CHAPTER 34 Indications and Patient Selection in Obstructive Coronary Disease
Since the advent of ECG-synchronized cardiac CT, this modality has become a rapidly evolving tool for noninvasive imaging of the coronary arteries.1,2 Advances in technology with fast data acquisition, improvements in temporal resolution, and high spatial resolution have contributed to the widespread acceptance of this test in a number of clinical scenarios. On the other hand, because of the rapid succession of technologic innovations, several scanner generations are currently in simultaneous clinical use.3 During the last decade, research has mainly been restricted to feasibility testing in tune with the rapid, roughly triennial, introduction of new multidetector-row CT (MDCT) scanner generations. Only recently do we see more emerging data of the greater impact of this potentially disruptive technology on the general field of health care. Albeit scarce, we see increasing evidence illustrating the cost-effectiveness of this test in the management of coronary heart disease.4 After years during which technologic development was focused almost exclusively on improved image quality and increased spatial resolution, particular attention is now being paid to mechanisms of radiation protection.5 Evidence-based guidelines have recently been implemented2,4 to steer the appropriate use and to avoid overuse of this test. This contribution aims to summarize the current indications for the use of coronary CT angiography (CTA) for assessment of obstructive native coronary artery disease (CAD).
OVERVIEW OF EVIDENCE AND TRENDS
Significant obstructive CAD is defined as a diameter stenosis of 70% or more in at least one major epicardial artery and as a 50% reduction in the case of the left main coronary artery. At coronary CTA, the assessment for hemodynamically significant coronary artery stenosis is roughly analogous to that of conventional invasive coronary angiography, which in clinical reality is ordinarily based on visual assessment and subjective estimation of stenosis severity. More objective assessment with quantitative catheter angiography is typically not routinely performed and is reserved for equivocal cases or research applications. Similarly, routine clinical coronary CTA evaluation of stenosis severity is ordinarily performed visually and subjectively, although virtually every currently available image display and analysis software platform provides semiautomated measurement tools for stenosis grading. These allow quantification of stenosis severity based on the ratio of stenosed arterial lumen relative to adjacent prestenotic and poststenotic segments with normal luminal diameters, similar to the standards as established, for instance, for intravascular ultrasound.6
A number of meta-analyses present an overview of the diagnostic performance of ECG-gated coronary CTA,7,8 and the number of studies is rapidly growing. One large analysis performed a systematic review of studies based on different scanner generations,3 illustrating the continuously improving image quality but also the growing experience of observers, as demonstrated by the high percentage of segments considered assessable with more recent scanner generations. Most important, however, a consistent performance with regard to the well-established high negative predictive value of this test, and thus the reliable exclusion of significant stenosis, is observed across scanner generations.
At present, the use of 16-slice MDCT with a gantry rotation speed of less than 500 ms is considered to meet the minimal requirements for successful ECG-gated imaging of the coronary arteries.2
False-negative findings, however rare, do occur. With regard to the clinically relevant per-patient analyses, many studies report between one and three patients who are missed by coronary CTA who have stenosis of more than 50%, a number that is fairly consistent across scanner generations, including the recently introduced dual-source CT (DSCT) technology.10 However the majority of missed stenoses were located in distal segments that are not amenable to revascularization.2
Noninvasive coronary CTA tends to overestimate disease. Main causes of false-positive findings are calcifications of the vessel wall and limited visualization of small-vessel lumens due to partial volume effects.9 The average positive predictive value is therefore moderate. Although average positive predictive values are reported to be higher with 64-slice CT,7 reports are ambiguous even with regard to the latest scanner types.10 Thus, coronary CTA should still be mainly regarded as a reliable tool to rule out significant obstructive disease rather than a means to estimate the severity of detected lesions.
Early evidence with use of 16-slice MDCT was characterized by excluding small vessels (<1.5 mm or <2 mm) or vessel segments with heavy calcifications or motion artifact from analysis, an approach that is not clinically feasible. More recently, in the majority of studies using 64-slice scanners, the entire coronary tree is considered for assessment. The number of nonassessable segments in the available literature has dropped from a mean 12% with 16-slice CT to 4% with 64-slice CT.7,8
SEGMENT-BASED AND PATIENT-BASED ANALYSIS
Indications
Throughout the available literature and for all scanner generations, it has been consistently found that ECG-synchronized coronary CTA has the ability to reliably rule out significant stenosis on the basis of the exceedingly high negative predictive value of this test. Selection of patients who most likely would benefit from noninvasive imaging of the coronary arteries is based on existing evidence on coronary CTA, known performance of conventional diagnostic procedures, and potential impact on patient management and risk estimation.4 Supported by broad consensus among experts, coronary CTA is recommended in symptomatic patients for the initial evaluation of chest pain in the setting of low to intermediate probability of CAD.3,5 In this context, coronary CTA is considered to have incremental value in patient management to inform the indication for invasive coronary angiography (Fig. 34-1). Of note, this target group (i.e., symptomatic patients with low to intermediate probability of CAD) is still different from the study populations on which most of the currently accepted clinical evidence is based. Invasive coronary angiography is typically not performed in patients with low probability of CAD.11 A few studies have closed this knowledge gap. Mejboom and colleagues10 investigated symptomatic patients at different levels of pretest likelihood and demonstrated that the performance of coronary CTA is inversely related to the probability of CAD. Coronary CTA was more accurate in patients with low to intermediate likelihood (Figs. 34-2 and 34-3) than in groups with high probability. The limited value in the latter group was explained by the high prevalence of advanced disease with heavy calcifications.
Therefore, symptomatic patients with high pretest probability are not appropriate candidates for noninvasive coronary CTA because this test is unlikely to add incremental value in their diagnostic work-up. Rather, invasive coronary angiography with the option of direct intervention and revascularization is the more appropriate initial procedure in the management of such patients (Fig. 34-4).9
Asymptomatic Patients
Cardiac CT is insufficiently validated for the application in asymptomatic individuals and is not currently recommended for this group.2,4 The characteristics of this test involving relatively high radiation doses and unclear cost/benefit currently do not support the use of coronary CTA as a screening modality.2,9 Whether coronary CTA may in the future have a role for better risk stratification in asymptomatic individuals at high Framingham risk12 or to detect silent CAD (e.g., in diabetic patients)13 remains to be established.
Estimation of Cardiovascular Risk and Pretest Probability for Coronary Artery Disease
An asymptomatic individual’s risk for future cardiovascular events is usually estimated on the basis of the traditional Framingham risk score, determining absolute and relative risk (compared with an age- and sex-adjusted low-risk group) of coronary heart disease.14 Risk is stratified to determine the appropriate level of aggressiveness in risk modification for disease prevention.
Pretest probability, on the other hand, is estimated in symptomatic patients, based on the quality of the chest pain (typical, atypical, and noncardiac quality; Table 34-1). Beyond symptoms, probability depends on age and gender (Table 34-2) and, as in the Duke score, may also include risk factors.12
| Typical angina (definite) |
| (1) Substernal chest discomfort with a characteristic quality and duration that is (2) provoked by exertion or emotional stress and (3) relieved by rest or nitroglycerin |
| Atypical angina (probable) |
| Meets 2 of the above characteristics |
| Noncardiac chest pain |
| Meets ≤1 of the typical angina characteristics |
From Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation 1999; 99:2829-2848.
Role of Coronary CT Angiography in the Context of Other Noninvasive Tests
Exercise testing and stress echocardiography are firmly established in the diagnostic algorithm of CAD, supported by extensive population-based evidence. Noninvasive stress testing provides functional and clinical information about the significance of myocardial hypoperfusion. Treadmill testing is a readily available low-cost procedure and is the primary tool in the diagnostic work-up of patients with suspected CAD. Similar to noninvasive angiography, exercise testing performs most accurately in patients with intermediate likelihood for CAD.13
Stress echocardiography is indicated as a second-line tool for patients who are unable to exercise.15 With recent developments in tissue Doppler imaging, improvements in the diagnostic performance of stress echocardiography have been reported. However, the modality is more sophisticated, and the diagnostic value depends on acoustic window and operator expertise. A considerable range of sensitivities and specificities is reported for both exercise testing and stress echocardiography to describe the accuracy of these tests for detection of significant CAD.15 Of note, the diagnostic accuracy of stress testing is recognized to be decreased in women, resulting in a higher rate of post-test referrals to invasive coronary angiography for women.16
Given the variable performance of noninvasive stress testing, particularly in women, a group of symptomatic and asymptomatic individuals is left in whom the probability after stress testing remains indeterminate. According to the National Cardiovascular Data Registry, a considerable part of invasive angiography is indicated by false-positive results of stress tests.17 Thus, most experts agree on the usefulness of coronary CTA in patients with inconclusive or uninterpretable stress test results.2,4
Patient-Related Considerations
Heavy Calcifications
Heavy calcifications tend to cause overestimation of the severity of lesions and are one of the most important sources of unassessable segments and false-positive findings at coronary CTA.2 The presence of calcium causes “blooming” artifacts that consequently may obscure noncalcified plaque components and portions of the residual vessel lumen, which in extreme cases (Fig. 34-5) may interfere with coronary CTA interpretation. Although extensive calcifications are recognized as a major limiting factor, detailed data (e.g., with calcium score subclasses) illustrating the impact on diagnostic performance of coronary CTA remain rare.18,19 Whereas calcifications are frequently reported to decrease the specificity of coronary CTA because of lesion overestimation, there appears to be no substantial negative effect on the overall clinically relevant high negative predictive value20,21 because of the aforementioned nesting effect. Particularly with newer scanner generations with improved temporal and spatial resolution combined with advanced visualization tools, most experts agree that extensive calcifications should no longer be considered a contraindication to coronary CTA. Moreover, if it is used appropriately in the low- to intermediate-risk groups (see earlier), the prevalence of excessive calcifications in the target group should be within limits (Fig. 34-6).
The known relative limitations due to heavy calcifications are unlikely to be overcome soon because voxel size as a determinant factor of blooming artifacts is at the limits of the technically feasible spatial resolution at sustainable radiation dose. Improvements might be expected from limiting motion artifacts at the interface of calcium and surrounding structures by high temporal resolution. According to observations using DSCT technology, a high negative predictive value can be maintained in the presence of severe calcifications.22
Heart Rate and Heart Rate Variability
The need for a defined temporal window per heart cycle to acquire sufficient data for image reconstruction makes temporal resolution the most important factor to determine diagnostic accuracy. For imaging of the coronary arteries, the reconstruction interval is preferably placed in the relatively quiet mid-diastole. Because the duration of diastole shows a nonproportional shortening in relation to systole at higher heart rates, a stable sinus rhythm with heart rates below 65 beats/min is recommended by many authors for both 16-slice MDCT and single-source 64-slice CT scanners23; some guidelines propose a limit of 70 beats/min.4 These relatively slow heart rates are ordinarily induced by pharmacologic rate control (e.g., β blockade), which may have the added benefit of reducing heart rate variability.24 However, faster and more irregular heart rates are considered relative contraindications to coronary CTA, at least with 64-slice single-source CT, so that diagnostic image quality ordinarily can be obtained in cases in which pharmacologic rate control fails.23
Currently available DSCT scanners offer a temporal resolution of 83 ms, further widening the scope of patients who are eligible for this test. Most published studies agree that DSCT allows investigation of patients with high and irregular heart rates with diagnostic results so that pharmacologic heart rate control can be abandoned.25 Recent investigations compared the performance of DSCT technology between groups with different heart rates and confirmed the robustness of this technology for exclusion of significant stenosis even at heart rates as high as 100 beats/min.25 There is initial evidence that the high temporal resolution of recent-generation scanners along with the ability of ECG editing may expand the use of this technology to the population of patients with atrial fibrillation, which traditionally had been considered a contraindication to the successful use of coronary CTA (Fig. 34-7).4
Obesity
Obesity is defined as a body mass index (BMI) above 30, and extreme obesity by a BMI of 40 or more. In 2004, the prevalence of obesity among adults in the United States was 32%, with an upward trend.26 Noninvasive visualization of the coronary arteries in obese patients represents a considerable challenge to ECG-gated coronary CTA. High image noise levels result in reduced spatial and contrast resolution (Fig. 34-8), with impaired evaluation of small vessels. Few studies evaluated the impact of obesity, as defined by a BMI above 30, on the diagnostic accuracy of coronary CTA. Raff and coworkers20 reported a reduced negative predictive value in a sample size of 35 patients. Strategies to reduce image noise comprise lowering of the pitch to accumulate data samples, increasing tube current, and modifying injection protocols with higher iodine concentration and high flow rates. Increasing kilovolt levels is not beneficial, given the loss of contrast resolution with high energy beams.23 An expert paper proposes a BMI limit of 40 or lower for reasonable image quality of noninvasive coronary arteriography (Fig. 34-9).4
Renal Insufficiency
The amount of intravenous iodinated contrast medium required for noninvasive coronary CTA could be substantially reduced with the introduction of faster scanners with shorter scan duration in the range of 6 to 12 seconds, depending on scanner type and scan length. Because ECG-gated CT of the heart ordinarily represents an elective examination, an even more intense risk/benefit analysis is necessary before indicating coronary CTA in patients with elevated serum creatinine levels of above 1.8 mg/dL.4
EMERGING APPLICATIONS
Evaluation of Acute Chest Pain in the Emergency Department Setting
Acute chest pain is the second most common reason for emergency department visits. A particular diagnostic challenge is the subset of patients who present with unspecific chest pain, with negative cardiac biomarkers, and without ECG changes. In this group of patients, there is a small but not negligible chance that the chest pain is of cardiac origin, constituting about 15% in this population according to current estimates.27 To identify this minority, traditional clinical guidelines recommend observation for 12 hours12 before resuming the diagnostic algorithm with a stress test. Fast scanners allow ECG-synchronized examination of the entire thorax with motion-free visualization of the cardiopulmonary vessels, enabling a “triple rule-out” protocol, that is, the exclusion of the major entities causing acute chest pain (obstructive CAD, acute aortic syndromes, pulmonary embolism).28,29 Other noncardiac causes of acute chest pain that are amenable to diagnosis at coronary CTA include the full spectrum of thoracic CT findings in this setting, such as pneumothorax, pneumonia, pleuritis, hiatal hernia, other disorders of the upper gastrointestinal tract, and malignant neoplasms. For type A aortic dissection, ECG-synchronized examinations allow better identification of intimal tear sites and motion-free depiction of the flap, which may involve a coronary ostium or cause coronary dissection. Diagnostic pitfalls arising from the artifactual impression of aortic dissection secondary to cardiac motion artifacts along the aortic root can be successfully avoided. Overall, however, acute aortic syndromes appear to be the least commonly encountered pathologic process in the patients undergoing coronary CTA assessment for acute chest pain.
Cardiac causes of acute chest pain are exacerbated chronic obstructive CAD and acute coronary syndrome, with plaque rupture and thrombus formation (Fig. 34-10). Data illustrating the utility of ECG-synchronized thoracic CTA for acute chest pain are evolving and encouraging yet limited in number at this time.30–32 Validation remains a problem related to the low prevalence of obstructive CAD in this population of patients. Even more than in the elective setting, the indication for noninvasive CTA in the emergency department must be stringent to avoid inappropriate application and overuse and must be restricted to patients with atypical chest pain with low to intermediate probability for CAD with negative biomarkers and ECG.31 Also in this population, patient management should be primarily based on the high negative predictive value for ruling out significant stenosis, and patients with equivalent findings at CT should undergo the traditional work-up for acute chest pain.
Preoperative Evaluation
Noncardiac Surgery
Guidelines recommend stratification of perioperative risk based on patient-related predictors and surgical risk.32 Preoperative invasive coronary angiography is routinely performed in patients with known cardiac conditions, such as heart failure, arrhythmia, or advanced valvular disease, to assess eligibility for and risk of surgery in such patients. Stress testing is recommended in the presence of less severe clinical risk factors before procedures that carry high (e.g., vascular surgery) or intermediate surgical risk. Noninvasive imaging with coronary CTA in the preoperative setting is not integrated in the current algorithm.4 However, because the American College of Cardiology/American Heart Association Guidelines for Perioperative Evaluation recognize the limitations of exercise testing for detection of significant coronary stenosis,32 preoperative spiral CTA might be considered to incrementally contribute for further work-up of patients with inconclusive stress test results before surgery.
Cardiac Surgery
A variety of studies investigated the performance of noninvasive coronary CTA in patients scheduled for heart valve surgery. In part, this relatively large evidence base might be due to the easily available reference standard because invasive coronary angiography is routinely performed before valve repair in the majority of patients.33 The presence of concomitant CAD has considerable impact on perioperative risk. Among patients with degenerative aortic valve disease, the prevalence of CAD is as high as 50% (Fig. 34-11).33 In patients with congenital valvular heart disease before surgery, current guidelines recommend preoperative invasive catheter angiography in a substantial portion of patients (men, ≥35 years; women, ≥35 years, with risk factors, and postmenopausal women).33 Among the population of patients scheduled to undergo heart valve surgery, individuals with low to intermediate probability for CAD might also benefit from noninvasive assessment of the coronary arteries, although this has not yet been implemented in current guidelines.
Vessel Wall Imaging
Coronary CTA allows unprecedented noninvasive imaging of the vessel wall comprising the entire coronary tree. During recent years, considerable efforts have been made to identify subjects at risk for future cardiovascular events. Calcium scoring has been established as a marker for atherosclerosis and has been advocated for cardiac risk stratification, but it shows only an approximate 20% of the total atherosclerotic plaque burden (Fig. 34-12).2,34
Invasive coronary angiography detects plaques that cause luminal compromise and cannot diagnose nonobstructive atherosclerotic disease. The high spatial and contrast resolution of intravascular ultrasound is unsurpassed, yet the modality, usually performed in combination with invasive coronary angiography, is expensive and invasive and requires considerable expertise.6
Several studies report on the feasibility of plaque characterization in comparison to intravascular ultrasound by defining CT attenuation criteria35 for lipid-rich, collagen-rich, and calcified plaques. However, a considerable overlap in attenuation values was found in noncalcified (lipid-rich versus fibrous) lesions. The limited reproducibility of plaque characterization in subsequent publications is attributable to scanner-dependent spatial resolution, patient geometry, and luminal opacification.36 Plaque characterization software has nonetheless become commercially available for routine applications, but its role in clinical routine is insufficiently established. The correlation between vessel wall disease and risk factors has attracted much scientific attention, but thus far the relationship between plaque characteristics and risk profiles remains inconclusive. Currently, noninvasive coronary CTA has no established role for cardiac risk stratification, although initial data on the predictive value of this test are currently emerging. Considering the relatively high radiation exposure currently still associated with coronary CTA and the lack of evidence, coronary CTA for assessment of the vessel walls is currently considered useful only within established indications, for example, to inform the indication for aggressive risk modification in patients with atypical chest pain and nonobstructive atherosclerosis (Fig. 34-13). Cardiac CT is not considered appropriate as a screening test and is not established for monitoring of response to medical treatment,4 although its use in such a manner for dedicated research applications has been described.
CONSIDERATIONS FOR PROCEDURE-RELATED RISKS
As with all medical testing, the specific risks of coronary CTA, such as adverse events related to contrast material and relatively high radiation dose, have to be weighed against the expected clinical benefit of performing coronary CTA. The lifetime risk for radiation-induced cancer is age and gender dependent.5 In this context, one must keep in mind that a population of patients with low to intermediate probability of CAD is more likely to be younger than the overall population of cardiovascular patients. Although female patients have lower age-related pretest likelihood for CAD compared with men, CAD is known to be under-diagnosed in the female population.16 Women more frequently present with protean symptoms of atypical chest pain. This has the potential to lead to preferred use of coronary CTA in this group. Consequently, radiation exposure should be kept as low as reasonably achievable by use of all available tools for radiation protection.
KEY POINTS
 Coronary CTA is indicated in symptomatic patients with low to intermediate probability for obstructive CAD.
Coronary CTA is indicated in symptomatic patients with low to intermediate probability for obstructive CAD. Coronary CTA is not indicated in symptomatic patients with high pretest probability of CAD. This group of patients should primarily undergo invasive angiography.
Coronary CTA is not indicated in symptomatic patients with high pretest probability of CAD. This group of patients should primarily undergo invasive angiography. Coronary CTA is currently not considered to be indicated in asymptomatic individuals for risk stratification or for screening.
Coronary CTA is currently not considered to be indicated in asymptomatic individuals for risk stratification or for screening. Application in specific scenarios, such as exclusion of obstructive disease before general surgery and evaluation of acute chest pain in the emergency department, can be considered within the context of currently established indications (i.e., in individuals with low to intermediate pretest likelihood).
Application in specific scenarios, such as exclusion of obstructive disease before general surgery and evaluation of acute chest pain in the emergency department, can be considered within the context of currently established indications (i.e., in individuals with low to intermediate pretest likelihood).Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol. 2006;48:1919-1928.
Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761-1791.
Flohr TG, Schoepf UJ, Ohnesorge BM. Chasing the heart. New developments for cardiac CT. J Thorac Imaging. 2007;22:4-16.
Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation. 1999;99:2829-2848.
Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475-1497.
Hoffmann U, Ferencik M, Cury MC, Pena AJ. Coronary CT angiography. J Nucl Med. 2006;47:797-806.
Mahnken AH, Muhlenbruch G, Gunther RW, Wildberger JE. Cardiac CT: coronary arteries and beyond. Eur Radiol. 2007;17:994-1008.
Schoepf UJ, Zwerner PL, Savino G, et al. Coronary CT angiography. Radiology. 2007;244:48-63.
Schroeder S, Achenbach S, Bengel F, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29:531-556.
White CS, Kuo D. Chest pain in the emergency department: role of multidetector CT. Radiology. 2007;245:672-681.
1 Becker CR, Knez A, Leber A, et al. Initial experiences with multi-slice detector spiral CT in diagnosis of arteriosclerosis of coronary vessels. Radiologe. 2000;40:118-122.
2 Budoff MJ, Achenbach S, Blumenthal RS, et al. American Heart Association Committee on Cardiovascular Imaging and Intervention; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761-1791.
3 Pugliese F, Mollet NR, Hunink MG, et al. Diagnostic performance of coronary CT angiography by using different generations of multisection scanners: single-center experience. Radiology. 2008;246:384-393.
4 Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475-1497.
5 Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317-323.
6 Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478-1492.
7 Hamon M, Lepage O, Malagutti P, et al. Diagnostic performance of 16- and 64-section spiral CT for coronary artery bypass graft assessment: meta-analysis. Radiology. 2008;247:679-686.
8 Abdulla J, Abildstrom SZ, Gotzsche O, et al. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007;28:3042-3050.
9 Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol. 2006;48:1919-1928.
10 Weustink AC, Meijboom WB, Mollet NR, et al. Reliable high-speed coronary computed tomography in symptomatic patients. J Am Coll Cardiol. 2007;50:786-794.
11 Meijboom WB, van Mieghem CA, Mollet NR, et al. 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J Am Coll Cardiol. 2007;50:1469-1475.
12 Ropers U, Ropers D, Pflederer T, et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J Am Coll Cardiol. 2007;50:2393-2398.
13 Scanlon PJ, Faxon DP, Audet AM, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation. 1999;99:2345-2357.
14 Gibbons RJ, Abrams J, Chatterjee K, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines. Committee on the Management of Patients With Chronic Stable Angina. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation. 2003;107:149-158.
15 Grundy SM, Pasternak R, Greenland P, et al. AHA/ACC scientific statement. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 1999;34:1348-1359.
16 Gibbons RJ, Balady GJ, Beasley JW, et al. ACC/AHA guidelines for exercise testing: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation. 1997;96:345-354.
17 Cheitlin MD, Armstrong WF, Aurigemma GP, et al. American College of Cardiology; American Heart Association; American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2003;108:1146-1162.
18 Mieres JH, Shaw LJ, Arai A, et al. Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111:682-696.
19 Brodoefel H, Burgstahler C, Tsiflikas I, et al. Dual-source CT: effect of heart rate, heart rate variability, and calcification on image quality and diagnostic accuracy. Radiology. 2008;247:346-355.
20 Raff GL, Gallagher MJ, O’Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552-557.
21 Mahnken AH, Mühlenbruch G, Günther RW, et al. Coronary arteries and beyond. Eur Radiol. 2007;17:994-1008.
22 Pundziute G, Schuijf JD, Jukema JW, et al. Impact of coronary calcium score on diagnostic accuracy of multislice computed tomography coronary angiography for detection of coronary artery disease. J Nucl Cardiol. 2007;14:36-43.
23 Schoepf UJ, Zwerner PL, Savino G, et al. Coronary CT angiography. Radiology. 2007;244:48-63.
24 Leschka S, Wildermuth S, Boehm T, et al. Noninvasive coronary angiography with 64-section CT: effect of average heart rate and heart rate variability on image quality. Radiology. 2006;241:378-385.
25 Johnson TR, Nikolaou K, Wintersperger BJ, et al. Dual-source CT cardiac imaging: initial experience. Eur Radiol. 2006;16:1409-1415.
26 Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;95:1549-1555.
27 Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163-1170.
28 White CS, Kuo D. Chest pain in the emergency department: role of multidetector CT. Radiology. 2007;245:672-681.
29 Hoffmann U, Nagurney JT, Moselewski F, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114:2251-2260.
30 Rubinshtein R, Halon DA, Gaspar T, et al. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007;115:1762-1768.
31 Goldstein JA, Gallagher MJ, O’Neill WW, et al. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863-871.
32 Eagle KA, Berger PB, Calkins H, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation. 2002;105:1257-1267.
33 American College of Cardiology/American Heart Association Task Force on Practice GuidelinesSociety of Cardiovascular AnesthesiologistsSociety for Cardiovascular Angiography and InterventionsSociety of Thoracic SurgeonsBonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84-e231.
34 Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
35 Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43:1241-1247.
36 Cademartiri F, La Grutta L, Palumbo AA, et al. Coronary plaque imaging with multislice computed tomography: technique and clinical applications. Eur Radiol. 2006;16(Suppl 7):M44-M53.

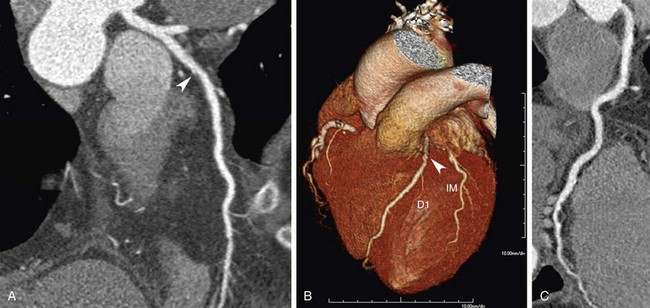
 FIGURE 34-1
FIGURE 34-1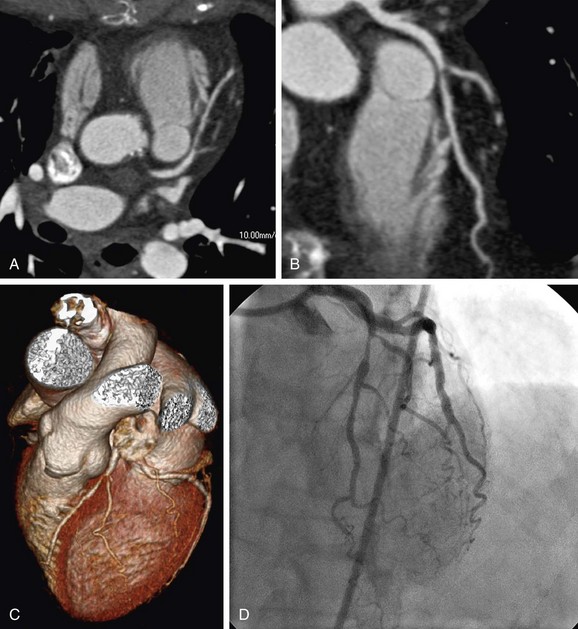
 FIGURE 34-2
FIGURE 34-2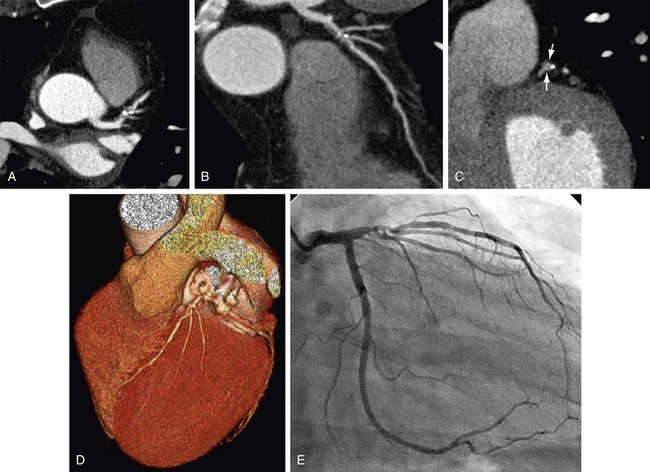
 FIGURE 34-3
FIGURE 34-3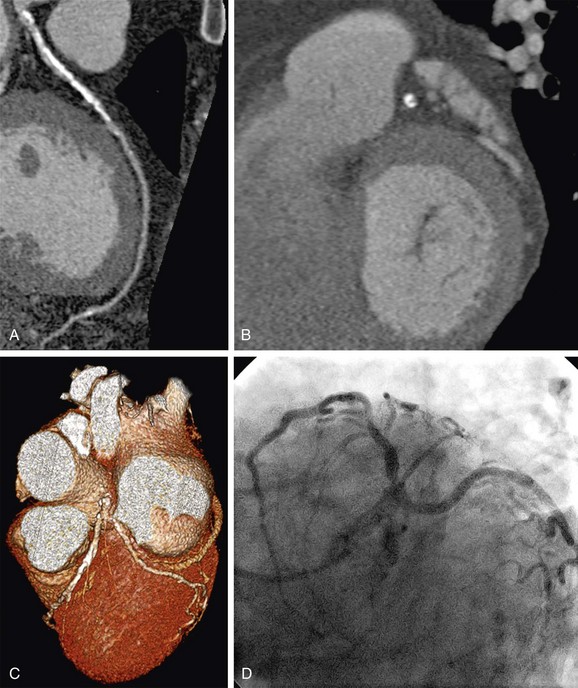
 FIGURE 34-4
FIGURE 34-4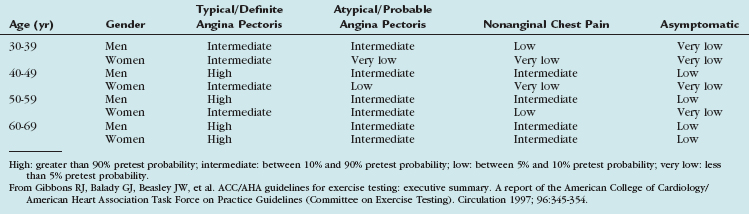
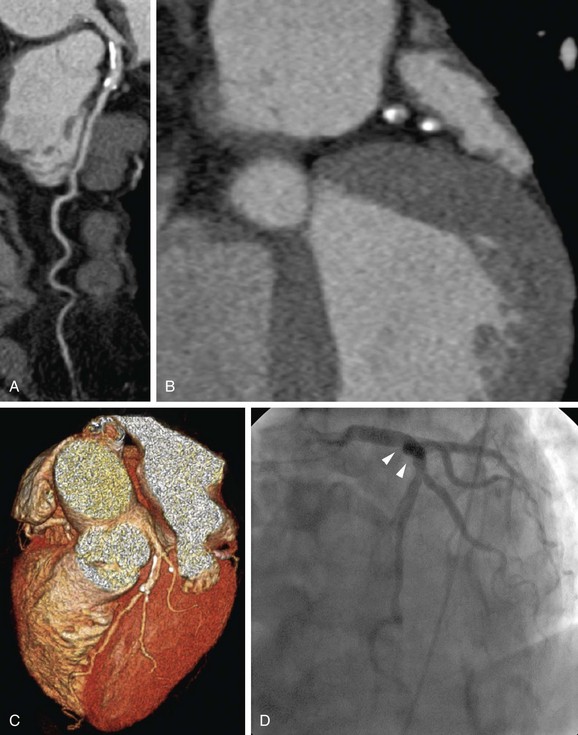
 FIGURE 34-5
FIGURE 34-5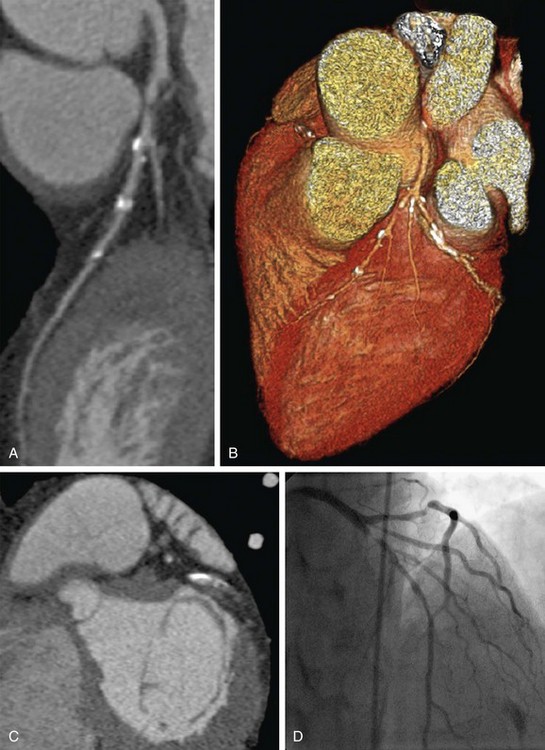
 FIGURE 34-6
FIGURE 34-6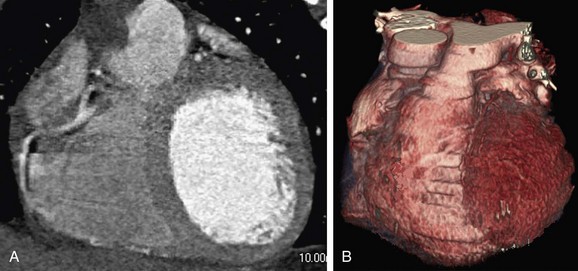
 FIGURE 34-7
FIGURE 34-7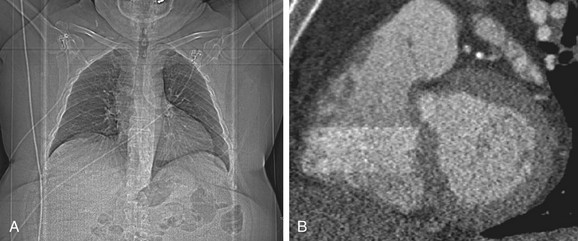
 FIGURE 34-8
FIGURE 34-8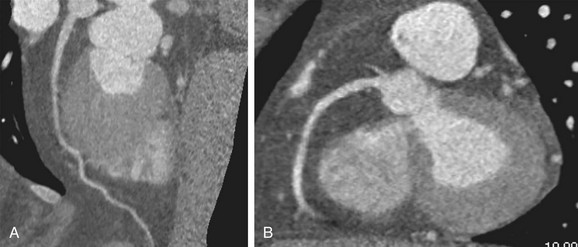
 FIGURE 34-9
FIGURE 34-9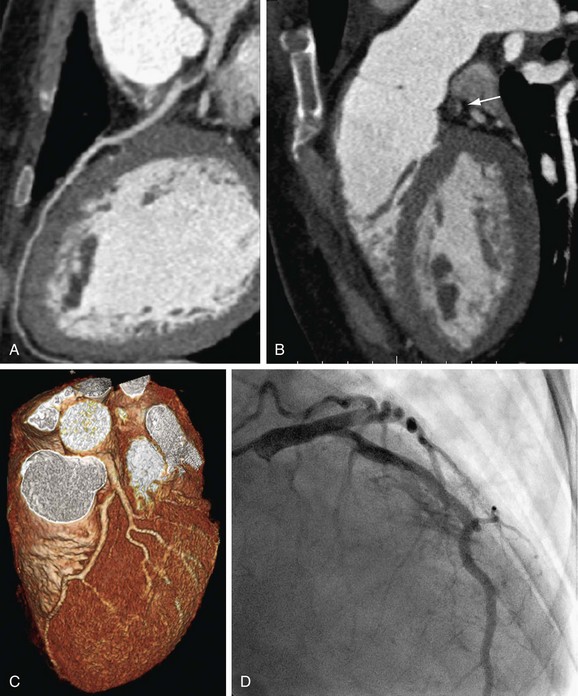
 FIGURE 34-10
FIGURE 34-10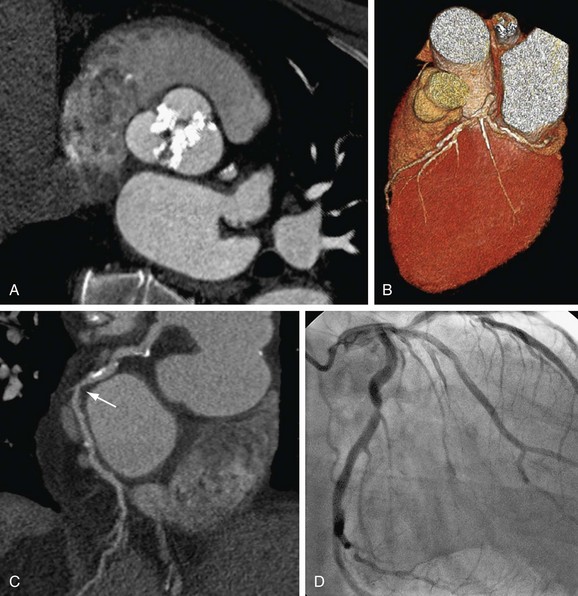
 FIGURE 34-11
FIGURE 34-11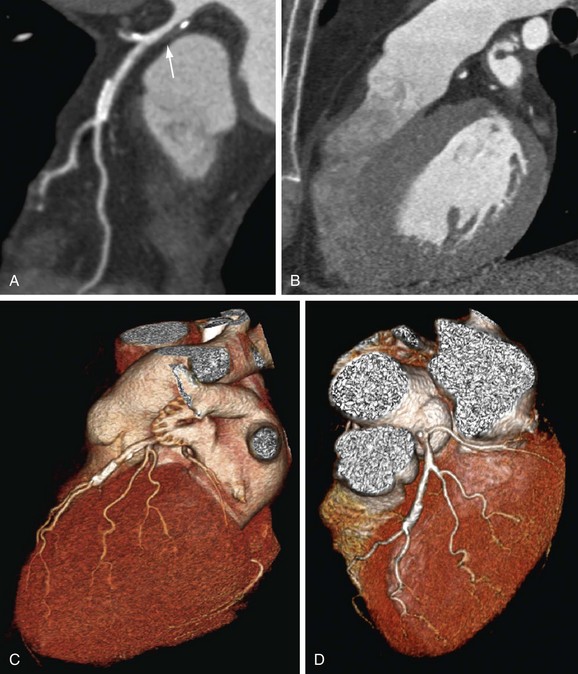
 FIGURE 34-12
FIGURE 34-12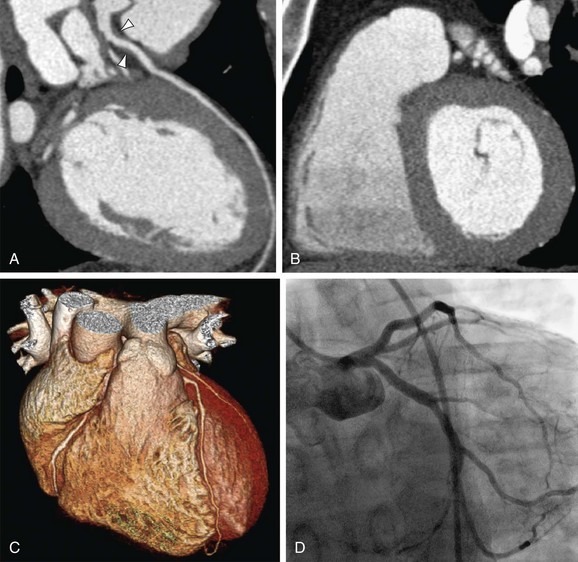
 FIGURE 34-13
FIGURE 34-13



