Chapter 32 Implantable Hearing Devices
SPECIFIC DEVICES
A variety of implantable devices have undergone investigation. Devices that are currently approved and that have been implanted into humans are reviewed here (Table 32-1). Patient selection criteria depend on the type of transducer and the method by which the sound is stimulated.
RION
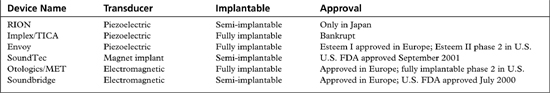
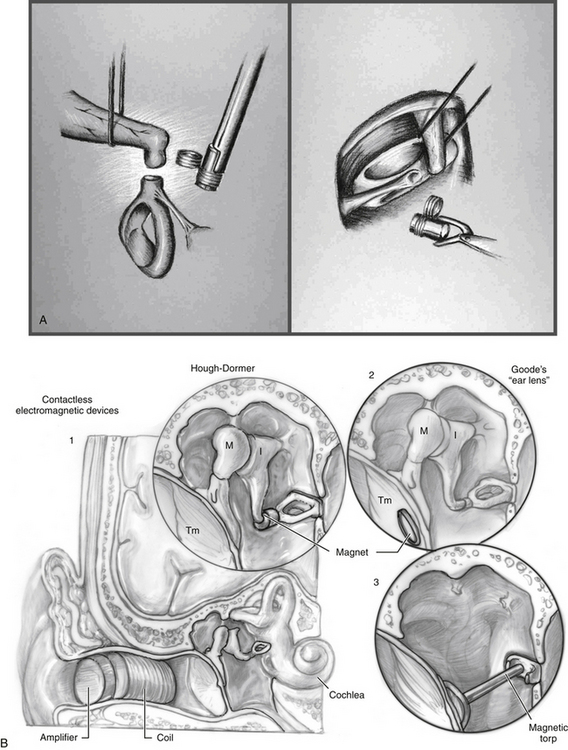
The RION (Fig. 32-1), developed at Ehime University and Teikyo University in Japan in collaboration with the Rion Company by Yanagihara and colleagues, was first implanted in 1984. This is a partially implantable middle ear device that uses a piezoelectric transducer approach. The device consists of a microphone, speech processor, and battery that are contained in an external behind-the-ear unit. The internal component consists of an ossicular vibrator and internal coil, which are coupled. The essential component is the vibratory element consisting of a bimorph, or two piezoelectric ceramic elements pasted together with opposite polarity, which have been coated with layers of biocompatible material. The free end of the bimorph is attached to the stapes, and is attached to a housing unit screwed into the mastoid cortex providing fixation. The bimorph vibrates in response to applied electric current.
The indications for the RION include mixed hearing loss and significant mastoid disease. Its primary use has been in patients with conductive hearing loss from chronic otitis media. Patient selection criteria are listed in Table 32-2. Frequency responses are attenuated after approximately 5000 kHz, so patients with significant sensorineural hearing loss above this frequency may not receive as much benefit as patients with pure conductive hearing loss. The RION has been implanted in patients in Japan, but is not currently available in the United States.
| Average bone conduction speech frequency hearing level (500, 1000, 2000 Hz); ≤50 dB |
| Moderate to severe deafness in contralateral ear |
| Intraoperative vibratory hearing test shows effectiveness of unit |
Totally Implantable Cochlear Amplifier
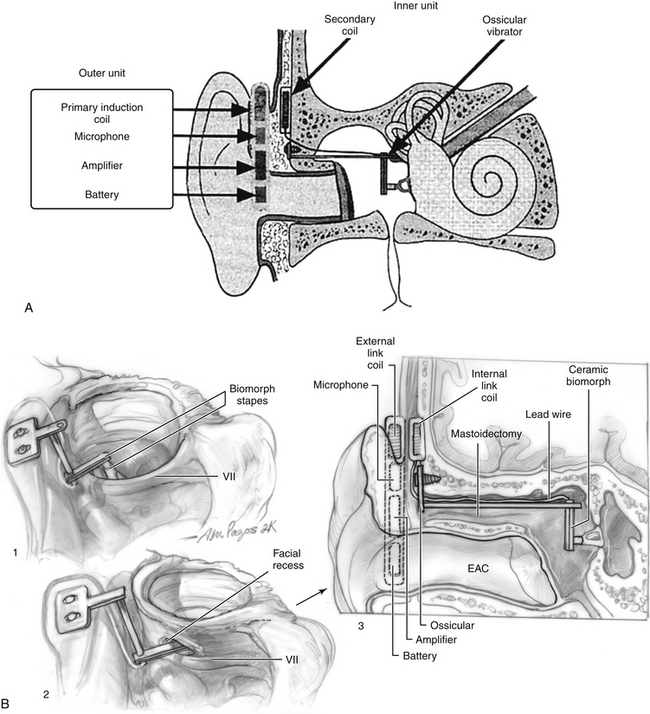
The Totally Implantable Cochlear Amplifier (TICA) device (Fig. 32-2) was developed at the University of Tubingen, Germany, in collaboration with Implex Corporation in Munich in the mid-1990s. The company went bankrupt, and the device is no longer available for use. The TICA is reviewed for historical purposes because it represents the first fully implantable device that was used in humans, and allowed the development of some important concepts that have been used elsewhere. The TICA was a fully implantable device that used a piezoelectric transducer to stimulate the ossicular chain. In addition, it used a microphone implanted in the ear canal that picked up sound. This sensor provided an electric input into the fully implanted unit or can, which was implanted subcutaneously in the mastoid area. The can was a hermetically sealed titanium container that included the speech processor, battery, and a receiving coil. The actuator attached to the body of the incus, causing vibration of the ossicular chain. The induction coil within the titanium can was used to receive electric impulses to permit recharging of the battery.
Vibrant Soundbridge
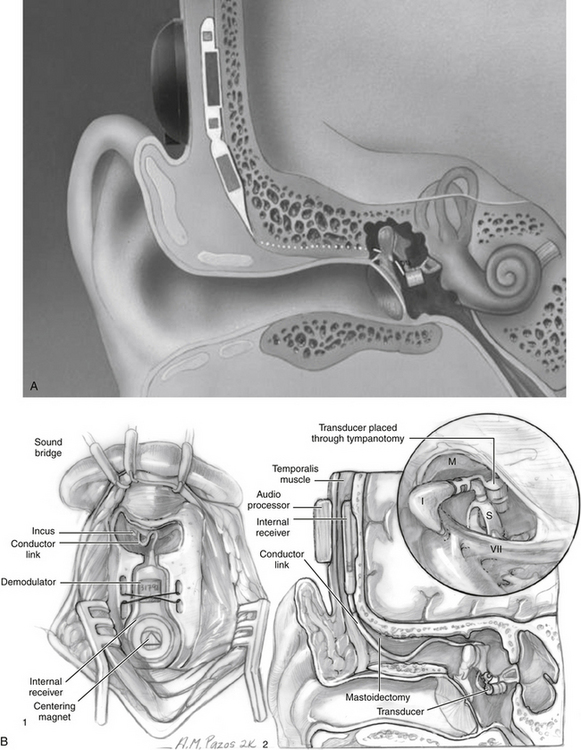
The Vibrant Soundbridge (Fig. 32-3) is the first FDA-approved implantable middle ear hearing device to treat sensorineural hearing loss, and has been implanted in thousands of patients worldwide. It is a partially implantable middle ear hearing device initially developed by Symphonix Devices, Inc. (San Jose, CA). Subsequently, Med-El Corporation of Innsbruck, Austria, took over the production and distribution of the device. The Vibrant Soundbridge is a semi-implantable hearing aid consisting of two parts: the speech processor worn externally, and the implantable vibrating ossicular prosthesis. The vibrating ossicular prosthesis is surgically placed subcutaneously in the postauricular area. The floating mass transducer (FMT) is connected to the internal receiver, and is attached to the stapes. The FMT is a unique electromagnetic transducer that contains a magnet of inertial mass within two electromagnetic coils. When activated, the magnet mass vibrates within the FMT between the two coils causing the entire unit to vibrate. Titanium strips are attached around the long process of the incus to hold the device in place. The FMT is oriented in the direction of the stapes so that the device vibrates directly into the inner ear, parallel to the plane of the stapes. The external auditory processor is held in place over the internal receiver by a magnet.
Placement of the internal device requires an outpatient mastoidectomy similar to cochlear implantation. The facial recess is widely opened to visualize the incudostapedial joint, and to allow the FMT to pass through easily. The FMT is crimped onto the incus after the vibrating ossicular prosthesis is embedded in the cortical bone in a seat behind the mastoid posterior to the sigmoid sinus. The external processor is attached 6 weeks after surgery, at which time the device is programmed. Table 32-3 lists current indications for the Vibrant Soundbridge.
| Adult (≥18 yr) |
| Word recognition score ≥50% |
| Normal middle ear function |
| Realistic expectations |
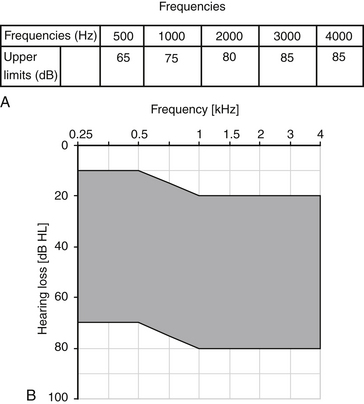
| Pure tone air conduction thresholds within frequencies shown in Figure 32-4 |
Soundtec
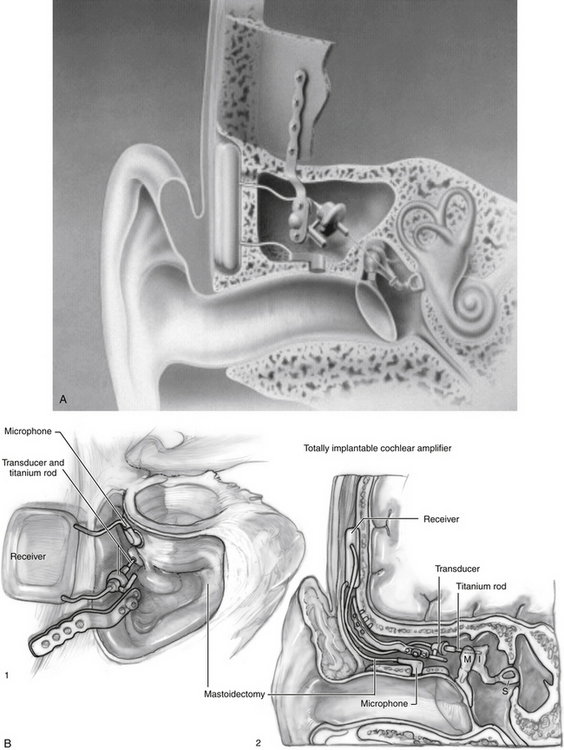
The Soundtec Direct Drive Hearing System (Fig. 32-5) system was developed by Hough of Oklahoma City, Oklahoma. Although new devices are no longer available, it is reviewed for historical purposes because many patients in the United States have this device. The Soundtec used an electromagnetic approach that separated the magnet and the induction coil. A tiny magnet, approximately the size of a grain of rice, was attached to the incudostapedial joint. The magnetic coil, which would drive the magnet, was located in the ear canal in a conventional in-the-ear mold. The sound processor, microphone, and battery were located in a behind-the-ear external unit and were linked to the coil in the external ear canal. The magnet was placed during an outpatient procedure via the transcanal approach, with the tympanic membrane elevated to attach the magnet to the incudostapedial joint A deep-seated ear canal fitting was required for the in-the-ear unit after complete healing occurred.
A total of 103 patients were implanted at 10 sites for the FDA trial before approval was given in 2001 for treatment of sensorineural hearing loss. The Soundtec direct system (see Fig. 32-4B) gave an average of 7.9 dB increase in functional gain over optimally fitted air conduction hearing aids. Patients’ AFAB and speech discrimination scores were much higher for the Soundtec direct system compared with the optimally fitted air conduction hearing aids. This device is no longer available for implantation.
Envoy Esteem
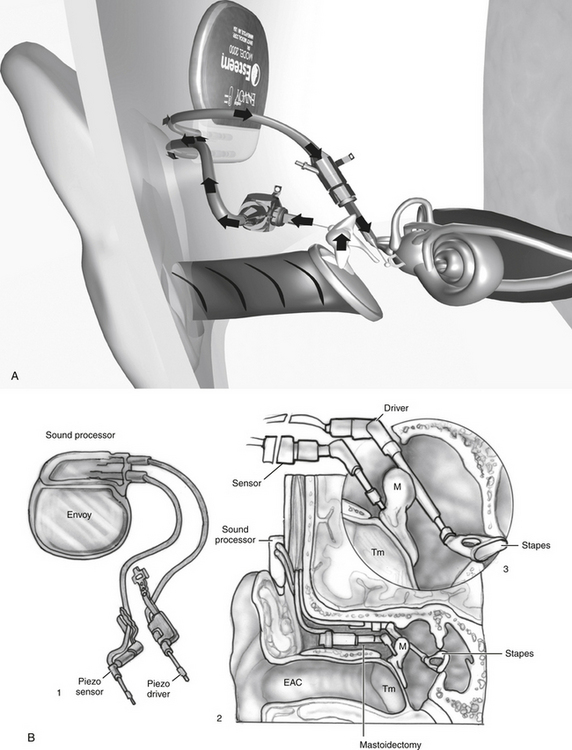
The Envoy Esteem (Fig. 32-6) has been developed by St. Croix Medical, Inc. (Minneapolis, MN). This is a fully implantable system that uses a piezoelectric transducer for reception and transduction. The transducer consists of two internal plates separated by a thin conduction material. The first transducer (sensor) detects movement of the malleus in response to sound stimulation. This sensor acts as a microphone sending signals to the processor. The second piezoelectric transducer (the driver) is placed on the stapes. The piezoelectric units use the bimorph design for the sensor and driver. One of the sensors is fixed to the malleus to detect vibration and stabilized by fixation to the cortical skull bone. A small amplifier in the base increases the gain. The fully implantable device also contains a speech processor powered by a lithium iodine battery.
MET
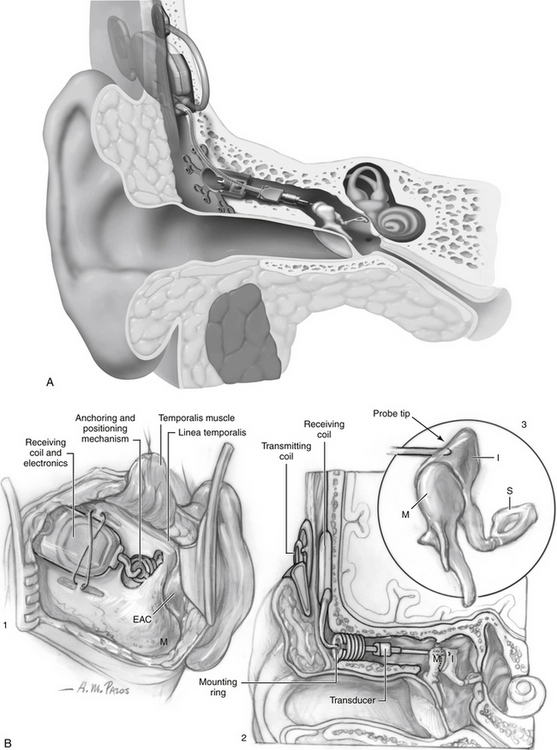
The MET (Fig. 32-7) is a fully implantable ossicular stimulator produced by Otologics (Boulder, CO). The initial device tested was a partially implantable device that consisted of an external digital speech processor and an implanted unit. The external components consisted of a microphone, speech processor, battery, and transmitter that were housed in a disc that fit in the internal implanted device. The external unit is held in place with magnets that align it to the internal unit, similar to a cochlear implant. The implanted components consist of a subcutaneous electric package containing a transcutaneous receiver and a transducer motor in a hermetically sealed case. The electromagnetic motor drives a biocompatible probe tip, which is placed in a hole in the body of the incus. Activation of the device causes mechanical motion of the probe tip, which vibrates the ossicular chain.
1. Fredrickson J.M., Coticchia J.M., Khosla S. Current status in the development of implantable middle ear hearing aids. In: Advances in Otolaryngology. St Louis, Mosby; 1996:189-204. vol. 10
2. Miller D.A., Fredrickson J.M. Implantable hearing aids. In: Valente M., editor. Audiology Treatment. New York: Thieme; 2000:489-510.
3. Wilska A. Einmethode zur bestimmung der horsch wellanamplituden des trommelfells bei verscheiden frequenzen. Skand Arch Physiol. 1935;72:161-165.
4. Rutschmann J. Magnetic audition: Auditory stimulation by means of alternating magnetic fields acting on a permanent magnet fixed to the eardrum. IRE Transactions Med Electron. 1959;6:22-23.
5. Fredrickson J.M., Tomlinson D.R., Davis E.R., Odkuist L.M. Evaluation of an electromagnetic implantable hearing aid. Can J Otolaryngol. 1973;2:53-62.
6. Fredrickson J.M., Coticchia J.M., Khosla S. Ongoing investigations into an implantable electromagnetic hearing aid for moderate to severe sensorineural hearing loss. Otolaryngol Clin North Am. 1995;28:107-120.
7. Maniglia A.J., Ko W.H., Garverick S.L., et al. Semi-implantable middle ear electromagnetic hearing device for sensorineural hearing loss. Ear Nose Throat J. 1997;76:333-338. 340-341
8. Dumon T., Zennaro O., Aran J.M., Bebear J.P. Piezoelectric middle ear implant preserving the ossicular chain. Otolaryngol Clin North Am. 1995;28:173-187.
9. Yanagihara N., Aritomo H., Yamanaka E., Gyo K. Implantable hearing aid: Report of the first human applicatoins. Arch Otolaryngol Head Neck Surg. 1987;113:869-872.
10. Yanagihara N., Sato H., Hinohira Y., et al. Long-term results using a piezoelectric semi-implantable middle ear hearing device: The Rion device E-type. Otolaryngol Clin North Am. 2001;34:389-400.
11. Zenner H.P., Leysieffer H. Total implantation of the Implex TICA hearing amplifier implant for high frequency sensorineural hearing loss: The Tubingen University experience. Otolaryngol Clin North Am. 2001;34:417-446.
12. Luetje C.M., Brackman D., Balkany T.J., et al. Phase III clinical trial results with the Vibrant Soundbridge implantable middle ear hearing device: A prospective controlled multicenter study. Otolaryngol Head Neck Surg. 2002;126:97-107.
13. Colletti V., Soli S.D., Carner M., Colletti L. Treatment of mixed hearing losses via implantation of a vibratory transducer on the round window. Int J Audiol. 2006;45:600-608.
14. Hough J., Dyer R., Matthews P., Wood M. Early clinical results: SOUNDTEC implantable hearing device phase II study. Laryngoscope. 2001;111:1-8.
15. First chronic implant performed. Envoy-Voices. Minneapolis, MN: St. Croix Medical; March 2000.