Infection and Inflammation
External Ear
Clinical Presentation and Etiologies: Children present with variable degrees of pain, as well as secretions that can be serous early and progress to frank purulence. External otitis is associated with swimming and can be caused by a number of pathogens, including fungi. Otomycosis is more common in the postsurgical ear and presents in a similar fashion to a bacterial infection.1
Imaging: The diagnosis of otitis externa should be made clinically; however, the diagnosis can be made in the evaluation for possible mastoiditis. Soft tissue swelling and inflammation of the external canal in isolation, without evidence of involvement of the parotid or mastoids, may be demonstrated on computed tomography (CT) (e-Fig. 11-1). The acute inflammatory stage can be classified from mild to severe, and when infection spreads to surrounding tissues, the condition is then termed malignant or necrotizing otitis externa.2 Langerhans cell histiocytosis can infiltrate the soft tissue of the external canal but is more often associated with bony involvement (Box 11-1).
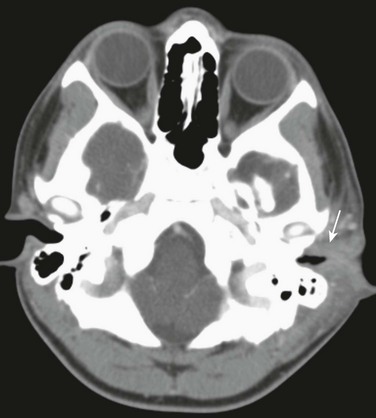
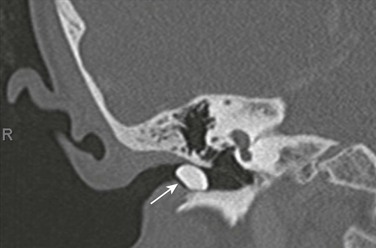
e-Figure 11-1 Otitis externa in a 13-year-old with ear pain and soft tissue swelling.
Axial postcontrast computed tomography through the level of the external canal shows extensive inflammation surrounding the left external canal (arrow) with normal aeration of the adjacent mastoids and a normal parotid gland (not shown).
Malignant (Necrotizing) Otitis Externa
Clinical Presentation and Etiologies: Usually, the presentation is acute, with associated systemic symptoms of fever and leukocytosis. Pseudomonas aeruginosa is the most common cause of this severe infection of the external canal. Diabetes is a common predisposing condition; however, any condition resulting in immunodeficiency can be associated with malignant otitis externa.3–5 Children are reported to have better outcomes compared with adults.
Imaging: CT shows more extensive inflammation within the external canal and can demonstrate bony destruction. Magnetic resonance imaging (MRI) with gadolinium demonstrates osteomyelitis, with increased signal on fat-suppressed T2-weighted images and abnormal enhancement, within bone. MRI is useful in confirming central skull base involvement, especially in the presence of multiple cranial nerve palsies, and in evaluating for intracranial complications. Facial nerve paralysis, intracranial extension, and additional cranial nerve involvement may result.5
Single photon emission CT (SPECT) technetium-99m bone scan is an effective way to confirm or exclude bone involvement in a patient whose condition evokes high clinical suspicion, and bone scan can be positive in the absence of osseous destruction on CT.6
Treatment: Several weeks of intravenous antibiotics are required for adequate treatment. High-resolution CT helps assess the extent of inflammation and surrounding bony involvement and is used to stratify patients into nonsevere and severe groups, with the latter offered early surgical intervention and debridement.7
Other Lesions Encountered Within the External Auditory Canal
Acquired cholesteatoma may present as debris or a mass within the external auditory canal (e-Fig. 11-2), as can keratosis obturans.8 Exostoses of the external canal may result from chronic inflammation caused by prolonged exposure to water. These lesions tend to be bilateral, broad based, and of bony density.
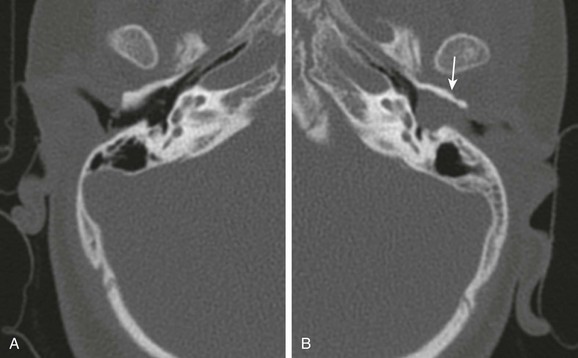
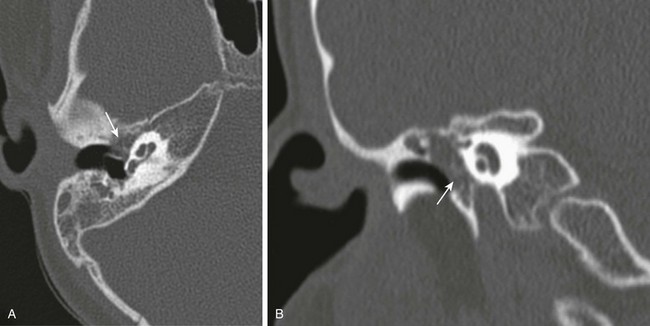
e-Figure 11-2 Acquired cholesteatoma in a 5-year-old with otorrhea.
A, Axial computed tomography image through the normal right external canal. B, Axial computed tomography image through the left external canal. A large soft tissue mass with erosion of the anterior wall (arrow) is present. The tympanic membrane bulges medially with findings representing an external canal cholesteatoma.
Foreign bodies and osteomas can also occur in the external canal, with osteomas appearing pedunculated and very dense (e-Fig. 11-3).9
Mastoid and Middle Ear
Otitis Media
Clinical Presentation and Etiologies: Otitis media is the most common childhood infection that is treated with antibiotics. The otoscopic findings are critical to the diagnosis. Acute otitis media typically presents with fever, ear pain, and a red tympanic membrane.10 The initial cause of the infection is likely viral, but it may be bacterial or represent a secondary bacterial infection. Antibiotic therapy does appear to be moderately more effective than no treatment. Complications may occur in up to 10%, and this rate may be increasing; this could be associated with more conservative treatment.11
Mastoiditis
Clinical Presentation and Etiologies: Acute mastoiditis is the most common complication of acute otitis media and usually presents with high fever and elevated systemic inflammatory markers. Bacterial infections are most commonly caused by Streptococcus pneumoniae and group A beta-hemolytic streptococci. Nonbacterial causes include tuberculosis and fungal infections. Disease in the very young or infections that are not responsive to antibiotic therapy should raise the suspicion of an atypical infection or possible Langerhans cell histiocytosis.12 Facial nerve paralysis is uncommon in bacterial mastoiditis and usually is temporary, which may suggest atypical etiologies. Complications of acute mastoiditis are common, reported in up to 25% of cases.13–15
Imaging: Mastoid air fluid levels can be seen in uncomplicated acute mastoiditis; however, the diagnosis remains a clinical diagnosis. Imaging becomes useful in evaluating for possible complications of acute mastoiditis, with CT being the primary acute imaging modality. CT facilitates the diagnosis of complications of mastoiditis with a high sensitivity and positive predictive value.16 MRI and magnetic resonance venography (MRV) are valuable in assessing intracranial involvement and associated dural sinus thrombosis.
The initial CT finding is decreased definition of the mastoid trabeculae caused by inflammatory hyperemia. As the trabeculae are absorbed and periostitis develops, coalescent mastoiditis develops with infected fluid within the mastoid.17 The subsequent development of a subperiosteal abscess is, by far, the most common complication and typically occurs in the postauricular region where bone is thin, termed the Macewen triangle. CT demonstrates a rim-enhancing fluid collection that is adhering close to bone; underlying bone is usually intact but may show focal destruction (Fig. 11-4). The abscess rarely may arise from the zygomatic root and present with an abscess anterior to the ear. The infection may also progress inferiorly through the mastoid tip, resulting in a Bezold abscess (e-Fig. 11-5). The eustachian tube allows infection to spread into the retropharyngeal space, and children with mastoiditis may present with a retropharyngeal abscess.
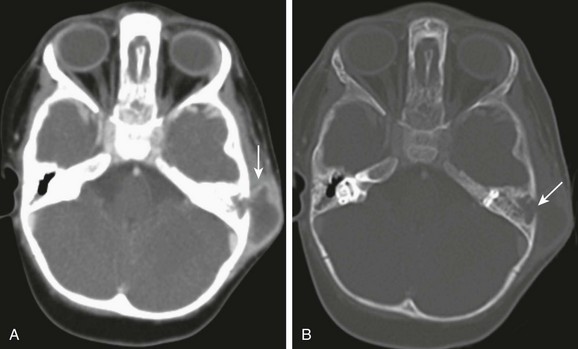
Figure 11-4 Mastoiditis in a 2-year-old with post auricular swelling.
A, Axial computed tomography image through the mastoids shows a large subperiosteal abscess (arrow). B, Axial computed tomography with bone windows demonstrates destruction of the lateral mastoid wall (arrow). No intracranial extension is present with a normally enhancing sigmoid sinus.
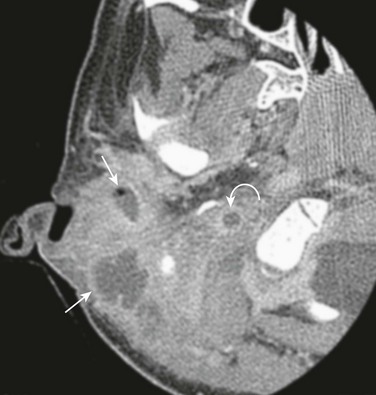
e-Figure 11-5 Complicated mastoiditis.
Axial postcontrast computed tomography shows a Bezold abscess inferior to the mastoids (arrows) with the anterior fluid collection containing air. Thrombosis of the internal jugular vein is also present (curved arrow). (Case courtesy of L Gibert Vezina MD.)
Mastoid infection may extend to the petrous apex and central skull base though the continuous mucosal spaces, resulting in petrous apicitis and osteomyelitis, respectively. Petrous apicitis classically presents as the clinical Gradenigo triad of purulent otorrhea, pain in the distribution of the fifth cranial nerve, and ipsilateral sixth cranial nerve palsy.18 CT demonstrates bony destruction and associated epidural empyema, but normal asymmetric pneumatization may make evaluation difficult. MRI demonstrates a peripherally enhancing fluid collection within the apex, and diffusion-weighted images show restricted diffusion with associated empyema or, less commonly, brain abscess (Fig. 11-6).
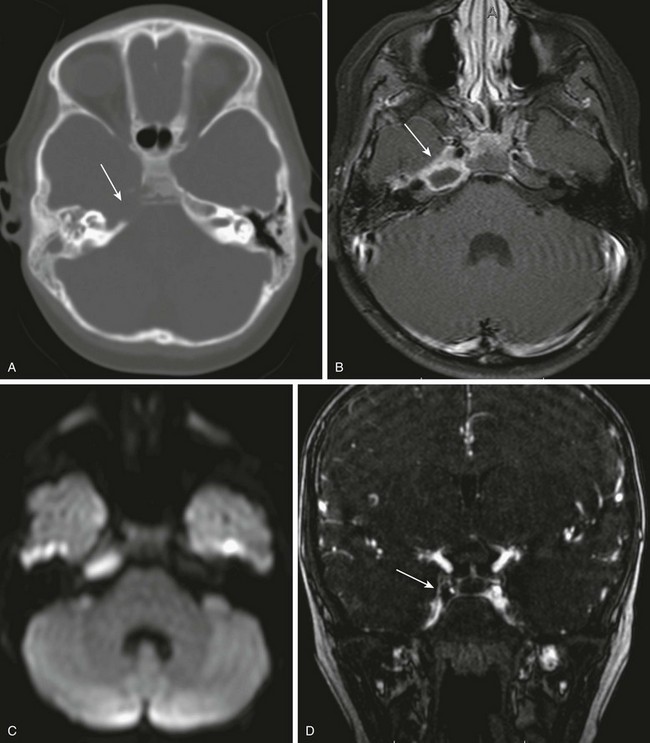
Figure 11-6 Petrous apicitis.
A 6-year-old with headache and right sixth nerve palsy. A, Axial computed tomography shows absence of the right petrous apex (arrow). B, Axial post-gadolinium T1-weighted images show a rim-enhancing fluid collection in the right petrous apex with associated dural enhancement. The left internal carotid artery is narrowed, likely relating to inflammation within the cavernous sinus (arrow). C, Restricted diffusion is present on the axial diffusion-weighted image. D, Gadolinium-enhanced magnetic resonance venography shows a filling defect in the right cavernous sinus consistent with thrombosis (arrow).
The major pathways that allow infectious intracranial extension include bone erosion, thrombophlebitis, and preformed pathways. The oval and round windows, cochlear and vestibular aqueducts, internal auditory canal, dehiscent tegmen, and patent petrosquamosal suture are preformed pathways that allow early or late intracranial extension. These pathways may lead to the development of suppurative labyrinthitis, as shown by abnormal enhancement of the internal auditory canal and membranous labyrinth (e-Fig. 11-7). Meningitis can occur via these pathways by spread to the subarachnoid space.17
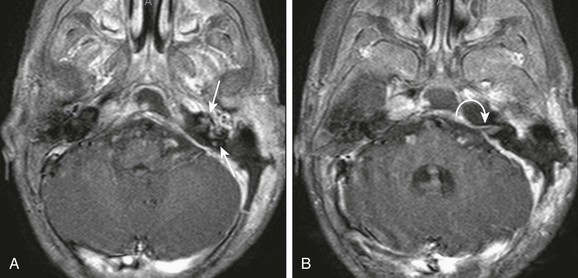
e-Figure 11-7 Suppurative labyrinthitis.
An 18-month-old with mastoiditis. A and B, Axial post-gadolinium T1-weighted images through the membranous labyrinth. Extensive peripheral enhancement throughout the mastoids and extension to the central skull base along with regional dural enhancement are present. Abnormal enhancement is present within the left internal auditory canal (curved arrow), cochlea, and vestibular system (arrows), consistent with suppurative labyrinthitis. A left-sided mastoidectomy has been performed.
Meningitis, epidural empyema, dural sinus thrombosis, and cerebellar or cerebral abscesses are the most common intracranial complications. Bony erosion commonly involves the relatively thin sigmoid plate (Trautmann triangle) and may result in an epidural empyema or anterior lateral cerebellar abscess. Usually, significant compression of the adjacent sigmoid sinus occurs, and it may be difficult to distinguish between extrinsic mass effect and thrombosis of the sinus. Erosion through the tegmen results in a middle cranial fossa epidural empyema and or temporal lobe abscess (Fig. 11-8). Veins allow organisms to readily traverse both bone and dura, resulting in thrombophlebitis and spread of infection. The sigmoid sinus is the most common to become thrombosed; however, the lateral, petrosal, and cavernous sinuses may be involved, especially with infection of the petrous apex.17,19,20 Venous sinus thrombosis may lead to venous infarctions or otitic hydrocephalus caused by impaired venous drainage.21 MRI and gadolinium-enhanced MRV can be helpful in diagnosing dural sinus thrombosis (e-Fig. 11-9). Diffusion-weighted images show purulent material to have increased signal, which may be especially helpful in postoperative imaging (Fig. 11-10 and Box 11-2).19
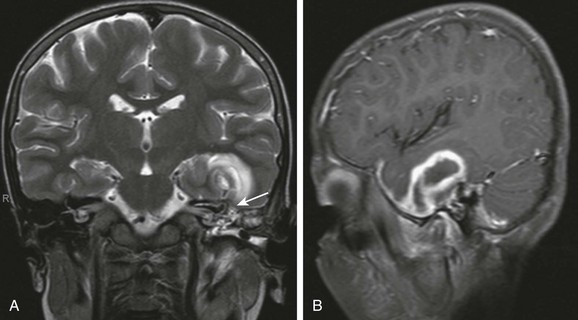
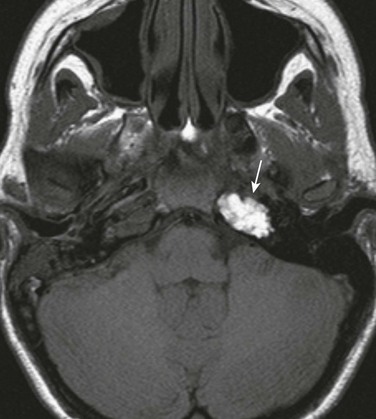
Figure 11-8 Brian abscess.
An 8-year-old with complicated mastoiditis. A, Coronal T2-weighted image shows fluid in the left mastoid and middle ear. A defect is present within the tegmen (arrow) associated with a large temporal intraaxial lesion. B, Left parasagittal post-gadolinium T1-weighted image demonstrates rim enhancement of the temporal mass, consistent with abscess.
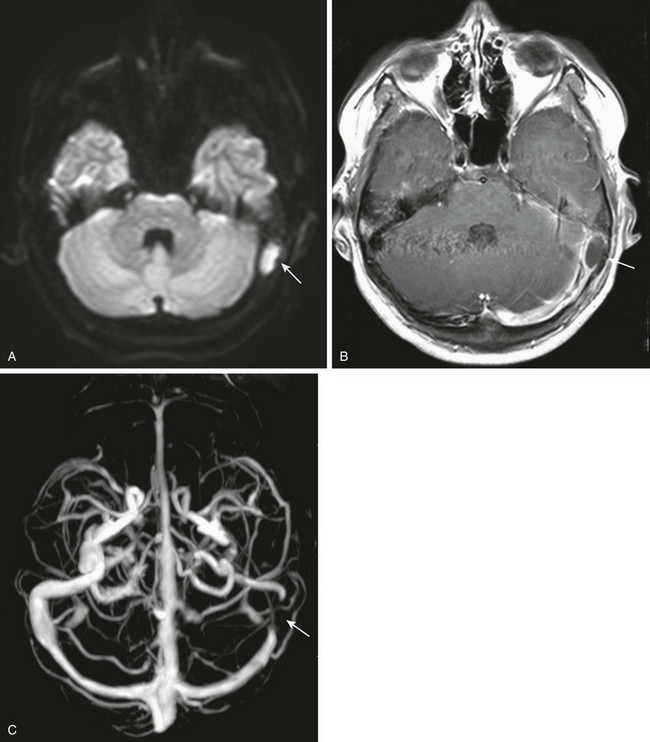
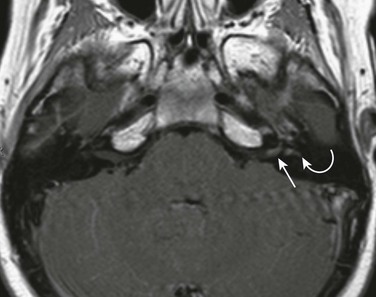
Figure 11-10 Epidural abscess.
An 11-year-old with mastoiditis. A and B, Axial diffusion-weighted and post-gadolinium T1-weighted image shows restricted diffusion in a rim-enhancing fluid collection (arrows). C, Axial maximum-intensity projection image from a gadolinium enhanced magnetic resonance venogram shows extrinsic mass effect on the sigmoid sinus, with no intrinsic filling defect (arrow). No thrombus was demonstrated after surgical drainage of the epidural abscess.
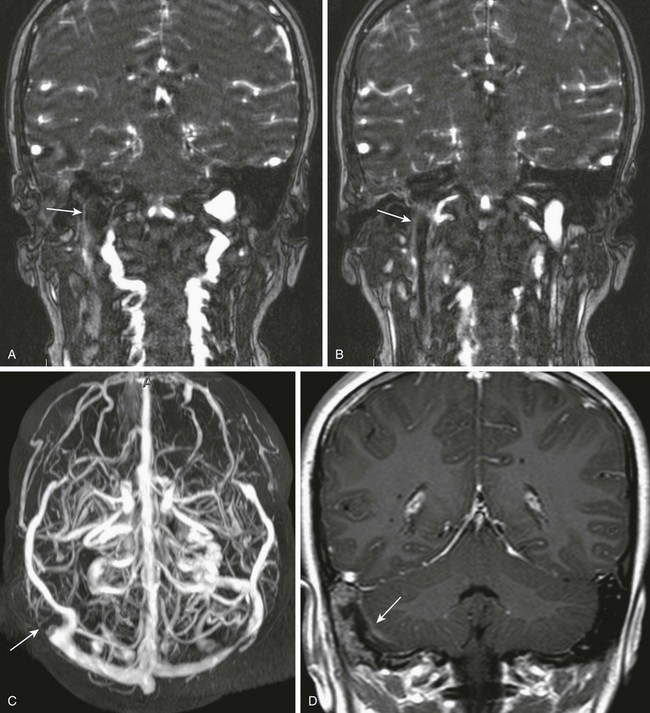
e-Figure 11-9 Venous thrombosis.
A 9-year-old with mastoiditis. A and B, Coronal source images from a gadolinium-enhanced magnetic resonance venogram show a central filling defect in the jugular bulb and high internal jugular vein (arrows), representing venous thrombosis. C, Maximum-intesity projection shows abrupt occlusion of the distal transverse sinus on the right (arrow). D, Coronal post-gadolinium T1-weighted image demonstrates a small coexisting epidural abscess (arrow).
Treatment: Subperiosteal abscess and other complications have traditionally been treated with drainage, cortical mastoidectomy, and ventilation tube.13,14 However, more conservative surgical management has been recently reported. Antibiotic therapy, followed by retroauricular puncture and grommet insertion, has been proven to be an effective alternative to surgical management of complicated mastoiditis.22,23 Intracranial involvement is treated more aggressively with neurosurgical consultation and drainage of extraaxial empyemas and intraparenchymal abscesses.
Chronic Infections
Clinical Presentation and Etiologies: Persistent retraction of the tympanic membrane and fluid within the middle ear cavity may be associated with chronic otitis media (COM). Tympanic membrane perforations from chronic middle ear infections typically involve the pars tensa. In contrast, retraction and perforation involving the pars flaccida portion of the tympanic membrane are usually caused by eustachian tube dysfunction. Children often present for imaging because of development of conductive hearing loss. Hearing loss may have been from ossicular erosions caused by chronic suppurative otomastoiditis or may be related to ossicular fixation, tympanosclerosis, or both.
Imaging: Ossicular erosions associated with COM frequently involve the distal portion of the long process of the incus and are associated with retraction rather than bulging of the tympanic membrane.24 Tympanosclerosis is caused by the deposition of hyalinized, often calcific, granulation tissue in the middle ear cavity. On CT, tympanosclerosis may appear as multiple middle ear masses with regions of increased density. Isolated involvement of the tympanic membrane may occur or be seen in conjunction with middle ear involvement (Fig. 11-11). Increased thickness of the ossicles may be present on imaging studies, suggesting osteitis, and CT classically demonstrates the ossicle that is not visualized too well.20,25
Treatment: Imaging is helpful in determining the extent of ossicular erosion and disease prior to surgical intervention in COM. High-resolution CT should be used selectively and only if complications are suspected.26 Surgical outcome is generally poor for tympanosclerosis but appears to depend on the extent of disease.27
Acquired Cholesteatoma
Clinical Presentation and Etiologies: Cholesteatoma is composed of squamous epithelium and keratin debris, most commonly introduced into the middle ear and mastoid via retractions or perforations in the tympanic membrane. A careful otoscopic examination of the ear is essential in the initial diagnosis of cholesteatoma, and CT is typically used to diagnose the extent of the lesion or associated complications.28 These can be subdivided into pars flaccida and pars tensa cholesteatoma. Pars flaccida lesions are caused by eustachian tube dysfunction and begin in the Prussak space before spreading into the epitympanum. Pars tensa lesions are the result of COM and perforations within the more inferior tympanic membrane, with extension of disease medial to the ossicles and into the oval window.
Imaging: High-resolution CT is the primary imaging modality in children. The differentiation of chronic otomastoiditis without cholesteatoma from COM with cholesteatoma may be difficult with CT. Cholesteatoma usually is associated with more extensive bony erosions, including short process of the incus, lateral wall of the attic, facial nerve canal, tegmen tympani, and superior margin of the external canal or scutum (e-Fig. 11-12). Mass effect is also a significant suggestive finding, with ossicular displacement and bulging of the tympanic membrane noted on CT.24 The ossicular chain typically lies an equal distance from the medial and lateral walls of the middle ear cavity. The position of the ossicles should be evaluated on all imaging studies, with the structures displaced medially with a pars flaccida and laterally with a pars tensa cholesteatoma (Fig. 11-13). Associated erosion of Korner septum in the mastoid antrum may also be seen. Erosion may also involve the bony separation of the middle ear cavity and the lateral semicircular canal, resulting in a fistula and sensorineural hearing loss. Cholesteatomas are histologically benign but may be locally aggressive and extend outside the confines of the temporal bone (Box 11-3).29,30
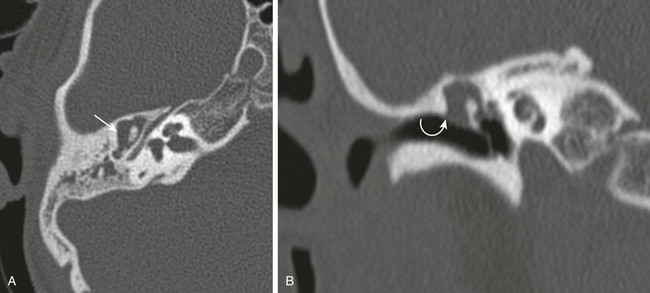
Figure 11-13 Cholesteatoma.
A 5-year-old with chronic ear infections. A and B, Axial and coronal high-resolution computed tomography images through the middle ear. The ossicles are displaced medially with abnormal soft tissue in Prussak space and epitympanum (arrow). The scutum is eroded (curved arrow). At surgery, a pars flaccida cholesteatoma was removed.
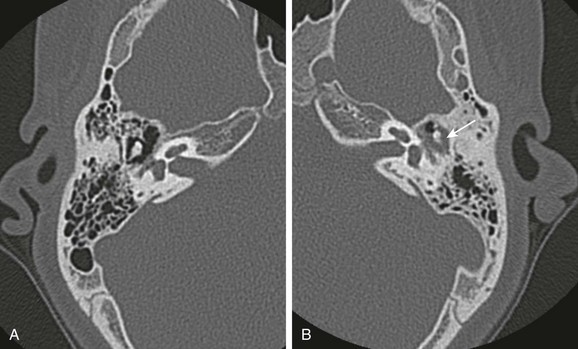
e-Figure 11-12 A 13-year-old with cholesteatoma.
A, Normal axial high resolution computed tomography right temporal bone. B, Axial high-resolution computed tomography left temporal bone. Significant erosion of the short process of the incus is present (arrow), with subtle medial displacement of the ossicles.
MRI is usually not necessary in the initial evaluation of a suspected cholesteatoma, unless intracranial involvement or labyrinthine fistula is suspected. CT evaluation of the postoperative ear is quite difficult, and differentiation of fluid from granulation tissue and recurrent cholesteatoma is nearly impossible. Echoplanar diffusion-weighted imaging was performed in the past, but small recurrent cholesteatomas were missed. Newer spin echo–based sequences have demonstrated cholesteatomas that are less than 5 mm in diameter.31 Increased signal compared with the brain on diffusion-weighted images is considered consistent with cholesteatoma. Increased signal on T2-weighted images and peripheral enhancement on post-gadolinium T1-weighted images are present (Fig. 11-14).32 Delayed imaging can be helpful as well, but not as useful in the pediatric population because of sedation issues.
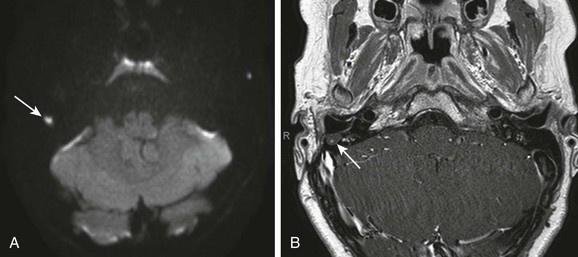
Figure 11-14 Recurrent cholesteatoma.
A 9-year-old after mastoidectomy for cholesteatoma. A, Axial diffusion-weighted image demonstrates a focus of increased signal within the right mastoid (arrow). B, Axial post gadolinium T1-weighted image shows the lesion to be fluid intensity without enhancement (arrow). Surgery confirmed a 5-mm cholesteatoma.
Treatment: High-resolution CT allows for improved operative management in patients with COM and suspected cholesteatoma. MRI, specifically diffusion-weighted imaging, has shown promise in evaluation of postoperative patients and may eventually replace the standard second-look surgery for residual cholesteatoma.32
Inner Ear and Petrous Temporal Bone
Clinical Presentation and Etiologies: Bacterial labyrinthitis usually is caused by extension from an acute otomastoiditis or petrous apicitis. Other causes of labyrinthitis include viral, syphilitic, posttraumatic with labyrinthine hemorrhage, and autoimmune disorders.29 Cholesteatoma with resultant translabyrinthine fistula may also result in labyrinthitis.30 In the pediatric population, congenital infections, classically cytomegalovirus, may lead to labyrinthitis and hearing loss, but evaluation of the inner ear in the nonacute setting is most often normal.
Sickle cell disease has a known association with sensorineural hearing loss and labyrinthine hemorrhage.33
Labyrinthitis Ossificans
Clinical Presentation and Etiologies: Labyrinthitis ossificans is the end result of a labyrinthine infection, hemorrhage, or toxic insult to the membranous labyrinth. Bacterial meningitis is the most common cause. A significant inflammatory process occurs and results in profound sensorineural hearing loss, which may occur as early as 2 weeks after the initial infection. Fibrous tissue is initially deposited within the membranous labyrinth, followed eventually by ossification.
Imaging: Acutely, MRI may demonstrate findings of labyrinthitis; however, the normal high T2-weighted signal within the cochlea and vestibular system is preserved. In the subacute fibrous phase of the inflammatory response, MRI shows loss of the normal high T2-weighted signal in the membranous labyrinth. Ossification occurs in the latter stages of labyrinthitis and is well demonstrated on CT (e-Fig. 11-15). Both scalar chambers of the cochlea that are visualized should be evaluated in these children, as the scala tympani may be involved in isolation (e-Fig. 11-16 and Fig. 11-17).35 CT shows increased density within the labyrinth late in the course of disease, but at this stage, cochlear implantation is more difficult. Complete ossification of the cochlea must be differentiated from cochlear aplasia, which is done by examining the cochlear promontory. The structure is absent in aplasia and normal in labyrinthitis ossificans.
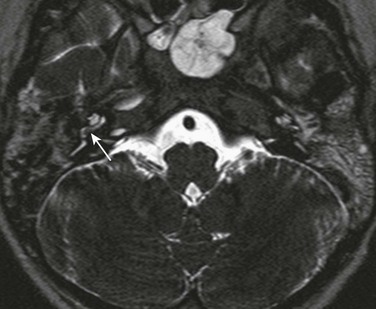
Figure 11-17 A 5-year-old with sensorineural hearing loss and meningitis.
Axial three-dimensional T2-weighted image through the right cochlea shows a T2-weighted hypointense plug at the junction of the scala tympani and vestibule (arrow), which was confirmed at cochlear implantation. Sphenoid sinus disease is also present.
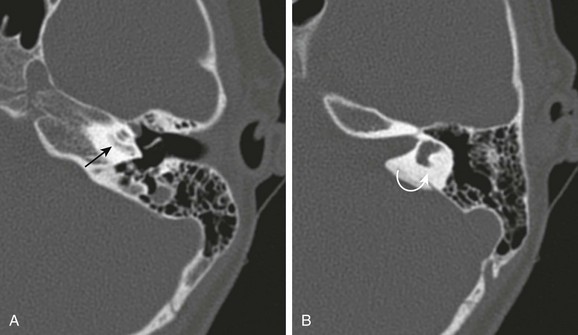
e-Figure 11-15 Ossifying labyrinthitis.
A 6-year-old with sensorineural hearing loss and remote history of prior pneumococcal meningitis. A and B, Axial computed tomography images through the cochlea and vestibule, respectively. Abnormal increased density within the cochlea (straight arrow) and obliteration of the lateral semicircular canal (curved arrow) are seen.
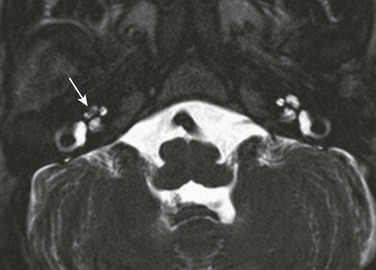
e-Figure 11-16 Labyrinthitis.
A 3-year-old with right sensorineural hearing loss and prior meningitis. Axial three-dimensional T2-weighted image through the cochlea shows low T2-weighted signal intensity within the middle and apical turns of the right cochlea (arrow), consistent with fibrous tissue and early labyrinthitis. Computed tomography was normal without ossification demonstrated (not shown).
Cholesterol Granuloma
Clinical Presentation and Etiologies: Cholesterol granuloma is the result of inflammation and obstruction that initiates repetitive hemorrhage and subsequent formation of granulation tissue. Sites of involvement include the middle ear cavity and petrous apex. Petrous apex cholesterol granuloma may be found incidentally or present with headaches and deficits in cranial nerves VI, VII, IX, X, XI, and XII.36
Imaging: The CT appearance is that of an expansile nonenhancing soft tissue mass with sharply marginated bone destruction. The mass is of high signal intensity on both T1- and T2-weighted MRI scans and low signal on diffusion-weighted images, which differentiates the lesion from a congenital or acquired cholesteatoma (e-Fig. 11-18).37
Facial Nerve
Clinical Presentation and Etiologies: Lyme disease is multisystemic and is caused by an infection by the tick-borne spirochete Borrelia burgdorferi. Three stages with influenza-like symptoms exist, with a classic rash occurring in stage 1. In stage 2, cardiac and neurologic symptoms occur. Stage 3 manifests with arthritic and chronic neurologic symptoms, sometimes years later. Clinical central nervous system involvement has been reported to occur in up to 15% of patients. Neurologic symptoms include myelopathies, encephalitis, pain syndromes, cerebellar dysfunction, movement disorders, and cranial nerve palsies.38,39 Bilateral facial nerve palsy is a common neurologic manifestation of Lyme disease and should suggest the diagnosis.
Imaging: Involvement of the brain may manifest with foci of increased T2-weighted signal, but MRI abnormalities in Lyme disease are rare. Small foci of increased T2-weighted signal are quite common, thus making the finding nonspecific.40,41 Post-gadolinium studies may show enhancement within these lesions, increasing the specificity. Meningeal enhancement may occur, but more commonly, abnormal enhancement of the involved cranial nerves is present. The facial and trigeminal nerves are most often involved, with bilateral facial nerve involvement being a classic presentation. (e-Fig. 11-19).42,43 Hearing loss has also been reported, possibly caused by cochlear nerve involvement. The differential diagnosis would include bilateral Lyme disease, autoimmune disease, demyelinating neuropathy (Miller-Fisher syndrome), and neoplastic etiologies (e-Fig. 11-20).
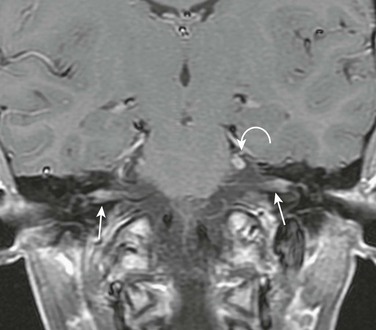
e-Figure 11-19 A 15-year-old with Lyme disease and multiple cranial palsies.
Coronal post-gadolinium T1-weighted image shows abnormal enhancement of the facial nerves within the internal auditory canals (arrows) and an enlarged, enhancing left trigeminal nerve (curved arrow). (Case courtesy L. Gibert Vezina, MD.)
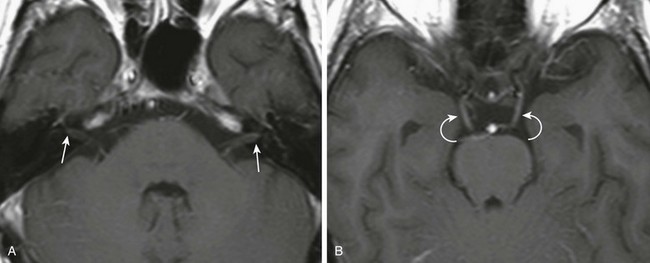
e-Figure 11-20 A 17-year-old with bilateral facial nerve palsy.
A and B, Axial post-gadolinium T1-weighted images. Abnormal enhancement of the intracanalicular facial nerves and labyrinthine segment on the right (arrows). Abnormal enhancement of both third nerves (curved arrows) is also present. The child was diagnosed with polyphenotypic leukemia.
Bell Palsy
Clinical Presentation and Etiologies: Bell palsy is the most common cause of acute lower motor neuron unilateral facial nerve palsy. A viral etiology has been postulated; however, ischemic and possibly autoimmune contributions are likely contributing factors.44 Bell palsy is a diagnosis of exclusion, and other potential etiologies of facial nerve paralysis in childhood include trauma, infection, neoplasm, and congenital anomalies. Virologic tests have suggested herpes simplex type 1 and varicella-zoster as possible etiologies.
Imaging: With typical Bell palsy, complete recovery usually occurs within 6 to 8 weeks, and imaging is not necessary in the acute phase. If prolonged paralysis or other neurologic signs are present, MRI evaluation is suggested.45 Imaging of Bell palsy demonstrates abnormal, asymmetric enhancement and mild enlargement of the facial nerve. It is important to realize that the normal facial nerve segments can enhance (geniculate ganglion, tympanic, and mastoid segments). The labyrinthine segment can show some mild enhancement. Any enhancement of the facial nerve within the internal auditory canal and strong enhancement of the labyrinthine segment is considered pathologic (Fig. 11-21).46,47 Mild enhancement of the normal canalicular facial nerve may be noted at 3-Tesla MRI.48
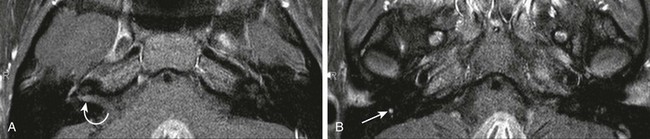
Figure 11-21 A 16-year-old female with recurrent left facial paralysis and likely Bell palsy.
A and B, Axial post-gadolinium T1-weighted fat-suppressed images. Abnormal enhancement of the right intracanalicular facial nerve (curved arrow) and asymmetric enhancement of the tympanic and descending facial nerve on the right (arrow) are seen. No mass is demonstrated and the right facial palsy eventually resolved.
Varicella zoster virus infection can also result in the more severe Ramsay Hunt syndrome, manifesting clinically with facial nerve palsy, sensorineural hearing loss, tinnitus, vertigo, ataxia, and a painful vesicular eruption within the region of the auricle.49 MRI demonstrates not only abnormal enhancement of the facial nerve but also the vestibulocochlear nerve and membranous labyrinth (e-Fig. 11-22).50
Treatment: The intensity of enhancement has been correlated with the outcome for these patients, using quantitative analysis.51 Antiviral agents and corticosteroids are the preferred treatment in the acute phase. Surgery to decompress the facial nerve is controversial when performed in patients with complete Bell palsy that has not responded to medical therapy. Imaging can potentially direct the surgeon to the more affected portion of the nerve.52
Dobben, GD, Raofi, B, Mafee, MF, et al. Otogenic intracranial inflammation: role of magnetic resonance imaging. Topics Magnetic Res Imaging. 2000;11:76–86.
Fernandez, RE, Rothberg, M, Ferencz, G, et al. Lyme disease of the CNS: MR imaging findings in 14 cases. AJNR Am J Neuroradiol. 1990;11:428–431.
Lemmerling, MM, De Foer, B, Verbist, BM, et al. Imaging of inflammatory and infectious diseases of the temporal bone. Neuroimag Clin North Am. 2009;19:321–337.
Oestreicher-Kedem, Y, Raveh, E, Kornreich, L. Complications of mastoiditis in children at the onset of the new millennium. Ann Otol Rhinol Laryngol. 2005;114:147–152.
Schwartz, KM, Lane, JI, Bolster, BD, et al. The utility of diffusion weighted imaging for cholesteatoma evaluation. AJNR Am J Neuroradiol. 2011;32:430–436.
References
1. Ho, T, Vrabec, JT, Yoo, D, et al. Otomycosis: clinical features and treatment indications. Otolaryngol Head Neck Surg. 2006;135:787–791.
2. Abramo, TJ, Beers, SL. Otitis externa review. Pediatr Emerg Care. 2004;20:250–253.
3. Franco-Vidal, V, Blanchet, H, Bebear, C, et al. Necrotizing external otitis: a report of 46 cases. Otol Neurotol. 2007;28:771–773.
4. Berenholz, L, Katzenell, U, Harell, M. Evolving resistant pseudomonas to ciprofloxacin in malignant otitis externa. Laryngoscope. 2002;112:1619–1622.
5. Kesser, BW, Carfrae, MJ. Malignant otitis externa. Otolaryngol Clin North Am. 2008;41:537–549.
6. Okapala, NCE, Siraj, QH, Nilssen, E, et al. Radiological and radionuclide investigation of malignant otitis externa. J Laryngol Otol. 2005;119:71–75.
7. Peleg, U, Perez, R, Raveh, D, et al. Stratification for malignant external otitis. Otolaryngol Head Neck Surg. 2007;137:301–305.
8. Yoon, YH, Park, CH, Kim, EH, et al. Clinical characteristics of external canal cholesteatoma in children. Otolaryngol Head Neck Surg. 2008;139:661–664.
9. Nemzek, WR, Swartz, JD. Temporal bone: inflammatory disease. In: Som PM, Curtin HAD, eds. Head and neck imaging. 4th ed. St. Louis, MO: Mosby; 2003:1173–1244.
10. Morris, PS, Leach, AJ. Acute and chronic otitis media. Pediatr Clin North Am. 2009;56:1383–1399.
11. Coker, TR, Chan, LS, Newberry, SJ. Diagnosis, microbial epidemiology, and antibiotic treatment of acute otitis media in children: a systematic review. JAMA. 2010;304:2161–2169.
12. Fernandez-Latorre, F, Menor-Serano, F, Alonso-Charterine, S, et al. Langerhan’s cell histiocytosis of the temporal bone in pediatric patients: imaging and follow-up. AJR. 2000;174:217–221.
13. Oestreicher-Kedem, Y, Raveh, E, Kornreich, L. Complications of mastoiditis in children at the onset of the new millennium. Ann Otol Rhinol Laryngol. 2005;114:147–152.
14. Glikkich, RE, Eavey, RD, Iannuzzi, RA, et al. A contemporary analysis of acute mastoiditis. Arch Otolaryngol Head Neck Surg. 1996;122:135–139.
15. Palva, T, Virtanen, H, Makinen, J. Acute and latent mastoiditis in children. J Laryngol Otol. 1985;99:127–136.
16. Migirov, L. Computed tomographic versus surgical findings in complicated acute otomastoiditis. Ann Otol Rhinol Laryngol. 2003;112:675–677.
17. Mafee, MF, Singleton, EL, Valvassori, GE. Acute otomastoiditis and its complications: role of CT. Radiology. 1985;155:391–397.
18. Gradeningo, G. Ueber Circumscripte leptomeningitis mit spinalen symptomen. Arch Ohrenheilk. 1904;52:60–62.
19. Dobben, GD, Raofi, B, Mafee, MF, et al. Otogenic intracranial inflammation: role of magnetic resonance imaging. Top Magn Res Imag. 2000;11:76–86.
20. Robson, CD, Robertson, RL, Barnes, PD. Imaging of pediatric temporal bone abnormalities. Neuroimaging Clin North Am. 1999;9:133–155.
21. Kuczkowski, J, Dubaniewicz-Wybieralska, M, Przewozny, T, et al. Otitic hydrocephalus associated with lateral sinus thrombosis and acute mastoiditis in children. Int J Pediatr Otorhinolaryngol. 2006;70:1817–1823.
22. Bakhos, D, Trijolet, JP, Moniniere, S, et al. Conservative management of acute mastoiditis in children. Arch Otolaryngol Head Neck Surg. 2011;137:346–350.
23. Zanetti, D, Nassif, N. Indications for surgery in acute mastoiditis and their complications in children. Int J Pediatr Otorhinolaryngol. 2006;70:1175–1182.
24. Mafee, MF, Aimi, K, Kahen, HL, et al. Chronic otomastoiditis: a conceptual understanding of CT findings. Radiology. 1986;160:193–200.
25. Palacios, E, Valvassori, G. Tympanosclerosis. (brief article) (statistical data included). Ear Nose Throat J. 2000;79:17.
26. Alzoubi, FQ, Odat, HA, Al-balas, HA, et al. The role of preoperative CT scan in patients with chronic otitis media. Eur Arch Otorhinolaryngol. 2009;266:807–809.
27. Yetiser, S, Hidir, Y, Karatas, E, et al. Management of tympanosclerosis with ossicular fixation: review and presentation of long-term results of 30 new cases. J Otolaryngol. 2007;36:303–308.
28. Isaacson, G. Diagnosis of pediatric cholesteatoma. Pediatrics. 2007;120:603–608.
29. Lemmerling, MM, De Foer, B, Verbist, BM, et al. Imaging of inflammatory and infectious diseases of the temporal bone. Neuroimaging Clin North Am. 2009;19:321–337.
30. Mafee, MF. MRI and CT in the evaluation of acquired and congenital cholesteatomas of the temporal bone. J Otolaryngol. 1993;22:239–248.
31. De Foer, B, Vercruysse, JP, Bernaerts, A, et al. Middle ear cholesteatoma: non-echo planar diffusion-weighted imaging versus delayed gadolinium-enhanced T1-weighted MR imaging—value in detection. Radiology. 2010;255:866–872.
32. Schwartz, KM, Lane, JI, Bolster, BD, et al. The utility of diffusion weighted imaging for cholesteatoma evaluation. AJNR Am J Neuroradiol. 2011;32:430–436.
33. Liu, BP, Saito, N, Wang, JJ, et al. Labyrinthitis ossificans in a child with sickle cell disease: CT and MRI findings. Pediatr Radiol. 2009;39:999–1001.
34. Hegarty, JL, Patel, S, Fischbein, N, et al. The value of enhanced magnetic resonance imaging in the evaluation of endocochlear disease. Laryngoscope. 2002;112:8–17.
35. Isaacson, B, Booth, T, Kutz, JW, et al. Labyrinthitis ossificans: how accurate is MRI in predicting cochlear obstruction? Otolaryngol Head Neck Surg. 2009;140:692–696.
36. Miyamoto, RC, Miyamoto, RT. Pediatric neurotology. Sem Pediatr Neurol. 2003;4:298–303.
37. Pisaneschi, MJ, Langer, B. Congenital cholesteatoma and cholesterol granuloma of the temporal bone: role of magnetic resonance imaging. Top Magn Res Imag. 2000;11:87–97.
38. Cryan, B, Wright, DJ. Lyme disease in paediatrics. Arch Dis Child. 1991;66:1359–1363.
39. Logigian, EL, Kaplan, RF, Steere, AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med. 1990;323:1438–1444.
40. Fernandez, RE, Rothberg, M, Ferencz, G, et al. Lyme disease of the CNS: MR imaging findings in 14 cases. AJNR Am J Neuroradiol. 1990;11:428–431.
41. Belman, AL, Coyle, PK, Roque, C, et al. MRI findings in children infected by Borrelia burgdorferi. Pediatr Neurol. 1992;8:428–431.
42. Vanzieleghem, B, Lemmerling, M, Carton, D, et al. Lyme disease in a child presenting with bilateral facial nerve palsy: MRI findings and review of the literature. Neuroradiology. 1998;40:739–742.
43. Agarwal, R, Sze, G. Neuro-Lyme disease: MR imaging findings. Radiology. 2009;253:167–173.
44. Yanagihara, N. Etiology and pathophysiology of Bell’s palsy. Ann Otol Rhino Laryngol. 1988;137(suppl):1–27.
45. Alaani, A, Hogg, R, Saravanappa, N, et al. An analysis of diagnostic delay in unilateral facial paralysis. J Laryngol Otol. 2005;119:184–188.
46. Tren, R, Dillon, WP, Jackler, RK. Contrast-enhanced MR imaging of the facial nerve in 11 patients with Bell’s palsy. AJNR Am J Neuroradiol. 1990;11:735–741.
47. Veillon, F, Ramos-Taboada, L, Abu-Eid, M, et al. Imaging of the facial nerve. Eur J Radiol. 2010;74:341–348.
48. Hong, HS, Yi, BH, Cha, JG, et al. Enhancement pattern of the normal facial nerve at 3.0 T temporal MRI. Br J Radiol. 2010;83:118–121.
49. Cha, HE, Baek, MK, Yoon, BK, et al. Clinical features and management of facial nerve paralysis in children; analysis of 24 cases. J Otol Laryngol. 2010;124:402–406.
50. Sartoretti-Schefer, S, Wichmann, W, Valavanis, A. Idiopathic, herpetic, and HIV-associated facial nerve palsies: abnormal enhancement patterns. AJNR Am J Neuroradiol. 1994;15:479–485.
51. Kress, B, Griesbeck, F, Stippich, C, et al. Bell palsy: quantitative analysis of MR imaging data as a method of predicting outcome. Radiology. 2004;230:504–509.
52. Grogan, PM, Gronseth, GS. Practice parameter: steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:830–836.