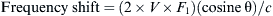Advances in Anesthesia, Vol. 28, No. 1, ** **
ISSN: 0737-6146
doi: 10.1016/j.aan.2010.07.004
Ultrasound in Central Venous Cannulation
More than 5 million central venous catheters (CVCs) are placed yearly [1] for a multitude of indications, and this number is expected to increase due to an aging population. With demands to prevent medical errors coupled with the improvement of portable ultrasound technology, the role of ultrasound guidance in central vein catheterization grows increasingly important. This article is not another meta-analysis, but looks at the significant history behind ultrasound’s prominence in guiding central venous access and provides the rationale as to why ultrasound should be adopted in current anesthesia practice. The second part of this article reviews the technical skills with which to achieve success with ultrasound in central venous access.
By the 1980s, ultrasound in central venous catheterization had already been well described [2,3]. Many of the initial studies comparing ultrasound to surface landmarks had differing methodologies that made generalization difficult. For example, the ultrasound-guided dynamic technique is likely superior to the static technique, that is, real-time guidance (visualization and identification of the relevant anatomic structures, tracking the progression of the needle, and confirmation of the guidewire within the target central vein) as compared with ultrasound being used for the sole purpose of anatomic identification and subsequent surface marking before needle insertion [4,5], yet each is considered ultrasound-assisted. In addition, there are differences in what constitutes a failure (number of attempts, an absolute time in procedure, or carotid puncture). Another confounding variation is which surface landmark approach ultrasound is to be judged against, as there are numerous landmark-based approaches. Even in this decade there are still studies [6,7] comparing the “superiority” of one landmark-based approach over another, yet it is uncertain how much operator experience and frequency of CVC placements contributes to success and complication.
Two meta-analyses are worth reviewing, as they are the most frequently cited, and often the basis for recommendations for the use of ultrasound guidance in central venous cannulation. Randolph and colleagues [8] identified 8 randomized controlled trials comparing ultrasound guidance versus traditional surface landmark-based techniques. There was a decrease in the number of placement failures in both internal jugular (relative risk [RR] 0.26; 95% confidence interval [CI] 0.11–0.58) and subclavian veins (RR 0.11; 95% CI 0.02–0.56) when ultrasound was used. The number of attempts before success also decreased with the use of ultrasound (RR 0.60; 95% CI 0.45–0.79). Of note, operator experience was not reported in many of the studies, and it was uncertain if there was any prior formal training or exposure to ultrasound concepts and equipment. In addition, the use of color flow Doppler and 2-dimensional (2D) ultrasound were mixed and considered to be equivalent.
In 2003, Hind and colleagues [9] performed a larger meta-analysis consisting of 18 trials (1646 patients) comparing 2D ultrasound with landmark-based techniques, specifically looking at failed catheter placement, catheter placement complications, number of attempts, and time to cannulation. Their results were more conclusive, as ultrasound decreased failed catheter placements both for internal jugular (RR 0.36; 95% CI 0.11–1.19) and for subclavian vein approaches (RR 0.09; 95% CI 0.02–0.38). As most of the data were based on the internal jugular approach, the time to catheterization with ultrasound was faster, 180 seconds, versus the landmark-based technique of 192 seconds (P <.0001). Though statistically significant, 12 seconds may not confer great time-saving advantage, as it may take at least that amount of time to locate the ultrasound machine. Fig. 1 shows a forest plot of the randomized controlled studies comparing failed catheter placement of 2D ultrasound versus the landmark technique at the internal jugular vein location.
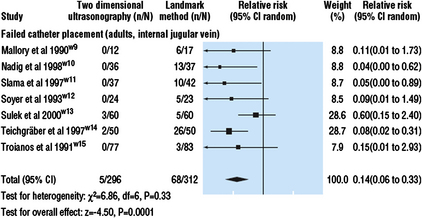
Fig. 1 Hind’s meta-analysis of ultrasound versus landmark technique for central venous access shows a success advantage for 2D ultrasound.
(Data from Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ 2003;327:361.)
Although not as extensively studied, the reviewers also supported ultrasound’s role in the subclavian and femoral venous approaches despite small sample sizes of 20 to 25 patients in each study arm. Similarly, ultrasound’s use in pediatrics was advocated in the internal jugular region based on data compiled from 3 studies with about 80 patients in each division. Newer small studies and reports have continued to show favorable outcomes with ultrasound-guided femoral [10] and subclavian vein [11] catheterizations.
These often quoted meta-analyses along with growing public and medical awareness of central line complications prompted the Agency for Healthcare Research and Quality (AHRQ) of the US Department of Health & Human Services in 2001 to issue the following statement after their own investigation: “Real-time US guidance for CVC insertion…improves catheter insertion success rates, reduces the number of venipuncture attempts prior to successful placement, and reduces the number of complications associated with catheter insertion” [12]. The British Committee for Standards in Haematology issued their guidelines [13] for central venous access insertions in 2007, recommending the use of ultrasound “for all routes of central venous catheterization,” and by 2008 the American College of Surgeons published guidelines that support the uniform use of real-time ultrasound guidance for the placement of CVCs in all patients [14]. A recent editorial has advocated that ultrasound for internal jugular central venous catheterization in the critical care setting should become the standard of care [15].
As practicing anesthesiologists, is there still a role for ultrasound? After all, the statements above did not deem it absolutely necessary for anesthesiologists to use this technology as the AHRQ wrote, “as experienced anesthesiologists can continue to place most CVCs without US guidance.” In fact, surveys on use suggest ultrasound use is actually low among anesthesiologists. Bailey and colleagues [16] sent out an electronic survey to all members of the Society of Cardiovascular Anesthesiologists and with a response rate of 35% showed that two-thirds never or almost never use ultrasound guidance versus 15% who always or almost always use it. While ultrasound availability was absent in 18% of respondents, the most common reason (46%) for not using ultrasound is the perception that it is not needed, despite almost 75% of the respondents having experienced a previous carotid puncture, 16.7% a pneumothorax, and 1.1% a stroke due to CVC placement.
Other investigators have also found that ultrasound use is low despite having access to an ultrasound machine. After the United Kingdom’s National Institute for Clinical Excellence recommended 2D ultrasound guidance, a survey of pediatric anesthetists in the United Kingdom also found lower than anticipated use. With a response rate of 63% and availability of ultrasound in 82% of the workplaces and 74% receiving 2D ultrasound training, only 26% of anesthetists with access to ultrasound used it on elective cases [17]. A separate postal survey in the United Kingdom found similar responses, with only 39% of pediatric anesthesiologists routinely using ultrasound guidance. The majority used either ultrasound or a landmark-based technique depending on the clinical circumstance, despite widespread access to and education in ultrasound [18]. However, the adaptation of this technology for central venous cannulation is taking place. A large German survey of anesthesia departments in 2007 revealed ultrasound use at 40%, much improved from 2003 when it was at 19% [19]. Thus the majority of CVCs are still placed via the landmark-based technique, and studies comparing landmark-based approaches continue to be published [7].
Ultrasound use in specialties outside of radiology is already widespread, including cardiology (transesophageal or transthoracic echocardiography), regional anesthesia for peripheral nerve blocks [20], and critical care and emergency medicine [21]. For example, transesophageal echocardiography has supplanted pulmonary artery catheterization for hemodynamic assessment, ultrasound-guided pericardiocentesis is superior to blind, electrocardiographic-guided, or fluoroscopically-guided techniques, and the focused abdominal sonogram for trauma (FAST) is often considered superior to diagnostic peritoneal lavage. Ultrasound in vascular access may be more readily embraced by emergency and internal medicine than by anesthesia. Nomura and colleagues [22] revealed a remarkable 90% ultrasound use among internal medicine and emergency medicine residents at a tertiary hospital, with most (88%) believing it to be easier than a landmark-based technique and 74% of respondents believing it should be used for all CVC placements. The investigators’ rationale for such high ultrasound use was attributed to increased adoption by the physicians-in-training versus those who have finished postgraduate training and, in addition, the presence of an emergency medicine ultrasound fellowship program.
Like many other great “discoveries” in medicine, it often takes many years before evidence-based practices are integrated at an institutional level. An editorial response to one of the surveys showing low ultrasound use suggested the implementation of hospital-wide clinical protocols for the routine use of ultrasound, as was successfully employed in the Veteran Affairs system [23]. Fig. 2 depicts the major steps in integrating evidence-based medicine in daily practice. The 2 arrows point to the adaptation points where most of medicine stands on ultrasound’s role in central venous access. Minimal new evidence from prospective randomized studies has been published on ultrasound for CVC placement in the last few years. Many local and national levels are developing the evidence-based clinical policies, on the plethora of existing data, and applying ultrasound in routine CVC placements. Of note, the American Society of Anesthesiologists (ASA) (Rupp, personal communication, 2010), National Institutes of Health, and the Cochrane Collaboration [24] are expected to release guidelines on ultrasound’s importance in central venous catheterization. It is very likely that their statements will further accelerate ultrasound adoption.
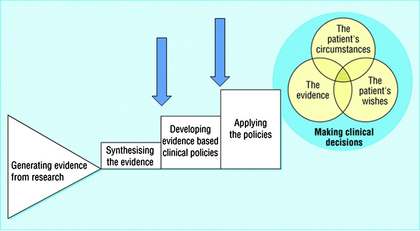
Fig. 2 Practicing evidence-based medicine requires much time and organization. The solid arrows point at the current state of ultrasound in CVC placement at many local and national levels.
(Data from Haynes B, Haines A. Barriers and bridges to evidence-based clinical practice. BMJ 1998;317:273.)
What really is, then, the incidence of complications of central venous catheterization? After all, complications [25] are often unnoticed unless severe, and more often than not they are underreported or not surveyed. Prior to ultrasound, the ASA Closed Claims project in 1996 gave some insight as to what constitutes complications [26] (Table 1). By self-reporting without an actual denominator, the registry helped promote awareness but did not create urgency despite the report of deaths associated with central venous cannulation, because these numbers appear small compared with the perceived number of central venous lines placed. In one of the earliest articles comparing ultrasound to landmark anatomy [27], carotid artery puncture occurred in 8.3% of patients and brachial plexus irritation occurred in 1.7% of patients in the traditional landmark-based technique versus 1.7% and 0.4% with ultrasound, respectively, in the “experienced” hands of cardiology fellows and attending physicians.
Table 1 The ASA Closed Claims project provides some of the dangers associated with central venous catheterization
| Complication | Total | Fatalities |
|---|---|---|
| Cardiac tamponade | 11 | 10 |
| Wire or catheter embolism | 12 | 0 |
| Vascular injuries (nonpulmonary artery): | 13 | 5 |
| Hemothonax | 6 | 4 |
| Hydrothonax | 3 | 1 |
| Carotid artery injury | 3 | 0 |
| Subclavian artery aneurysm | 1 | 0 |
| Pulmonary artery rupture | 2 | 2 |
| Pneumothorax | 7 | 1 |
| Air embolism | 2 | 2 |
| Fluid extravasation in neck | 1 | 0 |
| Total | 48 | 20 |
Data from Bowdle T. Central line complications from the ASA Closed Claims Project. ASA Newsl 1996;60:22.
Furthermore, being able to visualize the anatomic structures has other advantages. Asouhidou and colleagues [28] performed cadaveric dissections in 93 patients, and showed 3 internal jugular veins less than 6 mm in diameter with corresponding enlarged ipsilateral external jugular veins. These small internal jugular veins had no evidence of thrombosis, stenosis, and recent or previous cannulation, and traveled the usual anatomic course. This 3% of “absent” internal jugular veins was similar (2.5%) to that found in the 1991 ultrasound survey by Denys and Uretsky [29] of 200 patients in the intensive care unit and cardiac catheterization laboratory. With that discovery and an additional 2% of patients demonstrating the internal jugular vein located medial to the carotid artery, Denys and Uretsky recommended ultrasound examination before any attempted cannulation. Other investigators [30,31] have also confirmed the existence of smaller internal jugular veins, and consider this a “powerful predictor” for prolonged procedure time and carotid puncture. Mey and colleagues [30] concluded that an internal jugular vein smaller than or equal to 7 mm, when normally averaging 1.0 cm (range 0.46–3.6 cm), is an independent predictor of catheterization failure (P = .001).
Ultrasound also has benefit in reducing complications in children. In a 4-year retrospective study, Tercan and colleagues [32] compared pediatric (average age 3.3 years) with adult (56.3 years) patients and found similarly low complication rates (2.3% vs 2.4%) when ultrasound was used. No major mechanical complications such as pneumothorax or hemothorax were noted in 859 adult and 247 pediatric catheterization attempts.
Normal anatomic variations, probe position, and head rotation all influence the relationship of the internal jugular vein and carotid artery. For example, internal jugular vein duplication is estimated at 4 in 1000 patients [33]. As expected, the internal jugular vein is not always immediately anterior and lateral to the carotid artery as previously described [2]. Lin and colleagues [34] described variations in the position of the internal jugular vein in as many as 17.3% in 104 consecutive uremic patients for dialysis catheters. Another similar study in 450 nonuremic patients also noted that the “classic” anterior lateral position is found only in 79.3% on the right side and 83.5% on the left [35].
Head rotation (as is commonly used to help facilitate the classic surface landmark-based techniques in internal jugular venous cannulation) may potentially increase the risk of carotid injury. In a large ultrasound study, Troianos and colleagues [36] recorded the images of 1136 patients with normal surface anatomy for central venous placement to determine the relationship of the right internal jugular vein relative to the carotid artery. All patients were in the supine position without a pillow, with their heads rotated as far to the left as was comfortable. After independent scoring into 5 segments (completely lateral, up to 25% overlap, 25%–50%, 50%–75%, and >75% overlap) by 3 investigators, they concluded that the internal jugular vein overlies the carotid artery by 75% or more in 54% of all patients. Arai and colleagues [37] demonstrated in infants and children that head rotation to 45° as opposed to 0° at the cricoid level increases the overlap of the internal jugular vein to the carotid artery. Lieberman and colleagues [38] further investigated the influence of head rotation by comparing rotations at 0°, 15°, 30°, 45°, and 60° left of the midline in simulated catheterization of volunteer adults. This group found that the occurrence of carotid artery contact with the internal jugular vein is increased with excessive head rotation. Recommendations for optimal rotation in patients with body mass index (BMI) greater than 25 kg/m2 is 30° and 45° to 60° for patients with BMI <25 kg/m2. Fujiki and colleagues [39] also found a much higher degree of vessel overlap in obese patients when the head was rotated away from the neutral position. Thus, ultrasound may allow the operator to decide on the optimal approach, and the optimal neck positioning may indeed be neutral, contrary to prior practices for landmark-based approaches.
Of course, this would lead to the question, how often does carotid puncture occur and does it matter? The likely answer is it happens more frequently than reported. The Mayo Clinic answered this with a prospective study of 1011 consecutive cardiothoracic and vascular surgery patients, and found the incidence of carotid puncture with a “finder” needle at 9.3% with a landmark-based technique for internal jugular vein cannulation [40]. Damen and Bolton [41], in another large prospective study of 1400 patients scheduled for cardiac surgery, found an incidence of 4.8%, and in the pediatric population the reported incidence is 7% to 8% [42,43].
The adverse consequences of arterial punctures with a “finder” needle are likely less than that of dilation and placement of a large-bore catheter. Because of successful early detection of arterial puncture by pressure transduction or manometry in the Mayo study [40], actual carotid artery catheterization was avoided, with no neurologic or vascular complications noted within the first 24 hours. Thus, arterial puncture with a “small” needle, at least in this large study, did not have immediate obvious consequences of hematoma or neurologic events. However, once a large-bore (7–12F) introducer or catheter has been inserted, stroke or death is a potential complication, especially if the catheter is removed without repair by vascular surgery or interventional radiology. The Canadian Society for Vascular Surgery proposed an algorithm after retrospectively reviewing the management of catheter-related carotid injury at 3 Montreal centers in patients with inadvertent placement of large caliber (≥7F) catheters [44]. Because of its rarity, they also performed a literature review and, on evaluation together with the multicenter data, they discovered that patients who underwent open or endovascular repair had no complications versus a 47% complication rate, including death and stroke, if the catheter was removed and only direct external pressure then applied (P = .004). This prompted a letter to the editors [45] not congratulating the proposed action plan but stressing the importance of prevention and achieving competency in ultrasound-guided central venous cannulation via a rigorous education curriculum (composed of basic ultrasound physics, development of psychomotor skills, and simulation training), such that these algorithms would not have to be used.
Ultrasound by itself does not equate to increased safety. It is a tool that requires training and practice to understand its limitations. Misidentification of anatomic structures, a lack of understanding of applied ultrasound physics, and inadequate psychomotor skills to properly guide the needle to the intended target while avoiding nontarget structures can lead to complications even with ultrasound. The use of ultrasound for regional anesthesia, for example, is moving toward standardization of training processes in ultrasound [46], and whether this will promote or restrict ultrasound use is not known. Although there are no learning curve studies for central venous access, landmark or ultrasound, the increased adoption of ultrasound in regional anesthesia has given some insights on the learning curve of the psychomotor skills needed to guide a needle under ultrasound [47]. Novices quickly improved their speed and accuracy in a breast cyst aspiration model, with the most commonly committed error in 7 of 10 subjects being the failure to accurately image the needle while advancing, resulting in excessive depth and potential harm. Blaivas and Adhikari [48] designed a prospective observational study to measure the frequency of posterior vessel wall penetration for CVC for the internal jugular vein. In a vascular access mannequin, 16 of 25 residents (64%) who underwent a 2-day ultrasound course that included hands-on sessions inadvertently penetrated the posterior wall of the vein. Five of the residents (20%) mistakenly punctured the carotid artery. Most surprising, however, is that these residents had a median number of 8 previous ultrasound-guided cannulation attempts and their mean self-reported confidence of accurate placement was 8.0 out of 10 [48]. This study highlights that ultrasound use with inexperienced operators may contribute to a false sense of security and lack of self-awareness with regard to the limitations of their psychomotor skills and risk of injury to target and nontarget structures.
With ultrasound, physicians in multiple specialties all want to become proficient, yet there are no binding standards for education and training [49,51]. Short of a ban on ultrasound use by the national or local credentialing agencies, multiple specialties will continue to expand their use of ultrasound. Fortunately, many concepts, target identifications, and physical skills are now effectively taught in didactics and medical simulations [52,53] rather than by initial “practice” on actual patients, as was the case not too long ago [54].
The common assumption is that experience plays an important factor in success rate and complications, but proper education and training is likely more important because of the ability to recognize preventable dangers and incorrect placements [30]. Case reports on complications [55,56] while using ultrasound exist, and it may be useful to review Blaivas’ [56] compilations of videos on accidental arterial cannulation as an education module. The ability to interpret ultrasound images led to changes in management in 14% of the small series of patients studied by Gann and Sardi [57] undergoing port placement. Similarly, ultrasound detected asymptomatic thromboses in the internal jugular vein in 4 of 55 obese patients, and allowed for early detection and correction of malpositioned catheters [58]. The ability to visualize the guidewire in the lumen of the target venous structure before subsequent dilation and cannulation may serve to be a useful confirmatory and complementary step to pressure manometry in order to avoid unintended arterial cannulation [59].
As technology improves and the expense of machines decreases, cost analyses should strongly favor ultrasound in addition to the other evidence for use presented earlier. As early as 2003 Hind and colleagues [9] calculated a hypothetical cost saving of £2000 for every 1000 procedures even after incorporating machine acquisition and education costs. With Medicare and Medicaid no longer paying for complications, hospitals have refocused their efforts and budgets on prevention.
The investigators of the Third Sonography Outcomes Assessment Program (SOAP-3) Trial [60] compared the overall success rate of internal jugular venous cannulation with the use of anatomic landmark-based techniques (LM), ultrasound-guided static (S) techniques (quick-look visualization of the target vessels to evaluate the optimal approach and mark the skin over the intended needle insertion site), and dynamic (D) techniques (direct real-time visualization of needle advancement and entrance into the target vessel). The results, controlled for pretest difficulty assessment, were reported as odds improvement (95% CIs) over LM for D and S. D was shown to have an odds 53.5 (95% CI 6.6–440) times higher success than LM, and S had an odds 3 (95% CI 1.3–7) times higher success than LM, with an unadjusted success rate of cannulation of 98% (D), 82% (S), and 64% (LM). In addition, for first-attempt success, D had an odds of 5.8 (95% CI 2.7–13) times higher success compared with LM, and S had an odds of 3.4 (95% CI 1.6–7.2) times higher success compared with LM, with an unadjusted first-attempt success of 62% (D), 50% (S), and 23% (LM). The investigators concluded that both ultrasound techniques were superior to landmark-based techniques and that D was superior to S, but may require more training (Table 2). The second part of this article describes many of the technical skills needed to help employ ultrasound in CVC placement.
Technical considerations
Short-axis (SAX) and long-axis (LAX) views may be used to visualize and guide cannulation of the target vessel. The SAX view provides visualization of the target vessel in the transverse (axial) plane, and is obtained by placing the long axis of the transducer perpendicular to the long axis of the vessel. To obtain a SAX view of a vessel, the probe is positioned on the patient (Fig. 3) so that the target vessel is viewed as a dark, anechoic circular structure (Fig. 4). When the transducer is centered over the target vessel, the midpoint of the transducer serves as the reference point for introduction of the access needle. The LAX view provides visualization of the target vessel in the longitudinal (sagittal) plane and is obtained by placing the long axis of the transducer parallel to the long axis of the vessel, producing a sonographic image along the length of the vessel. For a long-axis view of a vessel, the probe is rotated 90° from the short-axis view (Fig. 5). The vessel will now appear as a dark, anechoic structure extending across the ultrasound screen (Fig. 6). The transducer is then oriented to view the vessel at widest anterior-posterior diameter. In contrast to the SAX view, the LAX view allows only a single vessel to be maintained in the imaging plane (field of view) and is not suitable to define the anatomic relationships between vessels.
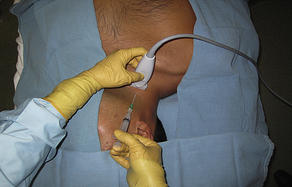
Fig. 3 Short-axis (vessel), out-of-plane (needle) internal jugular cannulation technique with linear array ultrasound transducer. Note: To better illustrate the probe position, the ultrasound probe cover has been removed. For actual placement of a central line, a complete sterile field is required, including a sterile ultrasound probe cover.
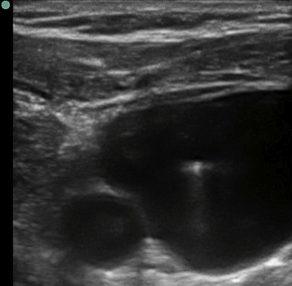
Fig. 4 Ultrasound image of a short-axis (vessel), out-of-plane (needle) internal jugular cannulation with a linear array transducer. Left side of image is medial, right side is lateral. Small anechoic (dark) circle in bottom left (medial) = carotid artery. Large anechoic (dark) area (internal jugular vein) has a hyperechoic (bright) dot (needle) in the vessel lumen.
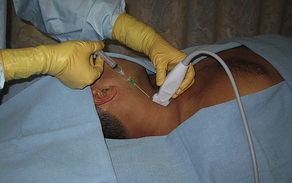
Fig. 5 Long-axis (vessel), in-plane (needle) internal jugular cannulation technique using a linear array transducer. The transducer is positioned to obtain an appropriate long-axis view of the target vessel.
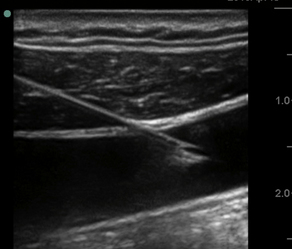
Fig. 6 Ultrasound image of long-axis (vessel), in-plane (needle) internal jugular cannulation technique using a linear array transducer. Cranial linear needle entry (right side of image) with tip of needle within lumen of internal jugular vein (dark, anechoic area). The needle tip is advanced in-plane aligned with the long axis of the transducer. Imaging the needle entry into the lumen of the vessel and aspiration of venous blood confirm access of the target vessel.
As with landmark-based techniques, Trendelenburg (head-down) positioning for internal jugular or subclavian line insertion is recommended for ultrasound-guided procedures. Increasing venous pressure from proper positioning results in larger vein targets, and the increased venous pressure may also limit the compression of the vein during needle entry [61].
As previously noted, ultrasound may be used for guidance to a central vein in 2 ways: static and dynamic. Static ultrasound uses the image to locate the vein. Once the vein is located, a mark is made on the patient. After the mark is made, the ultrasound is removed from the field and the procedure continues in a similar fashion to landmark techniques, but using the mark for the initial needle entry site. Dynamic ultrasound, sometimes referred to as real-time ultrasound, uses ultrasound during needle insertion to visualize the needle and the vessel together. Both of these techniques, static and dynamic, have been shown to improve initial success rates [60,62,63] as well as to decrease access time and arterial puncture [63,65]. Recent evidence indicates that ultrasound guidance also significantly improves the success rate and decreases the number of attempts and complications for related femoral vein dialysis catheter insertion [10].
Appropriate target vessels for central venous cannulation with ultrasound include the internal jugular vein, subclavian vein, and femoral vein. The internal jugular vein can be located on either side of the neck, in close proximity to the carotid artery. The internal jugular vein, though classically described as being superficial and lateral to the artery, can be found in various orientations to the carotid artery, even medial [66]. The subclavian vein is adjacent, caudal, and anterior to the subclavian artery. The subclavian vein can be located more easily with ultrasound laterally and then traced medially (Fig. 7). As the vein is traced medially, it will become shallower and then ultimately course underneath the clavicle (Fig. 8). Because the ultrasound beam cannot penetrate though bone, the needle entry site for the subclavian vein using ultrasound will likely be more lateral than with landmark approaches to the same vein without ultrasound. The femoral vein is a direct continuation of the popliteal vein and becomes the external iliac vein at the level of the inguinal ligament. The femoral vein is found medial to the artery in the inguinal region. The optimal cannulation site for femoral vein cannulation will be 2–3 cm distal to the inguinal ligament, ensuring that venipuncture occurs caudal to the inguinal ligament, which decreases the risk of a retroperitoneal hematoma in the event of puncture of the external iliac artery. As the femoral vein runs cranially, it will course deep into the pelvis along the anterior surface of the iliopsoas muscle to become the external iliac vein.
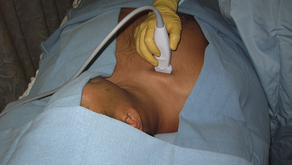
Fig. 7 Subclavian long-axis (vessel) ultrasound probe position just caudal to the clavicle, with linear array transducer.
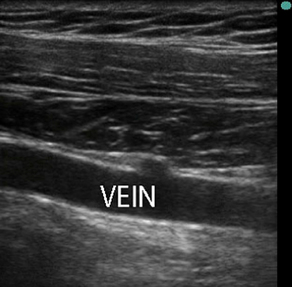
Fig. 8 Ultrasound image corresponding to transducer position illustrated in Fig. 7. The subclavian vein in long axis is anechoic (dark). The location of the subclavian vein on the left side of image is proximal and shallower (anterior) than the location on the right side (distal aspect of subclavian vein) of the image.
Selection of a catheter insertion site should ultimately depend on the indication catheter insertion and the characteristics of the patient. Although femoral venous cannulation has several practical advantages (directly compressible, away from the airway and pleura, does not typically require Trendelenburg position), it is associated with the higher catheter infection rates and more frequent thrombotic complications [67]. Therefore, in most controlled settings, selection of an internal jugular or subclavian central venous line is usually preferred. Ultrasound guidance for internal jugular cannulation is easier because the internal jugular vein is much shallower than the subclavian vein. However, if the practitioner has more experience with subclavian line placement, that may be the preferred insertion site.
Before cannulation, target vessels must be identified properly, especially to distinguish artery from vein. Using a SAX imaging plane, the artery and vein can be imaged simultaneously so each can be positively identified. Ultrasound characteristics of arteries include pulsatility, resistance to compression, and imaged walls that are often thicker than veins. Veins may also be pulsatile, especially if imaged proximally, but are often easy to compress with pressure exerted on the ultrasound transducer.
Color flow Doppler (CFD) allows for the identification and quantification of blood flow and can be helpful to distinguish an artery from a vein. The Doppler principle states that if an ultrasound pulse is sent out and strikes moving red blood cells, the ultrasound that is reflected back to the transducer will have a frequency that is different from the original emitted frequency. This change in frequency is known as the Doppler shift. The Doppler equation states that:
where V is the velocity of the red blood cells, F1 is the transmitted frequency, θ is the angle incidence of the ultrasound beam and the direction of blood flow, and c is the speed of the ultrasound in the media. It is this change in frequency that can be used to calculate (and subsequently displayed on the ultrasound screen) the presence (or absence) of blood flow and velocity. CFD on ultrasound is usually represented on the ultrasound screen as either red or blue. The red or blue color on the ultrasound image does not signify oxygenated (arterial) blood or deoxygenated (venous) blood. Rather, the color on the ultrasound image represents flow coming toward or away from the ultrasound transducer based on the Doppler principle. The brightness of the color, either red or blue, relates to the velocity of the blood flow. A brighter color represents a higher velocity of blood flow in that given direction. If a structure appears red when using CFD, this signifies that the blood flow is moving toward the transducer (the frequency of the returning signal is higher than the original emitted frequency). If a structure appears blue when using CFD, blood flow is moving away from the transducer (the frequency of the returning signal is lower than the original emitted frequency). A mix of color may signify turbulent blood flow, or flow that surpasses the maximum setting of the flow that can be measured (Nyquist limit). Sometimes there will be no color on the ultrasound image in a structure that may be a blood vessel. The angle between the flow of blood and the ultrasound beam must be either greater or less than 90°, as the cosine of 90° is 0; this means that the probe must be tilted in one direction or the other (away from perpendicular) to detect blood flow with CFD. A useful method to detect flow in blood vessels that are not identified by CFD is to use color power Doppler mode (CPD, which has a higher sensitivity for detecting low flow and is not dependent on θ). A useful clinical pearl to detect flow in the subclavian vein or femoral vein is to squeeze the distal extremity (arm or thigh, respectively) to increase venous return to the central venous system. Often, this will help identify a venous structure using color Doppler.
If dynamic needle visualization with ultrasound is used, it is important to visualize the needle image as best as possible as the needle approaches the target vessel, whether in-plane or out-of-plane. The ultrasound waves must contact the needle and be reflected back at the ultrasound transducer. If the needle is advanced to the target with a trajectory that is more parallel to the ultrasound beam, the ultrasound waves will not be reflected by needle well. The ultrasound image in this situation will result in a needle with low visibility. Therefore, clinically, it is important to advance the needle as perpendicular to the oncoming ultrasound beam as possible to image the needle well in both out-of-plane (single dot representing a cross section of the needle) or in-plane (entire needle shaft and tip). To keep the needle’s angle of incidence flat, start the needle entry site 1 to 3 cm away from the ultrasound probe for vessel cannulation (see Fig. 3).
In-plane techniques have the needle advancing to the target in the plane of the ultrasound beam. If performed correctly, in-plane approaches allow the entire needle (shaft and tip) to be visualized as it approaches and enters the target vessel. However, in-plane techniques can be difficult because keeping the needle and/or vessel in-plane throughout the procedure can be difficult. Also, arteries and veins often lie in close proximity. Any slight dislocation or tilt of the ultrasound transducer may inappropriately target the nearby artery, resulting in arterial cannulation, possibly leading to significant mechanical complications. In addition, not all vessels continue in a straight line. If vessels are tortuous, it may be difficult to keep the target in a long-axis view for an in-plane approach.
The out-of-plane needle technique most closely resembles needle insertion techniques used for vessel cannulation with landmark-based techniques. Some clinicians prefer out-of-plane needle technique because they are more comfortable with the traditional techniques to cannulate blood vessels (peripheral intravenous and arterial cannulation). However, out-of-plane needle techniques may not allow visualization of the tip of the needle at all times and therefore, potentially do not offer the same level of confidence that comes with always knowing where the needle tip is located. Robust technique is required to localize the needle tip with out-of-plane approaches. Two such techniques are described in the following paragraphs.
The out-of-plane technique to needle insertion appears simple, but can be difficult. The major disadvantage with the out-of-plane technique is that a needle will appear as a hyperechoic, bright dot on the ultrasound screen. This bright dot simply represents a cross section of the long axis of the needle (and is often assumed to be the tip of the needle). However, the dot can also be the shaft of the needle, and the tip can be much deeper than the bright dot on the ultrasound screen, giving the user a false belief that the tip is shallower than it actually is.
Optimal out-of-plane needle technique follows the tip of the needle as it is advanced through tissue toward the target vessel. There are 2 recommended techniques to follow the needle tip:
These 2 techniques require one important quality when looking for the bright (hyperechoic) dot of the needle: the bright dot must appear (as it crosses the plane of the ultrasound beam), then disappear, then appear again as it is advanced through the tissue. If the dot does not disappear, there is no way to rigorously confirm the dot is the needle tip.
Sliding Probe Technique
The needle is advanced out-of-plane until a bright dot is visualized above (shallow) the target. Once this dot is visualized, needle movement stops. The probe is then advanced forward (away from the needle) until the dot disappears. The needle is then advanced again, until the dot reappears. The dot should now be deeper and closer to the target. The probe is advanced until the dot disappears. The needle is then re-advanced. These steps are repeated until the dot is near the target vessel and subsequently enters the lumen of the target vessel (accompanied by presence of blood in the syringe while exerting continuous application of negative pressure). The dot must appear and disappear as the needle and probe are alternately advanced. This way, the tip is confirmed as it approaches the nerve or vessel target.
Tilting Probe Technique
Very similar to the sliding technique, this allows the probe to stay in one spot and may be useful in tighter areas where the probe cannot slide very far. The needle is advanced out-of-plane until a bright dot is visualized above (shallow) the target. Once this dot is visualized, needle movement stops. The ultrasound beam is then tilted forward (away from the needle) until the dot disappears. The needle is then advanced again, until the dot reappears. The dot should now be deeper and closer to the target. The ultrasound beam is again tilted forward until the dot disappears. The needle is then re-advanced. These steps are repeated until the dot is near the target. The dot must appear and disappear as the needle and probe are alternately moved. This way, the tip is confirmed as it approaches the nerve or vessel target.
As mentioned previously, despite all the advantages ultrasound confers to central line placement, morbidity and mortality persists. Therefore, confirmatory methods of accurate needle placement in a vein should be used including pressure measurement (waveform or manometry), continuous electrocardiography (ectopy), and/or ultrasound visualization of the guidewire, fluoroscopy, or venous blood gas.
Summary
Anesthesiology trains for the unexpected. Malignant hyperthermia, intraoperative awareness, and permanent neurologic injuries are examples where emphasis and education has taken place to decrease mortality and morbidity of infrequent but serious complications.
Complications exist for central venous catheterization, likely a few magnitudes more common than those mentioned earlier, and the evidence for the routine use of ultrasound-guided central venous access to decrease, though not necessarily eliminate complications is strong. The various techniques for ultrasound-guided central venous cannulation should be systematically taught within a structured educational curriculum along with hands-on simulation (in the form of phantom gels or specially designed mannequins) to optimize both procedural success and patient safety. Based on the increasing applications of ultrasound technology in the perioperative setting and the emerging evidence for its benefits, ultrasound-guided central venous cannulation will likely increase and potentially become the standard of care in the future.
References
[1] D. McGee, M. Gould. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123.
[2] S. Metz, J.C. Horrow, I. Balcar. A controlled comparison of techniques for locating the internal jugular vein using ultrasonography. Anesth Analg. 1984;63:673.
[3] M. Tryba, P. Kleine, M. Zenz. [Sonographic studies for optimizing the cannulation of the internal jugular vein]. Anaesthesist. 1982;31:626. [in German]
[4] K. Hosokawa, N. Shime, Y. Kato, et al. A randomized trial of ultrasound image-based skin surface marking versus real-time ultrasound-guided internal jugular vein catheterization in infants. Anesthesiology. 2007;107:720.
[5] Pittiruti M, LaGreca A, Scoppettuolo G, et al. Ultrasound-guided vs ultrasound-assisted central venous catheterization. In: 27th International Symposium on Intensive Care and Emergency Medicine. Brussels (Belgium): 2007. p. 158.
[6] M. Pittiruti, M. Malerba, C. Carriero, et al. Which is the easiest and safest technique for central venous access? A retrospective survey of more than 5,400 cases. J Vasc Access. 2000;1:100.
[7] W. Xiao, F. Yan, H. Ji, et al. A randomized study of a new landmark-guided vs traditional para-carotid approach in internal jugular venous cannulation in infants. Paediatr Anaesth. 2009;19:481.
[8] A.G. Randolph, D.J. Cook, C.A. Gonzales, et al. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24:2053.
[9] D. Hind, N. Calvert, R. McWilliams, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327:361.
[10] M.V. Prabhu, D. Juneja, P.B. Gopal, et al. Ultrasound-guided femoral dialysis access placement: a single-center randomized trial. Clin J Am Soc Nephrol. 2010;5:235.
[11] N. Sakamoto, Y. Arai, Y. Takeuchi, et al. Ultrasound-guided radiological placement of central venous port via the subclavian vein: a retrospective analysis of 500 cases at a single institute. Cardiovasc Intervent Radiol. 2010. [online]
[12] J. Rothschild. K.G. Shojania, B.W. Duncan, K.M. McDonald, et al, editors. Making health care safer: a critical analysis of patient safety practices [AHRQ Evidence Reports no. 43]. Rockville, MD: Association for Healthcare Research and Quality (AHRQ). 2001.
[13] L. Bishop, L. Dougherty, A. Bodenham, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol. 2007;29:261.
[14] Surgeons ACo: College’s Committee on Perioperative Care. Statement on recommendations for uniform use of real-time ultrasound guidance for placement of central venous catheters. Bull Am Coll Surg. 2008;93:35.
[15] D. Feller-Kopman. Ultrasound-guided central venous catheter placement: the new standard of care? Crit Care Med. 1875;33:2005.
[16] P.L. Bailey, L.G. Glance, M.P. Eaton, et al. A survey of the use of ultrasound during central venous catheterization. Anesth Analg. 2007;104:491.
[17] G. Tovey, M. Stokes. A survey of the use of 2D ultrasound guidance for insertion of central venous catheters by UK consultant paediatric anaesthetists. Eur J Anaesthesiol. 2007;24:71.
[18] M. Bosman, R.J. Kavanagh. Two dimensional ultrasound guidance in central venous catheter placement; a postal survey of the practice and opinions of consultant pediatric anesthetists in the United Kingdom. Paediatr Anaesth. 2006;16:530.
[19] W. Schummer, S.G. Sakka, E. Huttemann, et al. [Ultrasound guidance for placement control of central venous catheterization. Survey of 802 anesthesia departments for 2007 in Germany]. Anaesthesist. 2009;58:677. [in German]
[20] P. Marhofer, W. Harrop-Griffiths, S.C. Kettner, et al. Fifteen years of ultrasound guidance in regional anaesthesia: Part 1. Br J Anaesth. 2010;104:538-546.
[21] M.A. Johnson, L. McKenzie, S. Tussey, et al. Portable ultrasound: a cost-effective process improvement tool for PICC placement. Nurs Manage. 2009;40:47.
[22] J.T. Nomura, P.R. Sierzenski, J.E. Nace, et al. Cross sectional survey of ultrasound use for central venous catheter insertion among resident physicians. Del Med J. 2008;80:255.
[23] R. Bjerke. Survey of specialists shows we are not special. Anesth Analg. 2007;105:879. [author reply 879]
[24] Brass P, Hellmich M, Kolodziej L, et al. Traditional landmark versus ultrasound guidance for central vein catheterization (Protocol). The Cochrane Library; 2009 [online].
[25] R.J. Defalque, M.V. Fletcher. Neurological complications of central venous cannulation. JPEN J Parenter Enteral Nutr. 1988;12:406.
[26] T. Bowdle. Central line complications from the ASA Closed Claims Project. ASA Newsl. 1996;60:22.
[27] B.G. Denys, B.F. Uretsky, P.S. Reddy. Ultrasound-assisted cannulation of the internal jugular vein. A prospective comparison to the external landmark-guided technique. Circulation. 1993;87:1557.
[28] I. Asouhidou, K. Natsis, T. Asteri, et al. Anatomical variation of left internal jugular vein: clinical significance for an anaesthesiologist. Eur J Anaesthesiol. 2008;25:314.
[29] B.G. Denys, B.F. Uretsky. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19:1516.
[30] U. Mey, A. Glasmacher, C. Hahn, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer. 2003;11:148.
[31] A.N. Sibai, E. Loutfi, M. Itani, et al. Ultrasound evaluation of the anatomical characteristics of the internal jugular vein and carotid artery—facilitation of internal jugular vein cannulation. Middle East J Anesthesiol. 2008;19:1305.
[32] F. Tercan, L. Oguzkurt, U. Ozkan, et al. Comparison of ultrasonography-guided central venous catheterization between adult and pediatric populations. Cardiovasc Intervent Radiol. 2008;31:575.
[33] J.M. Prades, A. Timoshenko, J.M. Dumollard, et al. High duplication of the internal jugular vein: clinical incidence in the adult and surgical consequences, a report of three clinical cases. Surg Radiol Anat. 2002;24:129.
[34] B.S. Lin, C.W. Kong, D.C. Tarng, et al. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13:134.
[35] D. Dolla, F. Cavatorta, S. Galli, et al. Anatomical variations of the internal jugular vein in nonuremic outpatients. J Vasc Access. 2001;2:60.
[36] C.A. Troianos, R.J. Kuwik, J.R. Pasqual, et al. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85:43.
[37] T. Arai, Y. Matsuda, K. Koizuka, et al. Rotation of the head might not be recommended for internal jugular puncture in infants and children. Paediatr Anaesth. 2009;19:844.
[38] J.A. Lieberman, K.A. Williams, A.L. Rosenberg. Optimal head rotation for internal jugular vein cannulation when relying on external landmarks. Anesth Analg. 2004;99:982.
[39] M. Fujiki, C.G. Guta, H.J. Lemmens, et al. Is it more difficult to cannulate the right internal jugular vein in morbidly obese patients than in nonobese patients? Obes Surg. 2008;18:1157.
[40] W.C. OliverJr., G.A. Nuttall, F.M. Beynen, et al. The incidence of artery puncture with central venous cannulation using a modified technique for detection and prevention of arterial cannulation. J Cardiothorac Vasc Anesth. 1997;11:851.
[41] J. Damen, D. Bolton. A prospective analysis of 1,400 pulmonary artery catheterizations in patients undergoing cardiac surgery. Acta Anaesthesiol Scand. 1986;30:386.
[42] C. Rey, F. Alvarez, V. De La Rua, et al. Mechanical complications during central venous cannulations in pediatric patients. Intensive Care Med. 2009;35:1438.
[43] S.C. Nicolson, M.F. Sweeney, R.A. Moore, et al. Comparison of internal and external jugular cannulation of the central circulation in the pediatric patient. Crit Care Med. 1985;13:747.
[44] M.C. Guilbert, S. Elkouri, D. Bracco, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48:918.
[45] J. Smith. Regarding “Arterial trauma during central venous catheter insertion: case series, review, and proposed algorithm”. J Vasc Surg. 2009;49:1363. [discussion: 1363]
[46] B.D. Sites, V.W. Chan, J.M. Neal, et al. The American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anaesthesia and Pain Therapy Joint Committee recommendations for education and training in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2010;35:S74.
[47] B.D. Sites, J.D. Gallagher, J. Cravero, et al. The learning curve associated with a simulated ultrasound-guided interventional task by inexperienced anesthesia residents. Reg Anesth Pain Med. 2004;29:544.
[48] M. Blaivas, S. Adhikari. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37:2345.
[49] S. Price, G. Via, E. Sloth, et al. Echocardiography practice, training and accreditation in the intensive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;6:49.
[50] D. Thys, M. Abel, R. Brooker, et al. Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084.
[51] A.C. Lee, C. Thompson, J. Frank, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11:343.
[52] M. Ursino, J.L. Tasto, B.H. Nguyen, et al. CathSim: an intravascular catheterization simulator on a PC. Stud Health Technol Inform. 1999;62:360.
[53] J.H. Barsuk, W.C. McGaghie, E.R. Cohen, et al. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37:2697.
[54] Gawande A. The learning curve: like everyone else, surgeons need practice. That’s where you come in. New York: The New Yorker Magazine; 2002.
[55] A.J. Parsons, J. Alfa. Carotid dissection: a complication of internal jugular vein cannulation with the use of ultrasound. Anesth Analg. 2009;109:135.
[56] M. Blaivas. Video analysis of accidental arterial cannulation with dynamic ultrasound guidance for central venous access. J Ultrasound Med. 2009;28:1239.
[57] M. GannJr., A. Sardi. Improved results using ultrasound guidance for central venous access. Am Surg. 2003;69:1104.
[58] C. Brusasco, F. Corradi, P.L. Zattoni, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19:1365.
[59] M.B. Stone, A. Nagdev, M.C. Murphy, et al. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28:82.
[60] T.J. MillingJr., J. Rose, W.M. Briggs, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33:1764.
[61] S. Samy Modeliar, M.A. Sevestre, B. de Cagny, et al. Ultrasound evaluation of central veins in the intensive care unit: effects of dynamic manoeuvres. Intensive Care Med. 2008;34:333.
[62] D. Legler, M. Nugent. Doppler localization of the internal jugular vein facilitates central venous cannulation. Anesthesiology. 1984;60:481.
[63] R. Bansal, S.K. Agarwal, S.C. Tiwari, et al. A prospective randomized study to compare ultrasound-guided with nonultrasound-guided double lumen internal jugular catheter insertion as a temporary hemodialysis access. Ren Fail. 2005;27:561.
[64] D.L. Mallory, W.T. McGee, T.H. Shawker, et al. Ultrasound guidance improves the success rate of internal jugular vein cannulation. A prospective, randomized trial. Chest. 1990;98:157.
[65] D. Karakitsos, N. Labropoulos, E. De Groot, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10:R162.
[66] A.C. Gordon, J.C. Saliken, D. Johns, et al. US-guided puncture of the internal jugular vein: complications and anatomic considerations. J Vasc Interv Radiol. 1998;9:333.
[67] J. Merrer, B. De Jonghe, F. Golliot, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700.