Chapter 16 Implantation of Intrathecal Drug Delivery Systems
Indications
Implantation of an intrathecal drug delivery system is indicated for:
 Long-term infusion of baclofen for severe spasticity of spinal or cerebral origin that responds to baclofen
Long-term infusion of baclofen for severe spasticity of spinal or cerebral origin that responds to baclofenContraindications
Contraindications to implantation of an intrathecal drug delivery system are as follows:
Instrumentation
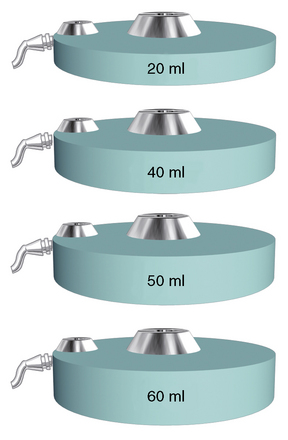
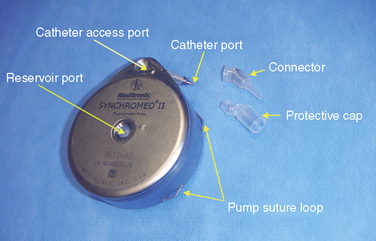
Figure 16–1 Various sizes of Tricumed intrathecal pumps (Tricumed Medizintechnik GmbH, Kiel, Germany).
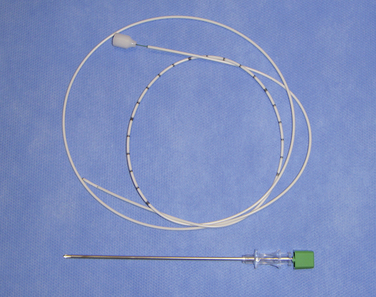
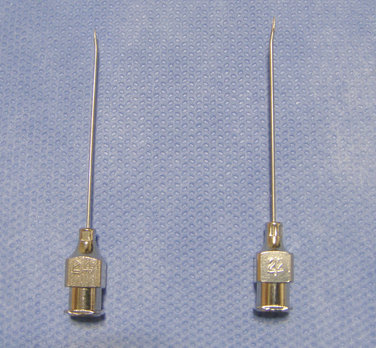
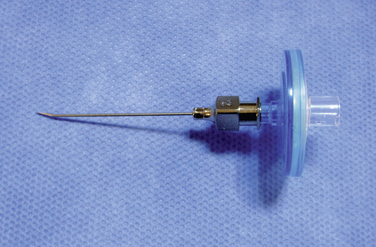
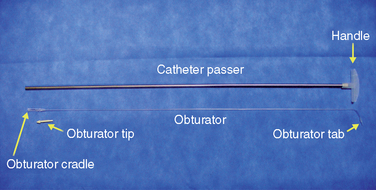
Figure 16–5 A catheter with a stylet (top) and a 15-gauge spinal needle (bottom) for spinal catheterization.
Drugs needed for implantation of an intrathecal drug delivery system are as follows:
Preoperative preparation
Physical examination should include the following actions:
 Nonsteroidal anti-inflammatory drug (NSAID), aspirin, and/or anticoagulant therapy should be stopped for 3 to 7 days before and after the procedure.
Nonsteroidal anti-inflammatory drug (NSAID), aspirin, and/or anticoagulant therapy should be stopped for 3 to 7 days before and after the procedure.Patient and caregiver education should be performed as follows:
 Explain that if the pump is not explanted at the patient’s death, the pump and alarm should be inactivated.
Explain that if the pump is not explanted at the patient’s death, the pump and alarm should be inactivated.Procedure
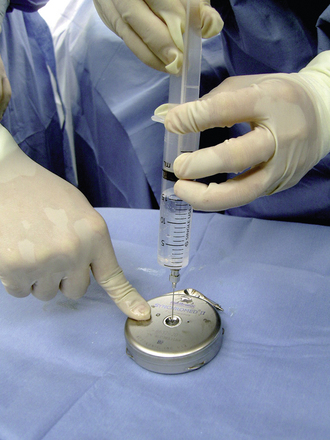
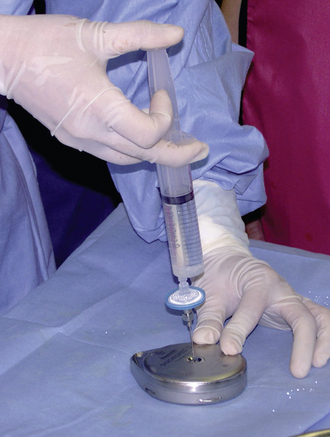
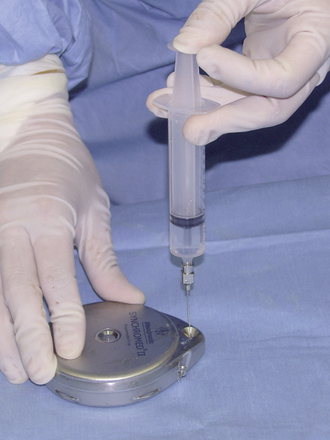
Step 1: Pump Preparation
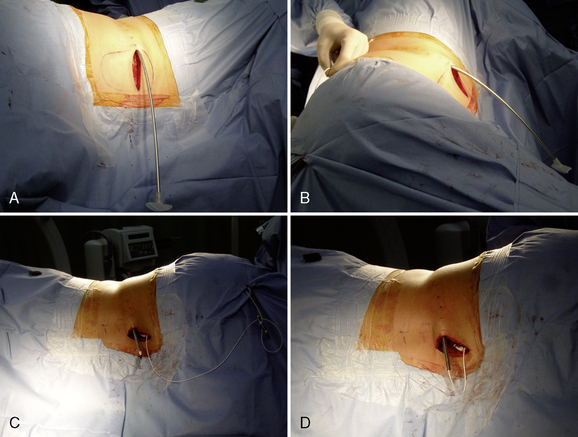
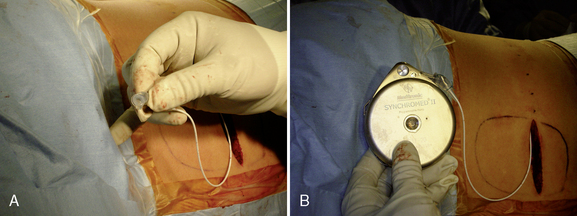
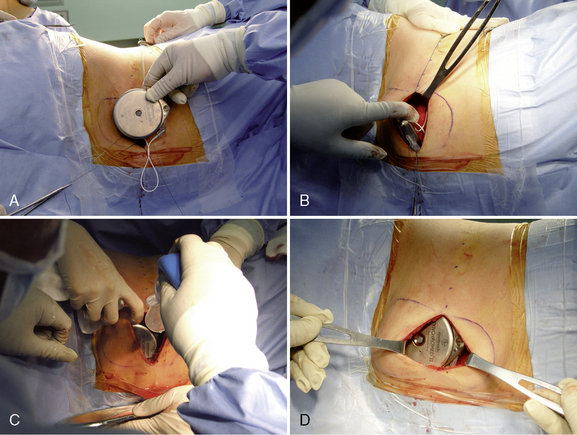
Step 2: Implant Procedure
The catheter is often implanted while an assistant prepares the pump.
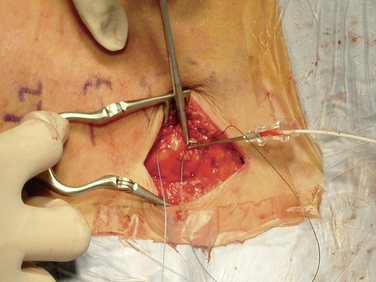
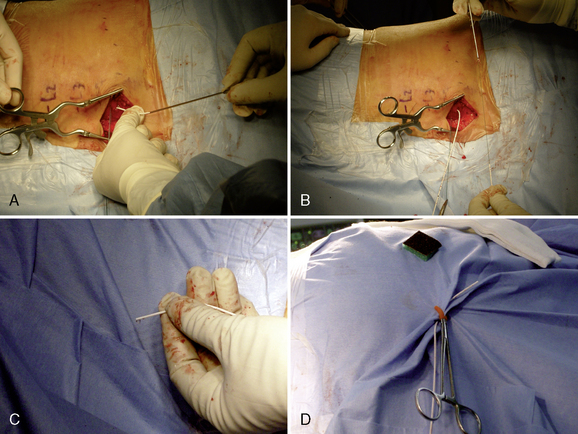
Figure 16–17 A pursestring suture is placed around the Tuohy needle and will be tied after the needle has been removed.
Postprocedural management
After implantation of an intrathecal drug delivery system, the following actions should be taken
Program the pump
Complete the four primary steps in determining the postoperative priming bolus, as follows:
Complications
The catheter problems that may occur are as follows:
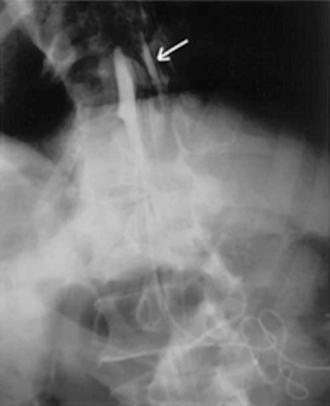
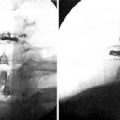
Figure 16–24 Standard radiograph with injection of contrast agent via the side port shows a leak in the subdural space (arrow).
(From Pasquier Y, Schnider A, Cahana A. Subdural catheter migration may lead to baclofen pump dysfunction. Spinal Cord 2003;41:700-702.)
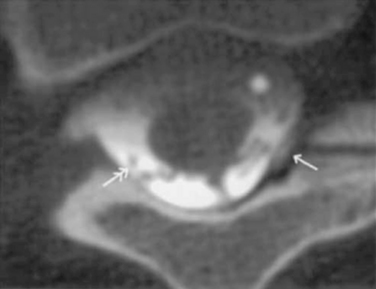
Figure 16–25 Computed tomography scan showing leak in the subdural space (single arrow) and catheter tip (double arrow).
(From Pasquier Y, Schnider A, Cahana A. Subdural catheter migration may lead to baclofen pump dysfunction. Spinal Cord 2003;41:700-702.)
The following pump problems may be seen after implantation of an intrathecal drug delivery system:
Another problem that may occur is drug underdose or overdose, for the following reasons:
 Reservoir depletion, mechanical failure, errors in pump preparation or programming, or errors in refilling of prescribed medications through the side-catheter access port or into pump pocket or subcutaneous tissue; such an underdose or overdose may be fatal.
Reservoir depletion, mechanical failure, errors in pump preparation or programming, or errors in refilling of prescribed medications through the side-catheter access port or into pump pocket or subcutaneous tissue; such an underdose or overdose may be fatal. Rapid pressure change, such as with sky diving, scuba diving, diathermy, or hyperbaric chamber therapy; such an underdose or overdose is temporary. Air travel, bathing, and living at high altitude will not affect pump function.
Rapid pressure change, such as with sky diving, scuba diving, diathermy, or hyperbaric chamber therapy; such an underdose or overdose is temporary. Air travel, bathing, and living at high altitude will not affect pump function.CASE STUDY 16.1 Cancer Pain
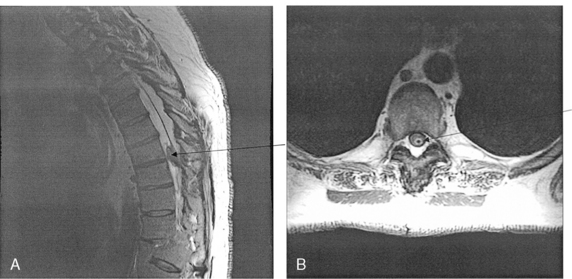
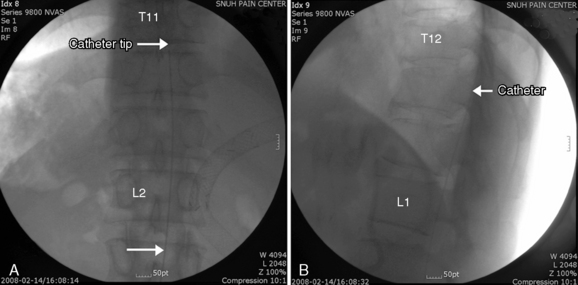
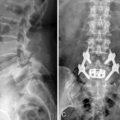
Implantation of the ITDDS was performed 1 day after the test trial of morphine. The skin entry point was the middle of the L4 body, and the intrathecal entry level was L2-L3 (Fig. 16-26). The catheter tip was located at T11 (Fig. 16-27).
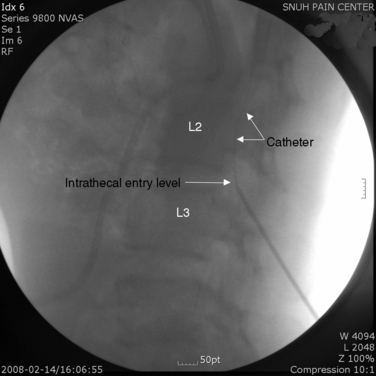
Figure 16–26 Intrathecal entry point at L2/3 disc level (single arrow) and catheter shadow in intrathecal area (split arrow).
CASTE STUDY 16.2 Complex Regional Pain Syndrome
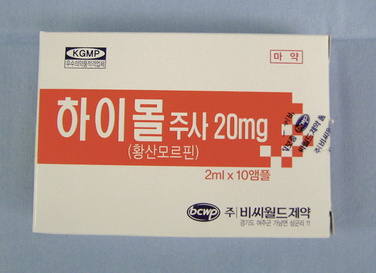
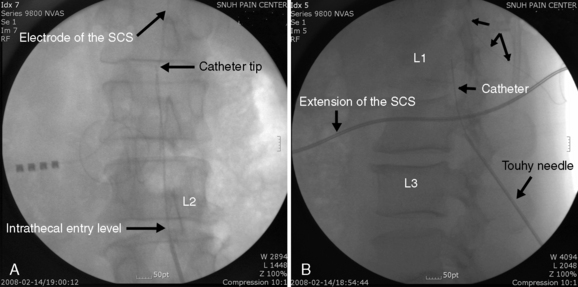
Implantation of the ITDDS was performed one day after the test trial of morphine. The skin entry point is middle of the L4 body and intrathecal entry level was L2-L3. The catheter tip was located at T11 (Fig. 16-28). The total opioid oral intake was calculated to be 154 mg/day of PO morphine: IR codone 30 mg/day PO, hydromorphone 6 mg/day PO, and fentanyl patch (50 μg/hr = 1.2 mg/day of patch). Morphine at 154 mg/day given orally is equivalent to 51 mg/day of IV morphine, 5 mg/day of epidural morphine, and 0.5 mg/day of intrathecal morphine. Highmol (20 mg/2 mL/ampoule) was diluted to 5 mg/mL with a preservative-free 0.9% normal saline, and the initial morphine delivery rate was 0.25 mg/day (half of the usual daily intake of opioids). It was increased gradually up to 1.0 mg/day with an interval of 12 hours, with the patient under close observation for opioid side effects.