., Ikeda M., Nozaki A., et al. Lactoferrin inhibits hepatitis C virus viremia in patients with chronic hepatitis C: a pilot study. Jpn J Cancer Res. 1999;90(4):367-371.
205 Yamauchi K., Hiruma M., Yamazaki N., et al. Oral administration of bovine lactoferrin for treatment of tinea pedis. A placebo-controlled, double-blind study. Mycoses. 2000;43(5):197-202.
206 King J.C.Jr., Cummings G.E., Guo N., Trivedi L., et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J Pediatr Gastroenterol Nutr. 2007;44:245-251.
207 Yaqoob P., Pala H.S., Cortina-Borja M., et al. Encapsulated fish oil enriched in a-tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions, Eur. J. Clin Invest. 2000;30:260-274.
208 Healy DA, Wallace FA, Miles EA, et al. The effect of low to moderate amounts of dietary fish oil
209 Simopoulos A.P. Essential fatty acids in health and chronic disease. AJCN. 1999;70:560S-569S.
210 Calder P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):197-202.
211 Badia E., Sacanella E., Fernadez-Sola J., Nicolas J.M., Antunez E., Rotilio D., de Gaetano G., Urbano-Marquez A., Estruch R. Decreased tumor-necrosis factor-induced adhesion of human monocytes to endothelial cells after moderate alcohol consumption. AJCN. 2004;80:225-230.
212 Blum S., Aviram M., Ben-Amotz A., Levy Y. Effect of a Mediterranean meal on post-prandial carotenoids, paraoxonase activity and C-reactive protein levels. Ann Nutr Metab. 2006;50:20-24.
213 Esmaillzadeh A., Kimiagar M., Mehrabi Y., et al. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007 Apr;137(4):992-998.
214 Giugliano D., Esposito K. Mediterranean diet and metabolic diseases. Curr Opin Lip. 2008;19:63-68.
215 Jenkins D.J., Josse A.R., Wong J.M., Nguyen T.H., Kendall C.W. The portfolio diet for cardiovascular risk reduction. Curr Atheroscler Rep. 2007;9(6):501-507.
216 Lopez-Garcia E., SchulzeMB Fung TT, Meigs J.B., Rifai N., Manson J.E., Hu F.B. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029-1035.
217 Nanri A., Yoshida D., Yamaji T., Misoue T., Takayanagi R. Kono, Dietary patterns and C-reactive protein in Japanese men and women. Am J Clin Nut. 2008;87(5):1488-1496.
218 Mena M.P., Sacanella E., Vazquez-Agell M., et al. Inhibition of circulating immune cell activation: a molecular anti-inflammatory effect of the Mediterranean diet. AJCN. 2009;89(1):248-256.
219 Kendall C.W., Jenkins D.J. A dietary portfolio: maximal reduction of low-density lipoprotein cholesterol with diet. Curr Atheroscler Rep. 2004;6(6):492-498.
220 Jenkins D., Kendall C., Josse A., et al. Almonds decrease post-prandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136:2987-2992.
221 Josse A., Kendall C., Augustin L., Ellis P., Jenkins D. Almonds and post-prandial glycemia—a dose-response study. Metabolism. 2007;56:400-404.
222 Salas-Salvadó J., Casas-Agustench P., Murphy M.M., et al. The effect of nuts on inflammation. 1:. Asia Pac J Clin Nutr. 2008;17(Suppl 1):333-336.
223 Shimizu M., Son D.O. Food-derived peptides and intestinal functions. Curr Pharm Des. 2007;13(9):885-895.
224 Cupisti A., Lorenzo G., D’Alessandro C., et al. Soy protein diet improves endothelial dysfunction in renal transplant patients. Nephrol Dial Transplant. 2007;22:229-234.
225 Chako B.K., Chandler R.T., Mundhekar A., et al. Revealing anti-inflammatory mechanisms of soy isoflavones by flow: modulation of leukocyte-endothelial interaction. Am J Physiol Heart Circ Physiol. 2005;289:H908-H915.
226 Ryan-Borchers T.A., Park J.S., Chew B.P., et al. Soy isoflavones modulate immune function in healthy post-menopausal women. AJCN. 2006;83(5):1118-1125.
227 Monobe M., Ema K., Kato F., et al. Immunostimulating activity of a crude polysaccharide derived from green tea extract. J Agric Food Chem. 2008;56(4):1423-1427.
228 Katiyer S.K. Skin photoprotection by green tea: antioxidant and immunomodulatory effects. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3(3):234-242.
229 Rowe C.A., Nantz M.P., Bukowski J.F., et al. Specific formulation of CS prevents cold and flu symptoms and enhances gamma, delta T-cell function: a RCT. J Am Coll Nutr. 2007;26(5):445-452.
230 Babu P.V., Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15(18):1840-1850.
231 Cao H., Kelly M.A., Kari F., Dawson H.D., Urban J.F.Jr., Coves S., Roussel A.M., Anderson R.A. Green tea increases anti-inflammatory tristetraprolin and decreases pro-inflammatory tumor necrosis factor mRNA levels in rats. J Inflamm (Lond). 2007 Jan 5;4:1.
232 Chen L., Zang H.Y. Cancer protective mechanisms of the green tea polyphenol (-) –epigallocatchin-3-gallate. Molecules. 2007;12:946-957.
233 Byrans J.A., Judd P.A., Ellis P.R. The effect of consuming instant black tea on postprandial plasma glucose and insulin concentrations in healthy humans. J Am Coll Nutr. 2007;26:471-477.
234 De Bacquer D., Clays E., Delanghe J., De Backer G. Epidemiological evidence for an association between habitual tea consumption and markers of chronic inflammation. Atherosclerosis. 2006;189(2):428-435.
235 Suganuma M, Sueoka E, Sueoka N, Okabe S, Fujiki H. Mechanisms of cancer prevention by tea poyphenols based on inhibition of TNF-alpha expression. Biofactors 200;13(1–4):67–72.
236 Bukowski J.F., Percival S.S. L-theanine intervention enhances human gammadeltaT lymphocyte function. Nutr Rev. 2008;66(2):96-102.
237 Jyonouchi H. Non-IgE mediated food allergy. Inflamm Allergy Drug targets. 2008;7(3):173-180.
238 Jyonoucchi H., Sun S., Itokazu N. Innate immunity associated with inflammatory responses with cytokine production against common dietary proteins in patients with ASD. Neuropsychobiologgy. 2002;46(2):76-84.
239 Diaz L.E., Montero A., Gonzalez-Gross M., et al. Influence of alcohol consumption on immumological status: a review. Eur J Clin Nutr. 2002;56(Suppl 3):S50-S53.
240 Magrone T., Candore G., Caruso C., et al. Polyphenols from red wine modulate immune responsiveness: biological and clinical significance. Curr Pharm Des. 2008;14(26):2733-2748.
241 Putics A., Vegh E.M., Csermely P., et al. Resveratrol induces the heat-shock response and protects human cells from severe heat stress. Antiox Redox Signal. 2008;10(1):65-75.
242 Falchetti R., Fuggetta M.P., Lanzilli G., et al. Effects of resveratrol on human immune cell function. Life Sci. 2001;70(1):81-96.
243 Chandra R.K. Nutrition and the immune system: an introduction. AJCN. 1997;66(2):260S-263S.
244 Harbige L.S. Nutrition and immunity with emphasis on infection and AI disease. Nutr Health. 1996;10(4):285-312.
245 Ferencik M., Ebringer L. Modulatory effects of Se and Zn on the immune system. Folia Microbiol (Praha). 2003;48(3):417-426.
246 Maggini S., Wintergerst E.S., Beveridge S., et al. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(Suppl 1):S28-S35.
247 Wintergerst E.S., Maggini S., Hornig D.H. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51(4):301-323.
248 Tsuji M., Suzuki K., Kinoshita K., et al. Dynamic interactions between bacteria and immune cells leading to IgA synthesis. Semin Immunol. 2008;20(1):59-66.
249 Van Eden W., Van der Zee R., Van Kooten P., et al. Balancing the Immune System: Th1 and Th2. Ann Rheum Dis. 2002;61(Suppl 2):25-28.
250 Mason K.L., Huffnagle G.B., Noverr M.C., et al. Overview of Gut immunology. Adv Exp Med Biol. 2008;635:1-14.
251 Forschelli M.L., Walker W.A. The role of GALT and mucosal defence. Br J Nutr. 2005;93:S41-S48.
252 Ogra P.L., Welliver R.C.Sr. Effects of early environment on mucosal immunologic homeostasis, subsequent bimmune responses and disease outcome. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:145-181.
253 Pai R., Kang G. Microbes in the Gut: A digestable account of host-symbiont interactions. Indian J Med Res. 2008;128(5):587-594.
254 Walker W.A. Mechanisms of action of probiotics. Clin Infect Dis. 2008;46(Suppl 2):S87-S91.
255 Suzuli K., Fagarasan S. How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008. [Epub ahead of print]
256 Mora J.R., von Andrian U.H. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009;21(1):28-35.
257 Bodera P. Influence of probiotics on the human immune system (GALT). Recent Pat Inflamm Allergy Drug Discov. 2008;2(2):149-153.
258 Bodera P., Chcialowski A. Immunomodulatory effect of probiotic bacteria. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):58-64.
259 Singh V., Singh K., Amdekar S., et al. Innate and specific gut-associated immunity and microbial interference. FEMS Immunol Med Microbiol. 2009;55(1):6-12.
260 Bellanti J.A., Zeligs B.J., Malka-Rais J., et al. Abnormalities of Th1 function in non IgE food allergy, coeliac disease and ileal lymphonodular hyperplasia: a new relationship? Ann Allergy Asthma Immunol. 2003;90(6 Suppl. 3):84-89.
261 Lin X.P., Magnusson J., Ahlstedt S., et al. Local allergic reaction in food-hypersensitive adults despite a lack of systemic food-specific IgE. J allergy Clin Immun. 2002;109(5):879-887.
262 Seksik P., Dray X., Sokol H., et al. Is there any place for alimentrary probiotics, prebiotics or synbiotics for patients with IBD? Mol Nutr Food Res. 2008;52(8):906-912.
263 Famularo G., Mosca L., Minisola G., et al. Probiotic lactobacilli: a new perspective for the treatment of IBD. Curr Pharm Des. 2003;9(24):1973-1980.
264 Chandran P., Satthaporn S., Robins A., et al. IBD: dysfunction of GALT and gut bacteria flora (II). Surgeon. 2003;1(3):125-136.
265 Mora J.R. Homimg imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008;14(2):275-289.
266 Mach T. Clinical usefulness of probiotics in IBD. J Physiol Pharmacol. 2006;57(Suppl 9):23-33.
267 Guarner F. Inulin and oligofructose: impact on intestinal diseases and disorders. Br J Nutr. 2005;93(Suppl 1):S61-S65.
268 Heilpern D., Szilagyi A. Manipulation of intestinal microbial flora for therapeutic benefit in IBD: Review of clinical trials of probiotics, prebiotics and synbiotics. Rev Recent Clin Trials. 2008;3(3):167-184.
269 Bohm S.K., Kruis W. Probiotics: do they help to control intestinal inflammation? Ann NY Acad Sci. 2006;1072:339-350.
270 Vitetta L., Sali A. Probiotics, prebiotics and gastrointestinal health. Medicine Today. 2008;9(9):65-70.
271 Goldin B.R., Gorbach S.L. Clinical indications for probiotics: an overview. Clin Infect Dis. 2008;46(Suppl 2):S96-S100.
272 Santosa S., Farnworth E., Jones P.J. Probiotics and their potential health claims. Nutr Rev. 2006;64(6):265-274.
273 Gosselink M.P., Schouten W.R., van Lieshout L.M., et al. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004;47(6):876-884.
274 Garrote J.A., Gomez-Gonzalez E., Bernardo D., et al. Coeliac disease pathogenesis: the proinflammatory cytokine network. J Pediatr Gastrointerol Nutr. 2008;47(Suppl 1):S27-S32.
275 du Toit G., Meyer R., Shah N., et al. Identifying and managing cow’s milk protein allergy. Arch Dis Child Educ Pract Ed. 2010;95(5):134-144.
276 Mazzatti D.J., Uciechowski P., Hebel S., et al. Effects of long-term zinc supplementation and deprivation on gene expression in human THP-1 mononuclear cells. J Trace Elem Med Biol. 2008;22(4):325-336.
277 Prasad A.S. Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr. 2009;28(3):257-265.
278 Heyland D.K., Jones N., Cvijanovich N.Z., et al. Zinc supplementation in critically ill patients: a key pharmaconutrient? JPEN J Parenter Enteral Nutr. 2008;32(5):509-519.
279 Mocchegiani E., Malavolta M., Muti E., et al. Zinc, metallothioneins and longevity: inter-relationships with niacin and selenium. Curr Pharm Des. 2008;14(26):2719-2732.
280 Franklin R.B., Costello L.C. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem. 2009 Jan 21. [Epub ahead of print]
281 Prassad A.S. Zinc in Human Health: Effect of Zinc on Immune Cells. Mol Med. 2008;14(5–6):353-357.
282 Prasad A.S. Zinc: Mechanisms of Host Defence. AJN. 2007;137:1345-1349.
283 Overbeck S., Uciechowski P., Ackland M.L., et al. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol. 2008;83:368-380.
284 Haase H., Mazzatti D.J., White A., et al. Differential gene expression after zinc supplementation and deprivation in human leukocyte subsets. Mol Med. 2007;13(7–8):362-370.
285 Haase H., Overbeck S., Rink L. Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol. 2008;43(5):394-408.
286 Wang L., Wildt K.F., Castro E., et al. The zinc-finger transcription factor Zbtb7b represses CD*-lineage gene expression in peripheral CD4+ T-cells. Immunity. 2008;29(6):876-887.
287 Hermann-Kleiter N., Gruber T., Lutz-Nicoladoni C., et al. The nuclear orphan receptor NR2F6 suppresses lymphocyte acrivation and T-helper 17-dependent autoimmunity. s.l.:. Immunity. 2008;29(2):205-216.
288 Haase H., Ober-Blobaum J.L., Engelhardt G., et al. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol. 2008;181(9):6491-6502.
289 Prasad A.S. Clinical, immunological, anti-inflammatory and anti-oxidant roles of zinc. Exp gerontol. 2008;43(5):370-377.
290 Uciechowski P., Kahmann L., Plumakers B., et al. Th1 and Th2 cell polarisation increases with ageing and is modulated by zinc supplementation. Exp Gerontol. 2008;43(5):493-498.
291 Varin A., Larbi A., Dedoussis G.V., et al. In vitro and in vivo effects of zinc on cytokine signalling in human T-cells. Exp Gerontol. 2008;43(5):472-482.
292 Mariani E., Neri S., Cattini L., et al. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK-cell function in healthy elderly: interactive influence of +647 MT1a and _174 IL-6 polymorphic alleles. Exp gerontol. 2008;43(5):462-471.
293 Haase H., Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20(3–4):579-585.
294 Finamore A., Massimi M., Conti Devirgillis L., et al. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J Nutr. 2008;138(9):1664-1670.
295 Vasto S., Mocchegiani E., Malavolta M., et al. Zinc and the inflammatory/immune response in ageing. Ann N Y Acad Sci. 2007;1100:111-122.
296 Scrimgeour A.G., Lukaski H.C. Zinc and diarrhoeal disease: current status and future perspectives. Curr Opin Clin Nutr Metab care. 2008;11(6):711-717.
297 Shen H., Oesterling E., Stromberg A., et al. Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappa B and PPAR signalling. J Am Coll Nutr. 2008;27(5):577-587.
298 Plum L.M., Rink L., Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Pub Health. 2010;7(4):1342-1365.
299 Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of Viamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85-94.
300 Lukacik M., thomas R.L., Aranda J.V. A meta-analysis of the effects of oral zinc in the treatment of acute and persistent diarrhoea. Pediatrics. 2008;121(2):326-336.
301 Walker C.L., Black R.E. Zinc for the treatment of diarrhoea: effect on diarrhoea morbidity, mortality and incidence of future episodes. Int J Epidemiol. 39(Suppl. 1), 2010. i63–9
302 Cuevas L.E., Koyanagi A. Zinc and Infection: A review. Ann Trop Paediatr. 2005;25(3):149-160.
303 McElroy B.H., Miller S.P. Effectiveness of zinc gluconate glycine lozenges (Cold-Eeze) against the common cold in school-aged subjects: a retrospective chart review. Am J Ther. 2002;9(6):472-475.
304 Silk R., LeFante C. Safety of zinc gluconate glycine (Cold-Eeze) in a geriatric population: a randomised placebo-controlled double-blind trial. Am J Ther. 2005;12(6):612-617.
305 McElroy B.H., Miller S.P. An open-label single-centre, phase IV clinical study of the effectiveness of zinc gluconate glycine lozenges (Cold-Eeze) in reducing the duration and symptoms of the common cold in school-aged subjects. Am J Ther. 2003;10(5):424-429.
306 Marshall S. Zinc gluconate and the common cold. review of RCTs. Can Fam Physician. 1998;44:1037-1042.
307 Garland M.L., Hagmeyer K.O. The role of zinc lozenges in treatment of the common cold. Ann Pharmacother. 1998;32(1):63-69.
308 Eby GA. Zinc ion availability – the determinant of efficacy in zinc lozenge treatment of common colds. J Antimicrobial Chemether. 1997;40(4):483-493.
309 Khalili H., Soudbakhsh A., Hajiabdolbaghi M., et al. Nutritional status and serum zinc and selenium levels in Iranian HIV infected individuals. BMC Infect Dis. 2008;8:165.
310 Becker Y. The changes in Th1 and Th2 cytokine balance during HIV-1 infection are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers-a review and hypothesis. Virus Genes. 2004;28(1):5-18.
311 Zhu B., Symonds A.L., Martin J.E., et al. Early growth response gene (Egr-2) controls the self-tolerance of T-cells and prevents the development of lupus-like auto-immune disease. J Exp Med. 2008;2005:2295-2307.
312 Rathinam C., Lassmann H., Mengel M., et al. Transcription factor Gfi1 restricts B-cell mediated autimmunity. J Immunol. 2008;181(9):6222-6229.
313 Wenzlau J.M., Juhl K., Yu L., et al. The cation efflux transporter ZnT8(Slc30A8) is a major auto-antigen in human Type 1 DM. Proc Natl Acad Sci USA. 2007;104(43):17040-17045.
314 Knip M., Siljander H. Auto-immune mechanisms in Type 1 DM. Autoimmun Rev. 2008;7(7):550-557.
315 Egefjord L., Petersen A.B., Rungby J. Zinc, alpha cells and glucagon secretion. Curr Diabetes Rev. 2010;6(1):52-57.
316 Gyulkhandanyan A.V., Lu H., Lee S.C., et al. Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem. 2008;283(15):10184-10197.
317 Moro J.R., Iwata M., von Andriano U.H. Vitamin effects on the immune system: vitamin A and D take centre stage. Nat Rev Immunol. 2008;8(9):685-698.
318 Pino-lagos K., Benson M.J., Noelle R.J. Retinoic acid in the immune system. Ann N Y Acad Sci. 2008;1143:170-187.
319 Kim C.H. Roles of retinoic acid in induction of immunity and immune tolerance. Endocr Metab Immune Disord Drug Targets. 2008;8(4):289-294.
320 Bos J.D., Spuls P.I. Topical treatments in psoriasis: today and tomorrow. Clin dermatol. 2008;26(5):432-437.
321 Strober W. vitamin A rewrites the ABCs of oral tolerance. Mucosal Immunol. 2008;1(2):92-95.
322 Ross A.C. Vit A supp and retinoic acid treatment in the regulation of antibody responses in vivo. Vitamin Horm. 2007;75:197-222.
323 Wiysonge C.S., Shey M.S., Sterne J.A., et al. Vit A supp for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. (4):2005. CD003648
324 Mehta S., Fawzi W. Effects of vitamins, inc Vit A, on HIV/AIDS patients. vitamin Horm. 2007;75:355-383.
325 Ahmad S.M., Haskell M.J., Raqib R., et al. Markers of innate immune fn are associated with vitamin A stores in men. J Nutr. 2009;139(2):377-385.
326 Albers R., Bol M., Bleumink R., et al. Effects of suppl with vitamins A, C, E, Se and Zn on immune function in a murine sterilised model. Nutrition. 2003;19(11–12):940-946.
327 Hoffman P.R., Berry M.J. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52(11):1273-1280.
328 Hoffman P.R. Mechanisms by which selenium influences immune responses. Arch Immunol Ther Exp. 2007;55(5):289-297.
329 Beck M.A., Levander O.A., Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133(5 Suppl. 1):1463S-1467S.
330 Rayman M.P. The argument for increasing selenium intake. Proc Nutr Soc. 2002;61(2):203-215.
331 Rayman M.P. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64(4):527-542.
332 Combs G.F.Jr., Gray W.P. Chemopreventative agents: Se. Pharmacol Ther. 1998;79(3):179-192.
333 Rayman M.P. The importance of selenium to human health. Lancet. 2000;356(9225):233-241.
334 Luty-Frackiewicz A. The role of selenium in cancer and viral infection prevention. Int J Occup Med Environ Health. 2005;18(4):305-311.
335 Hurwitz B.E., Klaus J.R., Llabre M.M., et L. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomised controlled trial. Arch Intern Med. 2007;167:148-154.
336 Naithani R. Organoselenium compounds in cancer chemoprevention. Mini Rev Med Chem. 2008;8(7):657-668.
337 Combs G.F.Jr., Clark L.C., Turnball B.W. An analysis of cancer prevention by selenium. Biofactors. 2001;14(1–4):153-159.
338 Sinha R., El-Bayoumy K. Apoptosis is a critical cellular event in cancer chemoprevention by selenium compounds. Curr Cancer Drug Targets. 2004;4(1):13-28.
339 Rikiishi H. Apoptotic cellular events for selenium compounds involved in cancer prevention. J Bioenerg Biomembr. 2007;39(1):91-98.
340 Deruelle F., Baron B. vitamin C: Is supplementation necessary for optimal health? J Altern Comp Med. 2008;14(10):1291-1298.
341 Padayatty S.J., Katz A., Wang Y., et al. Vit C as an anti-oxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):18-35.
342 Strohle A., Hahn A. vitamin C and immune function. Med Monatsschr Pharm. 2009;32(2):49-54.
343 Douglas R.M., Hemilia H., Chalker E., et al. Vit C for preventing and treating the common cold. Cochrane Database Syst Rev. (3):2007. CD000980
344 Sureda A., Tauler P., Aguilo A., et al. Influence of an anti-oxidant vitamin enriched drink on pre and post lymphocyte antiox system. Ann Nutr Metab. 2008;52(3):233-240.
345 Nakhostin-Roohi B., Babaei P., Rahmani-Nia F., et al. Effect of Vit C suppl on lipid peroxidation, muscle damage and inflammation after 30 min exercise at 75%VO2max. J Sports Med Phys Fitness. 2008;48(2):217-224.
346 Takeshita Y., Katsuki Y., Katsuda Y., et al. Vit C reversed malfunction of peripheral blood-derived MNCs in smokers through antiox properties. Circ J. 2008;72(4):654-659.
347 Van Hoydonck P.G., Schouten E.G., Manuel-Keenoy B., et al. Does Vit C supp influence the levels of circulating oxidised LDL, slCAM-1, sVCAM-1 and vWF-antigen in healthy male smokers? Eur J Clin Nutr. 2004;58(12):1587-1593.
348 Moyad M.A., Combs M.A., Vrablic A.S., et al. Vit C metabolites, independent of smoking status, sig enhance leukocyte, but not plasma ascorbate concentrations. Adv Ther. 2008;25(10):995-1009.
349 Holick M.F., Chen T.C. Vit D Deficiency: a world-wide problem with health consequences. AJCN. 2008;87(4):1080S-1086S.
350 Holick M.F. Sunlight and Vit D for bone health and prevention of AI diseases, cancers and CVD. AJCN. 2004;80(Suppl 6):1678S-1688S.
351 Holick M.F. Vit D: Importance in the prevention of cancers, Type 1 DM, heart disease and osteoporosis. AJCN. 2004;79(3):362-371.
352 Kurylowicz A., Bednarczuk T., Nauman J. The influence of Vit D deficiency on cancers and AI disease devt. Endokrynol Pol. 2007;58(2):140-152.
353 Wagner C.L., Taylor S.N., Hollis B.W. Does Vit D make the world go round? Breastfeed Med. 2008;3(4):239-250.
354 Bruce D., Ooi J.H., Yu S., Cantorna M.T. Vitamin D and host resistance to infection? Putting the cart in front of the horse. Exp Biol Med. 2010;235(8):921-927.
355 Holick M.F. Vitamin D: extraskeletal health. Endocrinol Metab Clin North Am. 2010;39(2):381-400.
356 Holick M.F. Vitamin D: a D-Lightful health perspective. Nutr Rev. 2008;66(10 Suppl. 2):S182-S194.
357 Holick M.F. vitamin D: important for prevention of OP, CVD, IDDM, AI diseases and some cancers. South Med J. 2005;98(10):1024-1027.
358 DeLuca H.F. Evolution of our understanding of vitamin D. Nutr Rev. 2008;66(10 Suppl. 2):S73-S87.
359 Reichrath J. The challenge resulting from + and – effects of sunlight: how much solar UV exposure is appropriate to balance between risks of vitamin D deficiency and skin cancer? Prog Biophys Mol Biol. 2006;92(1):9-16.
360 Peyrin-Biroulet L., Oussalah A., Bigard M.A. Crohn’s Disease: the hot hypothesis. Med Hypotheses. 2009;73(1):94-96.
361 Kamen D.L., Cooper G.S., Bouali H., et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmunity Reviews. 2006;5:114-117.
362 Melamid M.L., Michos E.D., Post W., et al. 25(OH)D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629-1637.
363 Holick M.F. Vit D and sunlight: strategies for Ca prevention and other health benefits. Clin J Am Soc Nephrol. 2008;3(5):1548-1554.
364 Holick M.F. vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol. 2006;92(1):49-59.
365 Mullin G.E., Dobs A. vitamin D and its role in Ca and immunity: a prescription for sunlight. Nutr Clin Pract. 2007;22(3):305-322.
366 Adams J.S., Ren S., Liu P.T., et al. vitamin D directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289-4295.
367 Enioutina E.Y., Bareyan D., Daynes R.A. TLR-induced local metabolism of D3 plays an important role in the diversification of adaptive immune responses. J Immunol. 2009;182(7):4296-4305.
368 van Etten E., Stoffels K., Gysemans C., et al. Regulation of Vit D homeostasis: implications for the immune system. s.l.: Nutr Rev. 2008;66(10 Suppl. 2):S125-S134.
369 Holick M.F. The vitamin D deficiency pandemic and consequences for nonskeletal health. Mol Aspects Med. 2008;29(6):361-368.
370 Autler P., Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Int Med. 2007;167:1730-1737.
371 Ardiszone S., Cassinotti A., Trabattoni D., et al. Immunomodulatory effects of vitamin D3 on Th1/Th2 cytokines in IBD: an in vitro study. Int J Immunopathol Pharmacol. 2009;2291):63-71.
372 Cantorna M.T., Zhu Y., Froicu M., et al. Vit D status, 1, 25(OH)D3 and the Immune system. AJCN. 2004;80(6 Suppl):1717S-1720S.
373 Cho W.C., Leung K.N. In vitro and in vivo immunomodulating and imunorestorative effects of A. membranaceus. J Ethnopharmacol. 2007;113(1):132-141.
374 Braun L. Astragalus membranaceous. JCM. 2008;7(3):44-47.
375 Liu Q.Y., Yao Y.M., Zhang S.W., Sheng Z.Y. Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB(low) DCs in vitro. J Ethnopharmacol. 2010 Jul 8.
376 Tan B.K. Immunomodulatory and antimicrobial effects of some TCM herbs: a review. Curr Med Chem. 2004;11(11):1423-1430.
377 Shao BM, Xu W, Dai H, et al. A study on the immune reseptors for polysaccharides from the roots of A. mem, a TCM herb.
378 Brush J., Mendenhall E., Guggenheim A., et al. The effect of Echinacea, Astragalus and Glycyrrhisa on CD69 expression and immune cell activation in humans. Phytother res. 2006;20(8):687-695.
379 Wei H., Sun R., Xiao W., et al. TCM Astragalus reverses predominance of Th2 cytokines and their up-stream transcript factors in lung cancer patients. Oncol Rep. 2003;10(5):1507-1512.
380 Yang Y., Wang L.D., Chen Z.B. Effects of A. membranaceus on TH cell subset function in children with recurrent tonsillitis. Zhongguo Dang Dai Er Ke Za Zhi. 2006;8(5):376-378.
381 Wang G., Liu C.T., Wang Z.L., et al. Effects of A. membranaceus inproducing T-helper cell type 1 polarisation and IFNgamma production by upregulating T-bet expression in patients with asthma. Chin J Integr Med. 2006;12(4):262-267.
382 Shan H.H., Wang K., Li W., et al. A. membranaceus prevents airway hyperreactivity in mice related to Th2 inhibition. J Ethnopharmacol. 2008;116(2):363-369.
383 Du Q., Chen Z., Zhou L.F., et al. Inhibitory effects of astragaloside IV on ovalbumin-induced chronic experiemental asthma. Can J Physiol Pharmacol. 2008;86(7):449-457.
384 Mao S.P., Cheng K.L., Zhou Y.F. Modulatory effects of A. membranaceus on Th1/Th2 cytokine in patients with herpes simplex keratitis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(2):121-123.
385 Chen W., Li Y.M., Yu M.H. Astragalus polysaccharides: an effective treatment for diabetes prevention in NOD mice. Exp Clin Endocrinol Diabetes. 2008;116(8):468-474.
386 Li R.J., Qiu S.D., Chen H.X., et al. Effect of A polysaccharide on pancreatic cell mass in type I diabetic mice. Zhongguo Zhong Yao Za Zhi. 2007;32(20):2169-2173.
387 Li R.J., Qiu S.D., Chen H.X., et al. The Immunotherapeutic effects of A polysaccharide in Type I diabetic mice. Biol Pharm Bull. 2007;30(3):470-476.
388 Li R.J., Qiu S.D., Chen H.X., et al. Immunomodulatory effects of A polysaccharide in diabetic mice. Zhong Xi Yi Jie He Xue Bao. 2008;6(2):166-170.
389 Cho W.C., Leung K.N. In vitro and in vivo anti-tumour effects of AM. Cancer Lett. 2007;252:43-54.
390 Block K.I., Mead M.N. Immune system effects of echinacea, ginseng and astragalus: a review. Integr Cancer Ther. 2003;2(3):247-267.
391 Dong J., Gu H.L., Ma C.T., et al. Effects of large dose of AM on the dendritic cell induction of peripheral mononuclear cell and antigen -presenting ability of dendritic cells in children with acute leukaemia. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25(10):872-875.
392 Taixang W., Munro A.J., Guanjian L. Chinese medical herbs for chemotherapy side-effects in colorectal cancer patients. Cochrane database Syust Rev. (1):2005. CD004540
393 McCulloch M., See C., Shu X.J., et al. Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small-cell lung cancer: meta-analysis of randomised trials. J Clin Oncol. 2006;24(3):419-430.
394 Su L., Mao J.C., Gu J.H. Effect of IV drip infusion of CTX with high-dose Astraglus injection in treating lupus nephritis. Zhong Xi Yi Jie He Xue Bao. 2007;5(3):272-275.
395 Hao Y., Qiu Q.Y., Wu J. Effect of Astragalus polysaccharides in promting neutophil-vascular endothial cell adhesion and expression of related adhesive molecules. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(5):427-430.
396 Lee S.J., Oh S.G., Seo S.W., et al. Oral administration of A. membranaceus inhibits the development of DNFB-induced dematitis in NC/Nga mice. Biol Pharm Bull. 2007;30(8):1468-1471.
397 Prieto J.M., Recio M.C., Giner R.M., et al. Influence of traditional Chinese anti-inflammatory medicinal plants on leukocyte and platelet functions. J Pharm Pharmacol. 2003;55(9):1275-1282.
398 Xu H.D., You C.G., Zhang R.L., et al. Effects of A polysaccharides and astragalosides on the phagocytosis of M. tuberculosis by macrophages. J Int Med Res. 2007;35(1):84-90.
399 Nergard C.S., Solhaug V. Cranberries for prevention of recurrent urinary tract infections. Tidsskr Nor Laegoforen. 2009;129(4):303-304.
400 Feldman M., Weiss E., Shemesh, et al. Crnberry constituents affect fructosyltransferase expression in Streptococcus mutans Altern Ther Health Med. 2009;15(2):32-38.
401 Liu Y., Gallardo-Moreno A.M., Pinzon-Arango P.A., et al. Cranberry changes the physicochemical surface properties of E. coli and adhesion with uroepithelial cells Colloids Surf B Biointerfaces. 2008;65(1):35-42.
402 Eydelnant I.A., Tufenkji N. Cranberry derived proanthocyanidins reduce bacterial adhesion to selected biomaterials Langmuir. 2008;24(18):10273-10281.
403 Magarinos H.L., Sahr C., Selaive S.D., et al. Prikl Biokhim Mikrobiol. 2008;44(3):333-336.
404 Lavigne J.P., Bourg G., Combescure C., et al. In vitro and in vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberyy) capsules Clin Microbiol Infect. 2008;14(4):350-355.
405 Jepson R.G., Craig J.C. Cranberries for preventing urinary tract infections. Cochrane Database Systematic Reviews. (1):2008 Jan 23. CD001321
406 Jepson R.G., Craig J.C. A systematic review of the evidence for cranberries and blueberries in UTI prevention Mol Nutr Food Res. 2007;51(6):738-745.
407 McMurdo M.E., Argo I., Phillips, et al. J Antimicrob Chemother. 2009;63(2):389-395.
408 Wing D.A., Rumney P.J., Preslicka C.W., et al. Daily cranberry juice for the prevention of asymptomatic bacteriura in pregnancy: a randomised, controlled pilot study J Urol. 2008;180(4):1367-1372.
409 Linde k, Barrett B., Wolkart K. Echinacea for preventing and treating the common cold Cochrane Database Systematic Reviews. (1):2006 Jan 25. CD000530
410 Barrett B. Medicinal properties of Echinacea: a critical review. Phytomedicine. 2003;10:66-86.
411 Vonau B., Chard S., Mandalia S., Wilkinson D., Barton S.E. Does the extract of the plant Echinacea purpurea influence the clinical course of recurrent gnital herpes? Int STD AIDS. 2001;12:154-158.
412 Freeman C., Spelman L. A critical evaluation of drug interactions with Echinacea spp. Mol Nutr Food Res. 2008;52(7):789-798.
413 Ragupathi G., Yeung K.S., Leung P.C., et al. Evaluation of widely consumed botanicals as immunological adjuvants. Vaccine. 2008;26:4860-4865.
414 Braun L., Honey. J Complem Med. 2003;V2 N1:54-56.
415 Lusby P.E., Coombes A., Wilkinson J.M. J. Wound Ostomy Continence Nurse. 2002;29(6):296-300.
416 Blair S. Honey and drug resistant pathogens. Paper presented at the Joint Scientific Meeting of the Australian Society for Microbiology, Cairns. July 2000:8-13.
417 Taormine Pj, Niermira B.A., Beuchat L.R., et al. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power Int J Food Microbiology. 2001;69(3):217-225.
418 Steinberg D., Kaine G., Gedalia I. Antibacterial effect of propolis and honey on oral bacteria. Am J Dent. 1996;9(6):236-239.
419 Jull A.B., Rodgers A., Walker N. Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews. 2008. Oct 8; (4):CD 05083
420 Jull A.B., Rodgers A., Paraq V., et al. Randomized trial of honey-impregnated dressing for venous ulcers Br J Surg. 2008;95(2):175-182.
421 Robson V., Dodd S., Thomas D. Standardised antibacterial honey (Medihoney) with standard therapy in wound care: a randomised clinical trial. J Adv Nurs. 2009;65(3):565-575.
422 Gethin G., Cowman S. Bacteriological changes in sloughy venous leg ulcers treated with manuka honey or hydrogel: an RCT. Journal of wound care. 2008;17(6):241-244.
423 Gethin G., Cowman S. Manuka honey versus hydrogel- a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers J Clin Nurs. 2009;18(3):466-474.
424 Gethin G., Cowman S., Coroy R. The impact of Manuka honey dressings on the surface pH of chronic wounds Int Wound J. 2008;5(2):185-194.
425 Evans J., Flavin S.. Honey: a guide for healthcare professionals Br J Nurs, 2008 Aug 14-Sep 10;17(15); S24, S26, S28–30
426 Hernandez-Reif M., Field T., Ironson G., et al. NK-cells and lymphocytes increase in women with breast cancer following massage therapy. Int J Neurosci. 2005;115(4):495-510.
427 Hernandez-Reif M., Ironson G., Field T., et al. Breast cancer patients have improved immune and neuroendocrine functions following massage therapy. J Psychosom Res. 2004;57(1):45-52.
428 Billhult A., Lindholm C., Gunnarsson R., et al. The effect of massage on cellular immunity, endocrine and psycholigical factors in women with BC- a RCT. Auton Neurosci. 2008;140(1–2):88-95.
429 Cabioglu M.T., Cetin B.E. Acupuncture and immune modulation. Am J Chin Med. 2008;36(1):25-36.
430 Peng G. Acupuncture and innate immunity. Zhen Ci Yan Jiu. 2008;33(1):49-52.
431 Du J. The messengers from PNS to CNS: involvement of neurotrophins and cytokines in the mechanisms of A. Zhen Ci Yan Jiu. 2008;33(1):37-40.
432 Kavoussi B., Ross B.E. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6(3):251-257.
433 Yamaguchi N., Takahashi T., Sakuma M., et al. A regulates leukocyte subpopulations in human peripheral blood. Evid Based Comp Altern Med. 2007;4(4):447-453.
434 Wang J.R., Chen X.R., Zhang Q., et al. Effect of moxibustion on immunological fn in the patient of AIDS in spleen-kidney yang deficiency. Zhongguo Zhen Jiu. 2007;27(12):892-894.