55 Indications for and Management of Tracheostomy
Tracheostomy is one of the most commonly performed surgical procedures in critically ill patients who require prolonged mechanical ventilation.1 A large body of literature describes the potential benefits, risks, and technical aspects of this procedure, but there is little guidance as to what constitutes optimal tracheostomy practice in the critically ill patient.2,3 This chapter reviews basic aspects of tracheostomy management, focusing in particular on indications, timing, technique, and postprocedure care.
 Indications for Tracheostomy
Indications for Tracheostomy
The presence of a “difficult airway” in a patient requiring prolonged mechanical ventilatory support constitutes an absolute indication for tracheostomy. Patients with so-called difficult airways include those with conditions such as significant maxillofacial trauma, angioedema, obstructing upper-airway tumors, or other anatomic characteristics that would render translaryngeal intubation technically difficult to perform in the event of inadvertent airway loss. Patients with difficult airways represent a small fraction of all individuals undergoing tracheostomy. More commonly, patients undergo this procedure for subjective indications (e.g., to facilitate ventilator weaning, to promote oral hygiene and pulmonary toilet, or to enhance comfort).4 Tracheostomy is most commonly performed in an elective fashion; accordingly, patients should be clinically optimized to minimize risk (e.g., minimal ventilatory support [FIO2 ≤ 50%, PEEP ≤ 7.5 cm H2O], hemodynamically stable, metabolic and hemostatic derangements corrected). Because many of the benefits of tracheostomy relative to prolonged translaryngeal intubation are unproven, unambiguous criteria for selecting patients for tracheostomy are lacking.3
 Timing of Tracheostomy in Acute Respiratory Failure
Timing of Tracheostomy in Acute Respiratory Failure
In the early years of critical care medicine, endotracheal tubes (ETTs) were composed of rigid materials and incorporated a low-volume, high-pressure pneumatic cuff. During this era, it became common practice to perform tracheostomy early—within 48 hours of initiating mechanical ventilation—in an effort to minimize laryngeal and tracheal injury associated with endotracheal intubation.5 With advances in ETT design, the trauma associated with prolonged translaryngeal intubation lessened.5 Further, a prospective study examining risks associated with tracheostomy suggested that this procedure was accompanied by high rates of morbidity and mortality.6 Accordingly, enthusiasm for the routine performance of tracheostomy waned. With refinement in techniques, perioperative complication rates associated with tracheostomy diminished. In addition, subsequent studies attempting to establish the relationship between prolonged translaryngeal intubation, prolonged tracheostomy, and laryngeotracheal damage produced conflicting findings.5 At present, no data clearly establish that translaryngeal intubation should be limited to any specific duration or that tracheostomy should be performed at any specific point in a patient’s course in an effort either to limit chronic laryngeal dysfunction or minimize tracheal injury.
Recent investigations examining timing of tracheostomy have focused on duration of mechanical ventilation and related measures of resource expenditure. Rodriguez et al. assigned 106 patients who developed acute respiratory failure following major trauma to either undergo tracheostomy within 7 days of intensive care unit (ICU) admission (“early” tracheostomy) or to tracheostomy at least 8 days following ICU admission (“late” tracheostomy). Compared to patients undergoing late tracheostomy, patients in the early tracheostomy group had a trend toward a lower incidence of pneumonia, as well as significant reductions in duration of mechanical ventilation, ICU length of stay, and hospital length of stay.7 Likewise, Lesnik et al. reported a retrospective analysis of 101 patients who developed acute respiratory failure following blunt trauma, comparing patients who underwent early tracheostomy (within 4 days of ICU admission) to late tracheostomy (>4 days following ICU admission). Compared to patients undergoing late tracheostomy, patients in whom tracheostomy was established early had a significantly shorter duration of mechanical ventilation and lower incidence of pneumonia.8 Others have likewise reported a benefit of early tracheostomy.9,10 In contrast, Blot et al. reported that neutropenic patients developing acute respiratory failure who underwent early tracheostomy (within 48 hours of intubation) had longer duration of mechanical ventilation and longer hospital length of stay than did patients who either underwent tracheostomy formation after 7 days or not at all.11 Given the conflicting results, variability in study quality, heterogeneity in populations enrolled, and inconsistency in endpoints studied, it is difficult to draw on the conclusions of these and similar studies to ascertain the optimal timing of tracheostomy creation. As a consequence, tracheostomy practice varies substantially.1
There are several reasons why tracheostomy may facilitate weaning from mechanical ventilation.5 Resistance to airflow in an artificial airway is proportional to air turbulence, tube diameter, and tube length. Air turbulence is increased in the presence of extrinsic compression and inspissated secretions.12 Airflow resistance and associated work of breathing should theoretically be less with tracheostomies than with ETTs because of an ETT’s rigid design, shorter length, and removable inner cannula (to allow for evacuation of secretions).12 Further, the presence of a tracheostomy may allow clinicians to be more aggressive in weaning patients from mechanical ventilation. Specifically, if a patient with a tracheostomy tube in place does not tolerate liberation from ventilatory support, he or she may be simply reconnected to the ventilator. In contrast, if a patient who is translaryngeally intubated does not tolerate extubation, he or she must be sedated and reintubated. This might represent a potential barrier to extubation in patients who are of marginal pulmonary status. Finally, patients with tracheostomies may receive less sedation than individuals with translaryngeal airways.13 Reduction in sedation may be accompanied by increases in mobility, differences in approaches to and success of weaning, and other factors that may shorten duration of ventilatory support.
Technical Considerations
Traditionally, tracheostomies have been performed in the operating room using standard surgical principles.14 In 1985, Ciaglia et al. described percutaneous dilational tracheostomy (PDT) in which tracheostomy is accomplished via a modified Seldinger technique, typically with the aid of bronchoscopy.15 PDT has subsequently gained wide acceptance and has become the predominate method of tracheostomy creation in many centers.16–18
There are several potential advantages of PDT relative to surgically created tracheostomies (SCT). PDT may be performed at the bedside, avoiding the inconvenience and risk of transporting a critically ill patient, as well as the expense of utilizing operating room resources. In a prospective randomized study comparing PDT and SCT, Freeman et al. found that PDT was associated with a reduction of approximately $1500 in patient charges per procedure.19 Other investigators have reported comparable findings.20 In addition, a meta-analysis of prospective trials comparing PDT with SCT suggests that PDT may be associated with fewer complications, specifically postprocedure bleeding and peristomal infection.21 The reduction in these complications may reflect that there is minimal dead space between the tracheostomy tube and adjacent pretracheal tissues following PDT, which may have a tamponading effect on minor bleeding and serve as a barrier to infection.21 Finally, PDT is relatively simple to learn. Individuals who have not received formal surgical training may become facile with this procedure and perform it safely and effectively.17,22
While there are many potential advantages of PDT, this procedure has been associated with a number of highly morbid complications, many of which (e.g., pretracheal insertion, tracheal laceration, esophageal perforation, pneumothorax, loss of airway) are unusual in surgically created tracheostomies.23–28 Accordingly, whereas PDT may be performed competently by those not trained in surgical techniques, persons who are expert at surgical airway management should be immediately available in the event complications arise.22
 Selection, Maintenance, and Care of Tracheostomy Tubes
Selection, Maintenance, and Care of Tracheostomy Tubes
Tracheostomy Tube Selection
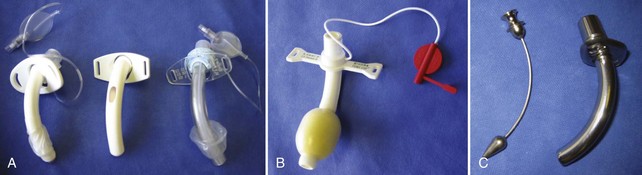
A detailed discussion of the various types and designs of tracheostomy tubes is beyond the scope of this text, but a working knowledge of tracheostomy tube features is essential to the competent care of patients who have undergone placement of these devices (Figure 55-1). Briefly, most tracheostomy tubes are manufactured from polyvinyl chloride, silicone, a combination of these materials, or metal. They are available in either single-lumen (no removable inner cannula) or dual-lumen (removable inner cannula) configurations. The purpose of the removable inner cannula is to facilitate cleaning of inspissated secretions that may lead to tube occlusion. Because silicone is relatively secretion resistant, tubes manufactured from this material frequently do not have an inner cannula. Tracheostomy tubes are available with and without pneumatic cuffs. The purpose of the cuff is to maintain a seal between the tube and the tracheal mucosa sufficient to prevent escape of air from around the tracheostomy tube during mechanical ventilation (i.e., cuff leak). Further, the cuff minimizes but does not prevent aspiration. Tracheostomy tubes with foam cuffs conform to a patient’s trachea and remain consistently inflated at low pressure. These tubes are indicated in patients who have sustained damage from excessive cuff pressure (e.g., tracheomalacia). Once a cuffed tracheostomy tube is no longer required—that is, the patient no longer requires mechanical ventilatory support and is not considered an aspiration risk—the cuffed tube is exchanged for a cuffless tube. Tracheostomy caps are generally provided with tracheostomy tubes for use in the decannulation process (see later discussion). Fenestrated tubes are used to promote speech and are generally used in individuals who tolerate liberation from mechanical ventilation for varying periods of time. Fenestrated tubes have an opening on their superior aspect such that when the inner cannula is removed, the cuff deflated, and the external orifice occluded (such as with a Passey-Muir type valve), air can pass the vocal cords, allowing phonation.
Monitoring Cuff Inflation Pressure
Tracheostomy tube pneumatic cuffs require monitoring to maintain an inflation pressure of approximately 20 to 25 mm Hg. Assuming a tracheostomy tube is of appropriate size, an insufficiently inflated cuff may both result in a sizable amount of air leaking around the cuff (“cuff leak”), rendering mechanical ventilation difficult, and provide poor protection against aspiration. Alternatively, excessive cuff pressures (exceeding 25 mm Hg) may result in compression of mucosal capillaries, giving rise to mucosal ischemia and attendant complications such as tracheomalacia and tracheal stenosis. The most reliable method of monitoring cuff inflation pressure is through direct measurement. Maneuvers such as pilot balloon palpation to estimate cuff pressure, or inflation of the cuff until end-inspiratory leaks are extinguished during positive-pressure ventilation, are not recommended because of their inaccuracy.29 Tracheal cuff inflation pressures should be measured and recorded on a regular basis for purposes of quality assurance.
Oral Nutrition
The presence of a tracheostomy provides opportunity for oral nutrition in the mechanically ventilated patient, with its attendant psychological benefits, but it also complicates alimentation because of the interference of the tracheostomy tube with mechanisms of normal swallowing and airway control.29 The presence of a tracheostomy inhibits physiologic upward movement of the larynx during deglutition, hinders glottic closure, and produces dysphagia due to mechanical compression of the esophagus. Further, an inflated tracheostomy balloon does not protect from aspiration. Patients with tracheostomies who are candidates for oral nutrition should mentate normally, have adequate oxygenation with low inspired oxygen concentrations (e.g., 30% FIO2), and possess sufficient ventilatory reserve such that they can physiologically tolerate an episode of aspiration during the introduction of oral feeding. Initial efforts at feeding should be carefully supervised.
 Complications
Complications
Tube Dislodgement
Although dislodgement of the tracheostomy tube may occur at any time following tracheostomy placement, this complication is most problematic in the immediate postoperative period, before the tracheostomy tract has matured. Factors predisposing to tracheostomy tube dislodgment include an inadequately secured tube, excessive coughing, and patient agitation. Tracheostomy tube dislodgement should be suspected when a patient is able to speak immediately following tracheostomy placement, the airway becomes obstructed, or respiratory distress develops. Because it may be technically difficult to reinsert the tracheostomy tube in this situation, the author recommends that the airway be reestablished via translaryngeal intubation. The tracheostomy should then be reinserted in the operating room with appropriate anesthetic assistance, lighting, and instrumentation. If tracheostomy tube dislodgement occurs once the tracheostomy track is sufficiently mature (that is, the tracheostomy track is at least 1 week old), it is generally technically feasible to reinsert the tracheostomy tube at the patient’s bedside as noted earlier (see Exchanging Tracheostomy Tubes).
Tracheoesophageal Fistula
Tracheoesophageal fistula requires surgical repair. Temporizing measures include positioning of an ETT cuff below the level of the fistula to limit aspiration, removal of nasogastric tubes, and placement of feeding gastrostomy tubes.30
Tracheoinnominate Artery Fistula
Tracheoinnominate artery fistula (TIF) is a rare complication following tracheostomy formation and theoretically results from pressure necrosis or injury to the trachea adjacent to the course of innominate artery.31 A number of risk factors have been postulated, including excessive tube movement, aberrant innominate artery anatomy, use of an excessively long or curved tracheostomy tube that erodes through the tracheal wall, inferior positioning of the tracheostomy tube, tracheal infection, and corticosteroid therapy.31 A TIF may become apparent as quickly as a few days or as late as several months following tracheostomy placement. The classic presentation is of a “sentinel bleed,” in which a large volume of blood emanates from the tracheostomy tube. Fiberoptic examination to evaluate for the presence of TIF should be performed in the operating room in the event airway manipulation results in massive hemorrhage. Temporizing measures in patients who develop massive bleeding include hyperinflation of the tracheostomy cuff, insertion of an ETT through the tracheostomy stoma in an effort to tamponade bleeding, or translaryngeal intubation and digital compression of the bleeding site through the tracheostomy stoma. Definitive repair entails median sternotomy, ligation of the innominate artery, and drainage of the mediastinum.
Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilational tracheostomy. Chest. 1985;87(6):715-719.
1989 Consensus conference on artificial airways in patients receiving mechanical ventilation. Chest. 1989;96(1):178-180.
Freeman BD, Borecki IB, Coopersmith CM, Buchman TB. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Crit Care Med. 2005;33:2513-2520.
Freeman B, Kennedy C, Robertson TE, et al. Tracheostomy protocol: experience with development and potential utility. Crit Care Med. 2008;36(6):1742-1748.
Describes a novel protocol-based approach to patient selection and timing of tracheostomy.
Heffner JE, Hess D. Tracheostomy management in the chronically ventilated patient. Clin Chest Med. 2001;22(1):55-69.
An excellent review of virtually all facets of tracheostomy care.
1 Freeman BD, Borecki IB, Coopersmith CM, Buchman TB. Relationship between tracheostomy timing and duration of mechanical ventilation in critically ill patients. Crit Care Med. 2005;33(11):2513-2520.
2 Fischler L, Erhart S, Kleger GR, Frutiger A. Prevalence of tracheostomy in ICU patients. A nation-wide survey in Switzerland. Intensive Care Med. 2000;26(10):1428-1433.
3 Freeman B, Kennedy C, Robertson TE, et al. Tracheostomy protocol: experience with development and potential utility. Crit Care Med. June 2008;36(6):1742-1748.
4 Consensus conference on artificial airways in patients receiving mechanical ventilation. Chest. 1989;96(1):178-180.
5 Heffner JE. Timing of tracheotomy in mechanically ventilated patients. Am Rev Respir Dis. 1993;147(3):768-771.
6 Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheotomy: a prospective study of 150 critically ill adult patients. Am J Med. 1981;70(1):65-76.
7 Rodriguez JL, Steinberg SM, Luchetti FA, Gibbons KJ, Taheri PA, Flint LM. Early tracheostomy for primary airway management in the surgical critical care setting. Surgery. 1990;108(4):655-659.
8 Lesnik I, Rappaport W, Fulginiti J, Witzke D. The role of early tracheostomy in blunt, multiple organ trauma. Am Surg. 1992;58(6):346-349.
9 Brook AD, Sherman G, Malen J, Kollef MH. A comparison of early- vs. late-tracheostomy in patients requiring prolonged mechanical ventilation. Am J Crit Care. 2000;9(5):352-359.
10 Rumbak MJ, Newton M, Truncale T, Schwartz SK, Adams JW, Hazard PB. A prospective randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004;32:1689-1694.
11 Blot F, Guiguet M, Antoun S, Leclercq B, Nitenberg G, Escudier B. Early tracheotomy in neutropenic, mechanically ventilated patients: rationale and results of a pilot study. Support Care Cancer. 1995;3(5):291-296.
12 Diehl JL, Atrous SE, Touchard D, Lemaire F, Brochard L. Changes in work of breathing induced by tracheostomy in ventilator dependent patients. Am J Respir Crit Care Med. 1999;159:383-388.
13 Nieszkowska A, Combes A, Luyt CE, Ksibi H, Trouillet JL, Gibert C, et al. Impact of trachestomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005;33:2527-2533.
14 Zollinger RMJr, Zollinger RM. Atlas of Surgical Operations, 8th ed. New York: McGraw-Hill, Inc.; 2002.
15 Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilational tracheostomy. Chest. 1985;87(6):715-719.
16 Hill BB, Zweng TN, Maley RH, Charash WE, Toursarkissian B, Kearney PA. Percutaneous dilatational tracheostomy: report of 356 cases. J Trauma. 1996;40(8):238-244.
17 Petros S, Engelmann L. Percutaneous dilatational tracheostomy in the medical ICU. Intensive Care Med. 1997;23:630-634.
18 Cooper RM. Use and safety of percutaneous tracheostomy in intensive care. Anaesthesia. 1998;53:1209-1227.
19 Freeman BD, Isabella K, Cobb JP, et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med. 2001;29(5):926-930.
20 Heikkinen M, Pertti A, Hannukainen J. Percutaneous dilational tracheostomy or conventional surgical tracheostomy? Crit Care Med. 2000;28(5):1399-1402.
21 Freeman BD, Isabella K, Lin N, Buchman TG. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 118, 2000. 1412-1218
22 Durbin CG. Questions answered about tracheostomy timing. Crit Care Med. 1999;27(9):2024-2025.
23 Kaylie DM, Wax MK. Massive subcutaneous emphysema following percutaneous tracheostomy. Am J Otolaryngol. 2002;23(5):300-302.
24 Briche T, Manach YL, Pats B. Complications of percutaneous tracheostomy. Chest. 2001;119(4):1282-1283.
25 Kaloud H, Smolle-Juettner F, Prause G, List WF. Iatrogenic rupture of the tracheobronchial tree. Chest. 1997;112:774-778.
26 Alexander R, Pappachan J. Timing of surgical tracheostomy after failed percutaneous tracheostomy (letter). Anaesth Intensive Care. 1997;25(1):91.
27 Douglas WE, Flabouris A. Surgical emphysema following percutaneous tracheostomy. Anaesth Intensive Care. 1999;27(1):69-72.
28 Malthaner RA, Telang H, Miller JD, McFadden S, Inculet RI. Percutaneous tracheostomy–is it really better? Chest. 1998;144(6):1771-1772.
29 Heffner JE, Hess D. Tracheostomy management in the chronically ventilated patient. Clin Chest Med. 2001;22(1):55-69.
30 Casson AG, Bethune DC. Acquired tracheoesophageal fistula. In: Pearson FG, Cooper JD, Deslauriers J, et al, editors. Thoracic Surgery. New York: Churchill Livingstone; 2002:341-346.
31 Myers EN, Stool SE. Complications of tracheostomy. In: Myers EN, Stool SE, Johnson JT, editors. Tracheotomy. New York: Churchill Livingstone; 1985:147-170.