CHAPTER 9 Implantation and placentation
IMPLANTATION
Implantation involves the initial attachment of the trophoblastic wall of the blastocyst to the endometrial luminal epithelium. The trophoblast lineage gives rise to three main cell types in the human placenta: the syncytiotrophoblast, which forms the epithelial covering of the villous tree and is the main endocrine component of the placenta; the villous cytotrophoblast cells, which represent a germinative population that proliferate throughout pregnancy and fuse to generate the syncytiotrophoblast; and extravillous trophoblast cells, which are non-proliferative and invade the maternal endometrium. The first two cell lines can be seen from stages 4 and 5 onwards. The cytotrophoblast cells that form the mural and polar trophoblast are cuboidal, and covered externally with syncytial trophoblast (syncytiotrophoblast), a multinucleated mass of cytoplasm that forms initially in areas near the inner cell mass after apposition of the blastocyst to the uterine mucosa (see Fig. 8.10).
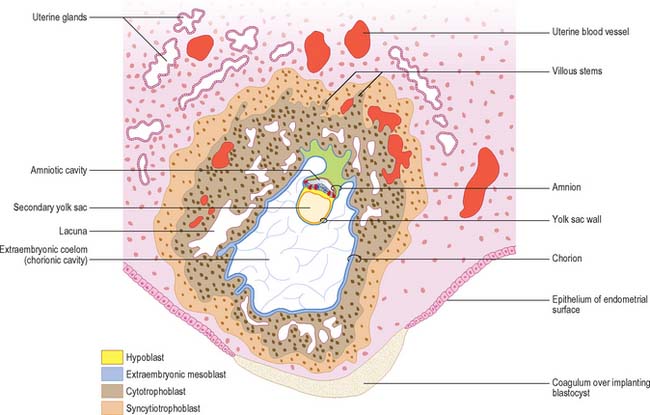
Implantation continues with erosion of maternal vascular endothelium and glandular epithelium, and phagocytosis of secretory products, until the blastocyst occupies an uneven implantation cavity in the stroma (interstitial implantation) (Fig. 9.1). In the early postimplantation phase, the maternal surface is resealed by re-epithelialization and the formation of a plug, which may contain fibrin. As the blastocyst burrows more deeply into the endometrium, syncytial trophoblast forms over the mural cytotrophoblast, but never achieves the thickness of the syncytial trophoblast over the embryonic pole.
Decidual differentiation is not evident in the stroma at the earliest stages of implantation, and it may not be until a week later that fully differentiated cells are present. During decidualization the interglandular tissue increases in quantity. It contains a substantial population of leukocytes (large granular lymphocytes, macrophages and T cells) distributed amongst large decidual cells: the most numerous are uterine natural killer (NK) cells which accumulate in the endometrium during the secretory phase of the cycle and persist until mid-pregnancy. Decidual cells are mesenchymally derived stromal cells which contain varying amounts of glycogen, lipid, and vimentin-type intermediate filaments in their cytoplasm. They are generally rounded, but their shape may vary depending on the local packing density. They may contain one, two or sometimes three nuclei and frequently display rows of club-like cytoplasmic protrusions enclosing granules. The cells are associated with a characteristic capsular basal lamina. Decidual cells produce a range of secretory products, including insulin-like growth factor binding protein 1 (IGF-BP1) and prolactin, which may be taken up by the trophoblast. These secretions probably play a role in the maintenance and growth of the conceptus in the early part of postimplantational development, and can be detected in amniotic fluid in the first trimester of pregnancy.
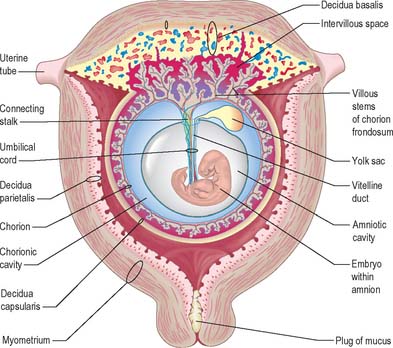
Extracellular matrix, growth factors and protease inhibitors produced by the decidua all probably modulate the degradative activity of the trophoblast and support placental morphogenesis and placental accession to the maternal blood supply. Once implantation is complete, distinctive names are applied to different regions of the decidua (Fig. 9.2). The part covering the conceptus is the decidua capsularis; that between the conceptus and the uterine muscular wall is the decidua basalis (where the placenta subsequently develops); and that which lines the remainder of the body of the uterus is the decidua parietalis. There is no evidence that their respective resident maternal cell populations exhibit site-specific properties.
DEVELOPMENT OF THE PLACENTA
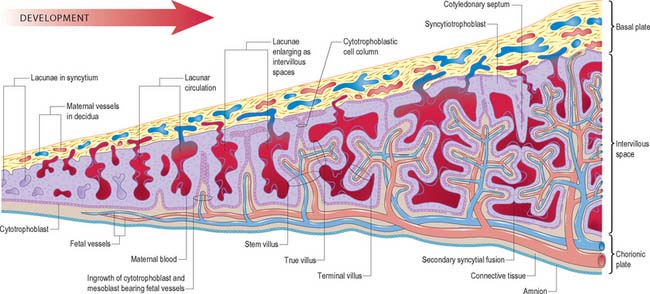
As the blastocyst implants, the syncytiotrophoblast invades the uterine tissues, including the glands and walls of maternal blood vessels (Fig. 9.1), and increases rapidly in thickness over the embryonic pole (Fig. 9.3). A progressively thinner layer covers the rest of the wall towards the abembryonic pole. Microvillus-lined clefts and lacunar spaces develop in the syncytiotrophoblastic envelope (days 9–11 of pregnancy) and establish communications with one another. Initially, many of these spaces contain maternal blood derived from dilated uterine capillaries and veins, as the walls of the vessels are partially destroyed. As the conceptus grows, the lacunar spaces enlarge, and become confluent to form an intervillous space.
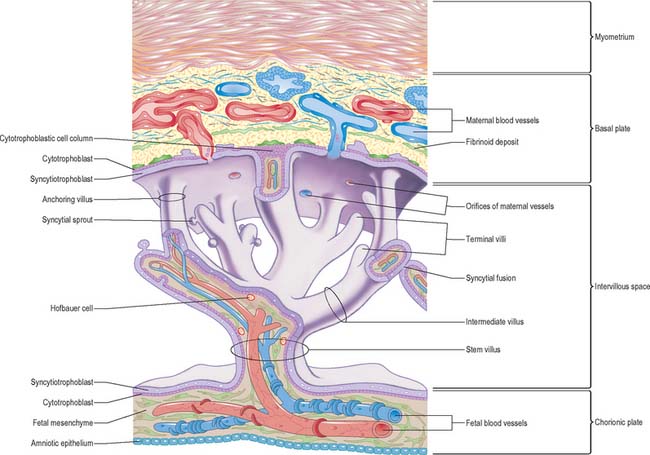
The projections of syncytiotrophoblast into the maternal decidua are called primary villi. They are invaded first with cytotrophoblast and then with extraembryonic mesenchyme (days 13–15) to form secondary placental villi. Fetal capillaries develop in the mesenchymal core of the villi. The cytotrophoblast within the villi continues to grow through the invading syncytiotrophoblast and makes direct contact with the decidua basalis, forming anchoring villi. Further cytotrophoblast proliferation occurs laterally so that neighbouring outgrowths meet to form a spherical cytotrophoblastic shell around the conceptus (Fig. 9.4). Lateral projections from the main stem villus form true and terminal villi.
With the onset of the embryonic heartbeat, a primitive circulation exists between the embryo and the yolk sac, succeeded later by that between the embryo and the placenta. The formed placenta is composed of a chorionic plate on its fetal aspect and a basal plate on the maternal aspect, and an intervening intervillous space containing villous stems with branches in contact with maternal blood (Fig. 9.4). Since maternal blood bathes the surfaces of the chorion which bound the intervillous space, the human placenta is defined as haemochorial. Different grades of fusion exist between the maternal and fetal tissues in many other mammals (e.g. epitheliochorial, syndesmochorial, endotheliochorial). The chorion is vascularized by the allantoic blood vessels of the body stalk, and so the human placenta is also termed chorio-allantoic (whereas in some mammals a choriovitelline placenta either exists alone or supplements the chorio-allantoic variety). In addition, the human placenta is defined as discoidal (in contrast to other shapes in other mammals) and deciduate because maternal tissue is shed with the placenta and membranes at parturition as part of the afterbirth.
Growth of the placenta
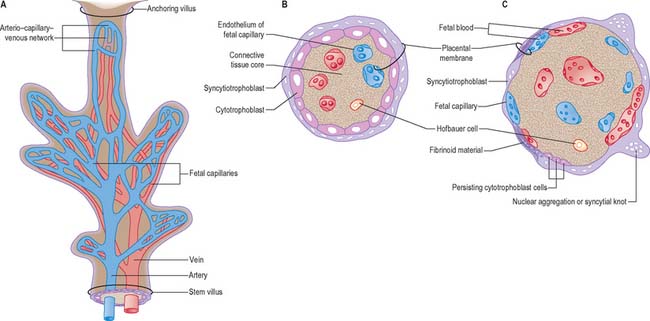
Expansion of the entire conceptus is accompanied by radial growth of the villi and, simultaneously, an integrated tangential growth and expansion of the trophoblastic shell. Eventually each villous stem forms a complex that consists of a single trunk attached by its base to the chorion, from which second and third order branches (intermediate and terminal villi) arise distally. Terminal villi are specialized for exchange between fetal and maternal circulations; each one starts as a syncytial outgrowth and is invaded by cytotrophoblastic cells, which then develop a core of fetal mesenchyme as the villus continues to grow. The core is vascularized by fetal capillaries (i.e. each villus passes through primary, secondary and tertiary grades of histological differentiation). The germinal cytotrophoblast continues to add cells that fuse with the overlying syncytium and so contribute to the expansion of the haemochorial interface. Terminal villi continue to form and branch within the confines of the definitive placenta throughout gestation, projecting in all directions into the intervillous space (Fig. 9.4).
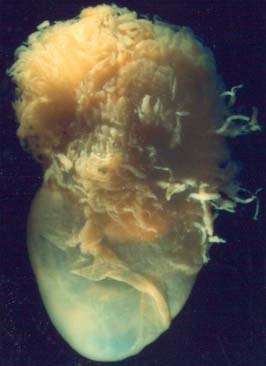
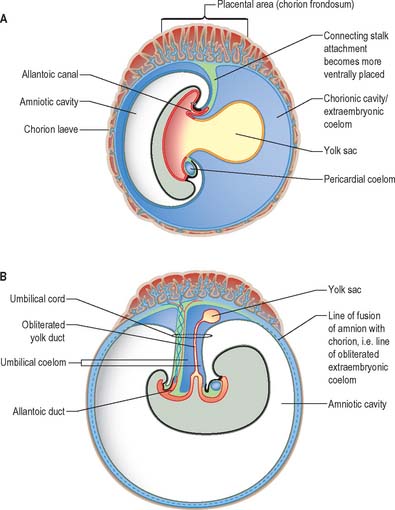
From the third week until about the second month of pregnancy, the entire chorion is covered with villous stems. They are thus continuous peripherally with the trophoblastic shell, which is in close apposition with both the decidua capsularis and the decidua basalis. The villi adjacent to the decidua basalis are stouter, longer and show a greater profusion of terminal villi. As the conceptus continues to expand, the decidua capsularis is progressively compressed and thinned, the circulation through it is gradually reduced, and adjacent villi slowly atrophy and disappear. This process starts at the abembryonic pole, and by the end of the third month, the abembryonic hemisphere of the conceptus is largely denuded. Eventually the whole chorion apposed to the decidua capsularis is smooth and is now termed the chorion laeve. In contrast, the villous stems of the disc-shaped region of chorion apposed to the decidua basalis increase greatly in size and complexity and the region is now termed the chorion frondosum (Fig. 9.3). The chorion frondosum and the decidua basalis constitute the definitive placental site (Fig. 9.2). Abnormalities in this process may account for the persistence of villi at abnormal sites on the chorionic sac and hence the presence of accessory or succenturiate lobes. At term, the placental diameter varies from 200 to 220 mm, the mean placental weight is 470 g, its mean thickness is 25 mm and the total villous surface area is 12–14 m2.
Chorionic plate
The chorionic plate is covered on its fetal aspect by the amniotic epithelium, on the stromal side of which is a connective tissue layer carrying the main branches of the umbilical vessels (Fig. 9.4 and Fig. 9.5). Subjacent to this is a diminishing layer of cytotrophoblast and then the inner syncytial wall of the intervillous space. The connective tissue layer is formed by fusion between the mesenchyme-covered surfaces of amnion and chorion: it is more fibrous and less cellular than Wharton’s jelly (of the umbilical cord), except near the larger vessels. The umbilical vessels radiate and branch from the cord attachment, with variations in the branching pattern, until they reach the bases of the trunks of the villous stems and then arborize within the intermediate and terminal villi. There are no anastomoses between vascular trees of adjacent stems. The two umbilical arteries are normally joined at, or just before they enter, the chorionic plate, by some form of substantial transverse anastomosis (Hyrtl’s anastomosis).
Basal plate
The basal plate, from fetal to maternal aspect, forms the outer wall of the intervillous space. The trophoblast and adjacent decidua are enmeshed in layers of fibrinoid and basement membrane-like extracellular matrix to form a complex junctional zone. In different places the basal plate may contain syncytium, cytotrophoblast or fibrinoid matrix, remnants of the cytotrophoblastic shell, and, at the site of implantation, areas of necrotic maternal decidua (the so-called Nitabuch’s stria) (Figs 9.4 and 9.5). Nitabuch’s stria and the decidua basalis contain cytotrophoblast and multinucleate trophoblast giant cells derived from the mononuclear cytotrophoblast population, which infiltrate the decidua basalis during the first 18 weeks of pregnancy. These cells penetrate as far as the inner one-third of the myometrium, but can often be observed at or near the decidual–myometrial junction. They are not found in the decidua parietalis or the adjacent myometrium, from which it may be inferred that the placental-bed giant cell represents a differentiative end stage in the extravillous trophoblast lineage. The striae of fibrinoid are irregularly interconnected and variable in prominence. Strands pass from Nitabuch’s stria into the adjacent decidua which contains basal remnants of the endometrial glands and large and small decidual cells scattered in a connective tissue framework that supports an extensive venous plexus.
From the third month onwards the basal plate develops placental or cotyledonary septa, which are ingrowths of the cytotrophoblast covered with syncytium that grow toward but do not fuse with the chorionic plate (Fig. 9.4). The septa circumscribe the maternal surface of the placenta into 15–30 lobes, often termed cotyledons. Each cotyledon surrounds a limited portion of the intervillous space associated with a villous trunk from the chorionic plate. From the fourth month these septa are supported by tissue from the decidua basalis. Throughout the second half of pregnancy the basal plate becomes thinned and progressively modified: there is a relative diminution of the decidual elements, increasing deposition of fibrinoid, and admixture of fetal and maternal derivatives.
Intervillous space
The intervillous space contains the main trunks of the villous stems and their arborizations into intermediate and terminal villi (Figs 9.4 and 9.5). A villous trunk and its branches may be regarded as the essential structural, functional and growth unit of the developing placenta.
Recent anatomic and in vivo studies have shown that human placentation is in fact not truly haemochorial in early pregnancy (Jauniaux et al 2003). From the time of implantation, the extravillous trophoblast not only invades the uterine tissues but also forms a continuous shell at the level of the decidua. The cells of this shell not only anchor the placenta to the maternal tissue but also form plugs in the tips of the utero-placental arteries (Burton et al 1999). The shell and the plugs act like a labyrinthine interface that filters maternal blood, permitting a slow seepage of plasma but no true blood flow into the intervillous space. This is supplemented by secretions from the uterine glands, which are discharged into the intervillous space until at least 10 weeks (Burton et al 2002). The comparison of these anatomical features with physiological data obtained in utero reveals that the architecture of the human first trimester gestational sac is designed to limit fetal exposure to oxygen to that which is strictly necessary for its development This creates a physiological placental hypoxia which may protect the developing embryo against the deleterious and teratogenic effects of oxygen, and also a uterine O2 gradient which exerts a regulatory effect on placental tissue development and function. In particular it influences cytotrophoblast proliferation and differentiation along the invasive pathway and villous vasculogenesis. At the end of the first trimester the trophoblastic plugs are progressively dislocated, allowing maternal blood to flow progressively more freely and continuously within the intervillous space. During the transitional phase of 10–14 weeks gestation, two-thirds of the primitive placenta disappears, the chorionic cavity is obliterated by the growth of the amniotic sac, and maternal blood flows progressively throughout the entire placenta (Jauniaux et al 2003). These events bring the maternal blood closer to the fetal tissues, facilitating nutrient and gaseous exchange between the maternal and fetal circulations.
Structure of a placental villus
Chorionic villi are the essential structures involved in exchanges between mother and fetus. The villous tissues separating fetal and maternal blood are therefore of crucial functional importance. From the chorionic plate, progressive branching occurs into the villous tree, as stem villi give way to intermediate and terminal villi. Each villus has a core of connective tissue containing collagen types I, III, V and VI, as well as fibronectin. Cross-banded fibres (30–35 nm) of type I collagen often occur in bundles, whereas type III collagen is present as thinner (10–15 nm) beaded fibres, which form a meshwork that often encases the larger fibres. Collagens V and VI are present as 6–10 nm fibres closely associated with collagens I and III. Laminin and collagen type IV are present in the stroma associated with the basal laminae that surround fetal vessels and also in the trophoblast basal lamina. Overlying this matrix are ensheathing cyto- and syncytiotrophoblast cells bathed by the maternal blood in the intervillous space (Figs 9.4, 9.5 and 9.6). Cohesion between the cells of the cytotrophoblast and also between the cytotrophoblast and the syncytium is provided by numerous desmosomes between the apposed plasma membranes.
FETAL MEMBRANES
The implanting conceptus consists initially of three cavities and their surrounding epithelia. The original blastocyst cavity, surrounded by trophoblast, is now termed the chorionic cavity (synonymous with extraembryonic coelom). It is a large cavity containing the much smaller amniotic cavity and secondary yolk sac (see Fig. 9.1). The apposition of the latter two cavities delineates the extent of the early embryo. The chorionic cavity becomes lined with extraembryonic mesoblast which is also reflected over the outer surface of the amnion and yolk sac. A fourth cavity, the allantois, develops later as a caudal hypoblastic diverticulum that becomes embedded within the extraembryonic mesenchyme, forming the connecting stalk of the embryo. It does not have a direct mesothelial covering.
Chorion
The chorion consists of developing trophoblast and extraembryonic mesothelium. It varies in thickness during development both temporally and spatially. It is thickest at the implantation site throughout gestation as the chorion frondosum and then the placenta, and thinner as gestation progresses over the remainder of its surface as the chorion laevae (Fig. 9.3). At term the chorion consists of an inner cellular layer containing fibroblasts and a reticular layer of fibroblasts and Hofbauer cells, which resembles the mesenchyme of an intermediate villus. The outer layer consists of cytotrophoblast 3 to 10 cells deep, resting on a pseudo-basement membrane, which extends beneath and between the cells. Occasional obliterated villi within the trophoblast layer are the remnants of villi present in the chorion frondosum of the first trimester. Although the interface between the trophoblast and decidua parietalis is uneven, no trophoblast infiltration of the decidua parietalis occurs.
Yolk sac
As the secondary yolk sac forms it delineates a cavity lined with parietal, and perhaps visceral, hypoblast, which is continuous with the developing endoderm from the primitive streak (Ch. 10). The secondary yolk sac is the first structure that can be detected ultrasonographically within the chorionic cavity (Jauniaux et al 1991). Its diameter increases slightly between 6 and 10 weeks of gestation, reaching a maximum of 6–7 mm after which its size decreases.
The inner cells of the yolk sac (denoted endoderm in many studies, although this layer is restricted to the embryo itself) display a few short microvilli and are linked by juxtaluminal tight junctions (Jones & Jauniaux 1995). Their cytoplasm contains numerous mitochondria, whorls of rough endoplasmic reticulum, Golgi bodies and secretory droplets, giving them the appearance of being highly active synthetic cells. With further development the epithelium becomes folded to form a series of cyst-like structures or tubules, only some of which communicate with the central cavity. The cells synthesize several serum proteins in common with the fetal liver, such as alpha-fetoprotein (AFP), alpha-1-antitrypsin, albumin, pre-albumin and transferrin (Jauniaux & Gulbis 2000). With rare exceptions, the secretion of most of these proteins is confined to the embryonic compartments.
The yolk sac becomes coated with extraembryonic mesenchyme, which forms mesenchymal and mesothelial layers. A diffuse capillary plexus develops between the mesothelial layer and the underlying secondary yolk sac wall, and subsequently drains through vitelline veins to the developing liver. The mesothelial layer bears a dense covering of microvilli: the presence of numerous coated pits and pinocytotic vesicles gives it the appearance of an absorptive epithelium (Jones & Jauniaux 1995).
The secondary yolk sac plays a major role in the early embryonic development of all mammals. In laboratory rodents it has been demonstrated as one of the initial sites of haematopoiesis. Recent human data indicate that it has an absorptive role for molecules of maternal and placental origin found in the chorionic cavity (Gulbis et al 1998) and mediates the main movement of molecules passing from the chorionic cavity to the yolk sac and subsequently to the embryonic gut and circulation.
Allantois
The allantoenteric diverticulum (see Fig. 10.1) arises early in the third week as a solid, endodermal outgrowth from the dorsocaudal part of the yolk sac into the mesenchyme of the connecting stalk. It soon becomes canalized. When the hindgut is developed, the proximal (enteric) part of the diverticulum is incorporated in its ventral wall. The distal (allantoic) part remains as the allantoic duct and is carried ventrally to open into the ventral aspect of the cloaca or terminal part of the hindgut (Fig. 9.7A). The allantois is a site of angiogenesis, giving rise to the umbilical vessels which connect to the placental circulation. The extraembryonic mesenchyme around the allantois forms the connecting stalk, which is later incorporated into the umbilical cord.
Amnion (chorio-amnion)
The original amniotic cells develop from the edges of the epiblast of the embryonic disc which ultimately form the interface with the skin at the umbilical region. Between the 10th and 12th weeks of pregnancy the amniotic cavity expands until it makes contact with the chorion to form the chorio-amnion, an avascular membrane which persists to term. The amniotic membrane extends along the connecting stalk and forms the outer covering of the umbilical cord. After birth, the site of this embryonic/extraembryonic junction is important, because the extraembryonic cell lines will die, causing the umbilical cord to degenerate and detach from the body wall. In cases of anomalous development of the ventral body wall, e.g. gastroschisis and exomphalos, the reflections of the amnion along the forming umbilical cord may be incomplete (see below).
AMNIOTIC FLUID
A volume of amniotic fluid in excess of 2 litres is generally considered to be abnormal and constitutes polyhydramnios. A deficiency is termed oligohydramnios and absent amniotic fluid is anhydramnios. (For information about amniotic fluid volume and ranges in gestation consult Brace & Wolf 1989.) Oligohydramnios in the second or third trimester is usually the result of premature rupture of the membranes, uteroplacental insufficiency or urinary tract malformations, e.g. bilateral renal agenesis or obstruction of the lower urinary tract. The major concern with oligohydramnios at less than 20 weeks is the significant risk of pulmonary hypoplasia and neonatal death. The mechanism for the development of pulmonary hypoplasia is poorly understood but loss of lung fluid, and chest compression are contributing factors. Conversely, increased amniotic fluid volume (polyhydramnios) is found essentially in two major circumstances: reduced fetal swallowing or absorption of amniotic fluid and increased fetal urination. Reduced fetal swallowing may be due to congenital malformations e.g. anencephaly, upper intestinal tract obstruction (oesophageal atresia), compressive pulmonary disorders (congenital diaphragmatic hernia) and neuromuscular impairment of swallowing. The cause for abnormal amniotic fluid volume can often be elucidated with detailed prenatal ultrasound examination.
UMBILICAL CORD
The formation of the connecting stalk is described in Chapter 10, and the early formation of the umbilical cord is described on p. 1209. The umbilical cord ultimately consists of an outer covering of flattened amniotic epithelial cells and an interior mass of mesenchyme of diverse origins (Fig. 9.7). It contains two tubes of hypoblastic origin, the vitelline-intestinal and allantoic ducts, and their associated vitelline and allantoic (umbilical) blood vessels. The yolk stalk and continuing duct extend the length of the cord whereas the allantoic duct extends only into its proximal part.
The mesenchymal core is derived from the somatopleuric extraembryonic mesenchyme covering the amniotic folds, splanchnopleuric extraembryonic mesenchyme of the yolk stalk (which carries the vitelline vessels and clothes the yolk duct), and similar allantoic mesenchyme of the connecting stalk (which clothes the allantoic duct and initially carries two umbilical arteries and two umbilical veins). These various mesenchymal compartments fuse and are gradually transformed into the loose connective tissue (Wharton’s jelly) that characterizes the more mature cord. The tissue consists of widely spaced elongated fibroblasts separated by a delicate three-dimensional meshwork of fine collagen fibres, which contains a variety of hydrated glycosaminoglycans, and is particularly rich in hyaluronic acid.
Brace RA, Wolf EJ. Normal amniotic fluid volume changes throughout pregnancy. Am J Obstet Gynaecol. 1989;161:382-388.
Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy; the Boyd Collection revisited. Am J Obstet Gynecol. 1999;181:718-724.
Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002;87:2954-2959.
Gulbis B, Jauniaux E, Cotton F, Stordeur P. Protein and enzyme pattern in the fluid cavities of the first trimester human gestational sac: Relevance to the absorptive role of the secondary yolk sac. Mol Hum Reprod. 1998;4:857-862.
Jauniaux E, Gulbis B, Burton GJ. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus: A review. Placenta-Trophoblast Res. 2003;24:S86-S93.
Jauniaux E, Jurkovic D, Henriet Y, Rodesch F, Hustin J. Development of the secondary human yolk sac: Correlation of sonographic and anatomic features. Hum Reprod. 1991;6:1160-1166.
Jauniaux E, Gulbis B. Fluid compartments of the embryonic environment. Hum Reprod Update. 2000;6:268-278.
Jones CPJ, Jauniaux E. Ultrastructure of the materno-embryonic interface in the first trimester of pregnancy. Micron. 1995;2:145-173.
Takashina S, Ise H, Zhao P, Akraike T, Nikaido T. Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct Funct. 2004;3:13-84.