CHAPTER 3 Indications for Unicompartmental Knee Arthroplasty
 Principal indications for medial UKA are anteromedial osteoarthritis and avascular necrosis (also called spontaneous osteonecrosis of the knee).
Principal indications for medial UKA are anteromedial osteoarthritis and avascular necrosis (also called spontaneous osteonecrosis of the knee).Introduction
This chapter provides an overview of the indications and contraindications for unicompartmental knee arthroplasty (UKA), with specific reference to the Oxford UKA. The Oxford UKA has a fully congruent, freely mobile meniscal bearing that is free to slide and rotate between the congruent surfaces of the spherical femur and flat tibia, and this congruency is maintained in all positions throughout the range of movement of the knee joint.1 These unique design features help in minimizing wear2 and also make the implant “patella friendly.” Therefore, the indications outlined in this chapter have a specific reference to (or evidence for) the Oxford UKA, and generalization of all these indications for any other design of UKA may not be possible.
Indications
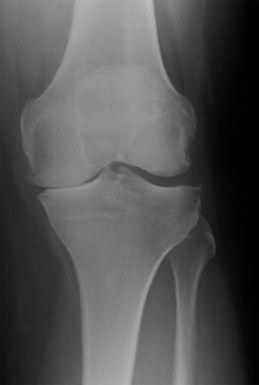
The principal indications for a medial Oxford UKA are anteromedial osteoarthritis (AMOA)3 (Fig. 3–1), and avascular necrosis (also known as spontaneous osteonecrosis of the knee, or SONK)1 (Fig. 3–2). AMOA, the most common indication for UKA, is a distinct entity, and it can be recognized by a consistent association between the clinicoradiologic signs and the pathologic lesions that cause them.1
Correlations
Intact cruciate ligaments and MCL can explain the symptoms and physical signs.1 Cruciate ligaments maintain the normal pattern of roll-back (“physiological roll-back”) of the femur on the tibia in the sagittal plane and thereby preserve the distinction between the damaged contact areas in extension (the anterior tibial plateau and the distal surface of the medial femoral condyle) and the intact contact areas in flexion (the posterior tibial plateau and the posterior surface of the femoral condyle). The shortened posterior capsule causes the flexion deformity. The varus deformity of the extended leg is caused by loss of cartilage and bone from the contact areas in extension. The angle of varus will depend on the amount of bone loss. To expose bone on both surfaces, the total thickness of cartilage lost is about 5 mm, causing about 5° of varus. At least this degree of deformity is usual on presentation because pain seldom becomes severe until there is bone-on-bone contact during weight bearing. Thereafter, each millimeter of bone eroded will increase the deformity by about 1°.
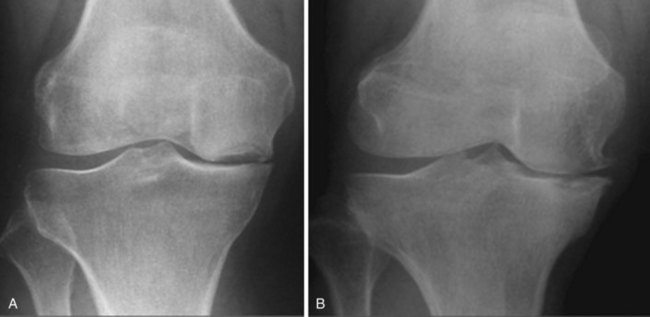
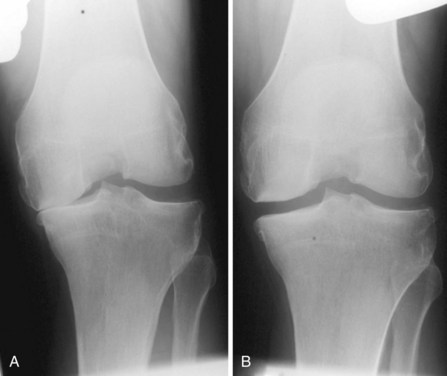
A diagnosis of AMOA is usually based on clinical findings as described above, although supportive evidence from radiographs is useful. Good-quality weight-bearing anteroposterior and lateral radiographs of the knee will help establish the presence of bone-on-bone appearance in the medial compartment and a varus deformity, which is usually present. If for some reason the radiograph does not confirm the presence of bone on bone in the affected medial compartment—that is, there is full-thickness cartilage loss (FTCL) over the femur as well as the tibia in the affected compartment—one can confirm the same by other investigations such as a varus stress view (Fig. 3–3A). In this view, the surgeon (or his or her assistant/radiographer) gives a varus stress to the knee under examination and takes an anteroposterior radiograph with the knee flexed to 20° to allow relaxation of the posterior capsule. After performing the varus stress radiograph, it is a good practice to obtain a valgus stress view (Fig. 3–3B). The valgus stress view allows confirmation of the presence of full-thickness cartilage in the lateral compartment, which is a prerequisite before proceeding to UKA. Some surgeons prefer to perform a Rosenberg view, which is equally useful in confirming the presence of FTCL in the medial compartment. If all these investigations fail to confirm the presence of FTCL in the affected medial compartment, the surgeon should perform an arthroscopy of the affected knee. If any of these investigations confirmed FTCL on both femur and tibia and the patient’s symptoms are bad enough to undergo knee replacement, then the surgeon can proceed to perform a UKA. If, indeed, this is not the case, then one should not perform UKA as the results are unreliable. We have not found other investigations (e.g., magnetic resonance imaging, computed tomography, or bone scan) to be of any specific value to confirm the presence of FTCL in the medial compartment; however, with improving imaging technology, this remains a possibility.
Anterior Cruciate Ligament
The anatomic state of the ACL at the time of surgery is an important determinant in the long-term outcome of UKA, as shown by Goodfellow et al. in 1992.1 They reported a sixfold difference in the 7-year cumulative survival of the Oxford UKA between knees with or without a functioning ACL at the time of surgery, irrespective of the primary disease and of all the other variables measured. In patients with AMOA, the ACL is invariably intact. White et al. described 46 medial tibial plateaus excised sequentially from a series of osteoarthritic knees treated by Oxford UKA, all of them with an intact ACL and with cartilage erosion exposing bone (Ahlbäck stages 2, 3, and 4).3 The erosions were all anterior and central. These rarely extended to the posterior quarter of the plateau and never reached the posterior joint margin. Similar findings have been confirmed by other investigators. Harman et al.4 examined the tibial plateau excised from 143 osteoarthritic knees during operations for total knee arthroplasty (TKA). They found that wear in ACL-deficient knees was located a mean 4 mm more posterior on the medial plateau than wear in ACL-intact knees. The ACL-deficient knees also exhibited more severe varus deformity. The site and extent of the tibial erosions can be reliably determined from lateral radiographs. Based on this, Keyes et al.5 studied the preoperative lateral radiographs of 50 osteoarthritic knees in which the state of the ACL had been recorded at surgery. Using four blind observers, they found a 95% correlation between preservation of the posterior part of the medial tibial plateau on radiograph and an intact ACL at surgery, and a 100% correlation of erosion of the posterior plateau on the radiograph with an absent or badly damaged ACL. These correlations show that, as long as the ACL remains intact, the tibiofemoral contact areas in flexion remain distinct from the areas of contact in extension. Progressive loss of bone causes the varus deformity in extension to increase but, while the ACL continues to function, this deformity corrects spontaneously in flexion and structural shortening of the MCL does not occur. If not treated in time, the deterioration observed in the ACL usually progresses via the following sequence1: normal → loss of synovial covering, → usually starting distally, → longitudinal splits in the substance of the exposed ligament, → stretching and loss of strength of the collagen bundles, which results in the ligament becoming “friable and fragmented.” The ACL will eventually rupture and disappear.
For the purpose of performing an Oxford UKA, we believe that, as long as the ACL is functionally intact (i.e., normal ACL or ACL with loss of synovial covering or longitudinal splits in the substance of the exposed ACL), an Oxford UKA may be safely performed. If the ACL is functionally impaired, this event will cause the transition from AMOA to the posteromedial form of the disease, with posterior subluxation of the femur and structural shortening of the MCL. Deschamps and Lapeyre6 observed that the absence of the ACL in an osteoarthritic knee was associated with the posterior subluxation of the femur on the tibia in extension. This subluxation results in the abrasion of the cartilage at the back of the tibial plateau by the exposed bone on the inferior surface of the femoral condyle. Thereafter, in flexion the cartilage on the posterior surface of the femoral condyle gets destroyed by abrasion on the tibial plateau, which is now devoid of any cartilage. The varus deformity is also therefore present in flexion as well as in extension and the MCL shortens structurally.
“Contraindications”
We believe that there is virtually no contraindication for performing Oxford UKA in a patient with AMOA. This may sound contentious, but we will try to provide evidence for the same. Any patient with bone-on-bone AMOA and significant pain can be offered a UKA and patient’s age, activity level, extent of obesity, chondrocalcinosis, patellofemoral arthritis, and/or preoperative site of pain can be safely ignored. This is contradictory to the recommendations made by Kozinn and Scott back in 1989.7 They suggested that patients who were younger than 60, patients with weight greater than 82 kg, patients with exposed bone in the patellofemoral compartment, or patients who are physically active or perform heavy labor should not be offered a UKA. They also suggested chondrocalcinosis to be a relative contraindication. It must be pointed out that these strict selection criteria were based on their experience with fixed-bearing UKAs and in general are thought to be more intuitive rather than evidence based. The Oxford Group have ignored these so-called contraindications over the past 25 years, and our data presented here support our stance. Since 1998, when the Phase III Oxford UKA was introduced (implanted using minimally invasive surgical technique), we have collected preoperative and subsequent follow-up clinical and radiologic data on a cohort of 1000 Oxford UKAs.
Exposed Bone in Patellofemoral Joint
In the consecutive series of 1000 UKAs, nearly one quarter of patients had the presence of exposed bone in the patellofemoral joint (PFJ) either on the patella or on the trochlea or on both sides. When compared to the patients without the presence of FTCL in the PFJ, no significant difference was noted in the clinical scores or in survivorship. In 2007, our group published its experience of Oxford UKA with specific reference to the intraoperative status of the PFJ in a cohort of 824 consecutive knees.8,9 In that series we had noted the presence of FTCL on the trochlea surface in 13% of cases, on the medial facet of the patella in 9%, and on the lateral facet in 4% of cases. No significantly worse outcome was noticed in these cases as compared to those without any patellofemoral arthritis. Similarly, the presence of preoperative anterior knee pain and/or radiologic evidence of degeneration of the PFJ was also assessed in a separate cohort of 100 consecutive knees. Fifty-four percent of patients had preoperative anterior knee pain. The clinical outcome in these patients was independent of the presence or absence of preoperative anterior knee pain. The presence of degenerative changes seen on the preoperative radiographs (in the PFJ as seen on skyline radiographs) did not show any significant difference in the clinical outcome. This was particularly evident in patients with medial patellofemoral degeneration. However, for some outcome measures in patients with lateral femoral patellofemoral degeneration, the Oxford knee score (OKS) tended to be 38 (lateral PFJ arthritis) versus 41 (normal lateral PFJ). We therefore recommend that, if there is severe damage to the lateral part of the PFJ with bone loss, grooving, or subluxation, a TKA should be performed.
Age
Some surgeons may consider the young age (age < 60) or old age (age > 80) of a patient as a contraindication to UKA. Wear and component loosening are concerns in the young while unnecessary risk of revision surgery is a concern in the old. The unique design features of the Oxford UKA minimize the wear, and the wear is independent of bearing thickness. This means that one can use a bearing as thin as 3 mm without any added risks of catastrophic wear or bearing fracture. This ensures the surgery to be bone conserving, which is an important advantage especially in the young. Various studies involving national joint registries have shown significantly lower complication rates with the use of UKA as compared to TKA, with particular reference to lower mortality, lower infection rate, and reduced need for blood transfusion. Hospital stay is reduced, range of movement is better, and faster recovery makes UKA an ideal implant in the elderly. In our cohort of 1000 UKAs, 25% of patients were younger than 60 at the time of the index procedure. At the last follow-up, no statistically significant difference was noted in the clinical or functional outcome or the failure rate between patients in this group and those over 60 years of age at the time of index procedure. In addition, Price et al. in 2005 compared the results of the Oxford UKA in patients younger and older than 60.10 The survival for the younger group of patients was 91% (95% confidence interval [CI], 12) while in the older group it was 96% (95% CI, 3). These results are comparable to those achieved with TKA in patients younger than 60 at the time of surgery and, in addition, the Hospital for Special Surgery score at 10-year follow-up was 94/100 for the younger patients as compared to 86/100 for the older patients.
Obesity
Fixed-bearing (particularly the all-polyethylene tibia) UKAs have not performed well in obese patients. This is due to the associated risk of catastrophic failure and/or tibial component loosening. The Oxford UKA has a fully congruent bearing with minimal wear and therefore wear is not an issue. Provision of a metal baseplate reduces the risk of tibial loosening. In the Oxford cohort, nearly 50% of patients’ weight was greater than 82 kg and therefore according to Kozinn and Scott’s criteria7 these patients would be considered as “less than ideal.” When this cohort of patients was compared to patients weighing less than 82 kg, no significant difference was noticed in any clinical or functional outcome or in the failure rate.11 Berend et al. retrospectively reviewed the early results of a consecutive series of fixed-bearing UKAs implanted via minimally invasive surgery using two implant designs (EIUS, Stryker Orthopaedics; and Repicci II, Biomet).12 At an average follow-up of 40.2 months, the authors had 16 failures from a consecutive series of 79 UKAs, with the most common reason for failure being tibial loosening in six cases. The authors concluded that a body mass index (BMI) greater than 32 increased the failure rate. More recently, the authors have published their results of the Oxford UKA and found that BMI greater than 32 did not increase the failure rate.12
Recently, we presented the impact of BMI on a consecutive series of nearly 600 Oxford UKAs with a minimum 5-year follow-up.13 The patients were divided into four groups: group 1 = BMI less than 25, group 2 = BMI 25–30, group 3 = BMI 30–35, and group 4 = BMI of 35 or greater. There was no significant difference in the 10-year survivorship among the four groups, although the numbers in group 4 were relatively small. Patients in groups 3 and 4 tended to have a lower preoperative OKS and lower OKS at the last follow-up, although the change in the OKS was similar to that in the other groups. Similarly, the functional American Knee Society score (AKSS) was lower in groups 3 and 4, although the change in the functional score was not significantly different. These results suggest that, for the Oxford UKA, obesity should not be considered a contraindication.
Chondrocalcinosis
Thirteen percent of cases from our Oxford UKA cohort showed the presence of chondrocalcinosis as evident on radiography and/or histology. Again no difference was noted in any of the clinical or functional outcomes or in the survivorship.11 Woods et al. in 1995 published the results of chondrocalcinosis in patients undergoing Oxford UKA.14 The survival rates between patients with and without chondrocalcinosis were not significantly different. Again there was no significant difference in clinical outcomes or radiologic outcomes.
Activity Level
It continues to be a contentious issue as to what activity levels are safe for patients undergoing knee arthroplasty. It obviously depends upon the type and frequency of activity as well as the type of implant. Nearly 10% of patients in our cohort have a Tegner activity level of 5 or more. (Tegner 5 activity level means that they are regularly involved in either heavy labor [building/forestry] or competitive [cycling/cross-country skiing] or recreational [jogging on uneven ground at least twice per week] sports). In this cohort of patients, the OKS and AKSS functional scores are superior with a lower failure rate, and the only failure we have noticed in this patient subgroup is in a patient who ruptured his ACL, which required subsequent reconstruction. If all these unnecessary contraindications (for the Oxford UKA) were adhered to (as suggested by Kozinn and Scott9), nearly 70% of these patients would not have been considered to be ideal candidates to undergo a UKA. There is, however, no difference in their functional outcome or failure rate as compared to the so-called ideal candidates, with survivorship being 96% at 12 years in those with or without accepted contraindications (not statistically different).
Spontaneous Osteonecrosis of the Knee
UKA is well suited to treat SONK, and various studies have shown excellent functional outcome and survivorship in these patients. From a technical point of view, implantation of the UKA can be demanding, and certain considerations must be taken into account when using the implant for osteonecrosis. The classic defect in the medial femoral condyle occurs in the weight-bearing area in extension: failure to identify the presence of a crater may result in the unwary surgeon recessing the spigot too deeply and thereby milling too much bone from the medial femoral condyle. This could then cause problems in balancing the extension gap. In addition, because of surrounding bony sclerosis, an attempt should be made to completely excavate any craters in the femoral condyle or to completely remove the osteonecrotic lesion in the medial tibial plateau so that normal bone can be used as a base for cement impregnation. It may be necessary for large craters to be filled with autologous bone graft harvested from the bone removed at surgery. Langdown et al. assessed the outcome of the Oxford UKA in 29 patients with end-stage SONK and compared the results with a matched group of patients with an Oxford UKA for AMOA.15 The clinical and functional outcomes were noted to be similar in these two groups.
Previous High Tibial Osteotomy
Rees et al. showed that the cumulative 10-year survivorship after a UKA in patients with previous high tibial osteotomy (HTO) is 66%, and this is significantly lower than in those with AMOA (96%).16 The authors noted persistent pain and/or early progression of osteoarthritis to the lateral compartment as the most common reasons for failure. We believe that the reason for the pain, lateral wear, and subsequent failure is that a medial UKA for primary AMOA results in correction of the varus deformity within the joint, restoring the leg to its predisease alignment. However, if the varus deformity has already been fully or partially corrected extra-articularly by an HTO, then any further change in alignment from a UKA can cause an overcorrection, which will increase loading of the lateral compartment. We therefore recommend that a previous HTO should be considered to be a contraindication to the use of an Oxford UKA. Knees in which symptoms recur after a previous HTO may be more effectively treated by TKA.
ACL Deficiency
The options for treatment of the young active patient with isolated symptomatic osteoarthritis of the medial compartment and preexisting deficiency of the ACL are limited. The potential longevity of the implant and the activity level of the patient may preclude TKA, and tibial osteotomy and UKA are unreliable because of the ligamentous instability. UKAs tend to fail because of wear or tibial loosening resulting from eccentric loading. In cases of primary traumatic ACL rupture with secondary arthritis of the medial compartment, the cartilage defect and bony erosion tend to be central and posterior on the tibia (posteromedial osteoarthritis). This is likely to be due to recurrent episodes of giving way, in which posterior femoral subluxation in the medial compartment places a heavy load on the posterior meniscus and posterior articular cartilage of the tibia, producing meniscal tears and the development of arthritis. In some cases the rest of the knee joint remains essentially intact, with no shortening of the MCL. This is probably because, in extension, the intact distal femoral cartilage is in contact with intact anterior tibial cartilage, so the varus deformity is corrected and the MCL is of normal length. It is in these patients, who are often young, that we would perform a combined ACL reconstruction followed by UKA.17 Depending on the presenting symptoms, the combined procedure can be done in one or two stages. The majority of patients present primarily with pain, and we tend to perform a combined single-stage procedure of ACL reconstruction and UKA under the same anesthetic, while in patients presenting with instability we tend to do a staged surgery—the first stage comprising ACL reconstruction followed by a subsequent UKA after a few months if the pain becomes a significant issue.
We have performed more than 50 cases with combined ACL reconstruction and UKA, and these patients have excellent clinical and functional outcomes with survivorship not dissimilar to those with AMOA and an intact ACL. We have also assessed in vivo functional kinematics, including bearing movement and femoral roll-back, in patients with an Oxford UKA with an intact ACL and an Oxford UKA with a reconstructed ACL. The kinematic patterns were similar in both groups and closely mimicked normal (native) knee kinematics.18
Summary
The indications and contraindications for UKA are design dependent and, for the Oxford UKA, virtually any patient with significant pain and bone-on-bone AMOA can undergo a UKA. In particular, one can ignore the age, activity level, presence of chondrocalcinosis, patellofemoral damage, preoperative site of pain, and obesity in these patients. If one uses the selection criteria recommended by Kozinn and Scott,7 UKA can only be offered to 2–10% of patients undergoing knee replacement, and this means that the surgeon is unable to gain experience and increase his or her skill in performing UKAs. When UKA is performed on an infrequent basis, the results may be suboptimal. In contrast, with the Oxford philosophy one can offer a UKA to about one in three patients undergoing a knee replacement (and maybe even higher, as suggested by some groups); this will allow the surgeon to gain experience and the patients to benefit from UKA.
1 Goodfellow J, O’Connor J. The anterior cruciate ligament in knee arthroplasty: a risk factor with unconstrained meniscal prosthesis. Clin Orthop. 1992;276:245-252.
2 Psychoyios V, Crawford RW, O’Connor JJ, Murray DW. Wear of congruent meniscal bearings in unicompartmental knee arthroplasty: a retrieval study of 16 specimens. J Bone Joint Surg [Br]. 1998;80:976-982.
3 White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg [Br]. 1991;73:582-586.
4 Harman MK, Markovich GD, Banks SA, Hodge WA. Wear patterns on tibial plateaus from varus and valgus osteoarthritic knees. Clin Orthop. 1998;325:149-158.
5 Keyes GW, Carr AJ, Miller RK, Goodfellow JW. The radiographic classifications of medial gonarthrosis. Correlation with operation methods in 200 knees. Acta Orthop Scand. 1992;63:497-501.
6 Deschamps G, Lapeyre B. Rupture of the anterior cruciate ligament: a frequently unrecognised cause of failure of unicompartmental knee prostheses. Fr J Orthop Surg. 1987;1:323-330.
7 Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg [Am]. 1989;71:145-150.
8 Beard DJ, Pandit H, Gill HS, et al. The influence of the presence and severity of pre-existing patellofemoral degenerative changes on the outcome of the Oxford medial unicompartmental knee replacement. J Bone Joint Surg [Br]. 2007;89:1597-1601.
9 Beard DJ, Pandit H, Ostlere S, et al. Pre-operative clinical and radiological assessment of the patellofemoral joint in unicompartmental knee replacement and its influence on outcome. J Bone Joint Surg [Br]. 2007;89:1602-1607.
10 Price AJ, Dodd CA, Svard UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg [Br]. 2005;87:1488-1492.
11 Pandit H, Jenkins C, Gill HS, et al, Unnecessary contraindications for mobile bearing unicompartmental knee replacement. Accepted for publication in, J Bone Joint Surg, Jan 2011.
12 Berend KR, Lombardi AVJr, Adams JB. Total knee arthroplasty in patients with greater than 20 degrees flexion contracture. Clin Orthop Relat Res. 2006;452:83-87.
13 Ferguson J, Pandit H, Price AJ, et al. The impact of body mass index on the outcome of the unicompartmental knee arthroplasty. Oxford: BASK; March 2010.
14 Woods DA, Wallace DA, Woods CG, et al. Chondrocalcinosis and medial unicompartmental knee arthroplasty. Knee. 1995;2:117-119.
15 Langdown AJ, Pandit H, Price AJ, et al. Oxford medial unicompartmental arthroplasty for focal spontaneous osteonecrosis of the knee. Acta Orthop. 2005;76:688-692.
16 Rees JL, Price AJ, Lynskey TG, et al. Medial unicompartmental arthroplasty after failed high tibial osteotomy. J Bone Joint Surg [Br]. 2001;83:1034-1036.
17 Pandit H, Beard DJ, Jenkins C, et al. Combined anterior cruciate reconstruction and Oxford unicompartmental knee arthroplasty. J Bone Joint Surg [Br]. 2006;88:887-892.
18 Pandit H, Van Duren BH, Gallagher JA, et al. Combined anterior cruciate reconstruction and Oxford unicompartmental knee arthroplasty: in vivo kinematics. Knee. 2008;15:101-106.